- 1Department of Neurology, University Hospital, Goethe University Frankfurt, Frankfurt, Germany
- 2Department of Anaesthesiology, Intensive Care and Pain Therapy, Sana Hospital Offenbach, Offenbach, Germany
- 3Department of Pediatric Cardiology and Congenital Heart Disease, Deutsches Herzzentrum der Charité, Berlin, Germany
- 4Faculty of Medicine, Goethe University Frankfurt, Frankfurt, Germany
- 5Department of Neurosurgery, University Hospital, Goethe University Frankfurt, Frankfurt, Germany
- 6Department of Traumatology, Hand and Reconstructive Surgery, University Hospital, Goethe University Frankfurt, Frankfurt, Germany
Introduction: Antiplatelet and anticoagulant therapies complicate the management and outcomes of traumatic brain injury (TBI) patients. This study evaluates clinical profiles and short-term outcomes focusing on prior antihemostatic therapy and tranexamic acid (TXA) use.
Patients and methods: We analyzed TBI patients admitted to University Hospital Frankfurt (2018–2021), assessing demographics, injury characteristics, clinical course, and short-term outcomes. The primary endpoint was hemorrhage progression; secondary endpoints included the modified Rankin Scale (mRS) at discharge, mortality and thromboembolic complications. Regression models identified predictors of functional outcome and mortality.
Results: Among 218 patients (median age 70 years, 35% female, median GCS at admission 7), 44% had prior antiplatelet or anticoagulation therapy. These patients were older, had higher pre-injury mRS scores, and more often sustained TBIs from falls. While hemorrhage progression was similar, they had worse mRS scores (p = 0.02) and higher mortality (p = 0.002). Coagulopathy (OR 1.11, CI 1.07–1.16, p < 0.001), injury severity (OR 2.25, CI 1.51–3.41, p < 0.001), and bleeding progression (OR 2.23, CI 1.48–3.41, p < 0.001) predicted poor functional outcomes. TXA was more often given to younger, severely injured patients but did not impact outcome.
Conclusion: This study underscores the need for tailored therapeutic approaches to improve survival and functional recovery in patients with pre-injury antiplatelet and anticoagulant therapies.
Introduction
Traumatic brain injury (TBI) constitutes a leading cause of death and disability worldwide, affecting approximately 69 million people worldwide each year (1–4). In recent decades, the epidemiology of TBI has shifted toward older patients (5, 6). Given the increasing age of TBI patients, comorbidities and the number of patients treated with antiplatelet or anticoagulant therapy rise proportionally (5–7). While in the acute setting of TBI, information on preexisting medication may not always be available at admission, previous studies demonstrated a high frequency of pharmacologically impaired platelet function in TBI patients with unknown preexisting medication (8). This frail patient population is particularly at risk for progressive hemorrhage, which occurs in 30 to over 70% of TBI patients (9–13) and predicts unfavorable clinical outcomes (14–16).
Clinically, hemorrhage progression may present with a decline in neurological status, such as increasing confusion, drowsiness, or focal neurological deficits. Management typically involves careful clinical monitoring and serial imaging. Therapeutic approaches in TBI can only address secondary injury mechanisms which also includes secondary hemorrhage (17–19). To this end, measures such as optimizing hemostasis (e.g., reversal therapies), consistent blood pressure management, and surgical approaches are applied. Regarding the optimization of coagulation, the CRASH−3 trial found that the risk of death was reduced in patients treated with tranexamic acid (TXA) which is why its early administration is currently recommended in patients with mild to moderate TBI, even in patients without prior antihemostatic therapy (20). Prompt recognition and intervention are crucial to improve outcomes as secondary hemorrhage significantly increases the risk of morbidity and mortality in the older patients following TBI.
The aim of the present study was to characterize outcomes following TBI in patients admitted to our hospital with a special focus on pre-existing antiplatelet or anticoagulant therapy and the chosen acute treatment approaches.
Patients and methods
This retrospective study analyzed data from patients with acute TBI, who were admitted to the Center of Neurology and Neurosurgery at Frankfurt University Hospital, Germany between 2018 and 2021. The study was approved by the institutional ethics committee (2022–913). Written informed consent was waived due to the study’s retrospective design.
We included patients with the diagnosis of acute TBI (ICD-10: S06.0 – S06. A) (21), categorizing TBI as acute if admission occurred within 24 h of the trauma.
The following clinical data were captured from medical records: age; sex; injury mechanism; modified Rankin Scale (mRS) prior to TBI; Glasgow Coma Scale (GCS) at admission, after 24 h and at discharge; Richmond Agitation-Sedation Scale (RASS) at admission and at discharge; severity of TBI classified by the GCS score at presentation after resuscitation (grade I “mild”: GCS score 13–15, grade II “moderate”: GCS score 9–12, and finally grade III “severe”: GCS score≤8); surgical interventions such as craniotomy or hemicraniectomy, implantation of an external ventricular drainage (EVD), lumbar drainage (LD) or intracranial pressure monitoring; previous antiplatelet or anticoagulant therapy; laboratory findings; the application of coagulation reversal therapies and/or measures of coagulation and hemostasis optimization such as prothrombin complex concentrate (PCC), fresh frozen plasma (FFP), TXA, vitamin k, Idarucizumab or Andexanet alfa, the application of platelet or erythrocyte concentrates (PC or EC); and finally imaging evidence of intracranial hemorrhage such epidural hematoma (EDH), subdural hematoma (SDH), subarachnoid hemorrhage (SAH) and intraventricular hemorrhage (IVH) and intracerebral hemorrhage (ICH); as well as an open/penetrating TBI and/or midline shift, and finally the restart of antiplatelet or anticoagulant therapies.
The primary endpoint was the rate of secondary hemorrhage progression defined as hemorrhagic lesion expansion measured in the follow-up imaging.
Secondary endpoints included functional outcome at discharge (mRS), mortality and thromboembolic complications (composite endpoint of pulmonary embolism, myocardial infarction, deep vein thrombosis and cerebral ischemic stroke).
Statistical analyses were performed using GraphPad Prism 10 Version 10.3.1 and GNU R 4.4.1 Ordinal and non-normal continuous data is depicted as median and corresponding interquartile range (IQR), normally distributed continuous data is depicted as mean ± standard deviation of the mean (SD) or standard error of the mean (SEM). Frequencies of nominal data are depicted as percentages. Data was assessed for normal distribution using the Kolmogorov–Smirnov-test. Intergroup differences of the included characteristics were evaluated using the t test, Mann–Whitney U test, χ2 or fisher’s test, or using the Kruskal-Wallis test if more than two groups were compared. A p-value < 0.05 was set as level of significance. To account for patient age, sex, and injury severity (GCS score at admission, TBI grade and evidence of open/penetrating mechanism), propensity score matching was applied. For predictor analysis, ordered multinomial and logistic regression models were performed.
Results
Study population
The search based on the inclusion criteria yielded 406 patients. After excluding 188 patients (8 duplicates; 5 with brain tumors, 8 with meningeomas, 5 with cerebral metastases, 1 with cerebral amyloid angiopathy, 32 patients without identifiable trauma, 95 with non-acute trauma and 34 with unknown latency between trauma and admission), the final analysis included 218 patients with acute TBI (Figure 1).
