- 1Department of Neurology, Hulunbuir People's Hospital, Hulunbuir, China
- 2Department of Geriatrics, Yantai Affiliated Hospital of Binzhou Medical University, Yantai, China
- 3Department of Neurology, Minhang Hospital, Fudan University, Shanghai, China
Objective: This study aimed to evaluate the influence of gender on the prognostic value of CD4+ Treg cells in patients with acute ischemic stroke.
Methods: A prospective cohort study was conducted at Minhang Hospital, enrolling 225 patients with acute ischemic stroke. CD4+ Treg cell counts were measured by flow cytometry within 24 h of admission, and stroke prognosis was assessed at 3 months using the mRS. Univariate and multivariable logistic regression models were used to identify prognostic factors, and an interaction analysis was conducted to examine whether gender moderated the effect of Treg cell levels on outcomes.
Results: Multivariable analysis revealed that infarct volume (OR = 1.08, 95% CI: 1.03–1.13, p = 0.0028), NIHSS score (OR = 1.30, 95% CI: 1.17–1.45, p < 0.0001), and WBC count (OR = 1.32, 95%CI: 1.05–1.67, p = 0.0172) were independent predictors of stroke prognosis. Higher CD4+ Treg cell counts were significantly associated with better prognosis in male patients (OR = 0.995, 95% CI: 0.992–0.999, p = 0.008), but showed no significant association in female patients (OR = 0.999, 95%CI: 0.998–1.001, p = 0.826). The interaction analysis confirmed that gender significantly moderated the relationship between CD4+ Treg cell counts and stroke prognosis (p = 0.0198). Additionally, segmented regression analysis revealed a nonlinear association between Treg cell counts and stroke prognosis in male patients, with specific thresholds indicating variable effects on prognosis.
Conclusion: Gender plays a critical role in modulating the immunoregulatory effects of CD4+ Treg cells on stroke prognosis, with male patients deriving significant benefit from higher Treg cell counts.
1 Introduction
Stroke remains a leading cause of mortality and disability worldwide, with ischemic stroke accounting for the majority of cases (1). Despite advances in acute stroke treatments such as thrombolysis and mechanical thrombectomy, substantial heterogeneity persists in long-term outcomes, with immune responses playing a key role (2). Regulatory T cells (Tregs), particularly the CD4 + subset, play a pivotal role in suppressing inflammation and promoting neurorepair after stroke (3, 4). Immunomodulation mediated by CD4+ Treg cells can attenuate excessive immune activation and subsequent central nervous system (CNS) damage by inducing anti-inflammatory cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), thereby facilitating functional recovery (5–7).
Gender differences not only influence stroke incidence and prognosis, but also modulate immune responses through T cell activity (8). Treg cell function is influenced by sex hormones such as estrogen and testosterone, with accumulating evidence implicating their roles in post-stroke inflammation (9). In addition, emerging studies suggest that genetic polymorphisms (e.g., FOXP3), gut microbiota composition, and lifestyle factors (e.g., stress, physical activity) may serve as sex-specific modulators of Treg-mediated immune responses (10, 11). Furthermore, the immunological effects of sex hormones appear to vary before and after menopause in women, with estrogen-driven Treg expansion predominantly occurring in the premenopausal phase (12, 13). However, the specific influence of gender on CD4 + Treg cell function and its relationship with stroke prognosis remains poorly understood. This study aims to investigate whether patient gender influences the modulatory role of CD4 + Treg cells in stroke prognosis, thereby providing insights into personalized immunotherapeutic strategies for post-stroke recovery.
2 Methods
2.1 Study design
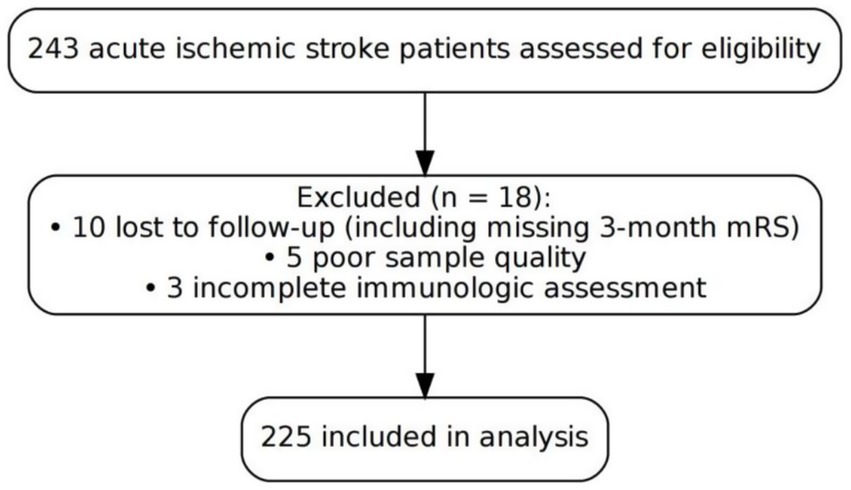
A single-center prospective cohort study was conducted at the Department of Neurology, Minhang Hospital, Fudan University, enrolling consecutive patients with acute ischemic stroke between January 2022 and December 2023. A total of 243 patients diagnosed with acute ischemic stroke were assessed for eligibility. Among them, 18 patients were excluded, including 10 lost to follow-up [e.g., missing 3-month modified Rankin Scale (mRS) data], 5 due to poor sample quality, and 3 due to incomplete immunologic assessment. A total of 225 patients were ultimately included in the analysis. All included participants met the eligibility criteria and completed both the 3-month follow-up and immunological testing (Figure 1).
