- 1Department of Neurological Sciences, Division of Epilepsy, University of Nebraska Medical Center, Omaha, NE, United States
- 2Department of Neurological Sciences, Division of Neuropsychology, University of Nebraska Medical Center, Omaha, NE, United States
- 3Department of Radiology, University of Nebraska Medical Center, Omaha, NE, United States
- 4Department of Neurological Sciences, Division of Neuroimmunology, University of Nebraska Medical Center, Omaha, NE, United States
Introduction: Autoimmune encephalitis (AE) is associated with severe cognitive disability. Brain metabolic dysfunction has been linked to encephalopathy in neurodegenerative disorders; however, its role in the development of cognitive loss in AE has not been studied. We hypothesized that cognitively impaired patients with AE will demonstrate altered brain metabolism and immune activation, and these measures will correlate with cognitive scores.
Methods: The hippocampal and cortical metabolites related to neuronal integrity, oxidative metabolism, and glial activation were assessed using single-voxel proton magnetic resonance spectroscopy (1H-MRS) in patients with AE, non-lesional temporal lobe epilepsy (TLE) and control subjects. Metabolite levels were correlated with neuropsychological test scores.
Results: We recruited patients with post-acute AE (n = 12), non-lesional TLE (n = 12), and control subjects (n = 11). Subjective cognitive complaints were reported by 83.3% of AE and all TLE patients. AE patients had fewer seizures and used fewer anti-seizure medications than TLE patients (p = 0.04, t-test and p = 0.03, post-hoc test). On neuropsychological testing, moderate and severe cognitive impairment was revealed in 58.3% of patients with AE and 41.6% of patients with TLE. Hippocampal myo-inositol (M-Ins) concentrations were higher in patients compared to control subjects, with a trend toward increase in AE and TLE relative to control (p = 0.046, ANOVA; p = 0.09 and p = 0.07 for AE and TLE vs. control, respectively; post-hoc tests). The concentration of creatine (tCr) and total choline (tCho) were significantly higher in patients with TLE compared to the controls (tCr: p = 0.007; tCh: p = 0.04; post-hoc tests). Elevated M-Ins in AE was associated with better attention but worse memory recognition scores (R2 = 0.38, p = 0.04 and R2 = 0.50, p = 0.02, respectively); higher tCr levels correlated with faster processing speed (R2 = 0.38; p = 0.04). The higher concentrations of tCr, tCho, and M-Ins in TLE have selectively correlated with worse measures of attention, processing speed, language, and memory.
Conclusions: Although AE and TLE patients report similar cognitive issues, their hippocampal metabolic signatures differ. The disease-specific changes in the measures of hippocampal inflammation and neuronal integrity can inform trajectories for cognitive recovery and be targeted therapeutically.
1 Introduction
Autoimmune encephalitis (AE) is an acute severe non-infectious brain inflammation associated with autoantibodies against cell surface proteins and intracellular targets (1). The AE syndromes share common manifestations of precipitous cognitive decline, psychosis, abnormal movements, and new-onset seizures (2). Once the acute life-threatening symptoms are controlled, patients face persistent cognitive loss which can continue for years and can be life-long (3, 4). Cognitive deterioration, particularly memory and language dysfunction results in loss of productivity and greatly impacts patients' quality of life. Considering that the incidence of AE has been consistently on the rise (5) and the success of existing therapies is modest, new therapeutic targets to preserve cognition are urgently needed.
The most common AE syndrome, anti-NMDA receptor (NMDAR) encephalitis, targets the hippocampus and other cortical areas. It leads to executive dysfunction and memory difficulties that persist for more than two years after the resolution of the acute phase (6, 7). The incomplete recovery of cognitive function is particularly detrimental to young patients who have a very high incidence of anti-NMDAR encephalitis as it limits their employment and other social opportunities. The anti-LGI1-antibody-associated encephalitis is another prevalent AE syndrome that affects limbic temporal lobe areas leading to severe permanent deficits in anterograde and episodic memory functions in up to one-third of patients (8, 9). Lastly, the anti-glutamic acid decarboxylase (GAD65)- associated AE which also has a predilection to the limbic areas, results in long-lasting executive dysfunction and prominent amnesia in recovering patients (10, 11). Chronic memory impairment is also present in AE syndromes for which the antibody targets have not been yet identified (i.e., antibody-negative AE) (12) suggesting that the pathophysiology of chronic cognitive loss could be shared among encephalitis syndromes.
The distinct pathophysiological mechanisms mediate the acute effects of autoantibodies supporting the unique clinical presentations of these syndromes. However, the chronic sequala of AE which persists upon removal of antibodies, could share the same pathophysiology. One potential contributor to the cognitive loss in AE could be chronic immune activation which may persist despite the immunotherapies. Neuroinflammation and brain metabolic dysfunction have been recently looked at in the context of cognitive dysfunction in patients with mild cognitive impairment (MCI) and Alzheimer's Disease (AD) (13, 14). However, it is not clear whether it contributes to cognitive loss in AE. In the present study, we used single-voxel proton magnetic resonance spectroscopy (1H-MRS) semi-Localized by Adiabatic Selective Refocusing (semiLASER) sequence (15) to assess relevant metabolite measures in AE and correlate them with patients' cognitive scores obtained during targeted neuropsychological evaluation. Compared to conventional sequences, semi-LASER provides improved accuracy and sensitivity in measuring metabolite concentrations, especially at higher field strengths and in brain structures with less uniform magnetic fields, such as the hippocampus (16). To determine if changes in metabolites could be influenced by the effects of anti-seizure medications (ASMs) and to establish the role of seizures in these changes, we compared the metabolic profile of patients with AE to that of patients with non-lesional temporal lobe epilepsy (TLE) and control subjects. Our focus was on the hippocampal-based cognitive measures and regional 1H-MRS markers of neuronal integrity, oxidative stress, and immune activation (17). We hypothesized that cognitively impaired patients with AE will demonstrate altered metabolism and enhanced immune activation in the hippocampus and these measures will correlate with their performance on cognitive tests.
2 Materials and methods
2.1 Participants
The study was approved by the Institutional Review Board (IRB) at the University of Nebraska Medical Center (UNMC); all the participants signed the informed consent. This prospective non-interventional cohort study followed the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) document (Supplementary Table 1). Male and female patients with a history of definite antibody-positive AE and probable antibody-negative AE established based on the accepted diagnostic criteria (2) were recruited from the Autoimmune Seizure Clinic and Neuroimmunology Clinic at UNMC between April 1, 2023, and June 30, 2024. The inclusion criteria were clinical recovery from acute encephalitis and eligibility for magnetic resonance imaging (MRI). Patients of both sexes with TLE without structural lesions on clinical MRI and subjective complaints of cognitive impairment were recruited from the epilepsy clinic at UNMC, the level 4 epilepsy center. The diagnosis of focal epilepsy was confirmed with ictal electroencephalography (EEG) recordings. The control subjects without a history of neurological diseases matched to the patients with respect to age, sex, and educational level were recruited from the Brain and Mind Health Registry at UNMC. The group size was determined based on the findings from the pilot study.
2.2 Clinical assessments
The demographic and clinical data of patients were extracted from the charts. The diagnosis of AE was confirmed by two neurologists experienced in autoimmune seizure disorders (O.T. and L. A.) (2); the disease duration was defined as the interval between the onset of symptoms and the time of the research imaging and cognitive tests. The patients' definite encephalitis type was supported by the antibody status of their cerebrospinal fluid (CSF) or serum (2). The clinical symptoms, seizure status, EEG and imaging findings at onset as well as utilization of ASM and immunotherapy agents were recorded at the last follow-up visit (Supplementary Tables 3, 4).
2.3 Neuropsychological evaluation
The targeted neuropsychological evaluation was conducted by a clinical neuropsychologist (A.M.) or neuropsychology postdoctoral fellow (K. G.) and included the assessment of attention, processing speed, executive functioning, language, visuoperception, memory, and recognition functions (Supplementary Table 2). The examiners were blinded to the disease status of the participants. Participant responses were scored using normative data to account for age, sex, and educational variability; the normative scores were standardized into z-scores for analysis. Additionally, each test and selected task components were separated into their respective domains and the mean z-score was calculated for each domain generating an estimate of domain functioning. Abnormal performance was defined as one or more scores falling at least 1.5 standard deviations (SD) below the mean (z score = ≤ -1.5) (6). Further, a cognitive impairment score, or a composite score, was assigned based on the overall number of affected domains, which was defined as mild (1 domain affected), moderate (2–3 domains affected), or severe (≥4 domains) (6). All participants were screened for depression, anxiety, and insomnia using the self-report questionnaires (Supplementary Table 2).
