Abstract
Objective:
Sudden sensorineural hearing loss (SSNHL) rapidly decreases hearing, often by more than 30 decibels within 3 days. While circulatory issues are suspected causes, the exact reason remains unclear. This study employs Arterial Spin Labeling to examine changes in cerebral blood flow and its relationship with hydration levels in SSNHL.
Methods:
A prospective study examined patients with SSNHL, dividing them into 20 right-sided SSNHL (RSSNHL), 22 left-sided SSNHL (LSSNHL), and 20 healthy controls (HC). Cerebral blood flow (CBF) data were obtained using MATLAB. Statistical analysis included one-way ANOVA with post hoc analysis among RSSNHL, LSSNHL, HC, and Pearson correlation to explore the relationship with clinical variables.
Results:
Compared to HC, patients with RSSNHL showed reduced CBF value in right medial superior frontal gyrus, Heschl's gyrus (HG), and left inferior temporal gyrus, conversely increased perfusion in left calcarine. In LSSNHL patients, CBF value was decreased in the left superior temporal gyrus (STG) and right middle temporal gyrus, with increased perfusion in the left temporal pole STG compared to HC (cluster level P < 0.05 after FDR correction). Furthermore, CBF in the right HG of RSSNHL and left STG of LSSNHL negatively correlated with blood viscosity (r = −0.621, P = 0.003; r = −0.560, P = 0.007) and urine specific gravity (r = −0.483, P = 0.031; r = −0.485, P = 0.022), and positively correlated with daily water intake (r = 0.650, P = 0.002; r = 0.568, P = 0.006).
Conclusion:
Cerebral perfusion changes were present in the temporal, frontal, and occipital lobes of SSNHL patients. Furthermore, insufficient water intake may be a potential cause of SSNHL. Drinking adequate water is vital in preventing and recovering from this condition.
Highlights
-
Arterial spin labeling analysis to observe cerebral blood flow rate alteration in Sudden sensorineural hearing loss (SSNHL) patients.
-
Addresses the possible pathophysiology mechanism of SSNHL.
-
Highlights the importance of intake of an adequate amount of water.
1 Introduction
Sudden sensorineural hearing loss (SSNHL) is a distressing condition often considered an otologic emergency due to its rapid onset (1). It is defined as a hearing loss of 30 dB or more for three consecutive frequencies that appears within 3 days and is accompanied by tinnitus, vertigo, and other discomforts. SSNHL is mostly unilateral (1, 2). The prevalence in adults is about 5–20 cases per 100,000 persons (2). Importantly, in 90% of SSNHL cases, despite adequate examination, the pathophysiology remains unclear, highlighting the complexity of this disease to understand (1, 3). Over the years, several potential hypotheses have been put forth, including vascular disorders (microthrombosis, vasospasm, hyperviscosity), infections (cytomegalovirus, meningitis, fungal meningitis, AIDS, mumps, syphilis), autoimmune disease (systemic lupus erythematosus, Behçet's disease, Cogan's syndrome), neural (retrochoclear neurodegeneration), and membrane rupture (2, 4, 5). The cause of idiopathic SSNHL and the proper treatment of its patients are still up for debate despite a great deal of research (3, 6). Even though many theories about the pathophysiology of SSNHL have been presented, the vascular theory is the most recognized (3). It is well-recognized that vascular risk factors can damage the vascular endothelium, leading to functional impairments in the inner ear (7). Based on vascular etiologic theory, acute vascular hemorrhage, vascular disease, vasospasm, or a change in blood viscosity (BV) could cause SSNHL (3, 5). Some research shows that BV is inversely related to blood flow (5), and elevated BV results in greater blood flow resistance, reducing cerebral blood flow (CBF) (8). Previous studies have revealed the vital role of measuring BV in patients with SSNHL, as changes in microcirculation may be involved in the etiology and correlating whole blood viscosity to decreased CBF (8, 9).
Furthermore, BV functions as an independent marker for the hydration status of individuals (10), and dehydration adversely affects both systemic and regional blood flow (11). Some research showed that dehydration affects the cardiovascular system, resulting in vascular insufficiency (10, 12), and has a detrimental effect on the frequency, severity, and prognosis of SSNHL (12). Previous research states that dehydration affects cognitive performance and indicates the importance of increasing fluid intake (13). In addition to hearing loss, an individual's quality of life is negatively impacted by a variety of non-auditory issues, including social isolation, depression, anxiety, and dementia (14). Asymmetrical hearing in patients with sudden sensorineural hearing loss (SSNHL) often causes localization problems, which can be irritating and even distressing for the affected individual. The prognosis concerning hearing recovery depends on various factors, including age at onset, degree of hearing loss, severity of vertigo, and time from onset to treatment visit (3). Therefore, these complexities highlight the ongoing need for research to determine contributing factors.
Recent advancements in imaging techniques, such as Arterial Spin Labeling (ASL), have enabled CBF measurements, offering new insights into changes in cerebral perfusion. ASL evaluates brain perfusion through magnetic resonance imaging (MRI), and its key advantage is that it does not require an external tracker, such as gadolinium. The labeled water molecules can move beyond the intravascular space (15). Compared to PET or contrast-enhanced MRI, ASL is non-invasive and produces quantitative CBF maps with excellent repeatability. Due to its sensitivity to changes in microvascular flow, ASL is especially good at detecting minute changes in perfusion inside the auditory cortices, such as the HG and STG (16).
In an fMRI study, SSNHL patients showed altered connections in the salience, default mode, and central executive networks, with decreased functional connectivity between the insula and other brain regions (14). A functional connectivity fMRI study highlighted that the reorganization of the anterior cingulate cortex and the disruption of multiple intrinsic networks are crucial in regulating cognitive, emotional, and auditory function processing in SSNHL patients (17). Furthermore, a study investigating the topological alterations within the complete brain network of SSNHL patients indicated modifications present in the white matter network (16).
