- 1Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2The Key Laboratory of Hunan Province for Integrated Traditional Chinese and Western Medicine on Prevention and Treatment of Cardio-Cerebral Diseases, College of Integrated Traditional Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 3The First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
Biological rhythms play a critical role in regulating human physiology and have been implicated in the onset, progression, and recovery of ischemic stroke (IS). This review summarizes recent experimental and clinical studies that associate circadian regulation with post-stroke blood–brain barrier (BBB) repair, focusing on the role of molecular clock components. Core clock components, including BMAL1 and CLOCK, influence BBB integrity by regulating tight junction protein expression, angiogenesis, neuroimmune responses, and neuroendocrine signaling. Finally, we discuss emerging chronotherapeutic strategies that integrate circadian biology into stroke rehabilitation.
1 Introduction
Ischemic stroke (IS), also referred to as cerebral infarction, arises from reduced or interrupted cerebral blood flow, leading to ischemic-hypoxic necrosis of brain tissue and subsequent neurological deficits. According to the Global Burden of Disease 2021 study, IS ranks as the second leading cause of death worldwide and remains a major cause of long-term disability (1). The pathophysiology of IS is initiated by cerebral hypoperfusion and progresses through multiple interconnected processes, including excitotoxicity (2–4), oxidative stress (5, 6), and neuroinflammation (7, 8). Acute cerebral ischemia/hypoxia rapidly depletes ATP, which in turn provokes persistent neuronal hyperexcitation and widespread apoptosis. At the same time, excessive generation of reactive oxygen species (ROS) promotes apoptotic signaling and cellular dysfunction, while activation of innate immunity maintains cytokine and chemokine release, thereby amplifying ischemic injury (9). Current therapeutic strategies for IS aim to restore cerebral perfusion as quickly as possible, mainly via intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) or by endovascular mechanical thrombectomy. When applied within the therapeutic window, these interventions improve blood and oxygen delivery to the ischemic penumbra, help preserve neuronal function, and reduce long-term disability (10, 11). However, because of the narrow therapeutic window and the risk of hemorrhagic complications, fewer than 10% of patients receive rt-PA, and less than half achieve successful reperfusion (12, 13). This underscores the urgent requirement for safer and more effective treatment options.
Disruption of the blood–brain barrier (BBB) is a key pathological feature of IS, primarily caused by degradation of tight junction (TJ) proteins and increased transcytosis, leading to vascular hyperpermeability (14). BBB breakdown triggers the release of damage-associated molecular patterns (DAMPs), such as vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and heat shock proteins (HSPs), from ischemic tissue, and promotes immune cell infiltration that accelerates neuronal necrosis (15). Necrotic neurons further release DAMPs, enhancing chemokine secretion by immune cells and perpetuating a cycle of vascular injury, neuroinflammation, and neuronal death. This pathological cascade aggravates ischemia–reperfusion injury and promotes secondary complications, including vasogenic edema and hemorrhagic transformation (16–18). Clinical studies have linked severe BBB disruption to unfavorable IS outcomes, such as higher NIH Stroke Scale (NIHSS) scores, poorer functional recovery on the modified Rankin Scale (mRS), and increased mortality (19). Therefore, therapies aimed at preserving BBB integrity may help reduce neurological deficits and improve long-term outcomes in patients with IS.
The BBB is a specialized neurovascular interface that regulates molecular and ionic exchange between the systemic circulation and the central nervous system (CNS), thereby maintaining cerebral homeostasis (20). The neurovascular unit constituting the BBB primarily comprises brain microvascular endothelial cells (BMECs), astrocytes, and pericytes interacting with neurons and microglia. These cellular components interact structurally and molecularly to establish and regulate barrier function (21, 22). Selective permeability of the BBB depends on tight junction complexes between endothelial cells, which are composed of transmembrane proteins (claudins, occludin, and junctional adhesion molecules [JAMs]) and cytoplasmic scaffolding proteins of the zona occludens (ZO) family. Together, these structures restrict paracellular diffusion. In addition, ATP-dependent transporters such as P-glycoprotein, together with vesicular mechanisms including adsorptive and receptor-mediated transcytosis, control transcellular molecular transport (23–25). Key mechanisms of BBB transport include receptor-mediated transcytosis (e.g., via transferrin receptors), efflux mediated by ATP-binding cassette (ABC) transporters such as BCRP, and increased paracellular permeability when tight junctions are disrupted (26). Mesenchymal stem cell-derived extracellular vesicles have been shown to help preserve BBB integrity and reduce the risk of hemorrhagic transformation (27). This is clinically important since symptomatic intracranial hemorrhage (sICH), although reported in only 2–7% of thrombolysis cases, is responsible for the majority of thrombolysis-related deaths (28). Recent studies have shown that endothelial clock genes (CLOCK/BMAL1) regulate the expression of tight junction proteins such as CLDN5 and OCLN, suggesting that circadian rhythms contribute to endogenous protection of BBB function (29).
Circadian rhythms are endogenous 24-h cycles regulated by the suprachiasmatic nucleus (SCN) in the hypothalamus, which synchronizes peripheral oscillators across mammalian tissues (30). Cell-autonomous rhythms are generated by transcription–translation feedback loops (TTFLs) involving core clock genes such as Clock, Bmal1, Per, and Cry (31). Clock genes are broadly expressed, not only in the SCN but also in other brain regions and peripheral organs (32, 33). At the molecular level, BMAL1 and CLOCK form heterodimers (CLOCK::BMAL1) that regulate transcription of downstream genes by binding to E-boxes, D-boxes, and Rev-erb/ROR response elements (34). Disruption of circadian rhythms contributes to pathological processes such as impaired DNA damage repair (35), altered metabolic and oxidative stress regulation (36), and dysregulated inflammatory and immune responses (37). Moreover, Circadian genes also regulate angiogenesis by influencing endothelial and pericyte functions, through mechanisms such as modulation of angiogenic factors (38), basement membrane degradation (39), extracellular matrix remodeling (40), and regulation of endothelial migration, proliferation, and pericyte recruitment (41).
Circadian rhythms influence the onset, progression, and clinical outcomes of IS by regulating diurnal blood pressure patterns, vascular tone, and platelet activity (42). Ambulatory blood pressure monitoring (ABPM) has provided strong evidence linking circadian disruption to stroke incidence. Abnormal circadian blood pressure rhythms are recognized as an independent risk factor for IS (43, 44). In rodent models, six weeks of environmental circadian disruption (ECD) increased infarct size and enhanced neuroinflammatory responses, thereby worsening stroke severity (45). Circadian-regulated molecules such as TNF-α, leptin, β-amyloid, delta sleep-inducing peptide (DSIP), and prostaglandin D2 (PGD2) are expressed in the central nervous system and may influence stroke pathogenesis by altering BBB circadian dynamics (46–50). However, the mechanisms by which circadian rhythms regulate BBB repair after ischemia and influence neurological recovery are still unclear. Further investigation in this area could provide the basis for chronotherapeutic approaches in stroke and identify novel strategies to protect the BBB through circadian regulation.
2 Circadian rhythms directly regulate BBB damage and repair following IS
2.1 Circadian rhythms directly regulate endothelial cell function
Endothelial cells (ECs) of the BBB display circadian rhythmicity and contain an intrinsic circadian regulatory system (51). At the molecular level, circadian regulation in ECs is governed by a transcription–translation feedback loop involving core clock genes such as CLOCK and BMAL1. Pan et al. reported that leptin transport across the BBB varies with time of day, being significantly higher at night than during the day (46). This variation correlates with circadian changes in the expression of endothelial transporters. Similarly, expression of the glucose transporter GLUT1 peaks during the circadian active phase (52). Members of the ABC efflux transporter family, which are highly expressed in BBB ECs, are regulated by both endothelial circadian clocks and neuronal activity patterns (53). Recent approaches integrating chemogenetic modulation (e.g., clozapine-N-oxide induction) with fluorescence-activated cell sorting and transcriptomic profiling have enabled detailed mapping of activity-dependent gene networks in endothelial cells, key BBB transporters such as P-glycoprotein show rhythmic expression coordinated with core clock genes, generating time windows that favor CNS drug penetration (54, 55). Transporter activity peaks during the circadian active phase (daytime in humans) and decreases during rest phases, inversely related to neuronal activity. Clinical studies have reported better safety outcomes for intravenous thrombolysis administered between noon and midnight compared with treatments given in the early morning (06:00–18:00) (56). These findings suggest that circadian timing should be considered in optimizing stroke treatment strategies.
ECs repair after ischemic stroke involves migration, proliferation, tube formation, and restoration of barrier function, each subject to time-dependent regulation that influences the course of BBB recovery (57). Astone et al. showed that the core clock gene BMAL1 regulates EC proliferation by modulating cell-cycle regulators, particularly cyclin D1 (58). In BMAL1-knockout mice, EC repair is impaired, leading to delayed BBB restoration after stroke (59). These findings indicate that clock genes are essential for endothelial regeneration. Pulido et al. observed that cerebrovascular EC proliferation depends on circadian phase and aligns with oscillations in core clock gene expression (55). When BMAL1 levels are high, ECs demonstrate increased migration and tube formation, which accelerates BBB repair. Recent studies suggest that cryptochrome (CRY) proteins regulate EC repair by coordinating the timing of proliferation, migration, and tube formation, thereby supporting neovascularization and vascular maturation (60). This regulation helps limit BBB hyperpermeability, providing a mechanism by which circadian disruption impairs endothelial repair and disturbs BBB homeostasis. Coordinated circadian control of EC repair is required to maintain barrier integrity, whereas its disruption results in persistent vascular leakage and impaired neurovascular recovery after stroke.
