- 1Department of Rehabilitation Medicine, Affiliated Huishan Hospital of Xinglin College, Nantong University, Wuxi Huishan District People's Hospital, Wuxi, Jiangsu, China
- 2Department of Pain Rehabilitation, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 3Department of Rehabilitation Medicine, The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Background: Traumatic peripheral nerve injuries of the upper limb often lead to substantial motor and sensory deficits, posing significant challenges to functional recovery and quality of life. Mirror therapy, a visually guided neurorehabilitation technique, has shown potential in enhancing upper limb function, yet its effectiveness in traumatic peripheral nerve injuries remains inconclusive.
Methods: This systematic review and meta-analysis followed PRISMA 2020 guidelines. Randomized controlled trials involving adult patients with upper limb traumatic peripheral nerve injuries treated with mirror therapy were identified through searches of seven major databases up to Augst 2025. Methodological quality was assessed using the PEDro scale, and pooled analyses were performed using standard mean differences with 95% confidence intervals.
Results: Seven clinical studies involving 112 participants were included and five randomized controlled trials contributed to the meta-analysis. Mirror therapy significantly improved hand function measured by the Rosen Score (SMD = 0.24; 95% CI: 0.02 to 0.46; p = 0.03; I2 = 0%). Improvements in grip strength (SMD = 0.45; p = 0.26) and sensory outcomes (SWM: SMD = 1.05; p = 0.07; 2PD: SMD = 0.45; p = 0.26) did not reach statistical significance. Pain-related outcomes were inconsistently reported. Subgroup analysis was not feasible due to intervention heterogeneity and limited sample sizes. Certainty of evidence was moderate for hand function and low to very low for other outcomes.
Conclusion: Mirror therapy may offer modest benefits in hand function recovery following upper limb traumatic peripheral nerve injury. However, current evidence is limited by small sample sizes, methodological heterogeneity, and low study quality. No significant effects were observed for sensory or pain-related outcomes. Further high-quality randomized controlled trials with standardized protocols and long-term follow-up are needed to establish the clinical efficacy and optimize the use of mirror therapy in this population.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023437659.
1 Introduction
Traumatic peripheral nerve injury (TPNI) has emerged as an important clinical and public health issue, with its incidence increasing alongside socioeconomic development. Contributing factors include motor vehicle accidents, penetrating trauma, lacerations, gunshot wounds, falls, burns, fractures, ischemia, traction, and crush injuries (1–4). Among all TPNI cases, injuries involving the upper extremities represent a substantial proportion, though prevalence and incidence vary across regions. For example, a 2019 study in the United States reported an incidence of 1.69% for upper limb TPNI (5), while a 2011 study in Iran reported a rate of 1.3% (6). In contrast, a 2022 study from South Korea documented a decline in incidence from 1.07% in 2008 to 0.79% in 2018 (7), suggesting regional and temporal variability. In upper limb TPNI, the radial, ulnar, and median nerves—as well as the brachial plexus—are most frequently involved. Among these, the radial nerve is most commonly affected, followed by the ulnar and median nerves (8). Such injuries are frequently associated with substantial motor and sensory dysfunction, persistent pain, and a decline in overall quality of life (9–11).
MT is a widely recognized rehabilitation technique, effective in promoting motor recovery and alleviating pain in patients with various neurological conditions (12, 13). It uses mirrors to create optical illusions that deceive the brain into perceiving motor and sensory feedback from the affected limbs (14). MT is based on the concept of mirror neurons in the brain, which activate during both action performance and observation (15). Mirror neurons are specialized cells that activate when a person performs an action or observes another performing the same action (16). MT aims to activate mirror neurons and stimulate neural pathways involved in motor control and sensory perception through the use of visual feedback via mirrors (15). In MT, the patient positions a mirror to reflect the unaffected limb, creating the illusion of normal movement in the injured limb. The patient then performs symmetrical movements of both limbs during a series of mirror exercises (17). A systematic review evaluating the effectiveness of MT in improving hand function and sensory recovery in individuals with TPNI is currently lacking. Therefore, a systematic review of the available studies was conducted to assess the effects of MT on these patients.
2 Methods
This study was conducted following the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and was registered with PROSPERO (registration no. CRD42023437659) (18). Ethical approval was not required.
2.1 Search strategy
Databases including PubMed, EMBASE, MEDLINE, the Cochrane Library, WANFANG DATA, CNKI and PEDro were systematically searched from their inception to Augst 5, 2025. Relevant studies examining the efficacy of MT for the treatment of TPNI were identified using specific keywords and controlled vocabulary tailored individually to each database. Detailed search strategies for each database are provided in Appendix 1.
2.2 Study selection and data collection
Study selection was carried out in two distinct phases. During the first stage, two reviewers (WL and LNJ) independently screened titles and abstracts based on the predefined eligibility criteria. If relevance could not be ascertained from the abstract alone, the full text was retrieved for further assessment. In the second stage, the same reviewers independently reviewed the full texts to confirm inclusion. Any disagreements were resolved through discussion, and unresolved cases were adjudicated by a third reviewer (DDL). Reference management and duplicate removal were performed using EndNote™ (version 20.5, Clarivate Analytics, Philadelphia, PA) in combination with manual verification.
Data extraction was initially conducted by one reviewer (DDL), and subsequently cross-checked by two independent reviewers (WL and LNJ) to ensure completeness and accuracy. Extracted variables were organized using a customized Microsoft Excel spreadsheet (Microsoft®, United States). Key data included: (i) sample size; (ii) participant demographics and clinical characteristics (e.g., age, sex, condition); and (iii) intervention details such as treatment protocol, dosage, primary outcome measures, evaluation time points, and principal findings. Any discrepancies in the data extraction process were discussed until consensus was reached.
2.3 Inclusion and exclusion criteria
This review included the following eligibility criteria: (1) randomized controlled trials (RCTs); (2) adult patients who had undergone upper limb peripheral nerve repair; (3) studies in which MT was the primary intervention. Exclusion criteria included: (1) studies involving pediatric populations (under 18 years of age); (2) studies that did not involve MT or primarily examined other interventions; (3) studies investigating treatments outside the scope of physical or occupational therapy; (4) studies involving patients with comorbidities or additional diagnoses that could confound treatment outcomes.
