Abstract
Background and purpose:
Endovascular treatment (EVT) is a standard therapy for acute ischemic stroke (AIS) caused by large vessel occlusion (LVO). However, the performance of EVT in primary stroke centers (PSCs) in China remains uncertain. This study aims to explore the performance of EVT in PSCs and compare it with that in comprehensive stroke centers (CSCs).
Methods:
We conducted a prospective registry of EVT at 11 CSCs and 26 PSCs in China. AIS patients with intracranial LVO who received EVT were divided into two groups based on the type of stroke center. We compared the AIS workflow, EVT procedural details, radiological, and clinical outcomes between the two groups.
Results:
From November 2021 to December 2022, 1,196 patients were enrolled, and 847 were included in the analysis. Overall, 84.8% of patients achieved successful reperfusion, and 46.3% achieved good clinical outcomes. Compared with patients treated at CSCs, those treated at PSCs had shorter onset-to-presentation time (OPT: 152 min vs. 268 min, p < 0.001) but longer door-to-puncture time (DPT: 112 min vs. 95 min, p < 0.001). Successful reperfusion rates were lower in PSCs (76.6% vs. 91.0%, p < 0.001), and mortality was higher (24.7% vs. 14.4%, p < 0.001). However, good clinical outcomes were similar between the two groups (44.3% vs. 47.8%, p = 0.309).
Conclusion:
In China, successful reperfusion rates were lower and mortality rates were higher despite shorter onset-to-presentation times in primary stroke centers. Additionally, door-to-puncture times were prolonged despite limited use of advanced brain imaging. These findings highlight the need for EVT skill training and improvement in the AIS workflow in primary stroke centers to enhance patient outcomes.
Introduction
Endovascular therapy (EVT) is the standard treatment for acute ischemic stroke caused by large vessel occlusion (AIS-LVO) (1). The efficacy of EVT is influenced by multiple factors, including the medical capabilities of hospitals and their staff, such as the use of EVT devices, the EVT skills of neuro-interventionalists, and the establishment of emergency treatment green channels for AIS. Additionally, other factors such as traffic conditions, public education, and awareness of AIS also play important roles, which may vary significantly between developed and developing countries. The RESILIENT randomized trial in Brazil demonstrated that patients with AIS-LVO in a developing country can still benefit from EVT (2).
Differences in stroke care are evident not only between developed and developing countries but also between county and municipal-level hospitals. Given China’s vast geographical expanse, there is significant variation in the construction of stroke centers, stroke burden, and the quality of stroke care across different regions (3). To improve the quality of stroke care, the guidelines for stroke center construction in China, issued in 2015, recommended establishing two levels of stroke centers: primary stroke centers (PSCs), typically county-level hospitals, and comprehensive stroke centers (CSCs), usually municipal or provincial hospitals (4). The efficacy of reperfusion treatments, including intravenous thrombolysis (IVT) and endovascular thrombectomy (EVT) for AIS is time depend (5). Therefore, reperfusion therapy should be performed as soon as possible. The “2019 Stroke Report in China” showed that EVT was increasingly performed in PSCs. However, differences in medical care capabilities, emergency treatment green channels for AIS, and traffic conditions may exist between PSCs and CSCs. We hypothesize that the AIS workflow and medical care capabilities of hospitals and their staff are suboptimal in PSCs. To explore these differences, we conducted a prospective real-world registry study, named the “Endovascular Treatment for Acute Ischemic Stroke in Chinese Municipal and County Hospitals (the ETERNITY registry),” from November 2021 to December 2022. We hope the findings will provide valuable information for improving stroke care quality in China.
Methods
Hospitals and participants
The ETERNITY registry is a nationwide, prospective, observational study of consecutive adult patients with AIS patients due to LVO who underwent EVT. The study was conducted across 11 CSCs(municipal hospital) and 26 PSCs(county hospitals) certified by the Ministry of Health China Stroke Prevention Project Committee (CSPPC) (6). CSCs serve as the backbone of the regional stroke care system. These centers, also known as advanced stroke centers, are tertiary hospitals responsible for stroke diagnosis, treatment, education, training, and scientific research. They are subject to national unified quality control management and actively promote the implementation of key stroke prevention recommendations. CSCs also facilitate the establishment of a two-way referral mechanism within their region to enhance overall stroke care (4). PSCs also referred to as Stroke Prevention Centers, adhere to standards that include establishing a green channel for multi-disciplinary collaboration in emergency management, participating in regional stroke classification treatment networks, conducting stroke prevention and secondary prevention programs, and ensuring endovascular thrombectomy capability (4).
This study included hospitals from 19 provinces and 1 municipality across China. The study protocol was approved by the Ethics Committees of local hospital and all participating centers. Written informed consent was obtained from patients or their legally authorized representatives. The study is registered at https://www.clinicaltrials.gov with the unique identifier number.
Patients were enrolled according to the following inclusion criteria: (1) age ≥18 years; (2) diagnosis of AIS based on imaging-confirmed intracranial LVO, including isolated cervical internal carotid artery occlusion or tandem occlusion, or occlusion in the intracranial internal carotid artery (ICA), middle cerebral artery (MCA, M1/M2), or anterior cerebral artery [ACA, A1/A2, 7]; (3) National Institutes of Health Stroke Scale (NIHSS) score ≥ 6; (4) Alberta Stroke Program Early CT Score (ASPECTS) ≥ 6;(5). initiation of any type of EVT, including mechanical thrombectomy or emergent angioplasty via balloon and stent; (6) onset-to-puncture time within 24 h, and if onset-to-presentation time (OPT) was within 6–24 h, computed tomography perfusion (CTP) should be performed and the results of CTP should fulfil the DAWN (7) or DEFUSE (8) criteria. The exclusion criteria were (1) no follow-up outcome data; (2) posterior circulation stroke and (3) no evidence of an LVO on angiogram.
Data collection
The baseline information included patients’ demographic data, medical history, physical examination findings, imaging results, and time-metric data. Treatment details included the administration and timing of IVT and EVT procedure information. Before enrollment, all sites were uniformly trained on an electronic data capture system, which allowed for data entry and electronic signatures. All the electronic data were captured with central quality checks by blinded statisticians to control for consistency, plausibility and completeness.
Clinical outcome assessment
The follow-up clinical examinations included NIHSS score assessments of the patients’ neurological function and modified Rankin Scales (mRS) assessments of the outcomes at 90 days, and a good outcome defined as a mRS score ≤ 2. The 90 days follow-up was ascertained using a standardized telephone interview performed by trained investigators blinded to the baseline and procedural data. The stroke subtypes were determined according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classifications (9). Symptomatic intracranial hemorrhage (sICH) was defined as an increase of ≥ 4 points in the NIHSS scores according to the Heidelberg criteria (10).
