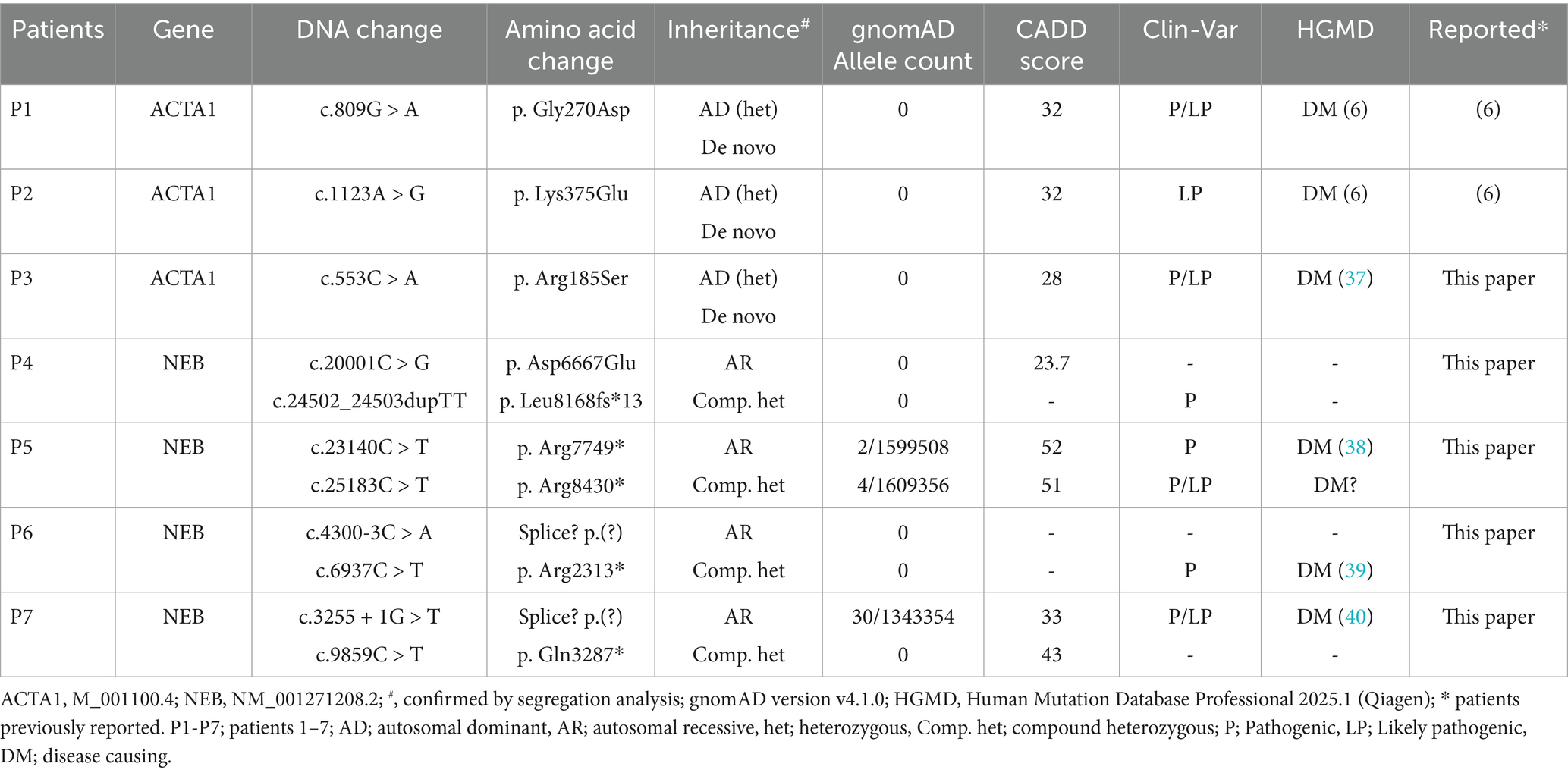
- 1Department of Laboratory Medicine, University of Gothenburg, Gothenburg, Sweden
- 2Cizre State Hospital, Sirnak, Türkiye
- 3Department of Pediatrics, University of Gothenburg, Gothenburg, Sweden
Background: Nemaline myopathy is a rare congenital muscle disorder characterized by the presence of nemaline rods, protein aggregates, in muscle fibers. Pathogenic variants in several genes, most commonly NEB and ACTA1, which encode thin filament proteins of the sarcomere, have been implicated in its etiology. Currently, there is no cure for nemaline myopathy, underscoring the need to identify disease-modifying targets for therapeutic development.
Methods: In this study, we employed quantitative nanoscale liquid chromatography–tandem mass spectrometry (LC-MS3) with labeled protein analysis on muscle tissue from five normal controls and seven infants diagnosed with nemaline myopathy due to NEB or ACTA1 pathogenic variants.
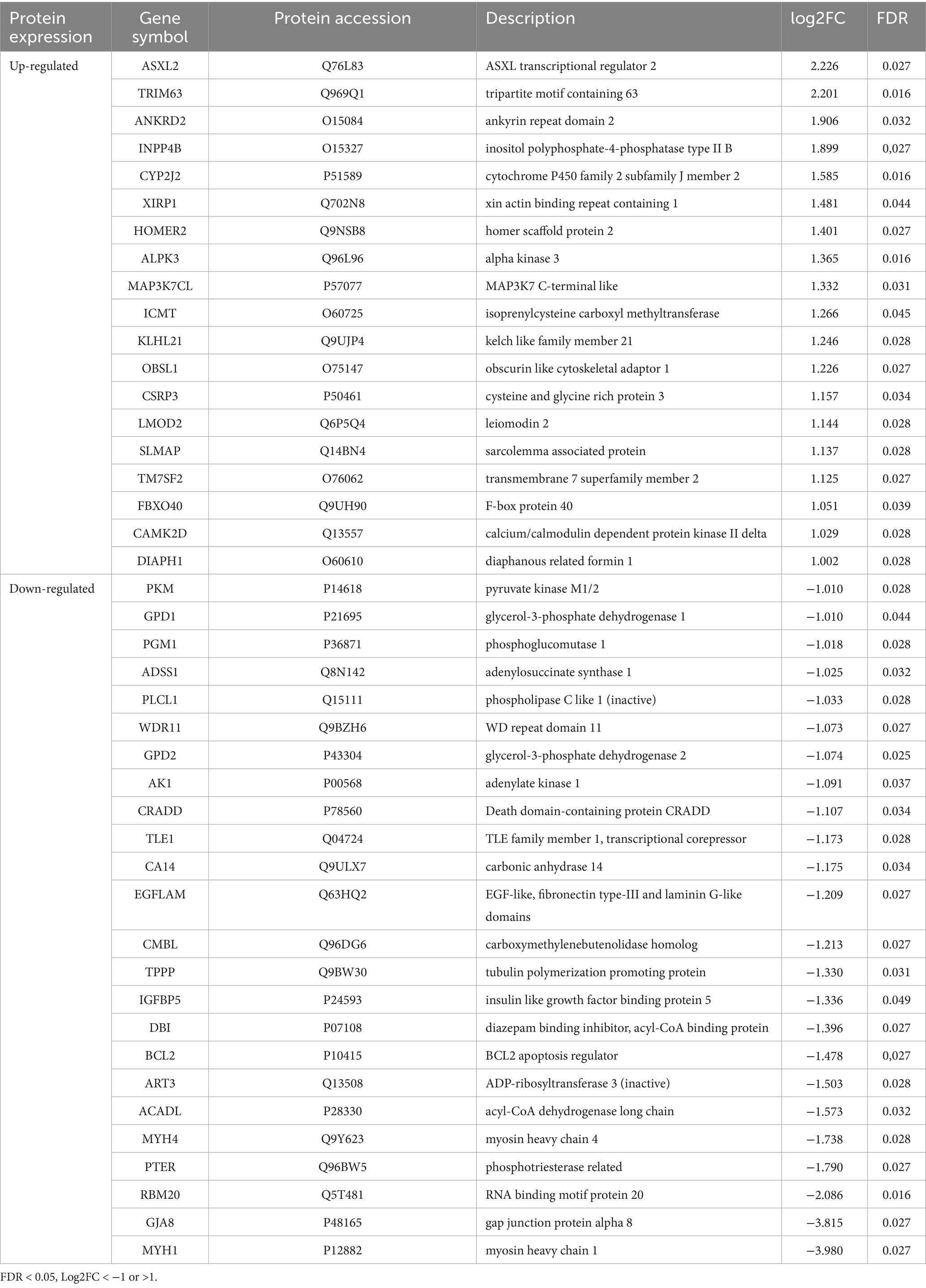
Results: We identified and quantified 4,846 proteins across all samples, with 183 proteins showing significant dysregulation. Protein–protein interaction analysis revealed nine upregulated, muscle-specific proteins: NRAP, FBXO40, TRIM63, TRIM54, ALPK3, XIRP1, ANKRD2, LMOD2, and CSRP3. Further pathway analysis indicated upregulation of protein synthesis and proteasomal degradation processes, alongside downregulation of glycolysis. Notably, the dysregulated proteins and pathways were consistent across both genetic subtypes, suggesting shared molecular mechanisms underlying the disease.
