- 1Department of Neurology, The Second People’s Hospital of Hunan Province (Brain Hospital of Hunan Province), Changsha, China
- 2Department of Neurology, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, China
Background: The atherogenic index of plasma (AIP) and non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) are newly developed markers of lipid and glucose metabolism. Nevertheless, the associations between the AIP or NHHR and early neurological deterioration (END) following thrombolysis in patients with acute ischemic stroke (AIS) remain ambiguous.
Methods: 1,323 AIS patients who had intravenous thrombolysis between January 2018 and October 2024 were retrospectively analyzed. An increase in the National Institutes of Health Stroke Scale (NIHSS) score of > 4 within 24 h following thrombolysis was considered post-thrombolysis END. Logistic regression analysis was conducted to investigate the associations between the AIP and NHHR with post-thrombolysis END. Receiver operating characteristic (ROC) analysis was employed to evaluate the AIP and NHHR capacity to differentiate post-thrombolysis END.
Results: Of the 1,323 patients who were recruited, 1,125 (85.03%) had non-END and 198 (14.97%) had post-thrombolysis END. A binary logistic regression model demonstrated that the AIP [odds ratio (OR), 1.657; 95% confidence interval (CI) 1.441–1.875, p < 0.001] and NHHR (OR, 1.519; 95% CI, 1.342–1.812, p < 0.001) were independent factors for post-thrombolysis END. The area under the curve (AUC) values for the AIP, NHHR, and AIP combined with the NHHR for post-thrombolysis END were 0.753, 0.678, and 0.795, respectively.
Conclusion: Our study suggests that the AIP and NHHR could be used as prognostic indicators to predict post-thrombolysis END.
Introduction
Stroke is a significant global public health challenge, characterized by high morbidity and mortality rates (1), with acute ischemic stroke (AIS) constituting approximately 70–80% of all strokes (2). Intravenous recombinant tissue plasminogen activator in the early phase (≤4.5 h) has been proven as the first-line treatment for AIS (3, 4). Some patients may have favorable long-term outcomes after vascular thrombolysis and recanalization, but others will still experience early neurological deterioration (END), which is when their neurological deficits and symptoms get worse within 24 h of thrombolysis (5, 6). For AIS patients, END is associated with poor long-term outcomes and an increased risk of death and disability (7, 8). To enhance the clinical prognosis of patients, it is crucial to identify predictors of post-thrombolysis END, identify high-risk stroke patients early, and assist in assessing future preventative initiatives.
Given the role of lipid metabolism in vascular pathophysiology and inflammation, lipid-derived indices such as atherogenic index of plasma (AIP) and non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) have gained attention as potential predictors of adverse cerebrovascular outcomes. The AIP and NHHR are emerging developed markers of lipid and glucose metabolism. The AIP is the logarithmic transformation of the ratio of the level of triglyceride (TG) to the level of high-density lipoprotein-cholesterol (HDL-C), that is, log (TG/HDL-C) (9). Previous studies have pointed out the logarithmic conversion of the ratio of TG to HDL-C was negatively correlated with the diameter of low density lipoprotein particles, indirectly reflecting small and dense low density lipoprotein cholesterol (sdLDLC), that is highly sensitive to underlying insulin resistance, making it an excellent indirect biomarker for glucose metabolism dysregulation risk (10, 11). NHHR is an emerging comprehensive indicator of atherosclerotic lipid. Non-high-density lipoprotein cholesterol is considered a pivotal contributor to cardiovascular disease (12). HDL-C exerting protective effects primarily through cholesterol transfer reversal, anti-inflammatory, antioxidant, anti-apoptotic, and vasodilatory mechanisms (13). AIP has been employed to evaluate metabolic syndrome, insulin resistance, and atherogenic dyslipidemia (14–16). The early identification of individuals with a high risk of cardiovascular disease due to aberrant glucose metabolism will be facilitated by the monitoring of long-term AIP change (17). Multiple epidemiological studies have indicated a significant association between AIP and coronary heart disease (18) as well as symptomatic carotid stenosis (19). A high cumulative AIP is associated with an increased risk of ischemic stroke (20). AIS patients were prospectively enrolled, revealing that elevated AIP correlated with unfavorable outcomes in all stroke patients (21). A recent study also found that a higher AIP index was linked to END in AIS patients who had mechanical thrombectomy treatment for an urgent large vessel occlusion (22). However, there is no data explicitly evaluating the relationship between AIP and END following thrombolysis in individuals with AIS. NHHR is a novel, comprehensive biomarker of atherosclerotic lipids that possesses significant predictive value in individuals with cardiovascular disease (23–26). Despite the increasingly significant data that emphasizes the significance of the NHHR, its connection to stroke is remains insufficiently characterized. A prior study indicated that NHHR is associated with an increased prevalence of stroke and may become a new predictor of stroke among adults in the USA (27). However, to date, no study has simultaneously examined the association of AIP and NHHR with post-thrombolysis END following intravenous thrombolysis in AIS patients, particularly within the Asian population.
END is a clinically severe complication, leading to poor prognosis and causing a heavy burden on patients and families (28, 29). Additionally, the long-term prognosis of patients is correlated with the early neurological outcome following thrombolysis (8, 30). Therefore, the present study aimed to investigate the associations of AIP and NHHR with the risk of early neurological deterioration in AIS patients treated with intravenous thrombolysis.
Materials and methods
Study design and participants
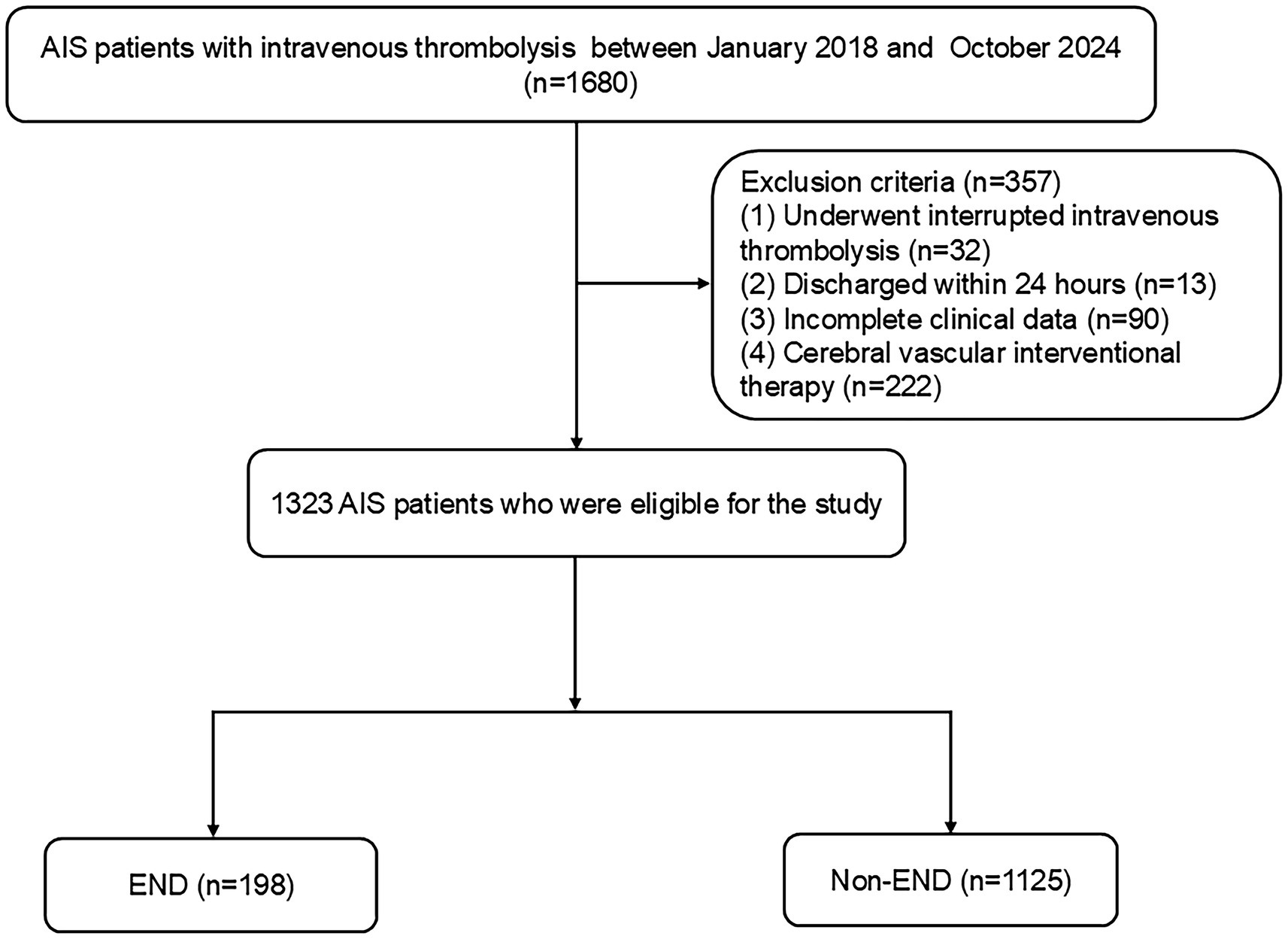
This multicenter retrospective analysis identified consecutive AIS patients who underwent intravenous thrombolysis within 4.5 h from two urban hospitals in China (center 1: Changsha Central Hospital; center 2: Hunan Province’s Second People’s Hospital). AIS was diagnosed based on the subsequent criteria: (1) Sudden onset; (2) localized neurological impairments (such as weakness or numbness on one side of the face or limb, language difficulties, etc.), with some presenting as generalized neurological deficits; (3) imaging showing lesions or symptoms/signs persisting for over 24 h; (4) elimination of nonvascular etiologies; and (5) brain CT/MRI to rule out cerebral hemorrhage (31). The inclusion criteria included: (1) admission within 4.5 h post-onset; (2) treatment with intravenous thrombolysis using r-tPA; and (3) participants aged 18 years or older. The exclusion criteria for patients included: (1) discharge within 24 h; (2) interruption of intravenous thrombolysis due to severe side effects; (3) incomplete clinical data; and (4) cerebral vascular interventional therapy. The Ethics Committee of Changsha Central Hospital and the Second People’s Hospital of Hunan Province authorized this study. From January 2018 to October 2024, 1,323 AIS patients were enrolled (Figure 1).
