- 1Department of Vascular and Functional Neurosurgery, National Center of Neurosurgery, Astana, Kazakhstan
- 2Department of Population Health Sciences, Faculty of Life Sciences and Medicine, King’s College London, London, United Kingdom
Background: Carotid-cavernous fistulas (CCFs) are abnormal connections between the carotid artery and/or its branches and the cavernous sinus, potentially resulting in significant complications, including visual impairment and neurological deficits. Timely diagnosis and appropriate therapeutic intervention are critical to minimizing the risk of adverse outcomes. Over the years, endovascular techniques have become the preferred method for treating CCFs, offering high success rates with fewer complications. This study aims to study the clinical and radiological outcomes of patients with CCFs treated with endovascular approaches, and to identify key prognostic factors associated with treatment efficacy and symptomatic resolution.
Methods: A retrospective analysis was conducted of patients diagnosed with carotid-cavernous fistulas (CCFs) and treated at the National Center for Neurosurgery in Astana, Kazakhstan, between 2008 and 2024. A total of 71 patients underwent endovascular embolization using either transarterial or transvenous techniques. According to the Barrow classification, type A CCF were observed in 62 patients (87.3%), type D in 7 patients (9.9%), and types B and C in 1 patient each (1.4%). Collected data included patient demographics, type of embolic materials utilized, number of procedures performed, treatment approach, and corresponding radiological and clinical outcomes.
Results: Among the 71 patients included in the study, 80.3% achieved complete clinical resolution, 15.5% demonstrated partial improvement, and 4.2% experienced no clinical benefit. The mean age was 35.7 ± 12.9 years, with 71.8% males. Clinical outcomes were significantly associated with complete occlusion following the initial intervention (p < 0.001), with 96% of these patients achieving full symptom resolution. Imaging follow-up was available in all patients, with complete occlusion confirmed in 65 cases (91.5%) on MRI at 6 months. Other variables, including distal internal carotid artery flow (p = 0.145), number of interventions (p = 0.838), treatment approach (p = 0.529), and type of embolic agent employed (p = 0.778), did not demonstrate a statistically significant association with clinical outcomes.
Conclusion: Endovascular embolization is a safe and effective first-line treatment modality for CCFs. Both transarterial and transvenous approaches offer comparable rates of clinical success. Achieving complete occlusion is a critical determinant of favorable clinical outcomes and is associated with a reduced risk of treatment-related complications.
1 Introduction
Carotid-cavernous fistulas (CCFs) are abnormal vascular connections between the carotid artery and/or its branches and the cavernous sinus. These lesions are categorized according to their etiology (traumatic or spontaneous), hemodynamic profile (high-flow or low-flow), and anatomical features. The most widely adopted classification system divides CCFs into direct and indirect types, with further sub classification based on flow dynamics and underlying etiological factors (1, 2).
The clinical presentation of CCFs is often variable and nonspecific; however, ophthalmic manifestations are the most common, primarily due to abnormal venous drainage from the orbit into the cavernous sinus (3). Early and accurate diagnosis, followed by prompt intervention, is crucial to preventing irreversible visual impairment and potentially life-threatening complications. The principal therapeutic objective in the management of CCFs is the complete occlusion of the fistulous connection while preserving physiological blood flow through the internal carotid artery (ICA) (4).
Historically, surgical ligation of the common carotid artery (CCA) represented the standard of care (5). However, the paradigm shifted significantly following Serbinenko’s introduction of detachable latex balloons in 1974 (6), which marked the advent of endovascular techniques in the treatment of cerebrovascular lesions. Since then, advancements in endovascular technology have substantially broadened the range of therapeutic options and have established endovascular embolization as the gold standard in CCF management, owing to its high efficacy and favorable safety profile.
Current endovascular strategies include transarterial or transvenous embolization, employing a range of embolic agents such as detachable coils (7), cyanoacrylates (8), or covered stents (9), each demonstrating variable rates of occlusion success. Although open surgical and radiosurgical interventions remain available, they are generally reserved as second-line or adjunctive therapies (10).
This study presents our institutional experience in the management of CCFs using various endovascular techniques and embolic materials. The aim is to analyze the various treatment strategies employed, classified by etiology, hemodynamic characteristics, and anatomical features, with the emphasis on long-term clinical and radiological outcomes during follow-up.
2 Materials and methods
2.1 Demographic and clinical characteristics
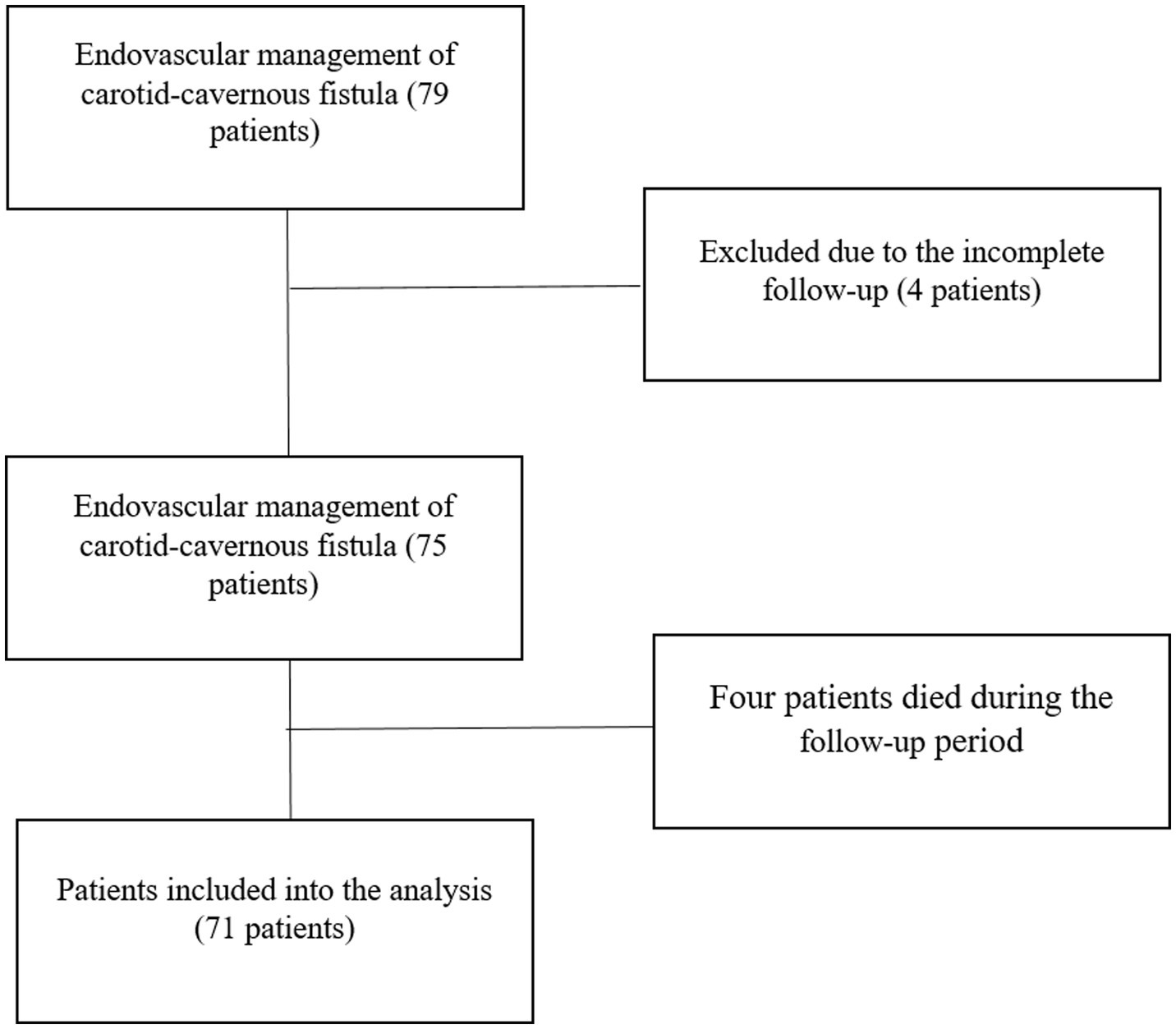
A retrospective review was conducted on all patients diagnosed with CCFs who underwent one or more endovascular treatment sessions at the National Center for Neurosurgery (Astana, Kazakhstan) between August 2008 and December 2024. A total of 79 patients who underwent embolization were initially identified for inclusion. Four patients were lost to follow-up due to unavailable follow-up MRI, and four had died by the time of data collection. Although the exact causes of death were not clearly defined, 3 of the 4 deceased patients were over the age of 60. Consequently, 71 patients were included in the final analysis (Figure 1).
