- 1Department of Neurology, Kindai University Faculty of Medicine, Osaka, Japan
- 2Department of Human Genetics, Yokohama City University Graduate School of Medicine, Yokohama, Japan
- 3Department of Clinical Genetics, Yokohama City University Hospital, Yokohama, Japan
- 4Division of Rehabilitation Medicine, Kindai University Hospital, Osakasayama, Japan
- 5Department of Rehabilitation Medicine, Kindai University Faculty of Medicine, Osakasayama, Japan
- 6Department of Rare Disease Genomics, Yokohama City University Hospital, Yokohama, Japan
Introduction: The genetic etiology of Parkinson’s disease (PD) is complex; approximately 10% of patients with PD have various gene mutations that lead to familial forms of the disease. Recent analyses of non-coding repeat regions revealed that many neurodegenerative diseases are associated with pathological expansions. We evaluated the genetic background of non-coding repeat expansions in Japanese patients with PD.
Methods: We collected blood samples from 203 Japanese patients with PD and analyzed various non-coding repeat genes, including ATXN8OS, RFC1, C9ORF72, NOTCH2NLC, BEAN1/TK2, and NOP56, using PCR-Sanger sequencing, repeat-primed PCR assay, and long-read sequencing.
Results: Three patients with PD (1.5%) were found to have heterozygous repeat expansions in ATXN8OS, the gene causative of spinocerebellar ataxia type 8 and is associated with long non-coding RNA. One (0.5%) patient had compound heterozygous repeat expansions (AAGGG and ACAGG) in RFC1, the gene causative of cerebellar ataxia, neuropathy, and vestibular areflexia syndrome, which encodes a DNA repair protein. No patient had repeat expansions in C9ORF72, NOTCH2NLC, BEAN1/TK2, or NOP56. All patients with ATXN8OS repeat expansions exhibited typical parkinsonism with relatively rare subjective dysphagia, which was confirmed by videofluoroscopic results. Functional imaging, such as dopamine-transporter single photon emission computed tomography, showed abnormal findings in patients with non-coding repeat expansions.
Discussion: Our findings revealed the importance of non-coding repeat expansions in Japanese patients with PD. This is the first study to show the positive result of non-coding repeat expansions in many patients with PD in Japan.
Introduction
Parkinson’s disease (PD) is clinically characterized by tremors, rigidity, and akinesia, which are later accompanied by postural instability. Radiological features were normal on conventional brain MRI but abnormal on dopamine transporter single-photon emission computed tomography or 123I-metaiodobenzylguanidine myocardial (MIBG) scintigraphy (1, 2). Dopamine replacement therapy is effective, at least in early-stage patients, but motor and non-motor complications, including reduced swallowing functions, become apparent in advanced stages. The progression, variation of symptoms, and response to treatment differ considerably between patients, suggesting that PD is associates with various implicated pathophysiological pathways (1). While PD mostly occurs sporadically, approximately 10% of patients with PD in Japan, as well as in Western countries, exhibit mutations in various genes that are responsible for familial PD. In addition, non-coding repeat expansions in genes causative of other neurodegenerative diseases have been reported worldwide in familial or sporadic PD. In contrast, no such findings have been established in Japan.
Spinocerebellar ataxia type 8 (SCA8) is an autosomal dominant neurodegenerative disease caused by non-coding CTA/CTG repeat expansions in ATXN8OS (ataxin 8 opposite strand). ATXN8OS is associated with long non-coding RNA, which, if not mutated, may not encode a functional protein (3, 4). The pathogenicity of the expanded allele has been proven using a transgenic mouse model (5). Many patients have pure cerebellar ataxia, whereas others have parkinsonism (6–8) or amyotrophic lateral sclerosis (9). Our preliminary analysis of 76 Japanese patients with PD did not reveal any ATXN8OS repeat expansions (8). To date, clinical data of only five PD patients exhibiting ATXN8OS repeat expansions have been reported globally, and none of these cases included imaging findings.
Cerebellar ataxia, neuropathy, and vestibular areflexia syndrome (CANVAS) have recently been attributed to biallelic non-coding pentanucleotide repeat expansions in RFC1 (replication factor C subunit 1) (10). Normal RFC1 encodes a DNA repair protein, the function of which is not altered by intronic repeat expansion (10). Pathogenic repeat configurations included AAGGG, ACAGG, AGGGC, AGAGG, and AAGGC (11). Recently, the disease entity has expanded to atypical phenotypes, including chronic or immune-mediated neuropathy without cerebellar ataxia or vestibular areflexia (12, 13). More recently, two reports from Northern Europe described that biallelic AAGGG repeat expansions are found in six patients with typical or early-onset PD (14, 15). A recent report from the US described that three patients with PD had presumably pathogenic biallelic AAGGG repeat expansions (16). Thus, RFC1 mutations were likely causative in 11 patients with PD, most of whom had biallelic AAGGG repeat expansions, with the exception of complex types of AGGGG and AAGGG repeats (17).
Other non-coding repeat genes associated with parkinsonism include C9ORF72 (Chromosome 9 open reading frame 72), causative for amyotrophic lateral sclerosis (ALS); NOTCH2NLC (notch 2 N-terminal like C) for neuronal intranuclear inclusion disease (NIID); BEAN1 (brain expressed associated with NEDD4 1)/TK2 (thymidine kinase 2) for spinocerebellar ataxia type 31 (SCA31); and NOP56 (nucleolar protein 56) for spinocerebellar ataxia type 36 (SCA36). In cohorts of Western countries, 1.1% of patients with PD had GGGGCC repeat expansions in C9ORF72 (18). Less than 1% of PD patients in China had NOTCH2NLC GCC repeat expansion (19). Several patients with ataxic BEAN1/TK2 repeat expansions also presented with parkinsonism; however, they did not fulfill the criteria for a diagnosis of PD (20). Patients with GGCCTG repeat expansions in NOP56 have abnormal dopamine transporter imaging findings consistent with those in patients with typical PD but lack clinical manifestation of parkinsonism (21).
The suppression of repeat expansion-associated toxicity and the removal of repeats are being actively investigated as a therapy for non-coding repeat diseases, such as C9ORF72-related ALS (22, 23). If such a therapy is established, it might be applicable to PD with non-coding repeats. In this report, we therefore analyzed non-coding repeat genes including ATXN8OS, RFC1, C9ORF72, NOTCH2NLC, BEAN1/TK2, and NOP56 in 203 Japanese patients with PD.
Materials and methods
Genetic testing
All patients and controls were Japanese and enrolled in this study from the Kinki region in Japan between 2005 and 2024. All patients were diagnosed with PD by board-certified neurologists in accordance with the United Kingdom Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria and had a Hoehn–Yahr grade of II–IV. A total of 203 patients with PD were included in the study, comprising 87 men and 116 women. The average age of the participants was 72 ± 11 years (mean ± SD). A control group consisting of 200 apparently healthy controls (116 men and 84 women; mean age ± SD, 71 ± 7 years) was also studied. Blood samples were collected from patients with PD who had no mutations in the ATXN1, ATXN2, or ATXN3 genes (24–27). DNA was extracted using a DNA extraction kit or Pure Gene Blood Core Kit (Qiagen Inc., Germantown, MD, United States). The region containing the CTA/CTG repeat of the ATXN8OS gene was amplified using PCR with primers as described (3, 28). The amplified products were purified using gel electrophoresis and subjected to Sanger sequencing. The normal number of CTA/CTG repeats in the ATXN8OS gene ranges from 15 to 50, while repeats of length 80 or more are pathogenic. In several reports, expansions of more than 50 CTA/CTG repeats, including intermediate expansions, were stated to cause ataxia at some point in life; however, there were no clinical details. BEAN1/TK2 was analyzed as described. C9ORF72 and NOP56 were analyzed by repeat-primed PCR assay as described (13, 27, 29). Primers used for the amplification of the short range of the repeat region in RFC1 were as previously described (13). When no normal size band was detected, the sample was subjected to repeat-primed polymerase chain reaction (PCR) for AAGGG (pathogenic), ACAGG (pathogenic), AGGGC (pathogenic), AGAGG (possibly pathogenic), AAGGC (possibly pathogenic), AAAGG (variable penetrance), AAAAG (likely non-pathogenic), AAAGGG (likely non-pathogenic), and AAGAG (likely non-pathogenic) repeat configurations. The primers used for repeat-primed PCR are described by Hirano et al. (13). Long-read sequencing was performed using the Revio system (PacBio, Menlo Park, CA) following the manufacturer’s protocol. To increase the depth of coverage, hifi_reads.bam and fail_reads.bam, which did not pass the HiFi Q20 threshold, were merged and aligned using pbmm2.1 Genotyping and visualization of repeats were performed using TRGT v0.9.0 and TRVZ v0.9.0.2 We performed exome sequencing in some patients with pathological expansions using a previously described method (30). We focused on the known familial PD genes and then extended the search to other possible genes in which mutations resided, as listed in the Supplementary Table S2.
