Abstract
Objective:
This study aimed to compare the efficacy and safety of high-intensity interval training (HIIT) versus conventional rehabilitation for improving lower limb function in post-stroke patients.
Methods:
A comprehensive literature search was conducted in PubMed, EMBASE, Web of Science, and Scopus from inception to January 2025. Only randomized controlled trials (RCTs) involving adults in post-stroke rehabilitation published in English were included, while grey literature was excluded. Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were calculated. The primary outcomes were 6-min walk test (6MWT), Self-Selected Speed (SSS) and the Fastest Speed (FS). The secondary outcomes were peak oxygen uptake (Peak VO2) and SF-36 scores. The experimental group received high-intensity interval training (which involved robotic-assisted, cycling-based, or treadmill protocols targeting ≥60% of Peak VO₂), and the control group received standard care or regular exercise.
Results:
This meta-analysis included 10 studies. The results showed that high-intensity interval training has demonstrated significant improvements in walking ability and cardiopulmonary function compared with controls. High-intensity interval training had positive effects on 6MWT [SMD = 0.25, 95% CI (−0.01, 0.52)], SSS [SMD = 0.65, 95% CI (0.26, 1.03)], FS [SMD = 0.49, 95% CI (0.10, 0.88)], SF-36 scores [SMD = 0.67, 95% CI (0.04, 1.21)] and Peak VO₂ [SMD = 0.29, 95% CI (0.04, 0.54)] in stroke patients. According to the analysis, HIIT participants demonstrated better rehabilitation outcomes in walking capacity, cardiorespiratory function and quality of life.
Conclusion:
HIIT may be a safe and effective therapy for specific post-stroke patients, but more high-quality research is needed to confirm its efficacy and optimize protocols.
Systematic review registration:
This systematic review was registered in PROSPERO (Unique Identifier: CRD42025637166). The protocol can be accessed at: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025637166.
Introduction
Post-stroke motor dysfunction (PSD) refers to persistent motor impairment, which is caused by damage to the neural structures responsible for motor control (1). To delineate our study population, we focused on patients with moderate functional impairment, defined as a modified Rankin Scale (mRS) score of 3–4. Globally, approximately 50%–75% of stroke survivors experience varying degrees of motor dysfunction, such as hemiplegia or balance impairment (2). Among the various impairments following stroke, lower limb dyskinesia is one of the primary factors hindering recovery. According to the Copenhagen Stroke Study (3), 22% of stroke survivors are unable to walk, 14% require assistance to walk, and fewer than 10% regain adequate walking speed and endurance. These impairments impose significant caregiving, financial, and psychological burdens on the families of stroke survivors. In the United Kingdom, the societal cost of post-stroke rehabilitation is projected to reach £75 billion in 2035 (UK) (4). Thus, restoring independent walking ability is essential for promoting functional recovery and reducing caregiver burden.
Previous studies have demonstrated that rehabilitation within the first 3 months after stroke plays a critical role in long-term motor function recovery (5). However, conventional stroke rehabilitation programs often provide suboptimal duration or intensity (6). Moreover, most conventional rehabilitation therapies rely on specialized equipment, are constrained by environmental factors, and are costly, placing a heavy burden on patients’ families (7). In response, the 2023 AHA/ASA stroke rehabilitation guidelines strongly endorse high-intensity, task-specific training, high-intensity interval training (HIIT) has emerged as a potential alternative (8).
The efficacy of high-intensity interval training (HIIT) in stroke recovery is supported by its multi-level physiological effects. Neurologically, HIIT promotes neuroplasticity and cortical reorganization by upregulating BDNF/TrkB signaling and modulating inhibitory circuits (9, 10). Systemically, it enhances cardiorespiratory fitness and cerebral blood flow, thereby improving the metabolic support for brain repair and collectively driving improvements in motor function.
Recent studies (11–13) have shown that high-intensity interval training (HIIT) is an effective intervention for patients in the 3–6 month recovery phase after stroke. HIIT is characterized by alternating bouts of higher-intensity exercise (≥60%–80% peak VO₂) and lower-intensity exercise (40%–50% for recovery). Compared to conventional rehabilitation, it can increase muscle strength, reduce spasticity, enhance endurance, and improve cardiopulmonary function, thereby supporting patients in daily life after recovery. Furthermore, the alternating structure of HIIT makes it less monotonous and more engaging than continuous exercise, reducing dropout rates by up to 30% and improving overall adherence (14).
However, some studies have suggested that high-intensity interval training (HIIT) may cause a rapid increase in heart rate, potentially leading to discomfort or safety concerns (15–19). Three of the original studies reported negative or non-beneficial outcomes in certain functional indicators, which may confound clinical decision-making. Therefore, this systematic review and meta-analysis aimed to comprehensively evaluate the safety and efficacy of HIIT in post-stroke rehabilitation to inform clinical practice.
Methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20) and was prospectively registered on the PROSPERO platform on January 22, 2025 (CRD42025637166). Since this study analyzes previously published data, ethical review is not required.
Search strategy
We systematically searched the following electronic databases from inception to January 2025: PubMed, EMBASE, Web of Science, Scopus, and Ovid/Medline. Our search employed MeSH and free-text terms, using the Boolean operator OR to encompass synonyms for “Stroke,” “High-Intensity Interval Training,” and “Randomized Controlled Trial,” and the operator AND to ensure results combined all three core concepts. The complete search strategy is available in the Supplementary Material S3.
Inclusion exclusion criteria
After completing the search, studies were screened based on the following inclusion and exclusion criteria: (1) Study design: only randomized controlled trials (RCTs) were included, (2) Participants: adults (≥18 years old) in the rehabilitation phase of acute, subacute, or chronic stroke, (3) The HIIT intervention had to report intensity, duration, and frequency, and could include devices such as treadmills or cycle ergometers, (4) The standard for low-to-moderate intensity continuous training is a peak oxygen uptake of <50%, or the provision of routine care or no intervention, (5) Outcomes: studies must report at least one of the following outcome measures—6-min walk test (6MWT), self-selected walking speed, fastest walking speed, peak VO₂, or SF-36 scores, studies with incomplete or unclear outcome data were excluded.
Exclusion criteria were defined as follows: (1) Studies not published in English, (2) Non-English studies, conference abstracts, case reports, and studies with missing or incomplete data were excluded.
Risk of bias
Two blinded independent researchers (XQ and YD) used the Cochrane Risk of Bias Tool [ROB 2.0 (Cochrane Collaboration, 2019, Odense, DK)] to evaluate the quality of included studies from seven dimensions: (1) Bias due to the randomization process, (2) Bias due to the allocation process, (3) Bias due to blinding deficiencies, (4) Bias due to the assessment of outcomes, (5) Bias due to follow-up, (6) Bias due to reporting of results, (7) Other biases. Any disagreements were resolved through consultation with a third reviewer.
Quality of evidence
Evidence quality was assessed using the GRADE framework, categorizing certainty as high, moderate, low, or very low based on risk of bias, inconsistency, indirectness, imprecision, and publication bias. All assessments were conducted using GRADEpro GDT (McMaster University, Hamilton, Canada).
Data extraction
Data extraction was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. A standardized Excel-based data extraction form was developed based on the Handbook. Prior to formal data extraction, the form was piloted on two randomly selected included studies to ensure clarity and consistency between reviewers. The first reviewer (XQ) extracted data from all eligible studies using the refined form, and the second reviewer (YD) independently verified the accuracy of the extracted data. Any discrepancies were resolved through discussion and consensus.
Data included study characteristics (author, year, sample size, mean age, time since stroke), intervention details (intensity, duration), and outcomes (6MWT, SSS, FS, peak VO₂, SF-36). If a study reported both pre- and post-intervention means and standard deviations (SDs), the mean difference and corresponding SD were calculated using the formulas recommended in the Cochrane Handbook for Systematic Reviews of Interventions. Specifically, when SDs were not directly available, they were imputed from 95% confidence intervals using the prescribed method.
Statistical analysis
All statistical analyses were meta-analyzed using STATA 18.0 and RevMan 5.3.4. For continuous variables, standardized mean difference (SMD) and 95% confidence interval (CI) were used for analysis. Heterogeneity was quantified using I2, categorized as low (<25%), moderate (25%–75%), or high (>75%). A fixed-effects model was applied when heterogeneity was low (I2 ≤ 50%); otherwise, a random-effects model was used to account for potential clinical variability among the trials. To evaluate the robustness of the results, sensitivity analyses were conducted. Publication bias was assessed using funnel plots and Egger’s test, with statistical significance set at p < 0.10 for the latter.
Results
Study screening, selection, and evaluation
As of January 2025, a preliminary total of 1,627 documents were identified through searches of five databases. After removing 674 duplicates, the remaining records were screened by title, abstract, and full text according to the inclusion and exclusion criteria. Additionally, we employed a backward citation tracing approach, screening the reference lists of included studies to identify two eligible studies. Ultimately, 10 studies were included in the quantitative synthesis. Figure 1 shows the PRISMA flow diagram of study selection.
Figure 1

Flow diagram of the selection process.
