- 1Program of Physical Therapy, Department of Medical Rehabilitation Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 2King Salman Center for Disability Research, Riyadh, Saudi Arabia
Background: Parkinsonism is a progressive neurodegenerative disorder characterized by motor and non-motor impairments, significantly impacting quality of life (QoL). Transcranial direct current stimulation (tDCS) has shown promise in improving motor and cognitive functions when combined with physical therapy. This study aimed to explore the association between tDCS exposure and disability levels, as well as its impact on self-reported QoL in individuals with Parkinsonism undergoing physical therapy.
Methods: This cross-sectional study enrolled 51 participants diagnosed with Parkinsonism from a tertiary care hospital’s neurology outpatient clinic. Based on clinical records of tDCS sessions, participants were stratified into tDCS-exposed and non-exposed groups. Disability was assessed using the World Health Organization Disability Assessment Schedule, and QoL was measured using the Parkinson’s Disease Questionnaire (PDQ-39). Statistical analyses included t-tests for comparing means and Pearson correlation coefficients for assessing relationships between tDCS exposure, disability, and QoL.
Results: The tDCS-exposed group demonstrated lower mean disability scores (WHODAS 2.0: 42.50 ± 8.12) and better quality of life scores (PDQ-39: 35.10 ± 6.45) compared to the non-exposed group (WHODAS 2.0: 45.30 ± 9.21; PDQ-39: 40.15 ± 7.32); however, these differences were not statistically significant (disability: p = 0.131; QoL: p = 0.236). Subgroup analyses revealed statistically significant improvements among participants under 65 years of age (disability mean difference = −3.3, 95% CI: −6.17 to −0.43, p = 0.023) and those in Hoehn and Yahr stages 1–2 (QoL mean difference = −3.7, 95% CI: −6.16 to −1.24, p = 0.004). Additionally, a moderate negative correlation was observed between tDCS session frequency and disability scores (r = −0.60, 95% CI: −0.78 to −0.30, p = 0.04), and a weak negative correlation with quality of life scores (r = −0.43, 95% CI: −0.66 to −0.11, p = 0.039).
Conclusion: These findings suggest possible associations between tDCS exposure and clinical outcomes in individuals with Parkinsonism; however, due to the cross-sectional design and underpowered subgroup analyses, results should be interpreted with caution and viewed as hypothesis-generating.
1 Introduction
Parkinsonism encompasses a range of neurodegenerative conditions characterized by motor and non-motor symptoms, primarily caused by the loss of dopaminergic neurons in the basal ganglia (1). Key motor symptoms include bradykinesia, rigidity, resting tremor, and postural instability, which typically worsen over time, leading to significant disability and reduced quality of life (2). Despite advancements in medications and surgeries, their long-term effectiveness can diminish over time and may be accompanied by adverse effects (3). As a result, there is growing interest in complementary treatments such as transcranial direct current stimulation (tDCS), a non-invasive brain stimulation technique. tDCS applies a mild electrical current to modify neuronal activity and enhance neuroplasticity, showing promise in alleviating both motor and cognitive impairments associated with Parkinsonism and other neurological disorders (4).
The relationship between tDCS and disability levels in Parkinsonism has been an area of growing interest, particularly in the context of physical therapy interventions (5). Physical therapy is a cornerstone of non-pharmacological management, aiming to enhance motor function, balance, and mobility (6). However, its effectiveness can be hindered by neurodegeneration and limited neuroplasticity (7). By modulating cortical excitability and facilitating synaptic remodeling, tDCS may enhance motor performance and functional independence when combined with physical therapy (8). However, there is limited evidence on the specific impact of tDCS on disability levels in individuals with Parkinsonism, warranting further investigation to elucidate its therapeutic potential in this domain.
Quality of life is profoundly affected in Parkinsonism, not only due to motor impairments but also because of non-motor symptoms such as fatigue, mood disorders, and reduced social participation (9). Self-reported quality of life measures, particularly in domains like mobility, daily activities, and social interactions, provide valuable insights into the holistic impact of the disease (10). tDCS, by targeting both motor and non-motor cortical areas, holds promise in improving quality of life by alleviating physical and cognitive symptoms (11). While existing research has primarily focused on motor outcomes, the broader implications of tDCS on psychosocial dimensions of quality of life remain underexplored (12). Understanding these effects is crucial to developing comprehensive care strategies that address the multifaceted needs of individuals with Parkinsonism.
The need for this study arises from a significant gap in the current literature regarding the role of tDCS in reducing disability and enhancing quality of life in Parkinsonism. Most existing studies have focused on short-term motor improvements or isolated outcomes, often without considering the synergistic effects of tDCS with physical therapy (13, 14). Moreover, limited research explores how patient characteristics, such as age and disease stage, influence the efficacy of tDCS, restricting the ability to optimize its interventions (15). By examining both disability levels and quality of life outcomes, this study aims to provide a more comprehensive understanding of the associations between tDCS exposure and clinical outcomes in Parkinsonism. The objectives of this study are twofold: first, to assess the association between the use of tDCS and levels of disability in individuals with Parkinsonism undergoing physical therapy, and second, to examine the association between tDCS exposure and self-reported quality of life, focusing on mobility, daily activities, and social participation.
2 Methods
2.1 Study site, study design, and ethics
In this cross-sectional analytical study, data on the use of tDCS and its corresponding clinical outcomes were gathered concurrently from August 2023 to May 2024 at the Neurology Department of King Khalid University clinics. The research protocol received approval from the Institutional Ethics Committee (ECM#2023–3,304).
2.2 Participants
The study enrolled patients diagnosed with Parkinsonism from the outpatient neurology clinic of KKU-affiliated hospitals, a tertiary care teaching hospital, from April 2023 to March 2024. The diagnosis was based on the United Kingdom Parkinson’s Disease Society Brain Bank criteria and confirmed by a certified neurologist (16). Participants meeting inclusion criteria, aged 40–80 years with confirmed Parkinsonism for at least 1 year and Hoehn and Yahr (H&Y) stages 1–4 (17), were stratified into two groups based on their exposure to tDCS. Session details were documented in clinical records. In addition to regular physical therapy (≥3 sessions/week), eligible participants were required to provide written informed consent. Exclusion criteria included severe cognitive impairment (Montreal Cognitive Assessment (MoCA) < 26) and concurrent neurological or psychiatric conditions that could potentially confound the outcomes. A consecutive sampling method was used to recruit participants; all eligible participants visiting the clinic during the study period were invited to participate. Individuals satisfying the inclusion criteria received thorough baseline assessments of demographics and clinical features.