2.2 Circadian rhythms directly regulate tight junction proteins
Circadian rhythms influence the expression of genes and proteins associated with tight junctions at both the transcriptional and translational levels. Among these, Claudin-5, Occludin, and zonula occludens-1 (ZO-1) are central to maintaining the structural integrity and function of tight junctions (23, 61).Claudin-5, a principal protein in inter-endothelial tight junctions, determines BBB selective permeabilitys (62). Occludin has been proposed as a marker of tight junction integrity, with reduced levels correlating with BBB disruption and cerebral edema (63). ZO-1 links transmembrane tight junction proteins to the actin cytoskeleton, and its expression and phosphorylation status are important for junction assembly and maintenance (64–66).Recent studies show that the expression of these tight junction proteins follows circadian oscillations (67).
Spadoni et al. reported that expression of claudin-5 and occludin depends on the core clock gene BMAL1. In wild-type mice, occludin mRNA shows clear circadian oscillation, which is lost in rhythm-disrupted mice and accompanied by impaired barrier integrity (68). Kyoko et al. found that claudin-5 expression in the intestinal vasculature is higher at night (active phase) than during the day (rest phase) (29). These findings support circadian regulation of claudin-5 (69) and suggest that the CLOCK/BMAL1 heterodimer regulates its transcription through E-box elements. Jensen et al. showed that loss of BMAL1 reduces claudin-5 expression and increases BBB permeability (70). Expression of the clock repressor Period2 (Per2) is inversely correlated with claudin-5 levels, supporting circadian regulation of tight junction dynamics (71). After ischemic stroke, tight junction repair is time-dependent, with efficiency differing across circadian phases (69). In rhythm-synchronized mice, occludin and claudin-5 show periodic changes in expression during the first 72 h after injury, whereas such oscillations are absent in rhythm-disrupted mice (72, 73). Together, these findings suggest that circadian regulation of tight junction proteins is an important mechanism for maintaining BBB integrity, and that targeting core clock components may help promote BBB recovery after ischemic stroke.
3 Circadian rhythms promote post-IS BBB repair through angiogenesis regulation
3.1 Circadian rhythms modulate pericyte phenotypic reprogramming to restore BBB integrity after IS
Angiogenesis after stroke is an important stage of BBB restoration, involving endothelial cell proliferation, migration, and pericyte recruitment. Circadian regulation of angiogenesis has been linked to core clock genes such as CLOCK and BMAL1 (70, 74). Post-stroke angiogenesis shows spatiotemporal heterogeneity (75). Immature neovessels often remain hyperpermeable, which aggravates BBB dysfunction, vasogenic edema, and neuroinflammation. Pericytes contribute to CNS homeostasis by regulating microcirculatory flow, controlling leukocyte entry, clearing neurotoxic metabolites, modulating circadian endothelial gene expression, and maintaining astrocytic end-foot polarization (76). As specialized capillary mural cells, they stabilize newly formed vessels during both developmental angiogenesis and post-injury repair. Functional vascular maturation requires adequate pericyte recruitment and continuous vessel wall coverage. Loss of pericytes alters endothelial gene expression and promotes pathological angiogenesis with persistent hyperpermeability (77). Jidigam et al. showed that BMAL1 regulates pericyte recruitment (78). In BMAL1-knockout mice, pericytes progressively detach, extracellular matrix deposition is reduced, and tight junctions disassemble, leading to unstable neovasculature and loss of BBB integrity (79). BMAL1 also regulates vascular maturation and stabilization by controlling the transcription of adhesion molecules and cytoskeletal regulators (80).
3.2 Circadian rhythms regulate VEGF–Mediated BBB repair following IS
VEGF shows circadian-regulated expression mediated by the molecular clock (81). It influences endothelial cell behavior and pericyte recruitment, and is a major determinant of angiogenic responses after stroke. Excessive VEGF expression enhances neuroinflammation and promotes the formation of immature, hyperpermeable vessels, resulting in tissue injury and worsening BBB dysfunction (82).BMAL1 binds E-box elements in the VEGFA promoter to activate its transcription, which stimulates endothelial proliferation, migration, and differentiation (83). Through VEGF signaling, BMAL1 helps regulate the balance between tip and stalk cells, which is required for stable vascular network formation. Conversely, the clock repressors PER2 and CRY1 suppress hypoxia-induced VEGFA transcription, contributing to circadian oscillations in VEGF expression (84). In hindlimb ischemia models, both genetic and environmental circadian disruption impair reparative neovascularization (74). Clinical studies report that shift workers show delayed vascular recovery after stroke, associated with disrupted VEGF rhythmicity and clock gene dysregulation (85).These findings suggest that the molecular clock regulates endothelial function and vascular repair by modulating VEGF signaling over time. Koyanagi et al. showed that BMAL1 coordinates diurnal VEGFA expression via hypoxia-inducible factor 1α (HIF-1α) in tumor models, and this mechanism also contributes to vascular repair after stroke (83). Fibroblast growth factors (FGFs), such as FGF21, show circadian expression and support vascular repair by enhancing VEGF signaling and promoting the activity of endothelial progenitor cells (EPCs) (86, 87).Together, these data suggest that therapeutic strategies targeting both clock components (CLOCK/BMAL1 or PER/CRY) and angiogenic factors (VEGFA/FGF) could enhance BBB recovery and improve outcomes after stroke. Such chronotherapeutic approaches may help preserve physiological angiogenic rhythms while limiting pathological vascular permeability in ischemic stroke.
4 Circadian rhythms regulate post-IS BBB repair via immune system modulation
4.1 Circadian rhythms orchestrate immune cell-mediated BBB repair following IS
After stroke, BBB dysfunction involves several inflammatory mechanisms, including increased matrix metalloproteinases (MMP-2/9), excessive ROS production, polarization of microglia toward pro-inflammatory states, and infiltration of peripheral leukocytes (18, 88–91). Thus, neuroinflammation strongly influences BBB recovery, and this process is under circadian regulation. The molecular clock regulates immune function by controlling leukocyte proliferation and migration, modulating phagocytic and cytotoxic activity, and influencing immune cell activation states. As a result, circadian regulation affects key immune processes such as pathogen defense, tissue surveillance, and homeostasis (92). Disruption of circadian rhythms disturbs the balance between pro- and anti-inflammatory cytokines, partly through altered immune cell trafficking and dysregulated clock proteins such as REV-ERBα/NR1D1 (93).Innate immune cells such as macrophages and NK cells contain functional circadian clocks, with the CLOCK:BMAL1 heterodimer regulating phagocytosis, cytokine production (e.g., IL-1β, TNF-α), and antimicrobial activity (94). Endothelial-specific BMAL1 deletion disrupts the rhythmic trafficking of immune cells to lymph nodes, highlighting the importance of circadian rhythms in regulating immune cell positioning (95).
The circadian system contributes to BBB repair after ischemic stroke by regulating neuroimmune interactions in a time-dependent manner across different pathological phases. During the acute phase (< 72 h post-stroke), microglia show circadian-dependent activation patterns, influencing the release of pro-inflammatory mediators (IL-6, ROS), modulating MMP-9 secretion, and thereby affecting the extent of BBB disruption (96, 97). Yenari et al. showed that suppression of microglial activation with minocycline reduced TNF-α and IL-1β secretion, promoted endothelial repair, decreased infarct volume and BBB leakage, and improved neurological outcomes (98). BMAL1 regulates the temporal pattern of MMP-9 expression by controlling neutrophil infiltration rhythms (99). Circadian-driven migration of Treg cells helps suppress excessive inflammation while maintaining MMP-9 activity, which supports BBB integrity (100, 101). Neurovascular unit crosstalk is also influenced by circadian regulation. Monocytes and macrophages promote endothelial proliferation and tight junction reassembly during specific circadian phases (102), while astrocyte-derived rhythmic inflammatory factors, such as basic fibroblast growth factor, can worsen BBB dysfunction through peripheral–central signaling (103). Novel immunotherapies that penetrate the BBB, such as neutrophil-hitchhiked bacterial outer membrane vesicles, are being investigated to enhance brain delivery of neuroprotective agents in stroke (104). Unresolved issues include how clock genes influence immune cell activation thresholds (e.g., Treg suppression) and what circadian windows (e.g., ZT4–8) are optimal for immunomodulation. Clarifying these mechanisms may guide the development of chronotherapy strategies for BBB repair.