Studies included in the systematic review
A total of 10 studies included 370 participants with the weighted mean age across all included studies was 61.1 ± 11.8 years. The mean time since stroke onset ranged from 17 to 88 months. Among them, 186 participants received high-intensity interval training (HIIT), while 184 received moderate-intensity continuous training (MICT) or usual care.
Interventions included treadmill-based (2, 21–25), robot-assisted (22), and cycling protocols (26) targeting 60%–85% of HRR or VO₂peak. The included studies reported exercise intensities ranging from 60% to 85% of HRR or VO₂peak, with total session durations ranging from 25 to 50 min (most commonly 30–40 min). Training frequency is 2–5 sessions per week, lasting 4–24 weeks (Table 1).
Table 1
| Study | Sample size (exp/con) (exp/con) | Mean age (exp/con) | DISEASE duration (exp/con) | Intervention/control | Exercise intensity | Course of treatment (exp/con) | Outcomes | Follow-up duration |
|---|---|---|---|---|---|---|---|---|
| Lapointe et al. (14) | 15/12 | 71.8 ± 9.9/69.6 ± 10.7 | 39.3 ± 61.0 mo | HIIT/routine care | 95% of PPO | 24 weeks | SBP/HAD/ Peak VO2/PPO | 48 weeks |
| Hsu et al. (26) | 10/13 | 58.5 ± 12.16/53.1 ± 9.65 | 38.5 ± 27.12/28.8 ± 42.01 mo | HIIT/MICT | 80% of peak VO₂ | 12 weeks | Peak VO2/AV O2diff/Δ [HHb]/BDNF | no mentioned |
| Hornby et al. (25) | 17/11 | 57 ± 9.72/66 ± 11.9 | 42 ± 45.71/20 ± 19.72 mo | HIIT(AIT+HIT)/HIT+normoxia | 75% of HRR | 5 weeks | Peak VO2/SSS/FS/6MWT | no mentioned |
| Yu et al. (24) | 14/16 | 61.86 ± 11.33/46.94 ± 15.61 | 511.07 ± 292.91/475.31 ± 411.42 days | HIIT(exowalk60min)/exowalk30min | No mentioned | 2 weeks/4 weeks | FAC/6MWT | no mentioned |
| Lee et al. (27) | 12/12/12/12 | 60.5 ± 10.6/65.3 ± 6 | 57.0 ± 54.2 mo | HIIT(Cycling)/sham cycling+sham PRT | 70% of peak VO₂ | 12 weeks | Peak VO2/PPO/6MWT | no mentioned |
| Hornby et al. (25) | 12/17/15 | 52 ± 13/57 ± 12 | 3.2 ± 1.8/3.7 ± 1.8 mo | HIIT/routine care | 70–80% of HRR | 10 weeks | SF-36/SSS | 8-12 weeks |
| Aidar et al. (29) | 11/11 | 51.7 ± 8/52.5 ± 7.7 | No mentioned | HIIT/routin | 70–80% of HRR | 12 weeks | SF-36 | no mentioned |
| Boyne et al. (21) | 27/28 | 63.8 ± 9.9/61.5 ± 9.9 | 2.7 ± 1.4/2.2 ± 1.2 year | HIIT/MAT | 60% of HRR | 12 weeks | VO2max/SSS/FS/6MWT | 12 weeks |
| Do et al. (22) | 11/11 | 61.8 ± 7.3/63.5 ± 8.1 | 88.0 ± 81.5/81.5 ± 52.4 mo | HIIT(RATW)/control | 70% of HRR | 8 weeks | VO2max/10MWT/FAC | 8 weeks |
| Moncion et al. (23) | 42/40 | 65.4 ± 8.9/64.4 ± 9.7 | 1.9 ± 1.3/1.7 ± 1.3 year | HIIT/MICT | 70–80% of HRR | 20 weeks | VO2max/6MWT/SBP | 8 weeks |
Characteristics of included studies.
VO2max, maximal oxygen consumption; Peak VO2, peak oxygen uptake; HRR, Heart Rate Reserve; SBP, systolic blood pressure; HAD, hospital anxiety and depression scale; PPO, peak power output; AVO2diff, arteriovenous O2 difference; BDNF, brain-derived neurotrophic factor; Δ[HHb], deoxyhemoglobin; SSS, self-selected speed; FS, fastest speed; 6MWT, 6-min walk test; SF-36, Medical Outcomes Short Form −36 questions; 10MWT, 10-meter walk test; FAC, functional ambulatory catego.
High intensity: Defined as meeting any of the following criteria: ≥70% heart rate reserve; ≥70% peak oxygen uptake; ≥95% peak power output (HIIT mode); Significantly higher single-session duration/dose compared to the control group while maintaining equivalent total training volume. Moderate/Low Intensity: Defined as: Approximately 30%–60% of heart rate reserve; Approximately 60% of peak oxygen uptake; Routine care/home exercise; Sham intervention (Table 2).
Table 2
| Study | HIIT intensity definition | Control intensity definition |
|---|---|---|
| Lapointe et al. (14) | 95% of PPO interspersed with a 60-s recovery | Usual care without any additional physical activity |
| Hsu et al. (26) | 80% of peak VO₂ low-intensity recovery periods at 40% VO2 peak | 60% of peak VO₂ |
| Hornby et al. (25) | 75% of HRR | Constant oxygen exposure |
| Yu et al. (24) | Exowalk/60 min | Exowalk/30 min |
| Lee et al. (27) | 70% of peak VO₂ | Sham cycling |
| Hornby et al. (25) | 70–80% of HRR | 30–40% of HRR |
| Aidar et al. (29) | Structured strength training | No strength training |
| Boyne et al. (21) | 60% of HRR | 40% ± 5% of HRR |
| Do et al. (22) | 70% of HRR | Routine care |
| Moncion et al. (23) | 80% of HRR | 40%–59% of HRR |
Summary of operational definitions for high-intensity and moderate/low-intensity training in the included studies.
Quality assessment and risk of bias
Risk of bias was assessed across seven Cochrane domains using RevMan 5.4. Overall, the included studies generally performed well in random sequence generation but exhibited a high risk of bias in allocation concealment and blinding (Figure 2; Table 3).
Figure 2
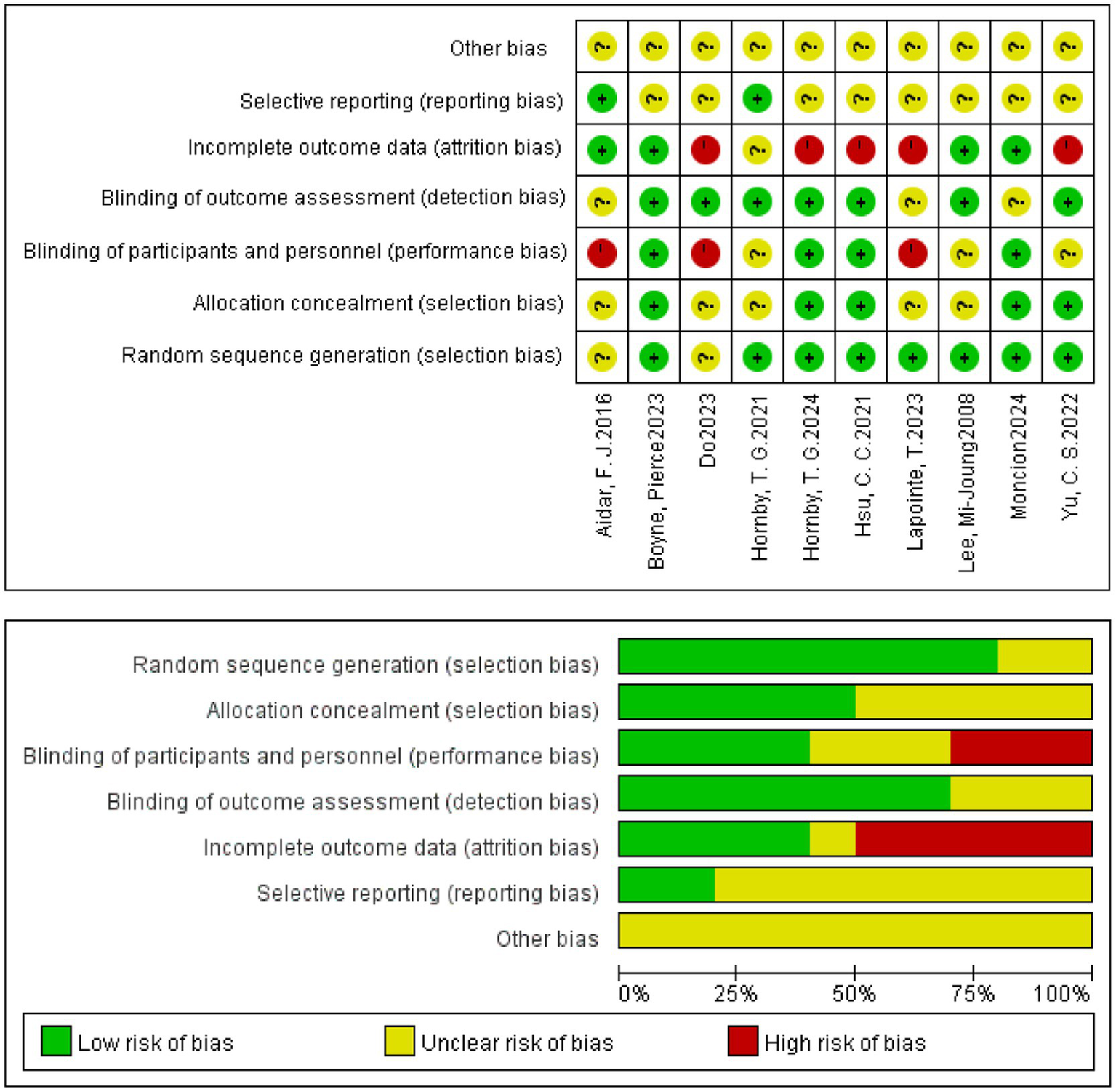
Risk of bias summary graph for included studies.
