- 1Department of Neurosurgery, Dongfang Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian, China
- 2Fujian Provincial Clinical Medical Research Center for Minimally Invasive Diagnosis and Treatment of Neurovascular Diseases, Fuzhou, China
- 3Department of Science and Education, 900th Hospital of PLA Joint Logistic Support Force, Fuzhou, Fujian, China
- 4Department of Epidemiology and Health Statistics, School of Public Health, Fujian Medical University, Fuzhou, Fujian, China
- 5Aberdeen Medical School, College of Life Sciences and Medicine, University of Aberdeen, Aberdeen, United Kingdom
- 6Department of Neurosurgery, Fuzhou University Affiliated Provincial Hospital, Fujian, China
- 7Department of Neurosurgery, Fuzong Clinical Medical College of Fujian Medical University, Fuzhou, Fujian, China
Background: In cerebrovascular diseases (CVD), the management strategies for ischemic stroke (IS) and cerebral venous thrombosis (CVT) have significant differences, but the underlying inflammation-driven mechanisms in these two conditions have not been fully translated into individualized intervention criteria.
Methods: We searched PubMed, Embase, Web of Science and Cochrane Library through February 1, 2025, and included 18 eligible studies in a Bayesian network meta-analysis following PRISMA-NMA. The data were processed using Revman (version 5.4.1) and R (version 4.3.3). The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) method was used to assess the quality of evidence. This study was registered in PROSPERO (CRD42024539498).
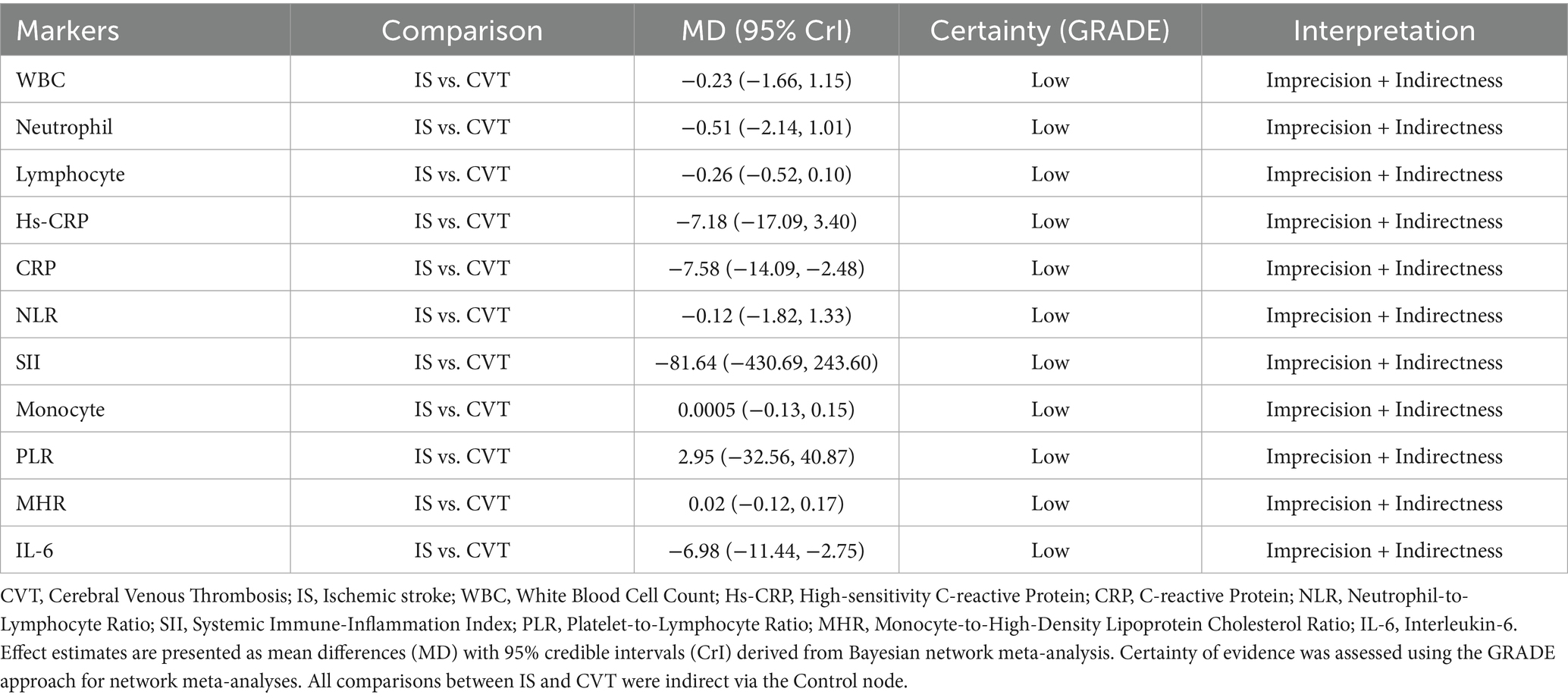
Results: In total, 18 studies were included in the review. The results showed that acute-phase inflammatory markers were significantly elevated in both CVT and IS. CVT was associated with a relatively stronger systemic inflammatory response, while lymphocyte counts were reduced in both, suggesting a immunosuppressive phenomenon in cerebral thrombotic disease. This network Meta-Analysis showed that CRP (MD = 7.58, 95% CI: 2.48–14.09) and IL-6 (MD = 6.98, 95% CI: 2.75–11.44) were more significantly elevated in the acute phase in CVT patients than in IS patients, suggesting they could serve as key inflammatory markers for differentiating the two conditions.
Conclusion: Inflammatory markers exhibit both specific differences and shared characteristics in CVT and IS. CRP and IL-6 were higher in CVT than in IS in Bayesian NMA, suggesting potential adjunctive markers for differential diagnosis; however, these findings are hypothesis-generating and require prospective validation, and neuroimaging remains the diagnostic gold standard.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024539498, CRD42024539498.
Introduction
Cerebrovascular diseases (CVD) are major causes of disability and mortality worldwide; two major types are ischemic stroke (IS) and cerebral venous thrombosis (CVT). Although both are characterized by vascular occlusion (blood vessel blockage), their pathological mechanisms, risk factors, and clinical management have significant difference.
IS is caused mainly by the rupture of atherosclerotic plaques or cardiac embolism leading to cerebral blood flow interruption. It is the second leading cause of death and disability worldwide, with an annual incidence of 200 per 100,000, of which more than 75 per cent occurs in people over 60 years of age (1). Recent studies have revealed that ischemia–reperfusion injury activates microglia through the TLR4/NF-κB pathway, releasing proinflammatory factors such as IL-1β and TNF-α, which drive microglia toward proinflammatory (M1-type) polarization, resulting in an “inflammation-thrombosis vicious cycle” (2, 3). Although antithrombotic therapy reduces the acute-phase mortality rate, survivors still have a risk of recurrence, suggesting that the chronic inflammation-driven pathological process requires clinical intervention.
Unlike IS, CVT is a rare cerebrovascular disease of the intracranial venous system (annual incidence of about 1.3/100,000 people), which affects mainly young women (the male-to-female ratio is 1:3) and is characterized by endothelial damage to the venous sinuses, hypercoagulability, and stagnant blood flow (4, 5). The thrombi in CVT are fibrin-rich and their formation is closely related to the activation of the coagulation cascade driven by the monocyte-tissue factor (TF) axis and the inflammatory response mediated by the interleukin (IL)-6/STAT3 pathway (6). Although anticoagulation significantly affects prognosis, many patients may experience chronic symptoms such as cognitive impairment or headache after treatment, possibly related to a persistent low inflammatory response (e.g., mildly elevated IL-6 and CRP) (5).
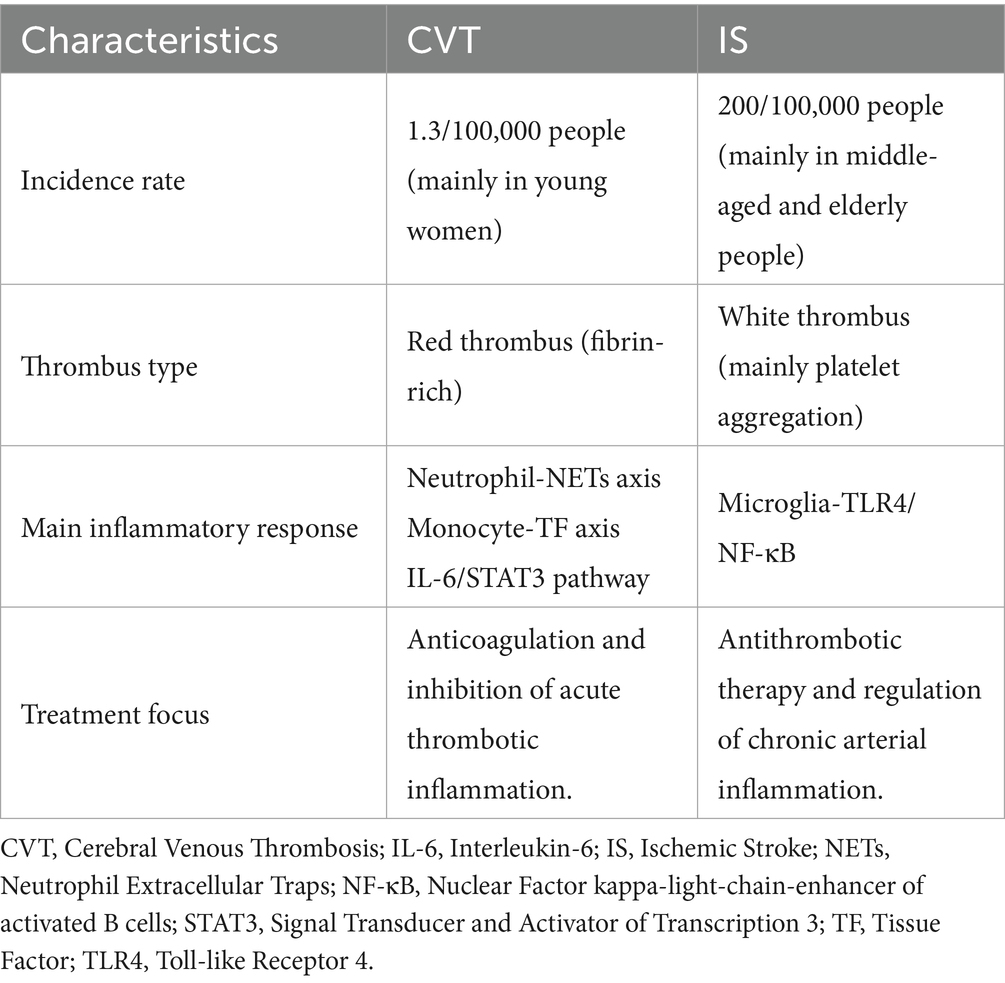
Although both IS and CVT involve “inflammation-thrombosis interaction” pathways, their specific mechanisms are significantly different (Table 1). Commonalities: Systemic inflammatory responses drive endothelial injury and thrombus expansion, forming a vicious cycle. Differences: In IS, arterial thrombosis with inflammation concentrated in the ischaemic region. In CVT, venous thrombosis is associated with more a more extensive inflammatory response and a high risk of chronicity.
