Abstract
Background:
While case studies have suggested that only a minority of patients with putaminal lesions develop parkinsonism, existing data are limited by involvement of adjacent brain regions, brief follow-up period, and lack of systematic imaging. As a result, the true incidence and nature of parkinsonism following isolated striatal infarcts remain unknown. This study aimed to assess the incidence, timing, and clinical features of parkinsonism following isolated striatal infarcts using comprehensive imaging and longitudinal clinical assessments.
Methods:
We conducted a prospective cohort study at Oslo University Hospital, including patients treated with intravenous thrombolysis and/or mechanical thrombectomy for acute ischemic stroke resulting in isolated striatal infarcts. Patients with NIHSS scores of six or above were excluded to reduce the influence of other neurological deficits. Clinical evaluations included NIHSS, MDS-UPDRS, and MoCA scales at 3 months and 1 year. Brain MRI was performed at 3 months and [123I]FP-CIT SPECT imaging at 1 year to assess dopaminergic integrity.
Results:
Fifteen patients (median age 61) with unilateral striatal infarcts were included between June 2020 and January 2023. The median NIHSS score was one at both follow-ups. By 3 months, 27% (4/15) of patients developed parkinsonism, increasing to 67% (10/15) at 1 year. MDS-UPDRS motor scores showed a progressive increase over time, with contralateral akinetic-rigid symptoms predominating. Cognitive performance remained stable, with no significant changes in MoCA scores. Both the volume and location of the infarct appeared to influence the likelihood of developing motor symptoms. All patients showed reduced [123I]FP-CIT uptake in the infarcted striatum.
Conclusion:
This is the first study to systematically investigate delayed-onset parkinsonism in patients with isolated striatal infarcts, and our findings indicate that it may occur more frequently than previously recognized. These results challenge existing assumptions and highlight the potential value of repeated, targeted assessments in this population to improve detection and management of post-stroke parkinsonism.
1 Introduction
While it is widely known that lesions of the nigrostriatal tract can cause parkinsonism (1–3), there are a number of observations suggesting that putaminal lesions alone most often are not sufficient to cause parkinsonism, questioning the validity of the current models of the nigrostriatal tract in parkinsonism. In an influential meta-analysis among patients with putaminal lesions (all bilateral), only 2/20 patients had parkinsonism (4). In another study, which included nine patients with isolated putaminal infarcts, no patients exhibited parkinsonism when assessed during their hospital stay (5). Another study, however, found that in patients with CT findings of lacunar infarcts in either the caudate, lentiform nucleus or both, 17/45 patients (38%) had developed parkinsonism (6). In 2004, a study investigated the incidence of parkinsonism associated with striatal infarcts, focusing on lesions larger than 1.5 cm involving the basal ganglia and internal capsule (7). Here, the patients were examined on one occasion (5–15 months after the stroke), and only 1/11 patients developed parkinsonism.
Previous studies have faced several challenges. First, spontaneous striatal stroke lesions most often extend to the surrounding structures, and spontaneous isolated striatal strokes are rare. Lesions involving the structures surrounding the striatum might cause other symptoms, such as sensorimotor paresis and dystonia, which can complicate clinical evaluation or mask parkinsonism altogether. Second, post-stroke movement disorders have been reported with highly variable delays after the occurrence of stroke, and parkinsonism might not yet be present if assessing the patient too early. Third, most of the earlier studies were conducted decades ago when high-field strength MRI was not widely available, limiting the accuracy of stroke lesion localization. Finally, there are only a handful of cases of post-stroke parkinsonism scanned with molecular brain imaging techniques that allow for verification of a resulting striatal presynaptic dopamine defect (7, 8).
The modern era in neurology has opened new opportunities for studying isolated striatal infarcts. With the expanding use of intravenous thrombolysis (IVT) and mechanical thrombectomy (MT), more patients are likely to experience isolated striatal lesions. The basal ganglia are supplied by lenticulostriatal perforating arteries, which are terminal arteries branching from the proximal middle cerebral artery (9). The basal ganglia therefore lack collateral blood supply, making the striatum particularly vulnerable to ischemic damage. By the time reperfusion therapy is administered, an occlusion of the M1 segment of the middle cerebral artery can therefore cause irreversible striatal ischemia while sparing the cerebral cortex (10), resulting in isolated striatal lesions.
The aim of this study was to evaluate the incidence and clinical and imaging characteristics of parkinsonism following isolated striatal stroke lesions. We conducted a prospective, observational cohort study of patients who had an isolated striatal infarct following a middle cerebral artery occlusion with successful reperfusion therapy. The patients were followed up for a year after the occurrence of the stroke with repeated clinical assessments, brain MRI, and [123I]FP-CIT SPECT.
2 Methods
2.1 Study population
Study participants had suffered from acute ischemic stroke and were admitted for MT evaluation at Oslo University Hospital, Rikshospitalet in Norway, a regional thrombectomy center as well as a tertiary referral hospital for movement disorders. Patients enrolled in the study had a residual striatal lesion identified on T2-FLAIR weighted images from standardized MRI stroke protocol 1 day post-stroke. The enrollment period was from 1 June 2020 to 31 January 2023. During this period, 439 patients received MT, with or without prior IVT. Out of these, 13 patients met the inclusion criteria for the study. Two additional patients, who were evaluated for MT but ultimately received IVT alone or IVT in combination with intracerebral stent, also met the inclusion criteria. Thus, a total of 15 participants were included. A simplified patient flow diagram of the enrollment process is presented in Figure 1.
Figure 1

Patient flow diagram of study inclusion. MT, mechanical thrombectomy. aPrecise data on the total number of patients not available. Created in BioRender. Holm, H. (2025) https://BioRender.com/ixdskjs.
The exclusion criteria were: (i) infarcts extending into adjacent structures, e.g., the pallidum or internal capsule, cortical infarcts identified on T2-FLAIR weighted images, or extensive white matter lesions, (ii) score of six or above using the National Institutes of Health Stroke Scale (NIHSS) at discharge, as higher scores may reflect deficits that interfere with reliable motor examination, and (iii) any comorbidity that could confound the clinical or radiological evaluation.
2.2 Clinical evaluation
NIHSS (range 0–42) (11) was used to determine inclusion in the study. Neurological examination was then performed approximately 3 months and again 1 year following the acute stroke. The primary outcome of the study was diagnosis of parkinsonism, which required bradykinesia plus rest tremor and/or rigidity (12). While not a formal criterion, all patients with parkinsonism in this study exhibited bradykinesia with decrement, consistent with clinical parkinsonism. A movement disorder specialist verified the diagnosis.
