- Departments of Biology and Biochemistry, Memorial University of Newfoundland, St. John's, NL, Canada
Fish are the most diversified group of vertebrates and, although progress has been made in the past years, only relatively few fish species have been examined to date, with regards to the endocrine regulation of feeding in fish. In fish, as in mammals, feeding behavior is ultimately regulated by central effectors within feeding centers of the brain, which receive and process information from endocrine signals from both brain and peripheral tissues. Although basic endocrine mechanisms regulating feeding appear to be conserved among vertebrates, major physiological differences between fish and mammals and the diversity of fish, in particular in regard to feeding habits, digestive tract anatomy and physiology, suggest the existence of fish- and species-specific regulating mechanisms. This review provides an overview of hormones known to regulate food intake in fish, emphasizing on major hormones and the main fish groups studied to date.
Introduction
Feeding is a complex behavior consisting of food ingestion itself as well as foraging or appetitive behaviors (which reflect motivation to consume food; Keen-Rhinehart et al., 2013; Woods and Begg, 2016). Feeding is ultimately regulated by central feeding centers of the brain, which receive and process information from endocrine signals from both brain and periphery. These signals consist of hormones that increase (e.g., orexin; neuropeptide Y-NPY) or inhibit, (e.g., cocaine and amphetamine regulated transcript-CART; proopiomelanocortin-POMC) feeding. Feeding centers are also influenced by metabolic and neural peripheral signals providing information on meal ingestion and nutritional status (Volkoff, 2006; Volkoff et al., 2009a,b; Rui, 2013; Sobrino Crespo et al., 2014).
Fish are the most diversified group of vertebrates, with 33,200 species identified to date (FishBase, 2016), the bony fish (teleosts) containing more than half of all vertebrate species (Nelson, 2006). However, only relatively few fish species have been examined to date, with regards to their physiology, in particular feeding. The large numbers of fish species, habitats, feeding habits and digestive tract anatomy and physiology, as well as the number of extrinsic and intrinsic factors affecting feeding behavior and physiology (Volkoff et al., 2009a; Hoskins and Volkoff, 2012) most probably result in complex species-specific feeding regulating mechanisms in fish, with a number of hormones and tissues involved.
Research on the endocrine regulation of feeding in fish has progressed in recent years. New fish appetite-regulating hormones and species other than traditional models (such as goldfish, salmon and zebrafish) are gradually being examined. In addition, traditional techniques such as brain lesions and injections and biochemical purification of peptides, although still useful and being used, have been complemented by new approaches such as gene expression studies, quantitative PCR, genomics (microarrays, RNA-seq), proteomics and metabolomics, transgenesis, gene knockout and silencing, and in vitro (cell and tissue culture, perifusion) studies.
The field of fish feeding endocrine physiology is evolving very rapidly and up-to-date reviews are often lacking. One of the first reviews on the endocrine regulation of feeding by R.E. Peter in 1979 (Peter, 1979) mostly focused on growth and growth hormone (GH) but predicted regions of the brain that might be responsible for feeding regulation in fish. In 1986, Matty's review described early data on the effects of GH, thyroid hormones, insulin, and gonadal steroids on feeding (Matty, 1986). Ten years later, Le Bail and Boeuf's review formulated hypotheses on mammalian hormones (e.g., leptin) that might putatively regulate feeding in fish (Le Bail and Boeuf, 1997). In the early twenty-first century, a number of reviews report recent advances on the field and include an increasing number of hormones (e.g., NPY, orexins, CART), some more comparative (Lin et al., 2000; de Pedro and Björnsson, 2001; Volkoff et al., 2005; Gorissen et al., 2006; Volkoff, 2011; Hoskins and Volkoff, 2012), some more focused on a single species (e.g., goldfish Matsuda, 2009; Matsuda et al., 2011a) or a particular group of fish (e.g., elasmobranchs Demski, 2012), some focused on growth (Won and Borski, 2013), and some on aquaculture and behavior (Papoutsoglou, 2012).
The purpose of this review is to provide an up-to-date, brief overview of the hormones regulating food intake in fish, emphasizing on recent studies, major brain hormones and the main fish groups studied thus far.
Overview of Regulation of Food Intake
In fish, as in mammals (Sobrino Crespo et al., 2014), feeding behavior is regulated by specific regions in the brain, the so-called feeding centers. Early pioneer studies using stimulation and lesion experiments in teleosts (reviewed in Peter, 1979) and elasmobranchs (reviewed in Demski, 2012) seemed to indicate that the hypothalamic area was involved in feeding and that the brain control of feeding in fish might use mechanisms similar to those in mammals. However, whereas in mammals, the feeding centers appear to be restricted to the hypothalamus, evidence indicates that they might be more widespread in fish brains (Cerda-Reverter and Canosa, 2009).
Feeding centers are under the influence of hormones produced by the brain and the periphery. Neurohormones secreted by the brain, in particular the hypothalamic area, regulate energy balance by inhibiting (anorexigenic factors) or stimulating (orexigenic factors) feeding. Peripheral chemical (e.g., glucose) or endocrine (e.g., gastrointestinal hormones) factors released in the blood cross the blood brain barrier and have a direct action on feeding centers. Peripheral sensory information (mechanical or endocrine) carried by the vagus nerve can also affect feeding centers, via innervation from the brainstem (Volkoff, 2011).
Hormones Involved in Food Intake
The list of hormones regulating feeding in vertebrates is long and increasingly so. Here, focus will be placed on major hormones and newly examined appetite-regulating factors (but not on their receptors), and the phylogeny of the fish species examined to date. Table 1, Figure 1 summarize the hormones that have been examined in fish and their possible effects on feeding.
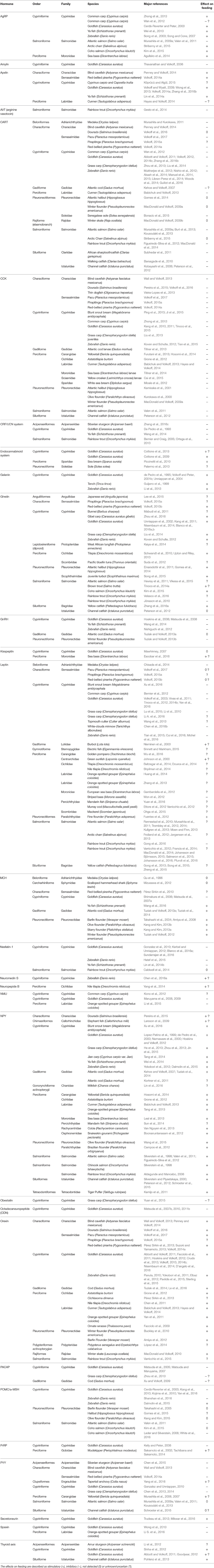
Table 1. List of major hormones (in alphabetical order) potentially involved in the regulation of feeding in fish (by order, family and species studied).
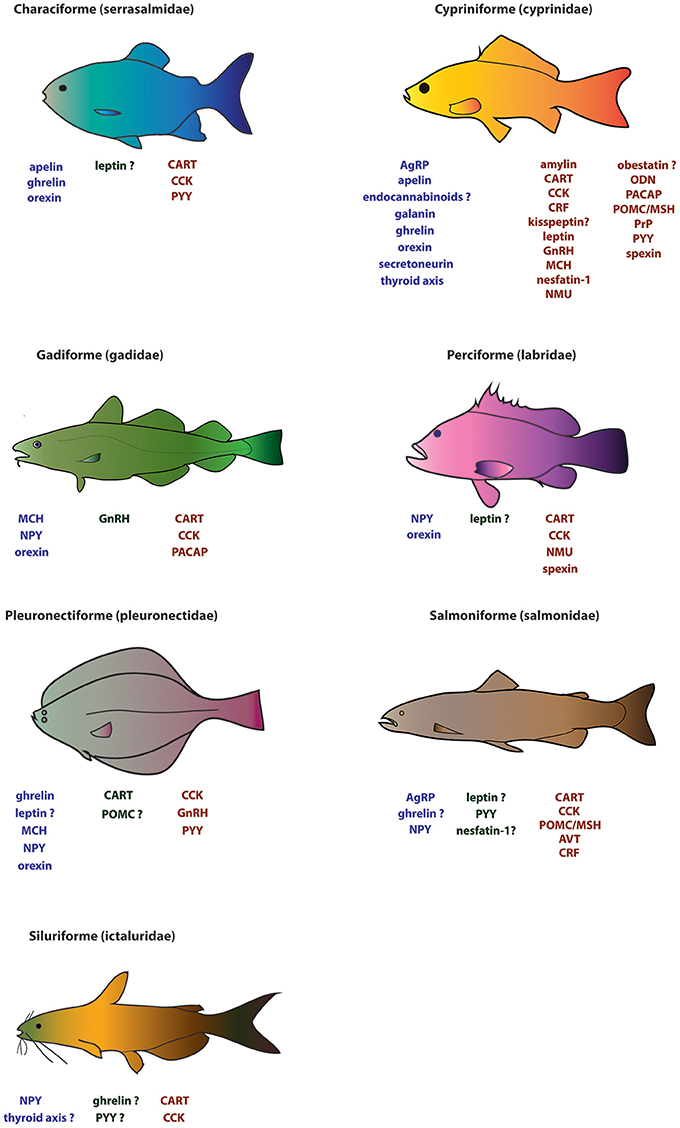
Figure 1. Major appetite regulators known for seven of the most studied representative fish families (serrasalmidae, cyprinidae, gadidae, labridae, pleuronectidae, salmonidae, and ictaluridae). Factors in blue (far left) and in red (far right) under the fish diagrams represent putative orexigenic and anorexigenic factors, respectively. Factors in green (middle) represent factors with no established effect on feeding. A “?” indicates uncertainty with regards to the role of a given factor in regulating feeding.
Major Appetite Regulating Factors
Central Orexigenic Factors
Agouti-related protein (or peptide, AgRP)
AgRP is a peptide released by hypothalamic NPY/AgRP neurons and is an endogenous antagonist of the melanocortin receptors MC3R and MC4R. AgRP plays a crucial role in the regulation of energy balance, as it increases food intake, by antagonizing the effects of the anorexigenic POMC product, α-melanocyte-stimulating hormone (α-MSH) (Sohn, 2015; Takeuchi, 2016).
In fish, AgRP has been identified in several species, including teleosts (e.g., goldfish Carassius auratus Cerdá-Reverter and Peter, 2003 and zebrafish Danio rerio Song et al., 2003, Atlantic salmon Salmo salar Murashita et al., 2009a, and seabass Dicentrarchus labrax Agulleiro et al., 2014, pufferfish Takifugu rubripes Klovins et al., 2004; Kurokawa et al., 2006), who have two genes products (AgRP1 and AgRP2; Cérda-Reverter et al., 2011) and Holocephali (Chimaeriforme, elephant fish Callorhinchus milii Västermark and Schioth, 2011).
AgRP appears to act as an orexigenic factor in Cypriniformes, as fasting increases hypothalamic AgRP expression in goldfish (Cerdá-Reverter and Peter, 2003), zebrafish (Song et al., 2003), and Ya fish Schizothorax prenanti (Wei et al., 2013). In addition, transgenic zebrafish overexpressing AgRP exhibit obesity, increased growth and adipocyte hypertrophy (Song and Cone, 2007). GH-transgenic common carp Cyprinus carpio, which display increased food intake, have higher hypothalamic AgRP1 mRNA expression levels than non-transgenic fish, further suggesting an orexigenic action (Zhong et al., 2013). However, this is contradicted by another study in carp showing that brain AgRP mRNA expression decreases after fasting and increases after re-feeding (Wan et al., 2012). In seabass (Perciforme), long-term fasting increases hypothalamic expression of AgRP1 but decreases that of AgRP2 (Agulleiro et al., 2014), suggesting an isoform-specific orexigenic action.
Within Salmoniformes, there is conflicting data with regards to the actions of AgRP. In Arctic charr Salvelinus alpinus, non-feeding fish have higher brain AgRP expression levels than feeding fish (Striberny et al., 2015) and transgenic coho salmon Oncorhynchus kisutch, which display increased feeding, have higher brain AgRP1 levels of mRNA than wild-type fish (Kim et al., 2015), suggesting an orexigenic role for AgRP. However, in Atlantic salmon, AgRP-1 brain mRNA levels decrease after fasting (Murashita et al., 2009a) and increase after feeding (Valen et al., 2011), rather pointing to an anorexigenic role.
Galanin
Galanin is a peptide expressed in both central nervous system and GIT, that regulates diverse physiological functions in mammals, including arousal/sleep, feeding, energy metabolism, and reproduction (Merchenthaler, 2010). Galanin and its receptors have been identified in a number of fish species (see review in Mensah et al., 2010). Central injections of galanin stimulate feeding in Cypriniformes (both goldfish de Pedro et al., 1995; Volkoff and Peter, 2001b, and tench, Tinca tinca Guijarro et al., 1999). In goldfish, brain galanin mRNA expression is not affected by fasting but increases post-prandially in unfed fish (Unniappan et al., 2004) and in zebrafish, fasting up-regulates brain mRNA expression of galanin receptors (Li et al., 2013). These data suggest that the galanin system is involved in the regulation of feeding in Cypriniformes, and perhaps other fish.
Melanin concentrating hormone (MCH)
Melanin concentrating hormone is a peptide originally isolated from the pituitary of chum salmon (Oncorhynchus keta) as a hormone involved in body color change (Kawauchi et al., 1983). MCH was later isolated in mammals and shown to stimulate feeding (Qu et al., 1996). In fish, the role of MCH as an appetite regulator is still unclear.
In Cypriniformes, early immunoreativity (ir) studies in goldfish showed the presence of MCH in neuron populations related to the regulation of feeding and of sleep and arousal (Huesa et al., 2005). In goldfish, central injections of MCH decrease feeding but have no effect on locomotor activity (Shimakura et al., 2006), anti-MCH serum treatments increase feeding (Matsuda et al., 2007a), and the number of certain hypothalamic neuronal cell bodies containing MCH-ir decreases in fasted fish (Matsuda et al., 2007a), altogether suggesting an anorexigenic role for MCH in this species. However, in Ya fish, MCH hypothalamic mRNA expression is higher in fasted compared to fed fish, suggesting an orexigenic role (Wang et al., 2016). Data on Gadiformes and Pleuronectiformes also seem to suggest an appetite-stimulating role for MCH: MCH brain mRNA levels increase during fasting in both Atlantic cod Gadus morhua (Tuziak and Volkoff, 2013a) and winter flounder Pseudopleuronectes americanus (Tuziak and Volkoff, 2012), and in cod fed diets with relatively high amounts of plant (camelina) material (Tuziak et al., 2014). In starry (Platichthys stellatus; Kang and Kim, 2013b), olive (Paralichthys olivaceus; Kang and Kim, 2013a) and Barfin (Verasper moseri; Takahashi et al., 2004) flounders, fish placed in light backgrounds have enhanced appetite and growth, which is concomitant with increased expression levels of MCH mRNA and/or numbers of MCH neurons in the brain. However, in medaka Oryzias latipes, transgenic fish overexpressing MCH have normal growth and feeding behavior (Qu et al., 1996) and in the scalloped hammerhead shark Sphyrna lewini, hypothalamic MCH mRNA levels are not affected by fasting (Mizusawa et al., 2012), suggesting little or no role of MCH in feeding regulation of Beloniformes and sharks.
Neuronal relationship between MCH- and NPY-containing neurons have been shown in goldfish (Matsuda et al., 2009) and MCH treatment increases orexin mRNA expression and decreases NPY mRNA expression in cultured goldfish forebrain slices (Matsuda et al., 2009), suggesting an interaction of MCH with appetite regulators in goldfish. Similarly, in red-bellied piranha Pygocentrus nattereri, orexin and MCH co-localize in pituitary and brain (Suzuki et al., 2007), and in Barfin flounder, close contacts are seen between orexin- and MCH-ir cell bodies and fibers in the hypothalamus, suggesting an interaction between the two systems and a possible role for MCH in the modulation of locomotion and feeding (Amiya et al., 2008).
Neuropeptide Y (NPY)
Neuropeptide Y (NPY) belongs to the NPY family of peptides, which also includes, peptide YY and pancreatic polypeptide (PP) (Holzer et al., 2012). Originally isolated from mammalian brain extracts (Tatemoto et al., 1982), NPY is one of the most abundant neuropeptides within the brain and has a major regulatory role in energy homeostasis and food intake (Loh et al., 2015).