Currently, in clinical practice, the diagnosis of these two diseases relies mainly on imaging evidence. However, it often faces issues such as insufficient sensitivity or delayed identification. Therefore, clarifying the differences in specific inflammatory markers between the two types of diseases is valuable for early differential diagnosis, targeted anti-inflammatory therapy and prognostic stratification. By systematically comparing their inflammatory marker profiles, this study aims to: reveal the differences in inflammatory marker profiles between IS and CVT, screen for disease-specific markers, and provide a low-cost diagnostic tool for resource-limited areas.
Methods
Reporting guidelines
This study followed the structure of the Preferred Reporting Items for Systematic Review incorporating Network Meta-analysis (PRISMA-NMA) (7). The study protocol was registered in advance with PROSPERO (CRD42024539498).
Search strategies
Several literature databases were searched in this study, namely PubMed, Embase, Web of Science and the Cochrane Library for publications published before February 2025. The keywords for the database searches included “cerebral venous thrombosis,” “cerebral venous sinus thrombosis,” “ischemic stroke,” “stroke,” “interleukin-6,” “C-reactive protein,” “high-sensitivity C-reactive protein,” “systemic immune-inflammation index,” “neutrophil to lymphocyte ratio,” “platelet to lymphocyte ratio,” “monocyte to high-density lipoprotein ratio” (the full search strategy can be found in Supplementary Table S1). The references of the retrieved articles were then thoroughly reviewed for additional reports that we may have missed in the search. The grey literature (e.g., conference abstracts, clinical trial registries) retrieved from the databases was not included, as it did not meet the predefined inclusion criteria for this network meta-analysis.
Study selection criteria
Two-stage screening by two reviewers; disagreements resolved by consensus/third reviewer;data-extraction double-entry. The enrolled studies included met the following criteria: (1) patients with a definitive diagnosis of IS or CVT; (2) inflammatory markers expressed as the means ± standard deviate (SD) or as median with interquartile range (IQR); (3) comparisons among IS patients, CVT patients and healthy controls. The exclusion criteria included: (1) patients with complications due to venous thrombosis or stroke, such as renal veins, mesenteric veins, stroke-associated pneumonia and poststroke depression; (2) inflammatory markers reported as median with range, median with 95% confidence interval (95%CI), mean with range or 95%CI, or as effect estimates (odds ratio and relative risk); (3) CVT or IS patients with transient risk factors (such as recent surgery, trauma, fracture, estrogen therapy, or newly diagnosed atrial fibrillation); (4) studies presenting data in non-extractable formats (e.g., only qualitative descriptions, non-numeric figures without raw values) or with incomplete outcome data that preclude valid statistical analysis.
Data extraction
The relevant data were extracted from each article via structured forms. The following information was retrieved for each article: study design, demographics, inflammatory markers, blood sample collection time and diagnosis time. The available markers included white blood cells (WBC), neutrophils, lymphocytes, monocytes, platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), high-sensitivity C-reactive protein (hs-CRP), C-reactive protein (CRP), IL-6, systemic immune inflammation index (SII), neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocytes-High-density lipoprotein ratio (MHR). Data were collected by four reviewers (X. Shen, L. Chen, L. Shen, and S. Chen), and if inconsistency existed between the two reviewers, the two other reviewers would re-examined the data and made a final decision based upon the majority.
Data pre-processing
Before conducting in-depth statistical analyses, rigorous preprocessing was performed on all biomarker data. Data cleaning involved outlier detection and normality testing of the data. The unit conversion of biomarkers (e.g., conversion of white blood cell counts to *10^9/L) was also performed at this stage to facilitate subsequent statistical analyses.
Descriptive statistics
Descriptive statistics were calculated for each inflammatory marker indicator in this study. The mean, standard deviation, median and interquartile range were calculated for each indicator. This provided the necessary overview and distribution of data for meta-analysis.
Data conversion and consolidation analysis
The mean (SD) was used for the pooled analysis, and the median (IQR) was converted to the mean (SD) for the meta-analysis in this study. Inflammatory marker data often exhibit non-normal distributions. To estimate the sample mean and standard deviation (SD) from medians and interquartile ranges (IQRs), we applied the Box-Cox (BC) transformation method proposed by McGrath et al. (8). The core assumption of this method can be summarized as follows: By optimizing the parameter λ to transform the data into a normal distribution, where λ is selected based on the quantile symmetry criterion (e.g., fλ(Q3) − fλ(Q2) = fλ(Q2) − fλ(Q1)), and inverting the original scale parameters are inverted via Monte Carlo simulation (8). This method demonstrates strong robustness for moderately skewed data (e.g., non-normally distributed data such as inflammatory marker date). Only medians with IQRs were converted to means and SDs using the McGrath Box-Cox approach; studies reporting medians with ranges or 95% CIs were not converted and were excluded from quantitative synthesis.
Data synthesis and analysis
All the statistical analyses were conducted via Review Manager (version 5.4.1) and R (version 4.3.3) in this study. The remaining variables were expressed as mean difference (MD), 95% confidence interval (CI), and dichotomous variables were expressed as OR, 95% CI to derive outcome statistics. A Bayesian network meta-analysis was performed to synthesize evidence across comparisons, with effect sizes reported as mean differences (MD) and 95% credible intervals (CrI). Retrospective case–control studies and prospective/retrospective cohort studies were be included in this study. To address potential variation among different types of studies, we used the Q - test (chi - square test) to assess data heterogeneity. If the I2 statistic was less than 50% of the value then there was no significant heterogeneity, justifying the use of a fixed-effects model for the calculation of the combined effect sizes; otherwise, a random-effects model was applied (9). To evaluate the stability of the statistical results, sensitivity analyses were conducted by excluding individual studies one by one to assess changes in the pooled effect size. The absence of significant alterations in the pooled effect size following the exclusion of any single study indicated good stability of the analysis results. For traditional meta-analysis outcomes, publication bias was visually inspected via funnel plots; for network meta-analysis outcomes, publication bias was evaluated via adjusted contrast funnel plots and Egger’s regression test (10). We conducted a network meta-analysis via Bayesian hierarchical modelling (“gemtc” package of R) to make pairwise comparisons between CVT, IS and controls.
Risk of bias assessment and quality of outcomes assessment
The Newcastle-Ottawa Scale (NOS) and was employed in this meta-analysis to assess the quality of non-randomized trials (11). Scores of 7–9, 4–6, and 4 were classified as having a low, moderate, or high risk of bias, respectively. In addition, the Risk of Bias in Individual Studies—Extended (RoB-I/E) tool was used to evaluate the risk of bias across seven domains (D1–D7) for all included studies, with each domain rated as low, some concerns, or high risk, and an overall risk of bias determined accordingly (12). The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) guidelines for systematic reviews and network meta-analyses were followed to assess the quality of outcomes (13).
Certainty assessment
For assessing imprecision, a mean difference threshold of ± 0.20 was considered clinically significant (14), which was also applied to heterogeneity, and the consistency of direct and indirect effects was evaluated against this threshold to assess incoherence in order to validate the consistency of the results of the different comparison pathways and to ensure the reliability of the network evidence.