Table 3
| Study | Randomization process | Allocation process | Blinding deficiencies | Assessment of outcomes | Follow-up | Reporting of results | Other bias |
|---|---|---|---|---|---|---|---|
| Lapointe et al. (14) | Low | Low | High | Low | Low | Unclear | Unclear |
| Hsu et al. (26) | Low | Low | Low | Low | Low | Unclear | Unclear |
| Hornby et al. (25) | Low | Low | Low | Low | Low | Unclear | Unclear |
| Yu et al. (24) | Low | Low | Unclear | Low | Low | Unclear | Unclear |
| Lee et al. (27) | Low | Unclear | Unclear | Low | Low | Unclear | Unclear |
| Hornby et al. (25) | Low | Unclear | Unclear | Low | Low | Low | Unclear |
| Aidar et al. (29) | Unclear | Unclear | High | Unclear | Low | Low | Unclear |
| Boyne et al. (21) | Low | Low | Low | Low | Low | Unclear | Unclear |
| Do et al. (22) | Unclear | Unclear | High | Low | Low | Unclear | Unclear |
| Moncion et al. (23) | Low | Low | Low | Unclear | Low | Unclear | Unclear |
Quality assessment and risk of bias.
Meta-analysis of results
Primary outcomes
6MWT (6-min walk test)
Five studies (2, 21, 23, 24, 27), involving a total of 219 patients, reported data on the 6-min walk test (6MWT). A fixed-effects model (I2 = 5.1%, p = 0.378) indicated no significant difference between HIIT and MICT. The pooled results demonstrated a small, non-significant improvement in 6MWT distance favoring HIIT over MICT [SMD = 0.25, 95% CI (−0.01, 0.52), Figure 3]. Although this result did not reach statistical significance, the point estimate supports the possibility of a positive trend. This uncertainty may be attributable to the limited sample size. This finding aligns with Luo et al. (28), who reported a significant improvement in walking capacity, lending support to the potential benefit of HIIT (28). Consequently, further large-scale RCTs are warranted to confirm the effect of HIIT on 6MWT performance.
Figure 3
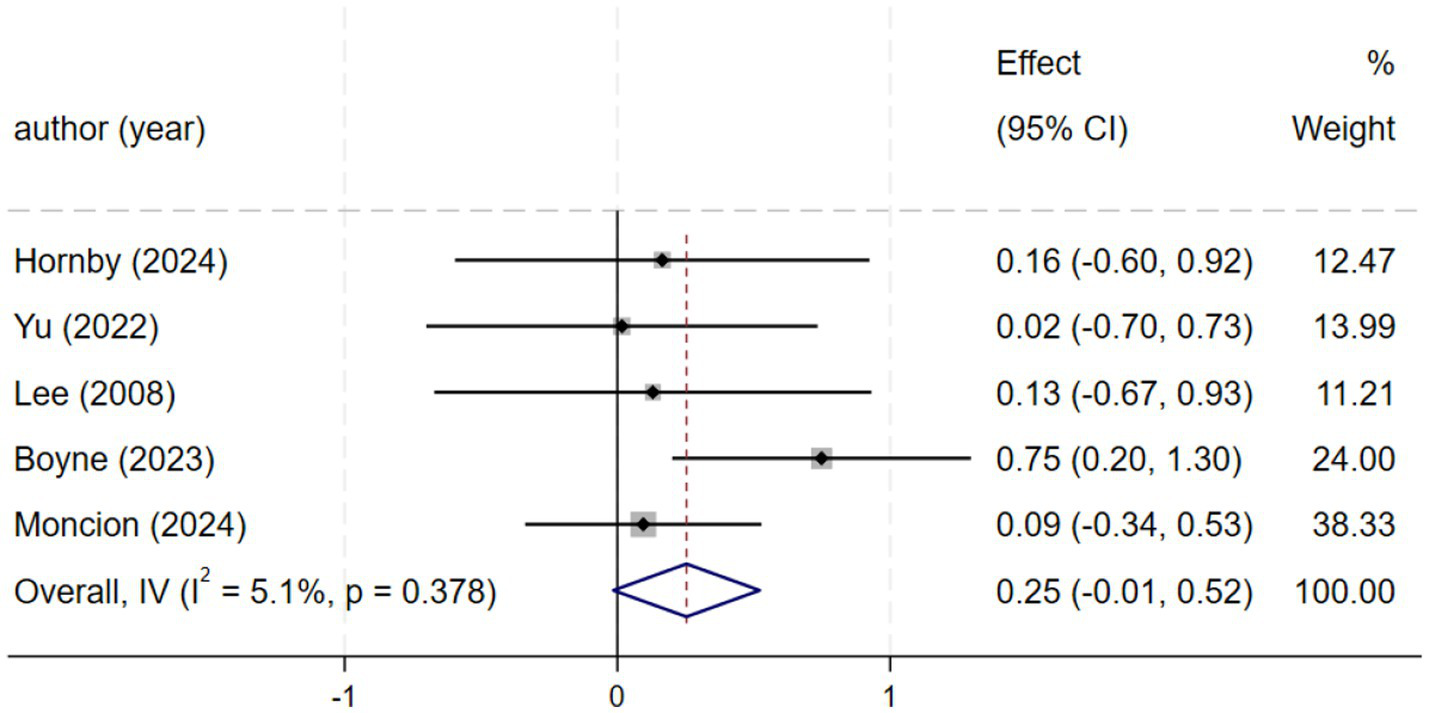
Forest plot of 6MWT.
SSS (speed of self-selection)
Three studies (2, 21, 25) reported self-selected walking speed. Homogeneity was observed among these studies (I2 = 0.0%, p = 0.442). Therefore, a fixed-effects model was chosen for the analysis of this outcome. Compared with the control group, HIIT demonstrated a positive effect in improving self-selected speed, with a moderate effect size [SMD = 0.65, 95% CI (0.26, 1.04), Figure 4] that suggests potential clinical relevance for functional mobility in stroke patients.
Figure 4
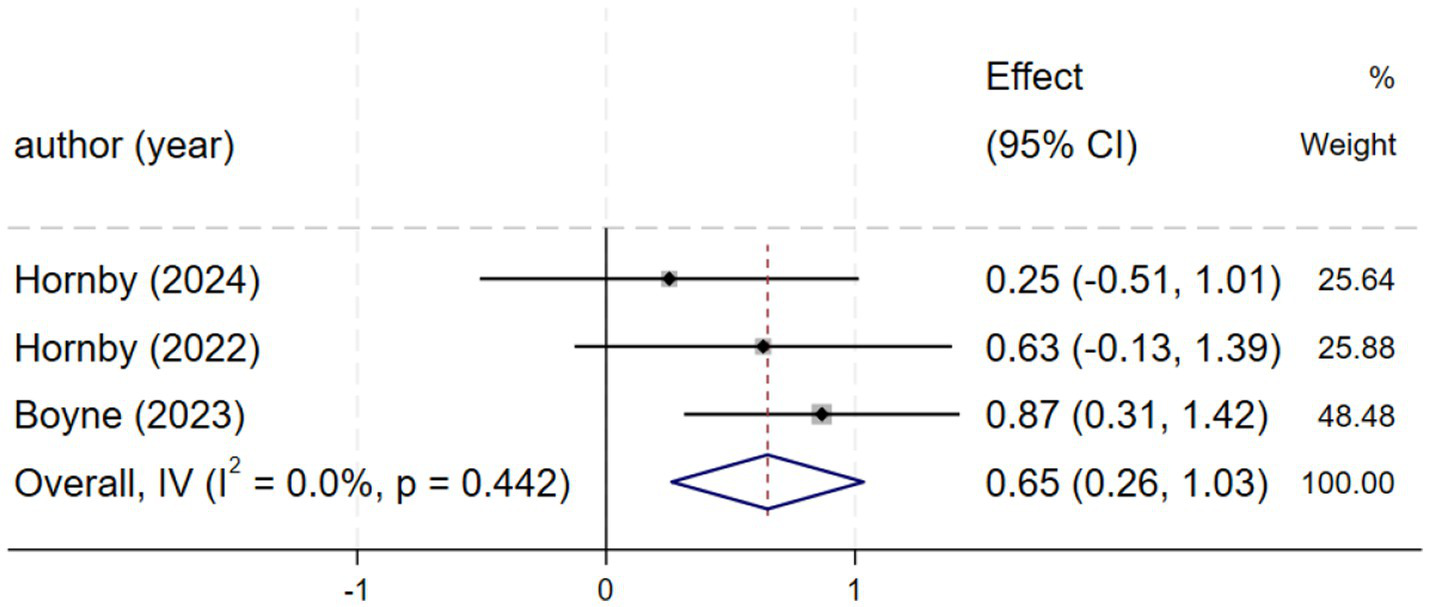
Forest plot of SSS.