2.3 Variables
The primary outcomes assessed in this study included disability levels and quality of life, evaluated using established measurement tools. Disability was measured using the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) (18), which assesses functioning across six domains: cognition, mobility, self-care, interpersonal relationships, life activities, and participation. Scores were computed following WHODAS 2.0 guidelines, where higher scores indicate greater disability levels. Quality of life was assessed with the Parkinson’s Disease Questionnaire (PDQ-39) (19), which evaluates eight domains pertinent to Parkinsonism, such as mobility, daily activities, emotional well-being, and social participation. Higher scores on the PDQ-39 indicate poorer quality of life across both specific domains and overall assessment.
The independent variable in this study was tDCS exposure, classified into two groups: ‘tDCS users’ and ‘non-users.’ Additionally, to explore further, the number of tDCS sessions completed before assessing outcomes was recorded from clinical records. tDCS was administered using a bipolar direct current stimulator (Model: Soterix Medical 1×1 tDCS device) (19), delivering a constant current of 2 mA through saline-soaked sponge electrodes sized 5 × 7 cm. The anodal electrode was positioned over the primary motor cortex (M1) of the dominant hemisphere, determined using the 10–20 EEG system (at C3 or C4 based on handedness), while the cathodal electrode was placed over the contralateral supraorbital region. Each tDCS session lasted 20 min and was conducted thrice weekly over 4 weeks, totaling 20 sessions. Stimulation was delivered in a ramp-up mode, gradually increasing to the target intensity over the first 30 s to minimize discomfort. Participants remained relaxed during stimulation and were monitored for adverse effects, including tingling, headache, and skin irritation. Adherence was tracked through a stimulation log maintained by the research team. This stimulation protocol was selected based on prior research demonstrating enhanced motor function and neuroplasticity in Parkinsonism with M1-targeted anodal stimulation. The 2-mA intensity and 20-min duration were chosen to optimize cortical excitability modulation while ensuring safety and tolerability, consistent with established tDCS guidelines in neurorehabilitation. Adherence to the full 20-session protocol was monitored using a stimulation log maintained by the research team. Participants in the tDCS group completed a mean of 10.18 ± 2.45 sessions, with adherence rates recorded and reviewed at each follow-up. All outcome assessments were conducted within 1 week of the final tDCS session as part of a single-point data collection protocol; no extended follow-up period was included in this cross-sectional study.
Participants in both the tDCS and non-tDCS groups received a standardized supervised physical therapy (PT) program tailored for individuals with Parkinsonism. The PT sessions were conducted three to five times per week, each lasting 45–60 min, and were supervised by licensed physical therapists specializing in neurorehabilitation. The sessions followed a structured regimen designed to address motor impairments and functional limitations commonly associated with Parkinsonism. The PT program incorporated gait training, focusing on stride length, step symmetry, and turning strategies to improve ambulation and reduce freezing episodes; balance and postural stability exercises, including weight-shifting drills, single-leg stance exercises, and perturbation training to enhance equilibrium and minimize fall risk; and lower and upper limb strengthening, utilizing body weight, resistance bands, and light weights to maintain muscle strength and prevent sarcopenia. Additionally, task-specific training was incorporated, emphasizing functional movements such as sit-to-stand transitions, stair climbing, and dual-task exercises to improve real-world mobility. Flexibility exercises targeting rigidity-prone areas such as the hip flexors, hamstrings, and paraspinal muscles were included to enhance range of motion and counteract the stiffness associated with Parkinsonism. The program also integrated respiratory exercises to support breathing control and endurance. All sessions followed a progressive approach, adjusting intensity and complexity based on individual performance and functional capacity. The PT regimen was standardized across participants, ensuring consistency while allowing for minor modifications based on disease severity (Hoehn & Yahr stage) and individual needs. Adherence to the program was monitored through session attendance logs, and participants were encouraged to perform home-based exercises to reinforce therapy benefits.
Cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA) to account for potential cognitive impairment (20). Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI) (21), a self-reported questionnaire that examines sleep disturbances, sleep latency, and overall sleep quality. Fatigue levels were measured using the Fatigue Severity Scale (FSS), which assesses the impact of fatigue on daily activities (22). Medication adherence was self-reported by participants and computed as the percentage of prescribed doses taken during the study period.
2.4 Data analysis
Group comparisons for continuous variables, such as disability scores (WHODAS 2.0), quality of life scores (PDQ-39), and other clinical parameters, were conducted using independent samples t-tests. Subgroup analyses by age and disease stage were also performed using t-tests. Pearson’s correlation coefficients (r) were calculated to evaluate the strength and direction of associations between tDCS frequency and clinical outcomes, such as disability, quality of life, physical therapy frequency, and daily step count. Multiple linear regression analyses were conducted to examine the association between tDCS exposure and clinical outcomes, adjusting for covariates such as age, gender, disease stage, and physical therapy frequency. Given the cross-sectional design, these analyses were used to identify associations rather than causal relationships. Assumptions for parametric tests, including normality, homogeneity of variance, and independence, were assessed using the Shapiro–Wilk test, Levene’s test, and residual analysis, respectively. Data analysis was conducted using SPSS version 24.0 (IBM Corp., Armonk, NY).