Data collected for each patient included demographic variables (age and sex), type of embolic materials employed (coils, cyanoacrylate glue, stents, or combinations thereof), endovascular approach (transarterial or transvenous), clinical presentation, and perioperative and postoperative complications. Clinical presentation was categorized into four groups based on symptomatology:
Ocular manifestations: vision loss, ocular pain, glaucoma, chemosis, exophthalmos.
Cavernous manifestations: ophthalmoplegia, diplopia, ptosis, anisocoria.
Cortical manifestations: intracranial hemorrhage, seizures, focal neurological deficits.
Petrosal manifestations: tinnitus.
CCFs were diagnosed using magnetic resonance imaging (MRI) and digital subtraction angiography (DSA) with a biplane system (Artis Zee Biplane; Siemens, Erlangen, Germany). A multidisciplinary team of neuroradiologists and neurosurgeons reviewed the images and classified CCFs according to the Barrow classification:
Type A: Direct fistulas.
Types B, C, and D: indirect fistulas.
Additionally, fistulas were categorized based on etiology (traumatic or spontaneous) and hemodynamic properties (high-flow or low-flow), determined through angiographic evaluation. The updated classification system proposed by Thomas et al. was also applied, incorporating venous drainage patterns and their correlation with clinical symptoms, treatment planning, and outcomes. Additionally, contralateral cavernous sinus filling was assessed.
2.2 Treatment
All patients underwent endovascular treatment, which involved transarterial or transvenous embolization of the CCF or occlusion of the ICA. The choice of treatment approach was selected by the complexity of the fistula and the patient’s clinical presentation. Interventions were performed as either single-stage or multistep procedures.
The most performed procedure was CCF occlusion via the ICA (Figure 2). Additional techniques included combined occlusion of the CCF and ICA (Figure 3), flow diverter-assisted CCF occlusion, and CCF occlusion via the superior ophthalmic vein (SOV) approach and the inferior petrosal sinus (IPS) approach.
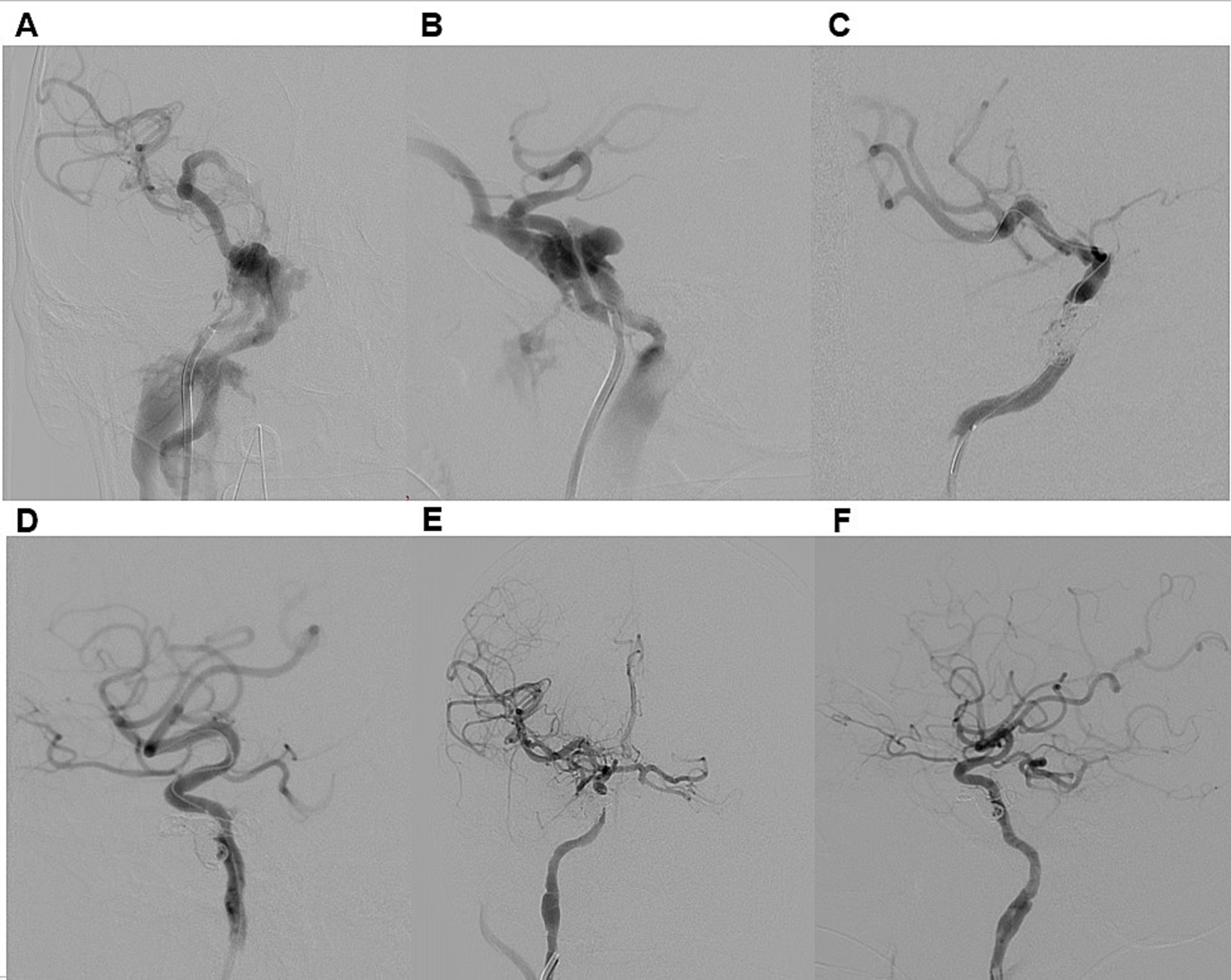
Figure 2. Illustrative case 1. A 34-year-old male presented with a six-month history of persistent pulsatile tinnitus, progressive proptosis, and conjunctival injection in the right eye, accompanied by visual impairment in the same eye after the motorcycle accident. DSA (A,B) confirmed a direct dural carotid-cavernous fistula on the right side, classified as Type A according to Barrow et al. and Type 5 according to the Thomas classification system. Venous drainage was retrograde through an enlarged right superior ophthalmic vein, the pterygoid venous plexus, and the right inferior petrosal sinus. Distal ICA flow was reduced, though both the anterior and posterior communicating artery were patent. The patient underwent transarterial embolization of CCF (C,D). A 6F guiding catheter was introduced, and a HyperGlide 4 × 10 mm balloon catheter was positioned in the cavernous segment of the right ICA for protection. Superselective catheterization of the fistula was performed using an Echelon 10 microcatheter, which was advanced into the fistula. Embolization was achieved with detachable coils. Post-procedure DSA confirmed complete obliteration of the CCF (E,F). At discharge, chemosis had regressed, and at the six-month follow-up, both exophthalmos and tinnitus had resolved.
