- 1Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD, United States
- 2Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD, United States
- 3Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD, United States
- 4Joint Trauma System, Joint Base San Antonio, San Antonio, TX, United States
- 5Department of Medicine, Uniformed Services University of the Health Sciences, Uniformed Services University, Bethesda, MD, United States
Background: A significant proportion of patients presenting with post-acute sequelae of COVID-19 (PASC) have been found to meet diagnostic criteria for certain disorders of the autonomic nervous system. Substantial gaps remain in our understanding of these conditions. Our objective is to evaluate demographic and medical factors associated with PASC dysautonomia in active duty US Service members (ADSM). Additionally we assessed for risk factors in those diagnosed with COVID-19 for PASC dysautonomia, and differences in those with PASC dysautonomia and non-PASC dysautonomia.
Methods: A matched case control dataset (n = 1,367,961) of ADSM diagnosed with COVID-19 matched with ADSM with no evidence of COVID-19 was utilized to assess associations of demographic and clinical factors with PASC dysautonomia. Logistic regression modeling was used to assess differences among those diagnosed with COVID-19. Conditional logistic regression modeling using propensity score weighting was used for comparisons between those with PASC dysautonomia and non-PASC dysautonomia.
Results: We identified 619,983 COVID-19 cases (158 PASC dysautonomia) and 747,978 controls (219 non-PASC dysautonomia). Among COVID-19 cases, factors positively associated with PASC dysautonomia were white, non-Hispanic race/ethnicity, female sex, younger age, northeast region, more severe COVID-19 infection, and comorbid depression or anxiety. Among those with dysautonomia, those with PASC dysautonomia were more likely to be of female sex, younger, in the northeast region, and less likely to have comorbid anxiety.
Conclusion: PASC dysautonomia is rare in ADSM but associated with increased care utility and often prolonged diagnostic pathways. Important demographic and COVID-19 specific risk factors are associated with the development of PASC dysautonomia. PASC dysautonomia has significant differences in risk factors as compared to non-PASC dysautonomia, warranting further examination. These findings may support clinician awareness and prognostication and prompt further research on the pathophysiology and management of these conditions.
Introduction
The COVID-19 pandemic has resulted in significant harms to the health of the world’s population and economy (1–3). To date the WHO has identified over 750 million diagnoses of COVID-19, resulting in over 7 million deaths (4). While recent strains of the SARS-COV-2 virus which causes COVID-19 has been associated with less virulent infections, there is still substantial morbidity and mortality from this now endemic pathogen (5, 6).
Early in the COVID-19 pandemic, reports began to identify individuals with persistent symptoms and/or symptoms developing after resolution of acute disease related to their COVID-19 infection (7, 8). This condition has been referred to as long COVID, long-haul COVID, post COVID condition, and post-acute sequelae of COVID (PASC, which will be used henceforth) (9). Symptoms associated with this condition appear widely variable, and encompass sustained symptoms similar to those seen in the acute phase of infection including cough, fatigue, alterations in sensation of taste and smell, as well as other atypical symptoms that typically onset after the acute phase of COVID-19 has ended including cognitive and psychological problems (‘brain fog’), palpitations and other cardiac symptoms, and neurologic symptoms (10–13). This heterogeneity in symptoms raises questions regarding differences in underlying disease pathophysiology between differing phenotypes of PASC (14, 15). Some risk factors that have been identified for PASC include female sex, Hispanic ethnicity, older age, underlying health conditions, including depression and anxiety, more severe acute COVID-19 infection, and being unvaccinated for COVID-19 (15, 16).
In some studies of PASC patients, a significant proportion meet diagnostic criteria for certain dysautonomia conditions (17, 18). Dysautonomia, or conditions that affect the function of the autonomic nervous system, which controls involuntary systems including heart rate and blood flow, are recently becoming increasingly identified following the onset of the COVID-19 pandemic (19, 20). While the underlying pathophysiology of COVID-19 induced dysautonomia (“PASC dysautonomia) remains uncertain, several hypotheses have been proposed. These include direct viral effects on the autonomic nervous system potentiating disordered regulation of neurotransmitters including serotonin, dysregulated inflammatory responses affecting the autonomic nervous system, and viral induced endothelial dysfunction impairing vascular function (21–25). The interplay between serotonin and other neurotransmitters with PASC, dysautonomia, and PASC dysautonomia are of particular interest as these conditions are frequently comorbid with anxiety and depression (26–28). There are several major gaps in our understanding of PASC dysautonomia (29). First, the prevalence of PASC dysautonomia is unclear, including in the Military Health System (MHS). Second, it is unclear whether PASC dysautonomia has distinct risk factors and pathophysiological mechanisms than dysautonomia not associated with COVID-19 (“non-PASC-dysautonomia”), and if differing approaches to clinical prediction and management are warranted for these individuals (29–31).
The purpose of this large scale (n = 1,367,961) EMR study was to evaluate dysautonomia in a study of individuals diagnosed with COVID-19 matched with individuals with no evidence of COVID-19 in the MHS. Objectives of the study included identification of demographic, military Service, and health related factors among individuals diagnosed with COVID-19 that increased the likelihood of developing dysautonomia after COVID-19 diagnosis. A further objective was to compare risk factors between PASC dysautonomia and non-PASC dysautonomia. Finally, we estimated the impact of PASC-dysautonomia, compared to those with a history of COVID-19 and no dysautonomia, through an analysis of associated medical care utility.
Methods
Study population
The population under consideration in this study was derived from the COVID-19 Military Registry Analysis Project (M-RAP), which identified diagnoses of COVID-19 in the MHS through data pulls from the DoD Military Health System Data Repository (MDR) from January 1st, 2020, through June 30th, 2022. Diagnoses of COVID-19 were determined via either a confirmatory laboratory diagnosis or ICD coding suggestive of COVID-19 (included in Supplementary Table S1) (32). The subset of the data examined for this analysis consisted of all active duty Service members diagnosed with COVID-19 in this period; for each individual with a COVID-19 diagnosis identified up to three individuals without a COVID-19 diagnosis were selected matched on region of residence, age (+/− 2 years), and gender. Medical claims data, pharmacy, and laboratory data for everyone identified were accessed from January 2017 through June 2023. All individuals included in the dataset were active duty MHS beneficiaries when diagnosed (or during the same month as the matched case was diagnosed for controls) and have no missing data for the variables of interest for this analysis.
Dysautonomia status
The presence of dysautonomia was determined based upon identification of medical encounters using an ICD code suggestive of a dysautonomia condition (a subset of G90 ICD codes, included in Supplementary Table S2). Probable diagnosis of dysautonomia was used for individuals with three encounters containing such codes, while a status of possible diagnosis of dysautonomia was used for those with at least one such encounter. Given the complex and likely disparate diagnostic pathways to a dysautonomia diagnosis in the MHS, no upper bound on the time from COVID-19 diagnosis to dysautonomia diagnosis was used to accommodate for potential late presentation to care and delays in diagnosis. For the purposes of evaluating dysautonomia in the setting of PASC in the subset of individuals diagnosed with COVID-19, the first encounter in which one of these codes had to be after the initial date of COVID-19 diagnosis for the participant to be considered to have PASC dysautonomia, whereas for controls without COVID-19 there was no limitation on the timing of dysautonomia.
Covariates
For all individuals included in the analysis demographic and military service variables were considered including sex, age, race/ethnicity, rank, military Service, geographical region (assessed by grouped US Department of Health and Human Services regions, and international classifiers). Additionally, the presence of potential associated illnesses was considered including depression, anxiety, diabetes, heart disease, and autoimmune disease. In addition to these factors, for those diagnosed with COVID-19 additional factors were considered related to their COVID infection including severity of infection, vaccination status, variant era of infection, and medication use within 3 months of infection that may be used for COVID-19 treatment, or be suggestive of dysautonomia including alpha agonists, beta blockers, corticosteroids, selective serotonin reuptake inhibiters, or stimulants. Severity of infection was categorized into groups with no additional medical visits within 14 days of COVID-19 diagnosis, those with at least 2 additional medical visits within 14 days of COVID-19 diagnosis but were not hospitalized, and those who were hospitalized within 30 days of COVID-19 diagnosis. The medication types were identified using the American Society of Health-System Pharmacists therapeutic classification schema (33).
Statistical analyses
Two analyses were conducted on this sample; a flowchart showing the participant groups and analyses performed is presented (Figure 1). Directed Acyclic Graphs (DAGS, Supplementary Figures S1, S2) were constructed to visualize rationale in developing models to assess dysautonomia risk factors among these groups. These DAGs were used to identify possible covariates which may be included in multivariate regression models, with further statistical criteria used for covariate selection. The first analysis compared those with a COVID-19 infection who were diagnosed with dysautonomia following their COVID-19 infection (PASC dysautonomia) to those diagnosed with COVID-19 without a dysautonomia diagnosis to ascertain risk factors for the development of PASC dysautonomia in terms of patient demographics and COVID-19 infection related variables. Bivariate analyses using logistic regression was performed to identify initial associations between each variable and dysautonomia status among those diagnosed with COVID-19. Variables that had adequate numbers in each group and resulted in a statistically significant effect with at least a 20% change in the odds ratio of dysautonomia in the bivariate analyses were used to develop a full model of dysautonomia associations. The included variables were first analyzed through a correlation matrix to assess collinearity, with prespecified limit of correlation coefficients >0.7 needing to be interrogated and corrected if identified. The final variables were then used to construct a full logistic regression model. Given the concern for low numbers of dysautonomia diagnoses in the military, an additional sensitivity analysis using Firth’s penalized likelihood method was performed on this model.
