- 1Division of Neurology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
- 2Department of Neurology, Rehabilitation Medicine, Memory and Geriatrics, Skane University Hospital, Lund, Sweden
- 3Department of Medicine, St Vincent’s Hospital, University of Melbourne, Melbourne, VIC, Australia
- 4Division of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden
- 5Department of Pharmacology, University of Gothenburg, Gothenburg, Sweden
- 6Department of Biomedical and Clinical Sciences, Linkoping University, Linköping, Sweden
Introduction: Recent studies suggest that the dopamine agonist (DA) rotigotine improves sleep among Parkinson disease (PD) patients. Parkinson’s KinetiGraph (PKG) offers a home-based alternative for evaluating sleep. We investigated the effect of rotigotine on sleep in PD patients with PKG and questionnaires. Secondarily, the effects of rotigotine on daytime sleepiness, motor symptoms, quality of life and correlations between PKG variables and rating scale results were investigated.
Method: Thirty-two PD patients with sleep disturbances (Clinical Global Impression-Severity (CGI-S) ≥ 3) were included in this observational study. Before start of treatment and during stable dose with rotigotine patients were assessed with Parkinson’s disease sleep scale 2 (PDSS-2), Epworth Sleepiness Scale (ESS), Parkinson’s disease quality of life questionnaire (PDQ-8), European Quality of life five dimensions (EQ-5D-5L) questionnaires and PKG recordings (24 h/day for 6 days). Clinicians evaluated sleep using CGI scales, and PD severity using Clinical Impression of Severity Index for Parkinson’s Disease (CISI-PD).
Results: Rotigotine did not significantly improve total PDSS-2 or PKG nighttime scores in the entire group, but PDSS-2 improved among patients with PDSS-2 ≥ 18 at baseline and for DA-naïve patients (p = 0.009 and p = 0.013). Treatment improved percent time tremor (PTT; p < 0.001), percent time immobile during daytime (PTID; p < 0.001), CISI-PD (p < 0.001), PDQ-8 (p = 0.014), and EQ-5D-5L (p = 0.002). No significant correlations were found between PTID and ESS-total (ρ = −0.046, p = 0.718) or between combined sleep score (CSS) and PDSS-2 total (ρ = −0.065, p = 0.612).
Conclusion: Rotigotine improved sleep in patients with a baseline PDSS-2 ≥ 18 and in DA-naïve patients, but not in the whole study group. Additionally, rotigotine seemed to improve motor function and quality of life. PTID improved with treatment. Whether the improved PTID reflects a positive impact on daytime sleepiness or just improved mobility and to what extent PKG nighttime scores accurately represent sleep variables remains to be investigated in further studies.
Introduction
Sleep disturbances and daytime sleepiness are common in patients with Parkinson disease (PD), can be disease- or treatment-related and significantly impact health related quality of life (1). Rotigotine, a non-ergot dopamine agonist (DA) delivered through a transdermal patch, provides stable plasma concentrations and consequently reduces motor fluctuations (2). It also shows promise in improving sleep-related issues, including night-time pain, nocturnal motor impairment (3), sleep fragmentation (4), and overall sleep quality (5). Furthermore, the Evidence Based Medicine in Movement Disorders committee considers rotigotine as possibly useful for improving sleep (6). In the RECOVER study, a four-week randomized controlled trial (RCT), rotigotine treatment led to improvements in 10 out of 15 items on the Parkinson’s disease sleep scale 2 (PDSS-2) (3). Long-term use of rotigotine demonstrated sustained effects on sleep and motor function in a one-year follow-up study (7). A recent meta-analysis has also concluded that rotigotine improves both motor symptoms and sleep quality among individuals with PD (8). Moreover, Calandra-Buonara et al. (5) found that rotigotine treatment had positive effects on nocturnal and diurnal sleep disturbances in PD patients, as per actigraphy recordings, although not reflected in ESS scores. However, in contrast to these studies, an RCT evaluating rotigotine’s effectiveness using the non-motor symptoms scale found no significant improvements in sleep or fatigue, as evaluated by the Non-Motor Symptoms Scale for Parkinson’s Disease (9). While antiparkinsonian treatment, especially DAs, can worsen excessive daytime sleepiness (EDS) (10), some studies have shown that rotigotine does not exacerbate daytime sleepiness (11–13), while others demonstrated an improvement in daytime sleepiness with rotigotine, as indicated by actigraphy and the Epworth sleepiness scale (ESS) (5, 14).
Polysomnography (PSG) is regarded as the gold standard for objective sleep measurement (15), but can be resource demanding and usually requires overnight hospital admission, which can impact sleep quality and make long-term monitoring challenging (16). The Parkinson’s KinetiGraph (PKG) is a measurement system used for ambulatory, objective assessment of PD motor symptoms, such as tremor, bradykinesia, dyskinesia, and motor fluctuations (17). Furthermore, studies have investigated the capability of PKG in assessing night sleep and daytime sleepiness in PD patients. McGregor et al. (18) found that PKG scores can differentiate with good sensitivity and specificity between normal and abnormal PSG studies, and that PKG provides a fairly accurate picture of wakefulness, sleep duration, sleep quality, and sleep fragmentation. Another study supports the use of PKG immobility and mobility segments as indicators of wakefulness and sleep and suggests that PKG can be used as a rough evaluation of night-time sleep and as a tool to determine whether PSG is needed (19). Kotschet et al. (20) found a correlation between daytime percent time immobile (PTID) measured with PKG and ESS, suggesting that it is a useful measure of daytime sleep, or at least somnolence in PD patients. However, Höglund et al. (21) could not detect any significant correlations between PTI and diary reported daytime sleepiness.
Most studies investigating the effects of rotigotine on sleep have relied on questionnaires (3, 4, 7, 8, 14), with only a few utilizing more objective methods (5, 22, 23). Therefore, further research is needed to objectively confirm rotigotine’s suggested sleep-enhancing effects.
The primary objective of this study was to investigate the effects of rotigotine on sleep in PD patients using well established rating scales and PKG recordings. Secondarily, it aimed to investigate the impact of rotigotine treatment on daytime sleepiness, quality of life and motor symptoms in PD patients. Furthermore, the study aimed to explore the correlations between PKG parameters used to evaluate sleep and daytime sleepiness, and corresponding questionnaires that evaluate these same parameters.
Materials and methods
This investigator-initiated, observational, prospective study is a collaboration within the Swedish Parkinson Research Network. Forty patients were included in the study and 32 patients completed the study.
Participation criteria
PD patients aged 18–85 who were experiencing sleep disturbances (Clinical Global Impression-Severity (CGI-S) ≥ 3) were included. The CGI-S scale was used as a quick and broad screening tool to capture both mild and severe sleep disturbances, allowing for inclusion of patients that more specific scales might miss. Its use also aligns with previous studies on rotigotine and sleep, ensuring comparability (3, 4). Participants were required to have maintained a stable PD treatment regimen for at least 28 days. Patients were excluded according to the following criteria: advanced therapies such as deep brain stimulation, apomorphine infusion, or levodopa infusion; oral DA administration the last 28 days; dementia or significant cognitive impairment; clinically significant prostate problems causing sleep problems, sleep apnea syndrome, or other diagnosed non-PD-related conditions significantly impacting nocturnal sleep. Initiation or modification of sedatives or hypnotics during the study was not permitted.
Study design
The study flow chart is presented in Figure 1.
