- 1Shanxi Key Laboratory of Brain Disease Control, Department of Neurology, Shanxi Provincial People’s Hospital, Taiyuan, China
- 2Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 3Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 4China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 5Dharmais Cancer Hospital, National Cancer Center, Jakarta, Indonesia
Purpose: This study aims to investigate the relationship between smoking status, smoking index, and the outcomes of intravascular treatment for acute basilar artery occlusion within 24 h.
Methods: We retrospectively analyzed all consecutive patients hospitalized with acute basilar artery occlusion who underwent endovascular treatment within 24 h from January 2012 to July 2018 at Beijing Tiantan Hospital. Smoking status was categorized as never smoking, current smoking, or previous smoking. The smoking index (SI) was calculated as the daily smoking count multiplied by the number of smoking years. The primary outcomes were a 90-day modified Rankin Scale score shift analysis and mortality at 90 days.
Results: The overall study cohort comprised 59 never smokers, 58 former smokers, and 70 current smokers. No significant differences in primary outcomes were observed between smoking status and functional independence (OR, 1.611; 95% CI, 0.776–3.344) or death (OR, 0.461; 95% CI, 0.196–1.084). Multivariate analysis indicated that smoking status had limited relevance to functional independence (OR, 1.958; 95% CI, 0.781–4.907) and death (OR, 0.446; 95% CI, 0.169–1.178). The smoking index was independently associated with functional independence (OR, 1.095; 95% CI, 1.015–1.182) and death (OR, 0.844; 95% CI, 0.757–0.941). The smoking index demonstrated a dose–effect relationship with outcomes, being positively correlated with functional independence and negatively correlated with death.
Conclusion: Smoking status does not appear to influence prognosis. However, the smoking index may be associated with improved functional independence and a reduced risk of death, demonstrating a dose-effect relationship.
Introduction
Smoking is a major risk factor for ischemic stroke morbidity and mortality and is the leading cause of death worldwide. Smoking nearly doubles the risk of acute ischemic stroke (AIS). Despite its adverse health effects, some studies have identified a “smoking paradox,” suggesting that smokers may experience more favorable outcomes in AIS compared to nonsmokers (1). Additionally, a retrospective analysis study also showing that smoking was independently associated with lower inpatient mortality in acute ischemic stroke, potentially due to tobacco-induced changes in cerebrovascular vasoreactivity or residual confounding (2). Regarding endovascular treatments, Meseguer et al. (3) reported that intra-arterial rt-PA administration was more effective in achieving complete recanalization in both current and former smokers. Consistently, von Martia et al. (4) also found that smoking might predict excellent clinical outcomes after endovascular treatment (EVT), which is increasingly preferred for severe strokes with large artery occlusion. However, the reasons for this association are not fully understood, and conflicting evidence complicates the potential benefits of smoking on favorable outcomes after adjusting for confounders (5–7).
There is limited research on the relationship between smoking index and prognosis following EVT, specifically in patients with acute basilar artery occlusion (BAO). Therefore, this study aimed to retrospectively examine the smoking status and smoking index in patients with acute basilar artery occlusion who underwent endovascular treatment within 24 h.
Methods
Study design
We conducted a retrospective analysis of patients with BAO who underwent endovascular treatment (EVT) at our institution from January 2012 to July 2018. In accordance with current guidelines, intravenous thrombolysis (tissue plasminogen activator) was administered prior to EVT when indicated. Eligible patients received endovascular treatment within 24 h of symptom onset. Exclusion criteria included a modified Rankin Scale (mRS) score greater than 3 and incomplete information regarding cigarette smoking status, secondhand smoke exposure, and e-cigarette use.
Study population
All participants provided informed consent. Clinical baseline variables were recorded, including age, gender, vascular risk factors, clinical characteristics, blood pressure, the National Institutes of Health Stroke Scale (NIHSS), laboratory tests, and stroke types classified according to the TOAST criteria from the ORG 10172 trial for acute stroke treatment. Additionally, anatomical and morphological characteristics of BAO, ASINT/SIR, and details of EVT were documented. Clinical outcomes and mortality within 90 days were prospectively collected. The time of BAO incidence was recorded based on patient or witness reports; if these were unavailable, the time of the last patient observation was used. For patients who experienced a sudden loss of consciousness following mild symptoms, the estimated time of clinical deterioration was used to approximate the onset of BAO. Functional outcomes at 90 days were assessed using the mRS. Follow-up for baseline variables was conducted by trained interviewers via standardized telephone interviews.