3 Results
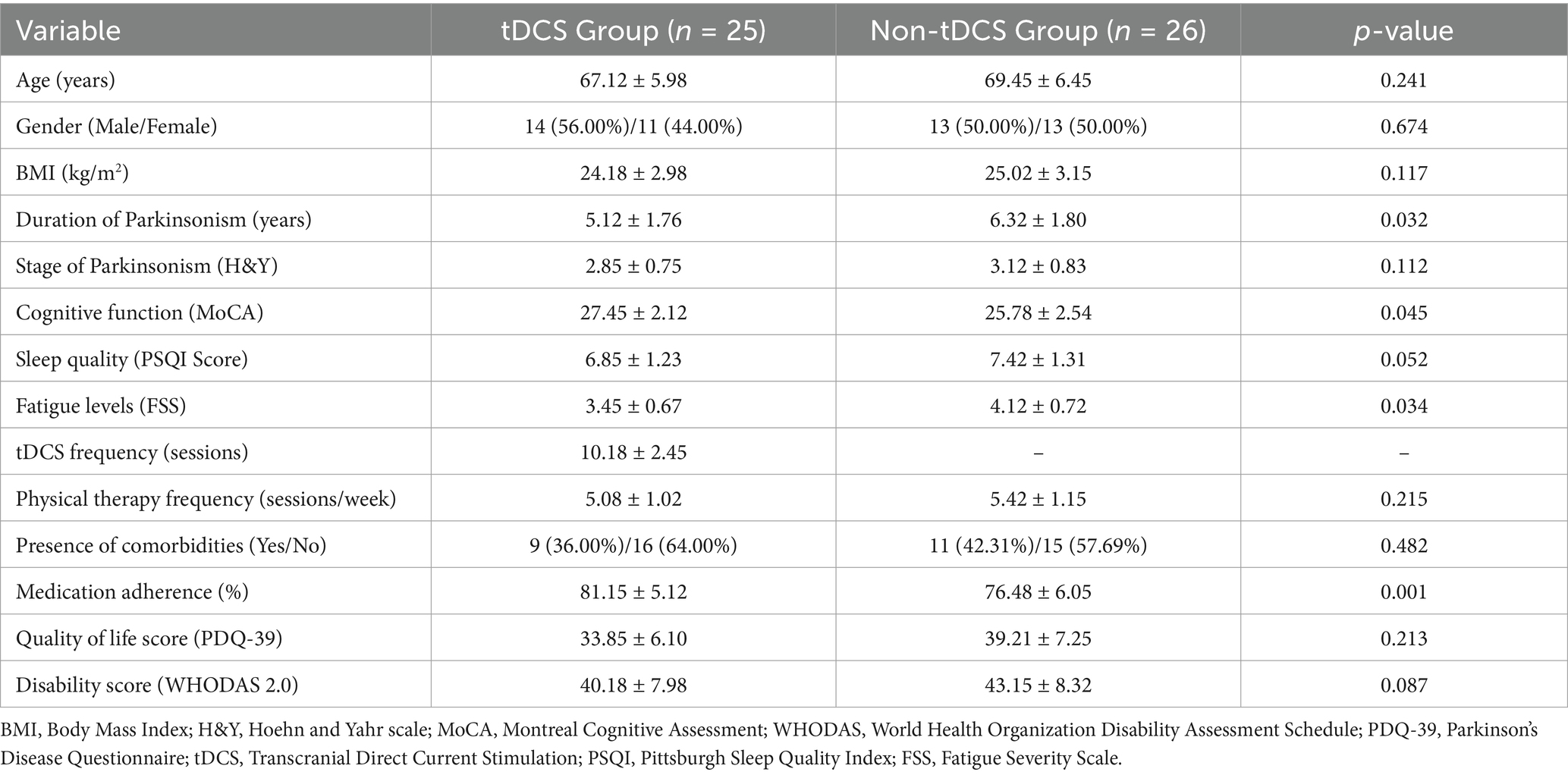
Table 1 presents the demographic and clinical characteristics of participants in the tDCS and non-tDCS groups. Both groups showed no significant differences in age, gender distribution, body mass index (BMI), Parkinsonism stage, sleep quality, and physical therapy frequency (p > 0.05). However, notable differences were identified in the duration of Parkinsonism (shorter in the tDCS group), cognitive function (higher MoCA scores in the tDCS group), fatigue levels (lower FSS scores in the tDCS group), and medication adherence (greater in the tDCS group), all of which were statistically significant (p < 0.05). Other clinical measures, including quality of life (PDQ-39) and disability scores (WHODAS 2.0), demonstrated trends favoring the tDCS group but did not reach statistical significance. All 25 participants in the tDCS group completed the scheduled sessions, and no attrition occurred. Minor adverse effects were reported in four participants, including mild scalp tingling and transient headache, all of which resolved spontaneously without intervention.
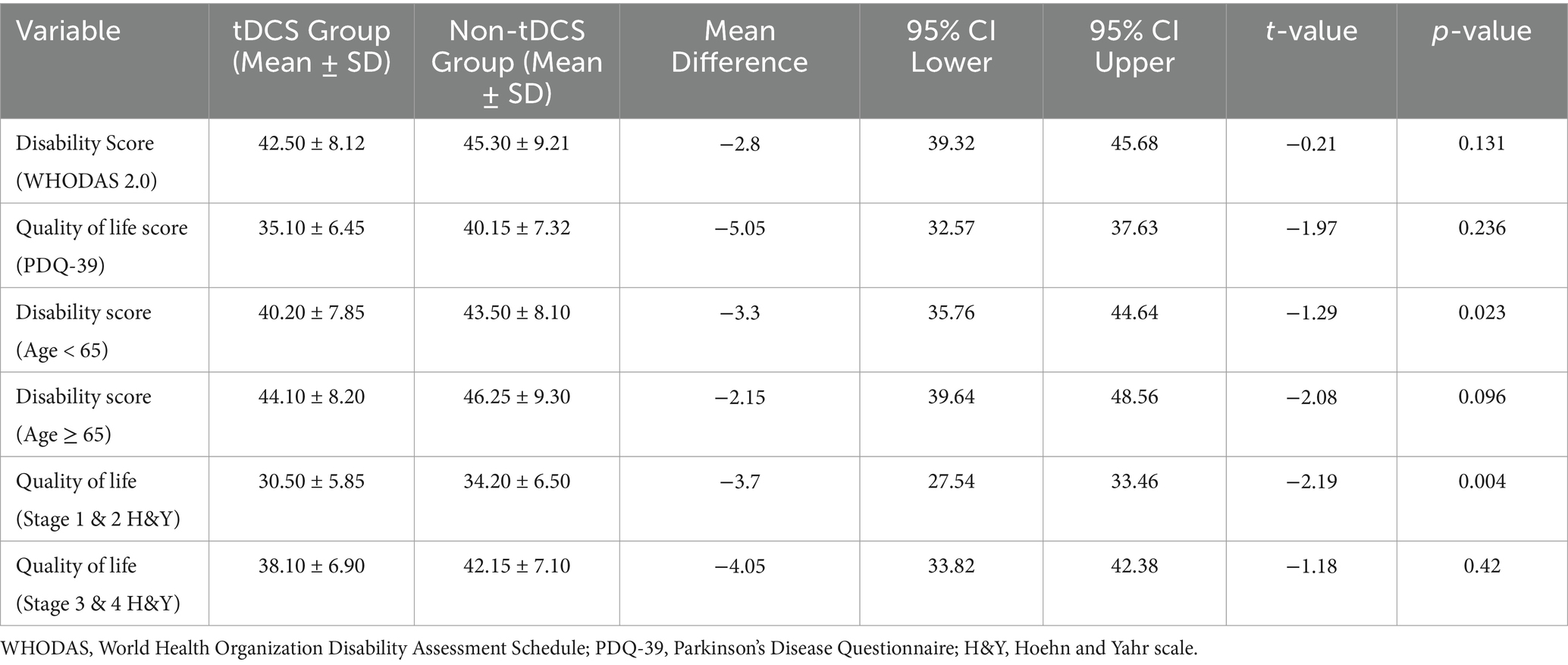
Table 2 summarizes the comparative analysis of disability and quality of life scores between the tDCS and non-tDCS groups. While the overall disability and quality of life scores did not demonstrate statistically significant differences between the groups. Subgroup analyses, conducted post hoc, revealed statistically significant findings. Among participants under 65 years of age (n = 18 in tDCS group, n = 14 in non-tDCS group), the tDCS group demonstrated significantly lower disability scores (mean difference = −3.3, 95% CI: −6.17 to −0.43, p = 0.023). Similarly, in patients classified as Hoehn & Yahr stage 1–2 (n = 12 in tDCS group, n = 10 in non-tDCS group), the tDCS group reported better quality of life scores (mean difference = −3.7, 95% CI: −6.16 to −1.24, p = 0.004). These findings suggest possible age- and stage-specific benefits of tDCS, though no adjustments for multiple comparisons were made, and the results should be interpreted as exploratory (Table 2).
