- 1Guangzhou Red Cross Hospital, Guangzhou, China
- 2Oncology Department, Shantou Central Hospital, Shantou, China
- 3967 Hospital of the Joint Logistics Support Force, Dalian, Liaoning, China
- 4The Second Medical Center, Chinese PLA General Hospital, Beijing, China
- 5Sun Yat-Sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
Objective: To explore and compare the effectiveness of various non-invasive brain stimulations (NiBS) on poststroke lower extremity disorders.
Methods: We searched for and gathered studies from Embase, PubMed, Web of Science, and Cochrane databases, with the most recent search carried out on 5 October 2024. All published studies meeting the eligibility criteria and investigating the effectiveness of NiBS in patients with poststroke lower limb disorders were included. A total of 29 studies involving 1,319 participants were reviewed. Two independent researchers extracted clinical characteristics and research data. Outcome measures included the Fugl–Meyer lower extremity scale, Barthel index, Berg balance scale (BBS), and timed up and go test. Standard pairwise meta-analysis results and treatment network geometry were generated using Stata MP version 15.0. Bayesian network analysis was conducted using R version 4.4.1 with the “BUGSnet” package.
Conclusion: The meta-analysis shows that low-frequency repetitive transcranial magnetic stimulation (LF-rTMS) and rTMS + transcranial direct current stimulation (tDCS) are effective neurostimulation therapies for enhancing poststroke lower limb motor function. Probability rankings indicate that, among all NiBS interventions examined, rTMS + tDCS may be the most effective. In terms of body balance, intermittent theta burst stimulation (iTBS) and LF-rTMS improved poststroke balance, with iTBS possibly being the most effective. For activities of daily living, iTBS, LF-rTMS, and rTMS + tDCS demonstrated beneficial effects, with LF-rTMS potentially being the most effective among them.
1 Introduction
As the population ages, the incidence of stroke continues to rise (1). Lower extremity dysfunction is a common post-stroke functional impairment. This dyskinesia persists for a long time, hindering daily activities, reducing muscle strength, and limiting work-related activities and social participation (2). Current rehabilitation approaches for post-stroke lower limb motor dysfunction mainly include repetitive task-oriented training, walking exercises, treadmill training, orthotics, and functional electrical stimulation (3). However, these traditional therapies are time-consuming and produce inconsistent results. Therefore, developing innovative treatment methods that enhance balance, walking ability, and performance of daily living activities is vital in stroke rehabilitation research.
Non-invasive brain stimulation (NiBS) includes emerging techniques used in neurorehabilitation to restore motor function after stroke by modulating the excitability of motor control centers (4). NiBS techniques include transcranial ultrasound stimulation, transcranial direct current stimulation (tDCS), and transcranial magnetic stimulation (TMS) (5). However, relatively few clinical studies have explored the effectiveness of transcranial ultrasound stimulation for poststroke motor function recovery (6). Based on various stimulation patterns, TMS techniques are classified into single-pulse TMS, dual-pulse TMS, repetitive TMS (rTMS), and the derived rTMS mode (theta burst stimulation, TBS) (7).
A considerable number of clinical studies have been published on treating poststroke lower limb movement disorders using NiBS techniques. These studies utilise different stimulation modes, including low-frequency rTMS (LF-rTMS), high-frequency rTMS (HF-rTMS), combined rTMS and transcranial direct current stimulation (rTMS + tDCS), intermittent TBS (iTBS), continuous TBS (cTBS), anodal tDCS (A-tDCS), dual-tDCS, and cathodal tDCS (C-tDCS). Reported outcomes include the Fugl–Meyer assessment for the lower extremity (FMA-LE), the Barthel index (BI), the Berg balance scale (BBS), and the timed up and go test (TUG) (8, 9). Based on these studies, several meta-analyses have evaluated the effectiveness of various NiBS therapies in treating post-stroke motor disorders (10, 11). Traditional meta-analyses, however, are limited to pairwise comparisons and cannot establish a comprehensive treatment hierarchy (network evidence), as their results are based on direct comparisons of relevant treatments. In contrast, network meta-analysis (NMA) is a relatively new statistical method that combines, compares, and integrates multiple interventions within a single analysis. Although a large number of traditional pairwise comparisons are needed to support such integration, NMA enables ranking of all interventions using both direct trial data and indirect evidence from cross-comparisons (12). To evaluate and compare the effectiveness of various NiBS treatments for lower extremity disorders in post-stroke patients, we conducted a literature search and synthesized the available evidence in this review.
2 Methods
The study protocol was registered in PROSPERO (CRD42024521395) on May 20, 2024.1 We prepared the NMA following the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) statement (13).
2.1 Eligibility criteria
Studies meeting the following criteria were included in the meta-analysis: (1) participants diagnosed with lower limb paralysis after stroke; (2) intervention involving NiBS, including rTMS, tDCS, specialized modes of rTMS, and the combined use of multiple NiBS techniques (no relevant studies identified for other NiBS modalities); (3) comparison using placebo conditions, such as sham stimulation or blank controls; (4) outcomes measured with TUG, FMA-LE, BI, and BBS; and (5) research limited to randomized controlled trials (RCTs).
Studies were excluded for the following reasons: (1) recruiting ineligible participants, such as healthy populations or animals; (2) using unrelated interventions, like invasive deep brain stimulation; (3) having unclear stimulation patterns; (4) when research data was inaccessible or incomplete; (5) being published as meetings, case reports, or reviews; and (6) duplicate publications.
2.2 Data sources and searches
We searched for relevant literature in the following databases, with the last search ending on October 5, 2024: PubMed, Embase, the Cochrane Library, and Web of Science. The keywords, including MeSH terms related to the lower extremities, stroke, tDCS, and TMS, are listed in the Supplementary file.
2.3 Data collection and analysis
Two independent researchers (DEL and LJY) screened potentially relevant studies based on titles, abstracts, and full texts. In cases of disagreements, a third researcher was consulted to make the final decision. After scanning the included studies, the following information was extracted: publication date, author names, stimulation area, stroke subtype (ischemic/hemorrhagic), time of onset, sex, sample size, age, and adverse effects.
2.3.1 Quality assessment
We used Review Manager (version 5.4), based on the Cochrane risk of bias assessment tool, to assess risk of bias in RCTs across seven domains (14). Two independent researchers (DEL and LJY) assessed the studies according to these domains, which are listed in Supplementary file 2. To determine potential publication bias among the included studies, we applied Egger’s test using Stata MP (version 15). A p-value <0.05 was considered to indicate that the results of the meta-analysis were unreliable (15).
2.3.2 Outcomes and effect measures
Four outcomes were used to evaluate the effectiveness of NiBS for poststroke lower extremity movement disorders: FMA-LE, TUG, BI, and BBS. For a thorough assessment of lower extremity motor recovery, the primary outcome was the FMA-LE, a tool commonly used to assess motor function in patients with stroke or other central nervous system diseases. This scale thoroughly evaluates lower limb function, with higher scores indicating better recovery. Secondary outcomes included the TUG, BI, and BBS. The TUG is a quick assessment test that measures walking ability by recording the time needed to complete the test. Shorter times reflect better walking function. The BBS is a detailed scale used to assess body balance function, with higher scores indicating better balance performance. The BI is a widely used tool to evaluate activities of daily living and is mainly useful for detecting changes in independent living abilities of elderly individuals before and after treatment. Higher BI scores suggest better performance in activities of daily living.
For all outcomes treated as continuous variables, we set the mean difference (MD) as the effect size, with a 95% confidence interval (CI). To calculate the effect measures for continuous outcomes, the outcomes before and after NiBS were recorded as means and standard deviations.
2.3.3 Geometry of the network
Network graphs were established to visualize the characteristics of the included NiBS techniques and to compare them with the placebo group. Each node in the network graph represents an NiBS technique. Node size indicates the number of subjects, and the lines between nodes represent random comparisons between intervention measures.
2.4 Statistical analysis
2.4.1 Methods for direct treatment comparisons
Based on the results of statistical heterogeneity, we applied a random-effects model to assess the direct relative effects between competing NiBS techniques and the placebo using Stata MP version 15.0.
2.4.2 Methods for indirect and mixed comparisons
Bayesian network analysis, based on the Markov chain Monte Carlo algorithm, was applied to assess the effectiveness of each NiBS therapy by R version 4.4.1 with the “BUGSnet” package. We applied the deviance information criterion (DIC) to guide model selection between fixed- and random-effects approaches, and the model with the lower DIC was chosen to ensure a better fit. All NiBS techniques were ranked according to their P-scores, which ranged from 0 to 1. The results are shown in a surface under the cumulative ranking curve (SUCRA) plot. Comparison results are reported as MD with 95% credible intervals, presented in a league table.