Conclusion: This proteomic profiling study has identified key dysregulated proteins and pathways in infantile nemaline myopathy. These findings advance our understanding of the disease’s molecular basis and highlight candidate targets for future therapeutic intervention.
1 Introduction
Nemaline myopathy is one of the most common forms of congenital myopathy and is characterized by the presence of numerous small protein aggregates named nemaline rods in the muscle fibers (1). Nemaline myopathies have traditionally been classified into different types based on their clinical presentation (2, 3). At least 12 different genes have been associated with nemaline myopathy, NEB encoding nebulin and ACTA1 encoding alpha-actin are the most prevalent (4). Nemaline myopathy caused by pathogenic NEB variants usually show recessive inheritance while ACTA1 associated nemaline myopathy is usually caused by dominant, mostly de novo, variants (3). All nemaline myopathies seem to be associated with proteins involved in the structure and function of the thin filaments of the sarcomere (3). In spite of the many genes involved there are morphological similarities with regard to the common pathological hallmark, the nemaline rods, but there are also differences with regard to fiber type composition, severity of morphological changes as well as age-related changes (5, 6). Currently, there are no therapies available for nemaline myopathy. Proteomic analysis of affected muscle tissue is an emerging research field that may, together with genomics, help identify dysregulated proteins and protein networks to reveal pathobiological mechanisms, novel biomarkers, and identify potential therapeutic interventions in neuromuscular disorders (7). In this study, we used a proteomic approach to identify dysregulated proteins and altered cellular pathways in muscle at infancy of the two major genetic forms of nemaline myopathy with similar clinical phenotype and muscle biopsy histopathology. This work identified several dysregulated proteins, which are potential targets to treat nemaline myopathy.
2 Materials and methods
2.1 Material
Muscle biopsy specimens from seven infant patients diagnosed with intermediate congenital nemaline myopathy (2) were included in this study (Table 1). The mean age of the patients at biopsy was 5.4 months (range, 2–19 months). Three patients had de novo dominant ACTA1 variants, and 4 patients were compound heterozygous for NEB variants (Table 1). Skeletal muscle controls included individuals with normal muscle biopsies who had been investigated for a possible mitochondrial disease, but in whom the clinical, biochemical and pathological investigations excluded muscle disease. The mean age of the controls at biopsy was 3.6 months (range, 1–9 months).
This study was conducted according to the Declaration of Helsinki and approved by the Swedish Ethical Review Authority, approval number 2022–00026-01.
2.2 Muscle biopsy and histopathological investigations
For histopathological investigations, open biopsy of the vastus lateralis muscle was performed. Specimens were mounted on cork plates and snap frozen in isopentane in liquid nitrogen. One specimen in each case was fixed in buffered glutaraldehyde for electron microscopy. Routine methods were applied for morphological and enzyme histochemical investigations (8). Fiber typing was assessed by myofibrillar ATPase staining or myosin heavy chain immunohistochemistry (8, 9).
2.3 Proteomic investigations
Skeletal muscle protein extracts from 7 patients and 5 normal controls were prepared from fresh frozen muscle biopsies. For quantitative analysis the proteins were labeled using TMTpro 18-plex isobaric mass tagging reagents (Thermo Fischer Scientific) and analyzed by nanoscale liquid chromatography–tandem mass spectrometry (LC-MS3) according to details described in Supplementary material. Raw files were processed and analyzed with Proteome Discoverer against UniProt Swiss-Prot Homo Sapiens using Sequest as a search engine. The gene symbols are used throughout the manuscript to describe the encoded proteins. Protein data were partly analyzed with the software Omics Playground (BigOmicsAnalytics, v3.5.24) (10). To identify differentially expressed proteins, the data was log2-transformed, and then, for each protein, log2 fold change (log2FC) and p-values were computed using Welch’s t-test for patients versus controls. To control for multiple comparisons, the Benjamini-Hochberg method was used to adjust the p-values, and proteins with a false discovery rate (FDR) less than 0.05 were considered significant.
Pathways analysis and protein–protein interaction analysis were performed by applying different search tools and web resources such as ProteoMap website1 (11) and STRING website2 (12).
3 Results
3.1 Histopathological investigation
Histopathological investigation showed a similar pattern of nemaline myopathy in all cases, (Figures 1, 2). Nemaline rods were seen in most fibers but mainly in small type 1 fibers. Both type 1 and type 2 fibers were present in all cases, with some variability of fiber type predominance, and also in different regions of the same biopsy. In addition to nemaline rods, other structural alterations of the myofibrils were also frequent.
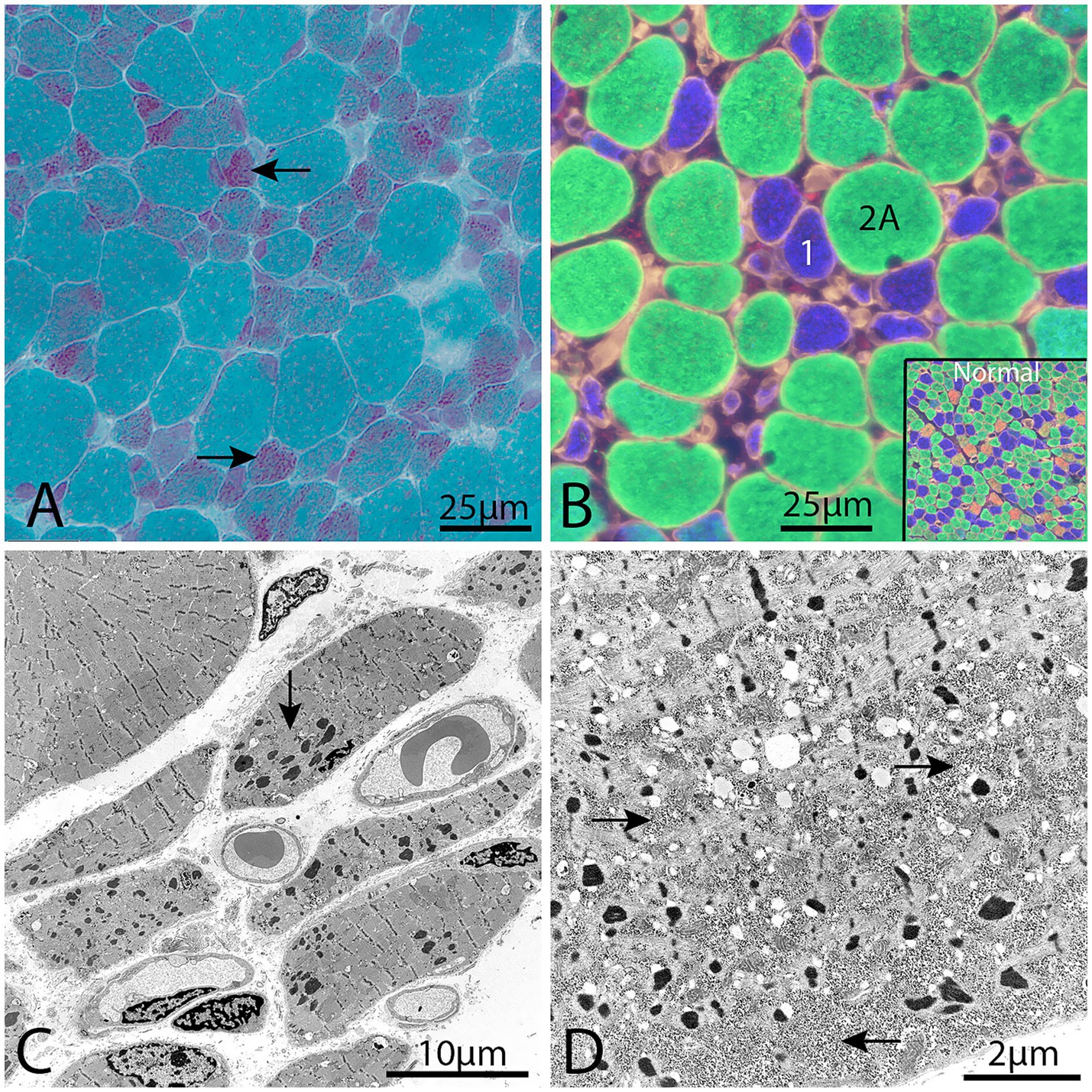
Figure 1. Representative example of a muscle biopsy of a patient with nemaline myopathy caused by NEB variants. (A) There is an increased fiber size variability and the majority of fibers show nemaline rods, especially the small fibers (arrows), Gomori trichrome. (B) Immunofluorescence of myosin heavy chain isoforms (see Material and methods). The small fibers are type 1 fibers (blue) and the large fibers are type 2A fibers (green). No type 2X fibers (red) are present, compared with inset showing normal muscles. (C) Electron micrograph showing nemaline rods and disrupted myofibrils in the muscle fibers. (D) Electron micrograph showing glycogen accumulation (arrows) between myofibrils and nemaline rods in a muscle fiber.