Standard protocol approvals, registrations, and patient consents
This study was approved by the Institutional Review Boards of Kindai University (IRB# 16–011) and Yokohama City University (B230600048-Revised). All participants provided written informed consent to publish their clinical data.
Evaluation of parkinsonism
Parkinsonism was evaluated in mutation-positive patients using part III (motor examination) of the Unified PD Rating Scale (UPDRS-III). All patients with ATXN8OS mutations were evaluated before and after dopaminergic treatment during “on” time.
Results
Results of genetic testing of PCR and sequencing analyses
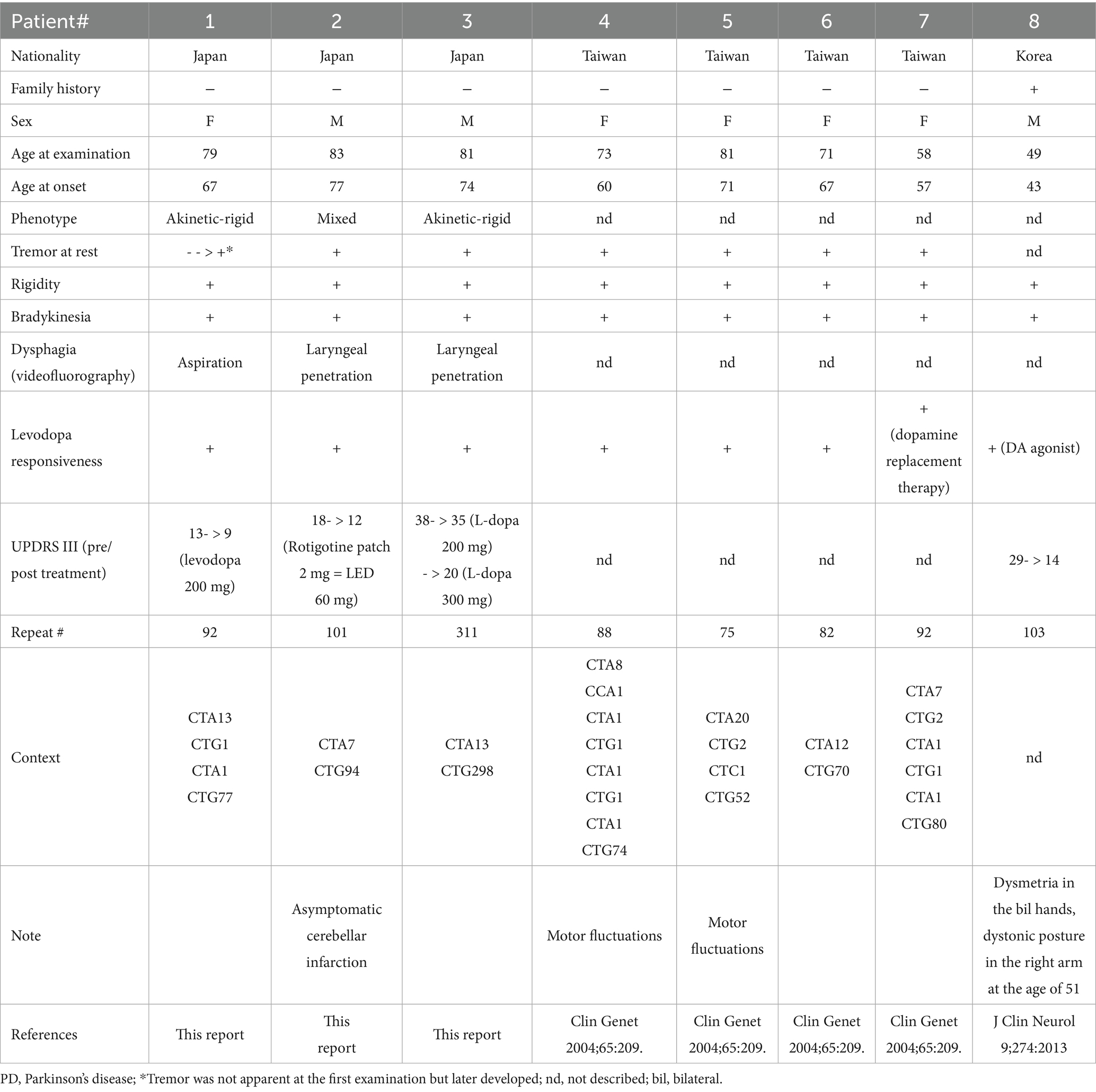
Genetic testing revealed that three patients with PD had heterozygous ATXN8OS mutations (Figure 1). Short-range PCR for RFC1 failed to identify a normal-size allele in one patient, and repeat-primed PCR revealed that the patient had compound heterozygous repeat expansions, AAGGG repeat and ACAGG repeat, in RFC1 (Figure 2A). No other genes were mutated. None of the controls had mutations in the genes tested in this study. The control group had 26 ± 4 repeats (mean ± SD), ranging from 18 to 32, in the ATXN8OS gene. Patient 1 had 92 repeats (CTA13CTG1CTA1CTG77), Patient 2 had 101 repeats (CAT7CTG94), and Patient 3 had 311 repeats (CTA13CTG298).
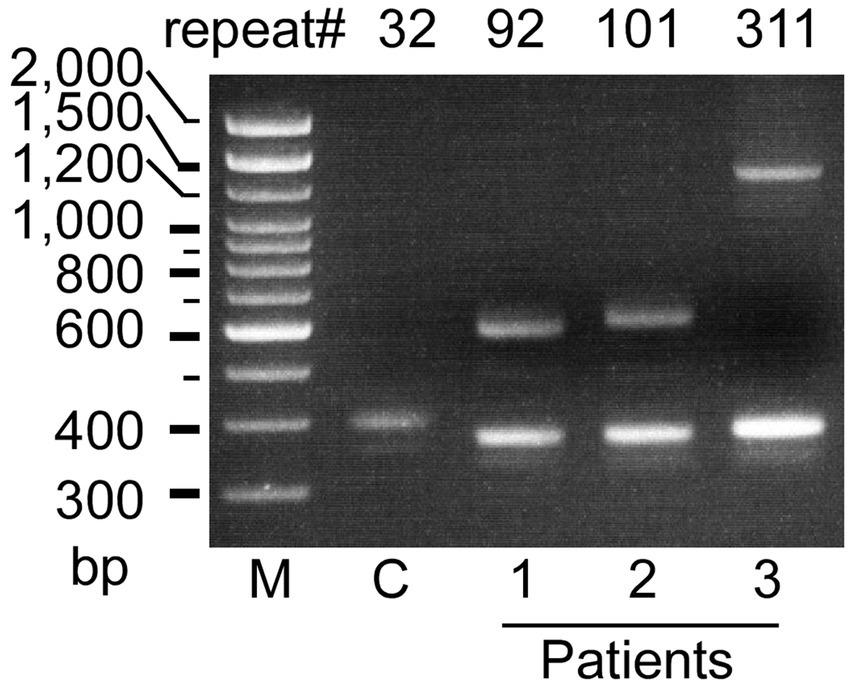
Figure 1. Agarose gel electrophoretic analysis of the ATXN8OS gene in patients with Parkinson’s disease (Pt 1–3). Three patients had expansions of the CTA/CTG repeat as indicated, whereas controls (C) had a normal repeat size. M, 100 base pair (bp) marker.