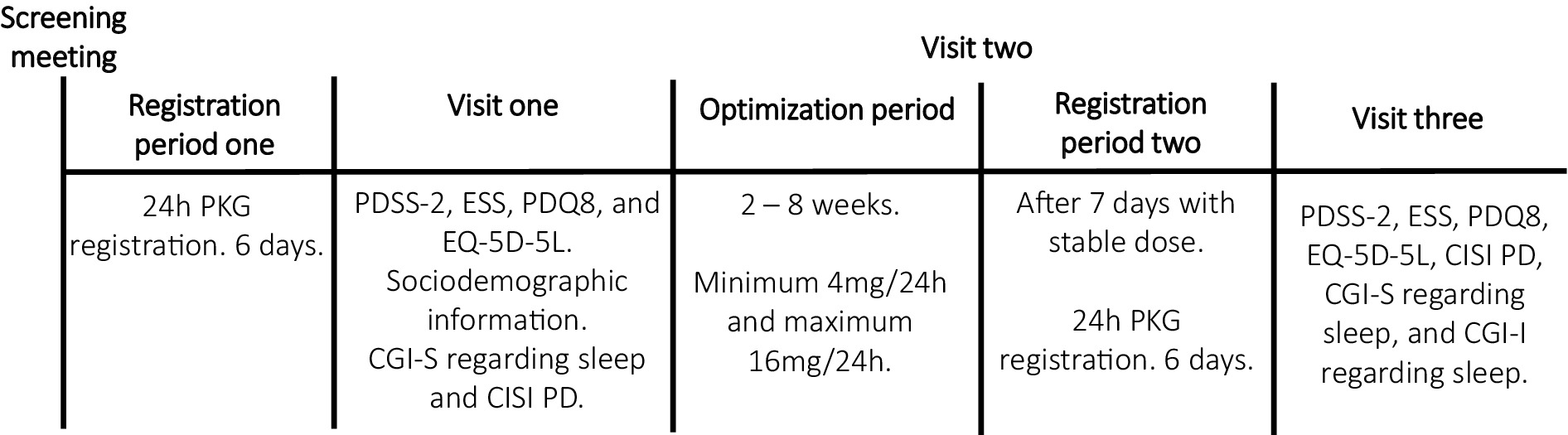
Figure 1. Explained study design. PKG, Parkinson’s KinetiGraph; PDSS-2, Parkinson’s disease sleep scale 2; ESS, Epworth Sleepiness Scale; PDQ8, Parkinson’s disease quality of life questionnaire; EQ-5D-5L, European Quality of life five dimensions with five levels; CGI-S, Clinical Global impressions severity; CISI-PD, Clinical Impression of Severity Index for Parkinson’s Disease; CGI-I, Clinical Global impressions improvement.
PD patients with sleep disturbances, already scheduled to start rotigotine treatment, were evaluated based on eligibility criteria. Demographic and clinical data were gathered, and levodopa equivalent doses (LED) were computed following Tomlinson et al. (24). Before initiating rotigotine, eligible patients underwent the first PKG recording, wearing the device continuously on their most affected side for 24 h over six consecutive days. After this recording, participants completed the PDSS-2 (25), ESS (26), Parkinson’s disease quality of life questionnaire (PDQ-8) (27), and European Quality of life five dimensions (EQ-5D-5L) questionnaires (28). Study clinicians also assessed the patients’ sleep through interviews using the CGI-S and evaluated disease severity using the Clinical Impression of Severity Index for Parkinson’s Disease (CISI-PD) (29).
Study clinicians titrated and optimized rotigotine per the study protocol’s titration plan, starting patients at 2 mg with weekly increases of 2 mg. Weekly follow-ups in-person or by phone, aimed to identify a dose that provided adequate motor symptom relief without bothersome side effects. If side effects occurred, the dose was reduced by 2 mg to the last tolerable level. The up-titration rate could be slightly adjusted based on factors like tolerance, prior medications, and clinical response, with target dosages ranging from 4 mg to 16 mg (2, 30). After maintaining the achieved dose for at least 1 week, another PKG recording period followed. Following this second recording, the clinician evaluated the patient’s sleep improvement using CGI - Improvement (CGI-I) scale (29), and all questionnaires and clinical scales that were assessed at baseline were completed again.
The PKG system and glossary of PKG terms
The PKG, developed by Global Kinetics Corporation, is a wrist-worn device worn for up to 10 days (18), using accelerometer data to generate continuous variables analyzed by specialized algorithms (31). For this study, patients wore the PKG on their most affected side for 6 days. A detailed glossary including all presented PKG variables are available in the Supplementary material.
Percent time immobile
PTID is a measure of daytime immobility that has shown concordance with the detection of daytime sleep by PSG (20). High PTI values have been associated with higher ESS scores in PD patients (20).
Combined sleep score
Combined sleep score (CSS) quantifies sleep quality by normalizing and scoring nighttime PTI, PTSN (percent time sleep during night-time), and SQ (sleep quality) variables. Higher values indicate better sleep, and CSS has been shown to a broad inverse correlation with PDSS-2 (18).
Statistical analyses
Co-author MH compiled the PKG data. Descriptive statistics are presented with median and interquartile ranges (IQR) or mean and range. Since the data was not normally distributed, changes in PKG variables and questionnaires before and with treatment were assessed using the Wilcoxon signed rank test. Two-sided p-values were used, and statistical significance was defined as p ≤ 0.05. Since the data was not normally distributed, the Spearman correlation test was used to investigate the correlation between PTID and ESS, as well as between PDSS-2 and CSS. Statistical analyses were performed using SPSS Statistics version 29 (IBM Corp) and Microsoft Excel version 365 (Microsoft Corporation). Graphs were created using GraphPad Prism version 9.0 (GraphPad Software, Inc).
Primary outcomes
The primary outcomes of the study were changes in total PDSS-2 and CSS; p-value was adjusted to 0.025 after Bonferroni correction.
Secondary outcomes
Secondary outcomes and post-hoc analyses were considered exploratory and therefore we did not correct for multiple comparisons (32). Secondary outcomes were the CGI-I regarding sleep, the remaining daytime and nighttime PKG scores, the ESS, the CISI-PD questionnaire, the PDQ-8, the EQ-5D-5L questionnaire, and analyses of correlations between PDSS-2 and CSS as well as between ESS and PTId. Post-hoc analyses examined the effects of baseline PDSS-2 and previous history of DA use on PDSS-2 outcomes.
Results
Participant characteristics
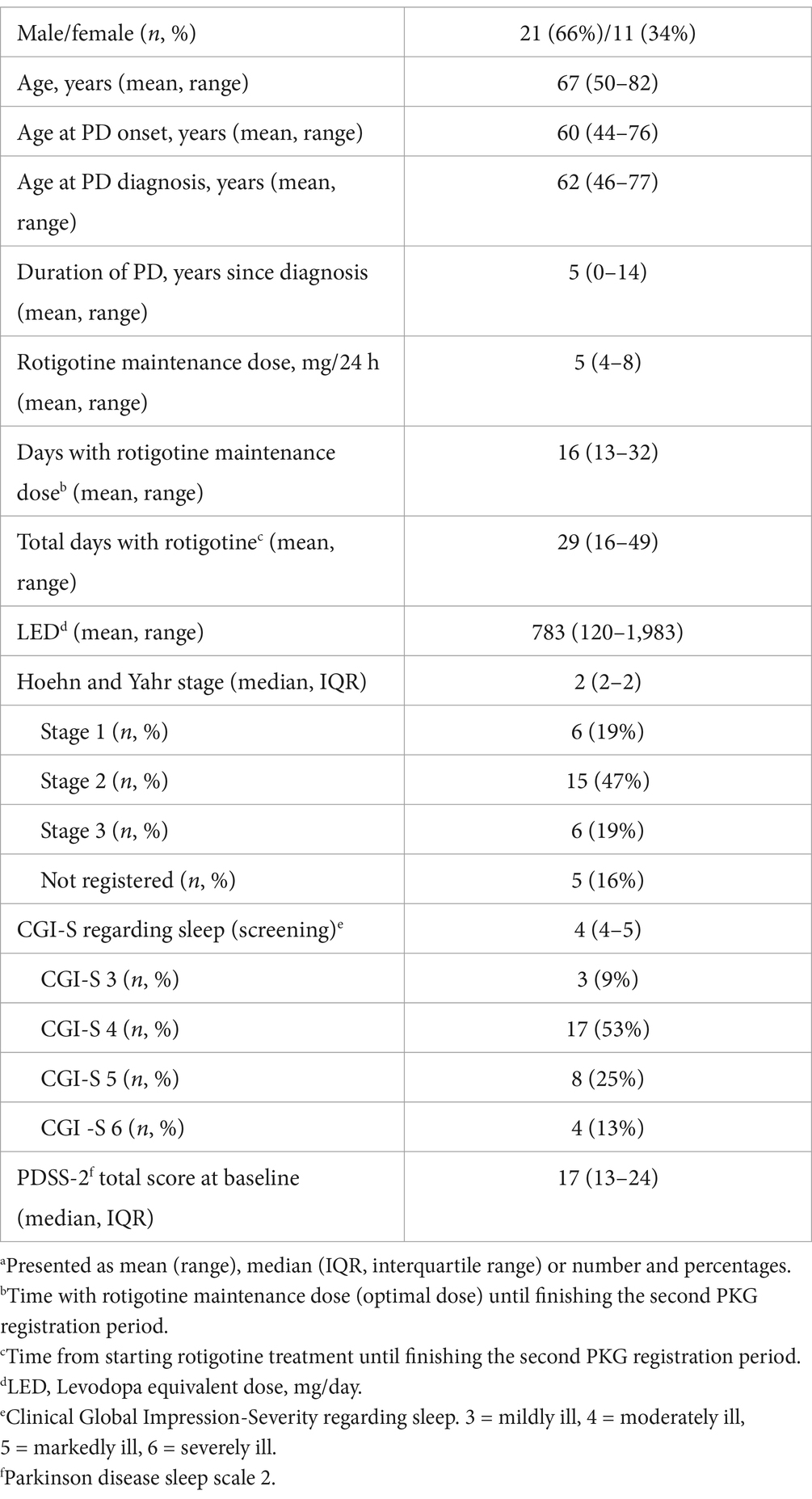
Figure 2 illustrates participant inclusion and discontinuation. Of 40 participants, 32 completed the study: 29 from Skane University Hospital, one from Sahlgrenska University Hospital, and two from Linköping University Hospital. All patients were on concomitant anti-parkinsonian medication. Sixteen patients were DA-naïve, while 16 had prior DA treatment, which was discontinued for at least 28 days prior to study inclusion. Baseline evaluations were conducted without DA treatment. The mean age was 67 years (range: 50–82), mean LED was 783 mg (range: 120–1983), mean time since diagnosis was 5 years (range: 0–14), and the median baseline CGI-S score for sleep was 4 (IQR: 4–5).