Data collection
The clinical evaluations were conducted by expert neurologists in a blinded manner. This study used secondary data from medical records. We collected data from each participant, which including age, sex, body mass index, stroke risk factors (hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, current alcohol consumption, and smoking), and clinical features (severity of stroke at admission, admission systolic blood pressure (SBP) and diastolic blood pressure (DBP), onset to treatment time (OTT), and stroke subtypes). The stroke subtype was identified using transcranial Doppler, carotid ultrasonography, echocardiography, electrocardiography, magnetic resonance imaging, and computed tomography. The parameters of the Trial of Organization 10,172 in Acute Stroke Treatment were used to categorize the stroke subtype. The demographic data, baseline clinical parameters, clinical diagnoses, and treatment plans were carefully collected using a standardized case-report form.
Blood samples were collected from all patients at 6–7 a.m. following a minimum fasting period of 8 h. For standard blood tests, two milliliters of EDTA-anticoagulated whole blood were utilized (BZ6800, China). For the standard biochemical analysis, 5 mL of blood containing coagulant were employed (HITACHI 7600, Japan). Triglycerides (TG), total cholesterol (TC), neutrophils, platelets, monocytes, white blood cells (WBC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and fasting blood glucose (FBG) levels were assessed in blood samples. All the indicators were tested using commercial kits, which were operated by qualified professionals in accordance with the specifications. Each of the blood tests was performed three times. The following formula was used to calculate AIP: Log [TG (mmol/L)/HDL-C (mmol/L)] (9). The NHHR index was computed using the following calculation: (TC-HDL-C)/HDL-C (27).
Definition of post-thrombolysis early neurological deterioration and symptomatic intracranial hemorrhage
The certified neurologists at both centers were blinded to our study and underwent standardized training for the assessment of NIHSS scores. An increase in the National Institutes of Health Stroke Scale (NIHSS) score of >4 within 24 h following thrombolysis was considered post-thrombolysis END (32, 33). Within 24 h following thrombolysis, a clinical deterioration of at least four points on the NIHSS score was considered symptomatic intracranial hemorrhage (sICH), which could be caused by an intraventricular hemorrhage, subarachnoid hemorrhage, or parenchymal hematoma (34).
Statistical analysis
Data analysis was conducted using SPSS 25.0 (IBM SPSS Statistics software, Version 25.0). The Kolmogorov–Smirnov test was employed to assess if the distribution of all the data was normal. If the continuous variables were regularly distributed, they were shown as mean ± SD; if not, they were shown as median (quartile). For categorical factors, results are shown as percentages. The chi-squared test or Fisher’s exact test was employed to analyze categorical data, while the Student’s t test or Mann–Whitney U test was utilized to assess disparities in baseline features of continuous variables among groups. Collinearity diagnostics were used to detect the presence of multicollinearity between independent variables. Binary logistic regression analysis for risk factors for post-thrombolysis END. To evaluate the overall discriminative power of the AIP and NHHR for post-thrombolysis END, a receiver operating characteristic (ROC) curve was created using a MedCalc 15.6.0 (MedCalc Software Acacialaan 22, B-8400 Ostend, Belgium) packet program. A two-tailed value of p < 0.05 was considered significant.
Results
Characteristics of clinical and demographic factors in AIS patients experiencing post-thrombolysis END compared to non-END
Table 1 provides the baseline information of all research participant. This study identified post-thrombolysis END in 198 patients (14.97%) and non-post-thrombolysis END in 1,125 patients (85.03%). In the post-thrombolysis END group, the NIHSS score after rt-PA for 24 h (p < 0.001), diabetes mellitus (p = 0.003), WBC (p = 0.014), neutrophils (p = 0.003), FBG (p < 0.001), TC (p = 0.001), TG (p < 0.001), AIP (p < 0.001),and NHHR (p < 0.001) were significantly greater than those in the post-thrombolysis non-END group, whereas HDL-C (p < 0.001) was significantly lower than those in the post-thrombolysis non-END group. The proportion of patients having symptomatic intracranial hemorrhage (sICH) in the post-thrombolysis END group was 26.77% (53/198). Furthermore, there was a significant difference in stroke subtype (p = 0.003) between the two groups. Figure 2 shows the AIP, NHHR, WBC, neutrophils, FBG, TC, TG, and HDL-C for the two groups.
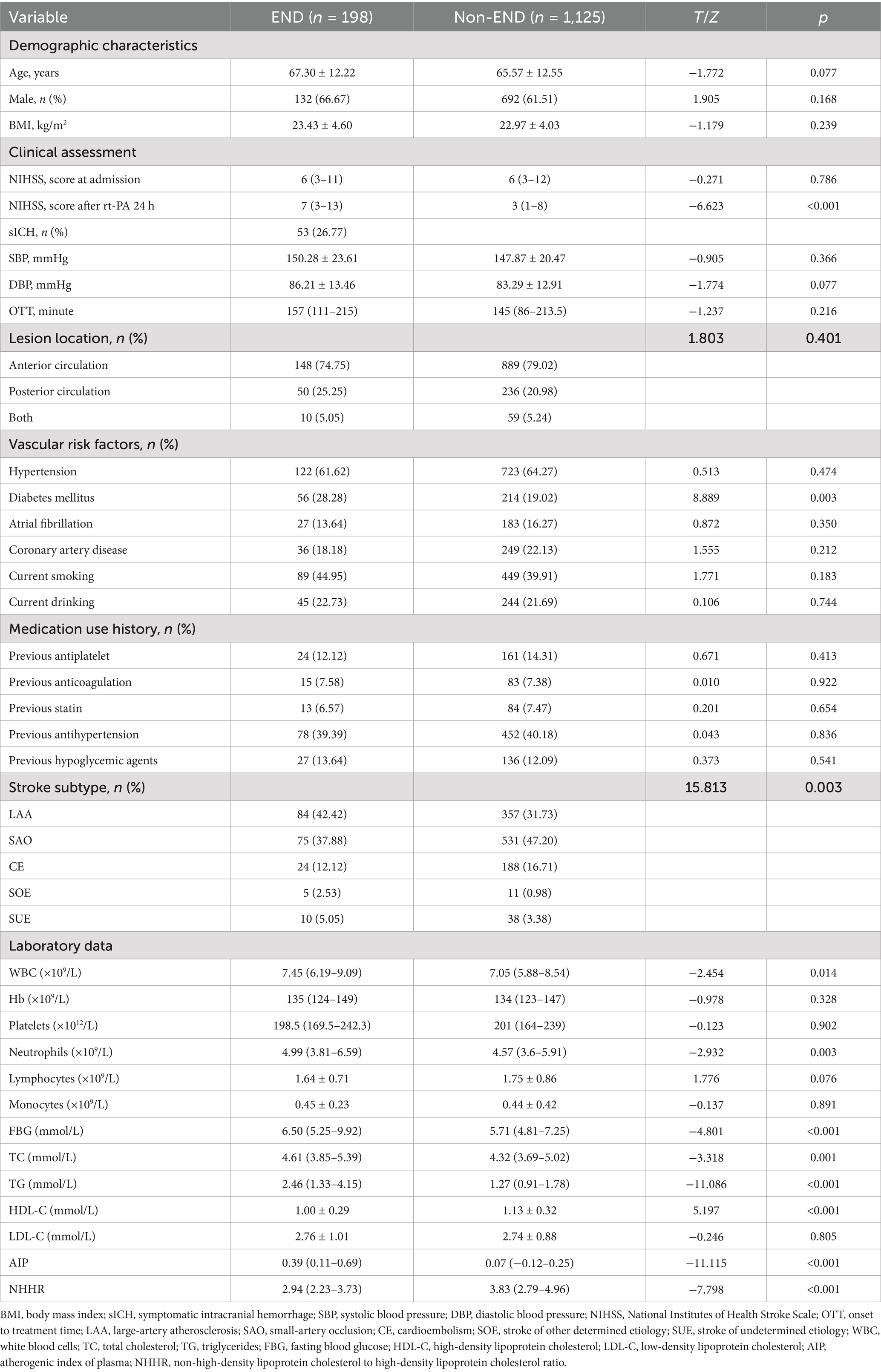
Table 1. Characteristics of clinical and demographic factors in AIS patients experiencing post-thrombolysis END compared to non-END.
