Abstract
Objective:
This study aimed to evaluate the efficacy and safety of flow diverters (FDs) in the treatment of small intracranial aneurysms (≤10 mm).
Methods:
PubMed, Embase, Web of Science, and Cochrane Library were comprehensively searched up to July 2025. Eligible studies included retrospective cohort studies reporting angiographic and clinical outcomes of FD treatment in small intracranial aneurysms. Data analysis was conducted using STATA 15.0. Pooled proportions and 95% confidence intervals (CIs) were calculated using Freeman–Tukey double arcsine transformation. Publication bias was evaluated using funnel plots and Egger’s test.
Results:
Ten retrospective studies comprising 2,275 patients with 1,938 aneurysms were included. The pooled complete or near-complete occlusion rate was 86% (95% CI: 80–92%). The treatment-related mortality rate was 1% (95% CI: 0–2%), and the hemorrhagic event rate was 1% (95% CI: 1–2%). The ischemic event rate was 2% (95% CI: 1–3%), and the stroke rate was 3% (95% CI: 1–5%). The overall complication rate was 9% (95% CI: 5–12%), while 98% (95% CI: 94–100%) of patients achieved favorable functional outcomes. Egger’s test showed no significant publication bias (p = 0.791).
Conclusion:
FDs appear to be both effective and safe for the treatment of small intracranial aneurysms, achieving high occlusion rates and favorable functional outcomes with low rates of mortality and complications. However, given the high heterogeneity and retrospective nature of the included studies, further large-scale prospective studies are warranted to confirm these findings and refine treatment strategies.
1 Introduction
Intracranial aneurysms are among the most common cerebrovascular disorders, with a prevalence of approximately 3.2% in the adult population (1). Rupture can lead to aneurysmal subarachnoid hemorrhage (aSAH), which carries a high case fatality rate, the 30-day in-hospital mortality is about 20%, and prehospital deaths further increase the overall burden (2). With advances in neuroimaging, an increasing number of SIAs (≤10 mm in diameter) have been detected, accounting for 70–80% of unruptured aneurysms (3). Although their rupture risk is generally lower than that of large or giant aneurysms, rupture of small intracranial aneurysms (SIAs) can result in devastating consequences. The Unruptured Cerebral Aneurysm Study of Japan (UCAS Japan) reported an annual rupture rate of 0.36% for aneurysms <7 mm, yet emphasized the poor prognosis once rupture occurs, underscoring the clinical challenges in managing SIAs (4).
Current treatment options include microsurgical clipping and endovascular coiling. Microsurgical clipping achieves high occlusion rates but requires craniotomy, with greater risks in anatomically complex cases (5). Endovascular coiling offers a minimally invasive alternative, particularly wide-necked or morphologically complex aneurysms remain prone to incomplete occlusion and recurrence (6, 7).
Flow diverters (FDs) represent an alternative therapeutic strategy by reconstructing parent vessel hemodynamics, promoting intra-aneurysmal thrombosis, and facilitating vessel wall remodeling, leading to progressive occlusion. Their efficacy and feasibility in large and giant aneurysms are well established (8, 9). More recently, evidence has accumulated in SIAs. The prospective multicenter PREMIER trial (≤12 mm, predominantly small-to-medium aneurysms) demonstrated high complete occlusion rates and low permanent neurological morbidity, findings later supported by other series (10–12). However, systematic evaluations of long-term outcomes and complication profiles in SIAs remain limited.
Therefore, this study conducted a systematic review and single-arm meta-analysis to comprehensively evaluate the efficacy and safety of FD treatment in SIAs, aiming to provide evidence-based insights for clinical practice and future research.
2 Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13) and was prospectively registered in the PROSPERO database (registration number: CRD420251136570).
2.1 Search strategy
PubMed, Web of Science, EMBASE, and the Cochrane Library were searched from inception to July 15, 2025. Search terms combined MeSH and free-text words: “Intracranial Aneurysm” OR “Small intracranial aneurysms” AND “Flow diverter” OR “Pipeline Embolization Device.” The full search strategy is provided in Supplementary Table S1.
2.2 Eligibility criteria
Inclusion criteria: (1) adult patients with SIAs (≤10 mm); (2) treatment with an FD (e.g., Pipeline Embolization Device (PED), Pipeline Flex, FRED X); (3) reporting at least one of the following outcomes: complete occlusion (Raymond–Roy Grade I), incomplete/partial occlusion, perioperative complications (ischemia, hemorrhage, thrombosis), mortality, recurrence, or functional outcome (modified Rankin Scale, mRS); (4) ≥ 20 patients with SIAs; and (5) prospective or retrospective observational studies.
Exclusion criteria: large or giant aneurysms (>10 mm); non-intracranial aneurysms; non-FD interventions; insufficient data; case reports, reviews, meta-analyses, editorials, conference abstracts; and duplicate or overlapping cohorts.
2.3 Study selection and quality assessment
All records were imported into EndNote 21. Duplicates were removed, and two reviewers independently screened titles/abstracts and full texts, with disagreements resolved by a third reviewer. Quality assessment was performed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Series (14).
2.4 Data extraction
Two reviewers independently extracted data using a standardized electronic form, including study characteristics (author, year, country), patient demographics, aneurysm features, treatment details.
2.5 Statistical analysis
Analyses were performed using Stata 15.0. Outcomes were expressed as proportions, and pooled using single-arm meta-analysis. To stabilize variance, the Freeman–Tukey double arcsine transformation was applied. Pooled estimates with 95% CIs were reported. Heterogeneity was assessed with Cochran’s Q test and the I2 statistic; random-effects models (DerSimonian–Laird) were applied when I2 > 50%, otherwise fixed-effects models were used. Publication bias was assessed with funnel plots and Egger’s test.
3 Results
3.1 Study selection
From 14,782 records, 5,476 duplicates were removed, leaving 9,306 for screening. After excluding 9,205 based on titles/abstracts, 101 full texts were assessed, and 10 studies met inclusion criteria (shown in Figure 1).
Figure 1

PRISMA flow diagram of study selection.
3.2 Study characteristics
The 10 retrospective studies (3, 15–23) included 2,275 patients with 1,938 SIAs. Five studies used PED, two FRED X, one PED Flex, and two reported mixed FD devices (SILK, Derivo, Surpass). Studies were conducted in the United States, Turkey, China, and Brazil. Study characteristics are summarized in Table 1.