Results
Literature characteristics
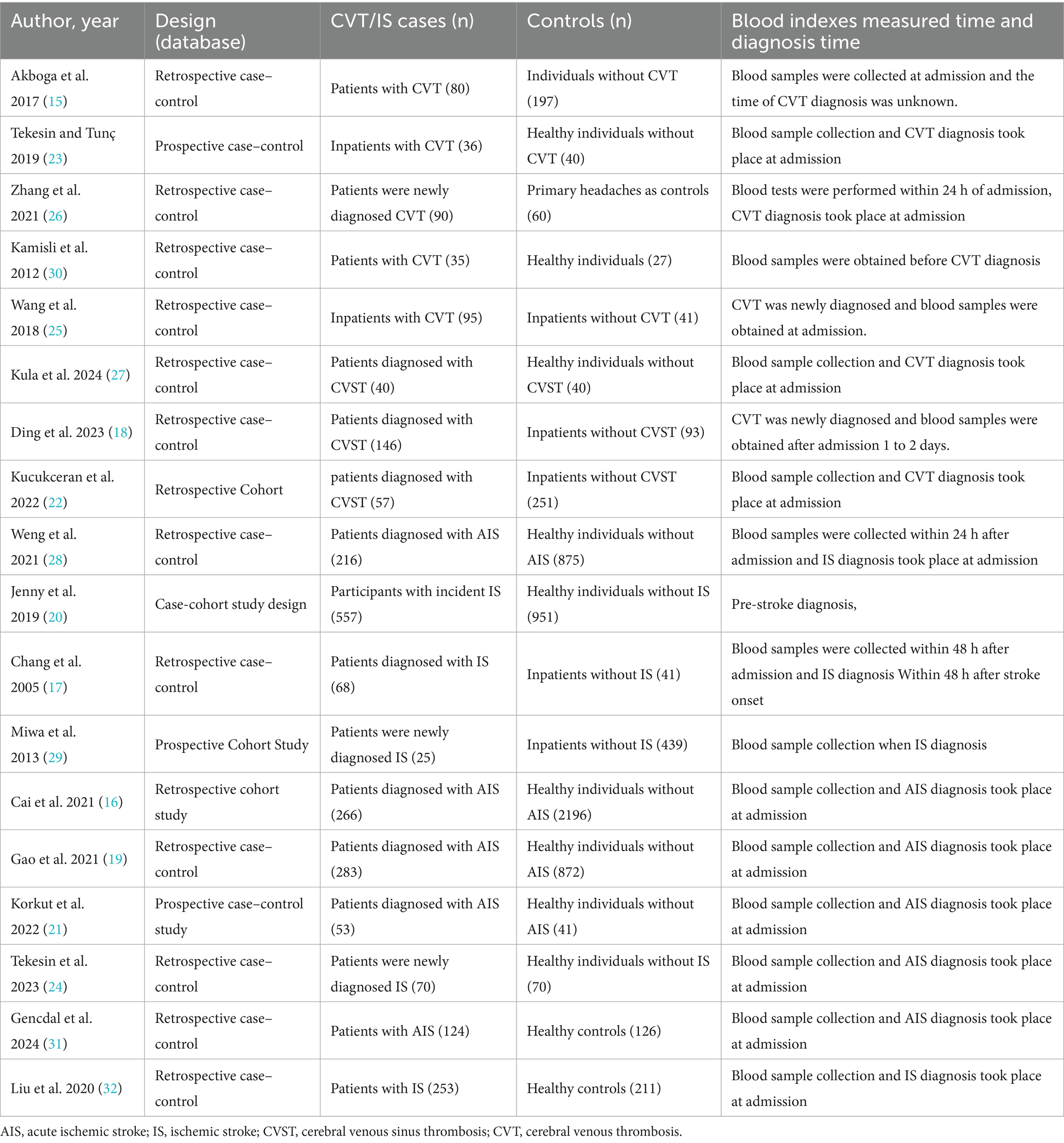
From the initial 5,822 records identified, 1,676 duplicates were removed. The remaining 4,146 records were screened for title and abstract, and then 484 selected articles were reviewed in full text. Ultimately, 18 articles were eligible for inclusion in this study (15–32). Study selection is summarized in the PRISMA 2020 flow diagram (Figure 1). The characteristics of the studies included are summarised in Table 2.
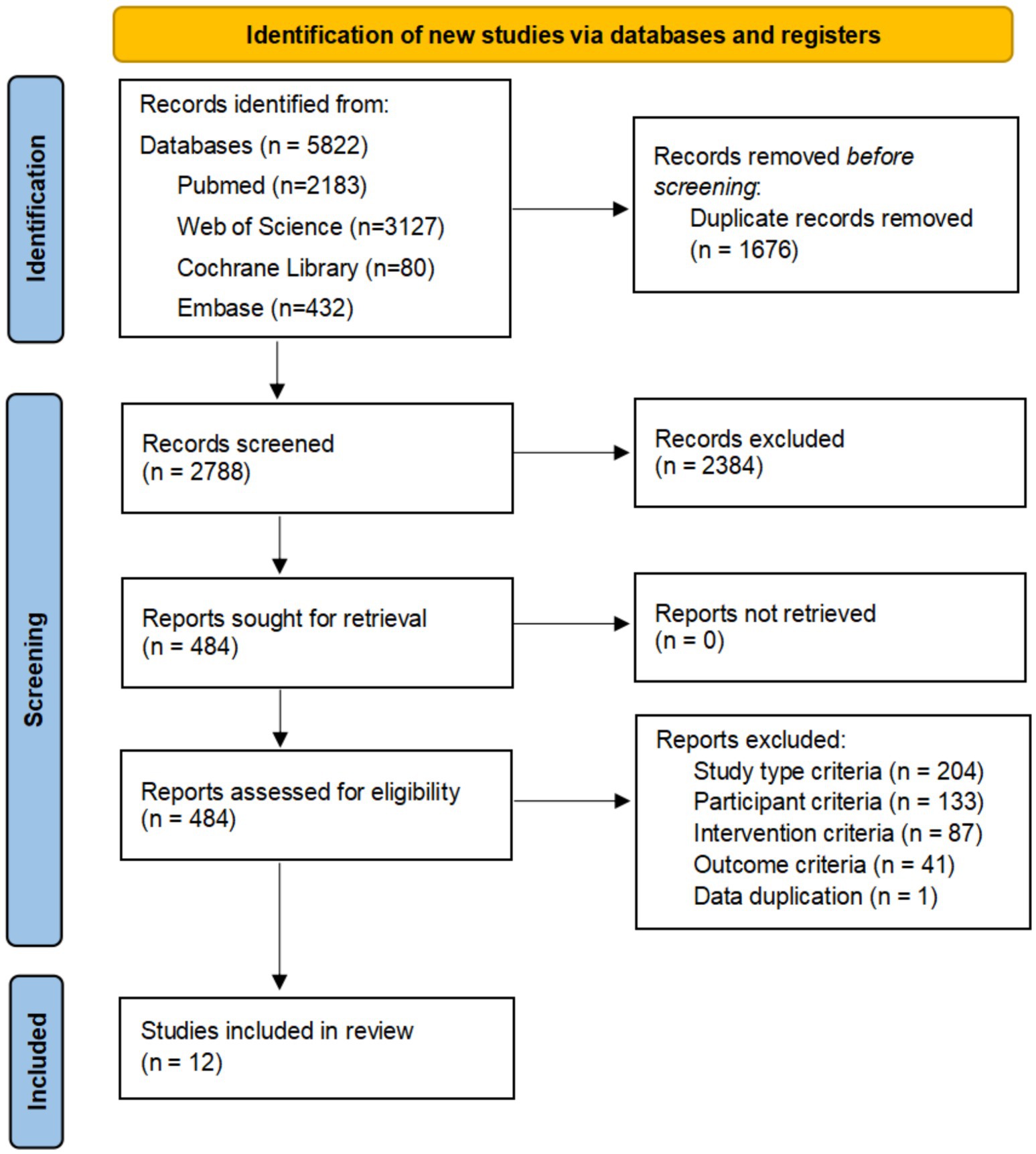
Figure 1. Flowchart of all studies identified, included and excluded in this systematic review and meta-analysis. Initially, 5,822 records were identified, with 1,676 duplicates removed, leaving 2,788 records screened. Of these, 2,384 were excluded, 484 reports sought and assessed for retrieval. No reports were lost. Exclusions included criteria like study type (204), participant (133), intervention (87), outcome (41), and data duplication (1). Twelve studies were included in the review.
Quality assessment
The NOS was used to assess the risk of bias for the 18 included studies. Two reviewers independently scored each study, with discrepancies resolved through discussion. Fourteen studies (77.8%) were rated as low risk of bias, and four (22.2%) as moderate risk, with no studies classified as high risk. Detailed scores for the “selection,” “comparability,” and “outcome/exposure” domains, along with total scores, are presented in Table 3. In addition, the RoB-I/E assessment showed that the majority of studies had low or moderate risk of bias, with only five studies rated as high risk. Risk-of-bias domains (D1–D7) are defined in the Figure 2. Most studies performed well across randomization and other bias domains, although some studies had limitations in randomization or allocation concealment (17, 21, 24, 26, 30). Overall, the included studies presented an acceptable risk of bias, which should be considered when interpreting the results.
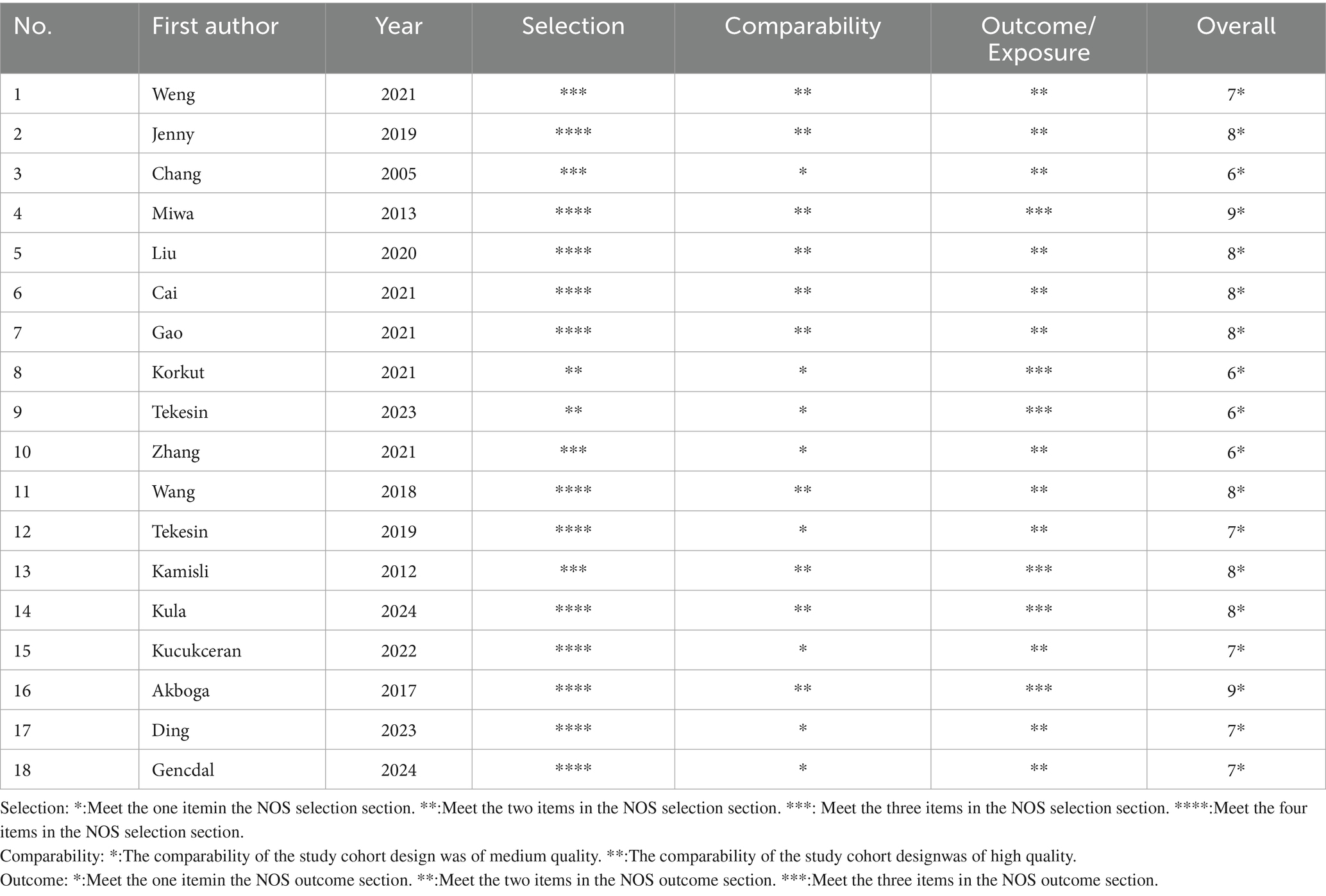
Table 3. Newcastle-Ottawa Scale for risk of bias assessment of the included studies (scores ≥ 7–9, 4–6, <4 are considered low, intermediate, and high risk, respectively).