Although reports for NPY-like ir in fish brain and other tissues appear in the 1980's (e.g., Osborne et al., 1985; Danger et al., 1990), the first fish NPY cDNAs were reported in goldfish and the electric ray Torpedo marmorata (elasmobranch, Torpediniformes; Blomqvist et al., 1992). One of the first studies showing the role of NPY in regulating in fish was that of Silverstein et al., showing by in situ hybridization (ISH) that, in chinook salmon (Oncorhynchus tshawytscha) and coho salmon, NPY-like mRNA signal areas were greater in fasted than fed fish (Silverstein et al., 1998). The first in vivo injection studies were performed in goldfish (Lopez-Patino et al., 1999; de Pedro et al., 2000; Narnaware et al., 2000) and channel catfish Ictalurus punctatus (Silverstein and Plysetskaya, 2000). Since then, NPY has been one of the most studied appetite-regulating hormones in fish. It has been cloned and/or shown to regulate feeding in several groups, including Characiformes (Pereira et al., 2015), Cypriniformes [(e.g., goldfish, zebrafish (Yokobori et al., 2012), blunt snout bream Megalobrama amblycephala (Xu et al., 2016), grass carp Ctenopharyngodon idellus (Jin et al., 2015), Jian carp (Cyprinus carpio) (Tang et al., 2014), Ya fish (Wei et al., 2014)], Gadiformes (Atlantic cod Kortner et al., 2011; Tuziak et al., 2014); Gonorynchiformes (milkfish Chanos chanos, Lin et al., 2016); Perciformes (yellowtail Seriola quinqueradiata Hosomi et al., 2014, Astatotilapia burtoni Grone et al., 2012, cunner Tautogolabrus adspersus Babichuk and Volkoff, 2013, orange-spotted grouper Epinephelus coioides Tang et al., 2013, sea bass Leal et al., 2013, mandarin fish, Siniperca chuatsi Sun et al., 2014, cobia Rachycentron canadum Van Nguyen et al., 2013, gourami Trichogaster pectoralis Boonanuntanasarn et al., 2012); Pleuronectiformes (olive flounder Wang et al., 2015, winter flounder MacDonald and Volkoff, 2009a, Brazilian flounder Paralichthys orbignyanus Campos et al., 2012), Salmoniformes (e.g., rainbow trout Oncorhynchus mykiss Aldegunde and Mancebo, 2006, Atlantic salmon Valen et al., 2011; Kim et al., 2015), Siluriformes (channel catfish, Peterson et al., 2012; Schroeter et al., 2015); Tetraodontiformes (tiger puffer Takifugu rubripes Kamijo et al., 2011) as well as elasmobranchs [(e.g., winter skate Leucoraja ocellata, Rajiforme (MacDonald and Volkoff, 2009b) and spotted catshark (Scyliorhinus canicula, Carcharhiniforme) Mulley et al., 2014)] and holocephalans (elephant fish Chimaeriformes; Larsson et al., 2009). The majority of these studies indicate that NPY has a widespread distribution and is present in both brain and intestinal tract, that it acts as an orexigenic factor and that its expression is affected by feeding and fasting.
Orexin
Orexins (also called hypocretins) are neuropeptides originally isolated in rats (Sakurai, 2014), that have since been identified in several fish species. The first direct evidence of an orexigenic action of orexins was shown via intracerebroventricular (ICV) injections in goldfish (Volkoff et al., 1999). As in mammals (Tsujino and Sakurai, 2009; Sakurai, 2014), orexins increase not only appetite and feeding behavior but also locomotor activity and reward-seeking/foraging behavior in fish (Panula, 2010).
In both goldfish (Volkoff et al., 1999; Nakamachi et al., 2006; Facciolo et al., 2011) and zebrafish (Danio rerio) (Yokobori et al., 2011) (Cypriniformes), and cavefish (Astyanax mexicanus) (Characiforme) (Penney and Volkoff, 2014), orexin injections increase searching/feeding behaviors. In orange-spotted grouper (Perciforme), intraperitoneal (IP) orexin injections increase hypothalamic mRNA expression levels of NPY, a major appetite stimulator (Yan et al., 2011), further suggesting an orexigenic role. However, in ornate wrasse (Thalassoma pavo) (Perciforme), orexin IP injections induce increases in locomotion but decreases in feeding (Facciolo et al., 2009), suggesting that the major role of orexin might be induction of hyperactivity rather than increasing food ingestion. Indeed, in goldfish, hypothalamic orexin mRNA expression levels peak when fish are active prior to a scheduled meal (Hoskins and Volkoff, 2012) and in zebrafish, increased locomotor activity is associated with increased activity of hypothalamic orexin neurons (Naumann et al., 2010) and larvae overexpressing orexin are hyperactive (Woods et al., 2014). Similarly, orexin expression decreases post-feeding in Characiformes [cavefish (Wall and Volkoff, 2013), dourado (Salminus brasiliensis) (Volkoff et al., 2016) and pacu (Piaractus mesopotamicus) (Volkoff et al., 2017)] and is higher at mealtime in orange-spotted grouper (Yan et al., 2011) and tilapia (Chen et al., 2011) (Perciformes), as well as Atlantic cod (Gadiforme) (Xu and Volkoff, 2007). In cod, orexin levels are also higher during daylight hours, when animals are active (Hoskins and Volkoff, 2012).
Fasting increases orexin brain mRNA expression in Cypriniformes (goldfish Abbott and Volkoff, 2011 and zebrafish Yokobori et al., 2011), Characiformes (cavefish Wall and Volkoff, 2013, dourado Volkoff et al., 2016, pacu Volkoff et al., 2017, and red-bellied piranha Volkoff, 2014a), and Pleuronectiformes (winter flounder Buckley et al., 2010 and Barfin flounder Amiya et al., 2012). In the mouth-brooding Astatotilapia burtoni (Perciforme), brain orexin mRNA levels increase in non-feeding females carrying eggs (Grone et al., 2012). In Atlantic cod (Gadiforme), orexin brain expression levels are higher in fish fed low rations than in fish fed high rations (Xu and Volkoff, 2007) or in fish fed the 30% camelina (plant) meal diet compared to fish fed a control (fish) diet (Tuziak et al., 2014), suggesting an effect of food quality and quantity on orexin expression. However, torpid cunner (Peciforme, labridae) undergoing a long-term fasting have low brain and gut orexin expression levels (Babichuk and Volkoff, 2013; Hayes and Volkoff, 2014), but this decrease might be due to a toprpor-induced general metabolic shutdown.
Anatomical studies provide further evidence for a role of orexin in nutrient digestion/abrorption and growth. In several fish species, e.g., pirapitinga (Piaractus brachypomus) (Characiforme) (Volkoff, 2015a), cunner (Perciforme) (Hayes and Volkoff, 2014) and rainbow trout (Salmoniforme) (Varricchio et al., 2015), orexin mRNA/protein expression is high in the gastrointestinal tract, suggesting a role of the orexin system in regulating feeding and digestive processes. Among Perciformes, in Japanese sea perch (Lateolabrax japonicus), orexin-like ir is present in pituitary GH-containing cells, suggesting a control of growth by the orexin system (Suzuki et al., 2007) and in Cichlasoma dimerus, orexin-ir fibers are present in both hypothalamus and in pituitary, suggesting a neuroendocrine control of pituitary secretions (Pérez Sirkin et al., 2013).
In addition to teleosts, orexin has been examined in the primitive bony fish birchir Polypterus senegalus and rope fish Erpetoichthys calabaricus (Chondrosteans, Polypteriformes) for which the brain orexin ir patterns are similar to that of other fish examined (López et al., 2014) and in the Chondrichthyan winter skate (Rajiforme), in which fasting increases hypothalamic orexin expression (MacDonald and Volkoff, 2010).
Overall, it appears that in all fish species studied to date, orexin is related to both food intake and appetitive/searching behavior and perhaps to growth.
Anorexigenic Factors
CART
CART is a peptide which transcript expression is regulated by administration of cocaine or amphetamine in rodents (Vicentic and Jones, 2007; Subhedar et al., 2014) and amphetamine in goldfish (Volkoff, 2013). CART acts as an anorexigenic factor in mammals (Larsen and Hunter, 2006), and was first identified and shown to be anorexigenic in goldfish (Volkoff and Peter, 2000, 2001a).
Two CART isoforms have been identified in goldfish (Volkoff and Peter, 2001a) and common carp (Wan et al., 2012), and 4 in zebrafish (Akash et al., 2014) whereas, to date, only one form has been isolated for grass carp (Zhou et al., 2013; Liu et al., 2014), Characiformes [pirapitinga (serrasalmidae) (Volkoff, 2015a), pacu (serrasasalmidae) (Volkoff et al., 2017) and dourado (characidae) (Volkoff et al., 2016), red bellied piranha (serrasalmidae) (Volkoff, 2014a)], Salmoniformes [Atlantic salmon (Murashita et al., 2009a), rainbow trout (Figueiredo-Silva et al., 2012), Arctic charr (Striberny et al., 2015) and lake trout (Salvelinus namaycush) (Volkoff et al., 2007)], Siluriformes (channel catfish Kobayashi et al., 2008), Gadiformes (Atlantic cod Kehoe and Volkoff, 2007), Perciformes (cunner Babichuk and Volkoff, 2013), winter flounder (MacDonald and Volkoff, 2009a) and Atlantic halibut (Hippoglossus hippoglossus) (Gomes et al., 2015) (Pleuronectiformes), venomous toadfish Thalassophryne nattereri (Batrachoidiforme) (Magalhaes et al., 2006), rainbow smelt (Osmerus mordax) (Osmeriforme), pufferfishes (Takifugu rubripes and Tetraodon nigroviridis, Tetraodontiforme) and stickleback Gasterosteus aculeatus (Gasterosteiforme) (cited in Murashita et al., 2009a). However, six forms of CART have been identified in the medaka (Beloniforme) (Murashita and Kurokawa, 2011) and seven forms in Senegalese sole Solea senegalensis (Pleuronectiforme), the highest number of CART genes reported to date in a vertebrate species (Bonacic et al., 2015). The only elasmobranch CART identified to date is that of winter skate (Rajiforme) (MacDonald and Volkoff, 2009b).
CART injections induce a decrease in food intake and an increase in locomotion in goldfish (Volkoff and Peter, 2000) and enhance responsiveness to sensory stimuli in zebrafish larvae (Woods et al., 2014), suggesting that CART is involved in feeding/searching behaviors in cyprinids.
Fasting/food restriction decreases CART brain expression in Cypriniformes (goldfish Volkoff and Peter, 2001a, zebrafish Nishio et al., 2012; Guillot et al., 2016 and common carp, Wan et al., 2012), most Characiformes (red-bellied piranha Volkoff, 2014a, and pacu Volkoff et al., 2017), most Salmoniformes (Atlantic salmon, Murashita et al., 2009a; Kousoulaki et al., 2013, rainbow trout Figueiredo-Silva et al., 2012), Atlantic cod (Kehoe and Volkoff, 2007), cunner (Perciforme) (Babichuk and Volkoff, 2013), medaka (CART3) (Murashita and Kurokawa, 2011), and Siluriformes (channel catfish Kobayashi et al., 2008, African sharptooth catfish Clarias gariepinus Subhedar et al., 2011), suggesting an anorexigenic role for CART in teleost fish. Postprandial increases in CART brain expression have been shown in Senegalese sole (CART1a, CART 2a and CART4) (Bonacic et al., 2015), pacu (Volkoff et al., 2017), dourado (Volkoff et al., 2016), channel catfish (Peterson et al., 2012) but not in cod (Kehoe and Volkoff, 2007).
However, in Arctic charr, CART hypothalamic expression is similar throughout the seasonal feeding cycles (Striberny et al., 2015) and fasting does not affect CART expression in either dourado (Volkoff et al., 2016), winter flounder (MacDonald and Volkoff, 2009a) or Atlantic halibut larvae (Gomes et al., 2015), and in lake trout, fish exposed to the pesticide tebufenozide and control fish have similar food intakes, despite higher CART mRNA brain expression levels in exposed fish (Volkoff et al., 2007). In winter skate, 2 weeks of fasting have no effects on brain CART expression (MacDonald and Volkoff, 2009b), suggesting that CART might not have a major feeding-regulating role in elasmobranchs.
CART expression does not appear to be affected by diet, as in both cod fed a camelina (plant) diet (Tuziak et al., 2014) or rotifers or zooplankton (Katan et al., 2016) and pacu fed soybean concentrate (Volkoff et al., 2017), similar CART brain expression are seen between experimental and control fish.
Overall, there is a large interspecific variation in the number of forms and responses to fasting in the CART system in fish, although most studies tend to show that CART is mostly a central factor that might act as an appetite inhibitor.
Pro-opiomelanocortin (POMC) family of peptides
Proopiomelanocortin (POMC) is a common precursor that is processed post-translationally to generate melanocortin peptides [α-, β-, and γ-melanocyte-stimulating hormone (α-, β-, γ-MSH)], adrenocorticotropic hormone (ACTH) and other hormones that include β-endorphin (β-END) and β-lipotropic hormone (β-LPH) (Adan et al., 2006; Takahashi, 2016). POMC is mainly produced in the vertebrate pituitary, but is also found in brain, in particular the arcuate nucleus (ARC) of the hypothalamus. Receptors for melanocortin peptides include five subtypes (MC1R- MC5R) (Takahashi, 2016). In mammals, POMC and α-MSH have been shown to be involved in the regulation of appetite and energy homeostasis: POMC neurons suppress appetite by releasing α-MSH, which is an agonist at the anorectic melanocortin-4 receptor (MC4R) (Adan et al., 2006; Cone, 2006; Sohn, 2015).
Teleost fish lack γ-MSH and the POMC gene encodes an extra MSH (δ-MSH) in elasmobranchs (Cérda-Reverter et al., 2011). Fish POMC was first identified in Salmoniformes (Kawauchi, 1983; Kitahara et al., 1988) and Cypriniformes (Arends et al., 1998), followed by the identification of several forms in other fish species. As in other vertebrates, fish POMC is mainly expressed in the pituitary gland, but also within the lateral tuberal nucleus, which is equivalent to the mammalian ARC (Cérda-Reverter et al., 2011). POMC, α-MSH and the MC4R have been shown to regulate feeding in a few fish species.
In goldfish, fasting does not seem to affect hypothalamic POMC mRNA expression levels (Cerdá-Reverter et al., 2003), but ICV administration of [Nle4, d-Phe7]- α-MSH, a melanocortin agonist, inhibits food intake (Cerdá-Reverter et al., 2003), suggesting the melanocortin system participates in central regulation of food intake in Cypriniformes (Cerdá-Reverter et al., 2003). In addition, ICV injections of a MSH (MC4R) receptor agonist (melanotan II) suppress hypothalamic NPY expression (Kojima et al., 2010), and hypothalamic α-MSH-containing neurons are in close contact to NPY-containing nerve fibers, suggesting that the anorexigenic actions of the melanocortin system are mediated in part by an inhibition of the NPY system. In zebrafish larvae, although early ISH studies could not detect fasting-induced changes in hypothalamic POMC transcript levels (Song et al., 2003), more recent qPCR studies indicate that POMCa expression decreases in starved fish (Shanshan et al., 2016). In addition, GH-transgenic zebrafish, who have increased feeding, display down-regulation of POMC (Dalmolin et al., 2015), consistent with an anorexigenic role for POMC-derived peptides in Cypriniformes.
Similarly, in salmonids, POMC/α-MSH appears to have an anorexigenic role. In coho salmon, IP injections of α-MSH decrease food intake (White et al., 2016), in rainbow trout, fasting induces a decrease in hypothalamic expression of POMC-A1 (but not POMC-A2 or POMC-B) (Leder and Silverstein, 2006), and in Atlantic salmon, expression of both POMC-A1 and POMC-B increase after feeding (Valen et al., 2011). Interestingly, α-MSH treatment does not affect feeding of GH-transgenic coho salmon (White et al., 2016), despite similar hypothalamic POMC and MC4R mRNA expression levels compared to non-transgenic fish (Kim et al., 2015), suggesting that the actions of α-MSH might be inhibited by high expression levels of GH and/or AgRP.
In both olive (Kang and Kim, 2015) and Barfin flounder (Takahashi et al., 2005) (Pleuronectiformes), pituitary POMC-C (isoforms 1, 2, and 3) mRNAs are not affected by fasting, suggesting pituitary POMC might not directly related to appetite regulation. However, in fasted halibut larvae, whole brain POMC-C mRNA expression is higher in unfed fish 30 min after re-feeding compared to continuously fed fish (Gomes et al., 2015), suggesting a short-term regulation of appetite. Given the small number of studies available, and the variation in experimental protocols (adults vs. larvae, pituitary vs. brain, long-term vs. short-term feeding), conclusions are difficult to drawn regarding the role of POMC in flatfish.
Major Peripheral Factors
Ghrelin
Originally discovered in rat stomach as an endogenous ligand to the GH secretagogue-receptor (Kojima et al., 1999) ghrelin is the only known orexigenic factor in the GIT of mammals (Higgins et al., 2007). In the 2000's, a ghrelin-like peptide which stimulated GH release was first described in Nile tilapia (Oreochromis mossambicus; Shepherd et al., 2000) and a ghrelin-ir peptide was first detected in burbot (Lota lota) plasma (Mustonen et al., 2002). Using goldfish as a model, Unniappan et al. provided the first fish ghrelin cDNA sequence and the first evidence of an orexigenic role for ghrelin in fish, as central injections of ghrelin stimulated food intake (Unniappan et al., 2002). Subsequent studies on several fish species reported sequences for ghrelin and confirmed its role as an appetite stimulator in fish (see Jönsson, 2013 for a review), including other Cypriniformes [e.g., goldfish (Kang et al., 2011; Nisembaum et al., 2014; Blanco et al., 2016a); gibel carp (Carassius auratus gibelio) (Zhou et al., 2016); Schizothorax davidi (Zhou et al., 2014)], Characiformes (red-bellied piranha Volkoff, 2015b), Perciformes (Nile tilapia Schwandt et al., 2010), for which fasting-induced and periprandial changes in expression/protein levels occur. In Salmoniformes, there is contradictory evidence. In rainbow trout, central ghrelin injections and long-term peripheral treatment both decrease food intake compared to controls (Jönsson et al., 2010) and in Atlantic salmon, ghrelin plasma levels are lower in fasted fish compared with fed fish (Hevrøy et al., 2011) and show no clear periprandial changes (Vikesa et al., 2015), suggesting that ghrelin might have little effect or an inhibitory effect on feeding of in salmonids. In contrast, in brown trout (Salmo truta), ghrelin treatment increases foraging activity (Tinoco et al., 2014a). In rainbow trout, ICV ghrelin injections induce changes in parameters related to hepatic lipid metabolism (Velasco et al., 2016), suggesting a role of ghrelin in metabolism and nutrient storage. In yellow catfish (Pelteobagrus fulvidraco) (Siluriforme), although fasting increases ghrelin expression (Zhang et al., 2016a), no periprandial differences in plasma or stomach ghrelin expression are observed (Peterson et al., 2012).
