- 1Department of Neurology, Second Hospital, Shanxi Medical University, Taiyuan, China
- 2College of Basic Medical Sciences, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Introduction: The correlation between serum homocysteine levels and post-stroke cognitive impairment (PSCI) remains inconsistent. This study aimed to investigate whether serum homocysteine levels are independently associated with PSCI and to assess the effects of renal function on this relationship.
Methods: A retrospective analysis was conducted in 608 patients with ischemic stroke. Homocysteine levels were obtained from inpatient medical records, and global cognitive function status 1 month after discharge was assessed using the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). The relationship between homocysteine levels and PSCI was evaluated using univariate and multiple linear and logistic regression analyses.
Results: The mean age of the patients was 66.6 ± 4.1 years, with 48% being female. The median homocysteine level was 13.8 μmol/L (interquartile range [IQR], 11.3–17.3 μmol/L), and 39.3% of patients had total homocysteine levels above the cutoff of 15 μmol/L. After full adjustment, a stronger positive association between homocysteine levels and PSCI was observed in patients with low estimated glomerular filtration rate (eGFR), with significant interactions between eGFR and MMSE scores (P for interaction = 0.005) and between eGFR and MoCA scores (P for interaction = 0.001). Joint analyses indicated that the highest risk of PSCI was in patients with eGFR < 90 ml/min/1.73 m2 and homocysteine levels ≥15 μmol/L (odds ratios [ORs] were 2.50 [95% CI: 1.49, 4.18; p < 0.001] for MMSE and 13.53 [95% CI: 6.64, 27.56; p < 0.001] for MoCA in the fully adjusted model).
Conclusion: These findings highlight the additive value of hyperhomocysteinemia and lower eGFR in predicting incident PSCI risk.
Introduction
Worldwide, PSCI is major sources of post-stroke morbidity and mortality (1) and is highly prevalent among stroke survivors in China (2). The risk of PSCI is a major concern for patients and their families. Therefore, understanding the mechanisms and modifiable determinants of PSCI is of clinical importance in developing preventive strategies and is key to delivering equitable health care to the Chinese population (3, 4).
Homocysteine is a non-essential sulfur-containing amino acid that is produced in the metabolic cycle by demethylation of methionine. It plays a central role in the methionine and folate cycles, and its metabolism is dependent on folate, vitamins B12 and B6. To date, some studies have suggested that elevated homocysteine levels are modifiable risk factors for Alzheimer’s disease (AD) and vascular dementia (VaD) (5–7). For more than four decades, hyperhomocysteinemia, defined as elevated serum homocysteine levels, has been widely recognized as a risk factor for vascular disease and VaD, supported by extensive clinical evidence (8–15). Recently, an umbrella review found hyperhomocysteinemia to be associated with cognitive impairment (16). An increasing number of reviews have shown that the plasma homocysteine level could be a potential biomarker for PSCI (17–19). A meta-analysis found higher homocysteine levels in individuals with AD and indicated that the homocysteine level can be used as an indicator to differentiate between AD and VaD (9). A new study suggests that high homocysteine levels are associated with the progression from mild cognitive impairment to dementia (20). One study demonstrated a correlation between elevated serum homocysteine levels and PSCI, with the former likely serving as a predictive factor for the latter (21). Another study found that elevated homocysteine levels were independently associated with cognitive impairment in a post-stroke population younger than 65 years (22).
The incidence of chronic kidney disease (CKD) is high among individuals with hyperhomocysteinemia, and hyperhomocysteinemia may be an independent risk factor for CKD as well as cardiovascular complications (23, 24). Recently, a study indicated that homocysteine is independently correlated with cognitive function in patients on maintenance hemodialysis (25). Studies have shown that hyperhomocysteinemia predicts an increased risk of inflammation and endothelial damage, which can lead to cardiovascular disease, stroke, and chronic kidney disease (26). Our prior investigation revealed that a decline in estimated glomerular filtration rate (eGFR) may serve as an effective indicator of cognitive impairment following a stroke (27).
Given the established relationships among hyperhomocysteinemia, chronic kidney disease (CKD), and cognitive function following stroke, it is crucial to investigate the precise interactions between homocysteine levels, kidney function indicators, and PSCI. Furthermore, understanding whether renal function indicators influence the association between homocysteine levels and PSCI is essential for the effective prevention and management of cognitive impairment following stroke. Therefore, this study aimed to investigate whether homocysteine levels are independently associated with PSCI and to assess the effects of renal function on this relationship.
Materials and methods
Data source
In this retrospective study, 608 patients aged 60 to 80 years with acute ischemic stroke, admitted within 72 h of stroke onset between January 2016 and December 2020, were analyzed. Data were obtained from hospital records and the outpatient cognitive assessment database, collected 1 month after discharge. Data collection began on September 1, 2019. During or after data collection, investigators had access to information that could identify individual patients. None of the patients had a history of severe cognitive impairment prior to stroke, and all were able to complete the assessment.
The study was conducted at the Department of Neurology, the Second Hospital of Shanxi Medical University, Taiyuan, a tertiary hospital in Shanxi, China. This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (No. 2019YX214) and was completed in accordance with the principles of the Declaration of Helsinki. Given the retrospective nature of the study and the anonymized data analysis, informed consent from the patients was not required.
Clinical and laboratory data
Clinical information obtained from hospital records included systolic and diastolic blood pressure at admission, self-reported demographic characteristics (age, sex, weight, height, education, smoking and drinking status, and history of hypertension and diabetes mellitus), and laboratory test results (homocysteine, blood lipids, fasting glucose, vitamin B12, folate, and creatinine levels). Fasting venous blood samples were collected on the morning following admission. Biochemical measurements were performed using automatic clinical analyzers (Beckman Coulter) at the core laboratory of the Second Hospital, Shanxi Medical University, Taiyuan, China. Serum homocysteine levels were measured using an enzymatic cycling method, and hyperhomocysteinemia was defined as a homocysteine concentration greater than 15 μmol/L. Serum vitamin B12 and folate levels were measured using a chemiluminescent immunoassay. Serum glucose levels were determined by the hexokinase method. The concentrations of serum creatinine and lipids were measured using enzymatic methods.