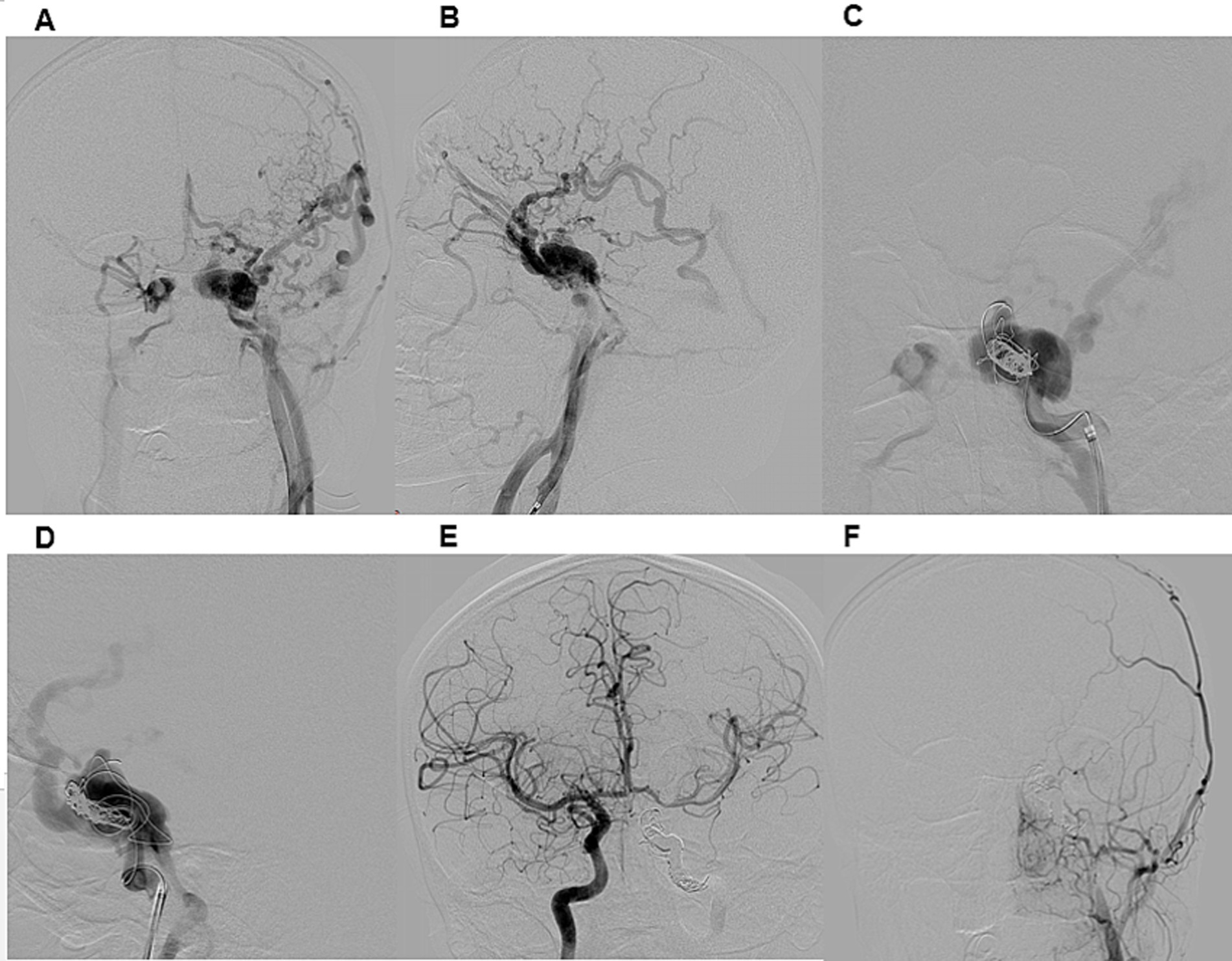
Figure 3. Illustrative case 2. A 21-year-old male presented with a seven-month history of conjunctival injection in the left eye, exophthalmos, and strabismus following a car accident. DSA (A,B) confirmed a direct CCF on the left side, classified as Type A according to Barrow et al. and Type 5 according to the Thomas classification system. Venous drainage was retrograde through an enlarged left superior ophthalmic vein, deep and superficial cortical veins, and the intracavernous sinus. Distal ICA flow was reduced, while both the anterior and posterior communicating artery were patent. A right ICA angiogram with compression (Matas maneuver) of the left CCA demonstrated sufficient collateral flow through the anterior communicating artery. The patient underwent transarterial embolization of the CCF and left ICA occlusion under general anesthesia (C,D). A 6F guiding catheter was introduced, and a Headway 17 microcatheter was advanced into the CCF. Embolization of both the CCF and ICA was performed using detachable coils. Post-procedural DSA confirmed complete occlusion of the left ICA and CCF (E,F). At discharge, chemosis had regressed, and at the three-month follow-up, exophthalmos and sixth nerve palsy had also resolved.
We assessed both the immediate and long-term efficacy and safety of various endovascular techniques and embolic materials.
2.3 Follow-up
The primary goal of CCF treatment was to occlude the pathological connections between the carotid artery and its branches and the cavernous sinus while preserving ICA patency. This aimed to reduce the risk of hemorrhage and improve clinical symptoms.
Clinical follow-up was conducted using a standardized 12-question survey administered via telephone interviews. Patients were assessed for: Ocular manifestations: persistence or resolution of vision loss, ocular pain, chemosis, and exophthalmos; Cavernous manifestations: Function of eye movement nerves (presence or absence of diplopia); Cortical manifestations: Occurrence of intracranial hemorrhage, seizures, or focal neurological deficits; Petrosal manifestations: Presence of tinnitus.
Ocular and cavernous sinus symptoms were evaluated by a neuro-ophthalmologist and neurosurgeons during follow-up visits, using comprehensive clinical examinations. Clinical outcomes were classified as follows: Positive outcome: Complete resolution of all symptoms; Moderate improvement: Partial resolution, which includes vision impairment; Negative outcome: No significant improvement in symptoms or the development of additional symptoms, including stroke.
Radiological outcomes were assessed by neurosurgeons and neuroradiologists. Complete obliteration of the CCF was defined as the absence of fistula filling on follow-up angiography performed after embolization. Long-term outcomes were evaluated based on follow-up radiological and clinical assessments.
In cases where recanalization was suspected on MRI during follow-up, diagnostic angiography was performed to confirm the findings. If recanalization was confirmed, a repeat endovascular procedure was carried out.
2.4 Statistical analysis
All statistical analyses were conducted using Stata version 18 MP (StataCorp LLC, College Station, TX, USA). Descriptive statistics were used to summarize demographic and clinical characteristics. Continuous variables were reported as means with standard deviations (mean ± SD), while categorical variables were presented as frequencies and percentages (N, %).
The Shapiro–Wilk test was applied to assess the normality of continuous variables. For comparisons across the three clinical outcome groups (“No improvement,” “Partial resolution,” “Resolution”), the Kruskal-Wallis test was used for non-normally distributed continuous variables, and Chi-square or Fisher’s exact test was used for categorical variables, as appropriate. Fisher’s exact test was applied when expected cell counts were <5.