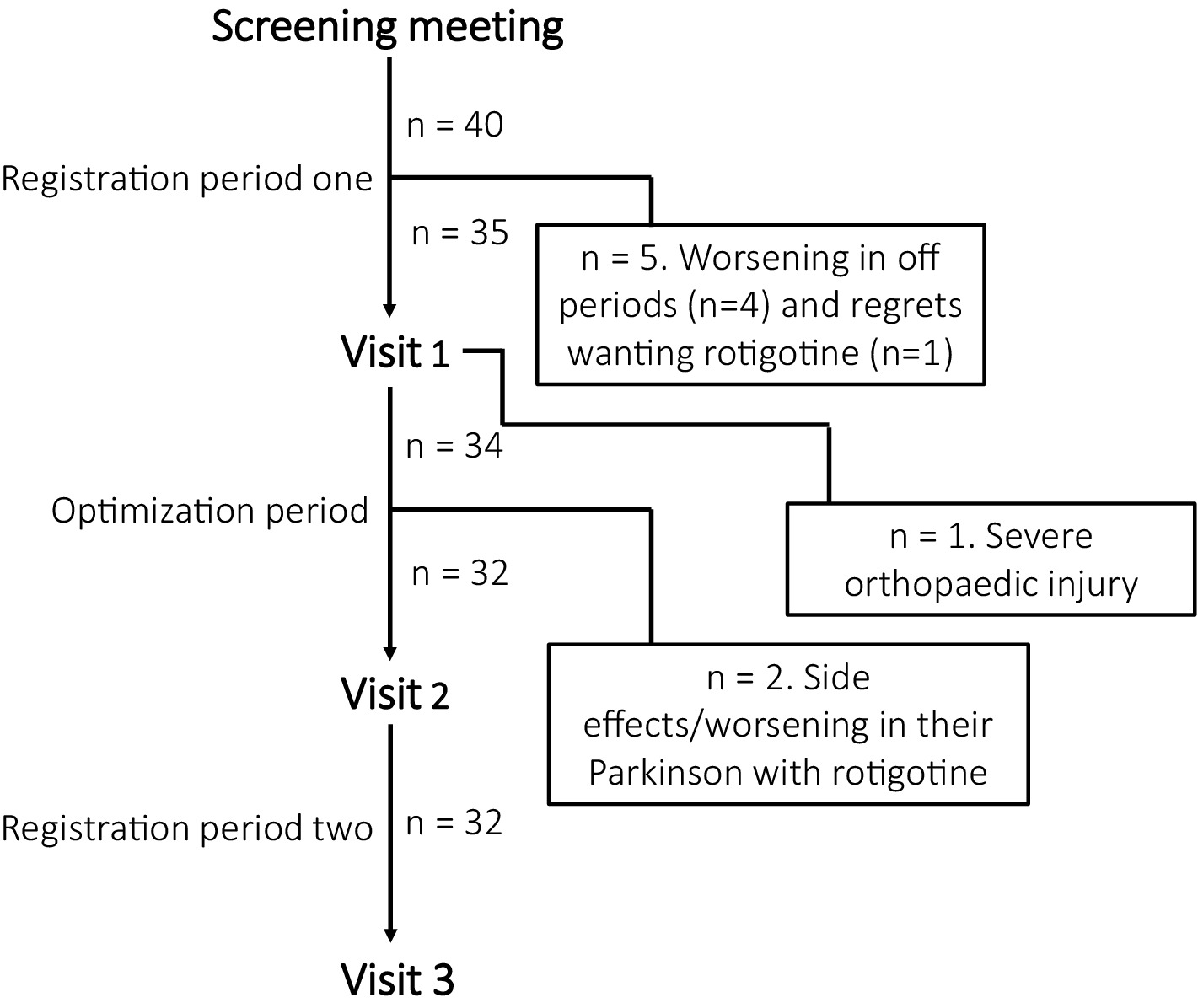
Figure 2. Participant inclusion and discontinuation reasons. During registration period one, four participants discontinued due to worsening off periods without dopamine agonist and before starting rotigotine. One patient was excluded during visit 1 due to a severe orthopedic injury that affected their sleep. During optimization period, one participant discontinued the study due to skin rash, and another experienced worsened PD symptoms with rotigotine.
Rotigotine usage and side effects
The mean maintenance rotigotine dose was 5 mg (range: 4–8) (Table 1). The average time on maintenance dose before finishing the second PKG registration was 16 days (range: 13–32), with a total treatment duration averaging 29 days (range: 16–49). Reported rotigotine side effects included nausea (n = 4), headache (n = 1), worsened tremor (n = 1), skin irritation/rash (n = 3), and worsened dyskinesia (n = 1).
Sleep and daytime sleepiness
Sleep
There was no significant improvement in PDSS-2 total score, with a median of 17 (IQR: 13–24) without rotigotine treatment and 13 (IQR: 11–24) with rotigotine treatment (p = 0.13) (Figure 3). Significant improvement was observed in PDSS-2 item 14 (sleepiness after waking) with a median of 2 (IQR: 1–3) before treatment and 1 (IQR: 1–3) with treatment (p = 0.02).
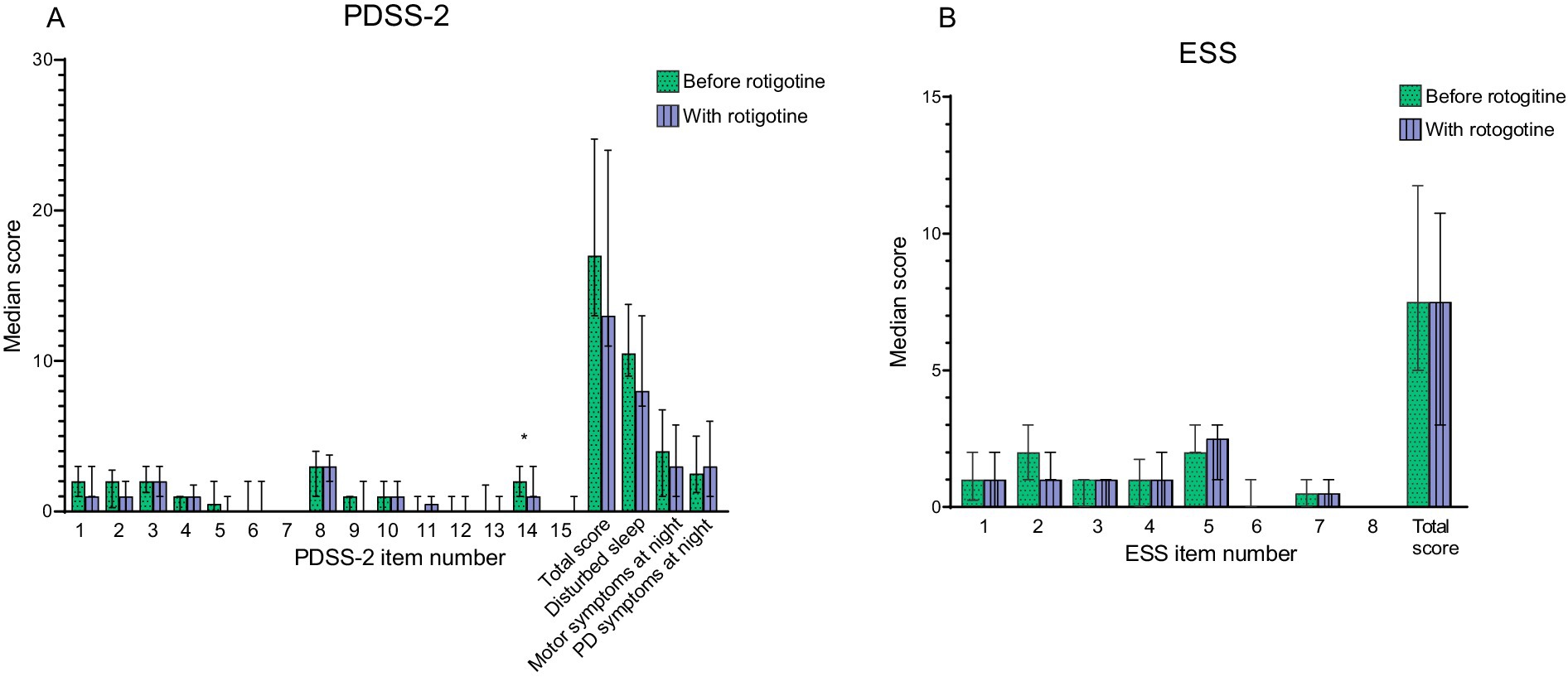
Figure 3. Sleep and daytime sleepiness before and with rotigotine treatment according to questionnaires. Median values are presented, and error bars represent the interquartile range. Green bars with dots indicate scores before rotigotine treatment, while purple bars with lines indicate scores with rotigotine treatment. The total score is the sum of all item scores. Statistical significance is denoted as *p ≤ 0.05, calculated using the Wilcoxon signed rank test. Bonferroni adjusted p value was set as p ≤ 0.025 for PDSS-2 (A) Parkinson’ s disease sleep scale (PDSS-2). Disturbed sleep include items 1, 2, 3, 8, and 14. Motor symptoms at night include items 4, 5, 6, 12, and 13. PD symptoms at night includes items 7, 9, 10, 11, and 15. Significant improvement was observed in question 14 (sleepiness after waking, p = 0.02) (B) Epworth Sleepiness Scale (ESS).