Most SSNHL research primarily focuses on topographical changes, structural alterations, particular regions of interest (ROIs), and functional brain connectivity networks, often neglecting possible perfusion changes that could clarify the pathophysiological processes involved. Although the theory of vascular compromise is well-established, limited studies examine how systematic hemodynamic changes influence SSNHL. Furthermore, despite the growing use of ASL in auditory research, the effects of SSNHL on cerebral perfusion and its underlying mechanisms are still poorly understood. ASL studies provide important insights into the mechanisms of vascular compromise and focus on cerebral perfusion (18). Our study fills the gap left by previous research, which ignores the importance of hydration. This discrepancy is essential since the brain-auditory axis is sensitive to changes in perfusion, and the cochlea requires sufficient CBF. By analyzing the variations in CBF values among SSNHL patients and investigating the possible contribution of dehydration to these fluctuations, this study sought to fill this information gap.
This research uses ASL imaging and a detailed analysis of 170 brain regions to compare and address the alteration in CBF value in SSNHL patients to healthy controls. The study aims to document the alterations in brain areas and identify the connection between hydration and SSNHL pathophysiology. Correlation analysis with the clinical data of the patients seeks to explore the differential mechanism of brain intrinsic function changes in patients with SSNHL.
2 Materials and methods
2.1 Subjects
This prospective study enrolled SSNHL patients admitted to the otorhinolaryngology department of Renmin Hospital of Wuhan University from August 2023 to June 2024. Participants included 23 healthy controls (HC) and 47 hearing loss patients. Following the Pure Tone Average (PTA) test, three patients were excluded due to other types of hearing loss. Based on the PTA results, 44 patients were divided into right-sided Sudden Sensorineural Hearing Loss (RSSNHL) and left-sided Sudden Sensorineural Hearing Loss (LSSNHL). The PTA test was administered to the HC group to ensure study standardization. Each SSNHL patient completed the Hearing Handicap Inventory for Adults (HHIA) questionnaire. An ASL scan was performed after laboratory testing for all participants, including SSNHL and HC groups. Following the exclusion of five subjects due to excessive head movement and motion artifacts, 42 SSNHL patients (19 male and 23 female, aged 19–70 years) and 20 healthy controls (10 male and 10 female, aged 19–68 years) were included in the final analysis. These groups were matched for age, gender, and educational level. Detailed information on the recruitment process and all the included steps are given in the flowchart (Figure 1). The inclusion criteria were as follows: 1) Acute hearing loss with no apparent cause, 2) hearing loss above 30 dB in three sequential frequencies, 3) symptom onset and diagnosis within 1 to 7 days, 4) age < 75 years (1, 5), and 5) no prior treatment (e.g., steroids).
Figure 1

Flowchart of subject recruitment.
Exclusion criteria included: 1) mixed hearing loss or Bilateral hearing loss, 2) history of sudden deafness, 3) ear disorders (intracochlear hemorrhage, Schwannoma, Meniere disease, or other infectious diseases), 4) claustrophobia or contraindication to MRI, 5) pregnancy, 6) prior ear surgery or brain trauma, and 7) disease duration >7 days were not included.
The Renmin Hospital of Wuhan University Medical Ethics Committee approved this study (WDRY2024-K275), following the principles of the Declaration of Helsinki.
2.2 Audiometry test
The Pure-Tone Average (PTA) was calculated to evaluate hearing thresholds through pure-tone audiometry. An otolaryngologist from our hospital performed the PTA across frequencies ranging from 0.125 to 8 kHz. The average PTA for SSNHL patients exceeded 30 dB HL across all frequencies, while the healthy control group had an average PTA of < 20 dB HL. Based on these PTA results, SSNHL patients were categorized into two subgroups: RSSNHL (n = 20) and LSSNHL (n = 22) (Figure 1).
2.3 Clinical assessment
Demographics, including age, gender, education level, handedness, related symptoms, and lifestyle factors (e.g., smoking and alcohol consumption), were collected from all SSNHL and HC groups. Clinical data such as disease duration and audiometry test for SSNHL patients. Laboratory tests were conducted to measure biochemical variables, including urine-specific gravity (Usg) and blood viscosity (BV) for both participants. The Hearing Handicap Inventory for Adults (HHIA) questionnaire was administered to assess the social and emotional impacts of hearing loss on daily life. The questionnaire consists of 25 items, with response scores of 0 for “No,” 2 for “Sometimes,” and 4 for “Yes.” Total scores were categorized as follows: 0–16 (No Handicap), 18–42 (Mild to Moderate Handicap), and 44 or above (Significant Handicap) (19).
2.4 Hydration assessment
Usg is a sensitive indicator of hydration status, with a standard reference range of 1.003–1.030 μmol/L (21). Due to its simplicity, speed, and affordability, Usg is a popular alternative for determining participants' hydration levels in clinical settings (20). Usg indicates that the patient's hydration level correlates with urine osmolality and illustrates the kidneys' capacity for concentration (21). All participants were instructed to continue fasting and refrain from drinking water before collecting urine and blood samples. Random morning samples (midstream urine) were taken from each participant during the baseline medical assessment. Blood Viscosity (BV), measured at different shear rates ranging from 0.1 s−1 to 1,000 s−1 (22), was also considered a biomarker of an individual's hydration status (10). BV was measured at various shear rates (low shear, medium shear, high shear) (23). Additionally, participants were asked questions regarding daily water consumption. The recommended average daily intake is 3.0 L for men and 2.5 L for women (24).
2.5 MRI acquisition
MRI scans were performed using a 3.0 Tesla MRI scanner (Signa Architect Air GE Healthcare, USA) with a 48-channel phased array head coil. The participants were given rubber-soft foam and earplugs to reduce equipment noise. ASL parameters Repetition time (TR) = 4,854 ms; echo time (TE) = 10.7 ms; slice thickness = 4 mm; FOV = 240 mm × 240 mm, Post label delay (PLD)= 2,025 ms; flip angle = 111; Labeling duration = 1,800 ms; Labeling offset: 90 mm; Readout method: 3D fast spin-echo and slice number = 36. All participants had MRI scans while keeping their eyes closed.
