Abstract
Background:
Stroke severity affects length of hospital stay and functional recovery in rehabilitation. Therefore, establishing baseline data of stroke severity is a crucial step. In 2017, neurorehabilitation researchers met at the Stroke Recovery and Rehabilitation Roundtable (SRRR) to build a consensus on new standards for stroke recovery research. Core outcomes for measurement in stroke trials resulted in the recommendation that severe stroke should be assessed using the NIHSS. This scoping review aims to provide an overview of the variety of measurements used in clinical research to assess severe stroke.
Methods:
RCTs and CCTs were identified by searching PubMed, CENTRAL, SSCI, and ICTRP, covering articles published between January 2018 and September 2024. Peer-reviewed articles in English focusing on rehabilitative interventions and patients aged 18 years or older who have been classified with a severe stroke. The articles included were analyzed according to used measurements and cut-off scores.
Results:
The initial search yielded 1,004 publications, of which 35 (3.6%) studies were deemed eligible. In total, 11 different measures were used to assess severe stroke. Most studies used the NIHSS (n = 14), followed by mRS (n = 6), the FMA upper extremity (n = 4), the original FMA (n = 4) and the (modified) BI (n = 3). Seven different cut-off scores for the NIHSS were identified, with the scale being most frequently used in clinical settings.
Conclusion:
This review indicates substantial variability in measurements and a diverse range of cut-off scores. Consequently, comparability of patients’ baseline stroke severity across studies is limited. Given the fact that the NIHSS is only partially used, future efforts should focus on barriers and challenges using the NIHSS.
1 Introduction
Strokes affect more than one billion people worldwide and are the leading cause of disability and the second leading cause of death (1). Post-stroke consequences can be reflected at every level of the International Classification of Functioning, Disability and Health (2). Different standardized measurements address these domains, capturing the complex impact of stroke on function, activity, and participation. An important factor influencing stroke survivors’ outcomes is stroke severity (3, 4). It is a key factor in hospital length of stay, which is one of the most important indicators for monitoring the utilization of hospital treatment (5). Unfortunately, the definition of stroke severity, especially severe stroke, is not used uniformly, with a wide range of different measures found (6–8).
In 2017, the measurement working group of the ‘Stroke Recovery and Rehabilitation Roundtable’ (SRRR) was established to develop standardized recommendations and establish guidelines for standardized measuring time points and metrics to be used in all adult stroke sensorimotor recovery research (9). According to the SRRR, the ‘National Institute of Health Stroke Scale’ (NIHSS) should be used as a baseline measurement to determine the severity of a stroke, providing a quantifiable measurement of post-stroke neurological impairments across domains as well as the severity of symptoms linked to cerebral infarcts (9).
The NIHS Scale ranges between 0 and 42 points, with a higher score indicating a higher stroke severity. Based on the work of Brott et al., the most prevalent cut-off values of the NIHSS for defining stroke severity are labeled as mild (1–4), moderate (5–14), severe (15–24) and very severe (25+) (10). Briggs et al. used a cut-off score of >16 points to define a severe stroke as well as >20/42 (11).
It is not known how the severity of stroke is currently classified in clinical research or whether they are measured using the recommended NIHSS. This scoping review aims to provide an overview of stroke severity measurements and cut-off scores used in clinical rehabilitation research.
2 Methods
2.1 Study design
This scoping review was conducted according to the Joanna Briggs Institute guideline for scoping research and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for reporting scoping (12). The study protocol was pre-registered on the Open Science Framework Platform.1
2.2 Information sources and search strategy
Searches were conducted between January 2018 and September 2024 using a specified search string (Table 1; Supplementary material). After an initial search, a comprehensive search strategy was developed and applied to MEDLINE via PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), the Social Sciences Citation Index (SSCI), and the International Clinical Trials Registry Platform (ICTRP).
Table 1
| MEDLINE via PubMed | |||
|---|---|---|---|
| 1 severe stroke[Title/Abstract] 2 stroke severity[Title/Abstract] 3 stroke disab*[Title/Abstract] 4 severe stroke impair*[Title/Abstract] 5 severe stroke limit*[Title/Abstract] 6 #1 OR #2 OR #3 OR #4 OR #5 7 Autogenic Training[MeSH Terms] 8 Combined Modality Therapy[MeSH Terms] 9 Exercise Movement Techniques[MeSH Terms] 10 Mentoring[MeSH Terms] 11 Nursing Care[MeSH Terms] 12 Patient Positioning[MeSH Terms] 13 Stroke Rehabilitation[MeSH Terms] 14 Teaching[MeSH Terms] 15 Transcutaneous Electric Nerve Stimulation[MeSH Terms] | 16 Video Games[MeSH Terms] 17 Virtual Reality Exposure Therapy[MeSH Terms] 18 aerobic exercise[Title/Abstract] 19 aerobic training[Title/Abstract] 20 biofeedback[Title/Abstract] 21 coaching[Title/Abstract] 22 cognitive behavioral therapy[Title/Abstract] 23 cognitive rehabilitation[Title/Abstract] 24 constraint induced therapy[Title/Abstract] 25 education[Title/Abstract] 26 electric stimulation therapy[Title/Abstract] 27 exercise[Title/Abstract] 28 exercise therapy[Title/Abstract] 29 functional electric stimulation[Title/Abstract] 30 health education[Title/Abstract] | 31 home care[Title/Abstract] 32 home rehabilitation[Title/Abstract] 33 intensive care[Title/Abstract] 34 mirror therapy[Title/Abstract] 35 mobilization[Title/Abstract] 36 motor relearning program[Title/Abstract] 37 movement therapy[Title/Abstract] 38 muscle training[Title/Abstract] 39 neuromuscular electric stimulation[Title/Abstract] 40 nursing[Title/Abstract] 41 occupational therapy[Title/Abstract] 42 patient education[Title/Abstract] 43 physical activity[Title/Abstract] 44 physical therapy modalities[Title/Abstract] 45 physical therapy speciality[Title/Abstract] | 46 robotics[Title/Abstract] 47 task specific training[Title/Abstract] 48 virtual reality[Title/Abstract] 49 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 50 #6 AND #49 51 #50 (Filter: Randomized Controlled Trial) 52 #51 (Filter: Date - Publication 01.01.2018 to 06.09) |
Search string.
2.3 Inclusion/exclusion criteria
All RCTs and CCTs published in peer-reviewed journals enrolled severe stroke patients aged ≥18 years undergoing a rehabilitative intervention (e.g., physiotherapy, speech-language therapy, occupational therapy, nursing, and neuropsychology) were included. Articles were excluded if they used pharmacological and surgical interventions, non-invasive brain stimulation and complementary or alternative medicine interventions. If one or more secondary analyses were published, it was checked whether the primary study had already been included, if not, the first publication of a secondary analysis was included.