2.4 Imaging studies
2.4.1 MRI and single-voxel 1H-MRS acquisition
All data were obtained on a Siemens Prisma VE11c 3.0 T scanner (Siemens Healthcare, Erlangen, Germany) equipped with 80 mT/m at 200 T/m/s high-performance gradients with high-order shim and highest density 32-channel head coil during one or two sessions within 1–2 weeks from the cognitive testing. Total scanning time was approximately 90 min. The 3D T1-weighted magnetization-prepared rapid gradient-echo sequence (MPRAGE, TR/TE = 2300/2.29 ms, FOV = 240 mm, 192 slices, voxel size = 0.9 × 0.9 × 1.0 mm) was used to acquire anatomical reference images used for volume of interest (VOI) placement. Single-voxel 1H-MRS data was acquired on the left and right hippocampi (VOI: 30 × 12 × 12 mm3; ≈4.3 ml) and left prefrontal cortex (VOI:16 × 16 × 16 mm3; ≈4.1 ml) using a short TE semiLASER sequence (TR/TE = 5000/30 ms, spectral points = 2048, spectral width = 6002.4 Hz, averages = 128) with 2 ms excitation and 4 ms Frequency Offset Corrected Inversion (FOCI) refocusing pulses (18–20) (Figure 1). Water suppression was achieved using Variable Power and Optimized Relaxation (VAPOR) delays (21). B0 shimming was performed with Fast, Automatic Shimming Technique by Mapping Along Projections (FASTMAP) (22). B1 pulses were manually calibrated for each VOI. Outer volume suppression (OVS) was used to suppress contamination from signals originating from outside the VOI. In addition to metabolite spectra (n = 128), water reference scans (n = 4) were acquired. Center frequency was 2.67 ppm for all metabolites and 4.67 ppm for water scans. Spectra were saved both as the sum and as single transients. The image analysis was performed by a neuroradiologist (M.W.) and a computational scientist with expertise in MRS (M.U.); all investigators were blinded to the participant's clinical status.
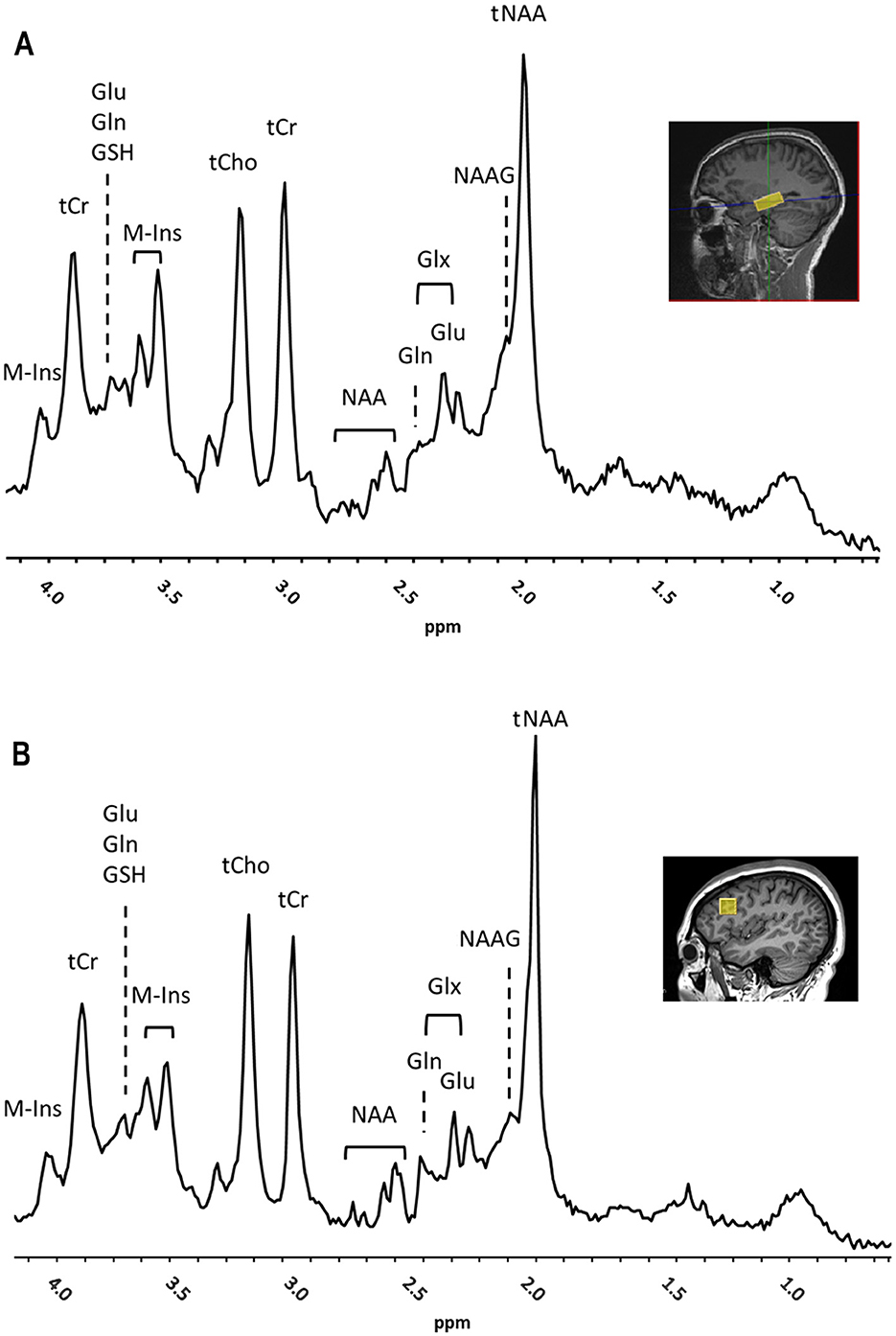
Figure 1. Representative 1H-MRS spectra and placement of volume of interest (VOI) (yellow box) for right hippocampus (A) and left prefrontal cortex (B) showed on a sagittal MRI (insets) from a subject with autoimmune encephalitis (AE). Main peaks for total N-acetyl aspartate and N-acetyl–aspartyl–glutamate (tNAA), total choline (tCho), total creatine (tCr), myo-Inositol (M-Ins), glutamate (Glu), glutamine (Gln), Glx, Glu and Gln, and glutathione (GSH), are labeled. Chemical Shift was expressed in parts per million (ppm).
2.4.2 1H-MRS analysis
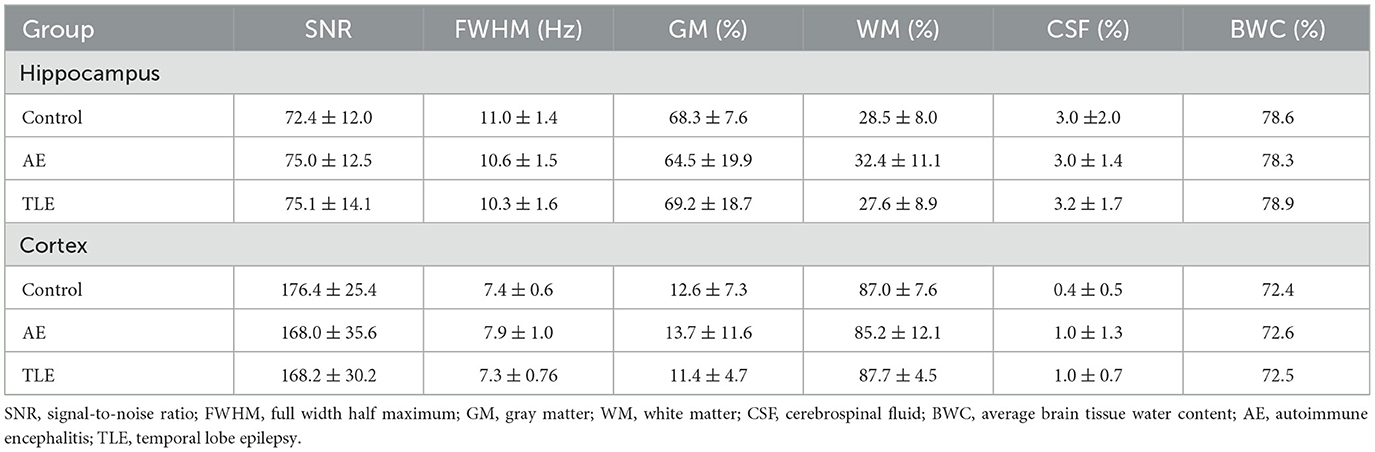
The metabolites that reflected the neuronal health and integrity (23), brain oxidative metabolism (24), and immune activation (25) were measured in the left and right hippocampus and left prefrontal cortex (Figures 1A, B). The total mean concentration of total N-acetyl-aspartate [tNAA: NAA and N-acetyl–aspartyl–glutamate (NAAG)], choline-containing compounds (tCho), creatine and phosphocreatine (tCr), glutamate (Glu), glutamine (Gln), myo-inositol (M-Ins) and glutathione (GSH) were calculated. The MRS data were preprocessed using MRSpa software in Matlab (26). For each VOI, the resulting 128 transients of the suppressed free induction decay (FID) signal were processed as follows: (1) Eddy current correction was applied using the unsuppressed “water” signal, (2) motion-corrupted transients were removed to ensure data quality, (3) frequency shift and phase variations in each transient were corrected, and (4) all corrected transients were added to generate a final FID. Average brain tissue water content was determined, assuming 81% of water in gray matter (GM), 71% in white matter (WM) and 100% in CSF using calculated within-VOI fractions of GM, WM, and CSF obtained from tissue segmentation (27, 28). The within-VOI average brain tissue water (BWC) and the mean CSF fraction were used to correct metabolite concentrations (29, 30). Fitting was carried out with a linear combination modeling in the LCModel software using a simulated basis set composed by 20 metabolite and 1 macromolecule baseline signals as pre-knowledge, and accounting for tissue type composition in the VOI (31, 32). Spectral analysis was conducted over the 0.5–4.2 ppm range, with zero- and first-order phases set to zero. Concentrations of metabolites were calculated relative to the concentration of water in the VOI in absolute units (in μmol/g). For each VOI, the signal-to-noise ratio (SNR) was measured as a ratio of the height of NAA peak at 2.02 ppm and root mean square of the noise (at ≈ [−2 0] ppm) in the metabolite spectra. In addition, the linewidth was calculated as full width at half maximum (FWHM) in the water spectra. SNR and FWHM were used as indicators of spectral quality; the data points were included if SNR ≥ 12 and FWHM < 0.1 ppm. Moreover, for each of the metabolite concentration measurements, the goodness of fit threshold for inclusion in the analysis (quantification reliability) was determined by a mean Cramer-Rao Lower Bounds (CRLB) ≤ 30% SD. One patient data set was excluded from further analysis due to poor spectra quality caused by movement. All MRS data were confirmed to meet the quality criteria based on the experts' consensus recommendations (16).