Radiological assessment
Baseline imaging assessments, including non-contrast CT, magnetic resonance (MR) imaging, CT angiography (CTA), MR angiography (MRA), and digital subtraction angiography (DSA), as well as post-procedural CT results, were evaluated by an imaging core laboratory using a standardized protocol (DISCOM). All analyses were performed by personnel blinded to clinical data and outcomes. All imaging assessments were independently conducted by two experienced neuroradiologists (each with over 5 years of experience), with a third neuroradiologist available for adjudication when discrepancies arose. The radiological evaluations encompassed the following: ASPECTS evaluated on plain CT for anterior circulation stroke, location of the occlusion site, presence of underlying ICAS (11, 12), baseline and postprocedural extended thrombolysis in cerebral infarction (eTICI) (13), tandem occlusion, and occurrence of ICH on post-treatment imaging. Successful reperfusion was defined as an eTICI ≥ 2b. Procedural-related complications such as vessel dissection, vasospasm, vessel perforation, embolization into new territory (ENT) and distal embolism(DE) (14) were also recorded. For patients whose images could not be obtained, site-reported data were used. The imaging review criteria of the local investigators were the same as those of the core laboratory.
Statistical analysis
Continuous data are presented as medians and interquartile ranges (IQRs) or means and standard deviations (SDs); between-group differences were tested by t tests and Mann–Whitney U tests, respectively. Categorical variables are presented as proportions and were compared using the χ2 test. Patients were classified into CSCs and PSCs group, baseline characteristic, radiological and clinical outcome were compared between two group. Typical variables related with prognosis (age, NIHSS, ASPEC, OPT, occlusion site) combined with stroke center type were included in multivariate logistic regression analyses. Odds ratios (ORs) were computed to determine the associated relationships between clinical outcome and stroke center type. All analyses were performed using SPSS (version 15.0; SPSS, Inc., Chicago, IL, United States), with a significance level of p < 0.05 (two-sided).
Results
Participated hospital and included patient
Between November 2021 and December 2022, totally 11 CSCs and 26 PSCs from 19 provinces and 1 municipality in China participated the study. In total, 1,196 subjects with AIS were registered (Supplementary Table 1). Of these, 349 were excluded: (1) 105 with posterior circulation stroke; (2) 56 with NIHSS scores < 6; (3) 75 with ASPECT < 6; (4) 54 without complete clinical information; (5) 31 who lacked complete imaging information and (6) 28 without 90-day follow-up data. Ultimately, this study included 847 patients, comprising 368 from PSCs and 479 from CSCs, respectively (Figure 1).
Figure 1

Study flow chart. NIHSS indicated National Institutes of Health Stroke Scale; ASPECT, Alberta Stroke Program Early CT Score.
Baseline characteristic of included patients
The baseline characteristic of included 847 patients were showed in Tables 1, 2, the mean age was 68 ± 12 years, 62%(525/847) were men, the median admission NIHSS scores was 14. Regarding vascular risk factors, 49.4% (418/847) had hypertension, 20.4% (173/847) had diabetes, 16.5% (33/847) had hyperlipidemia, 34.7% (294/847) had atrial fibrillation (AF), and 28.9% (245/847) were smokers. In terms of imaging modality, all patients underwent non-contrast CT, with a median ASPECTS of 8. Additionally, 66.1% (560/847) of patients underwent angiography imaging, and 37.0% (313/847) underwent perfusion imaging. According to the TOAST classification, 33.4% (283/847) had large-artery atherosclerosis (LAA), 48.2% (408/847) had cardioembolic (CE), and 18.4% (156/847) had other undefined strokes. Regarding occlusion site, 38.5% (326/847) were in the ICA, 58.6% (496/847) in the MCA, and 2.9% (25/847) in the ACA; 13.8% (117/847) showed tandem occlusion.
Table 1
| Parameters | All (n = 847) | PSCs (n = 368) | CSCs (n = 479) | p value |
|---|---|---|---|---|
| Age (mean ± SD) | 68 ± 12 | 68 ± 12 | 67 ± 12 | 0.408 |
| Male sex (n, %) | 525 (62.0%) | 230 (62.5%) | 295 (61.6%) | 0.786 |
| Risk factors (n, %) | ||||
| Hypertension | 418 (49.4%) | 183 (49.7%) | 235 (49.1%) | 0.847 |
| Diabetes | 173 (20.4%) | 73 (19.8%) | 100 (20.9%) | 0.710 |
| Hyperlipidemia | 33 (16.5%) | 10 (8.9%) | 23 (26.1%) | 0.001 |
| Atrial fibrillation | 294 (34.7%) | 151 (41.0%) | 143 (29.9%) | < 0.001 |
| Smoking | 245 (28.9%) | 122 (33.2%) | 123 (25.7%) | 0.017 |
| TOAST classification (n, %) | ||||
| LAA | 283 (33.4%) | 103 (28.0%) | 180 (37.6%) | 0.003 |
| Cardioembolic | 408 (48.2%) | 214 (58.2%) | 194 (40.5%) | < 0.001 |
| Other defined | 156 (18.4%) | 51 (13.9%) | 105 (21.9%) | 0.003 |
| Baseline NIHSS | 14 (11,19) | 15 (11,20) | 14 (10,18) | < 0.001 |
| Onset-to-presentation time (Median, IQR, min) | 205 (98,378) | 152 (73,251) | 258 (124,520) | < 0.001 |
| Within 3 h (n, %) | 376 (44.4%) | 213 (57.9%) | 163 (34%) | < 0.001 |
| Within 4.5 h (n, %) | 527 (62.2%) | 280 (76.1%) | 247 (51.6%) | < 0.001 |
| Within 6 h (n, %) | 617 (72.8%) | 310 (84.2%) | 307 (64.1%) | < 0.001 |
| Image modality | ||||
| CTA or MRA | 560 (66.1%) | 129 (35.1%) | 431 (90.0%) | < 0.001 |
| CTP or MRP | 313 (37.0%) | 9 (2.4%) | 304 (63.5%) | < 0.001 |
| ASPECTS (median, IQR) | 8 (7–10) | 9 (8–10) | 8 (7–10) | 0.071 |
| Intravenous thrombolysis (n, %) | 306 (36.1%) | 185 (50.3%) | 121 (25.3%) | < 0.001 |
| Occlusion site (n, %) | ||||
| ICA | 326 (38.5%) | 153 (41.6%) | 173 (36.1%) | 0.106 |
| MCA | 496 (58.6%) | 211 (57.3%) | 285 (59.5%) | 0.527 |
| ACA | 25 (2.9%) | 4 (1.1%) | 21 (4.4%) | 0.005 |
| Tandem occlusion (n, %) | 117 (13.8%) | 49 (13.3%) | 68 (14.2%) | 0.713 |
| DPT (median, IQR, min) | 101 (75,151) | 112 (78,161) | 95 (73,141) | <0.001 |
Demographics and characteristics of the included patients, stratified by stroke center type.
PSCs indicated primary stroke centers; CSCs, comprehensive stroke centers; SD, standard deviation; TOAST, Trial of Org 10,172 in Acute Stroke Treatment Large Artery Atherosclerosis; LAA, large-artery Atherosclerosis Study; NIHSS, National Institutes of Health Stroke Scale; IQR, interquartile range; CTA, computed tomography angiography; MRA, magnetic resonance angiography; CTP, computerized tomography perfusion; MRP, magnetic resonance perfusion; ASPECT, Alberta Stroke Program Early CT score; ICA, intracranial carotid artery; MCA, middle cerebral artery; ACA, anterior cerebral artery; DPT, door-to-puncture time.