In addition, body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Neurological function was assessed using the form of the National Institutes of Health Stroke Scale (NIHSS) score (28). The patients with acute ischemic stroke were diagnosed with large artery atherosclerosis, small artery occlusion, cardioembolism, other determined cause, or undetermined cause according to the Trial of Org 10,172 in acute stroke treatment (TOAST) classification (29). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (30).
Cognitive function assessment
All cognitive function assessments were performed by two trained neuropsychological evaluators using the MMSE and MoCA, 1 month after discharge from the hospital. MMSE and MoCA scores range from 0 to 30, with higher scores indicating better cognitive function. The cognitive domains assessed include concentration, attention, language, orientation, immediate and short-term recall, and the ability to follow simple verbal and written commands. Based on previous studies and the cognitive scores of patients, moderate-to-severe cognitive impairment 1 month post-stroke was defined as an MMSE score ≤20 or a MoCA score <17 (31).
Statistical analysis
Homocysteine levels were analyzed both as a continuous variable and as a categorical variable. Given the skewed distribution of serum homocysteine levels, a natural logarithmic transformation was applied prior to analysis. For normally distributed continuous data, mean ± SD values were used to describe the data, and independent-sample t-tests were employed to assess group differences. For skewed distributions, median values with interquartile ranges (IQR) were reported, and the Mann–Whitney U test was used to compare two groups. Categorical variables were presented as percentages, and differences between groups were assessed using the Chi-square test.
Multiple linear regression and logistic regression analyses were conducted to estimate the regression coefficients (β) and odds ratios (OR) for the association between homocysteine levels and post-stroke cognitive impairment. Patients were divided into two groups based on a cutoff of 15 μmol/L for homocysteine levels. Crude and multivariable-adjusted β and OR values with 95% confidence intervals (CIs) were calculated for cognitive scores and moderate-to-severe post-stroke cognitive impairment. These analyses were performed both categorically (using <15 μmol/L as the reference group) and continuously (per 1-unit increase in the homocysteine level, equivalent to a 2.7-fold increase).
Three models were constructed with progressively increased adjustments for potential confounding variables that could affect the association between homocysteine levels and cognitive function. The first model was Model 0 (unadjusted). Model 1 was adjusted for age, sex, BMI, education, history of diabetes and hypertension, stroke subtypes, NIHSS score, smoking and drinking status, systolic blood pressure, and serum levels of total cholesterol, triglycerides, vitamin B12, folate, and fasting glucose. Model 2 included further adjustment for eGFR. A two-tailed p-value of <0.05 was considered statistically significant. All analyses were performed using EmpowerStats software1 and the statistical package R.2
Results
Population characteristics
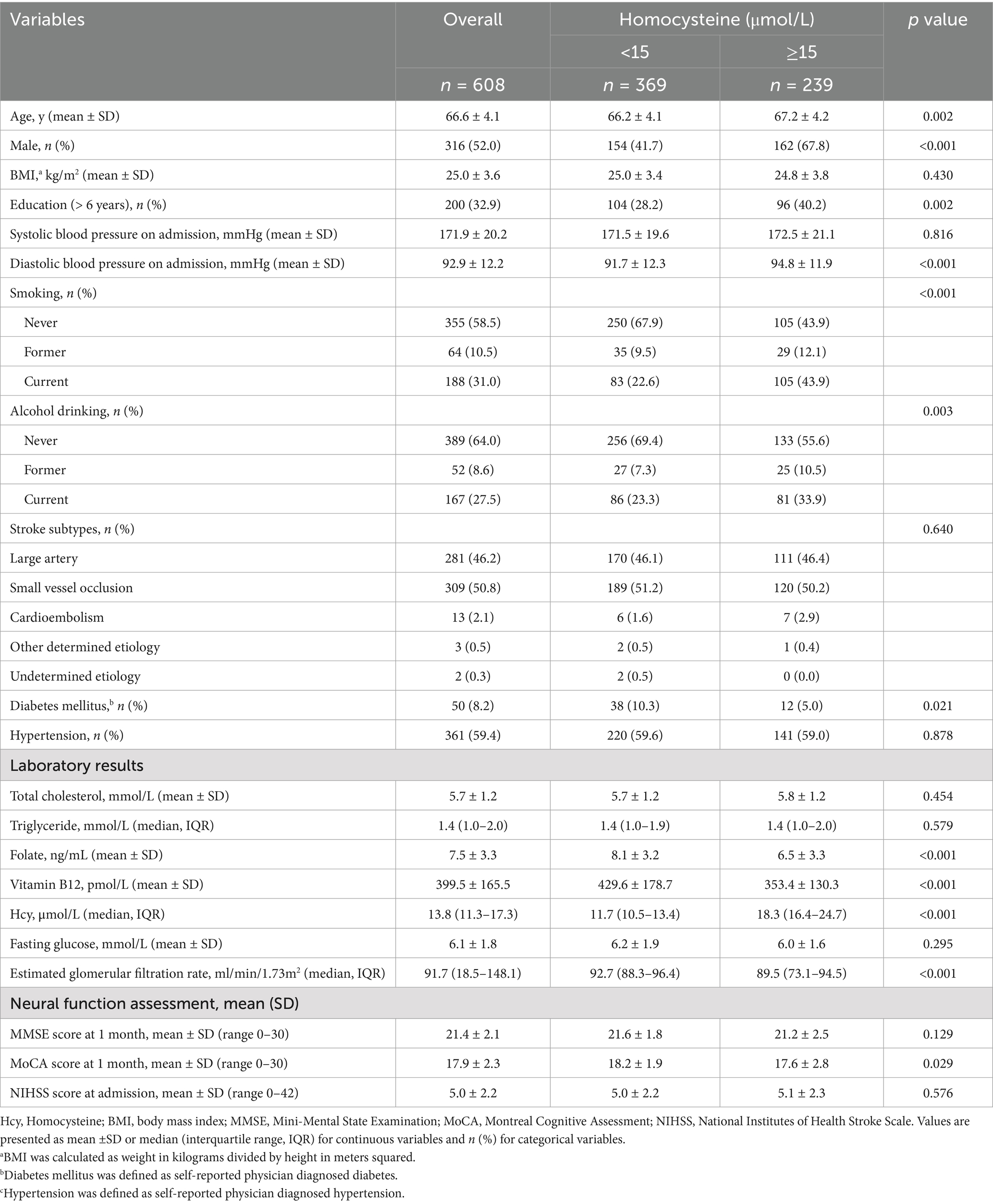
The clinical and demographic characteristics of all 608 patients, grouped by homocysteine levels, are presented in Table 1. The mean age of patients was 66.6 ± 4.1 years, and 48% were female. The median homocysteine level was 13.8 μmol/L (interquartile range [IQR]: 11.3–17.3 μmol/L). A total of 239 patients (39.3%) had total homocysteine levels higher than the cutoff of 15 μmol/L. Patients with higher homocysteine levels were more likely to be older, male, former or current drinkers, and smokers. They were also more likely to have high diastolic blood pressure, low folate and vitamin B12 levels, and low eGFR at admission, but less likely to have diabetes (Table 1).