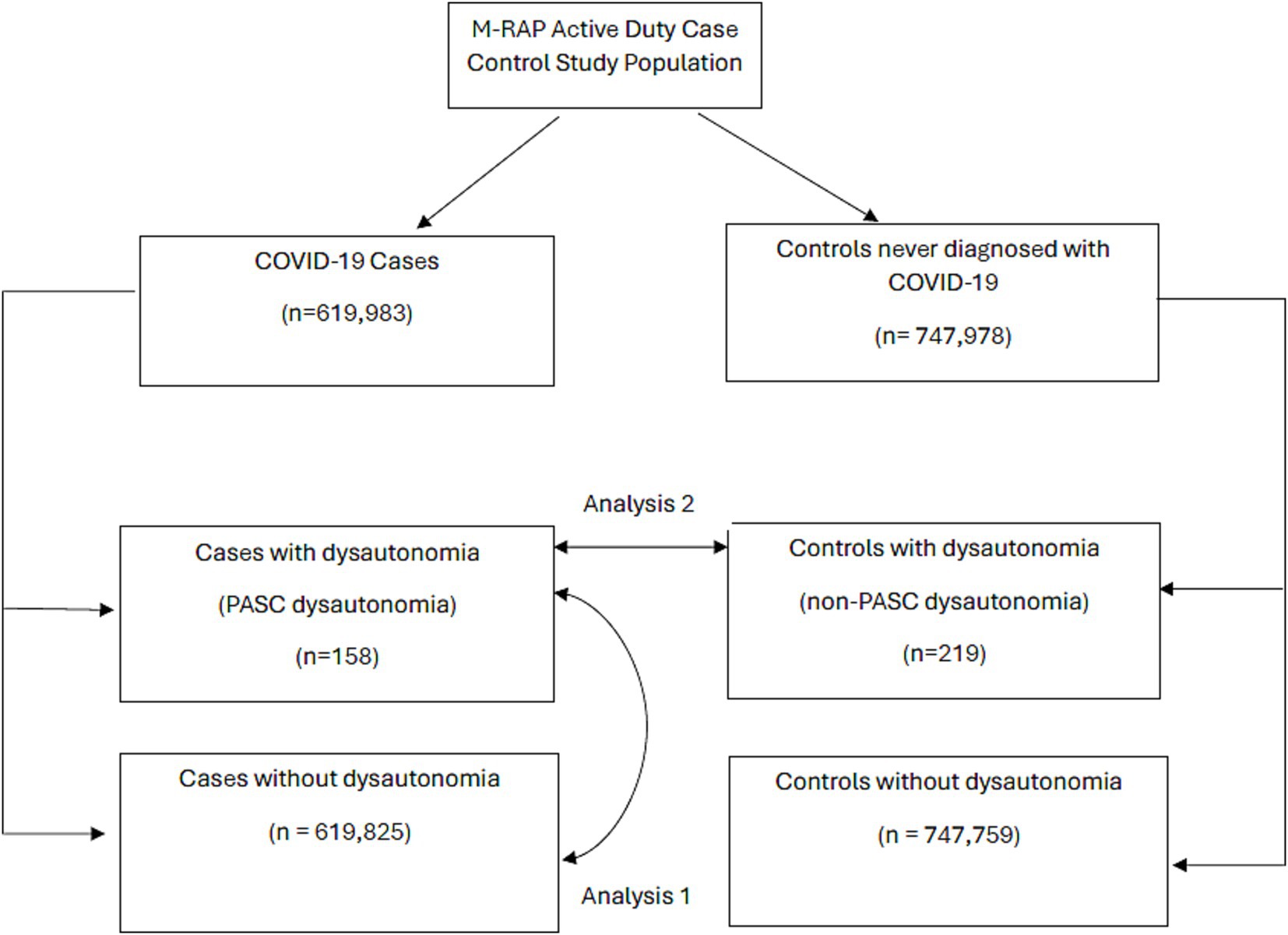
Figure 1. Flow diagram of military registry analysis project participants by COVID-19 case/control status, and dysautonomia status. Arrows are used to highlight the groups between which analysis 1 and analysis 2 were performed.
The next analysis evaluated those with PASC dysautonomia to those with non-PASC dysautonomia to assess if there were significant differences in demographic risk groups for the development of these COVID-19 associated conditions as compared to traditional diagnoses. As the data came from a matched case control dataset of individuals diagnosed with COVID-19 and controls with no evidence of COVID-19 matched on age, sex, and region, this analysis utilized a propensity score weighting using inverse probability weights using all variables under consideration in developing propensity scores for each individual. The analysis was performed using logistical regression conditioned on the matched variables of age, sex, and region, and weighted using the inverse probability weights. This methodology was used in evaluating these groups to account for the matched design while also adjusting for potential confounding covariates which were not matched (34, 35). The validity of the propensity score weighting was evaluated using a covariate balance assessment of standard mean differences of covariates between groups. After applying the weighting, bivariate conditional logistic regression was performed assessing associations of demographic and comorbid medical conditions with PASC dysautonomia. Variables that had adequate numbers in each group and resulted in a statistically significant effect that resulted in at least a 20% change in the odds ratio of dysautonomia in the bivariate analyses were used to develop a full model of dysautonomia associations. The included variables were first analyzed through a correlation matrix to assess collinearity, with prespecified limit of correlation coefficients >0.7 needing to be interrogated and corrected if identified. The final variables were then used to construct a full conditional logistic regression model.
For all analyses, the primary assessment was made on participants deemed to have probable dysautonomia, and sensitivity analyses were performed using the definition of possible dysautonomia. Referent groups in analyses were generally selected as the group with the largest population, or at the lowest level of an ordinal variable. The goodness of fit of all regression models was assessed using the Hosmer-Lemeshow method. All analyses were conducted using SAS version 9.4 (SAS Institute Inc). This research received an exempt determination by the Uniformed Services University of the Health Sciences Human Research Protection Program.
Results
Demographics
A total of 1,367,961 active duty Service members were included in the analysis, comprising 619,983 individuals diagnosed with COVID-19 and 747,978 individuals with no documented COVID-19 infection. A breakdown of the demographics, Service-related variables, and medical comorbidities of these individuals is presented (Table 1). A total of 158 individuals diagnosed with COVID-19 were identified as probable PASC dysautonomia (551 possible PASC dysautonomia), while 219 individuals without documented COVID-19 infection were identified as probable non-PASC dysautonomia (692 possible non-PASC dysautonomia). Breakdowns of the demographics, Service-related variables, and medical comorbidities of those with PASC dysautonomia as compared to the remainder of those diagnosed with COVID-19 and non-PASC dysautonomia as compared to the remainder of those without a COVID-19 diagnosis are presented (Tables 2, 3, respectively). Breakdowns of the variables relating to COVID-19 infection, including severity, variant era of infection and vaccination status, and medications prescribed within 90 days before infection are also presented (Table 2). The median time after COVID-19 infection for the first encounter in which a dysautonomia code was used was 186 days (IQR: 59 to 319 days). The MHS utilization of individuals with PASC dysautonomia and those with COVID-19 infection never diagnosed with dysautonomia is presented in Figure 2. Individuals with PASC dysautonomia had increased utilization of medical services after their COVID-19 diagnosis, having a mean of 2.63 (SD 3.20) medical encounters per month after their COVID-19 infection, as compared to 0.79 (SD 1.03) encounters per month after COVID-19 infection for those not diagnosed with dysautonomia. They also tended to be prescribed more medications, having prescriptions written for them at a rate of 1.61 (SD 1.61) prescriptions per month, as compared to 0.56 (SD 0.74) prescriptions per month after COVID-19 infection for those not diagnosed with dysautonomia.
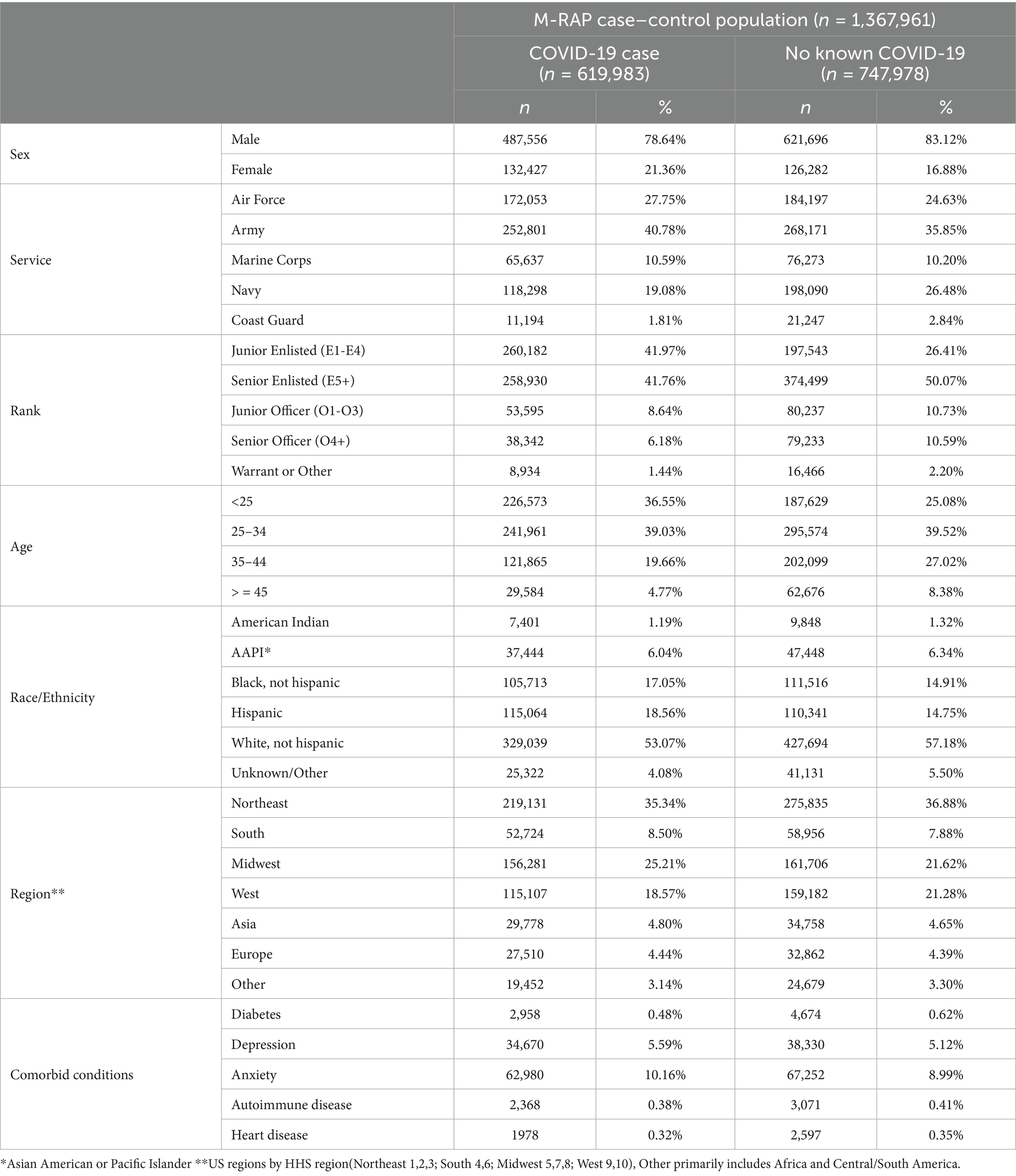
Table 1. Breakdown of relevant demographic and medical variables in M-RAP study population between those with a documented COVID-19 infection and those without documented COVID-19 infection.