2.6 Data processing
ASL data were collected using a GE MR scanner and processed using MATLAB software per established procedures (15) (Figure 2). The data processing steps included:
Figure 2

Data processing steps.
a.Converted 3D ASL DICOM files to NIfTI format, applied head motion correction, and co-registered T1 images with ASL data to ensure precise spatial normalization to Montreal Neurological Institute brain templates (MNI-152). b. Matlab-based Statistical Parametric Mapping (SPM12) was used to standardize the CBF value of each voxel, which was obtained by subtracting the mean CBF and dividing it by the standard deviation. The CBF value was smoothed with a Gaussian Kernel of 6 × 6 × 6 mm3. Anatomical Labeling (AAL3v1) atlas (25), based on MATLAB software, was used to segment and obtain normalized CBF value, followed by a one-way ANOVA to compare the three groups (RSSNHL, LSSNHL, HC), corrected using the False discovery rate (FDR)method, and followed by post hoc analyses, e. Overlaying and visualizing the results using AAL3v1 and Xjview.
2.7 Analysis of CBF in auditory and non-auditory brain regions
Based on previous studies indicating the primary auditory cortex as a central location for sound processing, the temporal lobe was selected as the ROI for analyzing both auditory and non-auditory brain regions (26). The AAL3V1 atlas further divided the temporal lobe into subregions as Heschl's gyrus (HG), superior temporal gyrus (STG), temporal pole superior temporal gyrus (TP-STG), middle temporal gyrus (MTG), temporal pole middle temporal gyrus (TP-MTG), and inferior temporal gyrus (ITG) (25). Since HG and STG are the primary auditory cortex, ROI analysis was focused on these areas for further Pearson correlation analysis.
2.8 Statistical analysis
Statistical analyses were conducted using MATLAB and SPSS. A one-way ANOVA test was used to compare the mean and standard deviation of the demographic and clinical data, as well as to compare the CBF values between the three groups: RSSNHL patients, LSSNHL patients, and the HC group using the False Discovery Rate (FDR) method. It was used to correct for multiple comparisons with a significance level of voxel-level P < 0.001 and cluster-level P < 0.05, followed by post hoc pairwise comparisons. The continuous variable obeys a normal distribution. The chi-square test was used for categorical data. A P-value < 0.05 was considered significant. Pearson correlation was used to assess the strength and correlation between perfusion change and clinical variables, with P < 0.05 considered significant. Linear regression was used to model the relationship between the variables further.
3 Results
3.1 Demographic and clinical data of SSNHL patients and HC
No significant differences were observed in age, gender, education level, handedness, hearing duration, PTA, and HHIA score between SSNHL patients and the HC group (Table 1). However, a significant difference was found in Usg and daily water consumption between these groups. All participants had normal kidney function test results.
Table 1
| Characteristics | RSSNHL (n = 20) | LSSNHL (n = 22) | HC (n = 20) | P-value |
|---|---|---|---|---|
| Age (years) | 39.10 ± 13.14 | 37.61 ± 11.63 | 34.20 ± 10.05 | 0.542 |
| Gender (M/F) | 9 M/11 F | 10 M/12 F | 10 M/10 F | 0.7 |
| Education level (years) | 11.17 ± 1.82 | 11.60 ± 1.72 | 10.95 ± 1.43 | 0.261 |
| Handedness (R/L) | 20/0 | 22/0 | 20/0 | - |
| Duration of hearing loss (Day) | 2.55 ± 1.2 | 2.12 ± 08 | - | - |
| HHIA score | 31.7 ± 10.27 | 30.5 ± 10.98 | - | - |
| PTA of affected ear (dB) | 58.28 ± 23.6 | 57.33 ± 13.9 | - | - |
| BV (low shear) | 21.56 ± 2.16 | 21.67 ± 2.25 | 19.32 ± 1.26 | < 0.001 |
| BV (medium shear) | 5.28 ± 0.80 | 4.91 ± 0.49 | 4.58 ± 0.41 | 0.002 |
| BV (high shear) | 4.36 ± 0.71 | 4.29 ± 0.65 | 3.81 ± 0.42 | 0.011 |
| Usg | 1.026 ± 0.007 | 1.027 ± 0.007 | 1.011 ± 0.002 | < 0.001 |
| Water intake per day (ml) | 1,090.48 ± 634 | 1,109.09 ± 516 | 2,395 ± 414 | < 0.001 |
Demographic and clinical characteristics.
Values are measured in mean ± SD. SSNHL, Sudden Sensorineural Hearing Loss; HC, Healthy Control; HHIA, Hearing Handicap Inventory for Adults; PTA, Pure tone average; Usg, Urine-specific gravity.
3.2 Cerebral perfusion comparison between three groups (LSSNHL vs. RSSNHL vs. HC)
One-way ANOVA with FDR correction, followed by post hoc analyses, revealed significant differences in cerebral perfusion across three groups.
Compared to HC, RSSNHL patients exhibited decreased CBF values in the right medial superior frontal gyrus (SFG), insula, HG, MTG, and left ITG, alongside increased perfusion in the left calcarine (Figure 3a, Table 2). The LSSHNL patients showed decreased CBF values in the left STG, left Rolandic operculum, and right MTG, and increased perfusion in the left TPSTG compared to the HC group (Figure 3b, Table 2). Compared to RSSNHL, LSSNHL patients had significantly decreased CBF values in the right STG (Figure 3c, Table 2).
Figure 3

(a) Alteration of perfusion in different brain regions between the RSSNHL and the HC group. Red indicates the brain areas with increased blood flow, and blue indicates those with decreased blood flow. (b) Alteration of perfusion in different brain regions between the LSSNHL and the HC group. Red indicates the brain areas with increased blood flow, and blue indicates those with decreased blood flow. (c) Alteration of perfusion in different brain regions between the RSSNHL and the LSSNHL group. Blue indicates those with decreased blood flow.