Table 2
| Procedure | All (n = 847) | PSCs (n = 368) | CSCs (n = 479) | p value |
|---|---|---|---|---|
| First line strategy (n, %) | ||||
| Stent | 677 (79.9%) | 316 (85.9%) | 361 (75.4%) | <0.001 |
| Aspiration | 143 (16.9%) | 52 (14.1%) | 91 (19.0%) | 0.061 |
| Angioplasty | 23 (2.7%) | 0 (0%) | 23 (4.8%) | <0.001 |
| Intra-arterial thrombolysis | 4 (0.5%) | 0 (0%) | 4 (0.8%) | 0.137 |
| Successful reperfusion (n, %) | 726 (85.7%) | 282 (76.6%) | 444 (92.7%) | <0.001 |
| PRT (mean ± SD) | 52 (37,76) | 54 (38, 75) | 51 (36, 76) | 0.585 |
| Procedure complication (n, %) | 59 (7.0%) | 29 (7.9%) | 30 (6.3%) | 0.359 |
Endovascular procedure details of the included patients, stratified by type of stroke center.
PSCs indicated primary stroke centers; CSCs, comprehensive stroke centers; PRT, puncture-to-reperfusion time; SD indicates standard deviation.
In terms of AIS workflow, the median OPT was 205 min, and 36.1% (306/847) received IVT. Among the 62.2% (527/847) of patients who presented within 4.5 h, about 58.1% (306/527) received IVT. The median door-to-puncture time (DPT) was 101 min. Overall, 85.7% (726/847) of patients achieved successful reperfusion, 7.0% (59/847) experienced procedure complications, 8.6% (73/847) had sICH, 46.3% (392/847) achieved good clinical outcomes, and 18.9% (160/847) died.
The proportion of patients eligible for EVT based on different selection criteria, including occlusion site, stroke severity (NIHSS and ASPECT), eligibility for intravenous thrombolysis, and OPT was showed Supplementary Table 2. Approximately 34.0% of the 847 patients from our registry meet the following strictest selection criteria: ICA and MCA M1 occlusion, NIHSS scores and ASPECTS ≥ 6, and OPT was within 6 h combined with intravenous thrombolysis.
Comparisons between the PSCs and CSCs
Baseline characteristics
Compared with patients from CSCs, those from PSCs had higher prevalence of AF (41.0% vs. 29.9%, p < 0.001) and smoking history (33.2% vs. 25.7%, p = 0.017). More patients from PSCs presented within 4.5 h (76.1% vs. 51.6%, p < 0.001) and 6 h (84.2% vs. 64.1%, p < 0.001) of symptom onset. Additionally, a higher proportion of patients from PSCs received IVT (50.3% vs. 25.3%, p < 0.001) and had CE subtype stroke (58.2% vs. 40.5%, p < 0.001). Conversely, fewer patients from PSCs had LAA (28.0% vs. 37.6%, p = 0.003) or other undefined subtype (13.9% vs. 21.9%, p = 0.003). Fewer patients from PSCs underwent CTA/MRA (35.1% vs. 90.0%, p < 0.001) or CTP/MRP (2.4% vs. 63.5%, p < 0.001). Baseline NIHSS scores were higher in PSCs (median 15 vs. 14, p < 0.001), while OPT was shorter (152 min vs. 258 min, p < 0.001; Table 1; Figure 2A). However, the DPT was longer (112 min vs. 95 min, p < 0.001) in PSCs (Figure 2A).
Figure 2
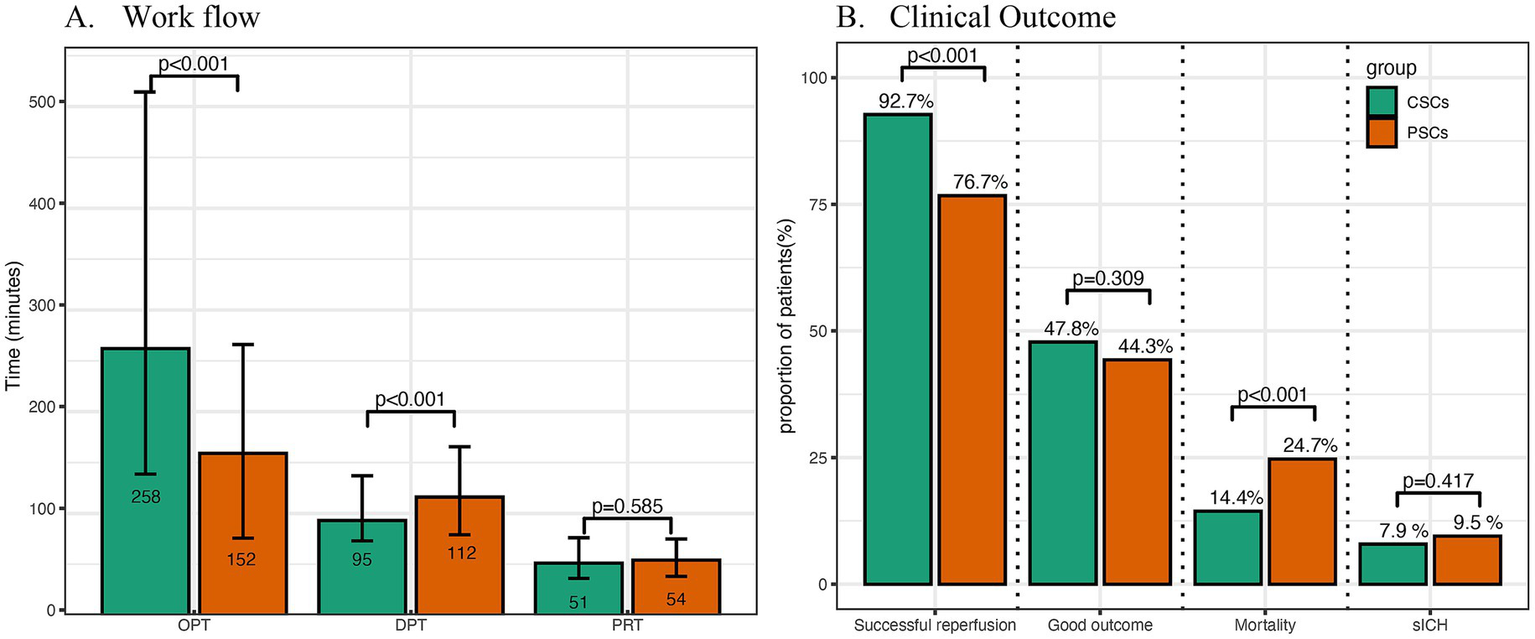
Comparison of workflow, radiological and clinical outcomes between primary and comprehensive stroke centers. OPT indicated onset-to-presentation time; DPT, door-to-puncture time; PRT, puncture-to-reperfusion time; CSCs, comprehensive stroke centers; PSCs, primary stroke centers; sICH, symptomatic intracranial hemorrhage.
EVT details (Table 2; Supplementary Table 3)
Compared with patients from CSCs, those from PSCs more frequently received stent retrievers (85.9% vs. 75.4%, p < 0.001) and less frequently underwent angioplasty as a first-line strategy (0% vs. 4.8%, p < 0.001). Successful reperfusion rates were lower in PSCs (76.6% vs. 92.7%, p < 0.001; Figure 2B). However, puncture-to-reperfusion time (PRT) (p = 0.585; Figure 2A) and procedure complication rates (p = 0.359) were similar between the two groups.