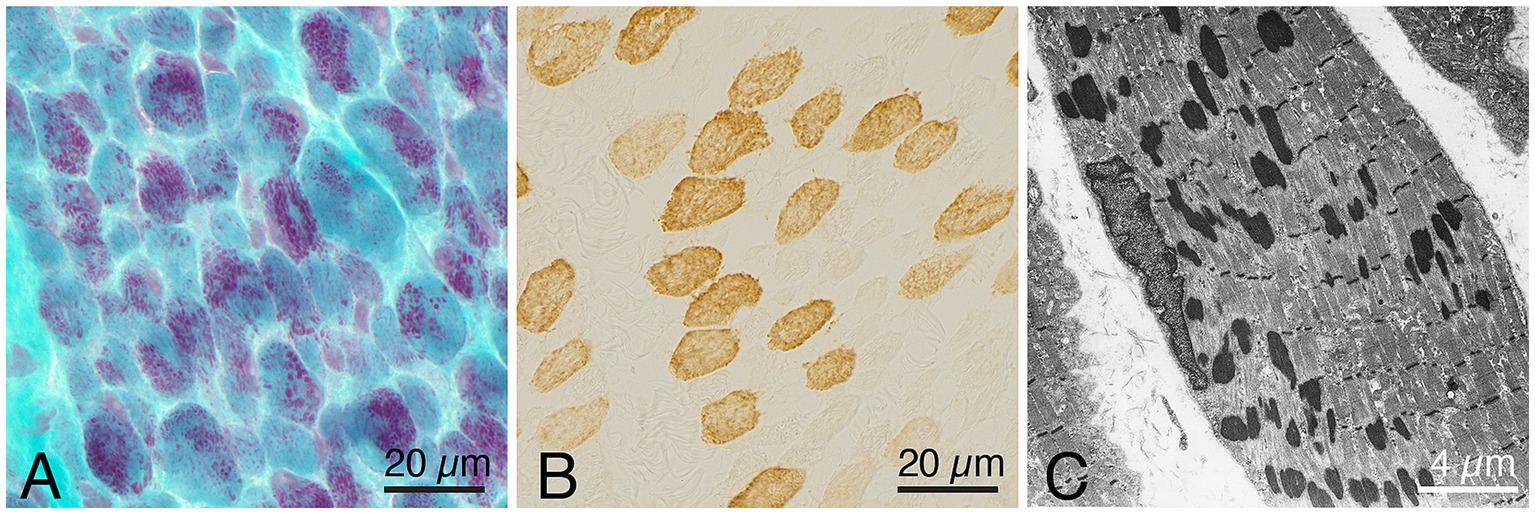
Figure 2. Representative example of a muscle biopsy of a patient with nemaline myopathy caused by an ACTA1 variant. (A) There is an increased fiber size variability, and the majority of fibers show nemaline rods, Gomori trichrome. (B) Immunohistochemistray of myosin heavy chain fast type, showing presence of both type 1 (unstained) and type 2 (stained) fibers. (C) Electron micrograph showing nemaline rods in a muscle fiber.
3.2 Proteomic investigation
The proteomic profiling was performed using quantitative analysis based on nanoscale liquid chromatography coupled to tandem mass tag labeling (TMT) and tandem mass spectrometry (LC-MS3). From the basic analysis of the proteomic data, 4,846 proteins were identified in all samples and quantified, of which 183 proteins were significantly and differently expressed in the nemaline myopathy patients compared to the control group [adjusted p-value (FDR) < 0.05]: 112 proteins were increased and 71 were decreased (Supplementary Table 1). Of the 183 proteins, 43 proteins were > 2 times up- or down-regulated (Table 2).
Proteomic investigation of the thin filament proteins including, the 12 nemaline myopathy associated proteins revealed for most proteins non-significant changes (Figures 3A,B). The only significantly upregulated protein was tropomodulin, which is involved in the regulation of thin filament length (13). The expression of proteins that were mutated in our patients, alpha-actin (ACTA1) and nebulin (NEB), were not significantly altered. Alpha-actinin-2 (ACTN2), a Z-disc protein and immunohistochemical hallmark of nemaline rods, was neither significantly upregulated.
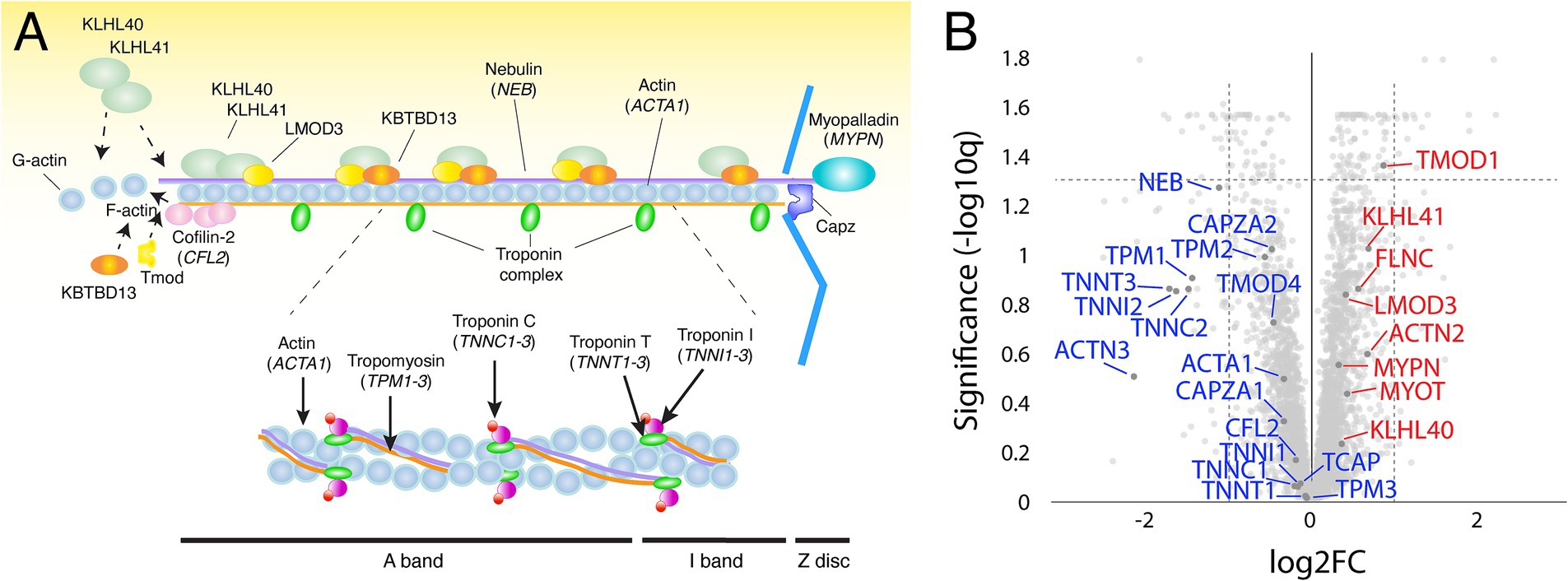
Figure 3. (A) Schematic illustration of the muscle thin filament in the sarcomere, including the 12 major nemaline myopathy-associated proteins. (B) Volcano plot with thin filament proteins and including the proteins associated with nemaline myopathy (in blue, down-regulated; in red, up-regulated). Proteins are given by their gene symbols.
A gene ontology (GO-term) based in silico analysis of dysregulated proteins was performed to elucidate the biological processes and subcellular compartments affected in patients with nemaline myopathy. The result of this overall proteomic profiling indicated some major alterations from the normal muscle. These included signs of increased protein turnover, as evidenced by augmented protein synthesis and increased degradation as revealed by the upregulation of proteins involved in ribosomes and proteasomal degradation (Figures 4A,C). On the downside of dysregulated proteins were glycolytic enzymes standing out as a major downregulated pathway (Figures 4B,C).