The clinical study was approved by the ethical review board of Minhang Hospital, Fudan University, Shanghai, China. All procedures were conducted in accordance with the ethical standards of the Declaration of Helsinki. Written informed consent was obtained from all participants or their legally authorized representatives.
2.2 Participants in the study
Inclusion criteria: (1) Patients aged ≥18 years, diagnosed with acute ischemic stroke according to International Stroke Association guidelines, confirmed by imaging (MRI or CT) (14); (2) Time from symptom onset to hospital admission ≤24 h (3) Patients with complete baseline data including clinical history, immune markers, and infarct volume measurement. (4) Patients with a pre-stroke mRS score<2, to ensure that baseline functional independence was preserved prior to the index stroke event.
Exclusion criteria were as follows: (1) A history of ischemic or hemorrhagic stroke, traumatic brain injury, or other central nervous system diseases likely to cause permanent neurological deficits; (2) Severe organ failure (e.g., cardiac, hepatic, or renal) or active systemic infection; (3) Inability to provide informed consent or to complete baseline clinical and laboratory assessments; (4) Loss to follow-up or failure to complete 3-month mRS evaluation.
2.3 Data acquisition
2.3.1 Clinical information
Demographic data (age, gender, body mass index [BMI], stroke history, smoking, and alcohol use) and clinical data (stroke subtype based on the Trial of Org 10,172 in Acute Stroke Treatment [TOAST] classification, and the presence of cardiovascular comorbidities such as hypertension, diabetes mellitus, dyslipidemia, or atrial fibrillation) were collected. The time of stroke onset was also recorded. Infarct volume on T2-weighted MRI was measured in cubic centimeters (cm3) using 3D reconstruction software.
2.3.2 Laboratory examination
Immune markers: Peripheral venous blood (5 mL) was collected from patients within 24 h of admission using ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes. Mononuclear cells were isolated by Ficoll density gradient centrifugation. The cells were resuspended in phosphate-buffered saline (PBS) containing 2% fetal bovine serum and stained with the following fluorochrome-conjugated monoclonal antibodies: CD3-fluorescein isothiocyanate (CD3-FITC), CD4-peridinin chlorophyll protein (CD4-PerCP), CD25-allophycocyanin (CD25-APC), and CD127-phycoerythrin (CD127-PE) (BD Biosciences, USA). Staining was performed at 4°C in the dark for 30 min. After washing, the cells were fixed in 1% paraformaldehyde and analyzed using a BD FACSCanto II flow cytometer. FlowJo software (version 10.6.2) was used for data analysis. TTreg cells were defined as CD3+CD4+CD25^high^CD127^low^. At least 50,000 CD3+ lymphocyte events were recorded per sample (Supplementary Figure 1).
Laboratory measurements: Blood samples were collected in the morning after an 8-h fast to assess biochemical markers, including hemoglobin concentration, platelet count, low-density lipoprotein (LDL), homocysteine, fasting blood glucose, and uric acid, using standard clinical chemistry methods.
2.3.3 Prognostic evaluation
mRS was used during telephone or clinic follow-up at 3 months to evaluate functional recovery after stroke. Favorable prognosis was defined as mRS score 0–2, and unfavorable prognosis was defined as mRS score 3–6.
2.4 Statistical evaluation
All statistical analyses were performed using R statistical software (version 4.2.1). For univariate comparisons, the Shapiro–Wilk test was used to assess the normality of continuous variables, and Levene’s test was applied to evaluate the homogeneity of variances. Based on these results, either the independent t-test or the Mann–Whitney U test was used for continuous variables, and the chi-square test was used for categorical variables. Multivariable analyses were performed using multivariable logistic regression, and interaction terms were incorporated into the models to test for moderation effects. Segmented regression analysis was conducted using the “segmented” package in R. Inflection points (breakpoints) were automatically identified by the maximum likelihood estimation algorithm, and separate regression coefficients were calculated for each interval. Statistical significance was defined as p < 0.05.