Table 1
| Study | N | n | Gender F/M | Size (mm) | Age (years) | Follow-up (m) | Rupture status | Circulation | DAPT | Flow-diverting stents | Country |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Akgul et al. (15) | 43 | 66 | 14/29 | ≤5 | 50.2 ± 12 | 26 (6–52) | Unrauptured + Ruptured | anterior circulation | Aspirin + Clopidogrel | SILK; Derivo; Pipeline; Surpass; FRED | Turkey |
| Brasiliense et al. (16) | 205 | 121 | 165/40 | ≤7 | 55.7 ± 14.4 | 1 | Unruptured Aneurysms | anterior + posterior circulation | NR | PED Flex | USA |
| Chalouhi et al. (17) | 100 | 100 | 89/11 | 5.2 ± 1.5 | 17–80 | 6.3 | Unruptured + Ruptured | anterior + posterior circulation | Aspirin + Clopidogrel | PED | USA |
| Clausen et al. (18) | 77 | 53 | 56/21 | ≤10 | 58.9 ± 17.75 | 6 | Unruptured + Ruptured | anterior + posterior circulation | Aspirin + Ticagrelor | FRED X | USA |
| Griessenauer et al. (19) | 117 | 149 | 100/17 | ≤7 | 54 ± 14.5 | 6 | Unruptured + Ruptured | anterior + posterior circulation | Aspirin + Clopidogrel | PED | USA |
| Kallmes et al. (20) | 793 | 349 | NR | 5.2 ± 2.2 | NR | 12 | Unruptured + Ruptured | anterior + posterior circulation | NR | PED | USA |
| Lin et al. (21) | 41 | 53 | 38/3 | 5.34 ± 0.3 | 54.9 ± 1.6 | 4.0 ± 1.9 | Unruptured + Aneurysms | anterior circulation | Aspirin + Clopidogrel | PED | USA |
| Roy et al. (22) | 154 | 162 | 126/28 | 5.9 ± 4.0 | 56.8 ± 12.84 | 12 | Unruptured + Ruptured | anterior + posterior circulation | Aspirin + Clopidogrel | FRED X | USA |
| Trivelato et al. (23) | 93 | 131 | 86/7 | 8.04 ± 0.53 | 52.50 ± 1.32 | 12 | Unruptured Aneurysms | Anterior + Posterior circulation | Aspirin + Clopidogrel | PED | Brazil |
| Zhang et al. (3) | 652 | 754 | 448/204 | 6.78 ± 2.67 | 53.9 ± 10.3 | 8.26 ± 5.91 | Unruptured + Ruptured | Anterior + Posterior circulation | aspirin + clopidogrel | PED Classic or PED Flex | China |
Basic characteristics information of included studies.
N of patients; n of small aneurysms (≤10 mm); NR: not report; DAPT: Dual Antiplatelet Therapy; FRED X: Flow-Redirection Endoluminal Device X; PED: Pipeline embolization device.
3.3 Quality assessment
Based on the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Series, 10 clinical studies were evaluated across ten domains assessing aspects such as case selection, disease evaluation, and data reporting. The results of quality assessment are provided in Table 2.
Table 2
| Query | Akgul et al. (15) | Brasiliense et al. (16) | Chalouhi et al. (17) | Clausen et al. (18) | Griessenauer et al. (19) | Lin et al. (21) | Kallmes et al. (20) | Roy et al. (22) | Trivelato et al. (23) | Zhang et al. (3) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4 | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall appraisal | Include | Include | Include | Include | Include | Include | Include | Include | Include | Include |
The results of JBI critical appraisal checklist for case series.
Query 1: Were there clear criteria for inclusion in the case series? Query 2: Was the condition measured in a standard, reliable way for all participants included in the case series? Query 3: Were valid methods used for identification of the condition for all participants included in the case series? Query 4: Did the case series have consecutive inclusion of participants? Query 5: Did the case series have complete inclusion of participants? Query 6: Was there clear reporting of the demographics of the participants in the study? Query 7: Was there clear reporting of clinical information of the participants? Query 8: Were the outcomes or follow up results of cases clearly reported? Query 9: Was there clear reporting of the presenting site(s)/clinic(s) demographic information? Query 10: Was statistical analysis appropriate?
3.4 Meta-analysis results
3.4.1 Complete or near-complete occlusion
Eight studies reported complete or near-complete occlusion (Raymond–Roy Grade I). The pooled analysis demonstrated an overall rate of 86% (95% CI: 80–92%). Significant heterogeneity was observed (I2 = 84.73%, p = 0.00). These findings indicate that most patients achieved favorable angiographic outcomes following FD treatment (shown in Figure 2).
Figure 2
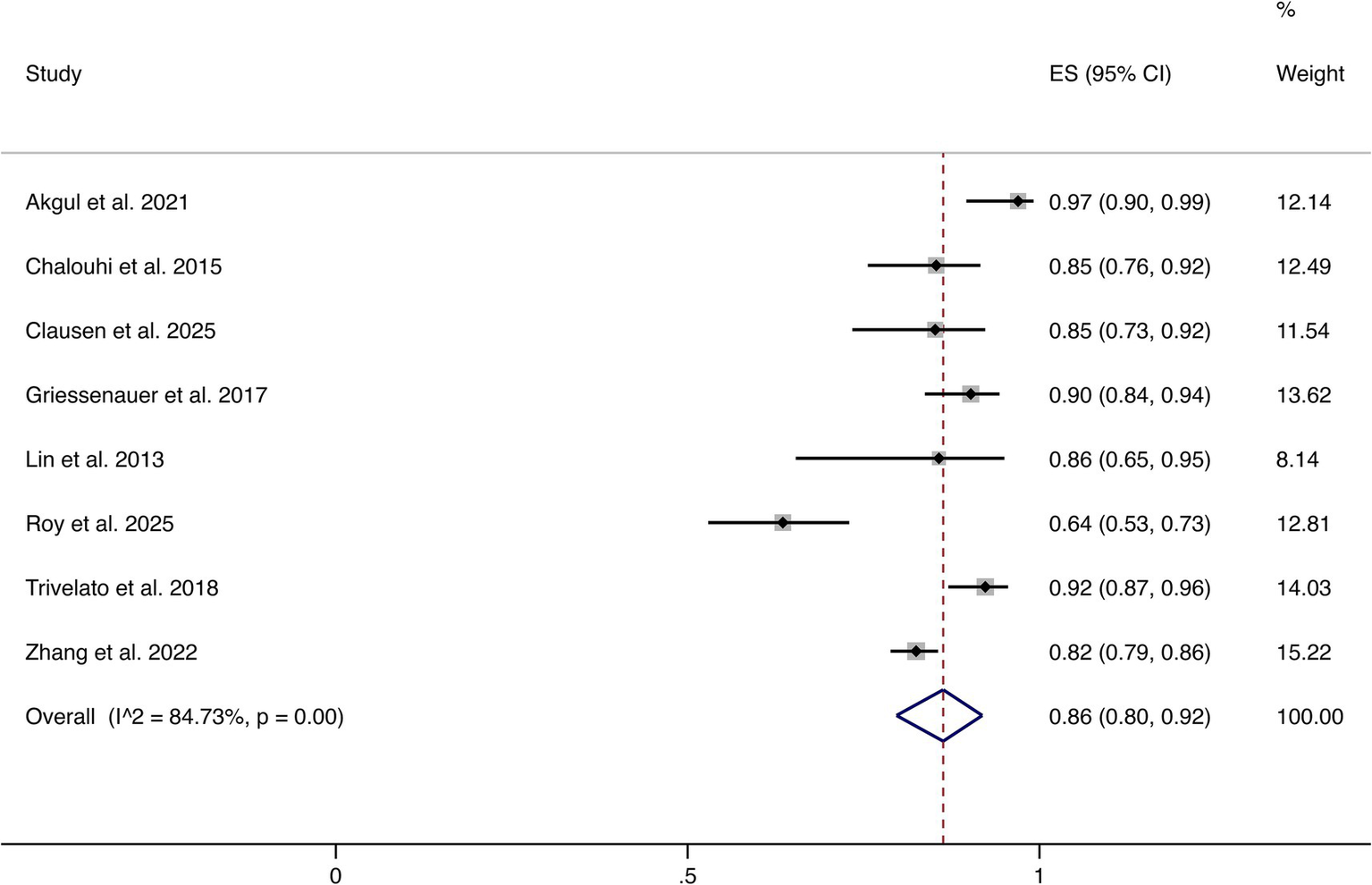
Forest plot of complete or near-complete occlusion.