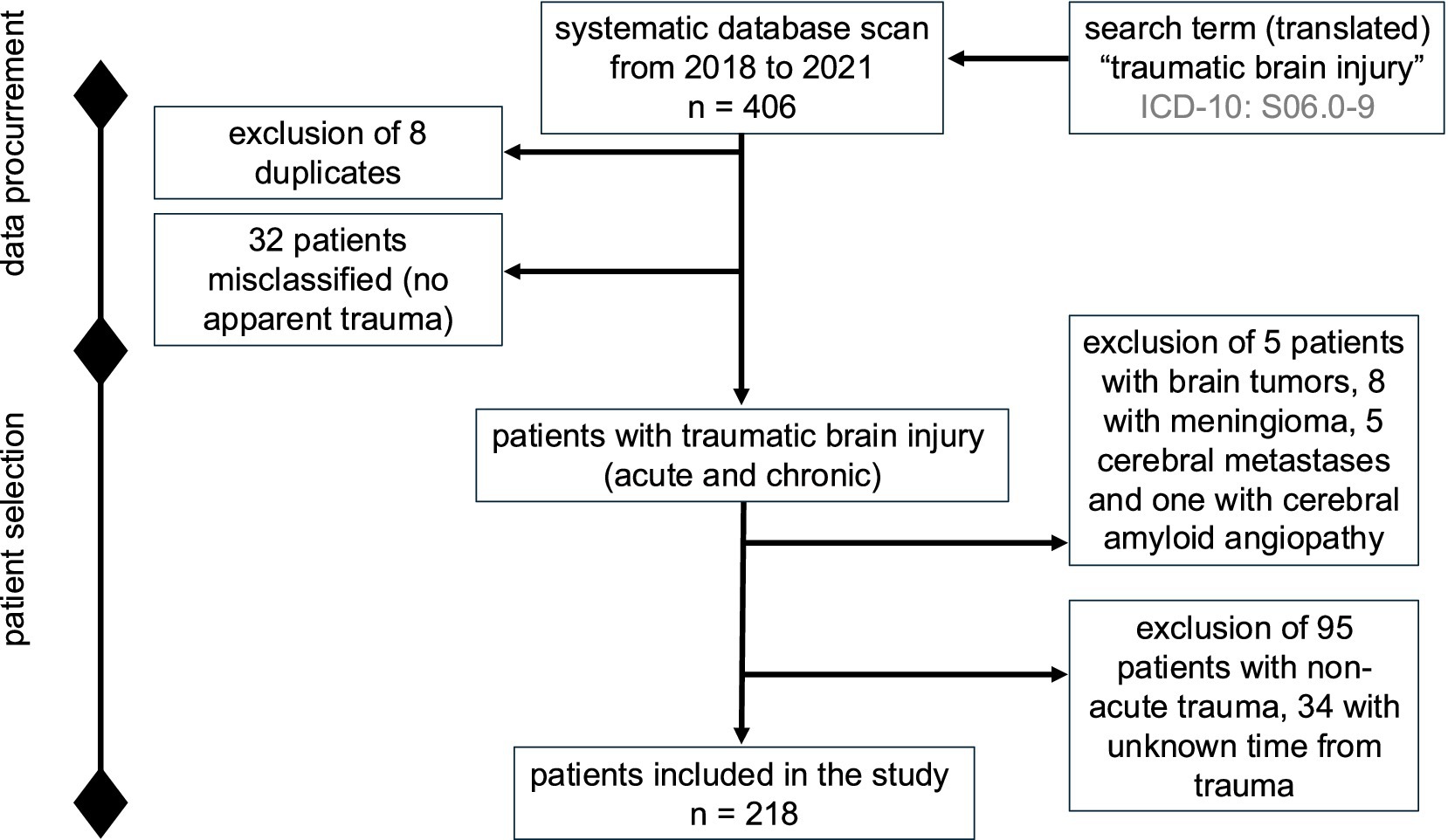
Figure 1. Study flowchart describing the data procurement and stepwise patient selection process intended for retrospective analysis.
The median age of the cohort was 70 (IQR 50–81) years, 76 (34.9%) patients were female. In 119 cases (54.6%), the injury mechanism was unspecified; however, falls accounted for 79 cases (36.2%). The median GCS score at admission was 7. Additional baseline characteristics are detailed in Table 1.
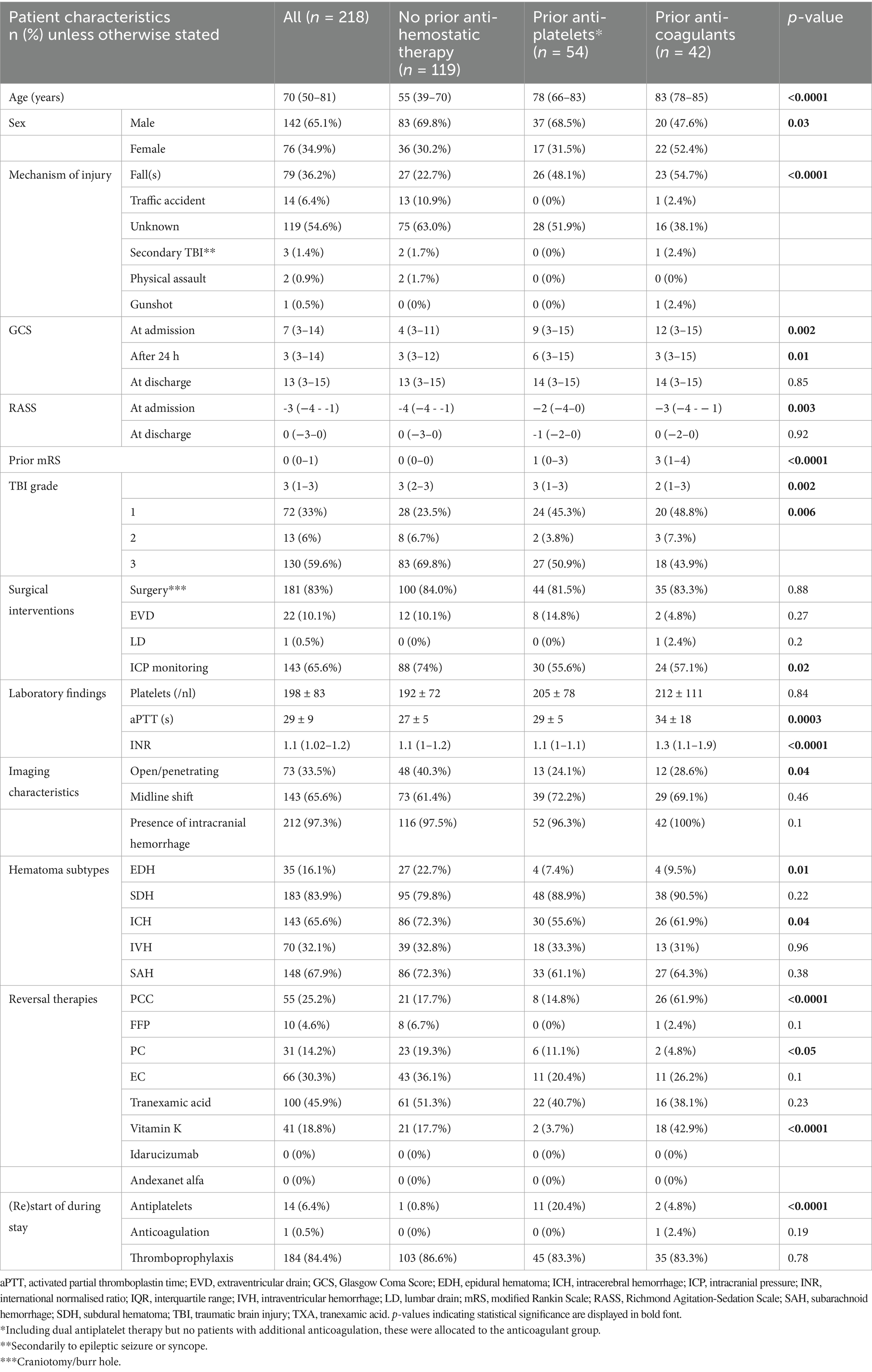
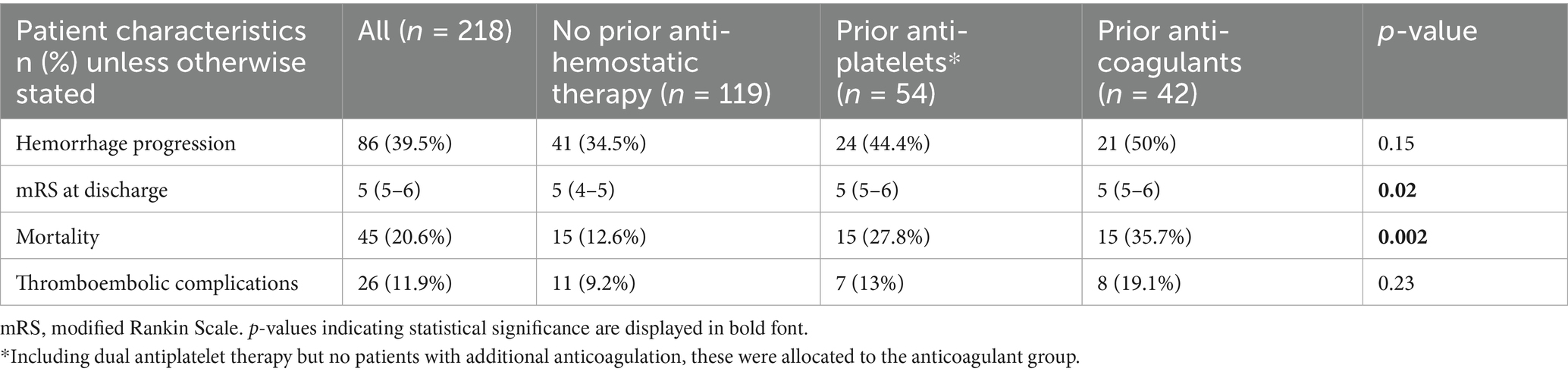
Table 1. Patient demographics, injury characteristics and clinical course depending on prior antihemostatic medication status.
Previous antihemostatic therapy
Of the total cohort, 96 patients (44%) had prior antiplatelet or anticoagulant therapy. In detail, 62 patients (28.4%) were on antiplatelet therapy, with 9 (4.1%) receiving dual antiplatelet therapy (DAPT). Among the patients with prior antiplatelet therapy, 8 also received anticoagulant therapy 3 with the vitamin k antagonist (VKA) phenprocoumon (Marcumar ®), 4 with direct oral anticoagulants (DOACs) and 1 with a therapeutic dose of low molecular weight heparin (LMWH). A total of 42 patients (19.3%) received prior anticoagulant therapy (including the aforementioned 8 patients with concomitant antiplatelet therapy), there were 13 patients on VKA, 24 on DOACs and 5 on therapeutic LMWH or unfractionated heparin (UFH). Patients on DOACs, LMWH, UFH and VKA were grouped together as receiving “prior anticoagulant therapy.” Data on previous medication was not available in 3 patients, who were excluded from the subsequent analysis.
Patient and injury characteristics by antihemostatic therapy status