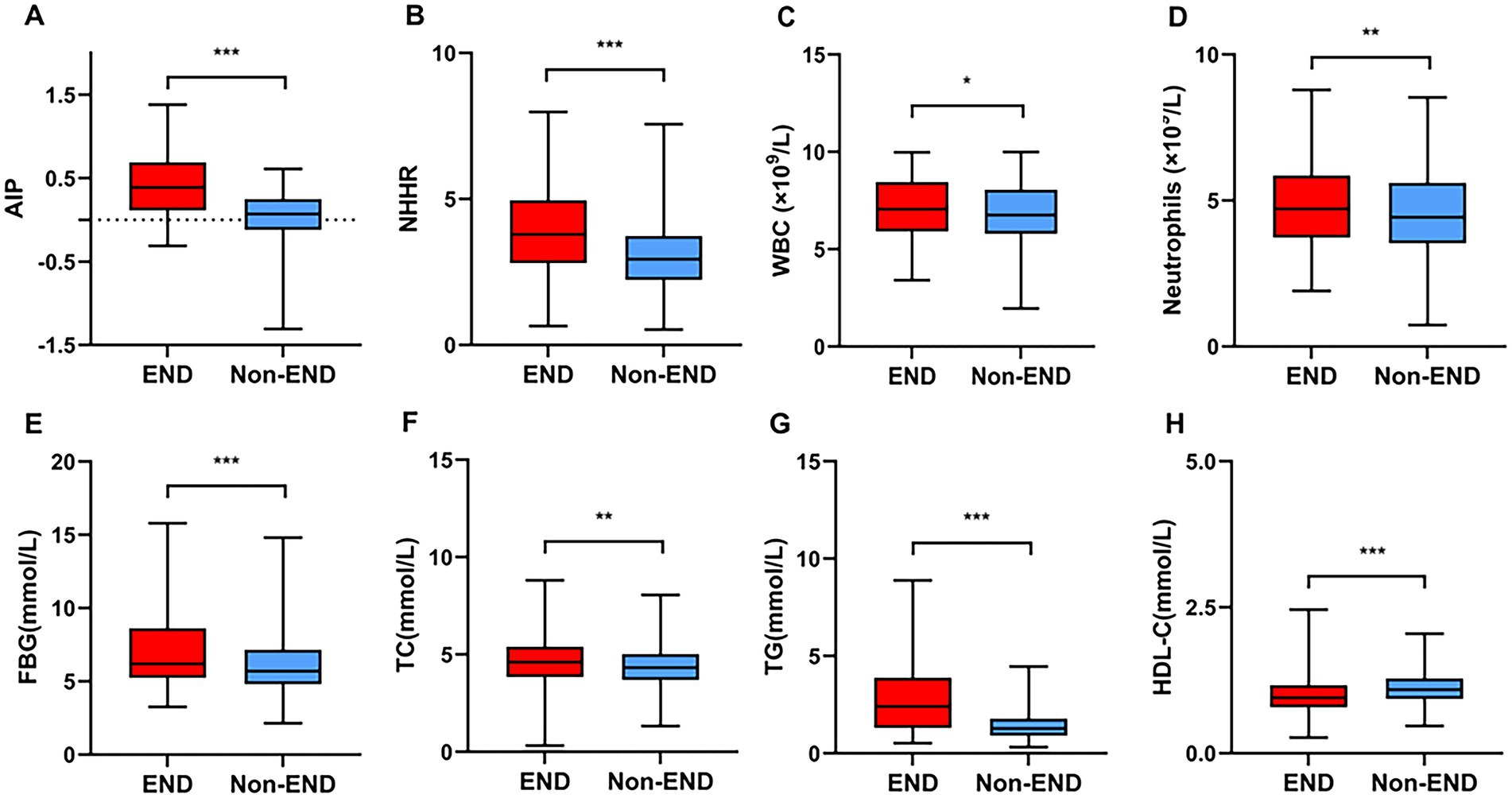
Figure 2. Comparisons of the AIP (A), NHHR (B), WBC (C), neutrophils (D), FBG (E), TC (F), TG (G), and HDL-C (H) between the END and non-END groups. ***p < 0.001, **p < 0.01, *p < 0.05.