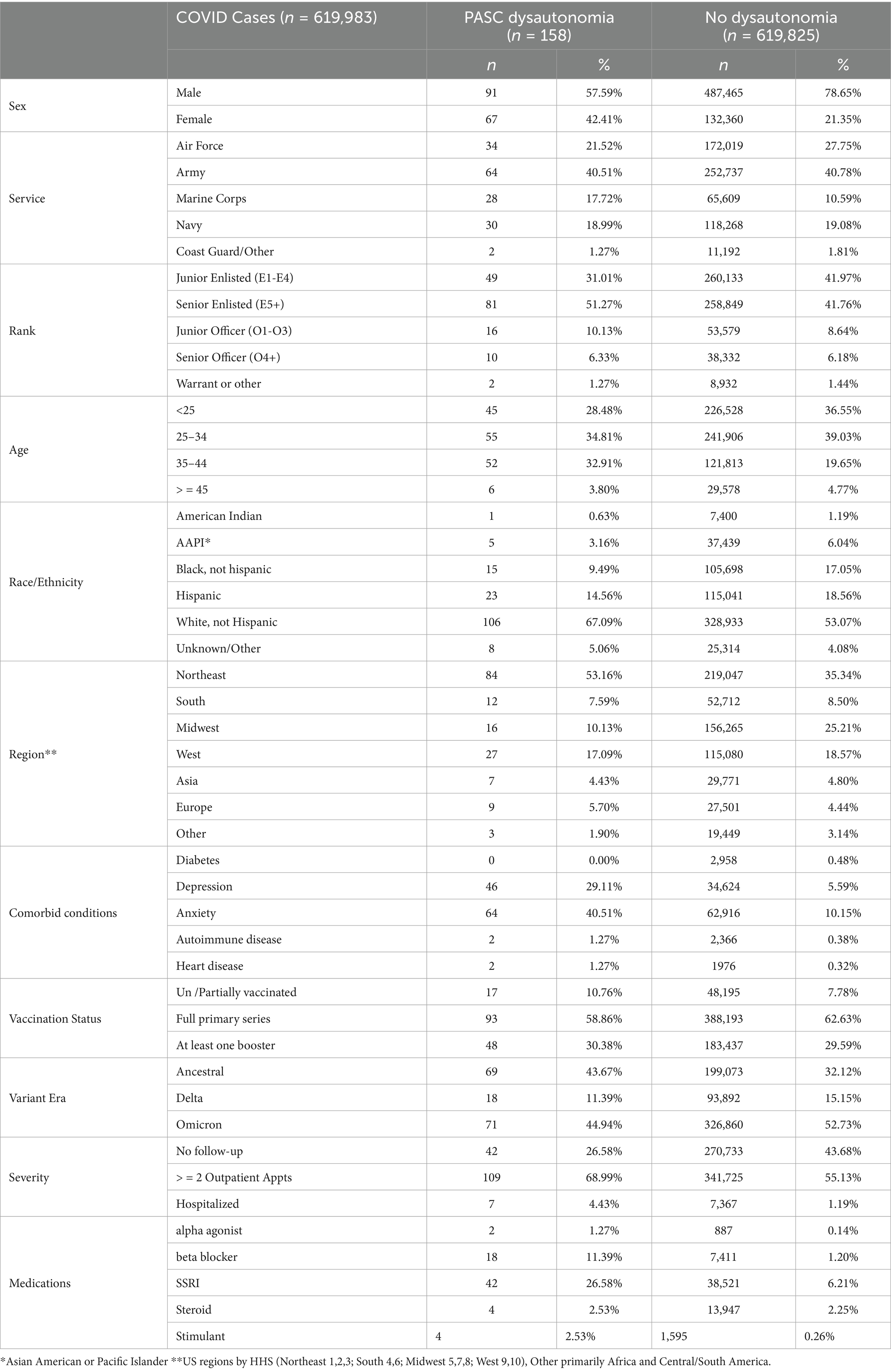
Table 2. Breakdown of relevant factors in COVID-19 cases between those with PASC dysautonomia and those without dysautonomia diagnosis.
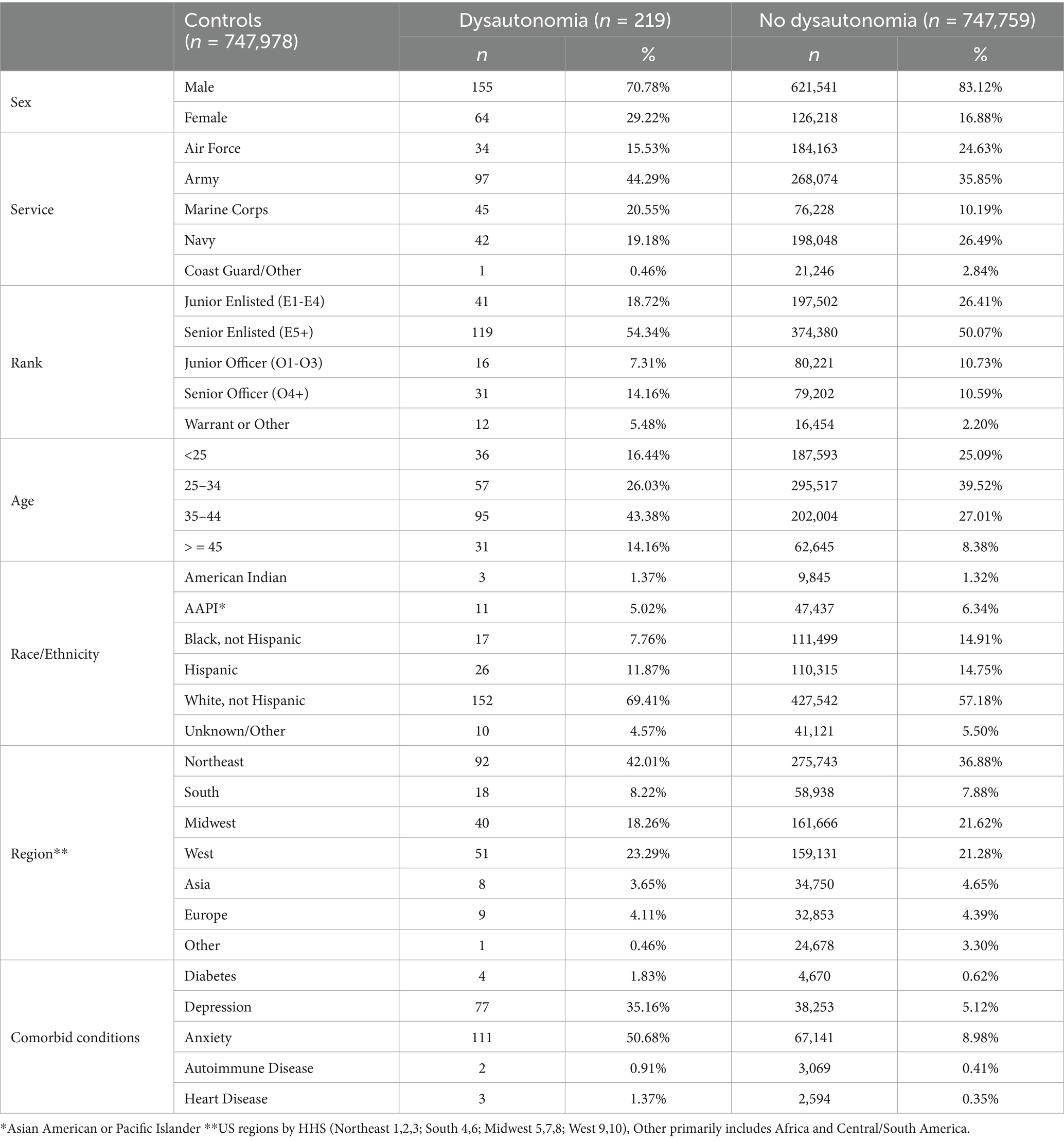
Table 3. Breakdown of studied demographic and medical variables in those with no history of COVID-19 infection, between those with non-PASC dysautonomia and those with no diagnosis of dysautonomia.