2.4 Study selection
The Rayyan management software (Rayyan Systems Inc., Cambridge, MA 02142, United States) was used to select the included articles (13). Two reviewers (KR, LK) independently screened titles and abstracts to exclude those that did not meet the inclusion criteria. In a second step, the same two researchers independently performed a full-text screening of the remaining studies etiology. Disagreements during the entire process were discussed with a third researcher (NS) until consensus was reached.
2.5 Data extraction
The data from each included study was extracted by KR and HB, using a data extraction framework. Conflicts were resolved by discussion with NS. According to the definition of scoping reviews, the methodological quality of the included studies was not evaluated. Following the JBI methodology for scoping reviews, a formal appraisal of methodological quality was not required (14).
Stroke severity measurement tools used in the included studies were grouped according to the International Classification of Functioning, Disability and Health into body function and body structures, activities, and participation (15).
3 Results
In total, 1,004 articles were identified for screening. After screening titles and abstracts, 646 articles were excluded due to exclusion criteria like pharmacological therapy or congress contribution without conclusive results. A total of 358 references remained and were screened for inclusion. The complete process for the inclusion of the final 35 publications is depicted in the PRISMA flow chart (Figure 1).
Figure 1

Study selection flow chart according to PRISMA 2020.
3.1 Description of included studies
Out of 1,004 articles screened, 35 articles were included. Geographically, most of the studies were conducted in Europe (38%), followed by America (24%), Asia (24%), and Australia (14%). Participants were recruited in various settings, which were categorized into three groups. Starting with the clinical setting (n = 15) and the rehabilitation setting (n = 14). The term “non-clinical/rehabilitative setting” (n = 10) was used to categorize various settings—such as laboratory or community settings—that did not align with either of the two primary categories. In two studies (16, 17) participants were recruited in two settings. In the studies by Mulder et al. (18) and Sakakibara et al. (19), participants were recruited from three different settings. Salazar et al. (20) did not provide any information about the setting.
Within these studies, participants were mostly included during the early subacute phase (n = 13), followed by chronic stroke phase (n = 8), hyperacute stroke phase (n = 8), late subacute (n = 3), and acute (n = 2). For two of these studies, the stroke phase was not specified.
Most interventions involved physical activation, including specific exercises, training programs, or early mobilization. Additionally, many interventions incorporated robotic-assisted technologies or other digital health solutions, such as mobile applications, health platforms, or virtual reality. Several studies implemented transcranial stimulation and brain-computer interfaces as part of the intervention. A smaller proportion received video-based education or adherence-enhancing strategies. Most of the control group received conventional therapy, standard hospital care, and home exercise program for the clinic or rehabilitation facility (Table 2).
Table 2
| Author, date, location | n | Setting | Phase | Measurement | Intervention |
|---|---|---|---|---|---|
| Aguiar et al. (2020) (25) Brazil, Canada | 22 | Non clinical/rehab | Chronic | FMA-UL FMA-LL | IG: aerobic treadmill training CG: outdoor-overground walking |
| Aprile et al. (2020) (47) Italy | 247 | Rehab | Early and late subacute | FMA-UL | IG: robotic and sensor-based device CG: conventional treatment: |
| Brunner et al. (2024) (29) Denmark | 40 | Rehab | Early subacute | NIHSS | IG: sessions with Brain-Computer Interfaces CG: standard physiotherapy and occupational therapy |
| Buvarp et al. (2023) (42) Sweden | 1.367 | Clinical, rehab | Hyperacute, acute | NIHSS | IG: increased physical activity CG: decreased physical activity |
| Chen et al. (2021) (27) China | 96 | Non clinical/rehab | Acute | BI | IG: goal-oriented Intervention CG: health education |
| Conroy et al. (2019) (24) United States | 45 | Clinical | Chronic | FMA | IG: robot-assisted arm training CG: therapist-assisted task training |
| Cumming et al. (2018, 2019) (48, 49), Walters et al. (2020) (50), Bernhardt et al. (2021) (51) United Kingdom, Australia, South East Asia | 2.104 | Clinical | Hyperacute | NIHSS | IG: very early and more frequent mobilization CG: usual care |
| De Bruyn et al. (2020) (30) Belgium | 40 | Rehab | Early subacute | FMA | IG: sensorimotor therapy (SENSe approach) and task specific exercises CG: cognitive table-top games with the non-affected UL and 30 min task-specific motor exercises |
| De Jong et al. (2018) (52) United Kingdom, Netherlands | 46 | Early subacute | FMA-UL | IG: PT therapy as recommended by the Dutch stroke guideline CG: OT therapy as recommended by the Dutch stroke guideline | |
| Ertas-Spantgar et al. (2024) (28) Germany | 24 | Rehab | Post-acute | BI | IG: usual therapy and experimental therapy by RehaGoal App CG: usual therapy |
| Frange et al. (2023) (21) Brazil | 8 | Clinical | Hyperacute | NIHSS | IG: performing calf muscle contractions by activity CG: inactivity |
| Kamal et al. (2020) (43) Pakistan | 310 | Clinical | Acute | NIHSS | IG: video-based education intervention CG: standard care |
| Kersey et al. (2023) (16) United States | 32 | CLINICAL and non clinical/rehab | Hyperacute, acute, early subacute | NIHSS | IG: patients receive strategy training using a mobile health platform (iADAPT) CG: patients receive strategy training using a workbook |
| Kim et al. (2020) (53) Korea | 21 | Rehab | Late subacute | FAC | IG: underwater gait training CG: overground gait training |
| Koo et al. (2018) (31) Korea | 24 | Clinical | Early subacute | mBI | IG: anodal transcranial direct current stimulation CG: sham stimulation |
| Logan et al. (2022) (54) England | 45 | Clinical | Hyperacute, acute | mRS | IG: functional standing frame program CG: usual physiotherapy |
| Le Franc et al. (2021) (55) France | 20 | Rehab | Chronic | FMA-UL | IG: tendor vibration and visual feedback; severe stroke group CG: tendor vibration and visual feedback; mild to moderate stroke group |
| Mahmood et al. (2022) (17) India | Rehab and non-clinical/rehab | Early subacute, chronic | FMA | IG: received additional adherence strategies CG: standard hospital care and home exercise program | |
