- 1Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Beijing Tiantan Hospital Affiliated to Capital Medical University, Beijing, China
- 3Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, China
Purpose: Migraine, a leading global cause of disability, affects over 1.1 billion individuals worldwide. Current acute pharmacotherapy is limited by contraindications and adverse events (AEs), underscoring the need for safer, more effective alternatives. The Sishun Formula (SSF), a traditional Chinese medicine (TCM) formulation targeting “Ying-Wei disharmony,” demonstrated promising preclinical and preliminary clinical results. This trial aims to rigorously evaluate SSF’s efficacy and safety for acute migraine attacks.
Patients and methods: This multicenter, randomized, double-blind, placebo-controlled trial will enroll 144 participants with acute migraine and “Ying-Wei disharmony” syndrome, randomized 1:1 to SSF or placebo. A single dose of SSF or a matched placebo is administered within 0.5 h of migraine onset. The primary outcome is the proportion achieving headache freedom at 2 h post-dose. Secondary outcomes include the proportion achieving headache relief at 2 h post-dose, absence of most bothersome associated symptoms, sustained relief/freedom at 24/48 h, the Visual Analogue Scale (VAS) score changes, time to efficacy onset, recurrence rates, and TCM syndrome improvement. Biomarkers [e.g., calcitonin gene-related peptide (CGRP)] and safety parameters (vital signs, laboratory tests, AEs) are monitored. Data analysis will employ SPSS 25.0 and R 4.3.1.
Conclusion: The findings are anticipated to deliver high-quality evidence validating SSF’s efficacy and safety, offering a promising therapeutic alternative for acute migraine attacks.
Clinical trial registration: http://itmctr.ccebtcm.org.cn/, identifier ITMCTR2025000187.
1 Introduction
Migraine ranks as the 3rd leading cause of disability-adjusted life-years (DALYs) among all nervous system disorders globally, and the top cause of DALYs in adolescents (5–19 years). It affects 14.9% of the global population (1.16 billion individuals), making it the most prevalent neurological condition by prevalence (1). Beyond its hallmark pain, the acute migraine attack is often associated with photophobia, phonophobia, nausea, and vomiting. These symptoms exacerbate disability, disrupt daily activities, and elevate risks of comorbid conditions such as anxiety, depression, and cardiovascular disorders (2). Guidelines indicate that acetaminophen, acetylsalicylic acid, ibuprofen, naproxen sodium, and triptans have the highest level of evidence for the treatment of acute migraine attacks (3, 4). In recent years, gepants have emerged as novel calcitonin gene-related peptide (CGRP) receptor antagonists offering a favorable safety profile for acute migraine treatment, particularly in patients with contraindications (5). Despite the availability of these medications, many patients experience inadequate relief from their symptoms (6). It was reported that a significant proportion of migraine sufferers (60–88%) failed to achieve pain freedom at 2 h after taking the triptans (7). Additionally, frequent use of these medications may lead to adverse events (AEs) such as medication-overuse headaches, gastrointestinal complications, and cardiovascular events (6, 8, 9), underscoring the urgent need for safer, more effective alternatives.
Traditional Chinese medicine (TCM) attributes migraine pathogenesis to imbalances in Ying and Wei, collectively termed “Ying-Wei disharmony” (10). According to TCM theory, Ying (nutritive Qi) and Wei (defensive Qi) govern physiological harmony, with their disharmony manifesting as disordered Qi-blood circulation and compromised visceral function, ultimately triggering migraine (11). In modern medicine, Ying and Wei are recognized as essential substances responsible for maintaining homeostasis of the vascular intima and adventitia, as well as balancing energy metabolism and immune defense (12). Ying-Wei imbalance is associated with key pathological mechanisms of migraine, including dysregulation of neuroinflammatory pathways, mitochondrial metabolic dysfunction, autonomic nervous system dysfunction, and impaired vasomotor activity (12).
Based on this theoretical framework and incorporating the extensive clinical expertise of TCM specialist Kegang Cao, we developed the Sishun Formula (SSF) (13). It is a pharmacologically optimized compound comprising five medicinal botanicals (constituent details provided in Table 1), each with documented analgesic and anti-inflammatory properties as evidenced by modern studies (14–18). Our preclinical studies in migraine-model rats demonstrated that SSF significantly reduced serum and trigeminal ganglion (TG) levels of CGRP while elevating 5-hydroxytryptamine (5-HT) concentrations, with concomitant downregulation of nuclear factor-kappa B (NF-κB) and cyclooxygenase-2 (COX-2) protein expression in TG tissues, thereby ameliorating neurogenic inflammation (19).
However, robust clinical validation through randomized controlled trials (RCTs) remains absent, limiting its integration into evidence-based practice. Therefore, this study will implement a multicenter, randomized, double-blind, placebo-controlled trial to further evaluate the efficacy and safety of SSF for acute migraine attacks. By employing the internationally recognized efficacy endpoints and integrating dynamic monitoring of neuropeptide biomarkers, we aim to elucidate the therapeutic mechanisms of SSF within a modern research framework and provide a safe and effective alternative option for migraineurs.