2.4.3 Segmentation
Tissue segmentation of within-VOI brain volume was performed using SPM12 software (33, 34) and a custom MATLAB script in order to determine and compare VOI tissue composition of GM, WM, and CSF between groups, as described in previous studies (28). The calculated within-VOI BWC and fraction of CSF were used to correct metabolite concentration values for each group.
2.5 Statistical analysis
The quantitative variables and categorical variables were compared using ANOVA and Fisher exact tests, respectively. The post hoc Tukey tests adjusted for pairwise comparisons were used when appropriate (GraphPad Prism 10.2.3, Boston, MA). The proportions of patients were compared using Fisher exact tests. The relationships between cognitive measures and brain metabolites were studied using linear regression analysis; Pearson correlation coefficients were computed. The fractions of WM, GM and CSF in the MRS VOI were compared between the control, AE and TLE groups using multivariate ANOVA. The SNR and FWHM values were compared between the groups using ANOVA.
3 Results
3.1 Patient characteristics
Two and one control subjects were excluded because of the abnormal cognitive scores and unacceptable 1H-MRS data quality, respectively yielding 11 subjects. Additional patients with cognitive complaints and AE or TLE were recruited (n = 12 per group). There were no significant differences concerning age, sex, handedness, and years of education between the three groups (Table 1). The durations of AE and TLE were comparable although it tended to be shorter in AE (p = 0.06; t-test; Table 1). The median seizure counts and ASM usage were significantly lower in AE compared to the TLE group (p = 0.04, t-test and p = 0.03, post-hoc test). The non-ASM usage was higher in the TLE group compared to the control (p = 0.02, post-hoc test; Table 1).
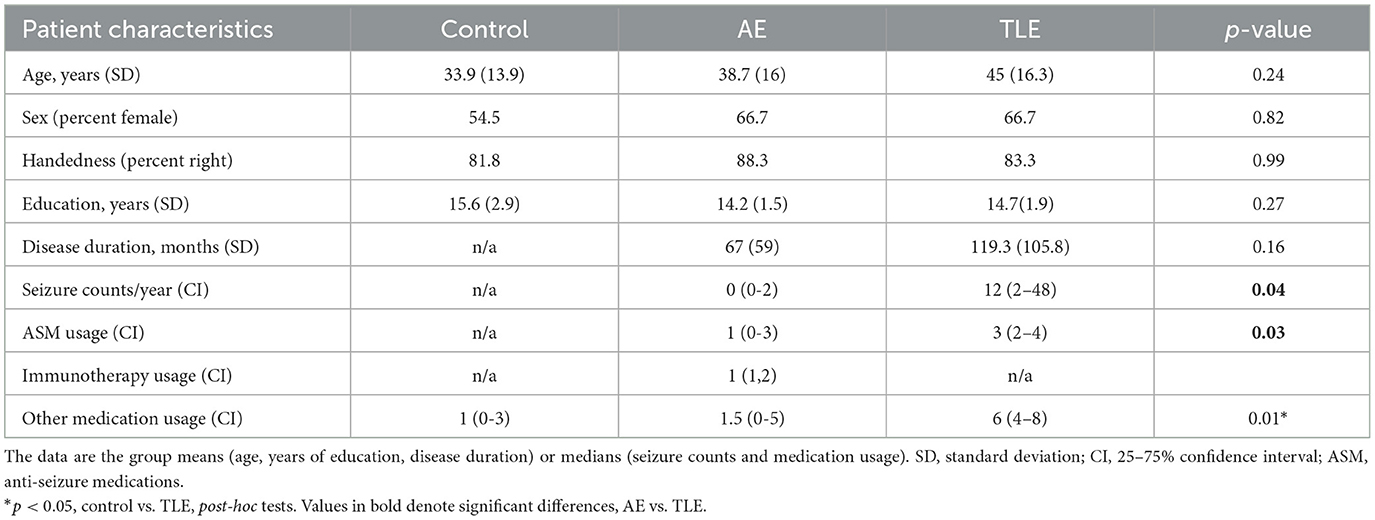
Table 1. Demographic and clinical characteristics of patients with autoimmune encephalitis (AE), temporal lobe epilepsy (TLE), and control subjects.
The mean age (SD) of patients with AE was 38.7 (16) years. The anti-NMDAR encephalitis was diagnosed in 5 patients while anti-LGI-1 encephalitis and GAD-65 encephalitis were present in 1 and 2 patients, respectively (Supplementary Table 3). Four patients had probable AE with no detectable antibodies (2). Among those, 3 patients have recovered from the new onset refractory status epilepticus (NORSE). The paraneoplastic etiology was ruled out in all patients with appropriate diagnostic tests. The mean disease duration from the onset of first symptoms to the time of study in patients with AE was 67 (59) months. The temporal lobe appearance in MRI was unremarkable in 10 patients but showed decreased size and increased signal intensity in bilateral hippocampi in one patient and decreased volume of the left amygdala in another patient (Supplementary Table 3). The annual seizure frequency (median; confidence interval, CI) was 0 (0–2) and the ASM usage was 1 (0–3). Patients were receiving immunotherapy with 1 (1,2) agents, including rituximab, intravenous immunoglobulin, and anakinra (Supplementary Table 3). All patients were living independently while 6 were employed and 1 was studying in college.
Patients with TLE had a mean age of 45 (16.3) years and suffered from epilepsy on average for 119.3 (105.8) months (Table 1). The disease was deemed to be medically refractory in 9 out of 12 (75%) patients (Supplementary Table 4). The MRI did not reveal structural abnormalities in the temporal lobes of patients with TLE except for a suspected left hippocampal cyst in one patient and bilateral temporal meningoceles in another patient (Supplementary Table 4). Patients reported a median annual count of 12 (2–48) seizures with various semiology and were on 3 (2–4) ASMs (Table 1).
3.2 Cognitive deficits, mood disturbances, and sleep disorders
Ten patients with AE (83.3%) and all patients with TLE reported subjective complaints of cognitive impairments. Upon formal testing, patients with AE and TLE showed deficits in multiple tests designed to evaluate attention, processing speed, executive function, language, learning, memory, and recognition (Figure 2A). Abnormal performance in patients with AE was most apparent on the tasks of attention, processing speed, language, visuospatial skills, learning, and recognition. The deficits in the TLE group were most prominent in the processing speed, language, visuospatial skills, memory, and recognition (Figure 2A). To determine the distribution of the overall cognitive performance in both groups, a composite qualitative severity score was assigned based on the number of domains that were deficient. The proportions of patients with normal performance and mild, moderate, and severe impairment were 25, 16.7, 33.3, and 25% in AE and 25, 33.3, 8.3, and 33.3% in the TLE group, respectively (Figure 2B) There were no significant differences between the distributions of these patients (p = 0.3; Fisher exact test).
