- 1Department of Rehabilitation Medicine, Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, China
- 2Department of Endocrinology, Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, China
- 3Department of Obstetrics and Gynecology, Quanzhou Women’s and Children’s Hospital, Quanzhou, China
- 4Department of Cardiovascular Surgery, Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, China
Background: Dual-site transcranial magnetic stimulation (TMS) has emerged as a promising neuromodulation technique in stroke rehabilitation. By targeting multiple brain regions, dual-site TMS may enhance neuroplasticity more effectively than single-site stimulation. However, its clinical efficacy remains uncertain.
Objective: To systematically evaluate the effects of dual-site TMS in improving motor function and activities of daily living (ADL) in patients with stroke.
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) following PRISMA guidelines. Seven electronic databases were searched from inception to February 19, 2024. Studies comparing dual-site TMS with single-site TMS, sham dual-site TMS, or routine rehabilitation in stroke patients were included. Outcomes included Fugl-Meyer Assessment (FMA), FMA-Upper Limb (FMA-UL), Action Research Arm Test (ARAT), Barthel Index (BI), Modified Barthel Index (MBI), Wolf Motor Function Test (WMFT), and others. Methodological quality was assessed using the PEDro scale. Meta-analyses were performed using a random-effects model.
Results: Fourteen RCTs involving 724 participants were included. Dual-site TMS significantly improved upper limb motor function compared with single-site TMS (MD = 7.07, 95% CI: 1.46 to 12.68, p < 0.001) and sham dual-site TMS (MD = 14.45, 95% CI: 6.23 to 22.66, p < 0.001). ADL outcomes also favored dual-site TMS over single-site TMS (MD = 9.90, 95% CI: 7.82 to 11.98, p < 0.001) and sham dual-site TMS (MD = 21.13, 95% CI: 9.37 to 32.88, p < 0.001). Subgroup analyses suggested enhanced benefits in subacute phase stroke and in protocols with >20 sessions. Sensitivity analysis confirmed robustness of findings. No serious adverse events were reported.
Conclusion: Dual-site TMS combined with routine rehabilitation is more effective than single-site TMS or sham dual-site TMS in improving motor function and ADL among stroke patients. These findings support its clinical application as an adjunct to conventional therapy. Further high-quality trials are needed to optimize stimulation protocols and confirm long-term effects.
Introduction
Stroke remains one of the leading causes of mortality and long-term disability globally, according to the systematic analysis for the Global Burden of Disease Study 2021, there were 93·8 million stroke survivors, 11·9 million new stroke events, and 160·5 million disability-adjusted life-years (DALYs) from stroke, comprising 5·6% of all DALYs from all causes, the fourth leading cause of DALYs (1). Motor deficits, including hemiparesis and impaired coordination, affect approximately 80% of stroke survivors, severely limiting upper and lower limb function and diminishing independence in activities of daily living (ADL) (2). These deficits contribute to reduced quality of life, increased caregiver burden, and substantial socioeconomic costs, underscoring the urgent need for effective rehabilitation strategies (3, 4).
Conventional interventions, such as physical therapy, constraint-induced movement therapy, and robotic-assisted training, aim to promote neuroplasticity and functional recovery (5). However, their efficacy is often modest, particularly in patients with severe motor impairment or chronic-phase stroke, due to limited neural reorganization capacity and suboptimal engagement of residual neural networks (6). Non-invasive brain stimulation techniques, notably transcranial magnetic stimulation (TMS), have emerged as promising adjuncts to traditional therapies (7). By modulating cortical excitability and enhancing synaptic plasticity, TMS can facilitate motor recovery through mechanisms such as long-term potentiation (LTP), interhemispheric inhibition rebalancing, and activation of diaschisis-related pathways (8). High-frequency (≥5 Hz) rTMS (repetitive Transcranial Magnetic Stimulation) applied to the ipsilesional motor cortex (M1) or low-frequency (≤1 Hz) rTMS targeting the contralesional M1 has shown moderate benefits in improving limb function (9). In addition, intermittent Theta Burst Stimulation (iTBS) and continuous Theta Burst Stimulation (cTBS) show positive effect in improving motor function outcomes for stroke patients (10, 11). Notably, cerebellar TMS interventions have also demonstrated efficacy in enhancing post-stroke balance control and ADL (12), further expanding the clinical applicability of TMS strategies in stroke rehabilitation.
Despite these advances, single-site TMS protocols exhibit variable clinical outcomes, likely due to their inability to address the complex, distributed neural networks disrupted by stroke (13, 14). Motor recovery relies not only on local cortical excitability but also on interregional connectivity between motor, premotor, and cerebellar regions (15). Dual-site TMS, which simultaneously or sequentially stimulates two distinct brain targets (e.g., bilateral M1, or M1-cerebellum), may amplify therapeutic effects by synchronizing neural oscillations, enhancing cross-hemispheric communication, and promoting network-level reorganization (16). Motor recovery after stroke relies on the brain’s capacity for plasticity, and non-invasive brain stimulation (NIBS) methods such as rTMS, have shown promise in enhancing this plasticity by modulating cortical excitability (17, 18). Preclinical studies suggest that dual-site stimulation may induce stronger and more durable neuroplastic changes compared to single-site protocols, potentially by co-activating complementary pathways involved in motor planning and execution (19). Dual-site TMS has emerged as a promising non-invasive therapeutic intervention in stroke rehabilitation.
Current evidence on dual-site TMS in stroke rehabilitation remains fragmented. Some randomized trials report superior motor and ADL outcomes with dual-site versus single-site TMS or sham stimulation (20–23), however, significant variability in study designs, intervention parameters, patient characteristics, and outcome measures has made it challenging to reach a consensus on its overall effectiveness in stroke rehabilitation. A systematic synthesis of existing data is critical to clarify the efficacy of dual-site TMS, identify optimal protocols, and inform clinical recommendations. This meta-analysis aims to address three key questions: (1) Does dual-site TMS yield greater improvements in motor function and ADL than single-site TMS? (2) Is dual-site TMS more effective than sham stimulation? (3) What factors may moderate treatment effects? By integrating findings from recent high-quality trials, this study will provide evidence-based recommendations to optimize TMS protocols, bridge translational gaps, and advance personalized neurorehabilitation strategies for stroke survivors.
Method
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.
Search strategy
A comprehensive literature search was performed in seven electronic databases, including PubMed, Embase, Cochrane Library (CENTRAL), Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang and Chinese Scientific Journal (VIP) from their inception to February 19, 2024. The following search items combined Medical Subject Headings and key words to identify appropriate studies: (“transcranial magnetic stimulation” or “magnetic stimulation transcranial” or “stimulation transcranial magnetic” or “theta burst stimulation” or “iTBS” or “cTBS” or “TMS” or “rTMS”) AND (“Stroke” or “cerebrovascular accident” or “CVA” or “cerebrovascular apoplexy” or “brain vascular accident” or “cerebrovascular stroke” or “cerebral stroke” or “cerebrovascular accident”). The full search strategy in PubMed database was available in Supplementary Table 1. The reference of all included studies were manually screened to identify any missed eligible study. Endnote X9 (Thomson Reuters) was used to manage all references.
Inclusion and exclusion criteria
Inclusion criteria were: ① target population: stroke survivor; ② interventions: any type of dual-site transcranial magnetic stimulation, including single-pulse TMS, rTMS, cTBS, and iTBS; ③ comparisons: dual-site TMS vs. single-site TMS/sham dual-site TMS/non-treatment, dual-site TMS + routine rehabilitation vs. single-site TMS + routine rehabilitation, dual-site TMS + routine rehabilitation vs. sham dual-site TMS + routine rehabilitation, dual-site TMS + routine rehabilitation vs. routine rehabilitation; ④ outcomes: at least one of motor function and activity of daily life measurements, such as Fugl-Meyer Assessment (FMA), Fugl-Meyer Assessment-upper limb (FMA-UL), Fugl-Meyer Assessment-lower limb (FMA-LL), Action Research Arm Test (ARAT), Ashworth or Modified Ashworth Scale (MAS), Wolf Motor Function Test (WMFT), modified Rankin Scale (mRS), Berg Balance Scale (BBS), Barthel Index (BI), Modified Barthel Index (MBI), and other motor function and ADL related outcomes; ⑤ study design: randomized controlled trials. Exclusion criteria were: ① animal model; ② repeated publications; ③ case reports, review, protocol, conference abstract, and letters to editor; ④ central-peripheral paired associative stimulation; ⑤ paired associated stimulation; ⑥ did not report motor function or ADL related outcomes.
