- 1Department of Neurology, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, Zhejiang, China
- 2Department of Endocrinology, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, Zhejiang, China
Stroke-associated pneumonia (SAP) is a frequent complication of acute ischemic stroke (AIS) that contributes to poor clinical outcomes. The systemic immune-inflammation index (SII), derived from neutrophil, lymphocyte, and platelet counts, may reflect post-stroke immune imbalance, but its role in predicting SAP remains unclear. In this retrospective study, we analyzed 1,767 AIS patients and evaluated the association between log₂-transformed SII and the occurrence of SAP using multivariable logistic regression, generalized additive models, and two-piecewise regression. SAP developed in 21.3% of patients during hospitalization. Higher SII levels were independently associated with increased SAP risk after adjustment for age, sex, vascular risk factors, comorbidities, baseline National Institutes of Health Stroke Scale (NIHSS) score, and dysphagia assessed by Kubota Water Drinking Test (KWDT). Patients in the highest SII quartile had a significantly greater likelihood of developing SAP compared to those in the lowest quartile (adjusted odds ratio = 2.03, 95% confidence interval: 1.21–3.38, p = 0.0069). A non-linear, threshold-dependent relationship was identified, with SAP risk increasing substantially beyond log₂-SII ≈ 8.5. Receiver operating characteristic (ROC) analysis demonstrated moderate predictive performance of SII for SAP (area under the curve (AUC) = 0.726), while C-reactive protein (CRP) showed superior discrimination (AUC = 0.826 p < 0.0001). Supplementary sensitivity analyses, including a fully adjusted model without NIHSS and KWDT and an alternative model replacing these with the A2DS2 score (Age, Atrial fibrillation, Dysphagia, Sex, Stroke Severity), showed consistent results, supporting the robustness of our findings. These findings suggest that SII may serve as a cost-effective and accessible biomarker to aid early identification of high-risk AIS patients.
Introduction
Stroke remains one of the leading causes of mortality and long-term disability worldwide, with acute ischemic stroke (AIS) accounting for approximately 80% of all cases (1). Despite advances in acute stroke management, complications during hospitalization, particularly stroke-associated pneumonia (SAP), continue to pose significant challenges (2). SAP occurs in 10 to 30% of AIS patients and is closely associated with prolonged hospital stays, increased healthcare costs, and worse functional outcomes, including higher mortality rates (3). Early identification of high-risk patients is essential to guide preventative interventions and improve prognosis (4).
Emerging evidence suggests that systemic inflammation plays a pivotal role in the development of SAP by disrupting immune homeostasis and enhancing susceptibility to pulmonary infections following AIS (5). Conventional inflammatory biomarkers such as CRP, white blood cell (WBC) count, and neutrophil-to-lymphocyte ratio (NLR) have been widely used to assess systemic inflammation; however, their predictive accuracy for SAP remains suboptimal (6). The systemic immune-inflammation index (SII) calculated from platelet count, neutrophil count, and lymphocyte count, is a novel composite marker that reflects the balance between pro-inflammatory and immune-regulatory responses (7). Recent studies have demonstrated its prognostic value in various cardiovascular and oncologic conditions, but its predictive utility in SAP remains underexplored (8). In this study, we focused on SII as a comprehensive marker of immune-inflammatory balance, and compared it with CRP, a widely used reference biomarker in stroke research, to assess whether SII provides additional or complementary prognostic value beyond CRP. Moreover, existing studies evaluating inflammatory markers in SAP have primarily focused on linear relationships, potentially overlooking complex non-linear and threshold effects (9). For example, Kuang et al. (10) examined the association between SII and SAP risk in a smaller, mixed cohort of acute stroke patients and reported a linear relationship, without investigating potential non-linear patterns or thresholds. Whether elevated SII levels exhibit a dose–response relationship or specific thresholds beyond which SAP risk dramatically increases has not been fully elucidated. Addressing these knowledge gaps is critical for refining clinical risk stratification and informing targeted preventative strategies (11).
We posited that early elevation of the SII, as a marker of post-stroke immune disequilibrium, would identify AIS patients at independently higher risk of SAP, and that the exposure–response might be non-linear with a clinically relevant threshold. To test this hypothesis, we investigated the association between SII and the development of SAP in patients with AIS by analyzing a large, retrospective cohort. Specifically, we examined the predictive value of log₂-transformed SII, explored potential non-linear and threshold effects through advanced modeling approaches, and compared the diagnostic performance of SII with established inflammatory biomarkers such as CRP.
Materials and methods
Study design and participants
This study utilized data from a previously established retrospective cohort investigating the prognosis of ischemic stroke. Patient data collected at The Quzhou Affiliated Hospital of Wenzhou Medical University (Quzhou People’s Hospital), Zhejiang, China, between September 2016 and September 2022 that met the inclusion criteria were included. All eligible patients were enrolled in a single-center retrospective cohort study and were subsequently classified into two groups based on the development of SAP during hospitalization: the SAP group and the non-SAP group. No case–control matching was performed, and group differences were addressed using multivariable models. This study was approved by the Ethics Committee of Quzhou People’s Hospital (Approval Number: 2023-151), which granted a waiver of informed consent due to the retrospective nature of the study and the use of de-identified data. The data were accessed for analysis on April 26, 2025. Prior to analysis, all data were anonymized, and researchers did not have access to any personally identifiable information.
Inclusion criteria were as follows: Patients were included if they met all of the following criteria: (1) Confirmed diagnosis of AIS within 24 h of symptom onset, with admission during the same time window; (2) Brain magnetic resonance imaging (MRI) performed within 48 h of symptom onset using standard sequences (including diffusion-weighted imaging, fluid-attenuated inversion recovery, susceptibility-weighted imaging, T1-weighted, T2-weighted, and magnetic resonance angiography), with radiological confirmation of acute infarction; (3) Blood samples for neutrophil-to-lymphocyte ratio (NLR) and platelet count were obtained within 48 h of symptom onset to assess the acute immune-inflammatory status; (4) Complete clinical and laboratory data available for calculation of the SII.
Exclusion criteria included: (1) Severe systemic comorbidities, including hepatic dysfunction (alanine aminotransferase >10 × or aspartate aminotransferase >3 × the upper limit of normal), renal impairment (serum creatinine >443 μmol/L), active malignancies, or hematologic disorders; (2) Cardiopulmonary insufficiency, including New York Heart Association class III–IV heart failure, left ventricular ejection fraction <40%, chronic obstructive pulmonary disease, or respiratory tract infection at admission; (3) Active infections at admission, including respiratory (e.g., community-acquired pneumonia [CAP]), urinary, or systemic infections; (4) Pregnancy or lactation; (5) Inability to reliably assess stroke severity due to coma, severe aphasia, or other neurologic deficits precluding use of the National Institutes of Health Stroke Scale (NIHSS); (6) Multiple AIS hospitalizations during the study period (only the first admission was included); (7) Missing laboratory data required for calculation of SII (i.e., Platelet count, Neutrophil count or Lymphocyte count); (8) Patients diagnosed with autoimmune diseases.
The detailed patient selection process is illustrated in Figure 1.
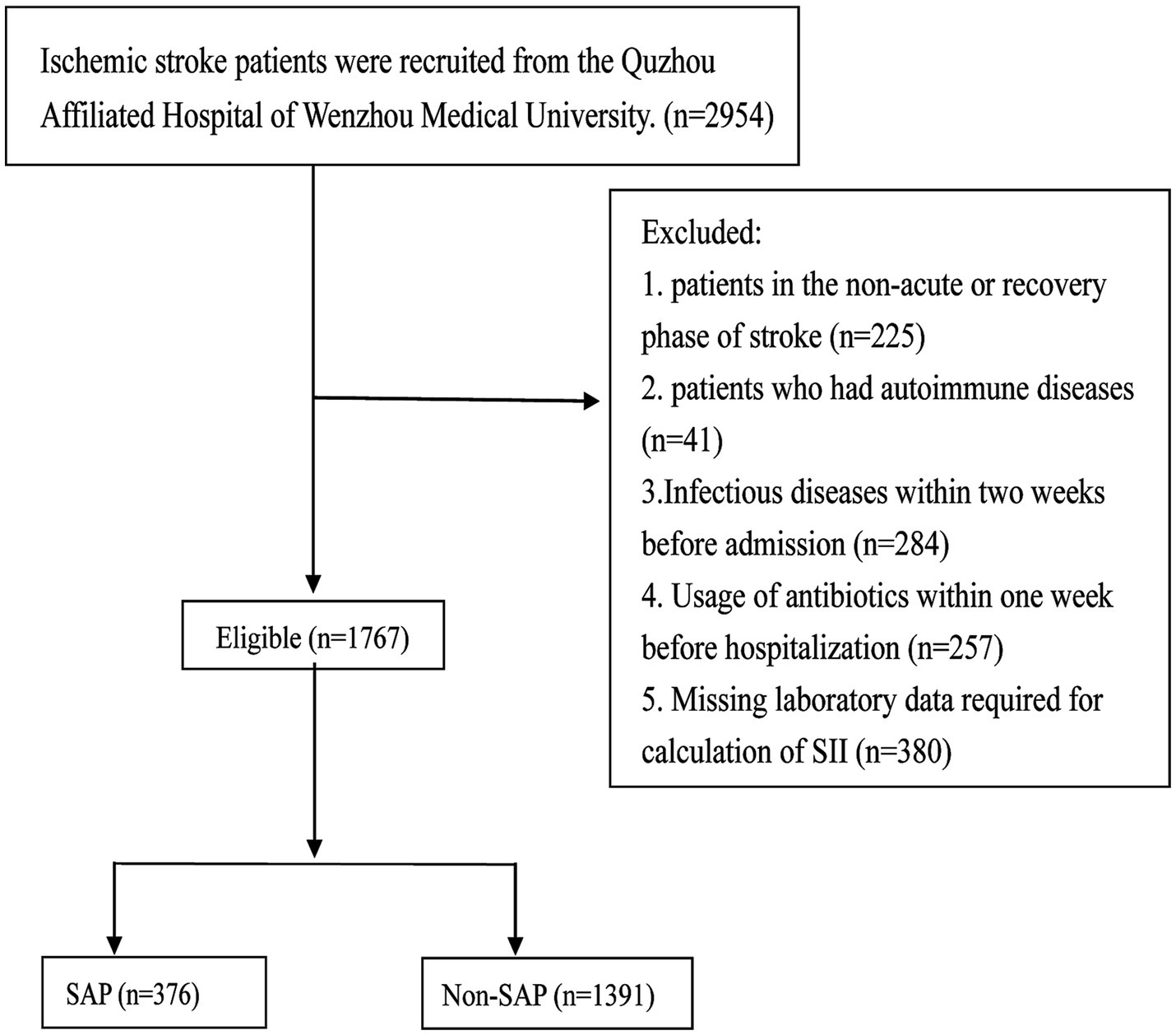
Figure 1. Patient selection flowchart for the study of the systemic immune-inflammation index (SII) and stroke-associated pneumonia (SAP). A total of 2,954 ischemic stroke patients were screened at the Quzhou Affiliated Hospital of Wenzhou Medical University. Patients were excluded if they were in the non-acute or recovery phase of stroke (n = 225), had autoimmune diseases (n = 41), had infectious diseases within 2 weeks prior to admission (n = 284), had received antibiotics within 1 week before hospitalization (n = 257), or had missing laboratory data required for SII calculation (n = 380). Ultimately, 1,767 eligible patients were included and stratified into the SAP group (n = 376) and the non-SAP group (n = 1,391). SII, systemic immune-inflammation index; SAP, stroke-associated pneumonia.
