- Department of Anesthesiology, Chongqing University Cancer Hospital, Chongqing, China
Introduction: This study aimed to extract, evaluate, and summarize the evidence related to the management of sleep disorders after malignant tumor surgery, providing a reference for evidence-based clinical practice.
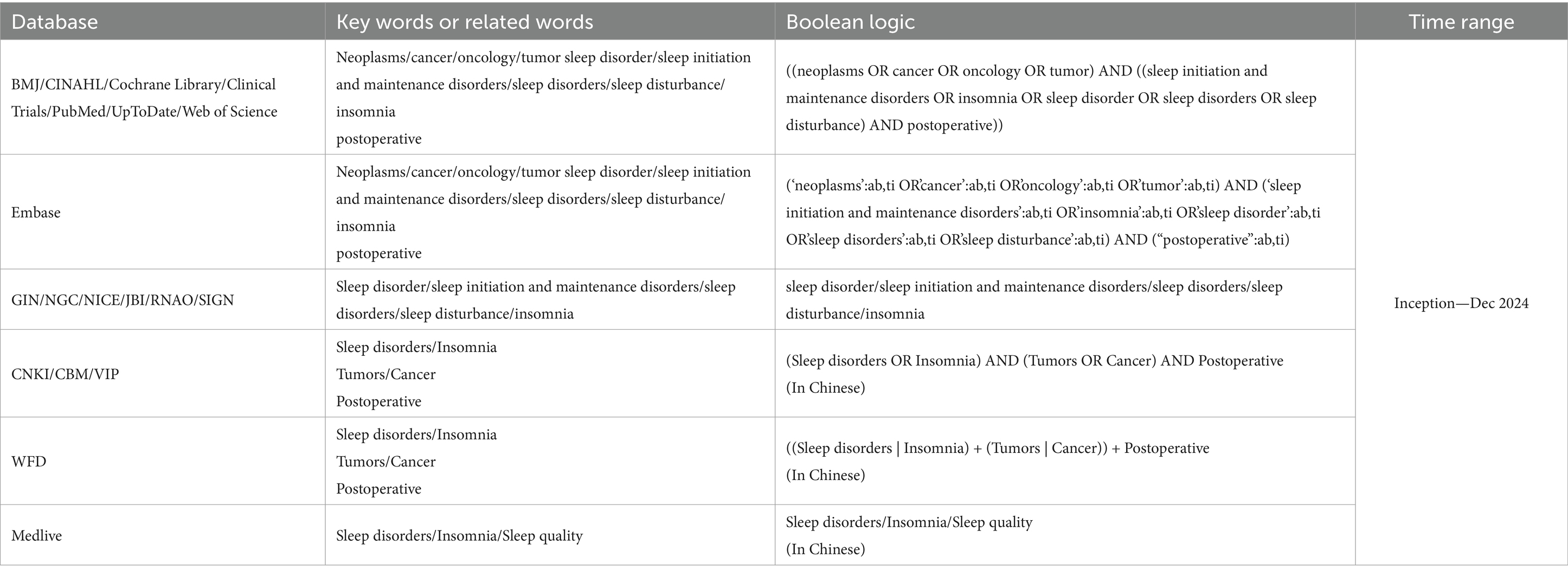
Methods: A systematic and hierarchical search strategy was used to identify relevant evidence from authoritative databases and resources. Following the “6S” evidence pyramid model, we conducted a comprehensive review of the following databases: BMJ Best Practice, CINAHL, Cochrane Library, Clinical Trials, Embase, PubMed, UpToDate, Web of Science, Guidelines International Network, National Guideline Clearinghouse, and the National Guidelines Database. Additionally, key institutional websites and specialized databases were consulted, such as the’ National Institute for Health and Care Excellence in the United Kingdom, ‘the Joanna Briggs Institute Centre for Evidence-Based Health Care in Australia, ‘the Registered Nurses’ Association of Ontario in Canada, the Scottish Intercollegiate Guidelines Network, China National Knowledge Infrastructure, China Biomedical Literature Database, Wan Fang Data, VIP Database, and Medlive. The search included clinical decisions, evidence summaries, guidelines, recommended practices, expert consensuses, systematic reviews, and randomized controlled trials. The retrieval period spanned from the inception of each database to December 31, 2024. Two researchers trained in evidence-based nursing independently evaluated the quality of the literature, extracted and synthesized the evidence, and incorporated expert recommendations as appropriate. This rigorous approach ensured comprehensive coverage of international and regional evidence-based resources, providing a solid foundation for our research.
Results: Finally, we screened 12 articles with high-quality results (including 10 guidelines and 2 expert consensuses), providing 37 pieces of evidence covering four aspects: risk factors, evaluation methods, intervention measures, and effect evaluation after intervention.
Discussion: The summarized evidence offers a reference for clinicians in managing sleep disorders in patients after malignant tumor surgery. However, the selection and application of evidence should be combined with specific circumstances to improve the postoperative rehabilitation of patients with malignant tumors.
1 Introduction
Sleep is a fundamental physiological requirement for human survival, essential for maintaining immune function, cognitive performance, emotional regulation, and physical recovery (1). Sustaining optimal sleep quality is particularly important during periods of physiological stress, such as surgery or illness.
Sleep disorders comprise a group of conditions characterized by disruptions in sleep quantity, quality, timing, or related behaviors that result in daytime impairment or distress (2). These conditions can be either acute or chronic and are typically categorized into insomnia, hypersomnia, circadian rhythm sleep–wake disorders, and sleep-related movement or respiratory disorders (2–4). According to the International Classification of Sleep Disorders, Third Edition (ICSD-3), acute sleep disturbances are often triggered by identifiable stressors and generally resolve within 3 months. In contrast, chronic disturbances persist beyond this period and are frequently associated with maladaptive cognitive and behavioral responses (5, 6).
Among patients with malignant tumors, sleep disturbances are highly prevalent and may be exacerbated by surgery, chemotherapy, radiotherapy, and the hospital environment (7). Insomnia is the most frequently reported subtype, particularly during the postoperative period. Contributing factors include pain, opioid use, environmental stimuli such as noise and light, and insufficient clinical attention to sleep-related issues (8–10). Studies have demonstrated that the prevalence of sleep disturbances varies depending on the type of surgery and the underlying malignancy (8, 9).
The pathophysiology of postoperative sleep disturbances is multifactorial. Biologically, surgical stress and sleep deprivation can trigger inflammatory responses, resulting in elevated levels of cytokines such as IL-1β, TNF-α, and IL-6, which negatively affect sleep quality (6, 11). Psychologically, factors such as anxiety, depression, and maladaptive coping mechanisms further contribute to the persistence of insomnia (5, 6). The “3-P” model—comprising predisposing, precipitating, and perpetuating factors—illustrates the complex interplay between biological vulnerability, perioperative stressors, and behavioral maladaptation in the development of chronic insomnia.