3 Results
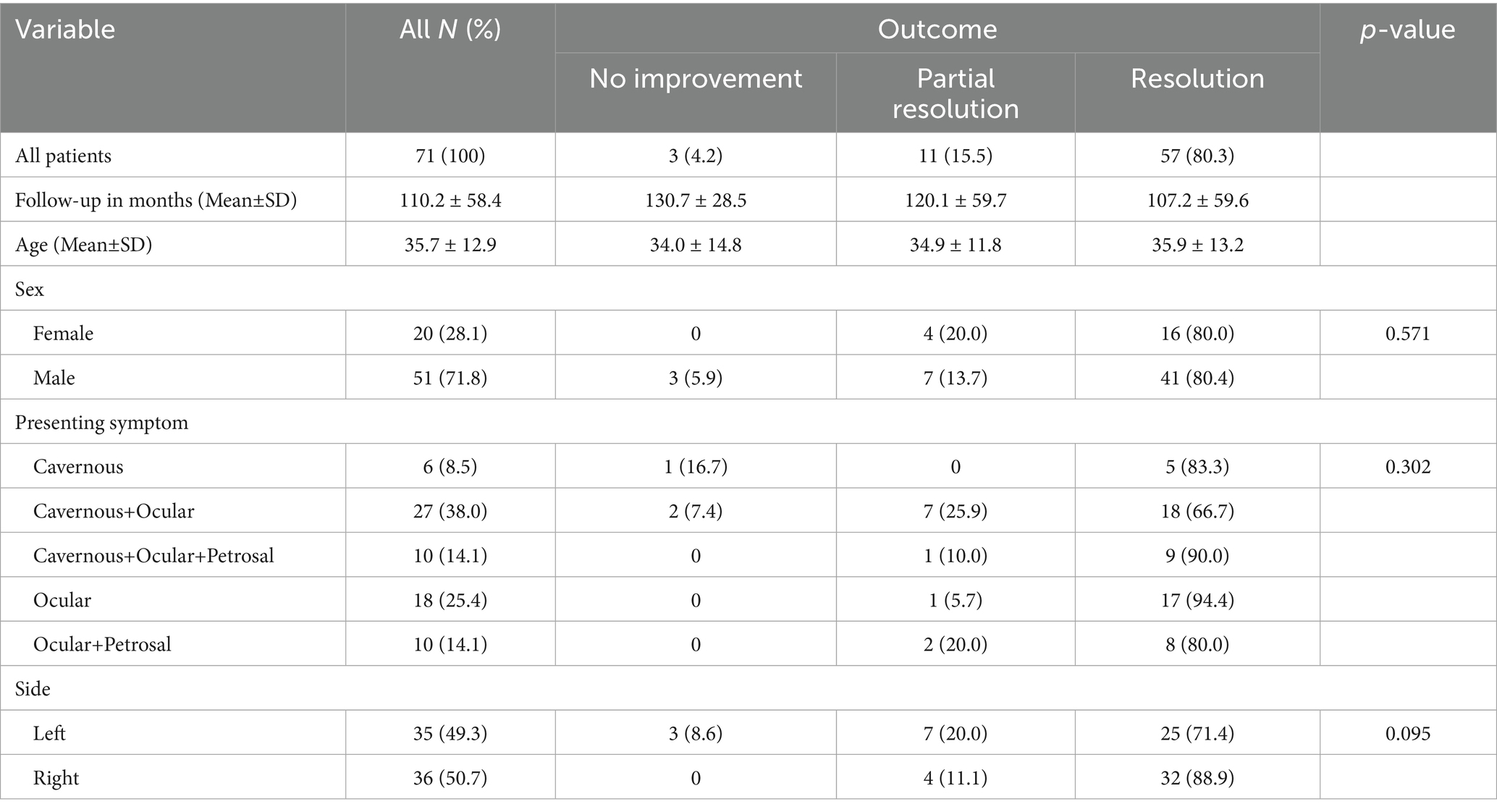
A total of 71 patients were included in the study (Table 1). The mean age was 35.7 ± 12.9 years, with no significant age difference across outcome groups (p = 0.97). Most patients were male (71.8%), and sex distribution did not differ significantly by outcome (p = 0.57). Presenting symptoms varied, with cavernous+ocular (38.0%) and ocular (25.4%) patterns being most frequent.
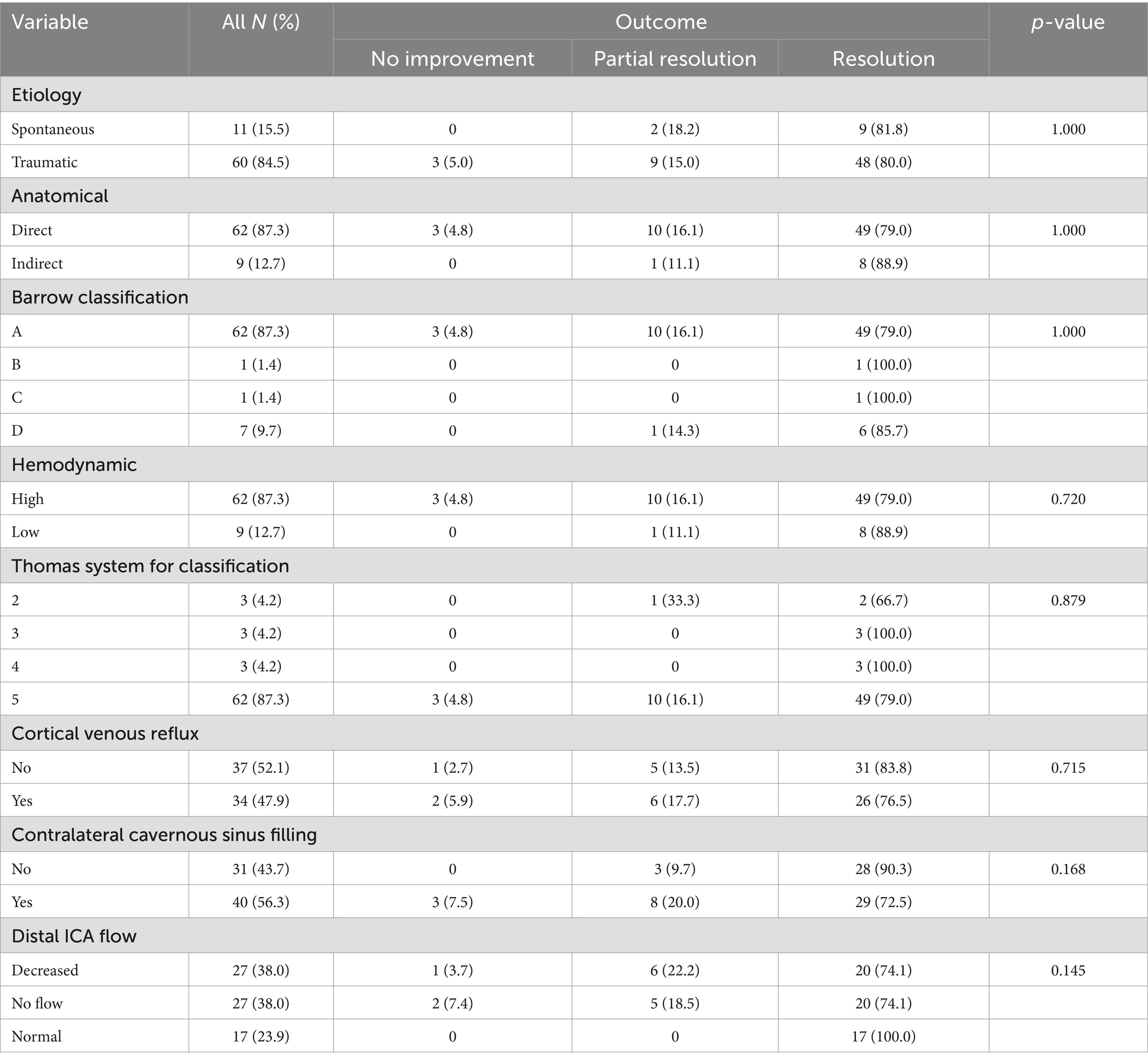
Out of 71 patients, most CCFs were traumatic in origin (60 cases, 84.5%), while 11 cases (15.5%) occurred spontaneously. Direct fistulas were seen in 62 patients (87.3%). According to the Barrow classification, type A was identified in 62 patients (87.3%), type D in 7 patients (9.9%), type B in 1 patient (1.4%), and type C in 1 patient (1.4%). Anatomical characteristics presented in the Table 2.