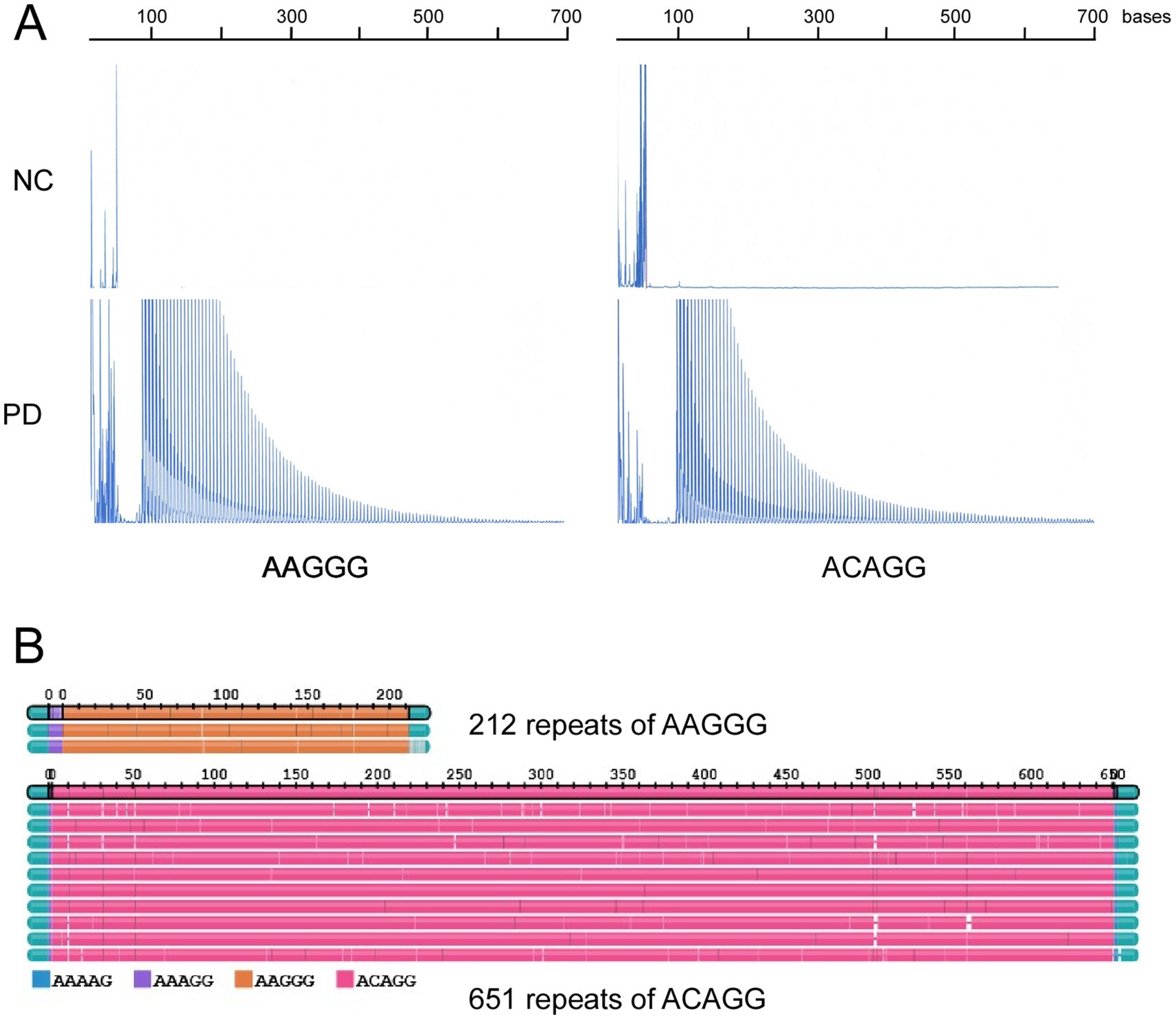
Figure 2. (A) Repeat-primed PCR results for RFC1. Pathological repeats of AAGGG or ACAGG were expanded in a patient with Parkinson’s disease (PD). (B) Long-read sequencing revealed 212 repeats of AAGGG and 651 repeats of ACAGG in the patient with PD.
Results of long-read sequencing
Long-read sequencing revealed that the patient with RFC1 had compound heterozygosity for 212 repeats of AAGGG and 651 repeats of ACAGG (Figure 2B). The pathogenic repeat length was originally described to be more than 400 (10), but several reports described that more than 100 were pathogenic (14, 15, 31).
Results of exome analyses
We performed exome sequencing on one of the three patients with ATXN8OS expansions (Patient 3) and the patient with biallelic RFC1 expansions. No pathological mutation was detected in either patient.
Clinical information about patients with ATXN8OS mutations
The clinical information of the three patients with ATXN8OS mutations in this study, along with previously reported patients, is summarized in Table 1 (14, 15). Our patients responded well to levodopa or the dopamine agonist rotigotine. They seemed to have typical PD symptoms. According to the reported study (32), the phenotypes of our patients were as follows: Patients 1 and 3 were akinetic-rigid type, and Patient 2 was mixed type (Table 1). No patient had cerebellar ataxia. Imaging findings of the three patients with ATXN8OS mutations are shown in Figure 3. Patient 1, with 92 repeats, had no obvious abnormality on MRI. Patient 2 with 101 repeats had an asymptomatic cerebellar infarction with non-specific atrophy in the cerebrum and cerebellum (Figure 3B). Patient 3, with 311 repeats in ATXN8OS, had no obvious abnormality on MRI but showed reduced striatal dopaminergic transporter uptake (Figure 3C). Other patients did not undergo dopaminergic transporter imaging or other functional imaging studies. All patients with ATXN8OS mutations reported swallowing difficulties or discomfort in the throat after eating, which was supported by videofluoroscopic results (Figure 4). Patient 1 exhibited aspiration (Figure 4A), and Patients 2 and 3 exhibited laryngeal penetration (Figs. 4B,C). No correlation was found between the number of repeats and severity or age at onset because the sample size was too small for statistical analysis.