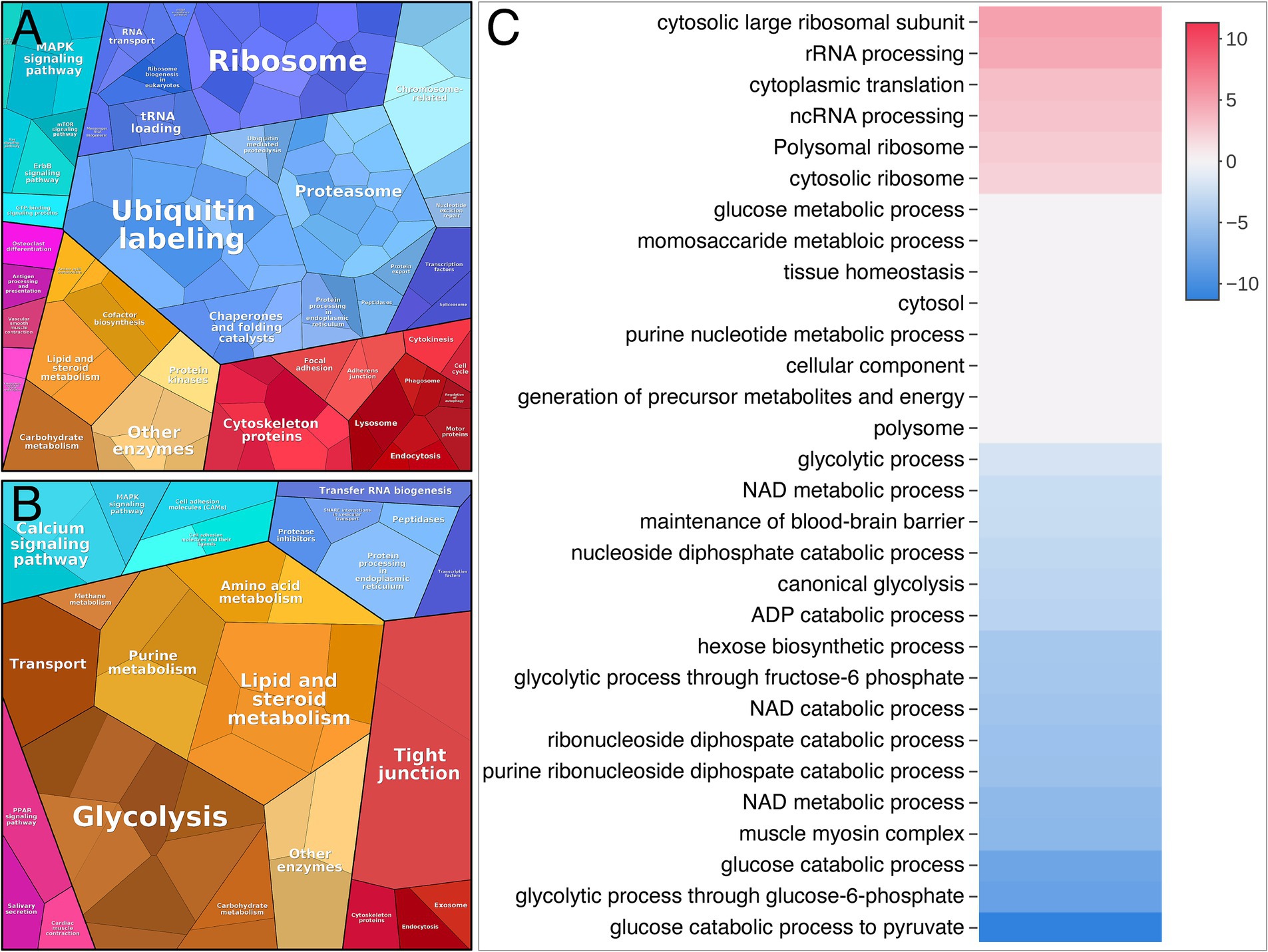
Figure 4. Proteome analysis visualized by Proteomap and Activation matrix analyses in patients compared to controls. (A) Proteomap showing that many up-regulated proteins are involved in ubiquitination, proteasomes and ribosomes. (B) Proteomap showing that many down-regulated proteins are involved in glycolysis. (C) Activation matrix (BigOmics Analytics) showing detected GO terms that are consistently up- or down-regulated, and are colored according to their upregulation (red) or downregulation (blue).
A major upregulated pathway was cytoplasmic translation with upregulated ribosomal proteins belonging to both the large and small ribosomal subunits (Figure 5A, cluster 1). The large subunit is comprised of ≈47 different proteins, most of which were identified and upregulated and 12 were significant (Figure 5B). A similar pattern was observed for the proteins comprising the cytosolic small ribosomal subunit (Supplementary Figure 1A). Another important upregulated pathway consisted of the proteasomal degradation system with several building blocks significantly increased (Figure 5A cluster 2; Figure 5C). Immunohistochemistry revealed that in a case where small fibers were generally more affected by structural alterations the proteasome 20S was accumulated in these small fibers (Supplementary Figure 2). A third cluster that was identified included the most significantly upregulated proteins (Figure 5A cluster 3; Figures 6A–C; Table 2). This cluster of nine proteins (NRAP, FBXO40, TRIM63, TRIM54, ALPK3, XIRP1, ANKRD2, LMOD2, and CSRP3) is a group of proteins that are highly expressed in muscle tissue and several of them are muscle-specific. They are important for the turnover and maintenance of sarcomere protein. The pattern was similar for both the ACTA1 and NEB patients (Figure 6C).
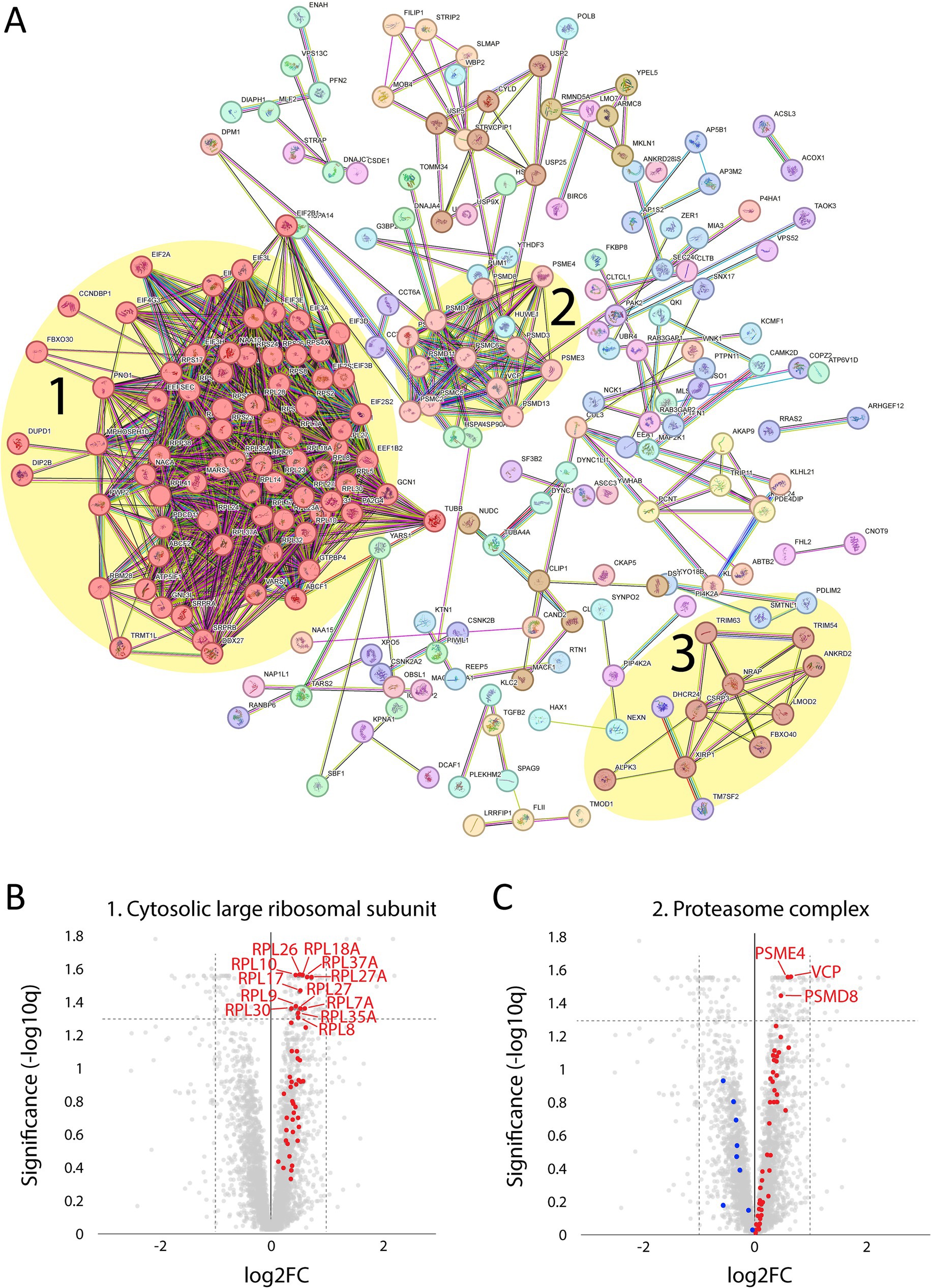
Figure 5. Proteomic analysis of up-regulated proteins. (A) From the STRING analysis, three clusters were detected and highlighted: 1, proteins involved in the cytosolic large ribosomal subunit; 2, proteins involved in the proteasome complex; 3, proteins involved in sarcomere turnover and maintenance (described in more detail in the figure 6; FDR = 0.1 Log2FC = 0.2, 256 proteins). (B) Volcano plot of cytosolic large ribosomal subunit proteins (GO:0022625) showing that all were up-regulated (marked in red). For those with FDR < 0.05, the gene symbols are included. (C) Volcano plot of proteasome complex (GO:0000502) showing most proteins are up-regulated (marked in red) and some down-regulated (marked in blue). For those with FDR < 0.05, the gene symbols are included.