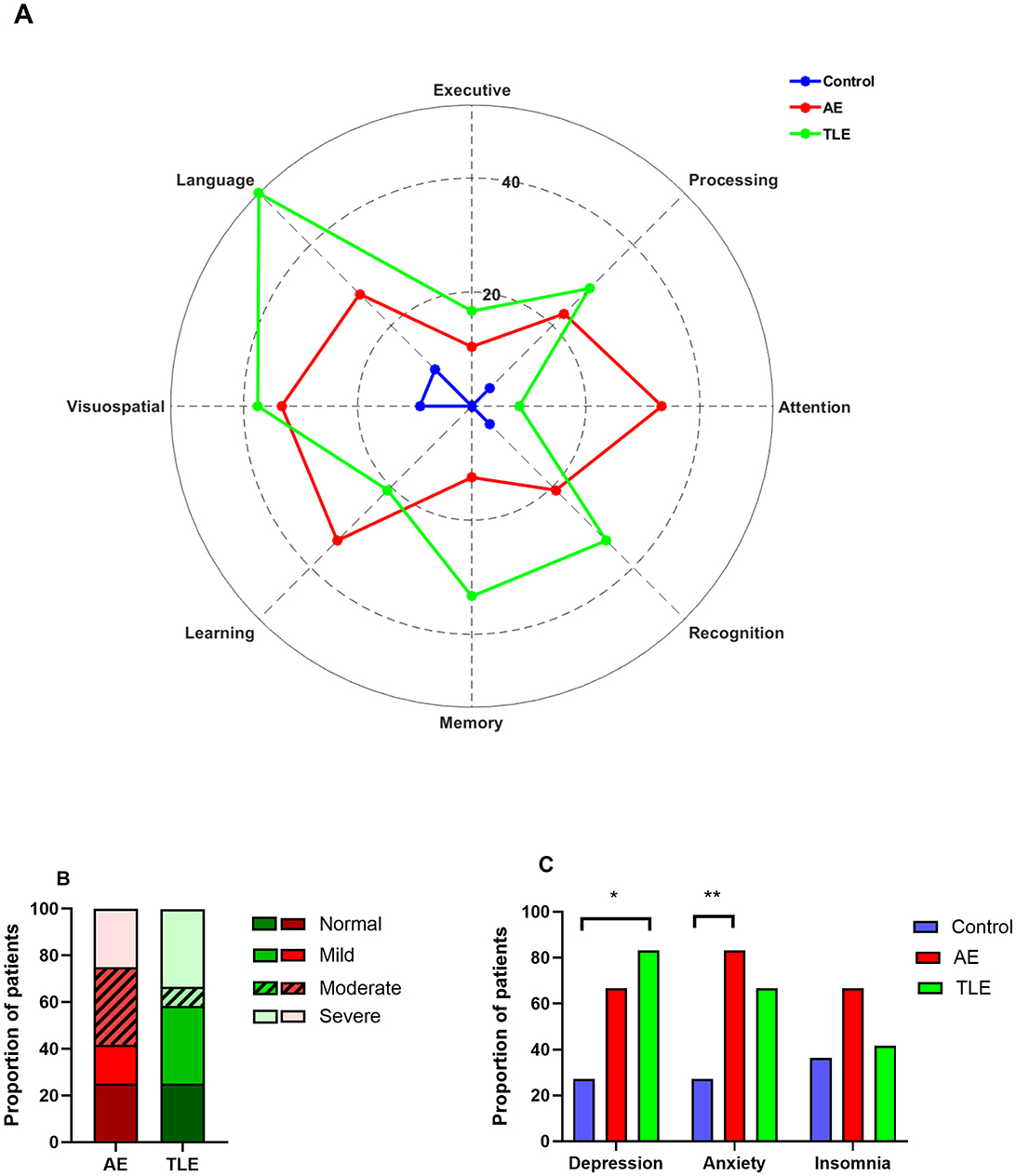
Figure 2. Cognitive, psychiatric and sleep disturbances in patients with autoimmune encephalitis (AE), temporal lobe epilepsy (TLE). (A) Distribution of patients with abnormal cognitive performance among AE, TLE and normal subjects. The data are mean proportions of patients with abnormal scores in each cognitive domain. The abnormal scores were defined as z ≤ −1.5. (B) The spectrum of the cognitive impairment severity in patients with AE and TLE. The data are the proportions of patients with different severity categories in two groups. (C) Psychiatric co-morbidities and insomnia profiles of patients with AE, TLE, and control subjects. The data are the proportions of patients. *, p < 0.05; **, p < 0.01; Fisher exact tests.
Given that psychiatric co-morbidities and insomnia are exceedingly prevalent in AE (9), we determined how these disorders have affected our populations. Overall, the proportions of patients with depression and anxiety differed significantly among the three groups (p = 0.03 and p = 0.03, respectively; Fisher exact tests). However, the proportions of patients with insomnia were comparable in three groups (p = 0.34, Fisher exact test). Specifically, patients with AE reported anxiety more often than control subjects but experienced depression and insomnia as frequently as control subjects (p = 0.01; p = 0.1; p = 0.22, respectively; Fisher exact tests; Figure 2C). On the other hand, patients with TLE had higher rates of depression compared to the control subjects but had similar rates of anxiety and insomnia (p = 0.01; p = 0.1 and p = 0.99, respectively; Figure 2C). The rates of depression, anxiety and insomnia were comparable in patients with AE and TLE (p = 0.64, p = 0.64; p = 0.41, respectively).
3.3 Brain metabolite measures
All metabolites of interest were reliably quantified, except for Gln, which had a mean CRLB > 30% and was excluded from the analysis (Supplementary Figures 2A, B). The concentrations of metabolites (in μmol/g) in the left and right hippocampus were comparable (tNAA: p = 0.42, tCho: p = 0.92, tCr: p = 0.30, Glu: p = 0.32, M-Ins: p = 0.36, GSH: p = 0.94; t-tests). Therefore, the mean values were used. The SNR and linewidth were of high quality and consistent across groups. The spectral SNRs (mean and SD) were 73.77 ± 11.70 and 74.27 ± 13.89 for the right and left hippocampus VOI, respectively. The spectral SNR for the cortical VOI was 165.13 ± 31.41. The FWHM values were 10.66 ± 1.44, 10.68 ± 1.60 and 7.33 ± 1.52 Hz for the right hippocampus, left hippocampus and cortical VOI, respectively. There were no differences between the SNR and FWHM values in the hippocampus of the control, AE, and TLE groups (p = 0.26 and p = 0.12, respectively; ANOVA). Furthermore, there were no differences in these values in the cortex of the control, AE, and TLE groups (p = 0.68 and p = 0.61, respectively; ANOVA). The average within-VOI tissue composition – fractions of GM, WM, and CSF- were similar among groups in both hippocampus (Control vs. AE: p = 0.50, Control vs. TLE: p = 0.57, AE vs. TLE: p = 0.43; multivariate ANOVA Wilk's Lambda test) and cortex (Control vs. AE: p = 0.54, Control vs. TLE: p = 0.40, AE vs. TLE: p = 0.94; multivariate ANOVA Wilk's Lambda test). Measured SNR, FWHM and within-VOI tissue composition values are shown in Table 2. The tCho tended to differ between the three comparison groups and were significantly higher in the TLE group compared to the control (p = 0.05, ANOVA; p = 0.04; post-hoc tests; Figure 3A; Supplementary Table 5). The concentrations of tCr in the hippocampus were significantly different in patients with AE, TLE, and normal subjects and were higher in the TLE groups compared to the control group (p = 0.01, ANOVA; p = 0.007; post-hoc; Figure 3A; Supplementary Table 5). The concentrations of M-Ins were significantly higher in both patient groups compared to the control group (p = 0.048 and p = 0.046, respectively; ANOVA). However, post-hoc analysis showed only a trend toward higher levels in AE and TLE compared to controls (p = 0.09 and p = 0.07; post hoc tests, respectively). On the other hand, the concentrations of tNAA, Glu, and GSH in the hippocampus were similar all comparison groups (p = 0.19; p = 0.82; p = 0.36; Figure 3A). The concentrations of tNAA, tCho, tCr, Glu, M-Ins, and GSH (in μmol/g) in the cortex were similar in all three groups (p = 0.25; p = 0.07; p = 0.44; p = 0.43, p = 0.71, and p = 0.77, respectively; Figure 3B; Supplementary Table 5).