Patients with prior antiplatelet or anticoagulant therapy were significantly older and had higher pre-injury mRS scores, particularly those on anticoagulant therapy (p < 0.0001; Table 1). Falls were more frequently the cause of TBI in these groups (p < 0.0001). Sex distribution differed slightly: females were under-represented in patients on antiplatelet therapy or no prior medication, but more prevalent among those on anticoagulation. As expected, these patients showed the highest values in INR and aPTT (p < 0.0001 and p = 0.0003, respectively). Patients on prior anticoagulant therapy more frequently received PCC and vitamin k (p < 0.0001).
Patients without prior antihemostatic therapy exhibited more severe injuries, as reflected by lower GCS scores at admission and after 24 h (p = 0.002 and p = 0.01, respectively), as well as higher TBI grades (p = 0.002). Accordingly, we found significantly more EDH and ICH in patients without prior antihemostatic therapy (p = 0.01 and 0.04, respectively) compared to patients on prior anticoagulant or antiplatelet therapy. Most patients across all groups underwent some form of surgical intervention, with significantly more ICP monitoring in patients without prior antihemostatic therapy (p = 0.02). Thrombosis prophylaxis with low-molecular-weight heparin (subcutaneous) was also carried out equally frequently during the hospital stay. Antiplatelet therapy was resumed in 20.4% of the patients with prior antiplatelet therapy, whereas anticoagulant therapy was restarted in only one case of the prior anticoagulant group.
Hemorrhage progression and functional outcomes
The overall occurrence of hemorrhage progression did not differ significantly by prior antihemostatic therapy. However, we observed worse functional outcomes (higher mRS scores) in patients on prior antiplatelet or anticoagulant therapy (p = 0.02; Table 2; Figure 2A). Intrahospital mortality was significantly higher in these patients (p = 0.002). Thromboembolic complications were similar across groups.