Analysis of logistic regression for risk factors associated with post-thrombolysis END
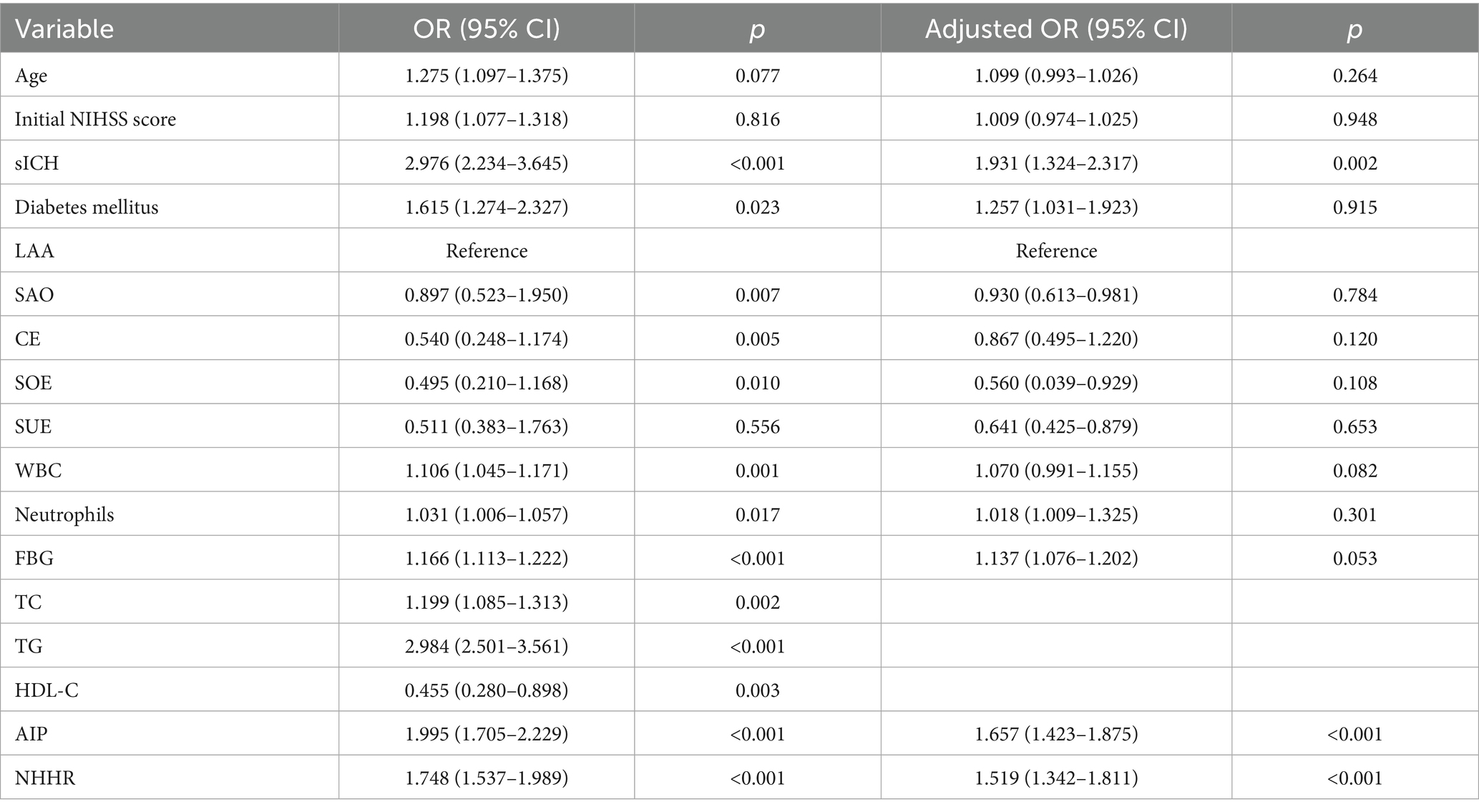
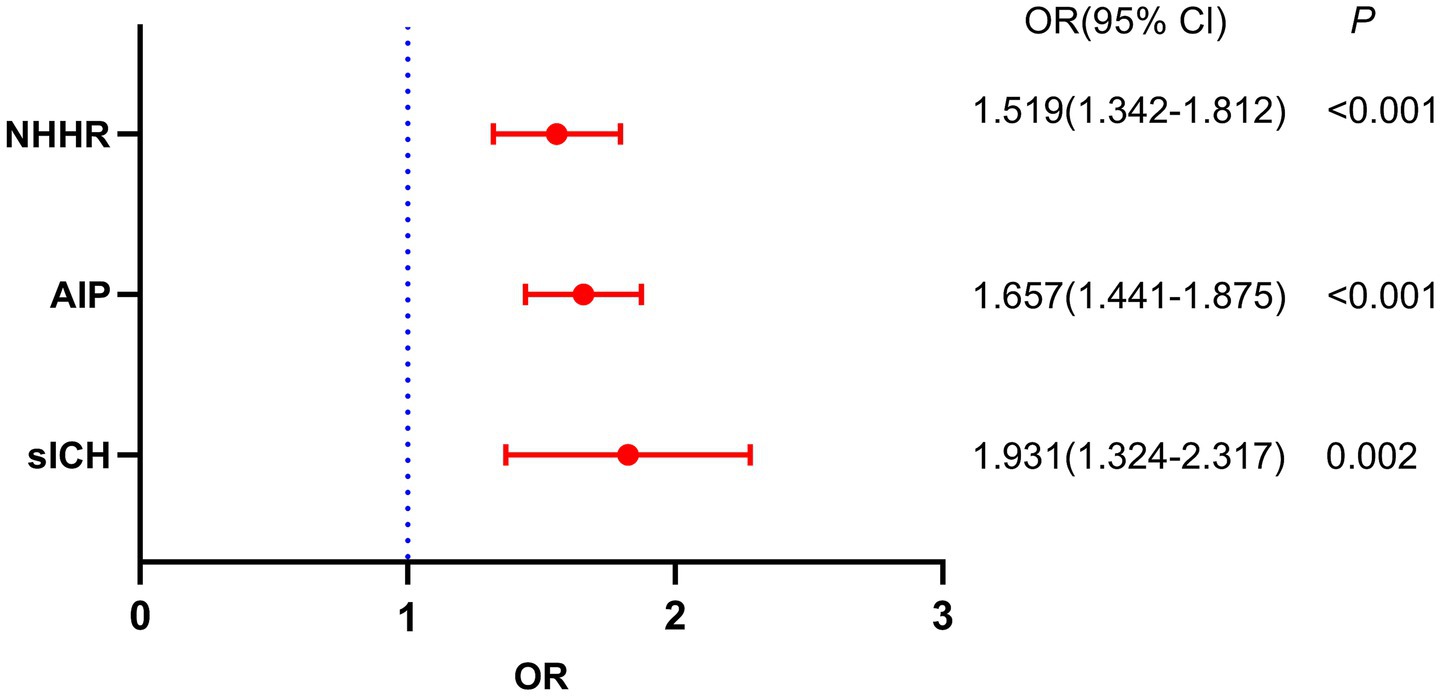
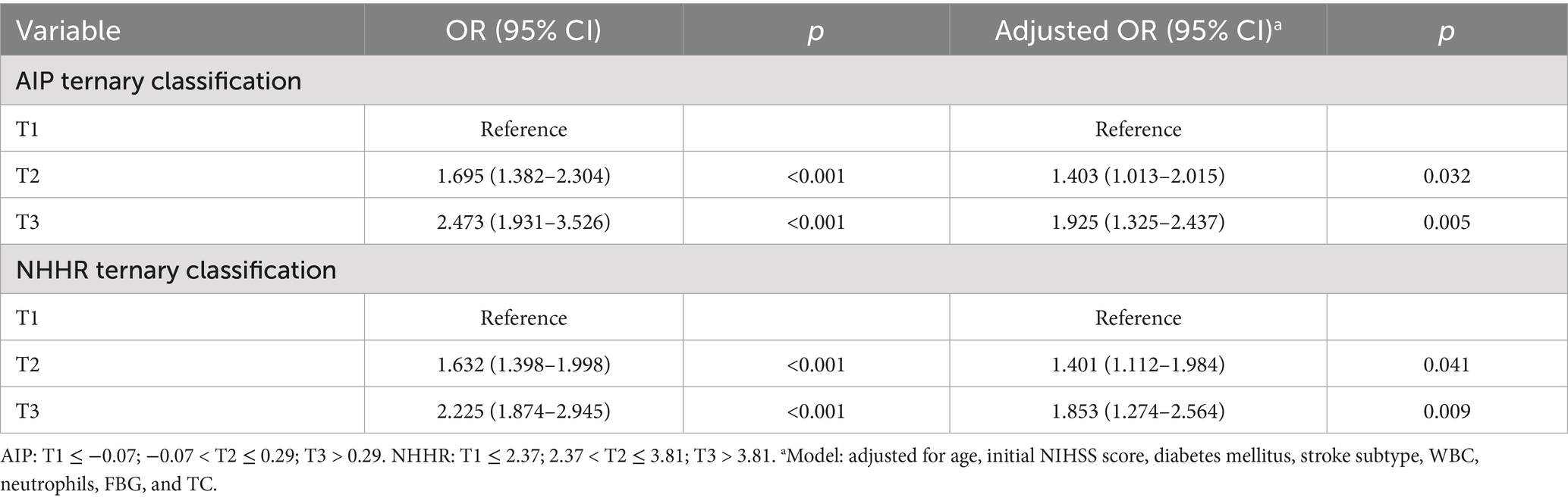
Table 2 shows the outcomes of the post-thrombolysis END crude models. The binary logistic regression model incorporated the statistically significant variables from Table 1 and determined independent risk factors for post-thrombolysis END. Multivariate analysis should incorporate sICH, age, and the NIHSS score, as these are significant determinants for post-thrombolysis END. There was no collinearity between the AIP and the NHHR (VIF = 1.3). However, TC (VIF = 35), TG (VIF = 43), and HDL-C (VIF = 29) were not included in the model because of collinearity with the AIP and NHHR. After controlling for age, initial NIHSS score, diabetes mellitus, stroke subtype, WBC, neutrophils and FBG, the AIP (OR, 1.657; 95% CI 1.432–1.875, p < 0.001), NHHR (OR, 1.519; 95% CI 1.342–1.811, p < 0.001) and sICH (OR, 1.931; 95% CI 1.324–2.317, p = 0.002) were found to be independent predictors for post-thrombolysis END (Figure 3). The AIP and NHHR were utilized as a classification variable based on tertiles. Following the adjustment for confounding variables, patients exhibiting elevated AIP levels (3th quartile vs. 1st quartile; OR, 1.925; 95% CI, 1.325–2.437, p = 0.005) and NHHR levels (3rd quartile vs. 1st quartile; OR, 1.853; 95% CI, 1.274–2.564, p = 0.009) demonstrated a heightened risk of post-thrombolysis END (Table 3).