Motor and non-motor symptoms of parkinsonism were further evaluated using the MDS Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) (13). All examiners had completed the official MDS-UPDRS training and certification program. MDS-UPDRS part III (range 0–132) represents the motor examination and was conducted by two examiners, one of them a movement disorder specialist who was blinded to the clinical data and imaging results. Each examiner performed the assessment separately, after which a consensus score was reached. The MDS-UPDRS III score was also divided into bradykinetic-rigid symptoms (items 2–8 and 14), axial symptoms (items 1, 3 (neck), and 9–13), and tremor (items 15–18) (14). MDS-UPDRS part I and II (both range 0–52) concern non-motor and motor experiences of daily living, respectively. Cognition was assessed using the Montreal Cognitive Assessment (MoCA; range 0–30), which is more sensitive than Mini-Mental State Examination in detecting mild cognitive impairment (15), especially in vascular dementia (16) and in patients with parkinsonism (17). A commonly used MoCA cutoff score to indicate cognitive impairment is 26/30 (15). The test was conducted exclusively with patients whose native language was Norwegian.
2.3 MRI
1.5 T brain MRI was performed at three-month follow-up to measure lesion volumes. All measurements were conducted by the same examiner to ensure consistency in the analyses. T2-FLAIR weighted images were selected to measure lesion volumes, and images with slice thickness of 1 mm were uploaded to ITK-SNAP, a software application which provides semi-automatic segmentation and 3D volume calculation of structures in medical images (18). Following auto-segmentation, manual adjustment were made to refine the segmented region-of-interest. Infarct volumes were calculated based on the number of hyperintense voxels. We then calculated an infarct volume proportion that describes the proportion of the lesion volume relative to the total volume of the putamen, caudate, and striatum as a whole on the contralateral side. Due to varying image quality and natural variation in basal ganglia volume, the infarct volume proportion was used for statistical analyses rather than absolute infarct volume, so that each patient served as their own control. All imaging data were also reviewed and described by board-certified neuroradiologists as part of routine clinical assessment, providing an additional level of validation.
2.4 SPECT imaging
SPECT imaging was performed at 1 year using the same device for all study participants (Discovery 670 Pro). Nuclear medicine specialists conducted both the visual inspection of image quality and the semi-quantitative analysis. Specific [123I]FP-CIT binding was calculated by using region-to-occipital-cortex ratio for six striatal subregions (caudate, anterior and posterior putamen). The classification of patients to normal and abnormal [123I]FP-CIT binding groups was based on DaTQuant, a commercial software provided by GE HealthCare (United States), which enables automatic semi-quantitative calculations of striatal uptake. The software compares patient values to a normative database of 118 healthy controls (73 men, 45 women, age range 31–84 years) from the Parkinson Progression Markers Initiative. Abnormal ligand uptake was defined as equal or more than two standard deviations below the reference mean.
2.5 Statistical analyses
Stata 18 was used for statistical analyses. Analyses were performed using complete case methodology. That is, participants with missing data for a given variable were excluded from that specific analysis, and no data imputation was performed. A summary of the number of participants with available data for each clinical test and imaging modality at three-month and one-year follow-up is provided in Supplementary Table 1.
The primary focus of this study was descriptive. All hypothesis testing was conducted using the bootstrap t-technique with 10,000 replications, which resamples the original dataset to generate confidence intervals and p-values without relying on parametric assumptions (19). Only main, predefined comparisons were tested statistically; all other subgroup or exploratory analyses are presented descriptively with confidence intervals only, due to limited statistical power. Given the small number of prespecified tests, no correction for multiple comparisons was applied. Associations between continuous variables were examined using Pearson’s correlation coefficients, with bootstrap-derived p-values. All statistical tests were two-tailed, and p-values < 0.05 were considered statistically significant.
3 Results
Fifteen patients were included into the study and no patients were lost to follow-up (Table 1). All participants had been successfully treated for M1 occlusions, including five patients also treated for additional occlusions of the internal carotid artery and/or M2 segment. The median NIHSS score was 14 at admission (range 6–21) and two at discharge (range 0–5).
Table 1
| Patient characteristics | Values |
|---|---|
| Age at baseline [yr in median (range)] | 61 (43–74) |
| Sex [n (%)] | |
| Male | 8/15 (53%) |
| Female | 7/15 (47%) |
| Stroke treatment [n (%)] | |
| Only IVT | 1/15 (6.7%) |
| Only MT | 4/15 (26.7%) |
| IVT and MT | 9/15 (60%) |
| IVT and intracerebral stent | 1/15 (6.7%) |
| Affected cortical hemisphere [n (%)] | |
| Right | 11/15 (73%) |
| Left | 4/15 (27%) |
| Affected part of striatum [n (%)] | |
| Putamen | 15/15 (100%) |
| Caudate | 14/15 (93%) |
| Time of follow-up [d after stroke in median (range)] | |
| 1st follow-up | 103 (87–159) |
| 2nd follow-up | 383 (341–487) |
Demographics.
d, days; IVT, intravenous thrombolysis; MT, mechanical thrombectomy; yr, years.
3.1 Parkinsonism and MDS-UPDRS III
At three-month follow-up, 4/15 patients (27%) had developed parkinsonism. By one-year follow-up, the prevalence of parkinsonism had increased to 10/15 (67%; Table 2). Patients who developed parkinsonism by 3 months were older than those who did not, but there was no age difference between the groups at 1 year. Across the whole cohort, the mean MDS-UPDRS III score increased significantly from three-month to one-year follow-up (p < 0.001; Figure 2). The score was notably higher in the parkinsonism group compared to the non-parkinsonism group at both time points. In the four patients with parkinsonism already at three-month follow-up, the MDS-UPDRS III score rose substantially from 3 months to 1 year. For the five patients who did not develop parkinsonism by the end of the follow-up, there also was a slight increase in the MDS-UPDRS III scores (Table 3).