2.4 Quality assessment
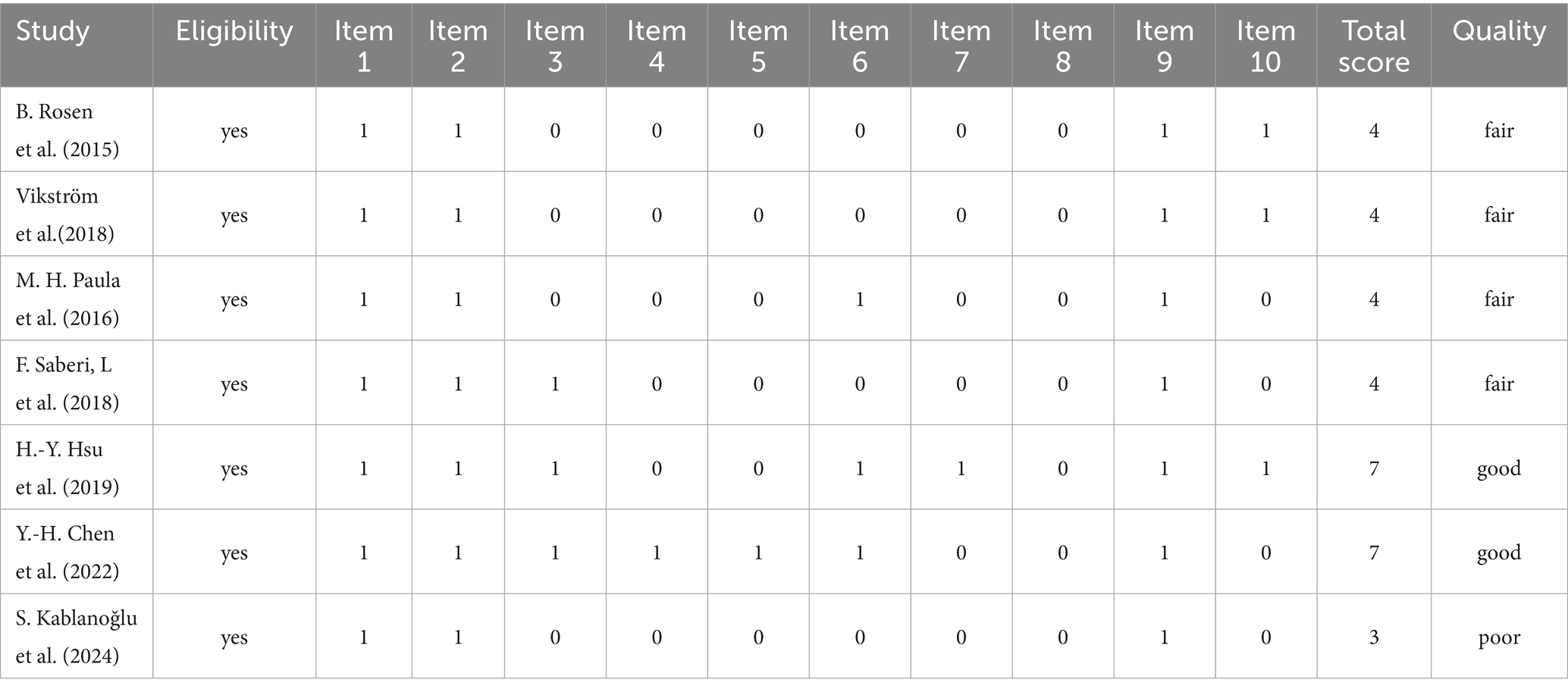
The methodological quality of the included studies was assessed using the PEDro Scale, an 11-item, standardized tool specifically designed for evaluating RCTs. This scale provides a comprehensive assessment of key dimensions such as internal validity, quality of reporting, and the interpretability of findings. Of the 11 items, 10 contribute to the final score, yielding a total score range from 0 to 10 (19). In this review, two authors independently conducted the quality assessments for each study. Discrepancies were resolved through discussion until consensus was reached, thereby improving the reliability of the evaluation process.
2.5 The risk of bias assessment
Risk of bias was assessed using the original Cochrane Risk of Bias (RoB 1.0) tool, in alignment with the methodological framework outlined in the Cochrane Handbook for Systematic Reviews of Interventions (20). This instrument evaluates five key methodological domains: random sequence generation, deviations from intended interventions, missing outcome data, outcome measurement, and selective outcome reporting. Each domain was rated as having a low risk, high risk, or some concerns, according to predefined judgment criteria. The assessments were independently performed by two reviewers. Any inconsistencies were addressed through discussion, with a third reviewer consulted in cases requiring further resolution to ensure judgment consistency.
3 Results
3.1 Study selection
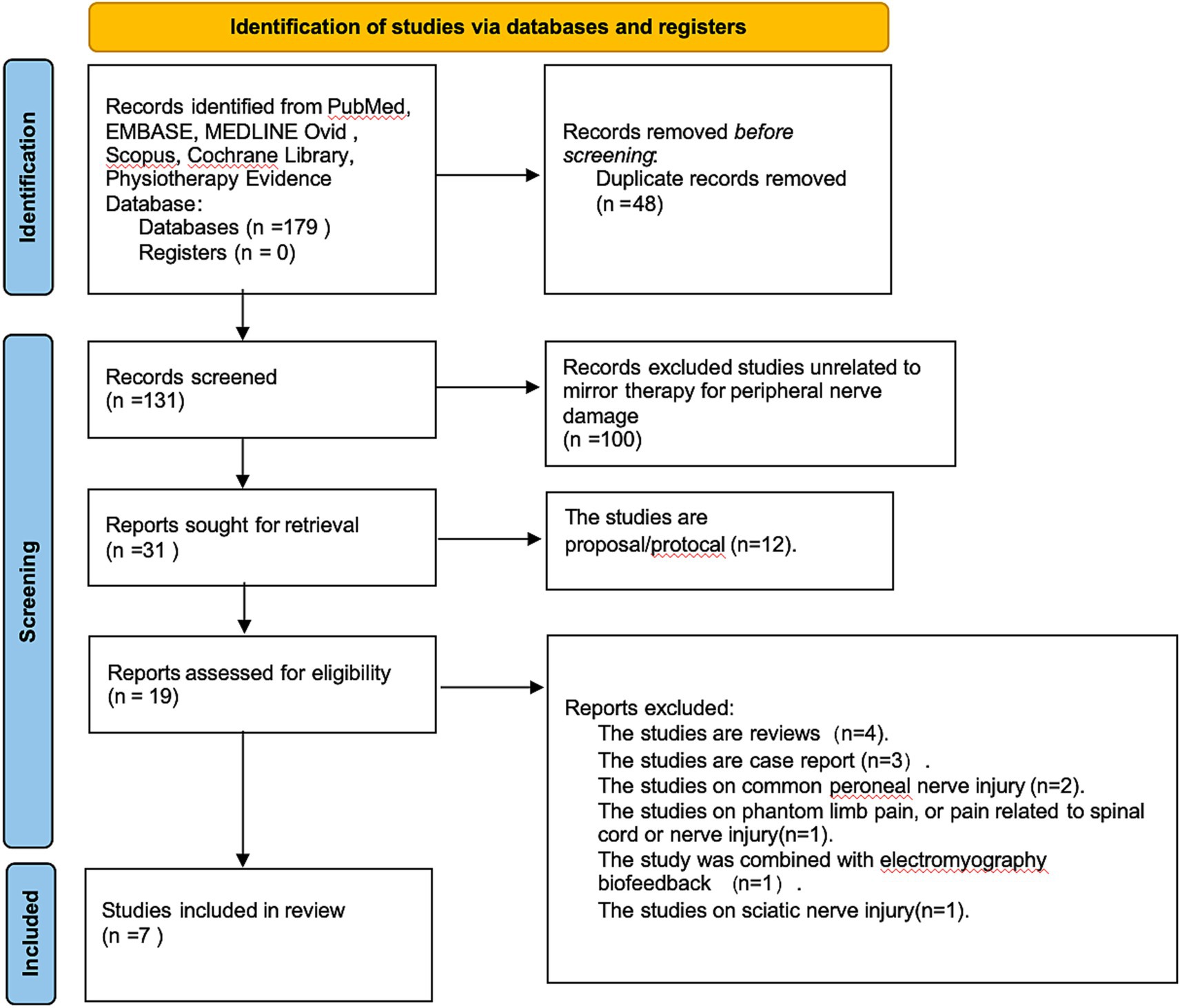
An initial pool of 179 records was retrieved from the selected databases. Following screening and eligibility assessment, seven studies met the inclusion criteria for this review (Figure 1). All included studies were published in English. Two trials were conducted in Taiwan (21, 22). The remaining five comprised trials from Turkey (23), Brazil (24), Iran (25), Sweden (26), and one conducted through a multinational collaboration across the United States, the Netherlands, and Sweden (27). Of the seven studies, five provided sufficient, harmonizable outcome data and were included in the meta-analysis. The other two were not quantitatively synthesized: in one, key outcome data could not be obtained despite attempts to contact the authors, and the other was a 4–9 year follow-up of a previously included trial. To avoid double counting and unit-of-analysis errors, both were included only in the narrative synthesis.