3.4.2 Incomplete occlusion (<90%)
Four studies reported incomplete occlusion (<90%). The pooled incidence was 7% (95% CI: 3–13%) with high heterogeneity (I2 = 70.16%, p = 0.02). This suggests that although most patients achieve complete or near-complete occlusion, a minority may experience incomplete occlusion, warranting careful clinical follow-up (shown in Figure 3).
Figure 3
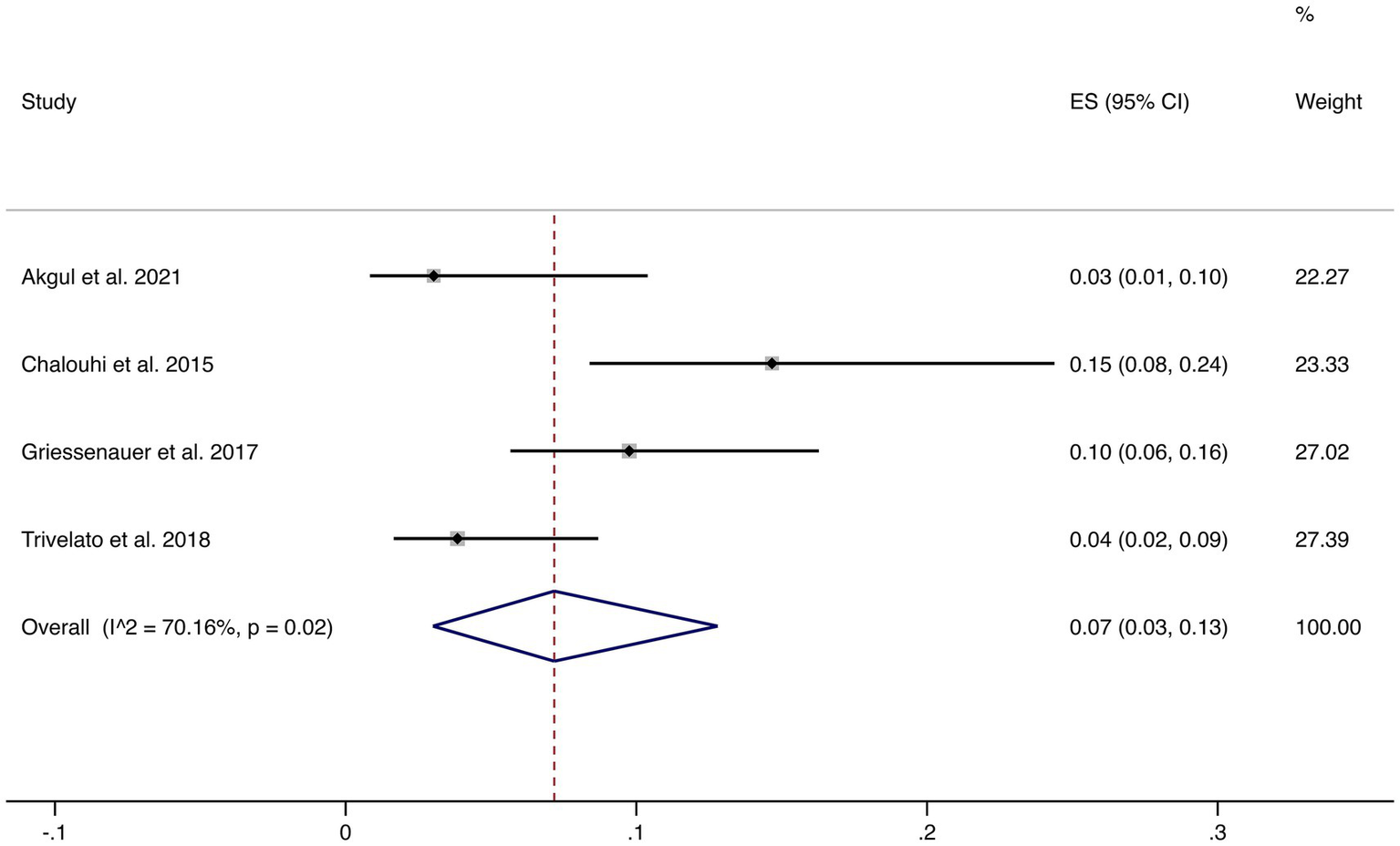
Forest plot of incomplete occlusion (<90%).
3.4.3 Mortality
Four studies reported mortality outcomes. The pooled mortality rate was 1% (95% CI: 0–2%) with low heterogeneity (I2 = 12.08%, p = 0.33), suggesting robust and consistent results. This indicates that FD treatment is associated with a very low risk of treatment-related death (shown in Figure 4).
Figure 4
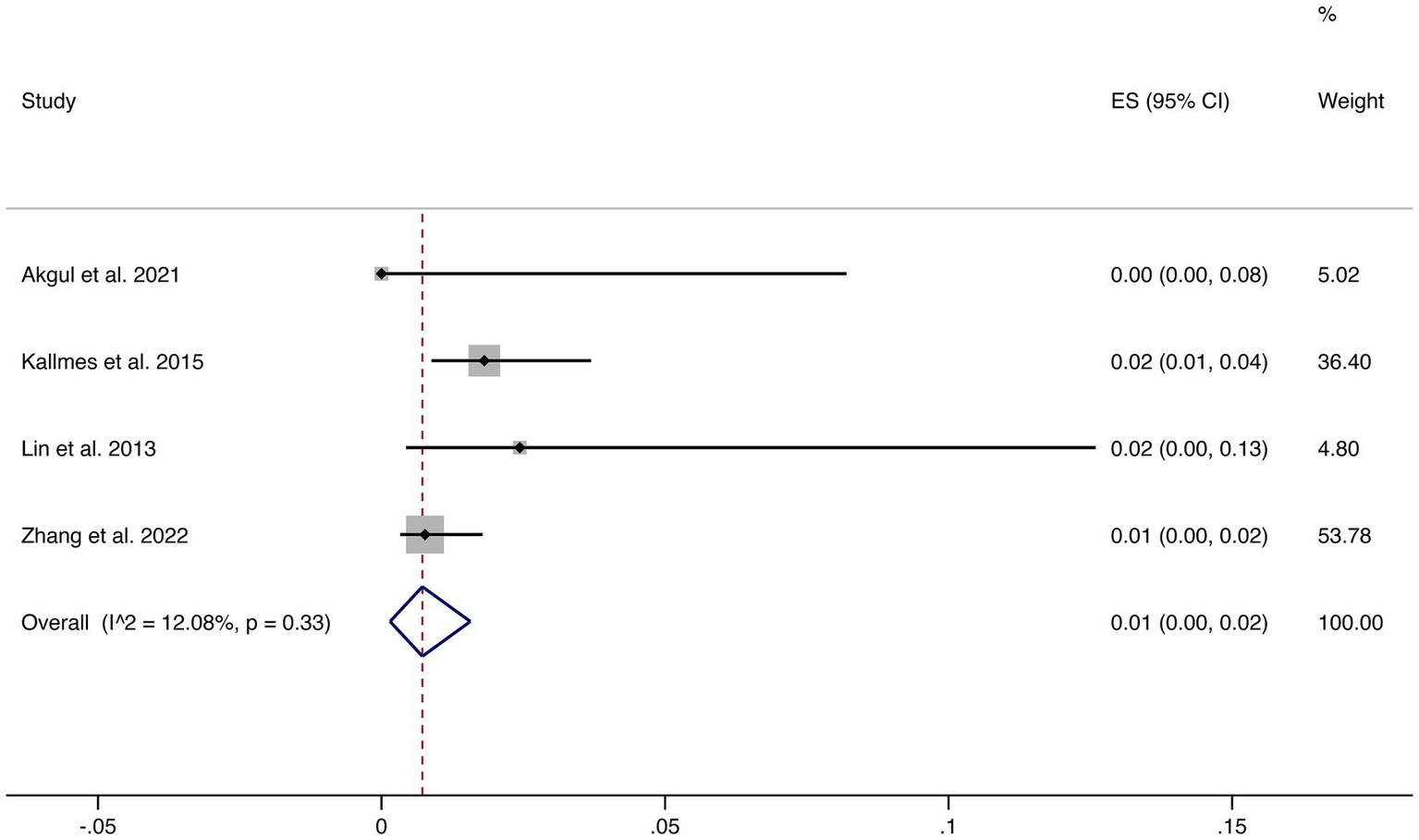
Forest plot of mortality.