Table 2
| Parkinsonism characteristics | 3 mos [n (%)] | 1 yr [n (%)] |
|---|---|---|
| Incidence of parkinsonism | 4/15 (27%) | 10/15 (67%) |
| Symptom type in patients with parkinsonism | ||
| Rigidity | 3/4 (75%) | 10/10 (100%) |
| Rest tremor | 1/4 (25%) | 2/10 (20%) |
| Symptom side compared to the lesion in patients with parkinsonism | ||
| Contralaterally | 4/4 (100%) | 9/10 (90%) |
| Bilaterally | 0/4 (0%) | 1/10 (10%) |
| Body parts involved in patients with parkinsonism | ||
| Only upper extremities | 3/4 (75%) | 6/10 (60%) |
| Upper and lower extremities | 1/4 (25%) | 4/10 (40%) |
Distribution of parkinsonism.
mos, months; yr, year.
Figure 2
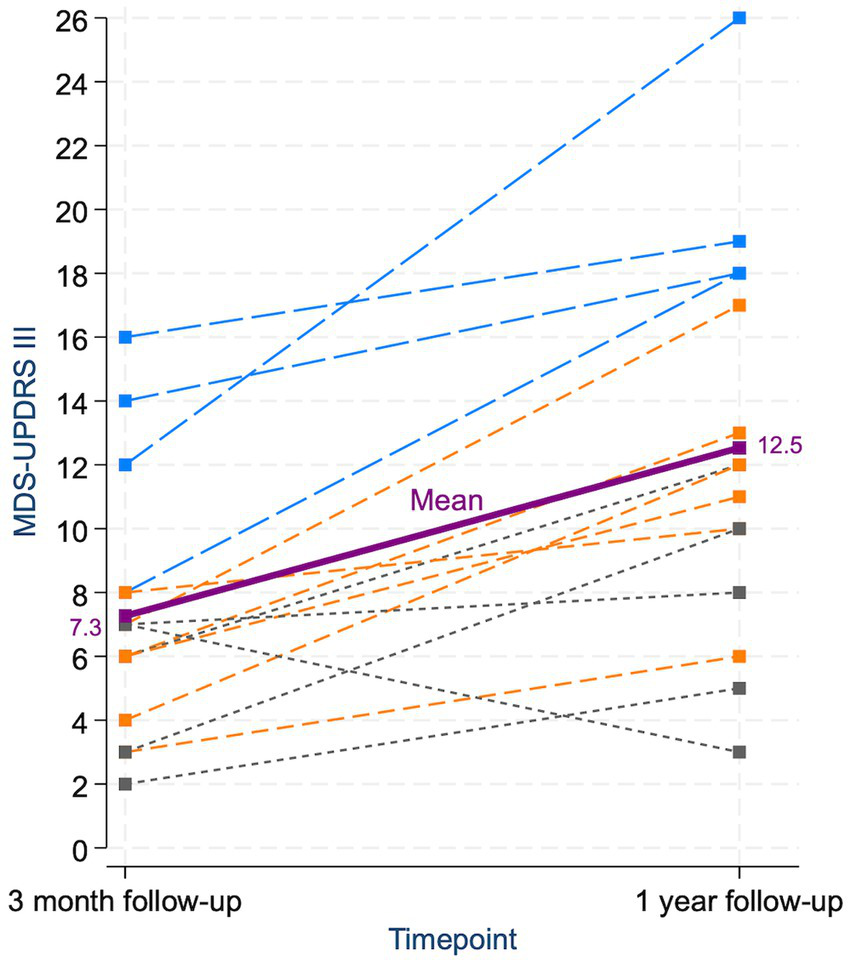
Individual and mean changes in MDS-UPDRS III scores from three-month to one-year follow-up. Each dashed line represents a single patient’s score trajectory. Patients with parkinsonism at both follow-ups are shown in blue, those who developed parkinsonism only at 1 year in orange, and those who never had parkinsonism in gray. The purple line and square markers represent the mean score at each timepoint. The increase in mean MDS-UPDRS III score from 3 months to 1 year was statistically significant. Only one patient showed a decrease in score; this patient exhibited isolated ipsilateral bradykinesia at 3 months, which had improved by 1 year, and never fulfilled criteria for parkinsonism.
Table 3
| Clinical characteristics | 3 mos | 1 yr |
|---|---|---|
| Age at baseline [median (range)] | ||
| Parkinsonism |
n = 4 73 (67–77) |
n = 10 61.5 (47–77) |
| No parkinsonism |
n = 11 57 (43–72) |
n = 5 61 (43–72) |
| MDS-UPDRS III score [mean (95% CI)] | ||
| Whole group | 7.3 (5.0–9.5) | 12.5 (9.1–15.9) |
| Parkinsonism vs. no parkinsonism | ||
| Parkinsonism | 12.5 (7.1–17.9) | 15.0 (10.9–19.1) |
| No parkinsonism | 5.4 (4.0–6.7) | 7.6 (3.1–12.1) |
| Parkinsonism and no parkinsonism consistently | ||
| Parkinsonism consistently (n = 4) | 12.5 (7.1–17.9) | 20.3 (14.1–26.4) |
| No parkinsonism consistently (n = 5) | 5.0 (2.1–7.9) | 7.6 (3.1–12.1) |
| MDS-UPDRS III symptom subscores [mean (95% CI)] | ||
| Bradykinesia + rigidity | ||
| Parkinsonism | 10.0 (7.5–12.5)a | 11.1 (9.0–13.2) |
| No parkinsonism | 4.9 (3.5–6.3) | 6.6 (3.5–9.7) |
| Axial symptoms | ||
| Parkinsonism | 2.7 (0–5)a,b | 2.9 (0–6)b |
| No parkinsonism | 0.5 (0–3)b | 0.8 (0–3)b |
| Tremor | ||
| Parkinsonism | 1.3 (0–4)a,b | 1.0 (0–5)b |
| No parkinsonism | 0.0 (0–0)b | 0.2 (0–1)b |
| MDS-UPDRS III score contra- and ipsilaterally to lesion in parkinsonism [mean (95% CI)] | ||
| Contralaterally | 9.3 (7–11)a,b | 9.3 (7.5–11.1) |
| Ipsilaterally | 0.7 (0–1)a,b | 1.7 (0.6–2.8) |
| MDS-UPDRS III score in upper and lower extremities in parkinsonism [mean (95% CI)] | ||
| Upper extremities | 6.3 (2.5–10.1)a | 7.1 (4.8–9.3) |
| Lower extremities | 3.7 (0.8–6.5)a | 4.0 (3.1–4.9) |
| MoCA score [mean (95% CI)] | ||
| Whole group | 28.0 (27.0–29.4)a | 28.5 (27.4–29.5)a |
| Parkinsonism vs. no parkinsonism | ||
| Parkinsonism | 26.5 (23.7–29.3) | 28.3 (26-30)a,b |
| No parkinsonism | 29.1 (28.3–30.0)a | 29.0 (28-30)a,b |
| NIHSS score [median (range)] | ||
| Whole group | 1 (0–2) | 1 (0–2) |
Descriptive clinical characteristics in patients with and without parkinsonism at 3 mos and 1 yr.