Both acute and chronic sleep disturbances after surgery have been associated with adverse clinical outcomes, including increased pain perception, delayed recovery, neurological complications, emotional distress, and reduced patient satisfaction (1, 12, 13). These consequences underscore the importance of early identification and targeted management of sleep disorders—particularly insomnia—in postoperative cancer care.
Nevertheless, the existing evidence on the management of postoperative sleep disturbances in cancer patients remains fragmented. Most available studies are observational in nature, involve small sample sizes, and exhibit considerable variability in assessment tools and intervention strategies. This inconsistency hinders the development of standardized clinical protocols and evidence-based recommendations.
Comprehensive and systematically developed evidence summaries are therefore urgently needed to support clinical decision-making and to establish a robust, unified foundation for practice. This study aimed to compile one of the most comprehensive available evidence on the management of sleep disorders following malignant tumor surgery. This study was registered through the Evidence-Based Nursing Center of Fudan University (registration number: ES20257203).
2 Materials and methods
2.1 Problem establishment
We employed the PIPOST tool developed by the Evidence-Based Nursing Center of Fudan University to construct our evidence-based question. P (Population): Patients diagnosed with malignant tumors undergoing surgery; I (Intervention): Management strategies for postoperative sleep disorders, including assessment and intervention; P (Professional): Physicians and nurses who implement the evidence; O (Outcome): Improvement of sleep quality and related symptoms; S (Setting): Clinical inpatient settings; T (Type of evidence): Clinical guidelines, expert consensuses, and systematic reviews. This structured approach ensured that our evidence synthesis remained patient-centered and clinically applicable (42, 43).
2.2 Evidence retrieval strategy
Following the “6S” evidence resource pyramid model (14), a structured and systematic search was conducted across multiple authoritative databases and resources, adhering to a hierarchical approach. The search encompassed the BMJ Best Practice, CINAHL, Cochrane Library, ClinicalTrials.gov, Embase, PubMed, UpToDate, Web of Science, Guidelines International Network, National Guideline Clearinghouse, and National Guidelines Database. Additionally, institutional archives and specialized databases were reviewed, including materials from the National Institute for Health and Care Excellence in the United Kingdom, Joanna Briggs Institute (JBI) Centre for Evidence-Based Health Care in Australia, Registered Nurses’ Association of Ontario in Canada, and the Scottish Intercollegiate Guidelines Network. For Chinese sources, we reviewed the China National Knowledge Infrastructure, China Biomedical Literature Database, Wanfang Data, VIP Database, and Medlive to ensure comprehensive coverage. The search period extended from the establishment of the database to December 31, 2024 (Table 1). When searching domestic and international websites or databases, the Chinese search terms included “Sleep disorders/insomnia, tumors/cancer, and postoperative,” whereas the English search terms were “neoplasms/cancer/oncology/tumor, sleep initiation and maintenance disorders/insomnia,” and “initiation and maintenance disorders/insomnia/sleep disorder/sleep disorders/sleep disturbance, postoperative.” Taking PubMed as an example, combination of subject terms and free-text words, the search formula: ((neoplasms OR cancer OR oncology OR tumor) AND ((sleep initiation and maintenance disorders OR insomnia OR sleep disorder OR sleep disorders OR sleep disturbance) AND postoperative)). To ensure a comprehensive and inclusive retrieval of evidence, the term “sleep disorders” was defined broadly to encompass any condition or symptom involving disturbances in sleep initiation, maintenance, duration, quality, or circadian regulation that result in daytime dysfunction. This included, but was not limited to, insomnia, poor sleep quality, sleep fragmentation, excessive daytime sleepiness, and circadian rhythm disruption (2). We adopted this inclusive approach to capture diverse clinical terminologies used in practice and across different guidelines, rather than relying solely on one diagnostic classification system.
2.3 Inclusion and exclusion criteria
2.3.1 Inclusion criteria
1. Studies focusing on patients with malignant tumors who underwent surgical intervention.
2. Evidence pertaining to the recognition of risk factors, evaluation techniques, management strategies, and assessment of their impact on postoperative sleep disturbances in patients with malignant tumors.
3. Acceptable categories of evidence encompass clinical practice guidelines, expert consensus documents, evidence synopses, and systematic reviews.
2.3.2 Exclusion criteria
(1) Literature that has been repeatedly published or updated; (2) studies with incomplete or inaccessible data; (3) Publications of low quality based on established evaluation criteria; (4) Documents that are guideline interpretations or implementation plans.
2.4 Data extraction and synthesis strategy
Two researchers trained in evidence-based systems independently completed an assessment of the quality of the included literature based on predetermined inclusion and exclusion criteria. Their assessments were cross-verified, and discrepancies between the two evaluators were resolved through consultation with a third expert in evidence-based medicine. Two researchers who systematically studied the evidence-based nursing course evaluated the included literature and cross-checked it using appropriate evaluation tools. Any disagreements were resolved by a third expert in evidence-based medicine, who reached a consistent conclusion.
We used thematic analysis to identify and summarize recurring topics across the included guidelines and expert consensus documents. Four major themes were extracted: risk factors, assessment methods, intervention strategies, and evaluation.
The extracted content included: (1) evidence-based recommendations related to the management of sleep disorders following malignant tumor surgery; (2) classification of each recommendation into one of four domains—risk factors, assessment tools, intervention strategies, and outcome indicators; (3) the level and grading of evidence, if available; and (4) reported or expected outcomes such as improvements in sleep quality, reduction in insomnia symptoms, and enhancement of postoperative recovery (e.g., pain relief, psychological well-being, or fatigue reduction). In this review, “relevant evidence” is defined as any recommendation that addresses sleep-related disturbances—such as insomnia, fragmented sleep, reduced sleep quality, or circadian disruption—within the perioperative or cancer care setting. For synthesis, a thematic categorization approach was used. All extracted recommendations were grouped into the four main evidence domains. Within each domain, recommendations were further organized according to the type of intervention (e.g., cognitive, behavioral, pharmacological) or evaluation method. Overlapping evidence from multiple sources was consolidated, while unique recommendations were retained as supplementary guidance.