3.4.4 Hemorrhagic events
Seven studies reported hemorrhagic complications. The pooled rate was 1% (95% CI: 1–2%), with very low heterogeneity (I2 = 2.70%, p = 0.40), indicating highly consistent findings across studies. These results confirm that hemorrhagic risk following FD treatment is low (shown in Figure 5).
Figure 5
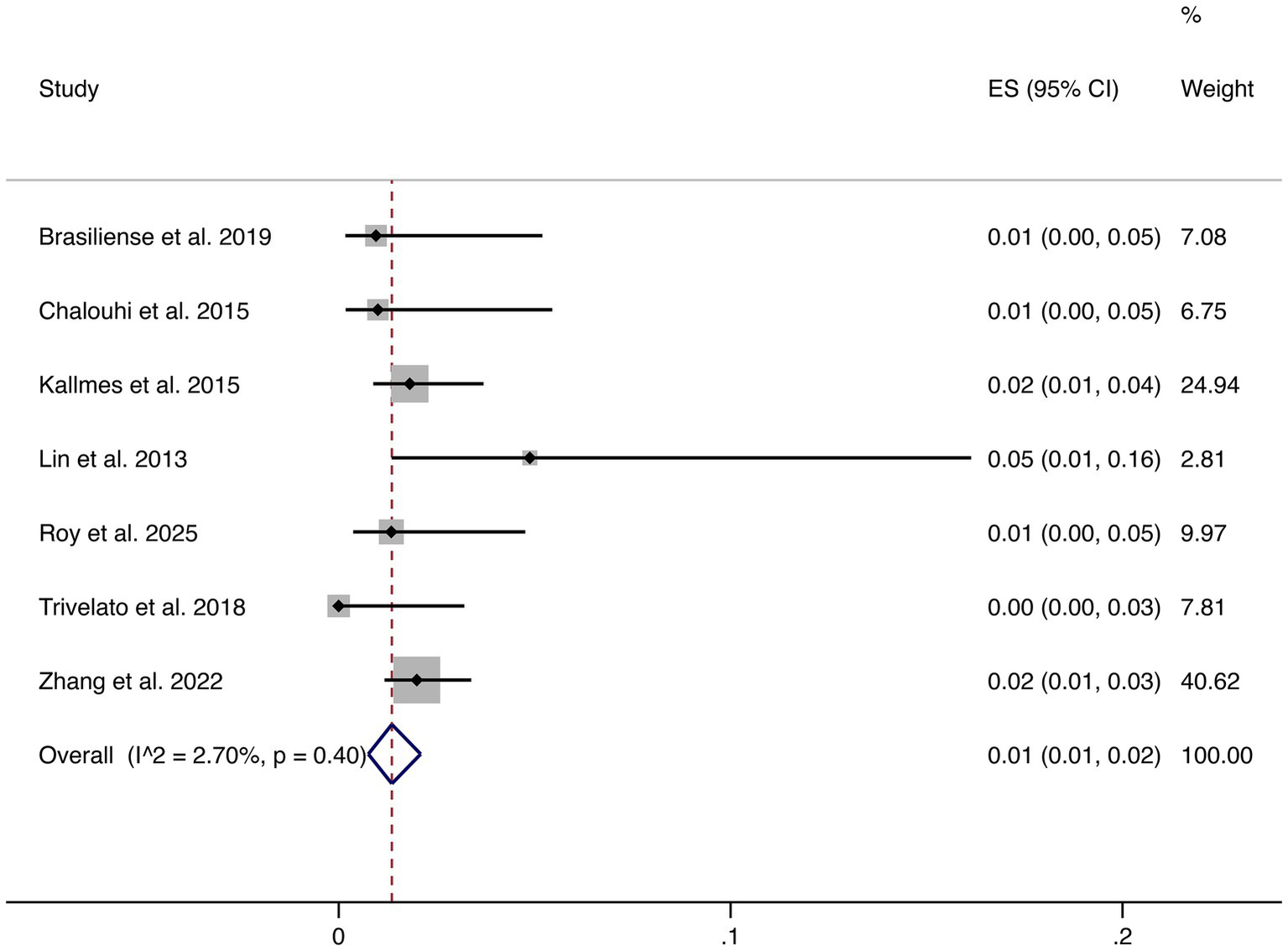
Forest plot of hemorrhagic events.
3.4.5 Stroke
Five studies reported stroke as an outcome. The pooled rate was 3% (95% CI: 1–5%) with moderate heterogeneity (I2 = 55.03%, p = 0.06). Although relatively uncommon, stroke remains a clinically relevant complication, underscoring the importance of perioperative risk management (shown in Figure 6).