CI, confidence interval; mos, months; yr, year. aScore not available for all patients; see Supplementary Table 1 for number of observations per variable. bCI exceeded the instrument’s valid range. Values are therefore presented as mean (range) instead.
9/10 patients experienced hemiparkinsonism on the contralateral side, whereas one patient presented with bilateral parkinsonism at one-year follow-up, albeit with predominant symptoms contralaterally to the infarct. The MDS-UPDRS III score was significantly higher for the contralateral side in patients with parkinsonism at 1 year (p < 0.001; Table 3). Patients with parkinsonism primarily exhibited bradykinesia and rigidity, with tremor being rare (Table 2). Consequently, the MDS-UPDRS III scores were high for bradykinesia and rigidity but low for tremor and axial symptoms (Table 3). All patients with parkinsonism showed symptoms in their upper extremities, with some also showing additional symptoms in their lower extremities (Table 2). The MDS-UPDRS III score was significantly higher for upper compared to lower extremities (p = 0.001; Table 3). Among the four patients with parkinsonism at three-month follow-up, dopaminergic treatment was initiated in one patient. This anecdotal case did not experience improvement with levodopa/carbidopa 100/25 mg administered three times daily and opted not to try a higher dosage.
3.2 MDS-UPDRS I-II and cognition
For MDS-UPDRS I and II, there were no significant changes in scores between 3 months and 1 year (p = 0.278 and p = 0.559, respectively), and no marked differences between patients with and without parkinsonism at either time point (Table A1). At three-month follow-up, only one patient had a MoCA score one point below the cutoff score. However, by 1 year, all patients achieved scores above cutoff. The mean MoCA score for the entire group was 28/30 both at 3 months and 1 year, with no significant difference observed between these time points (p = 0.617). At 3 months, the four patients with parkinsonism had a lower MoCA score compared to patients without parkinsonism, but this difference was only minor at 1 year (Table 3).
3.3 MRI
All patients had unilateral infarcts in the posterior putamen on MRI, with partial involvement of the caudate in all except one patient (Table 1). The infarct volume proportion (proportion of infarct volume relative to the contralateral total nucleus volume) was significantly higher in the putamen compared to the caudate (p = 0.005). Patients with parkinsonism had higher infarct volume proportions in the putamen compared to those without parkinsonism, whereas no substantial difference was observed in the caudate. The infarct volume proportion in the putamen had a positive and stronger correlation with MDS-UPDRS III score at 1 year compared to the caudate, although neither correlation was significant. In addition, neither the infarct volume proportion of the putamen nor the caudate was significantly correlated with the MoCA score (Table 4).
Table 4
| Groups | Infarct volume from total putamen volume [median (range)] | Infarct volume from total caudate volume [median (range)] |
|---|---|---|
| Whole group | 45% (2–100) | 19% (0–98) |
| Parkinsonism vs. no parkinsonism at 1 yr | ||
| Parkinsonism | 53% (5–100) | 19% (9–71) |
| No parkinsonism | 24% (2–97) | 24% (0–98) |
| Clinical scores | Correlation with infarct volume from total putamen volume [r (95% CI)] | p-value (r) | Correlation with infarct volume from total caudate volume [r (95% CI)] | p-value (r) |
|---|---|---|---|---|
| MDS-UPDRS III score at 1 yr | 0.39 (−0.29–0.80) | 0.164 | −0.02 (−0.51–0.45) | 0.923 |
| MoCA score at 1 yra | −0.13 (−0.61–0.42) | 0.618 | −0.02 (−0.54–0.45) | 0.930 |
Lesion sizes and their association with clinical scores.
CI, Confidence interval; r, correlation coefficient; yr, year. aScore not available for all patients; see Supplementary Table 1 for number of observations per variable.
3.4 SPECT
All patients showed reduced radiotracer uptake on [123I]FP-CIT SPECT at the stroke lesion site based on the visual evaluation (Figure 3). According to semi-quantitative regional analysis, 11/15 patients (73%) had abnormal SPECT imaging (Z-score ≤ −2 in at least one area), of which all included the posterior putamen. 8/10 patients with parkinsonism and 3/5 patients without parkinsonism at 1 year had abnormal semi-quantitative SPECT binding (Table 5). The Z-score was significantly lower on the infarcted side (p < 0.001), and it was lower in the putamen for patients with parkinsonism compared to those without (Table A2). There was a significant correlation between SPECT Z-score and infarct volume proportion for the striatum, indicating that the volume of the structural damage was coupled with the degree of dopamine deficit. However, there was no significant correlation between the MDS-UPDRS III score and striatal Z-scores for the striatum as a whole or for the posterior putamen (Table A3).
Figure 3
![Three-panel figure displaying brain imaging from a patient with an isolated striatal infarct. The left panel shows a T2-FLAIR MRI scan, demonstrating an infarct in the left posterior putamen. The middle panel presents a [¹²³I]FP-CIT SPECT image with color coding indicating reduced ligand uptake in the same region. The right panel displays a fused MRI and SPECT image, where the colored overlay highlights that the area of decreased ligand uptake corresponds closely with the vascular lesion seen on MRI.](https://www.frontiersin.org/files/Articles/1653832/xml-images/fneur-16-1653832-g003.webp)
MRI and [123I]FP-CIT SPECT in a patient with an isolated striatal infarct. (A) T2-FLAIR weighted image shows infarct in the left posterior putamen. (B) [123I]FP-CIT SPECT shows reduced ligand uptake in the left posterior putamen. (C) Fusion of MRI and [123I]FP-CIT SPECT shows that the reduced ligand uptake closely follows the limits of the vascular lesion.
Table 5
| [123I]FP-CIT binding | n (%) |
|---|---|
| Reduced binding based on visual evaluation | 15/15 (100%) |
| Reduced binding based on semi-quantative analysis (Z-score) | |
| Posterior putamen | 11/15 (73%) |
| Anterior putamen | 4/15 (27%) |
| Caudate | 4/15 (27%) |
| Reduced binding based on semi-quantative analysis (Z-score) for parkinsonism vs. no parkinsonism at 1 yr | |
| Parkinsonism | 8/10 (80%) |
| No parkinsonism | 3/5 (60%) |
[123I]FP-CIT binding.