Clinical outcomes (Tables 3, 4)
Table 3
| Outcomes | All (n = 847) | PSCs (n = 368) | CSCs (n = 479) | p value |
|---|---|---|---|---|
| sICH (n, %) | 73 (8.6%) | 35 (9.5%) | 38 (7.9%) | 0.417 |
| Good clinical outcome (n, %) | 392 (46.3%) | 163 (44.3%) | 229 (47.8%) | 0.309 |
| Mortality | 160 (18.9%) | 91 (24.7%) | 69 (14.4%) | <0.001 |
Clinical outcomes of the included patients, stratified by type of stroke center.
PSCs indicated primary stroke centers; CSCs, comprehensive stroke centers; sICH indicates symptomatic intracranial cerebral hemorrhage.
Table 4
| Parameter | Unadjusted OR (95% CI) | p | Model 1 OR (95% CI) | p | Model 2 OR (95% CI) | p |
|---|---|---|---|---|---|---|
| mRS 0–2 | 0.87 (0.66–1.14) | 0.309 | 1.00 (0.74–1.35) | 1.017 | 1.04 (0.75–1.43) | 0.832 |
| Mortality | 1.95 (1.39–2.77) | <0.001 | 1.83 (1.24–2.70) | 0.002 | 1.76 (1.17–2.66) | 0.007 |
| sICH | 1.22 (0.75–1.97) | 0.418 | 1.05 (0.63–1.77) | 0.842 | 1.03 (0.60–1.76) | 0.929 |
Multivariate logistic regression analyses relationship between clinical outcome and stroke center type.
mRS indicated modified Rankin Scale; sICH, symptomatic intracranial hemorrhage. CSCs as reference. Model 1:adjusted for age, NIHSS, ASPECT;Model 2:adjusted for age, NIHSS, ASPECT, occlusion site, and OPT.
The occurrence of symptomatic intracerebral hemorrhage (sICH) (p = 0.417) and the rate of good clinical outcomes (p = 0.309) were similar between CSCs and PSCs (Figure 2B). The distribution of 90-day modified Rankin Scale (mRS) scores across different types of stroke centers is shown in Figure 3. However, mortality rates were higher in PSCs (24.7% vs. 14.4%, p < 0.001). After adjusting for age, NIHSS, ASPECTS, occlusion site, and OPT, clinical outcomes, including good clinical outcomes [adjusted odds ratio (aOR) = 1.04, 95% confidence interval (CI), 0.75–1.43, p = 0.832] and sICH (aOR = 1.03, 95% CI, 0.63–1.76, p = 0.929), were comparable between CSCs and PSCs. However, the mortality rate remained higher in PSCs than in CSCs (aOR = 1.76, 95% CI, 1.17–2.66, p = 0.007; Figure 2B).
Figure 3
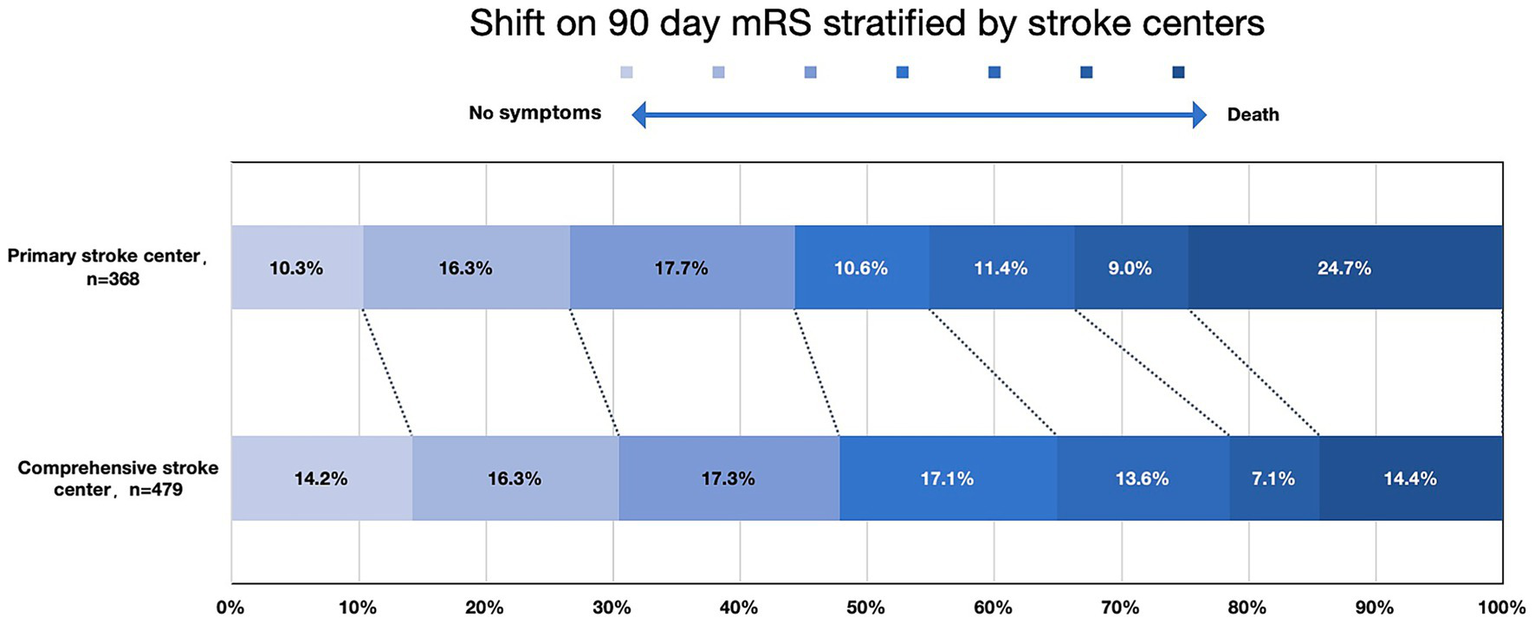
mRS score distribution at 90 days, stratified by stroke center mRS indicated modified Rankin Scale.
Comparisons of radiological and clinical outcomes between ETERNITY registry and ANGEL-ACT registry
Radiological and clinical outcomes were also compared between ETERNITY registry and ANGEL-ACT registry (Supplementary Table 4). Rate of good outcome and sICH was similar between two registries. Successful reperfusion rate was lowest in PSCs in Eternity registry, highest in CSCs in Eternity registry, general successful reperfusion rate in Eternity registry was comparable with ANGEL-ACT registry.
The clinical outcomes were similar across the predefined subgroups between two type of stroke center, except for patients with ASPECTS < 9 or who no received IVT had more favorable outcomes in CSCs than that in PSCs (Figure 4; Supplementary Figures 1–6).