Table 2
| Condition (CBF value) | Brain region | MNI coordinates (x,y,z) | Peak T score | Cluster size (voxels) |
|---|---|---|---|---|
| RSSNHL < HC | R-SFG (medial) | (10,50,6) | −4.5 | 75 |
| R-HG | (46,−16,5) | −5.3 | 564 | |
| R-Insula | (40,17,−5) | −3.37 | 380 | |
| R-MTG | (62,−42,5) | −5.7 | 90 | |
| L-ITG | (−58,−60,−20) | −2.9 | 150 | |
| RSSHHL>HC | L-Calcarine | (−20,−66,8) | 4.2 | 390 |
| LSSNHL < HC | L-STG | (−52,−2,−5) | −3.32 | 332 |
| L-Rolandic operculum | (−50,−7,10) | −4.34 | 300 | |
| R-MTG | (64,−42,6) | −4.2 | 70 | |
| LSSNHL>HC | L-TPSTG | (−44,22,−22) | 5.58 | 80 |
| LSSNHL < RSSNHL | R-STG | (46,−4,−10) | −5.4 | 434 |
Brain regions with statistically significant clusters of CBF between three groups (LSSNHL vs. RSSNHL vs. HC).
P < 0.05 was considered statistically significant after FDR correction.
CBF, Cerebral blood flow; SSNHL, Sudden Sensorineural Hearing loss; LSSNHL, Left sided Sudden Sensorineural Hearing loss; RSSNHL, Right sided Sudden Sensorineural Hearing loss; L, Left; R, Right; MNI, Montreal Neurologic Institute; SFG, Superior frontal gyrus; HG, Heschl's gyrus; MTG, Middle temporal gyrus; ITG, Inferior temporal gyrus; STG, Superior temporal gyrus; TP STG, Temporal pole superior temporal gyrus.
3.3 Pearson correlation analysis
The Pearson correlation analysis revealed distinct patterns of association between cerebral blood flow (CBF) in key auditory regions and hydration-related parameters among SSNHL subgroups.
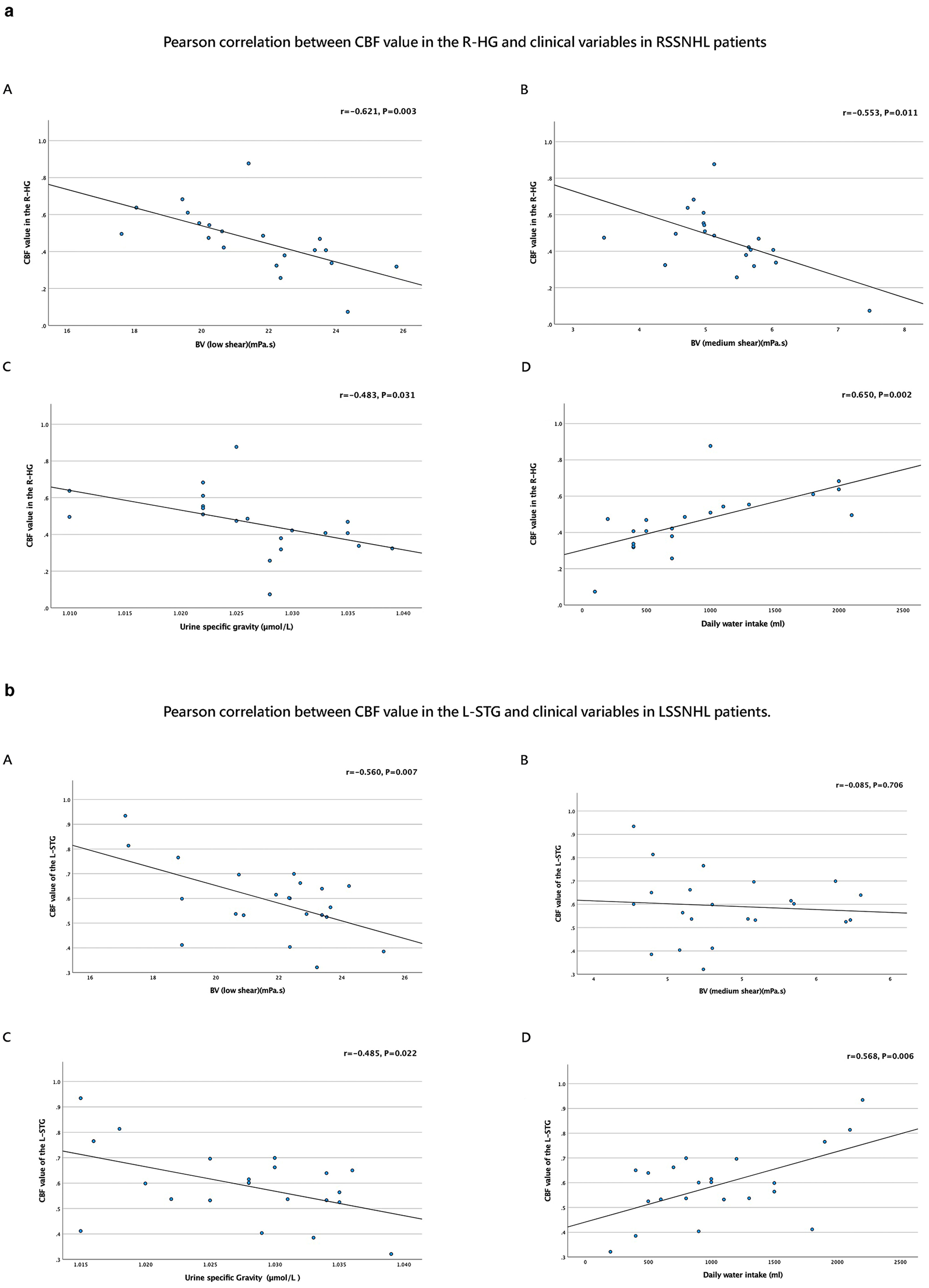
In RSSNHL patients, the right HG's CBF value was negatively correlated with BV at low and medium shear rates (mPa.s) and with Usg (μmol/L). Additionally, it showed a positive correlation with daily water intake (ml; Table 3, Figure 4a).
Table 3
| Group | Brain regions | BV (low shear) | BV (medium shear) | Urine specific gravity | Daily water intake (ml) |
|---|---|---|---|---|---|
| RSSNHL | R-HG | r = −0.621, P = 0.003 | r = −0.553, P = 0.011 | r = −0.483, P = 0.031 | r = 0.650, P = 0.002 |
| LSSNHL | L-STG | r = −0.560, P = 0.007 | r = −0.085, P = 0.706 | r = −0.485, P = 0.022 | r = 0.568, P = 0.006 |
Pearson correlation between SSNHL patients and clinical variables.