FS (fastest speed)
Three studies (2, 21, 27) reporting fastest speed demonstrated low heterogeneity (I2 = 25.9%, p = 0.259), justifying a fixed-effects model. The meta-analysis revealed that HIIT significantly increased fastest speed compared to control conditions, with a standardized mean difference [SMD = 0.49, 95% CI (0.10, 0.88), Figure 5].
Figure 5
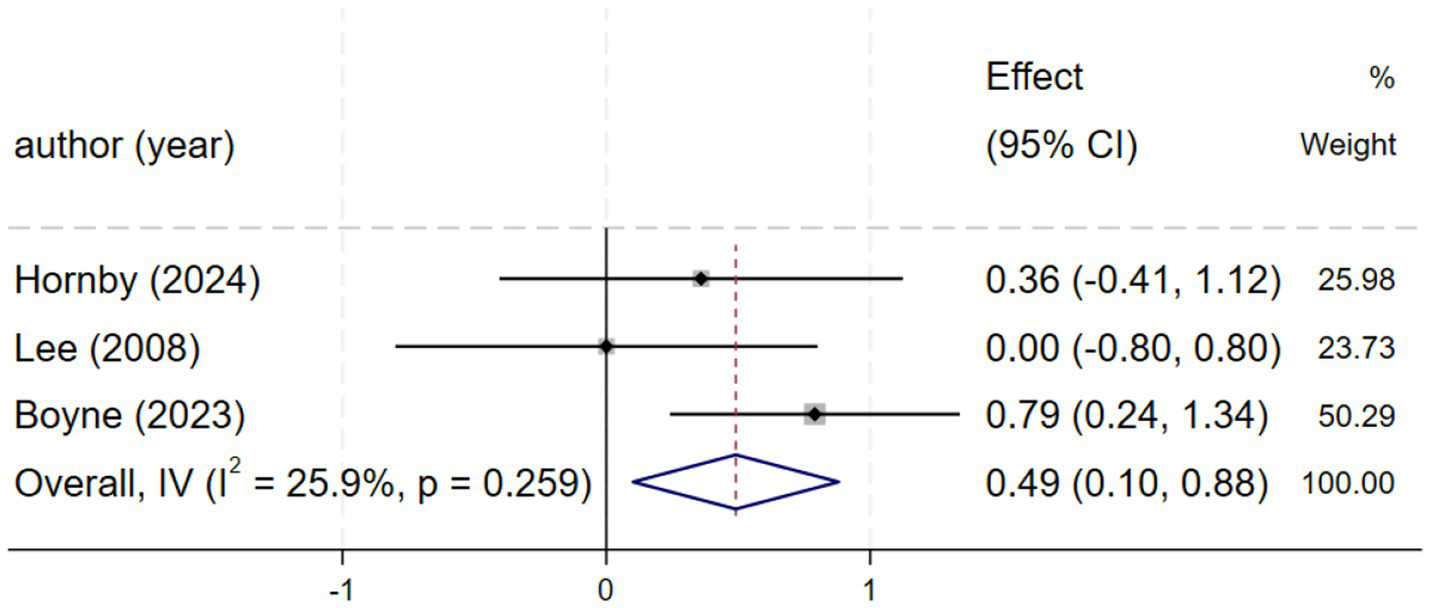
Forest plot of FS.
Secondary endings
Peak VO2 value
Seven studies (n = 261) demonstrated significant improvements in peak VO₂ [SMD = 0.29, 95% CI (0.04, 0.54); I2 = 0%, p = 0.630]. Although peak VO₂ was not a primary outcome, its improvement indicates enhanced cardiopulmonary capacity, supporting motor recovery (Figure 6).
Figure 6
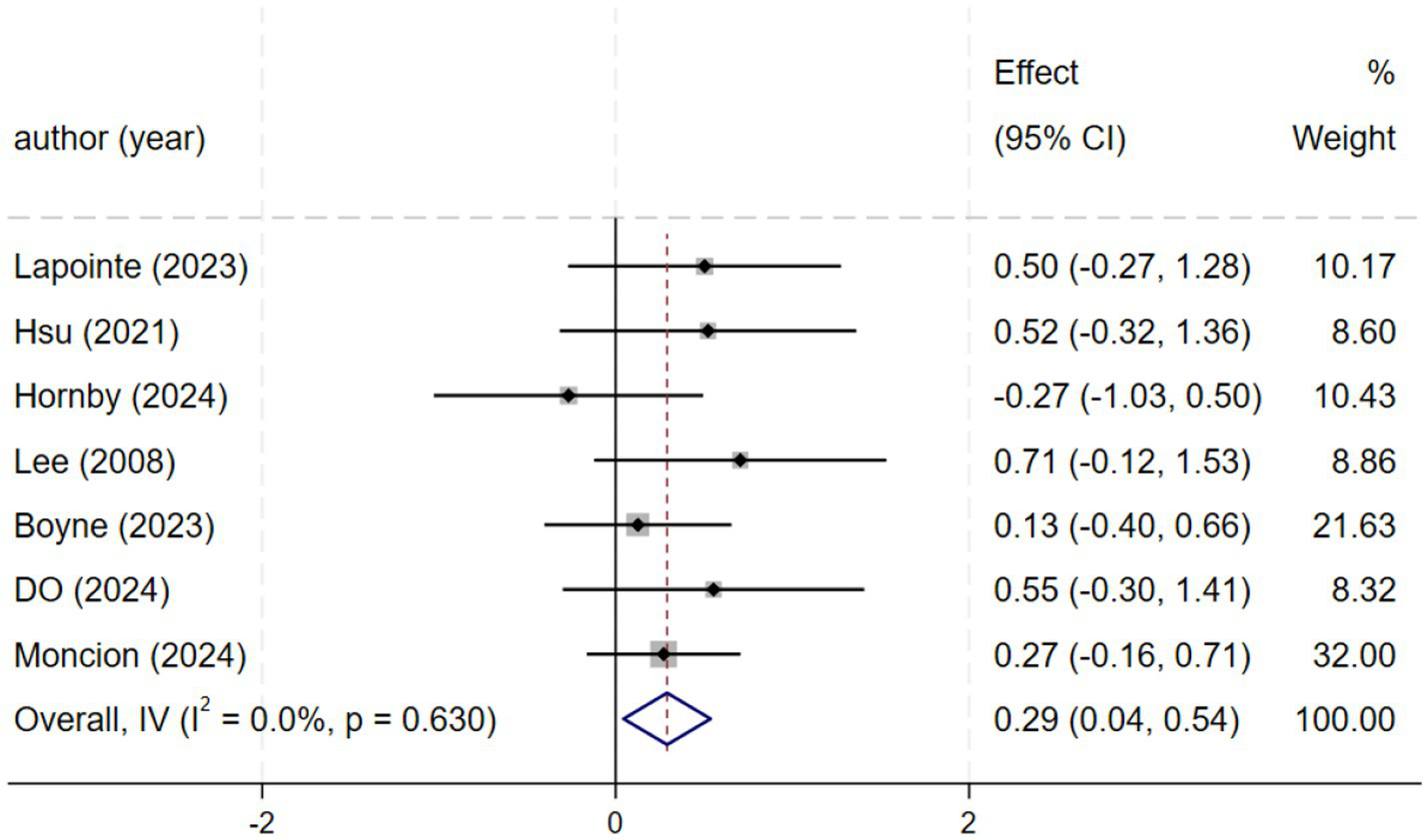
Forest plot of VO2.
SF-36
Three studies (25, 26, 29) reporting SF-36 scores demonstrated moderate heterogeneity (I2 = 39.0%, p = 0.194), and a fixed-effects model was consequently applied. The meta-analysis found a non-significant effect [SMD = 0.46, 95% CI (−0.02, 0.94), Figure 7]. Due to the wide confidence interval that spans the zero value, the effect estimate is subject to uncertainty. This finding is inconclusive regarding quality of life, and the observed effect may be a chance finding due to the limited statistical power of the included studies.