2.4.3 Assessment of statistical heterogeneity and inconsistency
For standard pairwise meta-analysis, we used the I2 statistic to assess statistical heterogeneity, with values over 50% indicating significant heterogeneity. For indirect and mixed comparisons, inconsistencies were assessed at both global and local levels. At the global level, inconsistency was evaluated by calculating the DIC from the inconsistency model and comparing it to the consistency model. A difference of less than 5 between the two models was deemed insufficient to indicate network inconsistency. To assess local inconsistency, leverage plots were created, and the scatter of data points was examined.
3 Results
3.1 Study selection
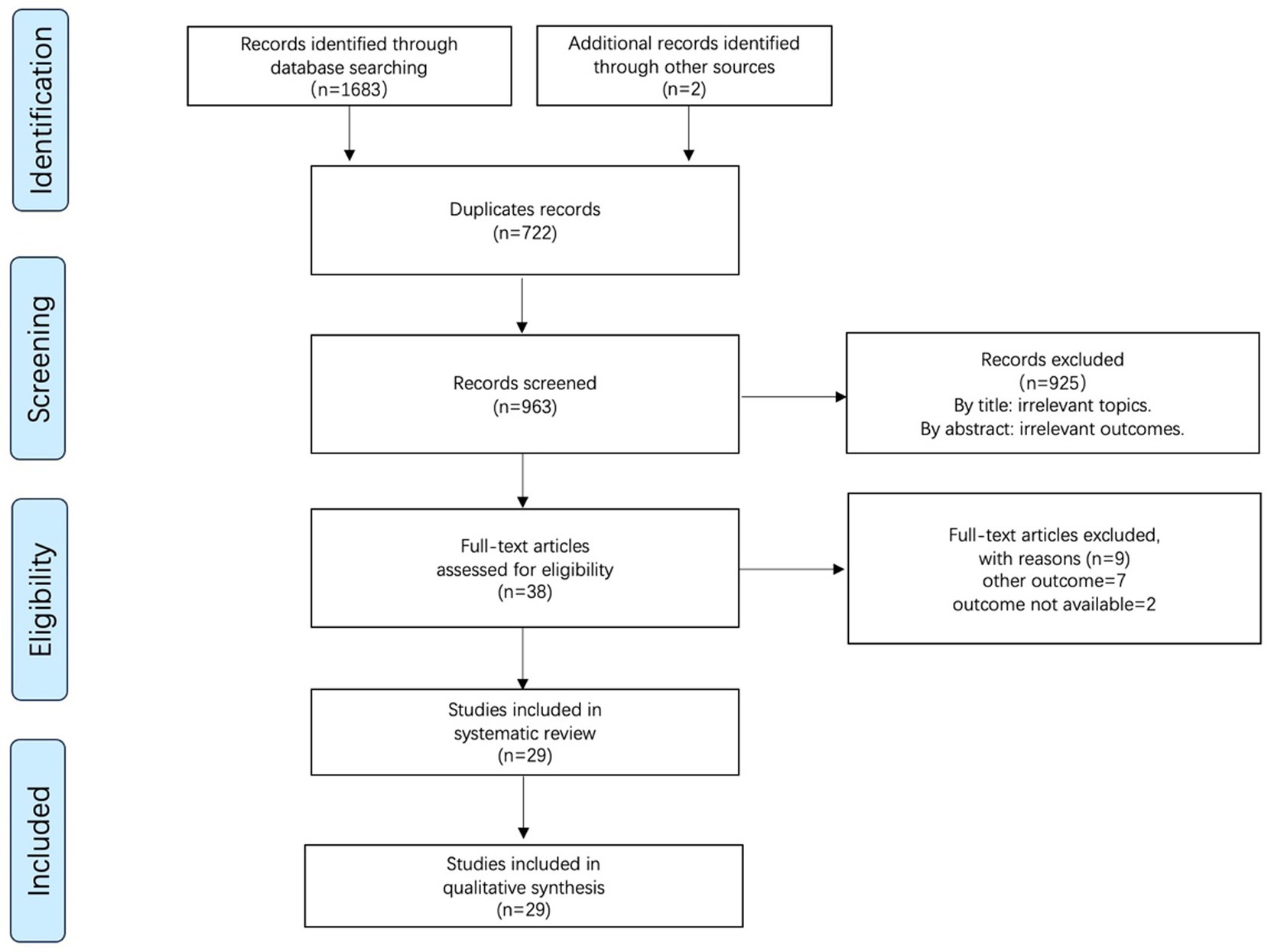
We collected 1,683 studies from four electronic databases: PubMed (n = 415), Embase (n = 352), WOS (n = 618), and Cochrane (n = 298). Additionally, two studies were included after reviewing other reviews. A total of 722 duplicate studies identified using Endnote’s duplicate citation checker were excluded. After reading and screening the titles and abstracts, 925 studies were excluded. Following full-text review of the remaining 38 studies, we excluded nine studies for the following reasons: other outcomes = 7 and unavailable outcome data = 2. Finally, 29 studies were included in the quantitative analysis. The PRISMA flow diagram for study selection is shown in Figure 1.
3.2 Study characteristics
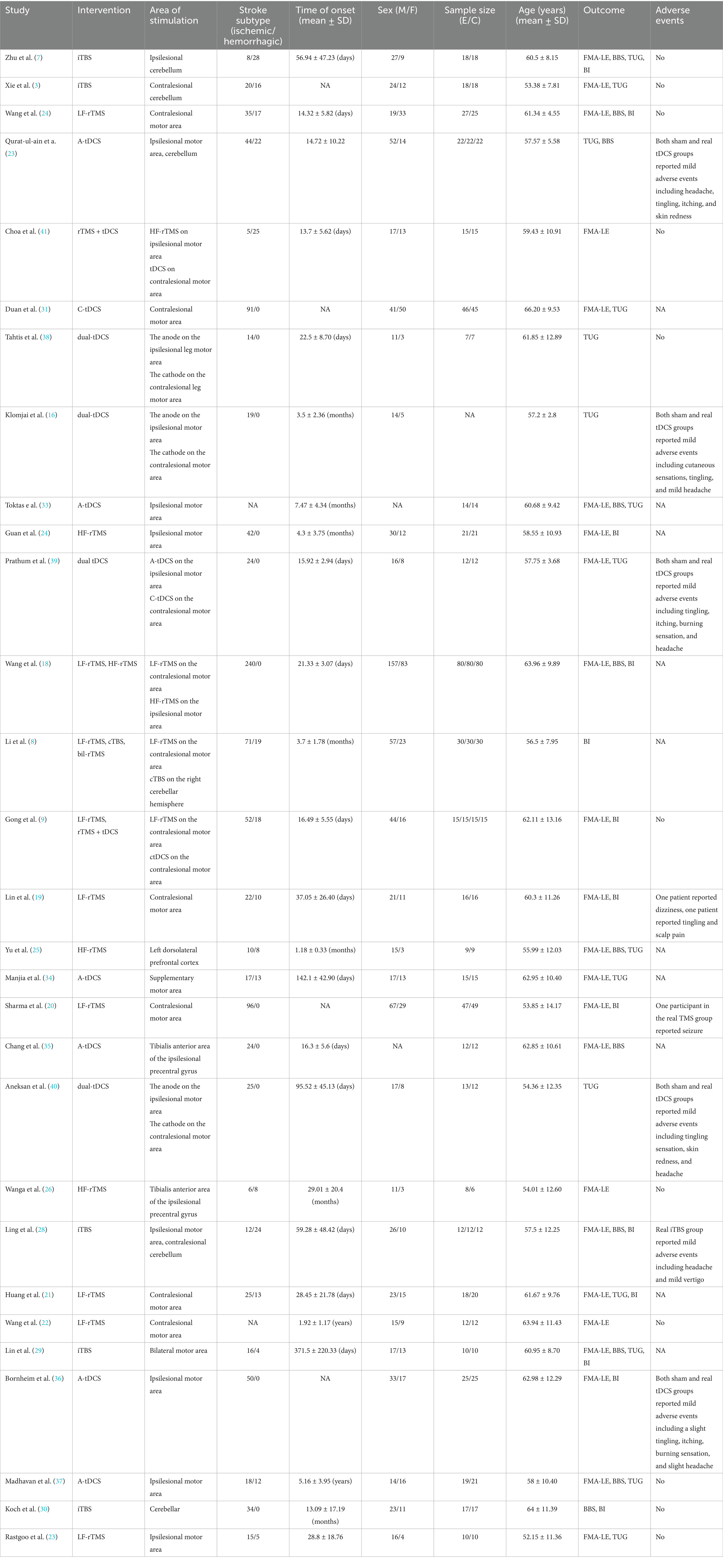
A comprehensive summary of the characteristics of the included studies is presented in Table 1. Of the 29 included studies, 28 were RCTs, except for 1 crossover trial (16). For the 29 studies involving 1,319 participants, LF-rTMS was used in 9 studies (8, 9, 17–23), HF-rTMS in 4 studies (18, 24–26), bil-rTMS in 1 study (8), iTBS in 5 studies (7, 27–30), cTBS in 1 study (8), C-tDCS in 1 study (31), A-tDCS in 6 studies (32–37), dual-tDCS in 4 studies (16, 38–40), and rTMS + tDCS in 2 studies (9, 41).