4.2 Circadian rhythms regulate inflammatory factor-mediated BBB repair following IS
Ischemia and hypoxia trigger a neuroinflammatory cascade in which the release of pro-inflammatory mediators disrupts the BBB. The resulting increase in BBB permeability further amplifies inflammation and aggravates neuronal injury. For example, TNF-α downregulates tight junction proteins such as occludin, thereby increasing endothelial permeability (105). IL-1β induces MMP-2/9-mediated degradation of basement membrane collagen (106). IL-6 upregulates VEGF expression and promotes vascular leakage (107), while IL-8 enhances neutrophil infiltration and aggravates BBB dysfunction (108). The secretion of pro-inflammatory cytokines follows circadian rhythms (109). Pharmacological and genetic studies in macrophages show that REV-ERBα links the circadian clock to inflammatory signaling by modulating the expression of innate immune genes such as IL-6 (110, 111). Candelario-Jalil et al. reported that anti-TNF-α therapy was more effective when administered during the nocturnal phase, suggesting that circadian regulation influences TNF-α-mediated inflammation (112). Ding et al. showed that BMAL1 regulates transcriptional activity by binding to cis-regulatory elements within inflammatory gene promoters, thereby controlling their circadian expression (113). Loss of BMAL1 disrupts circadian cytokine oscillations and prolongs inflammatory responses. Although the molecular mechanisms linking circadian rhythms to inflammatory mediators are not fully understood, circadian disruption is known to disturb cytokine regulation. These findings suggest that chrono-targeted strategies, such as timed inhibition of inflammatory pathways and preservation of endothelial tight junctions, may help reduce secondary injury after ischemic stroke.
5 Circadian rhythms regulate post-IS BBB repair via the neuroendocrine system
5.1 The neuroendocrine-circadian axis
The neuroendocrine system acts as a key interface linking circadian regulation to BBB homeostasis and contributes to barrier repair after ischemic stroke. As the main integrator of neural and hormonal signaling, it exhibits circadian oscillations in both hormone secretion and regulatory activity. The hypothalamus functions as the central regulatory hub, where specialized neurosecretory cells translate synaptic inputs into timed hormonal releases, particularly glucocorticoids under circadian control. Under the control of the SCN, these hypothalamic cells synchronize peripheral tissue clocks and help maintain systemic circadian coherence (114, 115). Recent evidence suggests that this temporal regulation involves not only the hypothalamic–pituitary–adrenal axis but also mesolimbic reward pathways, where circadian fluctuations in monoaminergic neurotransmission (particularly dopamine and serotonin) influence endocrine responses to environmental cues (116, 117). Experimental studies show bidirectional interactions between circadian and neuroendocrine systems, where neurotransmitter imbalances such as dopamine dysregulation impair clock function, and disruption of clock genes leads to neuroendocrine deficits (118, 119). Together, molecular clocks, neural circuits, and hormonal signals form an integrated neuroendocrine–circadian axis that coordinates physiological homeostasis and modulates stress responses.
5.2 The hypothalamic–pituitary–adrenal (HPA) axis
The neuroendocrine system regulates BBB integrity through signaling pathways that show clear circadian control (120). The HPA axis, functioning as the neuroendocrine system's master regulator, exhibits intrinsic circadian oscillations in both its basal tone and stress-responsive activity (121).Key HPA effector molecules, particularly glucocorticoids and angiotensin II, modulate BBB permeability in a phase-dependent manner by regulating tight junction proteins (claudin-5, ZO-1) and vascular permeability factors such as VEGF (122–125). Endogenous glucocorticoid secretion follows a conserved circadian pattern across species, with diurnal organisms (e.g., humans) showing peak concentrations during active phases (morning) and nocturnal species (e.g., rodents) displaying maximal levels during their active period (evening) (126).In humans, peak circadian cortisol secretion (approximately 06:00–10:00h) (127) temporally coincides with maximal expression of tight junction proteins (occludin, claudin-5), and optimal BBB integrity. Conversely, the nadir phase (approximately 22:00–02:00 h) is characterized by decreased junctional protein expression and elevated paracellular permeability (76, 128).This circadian variation represents an adaptive mechanism aligning BBB function with metabolic demands. The HPA axis also regulates circadian neuroimmune interactions. Glucocorticoids perform dual chrono-regulatory functions: entraining circadian oscillations in cytokine production (129–132), and exerting phase-dependent immunosuppression through inhibition of pro-inflammatory transcription factors (including NF-κB) (133–136).Preserved circadian cortisol rhythmicity following stroke acts as an endogenous brake on neuroinflammation, thereby limiting persistent BBB dysfunction (137).Clinical investigations demonstrate that blunted circadian cortisol rhythmicity (non-dipping pattern) shows significant positive correlation with both the extent and progression of post-stroke BBB pathology (138).These findings suggest that targeting clock genes to optimize the timing of neuroendocrine factor release (e.g., aligning glucocorticoid peaks with ischemic injury cycles) may provide a strategy for BBB stabilization.
5.3 Circadian rhythms modulate melatonin-mediated BBB repair after IS
Melatonin is an indoleamine hormone secreted by the pineal gland in a circadian pattern (139, 140). Melatonin has been shown to exert neuroprotective effects through anti-inflammatory and antioxidant actions, reduction of cerebral edema, preservation of cognitive function, and activation of endogenous repair mechanisms (141–145). Wang et al. reported that melatonin treatment reduced brain water content and improved BBB integrity compared with untreated controls (146). Experimental studies show that nighttime administration of melatonin increases the expression of occludin, claudin-5, and ZO-1, stabilizes the cytoskeleton, and reduces BBB leakage after ischemia. The protective effect is strongest when treatment is synchronized with endogenous secretion rhythms (26). Melatonin also improves endothelial function and protects against excitotoxicity-induced BBB disruption in neonatal rats (147). At the molecular level, melatonin directly binds to MMP-9, thereby inhibiting its activity and reducing ischemia/reperfusion-induced BBB hyperpermeability (148, 149). Together, these findings suggest that melatonin may serve as a therapeutic agent to mitigate post-ischemic BBB injury. Clinically, melatonin has been reported to reduce the risk of hemorrhagic transformation after tissue plasminogen activator (t-PA) therapy in ischemic stroke (150, 151), suggesting a potential role in improving thrombolytic safety through modulation of BBB permeability.
5.4 Circadian regulation of autonomic nervous system (ANS) activity to attenuating BBB dysfunction of IS
Sympathetic and parasympathetic activities exhibit circadian rhythmicity and modulate cerebrovascular function through neurovascular coupling mechanisms (152, 153).Sympathetic activity predominates during the daytime, whereas parasympathetic activity is enhanced at night (154). The vagus nerve, as the main parasympathetic effector, shows marked circadian fluctuations in activity (155). Clinical studies indicate that circadian disruption, such as shift work, attenuates the anti-inflammatory effects of vagal activity (156). Preclinical evidence demonstrates that non-invasive vagus nerve stimulation (nVNS) exerts neuroprotective effects, preserving BBB integrity and reducing infarct volume after ischemic injury (157). When applied during peak activity periods (nocturnal phase), VNS suppresses pro-inflammatory gene expression (e.g., IL-6), reduces inflammatory responses, and enhances BBB integrity through upregulation of ZO-1 and occluding (152, 158). Clinical data further reveal an inverse correlation between heart rate variability (HRV, an indicator of vagal activity) and the severity of BBB damage, suggesting that vagal activity contributes to BBB regulation through cerebrovascular dilation and hemodynamic control (159).
The molecular and cellular mechanisms by which circadian rhythms regulate neuroendocrine signaling are not yet fully understood, representing a priority for future investigation. Advancing chronotherapeutic delivery systems for neuroendocrine modulators may enable temporally optimized treatment strategies for ischemic stroke.
6 Summary
The circadian clock acts as a key temporal regulator that preserves the structural and functional homeostasis of the BBB through integrated molecular networks. Here, we summarize the molecular mechanisms through which circadian rhythms regulate BBB repair after ischemic stroke (Figure 1). Key mechanisms involve transcriptional regulation of tight junction proteins, circadian control of angiogenesis, modulation of neuroimmune responses, and coordination of neuroendocrine signaling. Both preclinical and clinical studies show that circadian disruption aggravates BBB injury, characterized by reduced expression of tight junction proteins (ZO-1, occludin, claudin-5), heightened neuroinflammation (TNF-α, IL-1β), MMP-9/2–mediated extracellular matrix degradation, and HPA axis dysregulation. Emerging chronotherapeutic strategies—including melatonin receptor agonists, time-restricted feeding, and targeted temperature modulation—show translational potential for enhancing BBB repair after stroke. Circadian regulation influences all phases of drug disposition (ADME) and receptor availability, thereby shaping therapeutic efficacy across time. Recent studies highlight circadian-targeted approaches for BBB repair after stroke, including mesenchymal stem cell–derived exosomes (160) and herbal compounds (161, 162), which act in part by modulating clock gene expression. Chronopharmacological dosing that aligns with circadian fluctuations in BBB permeability may improve treatment outcomes. For instance, scheduling thrombolytic therapy to coincide with peak permeability could reduce hemorrhagic complications (163, 164), while nanocarrier-based systems show enhanced BBB penetration during specific circadian windows (165, 166). Additional non-pharmacological interventions, including photobiomodulation (167–169) and circadian-aligned feeding (170–172), can restore endogenous rhythmicity and offer new avenues for neurorehabilitation.