2 Methods and analysis
2.1 Formulation preparation
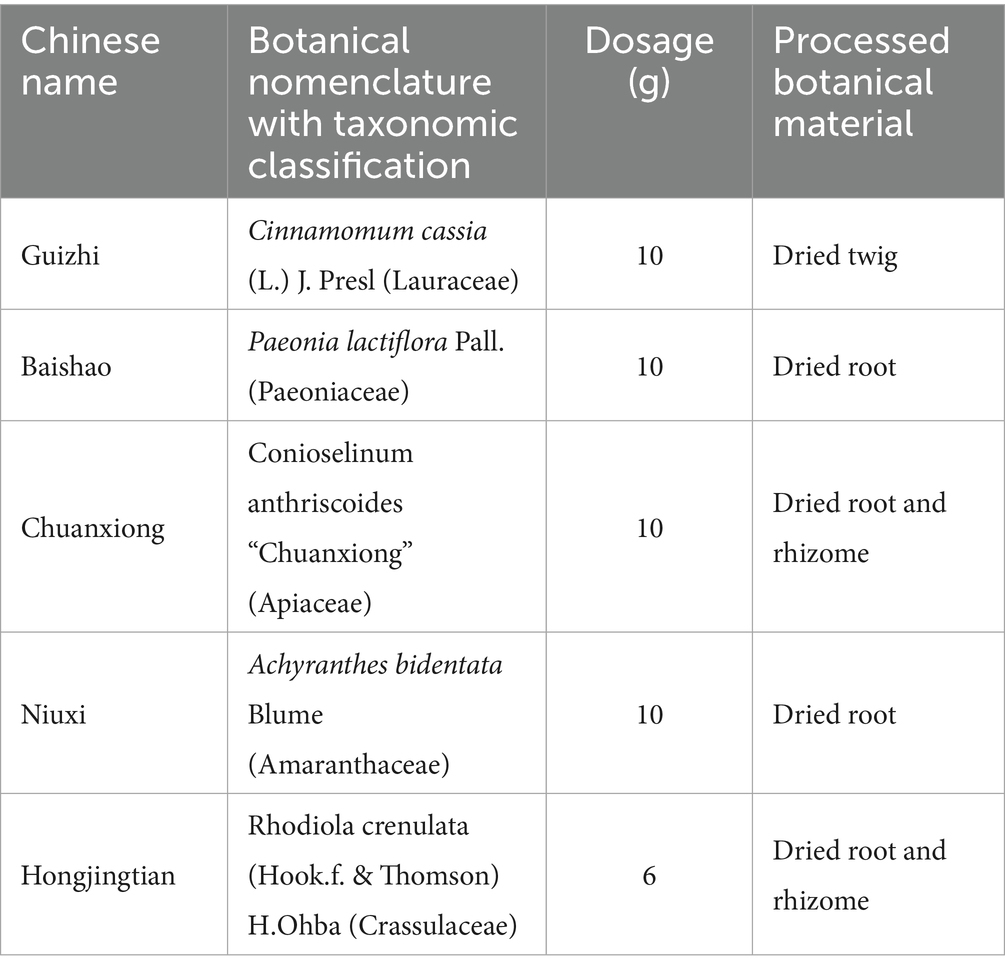
SSF, classified as a Type A botanical extract under pharmacopoeial standards (20), is a phytopharmaceutical formulation comprising five pharmacologically standardized botanical drugs processed through modern pharmaceutical techniques, including decoction, concentration, drying, and granulation (Supplementary material 1). The formula integrates Cinnamomum cassia (L.) J. Presl (Lauraceae; Guizhi-dried twig) (10 g), Paeonia lactiflora Pall. (Paeoniaceae; Baishao-dried root) (10 g), Conioselinum anthriscoides “Chuanxiong” (Apiaceae; Chuanxiong-dried root and rhizome) (10 g), Achyranthes bidentata Blume (Amaranthaceae; Niuxi-dried root) (10 g), and Rhodiola crenulata (Hook.f & Thomson) H.Ohba (Crassulaceae; Hongjingtian-dried root and rhizome) (6 g), harmonizing to regulate Ying, Wei, Qi, and Blood, and is therefore named “Sishun.” Quality control protocols employ high-performance liquid chromatography (HPLC) to validate botanical authenticity, quantify bioactive markers, and ensure batch-to-batch consistency, adhering to standard pharmacopoeial specifications for identity, purity, and extraction efficiency (21). Dosage parameters align with evidence-based clinical practices, utilizing processed botanical materials standardized to their pharmacologically active forms for optimized therapeutic delivery. The Chinese herbal components and dose of SSF are presented in Table 1.
2.2 Trial design
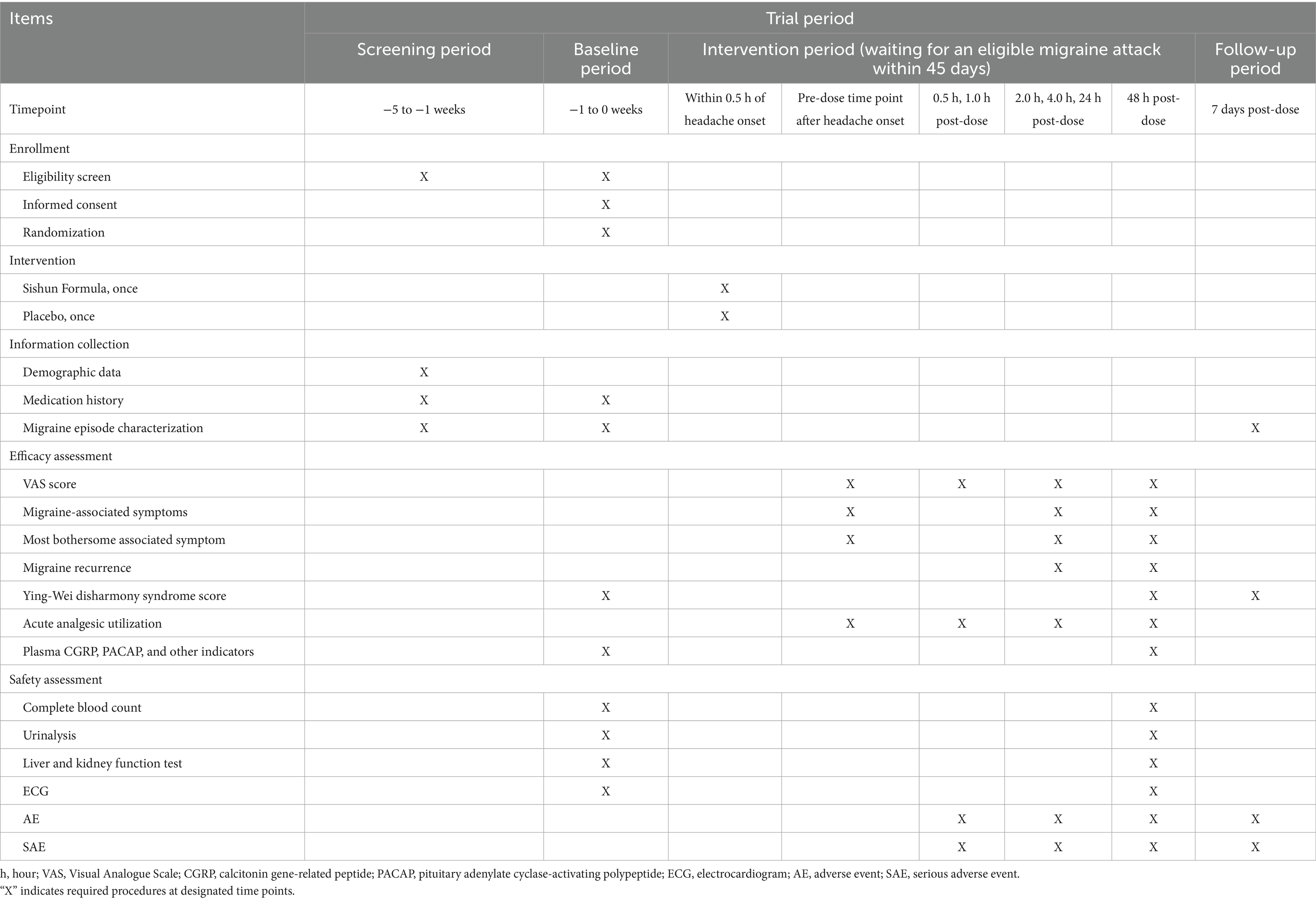
This is a multicenter, randomized, double-blind, placebo-controlled, single-attack clinical trial conducted across three centers: Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine, Xiyuan Hospital of China Academy of Chinese Medical Sciences, and Beijing Tiantan Hospital affiliated to Capital Medical University. A total of 144 eligible participants will be randomized in a 1:1 ratio to either the experimental group (SSF) or the control group (placebo). The study comprises a 4-week screening period, a 45-day intervention period, and a 7-day follow-up period. During the intervention phase, participants will administer SSF or placebo upon experiencing an eligible acute migraine attack, with efficacy and safety assessments conducted at the pre-dose time point after headache onset, 0.5 h, 1.0 h, 2.0 h, 4.0 h, 24 h, and 48 h post-dose. An eligible acute migraine attack must meet two criteria: (1) No prior use of migraine-specific medications (including all Western pharmaceuticals, TCM formulations in any dosage form, or TCM patent medicines explicitly approved for migraine treatment in their prescribing information) within the 24-h period preceding the current episode; (2) The current migraine episode is of moderate to severe intensity [Visual Analogue Scale (VAS) ≥4 scores].
The protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2025 guideline (22). The study flowchart is provided in Figure 1, with enrollment, intervention, and assessment timelines detailed in Table 2.
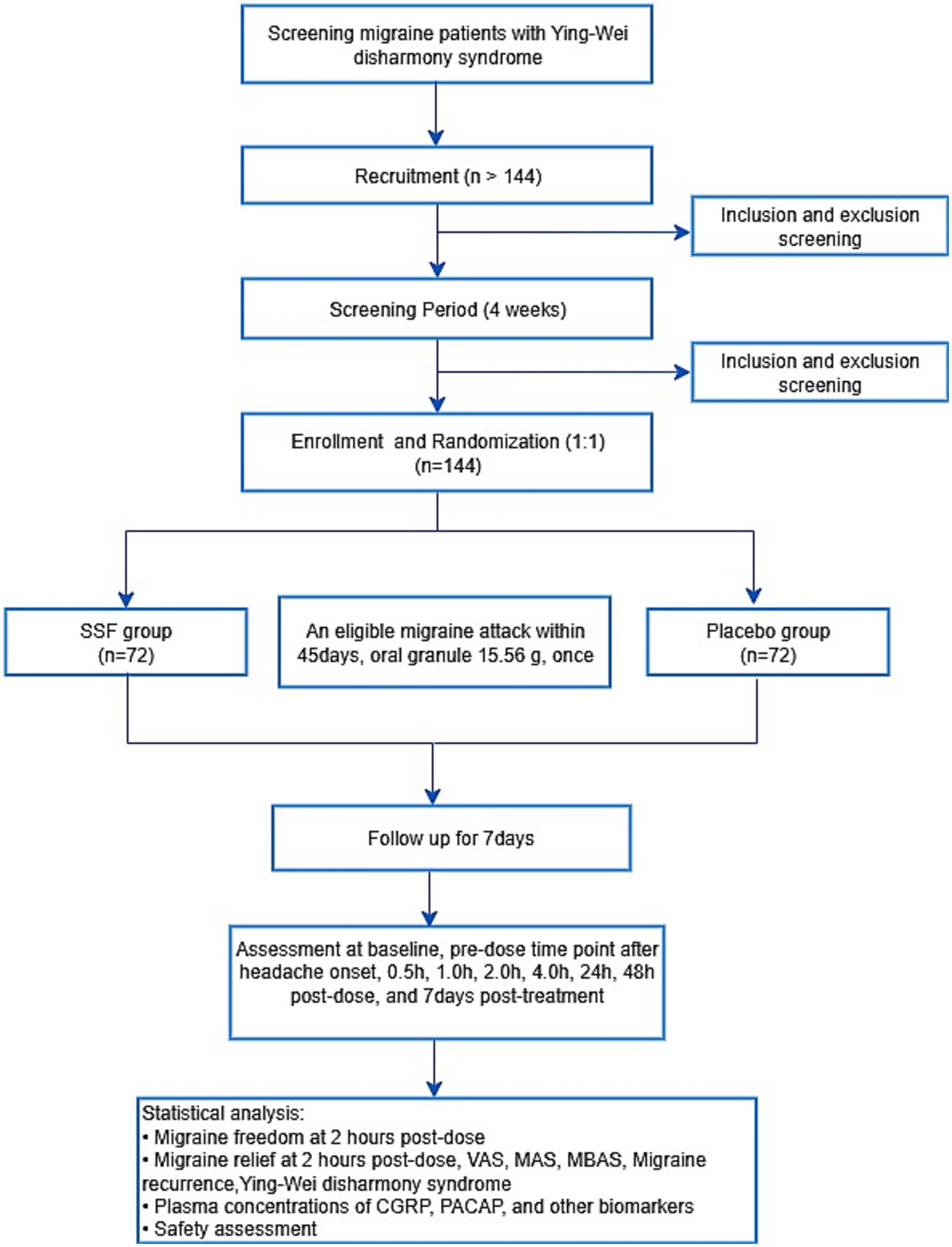
Figure 1. Study flowchart. SSF, Sishun Formula; VAS, Visual Analogue Scale; MAS, migraine-associated symptom; MBAS, most bothersome associated symptom; CGRP, calcitonin gene-related peptide; PACAP, pituitary adenylate cyclase-activating polypeptide.
2.3 Participants
2.3.1 Diagnostic criteria for migraine
According to the diagnostic criteria for “migraine without aura” and “migraine with typical aura” outlined in the International Classification of Headache Disorders, 3rd edition (ICHD-3) (23).
2.3.2 Diagnostic criteria for “Ying-Wei disharmony” syndrome
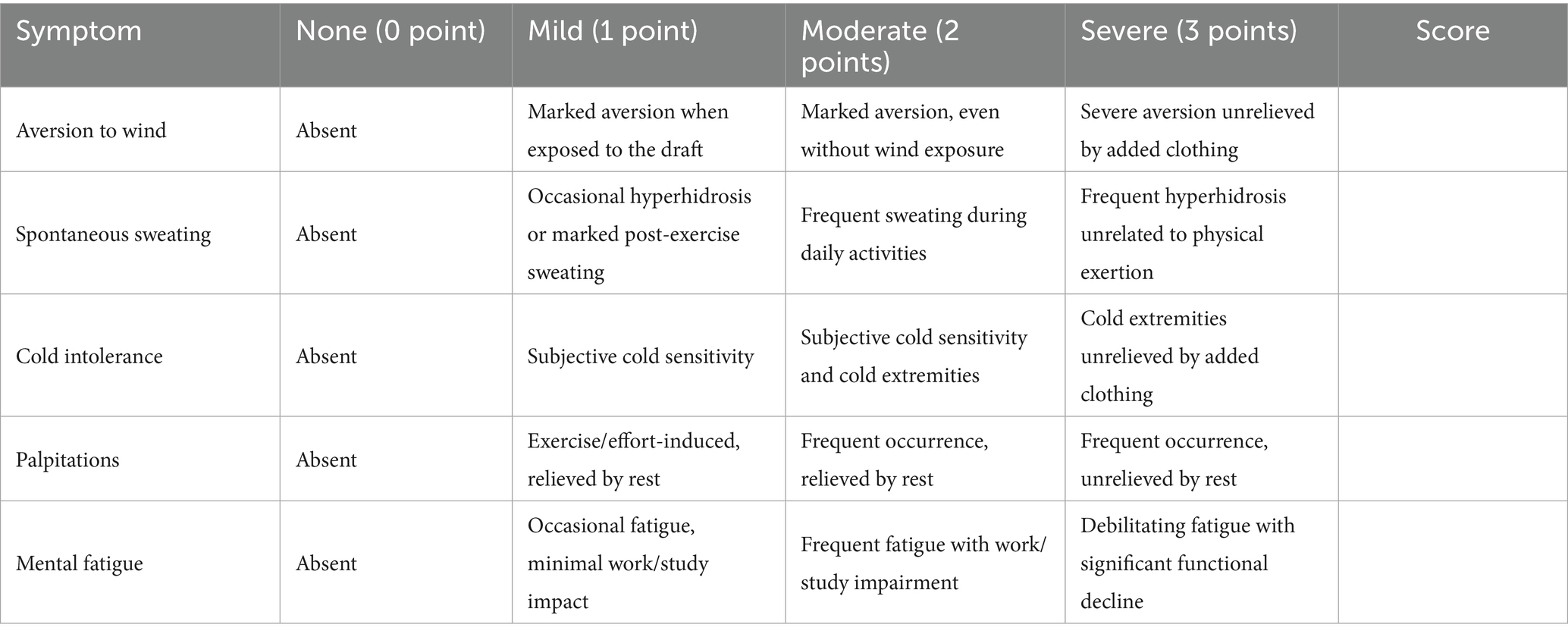
This syndrome is characterized by the presence of at least one of the following clinical features: (1) aversion to wind; (2) spontaneous sweating; (3) cold intolerance or cold extremities; (4) mental fatigue; and (5) palpitations. The differentiation of the syndrome will be independently assessed by two qualified researchers.