3.3 Quality assessment
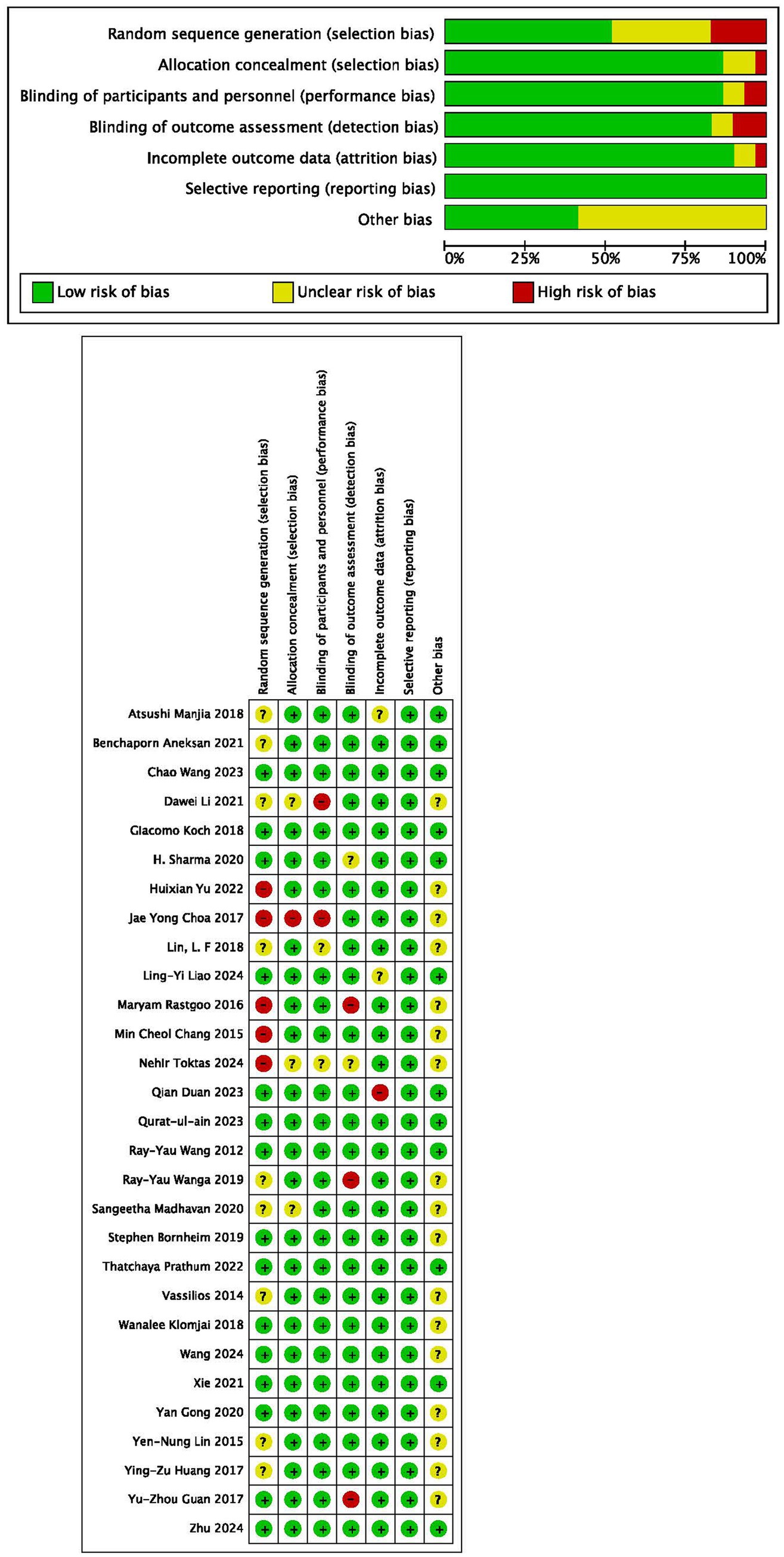
Among all the 29 selected studies included, 52% reported random sequence generation, 86% reported allocation concealment, 86% implemented blinding of participants and personnel, 83% implemented blinding of outcome assessment, and 90% provided incomplete outcome data (Figures 2A,B). Egger’s test results for different outcomes—FMA-LE (p = 0.586), TUG (p = 0.072), BBS (p = 0.542), and MBI (p = 0.298)—suggested a lack of evidence of publication bias.
3.4 Network geometry of interventions
A network graph illustrating different NiBS treatments for improving lower extremity motor function is presented in Figure 3.
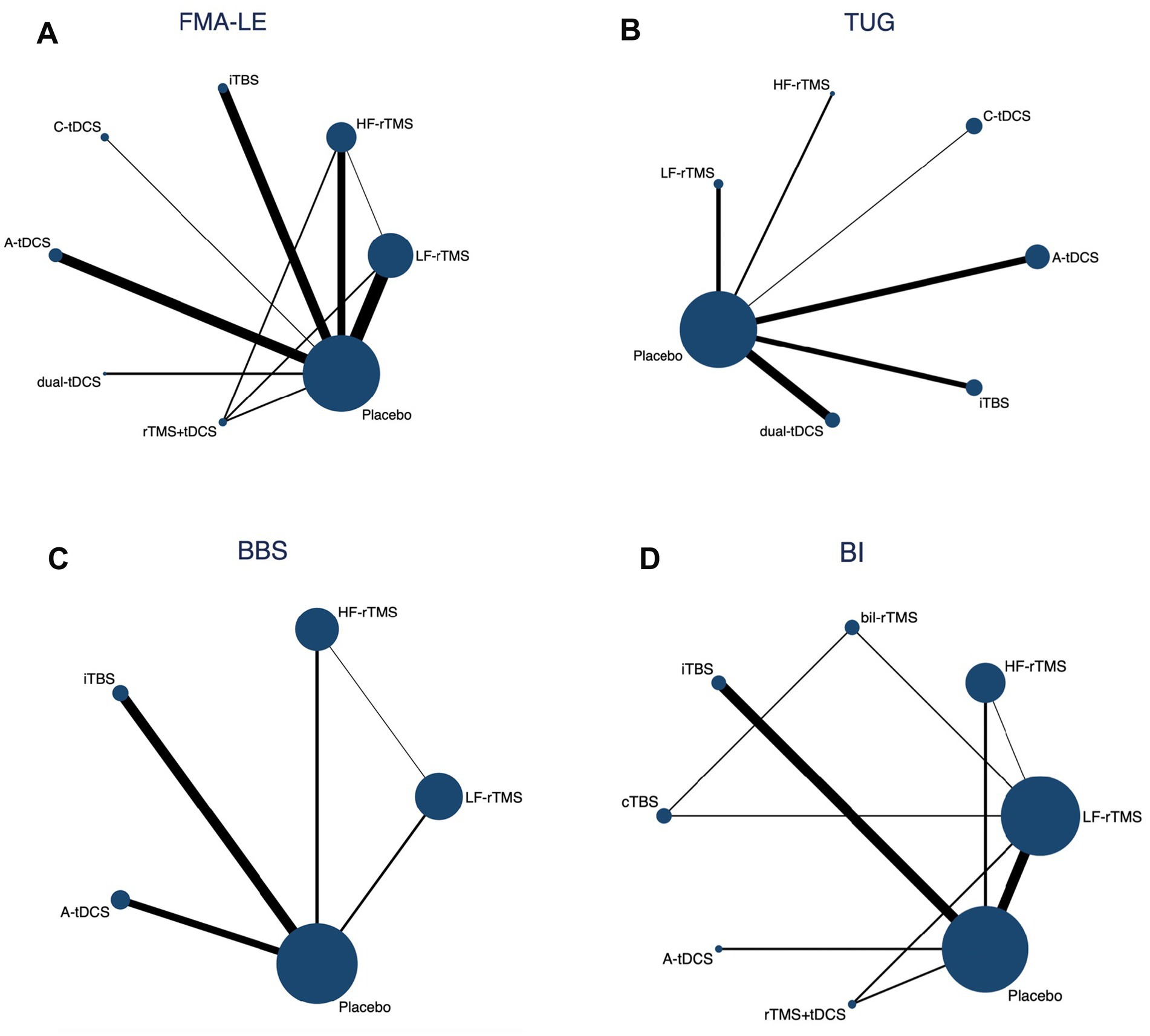
Figure 3. Network geometry of different outcome measures. Nodes are connected by a line when treatments are directly comparable. The width of each line is proportional to the number of randomized controlled trials, and the size of each node is proportional to the number of patients (sample size).