Baseline data collection
Baseline data were retrieved from medical records and included demographic and clinical characteristics at admission, such as age, sex, smoking status, hypertension, type 2 diabetes, AF and chronic obstructive pulmonary disease (COPD). Stroke severity was assessed using the NIHSS, swallowing difficulty was evaluated using the Kubota Water Drinking Test (KWDT), and consciousness disturbances were assessed using the Glasgow Coma Scale (GCS).
Blood samples were collected by trained nurses on the second morning after admission (6:00 AM) using vacuum tubes, stored at 4 °C, and processed within 2 h by certified laboratory technicians. Laboratory tests included: White blood cell count (WBC), Neutrophil-to-lymphocyte ratio (NLR), Platelet count, Aspartate transaminase (AST), Alanine transaminase (ALT), Glycated hemoglobin (HbA1c), Homocysteine (HCY), Serum creatinine (Scr), Albumin (ALB), Triglycerides (TG), Total cholesterol (TC), High-density lipoprotein (HDL-c), Low-density lipoprotein (LDL-c), The neutrophil-to-lymphocyte ratio (NLR) was calculated as neutrophil count divided by lymphocyte count. All laboratory results were reported using standard international units: WBC and PLT in ×109/L, CRP in mg/L, and NLR as a ratio. Reference ranges (e.g., WBC: 4.0–10.0 × 109/L, PLT: 150–400 × 109/L, CRP: <5 mg/L) were provided for clinical interpretation.
To ensure consistency in exposure measurement, only patients who underwent blood testing within 48 h of symptom onset were included. All NIHSS evaluations were performed by neurologists trained in standardized stroke assessment and blinded to laboratory data. Inter-rater reliability was maintained by duplicate scoring in a subset of cases.
Definitions
The diagnosis of SAP was determined independently by two attending neurologists following the Pneumonia in Stroke Consensus Group recommendations (12). If necessary, an attending respiratory physician was consulted for confirmation. To ensure incident SAP, patients with pneumonia present at admission (i.e., community-acquired pneumonia, CAP) were excluded, and only pneumonia developing after admission within the acute phase following stroke was classified as SAP.
The diagnostic criteria required at least one of the following:
Fever (>38 °C) without an alternative cause;
Abnormal WBC count (leukopenia <4 × 109/L or leukocytosis >12 × 109/L);
Altered mental status in patients ≥70 years without other causes.
Plus at least two of:
Purulent sputum or a change in sputum character;
Increased respiratory secretions or suction needs;
New or worsening cough, dyspnea, or tachypnea (>25 breaths/min);
Auscultatory findings of rales, crackles, or bronchial breath sounds;
Oxygen desaturation (PaO₂/FiO₂ ≤ 240) or increased oxygen requirement;
Radiologic confirmation was required with two consecutive chest X-rays showing new or progressive infiltrates, consolidation, or cavitation. In patients without prior pulmonary or cardiac disease, a single conclusive chest radiograph was deemed sufficient. Given the study’s focus on ischemic stroke-associated pneumonia, chest CT imaging was utilized instead of chest X-rays, providing superior diagnostic clarity. These criteria were selected for their relevance to the study population and their validated effectiveness in pneumonia diagnosis among stroke patients.
The SII was calculated as:
𝑆𝐼𝐼=Platelet count×Neutrophil count/Lymphocyte count.
The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula to assess renal function.
Statistical analysis
All statistical analyses were performed using R Studio (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) and EmpowerStats (version 2.0; https://www.empowerstats.net). EmpowerStats served as a user-friendly interface that automatically generates and executes standard R code. To ensure reproducibility, key analyses were also re-run directly in R, and the results were identical. The normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Normally distributed variables were expressed as mean ± standard deviation (SD) and compared with Student’s t-test, while non-normally distributed variables were presented as median (IQR) and compared using the Mann–Whitney U test. Categorical variables were analyzed using the Chi-square or Fisher’s exact test, as appropriate.
To examine the association between SII and SAP, SII values were log₂-transformed to reduce skewness and enhance interpretability. In addition to treating log₂-transformed SII as a continuous variable, we categorized it into quartiles based on its distribution to reduce the influence of extreme values, detect non-linear or threshold effects without prespecifying a cut-off, and facilitate risk comparison between groups in line with prior literature. We analyzed log₂-SII both as a continuous variable and under non-linear frameworks: the continuous approach enabled comparability with prior studies and provided an overall effect estimate, whereas non-linear analyses (GAM and two-piecewise logistic regression) were used to identify and characterize potential threshold-dependent relationships.
Multicollinearity among covariates was assessed using variance inflation factors (VIF). Logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Three models were constructed:
Model 1: Unadjusted;
Model 2: Adjusted for age and sex;
Model 3: Further adjusted for smoking status, hypertension, diabetes mellitus, atrial fibrillation (AF), chronic obstructive pulmonary disease (COPD), systolic and diastolic blood pressure (SBP, DBP), uric acid (UA), white blood cell count (WBC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), glycated hemoglobin (HbA1c), estimated glomerular filtration rate (eGFR), baseline NIHSS score (continuous), and KWDT results.
In supplementary sensitivity analyses, we examined two alternative adjustment strategies: (1) a fully adjusted model excluding NIHSS and KWDT; and (2) a model in which age, sex, AF, NIHSS, and KWDT were replaced by the A2DS2 score, to assess the robustness of the results.