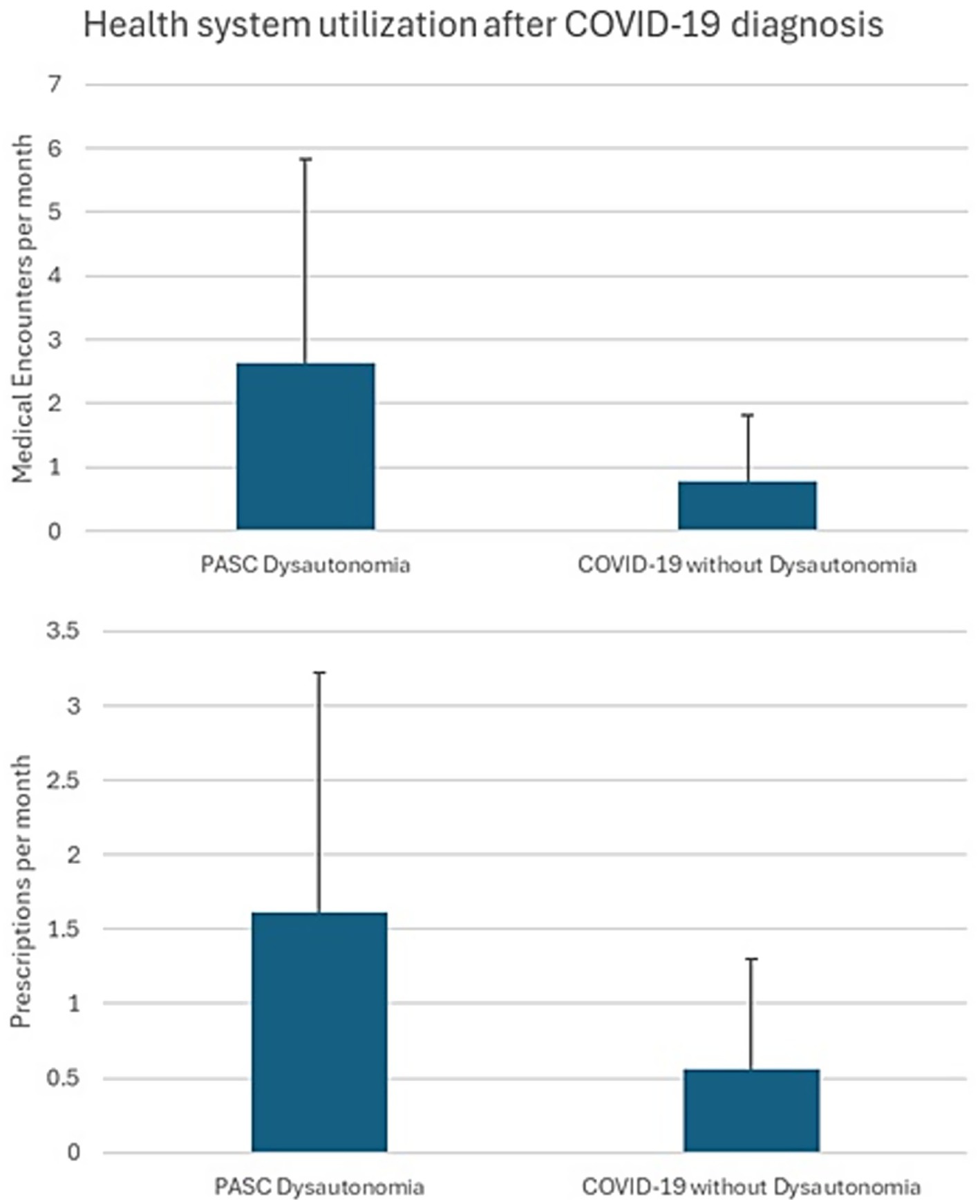
Figure 2. Military health system (MHS) utilization of individuals with PASC dysautonomia and those with COVID-19 infection never diagnosed with dysautonomia.
Analysis 1: PASC dysautonomia among those with COVID-19 infection
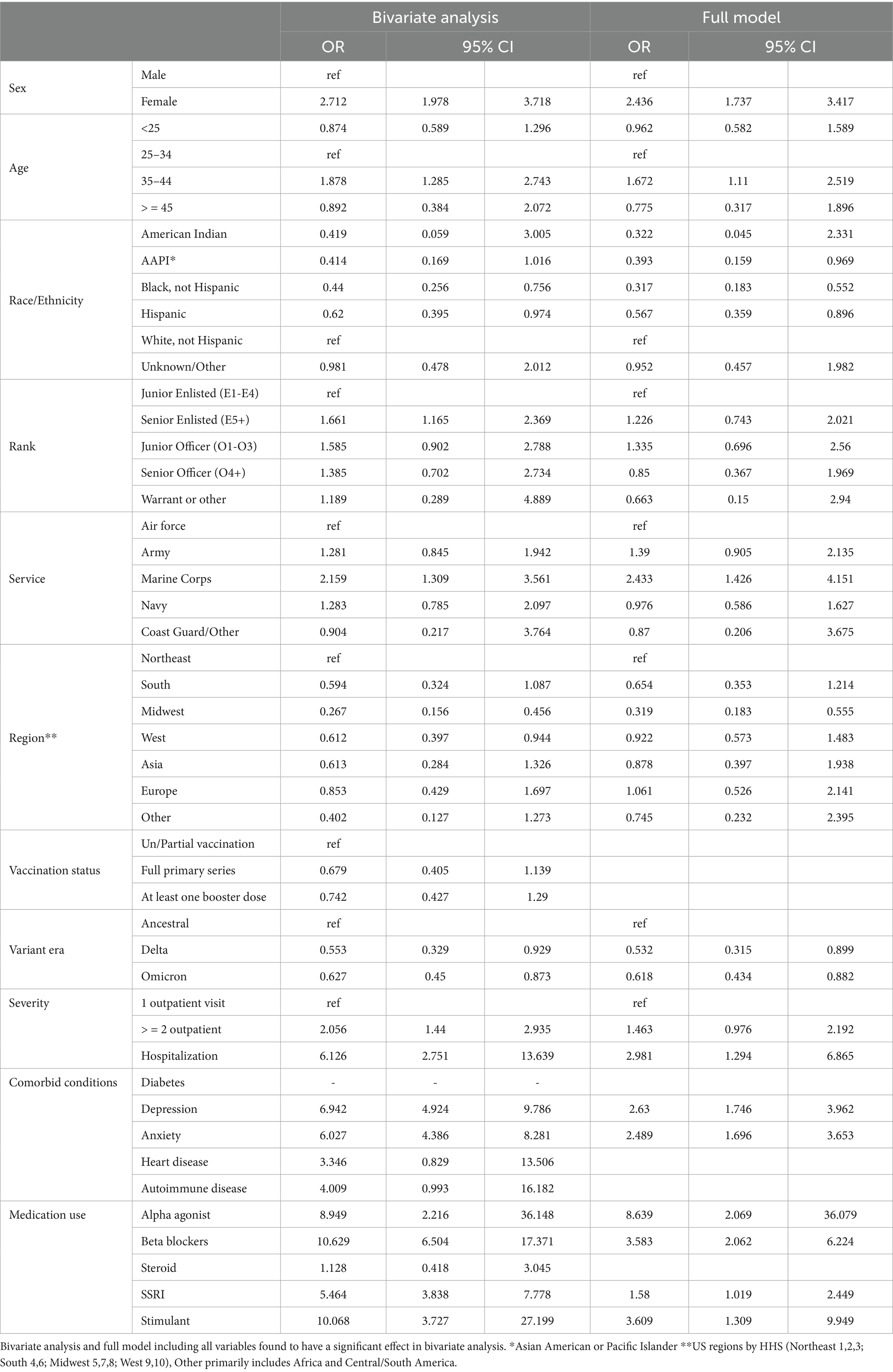
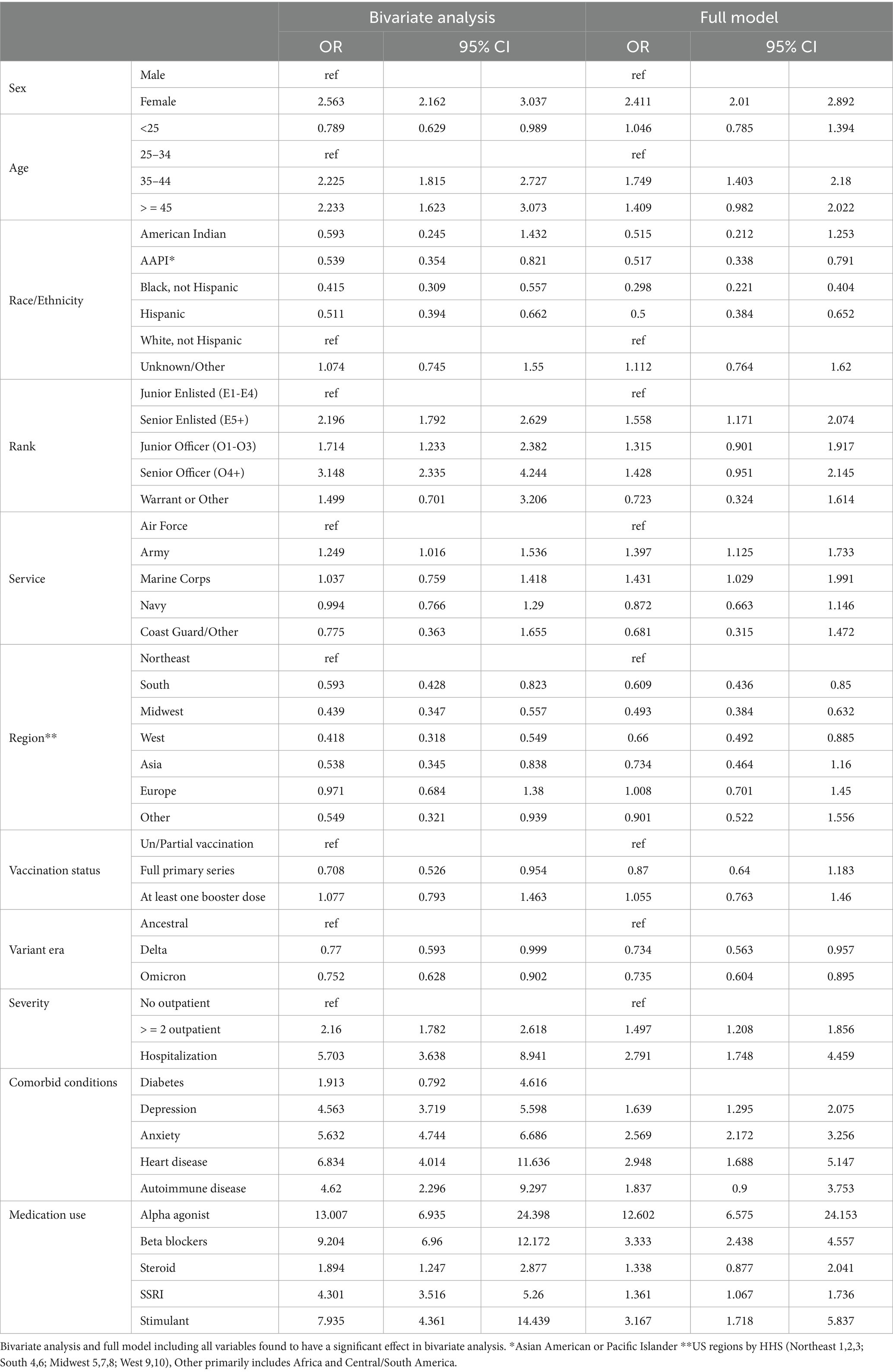
The bivariate analyses evaluating factors among those with COVID-19 infection associated with probable PASC dysautonomia are presented (Table 4). The majority of variables evaluated were found to have significant associations with PASC dysautonomia with the exception of comorbid heart disease, diabetes, autoimmune-related disease, prescription of corticosteroids before COVID infection, and vaccination status. Strikingly, the severity of COVID-19 infection showed a strong association with risk of dysautonomia that increased with the level of severity; additionally in relation to variant era of infection, with earlier variant eras of COVID-19 (prior to Delta variant) more associated with PASC dysautonomia. Further findings related to demographic status including higher risk among those who were white, non-Hispanic, women, aged 35–44, or residing in the Northeast. Prevalence of PASC dysautonomia among Marines also appears to be higher than other Services, and no significant effect associated with rank appears to be present. The prescription of beta blockers, alpha agonists, selective serotonin reuptake inhibitors, or stimulants within 90 days before COVID-19 infection also appears to have a strong positive association with PASC dysautonomia, along with the comorbid conditions of depression and anxiety. The results of the sensitivity analyses conducted following the same approach using the definition of possible PASC dysautonomia are presented (Table 5). Differences noted in the bivariate analyses include additional increased association with PASC dysautonomia in the >45 age group, in Service members in the Army, in ranks above junior enlisted, and among those with a history of heart disease or autoimmune disease and prescribed stimulants or steroids prior to COVID infection. Additionally, receiving the full primary series of COVID-19 vaccination has a protective effect in this group, however those with a booster dose did not have increased protection. In the full model many of these