Smoking definition
Smoking status was categorized into three groups: never smokers, current smokers, and former smokers. Current smokers were defined as individuals who smoked at least one cigarette per day and had been smoking continuously or cumulatively for at least 6 months prior to the stroke. Former smokers were those who had quit smoking for at least 6 months at the time of the survey. Individuals who had smoked less than 18 packs cumulatively and did not meet the criteria for current or former smoking were classified as never smokers. The smoking index (SI) was calculated as the product of daily cigarette consumption and the number of years of smoking (8).
Clinical outcomes
In this study, the primary outcome measures were functional independence, favorable outcomes, and mortality at 90 days postoperatively. Functional independence was defined as a mRS score of ≤2. A favorable outcome was similarly defined as an mRS score of ≤2.
Statistical analysis
Study data were collected using standardized forms, evaluated for completeness, and entered into SPSS for statistical analysis. Continuous variables were described using means (standard deviations) and/or medians (25th and 75th percentiles), while categorical variables were summarized using frequencies and/or proportions. Univariate analysis utilized independent sample t-tests or non-parametric tests (Mann–Whitney U test) to compare differences between means or medians, and Pearson chi-square tests or Fisher’s exact tests for comparing frequencies and proportions. Multivariate binary logistic regression was employed to determine independent predictors of 90-day outcomes, with adjusted odds ratios (OR) and 95% confidence intervals (CI) calculated. Variables with p-values ≤ 0.1 in univariate analysis or those showing more than a 10% variation in effect estimates were considered for inclusion in the multivariate model. Confounding factors were assessed, and collinearity was addressed. The smoking index, treated as a continuous variable, was analyzed using a spline smoothing function to evaluate its effect on thrombectomy outcomes in basilar artery occlusion (BAO). All tests were two-tailed, with statistical significance set at an alpha level of 0.05. Statistical analyses were conducted using the R software package (http://www-rprimt.org, R Foundation).
Results
Baseline characteristics of patients
From January 2012 to July 2018, a total of 187 patients with BAO ischemic stroke were enrolled in the study, all of whom received endovascular treatment within 24 h (Supplementary Figure 1). No patients were excluded. Based on smoking status, the cohort included 59 never smokers, 58 former smokers, and 70 current smokers. Patients were categorized according to their 90-day prognosis: 68 had a good prognosis (mRS 0–2), while 119 had a poor prognosis (mRS 3–6). Of the total, 149 patients survived and 38 patients died.
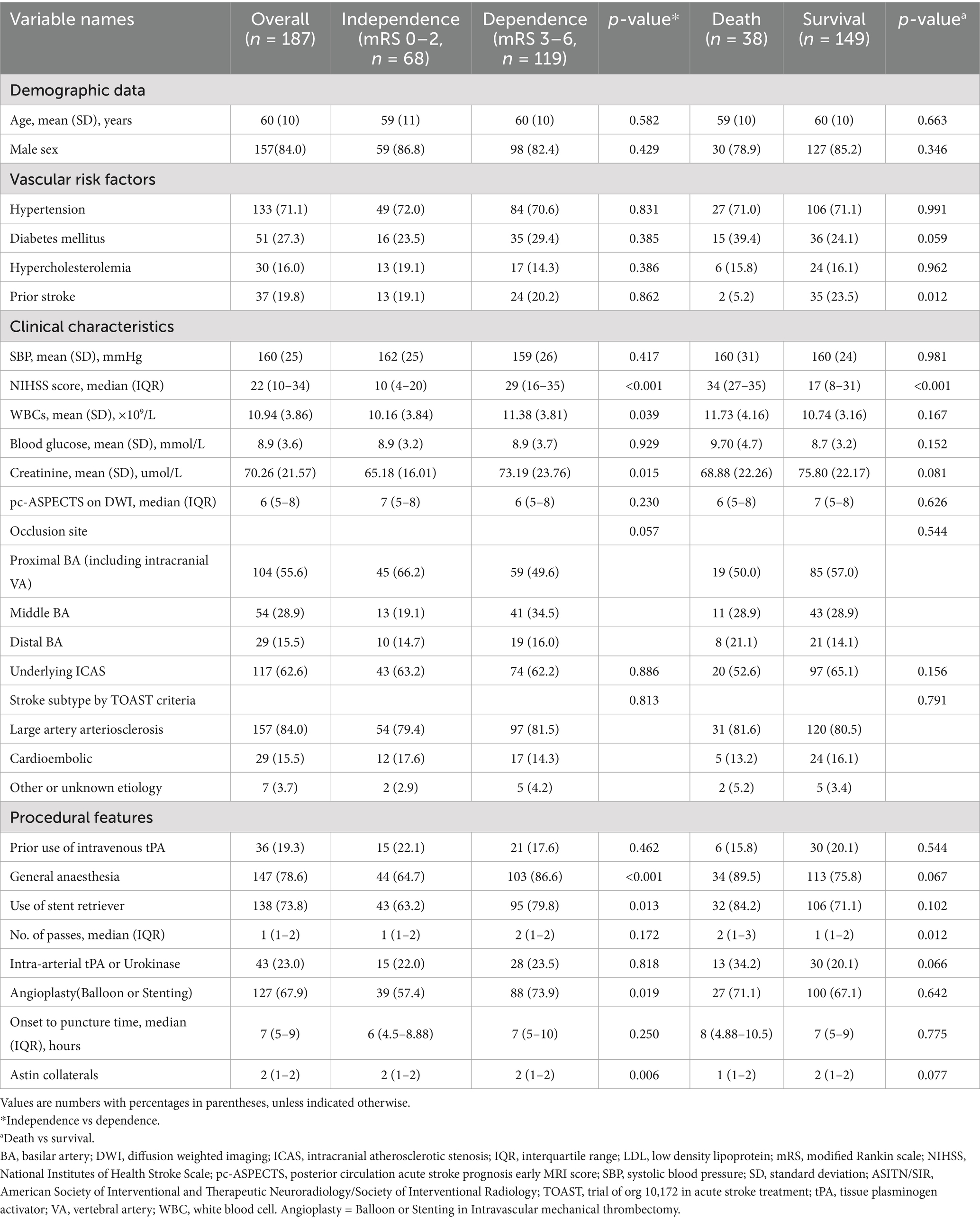