Generalized additive models (GAMs) with penalized splines were used to model the relationship between log₂-SII and SAP, allowing for flexible assessment of potential non-linear effects. To identify a threshold, we fitted two-segment logistic regression models across all possible cut points within the observed log₂-SII range. The cut point with the highest log-likelihood was selected as the threshold, and its 95% confidence interval was estimated using 1,000 bootstrap resamples. The predictive performance of SII alone and CRP alone was evaluated using univariate ROC curve analysis, with comparisons of area under the curve (AUC) performed via the DeLong test. To assess the incremental value of SII beyond established predictors, we constructed multivariable logistic regression models including both SII and CRP, and both SII and the A2DS2 score (treated as a continuous variable), and compared their AUCs with those of CRP alone and the A2DS2 score alone, respectively, using the DeLong test. A two-sided p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics by SAP status
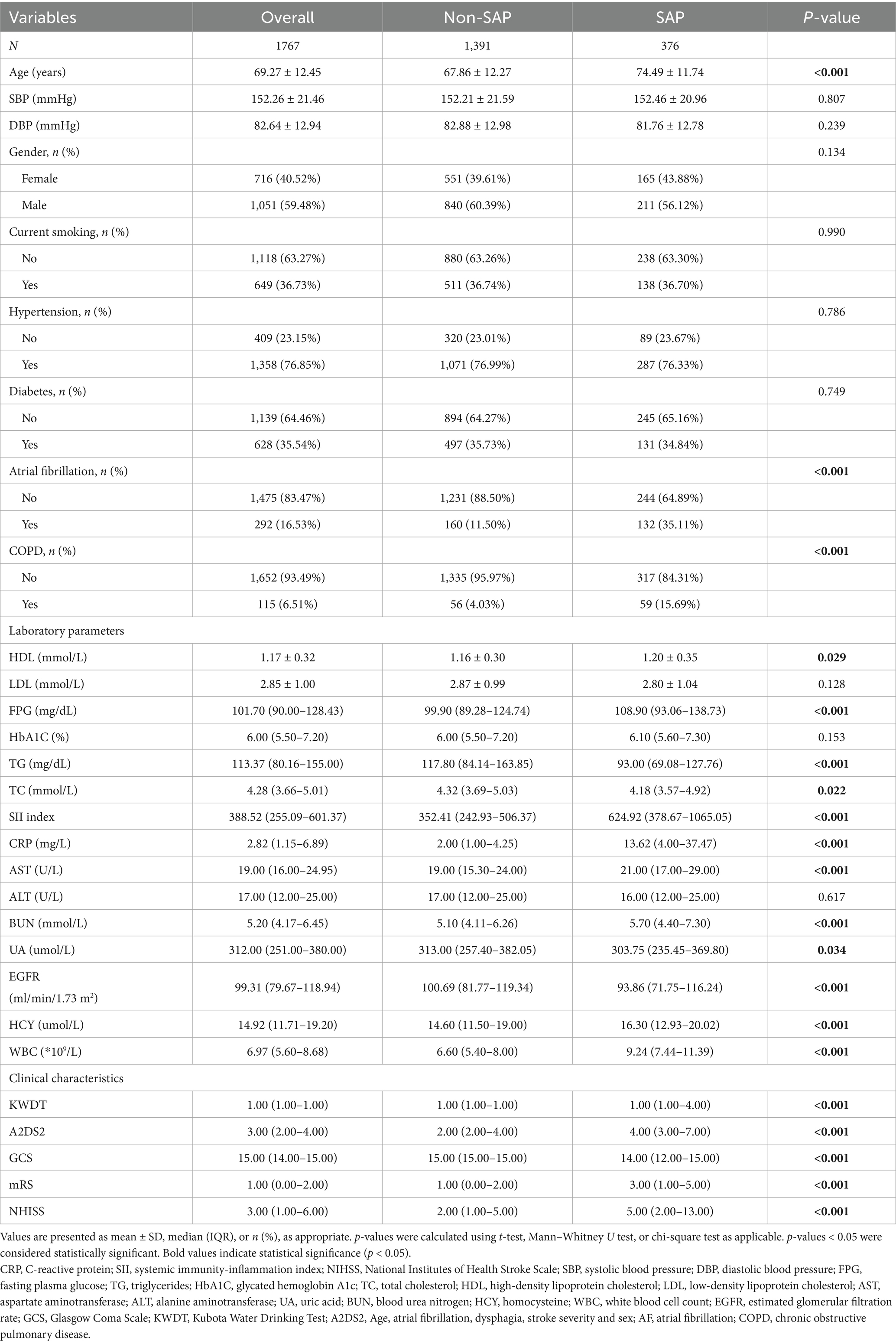
Among the 1,767 AIS patients, 376 (21.3%) developed SAP (Table 1). Compared with the non-SAP group, SAP patients were older (74.49 ± 11.74 vs. 67.86 ± 12.27 years, p < 0.001), and more frequently had atrial fibrillation (35.11% vs. 11.50%) and COPD (15.69% vs. 4.03%). No significant differences were observed in sex distribution or smoking status.
Laboratory findings showed elevated inflammatory markers in the SAP group, including SII (624.92 [378.67–1065.05] vs. 352.41 [242.93–506.37]), CRP, and WBC. Additionally, fasting glucose, BUN, AST, and homocysteine levels were higher, while triglycerides and eGFR were lower (all p < 0.05).
SAP patients exhibited more severe neurological impairment, as indicated by higher NIHSS and mRS scores and lower GCS scores (all p < 0.001). In addition, they also had higher A2DS2 scores, consistent with their increased risk of poststroke pneumonia (all p < 0.001).
Baseline characteristics by log₂-SII quartiles
Patient characteristics across log₂-SII quartiles are shown in Table 2. Age and diastolic blood pressure did not significantly differ among the groups. The incidence of SAP increased markedly across quartiles, from 11.1% in Q1–Q2 to 17.2% in Q3 and 45.7% in Q4 (p < 0.001). Higher SII quartiles were associated with increasing systolic blood pressure (p = 0.002) and a higher proportion of female patients (p = 0.023).
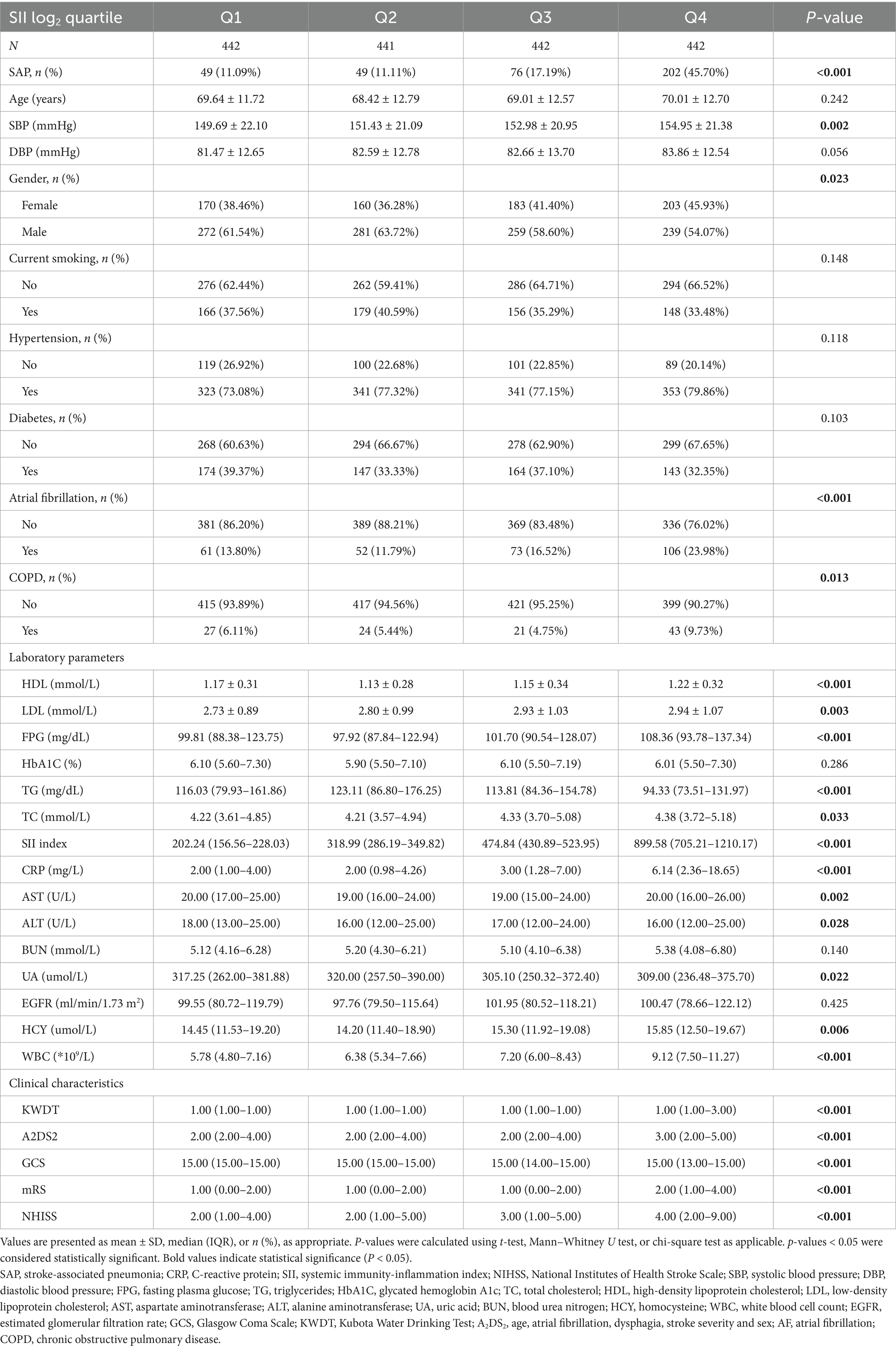
Table 2. Baseline characteristics of patients stratified by quartiles of log2-transformed systemic immunity-inflammation index (SII).
The prevalence of atrial fibrillation increased from 13.80% in Q1 to 23.98% in Q4 (p < 0.001), and COPD was more frequent in higher quartiles (p = 0.013). Laboratory findings showed progressively higher fasting glucose, LDL, CRP, and WBC counts and lower triglycerides across quartiles (all p < 0.001).
Neurological scores, including NIHSS, A2DS2, and mRS, increased with higher SII quartiles, while GCS scores decreased (all p < 0.001). Dysphagia was more common in the higher quartiles (p < 0.001).
Association between log₂-SII and SAP
Multivariable logistic regression analysis results are shown in Table 3. Compared to Q1, the unadjusted odds ratios (ORs) for SAP were 1.67 (95% CI: 1.13–2.45, p = 0.0091) for Q3 and 6.75 (95% CI: 4.75–9.59, p < 0.0001) for Q4. After adjustment for age and sex, the associations remained significant (Q3: OR = 1.74, 95% CI: 1.17–2.58, p = 0.0059; Q4: OR = 7.48, 95% CI: 5.20–10.75, p < 0.0001). In the fully adjusted model, only Q4 remained significantly associated with SAP (OR = 2.03, 95% CI: 1.21–3.38, p = 0.0069), while Q2 and Q3 were not statistically significant (both p > 0.05).