Subgroup and sensitivity analyses
Stratified analyses were conducted to determine if the relationship between AIP or NHHR and post-thrombolysis END was not influenced by some of the subgroups (Supplementary Figure 1). Our subgroup analyses revealed that the positive correlation was not significantly altered by stratification variables including age, sex, hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, smoking status, and alcohol use. AIP and NHHR were significantly associated with post-thrombolysis END in all subgroups, indicating that our study conclusion is robust and applicable to a wide range of populations. However, future external validation is warranted.
ROC curve analysis was conducted to assess the overall capacity to distinguish post-thrombolysis END
As seen in Figure 4, ROC curves were used to assess the AIP and NHHR’s overall discriminatory capacity in differentiating post-thrombolysis END. The AIP’s area under the curve (AUC) for determining post-thrombolysis END was 0.754 (95% CI, 0.729–0.778, p < 0.001), and the cut-off value was 0.46, with a sensitivity of 45.26% and a specificity of 95.99%. For the NHHR, the AUC was 0.678 (95% CI, 0.651–0.705, p < 0.001), and the cut-off value was 3.946, with a sensitivity of 47.09% and a specificity of 80.72%. Additionally, we conducted ROC curve analysis to assess the discriminatory power of the AIP and NHHR combination in distinguishing between the END group and the non-END group. The AUC for the combination of the NHHR and AIP was 0.795 (95% CI: 0.771–0.818, p < 0.001), and the cut-off value was 0.18, with a sensitivity of 66.67% and a specificity of 80.72%.
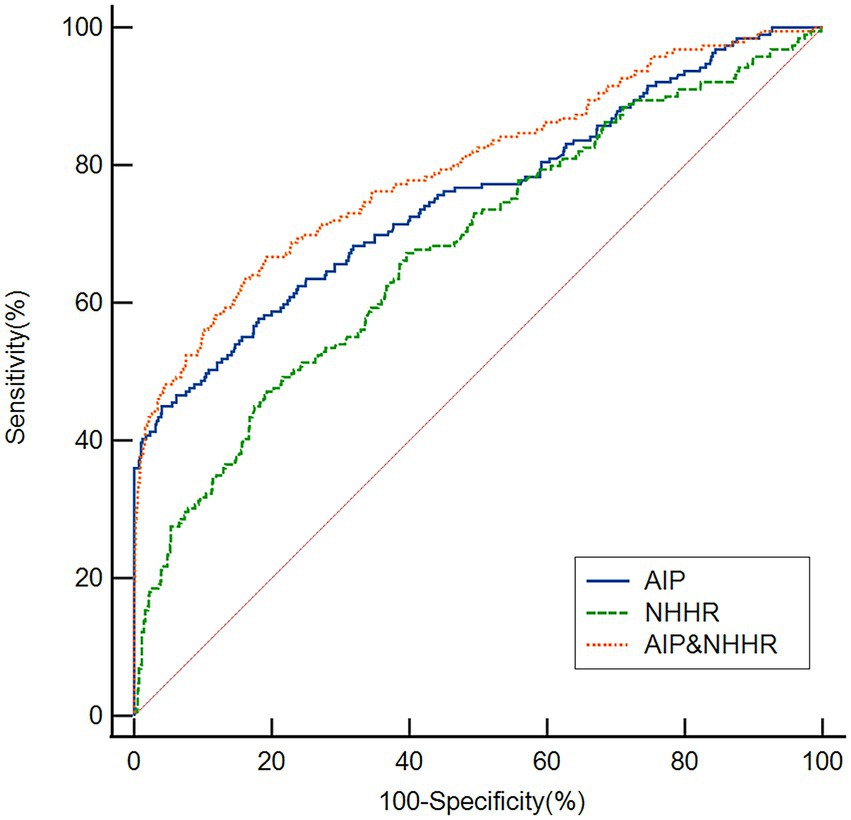
Figure 4. Based on ROC analysis, the AIP, NHHR and AIP and NHHR exhibited respectable post-thrombolysis END discriminating power with AUC values of 0.754, 0.678, and 0.795, respectively.
Discussion
The pathophysiology of END following a stroke is not completely understood. According to research, it might be intimately linked to the development of thrombosis, the shedding of atherosclerotic plaque, inadequate collateral circulation, oxidative stress, and inflammation (35). There is no particular treatment for END in clinical practice once it happens. Hence, the capacity of doctors to recognize stroke patients at high risk of END at an early stage is crucial.
This research represents the inaugural investigation into the relationship between AIP and post-thrombolysis END in AIS patients. 198 individuals (14.97%) in this study developed post-thrombolysis END, and the percentage matched those from other investigations (5, 6, 36). This investigation yielded some novel discoveries. Initially, we found that the AIP and NHHR in AIS patients with END were greater than those in AIS patients with non-END. Additionally, the binary logistic regression model indicated that the AIP and NHHR were independent factors for post-thrombolysis END. Lastly, according to ROC analysis, the AIP and NHHR showed decent post-thrombolysis END discriminating power. Together, these findings provide evidence that a higher AIP and NHHR are associated with post-thrombolysis END. The findings indicate that a higher AIP and NHHR correlate with post-thrombolysis END.
The AIP, a unique comprehensive lipid index that may accurately reflect the ratio of atherogenic to anti-atherogenic lipid particles, has lately drawn the attention of researchers (37). A meta-analysis indicated that elevated AIP serves as an independent prognostic factor in individuals with coronary artery disease (38). A previous study demonstrated that AIP serves as an independent factor for no-reflow in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention (39). A higher cumulative AIP was significantly associated with an increased risk of major adverse cardiac events, stroke and myocardial infarction independent of traditional cardiovascular risk factors (40). The Kailuan Study, which included 97,959 participants, demonstrated a significant association between high baseline and long-term AIP levels and an increased risk of ischemic stroke (41). An elevation in baseline AIP levels was significantly correlated with risk for ischemic stroke in middle-aged and elderly populations (1). Furthermore, an elevation in baseline AIP levels was significantly correlated with risk for ischemic stroke in middle-aged and elderly populations (29). Our study found that the AIP was significantly higher in the post-thrombolysis END group compared to the non-END group. A binary logistic regression model demonstrated that the AIP was an independent predictor of post-thrombolysis END. Moreover, when the AIP was employed as a categorical variable, our analysis, after controlling for confounding variables, indicated that an elevated AIP correlated with an increased likelihood of developing post-thrombolysis END. The findings suggest that elevated AIP during the acute phase of ischemic stroke may serve as a risk factor for post-thrombolysis END. Furthermore, the AIP demonstrated commendable post-thrombolysis END discriminative capability with AUC values of 0.754. These data may suggest that the AIP serves as a biomarker for post-thrombolysis END.
We suggested that this phenomenon could be explained by a number of possible mechanisms. First of all, the AIP reflects atherogenic dyslipidemia characterized by high TG, low HDL cholesterol, and the sdLDL, a subcomponent of LDL with pro-inflammatory and pro-atherogenic characteristics (11). TG rich lipoproteins, such as very low-density lipoprotein or chylomicron, are sufficiently small to penetrate the arterial intima (42, 43). These changes induce sustained low-grade inflammation and foam cell accumulation, culminating in atherosclerotic plaque destabilization and rupture (44). Additionally, sdLDL can promote the overproduction of reactive nitrogen and reactive oxygen species, thereby exacerbating endothelial cell damage (45). AIP indicates insulin resistance, which is closely associated with glucose metabolic dysfunction (46). Brain edema, symptomatic bleeding, and the progression of ischemia could result from these pathophysiological alterations (47). Finally, a variety of earlier studies have found strong correlations between AIP and a number of associated conditions, such as coronary heart disease, diabetes mellitus, hypertension, and current smoking, all of which have been identified as significant risk factors for END (48–51). These results highlight how AIP may mediate the existence of comorbidities and hence accelerate the progression of stroke.