2.5 Evidence quality evaluation criteria
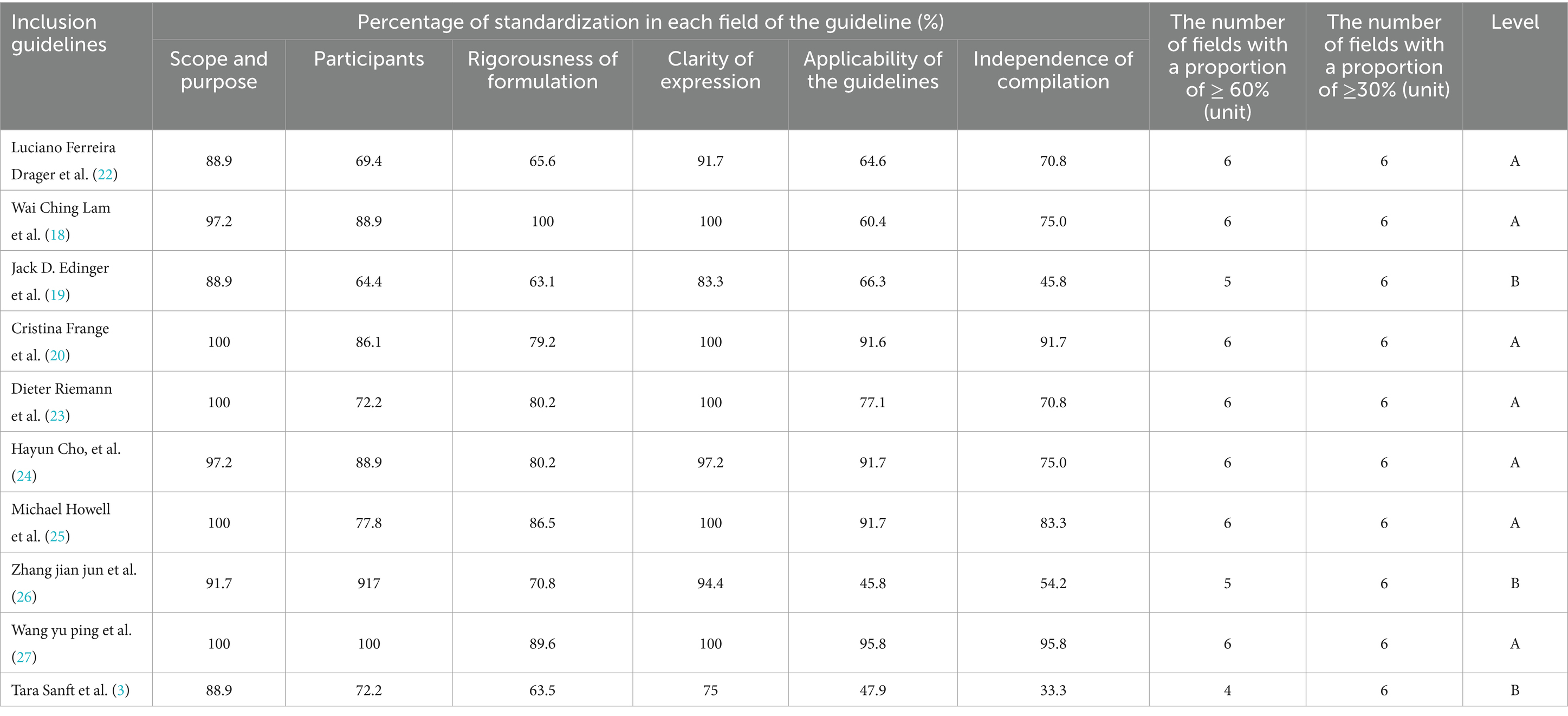
The quality of the guidelines was assessed using the AGREE II guideline Quality Assessment form (15). Agree II was revised in 2009 and updated in 2017 by the International Working Group for Research and Evaluation of Clinical Guidelines, comprised researchers from 13 countries, including Canada and the United Kingdom. This tool builds on the AGREE issued in 2003. The AGREE II comprises 23 items across six domains, with each item rated on a seven-point scale (1 = strong disagreement and 7 = strong agreement). The standardized score for each domain was calculated using the formula: [(actual score − minimum possible score) / (maximum possible score − minimum possible score)] × 100. All domain scores were evaluated collectively, and a unified threshold for the six domains was established through expert consensus. Guidelines received a quality rating of A if the standardized score in each domain was 70% or higher. If the standardized score ranged between 30 and 70%, the guideline quality was rated as B. To further enhance clarity and reduce redundancy, the quality rating criteria were reorganized as follows: guidelines earned an A rating when all six domains achieved a standardized score of at least 70%. Conversely, guidelines with a standardized score within the range of 30–70% in any domain were assigned a B rating. In this summary of evidence, the literature that failed to reach the level of grade B or above has been excluded.
The quality of the included guidelines was assessed using the JBI Center for Evidence-Based Health Care Expert Consensus Evaluation Criteria (2017 edition), which consists of six items and employs a grading system of “yes,” “no,” “unclear,” and “not applicable” (16). For systematic evaluation, the 2014 edition of the JBI Evidence-Based Practice Center Standards was used. This tool includes 11 items, each evaluated based on the criteria of “yes,” “no,” “unclear,” or “not applicable” (17). The evidence was synthesized by tracing back to the original source documents, and the appropriate JBI Center for Evidence-Based Health Care (2016 edition) tool was selected for quality assessment based on literature type.
Calculate the proportion of “Yes” responses and determine the overall rating based on the following criteria:
High quality (≥5 items rated “Yes”): The guideline meets quality standards in most key areas and is recommended for inclusion in the analysis.
Moderate quality (3–4 items rated “Yes”): The guideline meets quality standards in some areas but may have certain limitations, requiring cautious interpretation.
Low quality (≤2 items rated “Yes”): The guideline does not meet quality standards in most areas and is not recommended for inclusion in the analysis.