Figure 7
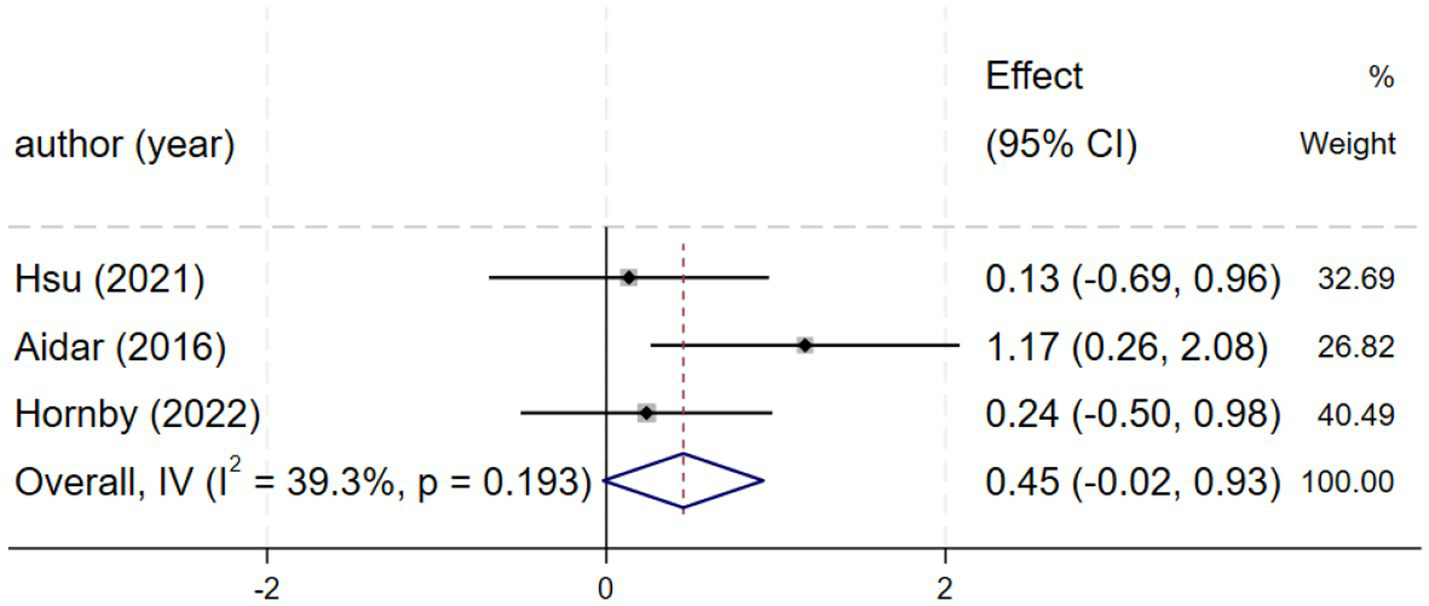
Forest plot of SF-36.
Publication bias and sensitivity analysis
Publication bias was assessed using funnel plots and Egger’s test. The results showed no significant publication bias for any outcome. However, the limited number of studies reduces the power of funnel plot asymmetry tests. (Figure 8): primary outcome 6MWT (p = 0.857, Figure 8b), SSS (p = 0.391, Figure 8c), FS (p = 0.185, Figure 8d), secondary outcomes VO2 (p = 0.420, Figure 8a), SF-36 (p = 0.360, Figure 8e).
Figure 8
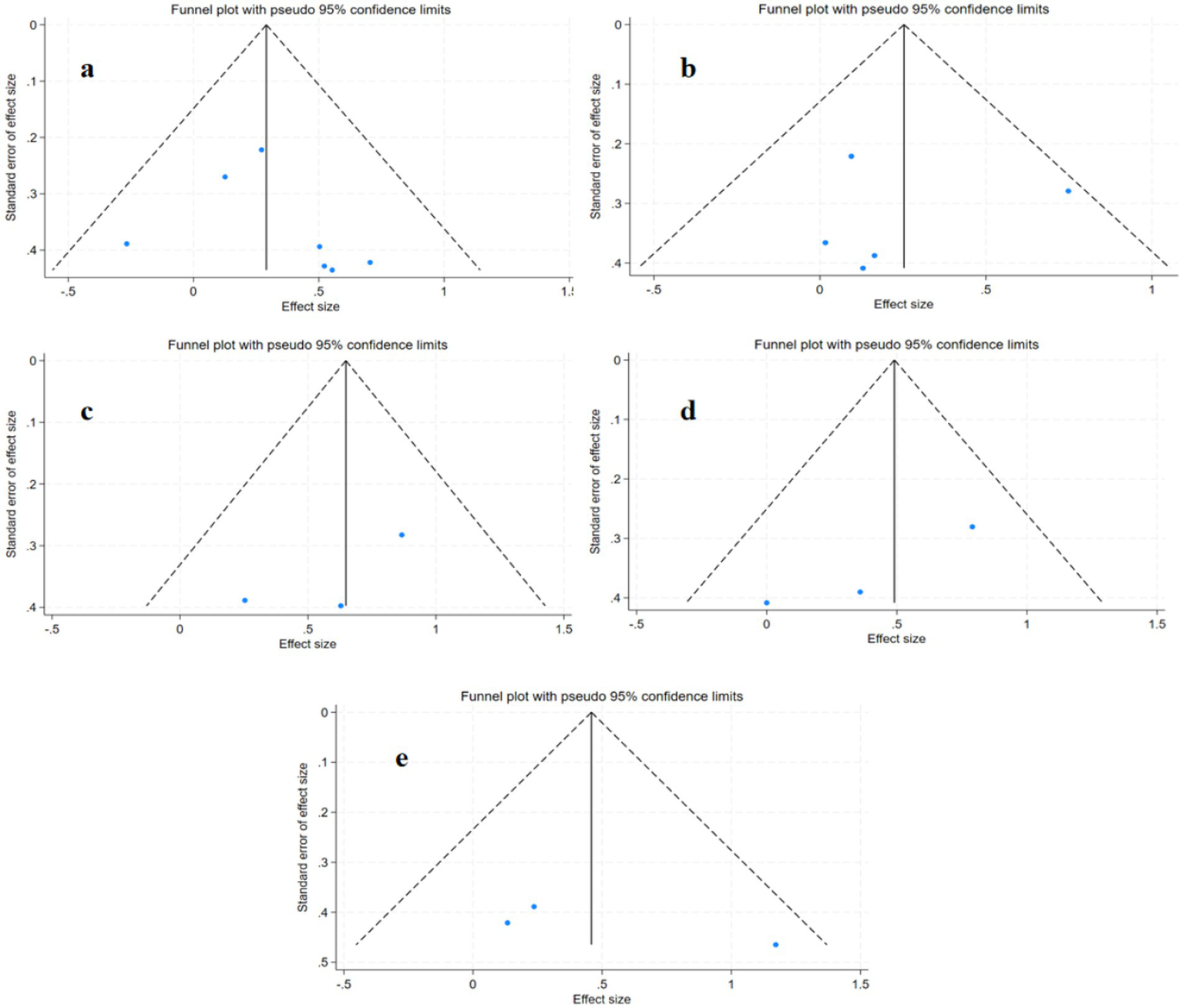
Publication bias plots for different outcome measures: (a) VO₂; (b) 6MWT; (c) SSS; (d) FS; (e) SF-36.
To assess the robustness of the results, we conducted a sensitivity analysis by sequentially excluding individual studies. After excluding the study by Boyne et al. (21), the combined effect size for the 6-min walk test and functional status showed a significant decline (Figure 9).
Figure 9
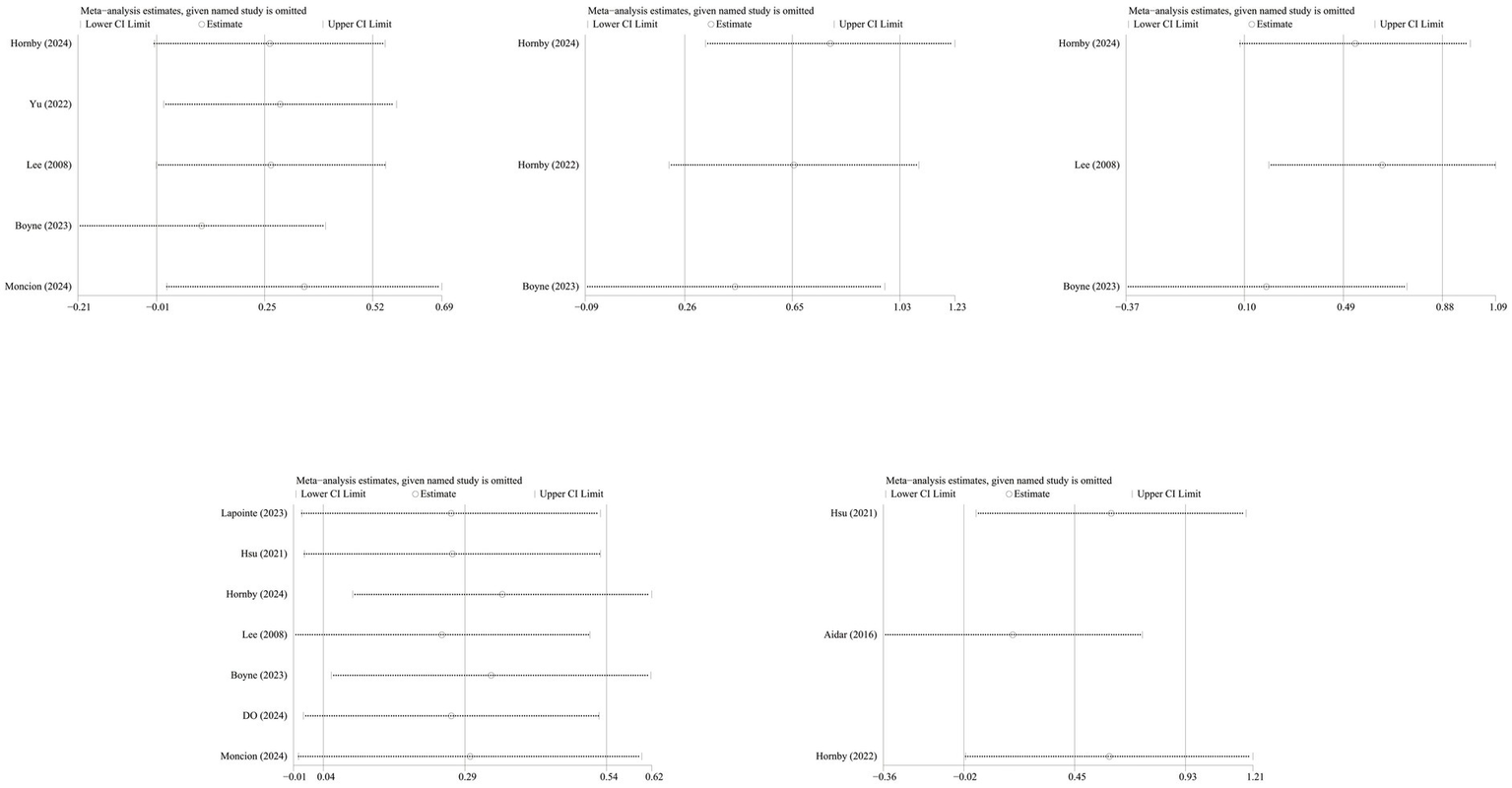
Sensitivity analysis.