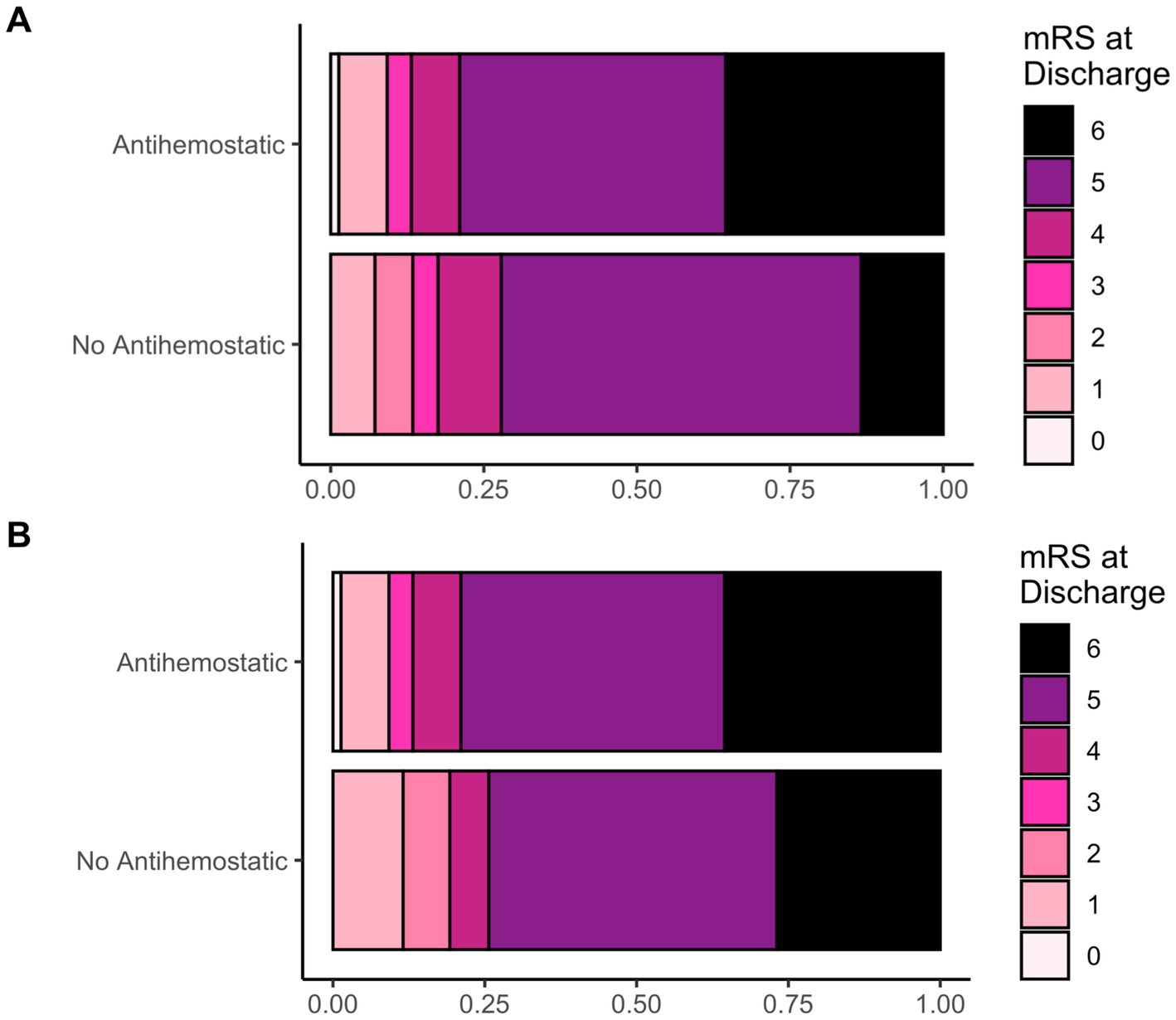
Figure 2. (A) Frequency distribution of the neurological outcome based on mRS at discharge (displayed as fractions) in patients with or without prior antihemostatic therapy (antiplatelet and anticoagulant together) and (B); after propensity score matching.
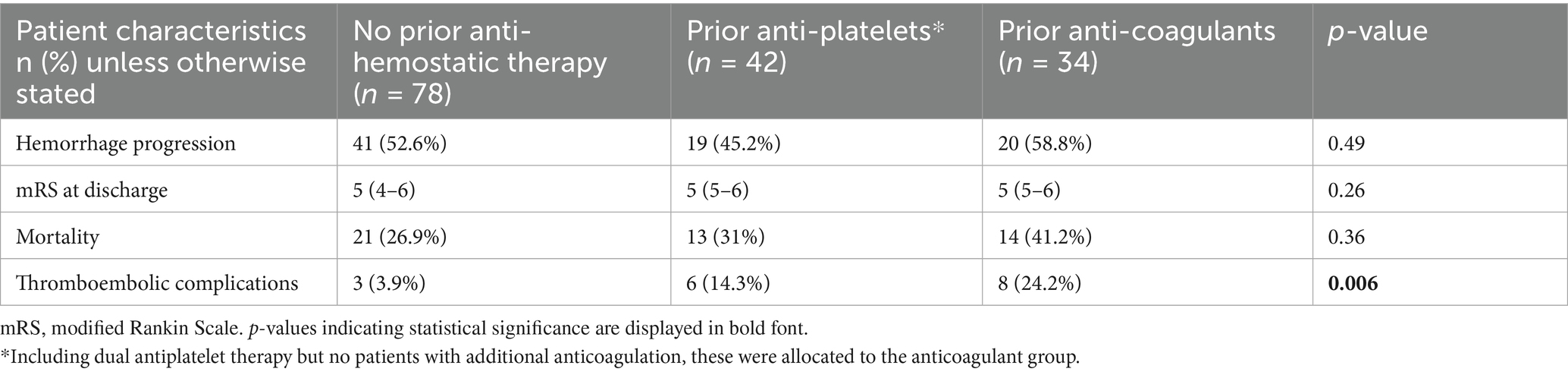
After propensity score matching for patient age, sex, GCS score at admission, TBI grade and open/penetrating injuries, mRS scores at discharge and mortality were no longer significantly different (Table 3; Figure 2B), while thromboembolic complications now reached statistical significance.
Predictors of functional outcomes and mortality
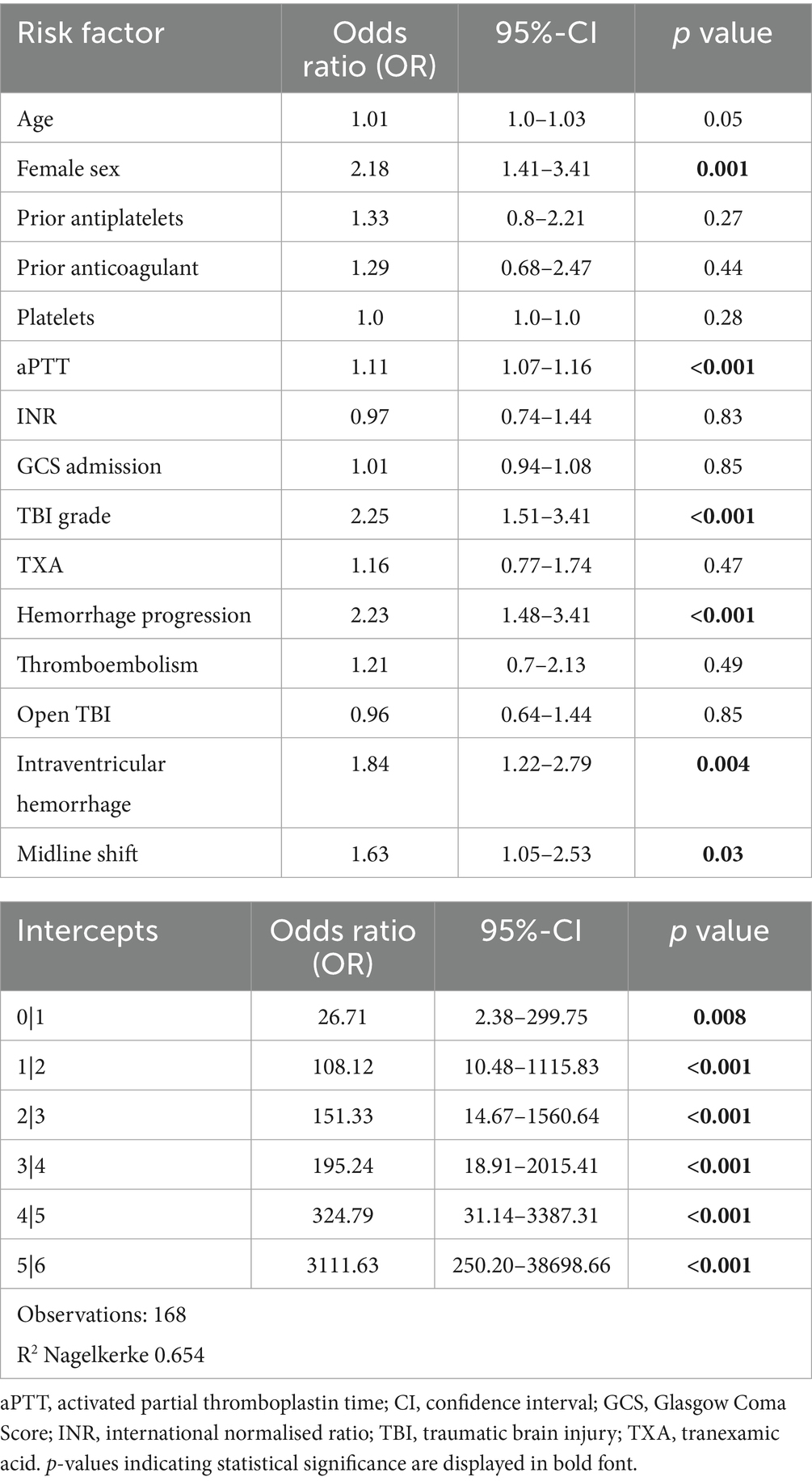
Ordered multinomial regression identified the following predictors of unfavorable outcomes (higher mRS scores): female sex (OR 2.18, CI 1.41–3.41, p = 0.001), prolonged aPTT at admission (OR 1.11, CI 1.07–1.16, p < 0.001), TBI grade (OR 2.25, CI 1.51–3.41, p < 0.001), midline shift (OR 1.63, CI 1.05–2.53, p = 0.03), intraventricular hemorrhage (OR 1.84, CI1.22–2.79, p = 0.004) and bleeding progression (OR 2.23, CI 1.48–3.41, p < 0.001, Table 4).