| Martins et al. (2020) (32) Brazil, Canada | 26 | Nonclinical/rehab | Chronic | FMA-UL FMA-LL | IG: task-specific circuit training CG: standard care |
| Middleton et al. (2019) (56) Australia | 970 | Clinical | Hyperacute to acute | LAMS | IG: Intervention stroke units with treatment protocols CG: Control group stroke units |
| Mulder et al. (2022) (18) Australia, Netherlands | 129 | Clinical, rehab and non-clinical/rehab | Early and late subacute | mRS | IG: 8-week caregiver-mediated exercises intervention CG: control intervention |
| Nagai et al. (2024) (57) Japan | 42 | Clinical | n.n. | mRS FIM motor item | IG: standing on unstable board for the nonparalyzed lower limbs CG: usual physical therapy |
| Ouyang et al. (2020, 2021) (44, 58) China, United Kingdom, Australia, India, Sri Lanka | 11.084 | Clinical | Hyperacute | NIHSS | IG: sitting up head position CG: lying flat |
| Radford et al. (2020) (22) n.n. | 46 | Clinical | n.n. | NIHSS | IG: vocational rehabilitation CG: usual care |
| Renner et al. (2020) (59) Germany | 69 | Rehab | Late subacute | FMA-UL MRC | IG: bilateral training CG: unilateral training |
| Reynolds et al. (2021) (33) Australia | 20 | Rehab | Early subacute | NIHSS mRS | IG: moderate-intensity fitness training CG: low-intensity exercise |
| Rose et al. (2022) (34) Australia, New Zealand | 116 | Non-clinical/rehab | Chronic | mRS | IG: Aphasia Therapies CIAT-Plus or M-MAT CG: usual care |
| Sakakibara et al. (2022) (19) Canada | 126 | Clinical, rehab and Non-clinical/rehab | Chronic | mRS | IG: Stroke Coach CG: Memory Training group |
| Salazar et al. (2024) (20) United States | 20 | n. n. | Chronic | NIHSS | IG: transcranial direct current stimulation CG: sham during image acquisition |
| Smith et al. (2021) (45) United States | 23 | Rehab | Early subacute | NIHSS | IG: bimanual lever-driven wheelchair CG: Conventional exercise group |
| Stockbridge et al. (2023) (35) United States | 51 | Clinical | Chronic | NIHSS | IG: transcranial direct current stimulation (tDCS) CG: standard care |
| Straudi et al. (2020) (26) Italy | 39 | Rehab | Early subacute | FMA-LL | IG: robot-assisted arm therapy and hand functional electrical stimulation CG: time-matched intensive conventional therapy |
| Threapleton et al. (2020) (23) United Kingdom | 33 | Clinical | All phases | NIHSS OSCPS | IG: virtual reality intervention CG: usual care |
| Tistad et al. (2018) (36) Sweden | 237 | Rehab | Early subacute | BI | IG: client-centered activities of daily living intervention CG: usual activities of daily living intervention |
| Watkins et al. (2022) (37) United Kingdom | 157 | Clinical | Hyperacute | NIHSS | IG: systematic voiding program for urinary incontinence CG: usual care |
Included studies, settings, stroke phases, stroke severity measurements and interventions of RCTs and CCTs.
BI, Barthel Index; CG, control group; FAC, Functional Ambulation Categories; FMA, Fugl-Meyer-Assessment; FMA-UL, Fugl-Meyer-Assessment upper extremity; FMA LL, Fugl-Meyer-Assessment lower extremity; IG, intervention group; LAMS, Los Angeles Motor Scale; mBI, Modified Barthel Index; mRS, modified Rankin Scale; NIHSS, National Institute Stroke Scale; OCSPC, Oxfordshire Community Stroke Project Classification.
3.2 Identified measures and framework conditions
Eleven different measures were used to assess stroke severity (Table 3). Most studies used the NIHSS (n = 14), followed by modified Rankin Scale (n = 6). The Fugl-Meyer-Assessment for the upper extremity (FMA-UL), and the original Fugl-Meyer-Assessment (FMA), were each used four times to address stroke severity. The Barthel Index (BI) was used in three studies, and the Functional Ambulation Categories (FAC) and the Los Angeles Motor Scale (LAMS) was used in one study. Six studies included two measures to assess stroke severity. The NIHSS was used in seven studies to identify the hyperacute phase.
Table 3
| Instrument of Measurement (abbrev.) | Original authors | Method of report | Components | Scoring system | Validation studies |
|---|---|---|---|---|---|
| National Institute of Health Stroke Scale (NIHSS) | Brott, T., Adams, H. P., Jr, Olinger, C. P., Marler, J. R., Barsan, W. G., Biller, J., Spilker, J., Holleran, R., Eberle, R., & Hertzberg, V. (1989). Measurements of acute cerebral infarction: a clinical examination scale. Stroke, 20(7), 864–870. https://doi.org/10.1161/01.str.20.7.864 | Observer Paper and Pencil | 15 Items Aphasia, Behavior, Cognition, Dysarthria, Vision Perception | 0 - 3 and 0 - 4 ordinal scale with written and numerical descriptors. Calculation of a total score 0 - 42. Higher scores indicate greater severity. | Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989 Jul;20(7):864-70. doi: 10.1161/01.str.20.7.864. PMID: 2749846. |
| Fugl-Meyer-Assessment (FMA) | Fugl-Meyer, A. R., Jääskö, L., Leyman, I., Olsson, S., & Steglind, S. (1975). The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian journal of rehabilitation medicine, 7(1), 13–31. | Observer Paper & Pencil | 155 Items Activities of daily linving, Functional Mobility, Pain Five domains (Motor function, Sensory function, Balance, Joint range of Motion, Joint pain), | 0 - 3 ordinal scale Calculation of a total score 0 – 226 | Fugl-Meyer AR et al.: The post-stroke hemiplegic patient. A method for evaluation of physical performance. Scand J Rehabil Med 1975, 7:13-31 |
| Fugl-Meyer upper extremity (FM UE) (as part of the FMA) | Fugl-Meyer, A. R., Jääskö, L., Leyman, I., Olsson, S., & Steglind, S. (1975). The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian journal of rehabilitation medicine, 7(1), 13–31. | Observer Paper & Pencil | A - Shoulder/Elbow /Forearm B – Wrist C – Hand D – Coordination/ Speed | 0 - 3 ordinal scale Calculation of a total score 0 - 66 | Fugl-Meyer AR et al.: The post-stroke hemiplegic patient. A method for evaluation of physical performance. Scand J Rehabil Med 1975, 7:13-31 |
| Fugl-Meyer lower extremity (FM LE) (as part of the FMA) | Fugl-Meyer, A. R., Jääskö, L., Leyman, I., Olsson, S., & Steglind, S. (1975). The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian journal of rehabilitation medicine, 7(1), 13–31. | Observer Paper & Pencil | E – Hip/Knee/Ancle F – Coordination/ Speed G - Balance | 0 - 3 ordinal scale Calculation of a total score 0 - 34 | Fugl-Meyer AR et al.: The post-stroke hemiplegic patient. A method for evaluation of physical performance. Scand J Rehabil Med 1975, 7:13-31 |