3.5 Synthesis of results
3.5.1 FMA-LE
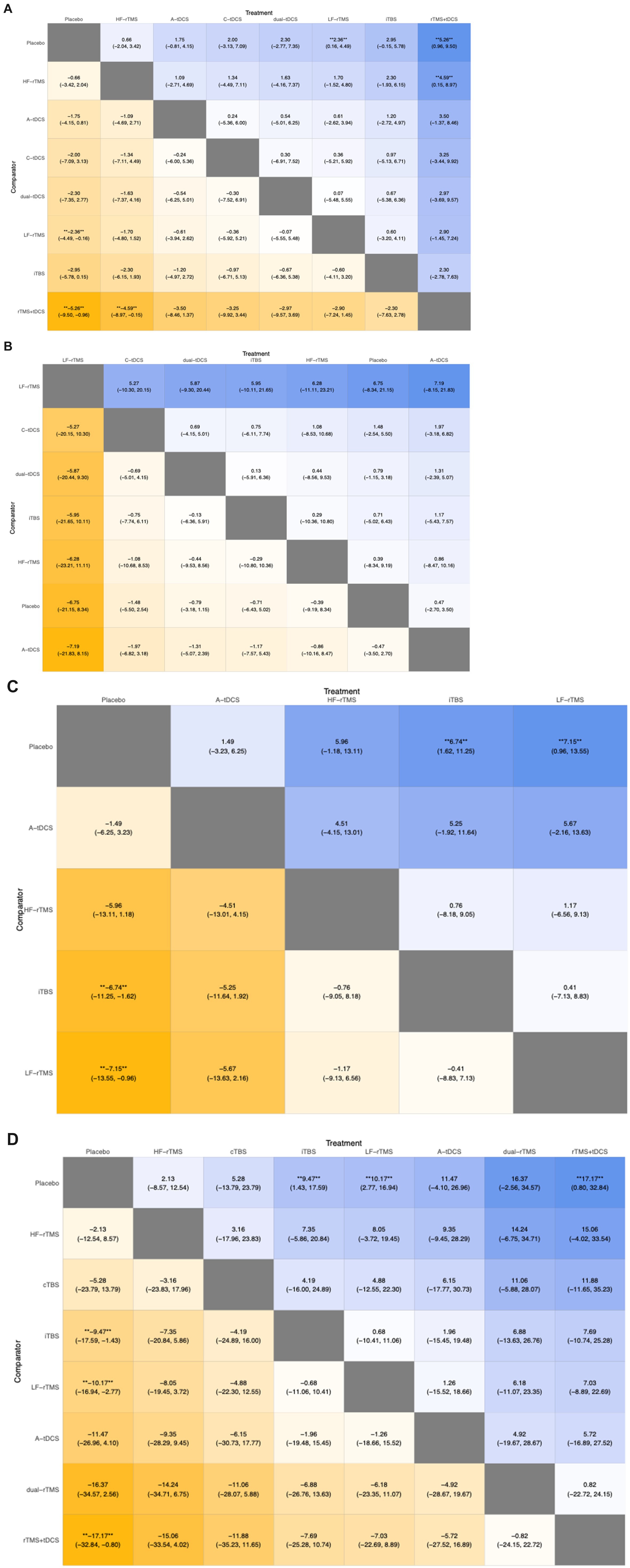
The NMA of NiBS treatments for lower extremity motor recovery, using FMA-LE as the outcome measure, included 23 studies. Pairwise meta-analysis suggested that LF-rTMS (MD, 2.58; 95% CI, 1.23 to 3.93), C-tDCS (MD, 2.00; 95% CI, 0.74 to 3.26), and dual-tDCS (MD, 2.30; 95% CI, 1.32 to 3.28) were significantly more effective than placebo (Figure 4A).
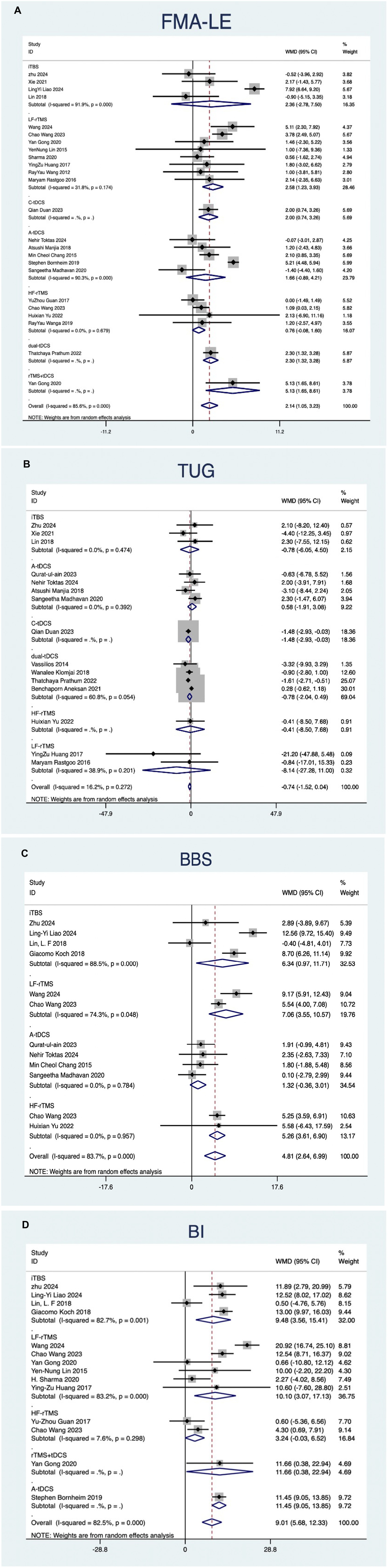
Figure 4. Forest plots of network meta-analyses for different outcome measures compared with placebo.
Regarding the NMA results, we compared the DIC of the fixed and random models. The DIC of the random model was lower than that of the fixed model (86.88 vs. 149.77) (Figure 5A1). We chose to use the random model for the NMA. The results indicated that LF-rTMS (MD, 2.36; 95% CI, 0.16 to 4.49) and rTMS + tDCS (MD, 5.26; 95% CI, 0.96 to 9.50) were significantly more effective than placebo (Figure 6A).
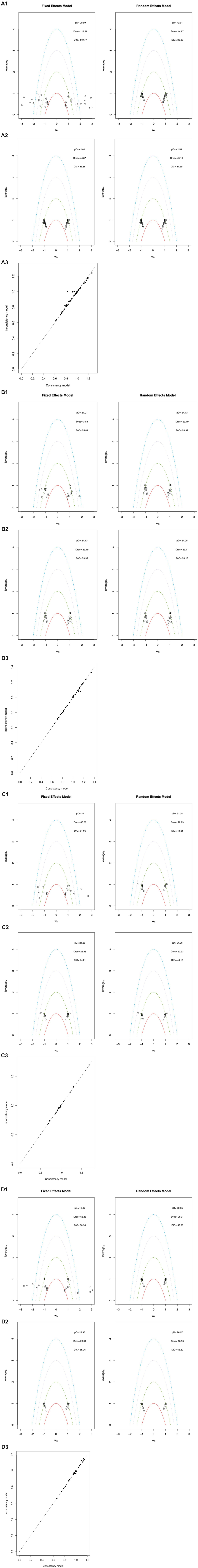
Figure 5. Leverage plots and fit statistics for different outcome measures. DIC, deviance information criterion.