Data extraction
Duplicate references were removed using EndNote X9. Two independent reviewers screened the titles and abstracts of the retrieved studies to exclude irrelevant records. Full texts of potentially eligible articles were then reviewed in detail to confirm inclusion. Relevant data were extracted into a standardized form, including study authors, year of publication, participant characteristics (sample size, age, sex, and stroke duration), stroke type, intervention details, outcome measures, and adverse events. Discrepancies were resolved through discussion with a third reviewer. When essential data were missing or unclear, study authors were contacted. If results were available only in graphical format and could not be obtained from the authors, values were estimated using GetData Graph Digitizer version 2.25.1
Risk of bias assessment
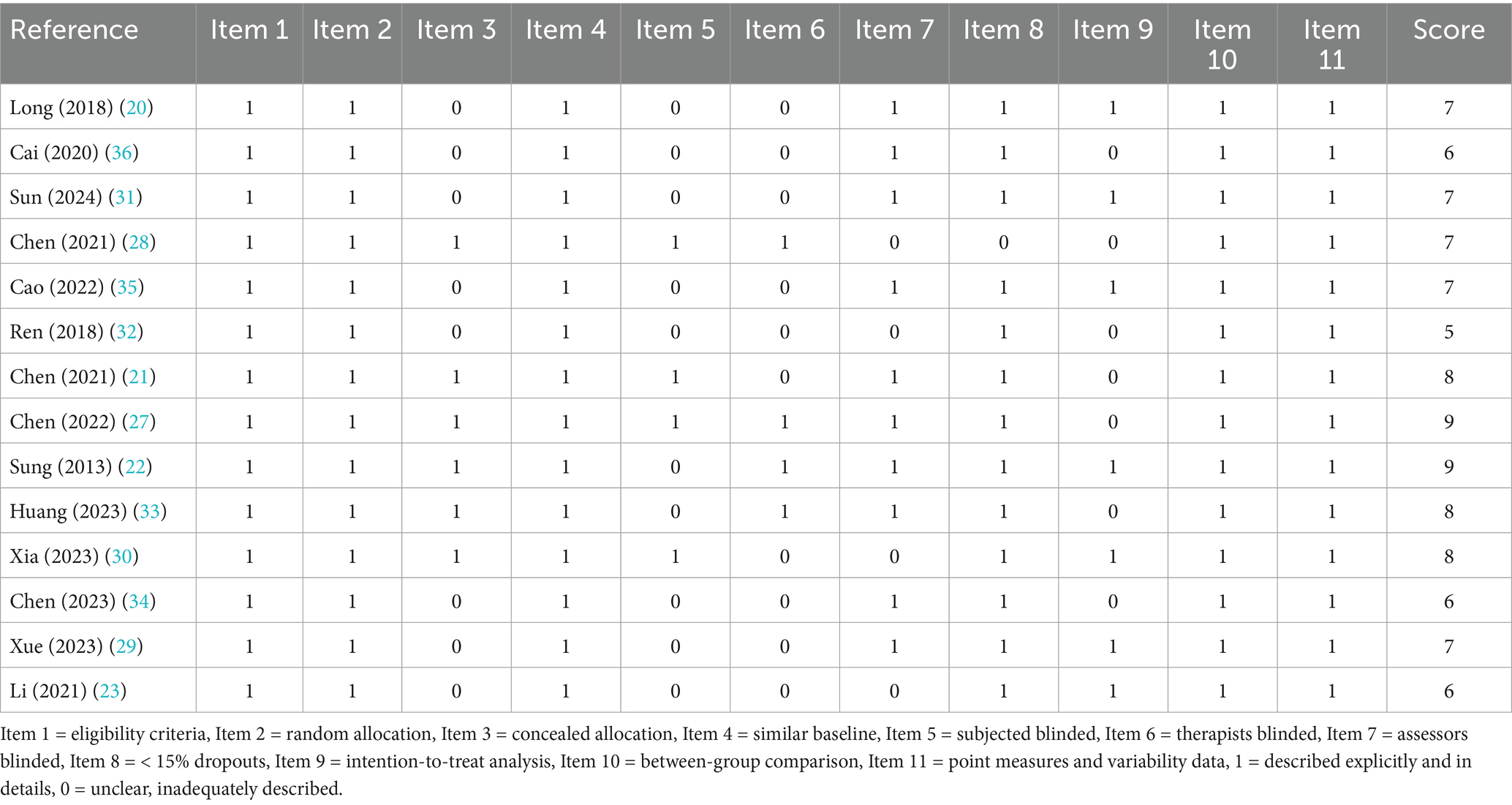
Methodological quality of the included studies was independently assessed by two reviewers using the Physiotherapy Evidence Database (PEDro) scale. The scale comprises 11 items evaluating aspects such as randomization, allocation concealment, baseline comparability, blinding (participants, therapists, assessors), follow-up adequacy, intention-to-treat analysis, between-group comparisons, and reporting of point estimates and variability. Each item was scored as 1 (criterion met) or 0 (criterion not met), yielding a maximum score of 10 (the first item is not included in the total score). Studies scoring ≥6 were considered high quality, scores of 4–5 were deemed moderate quality, and scores <4 were classified as low quality. Discrepancies were resolved through discussion with a third reviewer.
GRADE assessment
We used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to assess the certainty of evidence across studies for each outcome (24). The domains considered included risk of bias, inconsistency, indirectness, imprecision, and publication bias. The certainty ratings were categorized as high, moderate, low, or very low. These evaluations were independently conducted by two authors, and discrepancies were resolved by consensus.
Statistical analysis
For studies employing the same outcome measurement scale, meta-analyses were performed using Review Manager (RevMan) version 5.3. Continuous outcomes were summarized using the weighted mean difference (WMD) and corresponding 95% confidence intervals (CIs). The number of participants, post-intervention means, and standard deviations (SDs) in both experimental and control groups were extracted and analyzed. A random-effects model was applied given the expected heterogeneity among studies. Statistical significance was defined as a two-sided p < 0.05. Heterogeneity was assessed using the I2 statistic, with values interpreted as follows: low (<25%), moderate (25–50%), and high (>50%) (25). Subgroup analyses were conducted based on stroke phase (acute, subacute vs. chronic) and the total number of transcranial magnetic stimulation (TMS) sessions (≤10 vs. > 10 sessions). When meta-analyses included 10 or more studies, publication bias was evaluated using funnel plot asymmetry. In trials involving more than two groups with a shared control group, the shared group was evenly divided to prevent sample size inflation, allowing for independent pairwise comparisons. Sensitivity analyses were conducted by sequentially excluding individual studies to assess the robustness of the pooled estimates and explore potential sources of heterogeneity. Separate meta-analyses were conducted for each outcome measure when multiple instruments were used across studies. For outcomes reported in only a single study, results were summarized narratively rather than quantitatively. For studies reporting only mean values and 95% CIs, SDs were estimated by dividing the CI width by 3.92 and multiplying by the square root of the sample size. When only medians and interquartile ranges (IQRs) or ranges were reported, the means and SDs were approximated using established statistical methods depending on the data distribution and sample size (26).
Result
Study selection
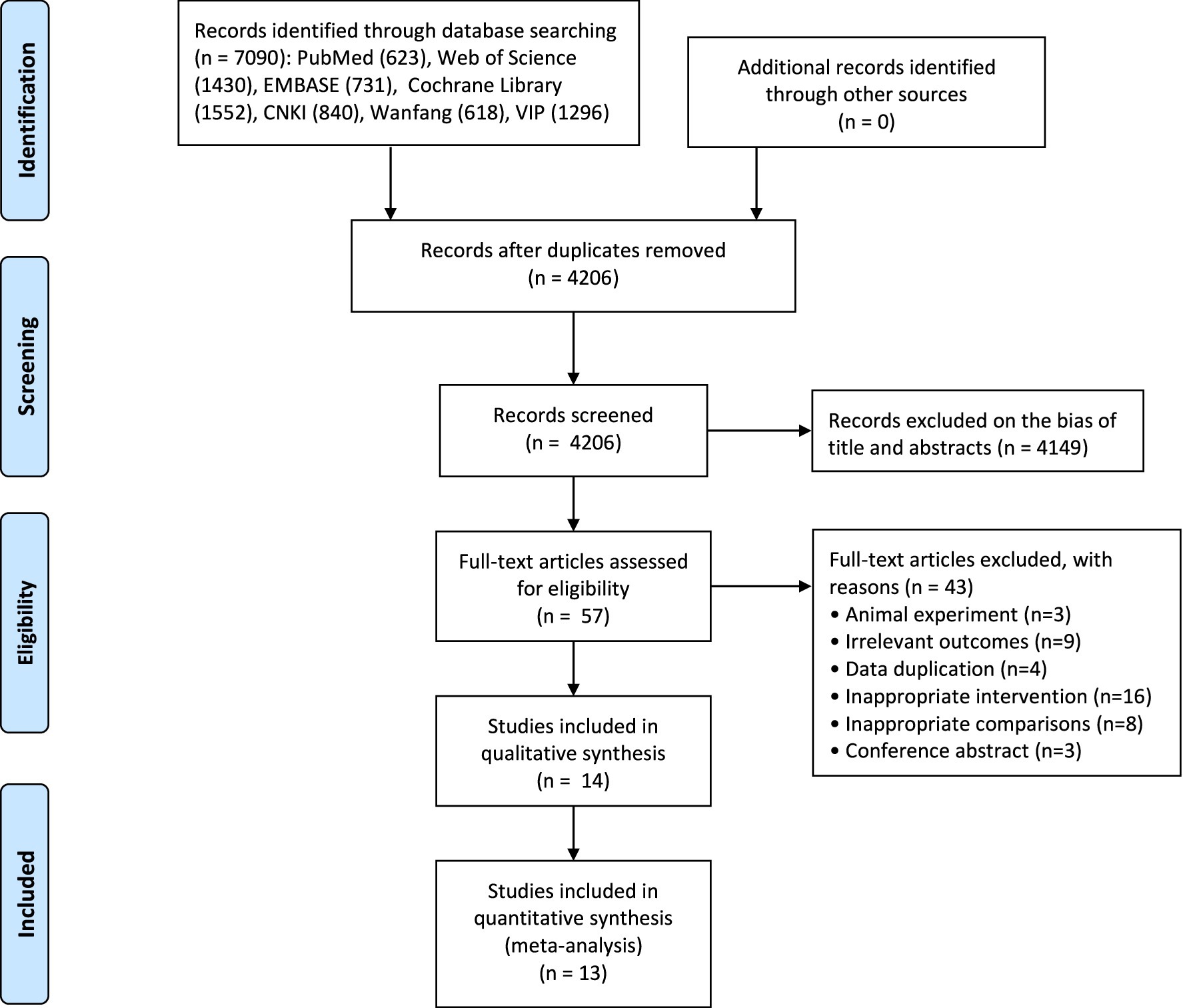
A total of 7,090 articles were retrieved from the database search, 57 of which were retained after removing duplicates and irrelevant records. During the detailed full-text screening, 14 studies (20–23, 27–36) were excluded for not meeting the inclusion criteria with a total of 724 participants. The detailed search and selection process were presented in the flow diagram (Figure 1).