| Barthel Index (BI) | Mahoney, F. I., & Barthel, D. W. (1965). Functional evaluation: the barthel index. Maryland state medical journal, 14, 61–65. | Performance Measure Paper & Pencil | 10 Items Activities of Daily Living Functional Mobility Gait | Items are rated based on the amount of assistance required to complete each activity, can choose between 0-5-10-15 | Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61-3. doi: 10.3109/09638288809164103. PMID: 3403500. |
| Modified Barthel Index (mBI) | Collin, C., Wade, D. T., Davies, S., & Horne, V. (1988). The Barthel ADL Index: a reliability study. International disability studies, 10(2), 61–63. https://doi.org/10.3109/09638288809164103 | Performance Measure Paper & Pencil | 10 Items Activities of Daily Living Functional Mobility Gait | Items are rated based on the amount of assistance required to complete each activity | Shah, S, Vanley, F., Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation https://doi.org/10.1016/0895-4356(89)90065-6 |
| Modified Rankin Scale (mRS) | van Swieten, J. C., Koudstaal, P. J., Visser, M. C., Schouten, H. J., & van Gijn, J. (1988). Interobserver agreement for the assessment of handicap in stroke patients. Stroke, 19(5), 604–607. https://doi.org/10.1161/01.str.19.5.604 | Observer | Single item | 6-point rankin scale fom 0=no symptoms to 5=severe disability: bedridden, incontinent, and requiring constant nursing care and attention | Rankin, J. (1957). Cerebral vascular accidents in patients over the age of 60. Scott Med J, 2, 200-215. |
| Functional Ambulation Categories (FAC) | Holden M.K., Gill K.M., Magliozzi M.R., Nathan J., Piehl-Baker L. Clinical gait assessment in the neurologically impaired Reliability and meaningfulness. Phys Ther. 1984; 64: 35-40 | Observer Paper & Pencil | Assessing walking ability in 6 broard categories | 0-5 Higher score indicates less dependency | Mehrholz, J., Wagner, K., Rutte, K., Meiner, D. and Pohl, M. Predictive validity and responsiveness of the Functional Ambulation Category in hemiparetic patients after stroke. Archives of Physical Medicine Rehabilitation, 2007, 88, 1314-1319. |
| Action Research Arm Test | Lyle R. C. (1981). A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International journal of rehabilitation research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation, 4(4), 483–492. https://doi.org/10.1097/00004356-198112000-00001 | Observer Paper & Pencil | Activities of Daily Living, Coordination, Dexterity, Upper Extremity Function 19 Items | 4-point ordinal scale Calculation of a total score 0 - 57 | Koh CL, Hsueh IP, Wang WC, Sheu CF, Yu TY, Wang CH, Hsieh CL. Validation of the action research arm test using item response theory in patients after stroke. J Rehabil Med. 2006 Nov;38(6):375-80. doi: 10.1080/16501970600803252. PMID: 17067971. Chen HF, Lin KC, Wu CY, Chen CL. Rasch validation and predictive validity of the action research arm test in patients receiving stroke rehabilitation. Arch Phys Med Rehabil. 2012 Jun;93(6):1039-45. doi: 10.1016/j.apmr.2011.11.033. Epub 2012 Mar 14. PMID: 22420887. |
| Oxfordshire Community Stroke Project Classification (OCSP) | Bamford, J., Sandercock, P., Dennis, M., Burn, J., & Warlow, C. (1991). Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet (London, England), 337(8756), 1521–1526. https://doi.org/10.1016/0140-6736(91)93206-o | classification of four sub-categories of cerebral infarction based on presenting symptoms and signs: | lacunar infarcts (LACI) total anterior circulation infarcts (TACI) partial anterior circulation infarcts (PACI) posterior circulation infarcts (POCI) | A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project 1981-86. 1. Methodology, demography and incident cases of first-ever stroke.J Neurol Neurosurg Psychiatry. 1988 Nov; 51(11): 1373–1380. | |
| Los Angeles Motor Scale (LAMS) | Llanes, J. N., Kidwell, C. S., Starkman, S., Leary, M. C., Eckstein, M., & Saver, J. L. (2004). The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehospital emergency care, 8(1), 46–50. https://doi.org/10.1080/312703002806 | Observer | 3-item prehospital scoring tool Facial droop Arm drift Grip strength | 0-1 0=absent +1= present/drifts down, weak grip +2=falls rapidly/no grip | Kim JT, Chung PW, Starkman S, et al. Field Validation of the Los Angeles Motor Scale as a Tool for Paramedic Assessment of Stroke Severity. Stroke. 2017; 48(2): 298-306. Nazliel B., Starkman S, Liebeskind DS, et al. A brief prehospital stroke severity scale identifies ischemic stroke patients harboring perstisting large arteroaö occlusions. Stroke. 2008; 39(8): 2264-7. |
Overview Stroke Measurements and ICF categories; n=11
A, Activity; BF, Body function; I, Impairment; ICF, International Classification of Functioning, Disability and Health; P, Participation.
The original FMA was used in all three settings, mainly during the chronic phase, as well as the combination of FMA-UL and the Fugl-Meyer-Assessment of the lower extremity (FMA-LL) (n = 3) and once during the early subacute phase. In contrast, the FMA-UL was used twice in a rehabilitation setting during early subacute and late subacute.
Most of the measures can be assigned to a single ICF level. The NIHSS, Oxfordshire Community Stroke Project Classification (OCSP), and Los Angeles Motor Scale (LAMS) correspond to body function and impairment. Only the FMA as well as FMA-UL and FMA-LL, and the Functional Independence Measure (FIM) motor items cover both body function and impairment as well as the activity level. The remaining five measures are classified under the activity level.
Figure 2 illustrates the relationships among the three domains—“setting,” “measurement,” and “stroke recovery phase”—using connections of varying widths, where the thickness of each connection reflects the number of shared elements between the domains.
Figure 2

Sankey diagram of setting, measurement, and phase. BI, Barthel Index; CG, control group; FAC, Functional Ambulation Categories; FMA, Fugl-Meyer-Assessment; FMA-UL, Fugl-Meyer-Assessment upper extremity; FMA LL, Fugl-Meyer-Assessment lower extremity; IG, intervention group; LAMS, Los Angeles Motor Scale; mBI, Modified Barthel Index; mRS, modified Rankin Scale; NIHSS, National Institute Stroke Scale; OCSPC, Oxfordshire Community Stroke Project Classification.
3.3 Cut-off scores
Study protocols provided different cut-off scores to assess severe stroke (Figures 3–5). For the NIHSS, the range for severe stroke was >5 and <20 (21) to 21–24 (22, 23). For the FMA the cut-off score applied was < 25 (24) and <50 (17). For the FMA-UL, the cut-off score was <30 (25) or ≤ 21 (26). For the BI, a cut-off score of ≤ 40 (27) or <30 (28) was used to assess severe stroke. In 11 studies (16, 20, 29–37), the cut-off scores for the evaluation of the severity of stroke were not specified.