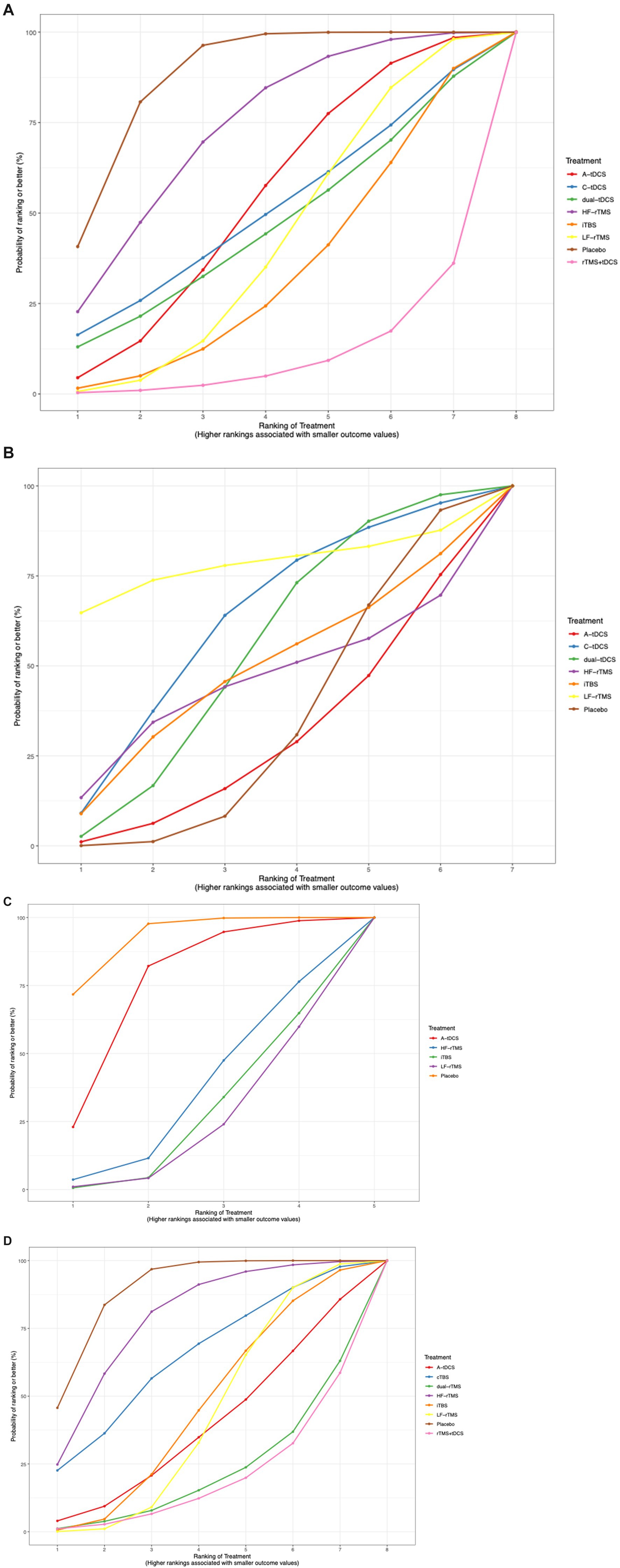
Figure 6. League table summarizing the results of the indirect comparisons of different outcome measures. Numbers in the cells denote the mean incidence risk rate (95% confidence interval). ** **p-value < 0.05.
The SUCRA plot ranked rTMS + tDCS as the most effective treatment for improving lower extremity motor function after stroke, followed by LF-rTMS, iTBS, A-tDCS, dual-tDCS, C-tDCS, and HF-rTMS (Figure 7A).
3.5.2 TUG
The NMA of NiBS treatments for improving walking function, using the TUG test as the outcome, included 15 studies. Pairwise meta-analysis suggested that no NiBS treatment was significantly more effective than placebo (Figure 4B).
For the NMA results, we compared the DIC of the fixed and random models. The DIC of the random model was lower than that of the fixed model (53.32 vs. 55.81) (Figure 5B1). We used the random model for the NMA. Results from the NMA suggested that no NiBS treatment was significantly more effective than placebo (Figure 6B).
The SUCRA plot indicated that LF-rTMS ranked highest for improving walking function in stroke, followed by HF-rTMS, C-tDCS, iTBS, dual-tDCS, and A-tDCS (Figure 7B).
3.5.3 BBS
The NMA of NiBS treatments for enhancing body balance function, using the BBS as the outcome, included 11 studies. Pairwise meta-analysis indicated that iTBS (MD, 6.34; 95% CI, 0.97 to 11.71), LF-rTMS (MD, 7.06; 95% CI, 3.55 to 10.57), and HF-rTMS (MD, 5.26; 95% CI, 3.61 to 6.90) were significantly more effective than placebo (Figure 4C).
For the NMA results, we compared the DIC of the fixed and random models. The DIC of the random model was lower than that of the fixed model (44.21 vs. 61.06) (Figure 5C1). We used the random model for the NMA. Results from the NMA showed that iTBS (MD, 6.74; 95% CI, 1.62 to 11.25) and LF-rTMS (MD, 7.15; 95% CI, 0.96 to 13.55) were significantly more effective than placebo (Figure 6C).
The SUCRA plot suggested that iTBS was the highest-ranked treatment for improving body balance function in stroke, followed by LF-rTMS, HF-rTMS, and A-tDCS (Figure 7C).
3.5.4 BI
The NMA of NiBS treatments for improving activities of daily living, using the BI as the outcome, included 13 studies. Pairwise meta-analysis showed that iTBS (MD, 9.48; 95% CI, 3.56 to 15.41), A-tDCS (MD, 11.45; 95% CI, 9.05 to 13.85), rTMS + tDCS (MD, 11.66; 95% CI, 0.38 to 22.94), and LF-rTMS (MD, 10.10; 95% CI, 3.07 to 17.13) were significantly more effective than placebo (Figure 4D).
For the NMA results, we compared the DIC values of the fixed and random models. The DIC of the random model was lower than that of the fixed model (55.26 vs. 88.36) (Figure 5D1). We selected the random model for the NMA. Results from the NMA indicated that iTBS (MD, 9.47; 95% CI, 1.43 to 17.59), LF-rTMS (MD, 10.17; 95% CI, 2.77 to 16.94), and rTMS + tDCS (MD, 17.17; 95% CI, 0.80 to 32.84) were significantly more effective than placebo (Figure 6D).
The SUCRA plot indicated that LF-rTMS was the most effective treatment for enhancing activities of daily living in stroke patients, followed by iTBS, rTMS + tDCS, dual-rTMS, A-tDCS, cTBS, and HF-rTMS (Figure 7D).
3.6 Assessment of statistical inconsistency
To evaluate global-level consistency, we compared the DIC between the consistency and inconsistency models. The results indicated that the difference in DIC was less than 5, with the consistency model showing a lower DIC than the inconsistency model across all selected outcomes (Figure 5). For local inconsistency, the leverage plots demonstrated that the data points were distributed along the slanting stitch, suggesting no evidence of inconsistency within any loop. Overall, the statistical assessment revealed no indication of inconsistency within the network.
3.7 Adverse effects
Only one case of seizure occurred after rTMS (20). No severe adverse events related to NiBS were reported in any of the included studies. Some studies reported mild adverse reactions, such as headaches, burning sensations, slight tingling, and itching, which resolved quickly after treatment and caused no long-term effects.
4 Discussion
To the best of our knowledge, this study represents the first NMA to examine the effectiveness of NiBS on poststroke lower extremity motor function. The analysis evaluated the efficacy of nine different NiBS treatments compared with placebo in 1319 participants with poststroke lower extremity disorders. For the primary outcome, measured using the FMA-LE, the NMA found that LF-rTMS and rTMS + tDCS were more effective than placebo. Pairwise meta-analysis also indicated that LF-rTMS, C-tDCS, and dual-tDCS were significantly more effective than placebo. Regarding walking function, assessed by the TUG test, both direct and indirect evidence showed that no NiBS intervention was more effective than placebo. The NMA assessment of body balance function revealed that iTBS and LF-rTMS were more effective than placebo. Pairwise meta-analysis suggested that iTBS, LF-rTMS, and HF-rTMS exceeded placebo in effectiveness. For activities of daily living, evaluated using the BI, direct evidence indicated that iTBS, A-tDCS, rTMS + tDCS, and LF-rTMS were more effective than placebo. The NMA results for BI demonstrated that iTBS, LF-rTMS, and rTMS + tDCS outperformed placebo.