2.3.3 Inclusion criteria
Eligible participants must fulfill all the criteria listed below:
1. Patients who both meet the diagnostic criteria for migraine and “Ying-Wei disharmony” syndrome.
2. Patients who are aged 18–65 years (inclusive).
3. Patients whose first migraine episode occurred before the age of 50.
4. Patients who have a documented migraine history of at least 1 year.
5. Patients who experience 2 to 8 moderate-to-severe migraine attacks (VAS ≥4 scores) within the past 4 weeks, with a minimum interval of 48 h between consecutive episodes.
6. Patients must be able to distinguish migraine from other types of headaches, such as tension-type headaches.
7. Patients who voluntarily participate in the study with written informed consent provided by the participant or their legal guardian.
2.3.4 Exclusion criteria
Participants meeting any of the following criteria will be excluded:
1. Patients with ≥15 migraine days within the past 4 weeks.
2. Patients who have used analgesics for ≥10 days within the past 4 weeks.
3. Patients with secondary headaches attributed to organic pathologies, including but not limited to subarachnoid hemorrhage, cerebral hemorrhage, cerebral infarction, vascular malformations, arteritis, hypertension, or atherosclerosis.
4. Patients with severe circulatory, digestive, respiratory, or hematopoietic system diseases or cancer.
5. Patients with a history of epilepsy, stroke, other neurological disorders, or psychiatric conditions.
6. Patients with severe liver diseases, or those presenting with abnormal liver function, defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels greater than 1.5 times the upper limit of the normal range (ULN).
7. Patients with severe kidney diseases, or those presenting with abnormal kidney function, defined as creatinine (Cr) levels exceeding the ULN.
8. Patients exhibiting significant anxiety or depression symptoms [Hamilton Anxiety Rating Scale (HAMA) ≥15 scores or Hamilton Depression Rating Scale (HAMD) ≥18 scores].
9. Patients with cognitive impairment, manifested as an inability to read or comprehend assessment scales, non-compliance with medical treatment, or inability to complete efficacy evaluations.
10. Patients using migraine prophylaxis at stable doses for <3 months.
11. Patients who are pregnant, lactating, or planning pregnancy.
12. Patients currently participating in other clinical trials.
2.3.5 Withdrawal/termination criteria
Participants will be discontinued from the study if any of the following criteria are met:
1. Patients who voluntarily request to discontinue their participation in the study.
2. Patients who are unable to follow the study procedures or become lost to follow-up during the trial period.
3. Patients who experience serious AEs.
4. Patients with pregnancy detected after trial enrollment.
5. Patients demonstrating absence of an eligible acute migraine attack within 45 days after enrollment.
2.4 Randomization and allocation
The randomization scheme was designed and managed by an independent statistical unit using block randomization methodology. A random allocation sequence was generated through the PROC PLAN procedure in the Statistical Analysis System (SAS) V9.4 software. The randomization procedure was supervised by statisticians from the Good Clinical Practice (GCP) Center at Dongzhimen Hospital, Beijing University of Chinese Medicine. Critical parameters, including the selected block size and randomization table, were sealed and stored at the GCP center of Dongzhimen Hospital. Study medication allocation across trial sites commenced with the lowest assigned identifier, with eligible participants receiving drugs sequentially according to enrollment chronology.
2.5 Blinding
Blinding was administered by an independent statistical unit without direct involvement in trial operations. The team reviewed the trial medications and placebos to ensure their consistency in appearance and odor. Subsequently, the team standardized the drugs’ packaging and labeling configurations, with preassigned randomization codes affixed to all pharmaceutical containers. The blinding protocols were thoroughly documented and archived under restricted access. Emergency unblinding is permitted when investigators deem immediate intervention imperative for participant safety. However, the reasons for unblinding, the date of the procedure, and the signature of the site principal investigator must be fully documented.
2.6 Intervention
A single dose of granule therapy is to be administered to both groups within 0.5 h following the onset of an eligible migraine attack. Patients in the treatment group are orally administered SSF granules (2 bags at 7.78 g each, with warm water) within 0.5 h of headache onset. Patients in the control group are orally administered placebo granules (2 bags at 7.78 g each, with warm water) within 0.5 h of headache onset. Both SSF and placebo granules were produced by Sichuan Neo-Green Pharmaceutical Technology Development Company Limited. The placebo was formulated with maltodextrin, coloring agents, bittering agents, and 5% of the SSF herbs to sensorily match the active granules in appearance, taste, and odor, thereby protecting blinding integrity while ensuring negligible pharmacological activity.
Additionally, researchers will provide migraine management counseling to both groups. During the clinical trial period, patients may utilize alternative acute analgesic treatments for headache episodes that do not meet eligibility criteria, provided they contact researchers for treatment registration. In cases where the trial drugs fail to alleviate symptoms during an eligible migraine attack (i.e., no improvement in headache severity, impairments in daily or occupational functioning, or mood alterations), researchers will maintain open communication with patients, permit the use of conventional Western acute analgesics under supervision, and provide guidance on medication administration and post-intervention monitoring. Participants will also be instructed to record the timing and type of rescue medication used.