Figure 3

Measurements assessing severe stroke used in clinical settings; inner ring: stroke recovery phase; middle ring: measurements; outer ring: cut-off scores. BI, Barthel Index; FAC, Functional Ambulation Categories; FMA, Fugl-Meyer-Assessment; FMA LL, Fugl-Meyer-Assessments lower limb; FMA-UL, Fugl-Meyer-Assessment upper limb; LAMS, Los Angeles Motor Scale; mBI, modified Barthel Index; mRS, modified Rankin Scale; NIHSS, National Institute Stroke Scale; n.n., not named.
Figure 4

Measurements assessing severe stroke used in rehabilitation settings; inner ring: stroke recovery phase; middle ring: measurements; outer ring: cut-off scores. BI, Barthel Index; FAC, Functional Ambulation Categories; FMA, Fugl-Meyer-Assessment; FMA LL, Fugl-Meyer-Assessments lower limb; FMA-UL, Fugl-Meyer-Assessment upper limb; LAMS, Los Angeles Motor Scale; mBI, modified Barthel Index; mRS, modified Rankin Scale; NIHSS, National Institute Stroke Scale; n.n., not named.
Figure 5
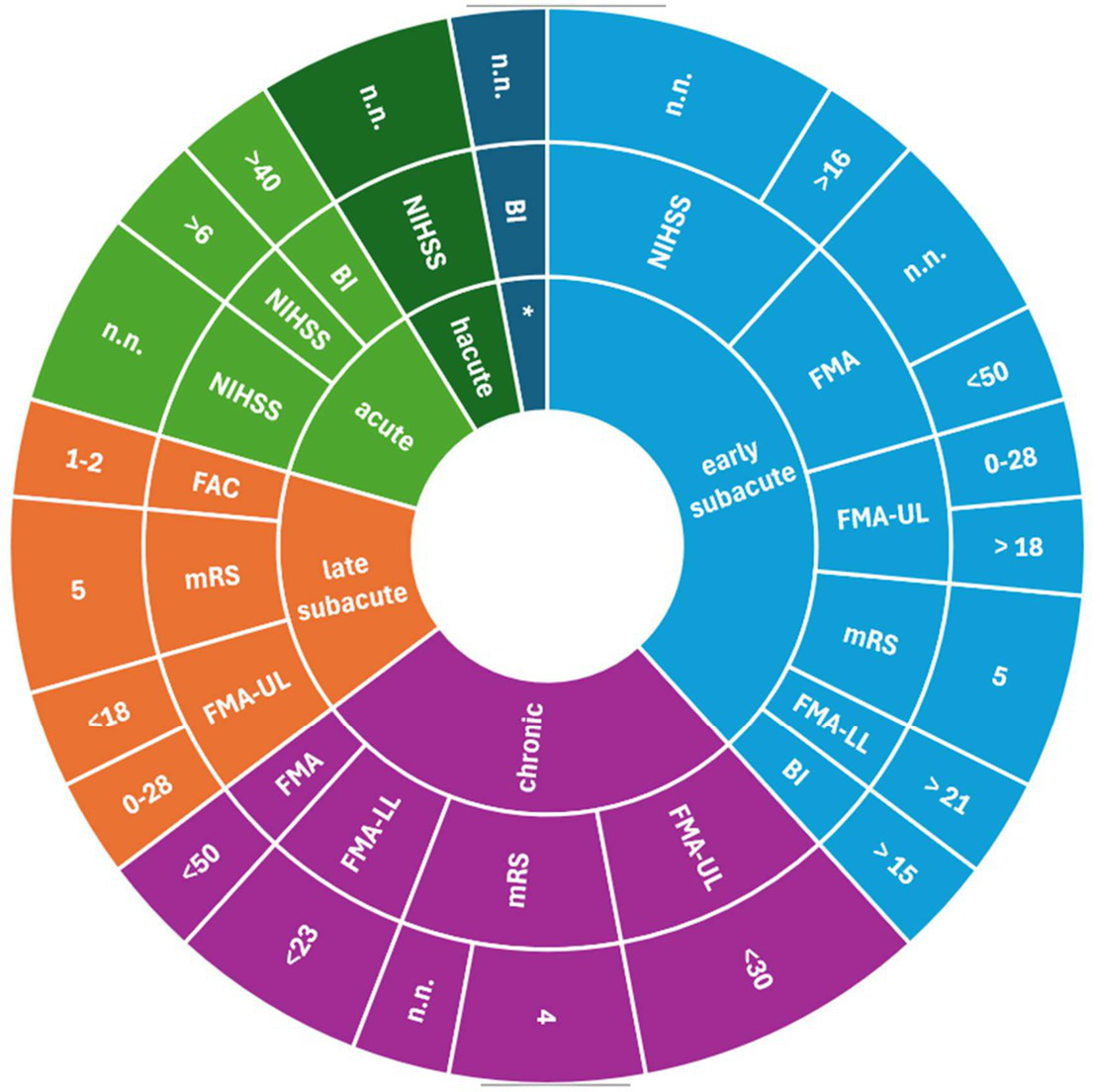
Measurements assessing severe stroke used in not clinical/rehabilitation settings; inner ring: stroke recovery phase; middle ring: measurements; outer ring: cut-off scores. BI, Barthel Index; FAC, Functional Ambulation Categories; FMA, Fugl-Meyer-Assessment; FMA LL, Fugl-Meyer-Assessments lower limb; FMA-UL, Fugl-Meyer-Assessment upper limb; LAMS, Los Angeles Motor Scale; mBI, modified Barthel Index; mRS, modified Rankin Scale; NIHSS, National Institute Stroke Scale; n.n., not named.
4 Discussion
Clinical manifestation of stroke varies depending on factors like etiology, localization, and stroke severity, with initial stroke severity known to be a crucial predictor of outcomes (38). Scales and measurements help to quantify the severity of stroke symptoms, aiding in treatment decisions. The focus of this scoping review was to give an overview of the measurements, and the cut-off scores used in clinical research to classify stroke severity.
Clinical symptoms undergo considerable changes over time. Guidelines consider these diverse areas of post-stroke disability and their associated symptoms beyond the acute phase of the disease. Stroke recovery includes the examination of level of consciousness, overall neurological impairment, motor function, balance, cognition, speech and language, Activities of Daily Living (ADL), depression, family functioning, and quality of life (39). For this study, research protocols focus on different outcomes, which require different methods and measures suitable for the individual research aim. On the one hand, the multitude of measures presented in this current review is not surprising; instead, they reflect the many different post-stroke symptoms and aims of stroke research. On the other hand, various measures to assess stroke lead to limitations in stroke research as the non-uniform use limits the ability to assemble treatment evidence across trials (40, 41). This review counted 11 measurements, underlining the lack of standardization. According to the roundtable, other outcome measures aligned with the trial’s purpose and target intervention can be added. The recommendation applies across all stages, from the hyperacute to the early and late subacute to the chronic phase. For the studies included in this review, it can be stated that this recommendation was not followed in 21 out of 35 studies.