Characteristics of the included studies
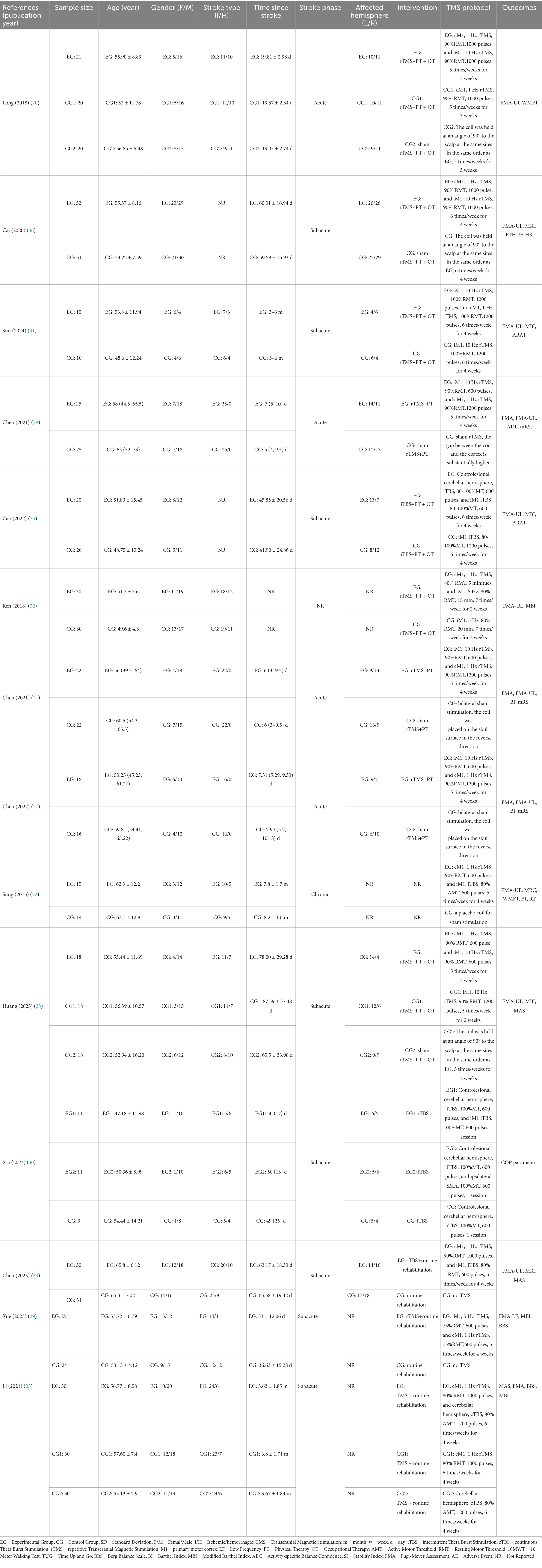
A total of 14 studies were included in the qualitative synthesis. The 14 included trials were published in 2013 (22), 2018 (20, 32), 2020 (36), 2021 (21, 23, 28), 2022 (27, 35), 2023 (29, 30, 33, 34), and 2024 (31) (Table 1). Individual study sample sizes ranged from 20 to 103. Mean ages of participants across studies ranged from 47 to 66 years. Time post-stroke varied widely, ranging from 6 days to 8 months. Most participants were in the subacute (1–6 months post-stroke) or chronic phase (>6 months post-stroke) of stroke. Male predominance was observed in most studies. Dual-site TMS protocols with routine rehabilitation were applied in the experimental groups. Interventions included sham dual-site TMS with routine rehabilitation, single-site TMS with routine rehabilitation, or routine rehabilitation alone in the control groups. Routine rehabilitation comprised individualized physical and occupational therapy, including passive and active motor exercises, task-oriented training, and activities of daily living. The TMS parameters including stimulation site, frequency, intensity, and duration, varied in different individual studies (Table 1).
Outcome Measures focused on motor recovery and activities of daily living (ADL), assessed by Fugl-Meyer Assessment (FMA), FMA-Upper Limb (FMA-UL), Wolf Motor Function Test (WMFT), Action Research Arm Test (ARAT), Modified Barthel Index (MBI), Barthel Index (BI), Modified Ashworth Scale (MAS), Berg Balance Scale (BBS), and others.
Methodological quality
According to PEDro scores (Table 2), thirteen studies were rated as high quality, and one as moderate quality. All studies reported comparable baseline characteristics between groups, between-group comparisons, and provided point estimates along with measures of variability. However, most studies did not meet the criteria for items related to participant blinding (item 5), therapist blinding (item 6), and concealed allocation (item 3), reflecting the methodological challenges commonly encountered in rehabilitation trials. Additionally, some studies lacked assessor blinding (item 7) and intention-to-treat analysis (item 9), which may have introduced bias into the results and affected the internal validity of the studies.
Outcome measures
FMA-UL
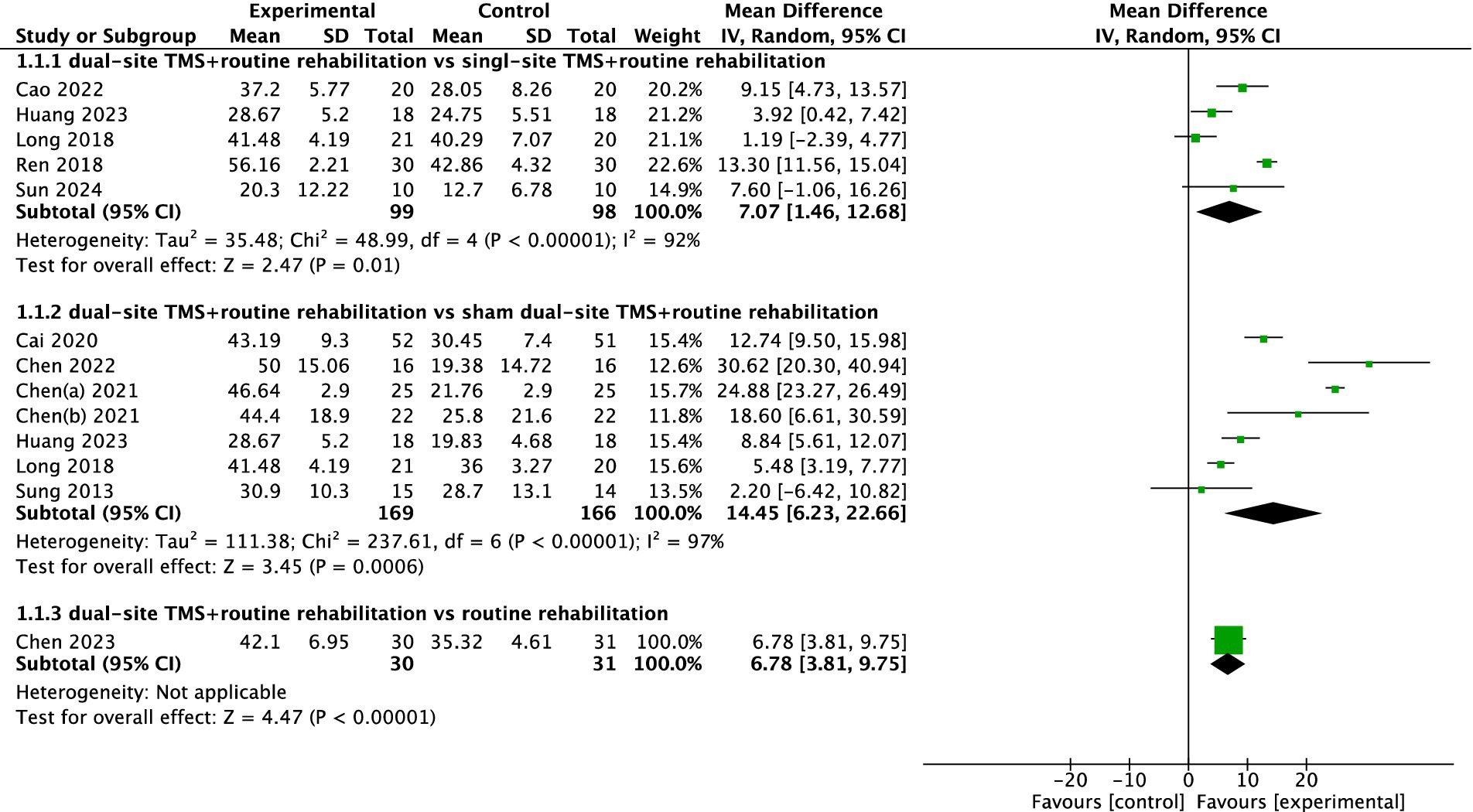
A total of eleven studies reported FMA-UL results. The pooled meta-analysis demonstrated that dual-site TMS combined with routine rehabilitation was significantly more effective than single-site TMS with routine rehabilitation in improving FMA-UL scores (MD, 7.07; 95% CI, 1.46 to 12.68; I2 = 92%, p < 0.001; Figure 2). Subgroup analyses indicated that patients in the subacute phase showed significant improvements in the experimental group (Table 3). Additionally, both treatment sessions ≤20 and >20 resulted in significant FMA-UL improvements in the experimental group (Table 3). When compared to sham stimulation with routine rehabilitation, dual-site TMS with routine rehabilitation also showed significant FMA-UL improvement (MD, 14.45; 95% CI, 6.23 to 22.66; I2 = 97%, p < 0.001; Figure 2). Subgroup analyses revealed that both acute and subacute patients experienced significant FMA-UL improvements in the experimental group, regardless of whether treatment sessions were ≤20 or >20 (Table 3). Only one study (34) reported that dual-site TMS with routine rehabilitation was significantly more effective than routine rehabilitation alone in improving FMA-UL scores (Figure 2).