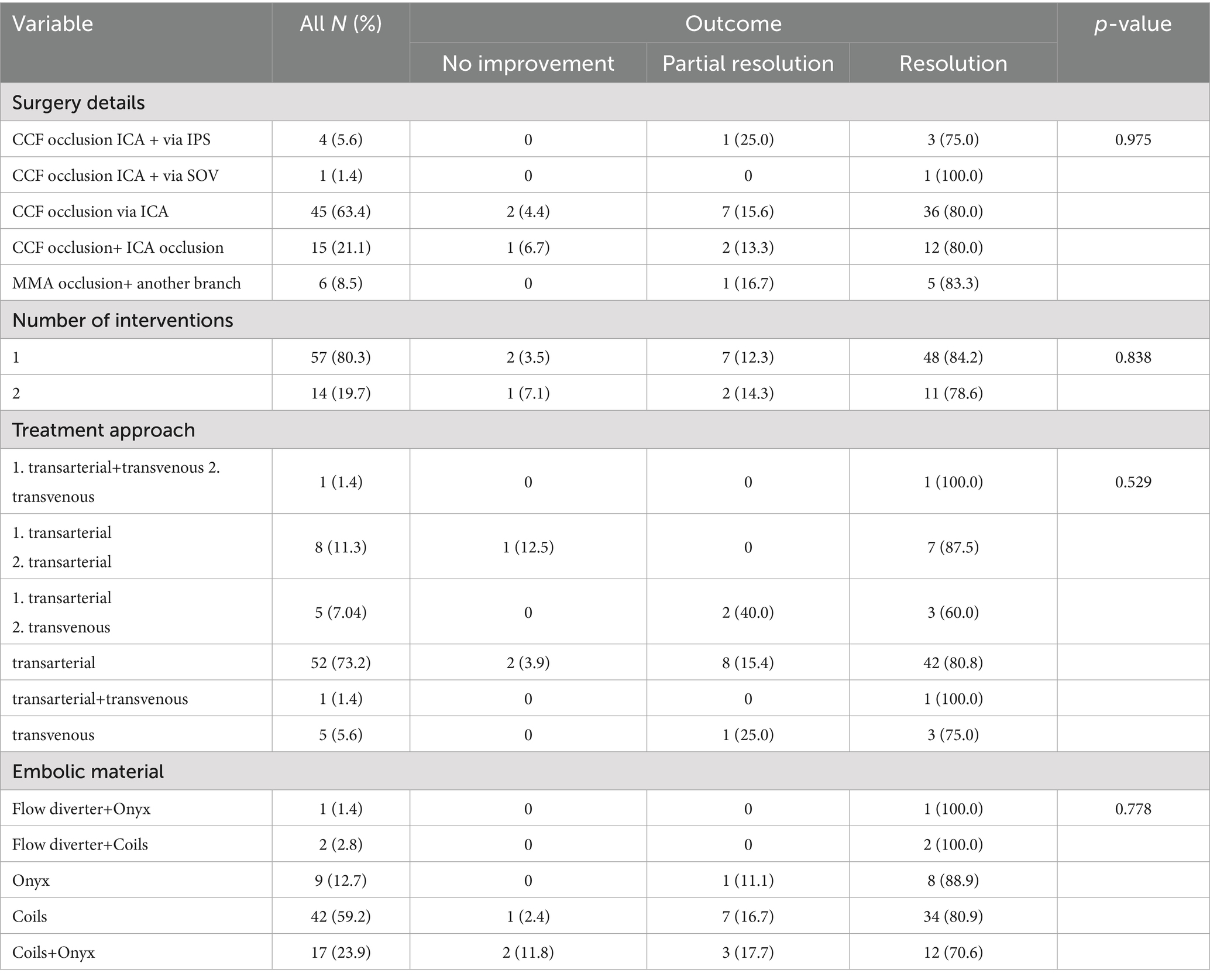
The most frequently performed surgical procedure was carotid-cavernous fistula occlusion via the internal carotid artery, accounting for 59.2% of cases (Table 3). Additional techniques included combined CCF and ICA occlusion (16.9%), CCF occlusion via the superior ophthalmic vein approach (7.0%), inferior petrosal sinus approach (4.2%), flow diverter-assisted CCF occlusion (4.2%), and middle meningeal artery occlusion (8.4%). We evaluated both the immediate and long-term efficacy and safety of different endovascular approaches (Supplementary Table 1) and embolic materials (Supplementary Table 2).
Out of 71 patients, 57 (80.3%) underwent a single intervention, while 14 (19.7%) required two interventions, indicating the necessity for staged interventions in complex cases. Among those who had one intervention, 84.2% achieved complete resolution, 12.3% had partial resolution, and 3.5% showed no improvement. Similarly, among patients with two interventions, 78.6% achieved complete resolution, 14.3% had partial resolution, and 7.1% showed no improvement.
Imaging follow-up was available for all 71 patients. Complete occlusion was confirmed in 65 of 71 patients (91.5%) on MRI performed at the 6-month follow-up. The remaining 6 patients (8.5%) showed signs of persistent CCF filling on MRI and subsequently underwent digital subtraction DSA for further evaluation (Table 4). The timing of MRI follow-up was uniformly 6 months for all patients. Clinical follow-up was available for a mean duration of 110.18 months (range: 6–193 months).
Clinical outcomes at discharge showed complete resolution in 20 patients (28.2%), partial resolution in 41 (57.7%), and no change in 10 (14.1%). Notably, all patients with complete resolution had no signs of recanalization at follow-up MRI. At the 6-month follow-up, clinical outcomes had improved, with complete resolution in 57 patients (80.3%), partial resolution in 11 (15.5%), and unchanged status in 3 (4.2%).
No significant associations were found between presenting symptoms, side of lesion, etiology, anatomical type, or Barrow classification and clinical outcome. Similarly, no associations were observed for hemodynamic profile, Thomas classification, cortical venous reflux, or contralateral cavernous sinus filling.
Among treatment-related variables, the occlusion status after the first surgery showed a strong and statistically significant association with clinical outcome (p < 0.001), with 96% of patients with complete occlusion achieving full resolution. Other factors such as distal ICA flow (p = 0.145), number of interventions (p = 0.838), treatment approach (p = 0.529), embolic material used (p = 0.778) were not significantly associated with clinical outcomes.
Periprocedural complications were observed in 3 cases (4.2%), which included: Coil migration with Solitaire extraction (1.4%), Petrosal ICA dissection (1.4%), and Onyx migration into the middle cerebral artery with Solitaire thromboextraction (1.4%). Despite these complications, no mortality was recorded. One case showed significant temporary worsening of cavernous symptoms (increased chemosis and exophthalmos) in a patient with an indirect CCF (Barrow type D) treated with transvenous embolization using a combination of coils and liquid embolic agents via the superior ophthalmic vein. The clinical worsening occurred on the third day post-procedure, and on the same day, a second transvenous coil embolization via the inferior petrosal sinus was performed, leading to symptom improvement.
4 Discussion
In this retrospective, single-center cross-sectional study, we assessed the periprocedural characteristics of endovascular treatment for CCFs, with particular focus on clinical outcomes. According to existing literature, spontaneous closure of direct high-flow CCFs is exceedingly rare, with an estimated incidence of approximately 0.05% of cases (11, 12). Consequently, active therapeutic intervention is generally warranted to prevent serious complications, including progressive visual loss and intracerebral hemorrhage (13, 14).
The decision to treat a dural CCF requires a careful assessment of the risks and benefits, balancing the severity of symptoms against the potential complications of intervention. The initial endovascular strategy is primarily determined by a detailed angiographic analysis of the fistula, particularly the arterial feeders and venous drainage routes (15). This evaluation often involves selective catheterization of the internal and external carotid arteries, along with a meticulous examination of the venous phase, to achieve a comprehensive understanding of the lesion’s angioarchitecture.
Historically, management options for direct CCFs were limited to either conservative observation or surgical trapping, such as ligation of the cervical or intracranial segments of the ICA or occlusion of the CCA (16). These approaches were associated with considerable risk, particularly ischemic complications resulting from compromised cerebral perfusion (17). The evolution of endovascular techniques has markedly transformed the management of CCFs. Open surgical procedures are now rarely indicated and are primarily employed as adjunctive strategies in combination with endovascular interventions, for instance, creating access routes via the transorbital approach (18, 19).
The choice of an optimal treatment strategy depends on several important factors, including the arterial supply, venous drainage pattern, flow dynamics of the fistula, and the patency of the circle of Willis (20). Among the various techniques available, the transarterial approach via the ICA remains the most frequently used (10). Over time, transarterial embolization with detachable platinum coils has proven to be highly effective, achieving both complete angiographic occlusion and favorable clinical outcomes (21). Consequently, it is now regarded as the first-line treatment for direct CCFs, offering a versatile and reliable therapeutic option (22). In most cases, balloon-assisted coiling of the cavernous sinus is routinely used to improve precision and control during coil deployment (23). Coils offer several advantages, including adaptability to different anatomical configurations and the ability to be repositioned during deployment if initial placement is suboptimal (24). However, challenges remain, such as the risk of coil migration and incomplete embolization, especially in cases with compartmentalized cavernous sinuses that require dense packing (25, 26).