2.7 Outcomes and measurements
Efficacy evaluation is conducted through a specifically designed migraine patient-reported form. Patients are instructed to document migraine severity and migraine-associated symptoms (MASs) at pre-dose and at 0.5, 1, 2, 4, 24, and 48 h post-dose (24). To ensure data accuracy, physicians will review all entries in the form. In cases of implausible or inconsistent data, the attending physician will further inquire with the patient to verify the reported information. Pain intensity is quantified using the VAS and categorized into four severity levels: 0 (no pain), 1–3 (mild pain), 4–6 (moderate pain), and 7–10 (severe pain). To ensure reliable self-assessment during migraine attacks, all participants receive structured pre-trial training on the use of the VAS. This training includes standardized verbal instructions and practical examples to enhance comprehension and consistency in real-time reporting. Additionally, a simplified VAS guide emphasizing anchor points (e.g., “0 = no pain,” “10 = worst imaginable pain”) is provided for quick reference. The MASs, including photophobia, phonophobia, nausea, and vomiting, are assessed via a binary scale (0 for absence; 1 for presence) (25). Detailed outcome measurements at each time point are summarized in Table 2.
2.7.1 Primary outcome
The primary endpoint is the proportion of patients achieving headache freedom at 2 h post-dose. Headache freedom is defined as the reduction in headache severity from baseline moderate or severe to no pain, as measured by the VAS.
2.7.2 Secondary outcomes
1. The proportion of patients achieving absence of the most bothersome associated symptom (MBAS) at 2 h post-dose. Patients will self-identify their MBAS from the following symptoms at migraine onset: photophobia, phonophobia, nausea, or vomiting.
2. The proportion of patients achieving headache relief at 2 h post-dose. Headache relief is defined as the reduction of initial pain intensity from moderate to severe levels down to mild or no intensity.
3. The proportion of patients sustaining headache relief from 2 to 24/48 h post-dose. Sustained headache relief from 2 to 24/48 h is defined as achieving headache relief without rescue medication use and the absence of moderate-to-severe headache recurrence during 2 to 24/48 h following initial treatment.
4. The proportion of patients sustaining headache freedom from 2 to 24/48 h post-dose. Sustained headache freedom from 2 to 24/48 h is defined as achieving headache freedom without rescue medication use, coupled with the absence of any headache recurrence (mild, moderate, or severe) during 2 to 24/48 h following initial treatment.
5. Change in VAS scores from pre-dose to 0.5, 1, 2, 4, 24, and 48 h post-dose.
6. Time to onset of efficacy, defined as the interval (in minutes) between drug administration and headache relief.
7. Headache recurrence rate between 2 and 48 h post-dose. Headache recurrence is defined as patients who achieved headache relief (freedom) at 2 h post-dose, reexperiencing moderate-to-severe headache (headache of any severity) within the 2 to 48 h post-dose window.
8. Change in TCM “Ying-Wei disharmony syndrome.” The syndrome will be assessed using the Ying-Wei Disharmony Assessment Scale (YWDAS) at baseline, 48 h post-dose, and 7 days post-dose. The scale comprises five core TCM syndromes, and the assessment framework is detailed in Table 3. Its development was grounded in clinical expertise from senior TCM practitioners and integrated classical and contemporary medical literature. Content validity was established through a rigorous review and consensus by a panel of five senior TCM experts. In a pilot study to assess feasibility and inter-rater reliability, all YWDAS assessments were independently performed by two TCM researchers who underwent standardized training using predefined scoring criteria to minimize assessment bias. The inter-rater reliability, assessed by the intraclass correlation coefficient (ICC) for absolute agreement, was 0.76 (95% CI: 0.64–0.84), indicating good agreement.
2.7.3 Exploratory indicators
We will assess changes in plasma concentrations of CGRP, PACAP, and other biomarkers. Blood samples will be collected at two specific timepoints: baseline and post-treatment. Serum levels of CGRP and PACAP will be quantified using enzyme-linked immunosorbent assays (ELISAs) that have been rigorously validated for clinical trial applications, ensuring reliability, sensitivity, and specificity. Furthermore, correlation analyses will be conducted to evaluate the association between changes in biomarker levels and clinical outcomes, particularly migraine intensity.
2.8 Safety assessments
Safety assessments involve the systematic monitoring of AEs and serious adverse events (SAEs), along with the evaluation of vital signs (including respiratory rate, heart rate, radial pulse, and blood pressure), routine laboratory parameters (such as complete blood count, urinalysis, liver and kidney function tests), and electrocardiograms (ECGs). All assessments are conducted at baseline and 48 h post-dose.
2.9 Sample size and recruitment
The sample size was calculated based on the primary outcome measure (the proportion of patients achieving headache freedom at 2 h post-dose). Sample size determination derived from the treatment effect of 35% versus 11% placebo response (based on unpublished data), calculated through binomial proportion comparison with one-sided α = 0.025, β = 0.1, and a 24% superiority margin. This calculation yielded a requirement of 60 participants per arm. To accommodate clinical enrollment uncertainties, including anticipated attrition and protocol deviations, the adjusted enrollment target was set at 144 participants, with 72 in each group. Patient recruitment will be conducted through neurology outpatient clinics and poster-based advertising, leveraging the high patient volume across three centers to ensure sufficient enrollment.
2.10 Data management
Data management is conducted using the Yidao Research Platform to ensure systematic oversight. Two trained researchers are responsible for entering research data accurately at specified follow-up intervals across all study centers. A comprehensive data cleaning process will be implemented, encompassing automated system checks and manual validation to identify inconsistencies, followed by iterative query resolution between data managers and investigators until all discrepancies are reconciled. Prior to database closure, a formal blind review will be conducted, with the data manager submitting a closure request accompanied by a blind review report and a database closure checklist. This checklist will confirm the completion of six critical criteria: full incorporation of all collected data (including resolved AEs), resolution of outstanding queries, verification of coding consistency, final review of anomalous data, archival compliance with standard operating procedures, and signed approval of the blind review report. Database closure will require documented authorization from stakeholders, after which editing permissions are revoked, with the locking timestamp serving as audit evidence. Post-closure errors will be rigorously documented and assessed for impact on safety/efficacy analyses through multidisciplinary consultation. Database modifications post-lock will require formal approval and restricted access, with relocking procedures mirroring initial protocols. Data integrity is further safeguarded through dual-entry validation, pre-processing audits for missing values and logical errors, and secure archival of source documents by the primary research unit, ensuring full traceability of all corrections via dated, initialed annotations.
2.11 Statistical analysis
All statistical analyses will be performed using SPSS 25.0 and R 4.3.1 software. Baseline characteristics will be presented through descriptive statistics [mean ± standard deviation or median (interquartile range) or frequency (percentage)]. For between-group balance testing, independent samples t-tests will be employed for normally distributed data with homogeneity of variance, while Mann–Whitney U tests are used otherwise. Categorical variables will be analyzed using chi-square tests or Fisher’s exact test based on expected frequencies. All predetermined potential confounders will be included as covariates in subsequent models for adjustment, regardless of their between-group balance status. Additionally, baseline variables showing significant imbalance will undergo sensitivity analyses to assess their confounding effects.