ADL
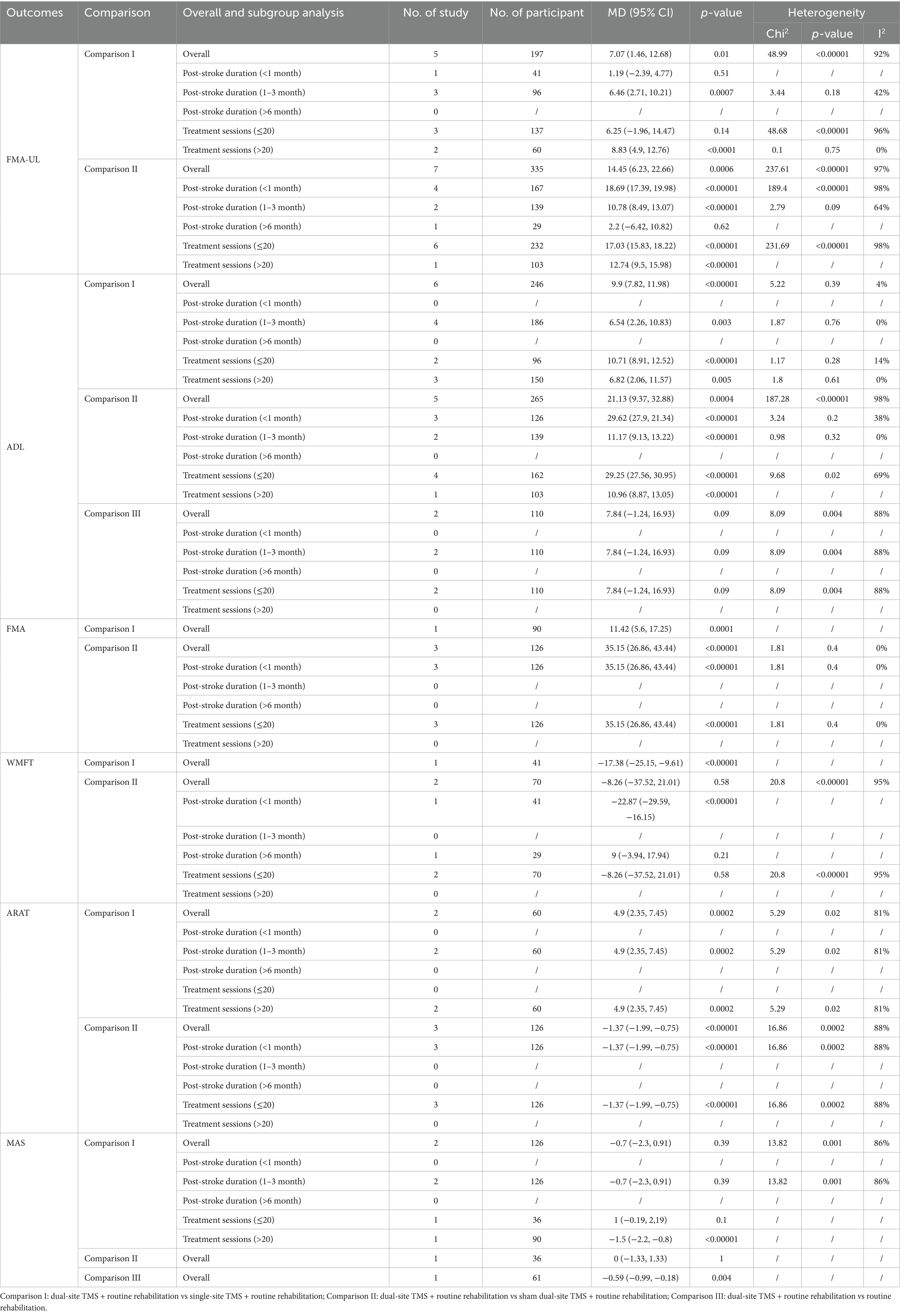
Eleven studies reported ADL-related outcomes, with two using the BI and nine using the MBI. The pooled meta-analysis indicated that dual-site TMS with routine rehabilitation was significantly more effective than single-site TMS with routine rehabilitation in improving ADL (MD, 9.90; 95% CI, 7.82 to 11.98; I2 = 4%, p = 0.39; Figure 3). Subgroup analyses showed that both treatment sessions ≤20 and >20 led to significant ADL improvements in the experimental group (Table 3). Compared to sham stimulation with routine rehabilitation, dual-site TMS with routine rehabilitation was significantly more effective in ADL (MD, 21.13; 95% CI, 9.37 to 32.88; I2 = 98%, p < 0.001; Figure 3). Subgroup analyses again showed significant improvements in both acute and subacute patients, regardless of treatment session number (Table 3). However, a pooled meta-analysis of two studies found that dual-site TMS with routine rehabilitation was not significantly more effective than routine rehabilitation alone in ADL (MD, 7.84; 95% CI, −1.24 to 16.93; I2 = 88%, p = 0.004; Figure 3).
FMA
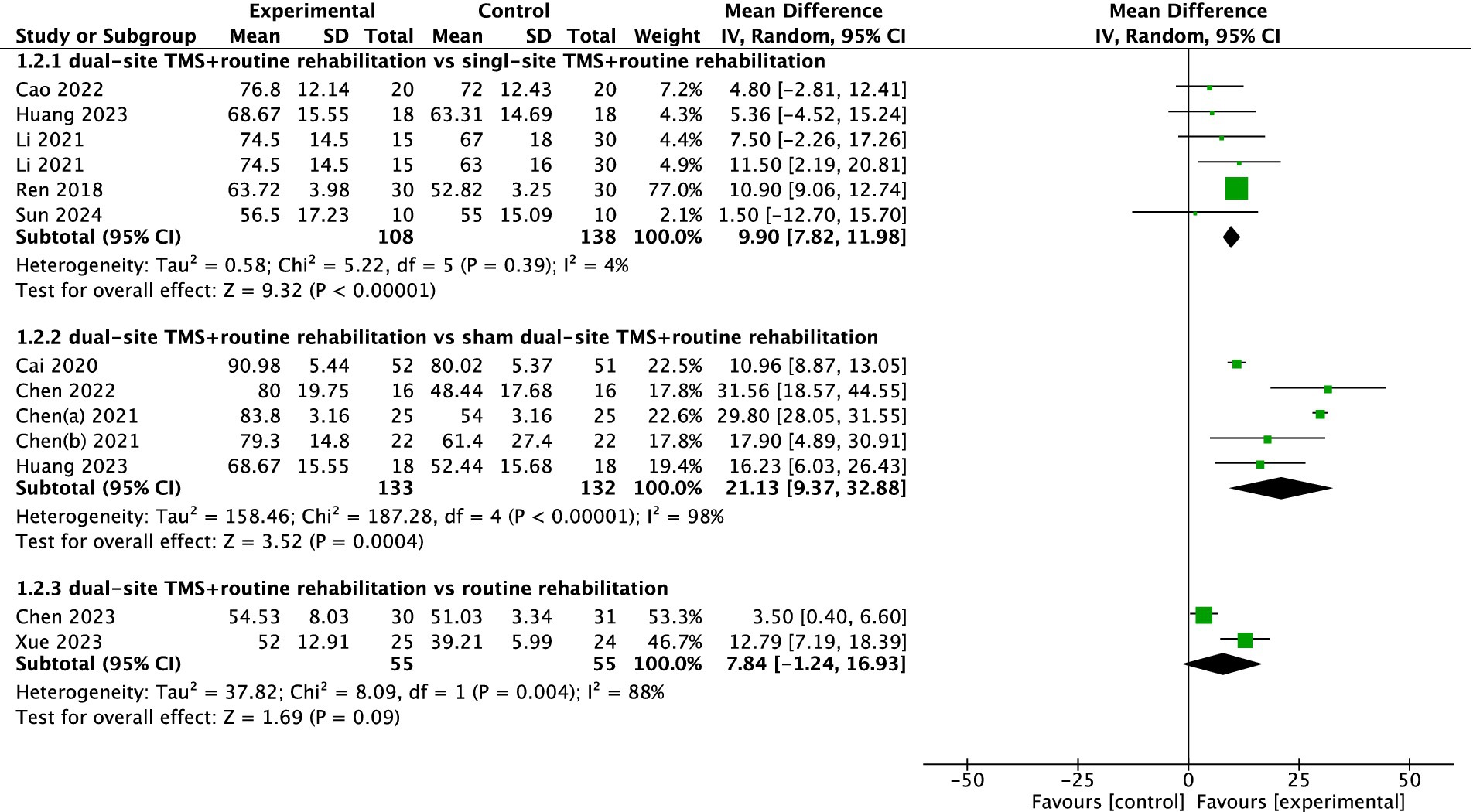
Four studies reported FMA results. The pooled meta-analysis showed that dual-site TMS with routine rehabilitation was significantly more effective than single-site TMS with routine rehabilitation (MD, 11.42; 95% CI, 5.60 to 17.25; I2 = 0%, p = 0.61; Figure 4). Similarly, dual-site TMS with routine rehabilitation was significantly more effective than sham stimulation with routine rehabilitation (MD, 35.15; 95% CI, 26.86 to 43.44; I2 = 0%, p = 0.40; Figure 4).