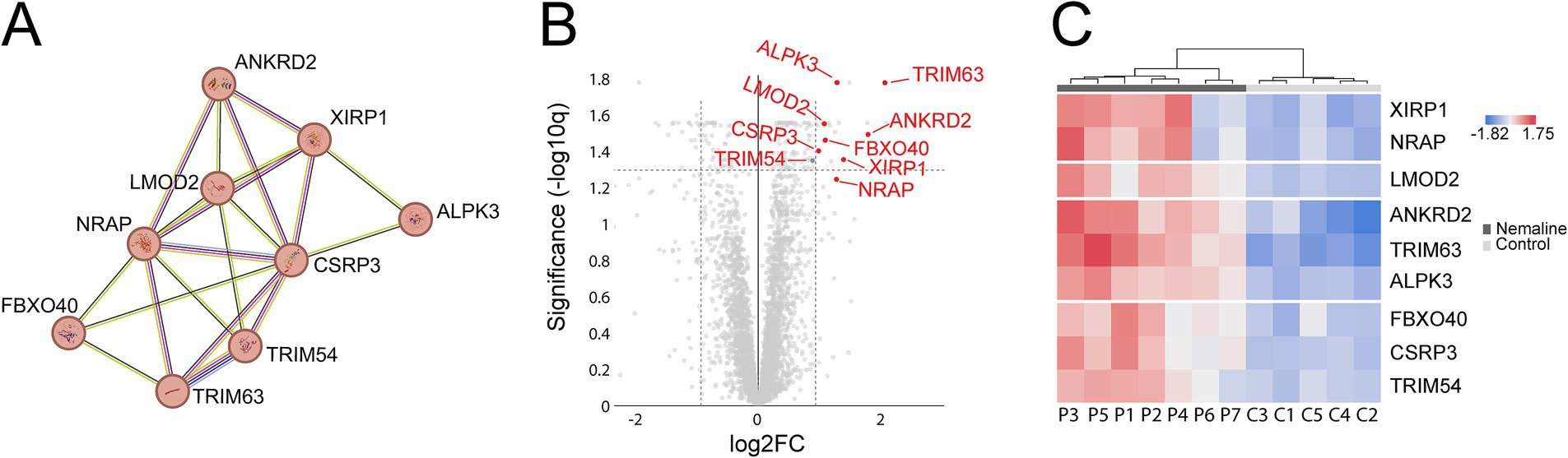
Figure 6. Detailed analysis of proteins included in cluster 3 from Figure 5. Proteins are given by their gene symbols. (A) Cluster 3 comprised of proteins involved in sarcomere turnover and maintenance. (B) Volcano plot showing that most of these proteins are highly and significantly upregulated. (C) Heat map showing the differentially expressed proteins in individual patients and controls colored according to their upregulation (red) or downregulation (blue). P, patient; C, control.
Analysis of downregulated proteins revealed the glycolysis and glycogenolysis as major dysregulated pathways where all enzymes involved were at the downside, whereas enzymes involved in the glycogen synthesis such as glycogenin-1 (GYG1), muscle glycogen synthase (GYS1) and branching enzyme (GBE1) seemed to be unaffected (Figure 7, cluster 1; Figure 8). The pattern was similar for both the ACTA1 and NEB patients (Figure 8C). Other energy metabolic pathways, especially in the mitochondria were analyzed in detail, such as the respiratory chain (Supplementary Figure 1C) but did not show any clear up- or down-regulation.
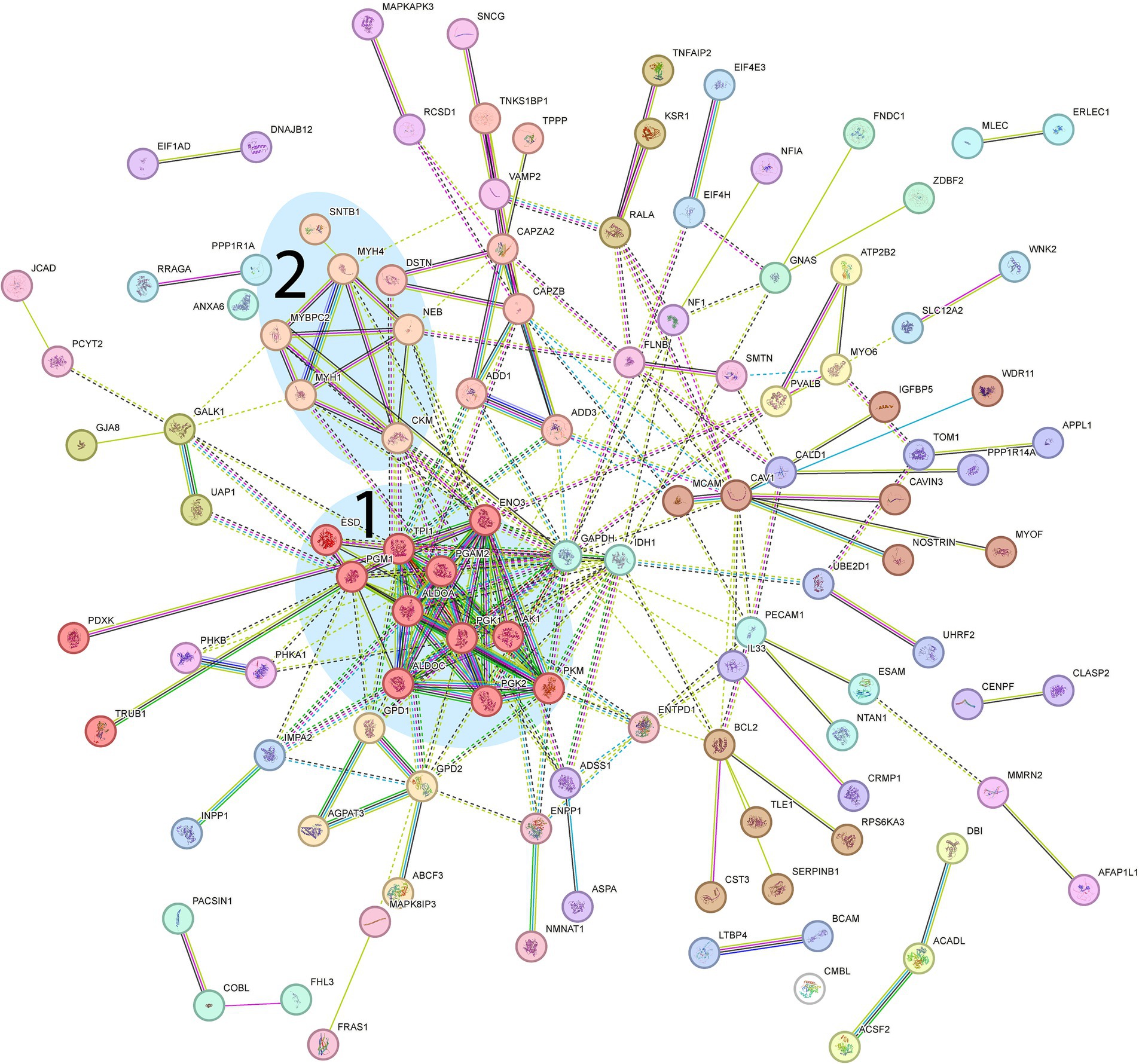
Figure 7. Proteomic analysis of down-regulated genes. From the STRING analysis, two clusters were detected and highlighted: 1, Proteins involved in glycolysis (further described in more detail in Figure 8); 2, Muscle-specific proteins involved in muscle contraction. (FDR = 0.1 Log2FC = 0.2, 158 proteins).