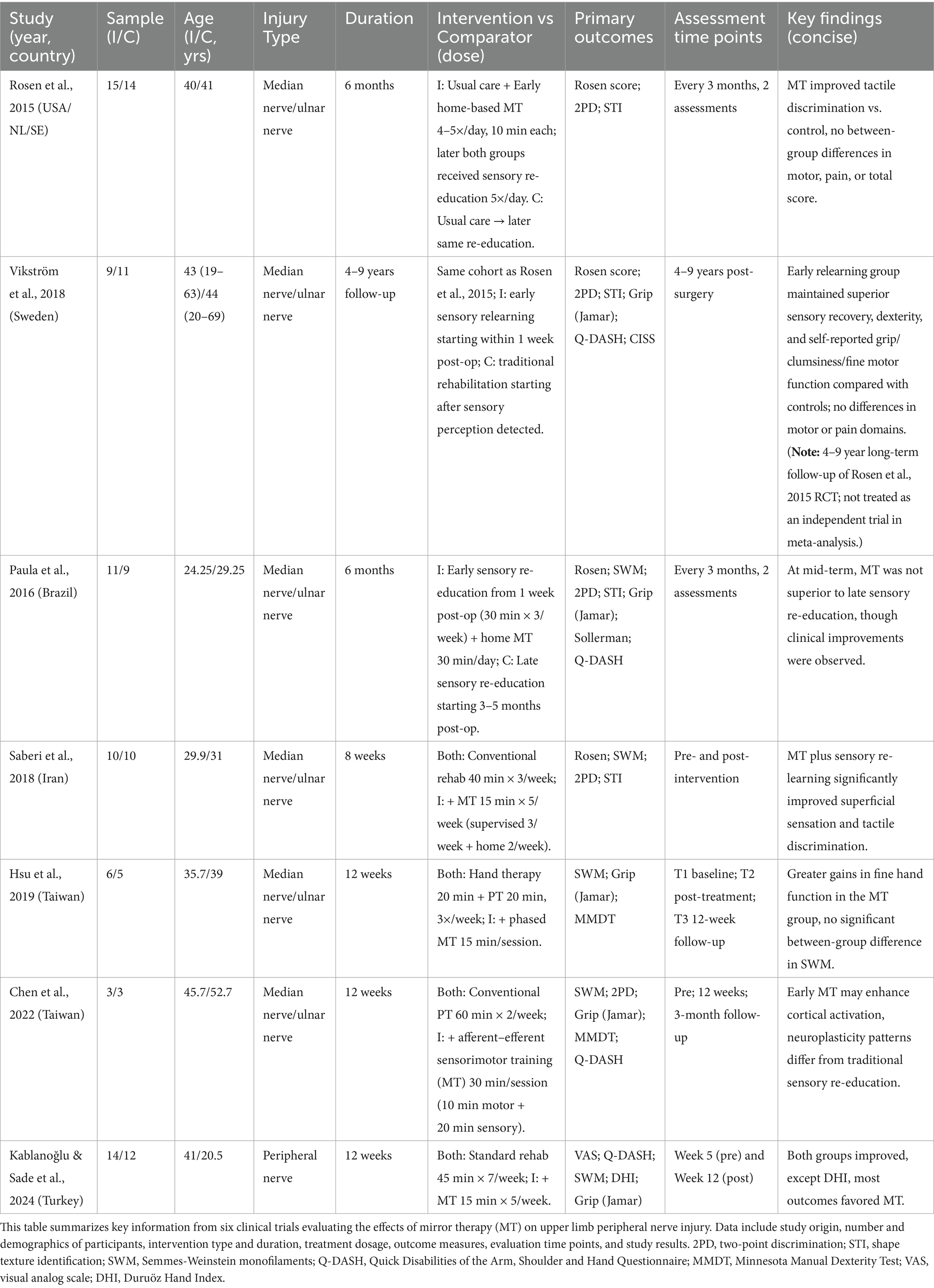
3.2 Characteristics of included studies
This review included seven studies (total n = 112) evaluating MT for upper-limb TPNI. Because several reports presented outcomes at multiple time points and, in some cases, across more than one publication, methodological quality was appraised at the study level. Sample sizes ranged from 6 to 29 participants. Mean age in intervention arms ranged from 24.25 to 45.70 years and in control arms from 20.50 to 52.70 years. Most injuries involved the median or ulnar nerve. Baseline characteristics (e.g., age) were generally comparable between groups.
For all included studies, we explicitly verified that participants assigned to MT also received the same conventional postoperative rehabilitation as the control group (e.g., routine physiotherapy/occupational therapy) with matched type, frequency, and dose. Accordingly, MT functioned strictly as an adjunct to standard care in every pooled trial, and the meta-analytic estimates therefore reflect the incremental effect of MT beyond conventional rehabilitation. Any deviations or uncertainties regarding co-interventions were recorded as potential sources of bias in the risk-of-bias assessment. For each study, we extracted country, participant demographics, MT delivery, session dose and duration, details of conventional therapy in each arm, comparator type, outcome measures, assessment time points, and main findings. Table 1 provides a structured summary.
3.3 Evidence quality evaluation
The methodological quality assessment, based on the PEDro scale, is summarized in Table 2. Scores ranged from 3 to 7 points. Accordingly, two studies were classified as high quality (21, 22), four as moderate quality (24–27), and one as low quality (23). This variability highlights methodological heterogeneity, which should be considered when interpreting the findings.
3.4 Risk of bias assessment results
Among the seven included studies, one was judged low risk of bias (21), whereas six were judged high risk of bias (22–27). The predominant concerns involved selection bias (item 2), performance bias (item 3), and detection bias (item 4), alongside a frequent problem of >20% missing outcome data without prespecified handling strategies—conditions that can undermine effect estimates and internal validity (Figure 2). Importantly, Rosen et al. (26) and Vikström et al. (27) report on the same cohort, with Vikström representing a post-hoc follow-up of the original Rosen trial. Accordingly, these two publications were treated as a single study for the purposes of risk-of-bias assessment. This study lacked assessor blinding and had substantial attrition. Saberi et al. (25) also showed high risk due to participant blinding, assessor blinding, and missing data. Paula et al. (24) and Kablanoğlu et al. (23) were primarily affected by missing data. Although Chen et al. (21) was rated overall low risk, minor concerns remained regarding blinding and attrition, but these did not alter the overall judgment.