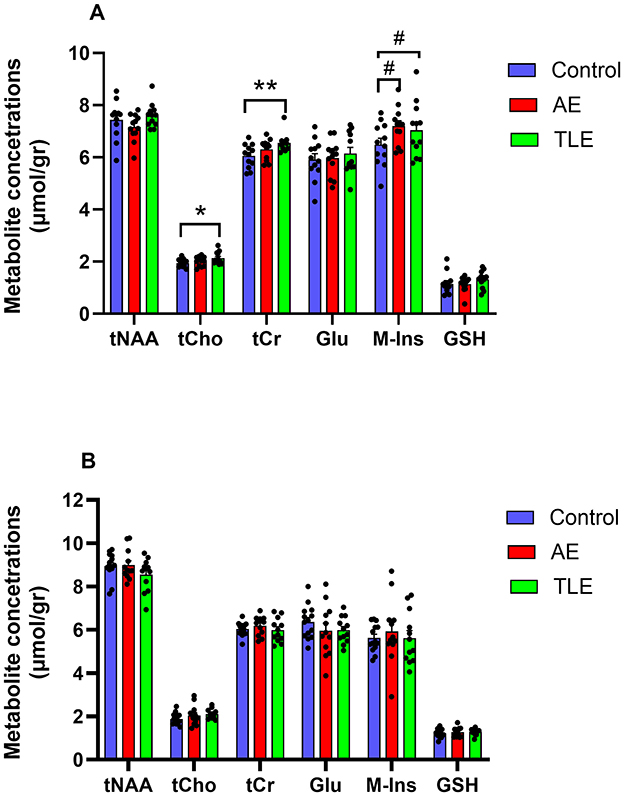
Figure 3. Metabolite profiles of patients with autoimmune encephalitis (AE), temporal lobe epilepsy (TLE), and control subjects. (A) The concentrations of myo-inositol (M-Ins) in the hippocampus were significantly higher in patients with AE compared to the control subjects while concentrations of total choline (tCh), total creatine (tCr), and myo-inositol (M-Ins) were significantly higher in patients with TLE compared to the control subjects. tNAA, total N-acetyl aspartate, and N-acetyl–aspartyl–glutamate; Glu, glutamate; GSH, glutathione. (B) The concentrations of metabolites in the cortex were comparable in all three groups. **, p < 0.01; *, p < 0.05; #, 0.5 ≤ p ≤ 0.1; post hoc tests. The data are mean concentrations (μmol/g) ± standard errors of the mean (SEM).
3.4 Correlations of brain metabolite concentrations with cognitive performance
One patient with AE was suffering from color vision deficiency and was an outlier in the measures involving visual function; therefore, he was removed from correlation analyses. Since we found the overall differences in the levels of tCho in patients with AE, TLE, and control subjects and based on the previous findings linking choline, creatine, and myo-inositol to cognitive function in neurodegenerative disorders (25), we examined the correlation of signals from these metabolites with cognitive measures in all three groups. There were no correlations between the hippocampal tCho levels and cognitive scores in AE or control subjects. However, in TLE, tCho negatively correlated with the measures of attention (R2 = 0.64, p = 0.002), processing speed (R2 = 0.56, p = 0.005), and language (R2 = 0.44, p = 0.02) such that higher metabolite levels were associated with worse cognitive performance (Supplementary Figures 1A–C; Supplementary Table 6). The hippocampal tCr levels negatively correlated with scores of attention, memory, and learning in TLE (R2 = 0.65, p = 0.002; R2 = 0.53, p = 0.007; R2 = 0.35, p = 0.04, respectively, Figures 4A–C; Supplementary Table 6). However, the tCr levels have positively correlated with measures of processing speed in AE (R2 = 0.38; p = 0.04; Figure 4D).
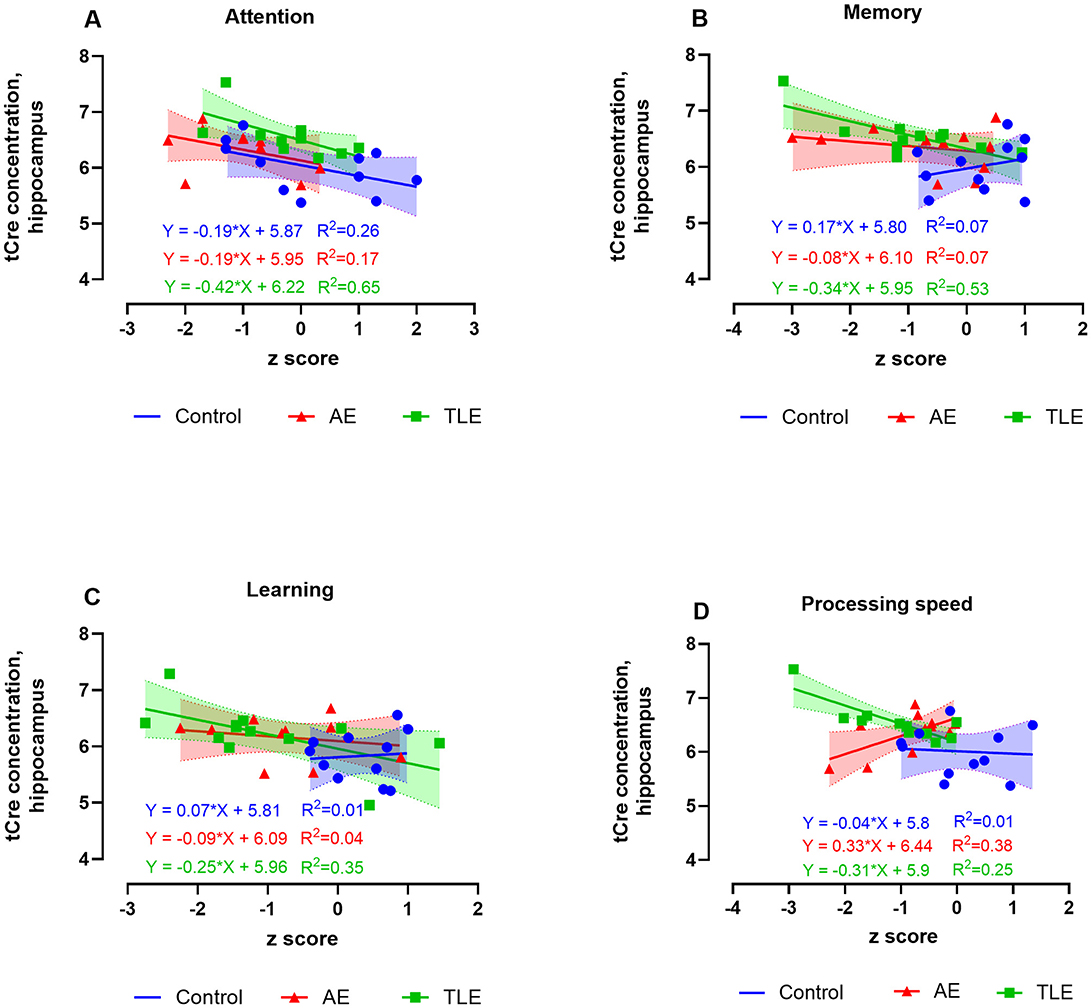
Figure 4. Relationship between the 1H-MRS measures of hippocampal total creatine (tCr) and cognitive performance in patients with autoimmune encephalitis (AE), temporal lobe epilepsy (TLE), and control subjects. The levels of tCr negatively correlated with scores attention, memory, and learning scores of attention in TLE (A, C, D), and positively correlated with the scores of processing speed in AE (B). The data are concentrations of metabolite (μmol/g) and mean standardized z scores for each cognitive domain.
The hippocampal concentrations of M-Ins positively correlated with attention and negatively correlated with recognition measures in AE such that lower and higher levels of M-Ins, respectively were accompanied by worse performance on these tasks (R2 = 0.38, p = 0.04 and R2 = 0.50, p = 0.02, respectively; Figures 5A, B; Supplementary Table 6). The M-Ins concentrations have negatively correlated with attention and processing speed in TLE (R2 = 0.51; p = 0.009 and R2 = 0.33; p = 0.05, respectively; Figure 5C; Supplementary Table 6). Curiously, the hippocampal M-Ins concentrations negatively correlated with attention and positively correlated with memory scores in control subjects (R2 = 0.50, p = 0.02 and R2 = 0.42, p = 0.03; Supplementary Table 6). There were no correlations between tCr, tCho, or M-Ins and other cognitive measures in AE, TLE, or control participants.