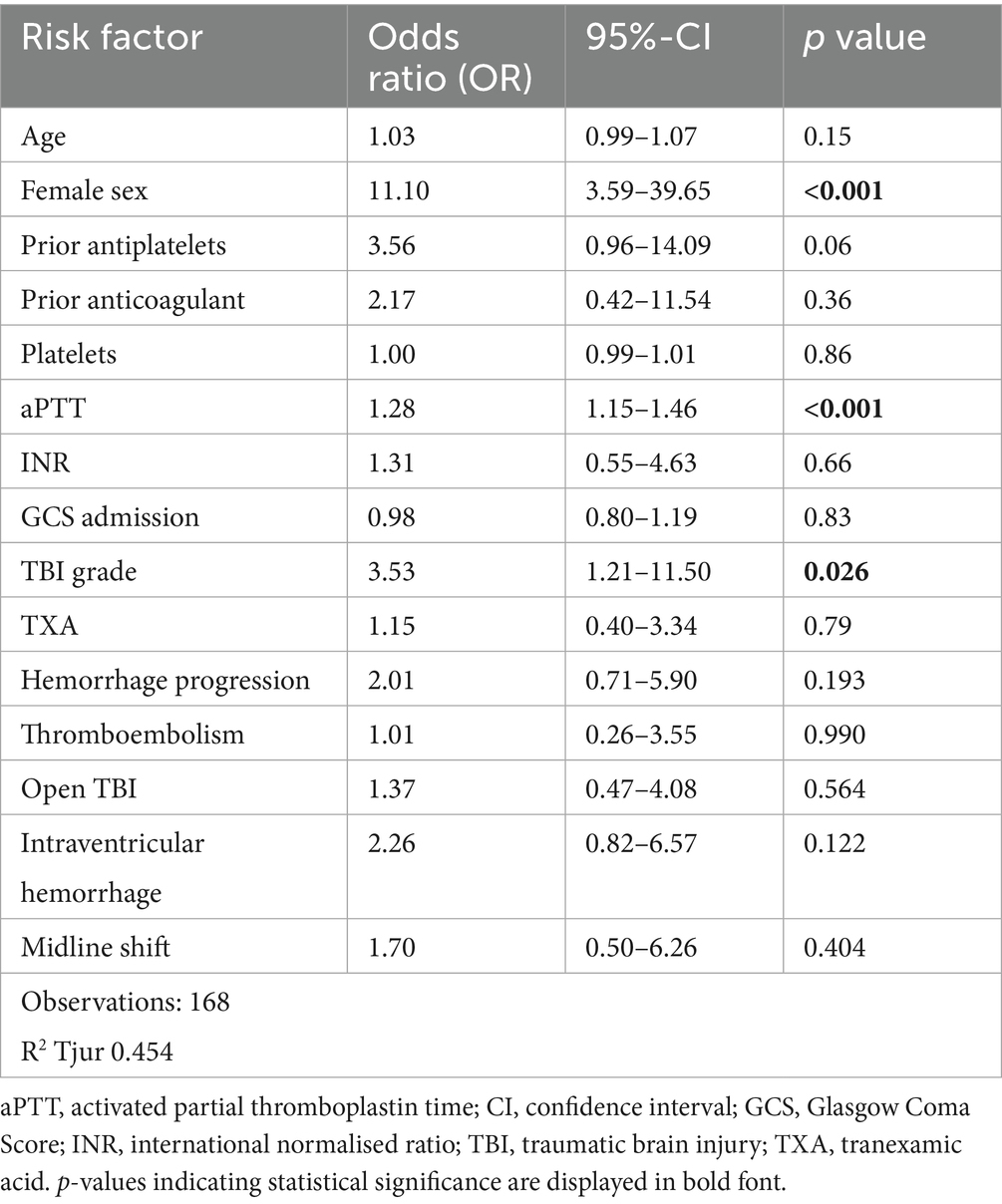
For mortality, logistic regression identified female sex (OR 11.1, CI 3.59–39.65, p < 0.001), TBI grade (OR 3.53, CI 1.21–11.5, p = 0.026) and prolonged aPTT (OR 1.28, CI 1.15–1.46, p < 0.001) as significant predictors (Table 5).
Baseline and injury characteristics by patient sex
In order to further investigate the sex-related effects on functional outcomes and mortality observed in the previous analysis, we conducted a secondary subanalysis in which we compared baseline characteristics and injury patterns between female and male patients. Female patients were on average older (p < 0.0001), showed a (non-significant) trend toward higher pre-injury mRS scores (p = 0.06), and more frequently received prior anticoagulant therapy (p = 0.01). Mechanisms of injury did not differ between sexes (p = 0.2); however female patients tended to present with a slightly higher incidence of midline shift (p = 0.03). Interestingly, surviving female patients demonstrated greater alertness (higher GCS scores and RASS scores) at discharge compared to males (p = 0.003 for the GCS score at discharge and p = 0.002 for the RASS score at discharge). Further sex-based differences are presented in Supplementary Table S1.