With that in mind, it must be additionally mentioned that completely different constructs are assessed when using the FAC, the FMA-UL or the ARAT, for example. It should be critically questioned whether it is sufficient to determine the severity of a stroke solely based on walking or upper limb function. For the sake of completeness, it must also be said that the NIHSS does not provide information on activities of daily living like walking or transfers, which are crucial factors for patients’ independence and thus for discharge. The results of this scoping review seem to reflect the lack of a single measure capturing all ICF levels. The second part of the current research question referred to quantifying severe stroke. Results showed that the cut-off scores used for identical measurements varied in the included studies. This is especially notable for the NIHSS and the FMA. Among the studies that reported cut-offs for the NIHSS, seven different ones were found. Buvarp et al. (42) indicated a cut-off for severe stroke at >6 points. Kamal et al. (43) at >9 points, Ouyang et al. (44) at >15, Smith et al. (45) at >16, Liu et al. (46) at >20, Frange (21) >5 and >20 and Radford et al. (22) between 21 and 24. Results with a value of 9 out of 42 points can hardly be comparable with one of 20. Similarly, different cut-off values can be found in the results of the FMA with cut-offs less than 25 or less than 50. The authors of the studies included refer to various sources. Without the authors giving more detailed reasons for the cut-off scores used, it can be assumed that the scores are adapted to the respective population and setting. A cut-off score could be comprehensible and appropriate for individual study, but it must be considered, as it limits quantitative synthesis.
4.1 Strengths and limitations
This is the first study about the realization of stroke measures focusing on assessing severe stroke and the used cut-off scores. One strength of this review is the comprehensive search strategy specific to non-medical therapeutic interventions in stroke rehabilitation. Furthermore, the research team provides a diverse educational/professional background in treating severe stroke patients.
A reason for the limited number of search results was the inclusion of CCT and RCT study types. Because there is extensive research in the neurorehabilitation of stroke, the quality of these studies also provided the opportunity to include studies that may be of interest for guideline recommendations.
This review’s wide range of measures reflects the diversity of existing tools for assessing stroke severity. These results highlight the variety of measures used in research and those used in clinical practice to evaluate severe stroke.
5 Conclusion
Using different instruments and cut-off scores to assess stroke severity, the measurements’ informative value is limited. It remains unclear what functional abilities the affected person has, as the measurements are based on non-standardized constructs. The categorization and standardization of stroke severity could facilitate communication between healthcare professionals, health insurance companies, and healthcare institutions. This is the case if there is a mutual understanding of stroke severity across all sectors. The use of the NIHSS as a basic instrument, as recommended by the Roundtable, and an instrument addressing the ICF level could reflect the actual situation of patients. Further research is required into obligatory, cross-setting cut-off scores.
Statements
Author contributions
KR: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. HB: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. NS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The open access publication was facilitated by the support of the Martin-Luther-University of Halle-Wittenberg Germany.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1631275/full#supplementary-material
Footnotes
1.^OSF, register number: 10.17605/OSF.IO/WYR5H, https://osf.io/wyr5h
References
1.
GBD. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2.
World Health Organization. Herausgeber, International classification of functioning, disability and health: ICF. Geneva: World Health Organization (2001). 299 p.
3.
AlmenkerkSVSmalbruggeMDeplaMFIAEefstingJAHertoghCMPM. What predicts a poor outcome in older stroke survivors? A systematic review of the literature. Disabil Rehabil. (2013) 35:1774–82. doi: 10.3109/09638288.2012.756941
4.
KerrDMFultonRLLeesKRfor the VISTA Collaborators. Seven-day NIHSS is a sensitive outcome measure for exploratory clinical trials in acute stroke: evidence from the virtual international stroke trials archive. Stroke. (2012) 43:1401–3. doi: 10.1161/STROKEAHA.111.644484
5.
García-RudolphACegarraBOpissoETormosJMBernabeuMSauríJ. Predicting length of stay in patients admitted to stroke rehabilitation with severe and moderate levels of functional impairments. Medicine (Baltimore). (2020) 99:e22423. doi: 10.1097/MD.0000000000022423
6.
McGlincheyMPJamesJMcKevittCDouiriASackleyC. The effect of rehabilitation interventions on physical function and immobility-related complications in severe stroke: a systematic review. BMJ Open. (2020) 10:e033642. doi: 10.1136/bmjopen-2019-033642
7.
RoesnerKSchefflerBKaehlerMSchmidt-MaciejewskiBBoettgerTSaalS. Effects of physical therapy modalities for motor function, functional recovery, and post-stroke complications in patients with severe stroke: a systematic review update. Syst Rev. (2024) 13:270. doi: 10.1186/s13643-024-02676-0
8.
TeasellRPereiraSCotoiA. The rehabilitation of severe stroke. In: Evidence-Based Review of Stroke Rehabilitation. (2018). Available online at: http://www.ebrsr.com/evidence-review/22-rehabilitation-severe-stroke (Accessed May 15, 2023).)
9.
KwakkelGLanninNABorschmannKEnglishCAliMChurilovLet al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Int J Stroke. (2017) 12:451–61. doi: 10.1177/1747493017711813
10.
BrottTAdamsHPOlingerCPMarlerJRBarsanWGBillerJet al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
11.
BriggsDEFelbergRAMalkoffMDBratinaPGrottaJC. Should mild or moderate stroke patients be admitted to an intensive care unit?Stroke. (2001) 32:871–6. doi: 10.1161/01.STR.32.4.871
12.
TriccoACLillieEZarinWO’BrienKKColquhounHLevacDet al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
13.
OuzzaniMHammadyHFedorowiczZElmagarmidA. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
14.
PetersMDJMarnieCTriccoACPollockDMunnZAlexanderLet al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. (2020) 18:2119–26. doi: 10.11124/JBIES-20-00167
15.
LeonardiMLeeHKostanjsekNFornariARaggiAMartinuzziAet al. 20 years of ICF-international classification of functioning, disability and health: uses and applications around the world. Int J Environ Res Public Health. (2022) 19:19. doi: 10.3390/ijerph191811321
16.
KerseyJKringleESetiawanIMAParmantoBSkidmoreER. Pilot RCT examining feasibility and disability outcomes of a mobile health platform for strategy training in inpatient stroke rehabilitation (iADAPT). Top Stroke Rehabil. (2023) 30:512–21. doi: 10.1080/10749357.2022.2077522
17.