In our cohort, coils were the sole embolic agent in 42 patients, predominantly those with traumatic CCFs. In 17 additional cases, coils were combined with Onyx to improve penetration and packing density. The rationale for this combined approach was based on the fistula’s complex morphology and evidence suggesting enhanced occlusion rates (27, 28). In our series, only one case of coil migration was observed. The dislocated coil was successfully retrieved using a stent retriever, and the patient experienced no neurological deterioration following the complication.
Several studies have reported favorable outcomes with the use of covered or flow-diverting stents for the treatment of CCFs via the internal carotid artery, often demonstrating high success rates (29–31). However, these procedures remain technically demanding, particularly in high-flow fistulas, where overlapping arterial and venous phases can hinder accurate navigation and precise stent deployment.
A major concern associated with covered stents is the risk of complications, including endoleaks, acute or delayed in-stent thrombosis, and in-stent stenosis (4, 32). While acute thrombosis is relatively rare, it has been documented in patients undergoing treatment for direct CCFs. Another important limitation is the high cost of covered stents, which substantially increases the overall cost of the procedure (17). This financial burden, combined with the technical challenges and risk of serious complications, limits the routine use of covered or flow-diverting stents in clinical practice (21). In our study, this approach was applied in only three patients, where flow-diverter stents were used in combination with coils and Onyx, depending on the individual angioarchitecture, without any complications.
Onyx embolization for CCFs was first introduced in 2004 (33), and subsequent studies have demonstrated excellent clinical and angiographic outcomes (34, 35). In our experience, Onyx was used as the sole embolic agent in nine patients with low-flow fistulas, while it was combined with detachable coils in 17 cases and with flow diversion in one case. Onyx offers several advantages as a liquid embolic agent: its nonadhesive nature minimizes the risk of microcatheter retention, allowing for prolonged injections and deeper penetration into the cavernous sinus, draining veins, and distal arterial feeders, areas that may be difficult to access with conventional coil embolization (36). Additionally, its controlled precipitation enables intermittent injections with periodic angiographic assessment for precise fistula occlusion (37). In our series, only one complication was observed, which is a migration of Onyx into the middle cerebral artery. The embolic material was successfully retrieved using a Solitaire stent retriever, and the patient experienced no neurological deterioration following the event.
Although transvenous embolization is commonly favored for indirect CCFs due to the relatively straightforward venous anatomy and high occlusion rates up to 94% in some studies (38), it carries a notable risk of complications, which may vary by center and reach up to 20% (39–41). Reported adverse events include intracerebral hemorrhage, ischemic stroke, cranial nerve palsies, trigeminal neuropathy, brainstem infarction, elevated intraocular pressure, and orbital hemorrhage, particularly when access is obtained via the superior or inferior ophthalmic veins (41). Rare complications have also been described, such as the syndrome of inappropriate antidiuretic hormone secretion (SIADH) (42). Additionally, bradycardia and asystole during Onyx embolization of CCFs have been documented (43), possibly due to direct stimulation of the trigeminal nerve, triggering a vagal response via the trigeminocardiac reflex during the transvenous approach.
Despite its effectiveness, the risk profile associated with transvenous embolization has led our center to favor the transarterial approach as the first-line treatment in most cases, particularly when the vascular anatomy permits safe arterial access. In patients with high-flow shunts or complex venous drainage patterns, a combined transarterial and transvenous embolization strategy may be required to achieve complete and durable occlusion (26). In our series, seven patients required this combined approach. One patient underwent a simultaneous procedure via the transfemoral artery and the inferior petrosal sinus, while six patients were treated in a staged manner, with transarterial embolization performed first, followed by transvenous embolization. Notably, no intraoperative complications, including those previously described, were observed in these cases.
ICA sacrifice in combination with CCF occlusion is a viable treatment option, particularly for patients with direct-type CCFs who demonstrate adequate collateral circulation during balloon test occlusion (BTO). During BTO, arterial pressure is typically reduced by approximately 20% to evaluate the adequacy of cerebral perfusion via the circle of Willis.
ICA sacrifice is generally reserved for cases involving complete ICA transection or a large fistulous defect that precludes successful cavernous sinus occlusion while preserving the parent artery. The need for ICA sacrifice should be considered in all patients with direct CCFs. A suspected ICA transection is typically indicated by failure to visualize the distal ICA on initial angiography; however, this finding may also result from high-flow shunting through the fistula (44). Persistent non-visualization of the distal ICA following partial coiling of the cavernous sinus strongly supports the diagnosis of complete transection, warranting occlusion of both the ICA and the cavernous sinus (45, 46).
In our series, the strategy of identifying ICA transection and proceeding with combined ICA and cavernous sinus occlusion was employed in 15 patients. Although the underlying indications varied, early evaluation of ICA integrity provided the flexibility to adapt the treatment plan accordingly.
At our institution, all vascular neurosurgeons are dual-trained and capable of performing emergency superficial temporal artery to middle cerebral artery (STA-MCA) bypasses. This surgical expertise is critical in cases where a false-negative BTO could lead to ischemic complications. Consequently, ICA sacrifice is a treatment option best suited for centers equipped with neurosurgeons experienced in microsurgical revascularization techniques.
According to the literature, endovascular treatment of carotid-cavernous fistulas (CCFs), irrespective of the specific technique or vascular access route employed, results in clinical improvement in approximately 60 to 95% of cases (17, 38, 47, 48). Ophthalmic symptoms such as proptosis, chemosis, and pulsatile tinnitus often resolve rapidly following embolization. In contrast, cranial nerve palsies and visual deficits tend to exhibit a more protracted course of recovery, frequently requiring several months to achieve full resolution (49).
In our cohort, among patients who achieved complete occlusion following the initial endovascular intervention, 96% experienced complete clinical resolution, while 4% demonstrated partial improvement. These findings suggest that early therapeutic intervention may play a critical role in the recovery of ocular symptoms and visual function. This hypothesis is further supported by a recent systematic review and meta-analysis conducted by Ali Al-Shalchy, which underscored the significance of timely treatment in optimizing visual outcomes in patients with CCF (50).
In total, complete symptom resolution was achieved in 57 patients (80.3%), while 11 patients (15.5%) demonstrated partial clinical improvement. These outcomes are consistent with findings from prior systematic reviews and meta-analyses, which have shown that clinical improvement following endovascular treatment of carotid-cavernous fistulas is not significantly influenced by the choice of embolic material, vascular access route, or the underlying anatomical or etiological classification (10, 51). Similarly, our data indicate that complete clinical recovery was strongly correlated with successful angiographic obliteration of the fistula during the initial intervention (20).
Importantly, no cases of procedure-related mortality or stroke were observed during the follow-up period, underscoring the safety and efficacy of endovascular treatment in this patient population.
Although this study provides important insights into the endovascular treatment of CCFs, there are a few limitations to consider. First, its retrospective, single-center design may limit the generalizability of the findings. Second, the 16-year study period spans significant advancements in endovascular techniques, embolic materials, and operator experience, which may have introduced heterogeneity in treatment approaches and outcomes, complicating direct comparisons. Finally, a major limitation of our study is the limited availability of long-term DSA follow-up. This may have led to underdetection of small residual or recurrent fistulas, which could contribute to late recurrences. Future prospective, multicenter studies with standardized outcome measures are needed to validate these results and optimize treatment strategies.