significant findings were attenuated to the point of being non-statistically significant, including the increased odds of PASC dysautonomia in the >45 age group, and ranks other than senior enlisted, comorbid autoimmune disease, concomitant use of steroids, as well as the protective effect of vaccination noted in the bivariate analysis. The results of the sensitivity analysis using the Firth’s penalized likelihood approach are provided in the Supplementary Table S3, with no deviations of note from our primary analysis.
Analysis 2: PASC dysautonomia and non-PASC dysautonomia
The bivariate analyses evaluating factors associated with PASC dysautonomia as compared to non-PASC dysautonomia are presented (Table 6). Those 45 and older, of senior officer rank and residing in the Midwest had significantly lower odds. No differences in sex, race/ethnicity, or Service were noted. Strikingly, in individuals with dysautonomia those with anxiety had 60.4% of the odds of PASC dysautonomia as compared to non-PASC dysautonomia, suggesting that anxiety is significantly less prevalent in those with PASC dysautonomia, with 40.51% of those with PASC dysautonomia having comorbid anxiety as compared to 50.68% of those with non-PASC dysautonomia. In the full model, the effect of rank was attenuated to not significant levels, and the general trends seen in the bivariate analysis persisted throughout, with augmentation of the effect size seen in those with anxiety. The results of these analyses using the sensitivity analyses definition of possible dysautonomia are presented (Table 7). In these analyses, women had increased odds of PASC dysautonomia, those aged 35–44 and greater than 45 had decreased odds, with no significant effects by race/ethnicity, and those residing in Asia or other (Africa / South America) were noted to have higher odds of PASC dysautonomia. Regarding Service specific variables, those in the Army and of Warrant Officer or other rank had decreased odds of PASC dysautonomia. Those with depression or anxiety also had decreased odds. In the full model many of these effects were attenuated, with no significant findings by military Service or comorbid anxiety.
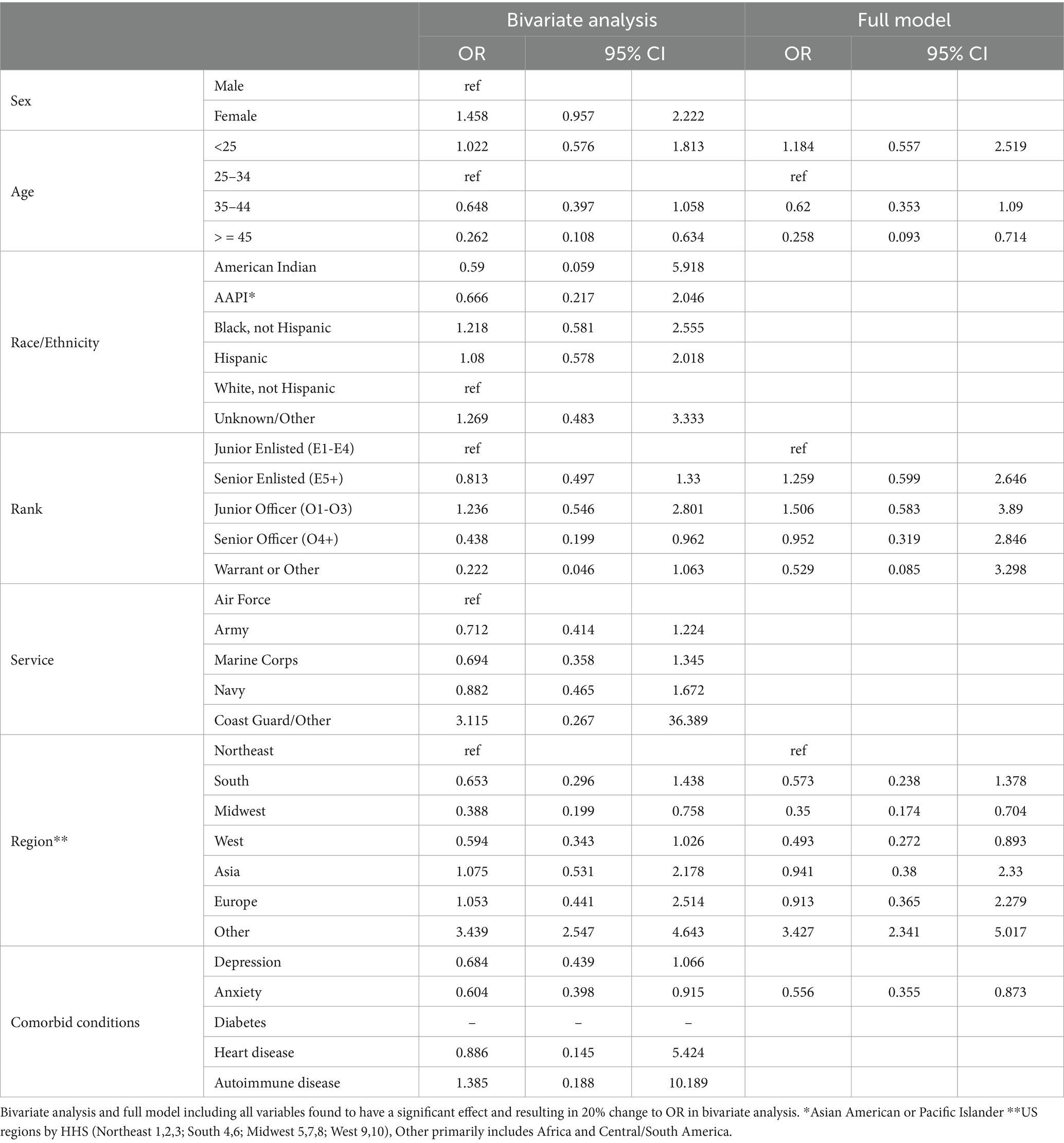
Table 6. Propensity score weighted conditional logistic regression of probable PASC dysautonomia vs. probable non-PASC dysautonomia.