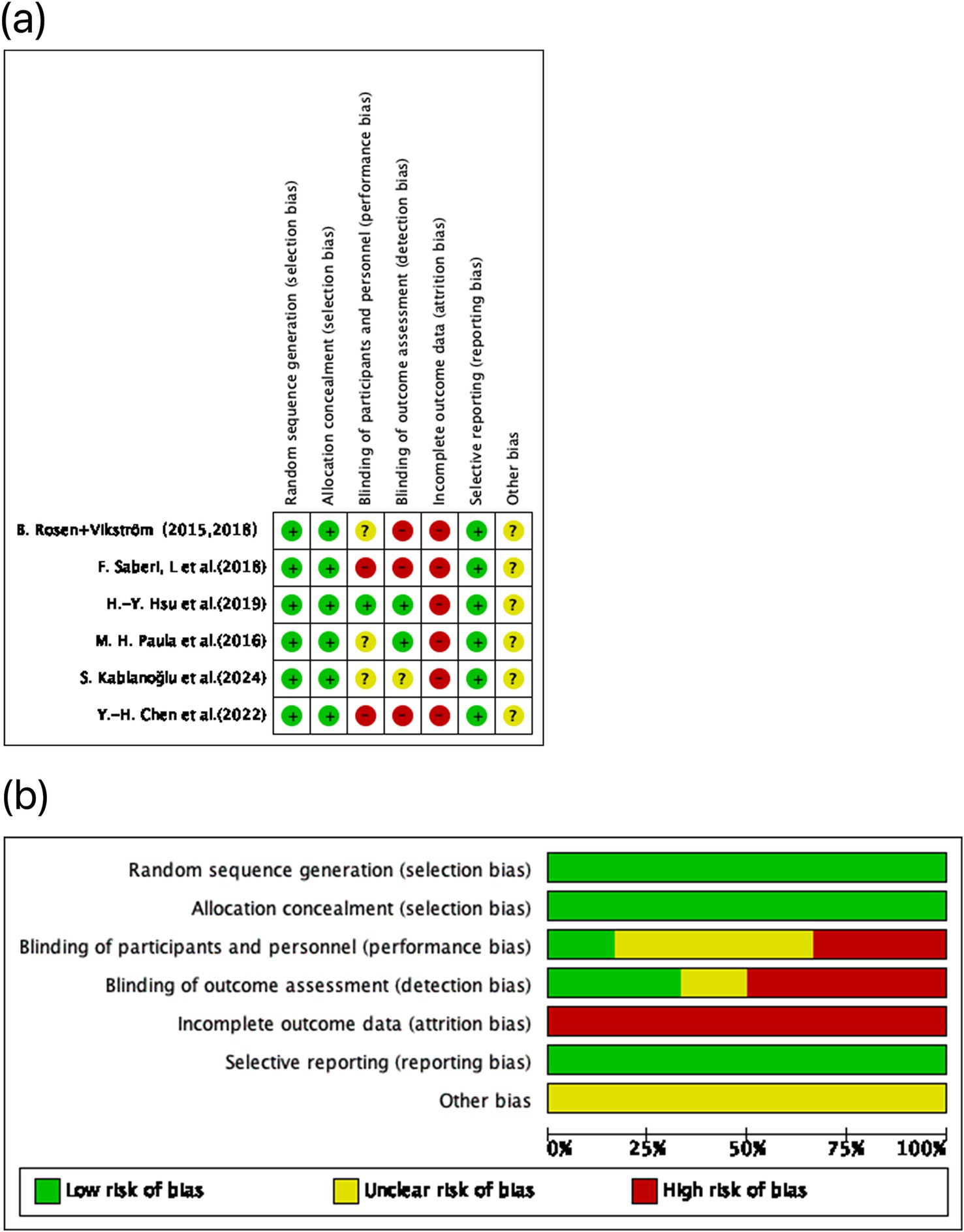
Figure 2. Risk of bias assessment for the included studies using the Cochrane RoB 1.0 tool. (A) Domain-level risk of bias ratings for each individual study, covering five key areas of potential methodological bias. (B) Aggregated distribution of risk of bias classifications across all included trials. Color coding indicates the risk level: green denotes low risk, yellow indicates some concerns, and red reflects high risk.
3.5 Clinical outcomes
3.5.1 Sensory testing
All seven studies assessed sensory function using the Semmes–Weinstein Monofilament (SWM) and two-point discrimination (2PD) tests (21–27). Three trials reported significantly greater improvements in the MT group than in controls (23, 25, 27), whereas three found no significant between group differences (21, 22, 24). At long term follow up (4–9 years after nerve repair), Vikström et al. reported superior performance in the early sensory relearning group on the Rosen sensory domain, particularly in discriminative touch/tactile gnosis and dexterity (26).
3.5.2 Pain testing
Three studies assessed pain (23, 25, 27). One trial showed a greater reduction in visual analog scale (VAS) pain with MT than with control (23), while two found no significant between-group differences (25, 27). Consistent with these findings, Vikström et al. detected no between group differences in the Rosen pain/discomfort domain or in cold sensitivity (CISS) at long-term follow-up (26).
3.5.3 Hand function assessment
Five studies assessed hand function using validated measures (21–24, 27). All five measured grip strength with a Jamar dynamometer; three favored MT (21–23), and two found no significant between-group differences (24, 27). Two studies used the Quick Disabilities of the Arm, Shoulder and Hand Questionnaire (Q-DASH); one favored MT (23) and one reported comparable outcomes (24). Manual dexterity, assessed with the Purdue Pegboard Test and the Minnesota Manual Dexterity Test (MMDT), favored MT in both trials that reported these outcomes (21, 22). One study also found better performance on the Pinch Holding Up Activity test after MT (21), whereas the Duruöz Hand Index (DHI) showed no between-group difference in a single study (23). At the long-term follow up, Vikström et al. found no between-group differences in the Rosen motor domain or in the total Rosen score. However, participants who received early sensory relearning reported fewer problems with grip function, clumsiness, and fine motor tasks, and DASH scores were similar between groups (26).
3.6 Meta-analysis
3.6.1 Effect of MT on sensory function compared to control group (measured using SWM and 2PD)
The pooled effect size for SWM was 1.05 (95% CI: −0.08 to 2.19, p = 0.07; Figure 3A), and for 2PD was 0.45 (95% CI: −0.34 to 1.24, p = 0.26; Figure 3B), both indicating non-significant effects. Heterogeneity was low for SWM (I2 = 10%) and not applicable for 2PD. The small sample sizes (n = 18 experimental; n = 17 control) may have limited the statistical power, potentially contributing to the non-significant results.