Figure 6
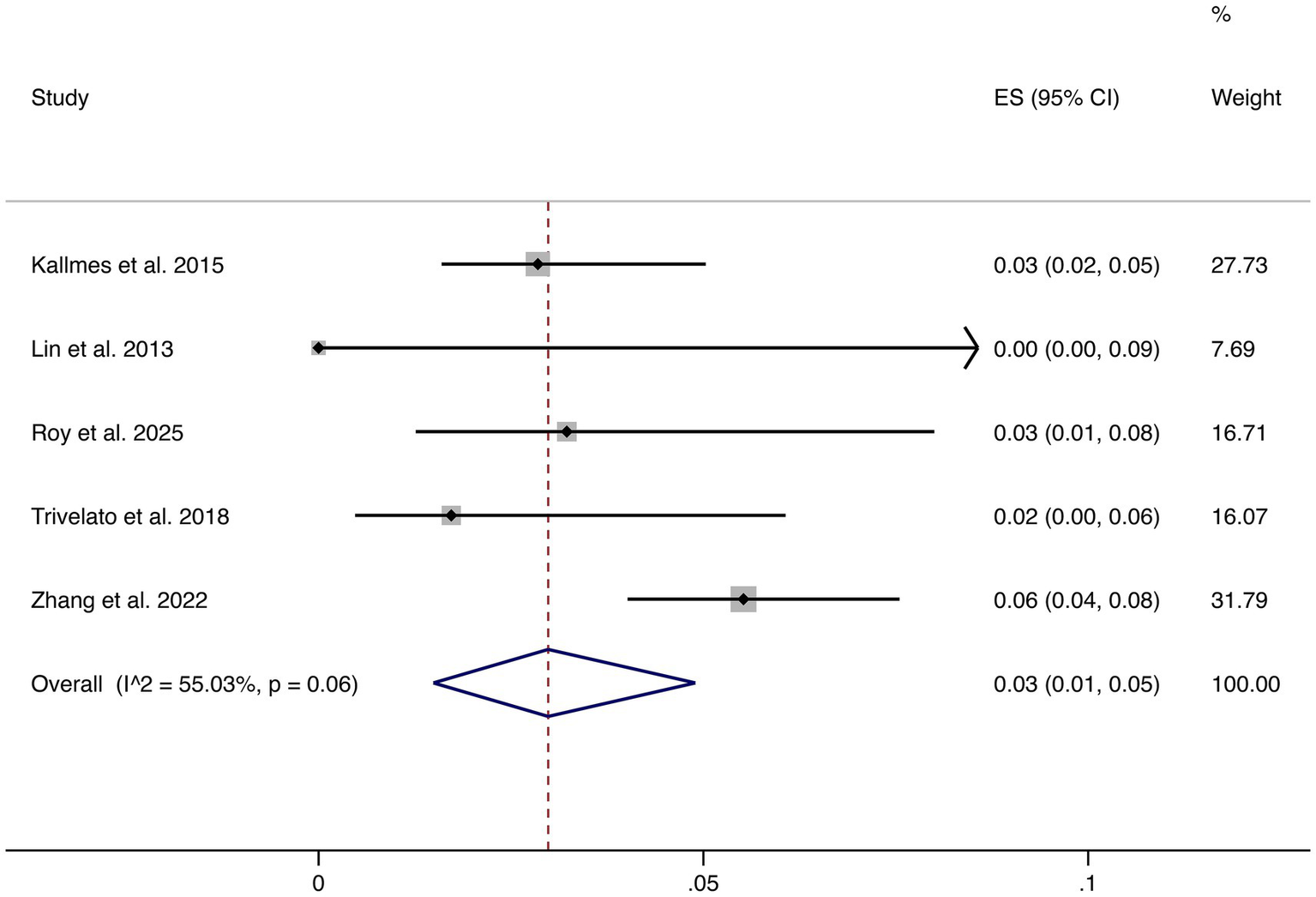
Forest plot of stroke.
3.4.6 Ischemic events
Six studies reported ischemic complications. The pooled rate was 2% (95% CI: 1–3%), with low heterogeneity (I2 = 0.00%, p = 0.70). This demonstrates that ischemic risk is consistently low across studies (shown in Figure 7).
Figure 7
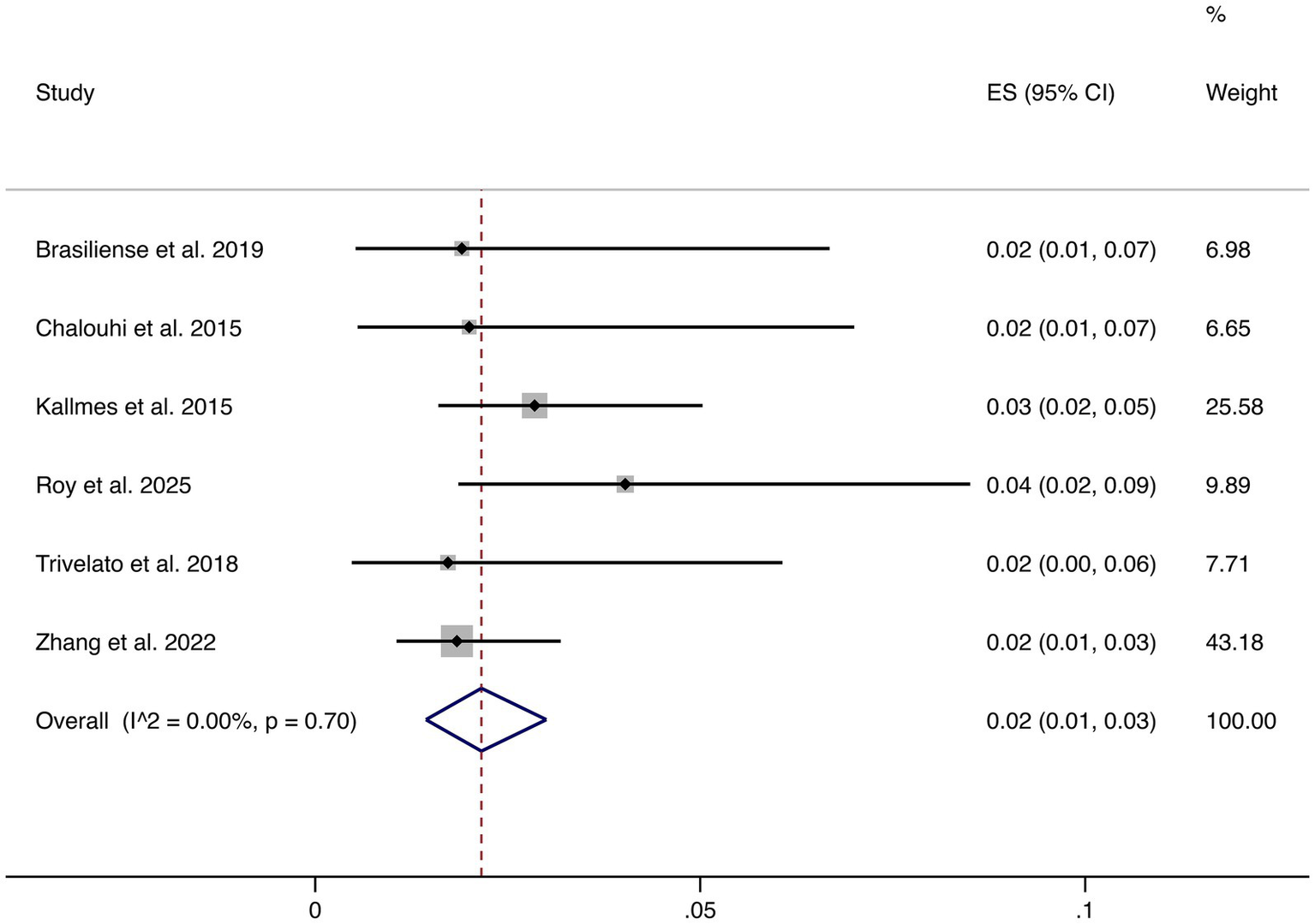
Forest plot of ischemic events.
3.4.7 Favorable functional outcomes
Four studies reported favorable functional outcomes (mRS 0–2). The pooled incidence was 98% (95% CI: 94–100%), although substantial heterogeneity was observed (I2 = 73.39%, p = 0.01). These results suggest that most patients maintained good neurological function following FD treatment (shown in Figure 8).