Yr, year.
4 Discussion
4.1 Interpretation of findings
There are several important findings in the present study. First, 27% (4/15) patients with isolated striatal infarcts developed parkinsonism by 3 months, increasing to 67% (10/15) by 1 year. Second, MDS-UPDRS motor score increased between 3 months and 1 year both in patients with and without parkinsonism at the first follow-up. Third, parkinsonism in these patients mainly manifested as contralateral akinetic-rigid symptoms in upper extremities, with only one patient with bilateral parkinsonism. Tremor and axial symptoms were rare. Fourth, the volume and location of the infarct appeared to influence the likelihood of developing motor symptoms. Although the putaminal lesion extended to the caudate in all but one of the 15 patients, none of the patients showed cognitive impairment, and there was no significant decline in MoCA scores in the one-year follow-up. Finally, all patients showed corresponding striatal dopamine deficit.
The incidence of parkinsonism following a striatal infarct was notably higher in our study compared to previous research. While 67% of our patients exhibited parkinsonism at 1 year, earlier studies reported incidences ranging from 0 to 38% (4–7). Several factors may explain this discrepancy. First, our study focused on patients with isolated striatal infarcts following successful IVT and/or MT, techniques that were not available at the time of the earlier studies. We used modern brain MRI rather than CT, which leads to more precise lesion localization (20). Notably, as our patients had isolated striatal infarcts and fulfilled inclusion criterion of NIHSS <6 (range 0–2), they did not exhibit motor impairments such as paresis or spasticity, minimizing the risk that parkinsonian symptoms were masked or confounded by other stroke-related deficits. In contrast, lesions in previous studies often extended to structures surrounding the striatum, potentially obscuring parkinsonism with other symptoms. Indeed, in one patient in the 2004 study, bradykinesia only became evident when the hemiparesis resolved (7). Second, the present study included repeated clinical examinations over a one-year period following the stroke, which may have enhanced the ability to detect parkinsonism compared to studies with shorter follow-up durations.
Our findings indicate that parkinsonism induced by isolated striatal lesions may follow a delayed and progressive course. Patients who did not meet the criteria for clinical parkinsonism at either of the time points still showed a slight increase in MDS-UPDRS III scores during follow-up (Figure 2). We hypothesize that these patients may develop clinical parkinsonism with a longer delay, but this remains to be confirmed by further follow-up.
In the chronic phase after an acute stroke, one might not typically expect further symptom development, in contrast to neurodegenerative diseases such as Parkinson’s disease (PD). Notably, the median NIHSS score was one (range 0–2) both at 3 months and 1 year, indicating that the symptom progression was not due to additional strokes. Possible explanations for the worsening of symptoms, however, is a retrograde denervation of nigrostriatal neurons and/or loss of compensatory mechanisms. Accordingly, a previous study showed degenerative changes in the substantia nigra 14 days after striatal infarcts (21), and the involvement of the nigrostriatal pathway is evidenced in the present study by the reduced striatal ligand uptake on [123I]FP-CIT SPECT. Similarly, numerous other studies have also demonstrated pre-synaptic dopaminergic dysfunction in patients with parkinsonism following infarcts involving the nigrostriatal tract (22–24). As we conducted SPECT imaging only cross-sectionally, repeated imaging in the future is needed to evaluate if the dopaminergic deficit is progressive and if this can explain the increasing symptoms in our patients.
Our patients primarily showed bradykinesia and rigidity, and to a much lesser extent tremor. This aligns with previous studies that have demonstrated that rest tremor is less frequent in patients with parkinsonism caused by cerebrovascular disease compared to PD. (25–28) Symptoms in our patients were mostly observed in the upper extremities, and gait disturbances were rare, apart from unilateral reduced arm swing in most patients. This pattern is consistent with what, by some, is termed vascular parkinsonism due to nigrostriatal lesions, as opposed to parkinsonism associated with extensive subcortical white matter lesions, which usually manifests with lower body symptoms and gait disorder (23). As expected, the majority of patients with parkinsonism developed symptoms contralaterally to the infarct. Only one patient, who did not exhibit signs of parkinsonism at 3 months, presented with bilateral symptoms at 1 year, including bradykinesia and rigidity in all four extremities, but still predominantly on the contralateral side. [123I]FP-CIT SPECT for this patient showed unilateral reduced ligand uptake corresponding to the vascular lesion. Notably, the one patient who developed parkinsonism in the 2004 study also exhibited bilateral symptoms with a unilateral infarct and corresponding unilateral reduced presynaptic dopamine function (7).
On MRI, the infarct volume in the putamen was higher in patients with parkinsonism compared to those without. Additionally, the proportion of infarct volume in the putamen moderately correlated with motor symptoms, while the caudate did not, although neither of these correlations reached significance. These findings align with the functional role of the striatal subregions: the putamen, particularly its posterior part, is well known to be activated during movement (29). In contrast, the caudate is more often associated with cognitive and affective function; however, based on the present findings, unilateral striatal infarcts affecting on average 1/4 of the caudate volume do not appear to be sufficient to cause clinically apparent cognitive deficits or progressive cognitive decline within 1 year after stroke.
All our patients had infarcts in the posterior putamen and reduced [123I]FP-CIT uptake in the same area (Figure 3), a region typically affected in PD. Although all patients showed reduced uptake on visual inspection, only 73% met the Z-score threshold for abnormality in DaTQuant. This discrepancy likely reflects the limitations of applying standardized Z-scores, developed for diffuse neurodegenerative conditions affecting entire anatomical regions, to focal infarcts involving only part of the striatum. As a result, DaTQuant may underestimate abnormalities in small, localized lesions such as those observed in this study. A representative case example illustrating this limitation is shown in Supplementary Figure 1. Notably, there was an apparent mismatch between imaging and clinical findings - i.e. reduced [123I]FP-CIT binding in some patients without clear parkinsonian symptoms. [123I]FP-CIT SPECT reflects presynaptic dopaminergic integrity, but not clinical expression, and a similar disconnect is well-documented in early PD, where abnormal scans can precede symptom onset (30, 31). In this context, the imaging confirms that the nigrostriatal pathway was structurally affected, even if clinical manifestations had not yet emerged. Longitudinal follow-up is needed to clarify whether these patients later develop symptoms.