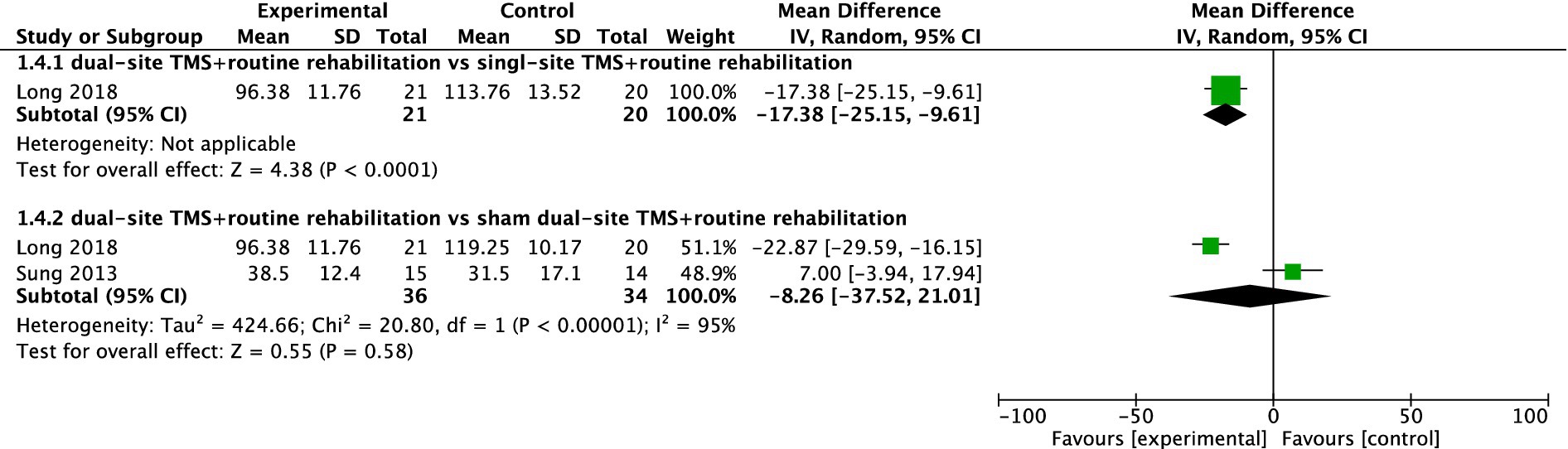
WMFT
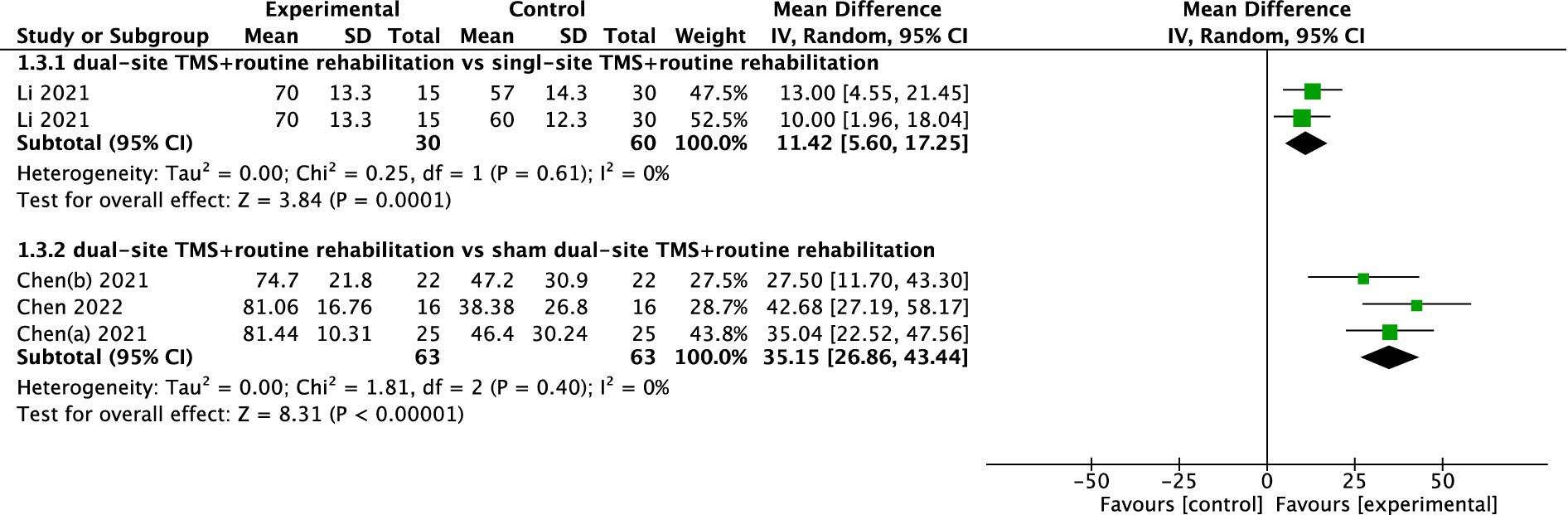
Two studies reported WMFT results. While one study found dual-site TMS with routine rehabilitation to be significantly more effective than single-site TMS with routine rehabilitation, the pooled meta-analysis of two studies showed no significant difference between dual-site TMS with routine rehabilitation and sham stimulation with routine rehabilitation (MD, −8.26; 95% CI, −37.52 to 21.01; I2 = 95%, p < 0.001; Figure 5).
ARAT
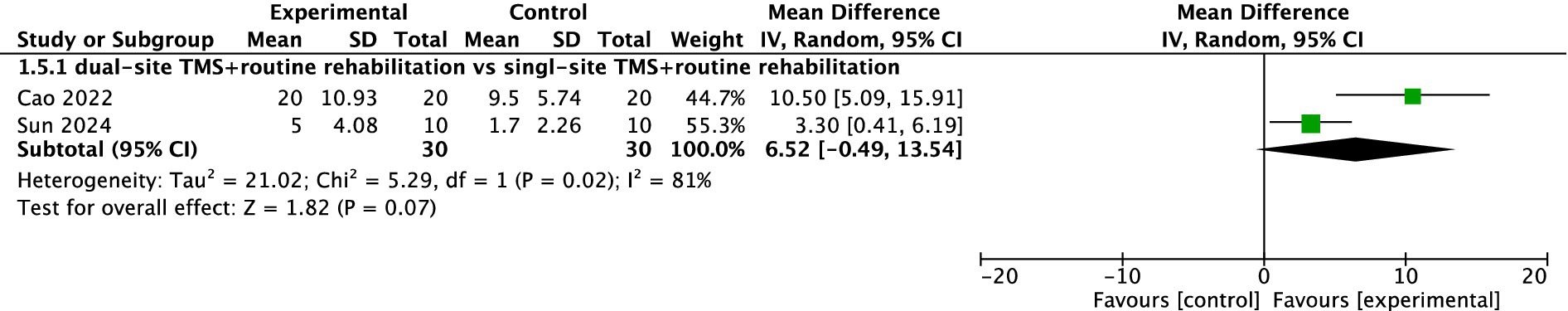
Two studies reported ARAT results. The pooled meta-analysis indicated no significant difference between dual-site TMS with routine rehabilitation and single-site TMS with routine rehabilitation (MD, 6.52; 95% CI, −0.49 to 13.54; I2 = 81%, p = 0.02; Figure 6).
mRS
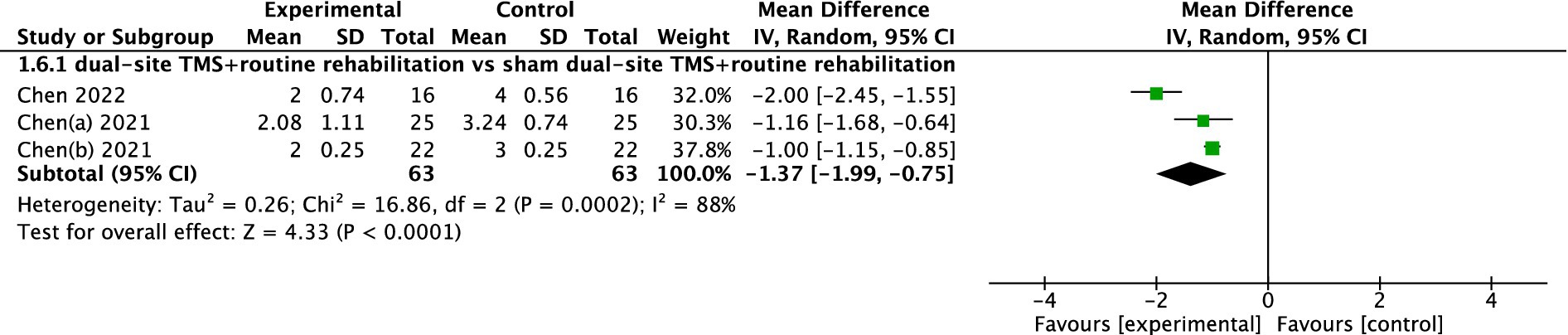
Three studies reported mRS results. The pooled meta-analysis demonstrated that dual-site TMS with routine rehabilitation was significantly more effective than sham stimulation with routine rehabilitation (MD, −1.37; 95% CI, −1.99 to −0.75; I2 = 88%, p < 0.001; Figure 7).