Figure 8
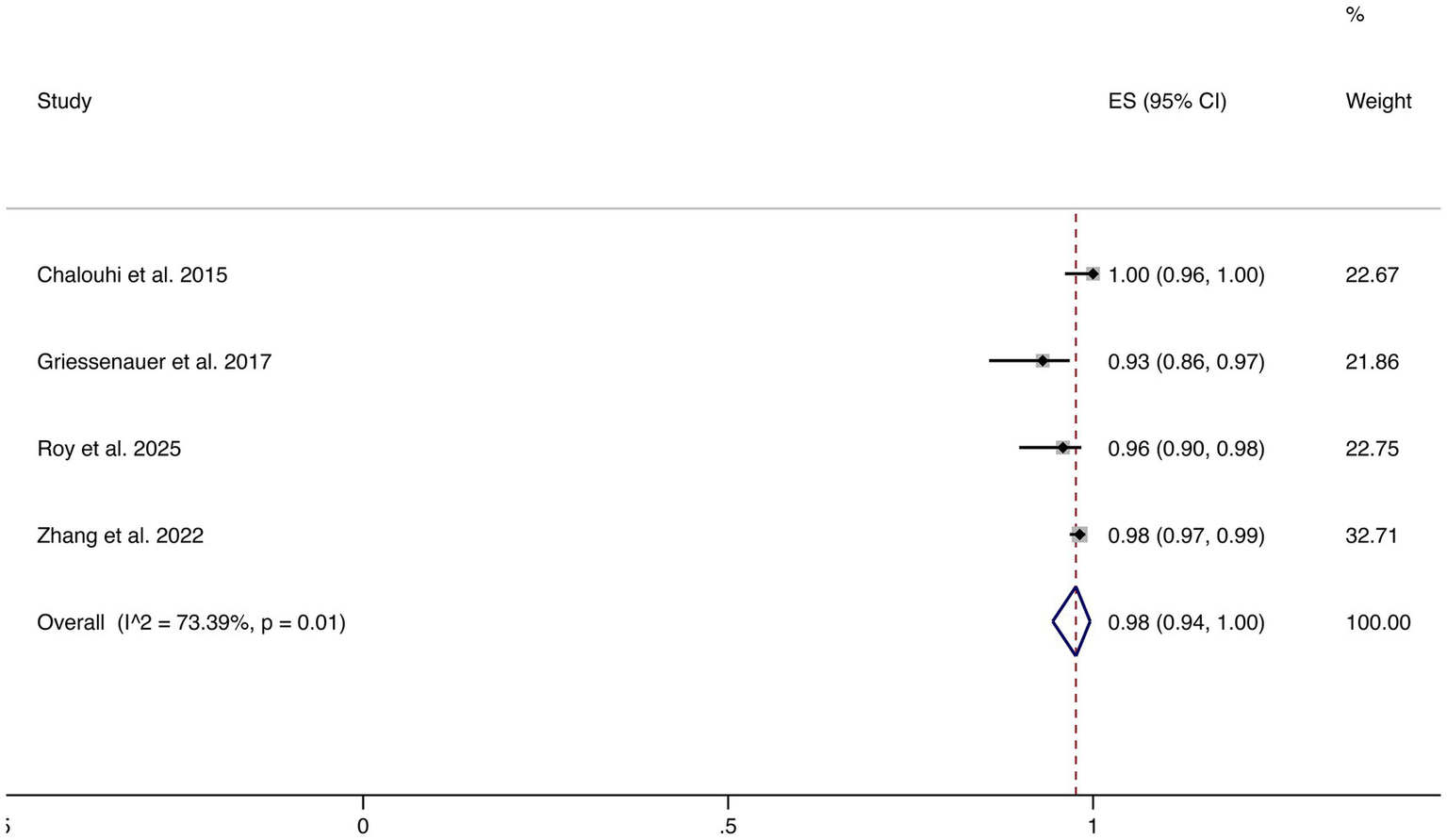
Forest plot of favorable functional outcomes.
3.4.8 Complications
Nine studies reported overall complications. The pooled complication rate was 9% (95% CI: 5–12%), with high heterogeneity (I2 = 82.26%, p = 0.00). This indicates considerable variability in reported complication rates across studies, highlighting that although FD treatment is generally safe, certain patients remain at risk of peri- or post-procedural complications that require clinical vigilance (shown in Figure 9).
Figure 9
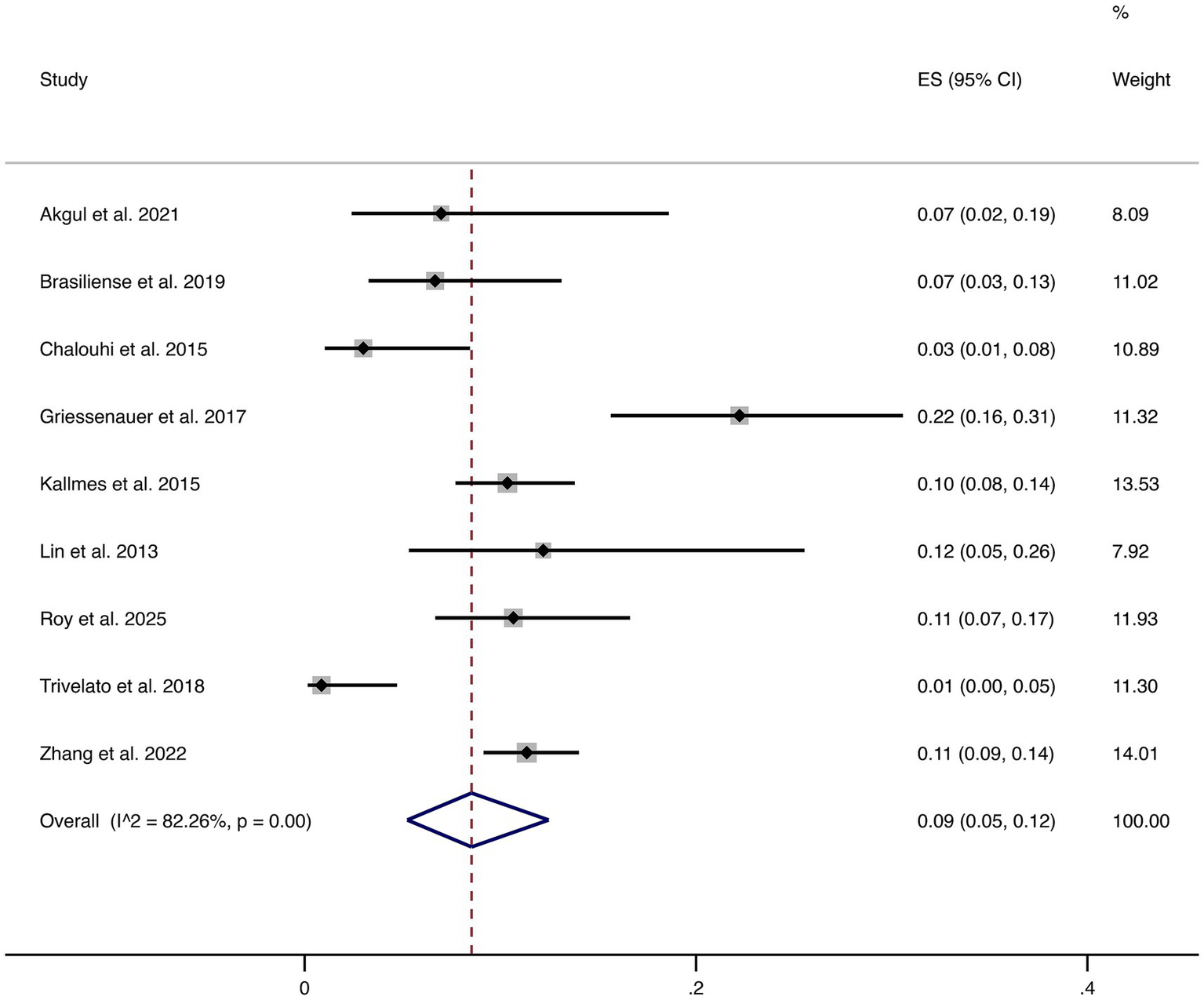
Forest plot of overall complications.