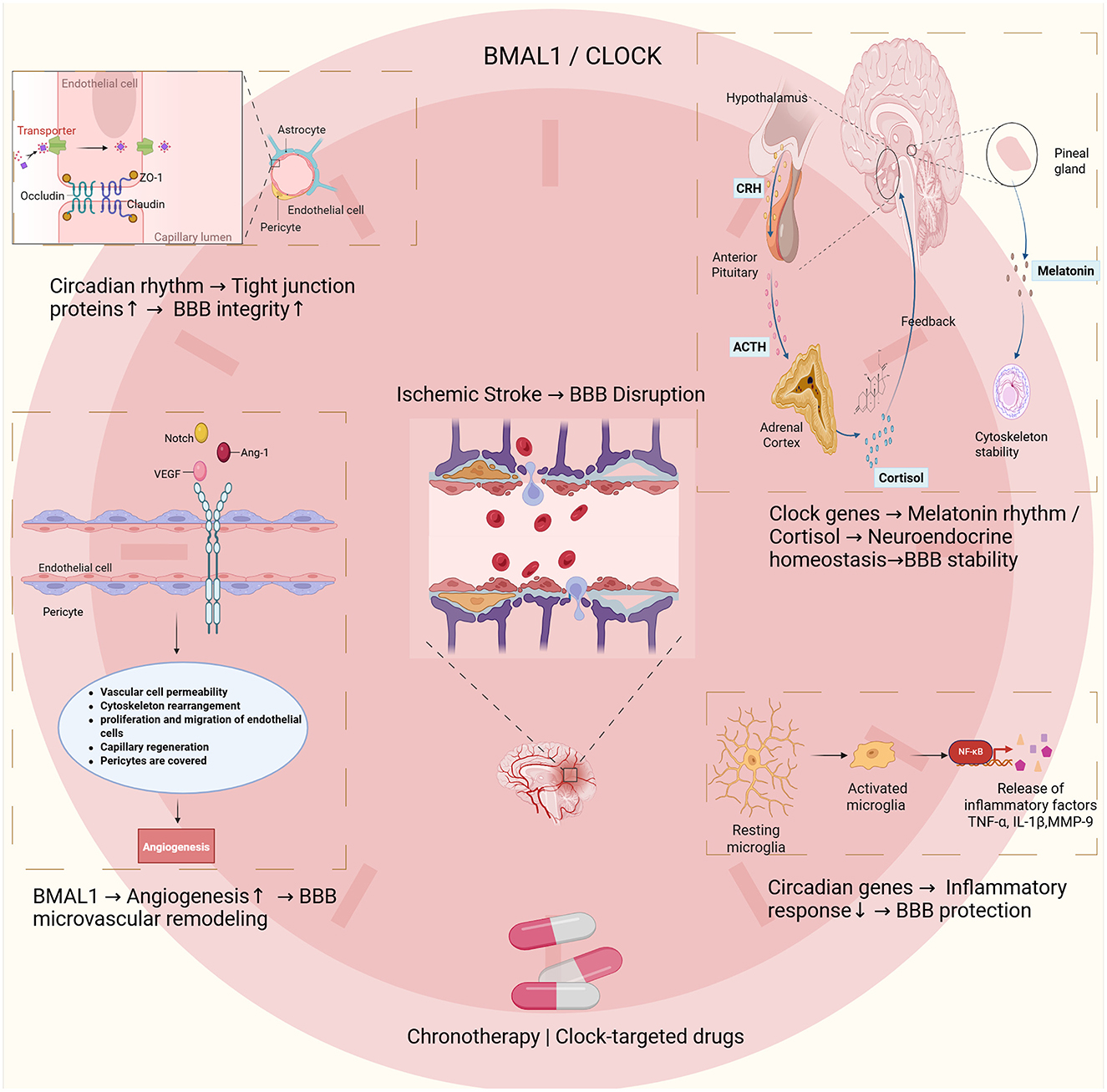
Figure 1. This schematic illustrates the interactions between circadian rhythms, blood–brain barrier (BBB) integrity, and ischemic stroke. The core circadian regulators BMAL1 and CLOCK play central roles in these processes. At the molecular level, circadian proteins maintain BBB integrity by regulating tight junction components such as occludin, claudin-5, and ZO-1. These effects are observed in endothelial cells, pericytes, and astrocytes. After ischemic injury, BMAL1 promotes angiogenesis by regulating vascular permeability, cytoskeletal remodeling, endothelial proliferation, and pericyte recruitment. These processes support microvascular remodeling and BBB restoration. Circadian genes also regulate neuroendocrine function, coordinating rhythmic melatonin and cortisol release. Clock genes modulate the hypothalamic–pituitary–adrenal (HPA) axis, mediated by CRH, ACTH, and cortisol, thereby influencing neuroendocrine balance and BBB function. Circadian regulation also limits neuroinflammation by restraining microglial activation and reducing the release of mediators such as TNF-α, IL-1β, and MMP-9. These mechanisms provide a rationale for developing chronotherapy and circadian-based pharmacological strategies to improve BBB outcomes after ischemic stroke.
Despite recent progress, the mechanistic links between circadian rhythms and BBB dynamics remain incompletely understood, and current animal models are limited by interspecies physiological differences. Standardized methods for evaluating BBB injury and repair after ischemia are also lacking, further complicated by interindividual variability and uncontrolled confounders. Bridging these gaps will require interdisciplinary approaches that integrate three complementary axes: mechanistic dissection of circadian–BBB interactions, clinical validation of chronotherapeutic strategies, and the development of translational technologies. Key translational needs include stroke models with greater human relevance, practical tools for BBB monitoring, and therapeutic protocols optimized for circadian timing. Future work should emphasize high-resolution mapping of BBB rhythmicity, precision chronotherapy tailored to individual circadian signatures, and the evidence-based incorporation of traditional Chinese medicine (TCM) into circadian biology frameworks. In conclusion, advancing our understanding of circadian regulation of BBB repair will not only deepen insight into stroke pathogenesis but also enable the design of temporally targeted treatments. Integrating modern chronobiology with traditional chronomedicine offers a promising path toward more precise and effective interventions, with the potential to improve post-stroke recovery and long-term outcomes.
Author contributions
YL: Writing – original draft. LLu: Writing – review & editing. QM: Writing – review & editing. XG: Funding acquisition, Writing – review & editing. YC: Funding acquisition, Writing – review & editing. SY: Funding acquisition, Writing – review & editing. MH: Supervision, Writing – review & editing. LLi: Conceptualization, Writing – review & editing. DZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (grant number 82104766), the Hunan Provincial Natural Science Foundation (grant numbers 2024JJ5313 and 2025JJ80981), the Health China-Buchang Zhiyuan Heart and Brain Health Public Welfare Project (grant number HIGHER2023041), and the Hunan University of Chinese Medicine Graduate Innovation Project (grant number 2024CX114).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. The authors acknowledge the use of ChatGPT (OpenAI, San Francisco, CA, USA) as an AI-assisted language editing tool for grammar correction, sentence restructuring and improvement of fluency during manuscript preparation. No AI tools were used to generate original scientific content, research ideas, data, or interpretations. All scientific concepts, analyses, and conclusions were conceived and developed solely by the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2021 Diseases and Injuries Collaborators (2024). Global incidence, prevalence, years lived with 747disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) 748for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 7491990-2021: a systematic analysis for the Global Burden of Disease Study. Lancet. (2021) 403:7502133–61. doi: 10.1016/S0140-6736(24)00757-8
2. Zong P, Feng J, Yue Z, Li Y, Wu G, Sun B, et al. Functional coupling of TRPM2 and extrasynaptic NMDARs exacerbates excitotoxicity in ischemic brain injury. Neuron. (2022) 110:1944–58.e8. doi: 10.1016/j.neuron.2022.03.021
3. Ayuso-Dolado S, Esteban-Ortega GM, Vidaurre ÓG, Díaz-Guerra M. A novel cell-penetrating peptide targeting calpain-cleavage of PSD-95 induced by excitotoxicity improves neurological outcome after stroke. Theranostics. (2021) 11:6746–65. doi: 10.7150/thno.60701
4. Yang XM, Yu H, Li JX, Li N, Li C, Xu DH, et al. Excitotoxic storms of ischemic stroke: a non-neuronal perspective. Mol Neurobiol. (2024) 61:9562–81. doi: 10.1007/s12035-024-04184-7
5. Mohamud Yusuf A, Borbor M, Hussner T, Weghs C, Kaltwasser B, Pillath-Eilers M, et al. Acid sphingomyelinase inhibition induces cerebral angiogenesis post-ischemia/reperfusion in an oxidative stress-dependent way and promotes endothelial survival by regulating mitochondrial metabolism. Cell Death Dis. (2024) 15:650. doi: 10.1038/s41419-024-06935-9
6. Kadri S, El Ayed M, Kadri A, Limam F, Aouani E, Mokni M. Protective effect of grape seed extract and orlistat co-treatment against stroke: Effect on oxidative stress and energy failure. Biomed Pharmacother. (2021) 136:111282. doi: 10.1016/j.biopha.2021.111282
7. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. (2021) 18:666–82. doi: 10.1038/s41569-021-00552-1
8. Denorme F, Portier I, Rustad JL, Cody MJ, de Araujo CV, Hoki C, et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. (2022) 132:e154225. doi: 10.1172/JCI154225
9. Kumari S, Dhapola R, Sharma P, Nagar P, Medhi B, HariKrishnaReddy D. The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor Rev. (2024) 78:105–19. doi: 10.1016/j.cytogfr.2024.06.002
10. Geng YQ, Qiu LN, Cheng YQ, Li JJ, Ma YL, Zhao CC, et al. Alleviating recombinant tissue plasminogen activator-induced hemorrhagic transformation in ischemic stroke via targeted delivery of a ferroptosis inhibitor. Adv Sci. (2024) 11:e2309517. doi: 10.1002/advs.202309517
11. Arkelius K, Wendt TS, Andersson H, Arnou A, Gottschalk M, Gonzales RJ, et al. LOX-1 and MMP-9 inhibition attenuates the detrimental effects of delayed rt-PA therapy and improves outcomes after acute ischemic stroke. Circ Res. (2024) 134:954–69. doi: 10.1161/CIRCRESAHA.123.323371