The NHHR is a developing comprehensive metric of atherosclerotic lipids (52). Prior research has demonstrated that when assessing the risk of non-alcoholic fatty liver disease (53) and chronic kidney disease (54), the NHHR performs better in terms of predictive and diagnostic capabilities than conventional blood lipid levels. Moreover, numerous recent studies have highlighted NHHR’s predictive worth and its strong prognostic value in people with cardiovascular disease (23–26). A prior study indicated that NHHR is associated with an increased prevalence of stroke and may become a new predictor of stroke (27). There are currently no studies that precisely examine how NHHR predicts the risk of post-thrombolysis END or how it is directly related to post-thrombolysis END. In this study, the NHHR was significantly higher in the post-thrombolysis END group compared to the non-END group. A binary logistic regression model demonstrated that the NHHR was an independent predictor of post-thrombolysis END. When the NHHR was employed as a categorical variable, it after controlling for confounding variables, indicated that an elevated NHHR correlated with an increased likelihood of developing post-thrombolysis END. Furthermore, the NHHR demonstrated commendable post-thrombolysis END discriminative capability with AUC values of 0.678. The findings suggest that elevated NHHR during the acute phase of ischemic stroke may serve as a risk factor for post-thrombolysis END. This connection can be explained pathophysiologically by the part lipoproteins play in atherosclerosis (55). Several studies have shown that non-HDL cholesterol, which comprises all atherogenic lipoproteins carrying apolipoprotein B, is more strongly linked to cardiovascular risk than LDL cholesterol alone (56, 57). On the other hand, because HDL cholesterol makes it easier for the body to eliminate cholesterol, it protects against atherosclerosis (58, 59). As a result, a higher NHHR indicates a greater proportion of atherogenic particles than protective ones, which may raise the risk of atherosclerotic events like post-thrombolysis END.
Our study’s sICH percentage in the END group was 26.77% (53/198), which is comparable to a previous study (33). Additionally, all AIS patients receiving thrombolysis had an overall sICH rate of 4.01% (53/1,323), which is in accordance with previous research (6, 34, 60). Similar to the previous study (6, 61), sICH was found to be an independent risk factor for post-thrombolysis END in this investigation after controlling for all other variables. Numerous studies exist on the early prediction of END. These factors include inflammation (neutrophil-to-Lymphocyte ratio, hypersensitive C-reactive protein, interleukin-6), protease (matrix metalloproteinase-9, alkaline phosphatase, lipoprotein-associated phospholipase A), coagulation (P-selectin and C-type lectin-like receptor 2, D-Dimer), metabolism (blood glucose, glycated albumin, blood Lipid, cystatin C, whole blood purine concentration, trimethylamine N-Oxide), oxidative stress and excitatory neurotoxicity (F2-Isoprostanes, microRNA-107, serum total bilirubin) (35). Additionally, the triglyceride-glucose (TyG) index and TG/HDL-C ratio are emerging as promising candidates for post-thrombolysis END (6). Other significant indicators for END include age, the NIHSS score, hypertension and hypotension, infarct location and large artery occlusion (28, 62). Owing to the vast heterogeneity in stroke progression, a single biomarker is unlikely to accurately predict the risk of progression. Combining multiple biomarkers is therefore necessary to enhance the capacity to estimate END. AIP and NHHR are easy to obtain markers in clinical practice and can be widely used. Moreover, no study to date has investigated the association between AIP/NHHR and END following intravenous thrombolysis in Asian AIS patients. This study indicates that the AIP and NHHR may serve as prognostic factors for predicting post-thrombolysis END. Our findings expand the understanding of the function of the AIP and NHHR in cerebrovascular disease and provide fresh perspectives on therapeutic approaches. However, our research found no significant correlation between age, the NIHSS score, and post-thrombolysis END. We think that the differences between various studies could be due to differences in the ethnicity of the research populations, sample sizes, medication status, and the severity of the condition. Furthermore, the AIP and NHHR demonstrated statistically significant AUC values, the practical clinical usefulness (especially given the moderate sensitivity) should be further explored. In our study, the AIP AUC = 0.754, but sensitivity = 45.26% limits its standalone screening value. Combined AIP + NHHR model yields AUC = 0.795. So, the combination of these two indicators may be clinical risk scores or decision tools in the future.
This study has the following limitations: (1) This study was cross-sectional and involved only Chinese patients receiving intravenous thrombolysis, indicating the presence of potential inherent biases. Moreover, there was a difference in sample size between END (n = 198) and non-END (n = 1,125) patients. Consequently, our findings necessitate verification in non-Chinese populations, and future research should involve larger-scale longitudinal cohort studies; (2) only the baseline AIP and NHHR levels were tested. There were no dynamic AIP and NHHR changes accessible during the hospital period; (3) because the data on the participants’ medicine (lipid-lowering, antihypertensive, and hypoglycemic medications) and lifestyle (physical activity, smoking status, and alcohol use) were self-reported, they were susceptible to recall bias; (4) this paper does not show the details of the infarction, including location and volume; (5) although numerous potential confounders were taken into account, the results could still have been impacted by unmeasured or residual confounders; and (6) there was no specific time of blood sample collection after intravenous thrombolysis. Future research needs to consider the specific time of blood sample collection after intravenous thrombolysis.
Conclusion
In conclusion, this study indicates that the AIP and NHHR may serve as prognostic factors for predicting post-thrombolysis END. The combination of these two indicators may be more beneficial in predicting post-thrombolysis END. Further research is necessary to validate these findings and elucidate the pathophysiology of post-thrombolysis END.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Changsha Central Hospital and the Second People's Hospital of Hunan Province. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YJ: Supervision, Writing – review & editing, Methodology, Formal analysis. FL: Supervision, Project administration, Conceptualization, Writing – review & editing, Methodology, Investigation, Software, Formal analysis, Writing – original draft, Data curation. MD: Methodology, Data curation, Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition, Resources. KS: Investigation, Software, Writing – original draft, Conceptualization, Funding acquisition, Methodology, Data curation. ZW: Conceptualization, Methodology, Resources, Funding acquisition, Writing – original draft, Validation, Data curation. WX: Conceptualization, Project administration, Data curation, Writing – original draft, Formal analysis. TF: Data curation, Methodology, Supervision, Writing – original draft. SC: Writing – original draft, Visualization, Data curation, Validation. CW: Data curation, Writing – original draft, Investigation. XM: Investigation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Natural Science Foundation of Hunan Province (grant no. 2023JJ40072), the Science and Technology Planning Project of Hunan Health Committee (grant no. D202303076376), the Key Project of Science and Technology Innovation in Hunan Province (grant no. 2020SK1012), and the Research Foundation of Hunan Brain Hospital (grant no. 2017B05).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1619727/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Subgroup analyses of AIP, NHHR and post-thrombolysis END.
References
1. Qu, L, Fang, S, Lan, Z, Xu, S, Jiang, J, Pan, Y, et al. Association between atherogenic index of plasma and new-onset stroke in individuals with different glucose metabolism status: insights from CHARLS. Cardiovasc Diabetol. (2024) 23:215. doi: 10.1186/s12933-024-02314-y
2. Kuriakose, D, and Xiao, Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci. (2020) 21:7609. doi: 10.3390/ijms21207609
3. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/str.0000000000000211
4. Marko, M, Posekany, A, Szabo, S, Scharer, S, Kiechl, S, Knoflach, M, et al. Trends of r-tPA (recombinant tissue-type plasminogen activator) treatment and treatment-influencing factors in acute ischemic stroke. Stroke. (2020) 51:1240–7. doi: 10.1161/strokeaha.119.027921
5. Gong, P, Liu, Y, Gong, Y, Chen, G, Zhang, X, Wang, S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. (2021) 18:51. doi: 10.1186/s12974-021-02090-6
6. Deng, M, Song, K, Xu, W, He, G, Hu, J, Xiao, H, et al. Association of higher triglyceride-glucose index and triglyceride-to-high-density lipoprotein cholesterol ratio with early neurological deterioration after thrombolysis in acute ischemic stroke patients. Front Neurol. (2024) 15:1421655. doi: 10.3389/fneur.2024.1421655
7. Weyland, CS, Mokli, Y, Vey, JA, Kieser, M, Herweh, C, Schönenberger, S, et al. Predictors for failure of early neurological improvement after successful Thrombectomy in the anterior circulation. Stroke. (2021) 52:1291–8. doi: 10.1161/strokeaha.120.030519
8. Mori, M, Naganuma, M, Okada, Y, Hasegawa, Y, Shiokawa, Y, Nakagawara, J, et al. Early neurological deterioration within 24 hours after intravenous rt-PA therapy for stroke patients: the stroke acute management with urgent risk factor assessment and improvement rt-PA registry. Cerebrovasc Dis. (2012) 34:140–6. doi: 10.1159/000339759
9. Nam, KW, Kwon, HM, and Lee, YS. Effect of atherogenic index of plasma and triglyceride-glucose index on early neurological deterioration of patients with large artery atherosclerotic ischemic stroke. Diabetol Metab Syndr. (2025) 17:123. doi: 10.1186/s13098-025-01684-x
10. Dobiásová, M, and Frohlich, J. The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: changes during lipanor therapy. Vnitr Lek. (2000) 46:152–6.