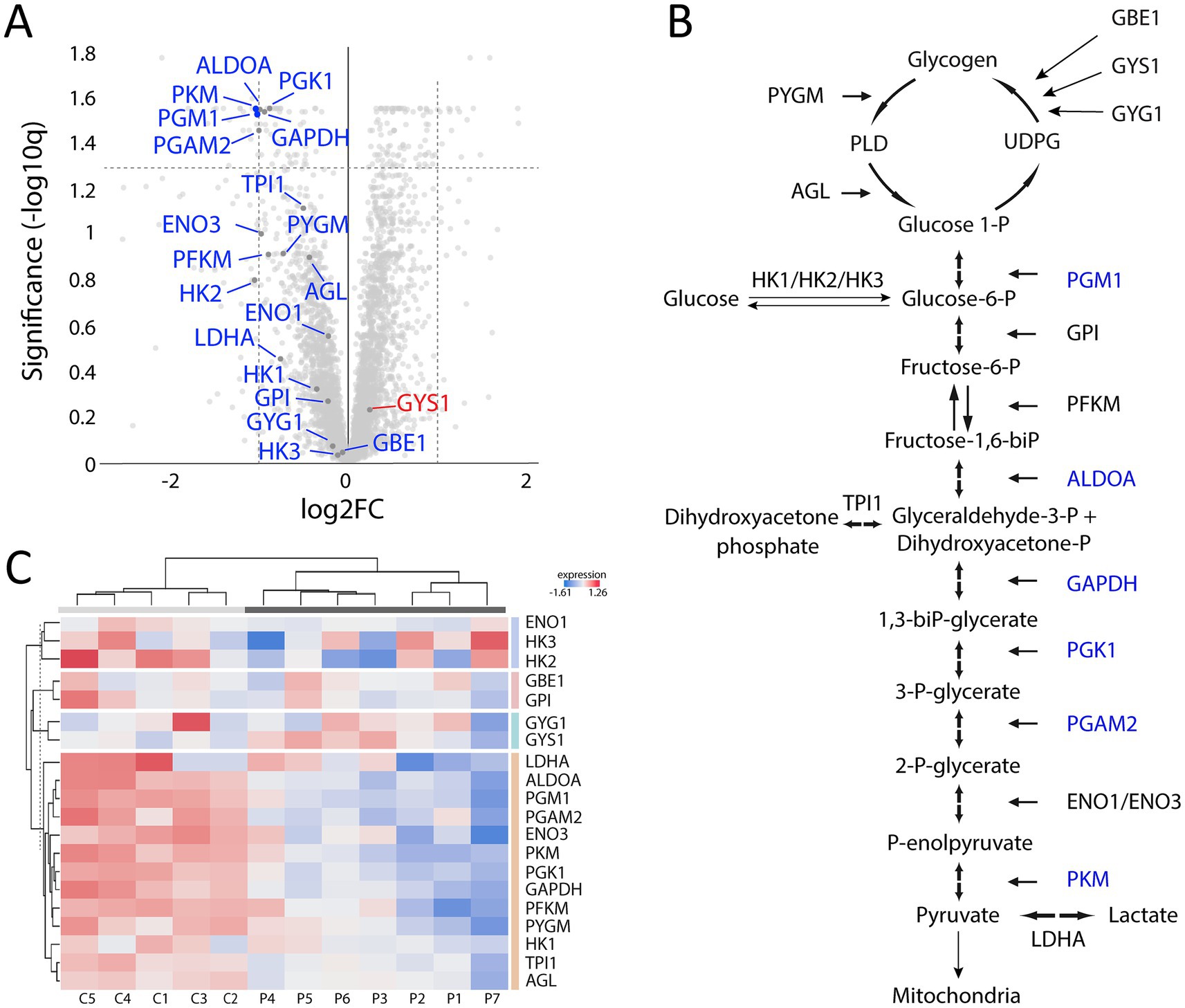
Figure 8. Proteomic analysis with a focus on glycogen metabolism and glycolysis. (A) Volcano plot of glycogen and glycolysis associated proteins showing that most of them are down-regulated (marked in blue) and that only GYS1 is up-regulated but not significantly. (B) Illustration of the glycogen metabolism and glycolysis with the significantly down-regulated proteins marked in blue. (C) Heat map showing the differentially expressed proteins in individual patients and controls, colored according to their upregulation (red) or downregulation (blue). Each column corresponds to one sample (P, patient; C, control). Dendrograms showing hierarchical clustering.
Another downregulated cluster was comprised of proteins mainly involved in muscle contraction within the sarcomeres (Figure 7, cluster 2). Among these proteins was myosin heavy chain 2X (MYH1), which is expressed in the fast type 2B (2X) fibers (14). MYH1 was the most downregulated of all proteins in the proteome.
Comparison of the results from the ACTA1 and NEB patient groups revealed no proteins with significantly different expression levels (Figure 9).
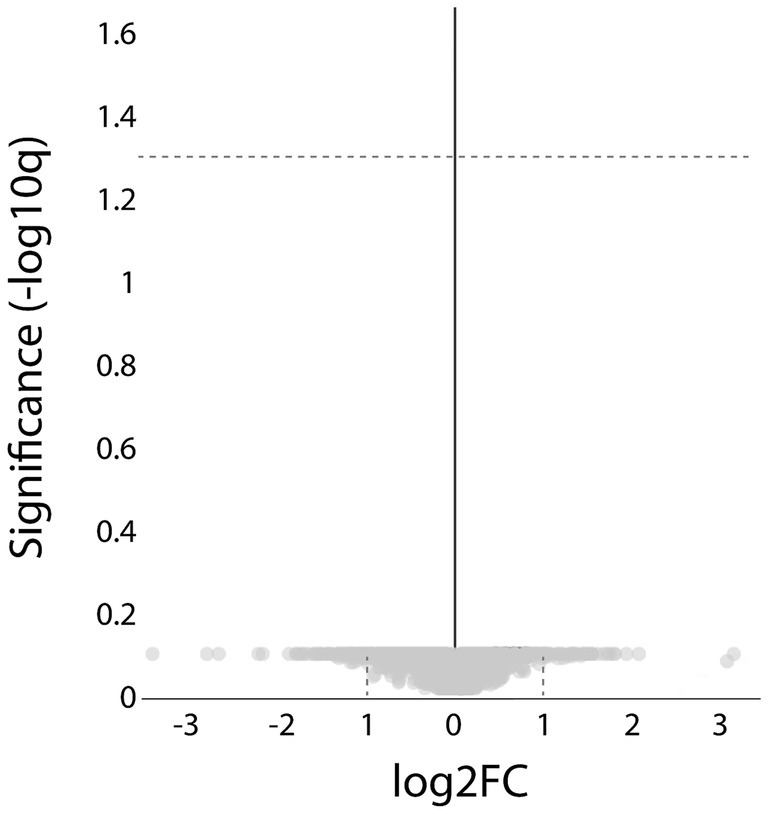
Figure 9. Proteomic analysis comparing the ACTA1 with the NEB cases. Volcano plot showing that no significant, differentially expressed proteins were detected (hatched line indicate FDR < 0.05).
4 Discussion
In this study, we investigated the proteomic profile in the two most common forms of nemaline myopathy caused by either NEB or ACTA1 gene variants. To reduce the influence of age on the results, only infants were included, and all analyzed muscle samples were obtained by open biopsy from living patients. We identified several dysregulated groups of proteins that may explain pathobiological events in nemaline myopathy.
Major upregulated proteins were important parts of protein synthesis and degradation, possibly indicating an increased turnover of proteins in nemaline myopathy. Ribosomal proteins were upregulated, including the building blocks of the large and small subunits of the ribosomes, indicating an increased number of ribosomes as a sign of increased protein synthesis. At the same time, the upregulated proteins, which are related to proteasomal degradation, indicate increased protein degradation, which may be related to the increased degradation or deficiency of mutated proteins in the thin filament and secondary degradation of other associated proteins in the sarcomeres. This hypothesis is supported by the loss of sarcomeres seen in large regions of affected muscle fibers, where the sarcomeres are replaced by rods (Figures 1, 2).
STRING analysis revealed that nine of the most upregulated proteins form a cluster (Figures 5A, 6). These proteins are highly expressed in muscle, and some of them are muscle-specific and involved in the structure and function of the sarcomeres.
FBXO40, TRIM54 and TRIM63 are muscle-specific E3 ubiquitin ligases. FBXO40 is a SCF E3 ubiquitin ligase involved in the regulation of the anabolic growth factor insulin like growth factor 1 (IGF1). This regulation occurs via ubiquitination and subsequent proteasomal degradation of insulin receptor substrate 1 (IRS1) in the IGF1/IRS1/PI3K/Akt pathway (15). Downregulation of Fbxo40 in mouse and pig results in muscle hypertrophy without any apparent pathology, and FBXO40 has been suggested as a target to treat human muscle disorders (16). It is therefore interesting that FBXO40 is highly upregulated both in NEB and ACTA1 associated nemaline myopathy as shown in the present study. Downregulating FBXO40 could potentially increase muscle mass and ameliorate the clinical phenotype.
TRIM54 and TRIM63 are involved in sarcomere protein regulation (17, 18), and pathogenic variants in TRIM54 and TRIM63 are associated with a protein aggregate myopathy and cardiomyopathy in mice and humans (18, 19). Our finding of upregulated sarcomere-associated ubiquitin ligases supports the concept of an increased protein turnover in nemaline myopathy. Except for FBXO40, the significantly upregulated E3 ubiquitin ligases appeared to be restricted to those involved in sarcomeric proteins, whereas others were either up- or down-regulated but not significantly (Supplementary Figure 1B).