Baseline characteristics of patients with different outcomes are presented in Table 1. Demographic data did not show significant differences between the groups. However, a history of prior stroke was more prevalent in the survivor group compared to the non-survivor group (23.5% vs. 5.2%). Other risk factors, including hypertension, diabetes, and hypercholesterolemia, did not differ significantly between prognosis groups. Regarding clinical features, patients in the dependence group had a higher National Institutes of Health Stroke Scale (NIHSS) score at admission (29 vs. 10) compared to the survivors, while the non-survivor group had an even higher NIHSS score (34 vs. 17). Additionally, the dependence group had elevated white blood cell counts (11.38 × 109/L vs. 10.16 × 109/L) and creatinine levels (73.19 μmol/L vs. 65.18 μmol/L). No significant differences were observed in systolic blood pressure, blood glucose levels, or diffusion-weighted imaging (DWI)-ASPECTS scores. The proportion of patients with poor prognosis was higher among those receiving general anesthesia (86.6% vs. 64.7%), with a statistically significant difference noted in the 90-day modified Rankin Scale (mRS) scores (p < 0.001). Conversely, no significant difference in prognosis was observed between survivors and non-survivors based on the type of anesthesia. Additionally, the use of stents (79.8% vs. 63.2%) and balloon dilation (73.9% vs. 57.4%) was more common in the poor prognosis group, with a significant difference in the 90-day mRS scores. However, no significant differences were found between survivors and non-survivors with respect to these interventions.
Smoking status and outcome
Endovascular treatment outcomes were analyzed based on smoking history, categorizing patients into three groups: (1) never smokers, (2) former smokers, and (3) current smokers. No significant differences were found in 90-day modified Rankin Scale (mRS) scores or survival rates among these groups. Univariate analysis revealed no significant association between smoking status and functional independence [odds ratio (OR), 1.611; 95% confidence interval (CI), 0.776–3.344] or mortality (OR, 0.461; 95% CI, 0.196–1.084), with p > 0.1. To account for potential confounding factors and collinearity, a multivariate logistic regression analysis was performed, including variables with significant univariate associations (p < 0.1) and those showing more than a 10% change in effect estimates. Factors considered included preoperative NIHSS score, preoperative white blood cell count, preoperative serum creatinine level, lesion site, DSA-based ASIPN/SIR collateral grading, general anesthesia, stent thrombectomy, and angioplasty (balloon dilation or stent implantation). The analysis showed that smoking status was not significantly associated with a good prognosis (mRS 0–2) or with mortality after adjusting for these factors. The presence of general anesthesia and the number of stents also did not significantly correlate with mortality.