Homocysteine levels and post-stroke cognitive impairment
Table 2 presents the results of analyses with unadjusted and adjusted models for continuous homocysteine levels and categorized homocysteine levels in relation to post-stroke cognitive impairment. When examined as a continuous variable (per 1-unit increase) in both the Model 0 and Model 1, the log-transformed increase in homocysteine level was significantly and negatively associated with MMSE and MoCA scores (for MMSE: β = −0.69, 95% CI: −1.16, −0.22, p = 0.004; for MoCA: β = −0.86, 95% CI: −1.38, −0.34, p = 0.001 in Model 1). However, after the inclusion of eGFR in Model 2, the association between the log-transformed homocysteine level and post-stroke cognitive impairment was no longer significant (for MMSE: β = 0.26, 95% CI: −0.18, 0.69, p = 0.253; for MoCA: β = 0.42, 95% CI: −0.04, 0.87, p = 0.073 in Model 2).
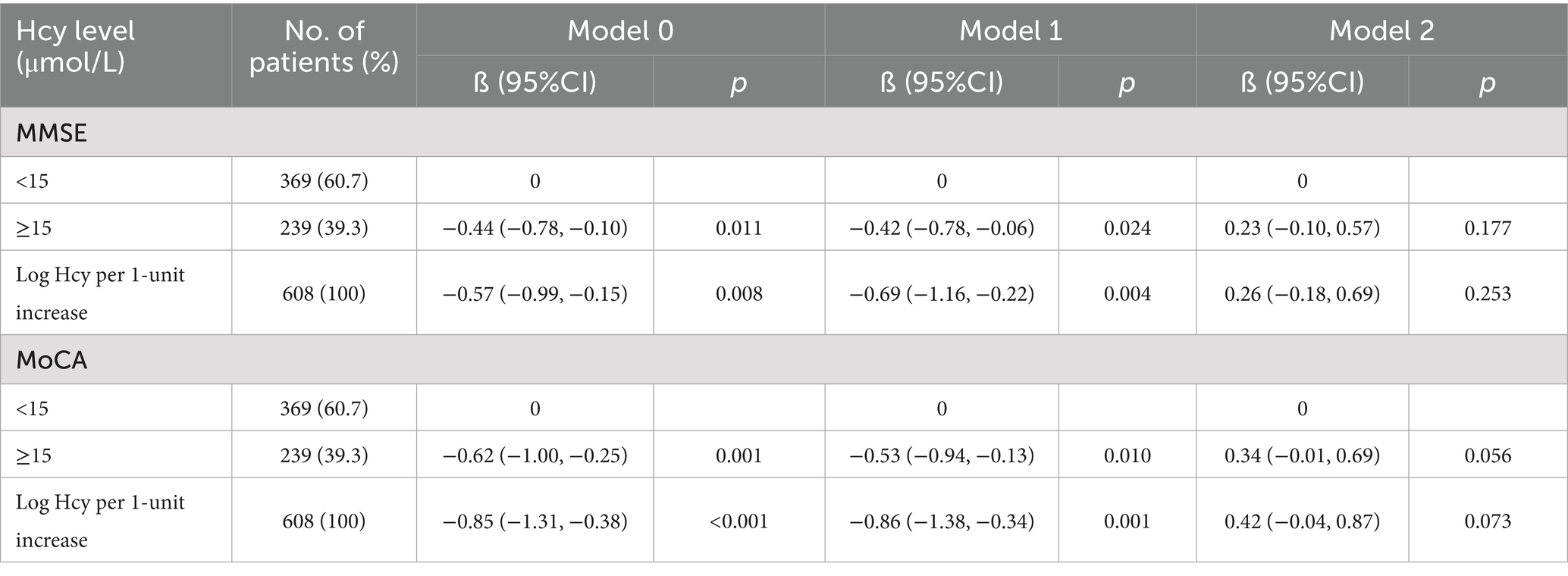
Table 2. Linear regression analysis of the association between serum homocysteine levels and post-stroke cognitive measures.
In categorical analysis, a significant association was observed for patients with homocysteine levels ≥15 μmol/L compared with those with homocysteine levels <15 μmol/L in the Model 0 and Model 1 (for MMSE: β = −0.44, 95% CI: −0.78, −0.10, p = 0.011 in the Model 0; β = −0.42, 95% CI: −0.78, −0.06, p = 0.024 in Model 1; for MoCA: β = −0.62, 95% CI: −1.00, −0.25, p = 0.001 in the Model 0; β = −0.53, 95% CI: −0.94, −0.13, p = 0.010 in Model 1). Similarly, the direction of the relationship between homocysteine levels and post-stroke cognitive impairment changed once eGFR was introduced into Model 2 (for MMSE: β = 0.23, 95% CI: −0.10, 0.57, p = 0.177; for MoCA: β = 0.34, 95% CI: −0.04, 0.87, p = 0.056 in Model 2).
Table 3 presents the incidence of moderate-to-severe cognitive impairment 1 month after stroke, stratified by different serum homocysteine levels. A total of 68 (56.7%) and 96 (42.7%) patients exhibited moderate-to-severe post-stroke cognitive impairment, based on MoCA and MMSE scores, respectively. In both the Model 0 model and Model 1, compared with patients in the homocysteine level < 15 μmol/L group, those with homocysteine levels ≥ 15 μmol/L had a higher risk of moderate-to-severe post-stroke cognitive impairment (unadjusted OR = 2.42, 95% CI: 1.62, 3.64; p < 0.001 for MoCA in Model 0; adjusted OR = 2.95, 95% CI: 1.81, 4.79; p < 0.001 for MoCA in Model 1). However, this association was not significant once eGFR was introduced into the model (OR = 1.19, 95% CI: 0.66, 2.15; p = 0.557 for MoCA in Model 2). Similar results were observed when homocysteine level was analyzed as a continuous variable. The log-transformed homocysteine level was not significantly associated with the risk of moderate-to-severe post-stroke cognitive impairment for MoCA scores in Model 2 (p = 0.955).