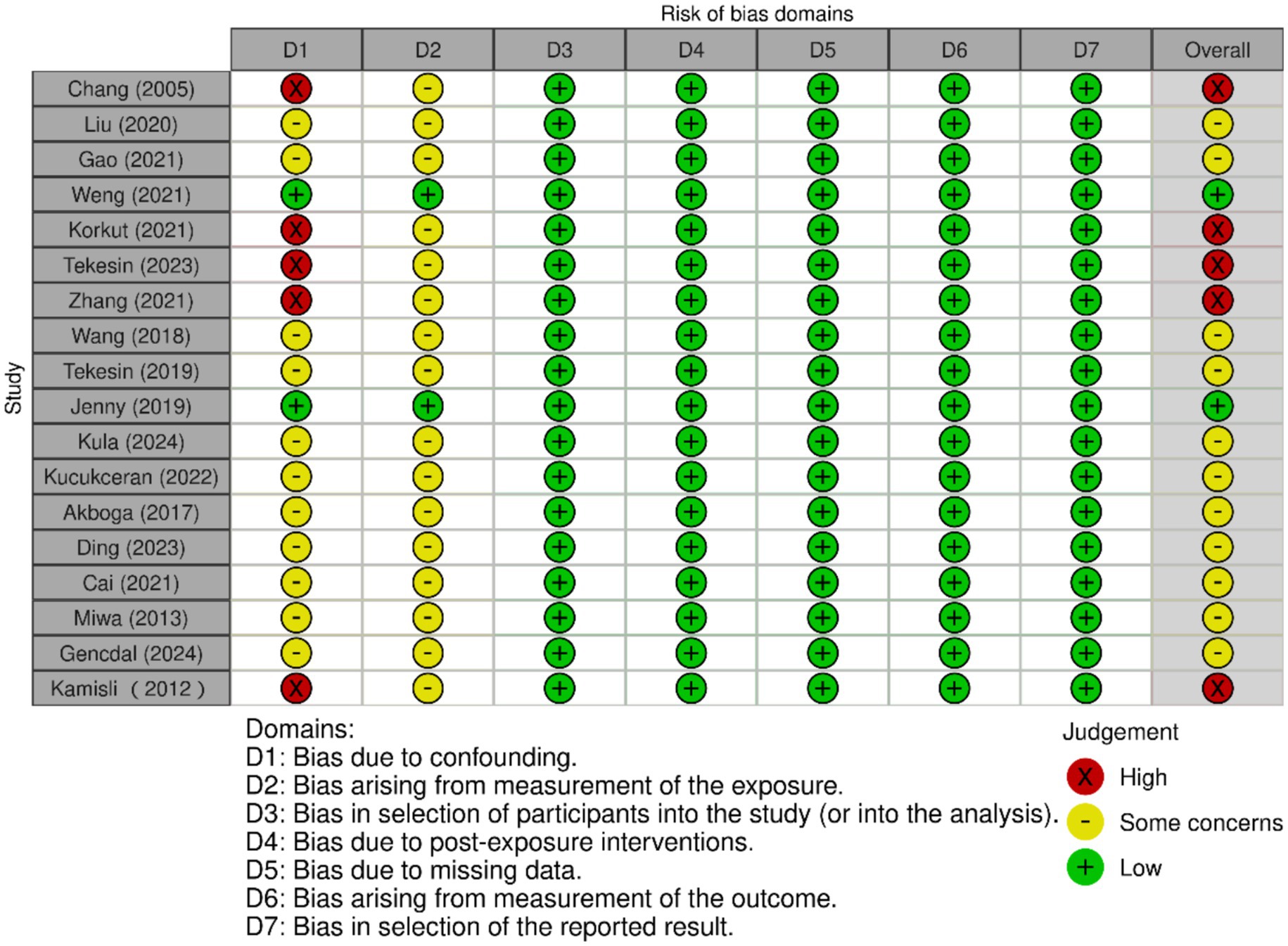
Figure 2. The forest plot of the risk of bias assessment for included studies. Columns labeled D1 to D7 represent different bias domains. Each cell uses traffic light symbols: red (X) for “High” risk, yellow (circle) for “Some concerns,” and green (plus) for “Low” risk. Studies are listed by author and year. Overall ratings are on the right, with a mix of judgments. A key below explains the symbols and domains.
Traditional pairwise meta-analysis
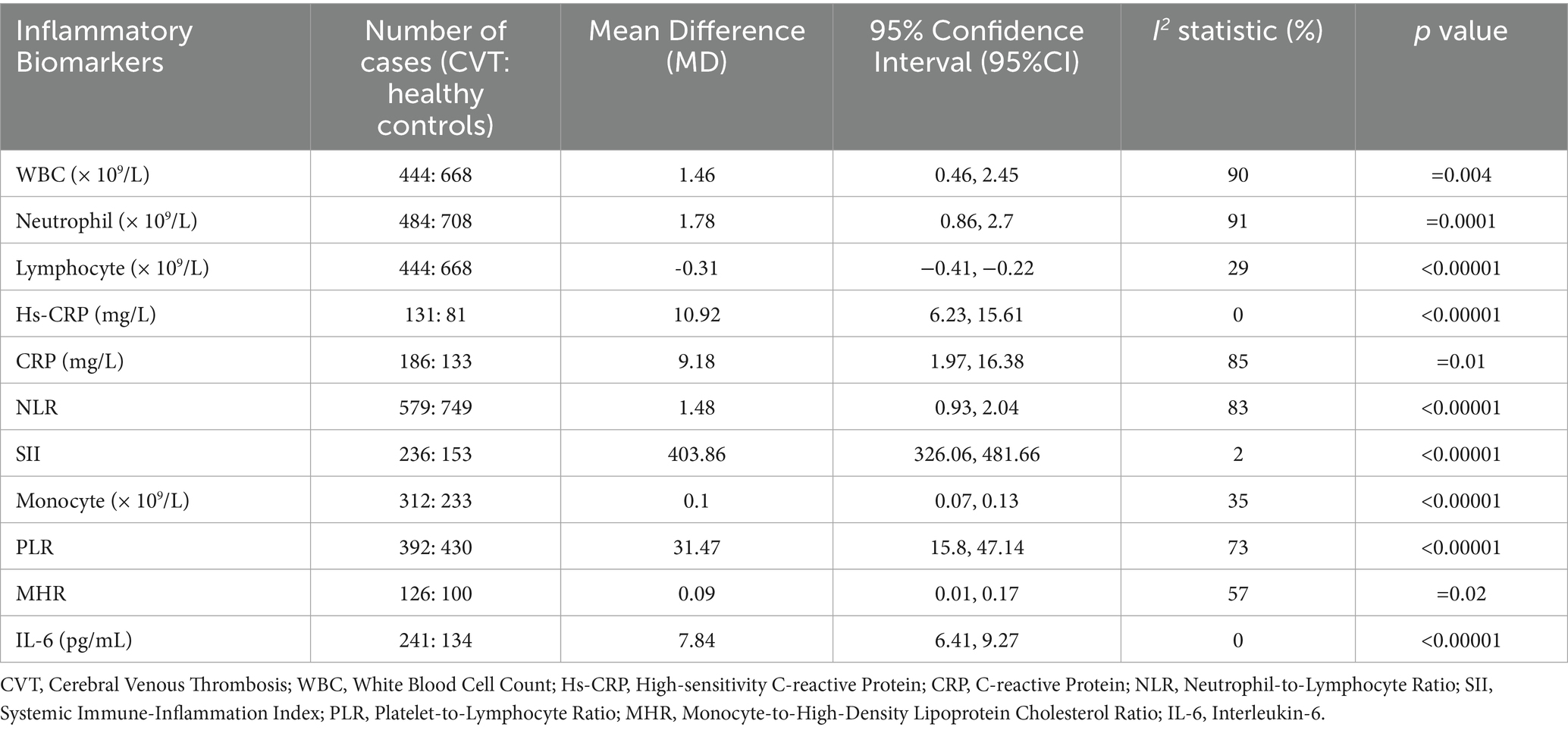
The following inflammatory markers were significantly elevated in CVT patients compared with control group (Table 4): WBC (MD = 1.46, 95%CI: 0.46–2.45), neutrophil (MD = 1.78, 95%CI: 0.86–2.7), hs-CRP (MD = 10.92, 95%CI: 6.23–15.61), CRP (MD = 9.18, 95%CI: 1.97–16.38), NLR (MD = 1.48, 95%CI: 0.93–2.04), SII (MD = 403.86, 95%CI: 326.06–481.66), PLR (MD = 31.47, 95%CI: 15.8–47.14), IL-6 (MD = 7.84, 95%CI: 6.41–9.27), monocytes (MD = 0.1, 95%CI: 0.07–0.13) and MHR (MD = 0.09, 95%CI: 0.01–0.17). Conversely, lymphocyte was significantly reduced in CVT patients (MD = −0.31, 95%CI: −0.41, −0.22). A funnel plot evaluating publication bias in the traditional meta-analysis of CVT (Supplementary Figure S1). Forest plots for the traditional meta-analyses of CVT and IS are provided in Supplementary Figure S2.