Adverse events, dropout rate, and compliance
The safety and compliance data from the included studies are summarized in Table 4. Most studies did not report serious adverse events associated with HIIT interventions. The dropout rates ranged from 0% to 30% in the intervention groups and from 0% to 25% in the control groups. Common reasons for discontinuation included changes in participants’ health status and external factors such as the COVID-19 pandemic. Overall compliance rates were generally high in the intervention groups, ranging from 77% to 99%.
Table 4
| Study | Total Participants (n) | Adverse events in the intervention group | Adverse events in the control group | Overall shedding rate (exp/con) | Primary causes of shedding | Compliance (%) |
|---|---|---|---|---|---|---|
| Lapointe et al. (14) | 36 | No serious adverse events | No serious adverse events | 21%/29% | Lack of interest (n = 6) Change in medical condition (n = 3) |
HIIT group (77%) |
| Hsu et al. (26) | 28 | No serious adverse events | No serious adverse events | 23%/13.3% | Recurrent stroke (n = 2) Unstable BP (n = 1) Hernia surgery (n = 1) Incomplete ex. (n = 1) |
No mentioned |
| Hornby et al. (25) | 35 | One serious adverse event was observed following AIH exposure | No serious adverse events | 15%/26.7% | Personal reasons (n = 5) Taking banned substances (n = 1) Dizziness reaction (n = 1) Personal reasons |
No mentioned |
| Yu et al. (24) | 36 | No serious adverse events | No serious adverse events | 22.2%/11.1% | FAC was level 1 (n = 2) FAC was level 6 (n = 4) |
No mentioned |
| Lee et al. (27) | 25 | No serious adverse events | No serious adverse events | 7.6%/0% | Changes in their health status (n = 1) | No mentioned |
| Hornby et al. (25) | 29 | No serious adverse events | No serious adverse events | 0%/0% | No detachment has occurred | No mentioned |
| Aidar et al. (29) | 27 | Three patients dropped out due to personal circumstances. | Two patients dropped out due to personal circumstances. | 0%/15.3% | Personal reasons (n = 5) | HIIT group (94%) Control group (No mentioned) |
| Boyne et al. (21) | 55 | No serious adverse events | No serious adverse events | 30%/18% | Participants voluntarily withdrew (n = 7) Back pain (n = 1) Recurrent hamstring strain (n = 1) COVID-19 (n = 4) |
HIIT group (82.3%) Control group (86.8%) |
| Do et al. (22) | 24 | No serious adverse events | No serious adverse events | 8.3%/8.3% | Unknown reason (n = 2) | No mentioned |
| Moncion et al. (23) | 82 | No serious adverse events | No serious adverse events | 21.4%/32,5% | Medical conditions (n = 9) COVID-19 (n = 6) Transportation issues (n = 2) Return to work (n = 2) Other rehabilitation (n = 1) Preference against intervention plan (n = 1) Unknown reasons (n = 1) Missing (n = 12) |
HIIT group (99%) Control group (99%) |
Summary of safety and compliance for interventions.
GRADE analysis for the certainty of the evidence
Although the included randomized controlled trials (RCTs) are considered the highest level of evidence, the quality of the results still requires cautious interpretation. Certainty for VO₂, SSS, and FS outcomes was rated as moderate due to imprecision, while 6MWT and SF-36 outcomes were downgraded to low due to heterogeneity (Figure 10).
Figure 10
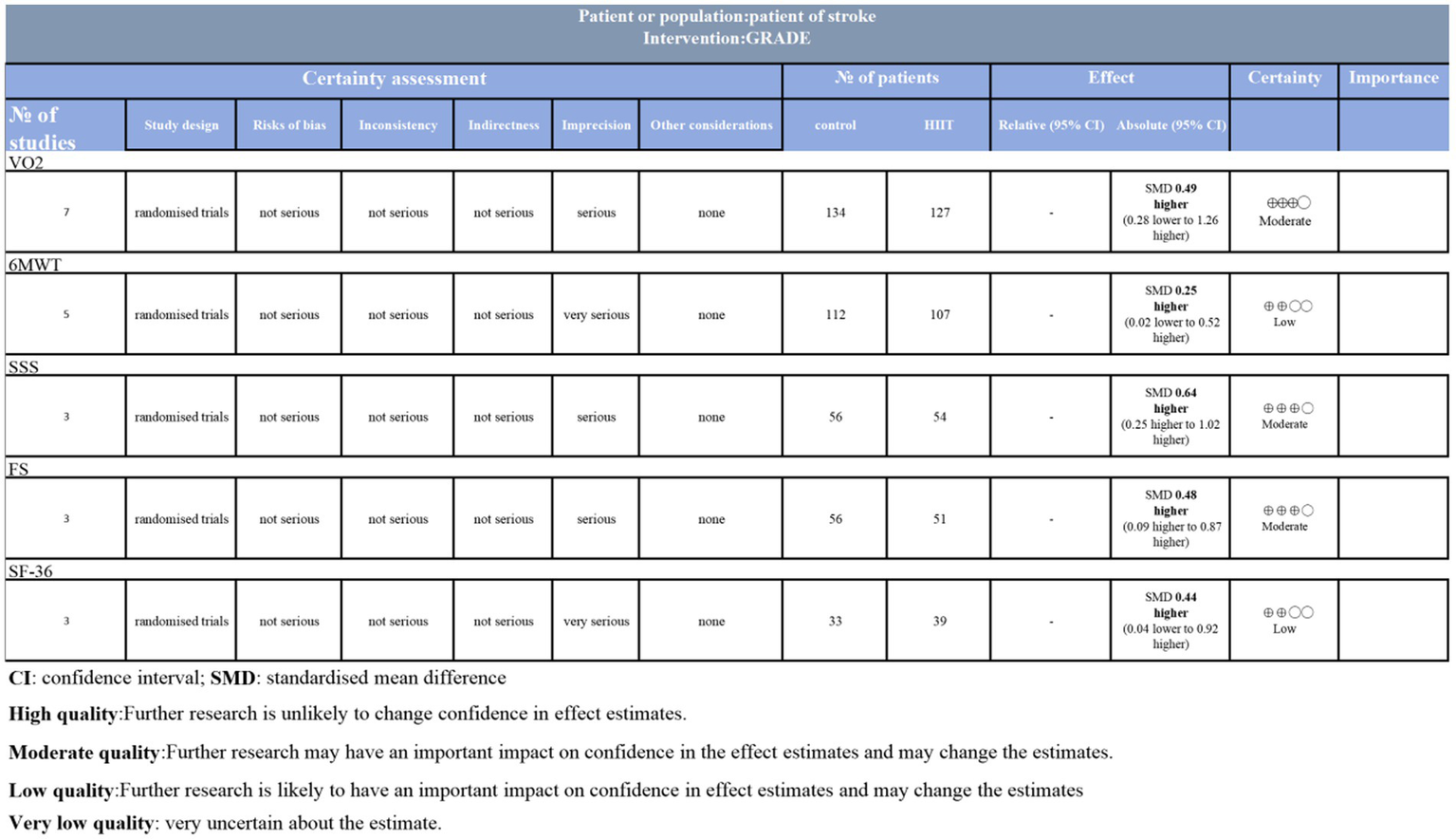
GRADE analysis and certainty of evidence.
Discussion
This meta-analysis of 10 RCTs supports the efficacy and safety of HIIT for improving functional recovery in post-stroke patients compared with conventional rehabilitation (30).