It thus seems that the role of ghrelin in the regulation of feeding and metabolism of fish is still unclear, and might be species- and form-specific, so that further studies on more species are required.
Anorexigenic Factors
Cholecystokinin (CCK)
In mammals, CCK inhibits food intake and induces the release of digestive enzymes from intestine/pancreas and gallbladder (Boguszewski et al., 2010; Dockray, 2012).
In fish, CCK was first shown to have a role in digestion, as, for example, it stimulated contraction of the gallbadder in coho (Vigna and Gorbman, 1977) and Atlantic (Aldman and Holmgren, 1987) salmon, as well as bluegill (Lepomis macrochirus), killifish (Fundulus heteroclitus), and the holostean bowfin (Amia calva) (Rajjo et al., 1988), stimulated lipase secretion in the stomachless killifish (Honkanen et al., 1988) and inhibited gastric secretion in Atlantic cod (Holstein, 1982). The first direct evidence of the actions of CCK on feeding was provided by injections in goldfish (Himick and Peter, 1994), followed by cloning of goldfish CCK cDNA (Peyon et al., 1998) and the demonstration of periprandial variations in CCK mRNA expression levels (Peyon et al., 1999). Subsequently, a number of studies have characterized CCK in several fish, including other Cypriniformes (e.g., common carp Zhong et al., 2013; zebrafish Koven and Schulte, 2012; Tian et al., 2015; grass carp; blunt snout bream Ping et al., 2013; Ji et al., 2015), Characiformes (e.g., cavefish Wall and Volkoff, 2013, dourado Pereira et al., 2015; Volkoff et al., 2016, thin dogfish Oligosarcus hepsetus Vieira-Lopes et al., 2013, pirapitinga Volkoff, 2015a, red-bellied piranha Volkoff, 2014a, pacu Volkoff et al., 2017), Salmoniformes (e.g., Atlantic salmon Valen et al., 2011), Gadiformes (Atlantic cod Tillner et al., 2013), Perciformes [e.g., yellowtail (Furutani et al., 2013; Hosomi et al., 2014); Astatotilapia burtoni (Grone et al., 2012); cunner (Babichuk and Volkoff, 2013; Hayes and Volkoff, 2014); sea bass (Tillner et al., 2014); yellow croaker (Larimichthys crocea) (Cai et al., 2015); white sea bream, Diplodus sargus (Micale et al., 2012, 2014)], Pleuronectiformes (e.g., winter flounder (MacDonald and Volkoff, 2009a), Atlantic halibut Kamisaka et al., 2001, olive flounder Kurokawa et al., 2000) and Siluriformes (channel catfish Peterson et al., 2012).
Overall, in all fish species studied to date, CCK appears to have similar roles in feeding and digestive processes to its role in mammals, i.e., it acts as a satiety/appetite-inhibiting factor and induces the release of digestive enzymes from the GIT.
Leptin
Leptin, a peptide originally cloned in obese ob/ob mice (Zhang et al., 1994), is secreted in mammals mainly by white adipose tissue, and its blood levels are proportional to body fat content (Park and Ahima, 2015). Leptin is a multifunctional hormone in both mammals (Park and Ahima, 2015) and fish (see review by Gorissen and Flik, 2014) and is involved in the regulation of not only food intake and body weight, but also reproduction, development and stress responses.
First hints of a role of leptin in fish were provided by reports of a decrease in feeding in goldfish ICV-injected with human leptin (Volkoff et al., 2003). The first fish leptin was identified in the pufferfish genome in 2005 by synteny studies (Kurokawa et al., 2005), followed by isolation of zebrafish, medaka, and carp leptins (Huising et al., 2006b). Since then, leptins have been identified in several fish species and shown to have multiple physiological functions (reviewed in Copeland et al., 2011; Angotzi et al., 2013; Londraville et al., 2014). As opposed to mammals who have a single leptin gene, several fish species have several leptin gene paralogs (e.g., lepA and lepB). Also in contrast to mammals, where subcutaneous fat is the main source of leptin, fish leptin is expressed in several tissues including liver and intestine, which is consistent with the fact that fish generally store lipids in intra-abdominal regions and liver (Birsoy et al., 2013).
Most studies on fish leptin have been conducted in Cypriniformes, in particular goldfish and zebrafish, and Salmoniformes. In goldfish, leptin injections decrease feeding and locomotor behavior (Volkoff et al., 2003; de Pedro et al., 2006; Vivas et al., 2011; Tinoco et al., 2012) in part by stimulating anorexigenic sytems (e.g., CART, CCK, and POMC) and inhibiting orexigenic ones (e.g., orexin, NPY, AgRP) (Volkoff et al., 2003; Yan et al., 2016). Similarly, in rainbow trout (Salmoniforme), central leptin administration suppresses food intake and increases the hypothalamic expressions of CART and POMC (Gong et al., 2016). Leptin treatment also inhibits feeding in grass carp (Li et al., 2010) (Cypriniforme) and increases energy expenditure in zebrafish larvae (Renquist et al., 2013). In Atlantic salmon (Salmoniforme), chronic IP treatment with leptin induces a decrease in growth rates (Murashita et al., 2011), and in hybrid striped bass (Morone saxatilis × Morone chrysops) (Perciforme), leptin treatment increases hepatic IGF-1 mRNA expression (Won et al., 2016), suggesting that leptin affects metabolism and growth.
Hepatic/gut/brain leptin increases in expressions are seen post-prandially in goldfish (Tinoco et al., 2012, 2014b), common carp (Huising et al., 2006a) and zebrafish (Tian et al., 2015) (Cypriniformes) as well as pacu (Volkoff et al., 2017) (Characiforme). However, in rainbow trout plasma leptin levels decrease post-feeding (Johansson and Björnsson, 2015).
There is a great variability in results with regards to fasting-induced changes in the leptin system. In goldfish, no significant differences in either brain or liver leptin expressions are seen between control, overfed and fasting fish, suggesting nutritional status does not affect the leptin system in goldfish (Tinoco et al., 2012). Similarly, leptin expression is not affected by fasting in the liver of common carp (Huising et al., 2006a) (Cyrpiniforme) and Nile tilapia (Shpilman et al., 2014) (Perciforme) or in the brains of red-bellied piranha (Volkoff, 2015b) and pacu (Volkoff et al., 2017) (Characiformes). However, fasting/food restriction increases hepatic leptin expression in white-clouds mountain minnow (Tanichthys albonubes, Cypriniforme; Chen et al., 2016b), in most Perciformes examined (orange-spotted grouper Zhang et al., 2013, mandarin fish Yuan et al., 2016, and mackerel Scomber japonicus Ohga et al., 2015, European sea bass Gambardella et al., 2012), in Arctic charr (Jørgensen et al., 2013) and Atlantic salmon (Rønnestad et al., 2010; Trombley et al., 2012; Moen and Finn, 2013) (Salmoniformes). In contrast, decreases in leptin expression are seen in liver of zebrafish (lepA) (Gorissen et al., 2009) and striped bass (Morone saxatilis) (lepB, perciforme) (Won et al., 2012) and intestine of red-bellied piranha (Volkoff, 2015b), and in blunt snout bream (Cypriniforme), higher feeding rates are associated with increased leptin pituitary expression (Xu et al., 2016). Whereas plasma leptin levels increase following fasting in rainbow trout (Salmeron et al., 2015; Johansson et al., 2016; Pfundt et al., 2016), Atlantic salmon (Trombley et al., 2012) and fine flounder Paralichthys adspersus (Pleuronectiforme) (Fuentes et al., 2012, 2013), they have been shown to decrease in earlier studies in fasted burbot (Lota lota) (Gadiforme) (Nieminen et al., 2003) and green sunfish (Lepomis cyanellus) (Perciforme) (Johnson et al., 2000).
In fish, leptin has been linked to metabolism. For example, in zebrafish, knocking down lepA decreases metabolic rate (Dalman et al., 2013) and in golden pompano, Trachinotus blochii (Perciforme), lepA gene polymorphisms are associated with different body weights, heights and lengths (Wu et al., 2016). Whereas in mammals, leptin acts as an adipostat and its plasma levels are proportional to the amount of body fat, there is little evidence for such a role in fish. In topmouth culter Culter alburnus (Cyprinoforme), leptin mRNA expression is lower in wild populations, who have more muscle fat content than cultured fish (Wang et al., 2013), in grass carp, fish fed high fat diets have higher leptin expression (Li A. et al., 2016) than control fish, and in medaka, leptin receptor null-mutants have higher food intake and larger deposits of visceral fat than that of wild-type fish (Chisada et al., 2014), suggesting a correlation between leptin levels and fat. However, results from other studies seem to contradict this hypothesis: leptin receptor null adult zebrafish do not exhibit increased feeding or adiposity (Michel et al., 2016); In rainbow trout, leptin levels are higher in lean fish than fat fish (Salmeron et al., 2015; Johansson et al., 2016; Pfundt et al., 2016), and in Arctic charr, neither hepatic leptin expression nor plasma leptin levels correlate with fish adiposity (Froiland et al., 2012; Jørgensen et al., 2013); In murray cod Maccullochella peelii peelii (Perciforme), fish fed different experimental diets containing fish oil with or without vegetable oil have similar leptin levels (Ettore et al., 2012; Varricchio et al., 2012); In yellow catfish (Siluriforme), IP injections of human leptin reduce hepatic lipid content and the activities of lipogenic enzymes (Song et al., 2015) but Zn deficiency, which tends to increase hepatic and muscle lipid contents, does not affect leptin mRNA levels (Zheng et al., 2015).
Zebrafish lacking a functional leptin receptor have alterations in insulin and glucose levels, suggesting a role of leptin in the control of glucose homeostasis (Michel et al., 2016), which is consistent with data showing that leptin gene expression is induced by glucose in grass carp (Lu et al., 2015) and that leptin injections increase plasma glucose levels in Nile tilapia (Baltzegar et al., 2014).
Interestingly, in the Gymnotiforme Eigenmannia virescens, intramuscular injections of leptin increase electric organ discharges (EOD) amplitude in food-deprived but not well-fed fish, suggesting that leptin mediates EOD responses to metabolic stress in electric fish (Sinnett and Markham, 2015).
Overall, there seems to be a great species-specific variability in the functions of leptin with regards to the regulation of feeding and metabolism in fish, perhaps due to different lipid metabolism and storage areas among fish species.
Peptide YY
Peptide YY consists of two forms, PYYa and PYYb (previously called PY) (Wahlestedt and Reis, 1993; Cerdá-Reverter and Larhammar, 2000; Sundström et al., 2013) and is a brain-gut peptide that acts as an anorexigenic signal in mammals (Blevins et al., 2008; Karra et al., 2009; Zhang et al., 2012). Interestingly, one of the first studies showing an effect of PYY on feeding in mammals used fish PYY (Balasubramaniam et al., 1992). PYY was first shown to be present in the gastrointestinal tract of fish by immunochemical methods in the 1980's (daddy sculpin Cottus scorpius and Baltic sea cod Gadus morhua callarias El-Salhy, 1984) and first cloned and detected in the brain by ISH in an Agnatha, the river lamprey (Lampetra fluviatilis; Söderberg et al., 1994). The first indirect evidence of a role for PYY in feeding in fish was provided in sea bass, in which PYY transcripts were detected in brain areas regulating feeding (Cerdá-Reverter et al., 2000) and the first direct evidence of an anorexigenic role for PYY in fish was provided by IP injections of goldfish PYY in goldfish (Gonzalez and Unniappan, 2010). Peripheral injections of species-specific PYY also decrease food intake in another cyprinid, the grass carp (Chen et al., 2013) and in Siberian sturgeon Acipenser baerii (Acipenseriformes) (Chen et al., 2015). However, in channel catfish (Siluriformes), human PYY injections do not affect food intake or plasma glucose levels or hypothalamic POMC expression (Schroeter et al., 2015), suggesting perhaps that species-specific PYYs are needed to elicit an effect on feeding.
Fasting induces decreases in brain PYY expression in both goldfish (Gonzalez and Unniappan, 2010) and Ya fish (Yuan et al., 2014) (Cypriniformes) and in PPY intestinal expression in red-bellied piranha (Characiforme,) (Volkoff, 2014a), suggesting a role in satiety. However, fasting does not affect brain PYY expression in either cavefish (Characiforme) (Wall and Volkoff, 2013) or red-bellied piranha (Volkoff, 2014a), either brain or gut PYY mRNA expression in Atlantic salmon (Salmoniforme) (Murashita et al., 2009b), and induces increases in PYY gut expression in both yellowtail (Perciformes) (Murashita et al., 2006, 2007) and Japanese grenadier anchovy Coilia nasus (Clupeiformes) (Yang et al., 2016).
PYY mRNA expression increases post-feeding in the brain of goldfish (Gonzalez and Unniappan, 2010) and Ya fish (Yuan et al., 2014), cave fish (Wall and Volkoff, 2013) and Siberian sturgeon (Chen et al., 2015), in the intestine of grass carp (Chen et al., 2014) and in whole larval Atlantic halibut (Pleuronectiformes) (Gomes et al., 2015). However, in Atlantic salmon, brain PYY expression shows no periprandial changes (Valen et al., 2011; Kousoulaki et al., 2013), perhaps suggesting that PYY does not play a major role as a short-term satiety factor in salmonids.
Overall, it appears that in most fish examined to date, PYY might acts as an anorectic/satiety peptide, although this does not seem to hold true for all fish species (e.g., salmon, yellowtail, or catfish).
Other Hormones and Systems
Hypothalamus-Pituitary-Thyroid Axis (HPT Axis)
The hypothalamic-pituitary-thyroid (HPT) axis regulates levels of thyroid hormones, which are essential for a number of biological functions, including food intake and energy expenditure. Hormones produced by the axis consist of thyrotropin releasing hormone (TRH), thyroid stimulating hormone (TSH) and thyroid hormones (triiodothyronine T3 and thyroxine T4) secreted by the hypothalamus, the pituitary and the thyroid gland, respectively (Fekete and Lechan, 2014).
In goldfish (Cypriniforme), ICV injections of TRH increase feeding and locomotor behaviors and the hypothalamic mRNA expressions of both orexin and CART (Abbott and Volkoff, 2011), and IP injections of T4 increase food intake and locomotion (Goodyear, 2012), suggesting an orexigenic role. Fasting increases TRH hypothalamic mRNA levels (Abbott and Volkoff, 2011), further suggesting that the HPT axis regulates feeding in goldfish. In Amur sturgeon, Acipenser schrenckii (Acipenseriforme), lower serum levels of thyroid hormones are seen in fish placed in high-density groups who display low feeding rates (Li et al., 2012). However, decreases in plasma levels of thyroid hormones are seen in fasted goldfish [T3] (Sinha et al., 2012) and in fasted channel catfish [T4 and T3] (Gaylord et al., 2001), suggesting that food deprivation might decrease the activity of the HPT at the level of thyroid hormone synthesis and secretion, similar to what is observed in mammals (Boelen et al., 2008). A decrease in circulating thyroid hormones might inhibit the thyroid hormone negative feedback action on hypothalamic cells and contribute to the increase in hypothalamic TRH expression levels seen in goldfish. Overall, these data suggest that, in fish, TRH and thyroid hormones might affect feeding and metabolism and that nutritional status might affect the HPT axis.
Reproductive Hypothalamus-Pituitary-Gonad (HPG) Axis
Gonadotropin releasing hormone (GnRH)
GnRH is a hypothalamic hormone that stimulates the release of pituitary gonadotropins, which in turn stimulate the release of gonadal steroids. Three major forms of GnRH are present in fish, GnRH 1, 2, and 3 (Roch et al., 2014). GnRH appears to act as an anorexigenic hormone, as in goldfish, ICV injections with GnRH2 not only stimulate spawning (Hoskins et al., 2008) but also decrease food intake (Hoskins et al., 2008; Matsuda et al., 2008) and hypothalamic orexin mRNA expression (Hoskins et al., 2008). Similarly, in zebrafish, ICV injections of GnRH2 decrease food intake (Nishiguchi et al., 2012). In addition, in goldfish, treatment with orexin stimulate feeding, inhibit spawning behavior, and decrease brain GnRH2 expression, suggesting a coordinated control of feeding and reproduction by the orexin and GnRH systems (Hoskins et al., 2008).