3 Results
3.1 Baseline characteristics
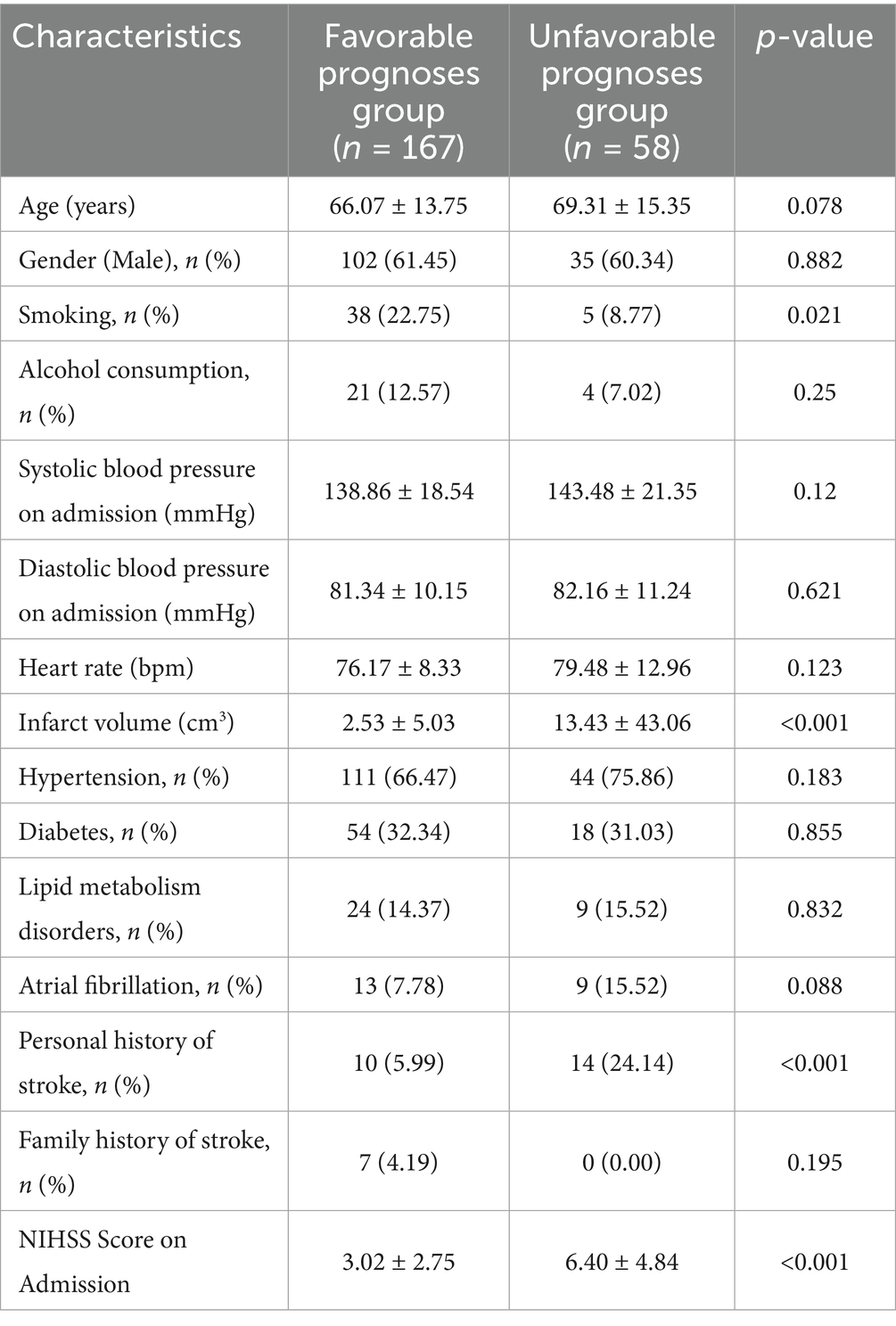
A total of 225 patients with acute ischemic stroke were included in the final analysis. Based on the 3-month mRS scores, 167 patients were classified as having a favorable prognosis (mRS 0–2), and 58 as having an unfavorable prognosis (mRS 3–6). There were no differences between groups in terms of age (66.07 ± 13.75 years vs. 69.31 ± 15.35 years, p = 0.078) and sex distribution (males, 61.45% vs. 60.34%, p = 0.882). Smoking status and infarct volume differed significantly between the two groups (p = 0.021 and p < 0.001, respectively), with the favorable prognosis group having a significantly smaller infarct volume (2.53 ± 5.03 vs. 13.43 ± 43.06 cm3, p < 0.001). On admission, the National Institutes of Health Stroke Scale (NIHSS) scores were statistically lower in the favorable outcome group (3.02 ± 2.75 vs. 6.40 ± 4.84, p < 0.001). The number of CD4+ Treg cells significantly increased in patients with a favorable prognosis (501.77 ± 187.24 vs. 450.90 ± 201.55, p = 0.033), and WBC counts decreased (7.39 ± 1.45 vs.7.95 ± 1.56, p = 0.013) (Table 1).
3.2 Multivariable logistic regression results
In the multivariable logistic regression analysis, we adjusted for the following confounders: age, gender, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, prior stroke history, smoking, and alcohol intake. The analysis demonstrated a significant correlation between infarct volume and poor prognosis (OR = 1.08, 95%CI = 1.03–1.13, p = 0.0028), indicating that each 1cm3 increment in infarct volume corresponds to an 8% elevation in the risk of unfavorable prognosis. NIHSS score on admission had a strong correlation with prognosis (OR = 1.30, 95%CI = 1.17–1.45, p < 0.0001), indicating that each 1-point increment in NIHSS score corresponds to a 30% elevation in the chance of unfavorable prognosis. The white blood cell (WBC) count was an independent and significant predictor in the adjusted model (OR = 1.32, 95%CI = 1.05–1.67, p = 0.0172), with a one-unit increase corresponding to a 32% increase in the odds for poor prognosis. The influence of CD4+ Treg cells neared significance (OR = 1.00, 95%CI = 1.00–1.00, p = 0.0774) (Table 2).