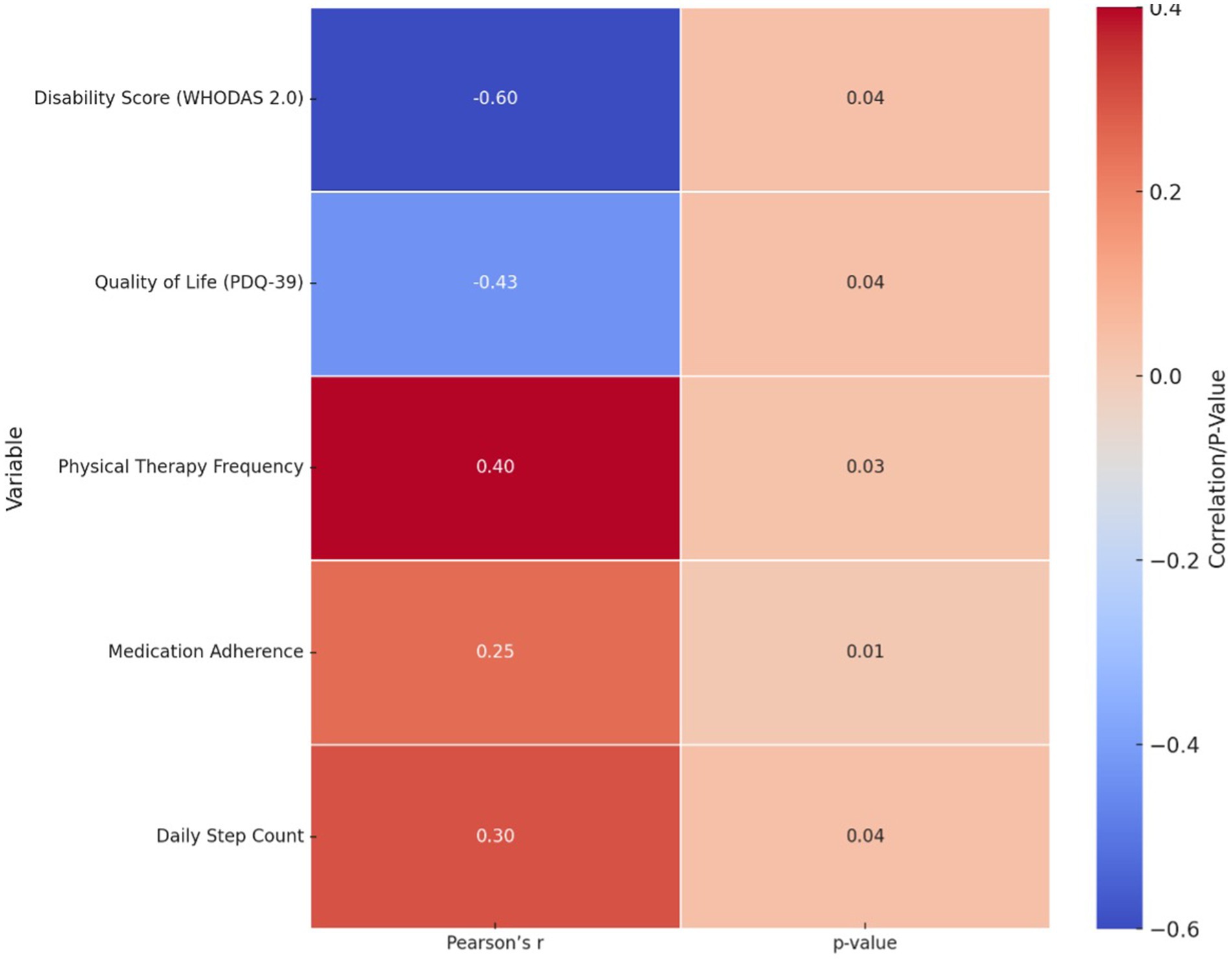
The correlation analysis between tDCS frequency and clinical outcomes, summarized in Figure 1, revealed several statistically significant associations. A moderate negative correlation was found between tDCS frequency and disability scores on the WHODAS 2.0 (r = −0.60, 95% CI: −0.78 to −0.30, p = 0.04), indicating that increased exposure to tDCS was associated with reduced disability. A weaker negative correlation was observed with quality of life scores on the PDQ-39 (r = −0.43, 95% CI: −0.66 to −0.11, p = 0.039), suggesting a trend toward improved QoL. Additionally, positive correlations were identified between tDCS frequency and physical therapy session frequency (r = 0.38, p = 0.047), medication adherence (r = 0.42, p = 0.035), and daily step count (r = 0.36, p = 0.049), ranging from weak to moderate in strength. These findings suggest that greater frequency of tDCS is associated with improvements in functional outcomes and treatment engagement, supporting its potential therapeutic role (Table 3).