Figure 4
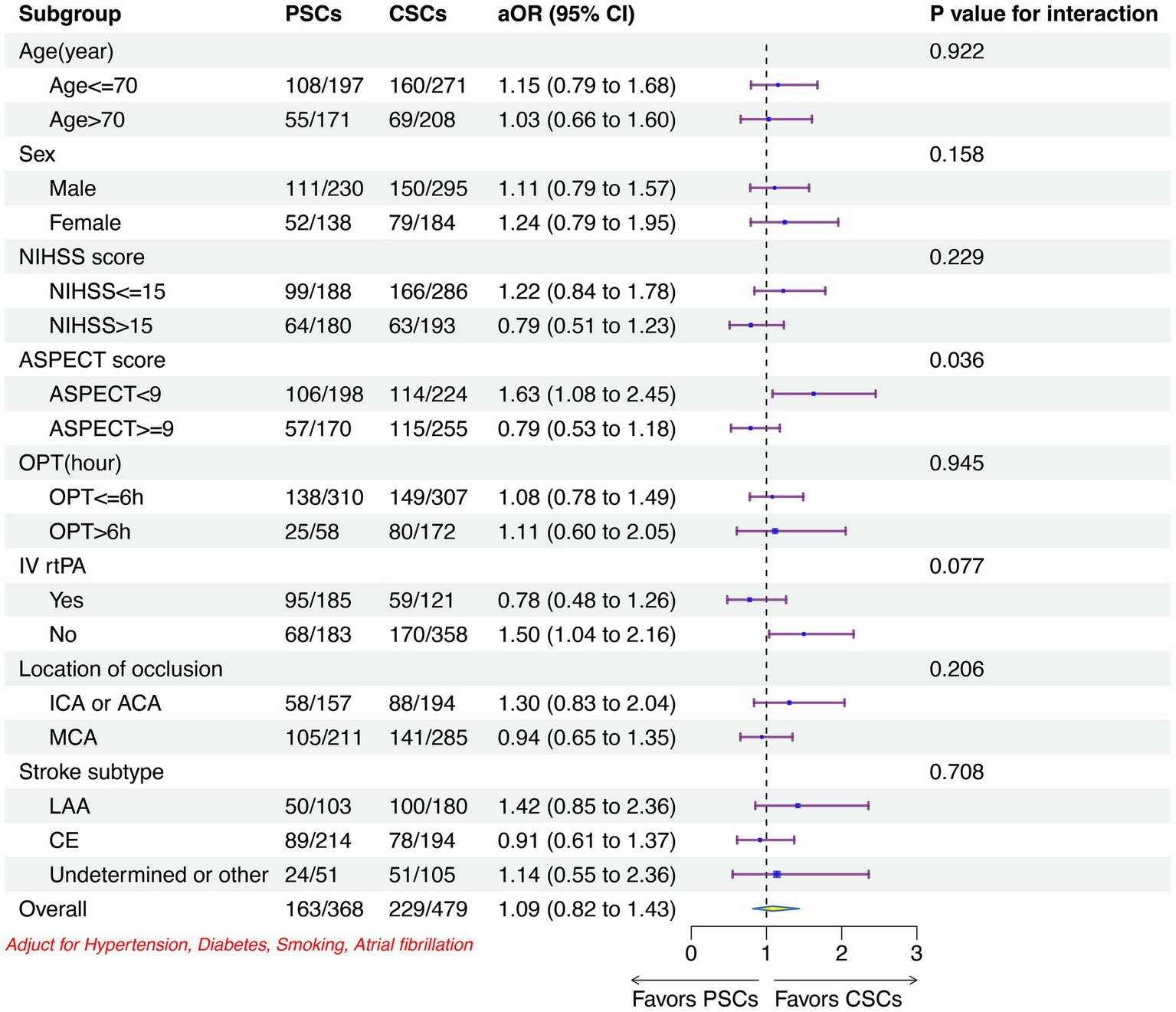
Subgroup analyses of the primary outcome between two stroke centers. CSCs indicated comprehensive stroke centers; PSCs, primary stroke centers; OR, odd ratio; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; ASPECT, Alberta Stroke; OPT, onset-to-puncture time; IV, intravenous; rtPA, recombined tissue plasminogen activator; ICA, intracranial carotid artery; ACA, anterior cerebral artery; MCA, middle cerebral artery; LAA, large artery atherosclerosis; CE, cardia embolism.
Discussion
To our best knowledge, the ETERNITY registry is the first real-world nationwide database with a broad geographic distribution of endovascular capable centers with relative few restrictions for patient selection. It provides an accurate reflection of the current status of EVT in clinical practice both in CSCs and PSCs in China. The registry specifically aims to explore differences in AIS workflow, patients’ characteristic, preprocedural brain image, EVT technique, clinical and radiological outcome between CSCs and PCSs.
From ETERNITY registry, we observed significant differences in patient characteristics between CSCs and PSCs. First, regarding TOAST classification, CE stroke was more common in PSCs, while LAA was more prevalent in CSCs. Second, OPT was shorter in PSCs, with a higher proportion of patients presenting within 4.5 h. Third, rate of IVT, IVT proportion was higher in PSCs. CE strokes typically present with a sudden onset of neurological deficits that are maximal at onset, whereas LAA strokes may have a less defined, waxing and waning course (15). Due to the characteristic onset of CE, patients with CE etiology may be referred to nearby hospitals, which are often PSCs. Combined with better traffic conditions, this may contribute to shorter OPTs and a higher proportion of patients presenting to PSCs within 4.5 h, potentially eligible for IVT.
IVT is a cornerstone treatment for AIS treatment, significantly improving outcomes when administered within 4.5 h of symptom onset (16). However, its utilization in China remains suboptimal. Only 10–20% of AIS patients in China arrive at a hospital within 3 h, and fewer than 3% receive IVT (17). This underuse can be attributed to multiple factors, including delayed symptom recognition, limited awareness among healthcare providers, inefficient in-hospital AIS workflow, and inadequate medical resources. In the ETERNITY registry, approximately 60% of patients arrived at the hospital within 4.5 h, and over 50% received IVT. This higher rate of IVT use in PSCs compared to previous studies (4, 18, 19) likely reflects improvements in stroke care due to the construction of stroke centers in China. The higher proportion of IVT in PSCs may also be influenced by the characteristics of cardioembolic (CE) stroke. CE strokes often present with sudden, severe neurological deficits, prompting quicker hospital presentation. Additionally, better traffic conditions in urban areas where PSCs are often located facilitate faster hospital arrival. The underuse of IVT in eligible population may also be influenced by the perception that direct mechanical thrombectomy (MT) without prior IVT is non-inferior to bridging therapy, as suggested by the DIRECT MT trial, although with a broad non-inferiority margin (20). Moreover, the IRIS meta-analysis showed no superiority of thrombolysis in later time windows (after 2.5 h), further complicating the decision-making process for IVT administration. In summary, while IVT is a highly effective treatment for AIS, its use in China is still limited. The ETERNITY registry highlights improvements in IVT utilization in PSCs, likely due to enhanced stroke center infrastructure and the characteristics of CE stroke. However, continued efforts are needed to optimize IVT delivery and improve stroke outcomes nationwide.