Among the 15 patients with clinically significant sleep disturbances (PDSS-2 ≥ 18) before rotigotine treatment (33), the PDSS-2 total score improved from a median of 25 (IQR: 21–27.5) to a median of 20 (IQR: 12–25) (p = 0.009). PDSS-2 subtotals for disturbed sleep (items 1, 2, 3, 8 and 14) improved from a median of 13 (IQR: 11.5–14.5) to a median of 8 (IQR: 8–13) (p = 0.013), and subtotals for PD symptoms at night (items 7, 9, 10, 11, and 15) improved from a median of 5 (IQR: 4–8.5) to a median of 4 (IQR: 2.5–7) (p = 0.041). All participants were evaluated without DA treatment at baseline, though 16 of them had previously been treated with DA. Explorative subgroup analysis showed that DA- naïve patients improved significantly in total PDSS-2 from a median of 17.5 (IQR: 13–25) to 12.5 (IQR:10–23) (p = 0.013) with rotigotine treatment, while no significant improvement was detected for patients previously on oral DA.
The median score on CGI-S regarding sleep improved from 4 to 3 (p < 0.001) with rotigotine treatment. According to CGI-I for sleep; one patient improved very much, five improved much, 13 improved minimally, eight did not change, two got minimally worse and three got much worse. No significant improvement was observed in the PKG nighttime scores (Figure 4B).
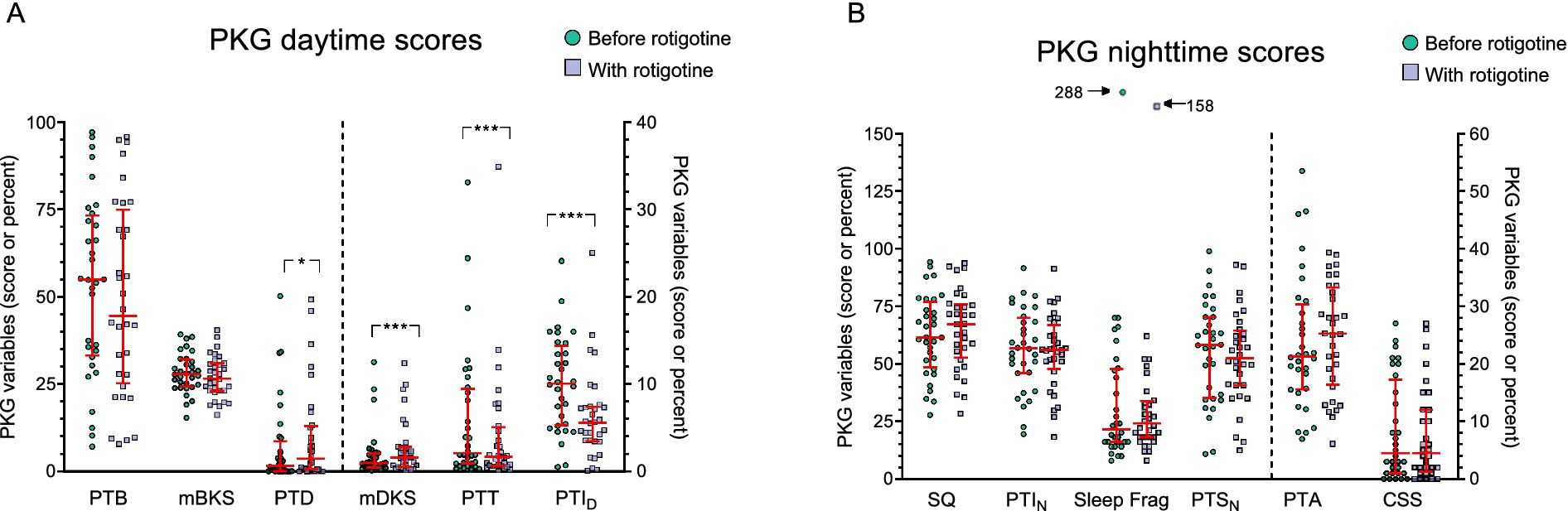
Figure 4. PKG scores before and with Rotigotine treatment. Median values are presented, and error bars represent the interquartile range. Green circles indicate scores before rotigotine treatment, while purple squares indicate scores with rotigotine treatment. The left side of the figures is separated by a dotted line and corresponds to the left Y axis, while the right side relates to the right Y axis. Statistical significance is denoted as *p ≤ 0.05, ***p ≤ 0.001, calculated using the Wilcoxon signed rank test. (A) Daytime PKG scores, assessed from 09:00 to 18:00, include the following parameters: PTB, percent time in bradykinesia; mBKS, median bradykinesia score during daytime; PTD, percent time in dyskinesia; mDKS, median dyskinesia score; PTT, percent time in tremor; PTID, percent time immobile during daytime. PTT and PTID showed a significant improvement with rotigotine (p < 0.001 and p < 0.001), while PTD and mDKS demonstrated a significant deterioration with treatment (p = 0.016 and p = 0.001). (B) Night-time PKG scores, assessed from 23:00 to 06:00, include the following parameters: SQ, sleep quality; PTIN, percent time immobile during night-time; Sleep Frag, Sleep fragments (the median duration of sleep fragments in minutes); PTSN, percent time asleep during night-time; PTA, Night percent time active score; CSS, Combined sleep score.
Daytime sleepiness
Median ESS total score was 7.5 both before and with rotigotine treatment (IQR before: 5–11.3; IQR after: 3–10.3) (p = 0.358) (Figure 3). Figure 4A presents the daytime PKG scores. Based on PKG data, significant improvements were observed in PTID with a median of 10 (IQR: 5–14) before rotigotine and a median of 6 (IQR: 3–7) with rotigotine (p < 0.001). Twelve patients transitioned from high to normal PTID scores (target score ≤10%) (34).
Motor symptoms and quality of life
Quality of life
Median PDQ-8 score improved from 9.5 (IQR: 6–13) to 7.5 (IQR: 3–12) (p = 0.014), particularly in terms of decreased feelings of depression (question 3 on PDQ-8, p = 0.007) (Figure 5). The median EQ-5D-5L Time Trade Off (TTO) improved from 0.79 (IQR: 0.7–0.9) to 0.84 (IQR: 0.8–0.9) (p = 0.002), and the median VAS score improved from a median of 60 (IQR: 44.5–70) to 67.5 (IQR: 52–76) (p = 0.002).