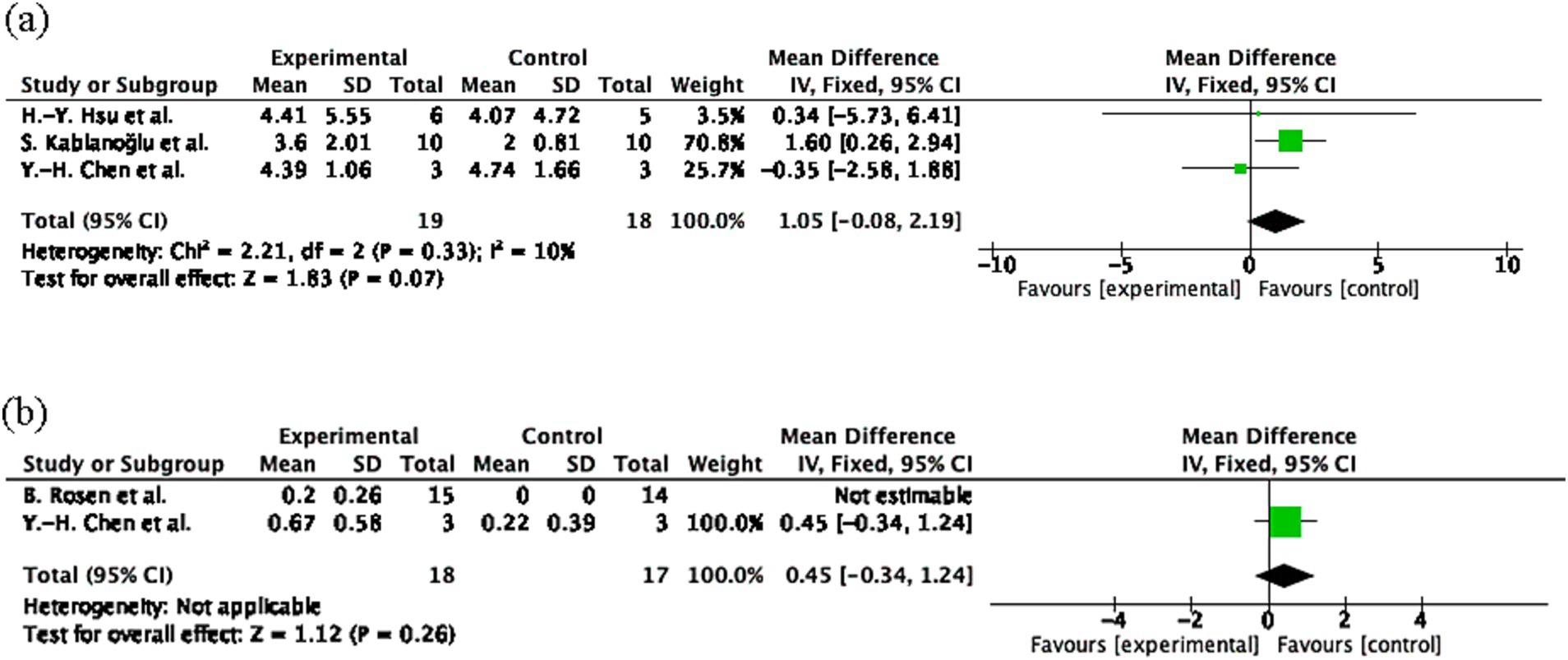
Figure 3. Sensory outcome measures following mirror therapy intervention. (A) Comparison of Semmes-Weinstein monofilament (SWM) test results before and after treatment in the mirror therapy and control groups. (B) Comparison of two-point discrimination (2PD) before and after treatment in the mirror therapy and control groups. Forest plots illustrate the efficacy of mirror therapy compared to control interventions. Statistical significance was defined as p < 0.05.
3.6.2 Effect of MT on hand function compared to control group (measured using Q-DASH and Jamar dynamometer)
The pooled effect size for Q-DASH was 3.78 (95% CI: −8.07 to 15.63, p = 0.53; Figure 4A) and for Jamar Handgrip Strength was 0.45 (95% CI: −0.34 to 1.24, p = 0.26; Figure 4B), both indicating non-significant effects. Heterogeneity was moderate for DASH (I2 = 54%) and not applicable for Jamar. The limited sample sizes (Q-DASH: n = 24 experimental; n = 22 control; Jamar: n = 18 experimental; n = 17 control) and one non-estimable study in the Jamar analysis may have reduced the statistical power.
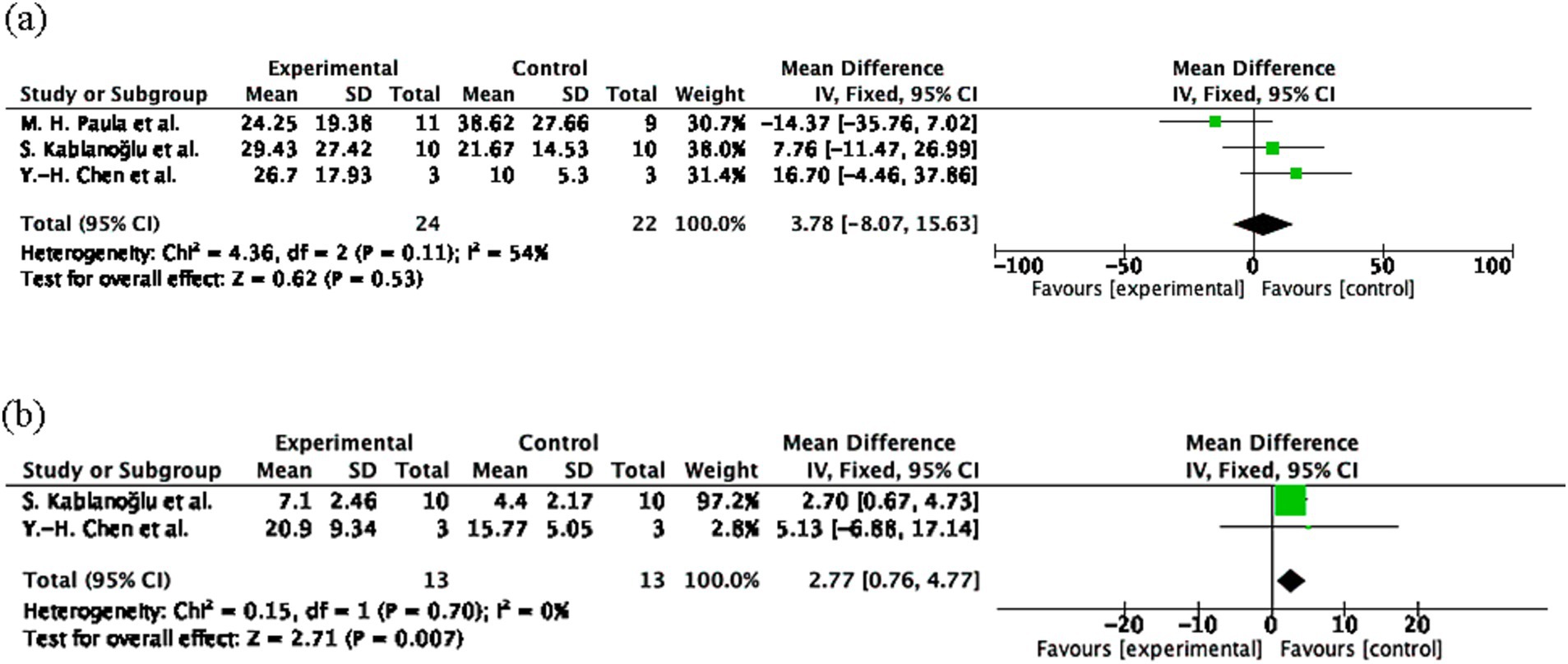
Figure 4. Hand function outcomes following mirror therapy intervention. (A) Comparison of Quick Disabilities of the Arm, Shoulder and Hand Questionnaire (Q-DASH) test results before and after treatment in the mirror therapy and control groups. (B) Comparison of Jamar dynamometer before and after treatment in the mirror therapy and control groups. Forest plots illustrate the efficacy of mirror therapy compared to control interventions. Statistical significance was defined as p < 0.05.