Efficacy analyses will be performed on both intention-to-treat (ITT) and per-protocol (PP) populations, with the ITT set including all randomized participants analyzed according to their allocated groups, regardless of protocol deviations, while the PP set will consist of participants who complied with the trial procedures without major deviations. For efficacy assessment, the use of rescue medication before the 2-h time point will be considered a treatment failure for the primary and key secondary endpoints (e.g., headache freedom, headache relief, absence of most bothersome symptom) (26). Accordingly, participants will be included in the 2-h efficacy analysis only if they have not used rescue medication before that time point, even if medication is taken afterward. Similarly, the assessment of sustained outcomes (e.g., sustained headache freedom at 24/48 h) will require no use of rescue medication within the respective 24/48-h timeframe.
The primary efficacy endpoint (2-h headache freedom) will be compared between groups using the chi-square test or Fisher’s exact test, with reporting of risk difference (RD) and relative risk (RR) accompanied by 95% confidence intervals (CIs). For secondary endpoints, categorical variables will be analyzed through logistic regression with baseline covariate adjustment, while continuous variables (e.g., VAS scores) will be assessed using a mixed-effects model for repeated measures (MMRM). Time to onset of efficacy will employ Kaplan–Meier curves with the Log-rank test and Cox proportional hazards models to estimate hazard ratios. To address missing data, multiple imputation will be employed under the assumption of missing at random, using fully conditional specification with predictive mean matching for continuous variables and logistic regression for categorical variables, incorporating baseline and post-baseline covariates; 20 imputed datasets will be generated, and results will be pooled using Rubin’s rules. To control for multiplicity, secondary endpoints will be grouped a priori into clinically coherent families, each tested using the Holm–Bonferroni method to control the family-wise error rate. The predefined families are: (1) a 2-h symptom response family comprising absence of the most bothersome symptom and headache relief at 2 h; (2) a sustained efficacy family consisting of sustained headache relief and sustained headache freedom from 2 to 24/48 h; and (3) a time-to-event and recurrence family including time to onset of efficacy and headache recurrence rate. Changes in VAS scores and the TCM syndrome score will be analyzed exploratorily and are not included in the adjustment. Safety analyses will be conducted in the safety set (SS), which includes all randomized participants receiving at least one dose of the study medication. This population will be summarized using descriptive statistics for adverse events, vital signs, and laboratory parameters, with between-group comparisons following the methods used for baseline characteristics.
2.12 Patient and public involvement statement
In the early stage of protocol development, structured surveys were administered to migraine patients to assess their therapeutic needs and preferences regarding TCM for acute pain management. Patients or the public are not involved in the conduct, reporting, or dissemination plans of our study.
3 Discussion
Migraine, a leading cause of global disability, profoundly impacts patients’ quality of life and socioeconomic productivity due to its debilitating symptoms and high recurrence rates (27, 28). Migraine attacks involve a complex interplay of multiple pathophysiological mechanisms, including aberrant neuronal hyperexcitability, neurogenic inflammatory responses, and subsequent central sensitization, ultimately culminating in severe cephalalgia and associated symptoms (29, 30). Current first-line therapies, including triptans and non-steroidal anti-inflammatory drugs (NSAIDs), do not always yield satisfactory outcomes, and prolonged use of these medications may precipitate AEs such as medication-overuse headache and cardiovascular complications (31–33).
TCM, with its holistic approach and emphasis on restoring physiological harmony, offers a promising therapeutic avenue for migraine, and its clinical efficacy has been validated by numerous studies (34, 35). Emerging evidence indicates that certain TCM formulations and herbal extracts exert multimodal effects, including inhibition of neurogenic inflammation, regulation of vascular tone, and neuroprotection, thereby mitigating pathophysiological processes associated with migraine (36, 37). However, although a variety of Chinese patent medicines are available for acute migraine treatment, most are based on other TCM etiological frameworks such as “liver Yang uprising” or “wind-phlegm obstruction” (38). In contrast, SSF is one of the few formulas specifically developed to regulate Ying and Wei. Rooted in the theory of “Ying-Wei disharmony,” SSF integrates five botanicals with documented anti-inflammatory and neuromodulatory properties. Preclinical studies demonstrated SSF’s ability to suppress neurogenic inflammation by modulating CGRP, 5-HT, and NF-κB pathways (19). Additionally, no AEs were observed following the administration of SSF (13). Therefore, there is a critical need for standardized RCTs to validate the efficacy and safety of SSF.
Conducting rigorous RCTs for TCM formulas poses unique challenges, particularly in reconciling TCM’s individualized “syndrome differentiation” approach with standardized trial protocols (39). To address this, our study employs dual diagnostic criteria, specifically the ICHD-3 for migraine diagnosis and a validated “Ying-Wei disharmony” syndrome scale, to ensure precise patient stratification. The selection of outcome measures in this trial aligns with internationally recognized endpoints for acute migraine RCTs (26). The primary outcome, 2-h headache freedom, reflects the gold standard for acute migraine efficacy, capturing the proportion of participants transitioning from moderate/severe pain to complete resolution. This metric is widely endorsed due to its direct clinical relevance, as pain freedom within 2 h signifies rapid therapeutic action, a critical determinant of patient satisfaction and functional recovery. Secondary outcomes further elucidate the multidimensional impact of SSF. The absence of the MBAS at 2 h addresses the heterogeneity of migraine experiences, empowering patients to self-identify their predominant symptom (photophobia, phonophobia, nausea, or vomiting), thereby personalizing efficacy assessment and aligning with patient-centered care paradigms. Sustained relief and freedom from 2 to 24/48 h post-dose evaluate therapeutic durability, distinguishing transient symptom suppression from prolonged efficacy, which serves as key indicators of treatment robustness. Serial VAS scores tracking at predefined intervals (0.5, 1, 2, 4, 24, and 48 h post-dose) provides granular insight into the temporal trajectory of pain reduction, enabling precise characterization of SSF’s onset and maintenance of effect. Additionally, the inclusion of headache recurrence rates and time-to-efficacy onset addresses gaps in conventional trials by quantifying relapse risks and speed of action, both pivotal for clinical decision-making. These outcomes collectively mirror endpoints validated in recent migraine trials (40–42), ensuring methodological rigor and facilitating cross-trial comparisons. This trial also incorporates the TCM-specific syndrome assessment and neuropeptide biomarker analyses to conventional efficacy endpoints, offering a holistic evaluation of SSF’s therapeutic profile. Despite these strengths, limitations warrant consideration. Firstly, the single-dose design focuses on acute attack management but does not address SSF’s long-term prophylactic potential or cumulative effects. Secondly, evaluating headache recurrence rates within 48 h post-dose may underestimate the actual recurrence risk, necessitating extended observation in future studies. Furthermore, by restricting the sample to patients with a specific TCM syndrome, our findings may have limited generalizability to the broader migraine population, particularly in non-TCM clinical settings. This key limitation should be considered when interpreting the results and extrapolating them to other practice contexts.