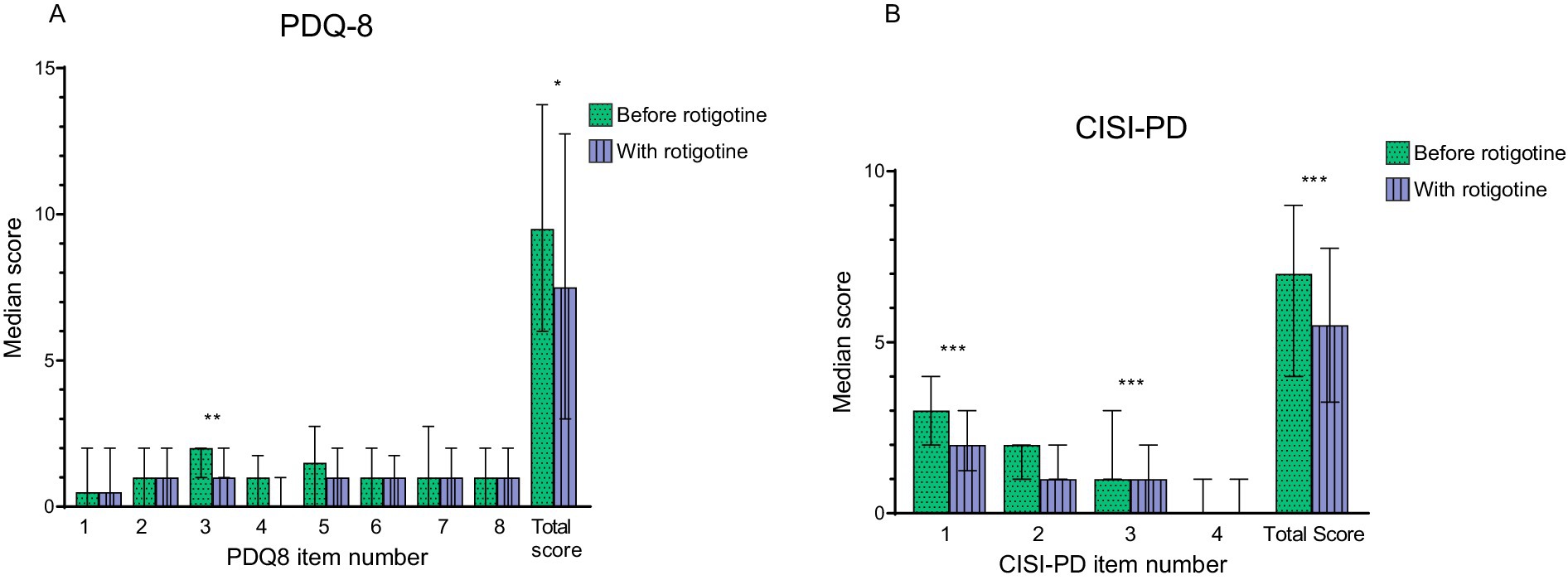
Figure 5. Motor complications and quality of life before and with rotigotine treatment according to questionnaires. Median values are presented resented, and error bars represent the interquartile range. Green bars with dots indicate scores before rotigotine treatment, while purple bars with lines indicate scores with rotigotine treatment. The total score is the sum of all item scores. Statistical significance is denoted as *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, calculated using the Wilcoxon signed rank test. (A) Parkinson’s Disease Quality of Life Questionnaire (PDQ-8). Significant improvements were observed in question 3 (felt depressed, p = 0.07), and the total score (p = 0.014). (B) Clinical Impression of Severity Index for Parkinson’s Disease (CISI-PD). Significant improvements were observed in question 1 (motor signs), question 3 (motor complications including dyskinesia and fluctuations), and the total score (p = 0.001, p < 0.001, and p < 0.001).
PD severity
Median CISI-PD score improved from 7 (IQR: 4–9) to 5.5 (IQR: 6–14) (p < 0.001) (Figure 5). More specifically, there was a reduction in motor signs according to question 1 (p = 0.001) and of motor complications according to question 3 (p < 0.001).
Daytime PKG score
Figure 4A presents the daytime PKG scores. Percent time tremor (PTT) showed a significant improvement with a median score of 2.1 (IQR: 0.9–9.2) before treatment and 1.7 (IQR: 0.7–4.8) with treatment (p < 0.001). Ten patients fell within the PTT target range (≤1%) before treatment, and the number increased to 13 with treatment. Median dyskinesia score (mDKS) and percent time in dyskinesia (PTD) increased after rotigotine treatment; mDKS from 0.95 (IQR: 0.5–2) to 1.6 (0.7–2.7) (p = 0.001), and PTD from 1.7 (IQR: 0–6.7) to 3.7 (IQR: 0.6–12.7) (p = 0.016). In one patient mDKS increased to >9 during rotigotine treatment, indicating uncontrolled dyskinesia that were not present before (34). There was a trend toward improvement in median bradykinesia score (mBKS) with a median of 27.9 (IQR: 24.6–31.6) before rotigotine and 26.5 (IQR: 23–30.8) with rotigotine (p = 0.106). Before rotigotine treatment 23 patients had mBKS scores >25 indicating uncontrolled bradykinesia, and in 4 of them (17%) mBKS ≤25 was achieved during rotigotine treatment. No patients deteriorated in BKS during treatment.
Correlations between PKG scores and questionnaires
No correlation was observed between total PDSS-2 and CSS (ρ = −0.065, p = 0.612) (Figure 6A). However, there was a negative weak correlation (ρ = −0.325, p = 0.009) between CSS and PDSS-2 question 2 (difficulty falling asleep; Figure 6B). No significant correlations were identified between the CSS and any other PDSS-2 items. There was no significant correlation between ESS and PTID (ρ = 0.046, p = 0.718) (Figure 6C). The PTID score was not significantly higher in patients with ESS score ≥10 compared with patients with an ESS score <10 (Mann Whitney Test; p = 0.967) and patients with PTID > 10 did not exhibit significantly higher ESS scores (Mann Whitney Test; p = 0.873).
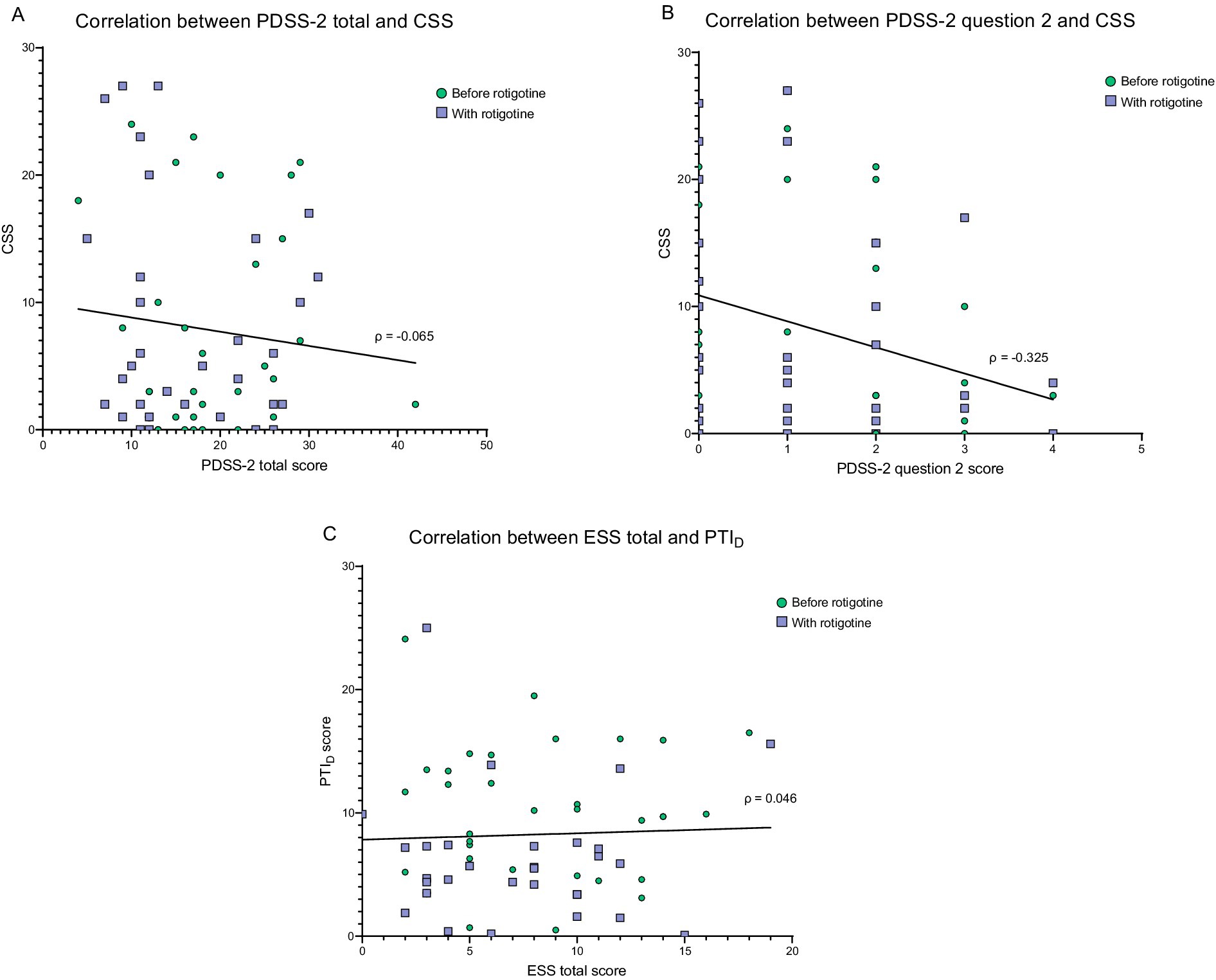
Figure 6. Correlation between PKG score and questionnaires. Green dots = before rotigotine treatment. Purple squares = with rotigotine treatment. The lines represent simple linear regressions. (A) Correlation between PDSS-2 total score and combined sleep score (CSS). PDSS-2 stands for Parkinson’s disease sleep scale 2, and CSS is measured with PKG. The Spearman correlation coefficient was −0.065 (p = 0.612). Simple linear regression gives the equation Y = −0.1115*X + 9.934 (p = 0.415). (B) Correlation between PDSS-2 question 2 (difficulty falling asleep) and combined sleep score (CSS). The Spearman correlation coefficient was −0.325 (p = 0.009). Simple linear regression gives the equation Y = −2.051*X + 10.88 (p = 0.0138). No significant correlations were identified between the CSS and any other PDSS-2 items. (C) Correlation between ESS total score and PTID. ESS refers to the Epworth Sleepiness Scale, while PTID represents the percentage of time immobile during the daytime measured with PKG, which estimates daytime sleepiness. The Spearman correlation coefficient was 0.046 (p = 0.718). Simple linear regression gives the equation Y = 0.05145*X + 7.833 (p = 0.749).