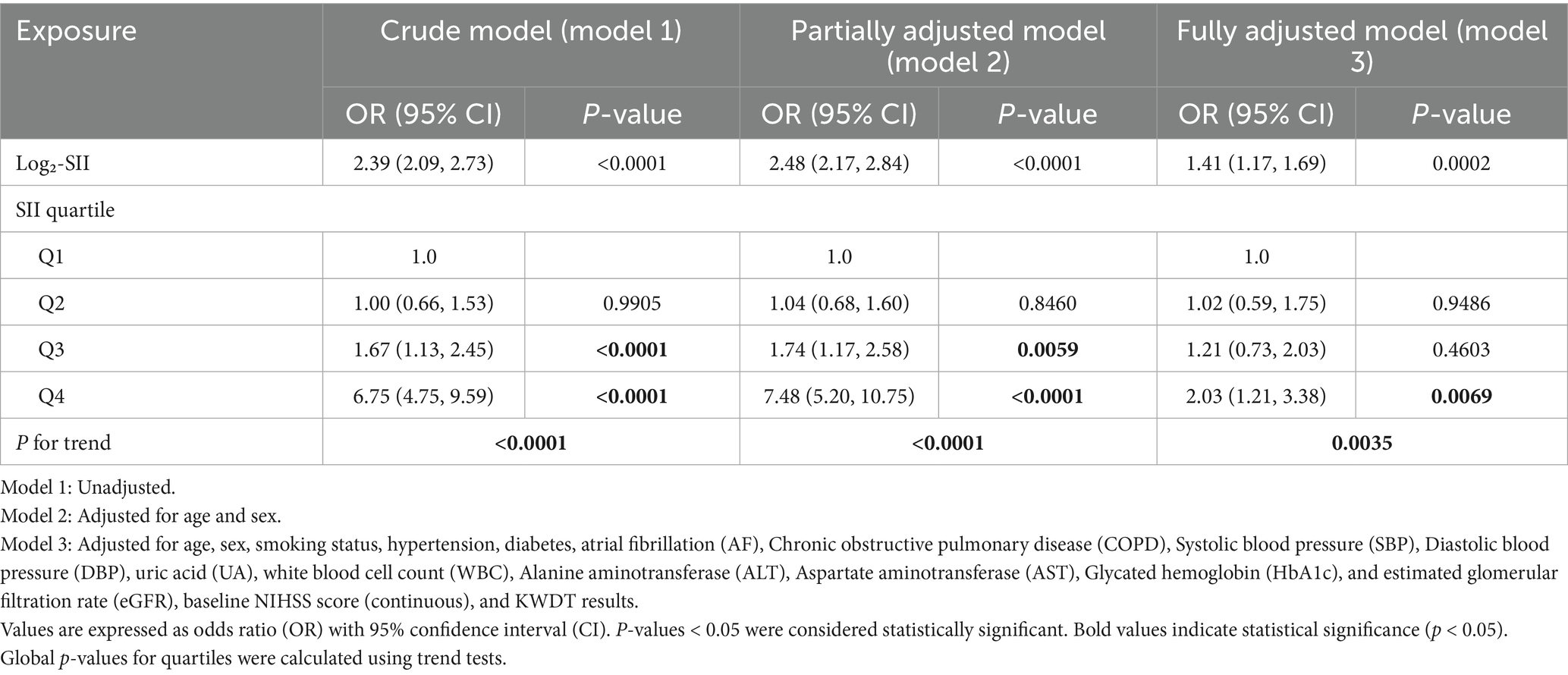
Table 3. Multivariable logistic regression analysis of the association between log2-transformed SII quartiles and stroke-associated pneumonia (SAP).
When modeled as a continuous variable, log₂-SII was significantly associated with SAP across all models. The unadjusted OR was 2.39 (95% CI: 2.09–2.73, p < 0.0001); adjusted ORs were 2.48 (95% CI: 2.17–2.84, p < 0.0001) in Model 2 and 1.41 (95% CI: 1.17–1.69, p = 0.0002) in Model 3. All variance inflation factors (VIFs) were below 3, indicating no evidence of multicollinearity among the covariates (Supplementary Table 2).
In sensitivity analyses, the association between log₂-SII and SAP remained consistent when using a fully adjusted model without NIHSS and KWDT (Supplementary Table 1A) and when replacing age, sex, AF, NIHSS, and KWDT with the A2DS2 score (Supplementary Table 1B). The effect estimates were comparable to those observed in the main model, supporting the robustness of our findings.
Subgroup analyses
To assess the consistency of the association between elevated SII and SAP, subgroup analyses were conducted across key clinical variables (Figure 2). The association remained significant in both age groups (<70 years: OR = 2.26, 95% CI: 1.81–2.80; ≥70 years: OR = 2.50, 95% CI: 2.11–2.97) and in both sexes (female: OR = 2.24, 95% CI: 1.84–2.73; male: OR = 2.50, 95% CI: 2.09–2.98). Similar results were observed in patients with and without smoking history, hypertension, diabetes, atrial fibrillation, and COPD. All p values for interaction were greater than 0.05, indicating that the effect of SII on SAP risk was consistent across clinical subgroups.
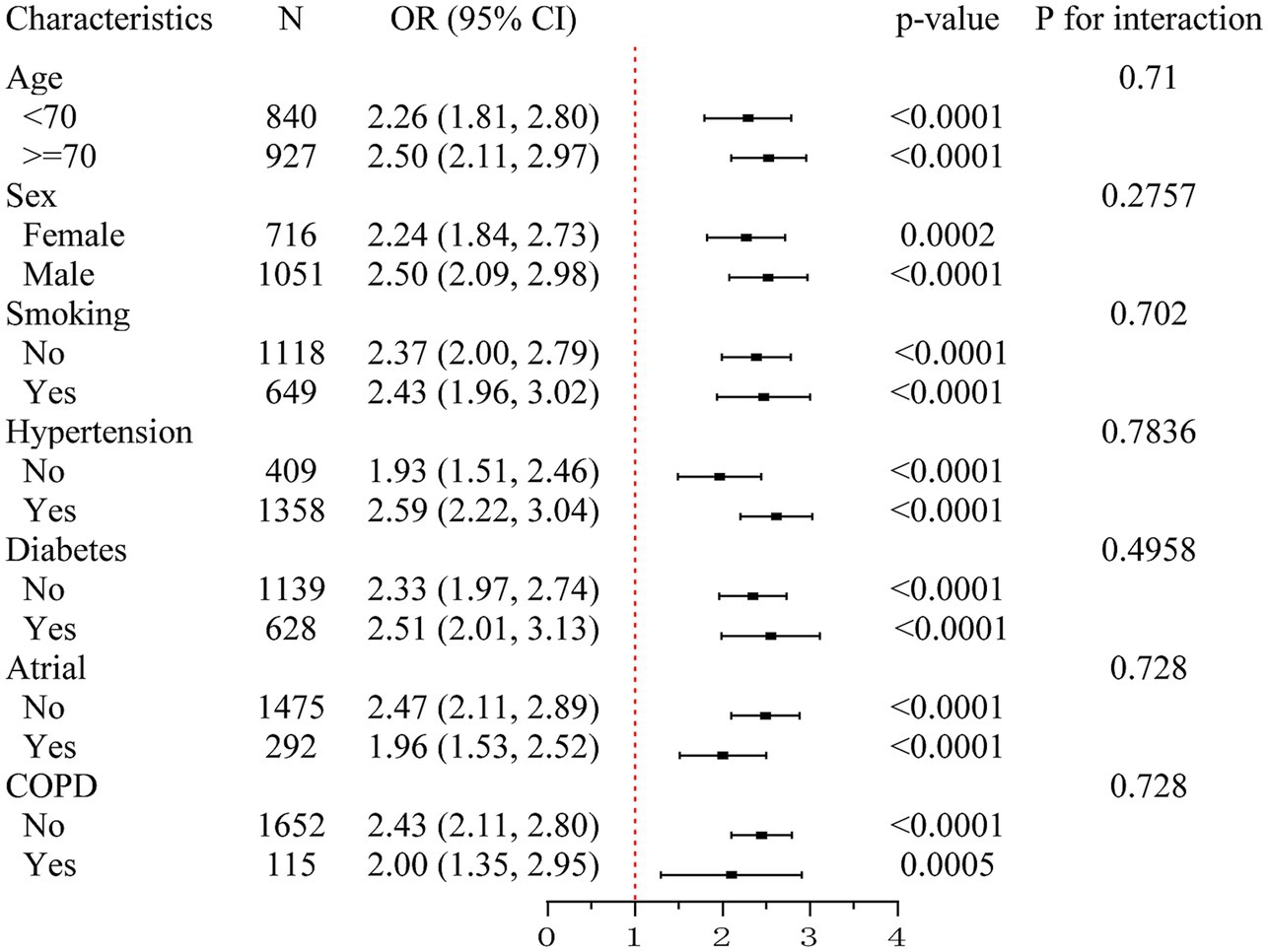
Figure 2. Subgroup analysis of the association between systemic immune-inflammation index (SII) and stroke-associated pneumonia (SAP). This forest plot illustrates odds ratios (ORs) and 95% confidence intervals (CIs) for the association between elevated SII and the risk of SAP across predefined clinical subgroups, including age (<70 vs. ≥70 years), sex, smoking status, hypertension, diabetes, atrial fibrillation, and chronic obstructive pulmonary disease (COPD). Elevated SII was consistently associated with increased SAP risk in all subgroups. No significant interactions were detected (all P for interaction > 0.05), suggesting stability of the association across strata. SII, systemic immune-inflammation index; SAP, stroke-associated pneumonia; OR, odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Predictive performance of SII and CRP for stroke-associated pneumonia
ROC curve analysis demonstrated that both log₂-transformed SII and CRP had predictive value for SAP in patients with acute ischemic stroke. As shown in Figure 3a, the area under the curve (AUC) was 0.726 (95% CI: 0.694–0.758) for SII and 0.826 (95% CI: 0.800–0.851) for CRP, with a statistically significant difference between the two (p < 0.0001) indicating that CRP had a significantly greater discriminatory capacity. At the optimal cutoff point, SII had a sensitivity of 59.6% and a specificity of 79.3%. For CRP, the sensitivity was 66.8% and the specificity was 86.4%. The overall accuracy was 75.1% for SII and 82.2% for CRP.