In winter flounder, fasting reduces both brain GnRH2 and GnRH3, but not GnRH1, mRNA expression levels (Tuziak and Volkoff, 2013b) and in zebrafish, GnRH2 brain mRNA levels increase in overfed fish (Nishiguchi et al., 2012). However, in Atlantic cod, neither GnRH2 nor GnRH3 brain transcripts are influenced by food deprivation (Tuziak and Volkoff, 2013a), suggesting that the role of GnRHs in the regulation of feeding might be species- and form-specific.
RFamides
RFamide peptides, first isolated in invertebrate species in the late 1970's and later found in vertebrates, act as neurotransmitters and neuromodulators. In vertebrates, the RFamide peptide family consists of PRL-releasing peptides (PrRP), PQRFamide peptides (neuropeptide FF, NPFF), pyroglutamylated RFamide peptide (QRFP)/26RFamides, LPXRFamide peptides (gonadotropin-inhibitory hormone, GnIH, in lower vertebrates, RFamide-related peptide-3, RFRP-3, in mammals) and kisspeptins (Tsutsui and Ubuka, 2013; Osugi et al., 2016). RFamides have been shown to regulate several physiological functions in vertebrates, including feeding (Bechtold and Luckman, 2007; Quillet et al., 2016). A number of RFamides have been identified in fish, although most have been examined for their role in reproduction and are not yet well characterized with regards to their potential role as feeding regulators.
In goldfish, IP or ICV administration of PrRP decrease food intake, and hypothalamic PrRP mRNA expression increases post-prandially and after food deprivation, suggesting an anorexigenic role for PrRP in goldfish (Kelly and Peter, 2006). In line with this hypothesis, in the euryhaline fish mudskipper (Periophthalmus modestus, Perciforme, gobidae), freshwater fish have lower food intake/growth rates than saltwater fish and higher brain and intestine PrRP mRNA expressions, suggesting that PrRP is involved in the regulation of feeding and energy homeostasis in this species (Sakamoto et al., 2002; Tachibana and Sakamoto, 2014).
Two neuropeptide FF receptor 1 (NPFFR1) genes have been identified in carp and shown to display variations in expression associated with growth-related traits (Peng et al., 2016). As NPFF1 is receptor for neuropeptide FF (NPFF) and the LPXRFamide peptide RFamide-related peptide (RFRP), which are involved in control of feeding behavior in both invertebrates and vertebrates, these data suggest that NPFFR1s might be related to the regulation of growth and body weight in common carp (Peng et al., 2016). Similarly, in seabass, LPXRFamide-ir cells and/or fibers are present in feeding, gustatory, sensory, and behavioral centers of the brain, suggesting that it could be involved in the regulation of foraging/feeding behavior (Paullada-Salmerón et al., 2016).
In goldfish, hypothalamic expression of 26RFa increases in fasted animals (Liu et al., 2009) and IP injections of human RFRP-3 decrease food intake (Mawhinney, 2007), indicating that these neuropeptides might regulate food intake and energy balance in cyprinid fish.
In sea bass, food-restricted male fish display an increase in both kisspeptin and kisspeptin receptor expressions in both pituitary and hypothalamus (Escobar et al., 2016), suggesting the kisspeptin system is affected by nutritional status. However, in goldfish, IP injections of mammalian kisspeptin appear to have no effect on feeding (Mawhinney, 2007).
CRF and the Hypothalamus-Pituitary-Interrenal (HPI) Axis
The major endocrine components of the hypothalamic–pituitary–adrenal (HPA) axis (or interrenal, HPI in lower vertebrates) are hypothalamic corticotropin-releasing factor (CRF, or corticotropin-releasing hormone, CRH), pituitary adrenocorticotropin (ACTH) and glucocorticoids (e.g., cortisol, corticosterone) from the adrenal/interrenal gland. CRF mediates the release of ACTH, which in turn stimulates the release of steroids by the adrenal/interrenal gland (Smith and Vale, 2006). The HPI axis regulates numerous physiological functions, including metabolic functions (e.g., blood glucose levels during fasting), food intake, reproduction, growth, and immunity. Urocortins (UCN) 1 (also termed urotensin 1 in fishes), 2, and 3 belong to a recently discovered family of CRF-related peptides, which functions are still not well characterized (Majzoub, 2006).
The role of the HPI axis in the regulation of feeding of fish has been examined in several fish species. In goldfish, ICV injections of CRF decrease feeding (De Pedro et al., 1993) and increase locomotor activity (Matsuda et al., 2013). In Ya fish, fasting decreases CRF brain expression levels (Wang et al., 2014) and goldfish exposed to the toxin fluoxetine have low food intake and increased brain expression of CRF (Mennigen et al., 2010), further suggesting an anorexigenic role for CRF in cyprinids. In goldfish, feeding fish with a diet containing low cortisol levels or implanting fish with cortisol-containing pellets result in higher food intake and CRF mRNA levels, compared to controls (Bernier et al., 2004). These results and others suggest that stress, cortisol and CRF can modulate food intake in Cypriniformes (Bernier et al., 2004).
In rainbow trout, CRF and urotensin 1 are anorexigenic, as ICV injections of either peptides inhibit feeding (Ortega et al., 2013). In addition, hypoxia stress suppresses appetite and increases forebrain CRF and urotensin mRNA levels, suggesting that, in Salmoniformes, CRF-related peptides might mediate the hypoxia-induced reduction in food intake (Bernier and Craig, 2005).
In Siberian sturgeon, IP injections of urocortin 3 inhibit feeding, and UCN3 brain mRNA expression levels increase post-feeding and decrease during fasting, suggesting that UCN3 acts as a satiety/anorexigenic factor in fish (Zhang et al., 2016c).
For more extensive reviews on the regulation of feeding by the HPI, please refer to previously published works, including (Bernier and Peter, 2001; Bernier, 2006; Flik et al., 2006; Lowry and Moore, 2006; Backström and Winberg, 2013).
“Novel” Appetite-Regulating Peptides
Amylin
Amylin (or islet amyloid polypeptide, IAPP), a hormone co-secreted with insulin from pancreatic β-cells, inhibits feeding in mammals (Riediger et al., 2003). In fish, the role of amylin in feeding has only been examined in goldfish. In this species, IP or ICV amylin treatments decrease food intake whereas ICV injections of an amylin receptor antagonist (AC 187) stimulate feeding (Thavanathan and Volkoff, 2006), suggesting an anorexigenic role for amylin in fish.
Apelin
Apelin is a peptide first identified in bovine stomach as a ligand for the orphan receptor APJ, with close identity to the angiotensin II (Ang II) receptor (Tatemoto et al., 1998; Habata et al., 1999) and subsequently shown to be involved in multiple physiological processes (see O'Carroll et al., 2013, for review) including feeding and metabolism in mammals: for example, apelin injections decrease food intake (O'Shea et al., 2003), and in adipocytes, apelin expression is inhibited by fasting (Boucher et al., 2005) and its secretion is regulated by insulin (Boucher et al., 2005).
In fish, apelin appear to be orexigenic: apelin injections increase food intake in goldfish (Volkoff and Wyatt, 2009) and cavefish (Penney and Volkoff, 2014). Fasting induces increases in brain apelin mRNA expression in Ya-fish (Lin et al., 2014a) and red-bellied piranha (Volkoff, 2014a). Moreover, in goldfish, the obesogen factor tributyltin (TBT) stimulates food intake and also increases brain apelin expression (Zhang et al., 2016b). In cavefish, IP injections of apelin increase orexin brain expression, and CCK injections induce a decrease in brain apelin expression (Penney and Volkoff, 2014), an indication that apelin interacts with other appetite regulators. Similarly, brain injections of the anorexigenic factor spexin reduce apelin brain expression (Wong et al., 2013) and in vitro treatment of brain fragments with apelin increase expressions of orexigenic peptides—i.e., orexin—and decrease CART expression (Volkoff, 2014b). Overall, the data suggest an orexigenic role for apelin in Cypriniformes. In cunner (Perciforme), summer fasting decreases intestinal apelin mRNA levels (Hayes and Volkoff, 2014), suggesting that GIT apelin might not be involved in the regulation of feeding. In common carp- but not in trout barb Capoeta trutta-, there is a negative correlation between apelin levels and body weight (Köprücü and Algül, 2015), suggesting that apelin might not be involved in metabolic processes leading to weight gain in some species.
Arginine Vasotocin
Arginine vasotocin (AVT) is the mammalian homolog of arginine vasopressin (AVP), and has been shown to have diverse and complex roles in fish physiology, including regulation of metabolic processes, stress responses and several behaviors (Balment et al., 2006). In rainbow trout, AVT treatments decrease feeding, and increase plasma levels of cortisol and glucose, brain serotonergic activity, and hypothalamic levels of POMC and CART, suggesting it acts as an anorexigenic factor in fish (Gesto et al., 2014).
Endocanabinoid System
In mammals, the endocannabinoid system (ECS), which consists of cannabinoid receptors (CB1 and CB2) and endogenous cannabinoids, is involved in the regulation of several physiological functions, including feeding and energy balance (Pagotto et al., 2006).
In goldfish, CB1 and CB2 are both expressed in brain, where CB1 co-localizes with NPY (Cottone et al., 2013). Treatment with low doses of the endocannabinoid receptor agonist anandamide (AEA) increases food intake (Valenti et al., 2005), and food deprivation increases CB1 and AEA brain mRNA levels (Cottone et al., 2009), suggesting the involvement of the ECS in the control of energy intake in Cypriniforme. Similarly, in sea bream Sparus aurata (Perciforme), AEA administered via water increases food intake and NPY brain mRNA levels (Piccinetti et al., 2010). In common sole, Solea solea (Pleuronectiforme), feeding fish with dietary nucleotides reduce CB1 brain transcript levels, suggesting that feeding and diets modulate the ECS (Palermo et al., 2013).
Nesfatin-1
Nesfatin-1, discovered in 2006 in mammals, is a peptide secreted from hypothalamic nuclei related to appetite regulation, from the precursor non-esterified fatty acid/nucleobinding 2 (NUCB2), and has been shown to reduce feeding and water intake in mammals (Ayada et al., 2015). In fish, the role of nesfatin-1 as an appetite regulator has been examined in Cypriniformes and Salmoniformes.
In goldfish, nesfatin-1 has been shown to be involved in the regulation of feeding and metabolism: nesfatin-1-like and ghrelin-like ir co-localize in both enteroendocrine and hypothalamic cells; IP or ICV injections of nesfatin-1 inhibit both food intake and brain expressions of ghrelin and NUCB2; and fasting increases both hepatic and hypothalamic NUCB2 mRNA levels (Gonzalez et al., 2010; Kerbel and Unniappan, 2012). In addition, NUCB2 mRNA levels increase in liver and hypothalamus in fish fed fat-enriched diets and decrease in gut after long-term feeding with a high-protein diet, suggesting that macronutrients regulate the expression of NUCB2/nesfatin-1 (Blanco et al., 2016a). In zebrafish, two isoforms of NUCB2 (NUCB2A and NUCB2B) exist, and both mRNAs decrease in the brain post-prandially and after food deprivation, suggesting an anorexigenic role for nesfatin-1 (Hatef et al., 2015). In Ya-fish, NUCB2A mRNA levels increase post-prandially in both hypothalamus and intestine, and fasting induces a decrease in NUCB2A mRNA levels in the hypothalamus, but an increase in the hepatopancreas, suggesting anorexigenic and metabolic roles (Lin et al., 2014b). However, in rainbow trout (Oncorhynchus mykiss), plasma nesfatin-1 levels are similar between fed and fasted females (Caldwell et al., 2014).
Neuropeptide B, Neuromedin S, and Neuromedin U
Neuropepide B (NPB), and neuromedins S (NMS) and U (NMU) are newly discovered mammalian short peptides that have been shown to affect feeding in fish.
NPB has been characterized in Nile tilapia (Perciforme), where it is expressed in brain and spinal cord. In this species, fasting increases NPB brain mRNA expression, and IP injections of NPB increase brain mRNA expression of NPY and CCK and inhibit pituitary GH expression, suggesting NPB is involved in feeding and growth in fish (Yang et al., 2014).
In both zebrafish (Chen et al., 2016a) and orange-spotted grouper (Li et al., 2015), an NMS-related protein (NMS-RP) has been identified that appears to act as an orexigenic factor. In both species, IP administration of species-specific NMS-RP increases both NPY and orexin expressions, and hypothalamic levels of NMS mRNA increase after food deprivation.
NMU has been characterized in Cypriniformes (carp, goldfish) and Perciformes (orange-spotted grouper). In both common carp (Kono et al., 2012) and goldfish (Maruyama et al., 2008), several forms of NMU (3–5) have been isolated and their mRNA expressions shown to decrease upon fasting, suggesting a role in feeding and metabolism (Kono et al., 2012). Similarly, in orange-spotted grouper, hypothalamic NMU mRNA levels decrease in fasted fish and increase post-feeding (Li et al., 2015), suggesting an anorexigenic role. In goldfish, central injections of NMU inhibit feeding and locomotor behaviors (Maruyama et al., 2008) and increase brain CRF mRNA expression levels (Maruyama et al., 2009) and in grouper, IP injections of NMU down-regulate hypothalamic NPY expression (Li et al., 2015), suggesting that the anorexigenic actions of NMU are mediated by the CRF system and an inhibition of the NPY system.
Obestatin
Obestatin, a gastrointestinal peptide discovered in 2005, is derived from the same precursor as ghrelin and inhibits food intake in mammals (Cowan et al., 2016). In grass carp, although IP injections of an obestatin-like peptide alone do not affect food intake or the expression levels of NPY, CART, or POMC, when co-injected with ghrelin, it blocks ghrelin-induced stimulation of appetite and up-regulation of expressions of NPY and NPY receptors (Yuan et al., 2015), suggesting that obestatin might inhibit the ghrelin system in Cypriniformes.
Octadecaneuropeptide
The octadecaneuropeptide (ODN) is a peptide belonging to the family of endozepines and is generated through the cleavage of diazepam-binding inhibitor (DBI) in the mammalian central nervous system (CNS) (Tonon et al., 2006). ODN acts as an inverse agonist of central-type benzodiazepine receptors (CBR) and inhibits food intake in rodents (do Rego et al., 2006).
Immunocytochemical methods first showed the presence in brain and pituitary of rainbow trout (Malagon et al., 1992) and more recently in the agnathan Atlantic hagfish, Myxine glutinosa (Myxiniforme, myxinidae; Candiani et al., 2000). Central injections of goldfish ODN inhibit food intake (Matsuda et al., 2007b) and stimulate locomotor activity (Matsuda et al., 2011b), and increase POMC brain mRNA levels (Matsuda et al., 2010), suggesting that the anorexigenic actions of ODN are in part mediated by the melanocortin system.
Pituitary Adenylate Cyclase Activating Polypeptide (PACAP)
Originally identified in the ovine hypothalamus (Miyata et al., 1989), pituitary adenylate cyclase-activating polypeptide (PACAP) belongs to the secretin/glucagon family of peptides that also includes secretin, glucagon, glucagon-like peptides and vasoactive intestinal peptide (Sherwood et al., 2000). In rodents, central injections of PACAP decrease food intake (Morley et al., 1992).
PACAP has been cloned in several fish, including Anguilliformes European eel (Anguilla anguilla) (Montero et al., 1998), Cypriniformes (e.g., zebrafish Sherwood et al., 2007, goldfish Matsuda et al., 1997), Gadiformes (cod Xu and Volkoff, 2009), Pleuronectiformes (e.g., olive flounder Nam et al., 2013), Salmoniformes (e.g., Atlantic salmon Parker et al., 1993), and Siluriformes (Thai catfish Clarias macrocephalus McRory et al., 1995, darkbarbel catfish Pelteobagrus vachelli Xu et al., 2012) as well as elasmobranchs (e.g., marbled electric ray Torpedo marmorata Agnese et al., 2016, stingray Dasyatis akajei Matsuda et al., 1998). In several fish, PACAP stimulates GH secretion by pituitary cells (see review in Gahete et al., 2009), but its role in regulating feeding is still unclear. In goldfish, central or peripheral PACAP injections inhibit food intake (Matsuda et al., 2005) and locomotor activity (Matsuda et al., 2006) and these actions might be mediated in part by the stimulation of POMC and CRH pathways (Matsuda and Maruyama, 2007). Similarly, in grass carp, central NPY injections decrease brain PACAP expression (Zhou et al., 2013), suggesting an anorexigenic role for PACAP in Cypriniformes. In Atlantic cod, PACAP inhibits intestinal smooth muscle contractions (Olsson and Holmgren, 2000), and although brain expression levels are not affected by 30 days of food deprivation, they increase after during the re-feeding period (Xu and Volkoff, 2009), suggesting that PACAP is involved in the regulation of feeding and digestive processes (Xu and Volkoff, 2009).
Secretoneurin
Secretoneurin (SN) is a short peptide derived from a secretogranin-II (SgII, also called chromogranin C) precursor protein (Zhao et al., 2009). In goldfish, ICV injections of the SN increase food intake and locomotor behavior (Trudeau et al., 2012), increase mRNA levels of hypothalamic NPY and decrease hypothalamic CART. In addition, fasting increases telencephalon SgII mRNA levels (Mikwar et al., 2016), suggesting that, in fish, SN might act as an orexigenic factor.