3 Results
3.1 Literature search and basic characteristics of the included literature
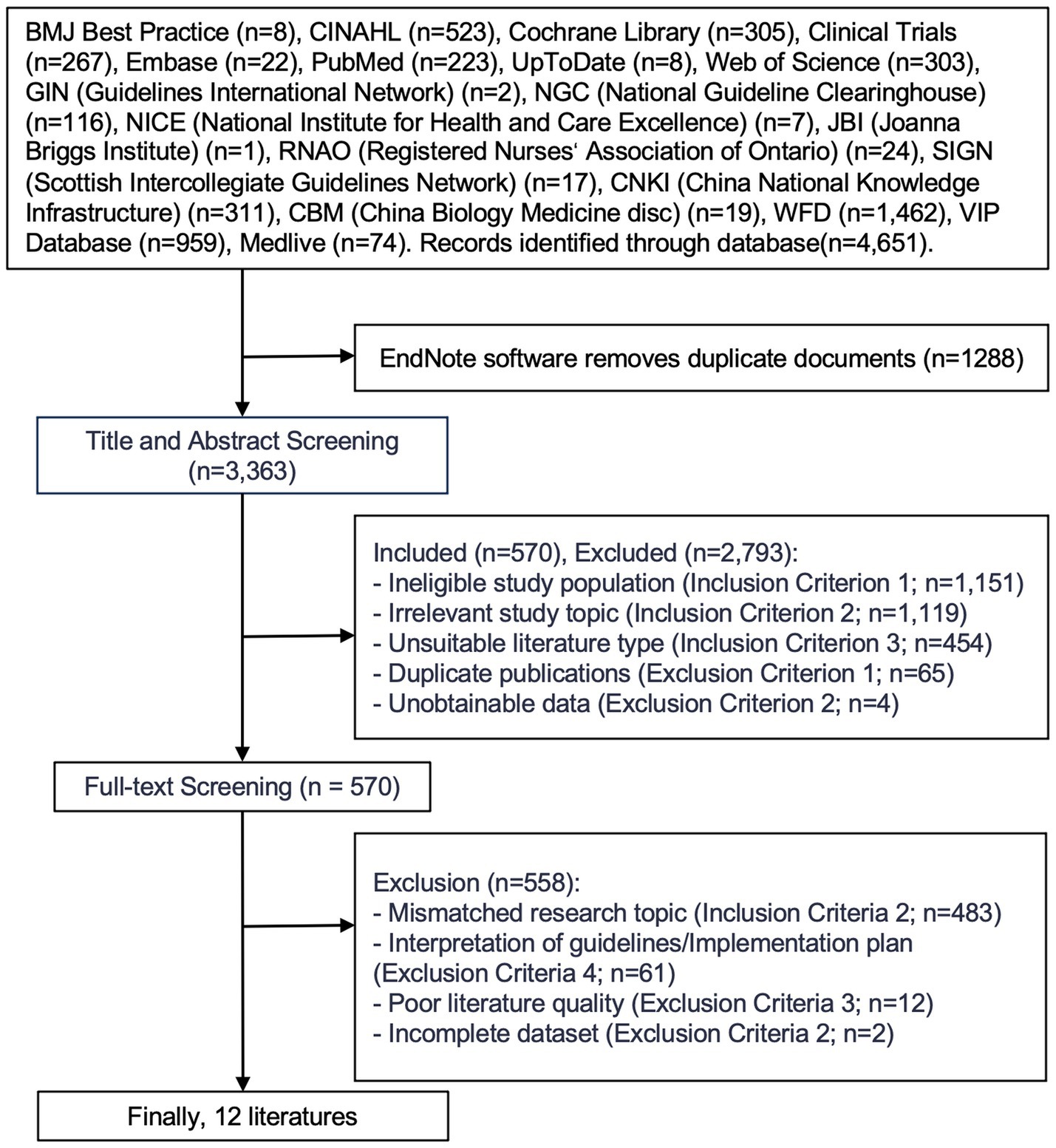
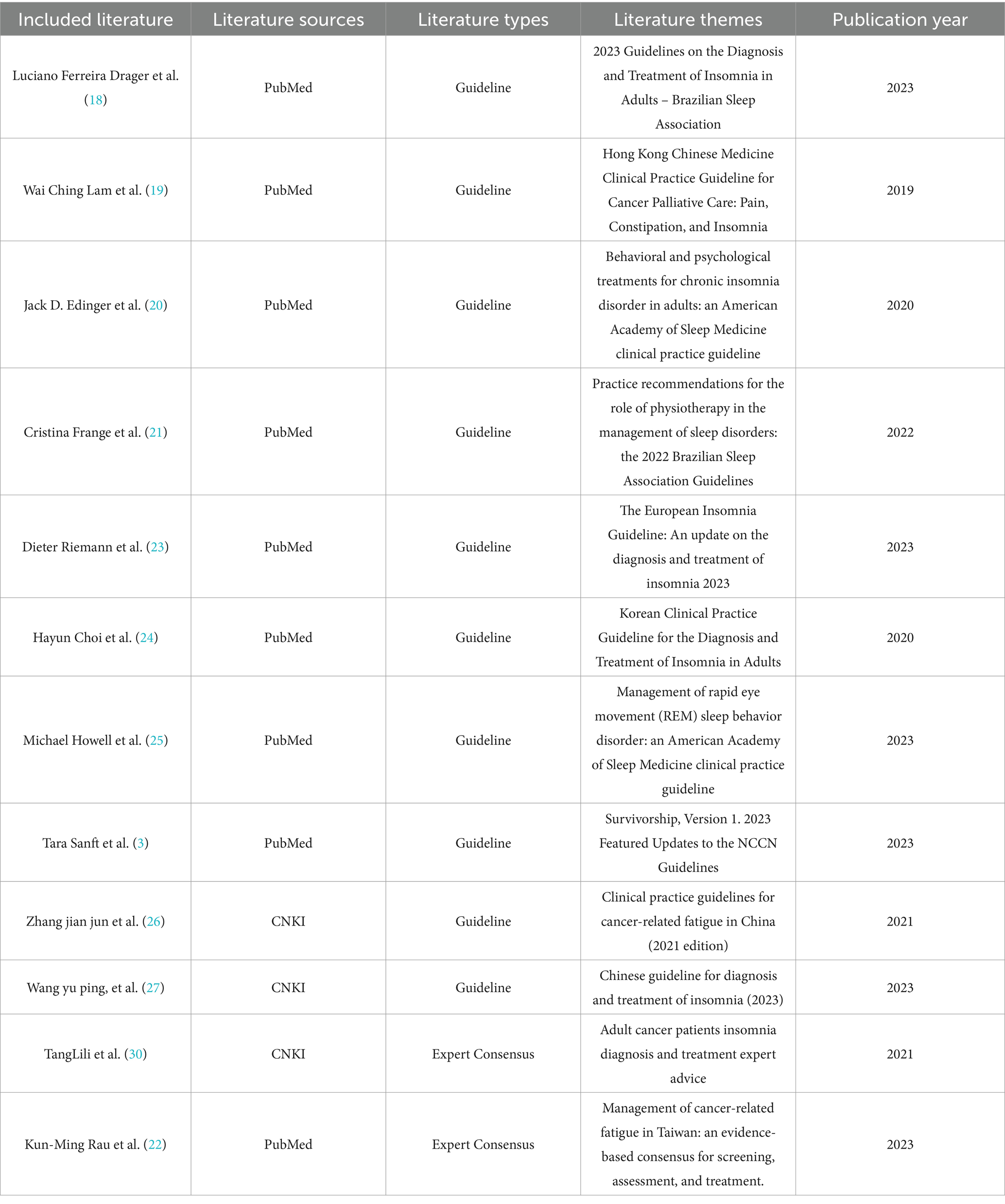
In total, 4,651 articles were initially identified through database searching. After removing duplicates, 3,363 articles remained. Following a review of titles and abstracts, 570 articles were selected for further screening. After a full-text review based on the inclusion and exclusion criteria, 12 articles were finally included in this review, consisting of 10 clinical practice guidelines and 2 expert consensus statements. The literature screening process is illustrated in Figure 1, and the main characteristics of the included studies are summarized in Table 2.