Figure 4
(a) Pearson correlation between the CBF value of R-HG and clinical variables in SSNHL. Pearson correlation analysis between (A) CBF value of R-HG and BV (low shear), (B) CBF value of R-HG and BV (medium shear), (C) CBF value of R-HG and Urine specific gravity, (D) CBF value of R-HG and Daily water intake (ml). (b) Pearson correlation between the CBF value of L-STG and clinical variables in LSSNHL. Pearson correlation analysis between (A) CBF value of L-STG and BV (low shea), (B) CBF value of L-STG and BV (medium shear), (C) CBF value of L-STG and Urine specific gravity, (D) CBF value of L-STG and Daily water intake (ml).
In LSSNHL patients, the left STG's CBF value showed a negative correlation with BV (low shear; mPa.s) and Usg (μmol/L). Conversely, it positively correlated with daily water consumption (ml; Table 3, Figure 4b).
No significant correlation was found between the CBF of healthy controls and clinical variables (all P > 0.05).
Also, no significant correlation was observed between the Hearing Handicap Inventory for Adults (HHIA) score and altered perfusion in these brain regions. Additionally, no significant correlation was observed between other brain regions and HHIA (all P > 0.05).
3.4 Linear regression analysis
In RSSNHL patients, R-HG showed a significant relationship between BV low shear, Usg, Daily water intake, Hematocrit, and PTA. In LSSNHL patients, L-STG also showed a substantial relationship between BV low shear, Usg, Daily water intake, Hematocrit and PTA, respectively (Table 4).
Table 4
| Brain region | Predictor | β (SE) | 95% CI | R 2 | F statistic | P-value |
|---|---|---|---|---|---|---|
| R-HG | BV (Low shear) | −0.0490 (0.015) | [−0.080, −0.018] | 0.386 | 11.29 | 0.003 |
| Urine specific gravity | −10.7987 (4.608) | [−20.480, −1.117] | 0.23 | 5.49 | 0.03 | |
| Daily water intake (mL) | 0.0002 (4.88e-5) | [7.47e-5, 0.0001] | 0.423 | 13.18 | 0.001 | |
| Hematocrit (Hct) | −0.1189 (0.031) | [−0.185, −0.053] | 0.445 | 14.44 | 0.001 | |
| PTA (dB) | −0.0086 (0.002) | [−0.012, −0.005] | 0.596 | 26.53 | 0.0001 | |
| L-STG | BV (Low shear) | −0.0357 (0.012) | [−0.060, −0.011] | 0.314 | 9.147 | 0.006 |
| Urine specific gravity | −9.6422 (3.890) | [−17.757, −1.527] | 0.235 | 6.143 | 0.022 | |
| Daily water intake (mL) | 0.0001 (4.62e-5) | [4.63e-5, 0.0002] | 0.323 | 9.535 | 0.005 | |
| Hematocrit (Hct) | −0.0847 (0.020) | [−0.125, −0.044] | 0.484 | 18.78 | 0.0003 | |
| PTA (dB) | −0.0057 (0.002) | [−0.009, −0.002] | 0.352 | 10.86 | 0.003 |
Linear regression between SSNHL patients and clinical variables.
4 Discussion
Water serves as the essence of life and constitutes the primary element in cells, tissues, and organs. Different organs vary in water content, with 83.0% found in blood, 74.8% in the brain, and 22.0% in the skeletal muscle system (27). This study aimed to investigate alterations in cerebral perfusion in auditory and non-auditory brain regions in individuals with SSNHL and evaluate how these changes relate to hydration. Prior research has not extensively explored this concept. One of the unique features of this study is its focus on the link between alterations in CBF value and hydration parameters in patients with SSNHL.
The primary auditory cortex (PAC) comprises Heschl's gyrus and the superior temporal gyrus (STG), both of which are essential for auditory processing. Anatomically, Heschl's gyrus—often the central auditory area—corresponds to Brodmann areas 41 and 42. Its primary function is the early processing of auditory information, such as sound intensity and frequency, and it is situated in the transverse temporal gyrus (28). Furthermore, the importance of the STG in auditory health has been highlighted by volumetric studies that have linked changes in the STG to several auditory diseases, such as tinnitus and hearing loss (29). These regions are responsible for higher-level auditory data processing, which leads to auditory perception (30, 31). This study found decreased CBF value in the affected side of SSNHL patients, specifically in the HG and STG regions integral to auditory processing. These findings align with other fMRI studies (32, 33), which suggest that alterations in the auditory cortex may disrupt the processing and timing of incoming auditory information (26, 34). Additionally, gray and white matter changes in the auditory cortex have been observed in voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) studies, pointing to a reduced executive control network in individuals with persistent hearing loss (35). Reduced perfusion in HG and STG may thus impair SSNHL patients' ability to process and interpret sound, leading to difficulties in speech comprehension, sound localization, and understanding spoken words (33).
The middle and inferior temporal gyrus plays a pivotal role in supporting visual perception, multimodal sensory integration, and the processing of linguistic and semantic memory (36). Gray matter volume decrease in MTG and ITG was reported in an fMRI investigation on brain structural and functional changes in people with unilateral SSNHL (37). Decreased CBF value in MTG and ITG was also found in this study, which might have affected their multimodal functions. The default mode network has been suggested to include the medial region of the superior frontal gyrus (SFG), which is frequently deactivated during cognitively relevant processing (38). Previous studies have identified the SFG as an essential component of the auditory processing network, integrating a wide range of sensory inputs and coordinating central nervous system activity (39). Decreased perfusion in this region may impair auditory processing and cognitive function in SSNHL patients. Another study of SFG, which plays a vital role in linguistic cognition (40), suggests that patients with SSNHL may have decreased functional connectivity in a SFG, and language memory might be impacted. Notably, the reduced perfusion in the STG and SFG, a critical area for multisensory integration, may help explain the non-auditory symptoms of SSNHL, including tinnitus (41).