MAS
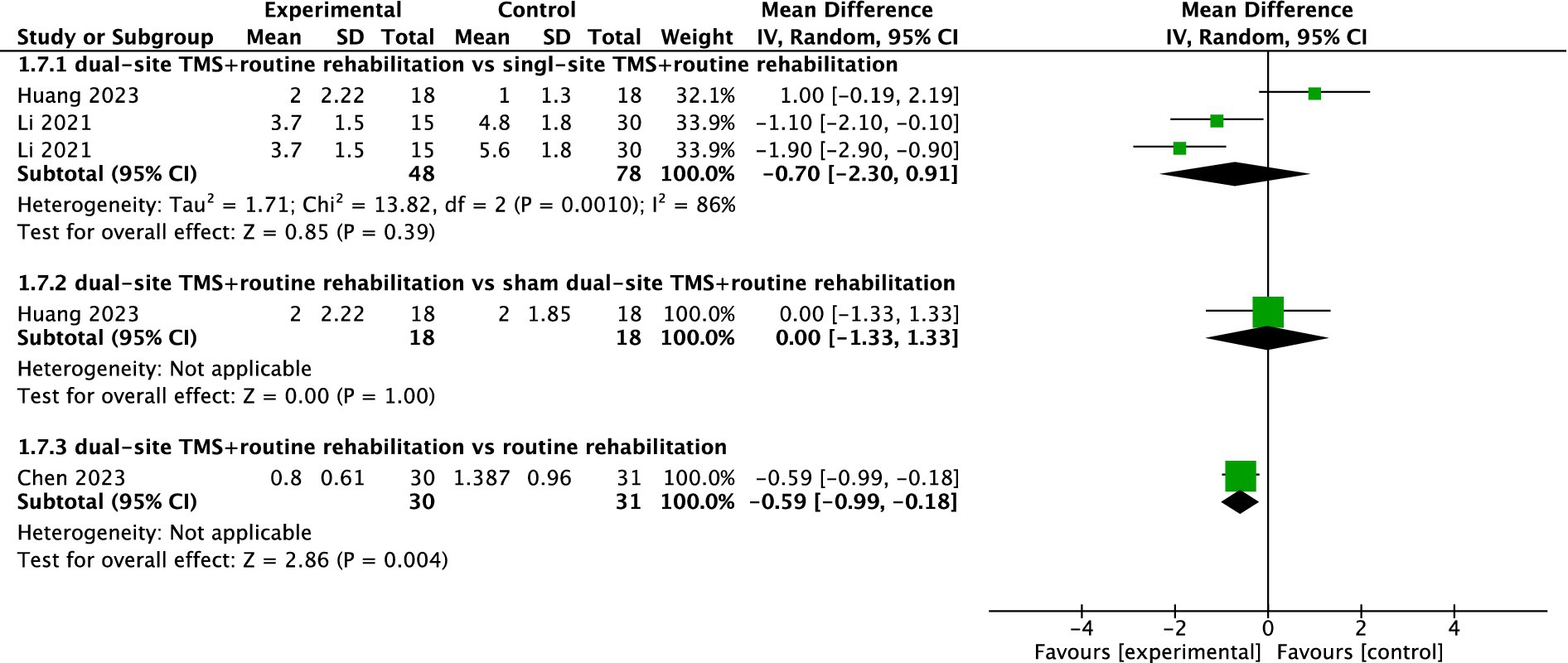
Three studies reported MAS results. The pooled meta-analysis showed no significant difference between dual-site TMS with routine rehabilitation and single-site TMS with routine rehabilitation (MD, −0.70; 95% CI, −2.30 to 0.91; I2 = 86%, p = 0.001; Figure 8). However, subgroup analyses indicated that treatment sessions >20 achieved significant MAS improvements in the experimental group (Table 3). One study (33) found no significant difference between dual-site TMS with routine rehabilitation and sham stimulation with routine rehabilitation in MAS. Another study (34) reported that dual-site TMS with routine rehabilitation was significantly more effective than routine rehabilitation alone in MAS.
Other outcomes
One study (29) showed that dual-site TMS with routine rehabilitation was significantly more effective than routine rehabilitation alone in FMA-LL. One study (36) showed that dual-site TMS with routine rehabilitation was significantly more effective than single-site TMS with routine rehabilitation in FTHUE-HK. One study (22) showed that dual-site TMS with routine rehabilitation was significantly more effective than sham TMS with routine rehabilitation in finger flexor MRC, simple reaction time task, and index finger tapping task. Another study (30) demonstrated that a single session of dual-site iTBS could immediately improve balance function in patients with eyes closed compared to single-site iTBS.
Sensitivity analysis and publication bias
Leave-one-out sensitivity analyses indicated that sequentially excluding each included study did not significantly alter the overall effect estimates, with most results remaining within the 95% confidence intervals. Notably, when excluding Cao et al. (35), the pooled meta-analysis showed that dual-site TMS with routine rehabilitation was not significantly more effective than single-site TMS with routine rehabilitation in FMA-UL (MD, 6.53; 95% CI, −0.51 to 13.56; I2 = 96%, p < 0.00001). These findings suggest that most meta-analysis results were robust and not unduly influenced by any single study. Publication bias was not formally assessed, as individual meta-analyses did not include 10 or more studies.
Adverse events and side effects
All participants tolerated DS-NIBS without significant adverse events. Four of the studies mentioned that subjects experienced transient mild headaches and slight tingling sensations during TMS stimulation (21, 27, 28, 36). However, the symptoms were well-tolerated and resolved immediately after treatment, and did not interfere with subsequent therapeutic sessions.
Quality of evidence
For FMA-UL, the evidence quality was low when comparing dual-site TMS combined with routine rehabilitation to single-site TMS with routine rehabilitation. However, it reaches moderate quality when compared to sham dual-site TMS with routine rehabilitation, and it drops to very low quality when compared to routine rehabilitation alone. This variation is influenced by factors such as serious inconsistency in some comparisons and extremely serious imprecision in others.
Regarding ADL, the evidence quality was low when dual-site TMS with routine rehabilitation is compared to single-site TMS with routine rehabilitation. It falls to very low quality when compared to both sham dual-site TMS with routine rehabilitation and routine rehabilitation alone. The main issues here include serious inconsistency and very serious imprecision.
For other outcomes, the evidence quality was very low across all comparisons, whether it’s with single-site TMS, sham dual-site TMS, or routine rehabilitation. Detailed ratings and justifications are provided in the Supplementary Table 2.
Discussion
This systematic review and meta-analysis provides compelling evidence for the effectiveness of dual-site transcranial magnetic stimulation (TMS) in enhancing motor function and activities of daily living (ADL) in stroke patients. Across 14 randomized controlled trials with 724 participants, dual-site TMS demonstrated significant advantages over single-site TMS and sham stimulation. Notably, improvements in upper limb motor function (MD, 7.07; 95% CI, 1.46 to 12.68; p < 0.001) and ADL (MD, 9.90; 95% CI, 7.82 to 11.98; p = 0.39) highlight its potential as a valuable adjunct to conventional rehabilitation therapies.
Dual-site TMS is an innovative neuromodulation technique used in stroke rehabilitation that targets multiple brain regions in close succession. This approach enhances understanding of brain network interactions and has shown potential to improve motor recovery in stroke survivors (37, 38). The efficacy of dual-site TMS could be attributed to its unique ability to modulate neural activity across distributed brain networks disrupted by stroke (39, 40). By sequentially stimulating two distinct brain regions, such as bilateral M1 or M1 and the cerebellum, dual-site TMS promotes synchronized neural oscillations and enhances interregional connectivity (41). This dual stimulation facilitates rebalancing of interhemispheric inhibition, which is often disrupted post-stroke, and activates diaschisis-related pathways that become inhibited secondary to the primary lesion (42, 43). Preclinical studies indicate that dual-site stimulation may induce more robust long-term potentiation (LTP) effects compared to single-site protocols, suggesting stronger neuroplastic changes underlying motor recovery (7).
A resting-state fMRI study revealed that rTMS increased functional activity and connectivity in motor-related brain regions for stroke patients, highlighting rTMS’s role in modulating neural networks to support motor rehabilitation (44). Stroke patients exhibit individualized cortical responses to rTMS, and that tailoring rTMS neuromodulation significantly improves motor imagery decoding and functional recovery (45). Cerebellar TMS enhanced balance and lower limb motor function in stroke patients, spontaneous neural activity alterations were identified in motor-related regions after stroke, including the precentral gyrus, putamen, thalamus, and paracentral lobule based on fMRI studies (46). Another systematic review of fMRI studies demonstrated that the neural mechanism of rTMS in improving motor function after stroke may be the activation and functional connectivity of motor-related brain areas, including enhancement of the activation of motor-related brain areas in the affected hemisphere, inhibition of the activation of motor-related brain areas in the unaffected hemisphere, and changing the functional connectivity of intra-hemispheric and inter-hemispheric motor networks (47).
Subgroup analyses indicated that dual-site TMS was especially effective during the acute and subacute phases of stroke recovery, with significant improvements in FMA-UL scores. This observation is consistent with the enhanced neuroplastic potential of the brain during the early post-stroke period, as supported by animal and human studies (48–50). Early intervention may therefore facilitate greater reorganization of motor pathways (51). Additionally, treatment outcomes were influenced by the number of TMS sessions. Both ≤20 and >20 sessions yielded significant functional gains; however, the >20 sessions subgroup demonstrated greater mean improvements. This finding supports the dose–response relationship of cumulative neuromodulatory effects on cortical excitability and synaptic plasticity (7, 52). These results underscore the importance of treatment duration and intensity in optimizing clinical efficacy”.
The frequency of rTMS plays a crucial role in modulating cortical excitability. Specifically, high-frequency rTMS (≥5 Hz) is generally excitatory and facilitates cortical activity, particularly over the ipsilesional motor cortex (M1), while low-frequency rTMS (≤1 Hz) exerts inhibitory effects, typically applied to the contralesional hemisphere to suppress maladaptive interhemispheric inhibition (7, 9). Dual-site TMS protocols strategically combine these two modalities to rebalance interhemispheric interactions—enhancing neural excitability in the affected hemisphere while concurrently reducing overactivity in the unaffected hemisphere. For instance, Long et al. (20) and Cai et al. (36) applied this dual-frequency strategy and reported significant improvements in upper-limb motor function in subacute stroke patients.
Stimulation intensity, typically expressed as a percentage of the RMT, plays a crucial role in treatment efficacy (53, 54). Higher intensities may produce more pronounced neurophysiological effects but also increase adverse event risks (55). Most studies used intensities ranging from 80 to 100% RMT without serious adverse effects. The duration of TMS sessions and overall treatment period also influence outcomes. Longer treatment durations may allow for more sustained neuroplastic changes (52). However, studies varied in their approaches, with treatment periods ranging from 2 to 4 weeks. Further research is needed to establish the ideal duration for dual-site TMS interventions.