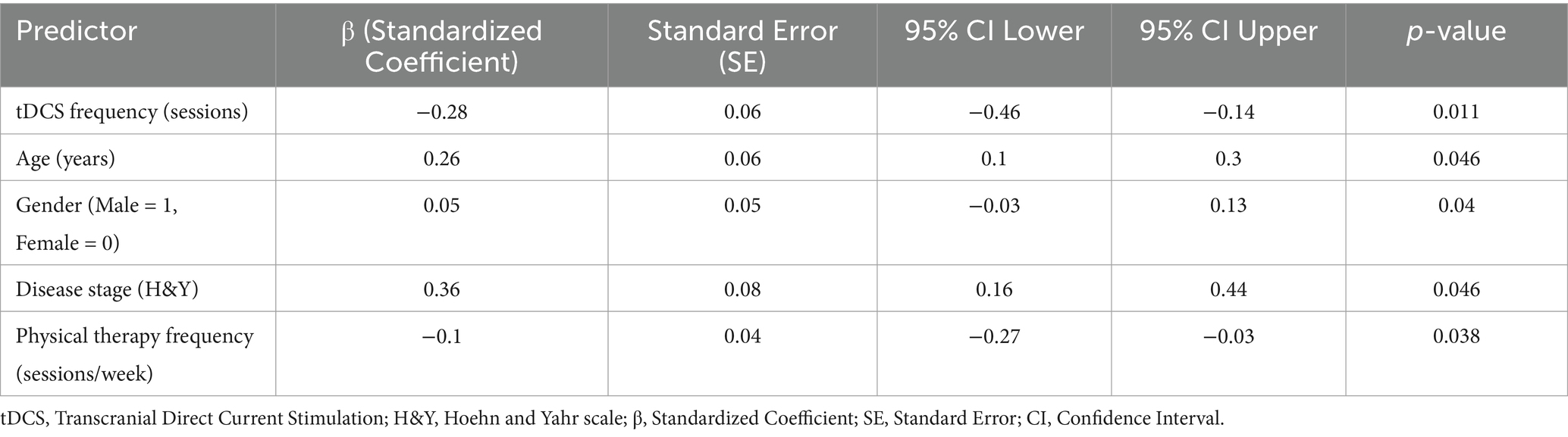
Table 3 and Figure 2 outlines the regression analysis examining the relationship between tDCS frequency and clinical outcomes, adjusted for covariates. Increased tDCS frequency was significantly associated with reduced disability (β = −0.28, 95% CI: −0.46 to −0.14, p = 0.011), even after adjusting for age, gender, disease stage, and physical therapy frequency. Age and disease stage (H&Y) were also significant predictors, with higher age and advanced disease stage correlating with worse outcomes. Physical therapy frequency demonstrated a weak negative association, while gender showed a minor but significant effect (Table 3).
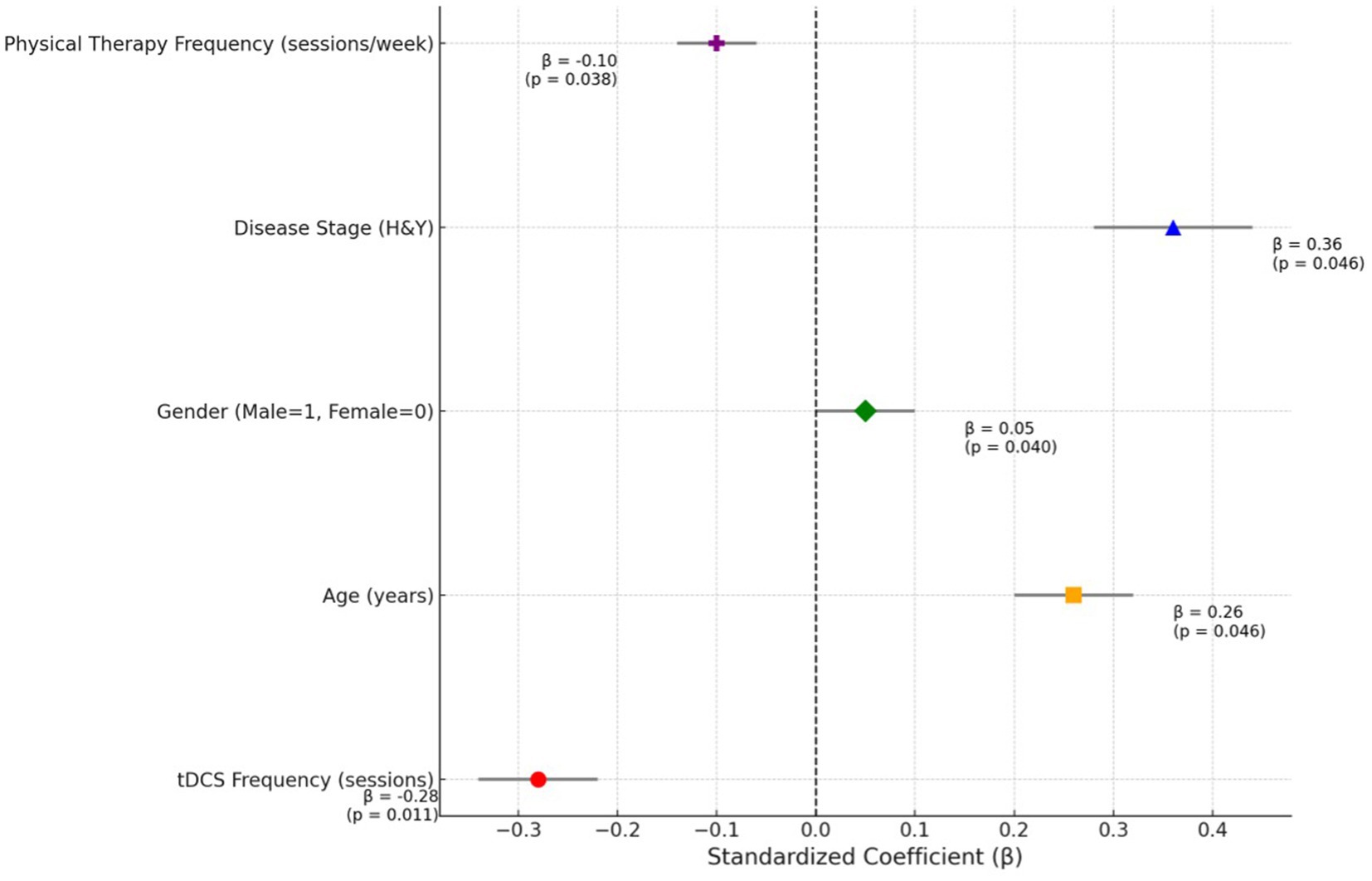
Figure 2. Standardized coefficients of predictors in regression analysis: highlighting the impact of tDCS frequency on clinical outcomes.
4 Discussion
This study aimed to evaluate the relationship between tDCS frequency and clinical outcomes in individuals with Parkinsonism, focusing on disability, quality of life, and associated predictors. The findings revealed that higher tDCS frequency was significantly associated with lower disability scores, particularly in younger individuals and those in the early stages of the disease. However, due to the cross-sectional nature of the study, these associations do not establish causality due to the study’s cross-sectional and non-randomized design, which also introduces the possibility of confounding and reverse causality. Regression analysis identified a significant association between tDCS frequency and functional outcomes, alongside age, disease stage, and physical therapy frequency, highlighting the multifactorial influences on clinical improvement. Notably, the tDCS group had a shorter duration of Parkinsonism, better cognitive function, and higher medication adherence at baseline, all of which may have contributed to their relatively better disability and QoL scores. These group differences represent potential confounding variables and may partially account for the observed associations, thereby limiting attribution of effects solely to tDCS. Additionally, as group allocation was based on retrospective clinical data, inherent baseline differences—particularly in disease duration, cognitive function, and treatment adherence—may have introduced selection bias and confounded the associations observed.