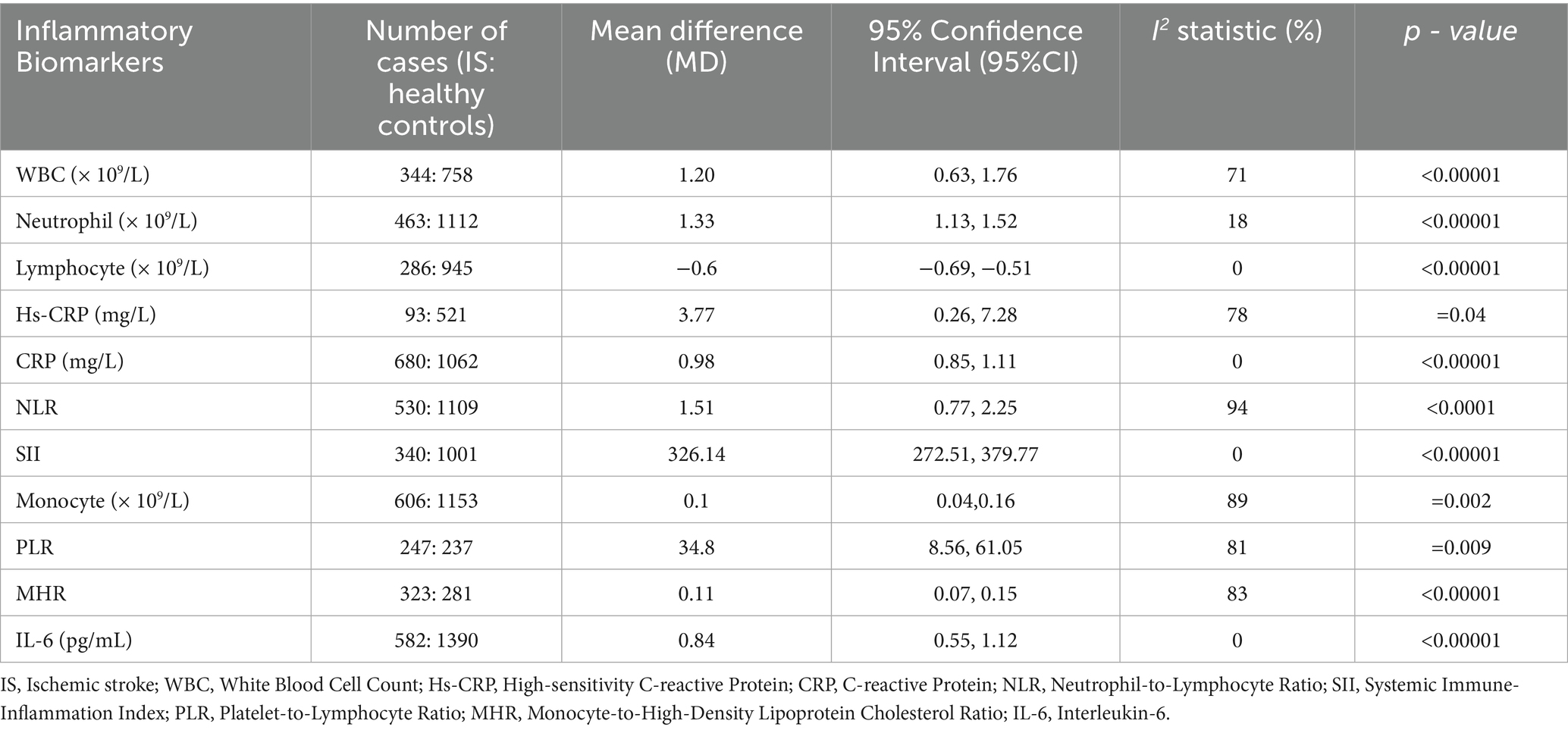
Compared with the healthy control group, the following inflammatory markers were significantly elevated in IS patients (Table 5): neutrophil (MD = 1.33, 95%CI: 1.13–1.52), hs-CRP (MD = 3.77, 95%CI: 0.26, 7.28), CRP (MD = 0.98, 95%CI: 0.85–1.11), NLR (MD = 1.51, 95%CI: 0.77–2.25), SII (MD = 326.14, 95%CI: 272.51–379.77), IL-6 (MD = 0.84, 95%CI: 0.55–1.12), MHR (MD = 0.84, 95%CI: 0.59–1.09), WBC (MD = 1.09, 95%CI: 0.81–1.38), monocytes (MD = 0.1, 95%CI: 0.04, 0.16), PLR (MD = 34.8, 95%CI: 8.56, 61.05). Notably, lymphocyte was also reduced in IS patients (MD = −0.60, 95%CI: −0.69, −0.51). A funnel plot evaluating publication bias in the traditional meta-analysis of IS (Supplementary Figure S3).
Network meta-analysis
CVT showed statistically significant differences in CRP (MD = 7.58, 95% CrI:2.48–14.09) and IL-6 (MD = 6.98, 95% CrI:2.75–11.44) compared with IS. Moreover, the network meta-analysis of CRP and IL-6 in the CVT group also showed significant differences compared with the healthy control group (MD = 8.71, 95% CrI:4.55–14.11), IL-6 (MD = 7.88, 95% CrI:4.86–11.22), suggesting that these two inflammatory biomarkers can serve as key inflammatory markers to distinguish between them. In contrast, comparisons of WBC (MD = −0.23, 95% CrI:−1.66, 1.15), Neutrophil (MD = −0.51, 95% CrI:−2.14, 1.01), Lymphocyte (MD = −0.26, 95% CrI:-0.52, 0.10), hs-CRP (MD = −7.18, 95% CrI:−17.09, 3.40), NLR (MD = −0.12, 95% CrI:−1.82, 1.33), SII (MD = −81.64, 95% CrI:−430.69, 243.60), Monocytes (MD = 0.0005, 95% CrI:−0.13, 0.15), PLR (MD = 2.95, 95% CrI:−32.56, 40.87), MHR (MD = 0.02, 95% CrI:−0.12, 0.17) between IS and CVT patients did not reject the null hypothesis (Table 6). Full network geometry and Forest plots are provided in the Supplementary Figures S4, S5. The main text reports key NMA contrasts with corresponding credible intervals.
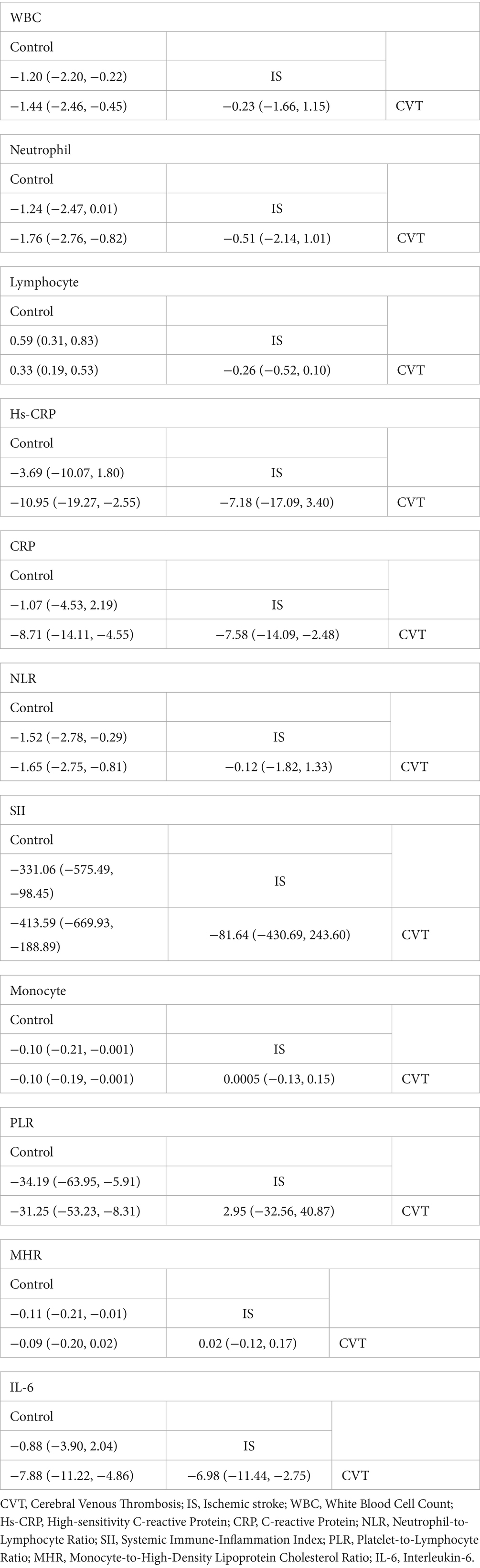
Table 6. The network meta-analysis for pair-comparisons of inflammatory biomarkers among the IS, CVT and controls.
Convergence and risk of bias
The results from the convergence tests (Supplementary Figures S7, S8) revealed that the trace plots of all the parameters stabilized without significant fluctuations in the later iterations, and the density plots exhibited a unimodal and tightly clustered distribution. The shrinkage factor approaches 1 in the final iterations, indicating good model convergence and reliable stability of the results. The funnel plots for the network meta-analysis (Supplementary Figure S6) showed symmetric patterns, and Egger’s regression test detected no significant publication bias (p > 0.05). Leave-one-study-out sensitivity analyses, conducted via both traditional and network meta-analysis, yielded results consistent with the primary analysis, with no notable changes in pooled effect sizes or confidence intervals.
Grade
According to the GRADE assessment, the overall certainty of evidence for all inflammatory markers comparing IS vs. CVT was low, primarily downgraded due to imprecision and indirectness (Table 7).
Certainty of evidence
The overall quality of evidence for the pooled results was rated as low, which was attributed primarily to inter-study bias risk, imprecise data, and inconsistent network comparisons. Inter-study bias stemmed from the significant influence of high-risk-of-bias studies on network estimates, which was particularly evident in indirect comparisons between CVT and IS. Imprecision arises from due to insufficient data to demonstrate conclusive effects, whereas inconsistency is caused by a lack of closed loops in the network structure, severely challenging the coherence of comparisons.