Spexin
Spexin (SPX) is a peptide identified in 2007 in mammalian adipose tissue. SPX expression is down-regulated in obese humans and rats, and subcutaneous injections of SPX reduce food intake and increase locomotion (Walewski et al., 2014).
In goldfish, SPX appear to act as an anorexigenic factor: brain injections of SPX inhibit both basal and NPY- or orexin-induced food consumption, decrease brain expressions of orexigenic factors (NPY, AgRP, and apelin) and increase that of anorexigenic factors (CCK, CART, POMC, MCH, and CRH), and brain SPX mRNA levels increase post-prandially (Wong et al., 2013). Similarly, in the orange-spotted grouper, IP administration of SPX increases hypothalamic mRNA levels of POMC and inhibits orexin expression, suggesting an anorexigenic role (Li S. et al., 2016). However, grouper SPX hypothalamic expression increases following long-term food deprivation (Li S. et al., 2016), suggesting that spexin might be a short-term satiety factor rather than a long-term hunger signal.
Concluding Remarks
Although the basic mechanisms regulating feeding seem to be relatively conserved between mammals and fish, it must be kept in mind that major physiological differences exist between these two groups. Fish are ectotherms and thus have lower metabolic rates than mammals and more sensitive to environmental changes, their physiology changing with their fluctuating surroundings. They also have different means of energy/nutrient storage (e.g., fat storage in liver rather than subcutaneous adipose tissue), and different growth patterns (as opposed to mammals, fish continue to grow after sexual maturity), suggesting that the endocrine regulation of energy balance, feeding and growth in fish differs from that of mammals.
Comparative studies at the genome level have revealed conserved sequences for appetite regulators across mammalian and fish species, indicating potentially conserved biological functions. Whereas the genome of all vertebrates is the result of two rounds (2R) of whole genome duplication (WGD) occurring in early vertebrate evolution, additional WGDs occurred in the teleost fish ancestor (3R) and most recently in certain teleost lineages (4R, e.g., salmonidae and cyprinidae), leading to the presence of increased gene copy numbers and multiple protein isoforms with potentially different physiological functions (Glasauer and Neuhauss, 2014), making the fish model potentially more complex. One must thus keep in mind that fish feeding-regulating hormones might not always have the same function as their mammalian homologs.
Fish are an extremely diversified group, with a great variability in feeding habits and requirements as well gut morphology and digestion processes. Fish can be carnivores, herbivores, omnivores or detritivores, with different feeding habits often seen within the same family (e.g., herbivore Mbuna cichlids and carnivore Nile tilapia in cichlidae; herbivore/omnivore pacu and carnivore piranha in serrasalmidae). Different fish species not only require different compositions of food, but also different amounts of food and feeding frequencies (Moore, 1941). Diet and feeding habits is reflected in the anatomy and physiology of the gastrointestinal tract. For example, carnivores or omnivores (such as most Characiformes and Siluriformes) have stomachs, pyloric caeca, and relatively short and straight intestines, whereas herbivores or detrivores (e.g., Cypriniformes and Cyprinodontiformes) may lack both stomach and caeca and have long and convoluted intestines (Leknes, 2015). Different diets and guts translate into different digestive enzyme profiles and different methods of nutrient storage (Day et al., 2011), as seen for lipids (e.g., in muscle in “oily” fish such as salmon and herring vs. liver in “lean” fish such as cod and flatfish), which usage might also be affected by reproductive stages and modes (guarding vs. non guarding; mature vs. immature; oviparous vs. viviparous).
Given the high diversity within fish, one should thus be careful when generalizing results from one species to all fish. Comparative studies establishing similarities and differences among species should be valuable to understand mechanisms regulating feeding. However, the large number of species poses the problem of the model species to choose. To date, most studies examining the neuroendocrine regulation of fish still use “classical” model species, i.e., cyprinids and salmonids. These somewhat differ from most fishes, as they display polyploidy, and might not represent a “perfect” model, but they are easily available and maintained, as their different holding conditions, habitats and diets, are well known. However, new species, in particular commercially important aquaculture species such as Perciformes (the largest teleost order) and Pleuronectiformes have recently been examined.
The increasing number of studies and species examined often generates conflicting and sometimes contradictory results. This variability might express true differences between species, but contradictory data also occur within same species. This variability might have several reasons. First, there is a great variability in the nature and nomenclature of isoforms examined (e.g., within CART forms). Second, when comparing studies, it is sometimes difficult to compare results obtained using different protocols (e.g., different lengths of fasting) and techniques (e.g., mRNA vs. protein vs. plasma levels), in particular because changes in gene expression do not necessarily translate into different protein levels or circulating levels. Finally, fish used between studies are often of different ages (e.g., larval vs. adult), sexual maturity (immature vs. mature spawning or non-spawning) or even environmental conditions (e.g., temperatures, photoperiods), all of these factors influencing feeding.
Even in mammals, the regulation of appetite is not yet fully understood. Using a comparative approach involving multiple fish species, perhaps choosing representative families/species from each fish group, and complementary methods might help us start drawing accurate models for the endocrine regulation of feeding in fish.
Author Contributions
HV designed this review, including table and figure, researched, acquired and analyzed all the information, drafted and revised the manuscript, and approved the version to be published. To HV's knowledge, information contained in this review and studies cited within it have been appropriately checked for accuracy or integrity.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many investigators have made contributions to the understanding of endocrinology of feeding in fish and some of their works are not cited in this review, due to space constraints. Research in the HV's laboratory is supported by a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant (# 261414-03).
References
Abbott, M., and Volkoff, H. (2011). Thyrotropin Releasing Hormone (TRH) in goldfish (Carassius auratus): role in the regulation of feeding and locomotor behaviors and interactions with the orexin system and cocaine- and amphetamine regulated transcript (CART). Horm. Behav. 59, 236–245. doi: 10.1016/j.yhbeh.2010.12.008
Adan, R. A., Tiesjema, B., Hillebrand, J. J., la Fleur, S. E., Kas, M. J., and de Krom, M. (2006). The MC4 receptor and control of appetite. Br. J. Pharmacol. 149, 815–827. doi: 10.1038/sj.bjp.0706929
Agnese, M., Valiante, S., Rosati, L., Andreuccetti, P., and Prisco, M. (2016). Pituitary adenylate cyclase-activating peptide (PACAP) and PAC1 receptor in the testis of cartilaginous fish Torpedo marmorata: a molecular and phylogenetic study. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 191, 26–35. doi: 10.1016/j.cbpb.2015.09.002
Agulleiro, M. J., Cortes, R., Leal, E., Rios, D., Sanchez, E., and Cerda-Reverter, J. M. (2014). Characterization, tissue distribution and regulation by fasting of the agouti family of peptides in the sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol. 205, 251–259. doi: 10.1016/j.ygcen.2014.02.009
Akash, G., Kaniganti, T., Tiwari, N. K., Subhedar, N. K., and Ghose, A. (2014). Differential distribution and energy status-dependent regulation of the four CART neuropeptide genes in the zebrafish brain. J. Comp. Neurol. 522, 2266–2285. doi: 10.1002/cne.23532
Aldegunde, M., and Mancebo, M. (2006). Effects of neuropeptide Y on food intake and brain biogenic amines in the rainbow trout (Oncorhynchus mykiss). Peptides 27, 719–727. doi: 10.1016/j.peptides.2005.09.014
Aldman, G., and Holmgren, S. (1987). Control of gallbladder motility in the rainbow trout, Salmo gairdneri. Fish Physiol. Biochem. 4, 143–155. doi: 10.1007/BF02110881
Amiya, N., Amano, M., Iigo, M., Yamanome, T., Takahashi, A., and Yamamori, K. (2008). Interaction of orexin/hypocretin-like immunoreactive neurons with melanin-concentrating hormone and alpha-melanocyte-stimulating hormone neurons in brain of a pleuronectiform fish, barfin flounder. Fish. Sci. 74, 1040–1046. doi: 10.1111/j.1444-2906.2008.01622.x
Amiya, N., Mizusawa, K., Kobayashi, Y., Yamanome, T., Amano, M., and Takahashi, A. (2012). Food deprivation increases the expression of the prepro-orexin gene in the hypothalamus of the Barfin flounder, Verasper moseri. Zool. Sci. 29, 43–48. doi: 10.2108/zsj.29.43
Angotzi, A. R., Stefansson, S. O., Nilsen, T. O., Rathore, R. M., and Rønnestad, I. (2013). Molecular cloning and genomic characterization of novel Leptin-like genes in salmonids provide new insight into the evolution of the Leptin gene family. Gen. Comp. Endocrinol. 187, 48–59. doi: 10.1016/j.ygcen.2013.03.022
Arends, R. J., Vermeer, H., Martens, G. J., Leunissen, J. A. M., Wendelaar Bonga, S. E., and Flik, G. (1998). Cloning and expression of two proopiomelanocortin mRNAs in the common carp (Cyprinus carpio L.). Mol. Cell. Endocrinol. 143, 23–31. doi: 10.1016/S0303-7207(98)00139-7
Ayada, C., Toru, U., and Korkut, Y. (2015). Nesfatin-1 and its effects on different systems. Hippokratia 19, 4–10.
Babichuk, N. A., and Volkoff, H. (2013). Changes in expression of appetite-regulating hormones in the cunner (Tautogolabrus adspersus) during short-term fasting and winter torpor. Physiol. Behav. 120C, 54–63. doi: 10.1016/j.physbeh.2013.06.022
Backstrom, T., and Winberg, S. (2013). Central corticotropin releasing factor and social stress. Front. Neurosci. 7:117. doi: 10.3389/fnins.2013.00117
Balasubramaniam, A., Rigel, D. F., Chance, W. T., and Fischer, J. E. (1992). Central and peripheral effects of sculpin pancreatic polypeptide and anglerfish peptide Y in rats. Pept. Res. 5, 106–109.
Balment, R. J., Lu, W., Weybourne, E., and Warne, J. M. (2006). Arginine vasotocin a key hormone in fish physiology and behaviour: a review with insights from mammalian models. Gen. Comp. Endocrinol. 147, 9–16. doi: 10.1016/j.ygcen.2005.12.022
Baltzegar, D. A., Reading, B. J., Douros, J. D., and Borski, R. J. (2014). Role for leptin in promoting glucose mobilization during acute hyperosmotic stress in teleost fishes. J. Endocrinol. 220, 61–72. doi: 10.1530/JOE-13-0292
Barsagade, V. G., Mazumdar, M., Singru, P. S., Thim, L., Clausen, J. T., and Subhedar, N. (2010). Reproductive phase-related variations in cocaine- and amphetamine-regulated transcript (CART) in the olfactory system, forebrain, and pituitary of the female catfish, Clarias batrachus (Linn.). J. Comp. Neurol. 518, 2503–2524. doi: 10.1002/cne.22349
Bechtold, D. A., and Luckman, S. M. (2007). The role of RFamide peptides in feeding. J. Endocrinol. 192, 3–15. doi: 10.1677/JOE-06-0069
Bernier, N. J. (2006). The corticotropin-releasing factor system as a mediator of the appetite-suppressing effects of stress in fish. Gen. Comp. Endocrinol. 146, 45–55. doi: 10.1016/j.ygcen.2005.11.016
Bernier, N. J., and Craig, P. M. (2005). CRF-related peptides contribute to stress response and regulation of appetite in hypoxic rainbow trout. Am. J. Physiol. 289, R982–R990. doi: 10.1152/ajpregu.00668.2004
Bernier, N. J., Gorissen, M., and Flik, G. (2012). Differential effects of chronic hypoxia and feed restriction on the expression of leptin and its receptor, food intake regulation and the endocrine stress response in common carp. J. Exp. Biol. 215, 2273–2282. doi: 10.1242/Jeb.066183
Bernier, N. J., and Peter, R. E. (2001). The hypothalamic-pituitary-interrenal axis and the control of food intake in teleost fish. Comp. Biochem. Physiol. Part B 129, 639–644. doi: 10.1016/S1096-4959(01)00360-8
Bernier, N. J., Bedard, N., and Peter, R. E. (2004). Effects of cortisol on food intake, growth, and forebrain neuropeptide Y and corticotropin-releasing factor gene expression in goldfish. Gen. Comp. Endocrinol. 135, 230–240. doi: 10.1016/j.ygcen.2003.09.016
Birsoy, K., Festuccia, W. T., and Laplante, M. (2013). A comparative perspective on lipid storage in animals. J. Cell Sci. 126(Pt 7), 1541–1552. doi: 10.1242/jcs.104992
Blanco, A. M., Bertucci, J. I., Delgado, M. A.J., Valenciano, A. I., and Unniappan, S. (2016a). Tissue-specific expression of ghrelinergic and NUCB2/nesfatin-1 systems in goldfish (Carassius auratus) is modulated by macronutrient composition of diets. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 195, 1–9. doi: 10.1016/j.cbpa.2016.01.016
Blanco, A. M., Gomez-Boronat, M., Redondo, I., Valenciano, A. I., and Delgado, M. J. (2016b). Periprandial changes and effects of short- and long-term fasting on ghrelin, GOAT, and ghrelin receptors in goldfish (Carassius auratus). J. Comp. Physiol. 186, 727–738. doi: 10.1007/s00360-016-0986-0
Blevins, J. E., Chelikani, P. K., Haver, A. C., and Reidelberger, R. D. (2008). PYY(3-36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides 29, 112–119. doi: 10.1016/j.peptides.2007.11.003
Blomqvist, A. G., Söderberg, C., Lundell, I., Milner, R. J., and Larhammar, D. (1992). Strong evolutionary conservation of neuropeptide Y: sequences of chicken, goldfish, and Torpedo marmorata DNA clones. Proc. Natl. Acad. Sci. U.S.A. 89, 2350–2354. doi: 10.1073/pnas.89.6.2350
Boelen, A., Wiersinga, W. M., and Fliers, E. (2008). Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid 18, 123–129. doi: 10.1089/thy.2007.0253
Boguszewski, C. L., Paz-Filho, G., and Velloso, L. A. (2010). Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynol. Polska 61, 194–206.
Bonacic, K., Martínez, A., Martín-Robles, Á. J., Muñoz-Cueto, J. A., and Morais, S. (2015). Characterization of seven cocaine- and amphetamine-regulated transcripts (CARTs) differentially expressed in the brain and peripheral tissues of Solea senegalensis (Kaup). Gen. Comp. Endocrinol. 224, 260–272. doi: 10.1016/j.ygcen.2015.08.017
Boonanuntanasarn, S., Jangprai, A., and Yoshizaki, G. (2012). Characterization of neuropeptide Y in snakeskin gourami and the change in its expression due to feeding status and melanocortin 4 receptor expression. Gen. Comp. Endocrinol. 179, 184–195. doi: 10.1016/j.ygcen.2012.07.024
Boucher, J., Masri, B., Daviaud, D., Gesta, S., Guigne, C., Mazzucotelli, A., et al. (2005). Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771. doi: 10.1210/en.2004-1427
Buckley, C., MacDonald, E. E., Tuziak, S. M., and Volkoff, H. (2010). Molecular cloning and characterization of two putative appetite regulators in winter flounder (Pleuronectes americanus): preprothyrotropin-releasing hormone (TRH) and preproorexin (OX). Peptides 31, 1737–1747. doi: 10.1016/j.peptides.2010.05.017
Burt, K., Hamoutene, D., Perez-Casanova, J., Gamperl, A. K., and Volkoff, H. (2013). The effect of intermittent hypoxia on growth, appetite and some aspects of the immune response of Atlantic salmon (Salmo salar). Aquac. Res. 45, 124–137. doi: 10.1111/J.1365-2109.2012.03211.X
Cai, Z. N., Li, W. J., Mai, K. S., Xu, W., Zhang, Y. J., and Ai, Q. H. (2015). Effects of dietary size-fractionated fish hydrolysates on growth, activities of digestive enzymes and aminotransferases and expression of some protein metabolism related genes in large yellow croaker (Larimichthys crocea) larvae. Aquaculture 440, 40–47. doi: 10.1016/j.aquaculture.2015.01.026
Caldwell, L. K., Pierce, A. L., Riley, L. G., Duncan, C. A., and Nagler, J. J. (2014). Plasma nesfatin-1 is not affected by long-term food restriction and does not predict rematuration among iteroparous female rainbow trout (Oncorhynchus mykiss). PLoS ONE 9:e85700. doi: 10.1371/journal.pone.0085700
Campos, V. F., Robaldo, R. B., Deschamps, J. C., Seixas, F. K., McBride, A. J. A., Marins, L. F., et al. (2012). Neuropeptide Y gene expression around meal time in the Brazilian flounder Paralichthys orbignyanus. J. Biosci. 37, 227–232. doi: 10.1007/s12038-012-9205-7
Candiani, S., Augello, A., Oliveri, D., and Pestarino, M. (2000). Immunoreactive endozepine-like peptides in the brain and pituitary of the Atlantic hagfish, Myxine glutinosa. Histochem. J. 32, 415–421. doi: 10.1023/A:1004091204806
Cérda-Reverter, J. M., Agulleiro, M. J. R. R. G., Sánchez, E., Ceinos, R., and Rotllant, J. (2011). Fish melanocortin system. Eur. J. Pharmacol. 660, 53–60. doi: 10.1016/j.ejphar.2010.10.108
Cerda-Reverter, J. M., and Canosa, L. F. (2009). “Neuroendocrine systems of the fish brain,” in Fish Physiology, eds N. J. Bernier, G. Van Der Kraak, A. P. Farrell, and C. J. Brauner (Academic Press), 3–74.