3.5 Publication bias
The funnel plot suggested some asymmetry, indicating the potential presence of publication bias or small-study effects. However, Egger’s regression test yielded p = 0.791, suggesting no statistically significant evidence of publication bias; thus, the results should be interpreted with caution.
4 Discussion
This meta-analysis included 10 retrospective studies comprising 2,275 patients with 1,938 SIAs. The pooled analysis demonstrated a complete or near-complete occlusion rate of 86%, indicating high efficacy of FDs in the treatment of small aneurysms. In addition, the rates of treatment-related mortality and hemorrhage were both 1%, ischemic events occurred in 2%, stroke in 3%, and favorable functional outcomes were achieved in 98% of patients. The overall complication rate was 9%. These findings suggest that FDs provide not only excellent angiographic outcomes but also favorable safety and functional profiles in the management of small intracranial aneurysms.
SIAs present unique therapeutic challenges due to their small size and complex anatomy. Conventional treatment approaches, such as microsurgical clipping or endovascular coiling, often face limitations including incomplete occlusion or recurrence. By reconstructing parent vessel hemodynamics, FDs promote intra-aneurysmal thrombosis and vessel wall remodeling, thereby achieving progressive occlusion. The present study indicates that in SIAs, FDs can achieve high occlusion rates without substantially increasing the risk of severe complications, which holds important clinical implications.
Our findings are largely consistent with previous prospective studies and meta-analyses, though several methodological differences should be considered. The PREMIER trial investigated unruptured wide-neck aneurysms ≤12 mm and reported complete occlusion rates of 76.8% at one year and 83.3% at three years, with a primary safety event rate of 2.8% (11, 24). However, the PREMIER cohort included both small (<10 mm) and medium-sized (10–12 mm) aneurysms, whereas our meta-analysis focused only on small aneurysms. Differences in aneurysm size, baseline characteristics, and patient selection likely account for part of the discrepancy. Bhatia et al. reported a major complication rate of only 0.9% in <10 mm aneurysms treated with the Pipeline Flex device, which is much lower than our pooled rate (25). This is largely due to methodological differences. Bhatia defined complications strictly as major adverse events (death, major ischemic stroke, or symptomatic intracranial hemorrhage), while our analysis included all reported complications, both major and minor. Their review also focused only on the second-generation Pipeline Flex, whereas our study covered multiple flow diverters across different devices and settings. Fiorella et al. conducted a meta-analysis of predominantly small to medium sized unruptured ICA aneurysms (26). They reported a 12-month complete occlusion rate of 74.9% and a composite primary safety event rate of 7.8%, providing performance benchmarks that are broadly in line with our findings. In our pooled analysis, the complete or near-complete occlusion rate was 86%, slightly higher than some prospective studies, while the overall complication rate was 9%. This higher rate likely reflects the greater heterogeneity of retrospective studies, variation in device type and operator experience, and differences in antiplatelet regimens. Nevertheless, when broken down by event type, treatment-related mortality and hemorrhagic events were 1%, ischemic events 2%, and stroke 3%. These remain lower than rates reported in cohorts of large or giant aneurysms. Finally, the proportion of patients with favorable functional outcomes reached 98%, reinforcing the clinical benefit of flow diverter treatment in small aneurysms.
This study has several limitations. First, all included studies were retrospective in design, which may introduce selection bias. Second, the relatively small sample sizes of some studies limit the robustness of the pooled estimates. Third, variability in follow-up duration and imaging assessment criteria across studies may have contributed to heterogeneity in the results. Fourth, most of the included studies mixed ruptured and unruptured small aneurysms, with only a few reporting results separately. Because of limited available data, we were unable to conduct a subgroup meta-analysis by rupture status. Future studies should stratify and report outcomes by rupture status to reduce heterogeneity and improve the quality of evidence. Therefore, future large-scale, multicenter, prospective randomized controlled trials with standardized imaging assessments and long-term follow-up are warranted to further validate the efficacy and safety of FDs in SIAs.
5 Conclusion
In this systematic review and single-arm meta-analysis, flow diverters demonstrated high efficacy and acceptable safety in the treatment of small intracranial aneurysms. With an overall complete occlusion rate of 86% and a favorable functional outcome rate of 98%, FD treatment offers a promising therapeutic option. Nonetheless, the high heterogeneity and retrospective design of included studies highlight the need for prospective, multicenter randomized trials to validate these findings and establish standardized clinical protocols.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. MN: Conceptualization, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. AR: Investigation, Methodology, Visualization, Writing – review & editing. ASe: Data curation, Investigation, Software, Writing – review & editing. MY: Methodology, Software, Visualization, Writing – review & editing. XG: Investigation, Methodology, Resources, Writing – review & editing. MA: Investigation, Methodology, Software, Writing – review & editing. YX: Investigation, Methodology, Validation, Writing – review & editing. HP: Data curation, Investigation, Methodology, Writing – review & editing. ASi: Investigation, Methodology, Software, Writing – review & editing. YH: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – review & editing. DY: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1706462/full#supplementary-material
References
1.
Vlak MH Algra A Brandenburg R Rinkel GJ . Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. (2011) 10:626–36. doi: 10.1016/S1474-4422(11)70109-0
2.
Asikainen A Korja M Kaprio J Rautalin I . Case fatality in patients with aneurysmal subarachnoid Hemorrhage in Finland: a Nationwide register-based study. Neurology. (2023) 100:e348–56. doi: 10.1212/WNL.0000000000201402
3.
Zhang H Li L Zhang H Liu J Song D Zhao Y et al . Small and medium-sized aneurysm outcomes following intracranial aneurysm treatment using the pipeline embolization device: a subgroup analysis of the plus registry. Front Neurol. (2022) 13:881353. doi: 10.3389/fneur.2022.881353
4.
Morita A Kirino T Hashi K Aoki N Fukuhara S Hashimoto N et al . The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. (2012) 366:2474–82. doi: 10.1056/NEJMoa1113260
5.
Deshmukh AS Priola SM Katsanos AH Scalia G Costa Alves A Srivastava A et al . The Management of Intracranial Aneurysms: current trends and future directions. Neurol Int. (2024) 16:74–94. doi: 10.3390/neurolint16010005
6.