MahmoodANayakPEnglishCDeshmukhAShashikiranUManikandanNet al. Adherence to home exercises and rehabilitation (ADHERE) after stroke in low-to-middle-income countries: a randomized controlled trial. Top Stroke Rehabil. (2022) 29:438–48. doi: 10.1080/10749357.2021.1940800
18.
MulderMNijlandRHMVloothuisJDMVan Den BergMCrottyMKwakkelGet al. Comparing two identically protocolized, multicentre, randomized controlled trials on caregiver-mediated exercises poststroke: any differences across countries? Akinwuntan AE, Herausgeber. PLoS One. (2022) 17:e0263013. doi: 10.1371/journal.pone.0263013
19.
SakakibaraBMLearSABarrSIGoldsmithCHSchneebergASilverbergNDet al. Telehealth coaching to improve self-management for secondary prevention after stroke: a randomized controlled trial of stroke coach. Int J Stroke. (2022) 17:455–64. doi: 10.1177/17474930211017699
20.
SalazarCAWelshJMLenchDHarmsenIEJensenJHGrewalPet al. Concurrent tDCS-fMRI after stroke reveals link between attention network organization and motor improvement. Sci Rep. (2024) 14:19334. doi: 10.1038/s41598-024-70083-5
21.
FrangeCEliasRMSiengsukonCCoelhoFMS. Physical activity for obstructive sleep apnea after stroke? A pilot study assessing the contribution of body fluids. Sleep Breath. (2023) 27:1343–50. doi: 10.1007/s11325-022-02735-7
22.
RadfordKGrantMSinclairEKettlewellJWatkinC. What is a return to work after stroke? 12-month outcomes in a feasibility trial. J Rehabil Med. (2020) 52:jrm00048. doi: 10.2340/16501977-2647
23.
ThreapletonKNewberryKSuttonGWorthingtonEDrummondA. Virtually home: feasibility study and pilot randomised controlled trial of a virtual reality intervention to support patient discharge after stroke. Br J Occup Ther. (2018) 81:196–206. doi: 10.1177/0308022617743459
24.
ConroySSWittenbergGFKrebsHIZhanMBeverCTWhitallJ. Robot-assisted arm training in chronic stroke: addition of transition-to-task practice. Neurorehabil Neural Repair. (2019) 33:751–61. doi: 10.1177/1545968319862558
25.
AguiarLTNadeauSBrittoRRTeixeira-SalmelaLFMartinsJCSamoraGARet al. Effects of aerobic training on physical activity in people with stroke: a randomized controlled trial. NeuroRehabilitation. (2020) 46:391–401. doi: 10.3233/NRE-193013
26.
StraudiSBaroniAMeleSCraigheroLManfrediniFLambertiNet al. Effects of a robot-assisted arm training plus hand functional electrical stimulation on recovery after stroke: a randomized clinical trial. Arch Phys Med Rehabil. (2020) 101:309–16. doi: 10.1016/j.apmr.2019.09.016
27.
ChenYWeiYLangHXiaoTHuaYLiLet al. Effects of a goal-oriented intervention on self-management behaviors and self-perceived burden after acute stroke: a randomized controlled trial. Front Neurol. (2021) 12:650138. doi: 10.3389/fneur.2021.650138
28.
Ertas-SpantgarFMüllerSVKorabovaSGabelASchieringIPapeAEet al. Errorless learning and assistive technology did not improve the negative prognosis for severe dressing impairment after stroke if persisting for two weeks: a randomized controlled trial. Appl Neuropsychol Adult. (2024) 31:939–47. doi: 10.1080/23279095.2022.2090839
29.
BrunnerILundquistCBPedersenARSpaichEGDosenSSavicA. Brain computer interface training with motor imagery and functional electrical stimulation for patients with severe upper limb paresis after stroke: a randomized controlled pilot trial. J NeuroEngineering Rehabil. (2024) 21:10. doi: 10.1186/s12984-024-01304-1
30.
De BruynNSaenenLThijsLVan GilsACeulemansEEssersBet al. Sensorimotor vs. motor upper limb therapy for patients with motor and somatosensory deficits: a randomized controlled trial in the early rehabilitation phase after stroke. Front Neurol. (2020) 11:597666. doi: 10.3389/fneur.2020.597666
31.
KooWRJangBHKimCR. Effects of anodal transcranial direct current stimulation on somatosensory recovery after stroke: a randomized controlled trial. Am J Phys Med Rehabil. (2018) 97:507–13. doi: 10.1097/PHM.0000000000000910
32.
MartinsJCNadeauSAguiarLTScianniAATeixeira-SalmelaLFDe Morais FariaCDC. Efficacy of task-specific circuit training on physical activity levels and mobility of stroke patients: a randomized controlled trial. NeuroRehabilitation. (2020) 47:451–62. doi: 10.3233/NRE-203207
33.
ReynoldsHSteinfortSTillyardJEllisSHayesAHansonEDet al. Feasibility and adherence to moderate intensity cardiovascular fitness training following stroke: a pilot randomized controlled trial. BMC Neurol. (2021) 21:52. doi: 10.1186/s12883-021-02052-8
34.
RoseMLNickelsLCoplandDTogherLGodeckeEMeinzerMet al. Results of the COMPARE trial of constraint-induced or multimodality aphasia therapy compared with usual care in chronic post-stroke aphasia. J Neurol Neurosurg Psychiatry. (2022) 93:573–81. doi: 10.1136/jnnp-2021-328422
35.
StockbridgeMDElmJBreiningBLTippettDCSebastianRCassarlyCet al. Transcranial direct-current stimulation in subacute aphasia: a randomized controlled trial. Stroke. (2023) 54:912–20. doi: 10.1161/STROKEAHA.122.041557
36.
TistadMFlinkMYtterbergCErikssonGGuidettiSThamKet al. Resource use of healthcare services 1 year after stroke: a secondary analysis of a cluster-randomised controlled trial of a client-centred activities of daily living intervention. BMJ Open. (2018) 8:e022222. doi: 10.1136/bmjopen-2018-022222
37.
WatkinsCTishkovskayaSBrownCSuttonCGarciaYSForshawDet al. Systematic voiding programme in adults with urinary incontinence following acute stroke: the ICONS-II RCT. Health Technol Assess. (2022) 26:1–88. doi: 10.3310/EFTV1270
38.
RostNSBottleALeeJRandallMMiddletonSShawLet al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc. (2016) 5:e002433. doi: 10.1161/JAHA.115.002433
39.
WinsteinCJSteinJArenaRBatesBCherneyLRCramerSCet al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2016) 47:e98–e169. doi: 10.1161/STR.0000000000000098
40.
BurtonJKFergusonEECBarughAJWalesbyKEMacLullichAMJShenkinSDet al. Predicting discharge to institutional long-term care after stroke: a systematic review and metaanalysis. J Am Geriatr Soc. (2018) 66:161–9. doi: 10.1111/jgs.15101
41.