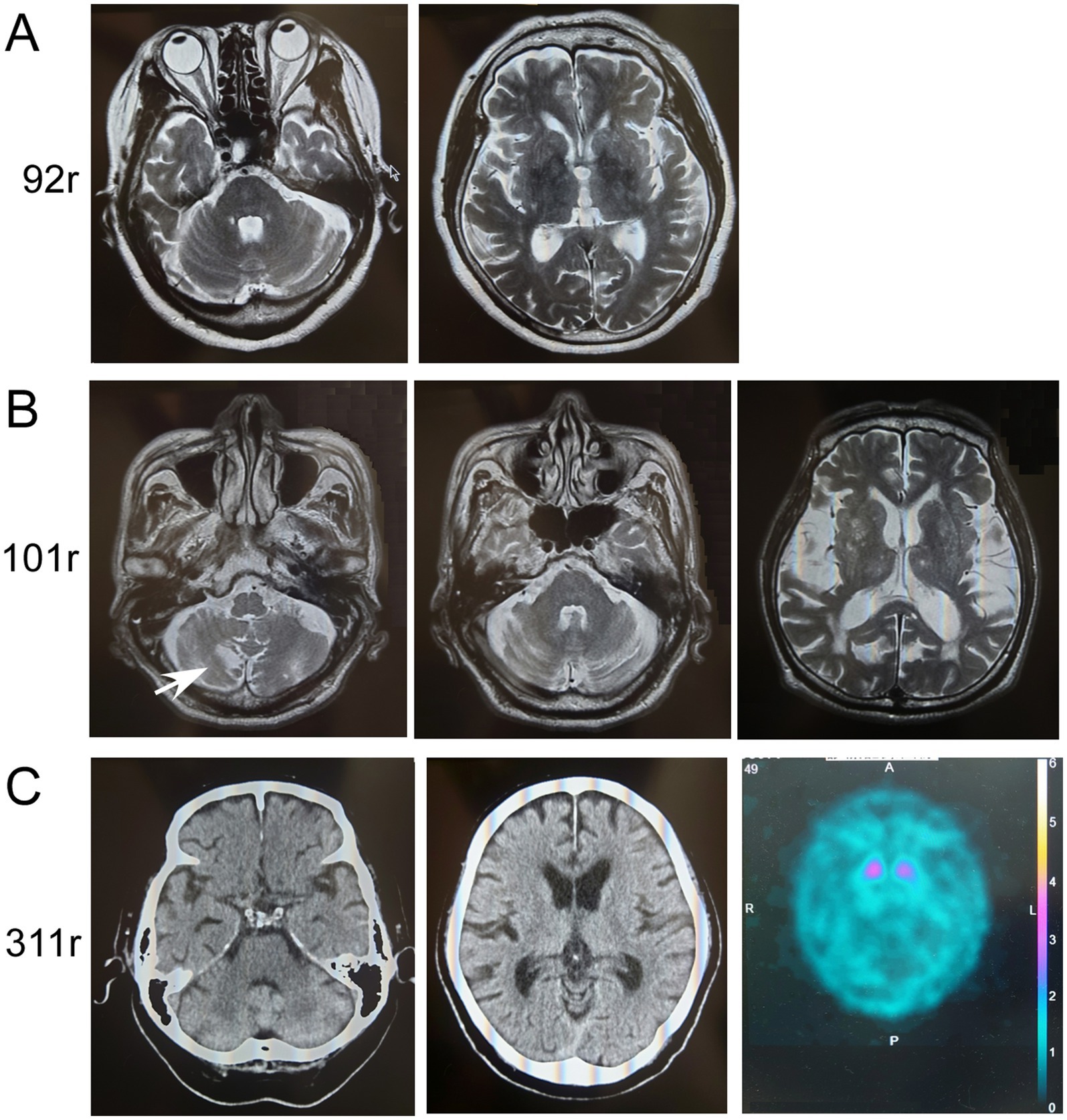
Figure 3. Imaging results of patients with ATXN8OS repeat expansions. (A) MRI of Patient 1 with 92 repeats (92r) showed no apparent atrophy of the cerebellum (upper panel) or cerebrum (lower panel). (B) MRI of Patient 2 with 101 repeats (101r) showed asymptomatic infarction of the cerebellum (arrow) and slight non-specific atrophy of the cerebellum or mild atrophy of the cerebrum with chronic ischemic lesions. (C) Computed tomography (CT) of the head in Patient 3 with 311 repeats (311r) showed no apparent atrophy of the cerebellum (left panel) or cerebrum (right panel). Dopamine-transporter single photon emission computed tomography showed marked reduction of the uptakes in the striatum. The specific binding ratio was 1.26 on the right striatum and 1.62 on the left.
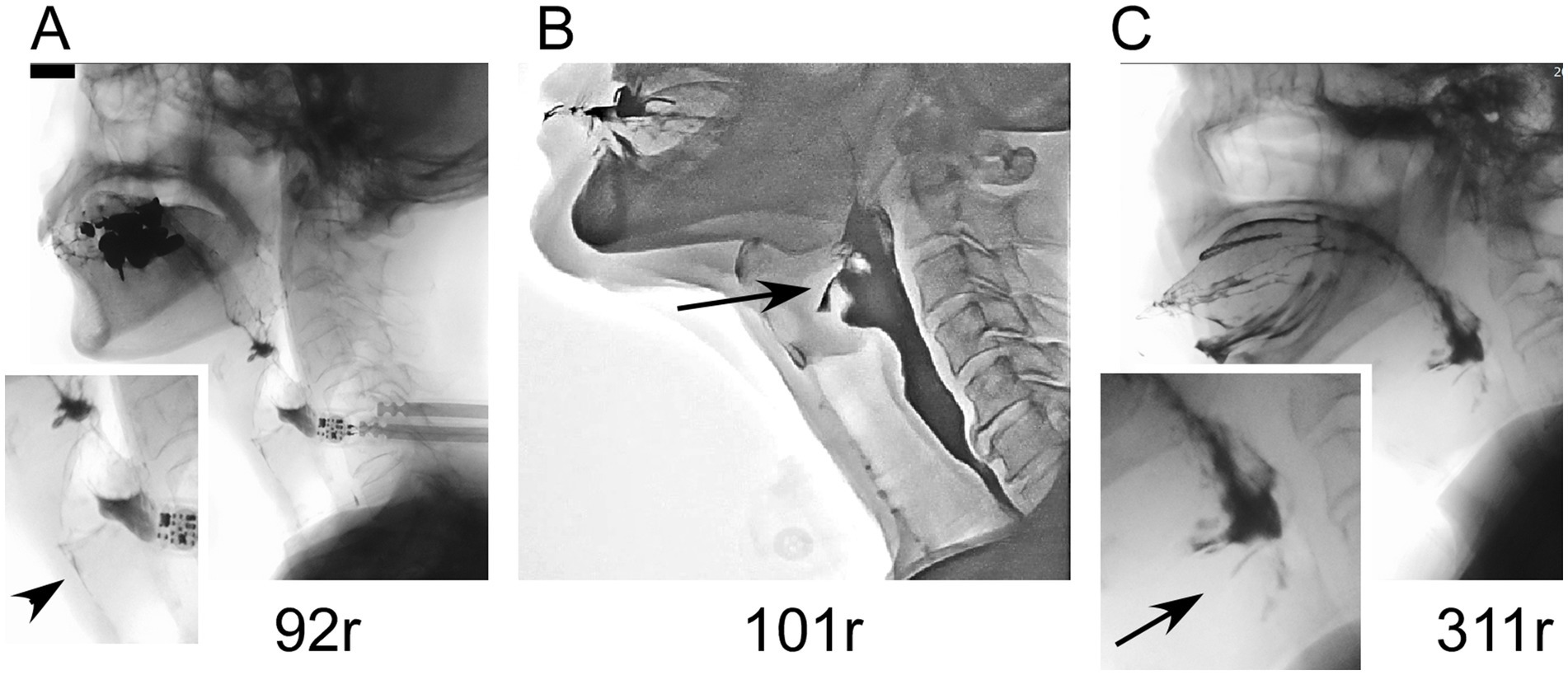
Figure 4. Videofluoroscopic results in patients with ATXN8OS repeat expansions. (A) A patient with 92 repeats underwent aspiration (arrowhead). (B) A patient with 101 repeats had laryngeal penetration. (C) A patient with 311 repeats also had laryngeal penetration.