As a key indicator of walking ability, gait speed was assessed under two conditions (11, 30): self-selected speed, which reflects a sustainable pace chosen to avoid fatigue, and fastest speed, which challenges physiological limits to enhance cardiopulmonary function and muscle metabolism (31).
Our study demonstrates that the results possess significant clinical significance (p < 0.05). Furthermore, improvements in SSS [MD = 0.13 m/s, 95% CI (0.06, 0.20)] and FS [MD = 0.15 m/s, 95% CI (0.05, 0.26)] both exceeded the recognized minimum clinically important difference (MCID) threshold (0.10 m/s) (32). Compared to MICT, HIIT provided significantly greater and clinically relevant improvements in both self-selected and fastest gait speeds. This consistent advantage across different measures of walking ability reinforces the findings of prior studies (30). Collectively, the evidence suggests that HIIT should be considered an effective component of stroke rehabilitation programs.
Although the improvement of HIIT on the 6MWT did not reach statistical significance, the observed trend may hold clinical significance and warrants further investigation. Compared to moderate-intensity continuous training (MICT), high-intensity interval training (HIIT) demonstrated significant improvements in walking distance, indicating its potential clinical value. Combined with prior evidence suggesting HIIT is safer and more compliant (33, 34), it should be considered in rehabilitation practice and subjected to in-depth research.
VO₂peak remains an important outcome to consider. Although it does not directly reflect lower-limb functional recovery, walking endurance—an essential component of walking ability—largely depends on cardiopulmonary capacity (33, 35). Studies have demonstrated a positive correlation between 6MWT distance and VO₂peak, indicating that improvements in VO₂peak can translate into greater walking endurance, a finding consistent with previous research (11, 30, 36, 37).
Although the SF-36 score did not reach statistical significance, it still indicated a positive impact on patients’ quality of life. Recovery of limb function not only reduces patients’ own burden but also alleviates stress on their families, thereby improving overall quality of life (38, 39). These findings are consistent with those reported by Reed et al. (33).
Although the results indicate that the intervention group showed overall positive efficacy, it is still necessary to assess the robustness of these findings and explore the sources of heterogeneity.
Robustness of research findings and sources of heterogeneity
Our sensitivity analysis identified that the robustness of the combined results for the 6-min walk test and functional status is low. After in-depth comparison, we hypothesize that heterogeneity likely stems from two sources: first, Boyne et al.’s study included patients with poorer baseline average walking ability, setting a higher “ceiling” for physiological improvement and potentially leading to greater functional gains following intervention.
Notably, the core training protocol employed by Boyne et al. was high-intensity interval training (HIIT)—walking at maximum safe speed for 30 s followed by 30–60 s of rest. In contrast, Lee et al. (27) separated training components by establishing a sham training control group. While this approach effectively isolated variables, the neuromuscular and metabolic stimulation intensity it provided was significantly lower than the protocol designed for functional impairment (walking) in Boyne et al.’s study. The superiority of this protocol in functional relevance and overall stimulation intensity likely explains its outstanding clinical outcomes.
Therefore, the heterogeneity of existing evidence does not negate the efficacy of HIIT but rather reveals that its effectiveness depends on highly specific training protocols and patient populations with improvement potential. For its clinical application, in addition to efficacy, safety and acceptability are of paramount importance.
Clinical feasibility of HIIT: safety and compliance analysis
Based on existing research, HIIT demonstrates good safety for stroke patients. The vast majority of studies reported no intervention-related serious adverse events, indicating low risk when HIIT is implemented under normal conditions.
Regarding tolerability, while dropout rates varied across studies, no consistent pattern of higher rates in intervention versus control groups emerged. High dropout rates were predominantly linked to non-directly related factors such as changes in individual health status or COVID-19 impacts, rather than training intensity itself, suggesting overall acceptable tolerability.
Adherence data showed that compliance rates in HIIT groups were generally high (e.g., 77% to 99%), indicating patients’ ability to persist with high-intensity training regimens. However, some studies did not report this data, which should be improved in future research.
It is important to note that, based on the GRADE assessment and the low robustness in sensitivity analyses, the overall certainty of evidence regarding the efficacy of HIIT is low to moderate. Therefore, any clinical recommendations based on these findings should be treated with caution.
Limitations
This study has several limitations. First, the included trials varied in study design, participant characteristics, interventions, and outcome measures, which may have influenced pooled effect estimates. Some studies exhibit a high risk of bias in allocation concealment and blinding (14, 25, 27, 29), The absence of blinding may lead participants and researchers to exhibit a halo effect on subjective outcome measures (such as functional scores), thereby increasing their tendency to report positive results. At the same time, inadequate allocation concealment may lead to researcher bias during patient inclusion, thereby undermining the effectiveness of randomization.
Therefore, subsequent studies should prioritize allocation concealment and ensure at least evaluator blinding, while increasingly utilizing objective physiological indicators (such as muscle strength testing and grip strength testing) to report patient outcomes.
Second, the relatively small cumulative sample size (n = 370) may have limited the statistical power to detect small but meaningful effects. Future research should focus on recruiting larger sample sizes to enhance statistical power, ensuring the ability to detect the smallest clinically meaningful differences.
Third, this study included only articles published in English, which have excluding non-English studies may bias results toward positive findings. Therefore, future meta-analyses should include a comprehensive, multilingual literature search to verify whether the positive effects of HIIT hold across a more diverse evidence base.
Fourth, variations in the definition of “intensity” between intervention and control groups—such as the use of different physiological metrics (e.g., %HRR vs. % VO₂peak), as well as differences in rehabilitation standards across studies, may have affected the objectivity and reliability of the analysis. Future studies should adopt standardized operational definitions of exercise intensity (e.g., consistently using %HRR) and provide detailed descriptions of control interventions. This will enhance the comparability of results across trials and strengthen the evidence base.
Fifth, transformations were applied to estimate means and standard deviations from studies that reported only medians and interquartile ranges (IQRs), which may have introduced additional heterogeneity and measurement error. Finally, most included studies recruited patients with mild to moderate post-stroke impairments, without considering individuals with other cardiovascular conditions.
Future research should adopt larger-scale, multicenter randomized controlled trials with long-term follow-up, enrolling patient populations with varying degrees of impairment. These studies should also explore neurophysiological indicators during rehabilitation (e.g., BDNF, cerebral perfusion, electromyographic activity). Furthermore, conducting cost-effectiveness and feasibility studies will be crucial to facilitate the translation of HIIT technology into routine clinical practice.
Conclusion
In conclusion, current evidence suggests this therapy may represent a safe and effective treatment strategy for specific post-stroke patients, but further high-quality studies are needed to validate these findings and optimize training parameters.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XW: Data curation, Formal analysis, Methodology, Software, Writing – original draft. DY: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing, Writing – original draft. QZ: Methodology, Project administration, Resources, Visualization, Writing – original draft. YX: Conceptualization, Data curation, Formal analysis, Project administration, Writing – review & editing. HH: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Hubei Province (2023AFD170) and Hubei Provincial Administration of Traditional Chinese Medicine 2025-2026 Chinese Medicine Research Program (ZY2025Q037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1695243/full#supplementary-material
References
1.
Langhorne P Bernhardt J Kwakkel G . Stroke rehabilitation. Lancet. (2011) 377:1693–702. doi: 10.1016/S0140-6736(11)60325-5
2.
Hornby TG Plawecki A Lotter JK Shoger LH Voigtmann CJ Inks E et al . Acute intermittent hypoxia with high-intensity gait training in chronic stroke: a phase II randomized crossover trial. Stroke. (2024) 55:1748–57. doi: 10.1161/STROKEAHA.124.047261
3.
Jørgensen HS Nakayama H Raaschou HO Olsen TS . Recovery of walking function in stroke patients: the Copenhagen stroke study. Arch Phys Med Rehabil. (1995) 76:27–32. doi: 10.1016/s0003-9993(95)80038-7
4.
Holmes R Ackerley S Fisher RJ Connell LA . Exploring variation in the six-month review for stroke survivors: a national survey of current practice in England. BMC Health Serv Res. (2025) 25:159. doi: 10.1186/s12913-025-12323-6
5.
Tao Y-X Wu Y-H Zhu G-Q Wang M . Efficacy of acupuncture in the treatment of limb dyskinesia after stroke: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2023) 27:10985–93. doi: 10.26355/eurrev_202311_34467
6.
Billinger SA Arena R Bernhardt J Eng JJ Franklin BA Johnson CM et al . Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2532–53. doi: 10.1161/STR.0000000000000022
7.
Coleman ER Moudgal R Lang K Hyacinth HI Awosika OO Kissela BM et al . Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep. (2017) 19:59. doi: 10.1007/s11883-017-0686-6
8.
Winstein CJ Stein J Arena R Bates B Cherney LR Cramer SC et al . Guidelines for adult stroke rehabilitation and recovery. Stroke. (2016) 47:e98–e169. doi: 10.1161/STR.0000000000000098
9.