Discussion
Sleep problems, as assessed by PDSS-2, showed a trend toward improvement with rotigotine treatment, but the finding was statistically significant only in patients with more severe baseline sleep disturbances (PDSS-2 ≥ 18) and among those who were DA-naïve. Clinician assessments using the CGI-S scale indicated sleep improvement, while PKG nighttime scores remained unchanged. PTID improved, suggesting rotigotine to reduce daytime sleepiness. Furthermore, motor symptoms and quality of life improved significantly with rotigotine treatment, as indicated by CISI-PD, PTT, PDQ-8, and EQ-5D-5L scores. No significant correlations were observed between PTID and ESS or between CSS and PDSS-2.
While previous studies have reported positive effects of rotigotine on sleep (5, 22, 23, 35), our study found no significant improvements in sleep for the entire study group, as measured by PDSS-2 and PKG nighttime scores. This may be due to the use of the CGI-S scale for patient inclusion, which is less comprehensive than PDSS-2, along with a low severity threshold for inclusion. The CGI-S was selected as a quick screening tool to include patients with both severe and mild sleep disturbances. Previous studies investigating rotigotine’s effect on sleep used similar inclusion methods, facilitating comparison (3, 4). While PD-specific scales like PDSS-2 identify PD-related sleep issues more accurately, setting a PDSS-2 cutoff could exclude patients with significant sleep disturbances. For instance, a patient with severe insomnia but no other issues could receive a relatively low PDSS-2 score. The CGI-S scale indicated significant sleep improvement, unlike PDSS-2 or PKG scores. The CGI-S scale relies on patient reports, and patients may have exaggerated sleep improvements during clinician discussions but not on questionnaires. Also, CGI values change differently from PDSS-2, which weighs all changes equally and linearly, potentially not reflecting real-life experiences.
In this study, the mean treatment duration was 29 days, with an optimal rotigotine dose maintained for an average of 16 days (range: 13–32), with the second registration the last week of maintenance dose. While one other study used a 2 week maintenance period (23), most studies assessed sleep after 4 weeks at the optimal dose (3, 5). A longer treatment duration may be needed to fully evaluate rotigotine’s effects. The mean rotigotine dose was 5 mg (range: 4–8 mg), while previous studies have reported doses ranging from 2 mg to 18 mg (3, 4, 8), with many averaging around 8–9 mg (4, 5, 23). Higher rotigotine doses might produce different outcomes. Few studies have assessed rotigotine’s dose-dependent effects on sleep. Anthony et al. (36) suggested 8 mg as the minimum dose to reduce “off” time but found no improvements in ESS or PDSS across 2–8 mg. While some studies reported sleep benefits at doses as low as 2 mg (13, 14), most showing improvements used higher doses (4, 5, 13, 23). The adaptive dose regimen in the current study was not specifically designed to address sleep problems, but the final dosing provides some indication about a dose threshold for positive effects in general. Further research is needed to clarify the dose–response relationship for sleep outcomes in PD.
The median baseline PDSS-2 score was 17, with 47% of patients scoring ≥18 before treatment. A cutoff of 18 points on the PDSS-2 is suggested as a marker for clinically relevant PD-specific sleep disturbances (33). Improvements in the PDSS-2 total score and the subtotals for “disturbed sleep” and “PD symptoms at night” were observed only in patients with a baseline PDSS-2 score of ≥18. These findings suggest that rotigotine’s sleep-enhancing effects are more pronounced in patients with severe sleep issues, consistent with Vallderiola et al. (37), who found that higher baseline PDSS-2 scores correlated with greater improvement of PDSS-2 score after 3 months of night-time rotigotine treatment. Identifying the patient groups whose sleep improves with rotigotine is crucial for physicians when determining who should be offered the treatment.
While all participants were evaluated without DA treatment at baseline, half had previously used oral DA. Subgroup analysis showed significant improvements in PDSS-2 total score for DA-naïve patients, while those with earlier oral DA showed no significant improvement. This contradicts Pagonabarraga et al. (4), who found no differences in PDSS-2 between DA- naïve patients and those switching from another DA to rotigotine. Possible explanations for this discrepancy include variations in sample sizes, differing durations of previous DA treatment, and individual patient characteristics. Further research is needed to assess rotigotine’s effect on sleep in relation to prior DA use and concurrent therapies.
No significant correlation was found between PDSS-2 and CSS, though a negative correlation was observed between CSS and PDSS-2 question 2 (difficulties falling asleep), indicating that CSS may be primarily influenced by sleep onset insomnia. Previous research has shown that CSS correlates with PDSS-2, especially in subscores related to sleep quality and quantity (18). PDSS-2 scores reflect contributing factors to sleep disturbances rather than sleep extent or quality, and the non-linear nature of PDSS-2 complicates the interpretation of correlation patterns (18). Thus, a strong linear correlation between CSS and PDSS-2 scores is unlikely. However, to support the reliance on PKG nighttime scores, a correlation between worsening PDSS-2 scores and CSS deterioration is expected, and such correlation was not seen in this study. The lack of correlation may be attributed to the subjective nature of the PDSS-2 or the need for a larger sample size. PSG is the gold standard for sleep assessment (15), and comparisons between PKG and PSG have suggested PKG to be a valuable tool for assessing sleep (18). Further studies are needed to determine the reliability of PKG nighttime scores for assessing sleep in PD patients, ideally incorporating both PSG and questionnaire assessments.
The reduction in the PKG PTID score, along with the normalization of PTID scores in 12 patients, suggests that rotigotine improves daytime immobility and potentially reduces daytime sleep episodes. Unlike other DAs, rotigotine has not been shown to worsen EDS or somnolence (11–13). Calandra-Buonara et al. (5) demonstrated through actigraphy that rotigotine decreased number and duration of daytime sleep episodes, while Steiger (38) observed a 70% reduction in daytime sleepiness with continuous dopaminergic treatment. Additionally, an open label study reported improvements in ESS score after 1 and 3 months of rotigotine treatment (14). These findings support the notion that rotigotine, with its continuous dopaminergic treatment (39), may have a positive effect on daytime sleepiness. Although PTID improved with rotigotine treatment, no significant change was observed in ESS score. This aligns with findings by Calandra-Buonara et al. (5), who found improved daytime sleepiness via actigraphy with rotigotine but no change in ESS scores. The subjective nature of the ESS, along with patients’ potential difficulty recalling symptoms over the past week and a tendency to under-report sleepiness due to unawareness of daytime naps, may affect its accuracy (40).
No significant correlation was found between PTID and ESS. Although an ESS score ≥10 indicates EDS (26), and Kotschet et al. (20) reported a significant association between ESS scores ≥10 and elevated PTID scores in PD patients, we observed no differences in PTID scores between patients with ESS scores above or below 10. Similarly, Höglund et al. (21) found no correlation between PKG score and self-evaluated daytime sleepiness. The lack of correlation between ESS and PTID may stem from the subjective nature of questionnaires, where patient ratings can vary despite similar symptoms. Also, one study showed that over a third of both PD patients and healthy individuals underreport brief naps with slow-wave sleep (40). Additionally, PTID measures immobility, and the CISI-PD scale indicates that rotigotine improved motor symptoms, likely leading to reduced immobility. Therefore, part of the discrepancy between ESS and PTID scores could, to some extent, be explained by PTID reflecting bradykinesia and immobility caused by motor symptoms, rather than daytime sleepiness. Larger studies comparing PKG data with questionnaires and PSG are needed to better evaluate PKG’s effectiveness in assessing sleep and daytime sleepiness in PD patients.