Clinical information about patients with RFC1 mutations
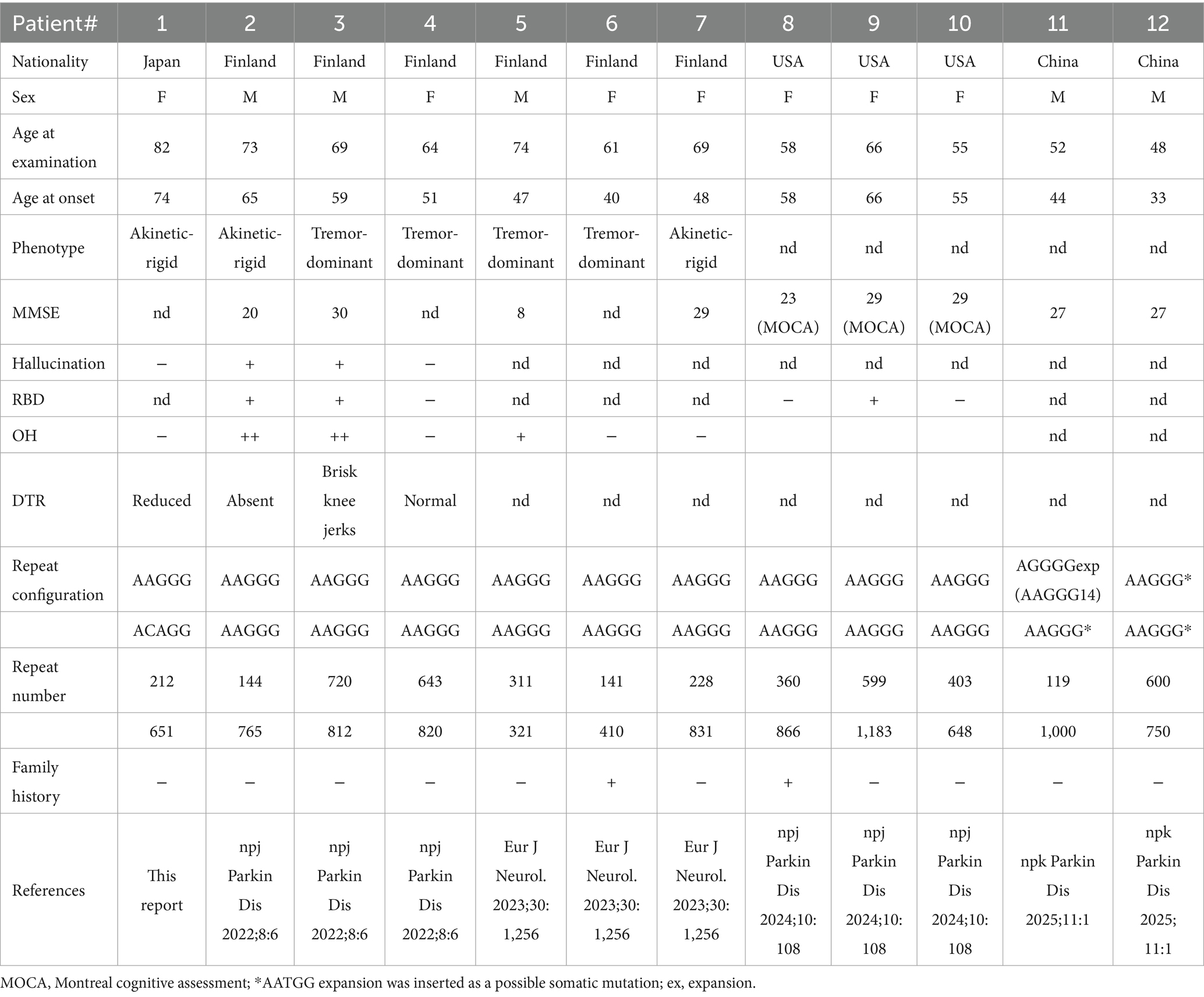
The clinical information of one patient with RFC1 mutations, along with previously reported patients, is summarized in Table 2 (15, 16). The patient had no cerebellar ataxia. Our patient had the oldest age of onset for the disease but had a typical akinetic-rigid phenotype. One of the four patients with biallelic AAGGG repeats reported in the US also had an additional LRRK2 mutation. This suggests that in this patient, the PD could have been caused by the LRRK2 mutation and not by the RFC1 repeat expansion and therefore was excluded from the table in this study. Imaging results of a patient with RFC1 repeat expansions are shown in Figure 5. The patient underwent MIBG scintigraphy and was found to have a normal heart/mediastinum (H/M) ratio but an increased washout ratio about 1 year after onset. No patients with RFC1 repeat expansions underwent videofluoroscopic analysis. No correlation was found between the number of repeats and age at onset because the sample size was too small for statistical analysis.
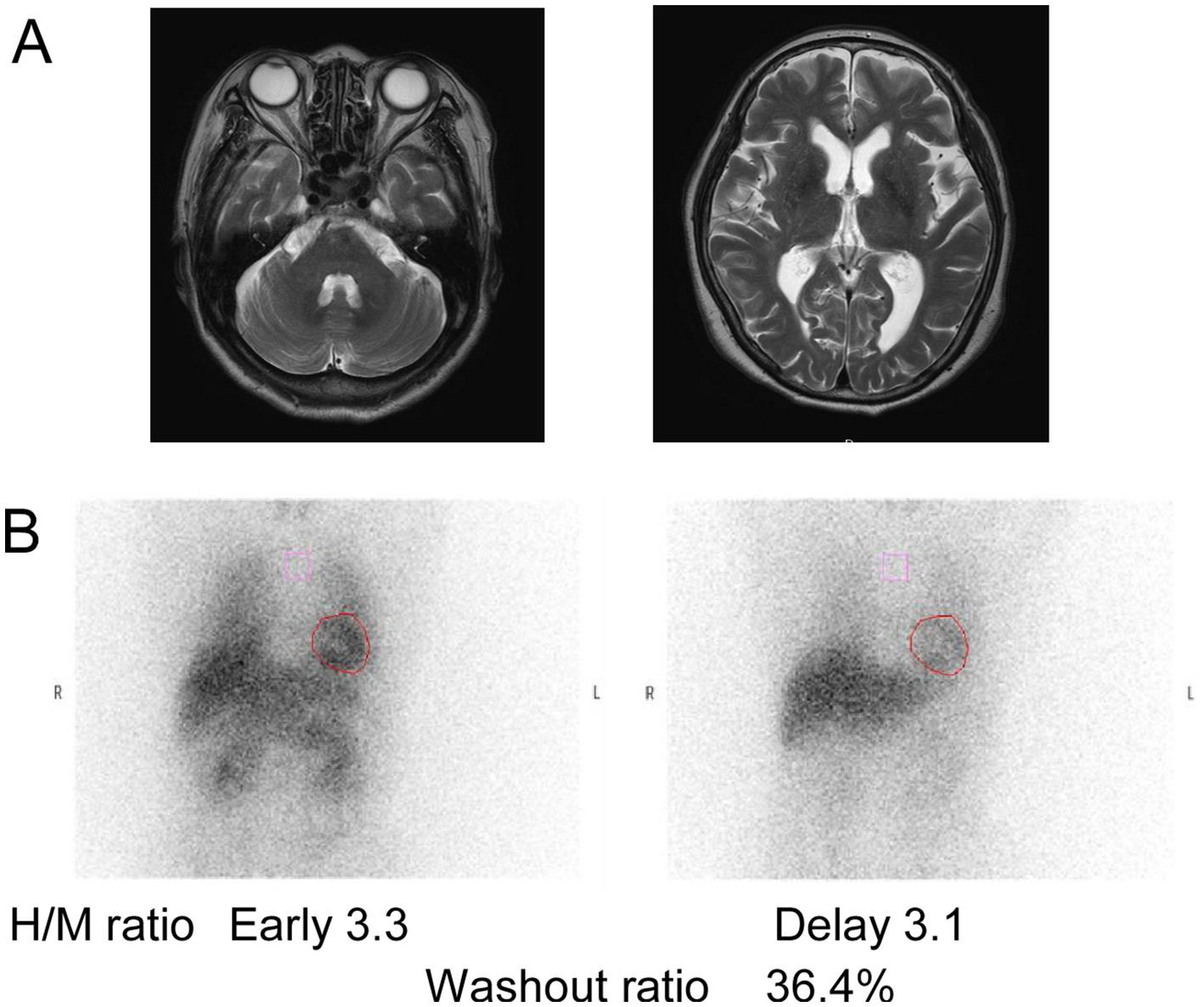
Figure 5. Imaging results of patients with RFC1 repeat expansions. (A) MRI of the patient with AAGGG and ACAGG repeat expansions. (B) 123I-metaiodobenzylguanidine myocardial scintigraphy performed on a patient about 1 year after onset showed normal values of heart/mediastinum (H/M) ratio. The H/M ratios were standardized under medium-energy collimator conditions (normal ≥ 2.2). Additionally, the washout ratio was found to have increased (normal ≤ 22%).