12. Zhu Z, Lu H, Jin L, Gao Y, Qian Z, Lu P, et al. C-176 loaded Ce DNase nanoparticles synergistically inhibit the cGAS-STING pathway for ischemic stroke treatment. Bioact Mater. (2023) 29:230–40. doi: 10.1016/j.bioactmat.2023.07.002
13. Broderick JP, Hill MD. Advances in acute stroke treatment 2020. Stroke. (2021) 52:729–34. doi: 10.1161/STROKEAHA.120.033744
14. Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. (2018) 315:C343–C356. doi: 10.1152/ajpcell.00095.2018
15. Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. (2019) 16:178. doi: 10.1186/s12974-019-1571-8
16. Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. (2019) 18:1058–66. doi: 10.1016/S1474-4422(19)30078-X
17. Leigh R, Seners P, Rousseau V, Christensen S, Albucher JF, Drif A, et al. Blood-brain barrier profile pretreatment is associated with hemorrhagic transformation after endovascular reperfusion. Ann Clini Transl Neurol. (2024) 11:3292–9. doi: 10.1002/acn3.52236
18. Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. (2019). Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 16:142. doi: 10.1186/s12974-019-1516-2
19. Rost NS, Cougo P, Lorenzano S, Li H, Cloonan L, Bouts MJ, et al. Diffuse microvascular dysfunction and loss of white matter integrity predict poor outcomes in patients with acute ischemic stroke. J Cereb Blood Flow Metabol. (2018) 38:75–86. doi: 10.1177/0271678X17706449
20. Pedder JH, Sonabend AM, Cearns MD, Michael BD, Zakaria R, Heimberger AB, et al. Crossing the blood-brain barrier: emerging therapeutic strategies for neurological disease. Lancet Neurol. (2025) 24:246–60. doi: 10.1016/S1474-4422(24)00476-9
21. Qi L, Wang F, Sun X, Li H, Zhang K, Li J. Recent advances in tissue repair of the blood-brain barrier after stroke. J Tissue Eng. (2024) 15:20417314241226551. doi: 10.1177/20417314241226551
22. Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. (2018) 135:311–36. doi: 10.1007/s00401-018-1815-1
23. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. (2010) 37:13–25. doi: 10.1016/j.nbd.2009.07.030
24. Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. (2015) 209:493–506. doi: 10.1083/jcb.201412147
25. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. (2019) 99:21–78. doi: 10.1152/physrev.00050.2017
26. Cuddapah VA, Zhang SL, Sehgal A. Regulation of the blood-brain barrier by circadian rhythms and sleep. Trends Neurosci. (2019) 42:500–10. doi: 10.1016/j.tins.2019.05.001
27. Li Y, Liu B, Zhao T, Quan X, Han Y, Cheng Y, et al. Comparative study of extracellular vesicles derived from mesenchymal stem cells and brain endothelial cells attenuating blood-brain barrier permeability via regulating Caveolin-1-dependent ZO-1 and Claudin-5 endocytosis in acute ischemic stroke. J Nanobiotechnology. (2023) 21:70. doi: 10.1186/s12951-023-01828-z
28. Zhong K, An X, Kong Y, Chen Z. Predictive model for the risk of hemorrhagic transformation after rt-PA intravenous thrombolysis in patients with acute ischemic stroke: A systematic review and meta-analysis. Clin Neurol Neurosurg. (2024) 239:108225. doi: 10.1016/j.clineuro.2024.108225
29. Kyoko OO, Kono H, Ishimaru K, Miyake K, Kubota T, Ogawa H, et al. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS ONE. (2014) 9:e98016. doi: 10.1371/journal.pone.0098016
30. Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. (1972) 42:201–6. doi: 10.1016/0006-8993(72)90054-6
31. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. (2002) 418:935–41. doi: 10.1038/nature00965
32. Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. (2018) 359:eaao0318. doi: 10.1126/science.aao0318
33. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. (2012) 35:445–62. doi: 10.1146/annurev-neuro-060909-153128
34. Fagiani F, Di Marino D, Romagnoli A, Travelli C, Voltan D, Di Cesare Mannelli L, et al. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transd Target Ther. (2022) 7:41. doi: 10.1038/s41392-022-00899-y
35. Shafi AA, McNair CM, McCann JJ, Alshalalfa M, Shostak A, Severson TM, et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat Commun. (2021) 12:401. doi: 10.1038/s41467-020-20513-5
36. Sun Q, Yang Y, Wang Z, Yang X, Gao Y, Zhao Y, et al. PER1 interaction with GPX1 regulates metabolic homeostasis under oxidative stress. Redox Biol. (2020) 37:101694. doi: 10.1016/j.redox.2020.101694
37. Zielinski MR, Gibbons AJ. Neuroinflammation, sleep, circadian rhythms. Front Cell Infect Microbiol. (2022) 12:853096. doi: 10.3389/fcimb.2022.853096
38. Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials. (2013) 34:9201–9. doi: 10.1016/j.biomaterials.2013.08.007
39. Preston R, Meng Q J, Lennon R. The dynamic kidney matrisome - is the circadian clock in control? Matrix Biol. (2022) 114:138–55. doi: 10.1016/j.matbio.2022.05.005
40. Streuli CH, Meng QJ. Influence of the extracellular matrix on cell-intrinsic circadian clocks. J Cell Sci. (2019) 132:jcs207498. doi: 10.1242/jcs.207498
41. Mastrullo V, van der Veen DR, Gupta P, Matos RS, Johnston JD, McVey JH, et al. Pericytes' circadian clock affects endothelial cells' synchronization and angiogenesis in a 3D tissue engineered scaffold. Front Pharmacol. (2022) 13:867070. doi: 10.3389/fphar.2022.867070
42. Faraci FM, Scheer FAJL. Hypertension: causes and consequences of circadian rhythms in blood pressure. Circ Res. (2024) 134:810–32. doi: 10.1161/CIRCRESAHA.124.323515
43. Crnko S, Du Pré BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol. (2019) 16:437–47. doi: 10.1038/s41569-019-0167-4
44. Webb AJS, Klerman EB, Mandeville ET. Circadian and diurnal regulation of cerebral blood flow. Circ Res. (2024) 134:695–710. doi: 10.1161/CIRCRESAHA.123.323049
45. Zhang Y, Zhao X, Guo C, Zhang Y, Zeng F, Yin Q, et al. The circadian system is essential for the crosstalk of VEGF-notch-mediated endothelial angiogenesis in ischemic stroke. Neurosci Bull. (2023) 39:1375–95. doi: 10.1007/s12264-023-01042-9
46. Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. (2001) 68:2705–14. doi: 10.1016/S0024-3205(01)01085-2
47. Pan W, Cornélissen G, Halberg F, Kastin AJ. Selected contribution: circadian rhythm of tumor necrosis factor-alpha uptake into mouse spinal cord. J Appl Physiol. (2002) 92:1357–62. doi: 10.1152/japplphysiol.00915.2001
48. Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, et al. Regulation of amyloid-β dynamics and pathology by the circadian clock. J Exp Med. (2018) 215:1059–68. doi: 10.1084/jem.20172347
49. Banks WA, Kastin AJ, Selznick JK. Modulation of immunoactive levels of DSIP and blood-brain permeability by lighting and diurnal rhythm. J Neurosci Res. (1985) 14:347–55. doi: 10.1002/jnr.490140307
50. Pandey HP, Ram A, Matsumura H, Hayaishi O. Concentration of prostaglandin D2 in cerebrospinal fluid exhibits a circadian alteration in conscious rats. Biochem Mol Biol Int. (1995) 37:431–7.