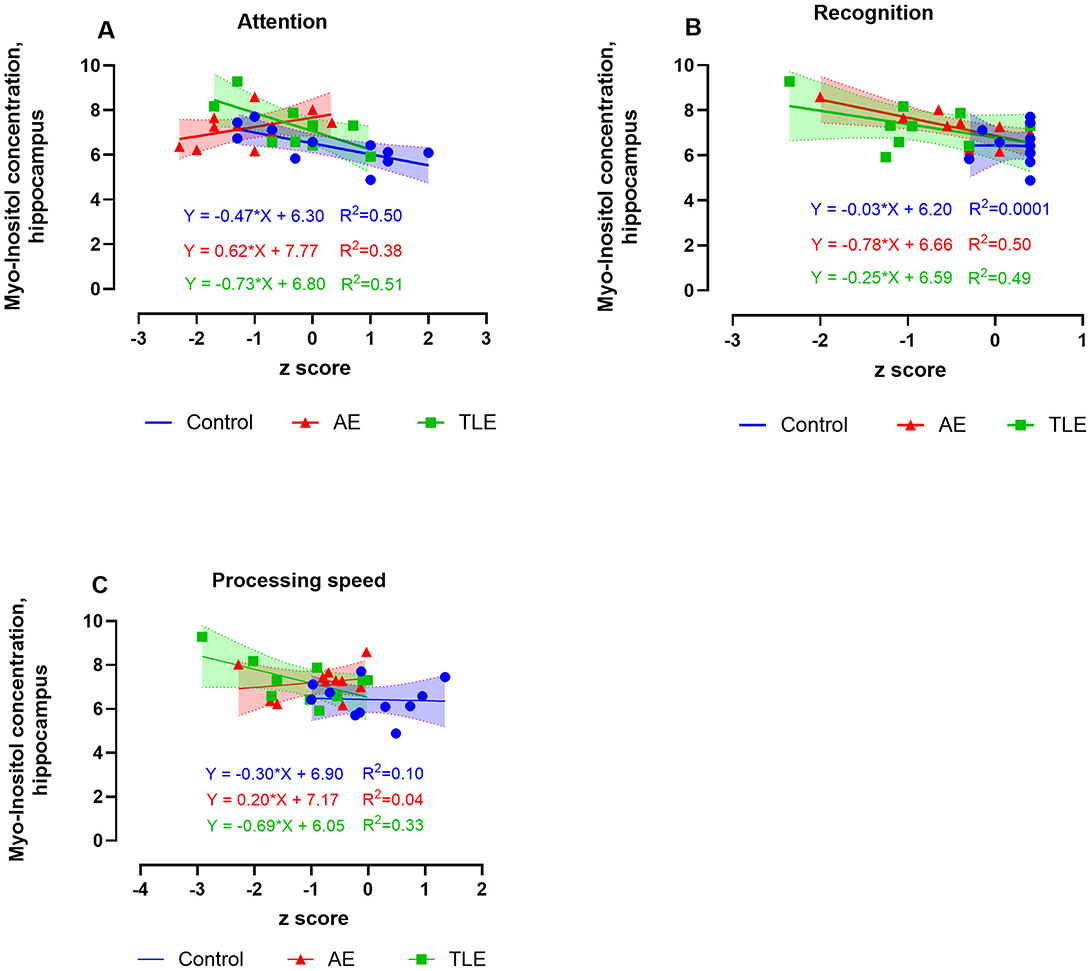
Figure 5. Relationship between the 1H-MRS measures of hippocampal myo-inositol (M-Ins) and cognitive performance in patients with autoimmune encephalitis (AE), temporal lobe epilepsy (TLE), and control subjects. The levels of M-ins positively correlated with attention (A) and negatively correlated with recognition (B). The M-Ins concentrations negatively correlated with attention and processing speed in TLE (C). The data are concentrations of metabolite (μmol/g) and mean standardized z scores for each cognitive domain.
In summary, we established that higher concentrations of M-ins in the hippocampus correlate with worse measures of attention and better recognition scores in patients with post-acute AE. On the other hand, high concentrations of tCho, tCr, and M-Ins selectively correlated with worse performance on attention, processing speed, language, memory, and learning tasks in TLE.
4 Discussion
In the present study, we applied the in vivo 1H-MRS to map the metabolite profiles of cognitively impaired patients with post-acute AE and compared them to those in patients with normal control subjects and patients with non-lesional TLE. Consistent with previous reports (4, 6, 12), patients with AE demonstrated a wide range of cognitive impairments, including attention, processing speed, language, visuospatial skills, learning, and recognition; the first three domains were particularly affected. Similarly, patients with non-lesional TLE have demonstrated impairment in multiple cognitive domains. Using the 1H-MRS, we showed that when compared to the control subjects, the concentrations of M-Ins were higher in both patient groups while concentrations of tCho and tCr were higher in the TLE group; these changes were specific to the hippocampus. The higher concentrations of M-ins correlated with worse recognition and better attention scores in AE. On the other hand, higher concentration M-Ins, tCr, and tCho have selectively correlated with worse performance on the tasks of attention, processing speed, language, and memory in TLE. These findings suggest that patients with AE and TLE have unique neuropsychological profiles and distinct metabolic spectra in the hippocampus. Further, our findings indicate that glial cell activation in the hippocampus contributes to the pathogenesis of cognitive failure in chronic AE and TLE.
One potential emerging mechanism of cognitive loss in chronic central nervous system (CNS) disorders is metabolic dysfunction which can be monitored dynamically using the 1H-MRS (35). We have established a new methodology of 1H-MRS by using a semiLASER sequence at the high field of 3 T which allowed us to enhance spectral quality, increase signal-to-noise ratio, and improve localization accuracy of brain metabolites compared to the conventional sequences. These attributes permitted precise quantification of metabolites in the hippocampus, a small and deep brain structure with a less uniform magnetic field (16). Our study achieved excellent spectral quality, characterized by increased SNRs and narrow linewidths, resulting in low CRLBs that permitted reliable quantification of metabolites with overlapping resonances or low concentrations, such as Glu and GSH. Additionally, the within-VOI tissue composition and average brain tissue water content were well aligned with previous reports, demonstrating consistent within-VOI tissue composition and accurate MRS voxel placement among groups (28, 36). Moreover, CSF correction, approximately 3% in the hippocampus and 1% in the cortex, had only a minor impact on metabolite quantification. Finally, the mean metabolite concentrations of tNAA, tCho, tCr, and M-ins in the control subjects in our study were consistent with those reported in previous studies (28, 36, 37).
To the best of our knowledge, this study was the first to systematically evaluate the region-specific spectra of metabolites in a cohort of AE patients. The M-Ins concentration tended to increase in the hippocampus during the post-acute AE phase which was consistent with a report of a serial 1H-MRS imaging in a patient with anti-NMDAR encephalitis (38). This metabolite serves several critical functions in the brain, including the regulation of cellular osmolarity, particularly during exposure to the hypotonic or hypertonic environments (39, 40). Furthermore, M-Ins acts as a precursor for the synthesis of the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) and more complex inositol phosphates that are supporting signal transduction and cellular signaling (41). A portion of M-Ins is synthesized de novo in astrocytes from glucose. Additionally, astrocytes express the sodium-dependent myo-inositol transporters SMIT1 and SMIT2, which regulate the myo-inositol uptake (42). Brain M-Ins levels are increased in the setting of acute hyperglycemia and chronic metabolic syndrome (43). Elevated brain levels of M-Ins have also been observed in AD. M-Ins is considered a glial marker due to its association with gliosis and inflammatory states (25, 44). Thus, our study provides indirect evidence of glial cell involvement in the pathophysiology of AE with cognitive loss. Given that our patients with AE had infrequent seizures, the observed increase in M-Ins was not likely caused by seizure activity. Future longitudinal studies with serial measurement using 1H-MRS can shed light on the utility of M-ins for monitoring the clinical recovery in transition to a latent phase of AE and its potential application as a disease biomarker.
Previous studies suggested that signal change from M-ins in the hippocampus of patients with epilepsy may reflect the direct effects of seizures and those of anti-seizure medications (ASMs) (45, 46). These findings have prompted us to compare the metabolic spectra in AE to those in non-lesional TLE with ongoing seizures. We found that despite the qualitative increase in M-Ins signal in TLE compared to the control subjects, the differences in metabolite concentrations only showed a trend. This could be due to larger than expected variability in the concentrations of M-Ins in this group requiring a larger sample size. The M-Ins signal in TLE also did not differ from that in AE. Since the TLE patients had higher seizure burden and ASM usage compared to AE patients, the augmented M-Ins signal in AE was not likely caused by seizure activity or ASM effects. Other studies in AE patients using the whole brain PET have shown hypermetabolism involving the mesial temporal lobe and widespread hypometabolism in the cortical areas (47). We did not detect any changes in the metabolite concentrations in the cortex. This could be explained by the differences in techniques and the timing of assessment with respect to the onset of AE. Indeed, FDG PET has normalized during clinical recovery in patients with anti-NMDAR encephalitis (48).
Quantitative changes in the hippocampal tNAA or tNAA/Cr ratio, the markers of neuronal loss, have been linked to cognitive deterioration in MCI, AD, and TLE (49, 50). Changes in other metabolites, such as Cr, Cho, and M-Ins have been related to cognitive loss in MCI, AD, and epilepsy indicating that metabolic compromise and immune activation disrupt cognition (49, 51). Our findings suggest that both AE and TLE are associated with elevated brain M-Ins; however, the data indicate that AE is primarily characterized by quantitative increase in M-Ins, whereas TLE shows more pronounced alterations in tCr and tCho. While M-Ins is widely recognized as an astrocytic marker, tCho has been linked to several processes, including membrane turnover, myelin integrity, microglial polarization, and astrocytic anti-inflammatory responses (52, 53). Elevated brain tCho levels have also been reported in traumatic brain injury and various neurodegenerative disorders (52, 53). These findings raise the intriguing possibility that, although astrogliosis is a common feature in both AE and TLE, it may occur through distinct pathophysiological mechanisms unique to each condition.