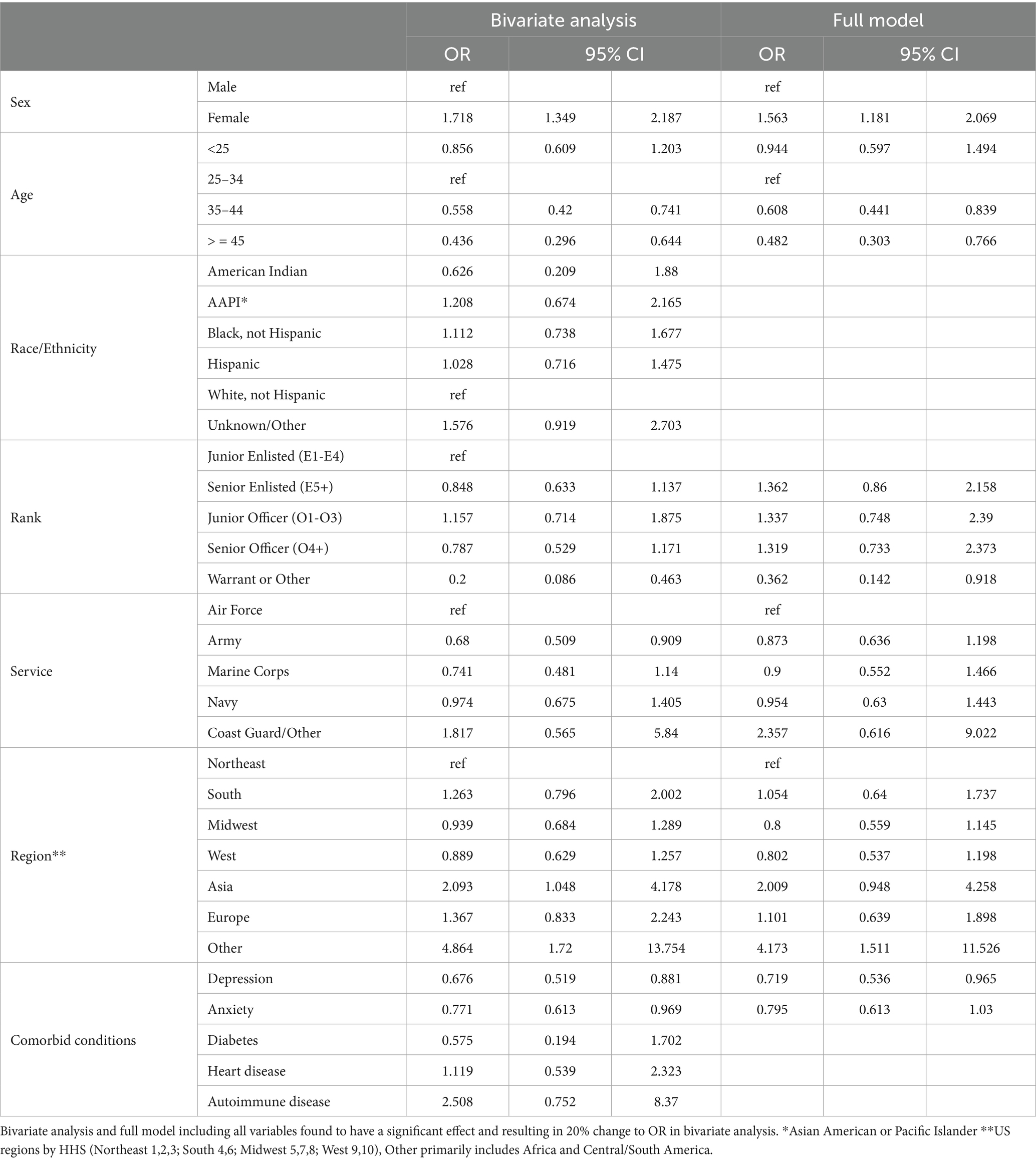
Table 7. Propensity score weighted conditional logistic regression of possible PASC dysautonomia vs. possible non-PASC dysautonomia.
Discussion
Our analysis of 1,367,91 active duty Service members identified 158 individuals with probable PASC dysautonomia diagnosed with COVID-19, as well as 219 individuals with probable non-PASC dysautonomia in those without evidence of COVID-19 infection. Factors that appeared to place individuals infected with COVID-19 at higher risk of PASC dysautonomia included female sex, age 35–44, service in the Marine Corps, residing in the Northeast, severity of infection, infection with COVID-19 prior to the delta variant era of SARS-CoV-2, comorbid depression or anxiety, and prescription of beta blockers, stimulants, selective serotonin reuptake inhibitors, or alpha agonists prior the time of their COVID-19 infection. Individuals with PASC dysautonomia as compared to those with non-PASC dysautonomia also were more likely to be residing in the Northeast, and were less likely to be above age 45, and to have comorbid anxiety. These risk factors may assist clinicians predict those more likely to develop this complication.
While an uncommon complication, we noted that those with PASC dysautonomia had markedly higher medical utilization after their COVID-19 diagnosis as compared to those diagnosed with COVID-19 without a PASC dysautonomia diagnosis, with the lowest medical utilization seen in those without documented COVID-19 infection without dysautonomia. These findings support that PASC dysautonomia is a risk for increased medical utilization within the MHS, and additionally that COVID-19 diagnosis in general also has some impact on increasing medical utilization rates after diagnosis. Those diagnosed with PASC dysautonomia had a median time from COVID-19 infection to dysautonomia diagnosis of approximately 6 months, contrasting to a previous study of individuals with a specific form of dysautonomia (postural orthostatic tachycardia syndrome), who reported a median time from symptom onset to diagnosis of 24 months (36). The relatively faster diagnoses of the individuals in our sample could be due to the increased visibility and attention to PASC syndromes during the height of the COVID-19 pandemic. Time to diagnosis may be further reduced with augmented messaging to MHS providers to raise awareness of this significant COVID-19 complication.
The findings of increased likelihood of PASC dysautonomia with increasing severity of infection are consistent with several other studies on PASC (37–40). Our analysis also indicated that earlier variant eras of SARS-CoV-2 may have presented higher likelihood of PASC dysautonomia; this may be consistent with earlier variants of SARS-CoV-2 being associated with more severe illness, or potentially be due to earlier infections accommodating longer study follow-up time to identify PASC cases (41). While the effect of vaccination was only found to be significant in the bivariate sensitivity analysis (and potentially impacted by confounding), other studies have noted that vaccination status may confer some protection against PASC dysautonomia through attenuation of risk for severe acute COVID-19 disease (7, 15).
Demographic factors affecting the likelihood of PASC dysautonomia have some overlap with other studies, most consistently showing that those with female sex, and white, non-Hispanic race/ethnicity having higher prevalence of PASC (16, 42, 43). We noted an association of PASC dysautonomia with residence in the Northeast. Potential rationale for these differences may include the geographic dispersion of active duty military personnel, with those Service members residing in the Northeast being otherwise different on important risk factors from those in other regions, or that the regional prevalence of PASC dysautonomia differs substantially from the distribution of other PASC syndromes.
Findings related to comorbid conditions also present some interesting associations. The finding of comorbid depression or anxiety being associated with a higher risk for PASC has been described before (44). These conditions have also been described as likely comorbid conditions in some dysautonomia syndromes (45). Our analyses found that, consistent with previous research, comorbid depression and anxiety were associated with development of PASC dysautonomia among those diagnosed with COVID-19. Interestingly, we found those with PASC dysautonomia were less likely to have these comorbid conditions as compared to those with non-PASC dysautonomia. These findings may indicate some difference in the pathophysiology in PASC associated dysautonomia from other non-PASC dysautonomia, raising questions around clinical management. The final finding of interest is of the increased likelihood of PASC dysautonomia among those with prescriptions of alpha agonists and beta blockers prior to diagnosis with COVID-19. This finding is interesting given that these medications are routinely used in the management of dysautonomia conditions (46). It is possible that these patients were being managed symptomatically for an undiagnosed dysautonomia prior to receiving an official diagnosis of this condition, or that a diagnosis of dysautonomia was not entered as an ICD-10 code. It is possible that their COVID infection potentiated these underlying symptoms leading to further engagement with the medical system and receipt of diagnosis. However, interpretations of these findings must be taken cautiously given the low numbers of individuals using these medications (two with PASC dysautonomia on alpha-agonists) (39).
Some of the strengths of this analysis include the broad population of U. S. Military Service members included in the data analysis comprising all identified individuals with documented COVID-19 infection in active duty Service members over the included study period. The design of the study further allowed for analysis not only of risks of PASC dysautonomia among individuals with documented COVID-19 infection, but also the identification of dysautonomia in those never diagnosed with COVID-19, allowing for comparisons of PASC dysautonomia to non-PASC dysautonomia. Additionally, the ability to combine multiple data sources to develop the MRAP dataset allowed for a more comprehensive evaluation of medical, demographic, and Service specific variables for evaluation in our analysis.