Hugues N Pellegrino C Rivera C Pin-Barre C Laurin J . Is high-intensity interval training suitable to promote neuroplasticity and cognitive functions after stroke?Int J Mol Sci. (2021) 22:3003. doi: 10.3390/ijms22063003
10.
Cai J Ji Q Xin R Zhang D Na X Peng R et al . Contralesional cortical structural reorganization contributes to motor recovery after sub-cortical stroke: a longitudinal voxel-based morphometry study. Front Hum Neurosci. (2016):10:393. doi: 10.3389/fnhum.2016.00393
11.
Helgerud J Hoydal K Wang E Karlsen T Berg P Bjerkaas M et al . Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. (2007) 39:665–71. doi: 10.1249/mss.0b013e3180304570
12.
Ross R Blair SN Arena R Church TS Despres J-P Franklin BA et al . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. (2016) 134:e653–99. doi: 10.1161/CIR.0000000000000461
13.
Gjellesvik TI Becker F Tjonna AE Indredavik B Lundgaard E Solbakken H et al . Effects of high-intensity interval training after stroke (the HIIT stroke study) on physical and cognitive function: a multicenter randomized controlled trial. Arch Phys Med Rehabil. (2021) 102:1683–91. doi: 10.1016/j.apmr.2021.05.008
14.
Lapointe T Houle J Sia Y-T Payette M Trudeau F . Addition of high-intensity interval training to a moderate intensity continuous training cardiovascular rehabilitation program after ischemic cerebrovascular disease: a randomized controlled trial. Front Neurol. (2023) 13:963950. doi: 10.3389/fneur.2022.963950
15.
Woodruffe S Neubeck L Clark RA Gray K Ferry C Finan J et al . Australian cardiovascular health and rehabilitation association (ACRA) core components of cardiovascular disease secondary prevention and cardiac rehabilitation 2014. Heart Lung Circ. (2015) 24:430–41. doi: 10.1016/j.hlc.2014.12.008
16.
Yue T Wang Y Liu H Kong Z Qi F . Effects of high-intensity interval vs. moderate-intensity continuous training on cardiac rehabilitation in patients with cardiovascular disease: a systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:845225. doi: 10.3389/fcvm.2022.845225
17.
Guiraud T Juneau M Nigam A Gayda M Meyer P Mekary S et al . Optimization of high intensity interval exercise in coronary heart disease. Eur J Appl Physiol. (2010) 108:733–40. doi: 10.1007/s00421-009-1287-z
18.
Juneau M Hayami D Gayda M Lacroix S Nigam A . Provocative issues in heart disease prevention. Can J Cardiol. (2014) 30:S401–9. doi: 10.1016/j.cjca.2014.09.014
19.
Guiraud T Nigam A Gremeaux V Meyer P Juneau M Bosquet L . High-intensity interval training in cardiac rehabilitation. Sports Med. (2012) 42:587–605. doi: 10.2165/11631910-000000000-00000
20.
Page MJ Moher D Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
21.
Boyne P Billinger SA Reisman DS Awosika OO Buckley S Burson J et al . Optimal intensity and duration of walking rehabilitation in patients with chronic stroke: a randomized clinical trial. JAMA Neurol. (2023) 80:342–51. doi: 10.1001/jamaneurol.2023.0033
22.
Do J Lim W-T Kim DY Ko EJ Ko M-H Kim GW et al . Effects of high-intensity interval robot-assisted gait training on cardiopulmonary function and walking ability in chronic stroke survivors: a multicenter single-blind randomized controlled trial. J Back Musculoskelet Rehabil. (2024) 37:1309–19. doi: 10.3233/BMR-230385
23.
Moncion K Rodrigues L De Las Heras B Noguchi KS Wiley E Eng JJ et al . Cardiorespiratory fitness benefits of high-intensity interval training after stroke: a randomized controlled trial. Stroke. (2024) 55:2202–11. doi: 10.1161/STROKEAHA.124.046564
24.
Yu CS Nam Y-G Kwon BS . Comparison of high-intensive and low-intensive electromechanical-assisted gait training by exowalk(R) in patients over 3-month post-stroke. BMC Sports Sci Med Rehabil. (2022) 14:126. doi: 10.1186/s13102-022-00515-0
25.
Hornby TG Rafferty MR Pinto D French D Jordan N . Cost-effectiveness of high-intensity training vs conventional therapy for individuals with subacute stroke. Arch Phys Med Rehabil. (2022) 103:S197–204. doi: 10.1016/j.apmr.2021.05.017
26.
Hsu C-C Fu T-C Huang S-C Chen CP-C Wang J-S . Increased serum brain-derived neurotrophic factor with high-intensity interval training in stroke patients: a randomized controlled trial. Ann Phys Rehabil Med. (2021) 64:101385. doi: 10.1016/j.rehab.2020.03.010
27.
Lee M-J Kilbreath SL Singh MF Zeman B Lord SR Raymond J et al . Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: a randomized sham exercise-controlled study. J Am Geriatr Soc. (2008) 56:976–85. doi: 10.1111/j.1532-5415.2008.01707.x
28.
Luo L Zhu S Shi L Wang P Li M Yuan S . High intensity exercise for walking competency in individuals with stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2019) 28:104414. doi: 10.1016/j.jstrokecerebrovasdis.2019.104414
29.
Aidar FJ de Oliveira RJ de Matos DG Mazini Filho ML Moreira OC de Oliveira CEP et al . A randomized trial investigating the influence of strength training on quality of life in ischemic stroke. Top Stroke Rehabil. (2016) 23:84–9. doi: 10.1080/10749357.2015.1110307
30.
Anjos JM Neto MG Dos Santos FS Almeida KDO Bocchi EA Lima Bitar YDS et al . The impact of high-intensity interval training on functioning and health-related quality of life in post-stroke patients: a systematic review with meta-analysis. Clin Rehabil. (2022) 36:726–39. doi: 10.1177/02692155221087082
31.
Miller A Reisman DS Billinger SA Dunning K Doren S Ward J et al . Moderate-intensity exercise versus high-intensity interval training to recover walking post-stroke: protocol for a randomized controlled trial. Trials. (2021) 22:457. doi: 10.1186/s13063-021-05419-x
32.
Perera S Mody SH Woodman RC . Meaningful change and responsiveness in common physical performance measures in older adults. J am Geriatr Soc. (2006) 54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x
33.
Reed JL Terada T Cotie LM Tulloch HE Leenen FH Mistura M et al . The effects of high-intensity interval training, Nordic walking and moderate-to-vigorous intensity continuous training on functional capacity, depression and quality of life in patients with coronary artery disease enrolled in cardiac rehabilitation: a randomized controlled trial (CRX study). Prog Cardiovasc Dis. (2022) 70:73–83. doi: 10.1016/j.pcad.2021.07.002
34.
Marzolini S Robertson AD MacIntosh BJ Corbett D Anderson ND Brooks D et al . Effect of high-intensity interval training and moderate-intensity continuous training in people with poststroke gait dysfunction: a randomized clinical trial. J Am Heart Assoc. (2023) 12:e031532. doi: 10.1161/JAHA.123.031532
35.
Flansbjer U-B Holmback AM Downham D Patten C Lexell J . Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. (2005) 37:75–82. doi: 10.1080/16501970410017215
36.
Tiozzo E Youbi M Dave K Perez-Pinzon M Rundek T Sacco RL et al . Aerobic, resistance, and cognitive exercise training poststroke. Stroke. (2015) 46:2012–6. doi: 10.1161/STROKEAHA.114.006649
37.
Michael K Macko RF . Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil. (2007) 14:5–12. doi: 10.1310/tsr1402-5
38.
Jiang L Ding H Ma Q Gao S Zhang X Chun B . Comparing the effectiveness of different exercise interventions on quality of life in stroke patients: a randomized controlled network meta-analysis. BMC Neurol. (2025) 25:24. doi: 10.1186/s12883-025-04035-5
39.
He X Ji J Pei Z Zhou T Fan H Guo L . Efficacy of pulmonary rehabilitation on health-related quality of life in patients with interstitial lung disease as assessed by SF-36: a systematic review and meta-analysis. Eur J Phys Rehabil Med. (2025) 61:313–34. doi: 10.23736/S1973-9087.25.08778-7
Summary
Keywords
post-stroke motor dysfunction, 6MWT, high-intensity interval training, rehabilitation, meta-analysis
Citation
Wu X, Yang D, Zhu Q, Xiao Y and Huang H (2025) The safety and efficacy of high-intensity interval training (HIIT) in post-stroke patients with moderate functional impairment: a systematic review and meta-analysis. Front. Neurol. 16:1695243. doi: 10.3389/fneur.2025.1695243
Received
11 September 2025
Revised
31 October 2025
Accepted
04 November 2025
Published
19 November 2025
Volume
16 - 2025
Edited by
António Miguel Monteiro, Instituto Politécnico de Bragança, Portugal
Reviewed by
Pedro Forte, Higher Institute of Educational Sciences of the Douro, Portugal
Luís Branquinho, Polytechnic Institute of Portalegre, Portugal
Updates
Copyright
© 2025 Wu, Yang, Zhu, Xiao and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Huang, huanghai@hbhtcm.com; Yao Xiao, xiaoyao@hbhtcm.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.