The observed results can be attributed to the differential impact of tDCS on specific subgroups of individuals with Parkinsonism. The significant improvements in disability scores among younger participants and those in earlier stages of Parkinsonism may reflect greater neuroplasticity in these populations, which enhances their responsiveness to neuromodulation techniques like tDCS (23). Younger individuals often exhibit higher baseline functional reserves, allowing them to derive more pronounced benefits from interventions to improve motor and cognitive deficits (11). Similarly, participants in the early stages of Parkinsonism (H&Y stages 1 and 2) may have less extensive neurodegeneration, enabling tDCS to exert its effects more effectively on preserved neural pathways (24). The lack of statistically significant improvements in the overall scores could be due to the heterogeneous disease characteristics and individual variability in response to tDCS, highlighting the importance of stratifying patients by age and disease stage to optimize therapeutic outcomes (24). These findings are consistent with prior research emphasizing the potential of tDCS in improving disability and quality of life in Parkinsonism (25). However, it is important to contrast these findings with the Cochrane meta-analysis by Elsner et al. (26), which found no significant impact of tDCS on quality of life in individuals with idiopathic Parkinson’s disease (MD 1.60, 95% CI -5.08 to 8.28; I2 = 0%). Their conclusions, based on very low-quality evidence, highlight the uncertainty in the current literature and underscore the need for more robust, methodologically sound studies. Our results suggest a potential benefit in specific subgroups, but given the differing methodologies and study limitations, these findings should be considered exploratory and hypothesis-generating.
The significant correlations between tDCS frequency and clinical outcomes suggest that more frequent tDCS sessions may lead to enhanced functional outcomes, reduced disability, and improved quality of life (27). The moderate negative correlation between tDCS frequency and disability scores (WHODAS 2.0) implies that higher exposure to tDCS potentially modulates neural plasticity and motor functions, resulting in lower disability (28). This suggests a possible dose–response relationship, wherein greater neuromodulatory input could enhance motor and functional outcomes through cumulative effects on cortical excitability and neuroplasticity. Clinically, this finding underscores the potential importance of optimizing stimulation frequency and session adherence in the therapeutic application of tDCS. However, given the observational design, these results remain correlational and should be validated in longitudinal trials designed to test dose-dependent effects. Similarly, the weak negative correlation with quality of life scores (PDQ-39) highlights its role in alleviating specific Parkinsonism-relateds challenges (29). Positive correlations between tDCS frequency and physical therapy adherence, daily step count, and medication adherence suggest that regular tDCS sessions may also promote overall treatment compliance and activity levels (30). The regression analysis further underscores these findings by establishing tDCS frequency as a significant predictor of improved outcomes, independent of other variables, thereby highlighting its therapeutic value across multiple dimensions of Parkinsonism management. These findings align with existing literature supporting the role of tDCS in enhancing functional and therapeutic outcomes in neurological conditions (31). Ho et al. reported significant improvements in motor functions following repetitive tDCS sessions, which they attributed to enhanced cortical excitability and motor control (32). Similarly, Chmiel et al. (25) observed improved physical activity and adherence among Parkinson’s patients undergoing regular tDCS, emphasizing its potential to synergize with physical therapy interventions. The age- and disease-stage-dependent effects noted in this analysis also resonate with findings from Farnad et al. (33), who described reduced neuroplasticity and responsiveness to neuromodulation in older individuals and those with advanced neurodegeneration. Collectively, these studies provide a robust foundation to justify the observed benefits of tDCS, reinforcing its role as a valuable adjunctive treatment in Parkinsonism. While some effect sizes were small to moderate, they may still be clinically meaningful in Parkinsonism, where even modest improvements in function or quality of life can enhance independence, reduce caregiver burden, and improve patient-centered outcomes. This underscores the potential utility of tDCS as a complementary intervention, even without large statistical effects.
4.1 Clinical significance
This study highlights the clinical significance of tDCS as a promising adjunctive intervention for individuals with Parkinsonism. The findings underscore the potential of tDCS to enhance functional outcomes, reduce disability, and improve quality of life, particularly among younger individuals and those in earlier disease stages (34). tDCS, particularly in younger individuals and those in earlier stages of Parkinsonism, may offer complementary benefits when integrated with physical therapy; however, these potential effects on treatment adherence and activity levels remain speculative and warrant confirmation through longitudinal studies (35). Identifying tDCS frequency as an independent predictor of clinical outcomes further reinforces its therapeutic importance and provides a rationale for integrating tDCS into personalized treatment plans (36). These results support the incorporation of tDCS into multidisciplinary care strategies for Parkinsonism, offering a non-invasive, cost-effective approach to improving patient-centered outcomes.
4.2 Limitations and future directions
This study is subject to several limitations, including its cross-sectional, non-randomized design, which limits causal inference and introduces potential confounding due to baseline group differences and retrospective assignment. Factors such as variability in therapy adherence, disease progression, and baseline functional status may have influenced the outcomes. Additionally, the small sample size, single-center setting, and absence of follow-up assessments restrict the generalizability and hinder evaluation of long-term effects or delayed adverse events. Future research should focus on large-scale, multicenter, randomized controlled trials with longitudinal follow-up to confirm these findings and explore causal relationships. Studies should also investigate patient-specific moderators of response, incorporate neurophysiological markers to elucidate mechanisms, and assess long-term safety and effectiveness of tDCS in diverse populations.
5 Conclusion
This study demonstrates that tDCS is significantly associated with improved clinical outcomes in individuals with Parkinsonism, particularly among younger patients and those in earlier stages of the disease. Higher tDCS frequency was correlated with reduced disability scores, improved quality of life, and enhanced adherence to physical therapy and medication regimens. Regression analysis identified significant associations between tDCS frequency and functional outcomes, adjusted for factors such as age and disease stage. These associations should be interpreted cautiously given the cross-sectional design. These findings highlight potential associations between tDCS exposure and clinical outcomes in individuals with Parkinsonism; however, the cross-sectional, non-randomized design precludes causal interpretations. Further longitudinal research is needed to confirm these associations and explore their implications for clinical practice.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the King Khalid University Ethics Committee (Approval number: ECM#2023-3304). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RR: Writing – original draft, Data curation, Writing – review & editing, Conceptualization, Validation, Project administration, Supervision, Methodology, Formal analysis. JT: Formal analysis, Resources, Data curation, Project administration, Visualization, Validation, Conceptualization, Methodology, Writing – original draft, Funding acquisition, Writing – review & editing, Supervision, Investigation, Software. IA: Methodology, Conceptualization, Data curation, Writing – review & editing, Formal analysis, Writing – original draft. VK: Data curation, Writing – original draft, Formal analysis, Visualization, Conceptualization, Writing – review & editing. SD: Writing – review & editing, Formal analysis, Writing – original draft, Methodology, Data curation, Conceptualization. KG: Project administration, Data curation, Writing – original draft, Formal analysis, Conceptualization, Writing – review & editing. PS: Formal analysis, Data curation, Writing – review & editing, Methodology, Writing – original draft, Software, Conceptualization. SA: Data curation, Conceptualization, Writing – review & editing, Writing – original draft. FA: Writing – original draft, Data curation, Writing – review & editing, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. King Salman Center for Disability Research, KSRG-2024-187.