3.6.3 Effect of MT on overall hand function recovery score compared to the control group (measured using the Rosen score)
The pooled effect size for the Rosen Score was 0.24 (95% CI: 0.02 to 0.46, p = 0.03; Figure 5), indicating a small but significant improvement in the experimental group. Heterogeneity was low (I2 = 0%, p = 0.39), suggesting consistent results. The study by Rosen et al. (n = 15 experimental; n = 14 control) contributed most to the overall effect (82.5%), while Paula et al. (n = 11 experimental; n = 9 control) had a smaller impact (17.5%). Despite the modest effect size, the narrow CI and absence of heterogeneity enhance result reliability.
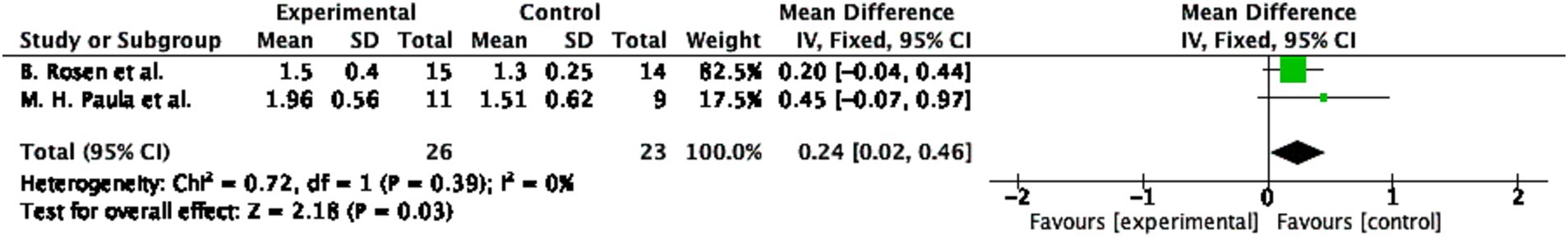
Figure 5. Overall hand function recovery following mirror therapy intervention. Comparison of the Rosen score results before and after treatment in the mirror therapy and control groups. Forest plots illustrate the efficacy of mirror therapy compared to control interventions. Statistical significance was defined as p < 0.05.
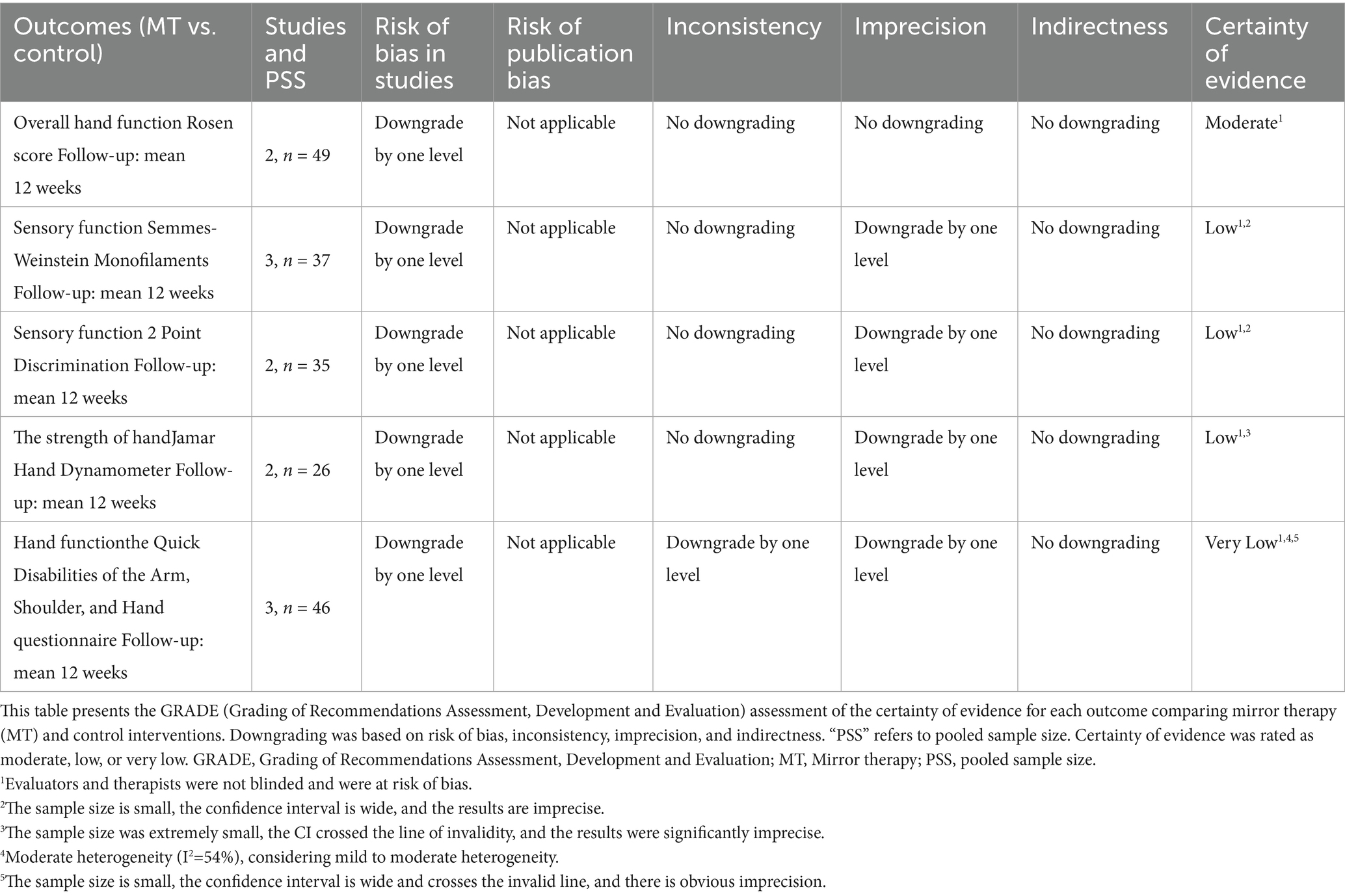
3.7 Certainty of evidence
Table 3 summarizes the GRADE assessment of evidence certainty. Sensory outcomes (SWM and 2PD) and handgrip strength (Jamar) were rated as low certainty due to risk of bias and imprecision from small sample sizes. The certainty for Q-DASH was very low, reflecting high bias risk, imprecision, and moderate heterogeneity. In contrast, the Rosen score showed moderate certainty, supported by consistent results, narrow confidence intervals, and low heterogeneity despite concerns about blinding.