Jin J Guo G Ren Y Yang B Wu Y Wang S et al . Risk factors for recurrence of intracranial aneurysm after coil embolization: a Meta-analysis. Front Neurol. (2022) 13:869880. doi: 10.3389/fneur.2022.869880
7.
Park DS Roh HG Chun YI Jeon YS . Efficacy of coil embolization in small, anterior circulation aneurysms in patients less than 40 years old. J Clin Med. (2024) 13:4764. doi: 10.3390/jcm13164764
8.
Booth TC Bassiouny A Lynch J Sonwalkar H Bleakley A Iqbal A et al . Outcome study of the pipeline vantage embolization device (second version) in unruptured (and ruptured) aneurysms (Pedvu(R) study). J Neurointerv Surg. (2024) 16:1136–44. doi: 10.1136/jnis-2023-020754
9.
Shehata MA Ibrahim MK Ghozy S Bilgin C Jabal MS Kadirvel R et al . Long-term outcomes of flow diversion for unruptured intracranial aneurysms: a systematic review and meta-analysis. J Neurointerv Surg. (2023) 15:898–902. doi: 10.1136/jnis-2022-019240
10.
Elek A Karagoz S Dindar GN Yucel S Cinar C Kusbeci M et al . Safety and efficacy of low profile flow diverter stents for intracranial aneurysms in small parent vessels: systematic review and meta-analysis. J Neurointerv Surg. (2025), 1–8. doi: 10.1136/jnis-2024-022834
11.
Hanel RA Cortez GM Lopes DK Nelson PK Siddiqui AH Jabbour P et al . Prospective study on embolization of intracranial aneurysms with the pipeline device (Premier study): 3-year results with the application of a flow diverter specific occlusion classification. J Neurointerv Surg. (2023) 15:248–54. doi: 10.1136/neurintsurg-2021-018501
12.
Li L Gao BL Shao QJ Zhang GL Wang ZL Li TX et al . Small unruptured intracranial aneurysms can be effectively treated with flow-diverting devices. Front Neurol. (2022) 13:913653. doi: 10.3389/fneur.2022.913653
13.
Page MJ Mckenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The Prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14.
Munn Z Barker TH Moola S Tufanaru C Stern C Mcarthur A et al . Methodological quality of case series studies: an introduction to the Jbi critical appraisal tool. Jbi Evid Synth. (2020) 18:2127–33. doi: 10.11124/JBISRIR-D-19-00099
15.
Akgul E Onan HB Bilgin SS Tahta A Khanmammadov E Gungoren FZ et al . Flow diverter stents in the treatment of cerebral aneurysms less than 5 mm. Turk Neurosurg. (2021) 31:31–7. doi: 10.5137/1019-5149.JTN.28762-19.2
16.
Brasiliense LBC Aguilar-Salinas P Lopes DK Nogueira D Desousa K Nelson PK et al . Multicenter study of pipeline flex for intracranial aneurysms. Neurosurgery. (2019) 84:E402–e409. doi: 10.1093/neuros/nyy422
17.
Chalouhi N Zanaty M Whiting A Yang S Tjoumakaris S Hasan D et al . Safety and efficacy of the pipeline embolization device in 100 small intracranial aneurysms. J Neurosurg. (2015) 122:1498–502. doi: 10.3171/2014.12.JNS14411
18.
Clausen TM Nakamura R Conching A Choi JW Zhang YJ Hui F et al . Flow diversion in the treatment of intracranial aneurysms using the novel Fred X device: an early experience from a single high-volume center. Interv Neuroradiol. (2025) 15910199251319059:10. doi: 10.1177/15910199251319059
19.
Griessenauer CJ Ogilvy CS Foreman PM Chua MH Harrigan MR He L et al . Pipeline embolization device for small intracranial aneurysms: evaluation of safety and efficacy in a multicenter cohort. Neurosurgery. (2017) 80:579–87. doi: 10.1227/NEU.0000000000001377
20.
Kallmes DF Hanel R Lopes D Boccardi E Bonafé A Cekirge S et al . International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. (2015) 36:108–15. doi: 10.3174/ajnr.A4111
21.
Lin LM Colby GP Kim JE Huang J Tamargo RJ Coon AL . Immediate and follow-up results for 44 consecutive cases of small (<10 mm) internal carotid artery aneurysms treated with the pipeline embolization device. Surg Neurol Int. (2013) 4:114. doi: 10.4103/2152-7806.117711
22.
Roy JM El Naamani K Amaravadi C Majmundar S Mouchtouris N Paul AR et al . Long-term safety and efficacy of the Fred X flow diverter for intracranial aneurysms: a multicenter study of 154 patients. J Neurosurg. (2025) 143:232–42. doi: 10.3171/2024.10.JNS241233
23.
Trivelato FP Salles Rezende MT Ulhôa AC Henrique De Castro-Afonso L Nakiri GS Abud DG . Occlusion rates of intracranial aneurysms treated with the pipeline embolization device: the role of branches arising from the sac. J Neurosurg. (2018) 130:543–9. doi: 10.3171/2017.10.JNS172175
24.
Hanel RA Kallmes DF Lopes DK Nelson PK Siddiqui A Jabbour P et al . Prospective study on embolization of intracranial aneurysms with the pipeline device: the Premier study 1 year results. J Neurointerv Surg. (2020) 12:62–6. doi: 10.1136/neurintsurg-2019-015091
25.
Bhatia KD Kortman H Orru E Klostranec JM Pereira VM Krings T . Periprocedural complications of second-generation flow diverter treatment using pipeline flex for unruptured intracranial aneurysms: a systematic review and meta-analysis. J Neurointerv Surg. (2019) 11:817–24. doi: 10.1136/neurintsurg-2019-014937
26.
Fiorella D Gache L Frame D Arthur AS . How safe and effective are flow diverters for the treatment of unruptured small/medium intracranial aneurysms of the internal carotid artery? Meta-analysis for evidence-based performance goals. J Neurointerv Surg. (2020) 12:869–73. doi: 10.1136/neurintsurg-2019-015535
Summary
Keywords
flow diverters, small intracranial aneurysms, intracranial aneurysms, FDs, meta-analysis
Citation
Maimaitiaili K, Nuermaimaiti M, Rouzi A, Seyiti A, Yasheng M, Guo X, Abulaiti M, Xiao Y, Pan H, Sidike A, Hu Y and Yilamu D (2025) Efficacy and safety of flow diverters in small intracranial aneurysms: a systematic review and single-arm meta-analysis. Front. Neurol. 16:1706462. doi: 10.3389/fneur.2025.1706462
Received
16 September 2025
Revised
23 October 2025
Accepted
07 November 2025
Published
26 November 2025
Volume
16 - 2025
Edited by
Osama O. Zaidat, Northeast Ohio Medical University, United States
Reviewed by
Muhammad Hussain Ahmadvand, Tehran University of Medical Sciences, Iran
Shenghao Ding, Renji Hospital, Shanghai Jiao Tong University, China
Updates
Copyright
© 2025 Maimaitiaili, Nuermaimaiti, Rouzi, Seyiti, Yasheng, Guo, Abulaiti, Xiao, Pan, Sidike, Hu and Yilamu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanbing Hu, 1428319488@qq.com; Dawutijiang Yilamu, kedawutjan@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.