3.3 Gender as a moderator of CD4+ Treg cell effect on stroke outcome
Interaction analysis revealed a significant gender-specific effect of CD4+ Treg cell counts on stroke prognosis (interaction p = 0.0198). In male patients, CD4 + Treg cells had a significant negative correlation with poor prognosis (adjusted OR = 0.995, 95%CI = 0.992–0.999, p = 0.008), indicating that each unit increase in CD4+ Treg cells diminishes the chance of poor prognosis by approximately 0.5%. No significant correlation between CD4+ Treg cells and prognosis was found in female patients (adjusted OR = 0.999, 95%CI = 0.998–1.001, p = 0.826) (Table 3).
Analysis of smooth curves in male patients revealed a variable correlation between CD4+ Treg cells and prognosis. In male patients, CD4+ Treg cell counts below 100 were associated with a higher likelihood of poor prognosis. The risk decreased significantly (p-trend<0.001) as long as the amount of CD4+ Treg cells was up to or above 295.74 Following additional increases to 415.52, the danger increased once more, followed by a decrease at 624.34, when the risk stabilized at low levels (Figure 2).
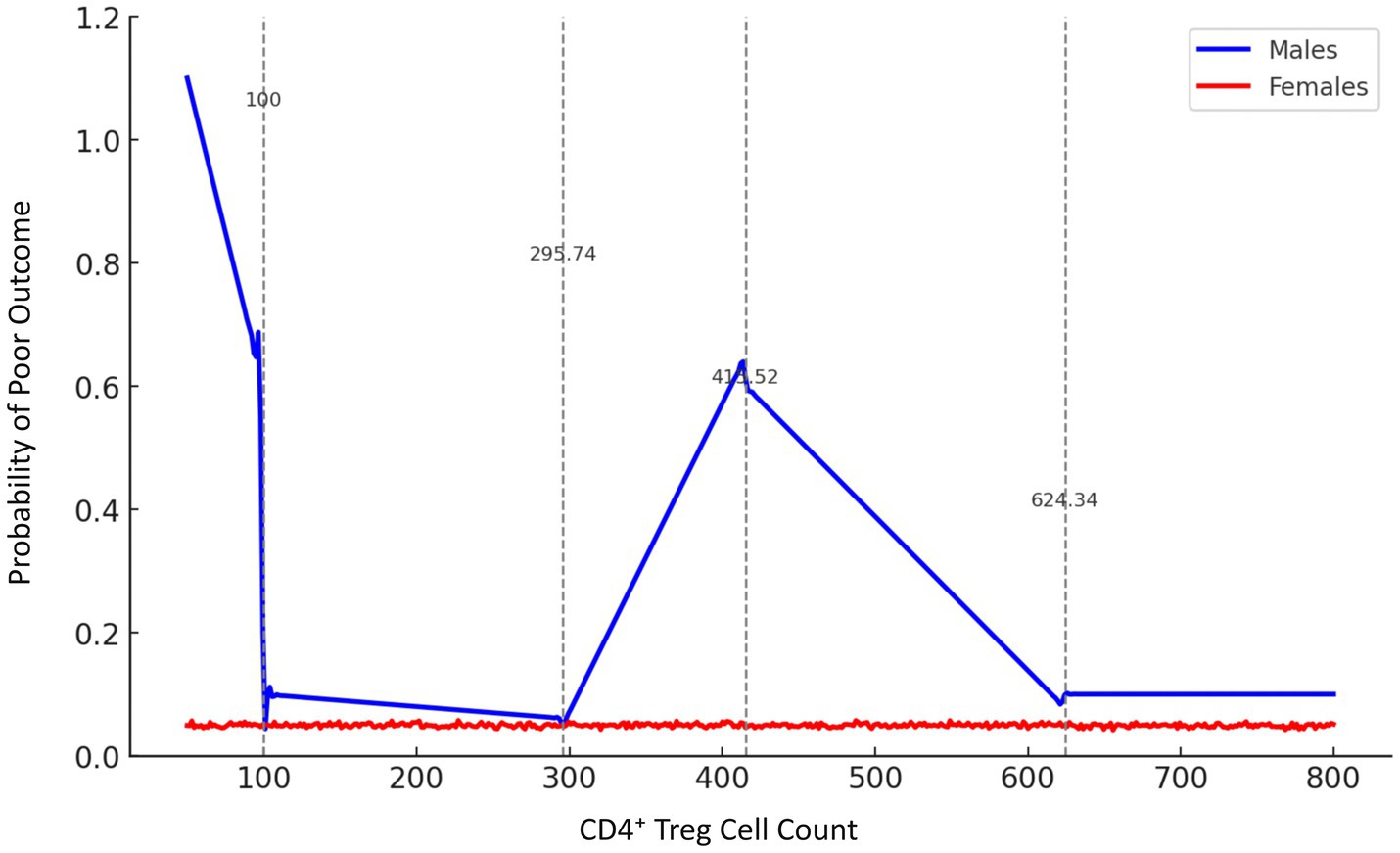
Figure 2. Gender-stratified smooth curve analysis of the association between CD4+ Treg cell count and stroke prognosis. Smooth curve fitting was performed using a generalized additive model (GAM), adjusting for age, infarct volume, NIHSS score, and other confounders. The blue line represents male patients, and the red line represents female patients. Vertical dashed lines indicate inflection points estimated by penalized spline regression at 100, 295.74, 415.52, and 624.34 cells/μL. In males, the relationship between CD4+ Treg cell count and poor prognosis probability was non-linear, while females showed no significant trend.