Cerdá-Reverter, J. M., and Larhammar, D. (2000). Neuropeptide Y family of peptides: structure, anatomical expression, function, and molecular evolution. Biochem. Cell Biol. 78, 371–392. doi: 10.1139/o00-004
Cerdá-Reverter, J. M., and Peter, R. E. (2003). Endogenous melanocortin antagonist in fish: structure, brain mapping, and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology 144, 4552–4561. doi: 10.1210/en.2003-0453
Cerdá-Reverter, J. M., Martinez-Rodriguez, G., Anglade, I., Kah, O., and Zanuy, S. (2000). Peptide YY (PYY) and fish pancreatic peptide Y (PY) expression in the brain of the sea bass (Dicentrarchus labrax) as revealed by in situ hybridization. J. Comp. Neurol. 426, 197–208. doi: 10.1002/1096-9861(20001016)426:2<197::AID-CNE3>3.0.CO;2-3
Cerdá-Reverter, J. M., Schioth, H. B., and Peter, R. E. (2003). The central melanocortin system regulates food intake in goldfish. Regul. Pept. 115, 101–113. doi: 10.1016/S0167-0115(03)00144-7
Chen, H., Huang, H., Chen, X., Deng, S. P., Zhu, C., Huang, H., et al. (2016a). Structural and functional characterization of neuromedin S in the teleost fish, zebrafish (Danio rerio). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 191, 76–83. doi: 10.1016/j.cbpb.2015.09.007
Chen, H., Zhang, X., Hao, J., Chen, D., Liu, J., Gao, Y., et al. (2015). Molecular cloning, expression analysis, and appetite regulatory effect of peptide YY in Siberian sturgeon (Acipenser baerii). Gene 563, 172–179. doi: 10.1016/j.gene.2015.03.028
Chen, T., Chen, S., Ren, C., Hu, C., Tang, D., and Yan, A. (2016b). Two isoforms of leptin in the White-clouds Mountain minnow (Tanichthys albonubes): differential regulation by estrogen despite similar response to fasting. Gen. Comp. Endocrinol. 225, 174–184. doi: 10.1016/j.ygcen.2015.08.002
Chen, W.-B., Wang, X., Zhou, Y.-L., Dong, H.-Y., Lin, H.-R., and Li, W.-S. (2011). Molecular cloning, tissue distribution and the expression in the regulation of food intake of prepro-orexin in Nile tilapia (Oreochromis niloticus). Zool. Res. 32, 285–292. doi: 10.3724/SP.J.1141.2011.03285
Chen, Y., Pandit, N. P., Fu, J., Li, D., and Li, J. (2014). Identification, characterization and feeding response of peptide YYb (PYYb) gene in grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 40, 45–55. doi: 10.1007/s10695-013-9822-6
Chen, Y., Shen, Y., Pandit, N. P., Fu, J., Li, D., and Li, J. (2013). Molecular cloning, expression analysis, and potential food intake attenuation effect of peptide YY in grass carp (Ctenopharyngodon idellus). Gen. Comp. Endocrinol. 187, 66–73. doi: 10.1016/j.ygcen.2013.03.029
Chisada, S.-I., Kurokawa, T., Murashita, K., Rønnestad, I., Taniguchi, Y., Toyoda, A., et al. (2014). Leptin receptor-deficient (knockout) medaka, Oryzias latipes, show chronical up-regulated levels of orexigenic neuropeptides, elevated food intake and stage specific effects on growth and fat allocation. Gen. Comp. Endocrinol. 195, 9–20. doi: 10.1016/j.ygcen.2013.10.008
Cone, R. D. (2006). Studies on the Physiological functions of the melanocortin system. Endocr. Rev. 27, 736–749. doi: 10.1210/er.2006-0034
Copeland, D. L., Duff, R. J., Liu, Q., Prokop, J., and Londraville, R. L. (2011). Leptin in teleost fishes: an argument for comparative study. Front. Physiol. 2:26. doi: 10.3389/fphys.2011.00026
Cottone, E., Guastalla, A., Pomatto, V., Campantico, E., Palermo, F., Magni, A. M., et al. (2009). Interplay of the endocannabinoid system with neuropeptide Y and corticotropin-releasing factor in the goldfish forebrain. Ann. N. Y. Acad. Sci. 1163, 372–375. doi: 10.1111/j.1749-6632.2009.04432.x
Cottone, E., Pomatto, V., Cerri, F., Campantico, E., Mackie, K., Delpero, M., et al. (2013). Cannabinoid receptors are widely expressed in goldfish: molecular cloning of a CB2-like receptor and evaluation of CB1 and CB2 mRNA expression profiles in different organs. Fish Physiol. Biochem. 39, 1287–1296. doi: 10.1007/s10695-013-9783-9
Cowan, E., Burch, K. J., Green, B. D., and Grieve, D. J. (2016). Obestatin as a key regulator of metabolism and cardiovascular function with emerging therapeutic potential for diabetes. Br. J. Pharmacol. 173, 2165–2181. doi: 10.1111/bph.13502
Crudo, M., Zizza, M., Panula, P., Canonaco, M., and Facciolo, R. M. (2013). Orexin-A influences mRNA expression of some glutamatergic receptor subtypes in Carassius auratus (Actinopterygii: Cyprinidae). Ital. J. Zool. 80, 329–336. doi: 10.1080/11250003.2013.823246
Cui, Y., Lv, S., Liu, J., Nie, S., Chen, J., Dong, Q., et al. (2016). Chronic perfluorooctanesulfonic acid exposure disrupts lipid metabolism in zebrafish. Hum. Exp. Toxicol. pii:0960327116646615. doi: 10.1177/0960327116646615
Dalman, M. R., Liu, Q., King, M. D., Bagatto, B., and Londraville, R. L. (2013). Leptin expression affects metabolic rate in zebrafish embryos (D-rerio). Front. Physiol. 4:160. doi: 10.3389/fphys.2013.00160
Dalmolin, C., Almeida, D. V., Figueiredo, M. A., and Marins, L. F. (2015). Food intake and appetite control in a GH-transgenic zebrafish. Fish Physiol. Biochem. 41, 1131–1141. doi: 10.1007/s10695-015-0074-5
D'angelo, L., Castaldo, L., De Girolamo, P., Lucini, C., Paolucci, M., Pelagalli, A., et al. (2016). Orexins and receptor OX2R in the gastroenteric apparatus of two teleostean species: Dicentrarchus labrax and Carassius auratus. Anat. Rec. 299, 1121–1129. doi: 10.1002/ar.23374
Danger, J. M., Tonon, M. C., Jenks, B. G., Saint-Pierre, S., Martel, J. C., Fasolo, A., et al. (1990). Neuropeptide Y: localization in the central nervous system and neuroendocrine functions. Fundam. Clin. Pharmacol. 4, 307–340. doi: 10.1111/j.1472-8206.1990.tb00497.x
Day, R. D., German, D. P., Manjakasy, J. M., Farr, I., Hansen, M. J., and Tibbetts, I. R. (2011). Enzymatic digestion in stomachless fishes: how a simple gut accommodates both herbivory and carnivory. J. Comp. Physiol. B 181, 603–613. doi: 10.1007/s00360-010-0546-y
De Pedro, N., Alonso-Gómez, A. L., Gancedo, B., Delgado, M. J., and Alonso-Bedate, M. (1993). Role of corticotropin-releasing factor (CRF) as a food intake regulator in goldfish. Physiol. Behav. 53, 517–520. doi: 10.1016/0031-9384(93)90146-7
de Pedro, N., and Björnsson, B. T. (2001). “Regulation of Food Intake by Neuropeptides and Hormones,” in Food Intake in Fish, eds. D. Houlihan, T. Boujard, and M. Jobling (Hoboken, NJ; New Jersey, NJ: Blackwell Science Ltd), 269–296.
de Pedro, N., Cespedes, M. V., Delgado, M. J., and Alonso-Bedate, M. (1995). The galanin-induced feeding stimulation is mediated via alpha 2-adrenergic receptors in goldfish. Regul. Pept. 57, 77–84. doi: 10.1016/0167-0115(95)91255-4
de Pedro, N., López-Patiño, M. A., Guijarro, A. I., Pinillos, M. L., Delgado, M. J., and Alonso-Bedate, M. (2000). NPY receptors and opioidergic system are involved in NPY-induced feeding in goldfish. Peptides 21, 1495–1502. doi: 10.1016/S0196-9781(00)00303-X
de Pedro, N., Martinez-Alvarez, R., and Delgado, M. J. (2006). Acute and chronic leptin reduces food intake and body weight in goldfish (Carassius auratus). J. Endocrinol. 188, 513–520. doi: 10.1677/joe.1.06349
Demski, L. S. (2012). The neural control of feeding in elasmobranchs: a review and working model. Environ. Biol. Fish. 95, 169–183. doi: 10.1007/s10641-011-9827-x
do Rego, J.-C., Orta, M.-H., Leprince, J., Tonon, M.-C., Vaudry, H., and Costentin, J. (2006). Pharmacological characterization of the receptor mediating the anorexigenic action of the octadecaneuropeptide: evidence for an endozepinergic tone regulating food intake. Neuropsychopharmacology 32, 1641–1648. doi: 10.1038/sj.npp.1301280
Dockray, G. J. (2012). Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 19, 8–12. doi: 10.1097/MED.0b013e32834eb77d
Douros, J. D., Baltzegar, D. A., Breves, J. P., Lerner, D. T., Seale, A. P., Grau, E. G., et al. (2014). Prolactin is a major inhibitor of hepatic Leptin A synthesis and secretion: studies utilizing a homologous Leptin A ELISA in the tilapia. Gen. Comp. Endocrinol. 207, 86–93. doi: 10.1016/J.Ygcen.2014.03.007
Einarsdottir, I. E., Power, D. M., Jonsson, E., and Bjornsson, B. T. (2011). Occurrence of ghrelin-producing cells, the ghrelin receptor and Na+, K+-ATPase in tissues of Atlantic halibut (Hippoglossus hippoglossus) during early development. Cell Tissue Res. 344, 481–498. doi: 10.1007/S00441-011-1158-X
Elbaz, I., Yelin-Bekerman, L., Nicenboim, J., Vatine, G., and Appelbaum, L. (2012). Genetic ablation of hypocretin neurons alters behavioral state transitions in zebrafish. J. Neurosci. 32, 12961–12972. doi: 10.1523/JNEUROSCI.1284-12.2012
El-Salhy, M. (1984). Occurrence of polypeptide YY (PYY) and pancreatic polypeptide (PP) in the gastrointestinal tract of the bony fish. Biomed. Res. 5, 441–444.
Escobar, S., Felip, A., Zanuy, S., and Carrillo, M. (2016). Is the kisspeptin system involved in responses to food restriction in order to preserve reproduction in pubertal male sea bass (Dicentrarchus labrax)? Comp. Biochem. Physiol. Part A 199, 38–46. doi: 10.1016/j.cbpa.2016.05.005
Ettore, V., Finizia, R., Elena, C., Giovanni, T., David, F., Paolo, D. G., et al. (2012). Immunohistochemical and immunological detection of ghrelin and leptin in rainbow trout Oncorhynchus mykiss and murray cod Maccullochella peelii peelii as affected by different dietary fatty acids. Microsc. Res. Tech. 75, 771–780. doi: 10.1002/jemt.21124
Facciolo, R. M., Crudo, M., Giusi, G., Alo, R., and Canonaco, M. (2009). Light- and dark-dependent orexinergic neuronal signals promote neurodegenerative phenomena accounting for distinct behavioral responses in the teleost Thalassoma pavo. J. Neurosci. Res. 87, 748–757. doi: 10.1002/jnr.21886
Facciolo, R. M., Crudo, M., Zizza, M., Giusi, G., and Canonaco, M. (2011). Feeding behaviors and ORXR-beta-GABA(A)R subunit interactions in Carassius auratus. Neurotoxicol. Teratol. 33, 641–650. doi: 10.1016/j.ntt.2011.09.008
Fekete, C., and Lechan, R. M. (2014). Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 35, 159–194. doi: 10.1210/er.2013-1087
Figueiredo-Silva, A. C., Saravanan, S., Schrama, J. W., Kaushik, S., and Geurden, I. (2012). Macronutrient-induced differences in food intake relate with hepatic oxidative metabolism and hypothalamic regulatory neuropeptides in rainbow trout (Oncorhynchus mykiss). Physiol. Behav. 106, 499–505. doi: 10.1016/j.physbeh.2012.03.027
FishBase (2016). World Wide Web Electronic Publication, eds R. Froese and D. Pauly. Available online at: http://www.fishbase.org, version (01/2016).
Flik, G., Klaren, P. H., Van den Burg, E. H., Metz, J. R., and Huising, M. O. (2006). CRF and stress in fish. Gen. Comp. Endocrinol. 146, 36–44. doi: 10.1016/j.ygcen.2005.11.005
Francis, D. S., Thanuthong, T., Senadheera, S. P. S. D., Paolucci, M., Coccia, E., De Silva, S. S., et al. (2014). n-3 LC-PUFA deposition efficiency and appetite-regulating hormones are modulated by the dietary lipid source during rainbow trout grow-out and finishing periods. Fish Physiol. Biochem. 40, 577–593. doi: 10.1007/S10695-013-9868-5
Froiland, E., Jobling, M., Bjornsson, B. T., Kling, P., Ravuri, C. S., and Jorgensen, E. H. (2012). Seasonal appetite regulation in the anadromous Arctic charr: evidence for a role of adiposity in the regulation of appetite but not for leptin in signalling adiposity. Gen. Comp. Endocrinol. 178, 330–337. doi: 10.1016/j.ygcen.2012.06.017
Fuentes, E. N., Kling, P., Einarsdottir, I. E., Alvarez, M., Valdes, J. A., Molina, A., et al. (2012). Plasma leptin and growth hormone levels in the fine flounder (Paralichthys adspersus) increase gradually during fasting and decline rapidly after refeeding. Gen. Comp. Endocrinol. 177, 120–127. doi: 10.1016/j.ygcen.2012.02.019
Fuentes, E. N., Safian, D., Einarsdottir, I. E., Valdes, J. A., Elorza, A. A., Molina, A., et al. (2013). Nutritional status modulates plasma leptin, AMPK and TOR activation, and mitochondrial biogenesis: implications for cell metabolism and growth in skeletal muscle of the fine flounder. Gen. Comp. Endocrinol. 186, 172–180. doi: 10.1016/j.ygcen.2013.02.009
Furutani, T., Masumoto, T., and Fukada, H. (2013). Molecular cloning and tissue distribution of cholecystokinin-1 receptor (CCK-1R) in yellowtail Seriola quinqueradiata and its response to feeding and in vitro CCK treatment. Gen. Comp. Endocrinol. doi: 10.1016/j.ygcen.2013.02.003
Gahete, M. D., Durán-Prado, M., Luque, R. M., Martínez-Fuentes, A. J., Quintero, A., Gutiérrez-Pascual, E., et al. (2009). Understanding the multifactorial control of growth hormone release by somatotropes. Ann. N. Y. Acad. Sci. 1163, 137–153. doi: 10.1111/j.1749-6632.2008.03660.x
Gambardella, C., Gallus, L., Amaroli, A., Terova, G., Masini, M. A., and Ferrando, S. (2012). Fasting and re-feeding impact on leptin and aquaglyceroporin 9 in the liver of European sea bass (Dicentrarchus labrax). Aquaculture 354–355, 1–6. doi: 10.1016/j.aquaculture.2012.04.043
Gaylord, T. G., MacKenzie, D. S., and Gatlin, D. M. (2001). Growth performance, body composition and plasma thyroid hormone status of channel catfish (Ictalurus punctatus) in response to short-term feed deprivation and refeeding. Fish Physiol. Biochem. 24, 73–79. doi: 10.1023/A:1011199518135
Gesto, M., Soengas, J. L., Rodriguez-Illamola, A., and Miguez, J. M. (2014). Arginine vasotocin treatment induces a stress response and exerts a potent anorexigenic effect in rainbow trout, Oncorhynchus mykiss. J. Neuroendocrinol. 26, 89–99. doi: 10.1111/jne.12126
Glasauer, S. M., and Neuhauss, S. C. (2014). Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genomics 289, 1045–1060. doi: 10.1007/s00438-014-0889-2
Gomes, A. S., Jordal, A. E., Olsen, K., Harboe, T., Power, D. M., and Ronnestad, I. (2015). Neuroendocrine control of appetite in Atlantic halibut (Hippoglossus hippoglossus): changes during metamorphosis and effects of feeding. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 183, 116–125. doi: 10.1016/j.cbpa.2015.01.009
Gomes, A. S., Kamisaka, Y., Harboe, T., Power, D. M., and Ronnestad, I. (2014). Functional modifications associated with gastrointestinal tract organogenesis during metamorphosis in Atlantic halibut (Hippoglossus hippoglossus). BMC Dev. Biol. 14:11. doi: 10.1186/1471-213X-14-11
Gong, N., Jonsson, E., and Björnsson, B. T. (2016). Acute anorexigenic action of leptin in rainbow trout is mediated by the hypothalamic Pi3k pathway. J. Mol. Endocrinol. 56, 227–238. doi: 10.1530/JME-15-0279
Gong, Y., Luo, Z., Zhu, Q.-L., Zheng, J.-L., Tan, X.-Y., Chen, Q.-L., et al. (2013). Characterization and tissue distribution of leptin, leptin receptor and leptin receptor overlapping transcript genes in yellow catfish Pelteobagrus fulvidraco. Gen. Comp. Endocrinol. 182, 1–6. doi: 10.1016/j.ygcen.2012.11.006
Gonzalez, R., and Unniappan, S. (2010). Molecular characterization, appetite regulatory effects and feeding related changes of peptide YY in goldfish. Gen. Comp. Endocrinol. 166, 273–279. doi: 10.1016/j.ygcen.2009.09.008
Gonzalez, R., Kerbel, B., Chun, A., and Unniappan, S. (2010). Molecular, cellular and physiological evidences for the anorexigenic actions of Nesfatin-1 in goldfish. PLoS ONE 5:e15201. doi: 10.1371/journal.pone.0015201
Goodyear, K. (2012). Effects of Thyroid Hormone Injections on Feeding and Appetite-Regulating Hormones in Goldfish (Carassius auratus). Honours thesis, Memorial University of Newfoundland.