Our findings corroborate and extend previous research on TMS in stroke rehabilitation. Earlier studies (56, 57) showed modest effects of single-site TMS on motor function and cognitive function, but dual-site TMS appears to offer enhanced benefits (58). For instance, a meta-analysis (9) found moderate effects of single-site rTMS on upper limb function, while our results indicate that dual-site TMS may provide superior outcomes. Additionally, another systematic review (16) reported that dual-site non-invasive brain stimulation outperformed single-site non-invasive brain stimulation in improving upper limb function and ADL.
Integrating dual-site TMS into clinical practice shows significant promise but presents challenges. Current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) recognize TMS as a potential adjunct to stroke rehabilitation, but evidence for dual-site TMS is still emerging (3). Our findings support considering dual-site TMS in clinical settings, particularly for subacute patients. However, standardized protocols and cost-effectiveness evaluations are needed before widespread adoption. Variability in the parameters of rTMS, including stimulation protocols, locations of stimulation, and frequency, further complicates the establishment of standardized treatment guidelines.
The dual-site TMS protocol in our meta-analysis involved stimulation over the ipsilesional M1 as at least one of the target sites, often in conjunction with either the contralateral M1 or the cerebellum. This variability may have contributed to some heterogeneity in treatment effects and should be more rigorously standardized in future trials. The M1 is the most frequently targeted region in post-stroke neuromodulation research due to its central role in initiating voluntary movement via the corticospinal tract (8, 57). Modulating excitability in the lesioned M1 (via high-frequency rTMS) or/and downregulating the contralesional M1 (via low-frequency rTMS) could restore interhemispheric balance and improve motor outcomes (8, 57).
Future research should prioritize more head-to-head comparisons of dual-site TMS protocols to determine optimal stimulation parameters and target regions. Long-term follow-up studies are necessary to assess durability of treatment effects. Incorporating advanced neuroimaging techniques like fMRI and DTI can provide insights into neural mechanisms and guide personalized treatment approaches. Additionally, exploring dual-site TMS in combination with other therapies, such as brain-computer interfaces (BCIs) or pharmacological agents, may further enhance rehabilitation outcomes.
Several limitations should be considered when interpreting our results. One limitation of this review is the absence of a prospectively registered protocol, which may raise concerns about selective reporting or methodological bias. Only 14 RCTs met our inclusion criteria, underscoring the need for larger, multi-center trials to confirm these findings. Heterogeneity among studies in participant characteristics, TMS protocols, and outcome measures may have influenced pooled effect estimates. Most studies did not adequately address participant or therapist blinding, potentially introducing biases. Short follow-up periods limit our ability to assess long-term efficacy.
Another major limitation of the studies included in our analysis is the homogeneity of the sample population, all participants were recruited in Chinese population, limiting generalizability to other ethnic and healthcare contexts. This raises concerns about the generalizability of the findings to other racial and ethnic groups. Future multi-center trials across diverse populations are warranted.
Conclusion
This systematic review and meta-analysis demonstrates that dual-site TMS combined with routine rehabilitation is effective in improving motor function and ADL in stroke patients. The findings highlight its potential to enhance neuroplasticity and functional recovery through dual-target stimulation. While the results are encouraging, further research is needed to optimize treatment protocols and address methodological limitations. Further research is required to establish the efficacy of dual-site TMS in larger, multicenter trials and to optimize treatment protocols, thereby ensuring robust evidence supporting its integration into standard rehabilitation practices.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JQ: Conceptualization, Writing – review & editing, Software, Writing – original draft, Funding acquisition, Formal analysis. ZH: Writing – original draft, Data curation, Conceptualization. JW: Data curation, Writing – original draft. YZ: Methodology, Formal analysis, Writing – original draft, Data curation. HZ: Writing – original draft, Data curation. SH: Supervision, Writing – original draft, Writing – review & editing, Software. LZ: Writing – review & editing, Supervision, Writing – original draft, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Science Foundation Project funded by the Science and Technology Department of Fujian Province (grant number: 2022J011466) and Quanzhou Science and Technology Program (grant number: 2024NY004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1630876/full#supplementary-material
Footnotes
References
1. Collaborators G. B. D. S. R. F. Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. (2024) 23:973–1003. doi: 10.1016/S1474-4422(24)00369-7
2. Bernhardt, J, Urimubenshi, G, Gandhi, DBC, and Eng, JJ. Stroke rehabilitation in low-income and middle-income countries: a call to action. Lancet. (2020) 396:1452–62. doi: 10.1016/S0140-6736(20)31313-1
3. Winstein, CJ, Stein, J, Arena, R, Bates, B, Cherney, LR, Cramer, SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2016) 47:e98–e169. doi: 10.1161/STR.0000000000000098
4. Gittler, M, and Davis, AM. Guidelines for adult stroke rehabilitation and recovery. JAMA. (2018) 319:820–1. doi: 10.1001/jama.2017.22036
5. Maier, M, Ballester, BR, and Verschure, P. Principles of neurorehabilitation after stroke based on motor learning and brain plasticity mechanisms. Front Syst Neurosci. (2019) 13:74. doi: 10.3389/fnsys.2019.00074
6. Hara, Y. Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch. (2015) 82:4–13. doi: 10.1272/jnms.82.4
7. Lefaucheur, JP, Aleman, A, Baeken, C, Benninger, DH, Brunelin, J, Di Lazzaro, V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin Neurophysiol. (2020) 131:474–528. doi: 10.1016/j.clinph.2019.11.002
8. Bai, Z, Zhang, J, and Fong, KNK. Effects of transcranial magnetic stimulation in modulating cortical excitability in patients with stroke: a systematic review and meta-analysis. J Neuroeng Rehabil. (2022) 19:24. doi: 10.1186/s12984-022-00999-4
9. Starosta, M, Cichon, N, Saluk-Bijak, J, and Miller, E. Benefits from repetitive transcranial magnetic stimulation in post-stroke rehabilitation. J Clin Med. (2022) 11. doi: 10.3390/jcm11082149
10. Vink, JJT, van Lieshout, ECC, Otte, WM, van Eijk, RPA, Kouwenhoven, M, Neggers, SFW, et al. Continuous Theta-burst stimulation of the Contralesional primary motor cortex for promotion of upper limb recovery after stroke: a randomized controlled trial. Stroke. (2023) 54:1962–71. doi: 10.1161/STROKEAHA.123.042924
11. Chen, K, Sun, M, and Zhuang, H. Effect of theta burst stimulation on lower extremity motor function improvement and balance recovery in patients with stroke: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). (2024) 103:e40098. doi: 10.1097/MD.0000000000040098
12. Wang, J, Wu, Z, Hong, S, Ye, H, Zhang, Y, Lin, Q, et al. Cerebellar transcranial magnetic stimulation for improving balance capacity and activity of daily living in stroke patients: a systematic review and meta-analysis. BMC Neurol. (2024) 24:205. doi: 10.1186/s12883-024-03720-1
13. Le, HT, Honma, K, Annaka, H, Shunxiang, S, Murakami, T, Hiraoka, T, et al. Effectiveness of transcranial magnetic stimulation on executive function, attention, and memory in stroke patients: a systematic review and Meta-analysis. Cureus. (2024) 16:e75194. doi: 10.7759/cureus.75194
14. Richards, LG, Stewart, KC, Woodbury, ML, Senesac, C, and Cauraugh, JH. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia. (2008) 46:3–11. doi: 10.1016/j.neuropsychologia.2007.08.013
15. Chen, S, Zhang, X, Chen, X, Zhou, Z, Cong, W, Chong, K, et al. The assessment of interhemispheric imbalance using functional near-infrared spectroscopic and transcranial magnetic stimulation for predicting motor outcome after stroke. Front Neurosci. (2023) 17:1231693. doi: 10.3389/fnins.2023.1231693
16. Ren, M, Xu, J, Wang, W, Shen, L, Wang, C, Liu, H, et al. Effect of dual-site non-invasive brain stimulation on upper-limb function after stroke: a systematic review and Meta-analysis. Brain Behav. (2024) 14:e70145. doi: 10.1002/brb3.70145
17. Simonetta-Moreau, M. Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann Phys Rehabil Med. (2014) 57:530–42. doi: 10.1016/j.rehab.2014.08.003
18. Kubis, N. Non-invasive brain stimulation to enhance post-stroke recovery. Front Neural Circuits. (2016) 10:56. doi: 10.3389/fncir.2016.00056
19. Taud, B, Lindenberg, R, Darkow, R, Wevers, J, Hofflin, D, Grittner, U, et al. Limited add-on effects of unilateral and bilateral transcranial direct current stimulation on Visuo-motor grip force tracking task training outcome in chronic stroke. A Randomized Controlled Trial. Front Neurol. (2021) 12:736075. doi: 10.3389/fneur.2021.736075
20. Long, H, Wang, H, Zhao, C, Duan, Q, Feng, F, Hui, N, et al. Effects of combining high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. Restor Neurol Neurosci. (2018) 36:21–30. doi: 10.3233/RNN-170733
21. Chen, QM, Yao, FR, Sun, HW, Chen, ZG, Ke, J, Liao, J, et al. Combining inhibitory and facilitatory repetitive transcranial magnetic stimulation (rTMS) treatment improves motor function by modulating GABA in acute ischemic stroke patients. Restor Neurol Neurosci. (2021) 39:419–34. doi: 10.3233/RNN-211195
22. Sung, WH, Wang, CP, Chou, CL, Chen, YC, Chang, YC, and Tsai, PY. Efficacy of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation to enhance motor recovery in hemiplegic stroke patients. Stroke. (2013) 44:1375–82. doi: 10.1161/STROKEAHA.111.000522
23. Li, D, Cheng, A, Zhang, Z, Sun, Y, and Liu, Y. Effects of low-frequency repetitive transcranial magnetic stimulation combined with cerebellar continuous theta burst stimulation on spasticity and limb dyskinesia in patients with stroke. BMC Neurol. (2021) 21:369. doi: 10.1186/s12883-021-02406-2
24. Higgins, J. P. T.Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Second edition. Wiley-Blackwell; Hoboken, NJ; (2019). xxviii–694.