This study's results are consistent with these findings, showing a decreased CBF, which may indicate a disruption in multimodal cognitive processes. In addition, a functional connectivity study that assessed the functional connectivity of brain networks in patients with SSNHL demonstrated that changes in connectivity are related to changes in auditory and cognitive function (32).
The insula, which separates the temporal lobe from the frontal and parietal lobes, plays a vital role in audio-visual and sensory integration and emotional processing. Previous research has shown hyperperfusion and decreased functional connectivity in the insula of SSNHL patients (14). However, this study observed reduced perfusion in the insula, which may suggest different pathophysiological mechanisms at play. The rostral temporal pole within the meso-temporal lobe is associated with higher cognitive functions such as emotion, social cognition, autobiographical memory, and multimodal sensory integration (42). Conversely, enhanced blood flow in the visual areas, notably the calcarine region, indicates the presence of cross-modal plasticity, which may compensate for auditory impairments. This study revealed hyperperfusion in the TP STG and calcarine regions, potentially indicating a compensation mechanism. The temporal pole, superior temporal gyrus, and calcarine areas may have higher CBF due to increased neuroplasticity. Patients with hearing loss can benefit from compensatory auditory processing in the temporal regions and cross-modal plasticity in the calcarine cortex, where the brain increases its dependence on visual information to compensate for auditory impairments.
This study found that the CBF value in the right HG in RSSNHL patients and the left STG in LSSNHL patients was negatively correlated with BV(low shear) and Usg. In RSSNHL patients, R-HG also showed a negative correlation with BV(medium shear). Furthermore, the left STG in LSSNHL patients and the right HG in RSSNHL patients positively correlated with daily water consumption. These findings suggest that increased BV, Usg, and decreased water intake may be associated with reduced CBF value in this critical auditory region. A few studies suggested that abnormalities in CBF in SSNHL are secondary to vascular anomalies and that impaired microvascular flow dynamics can significantly influence auditory processing (5). The results of our study support the “vascular compromise” theory, which postulates that dehydration (10) and BV hinder cochlear blood flow by causing microcirculatory sludging (9) extending to auditory cortices. This aligns with the study indicating that BV increases precede hearing loss (43). Changes in blood vessel tone and blood volume can result in congestion, lack of oxygen, and increased capillary permeability, all of which can negatively affect auditory processing (43). The results support this hypothesis, stating that higher BV appears to influence microvascular dynamics and immune responses, adversely affecting auditory processing.
Furthermore, the inverse relationship between hydration, as reflected by BV, Usg, fluid intake, and CBF, emphasizes the role of appropriate hydration in maintaining adequate cerebral perfusion. The BV-CBF association indicates that the “vascular steal” phenomenon, in which dehydration diverts blood from auditory circuits, is modulated by hydration (11). This study emphasizes the significant relationship between hydration status and perfusion in auditory brain regions, suggesting dehydration may contribute to impaired blood flow and auditory dysfunction in SSNHL patients. BV could also serve as a potential marker for recovery in SSNHL (9, 43), as dehydration status could be defined by BV and Usg (10, 24, 44). We can also establish that Usg is sensitive to changes in hydration (44). Our study is consistent with previous studies on water intake, emphasizing that dehydration leads to reduced cerebral blood flow and impaired cognitive function, as well as the importance of maintaining an appropriate daily water intake strategy and maintaining proper fluid intake to avoid physiological problems associated with insufficient body hydration (11). Linear regression analysis also provided further support for these results.
Recent brain imaging studies have also shown how dehydration affects blood vessel changes and cognitive performance, suggesting a possible treatment target for patients with SSNHL (13, 45). Additionally, clinical studies have proven that hydration status significantly impacts the outcome of SSNHL, highlighting the importance of maintaining adequate fluid intake to prevent dehydration-related physiological problems (12). This study shows that hydration status affects the prognosis and also the prevention of SSNHL.
5 Limitation
The main limitation of this study is its relatively small sample size, which diminishes the statistical power of the findings. A larger sample could yield more definitive results and allow for the identification of subtle differences in CBF among groups. Additionally, the cross-sectional design of this study restricts our ability to establish causal relationships. Although the study explored the connection between cerebral perfusion, hydration, and hearing loss, further longitudinal studies are needed to better understand the timeline and lasting effects of sudden sensorineural hearing loss (SSNHL) on brain functions and hydration levels. Ultimately, although this study reveals connections between dehydration and alterations in cerebral perfusion, further research is needed to investigate how dehydration affects neurotransmitter activity and cognitive processing in SSNHL patients. This will enhance our understanding of the specific mechanisms by which dehydration influences both auditory and mental functions. Our study does not prescribe a specific hydration protocol; however, ensuring sufficient water intake, screening SSNHL patients for dehydration, and promoting fluid consumption may serve as effective components of a hydration strategy.
6 Conclusion
This study provides new insights into cerebral perfusion changes in auditory and non-auditory brain regions of patients diagnosed with SSNHL. Reduced blood flow was observed in critical areas associated with auditory, cognitive, and emotional processing, as well as sensory compensation mechanisms, following hearing loss. These findings highlight the crucial role of adequate hydration in enhancing brain perfusion, which may facilitate recovery in patients with SSNHL. The study emphasizes the need for a deeper understanding of hydration's impact and potential benefits in the therapeutic management of SSNHL, extending beyond merely addressing symptoms. Further research is needed to investigate hydration strategies as part of a comprehensive treatment approach for SSNHL.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Renmin Hospital of Wuhan University (Approval number: WDRY2024-K275). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PS: Data curation, Formal analysis, Investigation, Writing – original draft. ZW: Investigation, Methodology, Project administration, Writing – review & editing. AZ: Data curation, Methodology, Resources, Writing – review & editing. XY: Resources, Validation, Visualization, Writing – review & editing. HL: Resources, Validation, Visualization, Writing – review & editing. MR: Data curation, Formal analysis, Writing – review & editing. CY: Formal analysis, Investigation, Writing – review & editing. JS: Formal analysis, Writing – review & editing. RY: Software, Writing – review & editing. JC: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We appreciate all the participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1647804/full#supplementary-material
Abbreviations
AAL3v1, Automated Anatomical Labeling atlas, version 3, release 1; BV, Blood viscosity; CBF, Cerebral blood flow; HC, Healthy controls; HCT, Hemocrit; HG, Heschl's gyrus; ITG, Inferior temporal gyrus; MTG, Middle temporal gyrus; PAC, Primary auditory cortex; SFG, Superior temporal gyrus; SSNHL, Sudden sensorineural hearing loss; STG, Superior temporal gyrus; TP STG, Temporal pole superior temporal gyrus; Usg, Urine specific gravity.