The reason why some people developed parkinsonism by 1 year after stroke and others not, remains unknown. In this investigation, the development of parkinsonism may be related to infarct volume, particularly within the putamen. Additionally, patients who developed symptoms early were older, suggesting that increasing age might be a risk factor for lesion-induced parkinsonism. For PD, male sex and increased age are well-established risk factors, whereas alcohol, tobacco smoke, coffee drinking, and physical activity are factors that are associated with reduced risk of developing the disease (32). It is not established if these factors purely affect the process of neurodegeneration in PD, or if they also apply to parkinsonism caused by cerebrovascular disease. Therefore, we did not adjust for any factors in our study. Although all patients had infarcts in the posterior putamen and reduced ligand uptake on [123I]FP-CIT SPECT in the same area, another possible explanation for the different outcomes might be impairment of different brain networks based on slight differences in infarct localization. As the putamen is part of the nigrostriatal pathway and corticostriatal motor loops, it is possible that striatal lesion-induced parkinsonism is actually mediated via the functional connections disrupted by the lesion rather than the anatomical location of the infarct (33). Notably, one-third of patients did not develop parkinsonism, despite similar infarct localization and imaging findings. This may suggest the presence of individual or network-level resilience factors, which warrant further investigation.
4.2 Limitations
Our study is limited by its small sample size, and all analyses should be interpreted with caution. The findings require replication in larger cohorts to assess their generalizability. Still, the consistently high proportion of patients who developed parkinsonism provides proof-of-concept that isolated striatal infarcts can lead to delayed-onset parkinsonism. While comorbid PD cannot be entirely ruled out, it is unlikely to explain the findings in 10/15 patients. This is supported by the correspondence between symptom laterality and lesion location, the close match between reduced [123I]FP-CIT uptake and the infarcted area, and the absence of a typical PD pattern - namely, bilateral asymmetric binding deficits with a posterior-to-anterior gradient. Furthermore, the clinical assessment relied on subjective scoring systems; however, to reduce bias, two examiners independently performed the MDS-UPDRS part III and agreed on a final score. Cognitive function was assessed using the MoCA, which, although widely used, does not provide a comprehensive evaluation of cognition and may overlook subtle or domain-specific impairments such as executive dysfunction, which is often associated with frontostriatal circuits (29). Future studies may include more targeted neuropsychological testing to assess these deficits. Lastly, some patients with isolated striatal infarcts might have been missed during enrollment, and the reported number should not be interpreted as representative of their true frequency in this population.
Another limitation is the measurement of infarct volume on MRI. This has several potential sources of error, such as suboptimal image quality and indefinite criteria for ischemic changes, and these findings should therefore be interpreted with caution.
4.3 Clinical implications
We believe the expanding use of IVT and MT (34, 35) will lead to an increase of isolated striatal infarcts, as the area is particularly vulnerable to ischemic damage and may be irreversibly damaged by the time reperfusion therapy is administered (10). Furthermore, with the decline in stroke mortality (36), more patients are prone to experience post-stroke complications, including movement disorders (37).
Based on our results, we suggest that the current approach to patients with striatal infarcts could merit reconsideration. Historically, limited attention has been given to screening patients with isolated striatal infarcts for parkinsonism due to the belief that it rarely develops. Our data, however, suggest that these patients may be at high risk of developing delayed, progressive parkinsonism and could benefit from targeted assessments for parkinsonism during both the subacute and chronic phase. This might prevent misdiagnosis, enhance patient understanding of their symptoms and prognosis, and enable them to explore treatment options, such as levodopa. Moreover, they may benefit from tailored physiotherapy and multidisciplinary follow-up, akin to what is increasingly provided to patients with idiopathic PD and atypical parkinsonism syndromes (38). Importantly, the results are not intended to generalize to broader stroke populations, as lesions involving structures adjacent to the striatum may lead to other motor deficits, potentially masking or confounding the clinical assessment of parkinsonism.
Finally, this study contributes to the understanding of potential pathophysiological mechanisms underlying parkinsonism following cerebrovascular disease, providing insights into a complex and less well-characterized area. These observations may serve as a foundation for further exploration of alternative treatment strategies. Future research should include larger sample sizes and consider applying lesion network mapping to investigate how striatal infarcts disrupt connected brain networks, combined with extended follow-up to better assess the progression of parkinsonism after isolated striatal infarcts.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Regional Committee for Medical and Health Research Ethics in South East Norway (Approval no. 187241). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft. ED: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. JJ: Visualization, Writing – review & editing. JC: Visualization, Writing – review & editing. CL: Conceptualization, Writing – review & editing. MS: Writing – review & editing. VG: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. SB: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by funding from The Research Council of Norway as part of The Medical Student Research Program (grant no. 271555/F20). HH has been supported by a grant from Reberg’s Trust for Parkinson research. The authors report no conflicts of interest relevant to this work.
Acknowledgments
The authors wish to thank study participants and their families, as well as Rajiv Advani at Oslo University Hospital for his assistance in patient inclusion. We also thank the Department of Biostatistics at the University of Oslo for their valuable advice.
Conflict of interest
ED has given lectures and/or been advisor with honorarium for AbbVie, Global Kinetics, Ipsen, and Nordic Infucare. JJ has received research funding from the Research Council of Finland, Finnish Medical Foundation, Sigrid Juselius Foundation, Signe & Ane Gyllenberg’s Foundation, Päivikki and Sakari Sohlberg’s Foundation, Finnish Parkinson Foundation, and Finnish Foundation for Alcohol Studies; lecturer honoraria from Insightec, Addiktum, Nordic Infucare, Lundbeck and Novartis, conference travel support from Abbvie, Abbott and Insightec, and consultancy fees from Summaryx, Adamant Health and TEVA Finland; is acting as an advisory board member for TEVA Finland; and owns stock of Neurologic Finland and Suomen Neurolaboratorio. VG has received travel grants, given lectures, and/or been advisor with honorarium for AbbVie, Global Kinetics, and Nordic Infucare. SB has received travel grants, given lectures, and/or been advisor with honorarium for Medtronic, Boston Scientific, AbbVie, and Nordic Infucare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. During the preparation of this work the authors used ChatGPT-4o in order to enhance readability and language quality. AI was not employed for scientific insights, data analysis, or interpretation. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1653832/full#supplementary-material
References
1.