4 Discussion
This systematic review and meta-analysis evaluated the effectiveness of MT for postoperative hand function recovery after upper-limb TPNI. MT is grounded in sensorimotor integration and neuroplasticity (28, 29) and has shown benefits in other neurological populations (12–15), yet the TPNI-specific evidence remains limited in quality and quantity. Of the pooled outcomes, only the Rosen score—an aggregate indicator of overall hand function—showed a statistically significant but small effect (SMD = 0.24; 95% CI, 0.02–0.46; p = 0.03) with low heterogeneity (I2 = 0%). Pooled results for grip strength and sensory function (SWM and 2PD) were not significant. Pain outcomes were inconsistently reported and could not be meaningfully synthesized. Most trials enrolled small samples (6–29 participants), which reduced statistical power, particularly for sensory and pain endpoints.
Follow-up duration also constrained inference. The trials contributing to the meta-analysis were predominantly short term (≤6 months) (21–24, 27). In contrast, Vikström et al. reported a randomized follow-up at 4–9 years. That study found sustained advantages for early sensory relearning on the Rosen sensory domain, particularly discriminative touch/tactile gnosis and dexterity, and fewer patient-reported problems with grip function, clumsiness, and fine motor tasks. There were no between-group differences in the Rosen motor domain, total Rosen score, Q-DASH, or CISS. Both groups improved from 6 months to long term. Because this follow-up extended a previously included trial, its outcomes were narratively synthesized to avoid double counting (26). These findings suggest that sensory-specific and patient-perceived benefits of early MT can persist even when global motor indices and pain measures do not differ.
The GRADE assessment supports cautious interpretation. Certainty was moderate for overall hand function and low to very low for other outcomes due to risk of bias, imprecision, and inconsistency. Common methodological limitations included unclear randomization, absence of blinding of participants, therapists, and assessors, incomplete outcome reporting, and heterogeneous intervention protocols, consistent with PEDro and Cochrane RoB 1.0 evaluations. Substantial variation in treatment parameters (frequency, duration, task design, and level of supervision) further limited comparability and precluded informative subgroup analyses.
Mechanistic data remain sparse. MT is hypothesized to engage visual feedback pathways and drive experience-dependent plasticity in sensorimotor networks (28, 29). Chen et al. reported increased cortical activation on fMRI immediately after nerve repair in patients receiving MT (22). Future trials should embed imaging or electrophysiological biomarkers to test mechanism and dose–response relationships.
Clinical findings were mixed across individual trials. Saberi et al. reported significant gains in sensory discrimination (25), whereas Hsu et al. (21) and Paula et al. (24) observed no material effects on sensory or pain outcomes. Kablano et al. found no significant differences in grip strength or pain but noted a trend toward better clinical responsiveness with MT (23). Differences in nerve type and lesion level, timing and intensity of MT, outcome instruments, and rehabilitation settings likely contributed to these discrepancies.
In summary, MT may yield modest improvements in overall hand function after TPNI, and early sensory-oriented protocols may confer durable sensory advantages on long-term observation. However, the small number of trials, methodological weaknesses, heterogeneity of interventions, and limited long-term data restrict confidence and generalizability. Future research should prioritize adequately powered randomized trials with standardized, reportable treatment parameters; rigorous bias control (allocation concealment, assessor blinding, prespecified outcomes, and complete data handling); consistent core outcome sets including patient-reported measures; and follow-up beyond 12 months. Work to identify patient-level moderators and to integrate mechanistic assessments (e.g., fMRI/fNIRS, quantitative sensory testing) will help clarify who benefits most and how MT should be optimized in peripheral nerve rehabilitation.
4.1 Limitations
This review has several limitations. (1) Small cumulative sample size (n = 112 across six RCTs; individual trials 6–29 participants), limiting the precision of pooled estimates, particularly for secondary endpoints such as sensory recovery and pain. (2) Substantial between trial heterogeneity in intervention protocols (duration, session frequency, task content, and degree of therapist involvement), constraining credible subgroup/meta regression analyses and limiting external validity. (3) Methodological limitations and risk of bias, including unclear sequence generation and allocation concealment, limited participant and/or assessor blinding, and incomplete outcome data; domain ratings on Cochrane RoB 1.0 and PEDro scores indicated predominantly “some concerns” to “high” risk. (4) Non-standardized outcome assessment, especially for pain, with inconsistent use of validated measures and objective quantification, hindering cross study comparability and potentially attenuating pooled effect estimates. (5) Short follow up durations (typically immediate post-intervention or early short-term only), precluding firm conclusions about the durability of treatment effects.
5 Conclusion
MT may be used as an adjunct to improve hand function after upper-limb TPNI, but current evidence supports only modest functional gains. The literature is based on few, heterogeneous trials with generally low methodological quality. Most pooled outcomes, particularly sensory function and grip strength did not reach statistical significance, and small sample sizes with variable treatment parameters contribute to imprecision and heterogeneity. Follow-up in the RCTs included in the meta-analysis was short. Although a 4–9-year randomized follow-up by Vikström et al. suggested durable sensory advantages with early sensory relearning, those data were narratively synthesized and not pooled, so long-term effects remain uncertain. Clinicians should therefore apply MT cautiously, preferably as part of a multimodal rehabilitation program rather than a stand-alone intervention.