D’NettoPRumbachADunnKFinchE. Clinical predictors of dysphagia recovery after stroke: a systematic review. Dysphagia. (2023) 38:1–22. doi: 10.1007/s00455-022-10443-3
42.
BuvarpDViktorissonAAxelssonFLehtoELindgrenLLundströmEet al. Physical activity trajectories and functional recovery after acute stroke among adults in Sweden. JAMA Netw Open. (2023) 6:e2310919. doi: 10.1001/jamanetworkopen.2023.10919
43.
KamalAKhojaAUsmaniBMagsiSMalaniAPeeraZet al. Effect of 5-minute movies shown via a mobile phone app on risk factors and mortality after stroke in a low- to middle-income country: randomized controlled trial for the stroke caregiver dyad education intervention (Movies4Stroke). J Med Internet Res. (2020) 8:e12113. doi: 10.2196/12113
44.
OuyangMZhangYWangXSongLBillotLRobinsonTet al. Quantifying regional variations in components of acute stroke unit (ASU) care in the international HeadPoST study. J Neurol Sci. (2020) 419:117187. doi: 10.1016/j.jns.2020.117187
45.
SmithBWLobo-PratJZondervanDKLewCChanVChouCet al. Using a bimanual lever-driven wheelchair for arm movement practice early after stroke: a pilot, randomized, controlled, single-blind trial. Clin Rehabil. (2021) 35:1577–89. doi: 10.1177/02692155211014362
46.
LiuLLuYBiQFuWZhouXWangJ. Effects of different intervention time points of early rehabilitation on patients with acute ischemic stroke: a single-center, randomized control study. Wang Q, Herausgeber. Biomed Res Int. (2021) 2021:1940549. doi: 10.1155/2021/1940549
47.
AprileIGermanottaMCrucianiALoretiSPecchioliCCecchiFet al. Upper limb robotic rehabilitation after stroke: a multicenter, randomized clinical trial. J Neurol Phys Ther. (2020) 44:3–14. doi: 10.1097/NPT.0000000000000295
48.
CummingTBBernhardtJLoweDCollierJDeweyHLanghornePet al. Early mobilization after stroke is not associated with cognitive outcome: findings from AVERT. Stroke. (2018) 49:2147–54. doi: 10.1161/STROKEAHA.118.022217
49.
CummingTBChurilovLCollierJDonnanGElleryFDeweyHet al. Early mobilization and quality of life after stroke: findings from AVERT. Neurology. (2019) 93:e717–28. doi: 10.1212/WNL.0000000000007937
50.
WaltersRCollierJMBraighi CarvalhoLLanghornePKatijjahbeMATanDet al. Exploring post acute rehabilitation service use and outcomes for working age stroke survivors (≤65 years) in Australia, UK and South East Asia: data from the international AVERT trial. BMJ Open. (2020) 10:e035850. doi: 10.1136/bmjopen-2019-035850
51.
BernhardtJBorschmannKCollierJMThriftAGLanghornePMiddletonSet al. Fatal and nonfatal events within 14 days after early, intensive mobilization Poststroke. Neurology. (2021) 96:e1156–66. doi: 10.1212/WNL.0000000000011106
52.
De JongLDVan WijckFStewartREGeurtsACHDijkstraPU. Content of conventional therapy for the severely affected arm during subacute rehabilitation after stroke: an analysis of physiotherapy and occupational therapy practice: content of conventional arm treatment after stroke. Physiother Res Int. (2018) 23:e1683. doi: 10.1002/pri.1683
53.
KimNHParkHYSonJKMoonYLeeJHChaYJ. Comparison of underwater gait training and overground gait training for improving the walking and balancing ability of patients with severe hemiplegic stroke: a randomized controlled pilot trial. Gait Posture. (2020) 80:124–9. doi: 10.1016/j.gaitpost.2020.05.022
54.
LoganAFreemanJKentBPoolerJCreanorSEnkiDet al. Functional standing frame programme early after severe sub-acute stroke (SPIRES): a randomised controlled feasibility trial. Pilot Feasibility Stud. (2022) 8:12. doi: 10.1186/s40814-022-01012-4
55.
Le FrancSBonanIFleuryMButetSBarillotCLécuyerAet al. Visual feedback improves movement illusions induced by tendon vibration after chronic stroke. J Neuroeng Rehabil. (2021) 18:948. doi: 10.1186/s12984-021-00948-7
56.
MiddletonSMcElduffPDruryPD’EsteCCadilhacDADaleSet al. Vital sign monitoring following stroke associated with 90-day independence: a secondary analysis of the QASC cluster randomized trial. Int J Nurs Stud. (2019) 89:72–9. doi: 10.1016/j.ijnurstu.2018.09.014
57.
NagaiKAmimotoKTeshimaMItoTNariyaHUenoRet al. Immediate effects of standing unstable board intervention on the non-paralyzed leg on sitting balance in severe hemiplegia: a randomized controlled trial. Top Stroke Rehabil. (2024) 31:446–56. doi: 10.1080/10749357.2024.2302730
58.
OuyangMRoffeCBillotLSongLWangXMuñoz-VenturelliPet al. Oxygen desaturation and adverse outcomes in acute stroke: secondary analysis of the HeadPoST study. Clin Neurol Neurosurg. (2021) 207:106796. doi: 10.1016/j.clineuro.2021.106796
59.
RennerCIEBrendelCHummelsheimH. Bilateral arm training vs unilateral arm training for severely affected patients with stroke: exploratory single-blinded randomized controlled trial. Arch Phys Med Rehabil. (2020) 101:1120–30. doi: 10.1016/j.apmr.2020.02.007
Summary
Keywords
stroke severity, outcome measure, cut-off scores, neurological rehabilitation, stroke phase, NIHSS
Citation
Roesner K, Brodowski H and Strutz N (2025) Measuring severe stroke: a scoping review of RCTs. Front. Neurol. 16:1631275. doi: 10.3389/fneur.2025.1631275
Received
19 May 2025
Accepted
07 July 2025
Published
30 July 2025
Volume
16 - 2025
Edited by
Mostafa Meshref, Al-Azhar University, Egypt
Reviewed by
Maha AbuZarifa, Al-Quds University, Palestine
Abdallah Khatatbeh, King Hussein Medical Center, Jordan
Updates
Copyright
© 2025 Roesner, Brodowski and Strutz.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katrin Roesner, katrin.roesner@uni-luebeck.de
†ORCID: Katrin Roesner, https://orcid.org/0000-0001-5700-3374
Hanna Brodowski, https://orcid.org/0000-0002-7930-241X
Nicole Strutz, https://orcid.org/0000-0002-4780-2188
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.