Orimo S Amino T Tanaka H Mitani K Ishiwata K Ishii K . A case of hemiparkinsonism following ischemic lesion of the contralateral substantia nigra: a PET study. Eur Neurol. (2004) 51:175–7. doi: 10.1159/000077666
2.
Ohta K Obara K . Hemiparkinsonism with a discrete lacunar infarction in the contralateral substantia nigra. Mov Disord. (2006) 21:124–5. doi: 10.1002/mds.20747
3.
Robles LA . Pure Hemiparkinsonism secondary to contralateral lacunar stroke in the substantia Nigra. J Stroke Cerebrovasc Dis. (2016) 25:e20–1. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.027
4.
Bhatia KP Marsden CD . The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. (1994) 117:859–76. doi: 10.1093/brain/117.4.859
5.
Russmann H Vingerhoets F Ghika J Maeder P Bogousslavsky J . Acute infarction limited to the lenticular nucleus: clinical, etiologic, and topographic features. Arch Neurol. (2003) 60:351–5. doi: 10.1001/archneur.60.3.351
6.
Reider-Groswasser I Bornstein NM Korczyn AD . Parkinsonism in patients with lucanar infarcts of the basal ganglia. Eur Neurol. (1995) 35:46–9. doi: 10.1159/000117089
7.
Peralta C Werner P Holl B Kiechl S Willeit J Seppi K et al . Parkinsonism following striatal infarcts: incidence in a prospective stroke unit cohort. J Neural Transm (Vienna). (2004) 111:1473–83. doi: 10.1007/s00702-004-0192-1
8.
Vaamonde J Flores JM Gallardo MJ Ibáñez R . Subacute hemicorporal parkinsonism in 5 patients with infarcts of the basal ganglia. J Neural Transm (Vienna). (2007) 114:1463–7. doi: 10.1007/s00702-007-0774-9
9.
Yonas H Wolfson SK Jr Dujovny M Boehnke M Cook E . Selective lenticulostriate occlusion in the primate. A highly focal cerebral ischemia model. Stroke. (1981) 12:567–72. doi: 10.1161/01.str.12.5.567
10.
Kaschner MG Lande R Rubbert C Caspers J Lee JI Gliem M et al . Predictors for basal ganglia viability after mechanical thrombectomy in proximal middle cerebral artery occlusion. Clin Imaging. (2019) 57:1–6. doi: 10.1016/j.clinimag.2019.04.013
11.
Lyden P Brott T Tilley B Welch KM Mascha EJ Levine S et al . Improved reliability of the NIH stroke scale using video training. NINDS TPA Stroke Study Group. Stroke. (1994) 25:2220–6. doi: 10.1161/01.str.25.11.2220
12.
Postuma RB Berg D Stern M Poewe W Olanow CW Oertel W et al . MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
13.
Goetz CG Tilley BC Shaftman SR Stebbins GT Fahn S Martinez-Martin P et al . Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
14.
Bjerknes S Toft M Brandt R Rygvold TW Konglund A Dietrichs E et al . Subthalamic nucleus stimulation in Parkinson's disease: 5-year extension study of a randomized trial. Mov Disord Clin Pract. (2022) 9:48–59. doi: 10.1002/mdc3.13348
15.
Nasreddine ZS Phillips NA Bédirian V Charbonneau S Whitehead V Collin I et al . The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
16.
Freitas S Simões MR Alves L Vicente M Santana I . Montreal cognitive assessment (MoCA): validation study for vascular dementia. J Int Neuropsychol Soc. (2012) 18:1031–40. doi: 10.1017/S135561771200077X
17.
Hoops S Nazem S Siderowf AD Duda JE Xie SX Stern MB et al . Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. (2009) 73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47
18.
Yushkevich PA Piven J Hazlett HC Smith RG Ho S Gee JC et al . User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. (2006) 31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015
19.
Zoubir AM Iskandler DR . Bootstrap methods and applications. IEEE Signal Process Mag. (2007) 24:10–9. doi: 10.1109/MSP.2007.4286560
20.
Czap AL Sheth SA . Overview of imaging modalities in stroke. Neurology. (2021) 97:S42–s51. doi: 10.1212/WNL.0000000000012794
21.
Nakane M Teraoka A Asato R Tamura A . Degeneration of the ipsilateral substantia nigra following cerebral infarction in the striatum. Stroke. (1992) 23:328–32. doi: 10.1161/01.STR.23.3.328
22.
Zijlmans J Evans A Fontes F Katzenschlager R Gacinovic S Lees AJ et al . [123I] FP-CIT spect study in vascular parkinsonism and Parkinson's disease. Mov Disord. (2007) 22:1278–85. doi: 10.1002/mds.21479
23.
Rektor I Bohnen NI Korczyn AD Gryb V Kumar H Kramberger MG et al . An updated diagnostic approach to subtype definition of vascular parkinsonism - recommendations from an expert working group. Parkinsonism Relat Disord. (2018) 49:9–16. doi: 10.1016/j.parkreldis.2017.12.030
24.
Ma KKY Lin S Mok VCT . Neuroimaging in Vascular Parkinsonism. Curr Neurol Neurosci Rep. (2019) 19:102. doi: 10.1007/s11910-019-1019-7
25.
Benítez-Rivero S Marín-Oyaga VA García-Solís D Huertas-Fernández I García-Gómez FJ Jesús S et al . Clinical features and 123I-FP-CIT SPECT imaging in vascular parkinsonism and Parkinson's disease. J Neurol Neurosurg Psychiatry. (2012) 84:122–9. doi: 10.1136/jnnp-2012-302618
26.
Rampello L Alvano A Battaglia G Raffaele R Vecchio I Malaguarnera M . Different clinical and evolutional patterns in late idiopathic and vascular parkinsonism. J Neurol. (2005) 252:1045–9. doi: 10.1007/s00415-005-0811-2
27.
Yamanouchi H Nagura H . Neurological signs and frontal white matter lesions in vascular parkinsonism. A clinicopathologic study. Stroke. (1997) 28:965–9. doi: 10.1161/01.str.28.5.965
28.
Inzelberg R Bornstein NM Reider I Korczyn AD . Basal ganglia lacunes and parkinsonism. Neuroepidemiology. (1994) 13:108–12. doi: 10.1159/000110367
29.
Lanciego JL Luquin N Obeso JA . Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. (2012) 2:a009621. doi: 10.1101/cshperspect.a009621
30.