Smoking index and outcome
Univariate analysis revealed that the smoking index was significantly associated with a good prognosis [odds ratio (OR) = 1.071; 95% confidence interval (CI) = 1.011–1.134; p < 0.05] and with mortality (OR = 0.862; 95% CI = 0.781–0.951; p < 0.05). In multivariate analysis, which adjusted for preoperative NIHSS score, white blood cell count, serum creatinine level, lesion site, DSA-based ASIPN/SIR collateral grading, general anesthesia, and angioplasty (excluding stent thrombectomy), the smoking index remained significantly associated with a good prognosis (OR = 1.019; 95% CI = 1.015–1.182; p < 0.05). This indicates a positive correlation between the smoking index and the likelihood of a good prognosis (mRS 0–2). Conversely, when adjusted for the same factors, the smoking index was also significantly correlated with reduced mortality (OR = 0.844; 95% CI = 0.757–0.941; p < 0.05), indicating a negative correlation with death. The smoking index, treated as a continuous variable, was further analyzed using spline smoothing. The results indicated a linear relationship between the smoking index and good prognosis, with higher smoking index values associated with a higher probability of a good prognosis (Figure 1). Conversely, there was an approximately linear negative correlation between the smoking index and mortality, with higher smoking index values associated with a lower probability of death (Figure 2).
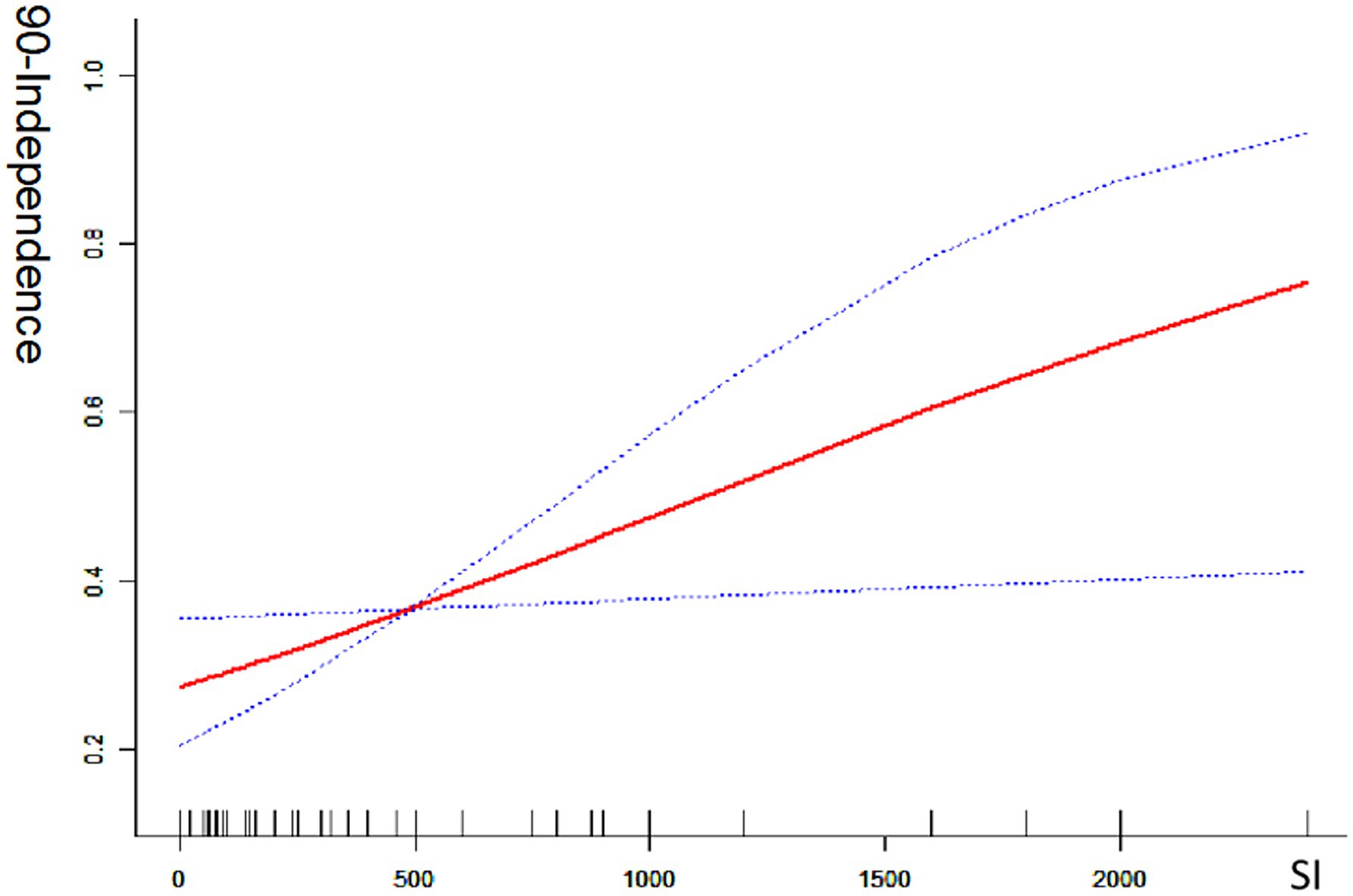
Figure 1. Good 90-day functional outcome with smoking index; 90-independence indicates modified Rankin scale (mRS 0–2) at 90 day ratio; SI indicates smoking index; smoking index (SI) = daily smoking count × number of smoking years.
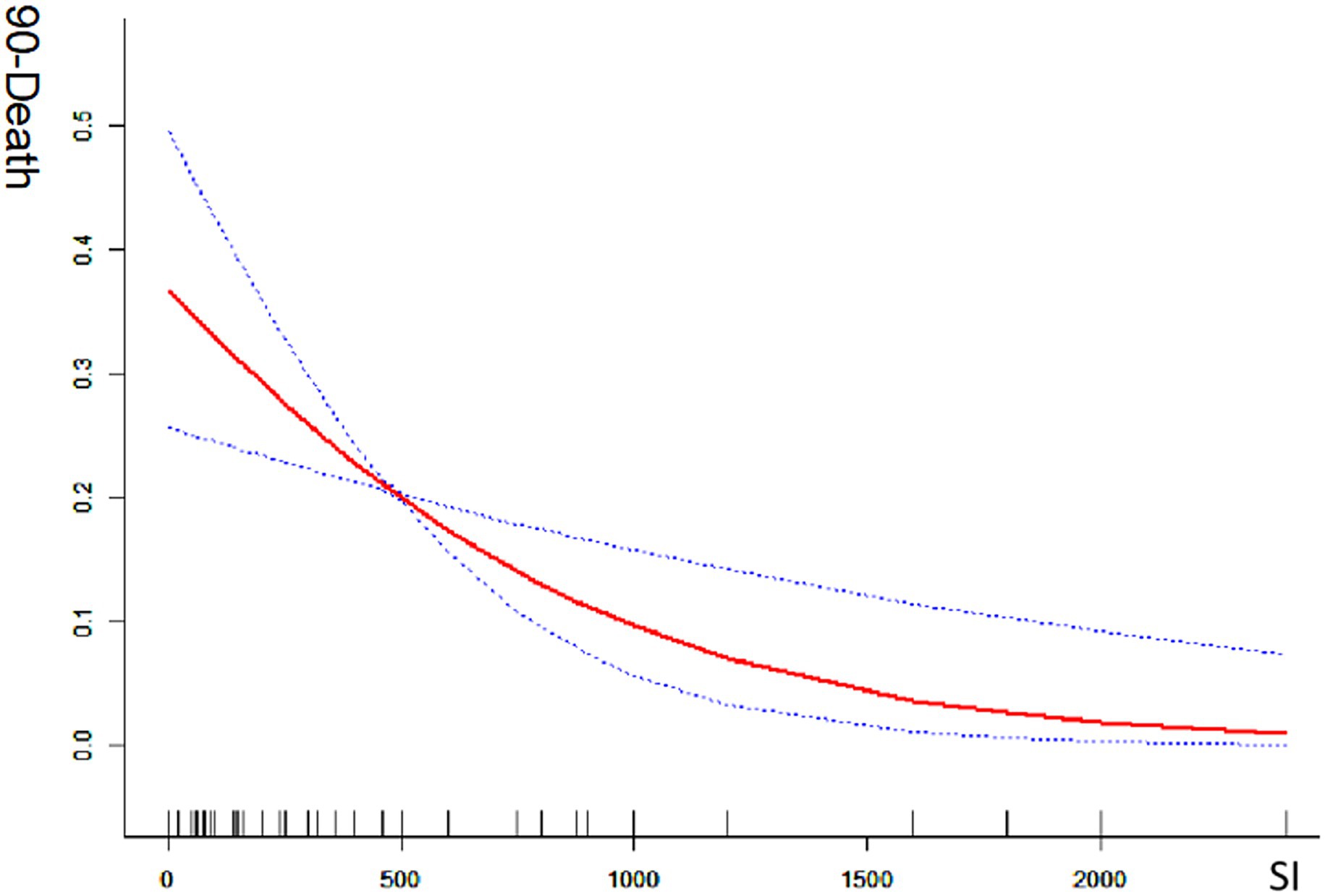
Figure 2. Mortality at 90-days with smoking index; 90-death indicates 90-day mortality ratio; SI indicates smoking index; smoking index (SI) = daily smoking count × number of smoking years.