Gorissen, M. H. A. G., Flik, G., and Huising, M. O. (2006). Peptides and proteins regulating food intake: a comparative view. Anim. Biol. 56, 447–473. doi: 10.1163/157075606778967829
Gorissen, M., and Flik, G. (2014). Leptin in teleostean fish, towards the origins of leptin physiology. J. Chem. Neuroanat. 61–62, 200–206. doi: 10.1016/J.Jchemneu.2014.06.005
Gorissen, M., Bernier, N. J., Nabuurs, S. B., Flik, G., and Huising, M. O. (2009). Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J. Endocrinol. 201, 329–339. doi: 10.1677/JOE-09-0034
Grone, B. P., Carpenter, R. E., Lee, M., Maruska, K. P., and Fernald, R. D. (2012). Food deprivation explains effects of mouthbrooding on ovaries and steroid hormones, but not brain neuropeptide and receptor mRNAs, in an African cichlid fish. Horm. Behav. 62, 18–26. doi: 10.1016/j.yhbeh.2012.04.012
Guijarro, A. I., Delgado, M. J., Pinillos, M. L., López-Pati-o, M. A., Alonso-Bedate, M., and De Pedro, N. (1999). Galanin and β-endorphin as feeding regulators in cyprinids: effect of temperature. Aquat. Res. 30, 483–489. doi: 10.1046/j.1365-2109.1999.00360.x
Guillot, R., Cortés, R., Navarro, S., Mischitelli, M., Garcia-Herranz, V., Sanchez, E., et al. (2016). Behind melanocortin antagonist overexpression in the zebrafish brain: a behavioral and transcriptomic approach. Horm. Behav. 82, 87–100. doi: 10.1016/j.yhbeh.2016.04.011
Habata, Y., Fujii, R., Hosoya, M., Fukusumi, S., Kawamata, Y., Hinuma, S., et al. (1999). Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta 1452, 25–35. doi: 10.1016/S0167-4889(99)00114-7
Hatef, A., Shajan, S., and Unniappan, S. (2015). Nutrient status modulates the expression of nesfatin-1 encoding nucleobindin 2A and 2B mRNAs in zebrafish gut, liver and brain. Gen. Comp. Endocrinol. 215, 51–60. doi: 10.1016/j.ygcen.2014.09.009
Hayes, J., and Volkoff, H. (2014). Characterization of the endocrine, digestive and morphological adjustments of the intestine in response to food deprivation and torpor in cunner, Tautogolabrus adspersus. Comp. Biochem. Physiol. Part A 170C, 46–59. doi: 10.1016/j.cbpa.2014.01.014
He, S., Liang, X.-F., Li, L., Sun, J., and Shen, D. (2013). Differential gut growth, gene expression and digestive enzyme activities in young grass carp (Ctenopharyngodon idella) fed with plant and animal diets. Aquaculture 410, 18–24. doi: 10.1016/j.aquaculture.2013.06.015
Hevrøy, E. M., Azpeleta, C., Shimizu, M., Lanzen, A., Kaiya, H., Espe, M., et al. (2011). Effects of short-term starvation on ghrelin, GH-IGF system, and IGF-binding proteins in Atlantic salmon. Fish Physiol. Biochem. 37, 217–232. doi: 10.1007/s10695-010-9434-3
Higgins, S. C., Gueorguiev, M., and Korbonits, M. R. (2007). Ghrelin, the peripheral hunger hormone. Ann. Med. 39, 116–136. doi: 10.1080/07853890601149179
Himick, B. A., and Peter, R. E. (1994). CCK/gastrin-like immunoreactivity in brain and gut, and CCK suppression of feeding in goldfish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 267, R841–R851.
Holstein, B. (1982). Inhibition of gastric acid secretion inthe Atlantic cod, Gadus morhua, by sulphated and desulphated gastrin, caerulein, and CCK-octapeptide. Acta Physiol. Scand. 114, 453–459. doi: 10.1111/j.1748-1716.1982.tb07009.x
Holzer, P., Reichmann, F., and Farzi, A. (2012). Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46, 261–274. doi: 10.1016/j.npep.2012.08.005
Honkanen, R. E., Crim, J. W., and Patton, J. S. (1988). Effects of cholecystokinin peptides on digestive enzymes in killifish in vivo. Comp. Biochem. Physiol. A 89, 655–660. doi: 10.1016/0300-9629(88)90849-3
Hoskins, L. J., and Volkoff, H. (2012). The comparative endocrinology of feeding in fish: insights and challenges. Gen. Comp. Endocrinol. 176, 327–335. doi: 10.1016/j.ygcen.2011.12.025
Hoskins, L. J., Xu, M., and Volkoff, H. (2008). Interactions between gonadotropin-releasing hormone (GnRH) and orexin in the regulation of feeding and reproduction in goldfish (Carassius auratus). Horm. Behav. 54, 379–385. doi: 10.1016/j.yhbeh.2008.04.011
Hosomi, N., Furutani, T., Takahashi, N., Masumoto, T., and Fukada, H. (2014). Yellowtail neuropeptide Y: molecular cloning, tissue distribution, and response to fasting. Fish. Sci. 80, 483–492. doi: 10.1007/s12562-014-0711-4
Huang, H., Wei, Y., Meng, Z. N., Zhang, Y., Liu, X. C., Guo, L., et al. (2014). Polymorphisms of leptin-b gene associated with growth traits in orange-spotted grouper (Epinephelus coioides). Int. J. Mol. Sci. 15, 11996–12006. doi: 10.3390/Ijms150711996
Huesa, G., van den Pol, A. N., and Finger, T. E. (2005). Differential distribution of hypocretin (orexin) and melanin-concentrating hormone in the goldfish brain. J. Comp. Neurol. 488, 476–491. doi: 10.1002/cne.20610
Huising, M. O., Geven, E. J. W., Kruiswijk, C. P., Nabuurs, S. B., Stolte, E. H., Spanings, F. A. T., et al. (2006a). Increased leptin expression in common carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology 147, 5786–5797. doi: 10.1210/en.2006-0824
Huising, M. O., Kruiswijk, C. P., and Flik, G. (2006b). Phylogeny and evolution of class-I helical cytokines. J. Endocrinol. 189, 1–25. doi: 10.1677/joe.1.06591
Ji, W., Ping, H.-C., Wei, K.-J., Zhang, G.-R., Shi, Z.-C., Yang, R.-B., et al. (2015). Ghrelin, neuropeptide Y (NPY) and cholecystokinin (CCK) in blunt snout bream (Megalobrama amblycephala): cDNA cloning, tissue distribution and mRNA expression changes responding to fasting and refeeding. Gen. Comp. Endocrinol. 223, 108–119. doi: 10.1016/j.ygcen.2015.08.009
Jin, Y., Tian, L. X., Xie, S. W., Guo, D. Q., Yang, H. J., Liang, G. Y., et al. (2015). Interactions between dietary protein levels, growth performance, feed utilization, gene expression and metabolic products in juvenile grass carp (Ctenopharyngodon idella). Aquaculture 437, 75–83. doi: 10.1016/j.aquaculture.2014.11.031
Johansson, M., and Björnsson, B. T. (2015). Elevated plasma leptin levels of fasted rainbow trout decrease rapidly in response to feed intake. Gen. Comp. Endocrinol. 214, 24–29. doi: 10.1016/j.ygcen.2015.02.020
Johansson, M., Morgenroth, D., Einarsdottir, I. E., Gong, N., and Bjornsson, B. T. (2016). Energy stores, lipid mobilization and leptin endocrinology of rainbow trout. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 186, 759–773. doi: 10.1007/s00360-016-0988-y
Johnson, R. M., Johnson, T. M., and Londraville, R. L. (2000). Evidence for leptin expression in fishes. J. Exp. Zool. 286, 718–724. doi: 10.1002/(SICI)1097-010X(20000601)286:7<718::AID-JEZ6>3.0.CO;2-I
Jönsson, E. (2013). The role of ghrelin in energy balance regulation in fish. Gen. Comp. Endocrinol. 187, 79–85. doi: 10.1016/j.ygcen.2013.03.013
Jönsson, E., Kaiya, H., and Björnsson, B. T. (2010). Ghrelin decreases food intake in juvenile rainbow trout (Oncorhynchus mykiss) through the central anorexigenic corticotropin-releasing factor system. Gen. Comp. Endocrinol. 166, 39–46. doi: 10.1016/j.ygcen.2009.11.001
Jørgensen, E. H., Martinsen, M., Strøm, V., Hansen, K. E., Ravuri, C. S., Gong, N., et al. (2013). Long-term fasting in the anadromous Arctic charr is associated with downregulation of metabolic enzyme activity and upregulation of leptin A1 and SOCS expression in the liver. J. Exp. Biol. 216(Pt 17), 3222–3230. doi: 10.1242/jeb.088344
Kaiya, H., Konno, N., Kangawa, K., Uchiyama, M., and Miyazato, M. (2014). Identification, tissue distribution and functional characterization of the ghrelin receptor in West African lungfish, Protopterus annectens. Gen. Comp. Endocrinol. 209, 106–117. doi: 10.1016/J.Ygcen.2014.07.021
Kamijo, M., Kojima, K., Maruyama, K., Konno, N., Motohashi, E., Ikegami, T., et al. (2011). Neuropeptide Y in Tiger Puffer (Takifugu rubripes): distribution, cloning, characterization, and mRNA expression responses to prandial condition. Zool. Sci. 28, 882–890. doi: 10.2108/zsj.28.882
Kamisaka, Y., Totland, G. K., Tagawa, M., Kurokawa, T., Suzuki, T., Tanaka, M., et al. (2001). Ontogeny of cholecystokinin-immunoreactive cells in the digestive tract of Atlantic halibut, Hippoglossus hippoglossus, larvae. Gen. Comp. Endocrinol. 123, 31–37. doi: 10.1006/gcen.2001.7653
Kang, D. Y., and Kim, H. C. (2013a). Functional characterization of two melanin-concentrating hormone genes in the color camouflage, hypermelanosis, and appetite of starry flounder. Gen. Comp. Endocrinol. 189, 74–83. doi: 10.1016/j.ygcen.2013.04.025
Kang, D. Y., and Kim, H. C. (2013b). Influence of density and background color to stress response, appetite, growth, and blind-side hypermelanosis of flounder, Paralichthys olivaceus. Fish Physiol. Biochem. 39, 221–232. doi: 10.1007/s10695-012-9693-2
Kang, D. Y., and Kim, H. C. (2015). Functional relevance of three proopiomelanocortin (POMC) genes in darkening camouflage, blind-side hypermelanosis, and appetite of Paralichthys olivaceus. Comp. Biochem. Physiol. Part B 179, 44–56. doi: 10.1016/j.cbpb.2014.09.002
Kang, K. S., Yahashi, S., Azuma, M., and Matsuda, K. (2010). The anorexigenic effect of cholecystokinin octapeptide in a goldfish model is mediated by the vagal afferent and subsequently through the melanocortin- and corticotropin-releasing hormone-signaling pathways. Peptides 31, 2130–2134. doi: 10.1016/j.peptides.2010.07.019
Kang, K. S., Yahashi, S., and Matsuda, K. (2011). The effects of ghrelin on energy balance and psychomotor activity in a goldfish model: an overview. Int. J. Pept. 2011:171034. doi: 10.1155/2011/171034
Karra, E., Chandarana, K., and Batterham, R. L. (2009). The role of peptide YY in appetite regulation and obesity. J. Physiol. 587, 19–25. doi: 10.1113/jphysiol.2008.164269
Katan, T., Nash, G. W., Rise, M. L., Hall, J. R., Fernandes, J. M. O., Boyce, D., et al. (2016). A little goes long way: improved growth in Atlantic cod (Gadus morhua) fed small amounts of wild zooplankton. Aquaculture 451, 271–282. doi: 10.1016/j.aquaculture.2015.09.014
Kawauchi, H. (1983). Chemistry of proopiocortin-related peptides in the salmon pituitary. Arch. Biochem. Biophys. 227, 343–350. doi: 10.1016/0003-9861(83)90462-9
Kawauchi, H., Kawazoe, I., Tsubokawa, M., Kishida, M., and Baker, B. I. (1983). Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature 305, 321–323. doi: 10.1038/305321a0
Keen-Rhinehart, E., Ondek, K., and Schneider, J. E. (2013). Neuroendocrine regulation of appetitive ingestive behavior. Front. Neurosci. 7:213. doi: 10.3389/fnins.2013.00213
Kehoe, A. S., and Volkoff, H. (2007). Cloning and characterization of neuropeptide Y (NPY) and cocaine and amphetamine regulated transcript (CART) in Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 146, 451–461. doi: 10.1016/j.cbpa.2006.12.026
Kelly, S. P., and Peter, R. E. (2006). Prolactin-releasing peptide, food intake, and hydromineral balance in goldfish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1474–R1481. doi: 10.1152/ajpregu.00129.2006
Kerbel, B., and Unniappan, S. (2012). Nesfatin-1 suppresses energy intake, co-localises ghrelin in the brain and gut, and alters ghrelin, cholecystokinin and orexin mRNA expression in goldfish. J. Neuroendocrinol. 24, 366–377. doi: 10.1111/j.1365-2826.2011.02246.x
Kim, J.-H., Leggatt, R. A., Chan, M., Volkoff, H., and Devlin, R. H. (2015). Effects of chronic growth hormone overexpression on appetite-regulating brain gene expression in coho salmon. Mol. Cell. Endocrinol. 413, 178–188. doi: 10.1016/j.mce.2015.06.024
Kitahara, N., Nishizawa, T., Iida, K., Okazaki, H., Andoh, T., and Soma, G.-I. (1988). Absence of a a-melanocyte-stimulating hormone sequence in proopiomelanocortin mRNA of chum salmon Oncorhynchus keta. Comp. Biochem. Physiol. Part B Comp. Biochem. 91, 365–370. doi: 10.1016/0305-0491(88)90155-1
Klovins, J., Haitina, T., Fridmanis, D., Kilianova, Z., Kapa, I., Fredriksson, R., et al. (2004). The melanocortin system in Fugu: determination of POMC/AGRP/MCR gene repertoire and synteny, as well as pharmacology and anatomical distribution of the MCRs. Mol. Biol. Evol. 21, 563–579. doi: 10.1093/molbev/msh050
Kobayashi, Y., Peterson, B. C., and Waldbieser, G. C. (2008). Association of cocaine- and amphetamine-regulated transcript (CART) messenger RNA level, food intake, and growth in channel catfish. Comp. Biochem. Physiol. Part A 151, 219–225. doi: 10.1016/j.cbpa.2008.06.029
Kojima, K., Amiya, N., Kamijo, M., Kageyama, H., Uchiyama, M., Shioda, S., et al. (2010). Relationship between alpha-melanocyte-stimulating hormone- and neuropeptide Y-containing neurons in the goldfish hypothalamus. Gen. Comp. Endocrinol. 167, 366–372. doi: 10.1177/0960327116646615
Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., and Kangawa, K. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660. doi: 10.1038/45230
Kono, T., Hamasuna, S., Korenaga, H., Iizasa, T., Nagamine, R., Ida, T., et al. (2012). The role of neuromedin U during inflammatory response in the common carp. Fish Shellfish Immunol. 32, 151–160. doi: 10.1016/j.fsi.2011.11.004
Köprücü, S., and Algül, S. (2015). Comparatively examining of the apelin-13 levels in the Capoeta trutta (Heckel, 1843) and Cyprinus carpio (Linnaeus, 1758). J. Anim. Physiol. Anim. Nutr. 99, 210–214. doi: 10.1111/jpn.12240
Kortner, T. M., Overrein, I., Oie, G., Kjorsvik, E., and Arukwe, A. (2011). The influence of dietary constituents on the molecular ontogeny of digestive capability and effects on growth and appetite in Atlantic cod larvae (Gadus morhua). Aquaculture 315, 114–120. doi: 10.1016/j.aquaculture.2010.04.008
Kousoulaki, K., Ronnestad, I., Olsen, H. J., Rathore, R., Campbell, P., Nordrum, S., et al. (2013). Krill hydrolysate free amino acids responsible for feed intake stimulation in Atlantic salmon (Salmo salar). Aquac. Nutr. 19(Suppl. 1, Sp. Iss. SI), 47–61. doi: 10.1111/anu.12094
Koven, W., and Schulte, P. (2012). The effect of fasting and refeeding on mRNA expression of PepT1 and gastrointestinal hormones regulating digestion and food intake in zebrafish (Danio rerio). Fish Physiol. Biochem. 38, 1565–1575. doi: 10.1007/s10695-012-9649-6
Kullgren, A., Jutfelt, F., Fontanillas, R., Sundell, K., Samuelsson, L., Wiklander, K., et al. (2013). The impact of temperature on the metabolome and endocrine metabolic signals in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 164, 44–53. doi: 10.1016/J.Cbpa.2012.10.005
Kurokawa, T., Murashita, K., and Uji, S. (2006). Characterization and tissue distribution of multiple agouti-family genes in pufferfish, Takifugu rubripes. Peptides 27, 3165–3175. doi: 10.1016/j.peptides.2006.09.013
Kurokawa, T., Suzuki, T., and Andoh, T. (2000). Development of cholecystokinin and pancreatic polypeptide endocrine systems during the larval stage of Japanese flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 120, 8–16. doi: 10.1006/gcen.2000.7512
Kurokawa, T., Uji, S., and Suzuki, T. (2005). Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides 26, 745–750. doi: 10.1016/j.peptides.2004.12.017
Larsen, P. J., and Hunter, R. G. (2006). The role of CART in body weight homeostasis. Peptides 27, 1981–1986. doi: 10.1016/j.peptides.2005.11.027
Larsson, T. A., Tay, B.-H., Sundstrom, G., Fredriksson, R., Brenner, S., Larhammar, D., et al. (2009). Neuropeptide Y-family peptides and receptors in the elephant shark, Callorhinchus milii confirm gene duplications before the gnathostome radiation. Genomics 93, 254–260. doi: 10.1016/j.ygeno.2008.10.001
Le, H. T., Angotzi, A. R., Ebbesson, L. O., Karlsen, O., and Ronnestad, I. (2016). The ontogeny and brain distribution dynamics of the appetite regulators NPY, CART and OX in larval Atlantic cod (Gadus morhua L.). PLoS ONE 11:e0153743. doi: 10.1371/journal.pone.0153743
Le Bail, P.-Y., and Boeuf, G. (1997). What hormones may regulate appetite in fish? Aquat. Living Resour. 10, 371–379.