The included publications were issued between 2019 and 2023, with the majority published in 2023 (n = 5), reflecting the timeliness and clinical relevance of the evidence. In terms of source distribution, nine articles were retrieved from PubMed, and three from CNKI, representing a blend of international and Chinese literature. These documents cover a wide range of themes related to postoperative sleep disturbances in patients with malignant tumors, including the diagnosis and treatment of insomnia, cancer-related fatigue, REM sleep behavior disorder, and comprehensive survivorship care. They were issued by authoritative institutions such as the American Academy of Sleep Medicine, the Brazilian Sleep Association, the National Comprehensive Cancer Network (NCCN), and national traditional Chinese medicine organizations, offering a diverse and multidisciplinary perspective on the subject.
3.2 Quality evaluation results of the included articles
3.2.1 Quality evaluation of clinical guidelines
Ten guidelines were included (3, 18–27). The evaluation results showed that the literature writing process was rigorous and the content was detailed, with a high overall quality, and all were eligible for inclusion (Table 3).
3.2.2 Quality evaluation of expert consensus
Two expert consensus documents were included (22, 27). Two expert consensus were evaluated using the JBI 2016 version of the expert consensus evaluation tool, and all six items were rated as “yes.” These expert consensuses had a high quality and were eligible for inclusion.
3.3 Evidence summary
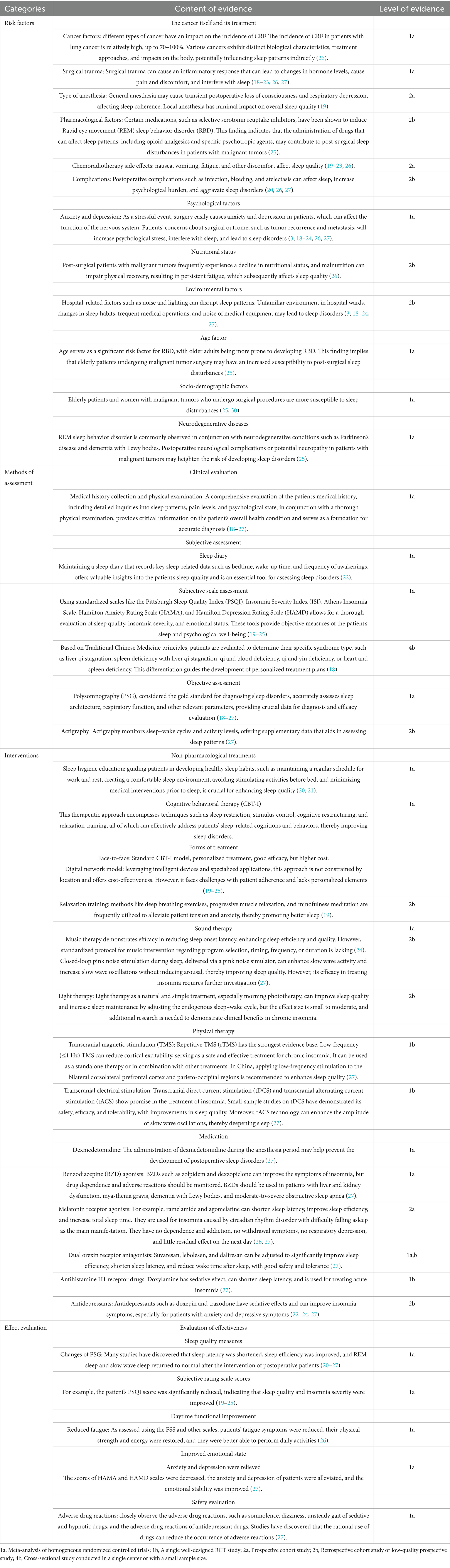
A total of 12 studies were included in the initial draft of the evidence summary (3, 18–27), from which 37 best-evidence statements were ultimately synthesized. The evidence spans four major domains: risk factors, assessment methods, intervention strategies, and evaluation of intervention outcomes. Table 4 provides a detailed breakdown of the extracted evidence. The levels of evidence were classified as follows: 1a-meta-analysis of homogeneous randomized controlled trials (RCTs); 1b-a single, well-designed RCT; 2a-prospective cohort study; 2b-retrospective cohort study or low-quality prospective study; and 4b-single-center or small-sample cross-sectional study.
Within the domain of risk factors, the literature identified both biological and psychosocial contributors to postoperative sleep disturbances in patients with malignant tumors (18–21). Key biological and treatment-related factors included cancer type, surgical trauma, anesthesia technique, pharmacological side effects (e.g., opioids, selective serotonin reuptake inhibitors), complications, and side effects of chemo-or radiotherapy. Psychosocial and environmental contributors such as anxiety, depression, nutritional status, hospital environment, and age-related vulnerability—particularly to REM sleep behavior disorder—were also frequently highlighted (18, 20, 22, 23). The level of supporting evidence for these factors ranged from 1a to 2b.
Assessment methods were divided into subjective and objective approaches. Subjective tools included sleep diaries and standardized instruments such as the Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD) (18, 19, 23). Objective assessments primarily included polysomnography (PSG)—regarded as the gold standard—and actigraphy (21, 24). In Chinese clinical guidelines, Traditional Chinese Medicine (TCM) syndrome differentiation was also used to guide personalized assessment and treatment (26).
Intervention strategies involved a combination of non-pharmacological and pharmacological approaches. Non-pharmacological treatments included cognitive behavioral therapy for insomnia (CBT-I), relaxation training, music therapy, and light therapy (19, 21, 22, 25). Physical therapies such as transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tDCS, tACS) were also reported (22, 25, 27). Pharmacological interventions included dexmedetomidine, benzodiazepine receptor agonists, melatonin receptor agonists, dual orexin receptor antagonists, antihistamines, and antidepressants (20, 21, 23).
The effectiveness of these interventions was evaluated based on improvements in PSG parameters, reductions in subjective sleep and emotional scores, relief of fatigue symptoms, and enhancement of daytime functioning (18, 22, 23, 25). Additionally, several studies emphasized the importance of monitoring adverse drug reactions to ensure treatment safety (20, 21).
In summary, the included studies provide comprehensive, high-quality, and multidisciplinary evidence for the assessment and management of sleep disturbances in postoperative cancer patients, thereby supporting evidence-based clinical decision-making (3, 18–27).
4 Discussion
4.1 Evidence synthesis process
This study adhered to stringent scientific standards, yielding high-quality evidence to guide clinical nursing practices. All research team members received comprehensive training in evidence-based nursing methodologies. Two researchers independently conducted evidence retrieval, quality assessment, data extraction, and evidence grading to ensure objectivity and reliability. Furthermore, sleep specialists oversaw quality control and reviewed the evidence to maintain scientific rigor of the synthesis process. Two expert consensuses were included, meeting all quality criteria. Ten guidelines were also included, and international peers rigorously reviewed and validated the evidence, ensuring their robustness and credibility (3, 18–27). These studies addressed risk factors, assessment methods, intervention strategies, and evaluation of sleep disorders following malignant tumor surgery. The research team critically evaluated the strengths and limitations of the evidence while considering the clinical context. Through a comprehensive analysis and synthesis, 37 optimal pieces of evidence were identified for managing postoperative sleep disorders. This evidence provides precise and scientifically sound guidance for enhancing the management of post-surgical sleep disorders, ultimately improving patient care and sleep quality. These four themes provide a structured and comprehensive overview of the current evidence and practical recommendations, contributing to a clearer understanding of postoperative sleep disorder management.