NRAP (nebulin-anchoring protein) is a nebulin family member and thin filament chaperone that is essential for normal muscle development. It is upregulated in KLHL41-associated nemaline myopathy, and it has been suggested that this upregulation was secondary to KLHL41 deficiency, since NRAP is normally controlled by KLHL41 through ubiquitination-mediated proteasomal degradation (20). In nebulin-deficient zebrafish, NRAP is also upregulated and shows aberrant localization, contributing to the pathology in that nemaline myopathy model (21). Genetic ablation of NRAP restored sarcomeric disorganization, reduced protein aggregates and improved muscle function in nebulin-deficient zebrafish (21). We show that upregulation of NRAP is observed in nebulin and alpha-actin associated nemaline myopathy in humans, indicating that it may be a universal phenomenon in nemaline myopathy, and NRAP reduction may be an effective therapeutic approach in nemaline myopathy.
Alpha-kinase 3 (ALPK3) is a phosphatase that binds to M-band proteins and interacts with sequestosome-1 (SQSTM1, p62) and MURF3 (TRIM54) (22). ALPK3 genetic variants have been associated with cardiomyopathy (23). Xin (XIRP1) is a marker of muscle degeneration and is found in protein aggregates in myofibrillar myopathies (24), and is accumulated in myofibrillar lesions in kyphoscoliosis peptidase (KY) deficiency, where nemaline rods also appear (25). Ankyrin repeat protein 2 (ANKRD2) is interacting with sarcomere proteins such as titin and telethonin and is involved in the mechanical stretch response (26). Leiomodin 2 (LMOD2) is an alpha-actin binding protein essential for the assembly and length of thin filaments (27) and genetic variants are associated with cardiomyopathy (28). Cysteine-rich protein 3 (CSRP3; MLP, muscle LIM protein) is a muscle-specific, Z-disc-associated protein that is important for maintaining muscle structure and function, and it may regulate autophagy by interaction with LC3 (29). It is essential for myofibrillar organization and pathogenic variants are associated with cardiomyopathy in mice and humans (30, 31).
These nine muscle-specific upregulated proteins described above are apparently involved in muscle fiber protein turnover but their role in the pathobiology of nemaline myopathy remains elusive. It may be speculated that one or several of these proteins can act as potential therapeutic targets, which warrants further investigations.
Downregulated proteins were mainly those involved in glycogenolysis and glycolysis. All the enzymes in these pathways were on the downside and six of them had FDR < 0.05. In contrary, the enzymes essential for glycogen synthesis, i.e., glycogenin-1 (GYG1), glycogen synthase (GYS1), and branching enzyme (GBE1) seemed not to be dysregulated. Interestingly, this finding is in accordance with a previous transcriptomic analysis on a group of nemaline myopathy patients with variable and partly unknown genetic backgrounds (32). It was speculated that the increased amount of glycogen, frequently seen in muscle fibers in nemaline myopathy, may be associated with this downregulation of glycolytic enzymes (Figure 1D) (33). We found no evidence of up or downregulation of proteins essential for mitochondrial energy metabolism, which can be seen in other forms of degenerative muscle disorders such as myopathy associated with sertraline medication (34) and in primary mitochondrial diseases (35). This result may also be compared to a study on single muscle fibers in ACTA1 and TNNT1 associated nemaline myopathy which indicated downregulation of energy metabolism in general (36). However, a direct comparison is not possible due to different methodological approaches. Another group of proteins that was downregulated was associated with muscle contraction in the sarcomeres. One of these, the myosin heavy chain type 2X (MYH1), which is present in the fast, glycolytic type 2B (2X) fibers, is downregulated, probably as a sign of absence or reduction of pure type 2B (2X) fibers in many congenital myopathies, including nemaline myopathy. Altered fiber type composition may sometimes explain some of the protein dysregulation identified by proteomic analyses (36). However, in our cases there was type 1 fiber predominance but also type 1 fiber hypoplasia, resulting in similar volumes of glycolytic type 2A and oxidative type 1 fibers, with some variation between individuals and different regions of the muscle specimen (see also Figures 1, 2).
The major dysregulated protein pathways identified in this study exhibited a similar pattern regardless of whether the mutated gene was ACTA1 or NEB. Furthermore, bioinformatic analysis revealed no proteins with significantly different expression levels between the two patient groups. This finding supports the concept that mutations in different genes may be associated with very similar phenotypes, not only from a clinical and morphological perspective but also regarding protein dysregulation. This finding may imply that there may be therapeutic approaches that can be efficient irrespective of the underlying genetic defect in nemaline myopathy.
In conclusion, we have identified some major dysregulated proteins and cellular pathways in the muscle of infants with nemaline myopathy. Our findings highlight the importance of proteomic profiling in understanding the pathobiology of nemaline myopathy. The identified proteins warrant further investigations as potential drug targets for the treatment of nemaline myopathy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Swedish Ethical Review Authority (approval number 2022–00026-01) and conducted accordance with the Declaration of Helsinki. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this study was conducted on de-identified and saved archival material.
Author contributions
CH-O: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. KV: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. EM: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. AO: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-1005700 to CH-O and ALFGBG-1005867 to AO). The Swedish Research Council (grant no 2021–02109 to AO). The Research Fund for Neuromuscular Disorders in West Sweden to CH-O and AO.
Acknowledgments
The authors acknowledge the Clinical Genomics Gothenburg core facility (CGG) and the Proteomics Core Facility (PCF) at the Sahlgrenska Academy, University of Gothenburg, Sweden with financial support from SciLifeLab.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1661747/full#supplementary-material
Footnotes
References
1. Shy, GM, Engel, WK, Somers, JE, and Wanko, T. Nemaline myopathy. A new congenital myopathy. Brain. (1963) 86:793–810. doi: 10.1093/brain/86.4.793
2. Wallgren-Pettersson, C, and Laing, NG. Report of the 70th ENMC international workshop: nemaline myopathy, 11-13 June 1999, Naarden, the Netherlands. Neuromuscul Disord. (2000) 10:299–306. doi: 10.1016/s0960-8966(99)00129-7
3. Laitila, J, and Wallgren-Pettersson, C. Recent advances in nemaline myopathy. Neuromuscul Disord. (2021) 31:955–67. doi: 10.1016/j.nmd.2021.07.012
4. Sewry, CA, Laitila, JM, and Wallgren-Pettersson, C. Nemaline myopathies: a current view. J Muscle Res Cell Motil. (2019) 40:111–26. doi: 10.1007/s10974-019-09519-9
5. Malfatti, E, Lehtokari, VL, Bohm, J, De Winter, JM, Schaffer, U, Estournet, B, et al. Muscle histopathology in nebulin-related nemaline myopathy: ultrastrastructural findings correlated to disease severity and genotype. Acta Neuropathol Commun. (2014) 2:44. doi: 10.1186/2051-5960-2-44
6. Ohlsson, M, Tajsharghi, H, Darin, N, Kyllerman, M, and Oldfors, A. Follow-up of nemaline myopathy in two patients with novel mutations in the skeletal muscle alpha-actin gene (ACTA1). Neuromuscul Disord. (2004) 14:471–5. doi: 10.1016/j.nmd.2004.05.016
7. Roos, A, Thompson, R, Horvath, R, Lochmuller, H, and Sickmann, A. Intersection of proteomics and genomics to "solve the unsolved" in rare disorders such as neurodegenerative and neuromuscular diseases. Proteomics Clin Appl. (2018) 12:1–6. doi: 10.1002/prca.201700073