There were, however, some key limitations in this study to consider as well. As a study including only active duty military personnel, there are concerns over generalizability to the general U. S. population given the age and sex distribution of active duty military Service members along with a generally high health status (47). It is possible that individuals presenting with potentially debilitating syndromes as might appear in dysautonomia may be deemed unfit for service and not fully captured in this analysis if they were unable to continue their service, although the number of separations in the U. S. Military due to Long COVID is currently described as very low (48). These limitations may have resulted in lower numbers of individuals diagnosed with dysautonomia in our analysis than might be expected in a similarly sizes sample of the general U. S. population. The use of the MDR provides a wealth of information on military medical encounters, however it remains administrative claims data which has significant limitations in interpretability and context of medical encounters. The lack of ability to access clinical documentation and elucidate important factors regarding diagnostic pathways, management, and outcomes for dysautonomia within the MHS may limit assessment of the overall impact of these diagnoses, and potential for biases in time to diagnosis after presentation with key symptoms suggestive of dysautonomia. A limitation in the analysis of this dataset also includes the time of observation of individuals in the study. All included individuals were active duty MHS beneficiaries for at least the time period from January 2020 through June 2023; therefore, individuals who left service prior to June 2023 were not included in the analysis, which could potentially bias the results if those who left the service in this timeframe were meaningfully different than those who did not. Also, while medical records were searched for all included individuals back to 2017, included individuals were not required to be active duty Service members back to this date potentially allowing for biases in length of observation prior to the COVID-19 pandemic. Additional analysis limitations include lack of assessment of interaction terms, and of the impact of repeated COVID-19 infections on dysautonomia status, which some studies have found to be associated with increased incidence of PASC syndromes (49). Finally, the low numbers of overall dysautonomia diagnoses are of concern given the small number of events for certain covariates of interest, however post hoc model diagnostics support the use of the a priori statistical analysis plan and sensitivity analysis using the Firth’s penalized likelihood approach provided similar results.
Next, steps for future research in this space would ideally integrate direct clinical evaluation and management of research participants to better elucidate underlying pathophysiologic mechanisms of PASC dysautonomia and identify potential treatment algorithms for management of these often-complex patients. Further development in this space is currently ongoing in several studies involved in evaluating and treating autonomic dysfunction as a sequelae of COVID-19, including the NIH RECOVER program for PASC research, and Uniformed Services University COVIVA study (50, 51). Further epidemiologic research should seek to further risk stratify individuals for PASC, and further breakdown risks using a syndromic approach as multiple findings in our analysis of PASC dysautonomia were discordant from other findings of risks for PASC overall. Further analysis within this data could attempt to access patient records to assess keywords and use other advanced textual analysis techniques to provide further insight into the clinical course of PASC dysautonomia within the MHS.
In conclusion, PASC dysautonomia is an emerging condition that appears to have some overlap, but also some significant differences in risk factors from non-PASC dysautonomia. Increased severity of COVID-19 infection appears to be a main driver in risk for PASC dysautonomia, with comorbid depression and anxiety also strongly correlated. Early identification and treatment of acute COVID-19 infection to prevent these PASC events is key, and research must focus on developing pathways for identification, typing, and management of PASC syndromes, including PASC dysautonomia to reduce the burden of these conditions on the population.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available from the United States Defense Health Agency. Restrictions apply to the availability of these data, which were used under federal Data User Agreements for the current study and so are not publicly available. Requests to access these datasets should be directed to ZGhhLm5jci5wY2wubWJ4LmRhdGEtc2hhcmluZ0BoZWFsdGgubWls.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
BP: Project administration, Visualization, Methodology, Formal analysis, Writing – review & editing, Investigation, Conceptualization, Writing – original draft. MC-K: Writing – review & editing, Validation, Methodology, Supervision, Investigation, Conceptualization. LS: Writing – review & editing, Methodology, Conceptualization. ES: Conceptualization, Methodology, Writing – review & editing. CS: Resources, Data curation, Writing – review & editing. BG: Resources, Data curation, Writing – review & editing. AM: Writing – review & editing. EP: Writing – review & editing. TK: Supervision, Writing – review & editing, Conceptualization. DS: Conceptualization, Writing – review & editing, Supervision. JM: Conceptualization, Supervision, Writing – review & editing. SP: Validation, Supervision, Methodology, Writing – review & editing, Conceptualization. TB: Conceptualization, Methodology, Writing – review & editing. DT: Supervision, Writing – review & editing, Funding acquisition, Resources, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by awards from the Defense Health Program (HU00012020067) and the National Institute of Allergy and Infectious Disease (HU00011920111). The protocol was executed by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USUHS) through a cooperative agreement by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been funded in part by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, under an interagency agreement (Y1-AI-5072).
Acknowledgments
The authors acknowledge critical advice from the Infectious Disease Clinical Research Program, Military Registry Analysis Project and faculty of the Uniformed Services University Department of Preventive Medicine and Biostatistics including Cara Olsen for review of statistical methods.
Conflict of interest
MC-K, LS, ES, AM, EP, and SP were employed by Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, Department of the Navy, the Department of the Air Force, the Department of Defense or the U. S. Government and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1653175/full#supplementary-material
References
1. Mallah, SI, Ghorab, OK, Al-Salmi, S, Abdellatif, OS, Tharmaratnam, T, Iskandar, MA, et al. COVID-19: breaking down a global health crisis. Ann Clin Microbiol Antimicrob. (2021) 20:35. doi: 10.1186/s12941-021-00438-7
2. Caylà, JA, Bellmunt, JM, Jansà, JM, Marco, A, and Millet, JP. Epidemiological evolution and economic impact of the COVID-19 pandemic in the European Union and worldwide and effects of control strategies on them: an ecological study. Med. Fam. SEMERGEN. (2024) 50:102274. doi: 10.1016/j.semerg.2024.102274
3. Faramarzi, A, Norouzi, S, Dehdarirad, H, Aghlmand, S, Yusefzadeh, H, and Javan-Noughabi, J. The global economic burden of COVID-19 disease: a comprehensive systematic review and meta-analysis. Syst Rev. (2024) 13:68. doi: 10.1186/s13643-024-02476-6
4. World Health Organization. WHO coronavirus (COVID-19) dashboard. Geneva: World Health Organization (2023).
5. Fernandes, Q, Inchakalody, VP, Merhi, M, Mestiri, S, Taib, N, Moustafa Abo El-Ella, D, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. (2022) 54:524–40. doi: 10.1080/07853890.2022.2031274
6. Maltezou, HC, Gamaletsou, MN, Lourida, A, Panagopoulos, P, Giannouchos, TV, Sourri, F, et al. Comparison of morbidity and absenteeism due to COVID-19 and seasonal influenza in a large cohort of health care personnel in the 2022 to 2023 season. Am J Infect Control. (2024) 52:1248–51. doi: 10.1016/j.ajic.2024.05.015
7. Yong, SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. (2021) 53:737–54. doi: 10.1080/23744235.2021.1924397
8. Nalbandian, A, Sehgal, K, Gupta, A, Madhavan, MV, McGroder, C, Stevens, JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
9. Parums, DV. Editorial: post-acute sequelae of SARS-CoV-2 infection (PASC). Updated terminology for the long-term effects of COVID-19. Med Sci Monit. (2023) 29:e941595. doi: 10.12659/MSM.941595
10. Astin, R, Banerjee, A, Baker, MR, Dani, M, Ford, E, Hull, JH, et al. Long COVID: mechanisms, risk factors and recovery. Exp Physiol. (2023) 108:12–27. doi: 10.1113/EP090802
11. Aiyegbusi, OL, Hughes, SE, Turner, G, Rivera, SC, McMullan, C, Chandan, JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. (2021) 114:428–42. doi: 10.1177/01410768211032850
12. Castanares-Zapatero, D, Chalon, P, Kohn, L, Dauvrin, M, Detollenaere, J, Maertens De Noordhout, C, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. (2022) 54:1473–87. doi: 10.1080/07853890.2022.2076901
13. Greenhalgh, T, Sivan, M, Perlowski, A, and Nikolich, JŽ. Long COVID: a clinical update. Lancet. (2024) 404:707–24. doi: 10.1016/S0140-6736(24)01136-X