References
1.
Chandrasekhar SS Tsai Do BS Schwartz SR Bontempo LJ Faucett EA Finestone SA et al . Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. (2019) 161:S1–S45. 10.1177/0194599819859885
2.
Ni W Song SP Jiang YD . Association between routine hematological parameters and sudden sensorineural hearing loss: a meta-analysis. J Otol. (2021) 16:47–54. 10.1016/j.joto.2020.07.006
3.
Giustino V Lorusso F Rizzo S Salvago P Martines F . Sudden sensorineural hearing loss. In: Sensorineural Hearing Loss: Pathophysiology, Diagnosis and Treatment. New York, NY: Nova Science Publishers, Inc. (2019). p. 207–16.
4.
Canapicchi R De Marchi D Lombardo F Fortunato S De Cori S Montanaro D et al . Sudden sensorineural hearing loss: MR imaging. Sage J. (2010) 23:161–71 10.1177/197140091002300203
5.
Lazarini PR Camargo ACK . Idiopathic sudden sensorineural hearing loss: etiopathogenic aspects. Braz J Otorhinolaryngol. (2006) 72:554–61. 10.1016/S1808-8694(15)31004-1
6.
Tripathi P Deshmukh P . Sudden sensorineural hearing loss: a review. Cureus. (2022) 14:e29458. 10.7759/cureus.29458
7.
Kanzaki S Sakagami M Hosoi H Murakami S Ogawa K . High fibrinogen in peripheral blood correlates with poorer hearing recovery in idiopathic sudden sensorineural hearing loss. PLoS ONE. (2014) 9:104680. 10.1371/journal.pone.0104680
8.
Grotta J Ackerman R Correia J Fallick G Chang J . Whole blood viscosity parameters and cerebral blood flow. Stroke. (1982) 13:296–301. 10.1161/01.str.13.3.296
9.
Ohinata Y Makimoto K Kawakami M Haginomori SI Araki M Takahashi H . Blood viscosity and plasma viscosity in patients with sudden deafness. Acta Otolaryngol. (1994) 114:601–7. 10.3109/00016489409126112
10.
Holsworth RE Cho YI Weidman J . Effect of hydration on whole blood viscosity in firefighters. Altern Ther Health Med. (2013) 19:44–9.
11.
Trangmar SJ González-Alonso J . New insights into the impact of dehydration on blood flow and metabolism during exercise. Exerc Sport Sci Rev. (2017) 45:146–53. 10.1249/JES.0000000000000109
12.
Abe Y Okada M Tanaka K Toyama K Miyamoto Y Hato N . The association between dehydration and the prognosis of sudden sensorineural hearing loss. Otol Neurotol Open. (2023) 3:e041. 10.1097/ONO.0000000000000041
13.
Secher M Ritz P . Hydration and cognitive performance. J Nutr Health Aging. (2012) 16:325–9. 10.1007/s12603-012-0033-0
14.
Xu XM Jiao Y Tang TY Zhang J Salvi R Teng GJ . Inefficient involvement of insula in sensorineural hearing loss. Front Neurosci. (2019) 13:133. 10.3389/fnins.2019.00133
15.
Clement P Petr J Dijsselhof MBJ Padrela B Pasternak M Dolui S et al . A beginner's guide to arterial spin labeling (ASL) image processing. Front Radiol. (2022) 2:929533. 10.3389/fradi.2022.929533
16.
Zou Y Ma H Liu B Li D Liu D Wang X et al . Disrupted topological organization in white matter networks in unilateral sudden sensorineural hearing loss. Front Neurosci. (2021) 15:666651. 10.3389/fnins.2021.666651
17.
Luan Y Wang C Jiao Y Tang T Zhang J Lu C et al . Abnormal functional connectivity and degree centrality in anterior cingulate cortex in patients with long-term sensorineural hearing loss. Brain Imaging Behav. (2020) 14:682–95. 10.1007/s11682-018-0004-0
18.
Wang Z . Arterial spin labeling perfusion MRI signal processing through traditional methods and machine learning. Investig Magn Reson Imaging. (2022) 26:220–8. 10.13104/imri.2022.26.4.220
19.
Aiello CP de Lima II Ferrari DV . Validity and reliability of the hearing handicap inventory for adults. Braz J Otorhinolaryngol. (2011) 77:432–8. 10.1590/S1808-86942011000400005
20.
Dong Y Chen S Yu Y Li W Xu Z Du J et al . Association between urine specific gravity as a measure of hydration status and risk of type 2 diabetes: the kailuan prospective cohort study. Nutrients. (2024) 16:1643. 10.3390/nu16111643
21.
Simerville JA Maxted WC Pahira JJ . Urinalysis: a comprehensive review. Am Fam Phys. (2005) 71:1153–62. Erratum in: Am Fam Phys. (2006) 74:1096. Available online at: https://www.aafp.org/pubs/afp/issues/2005/0315/p1153.html (Accessed August 29, 2025).
22.
Valério de Arruda M Cruz Silva A Fernandes Galduróz JC Ferreira Galduróz R . Standardization for obtaining blood viscosity: a systematic review. Eur J Haematol. (2021) 106:597–605. 10.1111/ejh.13594
23.
Tanaka K Ishida F Kawamura K Yamamoto H Horikawa D Kishimoto T et al . Hemodynamic assessment of cerebral aneurysms using computational fluid dynamics (CFD) involving the establishment of non-newtonian fluid properties. J Neurointerv Surg. (2018) 12:376–85. 10.5797/jnet.oa.2017-0088
24.