4 Conclusion
In conclusion, this trial will provide robust evidence on the efficacy and safety of SSF for the treatment of acute migraine attacks. The findings, whether demonstrating positive, neutral, or negative outcomes, will hold substantial clinical relevance for migraine management.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Dongzhimen Hospital, Beijing University of Chinese Medicine (Approval No. 2024DZMEC-508-02). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Formal analysis, Methodology, Writing – original draft, Data curation, Conceptualization, Writing – review & editing, Investigation. JL: Conceptualization, Investigation, Data curation, Writing – original draft, Methodology. HL: Investigation, Methodology, Supervision, Writing – review & editing. BZ: Writing – review & editing, Supervision, Investigation, Methodology. SW: Writing – original draft, Software, Methodology, Investigation, Data curation. LL: Investigation, Writing – original draft, Data curation, Software. XW: Data curation, Software, Writing – original draft, Investigation. KC: Conceptualization, Supervision, Investigation, Methodology, Writing – review & editing, Project administration, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (Grant No. 82474442), Capital’s Funds for Health Improvement and Research (Grant No. 2024-2-4193) and the 2024 Post-Support Program for Cultivation of Topnotch Innovative Talents (Grant No. 2024YTH0015).
Acknowledgments
We would like to express our gratitude to all medical staff and researchers involved in the trial process. We also thank all participants for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can befound online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1643130/full#supplementary-material
References
1. Steinmetz, JD, Seeher, KM, Schiess, N, Nichols, E, Cao, B, Servili, C, et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. (2024) 23:344–81. doi: 10.1016/S1474-4422(24)00038-3
2. Garcia-Azorín, D, Moya-Alarcón, C, Armada, B, and Del Río, MS. Societal and economic burden of migraine in Spain: results from the 2020 National Health and Wellness Survey. J Headache Pain. (2024) 25:38. doi: 10.1186/s10194-024-01740-3
3. Tzankova, V, Becker, WJ, and Chan, TLH. Diagnosis and acute management of migraine. Can Med Assoc J. (2023) 195:E153–8. doi: 10.1503/cmaj.211969
4. Puledda, F, Sacco, S, Diener, HC, Ashina, M, Al-Khazali, HM, Ashina, S, et al. International Headache Society global practice recommendations for the acute pharmacological treatment of migraine. Cephalalgia. (2024) 44:3331024241252666. doi: 10.1177/03331024241252666
5. Pellesi, L, Jedie, B, Barhum, F, Al-Abdullah, S, Martelletti, P, and Xiao, Z. Head-to-head relief: ubrogepant, rimegepant, and zavegepant in migraine treatment. Pain Manag. (2025) 15:279–84. doi: 10.1080/17581869.2025.2494494
6. VanderPluym, JH, Victorio, MCC, Oakley, CB, Rastogi, RG, and Orr, SL. Beyond the guidelines: a narrative review of treatments on the horizon for migraine in children and adolescents. Neurology. (2023) 101:788–97. doi: 10.1212/wnl.0000000000207677
7. Tfelt-Hansen, P, and Olesen, J. Taking the negative view of current migraine treatments: the unmet needs. CNS Drugs. (2012) 26:375–82. doi: 10.2165/11630590-000000000-00000
8. Koonalintip, P, Phillips, K, and Wakerley, BR. Medication-overuse headache: update on management. Life. (2024) 14:1146. doi: 10.3390/life14091146
9. Ong, JJY, and De Felice, M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics. (2018) 15:274–90. doi: 10.1007/s13311-017-0592-1
10. Jiaojiao, L, and Kegang, C. Harmonizing the Ying and Wei method for the treatment of migraine. Acta Chin Med. (2025) 40:537–41. doi: 10.16368/j.issn.1674-8999.2025.03.087
11. Xianming, L, Aijun, Z, Lihua, H, and Yuane, L. Acupuncture treatment of migraine based on the correlation between Ying-Wei and vasomotor function. Zhongguo Zhong Yi Yao Xin Xi Za Zhi. (2009) 16:93–4.
12. Xuran, Z, and Kegang, C. Dual treatment of migraine based on the Ying-Wei theory. J Beijing Univ Tradit Chin Med. (2025) 48:861–8.
13. Yao, Y. Randomized controlled clinical trial of Sishun Formula in the treatment of migraine during remission period. Beijing: Beijing University of Chinese Medicine (2020).
14. Youn, HS, Lee, JK, Choi, YJ, Saitoh, SI, Miyake, K, Hwang, DH, et al. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. (2008) 75:494–502. doi: 10.1016/j.bcp.2007.08.033
15. Qiuyan, W, Shixin, W, Fangyu, S, Miao, Z, Zihe, G, Fenghua, X, et al. Research progress on influencing factors of active components, pharmacological effects, and component changes in Baishao. Chin Tradit Herbal Drugs. (2025) 56:1817–29.
16. Yue, Y, Chunyan, M, Zixin, S, Yingxin, Z, and Gaoqi, L. Research progress on chemical constituents and pharmacological effects of Chuanxiong. Glob Tradit Chin Med. (2025) 18:635–41.
17. Yongguang, H, Yalan, T, Chaoyun, Z, and Hua, B. Research progress on chemical constituents and pharmacological effects of Niuxi and predictive analysis of its quality marker (Q-Marker). Nat Prod Res Develop. (2024) 36:1432–44. doi: 10.16333/j.1001-6880.2024.8.017
18. Yan, L, Huifeng, Z, and Xiaoguang, L. Research progress on the pharmacological effects of salidroside. J Jilin Med Univ. (2007) 3:175–7.
19. Zhang, K. Clinical efficacy of Sishun Formula in treating migraine and its regulatory effect on neurotransmitters and neurogenic inflammation. Beijing: Beijing University of Chinese Medicine (2021).
20. Heinrich, M, Jalil, B, Abdel-Tawab, M, Echeverria, J, Kulić, Ž, McGaw, LJ, et al. Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research-the ConPhyMP-guidelines. Front Pharmacol. (2022) 13:953205. doi: 10.3389/fphar.2022.953205
21. Hameedat, F, Hawamdeh, S, Alnabulsi, S, and Zayed, A. High performance liquid chromatography (HPLC) with fluorescence detection for quantification of steroids in clinical, pharmaceutical, and environmental samples: a review. Molecules. (2022) 27:1807. doi: 10.3390/molecules27061807
22. Chan, AW, Boutron, I, Hopewell, S, Moher, D, Schulz, KF, Collins, GS, et al. SPIRIT 2025 statement: updated guideline for protocols of randomized trials. Nat Med. (2025) 31:1784–92. doi: 10.1038/s41591-025-03668-w
23. Olesen, J. Headache classification Committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
24. Li, Y, and Wang, L. Changes in inflammatory factors in patients with osteoporotic vertebral compression fracture and influences of rehabilitation training on postoperative functional recovery and inflammation. J Musculoskelet Neuronal Interact. (2018) 18:272–9.