3.4 Segmented regression analysis of CD4+ Treg cell count and the stroke prognosis
Segmented regression analysis demonstrated that the effect of CD4+ Treg cells on the prognosis of stroke has significant heterogeneities over a number of thresholds. For the low range (CD4+ Treg ≤200), the count of CD4+ Treg cells showed a positive correlation with unfavorable prognosis and it exhibited an regression coefficient of 0.0017, p = 0.094. In the intermediate range (200 < CD4+ Treg ≤400), the regression coefficient was 0.0003, p = 0.606. In the elevated range (400 < CD4+ Treg ≤600), the regression coefficient was −0.0002, p = 0.700. In the most upper range (>600), the regression coefficient was −8.99e-06, p = 0.977 (Figure 3 and Tables 4, 5).
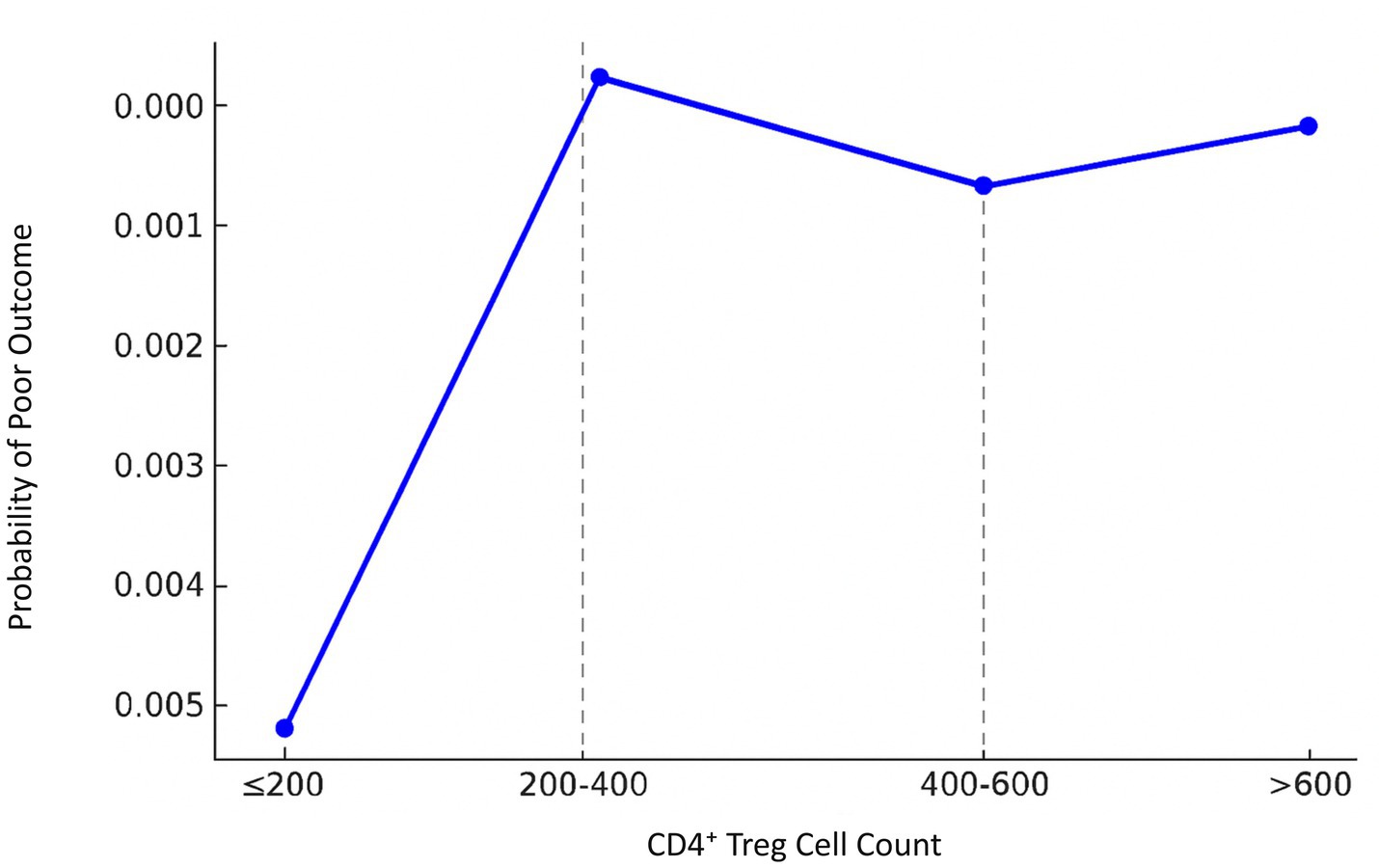
Figure 3. Segmented regression analysis of CD4+ Treg cell count and stroke prognosis in male patients. A segmented linear regression model was applied to assess the effect of CD4+ Treg count on the probability of poor outcome across four intervals (≤200, 200–400, 400–600, >600 cells/μL). Regression coefficients were estimated for each segment. Thresholds were determined using the segmented R package with maximum likelihood estimation. The model revealed significant heterogeneity in prognosis association across CD4+ Treg levels.