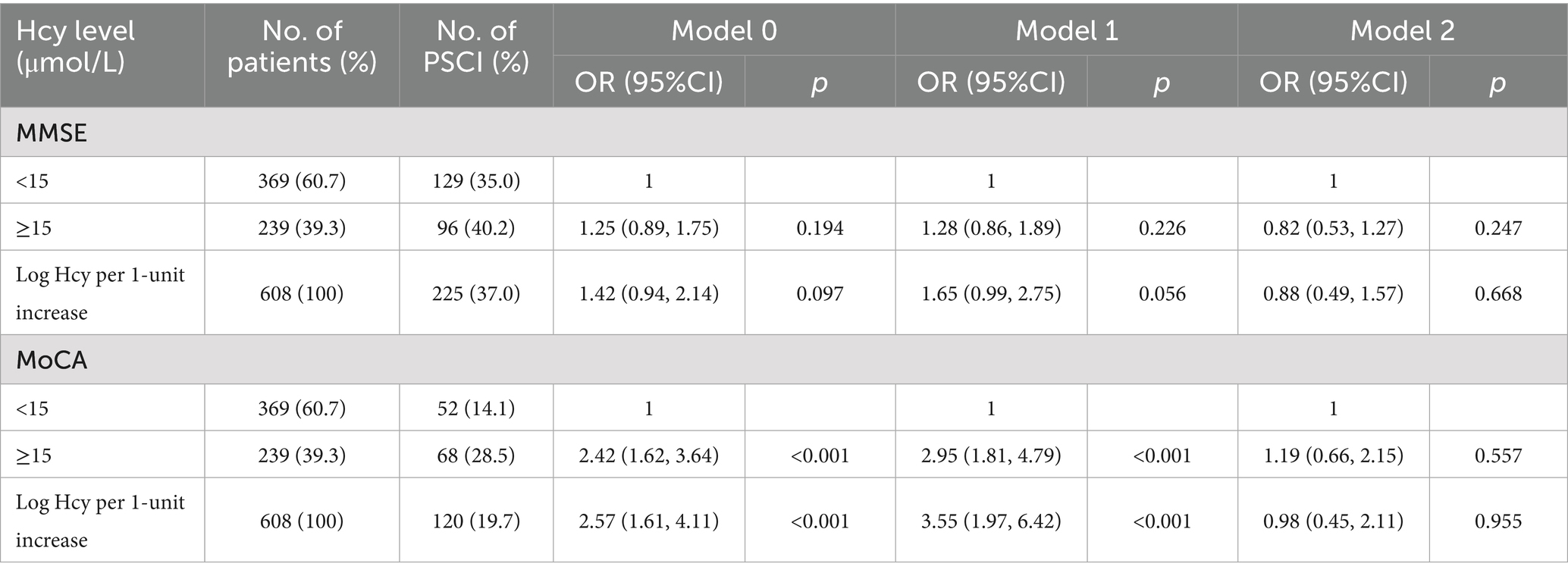
Table 3. Logistic regression analysis of the association between Hcy levels and moderate to severe post-stroke cognitive impairment.
Stratified analyses by potential effect modifiers
The relationship between homocysteine levels (≥15 μmol/L vs. <15 μmol/L) and post-stroke cognitive performance was further evaluated using stratified analysis (Table 4). A fully multivariable-adjusted significant negative association between elevated homocysteine levels and post-stroke cognitive performance was observed in patients with eGFR < 90 ml/min/1.73 m2, with significant interactions between eGFR and MMSE scores (P for interaction = 0.005) and MoCA scores (P for interaction = 0.001). In contrast, other variables, including sex, age, NIHSS score, systolic blood pressure, stroke subtypes, and blood glucose levels, did not significantly mediate the relationship between homocysteine levels and post-stroke cognitive impairment.
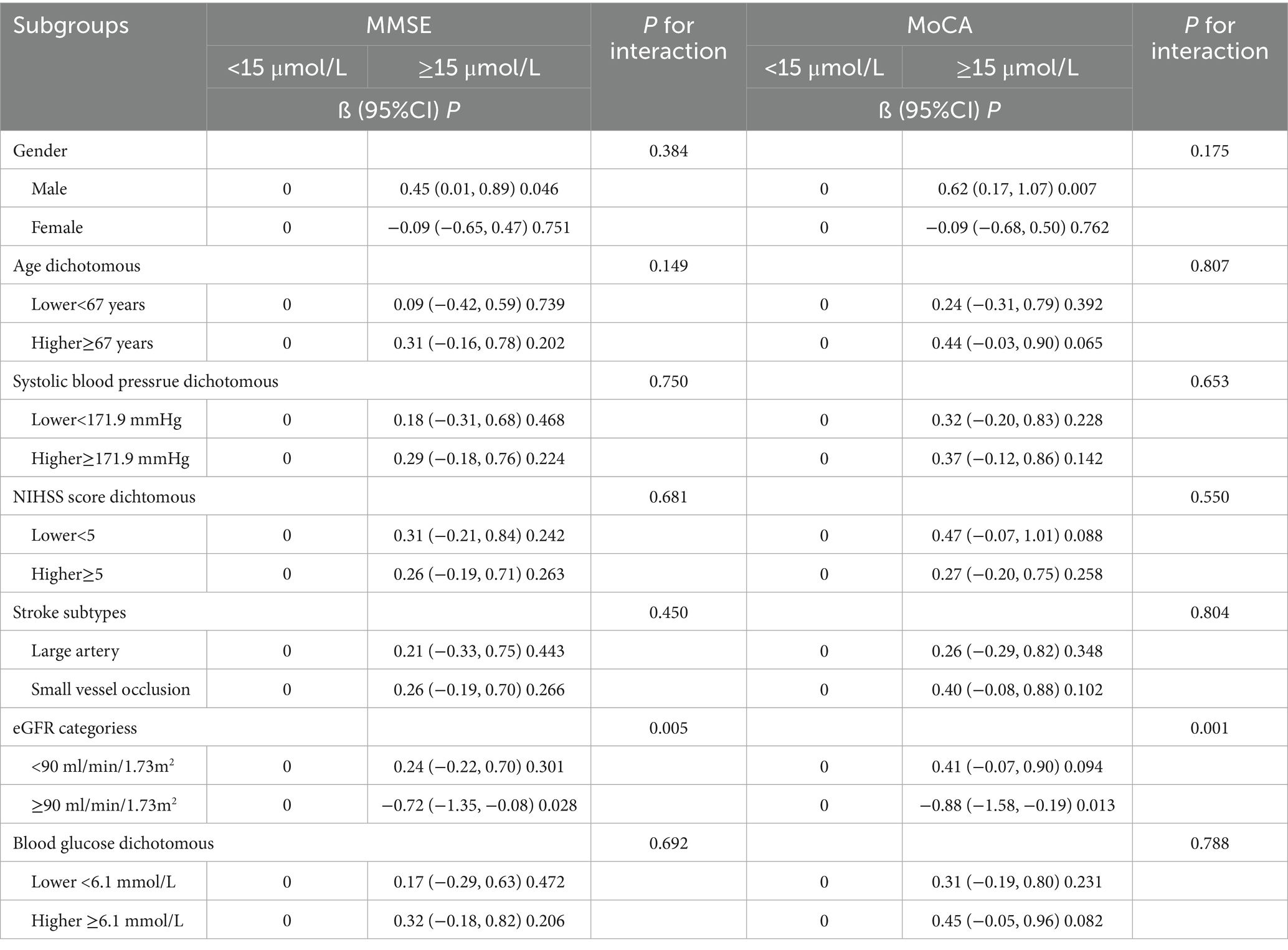
Table 4. Multivariable adjusted* linear regression of MMSE/MoCA scores with serum homocysteine within subgroups.