51. Zhang SL, Lahens NF, Yue Z, Arnold DM, Pakstis PP, Schwarz JE, et al. A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat Commun. (2021) 12:617. doi: 10.1038/s41467-020-20795-9
52. Rodríguez-Cortés B, Hurtado-Alvarado G, Martínez-Gómez R, León-Mercado LA, Prager-Khoutorsky M, Buijs RM. Suprachiasmatic nucleus-mediated glucose entry into the arcuate nucleus determines the daily rhythm in blood glycemia. Curr Biol. (2022) 32:796–805. doi: 10.1016/j.cub.2021.12.039
53. Wada Y, Inoko M, Ishihara K, Fukumoto K, Tsurudome Y, Horiguchi M, et al. Aging reduces ATP-binding cassette transporter expression in brain microvessels of mice. Pharmaceuticals. (2025) 18:191. doi: 10.3390/ph18020191
54. Savolainen H, Meerlo P, Elsinga PH, Windhorst AD, Dierckx RA, Colabufo NA, et al. P-glycoprotein function in the rodent brain displays a daily rhythm, a quantitative in vivo PET study. AAPS J. (2016) 18:1524–31. doi: 10.1208/s12248-016-9973-3
55. Pulido RS, Munji RN, Chan TC, Quirk CR, Weiner GA, Weger BD, et al. Neuronal activity regulates blood-brain barrier efflux transport through endothelial circadian genes. Neuron. (2020) 108:937–52.e7. doi: 10.1016/j.neuron.2020.09.002
56. Liu JA, Walton JC, DeVries AC, Nelson RJ. Disruptions of circadian rhythms and thrombolytic therapy during ischemic stroke intervention. Front Neurosci. (2021) 15:675732. doi: 10.3389/fnins.2021.675732
57. Rabinovich-Nikitin I, Kirshenbaum LA. BMAL1 regulates cell cycle progression and angiogenesis of endothelial cells. Cardiovasc Res. (2023) 119:1889–90. doi: 10.1093/cvr/cvad103
58. Astone M, Oberkersch RE, Tosi G, Biscontin A, Santoro MM. The circadian protein BMAL1 supports endothelial cell cycle during angiogenesis. Cardiovasc Res. (2023) 119:1952–68. doi: 10.1093/cvr/cvad057
59. Bhatwadekar AD, Beli E, Diao Y, Chen J, Luo Q, Alex A, et al. Conditional deletion of bmal1 accentuates microvascular and macrovascular injury. Am J Pathol. (2017) 187:1426–35. doi: 10.1016/j.ajpath.2017.02.014
60. Zhu JQ, Fan SR, Wei X, Zhang CX, Zhang DM, Chen MF, et al. Synthesis and biological evaluation of marine natural product, Cryptoechinuline D derivatives as novel antiangiogenic agents. Bioorg Med Chem Lett. (2022) 65:128717. doi: 10.1016/j.bmcl.2022.128717
61. Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB, et al. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology. (2021) 161:1924–39. doi: 10.1053/j.gastro.2021.08.047
62. Zhao Y, Gan L, Ren L, Lin Y, Ma C, Lin X. Factors influencing the blood-brain barrier permeability. Brain Res. (2022) 1788:147937. doi: 10.1016/j.brainres.2022.147937
63. Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood-brain barrier breakdown. Acta Neuropathol. (2008) 115:635–42. doi: 10.1007/s00401-008-0364-4
64. Li R, Liu Y, Wu J, Chen X, Lu Q, Xia K, et al. Adaptive metabolic responses facilitate blood-brain barrier repair in ischemic stroke via BHB-mediated epigenetic modification of ZO-1 expression. Adv Sci. (2024) 11:e2400426. doi: 10.1002/advs.202400426
65. Pimentel E, Sivalingam K, Doke M, Samikkannu T. Effects of drugs of abuse on the blood-brain barrier: a brief overview. Front Neurosci. (2020) 14:513. doi: 10.3389/fnins.2020.00513
66. Yan L, Dwiggins CW, Moriarty RA, Jung JW, Gupta U, Brandon KD, et al. Matrix stiffness regulates the tight junction phenotypes and local barrier properties in tricellular regions in an iPSC-derived BBB model. Acta Biomater. (2023) 167:109–20. doi: 10.1016/j.actbio.2023.06.003
67. Fellows RC, Chun SK, Larson N, Fortin BM, Mahieu AL, Song WA, et al. Disruption of the intestinal clock drives dysbiosis and impaired barrier function in colorectal cancer. Sci Adv. (2024) 10:eado1458. doi: 10.1126/sciadv.ado1458
68. Spadoni I, Pietrelli A, Pesole G, Rescigno M. Gene expression profile of endothelial cells during perturbation of the gut vascular barrier. Gut Microbes. (2016) 7:540–8. doi: 10.1080/19490976.2016.1239681
69. Hudson N, Celkova L, Hopkins A, Greene C, Storti F, Ozaki E, et al. Dysregulated claudin-5 cycling in the inner retina causes retinal pigment epithelial cell atrophy. JCI Insight. (2019) 4:e130273. doi: 10.1172/jci.insight.130273
70. Jensen LD, Cao Z, Nakamura M, Yang Y, Bräutigam L, Andersson P, et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep. (2012) 2:231–41. doi: 10.1016/j.celrep.2012.07.005
71. Carús-Cadavieco M, González de la Fuente S, Berenguer López I, Serrano-Lope MA, Aguado B, Guix F, et al. Loss of Cldn5 -and increase in Irf7-in the hippocampus and cerebral cortex of diabetic mice at the early symptomatic stage. Nutr Diabetes. (2024) 14:64. doi: 10.1038/s41387-024-00325-y
72. Xing C, Zhou Y, Xu H, Ding M, Zhang Y, Zhang M, et al. Sleep disturbance induces depressive behaviors and neuroinflammation by altering the circadian oscillations of clock genes in rats. Neurosci Res. (2021) 171:124–32. doi: 10.1016/j.neures.2021.03.006
73. Bishehsari F, Preuss F, Mirbagheri SS, Zhang L, Shaikh M, Keshavarzian A. Interaction of alcohol with time of eating on markers of circadian dyssynchrony and colon tissue injury. Chem Biol Interact. (2020) 325:109132. doi: 10.1016/j.cbi.2020.109132
74. Tsuzuki K, Shimizu Y, Suzuki J, Pu Z, Yamaguchi S, Fujikawa Y, et al. Adverse effect of circadian rhythm disorder on reparative angiogenesis in hind limb ischemia. J Am Heart Assoc. (2021) 10:e020896. doi: 10.1161/JAHA.121.020896
75. Kunze R, Marti HH. Angioneurins - Key regulators of blood-brain barrier integrity during hypoxic and ischemic brain injury. Prog Neurobiol. (2019) 178:101611. doi: 10.1016/j.pneurobio.2019.03.004
76. Schurhoff N, Toborek M. Circadian rhythms in the blood-brain barrier: impact on neurological disorders and stress responses. Mol Brain. (2023) 16:5. doi: 10.1186/s13041-023-00997-0
77. Zechariah A, ElAli A, Doeppner TR, Jin F, Hasan MR, Helfrich I, et al. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke. (2013) 44:1690–7. doi: 10.1161/STROKEAHA.111.000240
78. Jidigam VK, Sawant OB, Fuller RD, Wilcots K, Singh R, Lang RA, et al. Neuronal Bmal1 regulates retinal angiogenesis and neovascularization in mice. Commun Biol. (2022) 5:792. doi: 10.1038/s42003-022-03774-2
79. Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, et al. Disruption of Bmal1 Impairs Blood-Brain Barrier Integrity via Pericyte Dysfunction. J Neurosci. (2017) 37:10052–62. doi: 10.1523/JNEUROSCI.3639-16.2017
80. Crnko S, Cour M, Van Laake LW, Lecour S. Vasculature on the clock: circadian rhythm and vascular dysfunction. Vascul Pharmacol. (2018) 108:1–7. doi: 10.1016/j.vph.2018.05.003
81. Jensen LD, Cao Y. Clock controls angiogenesis. Cell Cycle. (2013) 12:405–8. doi: 10.4161/cc.23596
82. Tang J, Kang Y, Zhou Y, Shang N, Li X, Wang H, et al. TIMP2 ameliorates blood-brain barrier disruption in traumatic brain injury by inhibiting Src-dependent VE-cadherin internalization. J Clin Invest. (2023) 134:e164199. doi: 10.1172/JCI164199
83. Koyanagi S, Kuramoto Y, Nakagawa H, et al. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res. (2003) 63:7277–83.