Table 4. Multivariate logistic regression analysis of prognostic factors in patients with acute ischemic stroke.
4 Discussion
The present study explored the association of peripheral CD4+ Treg cells and prognosis in patients with acute ischemic stroke, and identified that higher circulating CD4+ Treg counts were associated with a better post-stroke prognosis. And importantly, gender was revealed as the significant moderator of the effect of CD4+ Treg cells. Exerting a more significant impact on prognosis in male patients, whilst no such effect was noted in females.
Our data confirm the essential role of CD4+ Treg cells in controlling immunological response post-stroke. Earlier studies support this premise. Cadavid M et al. demonstrated reduced neuroinflammation and improved stroke recovery after CD4+ Treg cell expansion in subjects experiencing acute ischemic stroke. Their later research revealed that increased numerical Treg cells were accompanied with improved neurological function at the time point of 90 days post-stroke (5). Li et al. also found a link between increased CD4+ Treg cell numbers and favorable 30-day post-stroke outcomes (15). The results are consistent with studies in our laboratory that suggest Treg cells suppress CNS injury by suppressing effector T cells and inflammatory mediators, thus promoting functional recovery (16–18).
The gender-specific regulation of Treg cell function distinguishes this study. In male patients, elevated CD4+ Treg cells were substantially correlated with improved prognosis (19). Yan et al. (20) found that male stroke patients with elevated testosterone levels exhibited significantly greater Treg cell numbers and improved neurological recovery compared to those with lower testosterone levels. With respect to male patients, testosterone levels and Treg cell counts showed a significant positive connection. Regression analysis revealed that higher testosterone levels enhanced Treg-mediated control of pro-inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (21). Guo and Yang et al. similarly found that testosterone not only raised Treg cell counts but also enhanced their suppressive capacity by TGF-β, hence reducing post-stroke brain damage (22, 23). The results suggest that testosterone may increase Treg-mediated immunological regulation, hence reducing neuroinflammation in male patients and improving stroke outcomes (18, 24).
In contrast, the relationship between CD4+ Treg cell counts and stroke prognosis in female patients is more complex. Although some studies have demonstrated a positive role of estrogen in enhancing Treg cell function, the results of the present study differed and failed to observe a significant correlation between the number of Treg cells and prognosis in female patients (25, 26). Animal studies by Ahnstedt et al. (27) found lower levels of Treg cell infiltration in female mice after stroke, and in the chronic phase of the disease Treg cells showed a different response to neurological inflammation was weakly regulated, which differed from immunoregulation during recovery in male mice. The lack of significant correlation between Treg cells and prognosis with the findings observed in female patients in this study is informative.
More importantly, a nonlinear relationship between CD4+ Treg cells and prognosis was identified via smooth curve methodology analysis as well as segmented regression in male patients. At low Treg levels (<100), the probability of poor prognosis was elevated; however, when Treg cell levels rose to roughly 295, the risk diminished. Subsequent increases to approximately 415 prompted a revival in risk; however, the risk subsequently diminished and stabilized at elevated levels (>624). This finding might suggest the dual role of Treg cells in post-stroke recovery. At low levels, there may be not enough Treg cells to regulate inflammation effectively, so this could cause stroke outcomes to become worse. Previous research showed that Treg cells could protect the CNS in the acute post-stroke phase by inhibiting inflammatory responses and reducing pro-inflammatory cytokines secretion, such as interleukin-17 (IL-17) and interferon-gamma (IFN-γ) (28–30). When the numbers of Treg cells are elevated to a certain threshold, they can carry out improved immunosuppression and consequent induction of neurorepair. This excessiveness in Treg cell numbers could in turn result in over-suppression of beneficial immune responses required for tissue clearance and repair (31–33). Shi et al. (34) suggested that over-inhibition of immune responses could be deleterious for neuroregeneration, translating into poor outcomes. Following the proliferation of Treg cells, immunological balance might be reconstituted and neural repair apparatus replenished. This discovery adds a new dimension to how Treg cells modulate post-stroke immune responses, offering us an important opportunity to understand the precise role in details. Future studies need to determine the optimal level of Treg cells in post-stroke repair, allowing for personalized regulation to achieve better stroke outcomes.
This study highlights the importance of CD4+ Treg cells in post-stroke immune modulation and the impact of gender on the effect of these cells on stroke outcome. Increases in Treg cell numbers contributed to significantly improved prognosis in male patients, but not female patients. It is likely that sex hormones modulate the efficacy of Treg cells by complex signaling pathways resulting in impact on stroke recovery. In addition, smoking status differed significantly between the prognosis groups at baseline, although it was not included in the main analysis. As a well-known vascular risk factor, smoking may influence stroke outcomes through inflammatory or endothelial mechanisms. This observation deserves attention in future investigations. In the future, large scale multicenter trials are needed to confirm these results and provide a stronger theoretical framework for individualized treatment of stroke.