Discussion
Currently, the exploration of the associations between inflammatory biomarkers and CVD continues to deepen. This study included 18 studies and aimed to explore the differences in inflammatory biomarkers between CVT and IS through a systematic review and network meta-analysis. This study transcended the limitations of traditional single-disease research. To our knowledge, this is the first NMA to compare the inflammatory markers of CVT and IS to reveal their common features and specificity in the “inflammation-thrombosis” interactions. However, the inevitable heterogeneity, unclear methodologies, and data transformations require cautious interpretation.
Our analysis revealed significantly elevated levels of IL-6, CRP, hs-CRP, SII, and PLR in the peripheral blood of CVT patients during acute phase. These findings indicate two key pathophysiological processes: (1) a robust systemic inflammatory response, and (2) amplified cross-talk between inflammation and coagulation cascades. This finding is in great agreement with earlier studies: the levels of hs-CRP and IL-6 in the acute stage of CVT are much higher than those in the chronic stage; SII is the strongest independent predictor for CVT, and its ability to predict CVT is better than NLR and PLR (25, 26). The results of this study further support the value of SII in evaluating the thrombo-inflammatory microenvironment. Therefore, for patients with unexplained headache, MRI/MRV is recommended as a priority to screen for CVT when SII is too high (26). As a pro-inflammatory cytokine, IL-6 induces hepatic synthesis of CRP and hs-CRP via the JAK–STAT pathway (33). These acute-phase proteins further to promote tissue factor expression and initiate the extrinsic coagulation pathway to facilitate thrombus formation (18). The significant elevation of SII and PLR reflects neutrophil and platelet overactivation and lymphocyte immunosuppression in the patients. Neutrophils activate endothelial cells and platelets by releasing neutrophil extracellular traps (NETs), reactive oxygen species (ROS), and proinflammatory factors (e.g., IL-6 and TNF-α) to promote coagulation (34, 35). Platelets are not only coagulation elements but also an important sources of inflammatory mediators and a key hubs linking the inflammatory response to thrombosis (5). An increased PLR is independently associated with the risk of recurrent venous thrombosis through a mechanism involving platelet-activated TF expression and increased fibrin production (36). In contrast, although monocytes and MHR showed statistical significance (p < 0.05), their effect sizes (MD = 0.1 and 0.09) were lower than that those of IL-6 and CRP. These findings suggest that monocyte-related inflammation may serve as a secondary driver in CVT. However, owing to the statistical significance of the sample size, its clinical value needs to be verified in a larger cohort.
In IS patients, elevated neutrophil levels signify their recruitment to arterial plaque rupture sites. At these sites, neutrophils release myeloperoxidase (MPO) and matrix metalloproteinases (MMPs), which exacerbate endothelial damage and degrade the collagen matrix of the plaque fibrous cap. This process leads to plaque structural instability and promotes ischemic reperfusion injury (37). This process leads to plaque structural instability and promotes ischaemia-reperfusion injury. Hs-CRP is strongly associated with plaque vulnerability, and its mild to moderate elevation indicates chronic inflammation within the plaque (38). Increases in SII and PLR signify synergistic neutrophil–platelet activation: SII indicates an immunocoagulation interaction in thrombus formation, and PLR denotes platelet hyperreactivity, both of which are involved in thromboembolism after plaque rupture (39). An elevated MHR highlights the important role of dysregulated lipid metabolism-inflammation axis interactions in driving atherosclerotic plaque instability. Nonetheless, the analysis of MHR is also limited by sample size, and IS subtype stratification (e.g., large-artery atherosclerosis, cardioembolic stroke, and small-vessel disease) was not performed. This methodological gap likely contributed to heterogeneous inflammatory marker profiles. Future investigations should combine advanced imaging (e.g., plaque component analysis) and molecular approaches (e.g., single-cell RNA sequencing) to dissect subtype-dependent inflammatory pathways, informing the development of targeted antithrombotic and anti-inflammatory strategies.
Compared with the largely focal arterial injury in IS, CVT induces venous congestion-driven endothelial hypoxia and diffuse BBB perturbation, amplifying systemic IL-6/CRP signals. Our network meta-analysis showed that CRP and IL-6 levels are significantly higher in CVT than in IS. This finding highlights a key difference in inflammatory pathways: in CVT, venous endothelial injury triggers a strong systemic acute-phase response, leading to marked elevations in CRP and IL-6. Several studies have shown that serum IL-6 and CRP levels are significantly higher in CVT patients than in healthy controls and are correlated with disease severity (40). Pathophysiologically, venous thrombosis induces endothelial hypoxia and venous congestion, which together provoke a robust systemic acute-phase response. Venous congestion due to impaired venous drainage further exacerbates endothelial injury, leading to a more pronounced local inflammatory response. Endothelial hypoxia in the setting of impaired venous drainage stimulates local IL-6 production and endothelial activation; IL-6 subsequently activates hepatic STAT3 signaling, markedly increasing CRP synthesis and creating an injury-cytokine → acute-phase protein cascade that also upregulates TNF-α and IL-1β (6, 33, 41). In addition, venous stasis and endothelial activation promote neutrophil recruitment and the formation of neutrophil extracellular traps (NETs), a thrombo-inflammatory process that amplifies both thrombus growth and local cytokine release and may further increase systemic IL-6 levels (34, 39). Venous congestion in CVT may produce a relatively more diffuse pattern of blood–brain barrier (BBB) perturbation than the typically focal BBB disruption seen in many cases of arterial ischemia (42); such diffuse BBB disturbance could facilitate translocation of brain-derived cytokines and inflammatory mediators into the systemic circulation and thereby magnify peripheral IL-6 and CRP signals in some patients. In IS, IL-6 is primarily secreted by activated microglia, astrocytes, and cells within arterial plaques in ischemic brain tissue (43). However, the selective permeability of the BBB restricts the diffusion of cytokines such as IL-6 into the systemic circulation, resulting in a significantly less pronounced increase in systemic IL-6 concentrations in IS patients compared to those with CVT (40, 43). This divergence stems from a fundamental difference in injury mechanisms. In CVT, venous thrombosis causes systemic endothelial hypoxia through impaired venous drainage. Conversely, IS results from localized arterial damage due to plaque rupture in cerebral arteries. These distinct pathological processes establish contrasting inflammatory profiles—CVT elicits a strong systemic acute-phase response, whereas IS is characterized by local inflammatory propagation. In IS patients, the mild elevation of CRP and IL-6, likely reflects a postischemic dynamic balance between central and peripheral inflammation: the activation of microglia in the brain results in the release of cytokines, including IL-6, which mediate the dual regulation of neurorestoration and inflammatory injury; concurrently, peripheral monocyte migration into the ischemic penumbra intensifies local oxidative stress, facilitating neuroimmune crosstalk (44). Although this chronic inflammatory state fails to initiate a strong systemic acute-phase response, sustained elevation of CRP is associated with plaque progression and long-term recurrence risk. This provides evidence for statin therapy to improve prognosis through regulating lipid metabolism and exerting anti-inflammatory effects (45). This pathophysiology explains why CRP/IL-6—but not NLR/PLR/SII—best discriminate CVT from IS in our analysis. Given the central role of IL-6 in driving thrombus formation, anti-IL-6 therapies may provide clinical benefits by blocking the IL-6 receptor to reduce inflammation and lower thrombotic risk (46). Recent CVT-specific clinical data show that short-term corticosteroid regimens combined with anticoagulation significantly reduce serum and CSF IL-6 and hs-CRP and are associated with improved functional outcomes, providing indirect clinical support for IL-6–mediated pathology in CVT (47). However, no IL-6–targeted agents have yet been evaluated in patients with CVT, therefore, future prospective randomized trials are warranted to determine the safety, optimal timing, and patient selection for potential anti-IL-6 or other anti-inflammatory interventions.
No significant intergroup differences were observed in other inflammatory markers, likely reflecting stress-induced inflammation inherent to CVD rather than thrombosis-type specific changes. This finding implies a shared core mechanism of systemic inflammation activation across arterial and venous thrombotic disorders. For example, neutrophils are associated with chemokine-mediated recruitment following venous endothelial injury in CVT, whereas in IS, they arise from ischaemic reperfusion injury. Despite distinct pathogenic pathways, neutrophils exhibit similar elevations in both diseases. The levels of novel inflammatory biomarkers, such as NLR, PLR, and SII are markedly increased in both CVT and IS, demonstrating their potential as markers to distinguish healthy individuals from CVD patients. High SII values can independently predict poor prognosis and monitor therapeutic efficacy in these patients (28, 41). These markers (SII, NLR and PLR) are recognized as critical indicators of cerebral arterial ischemia, potentially offering more accurate thrombosis diagnoses than immune cell counts alone (48). Our NMA findings further revealed that these novel inflammatory markers remain prominently elevated in affected patients. Importantly, neuroimaging remains the diagnostic gold standard for both IS and CVT, and the biomarkers identified in our analysis should be regarded as exploratory adjuncts that may complement but cannot substitute for imaging.
The inflammatory signatures of CVT and IS demonstrate both convergence and divergence. Shared mechanisms include activation of neutrophil–platelet crosstalk and systemic immunocoagulative responses, reflected by elevated SII, NLR, and PLR in both conditions. In contrast, CVT exhibits a more pronounced systemic acute-phase reaction, dominated by IL-6–driven CRP elevation and diffuse endothelial activation secondary to venous congestion. Conversely, IS is characterized by localized arterial inflammation, in which monocyte–macrophage and lipid-metabolism–related inflammation, reflected by indices such as MHR and markers linked to plaque vulnerability. Many other markers remain non-specific and overlapping; their elevation likely indexes a shared host response to vascular injury rather than pathognomonic disease-specific biology.