11. Kanonidou, C. Small dense low-density lipoprotein: analytical review. Clin Chim Acta. (2021) 520:172–8. doi: 10.1016/j.cca.2021.06.012
12. Carr, SS, Hooper, AJ, Sullivan, DR, and Burnett, JR. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. (2019) 51:148–54. doi: 10.1016/j.pathol.2018.11.006
13. Sun, L, Clarke, R, Bennett, D, Guo, Y, Walters, RG, Hill, M, et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med. (2019) 25:569–74. doi: 10.1038/s41591-019-0366-x
14. Dobiásová, M, and Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apo B-lipoprotein-depleted plasma (FER (HDL)). Clin Biochem. (2001) 34:583–8. doi: 10.1016/s0009-9120(01)00263-6
15. Igarashi, M, Hirata, A, Yamaguchi, H, Jimbu, Y, and Tominaga, M. Pioglitazone reduces atherogenic outcomes in type 2 diabetic patients. J Atheroscler Thromb. (2008) 15:34–40. doi: 10.5551/jat.e528
16. Kim, SW, Jee, JH, Kim, HJ, Jin, SM, Suh, S, Bae, JC, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. (2013) 168:2678–83. doi: 10.1016/j.ijcard.2013.03.027
17. Min, Q, Wu, Z, Yao, J, Wang, S, Duan, L, Liu, S, et al. Association between atherogenic index of plasma control level and incident cardiovascular disease in middle-aged and elderly Chinese individuals with abnormal glucose metabolism. Cardiovasc Diabetol. (2024) 23:54. doi: 10.1186/s12933-024-02144-y
18. Zhu, Y, Chen, M, Liu, K, Gao, A, Kong, X, Liu, Y, et al. Atherogenic index of plasma and the risk of in-stent restenosis in patients with acute coronary syndrome beyond the traditional risk factors. J Atheroscler Thromb. (2022) 29:1226–35. doi: 10.5551/jat.63136
19. Zhao, Z, Wang, H, Hou, Q, Zhou, Y, and Zhang, Y. Non-traditional lipid parameters as potential predictors of carotid plaque vulnerability and stenosis in patients with acute ischemic stroke. Neurol Sci. (2023) 44:835–43. doi: 10.1007/s10072-022-06472-3
20. Zheng, H, Wu, K, Wu, W, Chen, G, Chen, Z, Cai, Z, et al. Relationship between the cumulative exposure to atherogenic index of plasma and ischemic stroke: a retrospective cohort study. Cardiovasc Diabetol. (2023) 22:313. doi: 10.1186/s12933-023-02044-7
21. Liu, H, Liu, K, Pei, L, Li, S, Zhao, J, Zhang, K, et al. Atherogenic index of plasma predicts outcomes in acute ischemic stroke. Front Neurol. (2021) 12:741754. doi: 10.3389/fneur.2021.741754
22. Wu, H, Wang, W, Chen, S, Yan, E, Liu, L, Chen, J, et al. Association between the atherogenic index of plasma and early neurological deterioration in mechanical thrombectomy patients. J Stroke Cerebrovasc Dis. (2024) 33:107993. doi: 10.1016/j.jstrokecerebrovasdis.2024.107993
23. Zhang, S, and Zhu, Z. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and the risk of ischemic heart disease in type 2 diabetes mellitus participants: a large-scale cohort study from the UK biobank. Diabetol Metab Syndr. (2025) 17:99. doi: 10.1186/s13098-025-01646-3
24. Cui, Y, and Choi, M. Association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and angina pectoris in US adults: a cross-sectional retrospective study based on NHANES 2009-2018. Lipids Health Dis. (2024) 23:347. doi: 10.1186/s12944-024-02343-2
25. Wang, B, Li, L, Tang, Y, Lin, T, Wu, J, Wang, G, et al. Changes in non-high-density lipoprotein to high-density lipoprotein ratio (NHHR) and cardiovascular disease: insights from CHARLS. Lipids Health Dis. (2025) 24:112. doi: 10.1186/s12944-025-02536-3
26. Yu, B, Li, M, Yu, Z, Zheng, T, Feng, X, Gao, A, et al. The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) as a predictor of all-cause and cardiovascular mortality in US adults with diabetes or prediabetes: NHANES 1999-2018. BMC Med. (2024) 22:317. doi: 10.1186/s12916-024-03536-3
27. Ma, HX, Chen, HQ, and Wang, PC. Association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and stroke among adults in the USA: a cross-sectional NHANES study. Biomed Environ Sci. (2025) 38:37–46. doi: 10.3967/bes2025.001
28. Liu, H, Liu, K, Zhang, K, Zong, C, Yang, H, Li, Y, et al. Early neurological deterioration in patients with acute ischemic stroke: a prospective multicenter cohort study. Ther Adv Neurol Disord. (2023) 16:17562864221147743. doi: 10.1177/17562864221147743
29. Wang, Q, Jiang, G, Yan, L, Chen, R, Liu, Y, Liu, L, et al. Association of atherogenic index of plasma with early neurological deterioration in patients with acute ischemic stroke. Clin Neurol Neurosurg. (2023) 234:108014. doi: 10.1016/j.clineuro.2023.108014
30. Yeo, LL, Paliwal, P, Teoh, HL, Seet, RC, Chan, BP, Wakerley, B, et al. Early and continuous neurologic improvements after intravenous thrombolysis are strong predictors of favorable long-term outcomes in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:e590–6. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.024
31. Chinese Society of Neurology and Chinese Stroke Society. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. (2018) 51:666–82. doi: 10.3760/cma.j.issn.1006-7876.2018.09.004
32. Seners, P, Ben Hassen, W, Lapergue, B, Arquizan, C, Heldner, MR, Henon, H, et al. Prediction of early neurological deterioration in individuals with Minor stroke and large vessel occlusion intended for intravenous thrombolysis alone. JAMA Neurol. (2021) 78:321–8. doi: 10.1001/jamaneurol.2020.4557
33. Yu, WM, Abdul-Rahim, AH, Cameron, AC, Kõrv, J, Sevcik, P, Toni, D, et al. The incidence and associated factors of early neurological deterioration after thrombolysis: results from SITS registry. Stroke. (2020) 51:2705–14. doi: 10.1161/strokeaha.119.028287
34. Warach, SJ, Ranta, A, Kim, J, Song, SS, Wallace, A, Beharry, J, et al. Symptomatic intracranial hemorrhage with Tenecteplase vs Alteplase in patients with acute ischemic stroke: the comparative effectiveness of routine Tenecteplase vs Alteplase in acute ischemic stroke (CERTAIN) collaboration. JAMA Neurol. (2023) 80:732–8. doi: 10.1001/jamaneurol.2023.1449
35. Ji, X, Tian, L, Yao, S, Han, F, Niu, S, and Qu, C. A systematic review of body fluids biomarkers associated with early neurological deterioration following acute ischemic stroke. Front Aging Neurosci. (2022) 14:918473. doi: 10.3389/fnagi.2022.918473
36. Zhang, B, Lei, H, Ambler, G, Werring, DJ, Fang, S, Li, H, et al. Association between triglyceride-glucose index and early neurological outcomes after thrombolysis in patients with acute ischemic stroke. J Clin Med. (2023) 12:3471. doi: 10.3390/jcm12103471
37. Zhao, W, Gong, W, Wu, N, Li, Y, Ye, K, Lu, B, et al. Association of lipid profiles and the ratios with arterial stiffness in middle-aged and elderly Chinese. Lipids Health Dis. (2014) 13:37. doi: 10.1186/1476-511x-13-37
38. Rabiee Rad, M, Ghasempour Dabaghi, G, Darouei, B, and Amani-Beni, R. The association of atherogenic index of plasma with cardiovascular outcomes in patients with coronary artery disease: a systematic review and meta-analysis. Cardiovasc Diabetol. (2024) 23:119. doi: 10.1186/s12933-024-02198-y