14. Kersten, J, Baumhardt, M, Hartveg, P, Hoyo, L, Hüll, E, Imhof, A, et al. Long COVID: distinction between organ damage and deconditioning. J Clin Med. (2021) 10:3782. doi: 10.3390/jcm10173782
15. Crook, H, Raza, S, Nowell, J, Young, M, and Edison, P. Long covid—mechanisms, risk factors, and management. BMJ. (2021) 374:n1648. doi: 10.1136/bmj.n1648
16. Robertson, MM, Qasmieh, SA, Kulkarni, SG, Teasdale, CA, Jones, HE, McNairy, M, et al. The epidemiology of long coronavirus disease in US adults. Clin Infect Dis. (2023) 76:1636–45. doi: 10.1093/cid/ciac961
17. Kwan, AC, Ebinger, JE, Wei, J, Le, CN, Oft, JR, Zabner, R, et al. Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-Cov-2 infection. Nat Cardiovasc Res. (2022) 1:1187–94. doi: 10.1038/s44161-022-00177-8
18. Blitshteyn, S, and Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. (2021) 69:205–11. doi: 10.1007/s12026-021-09185-5
19. Dulal, D, Maraey, A, Elsharnoby, H, Chacko, P, and Grubb, B. Impact of COVID-19 pandemic on the incidence and prevalence of postural orthostatic tachycardia syndrome. Eur Heart J. (2025) 11:qcae111. doi: 10.1093/ehjqcco/qcae111
20. Seeley, MC, Gallagher, C, Ong, E, Langdon, A, Chieng, J, Bailey, D, et al. High incidence of autonomic dysfunction and postural orthostatic tachycardia syndrome in patients with long COVID: implications for management and health care planning. Am J Med. (2023) 138:354–361.e1. doi: 10.1016/j.amjmed.2023.06.010
21. Campen, C(L)MCV, and Visser, FC. Long-haul COVID patients: prevalence of POTS are reduced but cerebral blood flow abnormalities remain abnormal with longer disease duration. Healthcare. (2022) 10:2105. doi: 10.3390/healthcare10102105
22. Chadda, KR, Blakey, EE, Huang, CLH, and Jeevaratnam, K. Long COVID-19 and postural orthostatic tachycardia syndrome- is dysautonomia to be blamed? Front Cardiovasc Med. (2022) 9:860198. doi: 10.3389/fcvm.2022.860198
23. El-Rhermoul, FZ, Fedorowski, A, Eardley, P, Taraborrelli, P, Panagopoulos, D, Sutton, R, et al. Autoimmunity in long Covid and POTS. Oxf Open Immunol. (2023) 4:2. doi: 10.1093/oxfimm/iqad002
24. Kavi, L. Postural tachycardia syndrome and long COVID: an update. Br J Gen Pract. (2022) 72:8–9. doi: 10.3399/bjgp22X718037
25. Miglis, MG, Seliger, J, Shaik, R, and Gibbons, CH. A case series of cutaneous phosphorylated α-synuclein in long-COVID POTS. Clin Auton Res. (2022) 32:209–12. doi: 10.1007/s10286-022-00867-0
26. Fedorowski, A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. (2019) 285:352–66. doi: 10.1111/joim.12852
27. Chen, C, Haupert, SR, Zimmermann, L, Shi, X, Fritsche, LG, and Mukherjee, B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a Meta-analysis and systematic review. J Infect Dis. (2022) 226:1593–607. doi: 10.1093/infdis/jiac136
28. Gall, NP, James, S, and Kavi, L. Observational case series of postural tachycardia syndrome (PoTS) in post-COVID-19 patients. Br J Cardiol. (2022) 29:3. doi: 10.5837/bjc.2022.003
29. Goldstein, DS. Post-COVID dysautonomias: what we know and (mainly) what we don’t know. Nat Rev Neurol. (2024) 20:99–113. doi: 10.1038/s41582-023-00917-9
30. Raj, SR, Arnold, AC, Barboi, A, Claydon, VE, Limberg, JK, Lucci, VEM, et al. Long-COVID postural tachycardia syndrome: an American autonomic society statement. Clin Auton Res. (2021) 31:365–8. doi: 10.1007/s10286-021-00798-2
31. Leng, A, Shah, M, Ahmad, SA, Premraj, L, Wildi, K, Li Bassi, G, et al. Pathogenesis underlying neurological manifestations of long COVID syndrome and potential therapeutics. Cells. (2023) 12:816. doi: 10.3390/cells12050816
32. Craig-Kuhn, MC, Stewart, L, Sercy, E, Stern, C, Graham, B, Michel, A, et al. Comparing SARS-CoV-2 infections in the United States military health system and national data: Opportunities for future pandemic surveillance. (2024) Available online at: https://www.researchsquare.com/article/rs-5368521/v1 (Accessed Feb 7, 2025).
33. American Society of Health-System Pharmacists. AHFS Classification – Drug Assignments. (2021). Available online at: https://ahfsdruginformation.com/ahfs-classification-drug-assignments/ (Accessed Feb 7 2025).
34. Tchetgen Tchetgen, EJ. A general regression framework for a secondary outcome in case-control studies. Biostatistics. (2014) 15:117–28. doi: 10.1093/biostatistics/kxt041
35. Schifano, ED. A review of analysis methods for secondary outcomes in case-control studies. Commun Stat Appl Methods. (2019) 26:103–29. doi: 10.29220/CSAM.2019.26.2.103
36. Shaw, BH, Stiles, LE, Bourne, K, Green, EA, Shibao, CA, Okamoto, LE, et al. The face of postural tachycardia syndrome – insights from a large cross-sectional online community-based survey. J Intern Med. (2019) 286:438–48. doi: 10.1111/joim.12895
37. Hill, EL, Mehta, HB, Sharma, S, Mane, K, Singh, SK, Xie, C, et al. Risk factors associated with post-acute sequelae of SARS-CoV-2: an N3C and NIH RECOVER study. BMC Public Health. (2023) 23:2103. doi: 10.1186/s12889-023-16916-w
38. Luo, D, Mei, B, Wang, P, Li, X, Chen, X, Wei, G, et al. Prevalence and risk factors for persistent symptoms after COVID-19: a systematic review and meta-analysis. Clin Microbiol Infect. (2024) 30:328–35. doi: 10.1016/j.cmi.2023.10.016
39. Wu, Y, Sawano, M, Wu, Y, Shah, RM, Bishop, P, Iwasaki, A, et al. Factors associated with long COVID: insights from two Nationwide surveys. Am J Med. (2024) 137:515–9. doi: 10.1016/j.amjmed.2024.02.032
40. Yoo, SM, Liu, TC, Motwani, Y, Sim, MS, Viswanathan, N, Samras, N, et al. Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J Gen Intern Med. (2022) 37:1988–95. doi: 10.1007/s11606-022-07523-3
41. Petrone, D, Mateo-Urdiales, A, Sacco, C, Riccardo, F, Bella, A, Ambrosio, L, et al. Reduction of the risk of severe COVID-19 due to omicron compared to Delta variant in Italy (November 2021 – February 2022). Int J Infect Dis. (2023) 129:135–41. doi: 10.1016/j.ijid.2023.01.027
42. Toepfner, N, Brinkmann, F, Augustin, S, Stojanov, S, and Behrends, U. Long COVID in pediatrics—epidemiology, diagnosis, and management. Eur J Pediatr. (2024) 183:1543–53. doi: 10.1007/s00431-023-05360-y
43. Su, S, Zhao, Y, Zeng, N, Liu, X, Zheng, Y, Sun, J, et al. Epidemiology, clinical presentation, pathophysiology, and management of long COVID: an update. Mol Psychiatry. (2023) 28:4056–69. doi: 10.1038/s41380-023-02171-3
44. Wang, S, Quan, L, Chavarro, JE, Slopen, N, Kubzansky, LD, Koenen, KC, et al. Associations of depression, anxiety, worry, perceived stress, and loneliness prior to infection with risk of post–COVID-19 conditions. JAMA Psychiatry. (2022) 79:1081–91. doi: 10.1001/jamapsychiatry.2022.2640
45. Kakavand, B, Centner, A, Centner, S, and Hasan, S. The prevalence of anxiety and depression in children with postural orthostatic tachycardia syndrome (POTS): a retrospective study. Cureus. (2024) 16:e69941. doi: 10.7759/cureus.69941
46. Mar, PL, and Raj, SR. Postural orthostatic tachycardia syndrome: mechanisms and new therapies. Annu Rev Med. (2020) 71:235–48. doi: 10.1146/annurev-med-041818-011630
47. Olapeju, B, Ahmed, AE, Chu, K, Yoon, J, and Mancuso, J. Perceived health status and medical conditions among US active-duty service members. Psychol Res Behav Manag. (2023) 16:5121–38. doi: 10.2147/PRBM.S429341
48. Department of Defense. Report on the effects of long COVID on the readiness and retention of Servicemembers. Department of Defense. (2023). Available online at: https://www.health.mil/Reference-Center/Reports/2023/11/13/Effects-of-Long-COVID.
49. Hadley, E, Yoo, YJ, Patel, S, Zhou, A, Laraway, B, Wong, R, et al. Insights from an N3C recover EHR-based cohort study characterizing SARS-CoV-2 reinfections and long COVID. Commun Med. (2024) 4:129. doi: 10.1038/s43856-024-00539-2
50. RECOVER Clinical Trials. AUTONOMIC. Available online at: https://trials.recovercovid.org/autonomic (Accessed Feb 12, 2025).
51. Saunders, D, Arnold, TB, Lavender, JM, Bi, D, Alcover, K, Hellwig, LD, et al. Comparative cohort study of post-acute covid-19 infection with a nested, randomized controlled trial of Ivabradine for those with postural orthostatic tachycardia syndrome (the COVIVA study). medRxiv. (2023) 16:1550636. doi: 10.3389/fneur.2025.1550636
Keywords: COVID-19, long Covid, post-acute sequela of COVID-19, dysautonomia, case-control study, US military, autonomic disorders
Citation: Pierson BC, Craig-Kuhn MC, Stewart L, Sercy E, Stern CA, Graham B, Michel A, Parmelee E, Koehlmoos TP, Saunders D, Mancuso JD, Pollett S, Burgess T and Tribble DR (2025) Evaluating the risk and risk factors of dysautonomia as a post-acute sequelae of COVID-19: a secondary analysis of a matched case–control dataset. Front. Neurol. 16:1653175. doi: 10.3389/fneur.2025.1653175
Edited by:
Miriam Sklerov, University of North Carolina at Chapel Hill, United StatesReviewed by:
Osmar Antonio Jaramillo-Morales, University of Guanajuato, MexicoSean Apap Mangion, University College London, United Kingdom
Copyright © 2025 Pierson, Craig-Kuhn, Stewart, Sercy, Stern, Graham, Michel, Parmelee, Koehlmoos, Saunders, Mancuso, Pollett, Burgess and Tribble. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin C. Pierson, YmVuamFtaW4uYy5waWVyc29uLm1pbEBoZWFsdGgubWls
†ORCID: Benjamin C. Pierson, https://orcid.org/0009-0004-8642-293X
 Benjamin C. Pierson
Benjamin C. Pierson Megan Clare Craig-Kuhn
Megan Clare Craig-Kuhn Laveta Stewart2,3
Laveta Stewart2,3 Tracey Perez Koehlmoos
Tracey Perez Koehlmoos David Saunders
David Saunders David R. Tribble
David R. Tribble