4.2 Early identification of risk factors for postoperative sleep disorders in patients with malignant tumors is essential
Postoperative sleep disturbance in patients with cancer arise from multiply risk factors. Surgical intervention, chemotherapy, radiotherapy, and multiple therapeutic modalities can contribute to the onset and worsening of sleep disorders over time (7). Certain adjuvant medications may also impact sleep patterns; however, their influence generally diminishes as treatment progresses (28). Psychological factors, such as pre-existing anxiety and depression, significantly impair sleep quality, particularly if these conditions are present before the initiation of cancer treatment. Implementing emotion regulation strategies can help mitigate sleep disturbances (29). Regarding sleep-related cognition and behavior, negative thought patterns, including excessive worry before bedtime and catastrophic thinking, can lead to chronic insomnia (30, 31). Demographic characteristics also exert a substantial influence; with women, younger individuals, unemployed populations, and those from lower-income households being more susceptible. The impact of educational attainment on sleep outcomes remains inconsistent, potentially owing to variations in study populations, sample sizes, and treatment methods. Disease-specific factors and pre-treatment health status are critical determinants of sleep quality. More advanced disease stages were correlated with more severe sleep disturbances. Additional contributing factors include regional lymph node metastasis, pretreatment use of analgesics, higher number of comorbidities, poorer functional status, elevated body mass index, and preexisting sleep issues (29). Given the complexity of these risk factors, healthcare providers should leverage the evidence presented here for early identification. Targeted preventive measures should be implemented to address modifiable risk factors and enhance patient wellbeing.
4.3 Comprehensive assessment through subjective and objective evaluations
Evaluating sleep disorders encompass subjective and objective evaluations. Currently, most studies use subjective scales for evaluation. Among the various assessment tools, the Pittsburgh sleep quality index (PSQI) developed by Buysse et al. (32) is the most widely utilized internationally and domestically. Additionally, Lee’s general sleep disturbance scale (GSDS) is frequently employed and has demonstrated robust reliability and validity in cancer patient populations (33). Unlike the PSQI, the GSDS employs an 8-point scoring system that allows it to detect changes across different sleep factors more sensitively. Several studies have utilized the insomnia severity index and the Athens insomnia scale to assess insomnia. The insomnia severity index consists of seven items and focuses on assessing the severity of insomnia, emphasizing its adverse psychological impact. It has shown strong reliability and validity in patients with malignant tumors. The Athens insomnia scale, which includes eight items, primarily evaluates sleep quality, duration, and daytime dysfunction caused by insomnia (34). Its concise and practical nature enables accurate identification of insomnia cases. Polysomnography (PSG) provides comprehensive sleep measurements and is critical for diagnosing complex sleep disorders. However, PSG is generally unnecessary for evaluating insomnia symptoms and is unsuitable for the routine assessment of chronic insomnia. PSG should be considered in pathological sleepiness or report other sleep-related pathologies, such as sleep breathing disorders, periodic limb movements, or parasomnias (35). Some studies have combined objective measurement methods such as wrist actigraphy with subjective assessments using the PSQI to dynamically monitor patients’ sleep conditions and gather more comprehensive and objective data. However, most studies rely solely on subjective scales because of time and financial constraints, often lacking integration with objective assessments such as measurement instruments and biochemical indicators. Future research should adopt a combined approach of subjective and objective assessments (36). Initially, subjective scales were used to evaluate patients. If multiple pieces of evidence suggest the presence of related sleep disorders, objective assessments such as PSG should be employed to reflect the dynamic changes in sleep disorders more accurately.
4.4 Key considerations for developing effective intervention measures
Intervention strategies for postoperative sleep disorders in patients with malignant tumors can be broadly categorized into non-pharmacological and pharmacological approaches. Additionally, global consensus on which pharmacological treatment provides optimal efficacy or the best risk–benefit ratio is lacking. Cognitive behavioral therapy for Insomnia (CBT-I) is widely recognized as the first-line treatment (37). CBT-I and pharmacological interventions may produce comparable short-term outcomes; however, only CBT-I has demonstrated sustained long-term benefits post-treatment. Combining CBT-I with medication can accelerate the initial treatment response but may compromise the long-lasting positive effects of CBT-I (38). In specific cases, alternative interventions such as the use of dexmedetomidine during surgery, music therapy, traditional Chinese medicine, and physical rehabilitation exercises have shown efficacy in managing sleep disorders (27, 39). Following clinical assessment, when patients are in the early stages of sleep disorders, priority should be given to implementing psychological care interventions. If symptoms remain unalleviated, pharmacological treatment may then be introduced as an adjunctive measure for symptom control. When resources allow, cranial nerve stimulation therapy or a comprehensive treatment approach can be considered. Anesthesiologists must systematically refine perioperative pain management strategies to mitigate the risk of postoperative sleep disturbances. Psychological counseling should be integrated into routine postoperative care protocols as a key component of psychological intervention. Moreover, a systematic and standardized framework for managing sleep disorders should be developed, leveraging multidisciplinary expertise from fields such as anesthesiology, psychiatry, and nursing to foster interdisciplinary collaboration. A comprehensive approach should consider the patient’s medical condition, personal preferences, and available treatment resources to develop an intervention plan for postoperative sleep disorders in patients with malignant tumors.
4.5 Limitations
Despite considerable progress in the study of sleep disorders following malignant tumor surgery, several limitations remain. One of the primary challenges is the insufficient understanding of the complex interactions among various risk factors. In particular, the interplay between psychological and physiological elements—such as the inflammatory response induced by surgical trauma and psychological stress—has not been thoroughly elucidated (2, 28, 40). Further research is warranted to clarify these interrelationships, which may inform the development of more targeted and effective therapeutic strategies.
Cultural and regional differences in the management of sleep disorders also represent a critical area of concern. Variability in healthcare infrastructure, sociocultural attitudes, and accessibility of treatment may influence both the assessment and intervention approaches employed in different populations. Although the integration of multiple assessment modalities can enhance diagnostic accuracy, the validity and applicability of certain tools may vary across cultural and demographic contexts (19, 23, 25, 38, 39). Consequently, there is a pressing need to develop culturally sensitive, efficient, and clinically applicable assessment instruments that are adapted to specific populations, disease profiles, and healthcare workflows.