8. Dubowitz, V, Sewry, CA, and Oldfors, A. Muscle biopsy. A practical approach. 5th ed. Amsterdam: Elsevier (2021).
9. Hedberg-Oldfors, C, Eliasdottir, O, Geijer, M, Lindberg, C, and Oldfors, A. Dominantly inherited myosin IIa myopathy caused by aberrant splicing of MYH2. BMC Neurol. (2022) 22:428. doi: 10.1186/s12883-022-02935-4
10. Akhmedov, M, Martinelli, A, Geiger, R, and Kwee, I. Omics playground: a comprehensive self-service platform for visualization, analytics and exploration of big omics data. NAR Genom Bioinform. (2020) 2:lqz019. doi: 10.1093/nargab/lqz019
11. Liebermeister, W, Noor, E, Flamholz, A, Davidi, D, Bernhardt, J, and Milo, R. Visual account of protein investment in cellular functions. Proc Natl Acad Sci USA. (2014) 111:8488–93. doi: 10.1073/pnas.1314810111
12. Szklarczyk, D, Kirsch, R, Koutrouli, M, Nastou, K, Mehryary, F, Hachilif, R, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. (2023) 51:D638–46. doi: 10.1093/nar/gkac1000
13. Gokhin, DS, Ochala, J, Domenighetti, AA, and Fowler, VM. Tropomodulin 1 directly controls thin filament length in both wild-type and tropomodulin 4-deficient skeletal muscle. Development. (2015) 142:4351–62. doi: 10.1242/dev.129171
14. Murgia, M, Nogara, L, Baraldo, M, Reggiani, C, Mann, M, and Schiaffino, S. Protein profile of fiber types in human skeletal muscle: a single-fiber proteomics study. Skelet Muscle. (2021) 11:24. doi: 10.1186/s13395-021-00279-0
15. Shi, J, Luo, L, Eash, J, Ibebunjo, C, and Glass, DJ. The SCF-Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Dev Cell. (2011) 21:835–47. doi: 10.1016/j.devcel.2011.09.011
16. Zou, Y, Li, Z, Zou, Y, Hao, H, Li, N, and Li, Q. An FBXO40 knockout generated by CRISPR/Cas9 causes muscle hypertrophy in pigs without detectable pathological effects. Biochem Biophys Res Commun. (2018) 498:940–5. doi: 10.1016/j.bbrc.2018.03.085
17. Clarke, BA, Drujan, D, Willis, MS, Murphy, LO, Corpina, RA, Burova, E, et al. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. (2007) 6:376–85. doi: 10.1016/j.cmet.2007.09.009
18. Fielitz, J, Kim, MS, Shelton, JM, Latif, S, Spencer, JA, Glass, DJ, et al. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest. (2007) 117:2486–95. doi: 10.1172/JCI32827
19. Olive, M, Abdul-Hussein, S, Oldfors, A, Gonzalez-Costello, J, van der Ven, PF, Furst, DO, et al. New cardiac and skeletal protein aggregate myopathy associated with combined MuRF1 and MuRF3 mutations. Hum Mol Genet. (2015) 24:3638–50. doi: 10.1093/hmg/ddv108
20. Jirka, C, Pak, JH, Grosgogeat, CA, Marchetii, MM, and Gupta, VA. Dysregulation of NRAP degradation by KLHL41 contributes to pathophysiology in nemaline myopathy. Hum Mol Genet. (2019) 28:2549–60. doi: 10.1093/hmg/ddz078
21. Casey, JG, Kim, ES, Joseph, R, Li, F, Granzier, H, and Gupta, VA. NRAP reduction rescues sarcomere defects in nebulin-related nemaline myopathy. Hum Mol Genet. (2023) 32:1711–21. doi: 10.1093/hmg/ddad011
22. McNamara, JW, Parker, BL, Voges, HK, Mehdiabadi, NR, Bolk, F, Ahmad, F, et al. Alpha kinase 3 signaling at the M-band maintains sarcomere integrity and proteostasis in striated muscle. Nat Cardiovasc Res. (2023) 2:159–73. doi: 10.1038/s44161-023-00219-9
23. Agarwal, R, Wakimoto, H, Paulo, JA, Zhang, Q, Reichart, D, Toepfer, C, et al. Pathogenesis of cardiomyopathy caused by variants in ALPK3, an essential pseudokinase in the cardiomyocyte nucleus and sarcomere. Circulation. (2022) 146:1674–93. doi: 10.1161/CIRCULATIONAHA.122.059688
24. Kley, RA, Maerkens, A, Leber, Y, Theis, V, Schreiner, A, van der Ven, PFM, et al. A combined laser microdissection and mass spectrometry approach reveals new disease relevant proteins accumulating in aggregates of filaminopathy patients. Mol Cell Proteomics. (2013) 12:215–27. doi: 10.1074/mcp.M112.023176
25. Hedberg-Oldfors, C, Darin, N, Olsson Engman, M, Orfanos, Z, Thomsen, C, van der Ven, PF, et al. A new early-onset neuromuscular disorder associated with kyphoscoliosis peptidase (KY) deficiency. Eur J Hum Genet. (2016) 24:1771–7. doi: 10.1038/ejhg.2016.98
26. Mohamed, JS, Lopez, MA, Cox, GA, and Boriek, AM. Anisotropic regulation of Ankrd2 gene expression in skeletal muscle by mechanical stretch. FASEB J. (2010) 24:3330–40. doi: 10.1096/fj.10-158386
27. Chereau, D, Boczkowska, M, Skwarek-Maruszewska, A, Fujiwara, I, Hayes, DB, Rebowski, G, et al. Leiomodin is an actin filament nucleator in muscle cells. Science. (2008) 320:239–43. doi: 10.1126/science.1155313
28. Sono, R, Larrinaga, TM, Huang, A, Makhlouf, F, Kang, X, Su, J, et al. Whole-exome sequencing identifies homozygote nonsense variants in LMOD2 gene causing infantile dilated cardiomyopathy. Cells. (2023) 12:1–20. doi: 10.3390/cells12111455
29. Cui, C, Han, S, Tang, S, He, H, Shen, X, Zhao, J, et al. The autophagy regulatory molecule CSRP3 interacts with LC3 and protects against muscular dystrophy. Int J Mol Sci. (2020) 21:749. doi: 10.3390/ijms21030749
30. Salazar-Mendiguchia, J, Barriales-Villa, R, Lopes, LR, Ochoa, JP, Rodriguez-Vilela, A, Palomino-Doza, J, et al. The p.(Cys150Tyr) variant in CSRP3 is associated with late-onset hypertrophic cardiomyopathy in heterozygous individuals. Eur J Med Genet. (2020) 63:104079. doi: 10.1016/j.ejmg.2020.104079
31. Arber, S, Hunter, JJ, Ross, J Jr, Hongo, M, Sansig, G, Borg, J, et al. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. (1997) 88:393–403.
32. Sanoudou, D, Corbett, MA, Han, M, Ghoddusi, M, Nguyen, MA, Vlahovich, N, et al. Skeletal muscle repair in a mouse model of nemaline myopathy. Hum Mol Genet. (2006) 15:2603–12. doi: 10.1093/hmg/ddl186
33. Ryan, MM, Ilkovski, B, Strickland, CD, Schnell, C, Sanoudou, D, Midgett, C, et al. Clinical course correlates poorly with muscle pathology in nemaline myopathy. Neurology. (2003) 60:665–73. doi: 10.1212/01.wnl.0000046585.81304.bc
34. Hedberg-Oldfors, C, Lindgren, U, Visuttijai, K, Shen, Y, Ilinca, A, Nordstrom, S, et al. Lipid storage myopathy associated with sertraline treatment is an acquired mitochondrial disorder with respiratory chain deficiency. Acta Neuropathol. (2024) 148:73. doi: 10.1007/s00401-024-02830-x
35. Gangfuss, A, Hentschel, A, Rademacher, N, Sickmann, A, Stuve, B, Horvath, R, et al. Identification of a novel homozygous synthesis of cytochrome c oxidase 2 variant in siblings with early-onset axonal Charcot-Marie-tooth disease. Hum Mutat. (2022) 43:477–86. doi: 10.1002/humu.24338
36. Seaborne, RAE, Moreno-Justicia, R, Laitila, J, Lewis, CTA, Savoure, L, Zanoteli, E, et al. Integrated single-cell functional-proteomic profiling reveals a shift in myofibre specificity in human nemaline myopathy: a proof-of-principle study. J Physiol. (2025) 603:3033–48. doi: 10.1113/JP288363
37. Sparrow, JC, Nowak, KJ, Durling, HJ, Beggs, AH, Wallgren-Pettersson, C, Romero, N, et al. Muscle disease caused by mutations in the skeletal muscle alpha-actin gene (ACTA1). Neuromuscul Disord. (2003) 13:519–31. doi: 10.1016/s0960-8966(03)00101-9
38. Lehtokari, VL, Pelin, K, Sandbacka, M, Ranta, S, Donner, K, Muntoni, F, et al. Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum Mutat. (2006) 27:946–56. doi: 10.1002/humu.20370
39. Gorukmez, O, Gorukmez, O, and Topak, A. Clinical exome sequencing findings in 1589 patients. Am J Med Genet A. (2023) 191:1557–64. doi: 10.1002/ajmg.a.63190
Keywords: nemaline myopathy, pathology, proteomics, dysregulated proteins, proteinsynthesis, protein degradation, glycolysis
Citation: Hedberg-Oldfors C, Bedir AZ, Visuttijai K, Michael E and Oldfors A (2025) Proteomic analysis of nemaline myopathy in infants reveals distinct common dysregulated proteins and cellular pathways. Front. Neurol. 16:1661747. doi: 10.3389/fneur.2025.1661747
Edited by:
Gabriele Siciliano, University of Pisa, ItalyReviewed by:
Chiara Fiorillo, University of Genoa, ItalyAndreas Roos, Universitätsklinikum Essen, Germany
Jenni Laitila, University of Helsinki, Finland
Copyright © 2025 Hedberg-Oldfors, Bedir, Visuttijai, Michael and Oldfors. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anders Oldfors, YW5kZXJzLm9sZGZvcnNAZ3Uuc2U=
 Carola Hedberg-Oldfors
Carola Hedberg-Oldfors Ali Zeki Bedir
Ali Zeki Bedir Kittichate Visuttijai
Kittichate Visuttijai Eva Michael
Eva Michael Anders Oldfors1*
Anders Oldfors1*