39. Süleymanoğlu, M, Rencüzoğulları, İ, Karabağ, Y, Çağdaş, M, Yesin, M, Gümüşdağ, A, et al. The relationship between atherogenic index of plasma and no-reflow in patients with acute ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention. Int J Cardiovasc Imaging. (2020) 36:789–96. doi: 10.1007/s10554-019-01766-8
40. Liu, Z, Zhang, L, Wang, L, Li, K, Fan, F, Jia, J, et al. The predictive value of cumulative atherogenic index of plasma (AIP) for cardiovascular outcomes: a prospective community-based cohort study. Cardiovasc Diabetol. (2024) 23:264. doi: 10.1186/s12933-024-02350-8
41. Zhang, Y, Chen, S, Tian, X, Xu, Q, Xia, X, Zhang, X, et al. Elevated atherogenic index of plasma associated with stroke risk in general Chinese. Endocrine. (2024) 84:934–42. doi: 10.1007/s12020-023-03677-0
42. Hoshino, T, Ishizuka, K, Toi, S, Mizuno, T, Nishimura, A, Wako, S, et al. Prognostic role of hypertriglyceridemia in patients with stroke of atherothrombotic origin. Neurology. (2022) 98:e1660–9. doi: 10.1212/wnl.0000000000200112
43. Hoshino, T, Ishizuka, K, Toi, S, Mizuno, T, Nishimura, A, Takahashi, S, et al. Atherogenic dyslipidemia and residual vascular risk after stroke or transient ischemic attack. Stroke. (2022) 53:79–86. doi: 10.1161/strokeaha.121.034593
44. Peng, J, Luo, F, Ruan, G, Peng, R, and Li, X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. (2017) 16:233. doi: 10.1186/s12944-017-0625-0
45. Jin, X, Yang, S, Lu, J, and Wu, M. Small, dense low-density lipoprotein-cholesterol and atherosclerosis: relationship and therapeutic strategies. Front Cardiovasc Med. (2021) 8:804214. doi: 10.3389/fcvm.2021.804214
46. Qin, Z, Zhou, K, Li, Y, Cheng, W, Wang, Z, Wang, J, et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc Diabetol. (2020) 19:23. doi: 10.1186/s12933-020-0989-8
47. Thanvi, B, Treadwell, S, and Robinson, T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J. (2008) 84:412–7. doi: 10.1136/pgmj.2007.066118
48. Bhole, R, Nouer, SS, Tolley, EA, Turk, A, Siddiqui, AH, Alexandrov, AV, et al. Predictors of early neurologic deterioration (END) following stroke thrombectomy. J Neurointerv Surg. (2023) 15:584–8. doi: 10.1136/neurintsurg-2022-018844
49. Kim, JM, Bae, JH, Park, KY, Lee, WJ, Byun, JS, Ahn, SW, et al. Incidence and mechanism of early neurological deterioration after endovascular thrombectomy. J Neurol. (2019) 266:609–15. doi: 10.1007/s00415-018-09173-0
50. Zhang, M, Xing, P, Tang, J, Shi, L, Yang, P, Zhang, Y, et al. Predictors and outcome of early neurological deterioration after endovascular thrombectomy: a secondary analysis of the DIRECT-MT trial. J Neurointerv Surg. (2023) 15:e9–e16. doi: 10.1136/neurintsurg-2022-018976
51. Chen, NH, Zhang, YM, Jiang, FP, Liu, S, Zhao, HD, Hou, JK, et al. FLAIR vascular hyperintensity predicts early neurological deterioration in patients with acute ischemic stroke receiving endovascular thrombectomy. Neurol Sci. (2022) 43:3747–57. doi: 10.1007/s10072-021-05853-4
52. Lin, W, Luo, S, Li, W, Liu, J, Zhou, T, Yang, F, et al. Association between the non-HDL-cholesterol to HDL-cholesterol ratio and abdominal aortic aneurysm from a Chinese screening program. Lipids Health Dis. (2023) 22:187. doi: 10.1186/s12944-023-01939-4
53. Wang, K, Shan, S, Zheng, H, Zhao, X, Chen, C, and Liu, C. Non-HDL-cholesterol to HDL-cholesterol ratio is a better predictor of new-onset non-alcoholic fatty liver disease than non-HDL-cholesterol: a cohort study. Lipids Health Dis. (2018) 17:196. doi: 10.1186/s12944-018-0848-8
54. Chiu, H, Wu, PY, Huang, JC, Tu, HP, Lin, MY, Chen, SC, et al. There is a U shaped association between non high density lipoprotein cholesterol with overall and cardiovascular mortality in chronic kidney disease stage 3–5. Sci Rep. (2020) 10:12749. doi: 10.1038/s41598-020-69794-2
55. Lu, Y, Cui, X, Zhang, L, Wang, X, Xu, Y, Qin, Z, et al. The functional role of lipoproteins in atherosclerosis: novel directions for diagnosis and targeting therapy. Aging Dis. (2022) 13:491–520. doi: 10.14336/ad.2021.0929
56. Behbodikhah, J, Ahmed, S, Elyasi, A, Kasselman, LJ, De Leon, J, Glass, AD, et al. Apolipoprotein B and cardiovascular disease: biomarker and potential therapeutic target. Meta. (2021) 11:690. doi: 10.3390/metabo11100690
57. Johannesen, CDL, Langsted, A, Nordestgaard, BG, and Mortensen, MB. Excess apolipoprotein B and cardiovascular risk in women and men. J Am Coll Cardiol. (2024) 83:2262–73. doi: 10.1016/j.jacc.2024.03.423
58. Parhofer, KG. Increasing HDL-cholesterol and prevention of atherosclerosis: a critical perspective. Atheroscler Suppl. (2015) 18:109–11. doi: 10.1016/j.atherosclerosissup.2015.02.020
59. Ouimet, M, Barrett, TJ, and Fisher, EA. HDL and reverse cholesterol transport: basic mechanisms and their roles in vascular health and disease. Circ Res. (2019) 124:1505–18. doi: 10.1161/CIRCRESAHA.119.312617
60. Deng, M, Song, K, Tong, Y, Chen, S, Xu, W, He, G, et al. Higher fibrinogen and neutrophil-to-lymphocyte ratio are associated with the early poor response to intravenous thrombolysis in acute ischemic stroke. Front Neurol. (2024) 15:1291950. doi: 10.3389/fneur.2024.1291950
61. Mao, XL, He, SS, Lin, CD, Huang, XD, and Sun, J. Analysis of clinical characteristics and influencing factors of early neurological deterioration in patients with mild stroke by intravenous alteplase therapy. Neurologist. (2024) 29:275–9. doi: 10.1097/nrl.0000000000000553
Keywords: acute ischemic stroke, atherogenic index of plasma, non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio, early neurological deterioration, lipid metabolism, risk factor
Citation: Jiang Y, Deng M, Song K, Wang Z, Xu W, Feng T, Chen S, Wan C, Ma X and Li F (2025) Association between the atherogenic index of plasma and the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio with early neurological deterioration after thrombolysis. Front. Neurol. 16:1619727. doi: 10.3389/fneur.2025.1619727
Edited by:
Xintian Cai, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, ChinaReviewed by:
Lanjing Wang, Capital Medical University, ChinaCagdas Kaynak, Siirt University, Türkiye
Mathew John, Jubilee Mission Medical College and Research Institute, India
I. Nyoman Wande, Udayana University, Indonesia
Yudhisman Imran, Trisakti University, Indonesia
Copyright © 2025 Jiang, Deng, Song, Wang, Xu, Feng, Chen, Wan, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangyi Li, NDc1MDQwOTQyQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yan Jiang1†
Yan Jiang1† Mingzhu Deng
Mingzhu Deng Kangping Song
Kangping Song Zhen Wang
Zhen Wang Wei Xu
Wei Xu Fangyi Li
Fangyi Li