This study has several limitations. First, it was conducted at a single center with a relatively limited sample size, especially in the female subgroup, which may restrict the generalizability of the findings. Second, sex hormone levels such as estrogen and testosterone were not directly measured, limiting mechanistic interpretation of the gender-specific effects observed. Third, although smoking status differed between prognosis groups, it was not included in the main regression models and should be explored further in future studies. Lastly, the observational nature of this study precludes causal inference, and no experimental validation was performed. These limitations should be addressed in future prospective and mechanistic studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the ethical committee of Minhang Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HN: Conceptualization, Formal analysis, Writing – original draft. YG: Methodology, Project administration, Writing – review & editing. YL: Formal analysis, Writing – review & editing. SX: Data curation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Shanghai Municipal Health Commission Medical New Technology Research and Transformation Seed Project (Grant No. 2024ZZ2072 [to YL]); The Natural Science Research Project in Minhang District, Shanghai City (Grant No. 2023MHZ046 [to YL]); Training Program for High-Level Specialist Physicians under the Collaborative Health Service System of Education, Research, and Clinical Practice in Minhang District, Shanghai City (2024–2027) (Grant No. 2024MZYS09 [to YL]).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1626494/full#supplementary-material
References
1. Fan, J, Li, X, Yu, X, Liu, Z, Jiang, Y, Fang, Y, et al. Global burden, risk factor analysis, and prediction study of ischemic stroke, 1990-2030. Neurology. (2023) 101:e137–50. doi: 10.1212/WNL.0000000000207387
2. Duan, M, Xu, Y, Li, Y, Feng, H, and Chen, Y. Targeting brain-peripheral immune responses for secondary brain injury after ischemic and hemorrhagic stroke. J Neuroinflammation. (2024) 21:102. doi: 10.1186/s12974-024-03101-y
3. Liston, A, Dooley, J, and Yshii, L. Brain-resident regulatory t cells and their role in health and disease. Immunol Lett. (2022) 248:26–30. doi: 10.1016/j.imlet.2022.06.005
4. Wang, M, Thomson, AW, Yu, F, Hazra, R, Junagade, A, and Hu, X. Regulatory t lymphocytes as a therapy for ischemic stroke. Semin Immunopathol. (2023) 45:329–46. doi: 10.1007/s00281-022-00975-z
5. Santamaria-Cadavid, M, Rodriguez-Castro, E, Rodriguez-Yanez, M, Arias-Rivas, S, Lopez-Dequidt, I, Perez-Mato, M, et al. Regulatory t cells participate in the recovery of ischemic stroke patients. BMC Neurol. (2020) 20:68. doi: 10.1186/s12883-020-01648-w
6. Liu, Y, Dong, J, Zhang, Z, Liu, Y, and Wang, Y. Regulatory t cells: a suppressor arm in post-stroke immune homeostasis. Neurobiol Dis. (2023) 189:106350. doi: 10.1016/j.nbd.2023.106350
7. Kumari, S, Dhapola, R, Sharma, P, Nagar, P, Medhi, B, and HariKrishnaReddy, D. The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor Rev. (2024) 78:105–19. doi: 10.1016/j.cytogfr.2024.06.002
8. Banerjee, A, and McCullough, LD. Sex-specific immune responses in stroke. Stroke. (2022) 53:1449–59. doi: 10.1161/STROKEAHA.122.036945
9. Tariq, MB, Lee, J, and McCullough, LD. Sex differences in the inflammatory response to stroke. Semin Immunopathol. (2023) 45:295–313. doi: 10.1007/s00281-022-00969-x
10. Iozzo, M, Comito, G, Ippolito, L, Sandrini, G, Pardella, E, Pranzini, E, et al. Sex-related changes in lactate dehydrogenase a expression differently impact the immune response in melanoma. FEBS J. (2025) 292:3056–71. doi: 10.1111/febs.17423
11. Xiao, T, Lee, J, Gauntner, TD, Velegraki, M, Lathia, JD, and Li, Z. Hallmarks of sex bias in immuno-oncology: mechanisms and therapeutic implications. Nat Rev Cancer. (2024) 24:338–55. doi: 10.1038/s41568-024-00680-z
12. Mosconi, L, Nerattini, M, Matthews, DC, Jett, S, Andy, C, Williams, S, et al. In vivo brain estrogen receptor density by neuroendocrine aging and relationships with cognition and symptomatology. Sci Rep. (2024) 14:12680. doi: 10.1038/s41598-024-62820-7
13. Cai, S, Dai, S, Lin, R, Huang, C, Zeng, Y, Diao, L, et al. The effectiveness and safety of intrauterine infusion of autologous regulatory t cells (tregs) in patients with recurrent pregnancy loss and low levels of endometrial foxp3(+) cells: a retrospective cohort study. Am J Reprod Immunol. (2023) 90:e13735. doi: 10.1111/aji.13735
14. Mead, GE, Sposato, LA, Sampaio, SG, Yperzeele, L, Wu, S, Kutlubaev, M, et al. A systematic review and synthesis of global stroke guidelines on behalf of the world stroke organization. Int J Stroke. (2023) 18:499–531. doi: 10.1177/17474930231156753