Discussion
Our study demonstrates that a higher smoking index is associated with a better prognosis and lower in-hospital mortality. This association persists even after extensive adjustments for various covariates, extending the “smoking paradox” observed in other conditions. However, the available data is limited, and the underlying biological mechanisms explaining the observed benefits in smokers are not yet fully understood.
Many studies propose that the “smoking paradox” may be attributed to confounding factors, including the lower risk of acute ischemic stroke in younger and current smokers (3, 9–11). Additionally, male gender and older age are associated with poorer clinical outcomes (12, 13). Despite controlling for these confounding variables, some studies have found that in-hospital mortality remains lower in smokers compared to non-smokers (1, 2, 4). In our study, baseline characteristics, including age and risk factors, showed no significant differences between the groups, suggesting that the paradox cannot be fully explained or eliminated.
Our findings indicate that a higher smoking index is associated with better prognosis and lower in-hospital mortality. This observation may be attributed to smokers potentially having “more favorable” atherosclerotic profiles and, consequently, better outcomes compared to non-smokers (14). One possible explanation is that smoking induces a hypercoagulable state, which might enhance the effectiveness of thrombolytic therapy. This hypercoagulability is associated with increased endothelial dysfunction, elevated platelet activation and aggregation, higher thrombin generation, and elevated circulating fibrinogen levels, all of which can impair endogenous fibrinolysis (15–17). Conversely, non-smokers may experience more frequent occlusions due to the rupture or ulceration of atherosclerotic plaques, which form platelet-rich thrombi (18). These thrombi might respond more effectively to intravascular mechanical thrombectomy, potentially improving vascular recanalization rates and cerebral perfusion. As a result, smokers might achieve better prognoses and lower mortality rates following treatment. However, the current study lacks supporting evidence and further investigation is warranted to validate these findings.
A dose–response relationship has been established between the number of cigarettes smoked per day and circulating fibrinogen levels (19, 20). Our study indicates that a higher smoking index is associated with a better prognosis and lower mortality. This suggests that a certain level of smoking may confer a protective effect on the vessel wall, potentially enhancing the response to both spontaneous and therapeutic thrombolysis. Furthermore, smoking is associated with persistently elevated levels of carbon monoxide in the plasma, which may induce adaptive cellular responses akin to those seen with ischemic preconditioning. These responses could potentially make smokers more resilient to ischemic damage, thereby offering better preconditioning effects during ischemic episodes (21, 22).
We acknowledge several limitations in this study. First, the modest sample size, particularly in subgroup analyses, may reduce statistical power. Nevertheless, the consistency of results across both univariate and multivariate models, together with their concordance with prior studies, supports the reliability of our findings. While we consider this study an important contribution to the field, larger multicenter datasets are required for confirmation. Second, our mechanistic explanations remain speculative and are primarily based on prior literature. Future studies incorporating laboratory biomarkers (e.g., fibrinogen levels, platelet function assays) and imaging data (e.g., thrombus composition, collateral circulation quality) are needed to provide more direct evidence linking the smoking index to stroke outcomes. Third, as an observational study, it is subject to treatment biases that cannot be fully eliminated by multivariate adjustment. Fourth, we did not account for the use of other tobacco products, which may have introduced confounding if participants used these concurrently. Fifth, unmeasured factors such as rehabilitation patterns, socioeconomic status, and medical complications could also have influenced the outcomes. Finally, changes in smoking habits, including post-stroke cessation, were not assessed, which may have affected the results observed at 3 months. Despite these limitations, a key strength of our study lies in the application of advanced thrombectomy stents in real-world cases of basilar artery occlusion (BAO). By carefully adjusting for potential confounders, we sought to minimize the risk of false-positive findings. Further multicenter studies with larger sample sizes are warranted to validate these results.