25. Huedo-Medina, TB, Sanchez-Meca, J, Marin-Martinez, F, and Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. (2006) 11:193–206. doi: 10.1037/1082-989X.11.2.193
26. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
27. Chen, Q, Shen, W, Sun, H, Zhang, H, Liu, C, Chen, Z, et al. The effect of coupled inhibitory-facilitatory repetitive transcranial magnetic stimulation on shaping early reorganization of the motor network after stroke. Brain Res. (2022) 1790:147959. doi: 10.1016/j.brainres.2022.147959
28. Chen, Q, Shen, D, Sun, H, Ke, J, Wang, H, Pan, S, et al. Effects of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation on motor recovery in patients following acute cerebral infarction. Neuro Rehabilitation. (2021) 48:83–96. doi: 10.3233/NRE-201606
29. Xue, P, Yang, F, Jin, W, Chen, Z, Shen, Y, and Qian, L. Efficacy of bilateral repetitive transcranial magnetic stimulation combined with mirror visual feedback therapy for lower limb function recovery in patients with subacute stroke. Zhejiang Yi Xue. (2023) 45:80–3.
30. Xia, Y. Effects of cerebellum-cerebrum dual-targets transcranial magnetic stimulation on balance function and brain network in stroke patients: an fNIRS study. Master’s thesis. Shanghai: Shanghai University of Sport (2023).
31. Sun, X. The effect of bilateral repetitive transcranial magnetic stimulation on upper limb function recovery in stroke patient. Master’s thesis. Hangzhou: Zhejiang Chinses Medical University (2024).
32. Ren, P, Wang, X, Fan, Y, and Tan, J. Efficacy of low frequency combined with high frequency transcranial magnetic therapy for upper limb motor dysfunction after stroke. Acta Med Sin. (2018) 31:114–6. (in Chinese)
33. Huang, Y. Exploring the efficacy of bilateral rTMS on upper limb motor function and mechanism of neuroplasticity during stroke recovery period based on serum BDNF-related factors. Master’s thesis. Yinchuan: Ningxia Medical University (2023).
34. Chen, Y, Wang, Q, Cui, S, Li, Y, Zhang, S, Wei, Y, et al. Effect of bilateral sequential repetitive transcranial magnetic stimulation on motor function of upper limbs in stroke patients. Zhongguo Kangfu Lilun Yu Shijian. (2023) 29:926–32.
35. Cao, Z, Feng, H, Li, Y, Yang, J, Li, J, Wang, H, et al. Effects of multi-target iTBS between cerebral hemispheres on upper limb function in stroke patients. Zhongguo Kangfu Lilun Yu Shijian. (2022) 28:502–7. (in Chinese)
36. Cai, Q. Effect of repetitive transcranial magnetic stimulation on upper limb motor function in patients with cerebral infarction. Master’s thesis. Nanjing: Southeast University (2020).
37. Van Malderen, S, Hehl, M, Verstraelen, S, Swinnen, SP, and Cuypers, K. Dual-site TMS as a tool to probe effective interactions within the motor network: a review. Rev Neurosci. (2023) 34:129–221. doi: 10.1515/revneuro-2022-0020
38. Yuan, X, Yang, Y, Cao, N, and Jiang, C. Promotion of Poststroke motor-function recovery with repetitive transcranial magnetic stimulation by regulating the interhemispheric imbalance. Brain Sci. (2020) 10. doi: 10.3390/brainsci10090648
39. Tang, Z, Han, K, Wang, R, Zhang, Y, and Zhang, H. Excitatory repetitive transcranial magnetic stimulation over the Ipsilesional hemisphere for upper limb motor function after stroke: a systematic review and Meta-analysis. Front Neurol. (2022) 13:918597. doi: 10.3389/fneur.2022.918597
40. Siebner, HR, Funke, K, Aberra, AS, Antal, A, Bestmann, S, Chen, R, et al. Transcranial magnetic stimulation of the brain: what is stimulated?—a consensus and critical position paper. Clin Neurophysiol. (2022) 140:59–97. doi: 10.1016/j.clinph.2022.04.022
41. Chouinard, PA, and Paus, T. What have we learned from "perturbing" the human cortical motor system with transcranial magnetic stimulation? Front Hum Neurosci. (2010) 4:173. doi: 10.3389/fnhum.2010.00173
42. Hehl, M, Van Malderen, S, Geraerts, M, Meesen, RLJ, Rothwell, JC, Swinnen, SP, et al. Probing intrahemispheric interactions with a novel dual-site TMS setup. Clin Neurophysiol. (2024) 158:180–95. doi: 10.1016/j.clinph.2023.12.128
43. Fiori, F, Chiappini, E, Candidi, M, Romei, V, Borgomaneri, S, and Avenanti, A. Long-latency interhemispheric interactions between motor-related areas and the primary motor cortex: a dual site TMS study. Sci Rep. (2017) 7:14936. doi: 10.1038/s41598-017-13708-2
44. Zou, X, Zhu, J, Hu, S, Hou, Z, and Ma, G. Application of non-invasive brain stimulation combined with functional magnetic resonance imaging in post-stroke motor function rehabilitation. J Neurosci Methods. (2025) 421:110470. doi: 10.1016/j.jneumeth.2025.110470
45. Jia, T, Mo, L, McGeady, C, Sun, J, Liu, A, Ji, L, et al. Cortical activation patterns determine effectiveness of rTMS-induced motor imagery decoding enhancement in stroke patients. IEEE Trans Biomed Eng. (2025) 72:1200–8. doi: 10.1109/TBME.2024.3492977
46. Zeng, Y, Ye, Z, Zheng, W, and Wang, J. Efficacy of cerebellar transcranial magnetic stimulation for post-stroke balance and limb motor function impairments: Meta-analyses of random controlled trials and resting-state fMRI studies. Cerebellum. (2024) 23:1678–96. doi: 10.1007/s12311-024-01660-7
47. Tang, Z, Liu, T, Han, K, Liu, Y, Su, W, Wang, R, et al. The effects of rTMS on motor recovery after stroke: a systematic review of fMRI studies. Neurol Sci. (2024) 45:897–909. doi: 10.1007/s10072-023-07123-x
48. Nudo, RJ, Wise, BM, SiFuentes, F, and Milliken, GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. (1996) 272:1791–4. doi: 10.1126/science.272.5269.1791
49. Buma, F, Kwakkel, G, and Ramsey, N. Understanding upper limb recovery after stroke. Restor Neurol Neurosci. (2013) 31:707–22. doi: 10.3233/RNN-130332
50. Zeiler, SR, and Krakauer, JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol. (2013) 26:609–16. doi: 10.1097/WCO.0000000000000025
51. Marin-Medina, DS, Arenas-Vargas, PA, Arias-Botero, JC, Gomez-Vasquez, M, Jaramillo-Lopez, MF, and Gaspar-Toro, JM. New approaches to recovery after stroke. Neurol Sci. (2024) 45:55–63. doi: 10.1007/s10072-023-07012-3
52. Grefkes, C, and Fink, GR. Noninvasive brain stimulation after stroke: it is time for large randomized controlled trials! Curr Opin Neurol. (2016) 29:714–20. doi: 10.1097/WCO.0000000000000395
53. Berger, U, Korngreen, A, Bar-Gad, I, Friedman, A, Wolfus, S, Yeshurun, Y, et al. Magnetic stimulation intensity modulates motor inhibition. Neurosci Lett. (2011) 504:93–7. doi: 10.1016/j.neulet.2011.09.004
54. Turi, Z, Lenz, M, Paulus, W, Mittner, M, and Vlachos, A. Selecting stimulation intensity in repetitive transcranial magnetic stimulation studies: a systematic review between 1991 and 2020. Eur J Neurosci. (2021) 53:3404–15. doi: 10.1111/ejn.15195
55. Rossi, S, Hallett, M, Rossini, PM, and Pascual-Leone, A. Safety of T. M. S. C. G. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. (2009) 120:2008–39. doi: 10.1016/j.clinph.2009.08.016
56. Zhu, M, Huang, S, Chen, W, Pan, G, and Zhou, Y. The effect of transcranial magnetic stimulation on cognitive function in post-stroke patients: a systematic review and meta-analysis. BMC Neurol. (2024) 24:234. doi: 10.1186/s12883-024-03726-9
57. Xie, G, Wang, T, Deng, L, Zhou, L, Zheng, X, Zhao, C, et al. Repetitive transcranial magnetic stimulation for motor function in stroke: a systematic review and meta-analysis of randomized controlled studies. Syst Rev. (2025) 14:47. doi: 10.1186/s13643-025-02794-3
Keywords: transcranial magnetic stimulation, motor function, activities of daily living, stroke, systematic review
Citation: Qin J, Hong Z, Wang J, Zhang Y, Zhuang H, Hong S and Zheng L (2025) Effectiveness of dual-site transcranial magnetic stimulation on motor function and activities of daily living in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 16:1630876. doi: 10.3389/fneur.2025.1630876
Edited by:
Agnieszka Guzik, University of Rzeszow, PolandReviewed by:
Engy BadrEldin Saleh Moustafa, Cairo University, EgyptJukrapope Jitpimolmard, Ratchaphruek Hospital, Thailand
Rita Huan-Ting Peng, University of Illinois at Urbana-Champaign, United States
Copyright © 2025 Qin, Hong, Wang, Zhang, Zhuang, Hong and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanshan Hong, MTM2NzU5NDE5NjZAMTYzLmNvbQ==; Liling Zheng, emxsMTExMTExQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Jiawei Qin
Jiawei Qin Zhenzhen Hong2†
Zhenzhen Hong2†