TXA administration
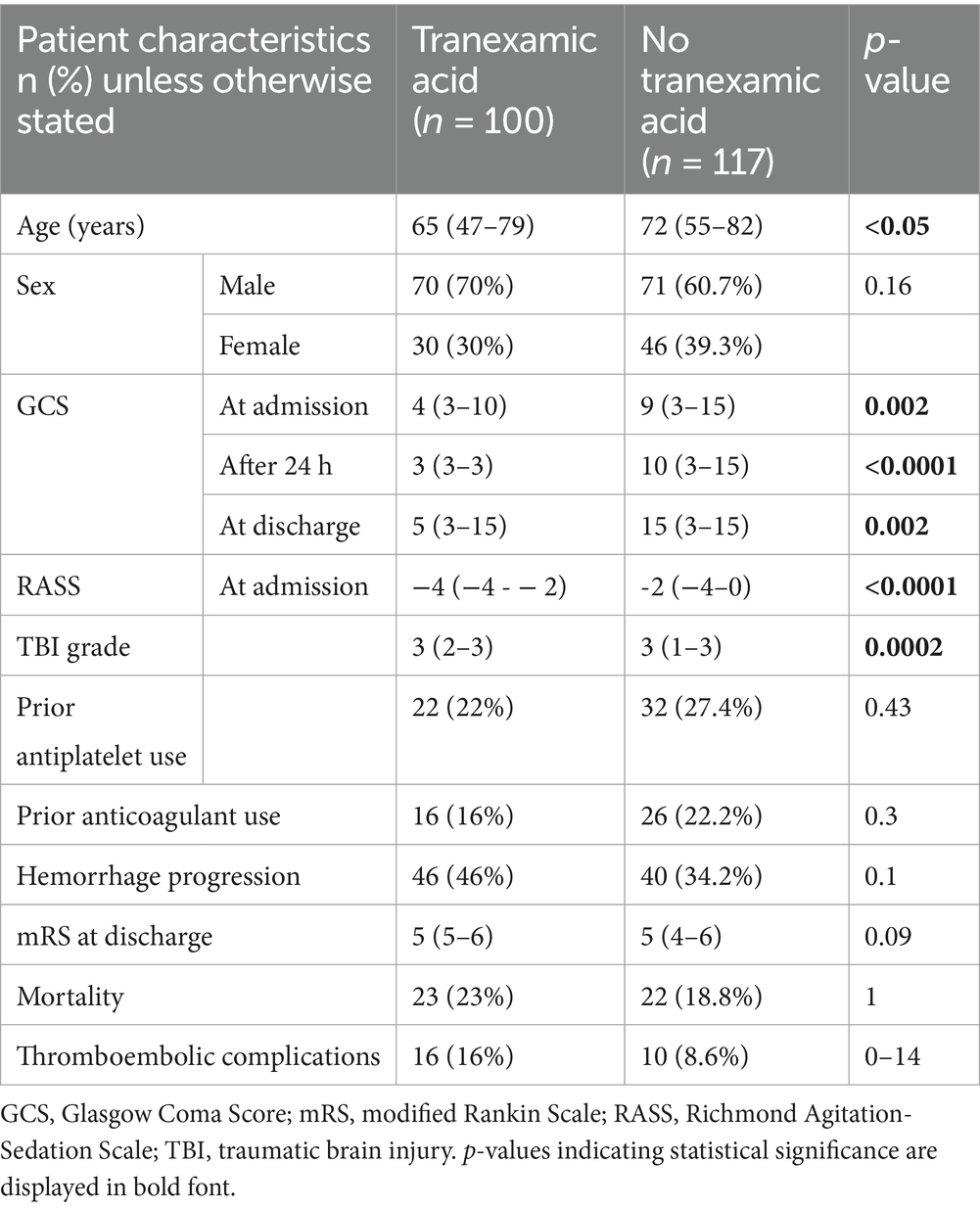
Finally, the use of TXA was examined. Patients receiving TXA (n = 100) were younger (p < 0.05) and had more severe injuries, as indicated by lower GCS scores at admission (p = 0.002), after 24 h (p < 0.0001) and at discharge (p = 0.002); lower RAAS scores at admission (p < 0.0001) and higher TBI grades (p = 0.0002). Sex distribution and prior antiplatelet or anticoagulant use did not differ. However, in terms of outcome parameters, hemorrhage progression, thromboembolic complications, intrahospital mortality and mRS scores at discharge did not differ significantly between the two groups. Data on TXA administration was missing for 1 patient, and 117 patients did not receive TXA (Table 6).
Discussion
Our tertiary trauma center predominantly manages severe TBIs with traumatic intracranial hemorrhages requiring urgent surgical intervention. However, even within this highly selective cohort, clear differences in the clinical profiles of TBI patients with and without prior antihemostatic therapy were observed. Patients with prior antiplatelet or anticoagulant therapy were older and had higher pre-injury disability as indicated by pre-injury mRS scores, reflecting the common overlap between advanced age, comorbidities, and the need for these medications. Falls were the predominant cause of TBI in this group, consistent with previous studies linking anticoagulant use to fall-related injuries among older populations (22, 23). While the rate of hemorrhage progression did not differ between patients with and without antihemostatic therapy, functional outcomes were worse. As such, patients with prior antiplatelet or anticoagulant therapy also exhibited significantly increased mortality rates, aligning with prior literature emphasizing the heightened risk of poor outcomes in patients with TBI under VKA (24, 25), but also DOACs, which may appear to be safer for this particular patient group (26–28).
After propensity score matching, however only thromboembolic complications remained significantly more frequent in patients with prior antiplatelet or anticoagulant therapy, which is not surprising when considering that these therapies are usually discontinued when traumatic intracranial hemorrhage is detected, at least temporarily. A significant body of literature exists, describing the influence of antiplatelets and anticoagulation therapies on the occurrence of traumatic intracranial bleeding (29–31). It is well established that they increase the risk of intracranial hemorrhage and rebleeding but do not necessarily correlate with poorer outcomes in mild and moderate to severe TBI requiring neurosurgical interventions, which our study now confirms (32–35). However, our findings underline the need for tailored clinical management and prevention strategies in this vulnerable population.
Non-modifiable factors like sex and TBI severity (indicated by TBI grade, IVH and midline shift) consistently predicted poor outcomes, while bleeding progression and coagulopathy (increased aPTT) were key modifiable contributors to mortality and morbidity. Thus, aggressive management strategies to mitigate bleeding complications in trauma patients, as the prevention of bleeding progression and managing coagulopathy remain crucial in improving outcomes (36, 37).
Considering the comparison between patient sexes, it is likely that the strong association of female sex with poorer functional outcomes, particularly reduced survival, observed in the predictor analysis may be attributable to confounding factors such as advanced patient age and pre-existing conditions. However, surviving females demonstrated a higher level of alertness at discharge compared to male patients. Previous literature regarding the impact of sex and gender on TBI outcomes is controversial: Some studies suggest that women of childbearing age may have a better prognosis due to hormonal benefits (38), while other studies show no significant differences or suggest that women may fare worse in certain cases, possibly due to hormonal fluctuations or higher prevalence of psychological consequences such as depression and anxiety disorders (39). Further research is needed to better understand gender- and sex-specific differences and to tailor treatment accordingly.
We here also investigated TXA administration and its potential benefits. This is of relevance as the hemostatic and immune systems interact in response to trauma, where plasmin stimulates complement activation. TXA competitively inhibits the conversion of plasminogen to plasmin, which, in turn, inhibits fibrin degeneration. This inhibition of fibrin degeneration may restrict the inflammatory response that can be provoked by fibrin degradation products. Patients receiving TXA presented with more severe injuries and worse baseline GCS scores. We did not detect a difference in hemorrhage progression or functional outcomes in the TXA group. These findings contradict the results of the CRASH-3 trial, which demonstrated that early TXA administration within 3 h of injury reduces mortality in TBI without increasing thromboembolic risks. However, patients with GCS of 3 and those with bilateral unreactive pupils at baseline were excluded. The effect was greatest for patients with mild and moderate head injuries but not apparent in those with severe head injuries (20). Our data showed that TXA was mainly given to severely affected patients, suggesting selection bias in its administration and explaining the lack of a statistically detectable effect. This emphasizes the need for standardized protocols to ensure that TXA benefits are consistently realized across diverse patient populations, as patients with mild and moderate injury who would benefit most from TXA appear to be less likely to receive it.