Conclusion
Our data suggest that smoking status itself does not significantly affect prognosis. However, the smoking index appears to be associated with improved functional independence and a lower risk of mortality, showing a dose–effect relationship. Further prospective, population-based studies are required to investigate these findings more comprehensively.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the ethics committees of Beijing Tiantan Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BH: Conceptualization, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. XT: Conceptualization, Methodology, Writing – review & editing. Raynald: Formal analysis, Investigation, Writing – review & editing, Writing – original draft. BJ: Formal analysis, Writing – review & editing, Data curation. DM: Supervision, Writing – review & editing. FG: Supervision, Writing – review & editing. NM: Writing – review & editing, Supervision. XS: Writing – review & editing, Supervision. ZM: Conceptualization, Supervision, Writing – review & editing, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Fundamental Research Program of Shanxi Province (202303021221231).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1623245/full#supplementary-material
References
1. Ovbiagele, B, and Saver, JLJN. The smoking–thrombolysis paradox and acute ischemic stroke. Neurology. (2005) 65:293–5. doi: 10.1212/01.wnl.0000168163.72351.f3
2. Ali, SF, Smith, EE, Bhatt, DL, Fonarow, GC, and LHJJotAHA, S. Paradoxical association of smoking with in-hospital mortality among patients admitted with acute ischemic stroke. J Am Heart Assoc. (2013) 2:e000171. doi: 10.1161/JAHA.113.000171
3. Meseguer, E, Labreuche, J, Gonzalez-Valcarcel, J, Sirimarco, G, Guidoux, C, Cabrejo, L, et al. The smoking paradox: impact of smoking on recanalization in the setting of intra-arterial thrombolysis. Cerebrovasc Dis Extra. (2014) 4:84–91. doi: 10.1159/000357218
4. von Martial, R, Gralla, J, Mordasini, P, El Koussy, M, Bellwald, S, Volbers, B, et al. Impact of smoking on stroke outcome after endovascular treatment. PLoS. (2018) 13:e0194652. doi: 10.1371/journal.pone.0194652
5. Zahger, D, Cercek, B, Cannon, CP, Jordan, M, Davis, V, Braunwald, E, et al. How do smokers differ from nonsmokers in their response to thrombolysis? (the TIMI-4 trial). Am J Cardiol. (1995) 75:232–6. doi: 10.1016/0002-9149(95)80026-o
6. Ferreiro, JL, Bhatt, DL, Ueno, M, Bauer, D, and DJJJotACoC, A. Impact of smoking on long-term outcomes in patients with atherosclerotic vascular disease treated with aspirin or clopidogrel: insights from the CAPRIE trial (Clopidogrel versus aspirin in patients at risk of ischemic events) (2014) 63:769–77. doi: 10.1016/j.jacc.2013.10.043
7. Cornel, JH, Ohman, EM, Neely, B, Clemmensen, P, Sritara, P, Zamoryakhin, D, et al. Impact of smoking status on platelet function and clinical outcomes with prasugrel vs. clopidogrel in patients with acute coronary syndromes managed without revascularization: insights from the TRILOGY ACS trial. Am Heart J. (2014) 168:76–87.e71. doi: 10.1016/j.ahj.2014.04.011