Data from the registry also highlight difference in AIS workflow between CSCs and PSCs. First, regarding brain imaging modalities, angiography and perfusion imaging were less frequently performed in PSCs. Second, DPT was longer in PSCs. Stroke imaging is crucial in AIS management (21). The role of brain imaging in AIS management includes identifying the infarct core (IC), tissue at risk, and arterial lesion (21). Non-contrast CT is an easily accessible and time-saving tool used in AIS management and can be performed at any time. However, it is suboptimal for identifying large vessel occlusions (LVOs) and is less sensitive in detecting extremely early ischemic changes (22). Clinician using NIHSS score ≥ 10 to identify LVO only have 48% sensitivity, potentially missing half of LVO cases (23). Therefore, invasive arterial imaging, such as CTA and MRA, is necessary in AIS management. CTA is the most accurate and efficient non-invasive method for confirming or excluding LVOs and is recommended to be performed as quickly as possible in patients with suspected LVOs (AHA Class I, Level of Evidence B) (23). Perfusion imaging is another important modality for detecting the IC and tissue at risk (24). CTA images analyzed on a PACS workstation with MIP and MPR capabilities may miss about 20% of LVOs (mostly M2 segment) during initial CTA evaluation (25, 26). Perfusion imaging with automated processing software can assist clinicians in easily identifying lesions (27). Since a complete imaging package with non-contrast CT, CTA, and CTP can be obtained in less than 6 min, published endovascular stroke intervention trials have shown that performing CTA imaging does not delay IV tPA infusion (22). Therefore, one-stop multimodal CTA and CTP should be recommended to be performed as soon as possible for better AIS management. However, less than half of AIS-LVO patients in PSCs underwent CTA/CTP. The reasons may include limited awareness among staff in PSCs, medical resource shortages, and in-hospital workflow inefficiencies. Despite the potential time-saving benefits from no advanced brain imaging, DPT was longer in PSCs. The reasons may include the lack of an unobstructed AIS in-hospital workflow, shortage of neuro-interventionists, and absence of a standardized workflow in the catheterization suite. Therefore, the AIS in-hospital workflow, including the emergency department, radiology department, and catheterization suite in PSCs, should be optimized according to the criteria proposed by the CSPPC to minimize door-to-CT, door-to-needle, and DPT time (6). Additionally, training of qualified personnel should be emphasized.
According to the registry, the successful reperfusion rate was lower in PSCs. Our registry showed that more than half of the cases were classified as CE according to the TOAST criteria. Previous studies had demonstrated that compared with no LAA, lower intraprocedural re-occlusion, higher successful reperfusion, shorter PRT (28) in CE stroke. However, data from registry did not show these promising results, on the contrary, low successful reperfusion rate was observed in PSCs. Many factors are associated with successful reperfusion, including occlusion site characteristics; EVT device use, including the use of a stent retriever or balloon guiding catheter; aspiration catheter use; EVT procedural technique (29–37); and neuro-interventionist skill. Effort should be made to improve neuro-interventionists’ capacity by training, enhance EVT success rate via virtual supervision by remote streaming support (38) or utilize remote robotic EVT technology (39). Additionally, it is crucial to ensure that a sufficient range of EVT devices is available to allow the use of appropriate devices according to lesion characteristics.
Despite the longer DPT and lower successful reperfusion rate in the PSCs, the rate of good functional outcomes was similar between PSCs and CSCs. Good functional outcome is associated with multiple factors, including the reperfusion status (13, 40), the onset-to-reperfusion time (41), procedural complications such as vessel perforation (42), and distal embolism (43). In PSCs, the short OPT may mitigate the impact of the lower successful reperfusion rate on overall clinical outcomes.
We also found that nearly one-quarter of thrombectomy cases died in PSCs, with mortality being higher in PSCs. The high mortality rate in PSCs may be attributed to the several factors: first, the high incidence of CE, which is associated with more severe complications and a higher incidence of adverse outcomes (44). Second, the low successful reperfusion rate, which is associated with lager infarct area. Third, post-procedure management. The post-procedure management of AIS, especially in patients with large infarcts is complex and includes blood pressure control, antiplatelet agent administration, brain edema control, and sICH management (42). Fourth, lack of stroke unit. Stroke unit care were associated with 15% lower odds of complications and 15% lower odds of mortality during hospitalization among patients who received thrombolytic therapy (6). Accordingly, to narrow the mortality gap between PSCs and CSCs, we must implement uniform, evidence-based post-reperfusion management protocols and guarantee equitable, round-the-clock access to dedicated stroke-unit beds.
Limitations
This study had several limitations. First, the registry includes data from only 26PSCs and does not represent all PSCs nationwide. However, we recruited hospitals from 7 regions in China and considered the population density and incidence of AIS morbidity in the different zones, which could provide a representative sample of PSCs nationwide to the greatest extent. Second, we included only AIS patients with anterior circulation LVO who met the 2018 AHA EVT guideline criteria. Hence, the EVT outcomes beyond the recommendations of the guidelines in the real world remain uncertain. Third, the registry included data from only 1 year. As AIS workflow and EVT skills improve annually, additional county hospitals should be recruited, and longer study times are needed to explore the differences between the two types of stroke centers.
Conclusion
Our nationwide real-world registry highlights several differences in EVT for AIS-LVO patients between PSCs and CSCs in China. Given that the clinical outcomes of AIS-LVO are closely associated with timely intervention and successful EVT, enhancing EVT skills through targeted training programs and standardizing stroke center construction are essential. With further improvements in in-hospital workflow and physician training at PSCs, we anticipate that clinical outcomes for AIS-LVO patients treated at PSCs will improve in the future.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committees of Zhangzhou Municipal Hospital, Fujian Medical University, and all participating centers (approval number: 2021KYB209). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
T-yY: Funding acquisition, Software, Resources, Conceptualization, Supervision, Project administration, Writing – review & editing, Visualization, Data curation, Writing – original draft, Methodology, Formal analysis, Validation, Investigation. SG: Writing – original draft, Software, Investigation, Formal analysis, Writing – review & editing, Methodology, Data curation, Project administration, Visualization, Conceptualization, Validation. MW: Project administration, Formal analysis, Resources, Data curation, Methodology, Writing – review & editing, Investigation. WeifH: Data curation, Investigation, Writing – review & editing, Resources, Project administration, Formal analysis, Methodology. YaW: Formal analysis, Methodology, Resources, Supervision, Project administration, Investigation, Data curation, Writing – review & editing. HaT: Writing – review & editing, Conceptualization. CY: Data curation, Writing – review & editing. LX: Data curation, Writing – review & editing. HuT: Writing – review & editing, Formal analysis. LG: Writing – review & editing, Conceptualization, Data curation. ShuZ: Writing – review & editing, Data curation, Conceptualization. LW: Writing – review & editing, Data curation. YiW: Data curation, Writing – review & editing. GY: Writing – review & editing, Data curation. JY: Data curation, Writing – review & editing. DJ: Writing – review & editing, Data curation. YS: Methodology, Writing – review & editing. ZZ: Writing – review & editing, Data curation. JS: Writing – review & editing, Data curation. ShiZ: Data curation, Writing – review & editing. WeidH: Writing – review & editing, Data curation. XW: Data curation, Writing – review & editing. DL: Data curation, Writing – review & editing. XL: Writing – review & editing. ZP: Software, Writing – review & editing. XZ: Data curation, Writing – review & editing. GH: Writing – review & editing, Data curation. RC: Data curation, Writing – review & editing. LZ: Formal analysis, Writing – review & editing. TN: Writing – review & editing, Validation. XB: Data curation, Writing – review & editing. LJ: Supervision, Resources, Conceptualization, Project administration, Writing – review & editing, Validation, Methodology, Formal analysis. W-hC: Writing – original draft, Resources, Investigation, Writing – review & editing, Visualization, Funding acquisition, Formal analysis, Validation, Data curation, Methodology, Project administration, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. W-hC received grants from National Health Commission Capacity Building and Continuing Education Center (grant no. GWJJ2021100203). T-yY received grants from Natural Science Foundation of Fujian Province (grant no. 2022 J01123138) and Beijing Health Promotion Association: Research on Appropriate Intervention Technique for High-risk Crowds of Stroke (BHPA2021IN002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GZ declared a past co-authorship with the author LJ to the handling editor.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1655954/full#supplementary-material
References
1.