With regard to intervention strategies, novel therapies such as transcranial alternating current stimulation and pink noise stimulation have shown preliminary promise in enhancing sleep quality. However, existing studies are constrained by limited sample sizes and a lack of large-scale, high-quality clinical trials. The long-term efficacy and safety profiles of these interventions remain uncertain and must be substantiated through further rigorous investigation before their integration into routine clinical practice can be recommended (11, 18, 41).
This review provides a comprehensive synthesis of the current evidence concerning risk factors, diagnostic tools, therapeutic interventions, and treatment outcomes associated with postoperative sleep disorders in patients with malignant tumors. Nonetheless, future research is essential to establish more robust and cohesive links among these domains, thereby improving clinical relevance and informing optimized, patient-centered care strategies.
5 Conclusion
This evidence summary integrates 37 high-quality studies on the management of postoperative sleep disorders in patients with malignant tumors, emphasizing the multifactorial complexity of sleep disturbances in this population. The interplay of biological factors such as surgical trauma and anesthesia effects, along with socio-psychological factors like opioid use and emotional distress, significantly elevates the risk of sleep disorders. Accurate assessment necessitates a combination of subjective scales and objective monitoring tools. Additionally, traditional Chinese medicine syndrome differentiation can facilitate individualized diagnosis. Non-pharmacological interventions, including cognitive behavioral therapy, music therapy, and light therapy, have been demonstrated to be safe and effective primary options, while pharmacological and complementary therapies serve as supplementary approaches. Effectiveness evaluation should encompass multiple dimensions, such as sleep quality, emotional well-being, and functional recovery. Future clinical practice should consider patient preferences and resource availability, promote multidisciplinary collaboration, optimize treatment strategies, and ultimately enhance patient quality of life and prognosis.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: this article aims to systematically summarize and synthesize the evidence presented in systematic reviews, clinical practice guidelines, expert consensus statements, and related literature. The current query is not applicable to this context. Requests to access these datasets should be directed to YL,MTU5MjMyOTUyMjVAMTYzLmNvbQ==.
Author contributions
CY: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. YL: Data curation, Methodology, Writing – original draft, Writing – review & editing. BX: Investigation, Writing – review & editing. JY: Methodology, Writing – review & editing. QZ: Data curation, Writing – review & editing. RT: Investigation, Writing – review & editing. HM: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CBT-I, Cognitive behavioral therapy for Insomnia; PSG, Polysomnography; GSDS, Lee’s general sleep disturbance scale; PSQI, Pittsburgh sleep quality index; JBI, Joanna Briggs Institute.
References
1. Lin, D, Huang, X, Sun, Y, Wei, C, and Wu, A. Perioperative sleep disorder: a review. Front Med. (2021) 8:640416. doi: 10.3389/fmed.2021.640416
2. Sateia, MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. (2014) 146:1387–94. doi: 10.1378/chest.14-0970
3. Sanft, T, Day, A, Ansbaugh, S, Armenian, S, Baker, KS, Ballinger, T, et al. NCCN guidelines insights: survivorship, version 1.2023. J Natl Compr Cancer Netw. (2023) 21:792–803. doi: 10.6004/jnccn.2023.0041
4. Voiss, P, Höxtermann, MD, Dobos, G, and Cramer, H. Cancer, sleep problems, and mind-body medicine use: results of the 2017 National Health Interview Survey. Cancer. (2019) 125:4490–7. doi: 10.1002/cncr.32469
5. Perlis, ML, Smith, MT, and Pigeon, WR. Treatment of chronic insomnia In: KL Lichstein and CM Morin, editors. Treatment of late-life insomnia. Thousand Oaks, CA: Sage Publications (1997). 93–109.
6. Irwin, MR, Olmstead, R, and Carroll, JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis. Biol Psychiatry. (2008) 64:521–6. doi: 10.1016/j.biopsych.2008.05.023
7. Savard, J, Ivers, H, Savard, MH, and Morin, CM. Cancer treatments and their side effects are associated with aggravation of insomnia: results of a longitudinal study. Cancer. (2015) 121:1703–11. doi: 10.1002/cncr.29244
8. Serbest, S, Tiftikçi, U, Askın, A, Yaman, F, and Alpua, M. Preoperative and post-operative sleep quality evaluation in rotator cuff tear patients. Knee Surg Sports Traumatol Arthrosc. (2017) 25:2109–13. doi: 10.1007/s00167-016-4228-5
9. Rhon, DI, Snodgrass, SJ, Cleland, JA, and Cook, CE. Comorbid insomnia and sleep apnea are associated with greater downstream health care utilization after arthroscopic hip surgery. Pain Physician. (2019) 22:E351–60. doi: 10.1093/pm/pnz155
10. Wolfe, RM, Pomerantz, J, Miller, DE, Weiss-Coleman, R, and Solomonides, T. Obstructive sleep apnea: preoperative screening and postoperative care. J Am Board Fam Med. (2016) 29:263–75. doi: 10.3122/jabfm.2016.02.150085
11. Mullington, JM, Simpson, NS, Meier-Ewert, HK, and Haack, M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. (2010) 24:775–84. doi: 10.1016/j.beem.2010.08.014
12. Halle, IH, Westgaard, TK, Wahba, A, Oksholm, T, Rustøen, T, and Gjeilo, KH. Trajectory of sleep disturbances in patients undergoing lung cancer surgery: a prospective study. Interact Cardiovasc Thorac Surg. (2017) 25:285–91. doi: 10.1093/icvts/ivx076
13. Caruana, N, McKinley, S, Elliott, R, and Gholizadeh, L. Sleep quality During and after cardiothoracic intensive care and psychological health During recovery. J Cardiovasc Nurs. (2018) 33:E40–9. doi: 10.1097/JCN.0000000000000499
14. DiCenso, A, Bayley, L, and Haynes, RB. Accessing pre-appraised evidence: fine-tuning the 5S model into a 6S model. Evid Based Nurs. (2009) 12:99–101. doi: 10.1136/ebn.12.4.99-b
15. Brouwers, MC, Kho, ME, Browman, GP, Burgers, JS, Cluzeau, F, Feder, G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. (2010) 182:E839–42. doi: 10.1503/cmaj.090449
16. McArthur, A, Klugarova, J, Yan, H, and Florescu, S. Chapter 4: Systematic reviews of text and opinion In: E Aromataris and Z Munn, editors. JBI Manual for Evidence Synthesis. JBI (2020). doi: 10.46658/JBIMES-20-05
17. Porritt, K, McArthur, A, Lockwood, C, and Munn, Z. JBI handbook for evidence implementation. Adelaide: Joanna Briggs Institute (2020).