Joint effects of homocysteine and eGFR levels on post-stroke cognitive impairment
Based on the data provided in Table 5, the joint analysis of serum homocysteine and eGFR levels on moderate to severe post-stroke cognitive impairment revealed significant insights into the risk factors associated with PSCI. The logistic regression analyses revealed that the highest risk of PSCI was in the group with eGFR < 90 ml/min/1.73 m2 and homocysteine levels ≥15 μmol/L. After adjusting for pertinent confounders, the odds ratios (ORs) were 2.50 (95% CI: 1.49, 4.18; p < 0.001) for MMSE and 13.53 (95% CI: 6.64, 27.56; p < 0.001) for MoCA.
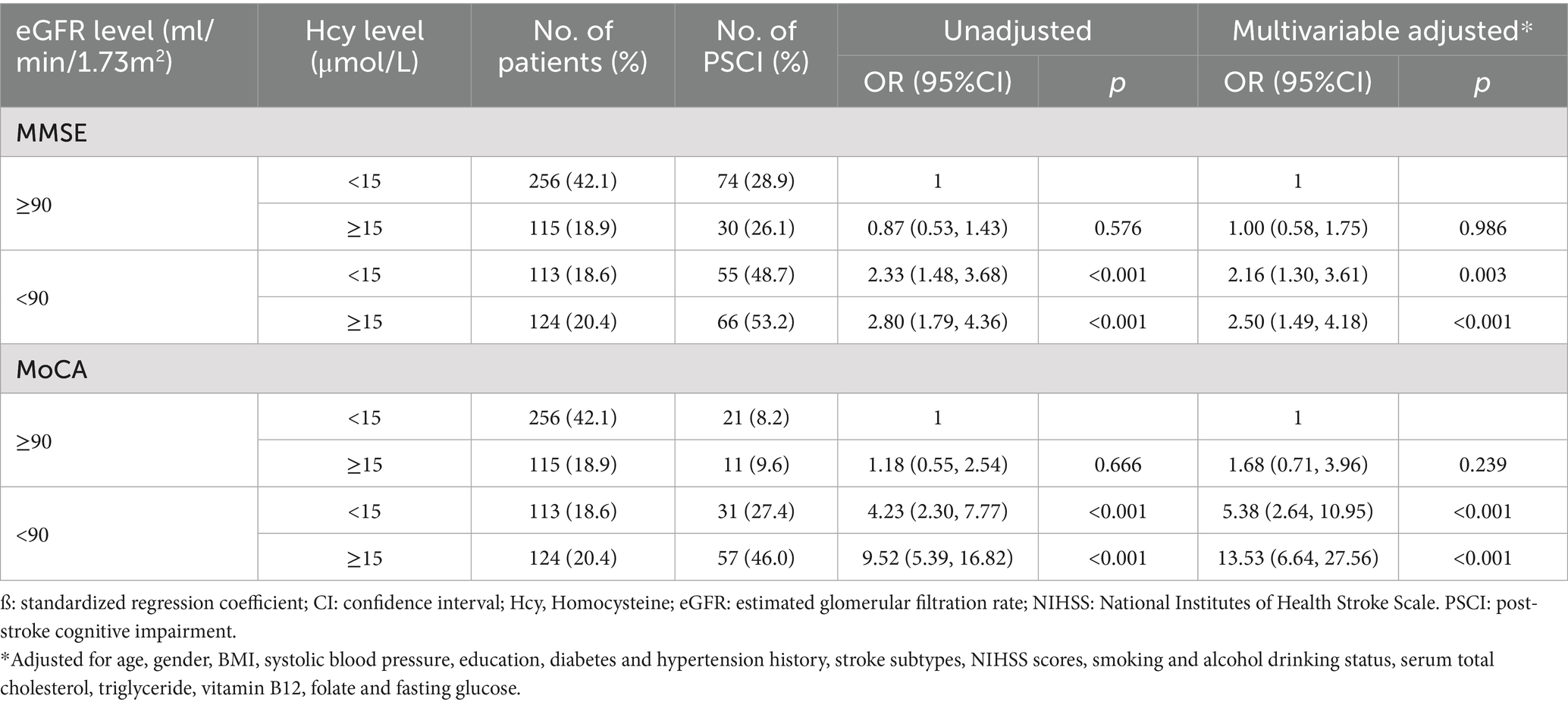
Table 5. Joint analysis of the serum homocysteine (<15 vs. ≥15) and eGFR (<90 vs. ≥90) levels on moderate to severe post-stroke cognitive impairment.