PTID improved with rotigotine treatment despite no improvement of nighttime PKG scores. Supporting this, Klingelhoefer et al. (19) found that nighttime PKG variables do not differentiate between patients with EDS and without EDS, and Liguori et al. (12) found no correlation between nocturnal sleep and daytime sleepiness. Conversely, McGregor et al. (18) showed that more severe daytime sleepiness was associated with poorer nighttime PKG scores. It is possible that daytime sleepiness can occur independently of nighttime sleep disturbances, though sleep disruptions likely contribute to it. Further research with multiple objective tools is needed to clarify the relationship between sleep disturbances and daytime sleepiness in PD.
Significant improvements in CISI-PD and PTT were observed with rotigotine treatment. Also, four patients who previously had uncontrolled bradykinesia according to the PKG achieved control within PKG target ranges (18, 34). These findings suggests that rotigotine reduces motor symptoms in PD patients, consistent with prior studies (35, 41). While PTD and mDKS increased significantly with rotigotine, they remained at low levels of clinically non-significant dyskinesia. Additionally, rotigotine significantly improved quality of life as measured by PDQ-8, and EQ-5D-5, aligning with previous research (3, 37).
This study has some limitations. A larger sample size could have improved the reliability of the results, and a longer treatment period or higher rotigotine doses might have led to different outcomes. Subjective questionnaires can be interpreted differently by patients and clinicians, potentially affecting the result. However, the same clinician rated the patient both before and with treatment, which is a strength. Moreover, specific tools for evaluating other sleep disturbances, such as restless legs syndrome and rapid eye movement sleep behavior disorders, were lacking. Also, while CISI-PD was used to assess motor symptoms, a more detailed scale like the Movement Disorder Society-Unified Parkinson’s disease rating scale might have offered richer clinical information. The lack of a placebo or control group introduces bias, as expectations may have influenced symptom assessments, which a double-blind design could have mitigated. Moreover, the observational study design led to a heterogeneous study group with varying PD duration, LED doses, and ages may have further impacted results, and a more homogeneous cohort might yield different findings. Additionally, using PSG to validate PKG and exclude patients with sleep apnea would have been preferable. However, it is a strength that the study investigated sleep with both subjective and objective tools. Furthermore, half of the patients had previously used a DA, which was tapered off and discontinued at least 28 days prior to study inclusion, to ensure comparability across baseline visits. This washout period may have altered baseline conditions, potentially influencing the findings.
Conclusion
In conclusion, while rotigotine did not significantly improve sleep in the entire group based on PDSS-2 or PKG nighttime scores, sleep improved in those with clinically relevant sleep disturbances (PDSS-2 ≥ 18) and in DA-naïve patients. Moreover, PKG measures showed reduced daytime immobility with rotigotine, suggesting a potential reduction in daytime sleepiness. Confirming this in future studies could establish rotigotine as a promising option for PD patients with daytime sleepiness. Additionally, rotigotine improved motor symptoms and quality of life. No correlations were seen between CSS and PDSS-2 or between ESS and PTID. Larger studies are needed to further explore correlations between PKG scores, PSG, and established rating scales.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Swedish Ethical Review Authority (Dnr 2019-01294). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. CJ: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MH: Resources, Software, Writing – review & editing. FB: Investigation, Project administration, Writing – review & editing. ND: Investigation, Project administration, Writing – review & editing. PO: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This is an academic study and all study-related costs including PKG, were covered by academic funds (Multipark).
Acknowledgments
The Restorative Parkinson Unit led by PO, thanks the Medical Faculty at Lund University, Multipark, the Swedish Parkinson Academy, the Swedish Parkinson Foundation, the Skåne University Hospital Foundation and Donations, and Global Kinetics Corporation for their support.
Conflict of interest
PO has received honoraria for lectures and expert advice from AbbVie, Bial, Britannia, Ever Pharma, Global Kinetics, Lobsor, Nordic Infucare, Stada, and Zambon. Hehas received funding from AbbVie, Lund University Medical Faculty, Multipark, the Swedish Parkinson Academy, Health Care Region Skåne and Åhlens Foundation. MH received has received funding from Global Kinetics and is an inventor of the PKG and a shareholder in Global Kinetics. He has financial interest in Global Kinetics. FB has received honoraria from Abbvie and Global Kinetics and in-kind research support from Global Kinetics and Sense4Care. He has received funding from Health Care Region Västra Götaland and the Swedish Parkinson Research Foundation. ND has received honoraria from AbbVie, H. Lundbeck AB and Nordic InfuCare AB. She has received funding from the Parkinson Research Foundation, Linköping University.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1591537/full#supplementary-material
References
1. Lajoie, AC, Lafontaine, A-L, and Kaminska, M. The Spectrum of sleep disorders in Parkinson disease: a review. Chest. (2021) 159:818–27. doi: 10.1016/j.chest.2020.09.099
2. Frampton, JE. Rotigotine transdermal patch: a review in Parkinson's disease. CNS Drugs. (2019) 33:707–18. doi: 10.1007/s40263-019-00646-y
3. Trenkwalder, C, Kies, B, Rudzinska, M, Fine, J, Nikl, J, Honczarenko, K, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord. (2011) 26:90–9. doi: 10.1002/mds.23441
4. Pagonabarraga, J, Piñol, G, Cardozo, A, Sanz, P, Puente, V, Otermín, P, et al. Transdermal Rotigotine improves sleep fragmentation in Parkinson's disease: results of the multicenter, prospective SLEEP-FRAM Study. Parkinsons Dis. (2015) 2015:131508:1–7. doi: 10.1155/2015/131508
5. Calandra-Buonaura, G, Guaraldi, P, Doria, A, Zanigni, S, Nassetti, S, Favoni, V, et al. Rotigotine objectively improves sleep in Parkinson's disease: an open-label pilot Study with Actigraphic recording. Parkinsons Dis. (2016) 2016:1–5. doi: 10.1155/2016/3724148
6. Seppi, K, Ray Chaudhuri, K, Coelho, M, Fox, SH, Katzenschlager, R, Perez Lloret, S, et al. Update on treatments for nonmotor symptoms of Parkinson's disease—an evidence-based medicine review. Mov Disord. (2019) 34:180–98. doi: 10.1002/mds.27602
7. Trenkwalder, C, Kies, B, Dioszeghy, P, Hill, D, Surmann, E, Boroojerdi, B, et al. Rotigotine transdermal system for the management of motor function and sleep disturbances in Parkinson’s disease: results from a 1-year, open-label extension of the RECOVER study. Basal Ganglia. (2012) 2:79–85. doi: 10.1016/j.baga.2012.05.009
8. Sun, W, Wang, Q, Yang, T, Feng, C, Qu, Y, Yang, Y, et al. A meta-analysis evaluating effects of the rotigotine in Parkinson's disease, focusing on sleep disturbances and activities of daily living. Neurol Sci. (2022) 43:5821–37. doi: 10.1007/s10072-022-06159-9
9. Antonini, A, Bauer, L, Dohin, E, Oertel, WH, Rascol, O, Reichmann, H, et al. Effects of rotigotine transdermal patch in patients with Parkinson's disease presenting with non-motor symptoms – results of a double-blind, randomized, placebo-controlled trial. Eur J Neurol. (2015) 22:1400–7. doi: 10.1111/ene.12757
10. Schütz, L, Sixel-Döring, F, and Hermann, W. Management of Sleep Disturbances in Parkinson's disease. J Parkinsons Dis. (2022) 12:2029–58. doi: 10.3233/JPD-212749