To enable definitive recommendations, future studies should employ adequately powered, high-quality randomized controlled trials with standardized, fully reported intervention protocols (e.g., clearly specified session frequency, duration, and intensity) and prespecified, clinically meaningful outcomes. Rigorous methods are essential, including allocation concealment, blinded outcome assessment, preregistration, and appropriate handling of missing data (e.g., intention-to-treat analyses). Trials should adopt harmonized core outcome sets that include both patient-reported and objective measures, use consistent assessment time points, and extend follow-up to ≥12 months. Identifying patient-level moderators—such as age, nerve involved and lesion level, injury severity, and timing post-surgery—will clarify who benefits most and inform stratified care. Embedding mechanistic assessments (e.g., fMRI/fNIRS, quantitative sensory testing) can elucidate dose–response relationships and neurophysiological pathways, supporting optimized, individualized use of MT in peripheral nerve rehabilitation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JX: Formal analysis, Methodology, Writing – original draft. DL: Data curation, Writing – original draft. LJ: Data curation, Investigation, Writing – original draft. WL: Formal analysis, Software, Writing – original draft. SY: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors gratefully acknowledge the support provided by the Affiliated Huishan Hospital of Xinglin College, Nantong University, and Wuxi Huishan District People’s Hospital. Special thanks are extended to the Department of Science and Education for their contributions to the development of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. Use of AI Statement: ChatGPT (OpenAI, San Francisco, CA; accessed August 2025) was used solely to assist with English grammar and stylistic editing. The AI did not contribute to study design, data collection, statistical analysis, or interpretation, nor did it generate original scholarly content. All revisions were reviewed and approved by the authors, who take full responsibility for the manuscript’s content; no identifiable or sensitive information was provided to the tool.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1689568/full#supplementary-material
References
1. Campbell, WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. (2008) 119:1951–65. doi: 10.1016/j.clinph.2008.03.018
2. Robinson, LR. Traumatic injury to peripheral nerves. Muscle Nerve. (2004) 57:173–86. doi: 10.1002/mus.27706
3. Sullivan, R, Dailey, T, Duncan, K, Abel, N, and Borlongan, CV. Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int J Mol Sci. (2016) 17:2101. doi: 10.3390/ijms17122101
4. Taylor, CA, Braza, D, Rice, JB, and Dillingham, T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. (2008) 87:381–5. doi: 10.1097/PHM.0b013e31815e6370
5. Tapp, M, Wenzinger, E, Tarabishy, S, Ricci, J, and Herrera, FA. The epidemiology of upper extremity nerve injuries and associated cost in US emergency departments. Ann Plast Surg. (2019) 83:676–80. doi: 10.1097/SAP.0000000000002083
6. Saadat, S, Eslami, V, and Rahimi-Movaghar, V. The incidence of peripheral nerve injury in trauma patients in Iran. Ulus Travma Acil Cerrahi Derg. (2011) 17:539–44. doi: 10.5505/tjtes.2011.75735
7. Kim, SJ, Kwon, YM, Ahn, SM, Lee, JH, and Lee, CH. Epidemiology of upper extremity peripheral nerve injury in South Korea, 2008 to 2018. Medicine (Baltimore). (2022) 101:e31655. doi: 10.1097/MD.0000000000031655
8. Bookman, J, and Hacquebord, J. Peripheral nerve injuries in the upper extremity. Bull Hosp Jt Dis. (2021) 79:11–6. doi: 10.1007/s00590-023-03751-3
10. McGillivray, MK, Haldane, C, Doherty, C, and Berger, MJ. Evaluation of muscle strength following peripheral nerve surgery: a scoping review. PM R. (2022) 14:383–94. doi: 10.1002/pmrj.12586
11. Duraku, LS, Hundepool, CA, and Moore, AM. Sensory nerve transfers in the upper limb after peripheral nerve injury: a scoping review. J Hand Surg Eur Vol. (2024) 49:946–55. doi: 10.1177/17531934231205546
12. Thieme, H, Mehrholz, J, Pohl, M, Behrens, J, and Dohle, CCochrane Stroke Group. Mirror therapy for improving motor function after stroke. Cochrane Database Syst Rev. (2012) 3:CD008449. doi: 10.1002/14651858.CD008449.pub2
13. Xie, HM, Zhang, KX, Wang, S, Wang, N, Wang, N, Li, X, et al. Effectiveness of mirror therapy for phantom limb pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2022) 103:988–97. doi: 10.1016/j.apmr.2021.07.810
14. Nogueira, NGHM, Parma, JO, Leão, SESA, Sales, IS, Macedo, LC, Galvão, ACDR, et al. Mirror therapy in upper limb motor recovery and activities of daily living, and its neural correlates in stroke individuals: a systematic review and meta-analysis. Brain Res Bull. (2021) 177:217–38. doi: 10.1016/j.brainresbull.2021.10.003
15. Yavuzer, G, Selles, R, Sezer, N, Sütbeyaz, S, Bussmann, JB, Köseoğlu, F, et al. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2008) 89:393–8. doi: 10.1016/j.apmr.2007.08.162
16. Cook, R, Bird, G, Catmur, C, Press, C, and Heyes, C. Mirror neurons: from origin to function. Behav Brain Sci. (2014) 37:177–92. doi: 10.1017/S0140525X13000903
17. Dohle, C, Püllen, J, Nakaten, A, Küst, J, Rietz, C, and Karbe, H. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair. (2009) 23:209–17. doi: 10.1177/1545968308324786
18. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gotzsche, PC, Ioannidis, JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Cashin, AG, and McAuley, JH. Clinimetrics: physiotherapy evidence database (PEDro) scale. Aust J Phys. (2020) 66:59. doi: 10.1016/j.jphys.2019.08.005
20. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JPT, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
21. Hsu, HY, Chen, PT, Kuan, TS, Yang, HC, Shieh, SJ, and Kuo, LC. A touch-observation and task-based mirror therapy protocol to improve sensorimotor control and functional capability of hands for patients with peripheral nerve injury. Am J Occup Ther. (2019) 73:7302205020p1–7302205020p10. doi: 10.5014/ajot.2018.027763
22. Chen, YH, Siow, TY, Wang, JY, Lin, SY, and Chao, YH. Greater cortical activation and motor recovery following mirror therapy immediately after peripheral nerve repair of the forearm. Neuroscience. (2022) 481:123–33. doi: 10.1016/j.neuroscience.2021.11.048
23. Kablanoğlu, S, Sade, I, Çekmece, Ç, Özdin, G, Buluç, L, and Dursun, N. The efficiency of mirror therapy in peripheral nerve injuries. Turk J Phys Med Rehabil. (2024) 70:81–9. doi: 10.5606/tftrd.2024.12648
24. Paula, MH, Barbosa, RI, Marcolino, AM, Elui, VMC, Rosén, B, and Fonseca, MCR. Early sensory re-education of the hand after peripheral nerve repair based on mirror therapy: a randomized controlled trial. Braz J Phys Ther. (2016) 20:58–65. doi: 10.1590/bjpt-rbf.2014.0130
25. Saberi, F, Lajevardi, L, Azad, A, Mirzaie, L, Taghizadeh, G, and Abdolrazaghi, HA. Can mirror visual feedback improve sensory relearning outcomes following median/ulnar nerve repair? Int J Ther Rehabil. (2018) 25:552–9. doi: 10.12968/ijtr.2018.25.10.552
26. Vikström, P, Rosén, B, Carlsson, IK, and Björkman, A. The effect of early relearning on sensory recovery 4 to 9 years after nerve repair: a report of a randomized controlled study. J Hand Surg Eur Vol. (2018) 43:626–30. doi: 10.1177/1753193418767024
27. Rosén, B, Vikström, P, Turner, S, McGrouther, DA, Selles, RW, Schreuders, TA, et al. Enhanced early sensory outcome after nerve repair as a result of immediate post-operative re-learning: a randomized controlled trial. J Hand Surg Eur Vol. (2015) 40:598–606. doi: 10.1177/1753193414553163
28. Hogan, MK, Hamilton, GF, and Horner, PJ. Neural stimulation and molecular mechanisms of plasticity and regeneration: a review. Front Cell Neurosci. (2020) 14:271. doi: 10.3389/fncel.2020.00271
Keywords: mirror therapy, traumatic peripheral nerve injury, sensory function, hand function, rehabilitation
Citation: Xu J, Lin D, Jian L, Liao W and Yang S (2025) Mirror therapy for postoperative functional recovery after surgical repair of upper-limb traumatic peripheral nerve injuries: a systematic review and meta-analysis. Front. Neurol. 16:1689568. doi: 10.3389/fneur.2025.1689568
Edited by:
Mamede De Carvalho, University of Lisbon, PortugalReviewed by:
Susana Pinto, Universidade de Lisboa, PortugalSerkan Kablanoğlu, Kocaeli Üniversitesi, Türkiye
Copyright © 2025 Xu, Lin, Jian, Liao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuming Yang, MTM2NjUxNzkyNTJAMTYzLmNvbQ==
 Jincheng Xu
Jincheng Xu Dandan Lin1
Dandan Lin1 Shuming Yang
Shuming Yang