84. Sato F, Bhawal UK, Kawamoto T, Fujimoto K, Imaizumi T, Imanaka T, et al. Basic-helix-loop-helix (bHLH) transcription factor DEC2 negatively regulates vascular endothelial growth factor expression. Genes Cells. (2008) 13:131–44. doi: 10.1111/j.1365-2443.2007.01153.x
85. Harding BN, Aguilar R, Espinosa A, Castaño-Vinyals G, Papantoniou K, Navarrete JM, et al. Disruption of cellular immune response among male rotating night shift workers in Spain- the HORMONIT study. Front Immunol. (2022) 13:776917. doi: 10.3389/fimmu.2022.776917
86. Hirai T, Nomura K, Ikai R, Nakashima KI, Inoue M. Baicalein stimulates fibroblast growth factor 21 expression by up-regulating retinoic acid receptor-related orphan receptor α in C2C12 myotubes. Biomed Pharmacother. (2019) 109:503–10. doi: 10.1016/j.biopha.2018.10.154
87. Li D, Xie K, Zhang L, Yao X, Li H, Xu Q, et al. Dual blockade of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF-2) exhibits potent anti-angiogenic effects. Cancer Lett. (2016) 377:164–73. doi: 10.1016/j.canlet.2016.04.036
88. Duris K, Splichal Z, Jurajda M. The role of inflammatory response in stroke associated programmed cell death. Curr Neuropharmacol. (2018) 16:1365–74. doi: 10.2174/1570159X16666180222155833
89. Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. (2019) 35:921–33. doi: 10.1007/s12264-019-00388-3
90. Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. (2007) 50:941–53. doi: 10.1016/j.neuint.2007.04.026
91. Zhu H, Hu S, Li Y, Sun Y, Xiong X, Hu X, et al. Interleukins and ischemic stroke. Front Immunol. (2022) 13:828447. doi: 10.3389/fimmu.2022.828447
92. Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. (2019) 20:227–41. doi: 10.1038/s41580-018-0096-9
93. Tomar MS, Mohit, Kumar A, Shrivastava. A circadian immunometabolism: a future insight for targeted therapy in cancer. Sleep Med Rev. (2025) 80:102031. doi: 10.1016/j.smrv.2024.102031
94. Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. (2007) 30:621–6. doi: 10.1248/bpb.30.621
95. Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol. (2018) 18:423–37. doi: 10.1038/s41577-018-0008-4
96. Madore C, Yin Z, Leibowitz J, Butovsky O. Microglia lifestyle stress, and neurodegeneration. Immunity. (2020) 52:222–40. doi: 10.1016/j.immuni.2019.12.003
97. Lu W, Wen J. Crosstalk among glial cells in the blood-brain barrier injury after ischemic stroke. Mol Neurobiol. (2024) 61:6161–74. doi: 10.1007/s12035-024-03939-6
98. Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. (2006) 37:1087–93. doi: 10.1161/01.STR.0000206281.77178.ac
99. Rosell A, Cuadrado E, Ortega-Aznar A, Hernández-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. (2008) 39:1121–6. doi: 10.1161/STROKEAHA.107.500868
100. Hand LE, Gray KJ, Dickson SH, Simpkins DA, Ray DW, Konkel JE, et al. Regulatory T cells confer a circadian signature on inflammatory arthritis. Nat Commun. (2020) 11:1658. doi: 10.1038/s41467-020-15525-0
101. Li P, Mao L, Liu X, Gan Y, Zheng J, Thomson AW, et al. Essential role of program death 1-ligand 1 in regulatory T-cell-afforded protection against blood-brain barrier damage after stroke. Stroke. (2014) 45:857–64. doi: 10.1161/STROKEAHA.113.004100
102. Medrano-Bosch M, Simón-Codina B, Jiménez W, Edelman ER, Melgar-Lesmes P. Monocyte-endothelial cell interactions in vascular and tissue remodeling. Front Immunol. (2023) 14:1196033. doi: 10.3389/fimmu.2023.1196033
103. Greene C, Connolly R, Brennan D. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat Neurosci. (2024) 27:421–32. doi: 10.1038/s41593-024-01576-9
104. Pan J, Wang Z, Huang X, Xue J, Zhang S, Guo X, et al. Bacteria-derived outer-membrane vesicles hitchhike neutrophils to enhance ischemic stroke therapy. Adv Mater. (2023) 35:e2301779. doi: 10.1002/adma.202301779
105. Gao H, Cao M, Chen P, Cooper DKC, Zhao Y, Wei L, et al. TNF-α promotes human antibody-mediated complement-dependent cytotoxicity of porcine endothelial cells through downregulating P38-mediated Occludin expression. Cell Commun Signal. (2019) 17:75. doi: 10.1186/s12964-019-0386-7
106. Mccoll BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. (2008) 28:9451–62. doi: 10.1523/JNEUROSCI.2674-08.2008
107. Choi BR, Johnson KR, Maric D, McGavern DB. Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature. Nat Immunol. (2023) 24:1110–23. doi: 10.1038/s41590-023-01521-1
108. Bernstein DL. Rom Let-7g* S, and miR-98 reduce stroke-induced production of proinflammatory cytokines in mouse brain. Front Cell Dev Biol. (2020) 8:632. doi: 10.3389/fcell.2020.00632
109. Chen S, Fuller KK, Dunlap JC, Loros JJ. A pro- and anti-inflammatory axis modulates the macrophage circadian clock. Front Immunol. (2020) 11:867. doi: 10.3389/fimmu.2020.00867
110. Everett LJ, Lazar MA. Nuclear receptor Rev-erbα: up, down, all around. Trends Endocrinol Metab. (2014) 25:586–92. doi: 10.1016/j.tem.2014.06.011
111. Tu Y, Yang Z, He Y, Wang T, Hua P, Yao Q, et al. Circadian rhythm disruption promotes M1 macrophages polarization exacerbating the inflammatory response in rosacea. Arch Dermatol Res. (2025) 317:658. doi: 10.1007/s00403-025-04060-x
112. Candelario-Jalil E, Dijkhuizen R M, Magnus T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, Imaging Modalities. Stroke. (2022) 53:1473–86. doi: 10.1161/STROKEAHA.122.036946
113. Ding J, Chen P, Qi C. Circadian rhythm regulation in the immune system. Immunology. (2024) 171:525–33. doi: 10.1111/imm.13747
114. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. (2005) 437:1257–63. doi: 10.1038/nature04284
115. Greco CM, Sassone-Corsi P. Circadian blueprint of metabolic pathways in the brain. Nat Rev Neurosci. (2019) 20:71–82. doi: 10.1038/s41583-018-0096-y
116. Belle MDC. Circadian tick-talking across the neuroendocrine system and suprachiasmatic nuclei circuits: the enigmatic communication between the molecular and electrical membrane clocks. J Neuroendocrinol. (2015) 27:567–76. doi: 10.1111/jne.12279
117. Olejniczak I, Begemann K, Wilhelm I, Oster H. The circadian neurobiology of reward. Acta Physiologica. (2023) 237:e13928. doi: 10.1111/apha.13928
118. Cervantes M, Lewis RG, Della-Fazia MA, Borrelli E, Sassone-Corsi P. Dopamine D2 receptor signaling in the brain modulates circadian liver metabolomic profiles. Proc Natl Acad Sci U S A. (2022) 119:e2117113119. doi: 10.1073/pnas.2117113119
119. Jones JR, Chaturvedi S, Granados-Fuentes D, Herzog ED. Circadian neurons in the paraventricular nucleus entrain and sustain daily rhythms in glucocorticoids. Nat Commun. (2021) 12:5763. doi: 10.1038/s41467-021-25959-9
120. Welcome MO, Mastorakis NE. Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol Res. (2020) 157:104769. doi: 10.1016/j.phrs.2020.104769
121. Focke CMB, Iremonger KJ. Rhythmicity matters: circadian and ultradian patterns of HPA axis activity. Mol Cell Endocrinol. (2020) 501:110652. doi: 10.1016/j.mce.2019.110652
122. Pfau SJ, Langen UH, Fisher TM, Prakash I, Nagpurwala F, Lozoya RA, et al. Characteristics of blood-brain barrier heterogeneity between brain regions revealed by profiling vascular and perivascular cells. Nat Neurosci. (2024) 27:1892–903. doi: 10.1038/s41593-024-01743-y
123. Wang Y, Sabbagh MF, Gu X, Rattner A, Williams J, Nathans J. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. Elife. (2019) 8:e43257. doi: 10.7554/eLife.43257
124. Anbalagan S, Gordon L, Blechman J, Matsuoka RL, Rajamannar P, Wircer E, et al. Pituicyte cues regulate the development of permeable neuro-vascular interfaces. Dev Cell. (2018) 47:711–726. doi: 10.1016/j.devcel.2018.10.017
125. Xie YY, Lu YW, Yu GR. The protective effects of hyperoside on Ang II-mediated apoptosis of bEnd.3 cells and injury of blood-brain barrier model in vitro. BMC Complement Med Therap. 22:157. doi: 10.1186/s12906-022-03635-9
126. Zhang SL, Yue Z, Arnold DM, Artiushin G, Sehgal A. A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell. (2018) 173:130–9. doi: 10.1016/j.cell.2018.02.017
127. Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. (2017) 38:3–45. doi: 10.1210/er.2015-1080
128. Clarke SA, Eng PC, Comninos AN, Lazarus K, Choudhury S, Tsang C, et al. Current challenges and future directions in the assessment of glucocorticoid status. Endocr Rev. (2024) 45:795–817. doi: 10.1210/endrev/bnae016
129. Shimba A, Cui G, Tani-Ichi S, Ogawa M, Abe S, Okazaki F, et al. Glucocorticoids Drive Diurnal Oscillations in T Cell Distribution and Responses by Inducing Interleukin-7 Receptor and CXCR4. Immunity. (2018) 48:286–98. doi: 10.1016/j.immuni.2018.01.004
130. Olejniczak I, Oster H, Ray DW. Glucocorticoid circadian rhythms in immune function. Semin Immunopathol. (2022) 44:153–63. doi: 10.1007/s00281-021-00889-2
131. Shimba A, Ejima A, Ikuta K. Pleiotropic effects of glucocorticoids on the immune system in circadian rhythm and stress. Front Immunol. (2021) 12:706951. doi: 10.3389/fimmu.2021.706951
132. Shimba A, Ikuta K. Immune-enhancing effects of glucocorticoids in response to day-night cycles and stress. Int Immunol. (2020) 32:703–8. doi: 10.1093/intimm/dxaa048
133. Ling LJ, Zhou Q, Zhang F, Lei WJ, Li MD, Lu JW, et al. The dual role of glucocorticoid regeneration in inflammation at parturition. Front Immunol. (2024) 15:1459489. doi: 10.3389/fimmu.2024.1459489
134. Jagot F, Gaston-Breton R, Choi AJ, Pascal M, Bourhy L, Dorado-Doncel R, et al. The parabrachial nucleus elicits a vigorous corticosterone feedback response to the pro-inflammatory cytokine IL-1β. Neuron. (2023) 111:2367–82.e6. doi: 10.1016/j.neuron.2023.05.009
135. Menezes-Garcia Z, Do Nascimento Arifa RD, Acúrcio L, Brito CB, Gouvea JO, Lima RL, et al. Colonization by Enterobacteriaceae is crucial for acute inflammatory responses in murine small intestine via regulation of corticosterone production. Gut Microbes. (2020) 11:1531–46. doi: 10.1080/19490976.2020.1765946
136. Madelaire CB, Silva DP, Titon SCM, Lamadrid-Feris F, Floreste FR, Titon B, et al. Contrasting effects of transdermal and implant corticosterone treatments in the American bullfrog wound healing. Philos Trans R Soc Lond B Biol Sci. (2023) 378:20220119. doi: 10.1098/rstb.2022.0119
137. Niklasson F, Agren H. Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood[J]. Biol Psychiatry. (1984) 19:1183–206.