11. Ohta, K, and Osada, T. Rotigotine transdermal patch does not make Parkinson disease patients sleepy during daytime. Clin Neuropharmacol. (2015) 38:231–5. doi: 10.1097/WNF.0000000000000110
12. Liguori, C, Mercuri, NB, Albanese, M, Olivola, E, Stefani, A, and Pierantozzi, M. Daytime sleepiness may be an independent symptom unrelated to sleep quality in Parkinson’s disease. J Neurol. (2019) 266:636–41. doi: 10.1007/s00415-018-09179-8
13. Rosa-Grilo, M, Qamar, MA, Taddei, RN, Pagonabarraga, J, Kulisevsky, J, Sauerbier, A, et al. Rotigotine transdermal patch and sleep in Parkinson's disease: where are we now? NPJ Parkinsons Dis. (2017) 3:28. doi: 10.1038/s41531-017-0030-4
14. Suzuki, K, Funakoshi, K, Fujita, H, and Hirata, K. The effect of Rotigotine on cognitive function, daytime sleepiness, and sleep problems in Parkinson disease: an open-label pilot Study. Clin Neuropharmacol. (2022) 45:61–4. doi: 10.1097/WNF.0000000000000501
15. Marino, M, Li, Y, Rueschman, MN, Winkelman, JW, Ellenbogen, JM, Solet, JM, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. (2013) 36:1747–55. doi: 10.5665/sleep.3142
16. Stavitsky, K, Saurman, JL, McNamara, P, and Cronin-Golomb, A. Sleep in Parkinson's disease: a comparison of actigraphy and subjective measures. Parkinsonism Relat Disord. (2010) 16:280–3. doi: 10.1016/j.parkreldis.2010.02.001
17. Krause, E, Randhawa, J, and Mehanna, R. Comparing subjective and objective response to medications in Parkinson's disease patients using the personal KinetiGraph™. Parkinsonism Relat Disord. (2021) 87:105–10. doi: 10.1016/j.parkreldis.2021.05.008
18. McGregor, S, Churchward, P, Soja, K, O’Driscoll, D, Braybrook, M, Khodakarami, H, et al. The use of accelerometry as a tool to measure disturbed nocturnal sleep in Parkinson’s disease. npj Parkinson's Dis. (2018) 4:1. doi: 10.1038/s41531-017-0038-9
19. Klingelhoefer, L, Rizos, A, Sauerbier, A, McGregor, S, Martinez-Martin, P, Reichmann, H, et al. Night-time sleep in Parkinson's disease - the potential use of Parkinson's KinetiGraph: a prospective comparative study. Eur J Neurol. (2016) 23:1275–88. doi: 10.1111/ene.13015
20. Kotschet, K, Johnson, W, McGregor, S, Kettlewell, J, Kyoong, A, O'Driscoll, DM, et al. Daytime sleep in Parkinson's disease measured by episodes of immobility. Parkinsonism Relat Disord. (2014) 20:578–83. doi: 10.1016/j.parkreldis.2014.02.011
21. Höglund, A, Hagell, P, Broman, JE, Pålhagen, S, Sorjonen, K, Fredrikson, S, et al. Associations between fluctuations in daytime sleepiness and motor and non-motor symptoms in Parkinson's disease. Mov Disord Clin Pract. (2021) 8:44–50. doi: 10.1002/mdc3.13102
22. Wang, Y, Yang, Y-C, Lan, D-M, Wu, HJ, and Zhao, Z-X. An observational clinical and video-polysomnographic study of the effects of rotigotine in sleep disorder in Parkinson’s disease. Sleep Breath. (2017) 21:319–25. doi: 10.1007/s11325-016-1414-0
23. Pierantozzi, M, Placidi, F, Liguori, C, Albanese, M, Imbriani, P, Marciani, MG, et al. Rotigotine may improve sleep architecture in Parkinson's disease: a double-blind, randomized, placebo-controlled polysomnograwphic study. Sleep Med. (2016) 21:140–4. doi: 10.1016/j.sleep.2016.01.016
24. Tomlinson, CL, Stowe, R, Patel, S, Rick, C, Gray, R, and Clarke, CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. (2010) 25:2649–53. doi: 10.1002/mds.23429
25. Trenkwalder, C, Kohnen, R, Högl, B, Metta, V, Sixel-Döring, F, Frauscher, B, et al. Parkinson's disease sleep scale—validation of the revised version PDSS‐2. Mov Disord. (2011) 26:644–52. doi: 10.1002/mds.23476
26. Johns, MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
27. Jenkinson, C, and Fitzpatrick, R. Cross-cultural evaluation of the short form 8-item Parkinson's disease questionnaire (PDQ-8): results from America, Canada, Japan, Italy and Spain. Parkinsonism Relat Disord. (2007) 13:22–8. doi: 10.1016/j.parkreldis.2006.06.006
28. Devlin, NJ, Shah, KK, Feng, Y, Mulhern, B, and van Hout, B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. (2018) 27:7–22. doi: 10.1002/hec.3564
29. Busner, J, and Targum, SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). (2007) 4:28–37.
30. Chen, JJ, Swope, DM, Dashtipour, K, and Lyons, KE. Transdermal rotigotine: a clinically innovative dopamine-receptor agonist for the management of Parkinson's disease. Pharmacotherapy. (2009) 29:1452–67. doi: 10.1592/phco.29.12.1452
31. Ghoraani, B, Galvin, JE, and Jimenez-Shahed, J. Point of view: wearable systems for at-home monitoring of motor complications in Parkinson's disease should deliver clinically actionable information. Parkinsonism Relat Disord. (2021) 84:35–9. doi: 10.1016/j.parkreldis.2021.01.022
32. Bender, R, and Lange, S. Adjusting for multiple testing—when and how? J Clin Epidemiol. (2001) 54:343–9. doi: 10.1016/S0895-4356(00)00314-0
33. Muntean, ML, Benes, H, Sixel-Döring, F, Chaudhuri, KR, Suzuki, K, Hirata, K, et al. Clinically relevant cut-off values for the Parkinson's disease sleep Scale-2 (PDSS-2): a validation study. Sleep Med. (2016) 24:87–92. doi: 10.1016/j.sleep.2016.06.026
34. Pahwa, R, Bergquist, F, Horne, M, and Minshall, ME. Objective measurement in Parkinson’s disease: a descriptive analysis of Parkinson’s symptom scores from a large population of patients across the world using the personal KinetiGraph®. J Clin Mov Disord. (2020) 7:5. doi: 10.1186/s40734-020-00087-6
35. Raeder, V, Boura, I, Leta, V, Jenner, P, Reichmann, H, Trenkwalder, C, et al. Rotigotine transdermal patch for motor and non-motor Parkinson's disease: a review of 12 Years' clinical experience. CNS Drugs. (2021) 35:215–31. doi: 10.1007/s40263-020-00788-4
36. Nicholas, AP, Borgohain, R, Chaná, P, Surmann, E, Thompson, EL, Bauer, L, et al. A randomized study of rotigotine dose response on 'off' time in advanced Parkinson's disease. J Parkinsons Dis. (2014) 4:361–73. doi: 10.3233/JPD-130320
37. Vallderiola, F, Compta, Y, Aparicio, J, Tarradellas, J, Salazar, G, Oliver, JM, et al. Effects of night-time use of Rotigotine on nocturnal symptoms in Parkinson's disease. Parkinson's Dis. (2015) 2015:1–6. doi: 10.1155/2015/475630
38. Steiger, M. Constant dopaminergic stimulation by transdermal delivery of dopaminergic drugs: a new treatment paradigm in Parkinson's disease. Eur J Neurol. (2008) 15:6–15. doi: 10.1111/j.1468-1331.2007.01674.x
39. Elshoff, JP, Braun, M, Andreas, JO, Middle, M, and Cawello, W. Steady-state plasma concentration profile of transdermal rotigotine: an integrated analysis of three, open-label, randomized, phase I multiple dose studies. Clin Ther. (2012) 34:966–78. doi: 10.1016/j.clinthera.2012.02.008
40. Merino-Andreu, M, Arnulf, I, Konofal, E, Derenne, JP, and Agid, Y. Unawareness of naps in Parkinson's disease and in disorders with excessive daytime sleepiness. Neurology. (2003) 60:1553–4. doi: 10.1212/01.WNL.0000058905.71369.97
Keywords: Parkinson’s disease, Parkinson’s KinetiGraph, rotigotine, sleep disturbances, Parkinson disease sleep scale-2, sleep quality, daytime sleepiness
Citation: Grigoriou S, Janz C, Horne M, Bergquist F, Dizdar N and Odin P (2025) Effects of rotigotine on sleep in Parkinson’s disease patients: a Parkinson’s KinetiGraph study. Front. Neurol. 16:1591537. doi: 10.3389/fneur.2025.1591537
Edited by:
Emilia Mabel Gatto, Sanatorio de la Trinidad Mitre, ArgentinaReviewed by:
Arturo Garay, Norberto Quirno Medical Education and Clinical Research Center (CEMIC), ArgentinaStefania Roxana Diaconu, Transilvania University of Brașov, Romania
Copyright © 2025 Grigoriou, Janz, Horne, Bergquist, Dizdar and Odin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carin Janz, Y2FyaW4uamFuekBtZWQubHUuc2U=
†These authors have contributed equally to this work and share first authorship
 Sotirios Grigoriou
Sotirios Grigoriou Carin Janz
Carin Janz Malcolm Horne
Malcolm Horne Filip Bergquist
Filip Bergquist Nil Dizdar
Nil Dizdar Per Odin
Per Odin