25. Croop, R, Goadsby, PJ, Stock, DA, Conway, CM, Forshaw, M, Stock, EG, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. (2019) 394:737–45. doi: 10.1016/S0140-6736(19)31606-X
26. Diener, HC, Tassorelli, C, Dodick, DW, Silberstein, SD, Lipton, RB, Ashina, M, et al. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: fourth edition. Cephalalgia. (2019) 39:687–710. doi: 10.1177/0333102419828967
27. Steiner, TJ, and Stovner, LJ. Global epidemiology of migraine and its implications for public health and health policy. Nat Rev Neurol. (2023) 19:109–17. doi: 10.1038/s41582-022-00763-1
28. Takeshima, T, Wan, Q, Zhang, YL, Zhang, Y, Komori, M, Stretton, S, et al. Prevalence, burden, and clinical management of migraine in China, Japan, and South Korea: a comprehensive review of the literature. J Headache Pain. (2019) 20:111. doi: 10.1186/s10194-019-1062-4
29. Dalkara, T, Kaya, Z, and Erdener, SE. Unraveling the interplay of neuroinflammatory signaling between parenchymal and meningeal cells in migraine headache. J Headache Pain. (2024) 25:124. doi: 10.1186/s10194-024-01827-x
30. Erdener, SE, Kaya, Z, and Dalkara, T. Parenchymal neuroinflammatory signaling and dural neurogenic inflammation in migraine. J Headache Pain. (2021) 22:138. doi: 10.1186/s10194-021-01353-0
31. Bentivegna, E, Galastri, S, Onan, D, and Martelletti, P. Unmet needs in the acute treatment of migraine. Adv Ther. (2024) 41:1–13. doi: 10.1007/s12325-023-02650-7
32. Ljubisavljevic, S, Ljubisavljevic, M, Damjanovic, R, and Kalinic, S. A descriptive review of medication-overuse headache: from pathophysiology to the comorbidities. Brain Sci. (2023) 13:1408. doi: 10.3390/brainsci13101408
33. Vécsei, L, Lukács, M, Tajti, J, Fülöp, F, Toldi, J, and Edvinsson, L. The therapeutic impact of new migraine discoveries. Curr Med Chem. (2019) 26:6261–81. doi: 10.2174/0929867325666180530114534
34. Huiting, Y, and Enlong, W. Research progress on traditional Chinese medicine treatment for migraine based on stage differentiation syndrome. Chin J Integr Med Cardio-Cerebrovas Dis. (2022) 20:1035–40.
35. Fan, D, Leng, W, and Zhang, LQ. Systematic evaluation and meta-analysis of flunarizine hydrochloride combined with traditional Chinese medicine decoction in the treatment of migraine headaches. Clinics. (2024) 79:100431. doi: 10.1016/j.clinsp.2024.100431
36. Wu, MR, Ou, Y, Han, R, Li, T-T, Wei, M-Y, Guo, H, et al. Traditional Chinese medicine use in the pathophysiological processes of migraine. World J Tradit Chin Med. (2025) 11:1–15. doi: 10.4103/wjtcm.wjtcm_90_24
37. Ramasamy, K, Gayathri, KP, Cheriyan, BV, Josephine, IG, and Deepti, VD. Evaluation of the neuroprotective effect of Lawsonia inermis flower extract in Alzheimer's disease-induced zebrafish model. World J Tradit Chin Med. (2024) 10:451–9. doi: 10.4103/wjtcm.wjtcm_67_24
38. Chinese Society of Neurology of Chinese Association of Integrative Medicine. Chinese guideline for the prevention and treatment of migraine with integrative medicine (2022). Chin J Integr Med. (2023) 43:517–26.
39. Jiang, M, Lu, C, Zhang, C, Yang, J, Tan, Y, Lu, A, et al. Syndrome differentiation in modern research of traditional Chinese medicine. J Ethnopharmacol. (2012) 140:634–42. doi: 10.1016/j.jep.2012.01.033
40. Lipton, RB, Dodick, DW, Ailani, J, Lu, K, Finnegan, M, Szegedi, A, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine the ACHIEVE II randomized clinical trial. JAMA. (2019) 322:1887–98. doi: 10.1001/jama.2019.16711
41. Yu, SY, Guo, AH, Wang, Z, Yu, S, Guo, A, Liu, J, et al. Rimegepant orally disintegrating tablet 75 mg for acute treatment of migraine in adults from China: a subgroup analysis of a double-blind, randomized, placebo-controlled, phase 3 clinical trial. J Headache Pain. (2024) 25:57. doi: 10.1186/s10194-024-01731-4
42. Croop, R, Madonia, J, Stock, DA, Thiry, A, Forshaw, M, Murphy, A, et al. Zavegepant nasal spray for the acute treatment of migraine: a phase 2/3 double-blind, randomized, placebo-controlled, dose-ranging trial. Headache. (2022) 62:1153–63. doi: 10.1111/head.14389
Glossary
5-HT - 5-Hydroxytryptamine
AE - Adverse event
ALT - Alanine aminotransferase
AST - Aspartate aminotransferase
CGRP - Calcitonin gene-related peptide
CI - Confidence interval
COX-2 - Cyclooxygenase-2
Cr - Creatinine
DALY - Disability-adjusted life-year
ECG - Electrocardiogram
ELISA - Enzyme-linked immunosorbent assays
GCP - Good Clinical Practice
HAMA - Hamilton Anxiety Rating Scale
HAMD - Hamilton Depression Rating Scale
HPLC - High-performance liquid chromatography
ICC - Intraclass correlation coefficient
ICHD-3 - International Classification of Headache Disorders, 3rd edition
ITMCTR - International Traditional Medicine Clinical Trial Registry
ITT - Intention-to-treat
MAS - Migraine-associated symptom
MBAS - Most bothersome associated symptom
MMRM - Mixed-effects model for repeated measures
NF-κB - Nuclear factor-kappa B
NSAID - Non-steroidal anti-inflammatory drug
PACAP - Pituitary adenylate cyclase-activating polypeptide
PP - Per-protocol
RD - Risk difference
RCT - Randomized controlled trial
RR - Relative risk
SAE - Serious adverse event
SAS - Statistical analysis system
SPIRIT - Standard Protocol Items: Recommendations for Interventional Trials
SS - Safety set
SSF - Sishun Formula
TCM - Traditional Chinese medicine
TG - Trigeminal ganglion
ULN - Upper limit of normal
VAS - Visual Analogue Scale
YWDAS - Ying-Wei Disharmony Assessment Scale
Keywords: Sishun Formula, acute migraine attack, randomized controlled trial, study protocol, traditional Chinese medicine
Citation: Zhang X, Liu J, Li H, Zhou B, Wang S, Li L, Wu X and Cao K (2025) Sishun Formula for acute migraine attack: study protocol for a double-blind, randomized, placebo-controlled trial. Front. Neurol. 16:1643130. doi: 10.3389/fneur.2025.1643130
Edited by:
Damiana Scuteri, University Magna Graecia of Catanzaro, ItalyReviewed by:
Xiao Ma, Chengdu University of Traditional Chinese Medicine, ChinaLanfranco Pellesi, University of Southern Denmark, Denmark
Abhigyan Datta, University of Minnesota Twin Cities, United States
Copyright © 2025 Zhang, Liu, Li, Zhou, Wang, Li, Wu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kegang Cao, YnVjbTEyM0AxMjYuY29t
 Xuran Zhang
Xuran Zhang Jiaojiao Liu1
Jiaojiao Liu1 Kegang Cao
Kegang Cao