Our study has important limitations. At first, as a retrospective, single-center study, the findings are inherently limited by the quality and completeness of the recorded data and are subject to selection bias influencing the cohort composition. In our tertiary care trauma center, many of the patients were secondary transfers requiring surgical intervention, which resulted in predominantly moderate to severe injuries. This reduces the generalizability of the findings to broader populations, including those with milder TBIs managed in non-tertiary settings. The study lacked information on critical variables in a significant proportion of patients. For example, the mechanism of injury was not documented in over half of the cases. The study primarily focused on short-term outcomes, such as intrahospital mortality, functional status at discharge, and immediate complications. Long-term follow-up, which is essential to understanding recovery trajectories, reintegration into daily life, and the sustained impact of therapeutic interventions, was not included. This restricts the ability to draw conclusions about the prolonged effects of factors like antihemostatic therapy or its reversal. Furthermore, information on the individual pharmacological response in the antiplatelet group would be of interest as this might also significantly affect patient outcomes and should be addressed in future studies. Lastly, the study grouped patients with different types of anticoagulants and antiplatelet therapies together, despite their varying mechanisms of action and potential risks. As such, phenprocoumon, DOACs, LMWH, and UFH were pooled for statistical analysis. This approach may obscure nuanced differences in how specific agents impact TBI outcomes and limit the applicability of findings to clinical decision-making for particular drugs.
From a clinical perspective, our findings still reinforce the importance of individualized care for TBI patients, particularly those on antiplatelet or anticoagulant therapy. Early and comprehensive coagulation management, including timely reversal of anticoagulation and cautious thromboprophylaxis, remains critical for optimizing outcomes.
Conclusion
In conclusion, this study underscores the complex interplay of age, anticoagulation, coagulopathy, and injury severity in determining TBI outcomes. It highlights the need for tailored therapeutic approaches and continued investigation into predictive factors and interventions, including TXA, to improve survival and functional recovery in this vulnerable population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Frankfurt University Hospital Ethics Committee (No. 2022–913). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
FL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. DM: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. KK: Formal analysis, Supervision, Writing – review & editing. DT: Validation, Writing – review & editing. JS: Writing – review & editing. SR: Writing – review & editing. JK: Writing – review & editing. CS: Writing – review & editing. CG: Writing – review & editing. FB: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
FL reports speakers’ honoraria from Laerdal. FB reports speakers’ honoraria, grants and personal from AstraZeneca, Bristol-Myers-Squibb, Laerdal, Medtronic Pfizer, Alexion, Boehringer Ingelheim and Stryker Neurovascular. JS reports travel reimbursements by Bayer AG. SR reports speakers’ honoraria from Astra Zeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1592583/full#supplementary-material
References
1. Peeters, W, Majdan, M, Brazinova, A, Nieboer, D, and Maas, AIR. Changing epidemiological patterns in traumatic brain injury: a longitudinal hospital-based study in Belgium. Neuroepidemiology. (2017) 48:63–70. doi: 10.1159/000471877
2. Roozenbeek, B, Maas, AIR, and Menon, DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. (2013) 9:231–6. doi: 10.1038/nrneurol.2013.22
3. Ramanathan, DM, McWilliams, N, Schatz, P, and Hillary, FG. Epidemiological shifts in elderly traumatic brain injury: 18-year trends in Pennsylvania. J Neurotrauma. (2012) 29:1371–8. doi: 10.1089/neu.2011.2197
4. Dewan, MC, Rattani, A, Gupta, S, Baticulon, RE, Hung, YC, Punchak, M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. (2019) 130:1080–97. doi: 10.3171/2017.10.JNS17352
5. Dams-O’Connor, K, Juengst, SB, Bogner, J, Chiaravalloti, ND, Corrigan, JD, Giacino, JT, et al. Traumatic brain injury as a chronic disease: insights from the United States traumatic brain injury model systems research program. Lancet Neurol. (2023) 22:517–28. doi: 10.1016/S1474-4422(23)00065-0
6. Giner, J, Mesa Galán, L, Yus Teruel, S, Guallar Espallargas, MC, Pérez López, C, Isla Guerrero, A, et al. Traumatic brain injury in the new millennium: new population and new management. Neurología. (2019) 37:383–89. doi: 10.1016/j.nrleng.2019.03.024
7. Baggiani, M, Guglielmi, A, and Citerio, G. Acute traumatic brain injury in frail patients: the next pandemic. Curr Opin Crit Care. (2022) 28:166–75. doi: 10.1097/MCC.0000000000000915
8. Prinz, V, Finger, T, Bayerl, S, Rosenthal, C, Wolf, S, Liman, T, et al. High prevalence of pharmacologically induced platelet dysfunction in the acute setting of brain injury. Acta Neurochir. (2016) 158:117–23. doi: 10.1007/s00701-015-2645-8
9. Narayan, RK, Maas, AIR, Servadei, F, Skolnick, BE, Tillinger, MN, and Marshall, LF. Progression of traumatic intracerebral hemorrhage: a prospective observational study. J Neurotrauma. (2008) 25:629–39. doi: 10.1089/neu.2007.0385
10. Oertel, M, Kelly, DF, McArthur, D, Boscardin, WJ, Glenn, TC, Lee, JH, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. (2002) 96:109–16. doi: 10.3171/jns.2002.96.1.0109
11. Kurland, D, Hong, C, Aarabi, B, Gerzanich, V, and Simard, JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma. (2012) 29:19–31. doi: 10.1089/neu.2011.2122
12. Tong, W-S, Zheng, P, Xu, J-F, Guo, YJ, Zeng, JS, Yang, WJ, et al. Early CT signs of progressive hemorrhagic injury following acute traumatic brain injury. Neuroradiology. (2011) 53:305–9. doi: 10.1007/s00234-010-0659-8
13. Chang, EF, Meeker, M, and Holland, MC. Acute traumatic intraparenchymal hemorrhage: risk factors for progression in the early post-injury period. Neurosurgery. (2006) 58:647–56. doi: 10.1227/01.NEU.0000197101.68538.E6
14. Juratli, TA, Zang, B, Litz, RJ, Sitoci, K-H, Aschenbrenner, U, Gottschlich, B, et al. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. J Neurotrauma. (2014) 31:1521–7. doi: 10.1089/neu.2013.3241
15. Carnevale, JA, Segar, DJ, Powers, AY, Shah, M, Doberstein, C, Drapcho, B, et al. Blossoming contusions: identifying factors contributing to the expansion of traumatic intracerebral hemorrhage. J Neurosurg. (2018) 129:1305–16. doi: 10.3171/2017.7.JNS17988
16. Yuan, F, Ding, J, Chen, H, Guo, Y, Wang, G, Gao, WW, et al. Predicting progressive hemorrhagic injury after traumatic brain injury: derivation and validation of a risk score based on admission characteristics. J Neurotrauma. (2012) 29:2137–42. doi: 10.1089/neu.2011.2233
17. Jamjoom, AAB, Rhodes, J, Andrews, PJD, and Grant, SGN. The synapse in traumatic brain injury. Brain. (2021) 144:18–31. doi: 10.1093/brain/awaa321
18. Cash, A, and Theus, MH. Mechanisms of blood-brain barrier dysfunction in traumatic brain injury. Int J Mol Sci. (2020) 21:3344. doi: 10.3390/ijms21093344
19. Corps, KN, Roth, TL, and McGavern, DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. (2015) 72:355–62. doi: 10.1001/jamaneurol.2014.3558