8. Organization WH. Guidelines for controlling and monitoring the tobacco epidemic World Health Organization (1998).
9. Kvistad, C, Oeygarden, H, Logallo, N, Thomassen, L, Waje-Andreassen, U, and HJAnS, N. Is smoking associated with favourable outcome in tPA-treated stroke patients? Acta Neurol Scand. (2014) 130:299–304. doi: 10.1111/ane.12225
10. Kufner, A, Nolte, CH, Galinovic, I, Brunecker, P, Kufner, GM, Endres, M, et al. Smoking-thrombolysis paradox: recanalization and reperfusion rates after intravenous tissue plasminogen activator in smokers with ischemic stroke. Stroke. (2013) 44:407–13. doi: 10.1161/STROKEAHA.112.662148
11. Aries, M, Uyttenboogaart, M, Koch, M, Langedijk, M, Vroomen, P, Luijckx, G, et al. Does smoking influence outcome after intravenous thrombolysis for acute ischaemic stroke? Eur J Neurol. (2009) 16:819–22. doi: 10.1111/j.1468-1331.2009.02596.x
12. Palnum, KD, Petersen, P, Sørensen, HT, Ingeman, A, Mainz, J, Bartels, P, et al. Older patients with acute stroke in Denmark: quality of care and short-term mortality: a nationwide follow-up study. Age Ageing. (2008) 37:90–5. doi: 10.1093/ageing/afm134
13. Smith, EE, Shobha, N, Dai, D, Olson, DM, Reeves, MJ, Saver, JL, et al. Risk score for in-hospital ischemic stroke mortality derived and validated within the get with the guidelines–stroke program. Circulation. (2010) 122:1496–504. doi: 10.1161/CIRCULATIONAHA.109.932822
14. Levine, SR (2005) Preclinical testing of thrombolytic therapy for acute ischemic stroke. Thrombolytic therapy for acute stroke. Totowa, New Jersey: Springer, pp. 65–76
15. Purcell, I, Newall, N, and Farrer, MJQ. Lower cardiac mortality in smokers following thrombolysis for acute myocardial infarction may be related to more effective fibrinolysis. QJM. (1999) 92:327–33. doi: 10.1093/qjmed/92.6.327
16. Zidovetzki, R, Chen, P, Fisher, M, and Hofman, FMJS. Nicotine increases plasminogen activator inhibitor-1 production by human brain endothelial cells via protein kinase C–associated pathway. Stroke. (1999) 30:651–5. doi: 10.1161/01.str.30.3.651
17. Messner, B, and Bernhard, DJA. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. (2014) 34:509–15. doi: 10.1161/ATVBAHA.113.300156
18. MA GJAJC. Effect of cigarette smoking on coronary potency after thrombolytic therapy for myocardial infarction. Am J Cardiol. (1993) 72:373–8. doi: 10.1016/0002-9149(93)91125-2
19. Heinrich, J, Balleisen, L, Schulte, H, Assmann, G, and van de Loo, JJAbiology tajov. Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb. (1994) 14:54–9. doi: 10.1161/01.atv.14.1.54
20. Stone, M, and JJTJotRCoGP, T. Plasma fibrinogen—a major coronary risk factor. J R Coll Gen Pract. (1985) 35:565–9.
21. Wang, Y, Reis, C, Applegate, R II, Stier, G, Martin, R, and JHJEn, Z. Ischemic conditioning-induced endogenous brain protection: applications pre-, per-or post-stroke. Exp Neurol. (2015) 272:26–40. doi: 10.1016/j.expneurol.2015.04.009
Keywords: smoking paradox, basilar artery occlusion, thrombectomy, smoking index, stroke
Citation: Han B, Tong X, Raynald, Jia B, Mo D, Gao F, Ma N, Sun X and Miao Z (2025) Impact of smoking status and smoking index on outcomes in patients with acute basilar artery occlusion. Front. Neurol. 16:1623245. doi: 10.3389/fneur.2025.1623245
Edited by:
Fulvio Tartara, University Hospital of Parma, ItalyReviewed by:
Bo Yang, Beijing Jiangong Hospital, ChinaYingkun He, Henan Provincial People's Hospital, China
Copyright © 2025 Han, Tong, Raynald, Jia, Mo, Gao, Ma, Sun and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongrong Miao, ZG9jdG9yemhvbmdyb25nbUB5ZWFoLm5ldA==; Xuan Sun, c3h1YW5AeWVhaC5uZXQ=
†These authors have contributed equally to this work
 Bin Han
Bin Han Xu Tong
Xu Tong Raynald
Raynald Baixue Jia
Baixue Jia Dapeng Mo
Dapeng Mo Feng Gao
Feng Gao Ning Ma
Ning Ma Xuan Sun
Xuan Sun Zhongrong Miao2,3,4*
Zhongrong Miao2,3,4*