5 Conclusion
Endovascular therapy is the treatment of choice for carotid-cavernous fistulas, offering high occlusion rates with a low risk of complications. Both transarterial and transvenous approaches demonstrate comparable efficacy, with no significant differences in clinical outcomes. These findings support the use of endovascular embolization as the first-line therapeutic strategy, with treatment individualized according to each patient’s vascular anatomy. Achieving complete occlusion significantly reduces the risk of serious complications, including visual impairment and ischemic events. Moreover, early intervention appears to play a key role in optimizing clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the National Center for Neurosurgery (Approval number: 1/4.04.2025). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CN: Methodology, Conceptualization, Software, Writing – original draft, Formal analysis. IM: Conceptualization, Writing – original draft, Data curation, Visualization. KM: Software, Writing – original draft, Formal analysis, Methodology. YK: Investigation, Writing – original draft, Data curation. RD: Data curation, Investigation, Writing – original draft. DK: Data curation, Writing – review & editing. SA: Project administration, Validation, Writing – review & editing. YM: Validation, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1625899/full#supplementary-material
References
1. Ringer, AJ, Salud, L, and Tomsick, TA. Carotid cavernous fistulas: anatomy, classification, and treatment. Neurosurg Clin N Am. (2005) 16:279–95. doi: 10.1016/j.nec.2004.08.004
2. Barrow, DL, Spector, RH, Braun, IF, Landman, JA, Tindall, SC, and Tindall, GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. (1985) 62:248–56. doi: 10.3171/jns.1985.62.2.0248
3. Alam, MS, Jain, M, Mukherjee, B, Sharma, T, Halbe, S, Jaisankar, D, et al. Visual impairment in high flow and low flow carotid cavernous fistula. Sci Rep. (2019) 9:12872. doi: 10.1038/s41598-019-49342-3
4. Ellis, JA, Goldstein, H, Connolly, ES, and Meyers, PM. Carotid-cavernous fistulas. Neurosurg Focus. (2012) 32:E9. doi: 10.3171/2012.2.FOCUS1223
5. Travers, B. A case of aneurism by anastomosis in the orbit, cured by the ligature of the common carotid artery. Med Chir Trans. (1811) 2:1–420.1.1. doi: 10.1177/095952871100200101
6. Serbinenko, FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. (1974) 41:125–45. doi: 10.3171/jns.1974.41.2.0125
7. Pashapour, A, Mohammadian, R, Salehpour, F, Sharifipour, E, Mansourizade, R, Mahdavifard, A, et al. Long-term endovascular treatment outcome of 46 patients with cavernous sinus Dural arteriovenous fistulas presenting with ophthalmic symptoms. A non-controlled trial with clinical and angiographic follow-up. Neuroradiol J. (2014) 27:461–70. doi: 10.15274/NRJ-2014-10079
8. Luo, CB, Teng, MMH, Chang, FC, and Chang, CY. Transarterial balloon-assisted n-butyl-2-cyanoacrylate embolization of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. (2006) 27:1535–40.
9. Gomez, F, Escobar, W, Gomez, AM, Gomez, JF, and Anaya, CA. Treatment of carotid cavernous fistulas using covered stents: midterm results in seven patients. AJNR Am J Neuroradiol. (2007) 28:1762–8. doi: 10.3174/ajnr.A0636
10. Texakalidis, P, Tzoumas, A, Xenos, D, Rivet, DJ, and Reavey-Cantwell, J. Carotid cavernous fistula (CCF) treatment approaches: a systematic literature review and meta-analysis of transarterial and transvenous embolization for direct and indirect CCFs. Clin Neurol Neurosurg. (2021) 204:106601. doi: 10.1016/j.clineuro.2021.106601
11. Kawaguchi, S, Sakaki, T, Morimoto, T, Hoshida, T, and Nakase, H. Surgery for dural arteriovenous fistula in superior sagittal sinus and transverse sigmoid sinus. J Clin Neurosci. (2000) 7:47–9. doi: 10.1054/jocn.2000.0711
12. Ahmed, AZ, Nassef, A, and Assad, RE. Endovascular treatment of carotid cavernous fistulae (CCF). Direct venous puncture using road mapping in dural CCF. Egypt J Radiol Nucl Med. (2013) 44:245–51. doi: 10.1016/j.ejrnm.2012.12.004
13. Alexander, MD, Halbach, VV, Hallam, DK, Cooke, DL, Ghodke, BV, Dowd, CF, et al. Long-term outcomes of endovascular treatment of indirect carotid cavernous fistulae: superior efficacy, safety, and durability of Transvenous coiling over other techniques. Neurosurgery. (2019) 85:E94–E100. doi: 10.1093/neuros/nyy486
14. Miyamoto, N, Naito, I, Takatama, S, Shimizu, T, Iwai, T, and Shimaguchi, H. Clinical and angiographic characteristics of cavernous sinus dural arteriovenous fistulas manifesting as venous infarction and/or intracranial hemorrhage. Neuroradiology. (2009) 51:53–60. doi: 10.1007/s00234-008-0468-5
15. Sur, S, Menaker, SA, Alvarez, C, Chen, S, Shah, SS, Peterson, EC, et al. Multimodal Management of Carotid-Cavernous Fistulas. World Neurosurg. (2020) 133:e796–803. doi: 10.1016/j.wneu.2019.10.004
16. Lang, M, Habboub, G, Mullin, JP, and Rasmussen, PA. A brief history of carotid-cavernous fistula. J Neurosurg. (2017) 126:1995–2001. doi: 10.3171/2016.5.JNS152372
17. Henderson, AD, and Miller, NR. Carotid-cavernous fistula: current concepts in aetiology, investigation, and management. Eye. (2018) 32:164–72. doi: 10.1038/eye.2017.240
18. Chen, CJ, Mastorakos, P, Caruso, JP, Ding, D, Schmitt, PJ, Buell, TJ, et al. Transorbital approach for endovascular occlusion of carotid-cavernous fistulas: technical note and review of the literature. Cureus. (2017) 9:e976. doi: 10.7759/cureus.976
19. Brenna, CTA, Priola, SM, Pasarikovski, CR, Ku, JC, Daigle, P, Gill, HS, et al. Surgical sparing and pairing endovascular interventions for carotid-cavernous fistula: case series and review of the literature. World Neurosurg. (2020) 140:18–25. doi: 10.1016/j.wneu.2020.05.013
20. Baharvahdat, H, Seraj, FQM, Al-Raaisi, A, Blanc, R, Najafi, S, Mirbolouk, MH, et al. Long-term outcome of endovascular treatment for indirect carotid-cavernous fistulas. Neurosurg Focus. (2024) 56:E5. doi: 10.3171/2023.12.FOCUS23795
21. Gemmete, JJ, Ansari, SA, and Gandhi, DM. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuroophthalmol. (2009) 29:62–71. doi: 10.1097/WNO.0b013e3181989fc0
22. Ide, S, Kiyosue, H, Tokuyama, K, Hori, Y, Sagara, Y, and Kubo, T. Direct carotid cavernous fistulas. J Neuroendovasc Ther. (2020) 14:583–92. doi: 10.5797/jnet.ra.2020-0131
23. Andrade, G, Ponte De Souza, ML, Marques, R, Silva, JL, Abath, C, and Azevedo-Filho, HRC. Endovascular treatment of traumatic carotid cavernous fistula with balloon-assisted sinus coiling. A technical description and initial results. Interv Neuroradiol. (2013) 19:445–54. doi: 10.1177/159101991301900407
24. Rahmatian, A, Yaghoobpoor, S, Tavasol, A, Aghazadeh-Habashi, K, Hasanabadi, Z, Bidares, M, et al. Clinical efficacy of endovascular treatment approach in patients with carotid cavernous fistula: a systematic review and meta-analysis. World Neurosurg X. (2023) 19:100189. doi: 10.1016/j.wnsx.2023.100189
25. Lee, SH, Park, H, Lee, K, Hwang, SH, Lee, CH, Kang, DH, et al. Venous outflow-targeted coil embolization of direct carotid-cavernous fistulas. Interv Neuroradiol. (2023) 29:251–9. doi: 10.1177/15910199221084787