Discussion
This study found that three patients with PD (1.5%) had heterozygous mutations in ATXN8OS and that one patient with PD (0.5%) had compound heterozygous mutations in RFC1. In contrast, no patient had a mutation in C9ORF72, NOTCH2NLC, BEAN1/TK2, or NOP56, some of which have been mutated in PD in other countries (18, 33). These findings suggest that pathogenic non-coding repeat expansions are occasionally associated with PD in Japan, with some racial or geographical differences. Especially, ATXN8OS mutations were more frequently found in patients with PD and related disorders in East Asia (6, 8, 34).
Interestingly, the three patients with ATXN8OS mutations reported subjective dysphagia, which was confirmed by the objective examination using videofluoroscopic analysis. Patients with PD without mutations also frequently have dysphagia, but only 39% of patients reported subjective dysphagia in our previous cohort (35). The swallowing disturbance found in ATXN8OS mutation-positive patients is reminiscent of our previous finding in ATXN8OS-related ALS, where three of the three mutation-positive patients had bulbar onset or rapid progression of dysphagia (9). We speculate that neurons associated with swallowing may be susceptible to the expanded repeats in ATXN8OS.
The role of expanding non-coding repeats in the etiology of PD remains unclear, but a recent pathological study on SCA8 revealed that all four patients had degeneration of the substantia nigra, which is a region that is also affected in PD. Tau pathology typical of supranuclear palsy (PSP) was found in only one patient (36), suggesting an unknown mechanism involved in the degeneration. In addition, a pathological study on a patient with CANVAS revealed depletion of the pars compacta of the substantia nigra with widespread Lewy bodies in the locus coeruleus and substantia nigra, regions affected in PD (37). The tendency for substantia nigra degeneration in such non-coding repeat diseases may be associated with striatal dopamine deficiency. Although the effect of non-coding repeat expansion on existing PD treatments is unknown, PD patients with non-coding repeats in the current and reported studies responded well to conventional dopamine replacement therapy, such as oral levodopa or dopamine agonists.
Imaging results, especially functional imaging, have been rarely reported in patients with non-coding repeat expansions. Dopamine-transporter single photon emission computed tomography in this study showed a marked reduction of striatal uptake, a cardinal feature of PD. MRI revealed no apparent atrophy of the cerebellum in the three patients with ATXN8OS mutations. This is compatible with the reported findings of ATXN8OS-related PD (6). Similarly, RFC1-related PD showed no apparent atrophy in the cerebellum. A patient with RFC1 repeat expansions a year after onset had an apparently normal H/M ratio in MIBG scintigraphy, which was supposed to be reduced in PD. However, H/M ratios are sometimes within normal ranges during the early phase of PD (2). In contrast, the increased washout ratio observed here was compatible with that in PD (38). These findings suggest that imaging findings in PD associated with non-coding repeat expansions are indistinguishable from those without repeat expansions.
Non-coding repeat expansions may have some common pathomechanisms, including the formation of RNA foci and repeat-associated non-ATG (RAN) translation (39). In SCA8, both mechanisms have been reported to be involved (5, 40, 41). In addition, the mechanism underlying the loss of function of genes with non-coding repeats has attracted much attention (22). A therapeutic approach to C9ORF72-related ALS may also be applicable to other non-coding repeat diseases. In C9ORF72-related ALS, the suppression of abnormal transcription by antisense oligonucleotides is an ongoing clinical project (42). A similar method was recently reported in SCA36 (43). In another study, the suppression of toxicity in an abnormal ATXN8OS transcript by the KH RNA-binding domain of Spoonbill in vivo exerted a therapeutic effect (44). Recently, excision of pathological repeats has been reported in experimental models of C9ORF72-related ALS (22). Although the ATXN8OS gene, which is reported to have bidirectional transcripts, may have a more complex pathomechanism (5), the suppression of at least one pathological pathway might help slow the disease process.
The pathogenesis of RFC1 repeat expansions remains to be elucidated, but recessive inheritance suggests the loss of function of RFC1. A patient with one truncated mutation in an allele and a repeat expansion in the other supports this notion. However, a loss-of-function mechanism of RFC1 has not been proven yet (10). A recent report showed RNA foci in the cerebellum, suggesting the dominant toxic function of the expanded repeat. The configuration of repeats has been shown to be crucial for pathogenicity in this gene, but of the pathogenic repeat configurations, AAGGG, ACAGG, AGGGC, or combinations thereof showed no clear genotype and phenotype relationship in total patients with CANVAS (11, 12, 45). Interestingly, our patient with PD had only ACAGG repeats and had the highest age of onset. The association between repeat configuration and age at PD onset should be assessed as more mutation-positive patients are identified. Thus, the pathogenesis of the RFC1 repeat expansion may involve multiple pathways.