Notably, both CVT and IS groups presented decreased lymphocyte counts. This shared feature may be associated with systemic inflammatory stress responses and immunological homeostasis disruption mediated by vascular injury. Vascular damage under both conditions triggers the innate immune response, leading to the release of proinflammatory cytokines such as IL-6 and TNF-α (49). These cytokines induce lymphocyte apoptosis and promote lymphocyte migration to thrombotic or ischemic foci, leading to a decrease in the number of circulating lymphocytes. IS is associated with more profound lymphopenia. This may be because arterial thrombosis-associated inflammation activates the hypothalamic–pituitary–adrenal (HPA) axis to augment cortisol-mediated immunosuppression. HPA axis activation not only directly inhibits B lymphocyte production but also elevates cortisol levels, which further suppresses lymphocyte proliferation and accelerates apoptosis, forming a “stress–immunosuppression” vicious cycle (50). Additionally, in the ischemic penumbra, bursts of oxygen free radicals, excessive microglial activation, and neutrophil infiltration trigger the release of chemokines (e.g., IL-6, TNF-α and CXCL10), facilitating lymphocyte migration into the brain and subsequent depletion (51). Thus, lymphopenia in CVT and IS likely reflects a combination of vascular injury, inflammatory signaling, and neuroendocrine regulation, with mechanistic disparities tied to thrombus etiology, inflammatory magnitude.
Research indicates that reduced peripheral blood lymphocyte counts in IS patients are significantly associated with the severity of neurological deficits and negatively correlated with infarct volume (52). Clinically, lymphopenia in IS has also been linked to worse neurological outcomes and higher rates of post-stroke infections (53, 54). This association may arise from increased lymphocyte apoptosis due to poststroke immunosuppression and impaired adaptive immunity (53, 54). In CVT patients, although the link between lymphocyte reduction and thrombus burden awaits confirmation, early evidence suggests that lymphopenia may signify a more aggressive thrombotic inflammatory phenotype (55). Abnormalities in lymphocyte count and derived indices such as NLR and PLR indicate a systemic “pro-inflammatory-anti-inflammatory” imbalance—for example, an elevated NLR combined with marked lymphocyte reduction suggests a paradoxical state of coexisting intense immune activation and suppression (41). When lymphopenia co-occurs with elevated IL-6 and CRP, the constellation may represent dysregulated immunity that could both exacerbate tissue injury and increase susceptibility to secondary infections such as pneumonia or urinary tract infection. Future cohorts should implement time-locked immune monitoring (including lymphocyte subsets) and capture secondary infections to delineate the clinical impact of immunosuppression following acute cerebrovascular events.
This study has several limitations. First, the limited number of studies included in the analysis (such as MHR and SII) precludes robust conclusions from the network meta-analysis. As a result, paired comparisons yielded imprecise effect size estimates, which should be interpreted as exploratory evidence rather than definitive findings in the current context. Second, although sensitivity analyses were conducted, inherent bias arose from heterogeneous study designs and variable data collection timepoints across the included studies, which may influence our conclusions. Third, conversion of medians to means for skewed data may introduce additional bias. Additionally, NMA is highly dependent on model specifications, which can increase variance in effect estimates, widen confidence intervals, and potentially compromise conclusion reliability. Finally, owing primarily to the insufficient sample size, subgroup analyses based on CVT and IS subtypes were not conducted. This omission led to excessive heterogeneity in some inflammatory markers. Moreover, geographical publication bias across the included studies and inconsistent measurement methods for certain markers also affected the accuracy and generalizability of the results. These constraints indicate that the present results are hypothesis-generating and require prospective, standardized validation before clinical application.
Conclusion
This study revealed differences and shared characteristics in the inflammatory marker profiles between CVT and IS. CVT is characterized by systemic inflammatory activation marked by elevated IL-6 and CRP, whereas IS involves localized arterial inflammation (monocytes, MHR) linked to atherosclerotic plaque instability. Network meta-analysis further confirmed that CRP and IL-6 can serve as specific biomarkers to distinguish between these two diseases. Markers such as SII, NLR, and PLR (due to their significant elevation) may have potential as exploratory screening indicators across CVD. The observation of lymphopenia in both CVT and IS patients suggests stress-induced immunosuppression, although the specific mechanisms and clinical implications require further exploration. Given the limitations of this study, these findings should be considered hypothesis-generating and require validation in prospective cohorts before clinical translation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XS: Conceptualization, Data curation, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LC: Data curation, Investigation, Writing – review & editing. LS: Data curation, Investigation, Writing – review & editing. SC: Data curation, Investigation, Writing – review & editing. SM: Data curation, Investigation, Writing – review & editing. LC: Data curation, Investigation, Writing – review & editing. SW: Funding acquisition, Methodology, Supervision, Writing – review & editing. ZL: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Joint Logistics Medical Key Specialty Project (LQZD-SW); Fujian Province Science and Technology Innovation Platform Project (2022Y2017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1634369/full#supplementary-material
References
1. Virani, SS, Alonso, A, Aparicio, HJ, Benjamin, EJ, Bittencourt, MS, Callaway, CW, et al. Heart disease and stroke Statistics-2021 update: a report from the American Heart Association. Circulation. (2021) 143:e254–743. doi: 10.1161/CIR.0000000000000950
2. Li, X, Yang, X, Lu, H, Wang, W, Cai, L, Chen, J, et al. Calycosin attenuates the inflammatory damage of microglia induced by oxygen and glucose deprivation through the HMGB1/TLR4/NF-κB signaling pathway. Acta Biochim Biophys Sin. (2023) 55:1415–24. doi: 10.3724/abbs.2023125
3. Li, Z, Zhao, M, Zhang, X, Lu, Y, Yang, Y, Xie, Y, et al. TJ-M2010-5, a novel CNS drug candidate, attenuates acute cerebral ischemia-reperfusion injury through the MyD88/NF-κB and ERK pathway. Front Pharmacol. (2022) 13:1080438. doi: 10.3389/fphar.2022.1080438
4. Coutinho, JM, Zuurbier, SM, Aramideh, M, and Stam, J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. (2012) 43:3375–7. doi: 10.1161/STROKEAHA.112.671453
5. Silvis, SM, de Sousa, DA, Ferro, JM, and Coutinho, JM. Cerebral venous thrombosis. Nat Rev Neurol. (2017) 13:555–65. doi: 10.1038/nrneurol.2017.104
6. Mackman, N, Tilley, RE, and Key, NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. (2007) 27:1687–93. doi: 10.1161/ATVBAHA.107.141911
7. Hutton, B, Salanti, G, Caldwell, DM, Chaimani, A, Schmid, CH, Cameron, C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
8. McGrath, S, Zhao, X, Steele, R, Thombs, BD, and Benedetti, Athe DEPRESsion Screening Data (DEPRESSD) Collaboration, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020) 29:2520–37. doi: 10.1177/0962280219889080
9. Higgins, JPT, Thompson, SG, and Spiegelhalter, DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. (2009) 172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x
10. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
11. Wells, G, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed March 19, 2025).
12. Sterne, JA, Hernán, MA, Reeves, BC, Savović, J, Berkman, ND, Viswanathan, M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
13. Salanti, G, Del Giovane, C, Chaimani, A, Caldwell, DM, and Higgins, JPT. Evaluating the quality of evidence from a network meta-analysis. PLoS One. (2014) 9:e99682. doi: 10.1371/journal.pone.0099682
14. Schünemann, HJ, Neumann, I, Hultcrantz, M, Brignardello-Petersen, R, Zeng, L, Murad, MH, et al. GRADE guidance 35: update on rating imprecision for assessing contextualized certainty of evidence and making decisions. J Clin Epidemiol. (2022) 150:225–42. doi: 10.1016/j.jclinepi.2022.07.015
15. Akboga, YE, Bektas, H, and Anlar, O. Usefulness of platelet to lymphocyte and neutrophil to lymphocyte ratios in predicting the presence of cerebral venous sinus thrombosis and in-hospital major adverse cerebral events. J Neurol Sci. (2017) 380:226–9. doi: 10.1016/j.jns.2017.07.036
16. Cai, H, Huang, H, Yang, C, Ren, J, Wang, J, Gao, B, et al. Eosinophil-to-neutrophil ratio predicts poor prognosis of acute ischemic stroke patients treated with intravenous thrombolysis. Front Neurol. (2021) 12:665827. doi: 10.3389/fneur.2021.665827
17. Chang, CY, Chen, JY, Ke, D, and Hu, ML. Plasma levels of lipophilic antioxidant vitamins in acute ischemic stroke patients: correlation to inflammation markers and neurological deficits. Nutrition. (2005) 21:987–93. doi: 10.1016/j.nut.2005.02.010
18. Ding, J, Pan, L, Lan, D, Chen, Z, Wang, Z, Zou, M, et al. Inflammatory markers differentiate cerebral venous sinus thrombosis from mimics. Thromb Haemost. (2023) 123:326–35. doi: 10.1055/a-1951-3402