Acknowledgments
The authors extend their appreciation to the King Salman center For Disability Research for funding this work through Research Group no KSRG-2024-187.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1601778/full#supplementary-material
Abbreviations
FSS, Fatigue Severity Scale; H&Y, Hoehn and Yahr Staging; MoCA, Montreal Cognitive Assessment; PDQ-39, Parkinson’s Disease Questionnaire; QoL, Quality of Life; SPSS, Statistical Package for the Social Sciences; tDCS, Transcranial Direct Current Stimulation; WHODAS 2.0, World Health Organization Disability Assessment Schedule 2.0; PSQI, Pittsburgh Sleep Quality Index.
References
1. Pagonabarraga, J, Tinazzi, M, Caccia, C, and Jost, WH. The role of glutamatergic neurotransmission in the motor and non-motor symptoms in Parkinson's disease: clinical cases and a review of the literature. J Clin Neurosci. (2021) 90:178–83. doi: 10.1016/j.jocn.2021.05.056
2. Blesa, J, Foffani, G, Dehay, B, Bezard, E, and Obeso, JA. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat Rev Neurosci. (2022) 23:115–28. doi: 10.1038/s41583-021-00542-9
3. Stocchi, F, Bravi, D, Emmi, A, and Antonini, A. Parkinson disease therapy: current strategies and future research priorities. Nat Rev Neurol. (2024) 20:695–707. doi: 10.1038/s41582-024-01034-x
4. Mosilhy, EA, Alshial, EE, Eltaras, MM, Rahman, MMA, Helmy, HI, Elazoul, AH, et al. Non-invasive transcranial brain modulation for neurological disorders treatment: a narrative review. Life Sci. (2022) 307:120869. doi: 10.1016/j.lfs.2022.120869
5. Oliveira, PCAD, Araujo, TABD, Machado, DGDS, Rodrigues, AC, Bikson, M, Andrade, SM, et al. Transcranial direct current stimulation on Parkinson's disease: systematic review and meta-analysis. Front Neurol. (2022) 12:794784. doi: 10.3389/fneur.2021.794784
6. Pozzi, C, Tatzer, VC, Strasser-Gugerell, C, Cavalli, S, Morandi, A, and Bellelli, G. Innovative non-pharmacological management of delirium in persons with dementia: new frontiers for physiotherapy and occupational therapy? Geriatrics. (2023) 8:28. doi: 10.3390/geriatrics8020028
7. Kumar, J, Patel, T, Sugandh, F, Dev, J, Kumar, U, Adeeb, M, et al. Innovative approaches and therapies to enhance neuroplasticity and promote recovery in patients with neurological disorders: a narrative review. Cureus. (2023) 15:e41914. doi: 10.7759/cureus.41914
8. Evancho, A, Tyler, WJ, and McGregor, K. A review of combined neuromodulation and physical therapy interventions for enhanced neurorehabilitation. Front Hum Neurosci. (2023) 17:1151218. doi: 10.3389/fnhum.2023.1151218
9. El-Mansoury, B, Maloui, AB, Draoui, A, Hamdan, YA, Oukhrib, M, Smimih, K, et al. Non-motor symptoms in Parkinson's disease: the other side of the disease In: Experimental and clinical evidence of the neuropathology of Parkinson’s disease. Hershey, Pennsylvania, USA: IGI Glolbal (2023). 25–45.
10. Carapellotti, AM, Rodger, M, and Doumas, M. Evaluating the effects of dance on motor outcomes, non-motor outcomes, and quality of life in people living with Parkinson’s: a feasibility study. Pilot Feasibility Stud. (2022) 8:36. doi: 10.1186/s40814-022-00982-9
11. Sanches, C, Stengel, C, Godard, J, Mertz, J, Teichmann, M, Migliaccio, R, et al. Past, present, and future of non-invasive brain stimulation approaches to treat cognitive impairment in neurodegenerative diseases: time for a comprehensive critical review. Front Aging Neurosci. (2021) 12:578339. doi: 10.3389/fnagi.2020.578339
12. Zakibakhsh, N, Basharpoor, S, Ghalyanchi Langroodi, H, Narimani, M, Nitsche, MA, and Salehinejad, MA. Repeated prefrontal tDCS for improving mental health and cognitive deficits in multiple sclerosis: a randomized, double-blind, parallel-group study. J Transl Med. (2024) 22:843. doi: 10.1186/s12967-024-05638-1
13. Bleton, J-P, Cossé, C, Caloc’h, AT, Moreno, S, Diverres, E, Derkinderen, P, et al. Combination of anodal tDCS of the cerebellum with a goal-oriented motor training to treat cervical dystonia: a pilot case series. Front Neurol. (2024) 15:1381390. doi: 10.3389/fneur.2024.1381390
14. González-Rodriguez, B, Serradell-Ribé, N, Viejo-Sobera, R, Romero-Muñoz, JP, and Marron, EM. Transcranial direct current stimulation in neglect rehabilitation after stroke: a systematic review. J Neurol. (2022) 269:6310–29. doi: 10.1007/s00415-022-11338-x
15. Nascimento, RMD, Cavalcanti, RL, Souza, CG, Chaves, G, and Macedo, LB. Transcranial direct current stimulation combined with peripheral stimulation in chronic pain: a systematic review and meta-analysis. Expert Rev Med Devices. (2023) 20:121–40. doi: 10.1080/17434440.2022.2039623
16. Khan, AM, Habib, MA, Hannan, M, and Huq, MR. Clinical presentation of Parkinson’s disease: experience of using Movement Disorder Society clinical diagnostic criteria for Parkinson’s disease. J Adv Med Med Res. (2021) 33:183–8.