Discussion
This study highlight the complex interplay between homocysteine levels and kidney function, as indicated by eGFR, on cognitive impairment post-stroke. Higher levels of homocysteine and reduced eGFR are significantly associated with an increased risk of PSCI, as observed in both the MMSE and MoCA assessments. These findings underscore the importance of managing plasma homocysteine levels and monitoring kidney function in stroke patients to mitigate the risk of cognitive decline (25, 32, 33).
To date, considerable data on homocysteine levels and PSCI have been published. However, the available data are controversial (34, 35) and the relationship with renal function remains to be elucidated. A systematic review study found a positive association between cognitive decline and elevated plasma homocysteine levels in the general population and patients with cognitive dysfunction. However, treatment with vitamin supplementation failed to show improved cognitive decline (14). A recent Chinese cohort of minor stroke/TIA patients (NIHSS 1.49 ± 1.31) reported that elevated baseline homocysteine (≥ 15 μmol/L) predicted PSCI at 12 months, but not at 3 months (MoCA < 22), in women (35). In contrast, our patients exhibited more severe neurological deficits (NIHSS 5.0 ± 2.2) and pronounced early cognitive impairment (MoCA < 17; MMSE < 20 at 1 month). Similarly, another Chinese study demonstrated that acute-phase hyperhomocysteinaemia (≥ 12 μmol/L) was independently associated with PSCI at 1 month in patients with baseline NIHSS 1 (IQR 0–3) and MMSE < 24 as the diagnostic threshold (22). Collectively, the discrepant findings across studies can be attributed to differences in initial stroke severity, cognitive assessment tools and diagnostic criteria, and the interval between stroke onset and evaluation. One study found that higher serum homocysteine levels and an increased vascular burden were negative related to executive function in patients with CKD (36). The Randomized FAVORIT Assisted Cognitive Trial found cognitive benefit in kidney transplant recipients with elevated baseline homocysteine levels with high doses of vitamin B supplementation (32). Our study revealed that patients with elevated homocysteine levels and lower eGFR exhibited a significant association with PSCI. These findings clearly showed the additive value of hyperhomocysteinemia and lower eGFR in predicting incident PSCI risk.
The exact pathophysiologic mechanisms that underlie the link between homocysteine levels, eGFR, and PSCI are not fully established and require further elucidation. Hyperhomocysteinemia induces oxidative stress and antagonizes the vasodilator properties of NO though the formation of S-nitrosohomocysteine, leading to endothelial dysfunction (37). Similarly, hyperhomocysteinemia causes vascular hypertrophy and remodeling, impairs the basic characteristics of blood vessels, and increases the stiffness of arteries (38). Furthermore, homocysteine has been shown to promote the proliferation of smooth muscle cells, leading to interactions with platelets, clotting factors, and lipids (39). Chronic elevated serum homocysteine alters functions of the vascular endothelial cells, and based on important pathobiochemical modifications, activates thiolation and homocysteinylation of plasma proteins and enzymes with a deleterious impact on cerebrovascular permeability and eventually on brain parenchyma (15).
The association between eGFR and homocysteine levels has been documented in numerous studies (24). Evidence have consistently shown a highly significant negative correlation between eGFR and homocysteine levels. This provides compelling indirect evidence that elevated homocysteine levels in patients with renal disease are closely linked to impaired kidney function (23). In our cohort, higher homocysteine levels were associated with lower eGFR. After adjustment for eGFR (Model 2), the β-coefficients for log-transformed homocysteine shifted from negative in Model 1 (MMSE: β = −0.69; MoCA: β = −0.86) to positive (MMSE: β = 0.26; MoCA: β = 0.42), and the association between homocysteine and PSCI became non-significant. These observations suggest that eGFR may mediate the relationship, although the underlying pathophysiological mechanisms remain to be elucidated. Organs such as the kidney, liver, gut, and pancreas contain the enzymes needed for homocysteine metabolism. The kidney plays an important role in clearing homocysteine from circulation. Patients with CKD and those who are predisposed to high blood Hcy have a markedly lower serum Hcy clearance (40). Moreover, CKD heightens the risk of elevated blood Hcy levels, which has been linked to a decrease in cognition (41). In addition, it is important to emphasize that decreased GFR is a well-known risk factor for cognitive impairment (42). The coexistence of low eGFR and hyperhomocysteinemia may also contribute to the accumulation of uremic toxins owing to the deterioration of the renal clearance function, causing direct neurotoxicity, or be accompanied by systemic hemodynamic impairment, both of which lead to the development of vascular cognitive impairment (26, 43). The additional detrimental effects of these markers on brain function remain unclear, warranting further research.
The strengths of this study include the assessment of cognitive function with both MoCA and MMSE, thus providing clinical accuracy to the analyses. In addition, we were able to control for many potential confounders including demographic and clinical indicators to reduce confounding effects. However, there were several limitations in our study. First, the homocysteine level was measured once, and thus possible intra-individual fluctuations were ignored. Second, we did not analyze impairment in specific cognitive domains, but only compared overall cognitive domains. Third, we lacked data on diet or post-diagnosis B-vitamin supplementation—both of which can alter homocysteine levels and bias the observed association. These variables will be collected in subsequent follow-up. Finally, cognitive assessment was assessed 1 month after discharge, and the incidence of PSCI was the highest 3 months after stroke (44). Further long-term follow-up should be performed.
Conclusion
Our findings indicates that the patients with hyperhomocysteinemia and low eGFR levels exhibited a significant association with PSCI. These results clearly showed the additive value of hyperhomocysteinemia and lower eGFR in predicting incident PSCI risk. These findings have important clinical implications. These findings underscore the importance of managing plasma homocysteine levels and monitoring kidney function in stroke patients to mitigate the risk of cognitive decline. Further investigation into the mechanisms behind elevated homocysteine and impaired kidney function could provide essential insights into prevention strategies for PSCI in post-stroke patients. Implementing interventions that target these modifiable risk factors could enhance cognitive outcomes following a stroke.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Hospital of Shanxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because given the retrospective nature of the study and the anonymized data analysis, informed consent from the patients was not required.