Waheed W Khan F Naud S Kasarskis E Matthews D Tandan R . Urine specific gravity to identify and predict hydration need in ALS. Amyotroph Lateral Scler Frontotemporal Degener. (2022) 23:407–14. 10.1080/21678421.2021.2013894
25.
Rolls ET Huang CC Lin CP Feng J Joliot M . Automated anatomical labelling atlas 3. Neuroimage. (2020) 206:116189. 10.1016/j.neuroimage.2019.116189
26.
Bueti D van Dongen EV Walsh V . The role of superior temporal cortex in auditory timing. PLoS ONE. (2008) 3:2481. 10.1371/journal.pone.0002481
27.
Zhang J Ma G Du S Zhang N . The relationships between water intake and hydration biomarkers and the applications for assessing adequate total water intake among young adults in Hebei, China. Nutrients. (2021) 13:3805. 10.3390/nu13113805
28.
Nakae S Kumon M Kojima D Higashiguchi S Ohba S Kuriyama N et al . Transsylvian and trans-heschl's gyrus approach for a left posterior insular lesion and functional analyses of the left heschl's gyrus: illustrative case. J Neurosurg Case Lessons. (2022) 3:CASE21622. 10.3171/CASE21622
29.
Wang X Xu P Li P Wang Z Zhao F Gao Z et al . Alterations in gray matter volume due to unilateral hearing loss. Sci Rep. (2016) 6:25811. 10.1038/srep25811
30.
Petrides M . On the evolution of polysensory superior temporal sulcus and middle temporal gyrus: a key component of the semantic system in the human brain. J Comp Neurol. (2023) 531:1987–95. 10.1002/cne.25521
31.
Wood KC Town SM Bizley JK . Neurons in primary auditory cortex represent sound source location in a cue-invariant manner. Nat Commun. (2019) 10:3019. 10.1038/s41467-019-10868-9
32.
Li J Zou Y Kong X Leng Y Yang F Zhou G et al . Exploring functional connectivity alterations in sudden sensorineural hearing loss: a multilevel analysis. Brain Res. (2024) 1824:148677. 10.1016/j.brainres.2023.148677
33.
Ponticorvo S Manara R Pfeuffer J Cappiello A Cuoco S Pellecchia MT et al . Cortical pattern of reduced perfusion in hearing loss revealed by ASL-MRI. Hum Brain Mapp. (2019) 40:2475–87. 10.1002/hbm.24538
34.
Armstrong NM An Y Doshi J Erus G Ferrucci L Davatzikos C et al . Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngol Head Neck Surg. (2019) 145:794–802. 10.1001/jamaoto.2019.1610
35.
Husain FT Medina RE Davis CW Szymko-Bennett Y Simonyan K Pajor NM et al . Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. (2011) 1369:74–88. 10.1016/j.brainres.2010.10.095
36.
Onitsuka T Shenton ME Salisbury DF Dickey CC Kasai K Toner SK et al . Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. (2004) 161:1603–11. 10.1176/appi.ajp.161.9.1603
37.
Yang M Chen HJ Liu B Huang ZC Feng Y Li J et al . Brain structural and functional alterations in patients with unilateral hearing loss. Hear Res. (2014) 316:37–43. 10.1016/j.heares.2014.07.006
38.
Li J Wen H Wang S Che Y Zhang N Guo L . Altered brain morphometry in cerebral small vessel disease with cerebral microbleeds: an investigation combining univariate and multivariate pattern analyses. Front Neurol. (2022) 13:819055. 10.3389/fneur.2022.819055
39.
Chen YC Feng Y Xu JJ Mao CN Xia W Ren J et al . Disrupted brain functional network architecture in chronic tinnitus patients. Front Aging Neurosci. (2016) 8:174. 10.3389/fnagi.2016.00174
40.
Li Z Zhu Q Geng Z Song Z Wang L Wang Y . Study of functional connectivity in patients with sensorineural hearing loss by using resting-state fMRI. Int J Clin Exp Med. (2015) 8:569–78.
41.
Xu ZG Xu JJ Chen YC Hu J Wu Y Xue Y . Aberrant cerebral blood flow in tinnitus patients with migraine: a perfusion functional MRI study. J Headache Pain. (2021) 22:61. 10.1186/s10194-021-01280-0
42.
Herlin B Navarro V Dupont S . The temporal pole: from anatomy to function—a literature appraisal. J Chem Neuroanat. (2021) 113:101925. 10.1016/j.jchemneu.2021.101925
43.
Hildesheimer M Bloch F Chava Muchnik M Rubinstein M . Blood viscosity and sensorineural hearing loss a chronic deficit in the labyrinthine. (1990) 116:820–3. 10.1001/archotol.1990.01870070068012
44.
Popowski LA Oppliger RA Lambert GP Johnson RF Johnson AK Gisolfi CV . Blood and urinary measures of hydration status during progressive acute dehydration. Med Sci Sports Exerc. (2001) 33:747–53. 10.1097/00005768-200105000-00011
45.
Masento NA Golightly M Field DT Butler LT Van Reekum CM . Effects of hydration status on cognitive performance and mood. Br J Nutr. (2014) 111:1841–52. 10.1017/S0007114513004455
Summary
Keywords
arterial spin labeling, blood viscosity, cerebral blood flow, dehydration, sudden sensorineural hearing loss
Citation
Shrestha P, Wen Z, Zheng A, Yang X, Liao H, Ramjaun MM, You C, Shrestha J, Yang R and Chen J (2025) Dehydration-associated cerebral hypoperfusion in sudden sensorineural hearing loss: an arterial spin labeling-based preliminary study. Front. Neurol. 16:1647804. doi: 10.3389/fneur.2025.1647804
Received
16 June 2025
Accepted
18 August 2025
Published
10 September 2025
Volume
16 - 2025
Edited by
Allison B. Reiss, New York University, United States
Reviewed by
Qinyang Shou, University of Southern California, United States
Yasunori Abe, Kurume University School of Medicine, Japan
Updates
Copyright
© 2025 Shrestha, Wen, Zheng, Yang, Liao, Ramjaun, You, Shrestha, Yang and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Chen whuchenjun@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.