15. Li, S, Huang, Y, Liu, Y, Rocha, M, Li, X, Wei, P, et al. Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke. J Cereb Blood Flow Metab. (2021) 41:2280–94. doi: 10.1177/0271678X21995694
16. Lei, TY, Ye, YZ, Zhu, XQ, Smerin, D, Gu, LJ, Xiong, XX, et al. The immune response of t cells and therapeutic targets related to regulating the levels of t helper cells after ischaemic stroke. J Neuroinflammation. (2021) 18:25. doi: 10.1186/s12974-020-02057-z
17. Xie, W, and Li, P. Visualizing regulatory lymphocytic responses to predict neurological outcome after stroke. CNS Neurosci Ther. (2021) 27:867–8. doi: 10.1111/cns.13698
18. Yuan, C, Shi, L, Sun, Z, Xu, F, Wang, C, Shan, J, et al. Regulatory t cell expansion promotes white matter repair after stroke. Neurobiol Dis. (2023) 179:106063. doi: 10.1016/j.nbd.2023.106063
19. Ahnstedt, H, and McCullough, LD. The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cell Immunol. (2019) 345:103960. doi: 10.1016/j.cellimm.2019.103960
20. Yan, J, Read, SJ, Henderson, RD, Hull, R, O'Sullivan, JD, McCombe, PA, et al. Frequency and function of regulatory t cells after ischaemic stroke in humans. J Neuroimmunol. (2012) 243:89–94. doi: 10.1016/j.jneuroim.2011.12.019
21. Mohamad, NV, Wong, SK, Wan, HW, Jolly, JJ, Nur-Farhana, MF, Ima-Nirwana, S, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. (2019) 22:129–40. doi: 10.1080/13685538.2018.1482487
22. Guo, S, and Luo, Y. Brain foxp3(+) regulatory t cells can be expanded by interleukin-33 in mouse ischemic stroke. Int Immunopharmacol. (2020) 81:106027. doi: 10.1016/j.intimp.2019.106027
23. Yang, SW, Cao, L, Yang, SW, Wang, SQ, Jia, WY, and Wang, C [imbalance of immunological functions of treg and tgf-beta1 aggravated cerebral ischemia damage in mice]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. (2011) 27:408–11.
24. Sciarra, F, Campolo, F, Franceschini, E, Carlomagno, F, and Venneri, MA. Gender-specific impact of sex hormones on the immune system. Int J Mol Sci. (2023) 24:24. doi: 10.3390/ijms24076302
25. Zhong, X, Sun, Y, Lu, Y, and Xu, L. Immunomodulatory role of estrogen in ischemic stroke: neuroinflammation and effect of sex. Front Immunol. (2023) 14:1164258. doi: 10.3389/fimmu.2023.1164258
26. Bravo-Alegria, J, McCullough, LD, and Liu, F. Sex differences in stroke across the lifespan: the role of T lymphocytes. Neurochem Int. (2017) 107:127–37. doi: 10.1016/j.neuint.2017.01.009
27. Ahnstedt, H, Patrizz, A, Chauhan, A, Roy-O'Reilly, M, Furr, JW, Spychala, MS, et al. Sex differences in t cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain Behav Immun. (2020) 87:556–67. doi: 10.1016/j.bbi.2020.02.001
28. Lu, T, Ma, L, Xu, Q, and Wang, X. Blood Th17 cells and IL-17A as candidate biomarkers estimating the progression of cognitive impairment in stroke patients. J Clin Lab Anal. (2022) 36:e24581. doi: 10.1002/jcla.24581
29. Malone, K, Shearer, JA, Waeber, C, and Moore, AC. The impact of fingolimod on treg function in brain ischaemia. Eur J Immunol. (2023) 53:e2350370. doi: 10.1002/eji.202350370
30. Jiang, C, Kong, W, Wang, Y, Ziai, W, Yang, Q, Zuo, F, et al. Changes in the cellular immune system and circulating inflammatory markers of stroke patients. Oncotarget. (2017) 8:3553–67. doi: 10.18632/oncotarget.12201
31. Iadecola, C, Buckwalter, MS, and Anrather, J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest. (2020) 130:2777–88. doi: 10.1172/JCI135530
32. Peng, L, Hu, G, Yao, Q, Wu, J, He, Z, Law, BY, et al. Microglia autophagy in ischemic stroke: a double-edged sword. Front Immunol. (2022) 13:1013311. doi: 10.3389/fimmu.2022.1013311
33. Zhang, Z, Lv, M, Zhou, X, and Cui, Y. Roles of peripheral immune cells in the recovery of neurological function after ischemic stroke. Front Cell Neurosci. (2022) 16:1013905. doi: 10.3389/fncel.2022.1013905
Keywords: CD4+ Treg cells, acute ischemic stroke, gender differences, stroke prognosis, immune modulation
Citation: Na H, Gu Y, Liu Y and Xia S (2025) The influence of gender on CD4+ Treg cell function in acute ischemic stroke prognosis. Front. Neurol. 16:1626494. doi: 10.3389/fneur.2025.1626494
Edited by:
Giuseppe Lanza, University of Catania, ItalyReviewed by:
Yuwen Xiu, Tulane University, United StatesAleksandra Golenia, Medical University of Warsaw, Poland
Copyright © 2025 Na, Gu, Liu and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiliang Xia, MTczNTY0MzIzMzBAc2luYS5jbg==
†These authors have contributed equally to this work
 Hui Na
Hui Na Yue Gu
Yue Gu Yang Liu
Yang Liu Shiliang Xia
Shiliang Xia