Powers WJ Rabinstein AA Chair V Ackerson T Adeoye OM Bambakidis NC et al . Guidelines for the early Management of Patients with Acute Ischemic Stroke: A guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. ASA J. (2018) 50:211. doi: 10.1161/STR.0000000000000211
2.
Martins SO Mont’Alverne F Rebello LC Abud DG Silva GS Lima FO et al . Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. (2020) 382:2316–26. doi: 10.1056/nejmoa2000120
3.
Wang YJ Li ZX Gu HQ Zhai Y Zhou Q Jiang Y et al . China stroke statistics: an update on the 2019 report from the National Center for healthcare quality Management in Neurological Diseases, China National Clinical Research Center for neurological diseases, the Chinese Stroke Association, National Center f. Stroke Vasc Neurol. (2022) 7:415–50. doi: 10.1136/svn-2021-001374
4.
Wang Y Li Z Zhao X Wang D Li H Xian Y et al . Stroke care quality in China: substantial improvement, and a huge challenge and opportunity. Int J Stroke. (2017) 12:229–35. doi: 10.1177/1747493017694392
5.
Saver JL . Time is brain--quantified. Stroke. (2006) 37:263–6. doi: 10.1161/01.STR.0000196957.55928.ab
6.
Shen Y Chao BH Cao L Tu WJ Wang LD . Stroke center care and outcome: results from the CSPPC stroke program. Transl Stroke Res. (2020) 11:377–86. doi: 10.1007/s12975-019-00727-6
7.
Nogueira RG Jadhav AP Haussen DC Bonafe A Budzik RF Bhuva P et al . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2017) 378:11–21. doi: 10.1056/NEJMoa1706442
8.
Albers GW Marks MP Kemp S Christensen S Tsai JP Ortega-Gutierrez S et al . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/nejmoa1713973
9.
Adams HP Bendixen BH Kappelle LJ Biller J Love BB Gordon DL et al . Classification of subtype of acute ischemic stroke. Stroke. (1993) 23:35–41. doi: 10.1161/01.STR.24.1.35
10.
Von Kummer R Broderick JP Campbell BCV Demchuk A Goyal M Hill MD et al . The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
11.
Chen W Wu Y Zhang M Yi T-Y Chen W-H Wu Y-M et al . Microcatheter “first-pass effect” predicts acute intracranial artery atherosclerotic disease-related occlusion. Neurosurgery. (2018) 84:1296–305. doi: 10.1093/neuros/nyy183
12.
Chen WH Yi TY Zhan AL Wu YM Lu YY Li YM et al . Stent-unsheathed effect predicts acute distal middle cerebral artery atherosclerotic disease-related occlusion. J Neurol Sci. (2020) 416:957. doi: 10.1016/j.jns.2020.116957
13.
Lecouffe NE Kappelhof M Treurniet KM Lingsma HF Zhang G van den Wijngaard IR et al . 2B, 2C, or 3: what should be the angiographic target for endovascular treatment in ischemic stroke?Stroke. (2020) 51:1790–6. doi: 10.1161/STROKEAHA.119.028891
14.
Zaidat OO Yoo AJ Khatri P Tomsick TA von Kummer R Saver JL et al . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. (2013) 44:2650–63. doi: 10.1161/STROKEAHA.113.001972
15.
Arboix A Oliveres M Massons J Pujades RG-EL . Early differentiation of cardioembolic from atherothrombotic cerebral infarction: a multivariate analysis. Eur J Neurol. (1999) 6:677–83. doi: 10.1046/j.1468-1331.1999.660677.x
16.
Powers WJ Rabinstein AA Ackerson T Adeoye OM Bambakidis NC Becker K et al . 2018 guidelines for the early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–e110. doi: 10.1161/STR.0000000000000158
17.
Wu S Wu B Liu M Chen Z Wang W Anderson CS et al . Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. (2019) 18:394–405. doi: 10.1016/S1474-4422(18)30500-3
18.
Man S Zhao X Uchino K Hussain MS Smith EE Bhatt DL et al . Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. (2018) 11:e004512. doi: 10.1161/CIRCOUTCOMES.117.004512
19.
Liu Z Gu H Wei M Feng X Yu F Feng J et al . Comparison between healthcare quality in primary stroke centers and comprehensive stroke centers for acute stroke patients: evidence from the Chinese stroke center alliance. Lancet Reg Health. (2023) 38:100863. doi: 10.1016/j.lanwpc.2023.100863
20.
Yang P Zhang Y Zhang L Zhang Y Treurniet KM Chen W et al . Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. (2020) 382:1981–93. doi: 10.1056/NEJMoa2001123
21.
Abdalkader M Siegler JE Lee JS Yaghi S Qiu Z Huo X et al . Neuroimaging of acute ischemic stroke: multimodal imaging approach for acute endovascular therapy. J Stroke. (2023) 25:55–71. doi: 10.5853/jos.2022.03286
22.
McTaggart RA Ansari SA Goyal M Abruzzo TA Albani B Arthur AJ et al . Initial hospital management of patients with emergent large vessel occlusion (ELVO): report of the standards and guidelines committee of the society of NeuroInterventional surgery. J Neurointerv Surg. (2017) 9:316–23. doi: 10.1136/neurintsurg-2015-011984
23.
Maas MB Furie KL Lev MH Ay H Singhal AB Greer DM et al . National institutes of health stroke scale score is poorly predictive of proximal occlusion in acute cerebral ischemia. Stroke. (2009) 40:2988–93. doi: 10.1161/STROKEAHA.109.555664
24.
Sui Y Chen W Chen C Chang Y Bivard A Wang P et al . CTP-defined large Core is a better predictor of poor outcome for endovascular treatment than ASPECTS-defined large Core. Stroke. (2024) 55:1227–34. doi: 10.1161/strokeaha.123.045091
25.
Fasen BACM Heijboer RJJ Hulsmans FJH Kwee RM . CT angiography in evaluating large-vessel occlusion in acute anterior circulation ischemic stroke: factors associated with diagnostic error in clinical practice. Am J Neuroradiol. (2020) 41:607–11. doi: 10.3174/AJNR.A6469
26.
Lin L Cheng X Bivard A Levi CR Dong Q Parsons MW . Quantifying reperfusion of the ischemic region on whole-brain computed tomography perfusion. J Cereb Blood Flow Metab. (2017) 37:2125–36. doi: 10.1177/0271678X16661338
27.