26. Fukawa, N, Nakagawa, N, Tsuji, K, Yoshioka, H, Furukawa, K, Nagatsuka, K, et al. Transarterial and Transvenous coil embolization of direct carotid-cavernous fistulas. J Neuroendovasc Ther. (2022) 16:127–34. doi: 10.5797/jnet.tn.2020-0115
27. Zaidat, OO, Lazzaro, MA, Niu, T, Hong, SH, Fitzsimmons, BF, Lynch, JR, et al. Multimodal endovascular therapy of traumatic and spontaneous carotid cavernous fistula using coils, n-BCA, Onyx and stent graft. J Neurointerv Surg. (2011) 3:255–62. doi: 10.1136/jnis.2010.003103
28. Ramalingaiah, AH, Prasad, C, Sabharwal, PS, Saini, J, and Pandey, P. Transarterial treatment of direct carotico-cavernous fistulas with coils and Onyx. Neuroradiology. (2013) 55:1213–20. doi: 10.1007/s00234-013-1224-z
29. Baranoski, JF, Ducruet, A, Przbylowski, CJ, Almefty, RO, Ding, D, Catapano, JS, et al. Flow diverters as a scaffold for treating direct carotid cavernous fistulas. J Neurointerv Surg. (2019) 11:1129–34. doi: 10.1136/neurintsurg-2019-014731
30. Stamatopoulos, T, Anagnostou, E, Plakas, S, Papachristou, K, Lagos, P, Samelis, A, et al. Treatment of carotid cavernous sinus fistulas with flow diverters. A case report and systematic review. Interv Neuroradiol. (2022) 28:70–83. doi: 10.1177/15910199211014701
31. Tiewei, Q, Ali, A, Shaolei, G, Feng, L, Zhongsong, S, Xuesong, L, et al. Carotid cavernous fistulas treated by endovascular covered stent grafts with follow-up results. Br J Neurosurg. (2010) 24:435–40. doi: 10.3109/02688697.2010.487127
32. Nurimanov, C, Makhambetov, Y, Kaliyev, A, and Akshulakov, S. A case report of multi-step management of extracranial carotid artery aneurysm and carotid-cavernous fistula combination in patients. Front Neurol. (2023) 14:1120786. doi: 10.3389/fneur.2023.1120786
33. Arat, A, Cekirge, S, Saatci, I, and Ozgen, B. Transvenous injection of Onyx for casting of the cavernous sinus for the treatment of a carotid-cavernous fistula. Neuroradiology. (2004) 46:1012–5. doi: 10.1007/s00234-004-1244-9
34. Barber, SM, Rangel-Castilla, L, Zhang, YJ, Klucznik, R, and Diaz, O. Mid- and long-term outcomes of carotid-cavernous fistula endovascular management with Onyx and n-BCA: experience of a single tertiary center. J Neurointerv Surg. (2015) 7:762–9. doi: 10.1136/neurintsurg-2014-011266
35. Al Saiegh, F, Baldassari, MP, Sweid, A, Bilyk, J, Mouchtouris, N, Hafazalla, K, et al. Onyx embolization of carotid-cavernous fistulas and its impact on intraocular pressure and recurrence: a case series. Oper Neurosurg. (2021) 20:174–82. doi: 10.1093/ons/opaa308
36. Zenteno, M, Santos-Franco, J, Rodríguez-Parra, V, Balderrama, J, Aburto-Murrieta, Y, Vega-Montesinos, S, et al. Management of direct carotid-cavernous sinus fistulas with the use of ethylene-vinyl alcohol (Onyx) only: preliminary results. J Neurosurg. (2010) 112:595–602. doi: 10.3171/2009.6.JNS09440
37. Elhammady, MS, Wolfe, SQ, Farhat, H, Moftakhar, R, and Aziz-Sultan, MA. Onyx embolization of carotid-cavernous fistulas. J Neurosurg. (2010) 112:589–94. doi: 10.3171/2009.6.JNS09132
38. El Naamani, K, Mouchtouris, N, Majmundar, S, Sah, E, Kaul, A, Sizdahkhani, S, et al. Comparison of the transarterial, transvenous, and superior ophthalmic vein approaches in the treatment of indirect carotid-cavernous fistulas. Neurosurg Focus. (2024) 56:E7. doi: 10.3171/2023.12.FOCUS23776
39. Yoshida, K, Melake, M, Oishi, H, Yamamoto, M, and Arai, H. Transvenous embolization of dural carotid cavernous fistulas: a series of 44 consecutive patients. AJNR Am J Neuroradiol. (2010) 31:651–5. doi: 10.3174/ajnr.A1882
40. Klisch, J, Huppertz, HJ, Spetzger, U, Hetzel, A, Seeger, W, and Schumacher, M. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery. (2003) 53:836–57. doi: 10.1227/01.NEU.0000083551.26295.AB
41. Kim, DJ, Kim, DI, Suh, SH, Kim, J, Lee, SK, Kim, EY, et al. Results of transvenous embolization of cavernous dural arteriovenous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol. (2006) 27:2078–82.
42. Chen, T, Kalani, MYS, Ducruet, AF, Albuquerque, FC, and McDougall, CG. Development of syndrome of inappropriate antidiuretic hormone secretion (SIADH) after Onyx embolisation of a cavernous carotid fistula. J Neurointerv Surg. (2017) 9:e3. doi: 10.1136/neurintsurg-2015-012104.rep
43. Amiridze, N, and Darwish, R. Hemodynamic instability during treatment of intracranial dural arteriovenous fistula and carotid cavernous fistula with Onyx: preliminary results and anesthesia considerations. J Neurointerv Surg. (2009) 1:146–50. doi: 10.1136/jnis.2009.000042
44. Hassan, T, Rashad, S, Aziz, W, Sultan, A, and Ibrahim, T. Endovascular modalities for the treatment of cavernous sinus arteriovenous fistulas: a single-center experience. J Stroke Cerebrovasc Dis. (2015) 24:2824–38. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.016
45. Coley, SC, Pandya, H, Hodgson, TJ, Jeffree, MA, and Deasy, NP. Endovascular trapping of traumatic carotid-cavernous fistulae. AJNR Am J Neuroradiol. (2003) 24:1785–8.
46. Dang, NV, Hai, NT, and Cuong, TC. Recurrent carotico-cavernous fistula after internal carotid artery ligation: a case with embolization of the fistula via contralateral internal carotid artery approach. Interv Neuroradiol. (2014) 20:482–6. doi: 10.15274/INR-2014-10021
47. Awad, A, Regenhardt, RW, Dmytriw, AA, Vranic, JE, Marciano, RD, Patel, AB, et al. Flow diversion in direct carotid-cavernous fistula refractory to multiple coil embolizations: case report and review of the literature. J Stroke Cerebrovasc Dis. (2022) 31:106840. doi: 10.1016/j.jstrokecerebrovasdis.2022.106840
48. Prasad, SN, Singh, V, Boruah, DK, Phadke, RV, Sharma, K, and Kannaujia, V. Endovascular Management of Direct Carotid-Cavernous Fistula: evolution of cost effective Sandwich technique. J Neurosci Rural Pract. (2020) 11:558–64. doi: 10.1055/s-0040-1714447
49. Tjoumakaris, SI, Jabbour, PM, and Rosenwasser, RH. Neuroendovascular management of carotid cavernous fistulae. Neurosurg Clin N Am. (2009) 20:447–52. doi: 10.1016/j.nec.2009.07.013
50. Al-shalchy, A, Al-Wassiti, AS, Hashim, MA, Al-Khazaali, YM, Talib, SH, Bani-Saad, AA, et al. Neuro-ophthalmic manifestations of carotid cavernous fistulas: a systematic review and Meta-analysis. Cureus. (2024) 16:e65821. doi: 10.7759/cureus.65821
Keywords: carotid-cavernous fistulas, endovascular treatment, transarterial approach, transvenous approach, long term results
Citation: Nurimanov C, Mammadinova I, Menlibayeva K, Kydyrmoldin Y, Duisengali R, Kerimbayeva D, Akshulakov S and Makhambetov Y (2025) Endovascular management of carotid-cavernous fistulas: a 16-year retrospective analysis of multimodal treatment strategies and long-term clinical outcomes. Front. Neurol. 16:1625899. doi: 10.3389/fneur.2025.1625899
Edited by:
Stephan Meckel, University of Freiburg Medical Center, GermanyReviewed by:
Qazi Zeeshan, University of Pittsburgh Medical Center, United StatesSitaram Barath, Geetanjali Medical College, India
Copyright © 2025 Nurimanov, Mammadinova, Menlibayeva, Kydyrmoldin, Duisengali, Kerimbayeva, Akshulakov and Makhambetov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iroda Mammadinova, aXJvZGFtYW1tYWRpbm92YUBnbWFpbC5jb20=
 Chingiz Nurimanov
Chingiz Nurimanov Iroda Mammadinova
Iroda Mammadinova Karashash Menlibayeva
Karashash Menlibayeva Yeldar Kydyrmoldin1
Yeldar Kydyrmoldin1 Yerbol Makhambetov
Yerbol Makhambetov