The positive correlation of M-Ins level with attention scores and tCr level with processing speed in AE in our study agrees with these findings and suggests a modulatory effect of hippocampal metabolites on these cognitive functions. Consistent with previous reports (54), we found a negative correlation of the hippocampal M-Ins, Cho, and Cr concentrations with the scores reflecting attention, processing speed, language, and memory functions in TLE. Along with the hippocampus, these cognitive domains are supported by other regions of the temporal, parietal, and frontal cortex, suggesting a much broader influence of metabolic disruption in the hippocampus on these cognitive functions in TLE. Taken together with previous studies, our current findings suggest that dysregulation of brain M-Ins may be a common feature of both AE and TLE-associated cognitive loss.
Some limitations of our study are worth noting. The main limitation was an inability to control the timing of brain imaging with respect to AE symptoms and initiation of immunotherapy. As such, current findings represent a cross-sectional assessment of metabolism in AE patients tested at different stages of post-acute recovery. In post hoc analyses of M-Ins and in comparisons of Glu levels across the three groups, we observed a trend toward increased metabolite levels. It is likely that the small sample size contributed to the inability to detect statistically significant differences. Due to this limitation, we were also unable to examine brain metabolite changes in specific AE subtypes or assess the impact of seizure lateralization in TLE. These factors may limit the generalizability of our findings. Future studies, preferably conducted through multicenter collaborations, will be necessary to address these important questions. Despite the different pathophysiology of acute AE, chronic cognitive impairment in AE appears to have a shared footprint. In patients with TLE, the effects of language dominance on cognitive function could not be assessed due to the small cohort size. Finally, our study was conducted at the level 4 epilepsy center which attracts the patients with the most severe AE and TLE. Therefore, the generalization of these findings to the broader patient populations in other clinical settings may be limited.
5 Conclusions
We found that despite similar subjective complaints of cognitive impairment, patients with AE and TLE have demonstrated distinct profiles of metabolites associated with neuronal dysfunction and glial activation in the hippocampus. The disease-specific metabolite changes have selectively modulated the cognitive function in AE and TLE.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Un of Neb Med Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
OT: Methodology, Writing – original draft, Conceptualization. LA: Project administration, Formal analysis, Writing – review & editing, Data curation. AM: Methodology, Writing – original draft, Formal analysis, Data curation. KG: Formal analysis, Data curation, Writing – original draft. MW: Data curation, Visualization, Formal analysis, Writing – review & editing. HG: Writing – review & editing, Visualization, Data curation. SL: Visualization, Data curation, Writing – review & editing. RZ: Visualization, Data curation, Writing – review & editing. TJ: Data curation, Writing – review & editing. MU: Writing – original draft, Visualization, Formal analysis, Methodology, Conceptualization, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. OT received salary and research support from the NIH P20GM130447 Cognitive Neuroscience and Development of Aging (CONDA) Award and the DHHS LB606 Nebraska Stem Cell Grant.
Author disclosures
OT received salary and research support from the NIH P20GM130447 Cognitive Neuroscience and Development of Aging (CONDA) Award and the DHHS LB606 Nebraska Stem Cell Grant. She has served on the Board of Directors for ABRET and the Medical and Scientific Advisory Board for the NORSE Institute. MW received consulting fees from ABC Medical Education, and payments for expert testimony from Erikson Sederstrom P. C., L.L.O., and Gordon Rees Scully Mansukhani. RZ received grants or contrast from Genentech, AAMAS, Sanofi, Novartis, Greenwich, Biogen, EMD Serono, Sun Pharma, and PCORI. She also received the consulting fee or honoraria for speaker engagement from Bayer, Biogen, Genentech, Celgene/Bristol Myers Squibb, Genzyme, Merck Serono, TEVA Neuroscience, TG Therapeutics, Greenwich, Novartis, and Sanofi. MU received support from the UNMC diversity grant fund 1709754086.
Acknowledgments
We thank the participants of the Brain and Mind Registry and patients from the Epilepsy and Autoimmune Seizure clinics at UNMC. We also thank James Brown for the excellent technical assistance as well as Ren Haasch and Nicolas Miller for their assistance with managing the study. We also thank Kaile Samson for the expert advice on statistical analysis. The MRS package was developed by Gülin Öz and Dinesh Deelchand and provided by the University of Minnesota under a C2P agreement. We thank Dinesh Deelchand for his technical assistance with the semiLASER protocol and data processing tools and procedures. We also thank Dr. Yan Zhang for assistance with tissue segmentation analysis and the Bioimaging Core at UNMC (RRID:SCR 022481) for the data processing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1597928/full#supplementary-material
References
1. Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. (2016) 12:1–13. doi: 10.3988/jcn.2016.12.1.1
2. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
3. Zhang W, Wang X, Shao N, Ma R, Meng H. Seizure characteristics, treatment, and outcome in autoimmune synaptic encephalitis: a long-term study. Epilepsy Behav. (2019) 94:198–203. doi: 10.1016/j.yebeh.2018.10.038
4. Griffith SP, Wesselingh R, Seery N, Rushen T, Kyndt C, Long B, et al. Characterizing cognitive function in patients with autoimmune encephalitis: an Australian prospective study. J Neurol. (2024) 271:310–24. doi: 10.1007/s00415-023-11967-w
5. Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. ClinInfectDis. (2012) 54:899–904. doi: 10.1093/cid/cir1038
6. Heine J, Kopp UA, Klag J, Ploner CJ, Prüss H, Finke C. Long-term cognitive outcome in anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol. (2021) 90:949–61. doi: 10.1002/ana.26241
7. Finke C, Kopp UA, Pruss H, Dalmau J, Wandinger KP, Ploner CJ. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. (2012) 83:195–8. doi: 10.1136/jnnp-2011-300411
8. Ariño H, Armangué T, Petit-Pedrol M, Sabater L, Martinez-Hernandez E, Hara M, et al. Anti-LGI1–associated cognitive impairment: Presentation and long-term outcome. Neurology. (2016) 87:759–65. doi: 10.1212/WNL.0000000000003009
9. Gibson LL, McKeever A, Coutinho E, Finke C, Pollak TA. Cognitive impact of neuronal antibodies: encephalitis and beyond. Transl Psychiatry. (2020) 10:304. doi: 10.1038/s41398-020-00989-x
10. Gagnon MM, Savard M. Limbic encephalitis associated with GAD65 antibodies: brief review of the relevant literature. Can J Neurol Sci. (2016) 43:486–93. doi: 10.1017/cjn.2016.13
11. Mueller C, Elben S, Day GS, Alves P, Hebert J, Tang-Wai DF, et al. Review and meta-analysis of neuropsychological findings in autoimmune limbic encephalitis with autoantibodies against LGI1, CASPR2, and GAD65 and their response to immunotherapy. Clin Neurol Neurosurg. (2023) 224:107559. doi: 10.1016/j.clineuro.2022.107559
12. Guevara-Silva E, Castro-Suarez S, Caparó-Zamalloa C, Cortez-Escalante J, Meza-Vega M. Cognitive impairment in adults with autoimmune encephalitis: experience from the Peruvian National Institute of Neurological Sciences. Neurol Perspect. (2022) 2:61–6. doi: 10.1016/j.neurop.2022.01.004
13. Ali F, Manzoor U, Bhattacharya R, Bansal AK, Chandrashekharaiah KS, Singh LR, et al. Brain metabolite, myo-inositol, inhibits catalase activity: a mechanism of the distortion of the antioxidant defense system in Alzheimer's disease. ACS Omega. (2022) 7:12690–700. doi: 10.1021/acsomega.1c06990
14. Jessen F, Block W, Träber F, Keller E, Flacke S, Lamerichs R, et al. Decrease of N-acetylaspartate in the MTL correlates with cognitive decline of AD patients. Neurology. (2001) 57:930–2. doi: 10.1212/WNL.57.5.930
15. Scheenen TWJ, Heerschap A, Klomp DWJ. Towards 1H-MRSI of the human brain at 7T with slice-selective adiabatic refocusing pulses. Magn Reson Mater Phy. (2008) 21:95–101. doi: 10.1007/s10334-007-0094-y
16. Öz G, Deelchand DK, Wijnen JP, Mlynárik V, Xin L, Mekle R, et al. Advanced single voxel1 H magnetic resonance spectroscopy techniques in humans: experts' consensus recommendations. NMR Biomed. (2021) 34:e4236. doi: 10.1002/nbm.4236
17. Dumas ME, Davidovic L. Metabolic profiling and phenotyping of central nervous system diseases: metabolites bring insights into brain dysfunctions. J Neuroimmune Pharmacol. (2015) 10:402–24. doi: 10.1007/s11481-014-9578-5
18. Deelchand DK, Berrington A, Noeske R, Joers JM, Arani A, Gillen J, et al. Across-vendor standardization of semi-LASER for single-voxel MRS at 3T. NMR Biomed. (2021) 34:e4218. doi: 10.1002/nbm.4218
19. Öz G, Tkáč I. Short-echo, single-shot, full-intensity 1H MRS for neurochemical profiling at 4T: validation in the cerebellum and brainstem. Magn Reson Med. (2011) 65:901–10. doi: 10.1002/mrm.22708
20. Ordidge RJ, Wylezinska M, Hugg JW, Butterworth E, Franconi F. Frequency offset corrected inversion (FOCI) pulses for use in localized spectroscopy. Magn Reson Med. (1996) 36:562–6. doi: 10.1002/mrm.1910360410
21. Tkáč I, Henry P, Andersen P, Keene CD, Low WC. Gruetter R. Highly resolved in vivo 1 H NMR spectroscopy of the mouse brain at 94 T. Magn Reson Med. (2004) 52:478–84. doi: 10.1002/mrm.20184
22. Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. (2000) 43:319–23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1
23. Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. (1998) 20:271. doi: 10.1159/000017321
24. Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. (2000) 62:649–71. doi: 10.1016/S0301-0082(99)00060-X
25. López-Gambero AJ, Sanjuan C, Serrano-Castro PJ, Suárez J, Rodríguez de Fonseca F. The biomedical uses of inositols: a nutraceutical approach to metabolic dysfunction in aging and neurodegenerative diseases. Biomedicines. (2020) 8:295. doi: 10.3390/biomedicines8090295
26. MRSpa software: Center for Magnetic Resonance Research, Radiology Department. University of Minnesota. Available online at: https://www.cmrr.umn.edu/downloads/mrspa/ (Accessed June 1, 2025).
27. Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. (2011) 32:802–10. doi: 10.1016/j.neurobiolaging.2009.05.002
28. Bednarík P, Moheet A, Deelchand DK, Emir UE, Eberly LE, Bareš M, et al. Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3 T: Reproducibility of MRS in the Human Hippocampus at 3 T. NMR Biomed. (2015) 28:685–93. doi: 10.1002/nbm.3309
29. Near J, Harris AD, Juchem C, Kreis R, Marjańska M, Öz G, et al. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts' consensus recommendations. NMR Biomed. (2021) 34:e4257. doi: 10.1002/nbm.4257
30. Quadrelli S, Mountford C, Ramadan S. Hitchhiker'S guide to voxel segmentation for partial volume correction of in vivo magnetic resonance spectroscopy. Magn Reson Insights. (2016) 9:1–8. doi: 10.4137/MRI.S32903
31. Provencher, Stephen. LCmodel software: [Internet]. Available online at: http://s-provencher.com/lcmodel.shtml (Accessed August 30, 2025).
32. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. (1993) 30:672–9. doi: 10.1002/mrm.1910300604
33. SPM12 - Statistical Parametric Mapping, Wellcome Centre for Human Neuroimaging, University College London, London UK. [Internet]. Available online at: https://www.fil.ion.ucl.ac.uk/spm/software/spm12/ (Accessed March 15, 2025).
34. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. (2005) 26:839–51. doi: 10.1016/j.neuroimage.2005.02.018
35. de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer's disease. Biochem Pharmacol. (2014) 88:548–59. doi: 10.1016/j.bcp.2013.12.012
36. Bednarík P, Henry PG, Khowaja A, Rubin N, Kumar A, Deelchand D, et al. Hippocampal neurochemical profile and glucose transport kinetics in patients with type 1 diabetes. J Clin Endocrinol Metab. (2020) 105:479–91. doi: 10.1210/clinem/dgz062
37. Pfyffer D, Zimmermann S, Simşek K, Kreis R, Freund P, Seif M. Magnetic resonance spectroscopy investigation in the right human hippocampus following spinal cord injury. Front Neurol. (2023) 14:1120227. doi: 10.3389/fneur.2023.1120227
38. Isik N, Candan F, Dincer A, Dama D, Seleker T, Aydin I. Serial cranial MR imaging and single voxel proton MR spectroscopy in paraneoplastic limbic encephalitis. Eur J Radiol Extra. (2004) 50:1–7. doi: 10.1016/j.ejrex.2003.12.005
39. Lohr JW, McReynolds J, Grimaldi T, Acara M. Effect of acute and chronic hypernatremia on myoinositol and sorbitol concentration in rat brain and kidney. Life Sci. (1988) 43:271–6. doi: 10.1016/0024-3205(88)90317-7
40. Sterns RH, Silver SM. Brain volume regulation in response to hypo-osmolality and its correction. Am J Med. (2006) 119:S12–6. doi: 10.1016/j.amjmed.2006.05.003
41. Kim H, McGrath BM, Silverstone PH, A. review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders—focus on magnetic resonance spectroscopy (MRS) studies. Hum Psychopharmacol. (2005) 20:309–26. doi: 10.1002/hup.693
42. Fu H, Li B, Hertz L, Peng L. Contributions in astrocytes of SMIT1/2 and HMIT to myo-inositol uptake at different concentrations and pH. Neurochem Int. (2012) 61:187–94. doi: 10.1016/j.neuint.2012.04.010
43. Kreis R, Ross BD. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology. (1992) 184:123–30. doi: 10.1148/radiology.184.1.1319074
44. Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. (1993) 15:289–98. doi: 10.1159/000111347
45. Wellard RM, Briellmann RS, Prichard JW, Syngeniotis A, Jackson GD. Myoinositol abnormalities in temporal lobe epilepsy. Epilepsia. (2003) 44:815–21. doi: 10.1046/j.1528-1157.2003.44102.x
46. Vadnal R, Parthasarathy R. Myo-inositol monophosphatase: diverse effects of lithium, carbamazepine, and valproate. Neuropsychopharmacology. (1995) 12:277–85. doi: 10.1038/sj.npp.1380262
47. Bergeret S, Birzu C, Meneret P, Giron A, Demeret S, Marois C, et al. Brain metabolic alterations in seropositive autoimmune encephalitis: an 18F-FDG PET study. Biomedicines. (2023) 11:506. doi: 10.3390/biomedicines11020506
48. Yuan J, Guan H, Zhou X, Niu N, Li F, Cui L, et al. Changing brain metabolism patterns in patients with ANMDARE: serial 18F-FDG PET/CT findings. Clin Nucl Med. (2016) 41:366–70. doi: 10.1097/RLU.0000000000001164
49. Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer's disease. Neurosci Lett. (2005) 384:23–8. doi: 10.1016/j.neulet.2005.04.035
50. Connelly A, Van Paesschen W, Porter DA, Johnson CL, Duncan JS, Gadian DG. Proton magnetic resonance spectroscopy in MRI-negative temporal lobe epilepsy. Neurology. (1998) 51:61–6. doi: 10.1212/WNL.51.1.61
51. Huang W, Alexander GE, Daly EM, Shetty HU, Krasuski JS, Rapoport SI, et al. High brain myo-inositol levels in the predementia phase of Alzheimer's disease in adults with down's syndrome: a 1H MRS study. Am J Psychiatry. (1999) 156:1879–86. doi: 10.1176/ajp.156.12.1879
52. Tayebati SK, Amenta F. Choline-containing phospholipids: relevance to brain functional pathways. Clin Chem Lab Med. (2013) 51:513–21. doi: 10.1515/cclm-2012-0559
53. Javaid S, Farooq T, Rehman Z, Afzal A, Ashraf W, Rasool MF, et al. Dynamics of choline-containing phospholipids in traumatic brain injury and associated comorbidities. Int J Mol Sci. (2021) 22:11313. doi: 10.3390/ijms222111313
Keywords: autoimmune encephalitis, temporal lobe epilepsy, cognitive loss, memory deficits, cerebral metabolism, myo-inositol, magnetic resonance spectroscopy
Citation: Taraschenko O, Arcot Jayagopal L, Mullane A, Greenman K, White M, Ghonim H, Lee S, Zabad RK, Jasinski T and Uberti M (2025) Hippocampal dysmetabolism contributes to cognitive loss in autoimmune encephalitis and focal temporal epilepsy. Front. Neurol. 16:1597928. doi: 10.3389/fneur.2025.1597928
Received: 21 March 2025; Accepted: 20 June 2025;
Published: 14 August 2025.
Edited by:
João M. N. Duarte, Lund University, SwedenReviewed by:
Yuhei Takado, National Institutes for Quantum and Radiological Science and Technology, JapanPetr Bednarik, Copenhagen University Hospital, Denmark
Copyright © 2025 Taraschenko, Arcot Jayagopal, Mullane, Greenman, White, Ghonim, Lee, Zabad, Jasinski and Uberti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olga Taraschenko, b2xoYS50YXJhc2NoZW5rb0B1bm1jLmVkdQ==
 Olga Taraschenko
Olga Taraschenko Lakshman Arcot Jayagopal
Lakshman Arcot Jayagopal Audrina Mullane2
Audrina Mullane2 Kyle Greenman
Kyle Greenman Matthew White
Matthew White Hesham Ghonim
Hesham Ghonim Rana Khalil Zabad
Rana Khalil Zabad Mariano Uberti
Mariano Uberti