19. Gao, B, Pan, W, Hu, X, Huang, H, Ren, J, Yang, C, et al. Neutrophil-related ratios predict the 90-day outcome in acute ischemic stroke patients after intravenous thrombolysis. Front Physiol. (2021) 12:670323. doi: 10.3389/fphys.2021.670323
20. Jenny, NS, Callas, PW, Judd, SE, McClure, LA, Kissela, B, Zakai, NA, et al. Inflammatory cytokines and ischemic stroke risk. Neurology. (2019) 92:e2375–84. doi: 10.1212/WNL.0000000000007416
21. Korkut, M, Selvi, F, and Bedel, C. Echocardiographic epicardial fat thickness and immature granulocyte are novel inflammatory predictors of acute ischemic stroke: a prospective study. Sao Paulo Med J. (2022) 140:384–9. doi: 10.1590/1516-3180.2021.0461.R1.16082021
22. Kucukceran, K, Ayranci, MK, Girisgin, AS, Kocak, S, Dundar, ZD, Ataman, S, et al. The role of the neutrophil-lymphocyte ratio in the diagnosis of cerebral venous sinus thrombosis. Bratisl Lek Listy. (2022) 123:840–5. doi: 10.4149/BLL_2022_134
23. Tekesin, A, and Tunç, A. Inflammatory markers are beneficial in the early stages of cerebral venous thrombosis. Arq Neuropsiquiatr. (2019) 77:101–5. doi: 10.1590/0004-282X20190001
24. Tekesin, A, Demiral, AB, and Yildirim, A. The importance of inflammatory biomarkers in ischemic stroke patients with carotid artery stenosis. North Clin Istanb. (2023) 10:784–9. doi: 10.14744/nci.2023.87003
25. Wang, L, Duan, J, Bian, T, Meng, R, Wu, L, Zhang, Z, et al. Inflammation is correlated with severity and outcome of cerebral venous thrombosis. J Neuroinflammation. (2018) 15:329. doi: 10.1186/s12974-018-1369-0
26. Zhang, X, Ding, R, Li, H, Liu, Y, Ou, W, Hu, J, et al. An association between inflammation and cerebral venous thrombosis: a retrospective study. J Stroke Cerebrovasc Dis. (2021) 30:106084. doi: 10.1016/j.jstrokecerebrovasdis.2021.106084
27. Kula, AY, Kurtoğlu, AV, Güzel, V, Balsak, S, Tak, AY, and Asil, T. Inflammatory biomarkers are correlated with thrombus burden in cerebral venous sinus thrombosis. Arq Neuropsiquiatr. (2024) 82:001–8. doi: 10.1055/s-0044-1787137
28. Weng, Y, Zeng, T, Huang, H, Ren, J, Wang, J, Yang, C, et al. Systemic immune-inflammation index predicts 3-month functional outcome in acute ischemic stroke patients treated with intravenous thrombolysis. Clin Interv Aging. (2021) 16:877–86. doi: 10.2147/CIA.S311047
29. Miwa, K, Tanaka, M, Okazaki, S, Furukado, S, Sakaguchi, M, Mochizuki, H, et al. Association between Interleukin-6 levels and first-ever cerebrovascular events in patients with vascular risk factors. Arterioscler Thromb Vasc Biol. (2013) 33:400–5. doi: 10.1161/ATVBAHA.112.300350
30. Kamisli, S, Çalışkan Kamışlı, Ö, Gonullu, S, Kaplan, Y, and Özcan, A. The prognostic value of increased leukocyte and neutrophil counts in the early phase of cerebral venous sinus thrombosis. Turk J Cerebrovasc Dis. (2012) 18:39–42. doi: 10.5505/tbdhd.2012.43531
31. Gencdal, I, Bayramoglu, B, Sahin, B, Baydili, KN, Sen, A, Ataklı, D, et al. Efficacy of novel haemogram parameters in the evaluation of inflammation in acute ischemic stroke. Neurol Asia. (2024) 29:895–904. doi: 10.54029/2024yxm
32. Liu, H, Zhan, F, and Wang, Y. Evaluation of monocyte-to-high-density lipoprotein cholesterol ratio and monocyte-to-lymphocyte ratio in ischemic stroke. J Int Med Res. (2020) 48:300060520933806. doi: 10.1177/0300060520933806
33. Ngwa, DN, Pathak, A, and Agrawal, A. IL-6 regulates induction of C-reactive protein gene expression by activating STAT3 isoforms. Mol Immunol. (2022) 146:50–6. doi: 10.1016/j.molimm.2022.04.003
34. Jin, J, Qiao, S, Liu, J, Li, W, Wang, F, Gao, X, et al. Neutrophil extracellular traps promote thrombogenicity in cerebral venous sinus thrombosis. Cell Biosci. (2022) 12:114. doi: 10.1186/s13578-022-00845-z
35. Manoj, H, Gomes, SM, Thimmappa, PY, Nagareddy, PR, Jamora, C, and Joshi, MB. Cytokine signalling in formation of neutrophil extracellular traps: implications for health and diseases. Cytokine Growth Factor Rev. (2025) 81:27–39. doi: 10.1016/j.cytogfr.2024.12.001
36. Murariu-Gligor, EE, Mureșan, S, and Cotoi, OS. From cell interactions to bedside practice: complete blood count-derived biomarkers with diagnostic and prognostic potential in venous thromboembolism. J Clin Med. (2025) 14:205. doi: 10.3390/jcm14010205
37. Woźniak, A, Satała, J, Gorzelak-Pabiś, P, Pawlos, A, Broncel, M, Kaźmierski, P, et al. OxLDL as a prognostic biomarker of plaque instability in patients qualified for carotid endarterectomy. J Cell Mol Med. (2024) 28:e18459. doi: 10.1111/jcmm.18459
38. Zhang, L, Xu, X, Zhang, X, Jiang, S, and Hui, P. Systemic immune-inflammation index is associated with ulcerative plaque in patients with acute ischemic stroke: a single center exploratory study. J Clin Ultrasound. (2024) 52:295–304. doi: 10.1002/jcu.23632
39. Döring, Y, Soehnlein, O, and Weber, C. Neutrophil extracellular traps in atherosclerosis and Atherothrombosis. Circ Res. (2017) 120:736–43. doi: 10.1161/CIRCRESAHA.116.309692
40. Aguiar de Sousa, D, Pereira‐Santos, MC, Serra‐Caetano, A, Lucas Neto, L, Sousa, AL, Gabriel, D, et al. Blood biomarkers associated with inflammation predict poor prognosis in cerebral venous thrombosis:: a multicenter prospective observational study. Eur J Neurol. (2021) 28:202–8. doi: 10.1111/ene.14526
41. Ding, J, Song, B, Xie, X, Li, X, Chen, Z, Wang, Z, et al. Inflammation in cerebral venous thrombosis. Front Immunol. (2022) 13:833490. doi: 10.3389/fimmu.2022.833490
42. Duan, J, Leng, X, Han, Z, Cai, Y, Wang, C, Rajah, G, et al. Identifying biomarkers associated with venous infarction in acute/subacute cerebral venous thrombosis. Aging Dis. (2021) 12:93–101. doi: 10.14336/AD.2020.0405
43. Guo, X, Liu, R, Jia, M, Wang, Q, and Wu, J. Ischemia reperfusion injury induced blood brain barrier dysfunction and the involved molecular mechanism. Neurochem Res. (2023) 48:2320–34. doi: 10.1007/s11064-023-03923-x
44. Li, S, Wang, Y, Wu, M, Younis, MH, Olson, AP, Barnhart, TE, et al. Spleen-targeted glabridin-loaded nanoparticles regulate polarization of monocyte/macrophage (Mo/Mφ) for the treatment of cerebral ischemia-reperfusion injury. Adv Mater. (2022) 34:e2204976. doi: 10.1002/adma.202204976
45. Kandelouei, T, Abbasifard, M, Imani, D, Aslani, S, Razi, B, Fasihi, M, et al. Effect of statins on serum level of hs-CRP and CRP in patients with cardiovascular diseases: a systematic review and Meta-analysis of randomized controlled trials. Mediat Inflamm. (2022) 2022:1–20. doi: 10.1155/2022/8732360
46. Monsour, M, Croci, DM, Agazzi, S, and Borlongan, CV. Contemplating IL-6, a double-edged sword cytokine: which side to use for stroke pathology? CNS Neurosci Ther. (2023) 29:493–7. doi: 10.1111/cns.14041
47. Hu, S, Gu, Y, Zhao, T, Zhang, K, Li, J, Zhou, C, et al. Steroids combined with anticoagulant in acute/subacute severe cerebral venous thrombosis. Chin Med J. (2025) 138:1825–34. doi: 10.1097/CM9.0000000000003502
48. Song, SY, Zhao, XX, Rajah, G, Hua, C, Kang, RJ, Han, YP, et al. Clinical significance of baseline neutrophil-to-lymphocyte ratio in patients with ischemic stroke or hemorrhagic stroke: an updated Meta-analysis. Front Neurol. (2019) 10:1032. doi: 10.3389/fneur.2019.01032
49. Lee, AH, Shin, HY, Park, JH, Koo, SY, Kim, SM, and Yang, SH. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci Rep. (2021) 11:543. doi: 10.1038/s41598-020-80748-6
50. Courties, G, Frodermann, V, Honold, L, Zheng, Y, Herisson, F, Schloss, MJ, et al. Glucocorticoids regulate bone marrow B Lymphopoiesis after stroke. Circ Res. (2019) 124:1372–85. doi: 10.1161/CIRCRESAHA.118.314518
51. Liu, L, Yang, C, Lavayen, BP, Tishko, RJ, Larochelle, J, and Candelario-Jalil, E. Targeted BRD4 protein degradation by dBET1 ameliorates acute ischemic brain injury and improves functional outcomes associated with reduced neuroinflammation and oxidative stress and preservation of blood-brain barrier integrity. J Neuroinflammation. (2022) 19:168. doi: 10.1186/s12974-022-02533-8
52. Li, S, Huang, Y, Liu, Y, Rocha, M, Li, X, Wei, P, et al. Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke. J Cereb Blood Flow Metab. (2021) 41:2280–94. doi: 10.1177/0271678X21995694
53. Telec, M, Frydrychowicz, M, Kazmierski, R, Wojtasz, I, Dworacki, G, Kozubski, W, et al. Circulating CD4+, CD8+, and double-negative T cells in ischemic stroke and stroke-associated infection: a prospective case-control study. Front Cell Neurosci. (2025) 19:1547905. doi: 10.3389/fncel.2025.1547905
54. Torres-Aguila, NP, Carrera, C, Giese, AK, Cullell, N, Muiño, E, Cárcel-Márquez, J, et al. Genome-wide association study of White blood cell counts in patients with ischemic stroke. Stroke. (2019) 50:3618–21. doi: 10.1161/STROKEAHA.119.026593
Keywords: cerebrovascular disorders, ischemic stroke, cerebral venous thrombosis, inflammation, inflammatory marker
Citation: Shen X, Chen L, Shen L, Chen S, Mu S, Chen L, Wang S and Li Z (2025) Inflammatory biomarkers in cerebral venous thrombosis versus ischemic stroke: a network meta-analysis. Front. Neurol. 16:1634369. doi: 10.3389/fneur.2025.1634369
Edited by:
Aleksandra Ekkert, Vilnius University, LithuaniaReviewed by:
Qiying Ye, Florida State University, United StatesVeysel Ozan Tanik, Ankara Etlik City Hospital, Türkiye
Copyright © 2025 Shen, Chen, Shen, Chen, Mu, Chen, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziqi Li, Y2hyaXN0aWFuemlxaV9sZWVAMTYzLmNvbQ==; Shousen Wang, d3Noc2VuQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xinlong Shen
Xinlong Shen Ling Chen3†
Ling Chen3† Shuwen Mu
Shuwen Mu Li Chen
Li Chen Shousen Wang
Shousen Wang Ziqi Li
Ziqi Li