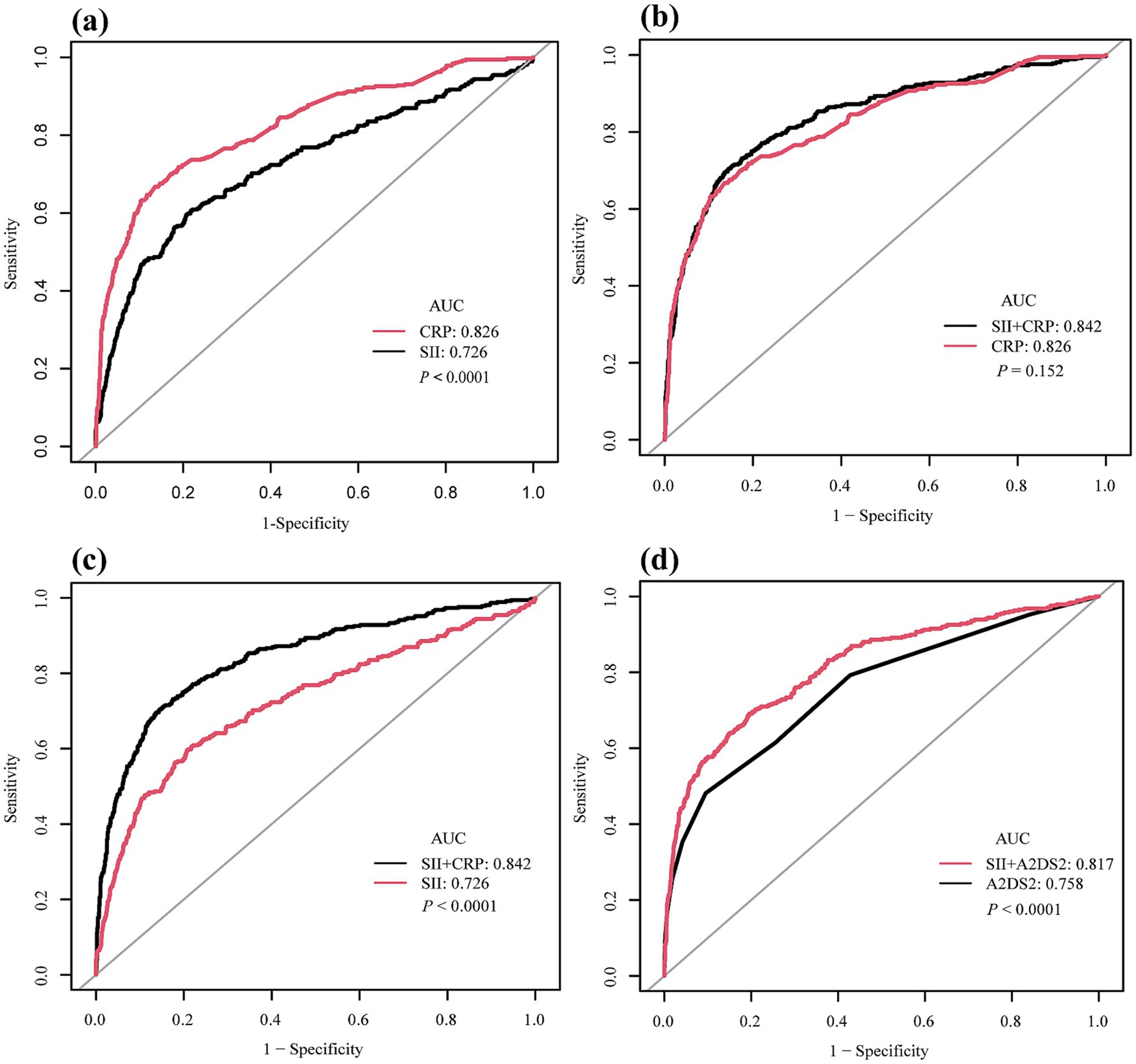
Figure 3. Receiver operating characteristic (ROC) curves for predictive performance of systemic immune-inflammation index (SII), C-reactive protein (CRP), and A2DS2 in relation to stroke-associated pneumonia (SAP). (a) Comparison of SII (AUC = 0.726, 95% CI: 0.694–0.758) and CRP (AUC = 0.826, 95% CI: 0.800–0.851) showing CRP had superior discriminatory ability (p < 0.0001). (b) ROC curves of CRP alone versus the combined model of SII + CRP. The combined model (AUC = 0.842, 95% CI: 0.817–0.866) was not significantly different from CRP alone (p = 0.152). (c) ROC curves of SII alone versus the combined model of SII + CRP. The addition of SII significantly improved predictive performance compared with SII alone (p < 0.0001). (d) ROC curves of A2DS2 score alone (AUC = 0.758, 95% CI: 0.729–0.787) versus the combined model of SII + A2DS2 (AUC = 0.817, 95% CI: 0.790–0.843), showing a significant improvement (p < 0.0001). ROC, receiver operating characteristic; AUC, area under the curve; SII, systemic immune-inflammation index; CRP, C-reactive protein; A2DS2, Age, Atrial Fibrillation, Dysphagia, Stroke Severity and Sex; SAP, stroke-associated pneumonia.
In an additional analysis, we constructed a multivariable model including both SII and CRP, which yielded an AUC of 0.842 (95% CI: 0.817–0.866). This was not significantly different from that of CRP alone (AUC 0.826, 95% CI: 0.800–0.851, p = 0.152) (Figure 3b). Similarly, the AUC of the combined model was significantly higher than that of SII alone (p < 0.0001) (Figure 3c). Similarly, when A2DS2 was analyzed as a continuous score, its AUC was 0.758 (95% CI: 0.729–0.787), and adding SII increased the AUC to 0.817 (95% CI: 0.790–0.843) (p < 0.0001) (Figure 3d).
Detailed diagnostic metrics for all models (AUC with 95% CI, sensitivity, specificity, and accuracy) are presented in Table 4.
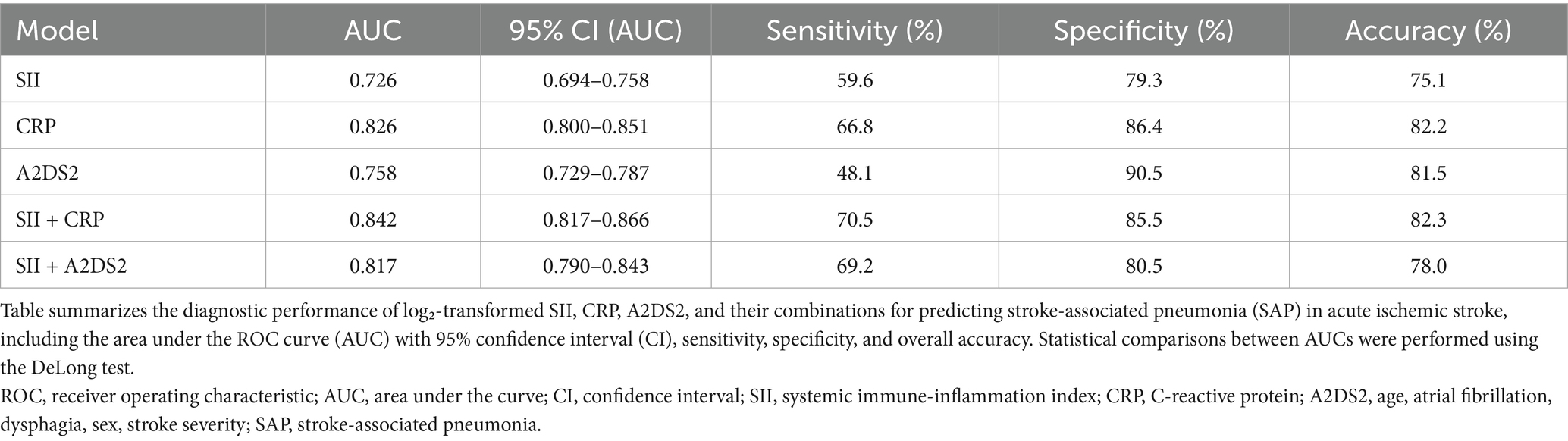
Table 4. Comparison of diagnostic performance of SII, CRP, A2DS2, and their combination models for predicting stroke-associated pneumonia.