20. The CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. (2019) 394:1713–23.
21. World Health Organization (WHO) (2019). International statistical classification of diseases and related health problems (ICD), 10th revision
22. Peeters, W, van den Brande, R, Polinder, S, Brazinova, A, Steyerberg, EW, Lingsma, HF, et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. (2015) 157:1683–96. doi: 10.1007/s00701-015-2512-7
23. Maas, AIR, Menon, DK, Manley, GT, Abrams, M, Åkerlund, C, Andelic, N, et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. (2022) 21:1004–60. doi: 10.1016/S1474-4422(22)00309-X
24. Della Pepa, GM, Covino, M, Menna, G, Auricchio, AM, Polli, FM, Manno, A, et al. Are oral anticoagulants a risk factor for mild traumatic brain injury progression? A single-center experience focused on of direct oral anticoagulants and vitamin K antagonists. Acta Neurochir. (2022) 164:97–105. doi: 10.1007/s00701-021-05066-w
25. Tykocki, T, and Guzek, K. Anticoagulation therapy in traumatic brain injury. World Neurosurg. (2016) 89:497–504. doi: 10.1016/j.wneu.2016.01.063
26. Batey, M, Hecht, J, Callahan, C, and Wahl, W. Direct oral anticoagulants do not worsen traumatic brain injury after low-level falls in the elderly. Surgery. (2018) 164:814–9. doi: 10.1016/j.surg.2018.05.060
27. Santing, JAL, Lee, YX, van der Naalt, J, van den Brand, CL, and Jellema, K. Mild traumatic brain injury in elderly patients receiving direct Oral anticoagulants: a systematic review and Meta-analysis. J Neurotrauma. (2022) 39:458–72. doi: 10.1089/neu.2021.0435
28. Cipriano, A, Pecori, A, Bionda, AE, Bardini, M, Frassi, F, Leoli, F, et al. Intracranial hemorrhage in anticoagulated patients with mild traumatic brain injury: significant differences between direct oral anticoagulants and vitamin K antagonists. Intern Emerg Med. (2018) 13:1077–87. doi: 10.1007/s11739-018-1806-1
29. Podolsky-Gondim, GG, Cardoso, R, Zucoloto Junior, EL, Grisi, L, Medeiros, M, de Souza, SN, et al. Traumatic brain injury in the elderly: clinical features, prognostic factors, and outcomes of 133 consecutive surgical patients. Cureus. (2021) 13:e13587. doi: 10.7759/cureus.13587
30. Scotti, P, Séguin, C, Lo, BWY, de Guise, E, Troquet, J-M, and Marcoux, J. Antithrombotic agents and traumatic brain injury in the elderly population: hemorrhage patterns and outcomes. J Neurosurg. (2020) 133:486–95. doi: 10.3171/2019.4.JNS19252
31. Vedin, T, Lundager Forberg, J, Anefjäll, E, Lehtinen, R, Faisal, M, and Edelhamre, M. Antiplatelet therapy contributes to a higher risk of traumatic intracranial hemorrhage compared to anticoagulation therapy in ground-level falls: a single-center retrospective study. Eur J Trauma Emerg Surg. (2022) 48:4909–17. doi: 10.1007/s00068-022-02016-8
32. O’Donohoe, RB, Lee, HQ, Tan, T, Hendel, S, Hunn, M, Mathews, J, et al. The impact of preinjury antiplatelet and anticoagulant use on elderly patients with moderate or severe traumatic brain injury following traumatic acute subdural hematoma. World Neurosurg. (2022) 166:e521–7. doi: 10.1016/j.wneu.2022.07.042
33. Greuter, L, Ullmann, M, Mariani, L, Guzman, R, and Soleman, J. Effect of preoperative antiplatelet or anticoagulation therapy on hemorrhagic complications in patients with traumatic brain injury undergoing craniotomy or craniectomy. Neurosurg Focus. (2019) 47:E3. doi: 10.3171/2019.8.FOCUS19546
34. Ott, MM, Eriksson, E, Vanderkolk, W, Christianson, D, Davis, A, and Scholten, D. Antiplatelet and anticoagulation therapies do not increase mortality in the absence of traumatic brain injury. J Trauma. (2010) 68:560–3. doi: 10.1097/TA.0b013e3181ad6600
35. Mathieu, F, Güting, H, Gravesteijn, B, Monteiro, M, Glocker, B, Kornaropoulos, EN, et al. Impact of antithrombotic agents on radiological lesion progression in acute traumatic brain injury: a CENTER-TBI propensity-matched cohort analysis. J Neurotrauma. (2020) 37:2069–80. doi: 10.1089/neu.2019.6911
36. Rakhit, S, Nordness, MF, Lombardo, SR, Cook, M, Smith, L, and Patel, MB. Management and challenges of severe traumatic brain injury. Semin Respir Crit Care Med. (2021) 42:127–44. doi: 10.1055/s-0040-1716493
37. Barletta, JF, Shirah, GR, Mangram, AJ, Sucher, JF, Hostert, SA, Bruce, K, et al. Reversal of pre-injury factor-Xa inhibitors with prothrombin complex concentrates in patients following traumatic brain injury. Clin Neurol Neurosurg. (2023) 235:108040. doi: 10.1016/j.clineuro.2023.108040
38. Späni, CB, Braun, DJ, and Van Eldik, LJ. Sex-related responses after traumatic brain injury: considerations for preclinical modeling. Front Neuroendocrinol. (2018) 50:52–66. doi: 10.1016/j.yfrne.2018.03.006
Keywords: traumatic brain injury, anticoagulation, (dual) antiplatelet therapy, intracranial hemorrhage, demographic change, falls, elderly
Citation: Lieschke F, Marggrander DT, Kohlhase KD, Touma D, Schaefer JH, Reitz SC, Konczalla J, Schindler CR, Grefkes C and Bohmann FO (2025) Coagulopathy, injury severity and bleeding progression but not prior antiplatelet and anticoagulation therapies drive prognosis in patients with moderate to severe traumatic brain injury. Front. Neurol. 16:1592583. doi: 10.3389/fneur.2025.1592583
Edited by:
Jin Lu, Shanghai Jiao Tong University, ChinaReviewed by:
Matthew J. Robson, University of Cincinnati, United StatesBradley Dengler, Uniformed Services University of the Health Sciences, United States
Copyright © 2025 Lieschke, Marggrander, Kohlhase, Touma, Schaefer, Reitz, Konczalla, Schindler, Grefkes and Bohmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ferdinand O. Bohmann, Qm9obWFubkBtZWQudW5pLWZyYW5rZnVydC5kZQ==
 Franziska Lieschke
Franziska Lieschke Daniel T. Marggrander2,3
Daniel T. Marggrander2,3 Konstantin D. Kohlhase
Konstantin D. Kohlhase Jan Hendrik Schaefer
Jan Hendrik Schaefer Sarah C. Reitz
Sarah C. Reitz Juergen Konczalla
Juergen Konczalla Cora R. Schindler
Cora R. Schindler Ferdinand O. Bohmann
Ferdinand O. Bohmann