Braak H Del Tredici K Rüb U de Vos RA Jansen Steur EN Braak E . Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. (2003) 24:197–211. doi: 10.1016/s0197-4580(02)00065-9
31.
Artzi M Even-Sapir E Lerman Shacham H Thaler A Urterger AO Bressman S et al . DaT-SPECT assessment depicts dopamine depletion among asymptomatic G2019S LRRK2 mutation carriers. PLoS One. (2017) 12:e0175424. doi: 10.1371/journal.pone.0175424
32.
Ascherio A Schwarzschild MA . The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. (2016) 15:1257–72. doi: 10.1016/S1474-4422(16)30230-7
33.
Joutsa J Horn A Hsu J Fox MD . Localizing parkinsonism based on focal brain lesions. Brain. (2018) 141:2445–56. doi: 10.1093/brain/awy161
34.
Adcock AK Schwamm LH Smith EE Fonarow GC Reeves MJ Xu H et al . Trends in use, outcomes, and disparities in endovascular Thrombectomy in US patients with stroke aged 80 years and older compared with younger patients. JAMA Netw Open. (2022) 5:e2215869. doi: 10.1001/jamanetworkopen.2022.15869
35.
Enriquez BA Tennøe B Nome T Gjertsen Ø Nedregaard B Sletteberg R et al . Mechanical thrombectomy in acute ischaemic stroke. Tidsskr Nor Laegeforen. (2022) 142:577. doi: 10.4045/tidsskr.21.0577
36.
Wu H Le Couteur DG Hilmer SN . Mortality trends of stroke and dementia: changing landscapes and new challenges. J Am Geriatr Soc. (2021) 69:2888–98. doi: 10.1111/jgs.17322
37.
Suri R Rodriguez-Porcel F Donohue K Jesse E Lovera L Dwivedi AK et al . Post-stroke movement disorders: the clinical, neuroanatomic, and demographic portrait of 284 published cases. J Stroke Cerebrovasc Dis. (2018) 27:2388–97. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.028
38.
Langbroek-Amersfoort A Schootemeijer S Bouten L Bloem BR De Vries NM . Exercise made accessible: the merits of community-based programs for persons with Parkinson's disease. Curr Neurol Neurosci Rep. (2023) 23:695–715. doi: 10.1007/s11910-023-01303-0
Appendix
Table A1
| Clinical scores | 3 mos [mean (95% CI)] | 1 yr [mean (95% CI)] |
|---|---|---|
| MDS-UPDRS I score | ||
| Whole group | 9.1 (4.4–13.8)a | 8.1 (3.5–12.7)a |
| Parkinsonism vs. no parkinsonism | ||
| Parkinsonism | 9.3 (5–15)a,b | 7.8 (0–17)a,b |
| No parkinsonism | 9.0 (0–26)a,b | 8.2 (0–24)b |
| MDS-UPDRS II score | ||
| Whole group | 5.1 (1.6–8.5)a | 5.3 (2.1–9.0)a |
| Parkinsonism vs. no parkinsonism | ||
| Parkinsonism | 6.3 (2–11)a,b | 5.8 (0–11)a,b |
| No parkinsonism | 4.7 (0–20)a,b | 4.2 (0–18)b |
MDS-UPDRS I and II at 3 mos and 1 yr.
CI, Confidence interval; mos, months; yr, year. aScore not available for all patients; see Supplementary Table 1 for number of observations per variable. bCI exceeded the instrument’s valid range. Values are therefore presented as mean (range) instead.
Table A2
| Area | Z score [mean (95% CI)] | |
|---|---|---|
| Ipsilateral to the lesion | Contralateral to the lesion | |
| Striatum | −1.4 (−2.5 – −0.3) | 1.4 (0.7–2.1) |
| Anterior putamen | −1.2 (−2.4 – −0.1) | 1.2 (0.5–2.0) |
| Posterior putamen | −2.9 (−3.6 – −2.1) | 1.0 (0.4–1.6) |
| Caudate | −0.5 (−1.8–0.8) | 1.6 (0.8–2.5) |
| Parkinsonism | No parkinsonism | |
| Striatum | −1.5 (−3.1–0.2) | −1.3 (−3.2–0.7) |
| Anterior putamen | −1.4 (−3.1–0.3) | −0.9 (−3.1–1.3) |
| Posterior putamen | −3.1 (−4.1 − −2.2) | −2.3 (−3.7 − −0.9) |
| Caudate | −0.3 (−2.2–1.6) | −0.9 (−2.8–1.0) |
[123I]FP-CIT binding in striatal subregions on ipsi- vs. contralateral side to the lesion.
CI, Confidence interval.
Table A3
| Imaging characteristics | Correlation with Z-score [r (95% CI)] | Correlation with MDS-UPDRS III score at 1 yr [r (95% CI)] | p-value (r) |
|---|---|---|---|
| Infarct volume proportion from total striatum volume | −0.73 (−0.88 – −0.44) | <0.001* | |
| Z-score in different regions | |||
| Striatum | −0.10 (−0.53–0.51) | 0.703 | |
| Anterior putamen | −0.15 (−0.58–0.47) | ||
| Posterior putamen | −0.19 (−0.60–0.31) | 0.405 | |
| Caudate | −0.0048 (−0.44–0.64) | ||
Correlation with [123I]FP-CIT binding.
CI, Confidence interval; r, correlation coefficient; yr, year. *p < 0.001.
Summary
Keywords
movement disorders, basal ganglia, corpus striatum, parkinsonian disorders, brain infarction
Citation
Holm H, Dietrichs E, Joutsa J, Connelly JP, Lund CG, Skjelland M, Gundersen V and Bjerknes S (2025) Delayed-onset parkinsonism is common after isolated striatal infarcts. Front. Neurol. 16:1653832. doi: 10.3389/fneur.2025.1653832
Received
25 June 2025
Accepted
03 October 2025
Published
20 November 2025
Volume
16 - 2025
Edited by
Pedro J Garcia-Ruiz, University Hospital Fundación Jiménez Díaz, Spain
Reviewed by
Timothy Michael Ellmore, City College of New York (CUNY), United States
Essam Al-Sibahee, College of Medicine, University of Baghdad, Iraq
Updates
Copyright
© 2025 Holm, Dietrichs, Joutsa, Connelly, Lund, Skjelland, Gundersen and Bjerknes.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hedda Holm, heddho@uio.no
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.