Non-linear association and threshold effect of SII on SAP risk
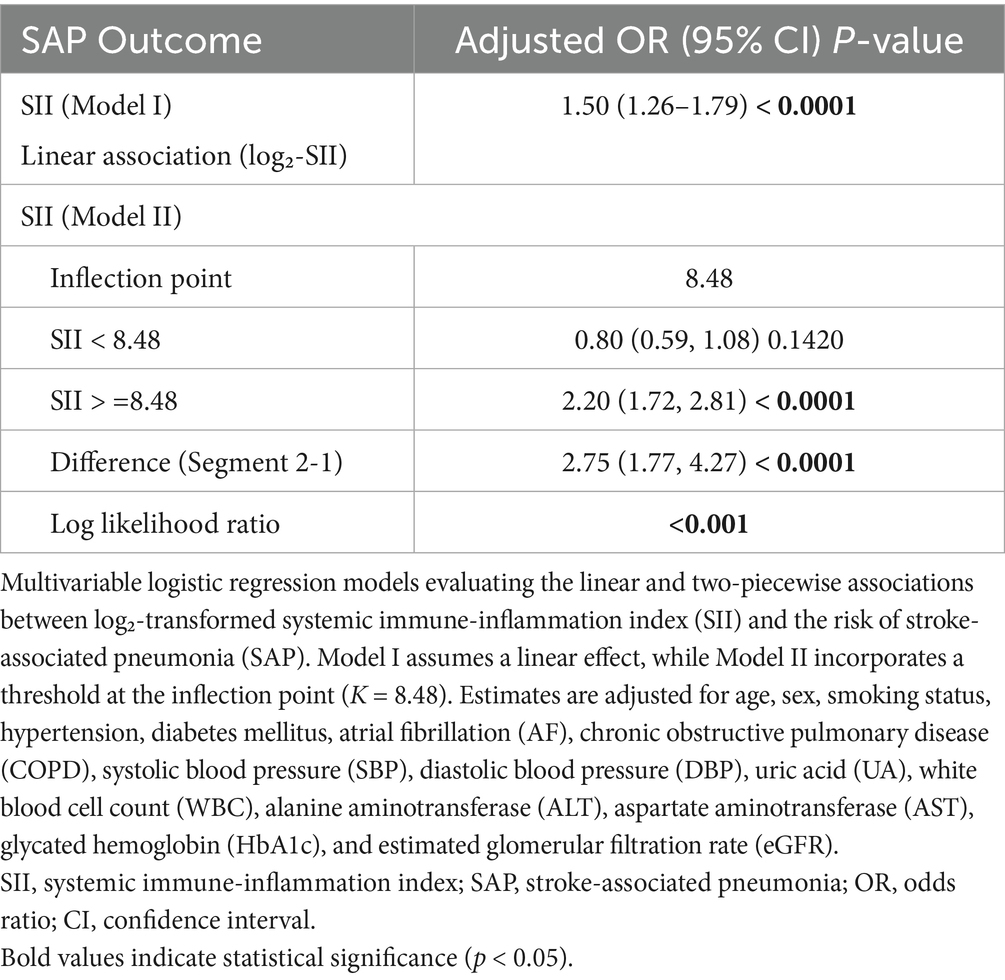
When log₂-SII was treated as a continuous variable in the fully adjusted logistic regression model, a significant overall positive association with SAP was observed (OR = 1.98 per 1-unit increase, 95% CI: 1.62–2.42, p < 0.001; Table 5), providing an overall effect estimate for comparability with prior studies.
A two-piecewise logistic regression model identified an inflection point at log₂-SII = 8.48. Below this threshold, SII was not significantly associated with SAP (OR = 0.80, 95% CI: 0.59–1.08, p = 0.1420), whereas above the threshold, a significant association was observed (OR = 2.20, 95% CI: 1.72–2.81, p < 0.0001). The difference in effect between segments was statistically significant (OR = 2.75, 95% CI: 1.77–4.27, p < 0.0001), and the log-likelihood ratio test supported the superiority of the threshold model over the linear model (p < 0.001) (Table 5). The threshold was determined by the cut point with the highest log-likelihood (95% CI obtained via 1,000 bootstrap resamples; see Methods).
Generalized additive model (GAM) analysis further supported a non-linear association between log₂-SII and SAP risk, revealing a flat trend at lower SII levels and a sharp increase beyond log₂-SII ≈ 8.5, consistent with the identified inflection point (Figure 4).
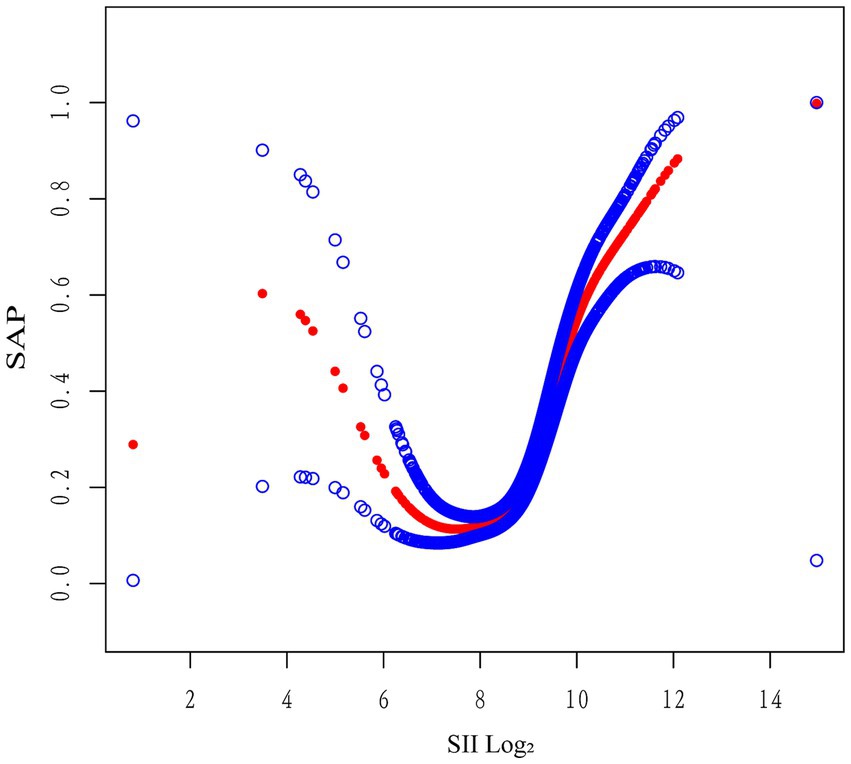
Figure 4. Generalized additive model illustrating the non-linear relationship between log₂-SII and SAP risk. The generalized additive model (GAM) depicts a non-linear association between log₂-transformed systemic immune-inflammation index (SII) and the probability of stroke-associated pneumonia (SAP). The SAP risk remained relatively stable at lower SII levels and increased sharply beyond log₂-SII ≈ 8.5, indicating a threshold-dependent relationship. The model was adjusted for relevant covariates described in the main analysis. SII, systemic immune-inflammation index; SAP, stroke-associated pneumonia; GAM, generalized additive model.
Discussion
In this retrospective cohort of 1,767 patients with AIS, elevated SII levels were independently associated with an increased risk of SAP. Patients in the highest SII quartile had a significantly greater risk of SAP (adjusted OR = 2.31, 95% CI: 1.44–3.70; p = 0.0005) compared to those in the lowest quartile. Furthermore, GAM and two-piecewise logistic regression identified a non-linear, threshold-dependent relationship between log₂-SII and SAP risk, indicating that the association becomes more pronounced beyond a specific threshold. To facilitate comparability with prior studies, we also reported the effect estimate from a model treating log₂-SII as a continuous predictor; this should be interpreted as an average effect across the exposure range, whereas the non-linear analyses (GAM and two-segment logistic regression) better characterize the threshold-dependent pattern observed (Table 5 and Figure 4).
The association between elevated SII and SAP risk may reflect stroke-induced immune dysfunction (13). AIS activates neuroendocrine responses such as the hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system, leading to increased levels of glucocorticoids and catecholamines (14, 15). These hormonal shifts promote lymphocyte apoptosis and suppression of adaptive immunity, characteristic of stroke-induced immunodepression syndrome (SIDS) (16, 17). By integrating neutrophil, platelet, and lymphocyte counts, SII captures this imbalance-reflecting both overactivation of innate immunity (neutrophilia and thrombocytosis) and suppression of adaptive responses (lymphopenia) (18–20).
Beyond stroke, SII has demonstrated prognostic relevance in various clinical conditions (21, 22). In cardiovascular disease, elevated SII levels have been independently associated with poor outcomes in coronary artery disease (23, 24), as well as with all-cause and cardiovascular mortality in large-scale cohorts (25–27). In oncology, SII has emerged as a reliable biomarker of systemic inflammation and prognosis across multiple malignancies, including hepatocellular, colorectal, and gynecologic cancers (22, 28–31). Furthermore, in sepsis, SII has shown predictive value for both disease severity and mortality, reinforcing its utility as a broad indicator of immune dysregulation (32, 33).
SII may also act as a surrogate marker for deeper immunopathological processes involved in the development of SAP (34, 35). Neutrophilia promotes the release of reactive oxygen species and proteases that damage the alveolar-capillary barrier (36, 37). Concurrent lymphopenia compromises adaptive immunity, while elevated platelet counts enhance inflammation through cytokine release (38, 39). This triad generates a pro-inflammatory yet immunosuppressed state, predisposing patients to pulmonary infections (40, 41).
Additional evidence from post-stroke immunology studies indicates that peripheral lymphocyte counts, particularly T cells, B cells, and NK cells, decline rapidly within hours to days after AIS onset due to apoptosis, redistribution to lymphoid organs, and functional exhaustion (42, 43). This lymphopenia, a hallmark of stroke-induced immunodepression syndrome (SIDS), compromises adaptive immunity and reduces pathogen clearance capacity (42, 44), thereby increasing susceptibility to infections such as SAP. In parallel, neutrophil and platelet activation further amplifies systemic inflammation and damages the alveolar-capillary barrier (45) (with NETs broadly detected in AIS thrombi (46)), creating a “double-hit” effect of immune suppression and inflammatory injury. These dynamic changes in immune cell populations directly influence SII values, as a rising SII often reflects both increased innate immune activation (neutrophilia, thrombocytosis) and marked adaptive immune suppression (lymphopenia) (42, 43), providing a plausible mechanistic link between elevated SII and heightened SAP risk in AIS patients.