The main stimulation modes of TMS included in this study were LF-rTMS and iTBS. For the recovery of hand motor function during the subacute phase of stroke, existing evidence and definite efficacy suggest a level A recommendation for LF-rTMS (42). A meta-analysis confirmed the therapeutic effect of LF-rTMS on lower limb movement disorders after stroke (3). Our research demonstrated that the effect of LF-rTMS on motor function recovery, body balance, and activities of daily living was superior to that of placebo in poststroke patients. iTBS, a novel TMS mode that functions in the opposite way of LF-rTMS, enhances nervous system excitability. iTBS should be considered a level B recommendation for treating lower-limb spasticity 字段 (42). Our investigation suggests that iTBS could improve activities of daily living and body balance in poststroke patients.
Regarding tDCS, previous meta-analyses and our own research have demonstrated its restorative effects in poststroke patients (11, 43). However, the number of RCTs assessing each effective tDCS mode was relatively small in this systematic review. Similarly, in the NMA of the primary outcome, although rTMS + tDCS appeared to be the most effective stimulation method, only two relevant RCTs were included (9, 41). Additional clinical studies are needed to evaluate the effects of tDCS in addressing lower extremity dysfunction after stroke.
To date, NiBS treatments for poststroke motor dysfunction mainly follow the interhemispheric inhibition model. This model indicates that the two hemispheres suppress each other’s excitability via nerve fiber bundles in the corpus callosum, maintaining a dynamic balance. After a stroke, the inhibitory effect of the affected hemisphere diminishes, disrupting this balance. The unaffected hemisphere then suppresses the excitability of the affected hemisphere through the corpus callosum, causing a decline in motor function (44). Nervous system excitability is affected by synaptic connections and efficacy, which NiBS modulates through mechanisms tied to long-term potentiation or depression (45, 46). To enhance poststroke limb dysfunction, inhibitory stimulation should be applied to the contralesional motor area (17, 31), whereas excitatory NiBS stimulation should focus on the ipsilesional motor area (4, 26, 36). Adhering to the interhemispheric inhibition model (HF-rTMS on the ipsilesional motor cortex and LF-rTMS on the contralesional side), one study investigated how rTMS influences motor function and cortical activation. Compared to the sham group, the real rTMS group exhibited motor improvements. fMRI data indicated a link between motor gains and increased cortical excitability caused by rTMS (47). Another study showed that applying A-tDCS to the primary motor cortex of stroke patients increased connectivity within the EEG network of the ipsilesional motor cortex. This heightened connectivity was linked to greater corticospinal excitability after A-tDCS (48). Notably, our NMA included a rare study exploring the effects of rTMS on the left dorsolateral prefrontal cortex (25), a region more commonly targeted to enhance cognitive function or treat depression (49). For poststroke motor dysfunction, the dorsolateral prefrontal cortex was rarely used as a stimulation target. Some included studies explored the improvement of poststroke lower limb dysfunction by using NiBS on the cerebellum (7, 27, 28, 30, 32). A study demonstrated that, compared to sham stimulation, cerebellar iTBS enhanced post-stroke body balance and lower limb function, along with an increase in motor-evoked potential amplitudes (28) regulatory center for movement. During exercise, the cerebellum receives and integrates information from the cerebral cortex, muscles, and joints. Based on this mechanism, the cerebellum presents a feasible target for modulating motor behavior and treating motor impairments caused by stroke (50). A study investigating poststroke dysphagia suggested that bilateral cerebellar iTBS can effectively enhance swallowing function (51). In treating post-stroke upper limb spasticity, cerebellar iTBS enhances the effects of conventional physical therapy (52). In a healthy population, another study found that active cerebellar rTMS restores swallowing accuracy and inhibitory effects caused by a cortical “virtual lesion” on pharyngeal motor-evoked potentials (53). In speech improvement, right cerebellar tDCS was found to significantly enhance phonemic fluency. This improvement is also linked to increased functional connectivity (54). Based on these promising findings, the cerebellum could be a crucial target for NiBS interventions in poststroke motor rehabilitation. However, more research is needed to develop a standardized approach to translate small-scale experimental results into a wide range of clinical practices (55).
Our investigation reported only one case of a severe adverse reaction (seizure) related to rTMS (20), Although causality between the seizure and rTMS treatment was not confirmed, numerous mild adverse events have been reported. These mainly involve skin sensations, are short in duration, and have no sequelae. According to the published TMS safety guidelines (56), seizure induction is the most severe acute adverse event; however, the risk of rTMS-induced seizures is definitely low. A review that included 41 reports published up to February 2020 examined TMS-induced seizures (57). Among these 41 reports, 13 involved healthy individuals, and 28 involved patients. Due to the inconsistent distribution of TMS patterns among the reports (19 HF-rTMS, 1 LF-rTMS, 8 single-pulse TMS, 9 deep TMS, 2 iTBS, 1 cTBS, and 1 unknown), it was difficult to identify a correlation between TMS-induced seizure and specific populations or TMS patterns. Regarding tDCS, our review found no severe adverse events and only mild adverse events similar to those of rTMS, with short duration and no sequelae. Previous safety guidelines have confirmed the safety of tDCS (58). However, given the widespread use of home-based tDCS devices (39), untrained application may cause burns, reduced accuracy, and other complications. Professional guidance is necessary before use. Theoretically, the combination of rTMS and tDCS could raise the incidence of severe adverse events (59); however, our review did not report any such cases (9, 41). Similarly, a study involving patients with depression reported no serious adverse events, except for increased scalp pain when rTMS was applied before tDCS (60). In a healthy population, another review found no serious adverse events related to combined interventions (61). In brief, there is no current evidence questioning the safety of the combination of tDCS and rTMS.
This study has several limitations. First, the analysis using TUG as the outcome measure indicated that, compared with the placebo group, NiBS did not appear to improve patients’ walking function. This result may be due to the fact that, in some of the included clinical studies, the baseline walking function of the experimental group was weaker than that of the control group (7, 29, 33). Second, previous studies reported varying efficacies of NiBS depending on the stage of stroke (5). Although our review included patients at different stages of stroke onset, a subgroup analysis of NiBS treatment effects by stroke stage was not performed due to limited relevant research. Additionally, the NMA did not encompass all NiBS interventions, such as tRNS, taVNS, and tACS. There is a lack of suitable studies on these interventions for lower-extremity motor function (11, 62).