Author contributions
CZ: Funding acquisition, Supervision, Writing – review & editing, Writing – original draft. XC: Investigation, Writing – original draft. CL: Formal analysis, Software, Writing – original draft. PM: Funding acquisition, Project administration, Writing – review & editing. HG: Investigation, Supervision, Writing – review & editing. BB: Data curation, Project administration, Writing – original draft. CX: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the Scientific Research Foundation for Doctors, the Second Hospital of Shanxi Medical University, China (grant number 201601-9) and the Natural Science Foundation of Shanxi Province, China (grant numbers 201801D221411 and 20210302124427).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1611140/full#supplementary-material
Footnotes
References
1. Rost, NS, and Brodtmann, A. Post-stroke cognitive impairment and dementia. Circ Res. (2022) 130:1252–71. doi: 10.1161/CIRCRESAHA.122.319951
2. Qu, Y, Zhuo, L, Li, N, Hu, Y, Chen, W, Zhou, Y, et al. Prevalence of post-stroke cognitive impairment in China: a community-based, cross-sectional study. PLoS One. (2015) 10:e0122864. doi: 10.1371/journal.pone.0122864
3. Plassman, BL, Williams, JW Jr, Burke, JR, Holsinger, T, and Benjamin, S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. (2010) 153:182–93. doi: 10.7326/0003-4819-153-3-201008030-00258
4. Rost, NS, Meschia, JF, Gottesman, R, Wruck, L, Helmer, K, and Greenberg, SM. Cognitive impairment and dementia after stroke: design and rationale for the DISCOVERY study. Stroke. (2021) 52:e499–516. doi: 10.1161/STROKEAHA.120.031611
5. Quadri, P, Fragiacomo, C, Pezzati, R, Zanda, E, Forloni, G, Tettamanti, M, et al. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr. (2004) 80:114–22. doi: 10.1093/ajcn/80.1.114
6. Flirski, M, and Sobow, T. Biochemical markers and risk factors of Alzheimer's disease. Curr Alzheimer Res. (2005) 2:47–64. doi: 10.2174/1567205052772704
7. Douaud, G, Refsum, H, de Jager, CA, Jacoby, R, Nichols, TE, Smith, SM, et al. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci USA. (2013) 110:9523–8. doi: 10.1073/pnas.1301816110
8. Hainsworth, AH, Yeo, NE, Weekman, EM, and Wilcock, DM. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta. (2016) 1862:1008–17. doi: 10.1016/j.bbadis.2015.11.015
9. Wang, B, Zhong, Y, Yan, H, and Cui, L. Meta-analysis of plasma homocysteine content and cognitive function in elderly patients with Alzheimer's disease and vascular dementia. Int J Clin Exp Med. (2014) 7:5118–23.
10. Jiang, B, Chen, Y, Yao, G, Yao, C, Zhao, H, Jia, X, et al. Effects of differences in serum total homocysteine, folate, and vitamin B12 on cognitive impairment in stroke patients. BMC Neurol. (2014) 14:217. doi: 10.1186/s12883-014-0217-9
11. Price, BR, Wilcock, DM, and Weekman, EM. Hyperhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front Aging Neurosci. (2018) 10:350. doi: 10.3389/fnagi.2018.00350
12. Holmen, M, Hvas, AM, and Arendt, JFH. Hyperhomocysteinemia and ischemic stroke: a potential dose-response association-a systematic review and meta-analysis. TH Open. (2021) 5:e420–37. doi: 10.1055/s-0041-1735978
13. Luzzi, S, Cherubini, V, and Falsetti, L. Homocysteine, cognitive functions, and degenerative dementias. State Art. (2022) 10:2741. doi: 10.3390/biomedicines10112741
14. Setién-Suero, E, Suárez-Pinilla, M, Suárez-Pinilla, P, Crespo-Facorro, B, and Ayesa-Arriola, R. Homocysteine and cognition: a systematic review of 111 studies. Neurosci Biobehav Rev. (2016) 69:280–98. doi: 10.1016/j.neubiorev.2016.08.014
15. Lehotský, J, Tothová, B, Kovalská, M, Dobrota, D, Beňová, A, Kalenská, D, et al. Role of homocysteine in the ischemic stroke and development of ischemic tolerance. Front Neurosci. (2016) 10:538. doi: 10.3389/fnins.2016.00538
16. Zhang, YR, Xu, W, Zhang, W, Wang, HF, Ou, YN, Qu, Y, et al. Modifiable risk factors for incident dementia and cognitive impairment: an umbrella review of evidence. J Affect Disord. (2022) 314:160–7. doi: 10.1016/j.jad.2022.07.008
17. Ma, Y, Chen, Y, Yang, T, He, X, Yang, Y, Chen, J, et al. Blood biomarkers for post-stroke cognitive impairment: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2024) 33:107632. doi: 10.1016/j.jstrokecerebrovasdis.2024.107632
18. Kim, KY, Shin, KY, and Chang, KA. Potential biomarkers for post-stroke cognitive impairment: a systematic review and meta-analysis. Int J Mol Sci. (2022) 23:2. doi: 10.3390/ijms23020602
19. Jiang, Z, Li, M, Wang, K, Duan, H, Zhang, B, and Fang, S. Potential biomarkers of post-stroke cognitive impairment in Chinese population: a systematic review and meta-analysis. Mol Neurobiol. (2025) 62:8670–94. doi: 10.1007/s12035-025-04792-x
20. Zuliani, G, Brombo, G, Polastri, M, Romagnoli, T, Mola, G, Riccetti, R, et al. High plasma homocysteine levels predict the progression from mild cognitive impairment to dementia. Neurochem Int. (2024) 177:105763. doi: 10.1016/j.neuint.2024.105763
21. Lan, C, Huang, Z, Luo, X, and Zhang, Y. The correlations between serum Hcy level and seizures and cognitive function in patients after stroke. Am J Alzheimers Dis Other Dement. (2022) 37:15333175221146738. doi: 10.1177/15333175221146738
22. Zhou, S, Chen, J, Cheng, L, Fan, K, Xu, M, Ren, W, et al. Age-dependent association between elevated homocysteine and cognitive impairment in a post-stroke population: a prospective study. Front Nutr. (2021) 8:691837. doi: 10.3389/fnut.2021.691837