18. Drager, LF, Assis, M, Bacelar, AFR, Poyares, DLR, Conway, SG, Pires, GN, et al. 2023 guidelines on the diagnosis and treatment of insomnia in adults-Brazilian sleep association. Sleep Sci. (2023) 16:507–49. doi: 10.1055/s-0043-1776281
19. Lam, WC, Zhong, L, Liu, Y, Shi, N, Ng, B, Ziea, E, et al. Hong Kong Chinese medicine clinical practice guideline for cancer palliative care: pain, constipation, and insomnia. Evid Based Complement Alternat Med. (2019) 2019:1038206. doi: 10.1155/2019/1038206
20. Edinger, JD, Arnedt, JT, Bertisch, SM, Carney, CE, Harrington, JJ, Lichstein, KL, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2021) 17:255–62. doi: 10.5664/jcsm.8986
21. Frange, C, Franco, AM, Brasil, E, Hirata, RP, Lino, JA, Mortari, DM, et al. Practice recommendations for the role of physiotherapy in the management of sleep disorders: the 2022 Brazilian sleep association guidelines. Sleep Sci. (2022) 15:515–73. doi: 10.5935/1984-0063.20220083
22. Rau, KM, Shun, SC, Hung, SH, Chou, HL, Ho, CL, Chou, CL, et al. An evidence-based consensus for screening, assessment and treatment of insomnia in cancer patients. Jpn J Clin Oncol. (2023) 53:46–56. doi: 10.1093/jjco/hyac164
23. Riemann, D, Espie, CA, Altena, E, Arnardottir, ES, Baglioni, C, Bassetti, CLA, et al. The European insomnia guideline: an update on the diagnosis and treatment of insomnia 2023. J Sleep Res. (2023) 32:e14035. doi: 10.1111/jsr.14035
24. Choi, H, Youn, S, Um, YH, Kim, TW, Ju, G, Lee, HJ, et al. Korean clinical practice guideline for the diagnosis and treatment of insomnia in adults. Psychiatry Investig. (2020) 17:1048–59. doi: 10.30773/pi.2020.0146
25. Howell, M, Avidan, AY, Foldvary-Schaefer, N, Malkani, RG, During, EH, Roland, JP, et al. Management of REM sleep behavior disorder: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2023) 19:759–68. doi: 10.5664/jcsm.10424
26. Zhang, J, and Qian, J. Clinical practice guidelines for cancer-related fatigue in China (2021 edition) [Chinese]. Chin J Cancer. (2021) 31:852–72. doi: 10.19401/j.cnki.1007-3639.2021.09.012
27. Subspecialty Group of Sleep Disorders, Chinese Society of Neurology. Diagnosis and treatment of adult insomnia in China (2023 edition) [Chinese]. Chin J Neurol. (2024) 57:560–84. doi: 10.3760/cma.j.cn113694-20240406-00209
28. Tian, J, Chen, GL, and Zhang, HR. Sleep status of cervical cancer patients and predictors of poor sleep quality during adjuvant therapy. Support Care Cancer. (2015) 23:1401–8. doi: 10.1007/s00520-014-2493-8
29. Santoso, AMM, Jansen, F, Lissenberg-Witte, BI, Baatenburg de Jong, RJ, Langendijk, JA, Leemans, CR, et al. Sleep quality trajectories from head and neck cancer diagnosis to six months after treatment. Oral Oncol. (2021) 115:105211. doi: 10.1016/j.oraloncology.2021.105211
30. Tang, L, Zhan, S, Yu, E, Sun, H, Wang, C, and Ji, A. Expert recommendations on the diagnosis and treatment of insomnia in adult cancer patients. Chin J Ment Health. (2021) 35:441–8.
31. Hiller, RM, Johnston, A, Dohnt, H, Lovato, N, and Gradisar, M. Assessing cognitive processes related to insomnia: a review and measurement guide for Harvey’s cognitive model for the maintenance of insomnia. Sleep Med Rev. (2015) 23:46–53. doi: 10.1016/j.smrv.2014.11.006
32. Sancho-Domingo, C, Carballo, JL, Coloma-Carmona, A, and Buysse, DJ. Brief version of the Pittsburgh sleep quality index (B-PSQI) and measurement invariance across gender and age in a population-based sample. Psychol Assess. (2021) 33:111–21. doi: 10.1037/pas0000959
33. Lee, KA. Self-reported sleep disturbances in employed women. Sleep. (1992) 15:493–8. doi: 10.1093/sleep/15.6.493
34. Soldatos, CR, Dikeos, DG, and Paparrigopoulos, TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. (2000) 48:555–60. doi: 10.1016/s0022-3999(00)00095-7
36. Dhruva, A, Paul, SM, Cooper, BA, Lee, K, West, C, Aouizerat, BE, et al. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Manag. (2012) 44:215–28. doi: 10.1016/j.jpainsymman.2011.08.010
37. Hertenstein, E, Trinca, E, Wunderlin, M, Schneider, CL, Züst, MA, Fehér, KD, et al. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: a systematic review and meta-analysis. Sleep Med Rev. (2022) 62:101597. doi: 10.1016/j.smrv.2022.101597
38. Perlis, ML, Posner, D, Riemann, D, Bastien, CH, Teel, J, and Thase, M. Insomnia. Lancet. (2022) 400:1047–60. doi: 10.1016/S0140-6736(22)00879-0
39. Wu, XH, Cui, F, Zhang, C, Meng, ZT, Wang, DX, Ma, J, et al. Low-dose Dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology. (2016) 125:979–91. doi: 10.1097/ALN.0000000000001325
40. Tejada, M, Viele, C, Kober, KM, Cooper, BA, Paul, SM, Dunn, LB, et al. Identification of subgroups of chemotherapy patients with distinct sleep disturbance profiles and associated co-occurring symptoms. Sleep. (2019) 42:zsz 151. doi: 10.1093/sleep/zsz151
41. Brouwers, MC, Kerkvliet, K, and Spithoff, K AGREE Next Steps Consortium. The AGREE reporting checklist: a tool to improve reporting of clinical practice guidelines. BMJ. (2016) 352:i1152. doi: 10.1136/bmj.i1152
42. Zhu, Z, Hu, Y, Xing, W, Zhou, Y, and Gu, Y. The composition of different types of evidence-based issues. J Nurses Train. (2017) 32:1991–4.
Keywords: sleep disorders, malignant tumor, evidence summary, evidence-based nursing, postoperative management
Citation: Yang C, Liu Y, Xiao B, Yang J, Zeng Q, Tan R and Ma H (2025) Evidence summary for the management of sleep disorders after malignant tumor surgery. Front. Neurol. 16:1580216. doi: 10.3389/fneur.2025.1580216
Edited by:
Zhaolan Hu, Central South University, ChinaReviewed by:
Angelica Quercia, University of Messina, ItalyDi Zhou, Fudan University, China
Xiuying Lu, Sichuan Cancer Hospital, China
Copyright © 2025 Yang, Liu, Xiao, Yang, Zeng, Tan and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongli Ma, NTI5NDQ5MzEzQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Chang Yang
Chang Yang Yuanfei Liu
Yuanfei Liu Bang Xiao
Bang Xiao Hongli Ma
Hongli Ma