In this study, the coincidental occurrence of SCA8 and PD was possible because mutations in the aforementioned genes have been infrequently found in controls (3, 4, 46). However, the repeat sizes found in patients with PD, 92–311 repeats, were not found in the reported control alleles in Japan (n = 654) (46). The relatively low prevalence of SCA8 (0.7/100,000) and PD (1.8/1,000) in Japan suggests that their coincidental coexistence is unlikely to occur in the three presumably unrelated patients. In contrast, because RFC1 mutations are infrequently found in controls, only one patient with PD may have coincident PD. Therefore, future studies on their correlation. In summary, this study reveals that a certain number of patients with PD were positive for non-coding repeat expansions and extends the geographic range of such patients to Japan. In the future, new therapies for non-coding repeat disease are expected to be developed for this disease group.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Kindai University institutional review board and Yokohama City University institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MH: Data curation, Methodology, Conceptualization, Investigation, Supervision, Funding acquisition, Writing – review & editing, Writing – original draft, Formal analysis. MS: Conceptualization, Writing – review & editing, Formal analysis, Data curation. SM: Writing – review & editing, Data curation. YY: Writing – review & editing, Data curation. CI: Data curation, Writing – review & editing. RY: Data curation, Writing – review & editing. KS: Data curation, Validation, Writing – review & editing, Project administration. AT: Writing – review & editing, Data curation. YH: Data curation, Writing – review & editing. EK: Writing – review & editing, Formal analysis. TM: Writing – review & editing, Formal analysis. KF: Data curation, Writing – review & editing. YM: Formal analysis, Writing – review & editing. NM: Writing – review & editing, Formal analysis, Data curation. YN: Resources, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partly supported by Grants-in-Aid for Scientific Research (C) (22 K07510 to MH), Grants-in-Aid for Scientific Research (A) (23H00459 to MH), AMED (JP24ek0109697 to MH), and Transformative Research Areas (A) (Multifaceted Proteins) (20H05927 to YN) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Takeda Japan Medical Office Funded Research Grant 2023 (to MH), and Kindai University Research Grant (KD2306 to MH). This work was also supported in part by AMED under grant numbers JP24ek0109674, JP24ek0109760, JP24ek0109617, JP24ek0109648, and JP24ek0109677 (N. Matsumoto); JSPS KAKENHI under grant numbers JP23K27520 (S. Miyatake), JP23K27568 and JP23K18278 (T. Mizuguchi), and JP24K02230 (N. Matsumoto); and the Takeda Science Foundation (T. Mizuguchi, N. Matsumoto). This study also received funding from Takeda Pharmaceuticals, Takeda Japan Medical Office Funded Research Grant 2023. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1606305/full#supplementary-material
Footnotes
1. ^https://github.com/PacificBiosciences/pbmm2
2. ^https://github.com/PacificBiosciences/trgt?tab=readme-ov-file
References
1. Wullner, U, Borghammer, P, Choe, CU, Csoti, I, Falkenburger, B, Gasser, T, et al. The heterogeneity of Parkinson's disease. J Neural Transm (Vienna). (2023) 130:827–38. doi: 10.1007/s00702-023-02635-4
2. Orimo, S, Ozawa, E, Nakade, S, Sugimoto, T, and Mizusawa, H. (123)I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson's disease. J Neurol Neurosurg Psychiatry. (1999) 67:189–94. doi: 10.1136/jnnp.67.2.189
3. Koob, MD, Moseley, ML, Schut, LJ, Benzow, KA, Bird, TD, Day, JW, et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet. (1999) 21:379–84. doi: 10.1038/7710
4. Day, JW, Schut, LJ, Moseley, ML, Durand, AC, and Ranum, LP. Spinocerebellar ataxia type 8: clinical features in a large family. Neurology. (2000) 55:649–57. doi: 10.1212/WNL.55.5.649
5. Moseley, ML, Zu, T, Ikeda, Y, Gao, W, Mosemiller, AK, Daughters, RS, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. (2006) 38:758–69. doi: 10.1038/ng1827
6. Wu, YR, Lin, HY, Chen, CM, Gwinn-Hardy, K, Ro, LS, Wang, YC, et al. Genetic testing in spinocerebellar ataxia in Taiwan: expansions of trinucleotide repeats in SCA8 and SCA17 are associated with typical Parkinson's disease. Clin Genet. (2004) 65:209–14. doi: 10.1111/j.0009-9163.2004.00213.x
7. Kim, JS, Son, TO, Youn, J, Ki, CS, and Cho, JW. Non-ataxic phenotypes of SCA8 mimicking amyotrophic lateral sclerosis and Parkinson disease. J Clin Neurol. (2013) 9:274–9. doi: 10.3988/jcn.2013.9.4.274
8. Samukawa, M, Hirano, M, Saigoh, K, Kawai, S, Hamada, Y, Takahashi, D, et al. PSP-phenotype in SCA8: case report and systemic review. Cerebellum. (2019) 18:76–84. doi: 10.1007/s12311-018-0955-0
9. Hirano, M, Samukawa, M, Isono, C, Saigoh, K, Nakamura, Y, and Kusunoki, S. Noncoding repeat expansions for ALS in Japan are associated with the ATXN8OS gene. Neurol Genet. (2018) 4:e252. doi: 10.1212/NXG.0000000000000252
10. Cortese, A, Simone, R, Sullivan, R, Vandrovcova, J, Tariq, H, Yau, WY, et al. Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nat Genet. (2019) 51:649–58. doi: 10.1038/s41588-019-0372-4
11. Dominik, N, Magri, S, Curro, R, Abati, E, Facchini, S, Corbetta, M, et al. Normal and pathogenic variation of RFC1 repeat expansions: implications for clinical diagnosis. Brain. (2023) 146:5060–9. doi: 10.1093/brain/awad240
12. Yuan, JH, Higuchi, Y, Ando, M, Matsuura, E, Hashiguchi, A, Yoshimura, A, et al. Multi-type RFC1 repeat expansions as the most common cause of hereditary sensory and autonomic neuropathy. Front Neurol. (2022) 13:986504. doi: 10.3389/fneur.2022.986504
13. Hirano, M, Kuwahara, M, Yamagishi, Y, Samukawa, M, Fujii, K, Yamashita, S, et al. CANVAS-related RFC1 mutations in patients with immune-mediated neuropathy. Sci Rep. (2023) 13:17801. doi: 10.1038/s41598-023-45011-8
14. Kytovuori, L, Sipila, J, Doi, H, Hurme-Niiranen, A, Siitonen, A, Koshimizu, E, et al. Biallelic expansion in RFC1 as a rare cause of Parkinson's disease. NPJ Parkinsons Dis. (2022) 8:6. doi: 10.1038/s41531-021-00275-7
15. Ylikotila, P, Sipila, J, Alapirtti, T, Ahmasalo, R, Koshimizu, E, Miyatake, S, et al. Association of biallelic RFC1 expansion with early-onset Parkinson's disease. Eur J Neurol. (2023) 30:1256–61. doi: 10.1111/ene.15717
16. Alvarez Jerez, P, Daida, K, Miano-Burkhardt, A, Iwaki, H, Malik, L, Cogan, G, et al. Profiling complex repeat expansions in RFC1 in Parkinson's disease. NPJ Parkinsons Dis. (2024) 10:108. doi: 10.1038/s41531-024-00723-0
17. Liu, P, Zhang, F, Chen, X, Zheng, X, Chen, M, Lin, Z, et al. Long-read sequencing revealed complex biallelic pentanucleotide repeat expansions in RFC1-related Parkinson's disease. NPJ Parkinsons Dis. (2025) 11:21. doi: 10.1038/s41531-025-00868-6
18. Kartanou, C, Kontogeorgiou, Z, Rentzos, M, Potagas, C, Aristeidou, S, Kapaki, E, et al. Expanding the spectrum of C9ORF72-related neurodegenerative disorders in the Greek population. J Neurol Sci. (2022) 442:120450. doi: 10.1016/j.jns.2022.120450