Koopman MS Berkhemer OA Geuskens RREG Emmer BJ van Walderveen M Jenniskens SFM et al . Comparison of three commonly used CT perfusion software packages in patients with acute ischemic stroke. J Neurointerv Surg. (2019) 11:1249–56. doi: 10.1136/neurintsurg-2019-014822
28.
Tsang ACO Orru E Klostranec JM Yang IH Lau KK Tsang FCP et al . Thrombectomy outcomes of intracranial atherosclerosis-related occlusions. Stroke. (2019) 50:1460–6. doi: 10.1161/STROKEAHA.119.024889
29.
Yi T-Y Chen W-H Wu Y-M Zhang MF Lin DL Lin XH . Another endovascular therapy strategy for acute tandem occlusion: protect-expand-aspiration-revascularization-stent (PEARS) technique. World Neurosurg. (2018) 113:e431–8. doi: 10.1016/j.wneu.2018.02.052
30.
Chen WH Yi TY Wu YM Zhang MF Lin DL Lin XH . Endovascular therapy strategy for acute embolic tandem occlusion: the pass-Thrombectomy-protective Thrombectomy (double PT) technique. World Neurosurg. (2018) 120:e421–7. doi: 10.1016/j.wneu.2018.08.096
31.
McTaggart RA Tung EL Yaghi S Cutting SM Hemendinger M Gale HI et al . Continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE): a technique which improves outcomes. J Neurointerv Surg. (2017) 9:1154–9. doi: 10.1136/neurintsurg-2016-012838
32.
Chen WH Yi T Wu YM Pan ZN Zheng XF Lin XH et al . Initial clinical experience of repeat Thrombectomy with a retrieval stent (RTRS) with continuous proximal flow arrest by balloon guide catheter for acute intracranial carotid occlusion. Behav Neurol. (2021) 2021:1–7. doi: 10.1155/2021/7607324
33.
Yi TY Wu YM Lin DL Pan ZN Zheng XF Gan J et al . Application of balloon AngioplaSty with the dIstal protection of stent retriever (BASIS) technique for acute intracranial artery atherosclerosis-related occlusion. Front Neurol. (2022) 13:543. doi: 10.3389/fneur.2022.1049543
34.
McTaggart RA McTaggart RA McTaggart RA Ospel JM Psychogios M-N Puri AS et al . Optimization of endovascular therapy in the neuroangiography suite to achieve fast and complete (expanded treatment in cerebral ischemia 2c-3) reperfusion. Stroke. (2020) 51:1961–8. doi: 10.1161/STROKEAHA.119.026736
35.
Yi HJ Sung JH Lee DH Song SY . Effectiveness and technical considerations of solitaire platinum 4×40 mm stent retriever in mechanical thrombectomy with solumbra technique. J Korean Neurosurg Soc. (2021) 64:30–8. doi: 10.3340/jkns.2020.0046
36.
Chen Z Liu Y Li B Yuan C Hou K Chen L et al . Comparing the conventional and balloon-guided catheter-assisted SWIM Technology for the Treatment of acute ischemic stroke. Front Neurol. (2022) 13:1–7. doi: 10.3389/fneur.2022.866673
37.
Nguyen TN Abdalkader M Qureshi MM Michel P Strambo D Strbian D et al . First-line stent retriever versus contact aspiration or combined technique for endovascular therapy of posterior cerebral artery occlusion stroke: the PLATO study. Stroke Vasc Interv Neurol. (2024) 4:1–14. doi: 10.1161/svin.123.001004
38.
Paech D Lehnen N Lakghomi A Schievelkamp A Gronemann C Bode FJ et al . School of thrombectomy—a 3-step approach to perform acute stroke treatment with simulator training and virtual supervision by remote streaming support (RESS). Clin Neuroradiol. (2023) 33:529–35. doi: 10.1007/s00062-022-01242-2
39.
Singer J Vanoosterhout S Madder R . Remote robotic endovascular thrombectomy for acute ischaemic stroke. BMJ Neurol Open. (2021) 3:e000141–6. doi: 10.1136/bmjno-2021-000141
40.
Liebeskind DS Bracard S Guillemin F Jahan R Jovin TG Majoie CB et al . ETICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. (2019) 11:433–8. doi: 10.1136/neurintsurg-2018-014127
41.
Khatri P Yeatts SD Mazighi M Broderick JP Liebeskind DS Demchuk AM et al . Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. (2014) 13:567–74. doi: 10.1016/S1474-4422(14)70066-3
42.
Yi T Chen W Wu Y Pan Z Lin X Lin D et al . Intra-arterial injection of thrombin as rescue therapy of vessel perforation during mechanical Thrombectomy for acute ischemic stroke. Brain Sci. (2022) 12:760. doi: 10.3390/brainsci12060760
43.
Todo A Minaeian A Sahni R Chao KH . Incidence and outcome of procedural distal emboli using the penumbra thrombectomy for acute stroke. J Neurointerv Surg. (2013) 5:135–8. doi: 10.1136/neurintsurg-2011-010216
44.
Wang Y Haddad Y Patel R Geng X du H Ding Y . Factors influencing the outcome of cardiogenic cerebral embolism: a literature review. Neurol Res. (2022) 44:187–95. doi: 10.1080/01616412.2021.1968704
45.
Jia B Ren Z Mokin M Burgin WS Bauer CT Fiehler J et al . Current status of endovascular treatment for acute large vessel occlusion in China: a real-world Nationwide registry. Stroke. (2021) 52:1203–12. doi: 10.1161/STROKEAHA.120.031869
Summary
Keywords
endovascular treatment, prognosis, large vessel occlusion, acute ischemia, primary stroke center, comprehensive stroke center
Citation
Yi T-y, Gan S, Wu M, Huang W, Wu Y, Tu H, Yang C, Xu L, Tan H, Gao L, Zhao S, Wei L, Wu Y, Yang G, Ye J, Ju D, Shao Y, Zhang Z, Su J, Zhao S, Huang W, Wu X, Lin D, Lin X, Pan Z, Zheng X, Hong G, Chen R, Zeng L, Nguyen TN, Bai X, Jiao L and Chen W-h (2025) Comparison of endovascular therapy care and outcome in primary and comprehensive stroke centers for acute ischemic stroke in China a real-world nationwide registry. Front. Neurol. 16:1655954. doi: 10.3389/fneur.2025.1655954
Received
29 June 2025
Accepted
06 October 2025
Published
10 November 2025
Volume
16 - 2025
Edited by
Stephan Meckel, University of Freiburg Medical Center, Germany
Reviewed by
Guang Zhang, First Affiliated Hospital of Harbin Medical University, China; Zhaolong Zhang, The Affiliated Hospital of Qingdao University, China
Updates
Copyright
© 2025 Yi, Gan, Wu, Huang, Wu, Tu, Yang, Xu, Tan, Gao, Zhao, Wei, Wu, Yang, Ye, Ju, Shao, Zhang, Su, Zhao, Huang, Wu, Lin, Lin, Pan, Zheng, Hong, Chen, Zeng, Nguyen, Bai, Jiao and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Jiao, liqunjiao@sina.cn; Wen-huo Chen, doctorwwenhuo@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.