4.1 Conclusion
The meta-analysis suggests that LF-rTMS and rTMS + tDCS are effective neurostimulation therapies for enhancing poststroke lower limb motor function. Probability ranking indicated that, among all the NiBS interventions analyzed, rTMS + tDCS may be the most effective. Concerning body balance function, iTBS and LF-rTMS improved poststroke balance, with iTBS potentially being the most effective. For activities of daily living, iTBS, LF-rTMS, and rTMS + tDCS demonstrated beneficial effects, with LF-rTMS possibly being the most effective among them.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
ED: Conceptualization, Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. JL: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. LZ: Formal analysis, Investigation, Project administration, Resources, Writing – review & editing. XZ: Conceptualization, Investigation, Writing – review & editing. ZW: Investigation, Software, Writing – review & editing. WX: Writing – original draft, Writing – review & editing. DJ: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by by Science Technology Projects in Guangzhou (2023A03J0532).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1664707/full#supplementary-material
Footnotes
Edited by:
Hongyu Xu, Virginia Commonwealth University, United StatesReviewed by:
Hai Li, Southern Medical University, ChinaReferences
1. Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Robinson, CA, Shumway-Cook, A, Matsuda, PN, and Ciol, MA. Understanding physical factors associated with participation in community ambulation following stroke. Disabil Rehabil. (2011) 33:1033–42. doi: 10.3109/09638288.2010.520803
3. Xie, YJ, Chen, Y, Tan, HX, Guo, QF, Lau, BW, and Gao, Q. Repetitive transcranial magnetic stimulation for lower extremity motor function in patients with stroke: a systematic review and network meta-analysis. Neural Regen Res. (2021) 16:1168–76. doi: 10.4103/1673-5374.300341
4. Di Pino, G, Pellegrino, G, Assenza, G, Capone, F, Ferreri, F, Formica, D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. (2014) 10:597–608. doi: 10.1038/nrneurol.2014.162
5. Ahmed, I, Mustafaoglu, R, Rossi, S, Cavdar, FA, Agyenkwa, SK, Pang, MYC, et al. Non-invasive brain stimulation techniques for the improvement of upper limb motor function and performance in activities of daily living after stroke: a systematic review and network meta-analysis. Arch Phys Med Rehabil. (2023) 104:1683–97. doi: 10.1016/j.apmr.2023.04.027
6. Beisteiner, R, Lozano, A, Di Lazzaro, V, George, MS, and Hallett, M. Clinical recommendations for non-invasive ultrasound neuromodulation. Brain Stimul. (2024) 17:890–5. doi: 10.1016/j.brs.2024.07.013
7. Zhu, PA, Li, ZL, Lu, QQ, Nie, YY, Liu, H, Kiernan, E, et al. Can cerebellar theta-burst stimulation improve balance function and gait in stroke patients? A randomized controlled trial. Eur J Phys Rehabil Med. (2024) 60:391–9. doi: 10.23736/S1973-9087.24.08307-2
8. Li, D, Cheng, A, Zhang, Z, Sun, Y, and Liu, Y. Effects of low-frequency repetitive transcranial magnetic stimulation combined with cerebellar continuous theta burst stimulation on spasticity and limb dyskinesia in patients with stroke. BMC Neurol. (2021) 21:369. doi: 10.1186/s12883-021-02406-2
9. Gong, Y, Long, XM, Xu, Y, Cai, XY, and Ye, M. Effects of repetitive transcranial magnetic stimulation combined with transcranial direct current stimulation on motor function and cortex excitability in subacute stroke patients: a randomized controlled trial. Clin Rehabil. (2021) 35:718–27. doi: 10.1177/0269215520972940
10. He, Y, Li, K, Chen, Q, Yin, J, and Bai, D. Repetitive transcranial magnetic stimulation on motor recovery for patients with stroke: a Prisma compliant systematic review and meta-analysis. Am J Phys Med Rehabil. (2020) 99:99–108. doi: 10.1097/phm.0000000000001277
11. Veldema, J, and Gharabaghi, A. Non-invasive brain stimulation for improving gait, balance, and lower limbs motor function in stroke. J Neuroeng Rehabil. (2022) 19:84. doi: 10.1186/s12984-022-01062-y
12. Saikaley, M, Pauli, G, Sun, H, Serra, JR, Iruthayarajah, J, and Teasell, R. Network meta-analysis of non-conventional therapies for improving upper limb motor impairment poststroke. Stroke. (2022) 53:3717–27. doi: 10.1161/STROKEAHA.122.040687
13. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
14. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
15. Sterne, JA, Sutton, AJ, Ioannidis, JP, Terrin, N, Jones, DR, Lau, J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
16. Klomjai, W, Aneksan, B, Pheungphrarattanatrai, A, Chantanachai, T, Choowong, N, Bunleukhet, S, et al. Effect of single-session dual-tDCS before physical therapy on lower-limb performance in sub-acute stroke patients: a randomized sham-controlled crossover study. Ann Phys Rehabil Med. (2018) 61:286–91. doi: 10.1016/j.rehab.2018.04.005
17. Wang, L, Wang, Z, Gao, F, Wu, J, and Tang, H. Clinical effect analysis of wearable sensor technology-based gait function analysis in post-transcranial magnetic stimulation stroke patients. Sensors. (2024) 24, 24–10. doi: 10.3390/s24103051
18. Wang, C, Zeng, Q, Yuan, Z, Wang, W, and Shen, M. Effects of low-frequency (0.5 Hz) and high-frequency (10 Hz) repetitive transcranial magnetic stimulation on neurological function, motor function, and excitability of cortex in ischemic stroke patients. Neurologist. (2023) 28:11–8. doi: 10.1097/NRL.0000000000000435
19. Lin, YN, Hu, CJ, Chi, JY, Lin, LF, Yen, TH, Lin, YK, et al. Effects of repetitive transcranial magnetic stimulation of the unaffected hemisphere leg motor area in patients with subacute stroke and substantial leg impairment: a pilot study. J Rehabil Med. (2015) 47:305–10. doi: 10.2340/16501977-1943
20. Sharma, H, Vishnu, VY, Kumar, N, Sreenivas, V, Rajeswari, MR, Bhatia, R, et al. Efficacy of low-frequency repetitive transcranial magnetic stimulation in ischemic stroke: a double-blind randomized controlled trial. Arch Rehabil Res Clin Transl. (2020) 2:100039. doi: 10.1016/j.arrct.2020.100039
21. Huang, YZ, Lin, LF, Chang, KH, Hu, CJ, Liou, TH, and Lin, YN. Priming with 1-Hz repetitive transcranial magnetic stimulation over contralesional leg motor cortex does not increase the rate of regaining ambulation within 3 months of stroke: a randomized controlled trial. Am J Phys Med Rehabil. (2018) 97:339–45. doi: 10.1097/PHM.0000000000000850
22. Wang, RY, Tseng, HY, Liao, KK, Wang, CJ, Lai, KL, and Yang, YR. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair. (2012) 26:222–30. doi: 10.1177/1545968311423265
23. Rastgoo, M, Naghdi, S, Nakhostin Ansari, N, Olyaei, G, Jalaei, S, Forogh, B, et al. Effects of repetitive transcranial magnetic stimulation on lower extremity spasticity and motor function in stroke patients. Disabil Rehabil. (2016) 38:1918–26. doi: 10.3109/09638288.2015.1107780
24. Guan, YZ, Li, J, Zhang, XW, Wu, S, Du, H, Cui, LY, et al. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: a one-year longitudinal randomized trial. CNS Neurosci Ther. (2017) 23:940–6. doi: 10.1111/cns.12762
25. Yu, H, Liu, S, Dai, P, Wang, Z, Liu, C, and Zhang, H. Effects of repetitive transcranial magnetic stimulation on gait and postural control ability of patients with executive dysfunction after stroke. Brain Sci. (2022) 12:1185. doi: 10.3390/brainsci12091185
26. Wang, RY, Wang, FY, Huang, SF, and Yang, YR. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: a double-blinded randomized controlled pilot trial. Gait Posture. (2019) 68:382–7. doi: 10.1016/j.gaitpost.2018.12.023
27. Xie, YJ, Wei, QC, Chen, Y, Liao, LY, Li, BJ, Tan, HX, et al. Cerebellar theta burst stimulation on walking function in stroke patients: a randomized clinical trial. Front Neurosci. (2021) 15:688569. doi: 10.3389/fnins.2021.688569
28. Liao, LY, Zhu, Y, Peng, QY, Gao, Q, Liu, L, Wang, QH, et al. Intermittent theta-burst stimulation for stroke: primary motor cortex versus cerebellar stimulation: a randomized sham-controlled trial. Stroke. (2024) 55:156–65. doi: 10.1161/STROKEAHA.123.044892
29. Lin, LF, Chang, KH, Huang, YZ, Lai, CH, Liou, TH, and Lin, YN. Simultaneous stimulation in bilateral leg motor areas with intermittent theta burst stimulation to improve functional performance after stroke: a feasibility pilot study. Eur J Phys Rehabil Med. (2019) 55:162–8. doi: 10.23736/S1973-9087.18.05245-0