23. Long, Y, and Nie, J. Homocysteine in renal injury. Kidney Dis. (2016) 2:80–7. doi: 10.1159/000444900
24. Chen, W, Feng, J, Ji, P, Liu, Y, Wan, H, and Zhang, J. Association of hyperhomocysteinemia and chronic kidney disease in the general population: a systematic review and meta-analysis. BMC Nephrol. (2023) 24:247. doi: 10.1186/s12882-023-03295-y
25. Feng, J, Lu, X, Wang, S, and Li, H. The assessment of cognitive impairment in maintenance hemodialysis patients and the relationship between cognitive impairment and depressive symptoms. Semin Dial. (2022) 35:504–10. doi: 10.1111/sdi.13031
26. Cianciolo, G, De Pascalis, A, Di Lullo, L, Ronco, C, Zannini, C, and La Manna, G. Folic acid and homocysteine in chronic kidney disease and cardiovascular disease progression: which comes first? Cardiorenal Med. (2017) 7:255–66. doi: 10.1159/000471813
27. Zhang, C, Xue, G, Hou, Y, Meng, P, Gao, H, Bai, B, et al. Association between kidney measurements and cognitive performance in patients with ischemic stroke. PLoS One. (2023) 18:e0292506. doi: 10.1371/journal.pone.0292506
28. Spilker, J, Kongable, G, Barch, C, Braimah, J, Brattina, P, Daley, S, et al. Using the NIH stroke scale to assess stroke patients. The NINDS rt-PA stroke study group. J Neurosci Nurs. (1997) 29:384–92. doi: 10.1097/01376517-199712000-00008
29. Adams, HP Jr, Bendixen, BH, Kappelle, LJ, Biller, J, Love, BB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
30. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YL, Castro, AF 3rd, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
31. Shi, D, Chen, X, and Li, Z. Diagnostic test accuracy of the Montreal cognitive assessment in the detection of post-stroke cognitive impairment under different stages and cutoffs: a systematic review and meta-analysis. Neurol Sci. (2018) 39:705–16. doi: 10.1007/s10072-018-3254-0
32. Scott, TM, Rogers, G, Weiner, DE, Livingston, K, Selhub, J, Jacques, PF, et al. B-vitamin therapy for kidney transplant recipients lowers homocysteine and improves selective cognitive outcomes in the randomized FAVORIT ancillary cognitive trial. J Prev Alzheimers Dis. (2017) 4:174–82. doi: 10.14283/jpad.2017.15
33. Lu, R, Fang, Y, Zhou, Y, Che, M, Shen, J, Liu, Q, et al. A pilot study of thiamin and folic acid in hemodialysis patients with cognitive impairment. Ren Fail. (2021) 43:766–73. doi: 10.1080/0886022X.2021.1914656
34. Cui, L, Lu, P, Li, S, Pan, Y, Wang, M, Li, Z, et al. Relationship among homocysteine, inflammation and cognitive impairment in patients with acute ischemic stroke and transient ischemic attack. Neuropsychiatr Dis Treat. (2021) 17:3607–16. doi: 10.2147/NDT.S333753
35. Li, R, Weng, H, Pan, Y, Meng, X, Liao, X, Wang, M, et al. Relationship between homocysteine levels and post-stroke cognitive impairment in female and male population: from a prospective multicenter study. J Transl Int Med. (2021) 9:264–72. doi: 10.2478/jtim-2021-0035
36. Yeh, YC, Huang, MF, Hwang, SJ, Tsai, JC, Liu, TL, Hsiao, SM, et al. Association of homocysteine level and vascular burden and cognitive function in middle-aged and older adults with chronic kidney disease. Int J Geriatr Psychiatry. (2016) 31:723–30. doi: 10.1002/gps.4383
37. Stamler, JS, Osborne, JA, Jaraki, O, Rabbani, LE, Mullins, M, Singel, D, et al. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. (1993) 91:308–18. doi: 10.1172/JCI116187
38. Wustmann, K, Klaey, M, Burow, A, Shaw, SG, Hess, OM, and Allemann, Y. Additive effect of homocysteine- and cholesterol-lowering therapy on endothelium-dependent vasodilation in patients with cardiovascular disease. Cardiovasc Ther. (2012) 30:277–86. doi: 10.1111/j.1755-5922.2011.00272.x
39. Li, H, Lewis, A, Brodsky, S, Rieger, R, Iden, C, and Goligorsky, MS. Homocysteine induces 3-hydroxy-3-methylglutaryl coenzyme a reductase in vascular endothelial cells: a mechanism for development of atherosclerosis? Circulation. (2002) 105:1037–43. doi: 10.1161/hc0902.104713
40. Karmin, O, and Siow, YL. Metabolic imbalance of homocysteine and hydrogen sulfide in kidney disease. Curr Med Chem. (2018) 25:367–77. doi: 10.2174/0929867324666170509145240
41. Roy, A, Roy, R, and Bhattacharya, P. The vicious consequences of chronic kidney disease on cognitive impairment and Alzheimer's disease. ACS Chem Neurosci. (2025) 16:1847–59. doi: 10.1021/acschemneuro.5c00050
42. Wang, F, Zhang, L, Liu, L, and Wang, H. Level of kidney function correlates with cognitive decline. Am J Nephrol. (2010) 32:117–21. doi: 10.1159/000315618
43. Franco, ÁO, Starosta, RT, and Roriz-Cruz, M. The specific impact of uremic toxins upon cognitive domains: a review. J Bras Nefrol. (2019) 41:103–11. doi: 10.1590/2175-8239-jbn-2018-0033
Keywords: serum homocysteine, estimated glomerular filtration rate, post-stroke cognitive impairment, acute ischemic stroke, adult
Citation: Zhang C, Cao X, Liu C, Meng P, Gao H, Bai B and Xue C (2025) Joint effects of elevated homocysteine levels and low eGFR on post-stroke cognitive impairment. Front. Neurol. 16:1611140. doi: 10.3389/fneur.2025.1611140
Edited by:
Nobuyuki Kobayashi, Mainrain Brain Inc., JapanReviewed by:
Yanyan Wang, Huazhong University of Science and Technology, ChinaDebabrata Chakraborty, Apollo Gleneagles Hospitals, India
Copyright © 2025 Zhang, Cao, Liu, Meng, Gao, Bai and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyan Zhang, bWFyemlwYW56Y3lAMTI2LmNvbQ==
 Chunyan Zhang
Chunyan Zhang Xueqin Cao1
Xueqin Cao1 Bo Bai
Bo Bai