Unlike CRP, SII reflects cellular immune dynamics by integrating neutrophils, platelets, and lymphocytes (47). This composite measure captures both innate activation and adaptive suppression (7). The threshold-dependent pattern observed in our study suggests that SAP risk rises notably only when this imbalance exceeds a critical level. CRP, as an acute-phase reactant produced by the liver in response to infection or tissue injury, often rises rapidly and directly with the onset of infection—this may explain its higher discriminatory performance for SAP in our cohort (e.g., Liu et al. found that CRP levels within 12 h of stroke onset were independently associated with poor outcomes) (48, 49). In contrast, SII reflects the underlying immune-inflammatory balance and may be more informative for early risk stratification, before overt infection occurs. Although other inflammatory markers such as WBC and NLR have been studied—NLR may help identify high-risk SAP patients (50)—these markers typically show modest and heterogeneous discrimination (6). Therefore, we prioritized SII as an integrative index and used CRP as a reference biomarker; WBC was adjusted for in models, and SII remained independently associated with SAP (Table 3; VIFs < 3).
Previous studies, such as Kuang et al. (10), have reported a linear association between SII and SAP risk in a mixed acute stroke population, but did not explore potential non-linear patterns or threshold effects and did not restrict the analysis to a well-defined cohort of AIS patients. By applying GAM and two-piecewise logistic regression, our study identified a distinct inflection point, revealing that the relationship between SII and SAP is not uniform across its range. Furthermore, our work extends prior findings by evaluating the added predictive value of SII beyond established clinical severity scores (NIHSS and A2DS2), thereby providing a more nuanced understanding of how immune imbalance contributes to SAP development and underscoring the value of advanced modeling in biomarker-based risk stratification.
These findings not only enhance our understanding of SII’s prognostic relevance but also support its potential integration into clinical risk models. Compared with biomarkers like CRP, SII is more accessible, cost-effective, and readily obtained from routine blood tests. Combining SII with validated scores such as A2DS2 could improve early SAP detection, particularly in resource-limited settings (51, 52). Future studies should explore dynamic SII monitoring and assess its predictive value across different stroke subtypes and care settings (53).
Several limitations should be acknowledged. First, the retrospective and single-center design may limit generalizability. Second, inflammatory markers were measured only once within 48 h of admission, precluding evaluation of temporal changes. Third, although our main models adjusted for baseline stroke severity (NIHSS) and dysphagia (KWDT), residual confounding by unmeasured aspects of severity (e.g., level of consciousness, aspiration risk, use of nasogastric/jejunal tubes, prolonged bed rest, and infarct location) cannot be fully excluded despite multivariable adjustment. In addition, we did not evaluate other inflammatory or immune biomarkers (e.g., cytokines or measures of immune cell function), which may provide complementary information beyond SII and CRP and should be explored in future work. Finally, long-term outcomes, such as SAP recurrence or post-discharge mortality, were not assessed. Prospective, multicenter studies are warranted to validate our findings and further refine the clinical utility of SII.
Conclusion
In this retrospective cohort of AIS patients, elevated SII levels were independently and non-linearly associated with SAP risk, exhibiting a clear threshold effect. Although CRP demonstrated superior discriminative ability, SII showed complementary prognostic value, particularly when combined with CRP or A2DS2, while remaining easily obtainable from standard blood counts. Integrating SII into existing clinical risk models may enhance early identification of high-risk individuals. Prospective, multicenter investigations are warranted to validate these findings and to explore whether dynamic SII monitoring can improve both short- and long-term outcomes in AIS populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Quzhou People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because informed consent was waived due to the retrospective nature of the study and the use of de-identified patient data.
Author contributions
TD: Conceptualization, Formal analysis, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MY: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. YZ: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. CZ: Conceptualization, Data curation, Investigation, Supervision, Visualization, Writing – review & editing. ZR: Conceptualization, Formal analysis, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1651656/full#supplementary-material
References
1. Fan, J, Li, X, Yu, X, Liu, Z, Jiang, Y, Fang, Y, et al. Global burden, risk factors analysis, and prediction study of ischemic stroke, 1990-2030. Neurology. (2023) 101: e137–e150. doi: 10.1212/WNL.0000000000207387
2. Zhang, X-P, Zhang, G, Nie, D, Chen, J, Liu, Z-Y, Wei, L, et al. Reducing the incidence of stroke-associated pneumonia: an evidence-based practice. BMC Neurol. (2022) 22:297. doi: 10.1186/s12883-022-02826-8
3. Zhang, J, He, Y, Song, X, and Bai, J. A nomogram based on nutritional status and A2DS2 score for predicting stroke-associated pneumonia in acute ischemic stroke patients with type 2 diabetes mellitus: a retrospective study. Front Nutr. (2022) 9:1009041. doi: 10.3389/fnut.2022.1009041
4. Zheng, X, Zheng, X, Zhou, J, Zhao, Z, Wang, F, Wang, F, et al. Using machine learning to predict stroke-associated pneumonia in Chinese acute ischaemic stroke patients. Eur J Neurol. (2020) 27:1656–63. doi: 10.1111/ene.14295
5. Wu, M, Liu, R, Liu, X, Chen, J, Zhu, X, Wang, Z, et al. Systemic immune-inflammation index and long-term mortality in patients with stroke-associated pneumonia. J Inflamm Res. (2023) 16:1581–93. doi: 10.2147/JIR.S399371
6. Xian, H, Yi, Y, Wang, X, Tu, J, Cheng, R, Luo, H, et al. Comparison of the predictive value of inflammatory biomarkers for the risk of stroke-associated pneumonia in patients with acute ischemic stroke. Clin Interv Aging. (2023) 18:1477–90. doi: 10.2147/CIA.S425393
7. Xia, Y, Xia, C, Wu, L, Li, Z, Li, H, and Zhang, J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12:1128. doi: 10.3390/jcm12031128
8. Tuzimek, A, Junka, A, Gąsior, J, Paleczny, J, Dąbrowski, M, Jankowski, P, et al. Investigation of the associations of novel inflammatory biomarkers—systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)—with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. (2022) 23:9553. doi: 10.3390/ijms23179553
9. Giustina, A, de Aguiar Costa, M, De Mello, MEL, Petronilho, F, Andreghetto, S, De Rezende, VL, et al. The risk of stroke-related pneumonia: a systematic review of peripheral immunodepression markers. Expert Rev Respir Med. (2025) 19:449–59. doi: 10.1080/17476348.2025.2481956
10. Kuang, S, Gu, S, Chen, H, Sun, H, Han, Y, Yang, X, et al. Predictive value of the systemic immune inflammation (SII) index for stroke-associated pneumonia. Brain Behav. (2023) 13:3302. doi: 10.1002/brb3.3302
11. Kuo, Y, Lee, J-D, Lee, T-H, Huang, Y-C, and Lee, M. Risk stratification model for post-stroke pneumonia in patients with acute ischemic stroke. Eur J Cardiovasc Nurs. (2019) 19:513–20. doi: 10.1177/1474515119889770
12. Smith, CJ, Kishore, AK, Vail, A, Chamorro, A, Garau, J, Hopkins, SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. (2015) 46:2335–40. doi: 10.1161/STROKEAHA.115.009617
13. Miró-Mur, F, Faura, J, Bustamante, A, and Montaner, J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. (2021) 18:127. doi: 10.1186/s12974-021-02177-0
14. Saha, C, Sarmah, D, Bhattacharya, P, Godse, P, Datta, A, and Sharma, M. Neuroendocrine regulation in stroke. Trends Endocrinol Metab. (2023) 34:260–77. doi: 10.1016/j.tem.2023.02.005
15. Sternberg, E, Tonelli, L, and Webster, J. Neuroendocrine regulation of immunity. Annu Rev Immunol. (2003) 20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914
16. Urra, X, Chamorro, Á, and Obach, V. Stroke induced immunodepression syndrome: from bench to bedside. Curr Mol Med. (2009) 9:195–202. doi: 10.2174/156652409787581574
17. Liu, D-D, Chu, S-F, Chen, C, Yang, Pf, Chen, N, and He, X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP). Neurochem Int. (2018) 114:42–54. doi: 10.1016/j.neuint.2018.01.002
18. Wang, L-X, Lu, A, Guo, J-W, Zhao, H, Yuan, F, Huang, Y-J, et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. (2023) 14:1115031. doi: 10.3389/fimmu.2023.1115031
19. Liu, Y, He, Z, Zhang, Y, Zhang, H, Yang, L, Liu, K, et al. Systemic immune-inflammation index (SII) and neutrophil-to-lymphocyte ratio (NLR): a strong predictor of disease severity in large-artery atherosclerosis (LAA) stroke patients. J Inflamm Res. (2025) 18:195–202. doi: 10.2147/JIR.S500474