30. Koch, G, Bonni, S, Casula, EP, Iosa, M, Paolucci, S, Pellicciari, MC, et al. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: a randomized clinical trial. JAMA Neurol. (2019) 76:170–8. doi: 10.1001/jamaneurol.2018.3639
31. Duan, Q, Liu, W, Yang, J, Huang, B, and Shen, J. Effect of cathodal transcranial direct current stimulation for lower limb subacute stroke rehabilitation. Neural Plast. (2023) 2023:1–10. doi: 10.1155/2023/1863686
32. Qurat Ul A,, Ahmad, Z, Ilyas, S, Ishtiaq, S, Tariq, I, Nawaz Malik, A, et al. Comparison of a single session of TDCS on cerebellum vs. motor cortex in stroke patients: a randomized sham-controlled trial. Ann Med. (2023) 55:2252439. doi: 10.1080/07853890.2023.2252439
33. Toktas, N, Duruturk, N, Guzel, S, Yuruk, O, and Ozen, S. The effect of transcranial direct current stimulation on balance, gait function and quality of life in patients with stroke. Neurol Res. (2024) 46:868–75. doi: 10.1080/01616412.2024.2362583
34. Manji, A, Amimoto, K, Matsuda, T, Wada, Y, Inaba, A, and Ko, S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci Lett. (2018) 662:302–5. doi: 10.1016/j.neulet.2017.10.049
35. Chang, MC, Kim, DY, and Park, DH. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimul. (2015) 8:561–6. doi: 10.1016/j.brs.2015.01.411
36. Bornheim, S, Croisier, JL, Maquet, P, and Kaux, JF. Transcranial direct current stimulation associated with physical-therapy in acute stroke patients - a randomized, triple blind, sham-controlled study. Brain Stimul. (2020) 13:329–36. doi: 10.1016/j.brs.2019.10.019
37. Madhavan, S, Cleland, BT, Sivaramakrishnan, A, Freels, S, Lim, H, Testai, FD, et al. Cortical priming strategies for gait training after stroke: a controlled, stratified trial. J Neuroeng Rehabil. (2020) 17:111. doi: 10.1186/s12984-020-00744-9
38. Tahtis, V, Kaski, D, and Seemungal, BM. The effect of single session bi-cephalic transcranial direct current stimulation on gait performance in sub-acute stroke: a pilot study. Restor Neurol Neurosci. (2014) 32:527–32. doi: 10.3233/RNN-140393
39. Prathum, T, Piriyaprasarth, P, Aneksan, B, Hiengkaew, V, Pankhaew, T, Vachalathiti, R, et al. Effects of home-based dual-hemispheric transcranial direct current stimulation combined with exercise on upper and lower limb motor performance in patients with chronic stroke. Disabil Rehabil. (2022) 44:3868–79. doi: 10.1080/09638288.2021.1891464
40. Aneksan, B, Sawatdipan, M, Bovonsunthonchai, S, Tretriluxana, J, Vachalathiti, R, Auvichayapat, P, et al. Five-session dual-transcranial direct current stimulation with task-specific training does not improve gait and lower limb performance over training alone in subacute stroke: a pilot randomized controlled trial. Neuromodulation. (2022) 25:558–68. doi: 10.1111/ner.13526
41. Cho, JY, Lee, A, Kim, MS, Park, E, Chang, WH, Shin, YI, et al. Dual-mode noninvasive brain stimulation over the bilateral primary motor cortices in stroke patients. Restor Neurol Neurosci. (2017) 35:105–14. doi: 10.3233/RNN-160669
42. Lefaucheur, JP, Aleman, A, Baeken, C, Benninger, DH, Brunelin, J, Di Lazzaro, V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin Neurophysiol. (2020) 131:474–528. doi: 10.1016/j.clinph.2019.11.002
43. Tien, HH, Liu, WY, Chen, YL, Wu, YC, and Lien, HY. Transcranial direct current stimulation for improving ambulation after stroke: a systematic review and meta-analysis. Int J Rehabil Res. (2020) 43:299–309. doi: 10.1097/MRR.0000000000000427
44. Palmer, JA, Wheaton, LA, Gray, WA, Saltao da Silva, MA, Wolf, SL, and Borich, MR. Role of interhemispheric cortical interactions in poststroke motor function. Neurorehabil Neural Repair. (2019) 33:762–74. doi: 10.1177/1545968319862552
45. Kakuda, W, Abo, M, Shimizu, M, Sasanuma, J, Okamoto, T, Yokoi, A, et al. A multi-center study on low-frequency rTMS combined with intensive occupational therapy for upper limb hemiparesis in post-stroke patients. J Neuroeng Rehabil. (2012) 9:4. doi: 10.1186/1743-0003-9-4
46. Chang, WH, Kim, YH, Yoo, WK, Goo, KH, Park, CH, Kim, ST, et al. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restor Neurol Neurosci. (2012) 30:179–89. doi: 10.3233/RNN-2012-110162
47. Du, J, Yang, F, Hu, J, Hu, J, Xu, Q, Cong, N, et al. Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. Neuroimage Clin. (2019) 21:101620. doi: 10.1016/j.nicl.2018.101620
48. Hordacre, B, Moezzi, B, and Ridding, MC. Neuroplasticity and network connectivity of the motor cortex following stroke: a transcranial direct current stimulation study. Hum Brain Mapp. (2018) 39:3326–39. doi: 10.1002/hbm.24079
49. Han, S, Li, XX, Wei, S, Zhao, D, Ding, J, Xu, Y, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Cell Rep Med. (2023) 4:101060. doi: 10.1016/j.xcrm.2023.101060
50. Ntakou, EA, Nasios, G, Nousia, A, Siokas, V, Messinis, L, and Dardiotis, E. Targeting cerebellum with non-invasive transcranial magnetic or current stimulation after cerebral hemispheric stroke-insights for Corticocerebellar network reorganization: a comprehensive review. Healthcare. (2022) 10:2401. doi: 10.3390/healthcare10122401
51. Rao, J, Li, F, Zhong, L, Wang, J, Peng, Y, Liu, H, et al. Bilateral cerebellar intermittent theta burst stimulation combined with swallowing speech therapy for dysphagia after stroke: a randomized, double-blind, sham-controlled, clinical trial. Neurorehabil Neural Repair. (2022) 36:437–48. doi: 10.1177/15459683221092995
52. Chen, Y, Wei, QC, Zhang, MZ, Xie, YJ, Liao, LY, Tan, HX, et al. Cerebellar intermittent Theta-burst stimulation reduces upper limb spasticity after subacute stroke: a randomized controlled trial. Front Neural Circuits. (2021) 15:655502. doi: 10.3389/fncir.2021.655502
53. Sasegbon, A, Watanabe, M, Simons, A, Michou, E, Vasant, DH, Magara, J, et al. Cerebellar repetitive transcranial magnetic stimulation restores pharyngeal brain activity and swallowing behaviour after disruption by a cortical virtual lesion. J Physiol. (2019) 597:2533–46. doi: 10.1113/JP277545
54. Turkeltaub, PE, Swears, MK, D'Mello, AM, and Stoodley, CJ. Cerebellar tDCS as a novel treatment for aphasia? Evidence from behavioral and resting-state functional connectivity data in healthy adults. Restor Neurol Neurosci. (2016) 34:491–505. doi: 10.3233/RNN-150633
55. Sasegbon, A, Niziolek, N, Zhang, M, Smith, CJ, Bath, PM, Rothwell, J, et al. The effects of midline cerebellar rTMS on human pharyngeal cortical activity in the intact swallowing motor system. Cerebellum. (2021) 20:101–15. doi: 10.1007/s12311-020-01191-x
56. Rossi, S, Antal, A, Bestmann, S, Bikson, M, Brewer, C, Brockmoller, J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. (2021) 132:269–306. doi: 10.1016/j.clinph.2020.10.003
57. Chou, YH, Ton That, V, Chen, AY, Sundman, M, and Huang, YZ. TMS-induced seizure cases stratified by population, stimulation protocol, and stimulation site: a systematic literature search. Clin Neurophysiol. (2020) 131:1019–20. doi: 10.1016/j.clinph.2020.02.008
58. Antal, A, Alekseichuk, I, Bikson, M, Brockmoller, J, Brunoni, AR, Chen, R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. (2017) 128:1774–809. doi: 10.1016/j.clinph.2017.06.001
59. Rossi, S, Hallett, M, Rossini, PM, and Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. (2009) 120:2008–39. doi: 10.1016/j.clinph.2009.08.016
60. Loo, C, Martin, D, Pigot, M, Arul-Anandam, P, Mitchell, P, and Sachdev, P. Transcranial direct current stimulation priming of therapeutic repetitive transcranial magnetic stimulation: a pilot study. J ECT. (2009) 25:256–60. doi: 10.1097/YCT.0b013e3181a2f87e
61. Karabanov, A, Ziemann, U, Hamada, M, George, MS, Quartarone, A, Classen, J, et al. Consensus paper: probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul. (2015) 8:993–1006. doi: 10.1016/j.brs.2015.06.017
Keywords: rTMS (repetitive transcranial magnetic stimulation), tDCS, stroke—diagnosis, lower limb and rehabilitation, NIBS (non-invasive brain stimulation)
Citation: Deng E, Li J, Zhang L, Zhou X, Wu Z, Xu W and Jin D (2025) Non-invasive brain stimulation for the improvement of lower extremity motor function in patients with stroke: a systematic review and network meta-analysis. Front. Neurol. 16:1664707. doi: 10.3389/fneur.2025.1664707
Copyright © 2025 Deng, Li, Zhang, Zhou, Wu, Xu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wuhua Xu, eGlvbmd3dWFkZkBzaW5hLmNvbQ==; Dongmei Jin, ZG1qaW5AMTI2LmNvbQ==
 Enliang Deng
Enliang Deng Jiayu Li2
Jiayu Li2 Dongmei Jin
Dongmei Jin