17. Ma, X, Li, S, Li, C, Wang, R, Chen, M, Chen, H, et al. Total cerebral small vessel score association with Hoehn and Yahr stage in Parkinson’s disease. Front Aging Neurosci. (2021) 13:682776. doi: 10.3389/fnagi.2021.682776
18. Ciamarra, P, Corbi, G, Gimigliano, F, Feola, A, and Campobasso, CP. The World Health Organization disability assessment schedule 2.0 (WHODAS 2.0) as a measure among elderly population. A review. Disabil Rehabil. (2024):1–8. doi: 10.1080/09638288.2024.2439567 [Epub ahead of print].
19. Schönenberg, A, and Prell, T. Measuring quality of life with the Parkinson’s disease Questionnaire-39 in people with cognitive impairment. PLoS One. (2022) 17:e0266140. doi: 10.1371/journal.pone.0266140
20. Su, Y, Dong, J, Sun, J, Zhang, Y, Ma, S, Li, M, et al. Cognitive function assessed by Mini-mental state examination and risk of all-cause mortality: a community-based prospective cohort study. BMC Geriatr. (2021) 21:524. doi: 10.1186/s12877-021-02471-9
21. Zitser, J, Allen, IE, Falgàs, N, Le, MM, Neylan, TC, Kramer, JH, et al. Pittsburgh sleep quality index (PSQI) responses are modulated by total sleep time and wake after sleep onset in healthy older adults. PLoS One. (2022) 17:e0270095. doi: 10.1371/journal.pone.0270095
22. Naik, H, Shao, S, Tran, KC, Wong, AW, Russell, JA, Khor, E, et al. Evaluating fatigue in patients recovering from COVID-19: validation of the fatigue severity scale and single item screening questions. Health Qual Life Outcomes. (2022) 20:170. doi: 10.1186/s12955-022-02082-x
23. Singh, S, Bhattacharjee, S, and Udupa, K. Neuromodulation interventions in paediatric neurological disorders: A contemporary review of scientific advancements. J Pediatr Neonat Med. (2024) 6:1.
24. Bosch-Barceló, P, Climent-Sanz, C, Martínez-Navarro, O, Masbernat-Almenara, M, Pakarinen, A, Ghosh, PK, et al. A treadmill training program in a gamified virtual reality environment combined with transcranial direct current stimulation in Parkinson’s disease: study protocol for a randomized controlled trial. PLoS One. (2024) 19:e0307304. doi: 10.1371/journal.pone.0307304
25. Chmiel, J, Kurpas, D, and Stępień-Słodkowska, M. The potential of transcranial direct current stimulation (tDCS) in improving quality of life in patients with multiple sclerosis: a review and discussion of mechanisms of action. J Clin Med. (2025) 14:373. doi: 10.3390/jcm14020373
26. Elsner, B, Kugler, J, Pohl, M, and Mehrholz, J. Transcranial direct current stimulation (tDCS) for idiopathic Parkinson's disease. Cochrane Database Syst Rev. (2016) 2016:CD010916. doi: 10.1002/14651858.CD010916.pub2
27. Rahimi, F, Nejati, V, Nassadj, G, Ziaei, B, and Mohammadi, HK. The effect of transcranial direct stimulation as an add-on treatment to conventional physical therapy on pain intensity and functional ability in individuals with knee osteoarthritis: a randomized controlled trial. Neurophysiol Clin. (2021) 51:507–16. doi: 10.1016/j.neucli.2021.06.002
28. Smashna, O. Transcranial electrical stimulation in post-traumatic stress disorder and BRAIN injury: possibilities of tuning neuronal networks. Med Perspect. (2024) 29:175–83. doi: 10.26641/2307-0404.2024.3.313673
29. Semmler, C, Stopic, V, Jost, ST, Fink, GR, Weiss, PH, and Barbe, MT. Preoperative motor deficits and depressive symptoms predict quality of life in patients with Parkinson’s disease at different time points after surgery for subthalamic stimulation: a retrospective study. Neurol Res Pract. (2024) 6:8. doi: 10.1186/s42466-023-00303-2
30. Yeroushalmi, S, Maloni, H, Costello, K, and Wallin, MT. Telemedicine and multiple sclerosis: a comprehensive literature review. J Telemed Telecare. (2020) 26:400–13. doi: 10.1177/1357633X19840097
31. Flöel, A. tDCS-enhanced motor and cognitive function in neurological diseases. NeuroImage. (2014) 85:934–47. doi: 10.1016/j.neuroimage.2013.05.098
32. Ho, K-A, Taylor, JL, Chew, T, Gálvez, V, Alonzo, A, Bai, S, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Stimul. (2016) 9:1–7. doi: 10.1016/j.brs.2015.08.003
33. Farnad, L, Ghasemian-Shirvan, E, Mosayebi-Samani, M, Kuo, M-F, and Nitsche, MA. Exploring and optimizing the neuroplastic effects of anodal transcranial direct current stimulation over the primary motor cortex of older humans. Brain Stimul. (2021) 14:622–34. doi: 10.1016/j.brs.2021.03.013
34. Lefaucheur, J-P, Antal, A, Ayache, SS, Benninger, DH, Brunelin, J, Cogiamanian, F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
35. do Prado De, M.M.R. (eds.). Handbook of research on advances in digital technologies to promote rehabilitation and community participation. Hershey, Pennsylvania, USA: IGI Global.
Keywords: Parkinsonism, tDCS, disability, quality of life, neuromodulation
Citation: Reddy RS, Tedla JS, Ahmad I, Kakaraparthi VN, Dixit S, Gular K, Samuel PS, Aljehani SM and Alarabi FA (2025) Association between transcranial direct current stimulation and disability and quality of life in individuals with Parkinsonism: cross-sectional study. Front. Neurol. 16:1601778. doi: 10.3389/fneur.2025.1601778
Edited by:
Giorgio Scivoletto, Santa Lucia Foundation (IRCCS), ItalyReviewed by:
Koji Ishikuro, University of Toyama University Hospital, JapanRabeet Tariq, Aga Khan University, Pakistan
Nicole Fritz, Universidad de Los Lagos, Chile
Tirath Patel, American University of Antigua, Antigua and Barbuda
Copyright © 2025 Reddy, Tedla, Ahmad, Kakaraparthi, Dixit, Gular, Samuel, Aljehani and Alarabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ravi Shankar Reddy, cnNoYW5rYXJAa2t1LmVkdS5zYQ==
 Ravi Shankar Reddy
Ravi Shankar Reddy Jaya Shanker Tedla
Jaya Shanker Tedla Irshad Ahmad
Irshad Ahmad Venkata Nagaraj Kakaraparthi
Venkata Nagaraj Kakaraparthi Snehil Dixit1
Snehil Dixit1 Kumar Gular
Kumar Gular Paul Silvian Samuel
Paul Silvian Samuel