19. Liu, P, Yang, D, Zhang, F, Chen, S, Xie, F, Luo, Y, et al. The role of NOTCH2NLC in Parkinson's disease: a clinical, neuroimaging, and pathological study. Eur J Neurol. (2022) 29:1610–8. doi: 10.1111/ene.15283
20. Norioka, R, Sugaya, K, Murayama, A, Kawazoe, T, Tobisawa, S, Kawata, A, et al. Midbrain atrophy related to parkinsonism in a non-coding repeat expansion disorder: five cases of spinocerebellar ataxia type 31 with nigrostriatal dopaminergic dysfunction. Cerebellum Ataxias. (2021) 8:11. doi: 10.1186/s40673-021-00134-4
21. Ohta, Y, Yamashita, T, Hishikawa, N, Sato, K, Matsuzono, K, Tsunoda, K, et al. Potential multisystem degeneration in Asidan patients. J Neurol Sci. (2017) 373:216–22. doi: 10.1016/j.jns.2017.01.003
22. Meijboom, KE, Abdallah, A, Fordham, NP, Nagase, H, Rodriguez, T, Kraus, C, et al. CRISPR/Cas9-mediated excision of ALS/FTD-causing hexanucleotide repeat expansion in C9ORF72 rescues major disease mechanisms in vivo and in vitro. Nat Commun. (2022) 13:6286. doi: 10.1038/s41467-022-33332-7
23. Cabrera, GT, Meijboom, KE, Abdallah, A, Tran, H, Foster, Z, Weiss, A, et al. Artificial microRNA suppresses C9ORF72 variants and decreases toxic dipeptide repeat proteins in vivo. Gene Ther. (2024) 31:105–18. doi: 10.1038/s41434-023-00418-w
24. Hirano, M, Nakamura, Y, Saigoh, K, Sakamoto, H, Ueno, S, Isono, C, et al. Mutations in the gene encoding p62 in Japanese patients with amyotrophic lateral sclerosis. Neurology. (2013) 80:458–63. doi: 10.1212/WNL.0b013e31827f0fe5
25. Hirano, M, Nakamura, Y, Saigoh, K, Sakamoto, H, Ueno, S, Isono, C, et al. VCP gene analyses in Japanese patients with sporadic amyotrophic lateral sclerosis identify a new mutation. Neurobiol Aging. (2015) 36:e1–6. doi: 10.1016/j.neurobiolaging.2014.10.012
26. DeJesus-Hernandez, M, Mackenzie, IR, Boeve, BF, Boxer, AL, Baker, M, Rutherford, NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. (2011) 72:245–56. doi: 10.1016/j.neuron.2011.09.011
27. Kobayashi, H, Abe, K, Matsuura, T, Ikeda, Y, Hitomi, T, Akechi, Y, et al. Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement. Am J Hum Genet. (2011) 89:121–30. doi: 10.1016/j.ajhg.2011.05.015
28. Isono, C, Hirano, M, Sakamoto, H, Ueno, S, Kusunoki, S, and Nakamura, Y. Differential progression of dysphagia in heredity and sporadic ataxias involving multiple systems. Eur Neurol. (2015) 74:237–42. doi: 10.1159/000442252
29. Renton, AE, Majounie, E, Waite, A, Simon-Sanchez, J, Rollinson, S, Gibbs, JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. (2011) 72:257–68. doi: 10.1016/j.neuron.2011.09.010
30. Hirano, M, Satake, W, Moriyama, N, Saida, K, Okamoto, N, Cha, PC, et al. Bardet-Biedl syndrome and related disorders in Japan. J Hum Genet. (2020) 65:847–53. doi: 10.1038/s10038-020-0778-y
31. Wan, L, Chen, Z, Wan, N, Liu, M, Xue, J, Chen, H, et al. Biallelic Intronic AAGGG expansion of RFC1 is related to multiple system atrophy. Ann Neurol. (2020) 88:1132–43. doi: 10.1002/ana.25902
32. Spiegel, J, Hellwig, D, Samnick, S, Jost, W, Mollers, MO, Fassbender, K, et al. Striatal FP-CIT uptake differs in the subtypes of early Parkinson's disease. J Neural Transm (Vienna). (2007) 114:331–5. doi: 10.1007/s00702-006-0518-2
33. Billingsley, KJ, Alvarez Jerez, P, Grenn, FP, Bandres-Ciga, S, Malik, L, Hernandez, D, et al. Profiling the NOTCH2NLC GGC repeat expansion in Parkinson's disease in the European population. Mov Disord. (2022) 37:2161–2. doi: 10.1002/mds.29155
34. Baba, Y, Uitti, RJ, Farrer, MJ, and Wszolek, ZK. Sporadic SCA8 mutation resembling corticobasal degeneration. Parkinsonism Relat Disord. (2005) 11:147–50. doi: 10.1016/j.parkreldis.2004.10.008
35. Hirano, M, Samukawa, M, Isono, C, Kusunoki, S, and Nagai, Y. The effect of rasagiline on swallowing function in Parkinson's disease. Heliyon. (2024) 10:e23407. doi: 10.1016/j.heliyon.2023.e23407
36. Yonenobu, Y, Beck, G, Kido, K, Maeda, N, Yamashita, R, Inoue, K, et al. Neuropathology of spinocerebellar ataxia type 8: common features and unique tauopathy. Neuropathology. (2023) 43:351–61. doi: 10.1111/neup.12894
37. Huin, V, Coarelli, G, Guemy, C, Boluda, S, Debs, R, Mochel, F, et al. Motor neuron pathology in CANVAS due to RFC1 expansions. Brain. (2022) 145:2121–32. doi: 10.1093/brain/awab449
38. Matsubara, T, Kameyama, M, Tanaka, N, Sengoku, R, Orita, M, Furuta, K, et al. Autopsy validation of the diagnostic accuracy of (123)I-Metaiodobenzylguanidine myocardial scintigraphy for Lewy body disease. Neurology. (2022) 98:e1648–59. doi: 10.1212/WNL.0000000000200110
39. Kearse, MG, and Todd, PK. Repeat-associated non-AUG translation and its impact in neurodegenerative disease. Neurotherapeutics. (2014) 11:721–31. doi: 10.1007/s13311-014-0292-z
40. Zu, T, Gibbens, B, Doty, NS, Gomes-Pereira, M, Huguet, A, Stone, MD, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci USA. (2011) 108:260–5. doi: 10.1073/pnas.1013343108
41. Ayhan, F, Perez, BA, Shorrock, HK, Zu, T, Banez-Coronel, M, Reid, T, et al. SCA8 RAN polySer protein preferentially accumulates in white matter regions and is regulated by eIF3F. EMBO J. (2018) 37:37. doi: 10.15252/embj.201899023
42. Sareen, D, O'Rourke, JG, Meera, P, Muhammad, AK, Grant, S, Simpkinson, M, et al. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med. (2013) 5:208ra149. doi: 10.1126/scitranslmed.3007529
43. Matsuzono, K, Imamura, K, Murakami, N, Tsukita, K, Yamamoto, T, Izumi, Y, et al. Antisense oligonucleotides reduce RNA foci in spinocerebellar ataxia 36 patient iPSCs. Mol Ther Nucleic Acids. (2017) 8:211–9. doi: 10.1016/j.omtn.2017.06.017
44. Tripathi, BK, Surabhi, S, Bhaskar, PK, Mukherjee, A, and Mutsuddi, M. The RNA binding KH domain of spoonbill depletes pathogenic non-coding spinocerebellar ataxia 8 transcripts and suppresses neurodegeneration in Drosophila. Biochim Biophys Acta. (2016) 1862:1732–41. doi: 10.1016/j.bbadis.2016.06.008
45. Ando, M, Higuchi, Y, Yuan, JH, Yoshimura, A, Higashi, S, Takeuchi, M, et al. Genetic and clinical features of cerebellar ataxia with RFC1 biallelic repeat expansions in Japan. Front Neurol. (2022) 13:952493. doi: 10.3389/fneur.2022.952493
Keywords: spinocerebellar ataxia type 8, repeat disease, parkinsonism, Canvas, RFC1 , dysphagia, videofluoroscopic analysis
Citation: Hirano M, Samukawa M, Miyatake S, Yamagishi Y, Isono C, Yoshikawa R, Saigoh K, Terayama A, Higashimoto Y, Koshimizu E, Mizuguchi T, Fujii K, Mitsui Y, Matsumoto N and Nagai Y (2025) Non-coding repeat analyses in patients with Parkinson’s disease. Front. Neurol. 16:1606305. doi: 10.3389/fneur.2025.1606305
Edited by:
Emilia Mabel Gatto, Sanatorio de la Trinidad Mitre, ArgentinaReviewed by:
Jacky Ganguly, Institute of Neurosciences, Kolkata (I-NK), IndiaShuang-Qi Gao, Sun Yat-sen University, China
Copyright © 2025 Hirano, Samukawa, Miyatake, Yamagishi, Isono, Yoshikawa, Saigoh, Terayama, Higashimoto, Koshimizu, Mizuguchi, Fujii, Mitsui, Matsumoto and Nagai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Makito Hirano, bWFoaXJhbm8tbmV1cm9AdW1pbi5hYy5qcA==
 Makito Hirano
Makito Hirano Makoto Samukawa1
Makoto Samukawa1 Kazumasa Saigoh
Kazumasa Saigoh Takeshi Mizuguchi
Takeshi Mizuguchi Naomichi Matsumoto
Naomichi Matsumoto Yoshitaka Nagai
Yoshitaka Nagai