138. Ge J, He ML, Tang Y, Liu YM, Jin J, Zhang D. Relationship between circadian rhythm disorder of blood pressure and ischemic stroke. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. (2020) 42:831–5.
139. Lee BH, Hille B, Koh DS. Serotonin modulates melatonin synthesis as an autocrine neurotransmitter in the pineal gland. Proc Natl Acad Sci U S A. (2021) 118:e2113852118. doi: 10.1073/pnas.2113852118
140. Gehrman PR, Anafi RC. Treatment of a patient with a circadian sleep-wake disorder using a combination of melatonin and metoprolol. J Clini Sleep Med. (2021) 17:2121–4. doi: 10.5664/jcsm.9410
141. Yawoot N, Sengking J, Govitrapong P, Tocharus C, Tocharus J. Melatonin modulates the aggravation of pyroptosis, necroptosis, and neuroinflammation following cerebral ischemia and reperfusion injury in obese rats. Acta Molecular Basis of Disease. (2023) 1869:166785. doi: 10.1016/j.bbadis.2023.166785
142. Yip HK, Dubey NK, Lin KC, Sung PH, Chiang JY, Chu YC, et al. Melatonin rescues cerebral ischemic events through upregulated tunneling nanotube-mediated mitochondrial transfer and downregulated mitochondrial oxidative stress in rat brain. Biomed Pharmacother. (2021) 139:111593. doi: 10.1016/j.biopha.2021.111593
143. Chen KH, Chai HT, Chen CH, Huang CR, Chiang JY, Sung PH, et al. Synergic effect of combined cyclosporin and melatonin protects the brain against acute ischemic reperfusion injury. Biomed Pharmacother. (2021) 136:111266. doi: 10.1016/j.biopha.2021.111266
144. Wang J, Gao S, Lenahan C, Gu Y, Wang X, Fang Y, et al. Melatonin as an antioxidant agent in stroke: an updated review. Aging Dis. (2022) 13:1823–44. doi: 10.14336/AD.2022.0405
145. Wang X, Wang Z, Cao J, Dong Y, Chen Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. (2023) 11:17. doi: 10.1186/s40168-022-01452-3
146. Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, et al. Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, mitochondria injury. Transl Stroke Res. (2018) 9:74–91. doi: 10.1007/s12975-017-0559-x
147. Moretti R, Zanin A, Pansiot J, Spiri D, Manganozzi L, Kratzer I, et al. Melatonin reduces excitotoxic blood-brain barrier breakdown in neonatal rats. Neuroscience. (2015) 311:26542996. doi: 10.1016/j.neuroscience.2015.10.044
148. Lee AH, Tai SH, Huang SY, Chang LD, Chen LY, Chen YN, et al. Melatonin improves vasogenic edema via inhibition to water channel Aquaporin-4 (AQP4) and metalloproteinase-9 (MMP-9) following permanent focal cerebral ischemia. Biomedicines. (2024) 12:2184. doi: 10.3390/biomedicines12102184
149. Alluri H, Wilson RL, Anasooya Shaji C, Wiggins-Dohlvik K, Patel S, Liu Y, et al. Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. PLoS ONE. (2016) 11:e0154427. doi: 10.1371/journal.pone.0154427
150. Kilic E, Kilic U, Reiter RJ, Bassetti CL, Hermann DM. Tissue-plasminogen activator-induced ischemic brain injury is reversed by melatonin: role of iNOS and Akt. J Pineal Res. (2005) 39:151–5. doi: 10.1111/j.1600-079X.2005.00228.x
151. Chen TY, Lee MY, Chen HY, Kuo YL, Lin SC, Wu TS, et al. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J Pineal Res. (2006) 40:242–50. doi: 10.1111/j.1600-079X.2005.00307.x
152. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. (2017) 96:17–42. doi: 10.1016/j.neuron.2017.07.030
153. Kaplan L, Chow B W, Gu C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci. (2020) 21:416–32. doi: 10.1038/s41583-020-0322-2
154. Taylor KS, Murai H, Millar PJ, Haruki N, Kimmerly DS, Morris BL, et al. Arousal from sleep and sympathetic excitation during wakefulness. Hypertension. (2016) 68:1467–74. doi: 10.1161/HYPERTENSIONAHA.116.08212
155. Chrobok L, Northeast RC, Myung J, Cunningham PS, Petit C, Piggins HD. Timekeeping in the hindbrain: a multi-oscillatory circadian centre in the mouse dorsal vagal complex. Commun Biol. (2020) 3:225. doi: 10.1038/s42003-020-0960-y
156. Lightman SL, Conway-Campbell BL. Circadian and ultradian rhythms: Clinical implications. J Intern Med. (2024) 296:121–38. doi: 10.1111/joim.13795
157. Yang Y, Yang LY, Orban L, Cuylear D, Thompson J, Simon B, et al. Non-invasive vagus nerve stimulation reduces blood-brain barrier disruption in a rat model of ischemic stroke. Brain Stimul. (2018) 11:689–98. doi: 10.1016/j.brs.2018.01.034
158. Brem S. Vagus nerve stimulation: Novel concept for the treatment of glioblastoma and solid cancers by cytokine (interleukin-6) reduction, attenuating the SASP, enhancing tumor immunity. Brain, Behav Immunity - Health. (2024) 42:100859. doi: 10.1016/j.bbih.2024.100859
159. Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Extreme dipping: is the cardiovascular risk increased? An unsolved issue. J Hypertension. (2019) 37:1917–26. doi: 10.1097/HJH.0000000000002099
160. Qiu L, Cai Y, Geng Y, Yao X, Wang L, Cao H, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate tPA-induced blood-brain barrier disruption in murine ischemic stroke models. Acta Biomater. (2022) 154:424–42. doi: 10.1016/j.actbio.2022.10.022
161. Zhu W, Gong A, Zhang B, Cheng H, Huang L, Wu X, et al. The chronobiological and neuroprotective mechanisms of resveratrol in improving sleep. Mediators Inflamm. (2025) 2025:4954030. doi: 10.1155/mi/4954030
162. Zheng Y, Ren X, Qi X, Song R, Zhao C, Ma J, et al. Bao Yuan decoction alleviates fatigue by restraining inflammation and oxidative stress via the AMPK/CRY2/PER1 signaling pathway. J Ethnopharmacol. (2024) 328:118058. doi: 10.1016/j.jep.2024.118058
163. Mendelson SJ, Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA. (2021) 325:1088–98. doi: 10.1001/jama.2020.26867
164. Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the american heart Association/American Stroke Association. Stroke. (2017) 48:e343–61. doi: 10.1161/STR.0000000000000152
165. Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Overcoming the blood-brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. (2018) 30:e1801362. doi: 10.1002/adma.201801362
166. Fornaguera C, Dols-Perez A, Calderó G, García-Celma MJ, Camarasa J, Solans C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood-brain barrier. J Control Release. (2015) 211:134–43. doi: 10.1016/j.jconrel.2015.06.002
167. West A, Jennum P, Simonsen SA, Sander B, Pavlova M, Iversen HK. Impact of naturalistic lighting on hospitalized stroke patients in a rehabilitation unit: design and measurement. Chronobiol Int. (2017) 34:687–97. doi: 10.1080/07420528.2017.1314300
168. Faulkner SM, Bee PE, Meyer N, Dijk DJ, Drake RJ. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: a systematic review and meta-analysis. Sleep Med Rev. (2019) 46:108–23. doi: 10.1016/j.smrv.2019.04.012
169. Huang X, Tao Q, Ren C. A comprehensive overview of the neural mechanisms of light therapy. Neurosci Bull. (2024) 40:350–62. doi: 10.1007/s12264-023-01089-8
170. Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, et al. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS ONE. (2013) 8:e67102. doi: 10.1371/journal.pone.0067102
171. Huang R, Chen J, Zhou M, Xin H, Lam SM, Jiang X, et al. Multi-omics profiling reveals rhythmic liver function shaped by meal timing. Nat Commun. (2023) 14:6086. doi: 10.1038/s41467-023-41759-9
Keywords: biological rhythms, ischemic stroke, blood-brain barrier, angiogenesis, immune system, neuroendocrine system
Citation: Liao Y, Luo L, Ma Q, Gao X, Chen Y, Yan S, He M, Liu L and Zhou D (2025) Research progress on the correlation between biological rhythms and the blood-brain barrier after ischemic stroke. Front. Neurol. 16:1627172. doi: 10.3389/fneur.2025.1627172
Received: 12 May 2025; Accepted: 12 September 2025;
Published: 26 September 2025.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Richard Leigh, Johns Hopkins University, United StatesOzge Altintas Kadirhan, Kirklareli University, Türkiye
Copyright © 2025 Liao, Luo, Ma, Gao, Chen, Yan, He, Liu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Desheng Zhou, ZGVzaGVuZ3pob3VAaG51Y20uZWR1LmNu; Lijuan Liu, bGlqdWFubGl1QGhudWNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yuanchen Liao
Yuanchen Liao Lei Luo1†
Lei Luo1† Desheng Zhou
Desheng Zhou