20. Buonacera, A, Stancanelli, B, Colaci, M, and Malatino, L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. (2022) 23:3636. doi: 10.3390/ijms23073636
21. Chang, Q, Meng, X, Gao, N, Wang, W-H, and Yang, R. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. (2018) 9:3295–302. doi: 10.7150/jca.25691
22. Yang, Y, Li, X, Hu, X, Zhang, R, and Meng, L. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J Transl Med. (2023) 21:79. doi: 10.1186/s12967-023-03924-y
23. Zhou, Y, Yang, S, Zhang, X, Huang, X, Zhao, Z, and Sun, T. Prognostic value of systemic immune-inflammation index in CAD patients: systematic review and meta-analyses. Eur J Clin Investig. (2023) 54:4100. doi: 10.1111/eci.14100
24. Şahinarslan, A, Kızıltunç, E, Nurkoç, S, and Candemir, M. Relationship between systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology. (2021) 72:575–81. doi: 10.1177/0003319720987743
25. Wang, S, and Zhang, G. Association between systemic immune-inflammation index and adverse outcomes in patients with acute coronary syndrome: a meta-analysis. Angiology. (2024) 21:33197241263399. doi: 10.1177/00033197241263399
26. Xie, Y, Zhou, Y, Xu, J, Xu, X, Chen, S, Kang, Y, et al. Association between systemic immune inflammation level and poor prognosis across different glucose metabolism status in coronary artery disease patients. J Inflamm Res. (2023) 16:4031–42. doi: 10.2147/JIR.S425189
27. Tong, X, Bai, X, Wang, H, Bu, G, and Nie, H. Systemic immune-inflammation index (SII) and the risk of all-cause, cardiovascular, and cardio-cerebrovascular mortality in the general population. Eur J Med Res. (2023) 28:575. doi: 10.1186/s40001-023-01529-1
28. Urbarova, I, Johansson, M, Byrne, K, Nøst, T, Guida, F, Sandanger, T, et al. Systemic inflammation markers and cancer incidence in the UK biobank. Eur J Epidemiol. (2021) 36:841–8. doi: 10.1007/s10654-021-00752-6
29. Wang, H, and Ji, Y. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: a meta-analysis. World J Surg Oncol. (2020) 18:197. doi: 10.1186/s12957-020-01974-w
30. Wang, X-Y, Chong, Y, Gu, L, Li, X, Chen, Y, He, D, et al. Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann Med. (2021) 53:1827–38. doi: 10.1080/07853890.2021.1991591
31. Ni, L, Huang, J, Kou, J, Li, J, and Wu, Z. Systemic immune-inflammation index predicts prognosis and responsiveness to immunotherapy in cancer patients: a systematic review and meta-analysis. Clin Exp Med. (2023) 23:3895–905. doi: 10.1007/s10238-023-01035-y
32. Mangalesh, S, Dudani, S, and Malik, A. The systemic immune-inflammation index in predicting sepsis mortality. Postgrad Med. (2022) 135:345–51. doi: 10.1080/00325481.2022.2140535
33. Wu, X, Sun, Y, Zhang, W, Chen, C, Yang, H-Q, Fang, B, et al. Systemic immune-inflammation index combined with quick sequential organ failure assessment score for predicting mortality in sepsis patients. Heliyon. (2023) 9:9526. doi: 10.1016/j.heliyon.2023.e19526
34. Samary, CS, Rocco, PRM, Silva, L, and Pelosi, P. Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy. Crit Care. (2016) 20:391. doi: 10.1186/s13054-016-1573-1
35. Bitar, A, Usman, A, Farha, RA, Khan, AH, and Zawiah, M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio, and platelet-lymphocyte ratio in stroke-associated pneumonia: a systematic review and meta-analysis. Curr Med Res Opin. (2023) 39:475–82. doi: 10.1080/03007995.2023.2174327
36. Grommes, J, and Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol Med. (2011) 17:293–307. doi: 10.2119/molmed.2010.00138
37. Chapman, E, Hackett, A, Wright, H, and Glennon-Alty, L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic Biol Med. (2018) 125:25–35. doi: 10.1016/j.freeradbiomed.2018.03.049
38. Kapur, R, Semple, J, Rebetz, J, and Maouia, A. The immune nature of platelets revisited. Transfus Med Rev. (2020) 34:209–20. doi: 10.1016/j.tmrv.2020.09.005
39. Thomas, M, and Storey, R. The role of platelets in inflammation. Thromb Haemost. (2015) 114:449–58. doi: 10.1160/TH14-12-1067
40. Ming, L, Jun, F, Wang, Q, and Jiang, Z. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: a systematic review and meta-analysis. Int Angiol. (2018) 37:4–11. doi: 10.23736/S0392-9590.17.03848-2
41. Xu, N, Chen, Ln, Luo, Z, and Zhang, W. Prognostic value of neutrophil: lymphocyte and platelet: lymphocyte ratios for 28-day mortality of patients with AECOPD. Int J Gen Med. (2021) 14:2839–48. doi: 10.2147/IJGM.S312045
42. Chamorro, A, Meisel, A, Planas, AM, Urra, X, van de Beek, D, and Veltkamp, R. The immunology of acute stroke. Nat Rev Neurol. (2012) 8:401–10. doi: 10.1038/nrneurol.2012.98
43. Meisel, C, Schwab, K, Fau-Prass, J, Prass, A, Fau-Meisel, K, Meisel, U, et al. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. (2005) 6, 775–786. doi: 10.1038/nrn1765
44. Dirnagl, U, Klehmet, J, Braun, JS, Harms, H, Meisel, C, Ziemssen, T, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. (2007) 38:770–3. doi: 10.1161/01.STR.0000251441.89665.bc
45. Zarbock, A, Polanowska-Grabowska, RK, and Ley, K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. (2007) 21:99–111. doi: 10.1016/j.blre.2006.06.001
46. Essig, F, Kollikowski, AM, Pham, M, Solymosi, L, Stoll, G, Haeusler, KG, et al. Immunohistological analysis of neutrophils and neutrophil extracellular traps in human Thrombemboli causing acute ischemic stroke. Int J Mol Sci. (2020) 21:7387. doi: 10.3390/ijms21197387
47. Zacher, J, Walzik, D, Zimmer, P, and Joisten, N. Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur J Appl Physiol. (2021) 121:1803–14. doi: 10.1007/s00421-021-04668-7
48. Emsley, HC, Smith, CJ, Gavin, CM, Georgiou, RF, Vail, A, Barberan, EM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. (2003) 139:93–101. doi: 10.1016/s0165-5728(03)00134-6
49. Liu, A., Bui, T., Nguyen, H.Van, Ong, B., Shen, Q., and Kamalasena, D. Serum C-reactive protein as a biomarker for early detection of bacterial infection in the older patient Age Ageing 39 (2010) 559–565 doi: 10.1093/ageing/afq067
50. Nam, KW, Kim, TJ, Lee, JS, Kwon, HM, Lee, YS, Ko, SB, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. (2018) 49:1886–92. doi: 10.1161/STROKEAHA.118.021228
51. Anusasnee, N, Na-Ek, N, Krongsut, S, and Soontornpun, A. Integrating the A2DS2 score with 24-hour ASPECTS and red cell distribution width for enhanced prediction of stroke-associated pneumonia following intravenous thrombolysis: model development and internal validation. Eur J Med Res. (2025) 30:28. doi: 10.1186/s40001-025-02282-3
52. Wu, X, Sun, J, Ni, J, and Shou, W. Prediction of stroke-associated pneumonia by the A2DS2, AIS-APS, and ISAN scores: a systematic review and meta-analysis. Expert Rev Respir Med. (2021) 15:1461–72. doi: 10.1080/17476348.2021.1923482
53. He, L, Zhou, Z, Zhou, M, Lei, Z, Gong, S, Guo, J, et al. Validation of risk scoring models for predicting stroke-associated pneumonia in patients with ischaemic stroke. Stroke Vasc Neurol. (2016) 1:122–6. doi: 10.1136/svn-2016-000025
Glossary
AIS - acute ischemic stroke
SAP - stroke-associated pneumonia
SII - systemic immune-inflammation index
CRP - C-reactive protein
GAM - generalized additive model
ROC - receiver operating characteristic
AUC - area under the curve
NIHSS - National Institutes of Health Stroke Scale
GCS - Glasgow Coma Scale
KWDT - Kubota Water Drinking Test
A2DS2 - Age, Atrial fibrillation, Dysphagia, Sex, Stroke severity
NLR - neutrophil-to-lymphocyte ratio
PLT - platelet count
WBC - white blood cell count
ALT - alanine aminotransferase
AST - aspartate aminotransferase
HbA1c - glycated hemoglobin
HCY - homocysteine
Scr - serum creatinine
ALB - albumin
TG - triglycerides
TC - total cholesterol
HDL-c - high-density lipoprotein cholesterol
LDL-c - low-density lipoprotein cholesterol
AF - atrial fibrillation
COPD - chronic obstructive pulmonary disease
SBP - systolic blood pressure
DBP - diastolic blood pressure
eGFR - estimated glomerular filtration rate
UA - uric acid
MRI - magnetic resonance imaging
CT - computed tomography
SD - standard deviation
OR - odds ratio
CI - confidence interval
Keywords: ischemic stroke, stroke-associated pneumonia, systemic immune-inflammation index, inflammatory biomarkers, risk stratification, non-linear modeling
Citation: Duan T, Yang M, Zhang Y, Zhu C and Rao Z (2025) Elevated systemic immune-inflammation index is associated with stroke-associated pneumonia in acute ischemic stroke: a retrospective cohort study. Front. Neurol. 16:1651656. doi: 10.3389/fneur.2025.1651656
Edited by:
Sheng Luo, The Second Affiliated Hospital of Guangzhou Medical University, ChinaReviewed by:
Naushad Ahmad Khan, Hamad Medical Corporation, QatarTieshi Zhu, Zhanjiang Central Hospital, China
DongMing Zhang, Second Affiliated Hospital of Guangzhou Medical University, China
Wenjun Zhang, Shaoyang University, China
Copyright © 2025 Duan, Yang, Zhang, Zhu and Rao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zichen Rao, cnpjMTUyMkB3bXUuZWR1LmNu
†ORCID: Yiming Zhang, https://orcid.org/0009-0006-2698-9883
Chunyan Zhu, https://orcid.org/0009-0006-1148-1753
Zichen Rao, https://orcid.org/0009-0005-6788-4829
 Tingting Duan1
Tingting Duan1 Yiming Zhang
Yiming Zhang Zichen Rao
Zichen Rao