Leal, E., Fernández-Durán, B., Agulleiro, M. J., Conde-Siera, M., Miguez, J. M., and Cerda-Reverter, J. M. (2013). Effects of dopaminergic system activation on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): a self-feeding approach. Horm. Behav. 64, 113–121. doi: 10.1016/j.yhbeh.2013.05.008
Leder, E. H., and Silverstein, J. T. (2006). The pro-opiomelanocortin genes in rainbow trout (Oncorhynchus mykiss): duplications, splice variants, and differential expression. J. Endocrinol. 188, 355–363. doi: 10.1677/joe.1.06283
Lee, N., Kim, D., Lee, B., Kim, S., and Kim, K. (2015). Distribution of ghrelin immunoreactivity in artificially reared Japanese eel, Anguilla japonica, leptocephalus and metamorphosed glass eel. J. Environ. Biol. 36, 521–529.
Leknes, I. L. (2015). Mucin in epithelial cells in oesophagus and stomach of black tetra, Gymnocorymbus ternetzi (Characidae, Teleostei). Zoomorphology 134, 269–277. doi: 10.1007/s00435-015-0256-9
Li, A., Yuan, X., Liang, X.-F., Liu, L., Li, J., Li, B., et al. (2016). Adaptations of lipid metabolism and food intake in response to low and high fat diets in juvenile grass carp (Ctenopharyngodon idellus). Aquaculture 457, 43–49. doi: 10.1016/j.aquaculture.2016.01.014
Li, D., Liu, Z. D., and Xie, C. X. (2012). Effect of stocking density on growth and serum concentrations of thyroid hormones and cortisol in Amur sturgeon, Acipenser schrenckii. Fish Physiol. Biochem. 38, 511–520. doi: 10.1007/s10695-011-9531-y
Li, G. G., Liang, X. F., Xie, Q. L., Li, G. Z., Yu, Y., and Lai, K. S. (2010). Gene structure, recombinant expression and functional characterization of grass carp leptin. Gen. Comp. Endocrinol. 166, 117–127. doi: 10.1016/j.ygcen.2009.10.009
Li, L., Wei, S., Huang, Q., Feng, D., Zhang, S., and Liu, Z. (2013). A novel galanin receptor 1a gene in zebrafish: tissue distribution, developmental expression roles in nutrition regulation. Comp. Biochem. Physiol. Part B 164, 159–167. doi: 10.1016/j.cbpb.2012.12.004
Li, S., Liu, Q., Xiao, L., Chen, H., Li, G., Zhang, Y., et al. (2016). Molecular cloning and functional characterization of spexin in orange-spotted grouper (Epinephelus coioides). Comp. Biochem. Physiolo. Part B 196–197, 85–91. doi: 10.1016/j.cbpb.2016.02.009
Li, S., Xiao, L., Liu, Q., Zheng, B., Chen, H., Liu, X., et al. (2015). Distinct functions of neuromedin U and neuromedin S in orange-spotted grouper. J. Mol. Endocrinol. 55, 95–106. doi: 10.1530/JME-15-0018
Libran-Perez, M., Velasco, C., Lopez-Patino, M. A., Miguez, J. M., and Soengas, J. L. (2014). Counter-regulatory response to a fall in circulating fatty acid levels in rainbow trout. Possible involvement of the hypothalamus-pituitary-interrenal axis. PLoS ONE 9:e113291. doi: 10.1371/journal.pone.0113291
Lin, F., Wu, H., Chen, H., Xin, Z., Yuan, D., Wang, T., et al. (2014a). Molecular and physiological evidences for the role in appetite regulation of apelin and its receptor APJ in Ya-fish (Schizothorax prenanti). Mol. Cell. Endocrinol. 396, 46–57. doi: 10.1016/j.mce.2014.08.009
Lin, F., Zhou, C., Chen, H., Wu, H., Xin, Z., Liu, J., et al. (2014b). Molecular characterization, tissue distribution and feeding related changes of NUCB2A/nesfatin-1 in Ya-fish (Schizothorax prenanti). Gene 536, 238–246. doi: 10.1016/j.gene.2013.12.031
Lin, X., Volkoff, H., Narnaware, Y., Bernier, N. J., Peyon, P., and Peter, R. E. (2000). Brain regulation of feeding behavior and food intake in fish. Comp. Biochem. Physiol. Part A 126, 415–434. doi: 10.1016/S1095-6433(00)00230-0
Lin, X., Wang, P., Ou, Y., Li, J., and Wen, J. (2016). An immunohistochemical study on endocrine cells in the neuroendocrine system of the digestive tract of milkfish Chanos chanos (Forsskal, 1775). Aquac. Res. [Epub ahead of print]. doi: 10.1111/are.12979
Liu, L., Liang, X.-F., Li, J., Yuan, X., Zhou, Y., and He, Y. (2014). Feed intake, feed utilization and feeding-related gene expression response to dietary phytic acid for juvenile grass carp (Ctenopharyngodon idellus). Aquaculture 424, 201–206. doi: 10.1016/j.aquaculture.2013.12.044
Liu, Y., Li, S. S., Huang, X. G., Lu, D. Q., Liu, X. C., Ko, W. H., et al. (2013). Identification and characterization of a motilin-like peptide and its receptor in teleost. Gen. Comp. Endocrinol. 186, 85–93. doi: 10.1016/J.Ygcen.2013.02.018
Liu, Y., Zhang, Y., Li, S., Huang, W., Liu, X., Lu, D., et al. (2009). Molecular cloning and functional characterization of the first non-mammalian 26RFa/QRFP orthologue in Goldfish, Carassius auratus. Mol. Cell. Endocrinol. 303, 82–90. doi: 10.1016/j.mce.2009.01.009
Loh, K., Herzog, H., and Shi, Y.-C. (2015). Regulation of energy homeostasis by the NPY system. Trends Endocrinol. Metabol. 26, 125–135. doi: 10.1016/j.tem.2015.01.003
Londraville, R. L., Macotela, Y., Duff, R. J., Easterling, M. R., Liu, Q., and Crespi, E. J. (2014). Comparative endocrinology of leptin: assessing function in a phylogenetic context. Gen. Comp. Endocrinol. 203, 146–157. doi: 10.1016/j.ygcen.2014.02.002
López, J. M., Sanz-Morello, B., and González, A. (2014). Organization of the orexin/hypocretin system in the brain of two basal actinopterygian fishes, the cladistians Polypterus senegalus and Erpetoichthys calabaricus. Peptides 61, 23–37. doi: 10.1016/j.peptides.2014.08.011
López-Patiño, M. A., Guijarro, A. I., Isorna, E., Delgado, M. J., Alonso-Bedate, M., and de Pedro, N. (1999). Neuropeptide Y has a stimulatory action on feeding behavior in goldfish (Carassius auratus). Eur. J. Pharmacol. 377, 147–153. doi: 10.1016/S0014-2999(99)00408-2
Lowry, C. A., and Moore, F. L. (2006). Regulation of behavioral responses by corticotropin-releasing factor. Gen. Comp. Endocrinol. 146, 19–27. doi: 10.1016/j.ygcen.2005.12.006
Lu, R.-H., Zhou, Y., Yuan, X.-C., Liang, X.-F., Fang, L., Bai, X.-L., et al. (2015). Effects of glucose, insulin and triiodothyroxine on leptin and leptin receptor expression and the effects of leptin on activities of enzymes related to glucose metabolism in grass carp (Ctenopharyngodon idella) hepatocytes. Fish Physiol. Biochem. 41, 981–989. doi: 10.1007/s10695-015-0063-8
Mabudi, H., Jamili, S., Majd, N. E., Vosoughi, G., Fatemi, M. R., and Rashed, S. (2011). The effects of ghrelin on ovary histology in Barbus sharpeyi. J. Anim. Physiol. Anim. Nutr. (Berl.) 95, 599–602. doi: 10.1111/j.1439-0396.2010.01089.x
MacDonald, E. E., and Volkoff, H. (2010). Molecular cloning and characterization of preproorexin in winter skate (Leucoraja ocellata). Gen. Comp. Endocrinol. 169, 192–196. doi: 10.1016/j.ygcen.2010.09.014
MacDonald, E., and Volkoff, H. (2009a). Cloning, distribution and effects of season and nutritional status on the expression of neuropeptide Y (NPY), cocaine and amphetamine regulated transcript (CART) and cholecystokinin (CCK) in winter flounder (Pseudopleuronectes americanus). Horm. Behav. 56, 58–65. doi: 10.1016/j.yhbeh.2009.03.002
MacDonald, E., and Volkoff, H. (2009b). Neuropeptide Y (NPY), cocaine- and amphetamine-regulated transcript (CART) and cholecystokinin (CCK) in winter skate (Raja ocellata): cDNA cloning, tissue distribution and mRNA expression responses to fasting. Gen. Comp. Endocrinol. 161, 252–261. doi: 10.1016/j.ygcen.2009.01.021
MacDonald, L. E., Alderman, S. L., Kramer, S., Woo, P. T. K., and Bernier, N. J. (2014). Hypoxemia-induced leptin secretion: a mechanism for the control of food intake in diseased fish. J. Endocrinol. 221, 441–455. doi: 10.1530/Joe-13-0615
Magalhaes, G. S., Junqueira-de-Azevedo, I. L. M., Lopes-Ferreira, M., Lorenzini, D. M., Ho, P. L., and Moura-da-Silva, A. M. (2006). Transcriptome analysis of expressed sequence tags from the venom glands of the fish Thalassophryne nattereri. Biochimie 88, 693–699. doi: 10.1016/j.biochi.2005.12.008
Majzoub, J. A. (2006). Corticotropin-releasing hormone physiology. Eur. J. Endocrinol. 155(suppl. 1), S71–S76. doi: 10.1530/eje.1.02247
Malagon, M., Vallarino, M., Tonon, M. C., and Vaudry, H. (1992). Localization and characterization of diazepam-binding inhibitor (DBI)-like peptides in the brain and pituitary of the trout (Salmo gairdneri). Brain Res. 576, 208–214. doi: 10.1016/0006-8993(92)90682-Y
Manuel, R., Gorissen, M., Roca, C. P., Zethof, J., van de Vis, H., Flik, G., et al. (2014). Inhibitory avoidance learning in zebrafish (Danio rerio): effects of shock intensity and unraveling differences in task performance. Zebrafish 11, 341–352. doi: 10.1089/zeb.2013.0970
Manuel, R., Gorissen, M., Stokkermans, M., Zethof, J., Ebbesson, L. O., Vis, H. V., et al. (2015). The effects of environmental enrichment and age-related differences on inhibitory avoidance in zebrafish (Danio rerio Hamilton). Zebrafish 12, 152–165. doi: 10.1089/zeb.2014.1045
Maruyama, K., Konno, N., Ishiguro, K., Wakasugi, T., Uchiyama, M., Shioda, S., et al. (2008). Isolation and characterisation of four cDNAs encoding neuromedin U (NMU) from the brain and gut of goldfish, and the inhibitory effect of a deduced NMU on food intake and locomotor activity. J. Neuroendocrinol. 20, 71–78. doi: 10.1111/j.1365-2826.2007.01615.x
Maruyama, K., Wada, K., Ishiguro, K., Shimakura, S.-I., Wakasugi, T., Uchiyama, M., et al. (2009). Neuromedin U-induced anorexigenic action is mediated by the corticotropin-releasing hormone receptor-signaling pathway in goldfish. Peptides 30, 2483–2486. doi: 10.1016/j.peptides.2009.08.013
Matsuda, K. (2009). Recent advances in the regulation of feeding behavior by neuropeptides in fish. Ann. N. Y. Acad. Sci. 1163, 241–250. doi: 10.1111/j.1749-6632.2008.03619.x
Matsuda, K., and Maruyama, K. (2007). Regulation of feeding behavior by pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal polypeptide (VIP) in vertebrates. Peptides 28, 1761–1766. doi: 10.1016/j.peptides.2007.03.007
Matsuda, K., Arimura, A., Shioda, S., Takei, Y., Shimomura, H., and Uchiyama, M. (1997). “Pituitary adenylate-cyclase activating polypeptide (PACAP) in fishes: purification, distribution and physiological function,” in Advances in Comparative Endocrinology, eds S. Kawashima and S. Kikuyama (Tome: Monduzzi Editore), 687–691.
Matsuda, K., Hagiwara, Y., Shibata, H., Sakashita, A., and Wada, K. (2013). Ovine corticotropin-releasing hormone (oCRH) exerts an anxiogenic-like action in the goldfish, Carassius auratus. Gen. Comp. Endocrinol. 188, 118–122. doi: 10.1016/j.ygcen.2013.01.001
Matsuda, K., Kang, K. S., Sakashita, A., Yahashi, S., and Vaudry, H. (2011a). Behavioral effect of neuropeptides related to feeding regulation in fish. Ann. N. Y. Acad. Sci. 1220, 117–126. doi: 10.1111/j.1749-6632.2010.05884.x
Matsuda, K., Kojima, K., Shimakura, S., Miura, T., Uchiyama, M., Shioda, S., et al. (2009). Relationship between melanin-concentrating hormone- and neuropeptide Y-containing neurons in the goldfish hypothalamus. Comp. Biochem. Physiol. Part A 153, 3–7. doi: 10.1016/j.cbpa.2008.10.002
Matsuda, K., Kojima, K., Wada, K., Maruyama, K., Leprince, J., Tonon, M. C., et al. (2010). The anorexigenic action of the octadecaneuropeptide (ODN) in goldfish is mediated through the MC4R- and subsequently the CRH receptor-signaling pathways. J. Mol. Neurosci. 42, 74–79. doi: 10.1007/s12031-010-9346-9
Matsuda, K., Maruyama, K., Nakamachi, T., Miura, T., and Shioda, S. (2006). Effects of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide on food intake and locomotor activity in the goldfish, Carassius auratus. Ann. N. Y. Acad. Sci. 1070, 417–421. doi: 10.1196/annals.1317.054
Matsuda, K., Maruyama, K., Nakamachi, T., Miura, T., Uchiyama, M., and Shioda, S. (2005). Inhibitory effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) on food intake in the goldfish, Carassius auratus. Peptides 26, 1611–1616. doi: 10.1016/j.peptides.2005.02.022
Matsuda, K., Nakamura, K., Shimakura, S.-I., Miura, T., Kageyama, H., Uchiyama, M., et al. (2008). Inhibitory effect of chicken gonadotropin-releasing hormone II on food intake in the goldfish, Carassius auratus. Horm. Behav. 54, 83–89. doi: 10.1016/j.yhbeh.2008.01.011
Matsuda, K., Shimakura, S. I., Miura, T., Maruyama, K., Uchiyama, M., Kawauchi, H., et al. (2007a). Feeding-induced changes of melanin-concentrating hormone (MCH)-like immunoreactivity in goldfish brain. Cell Tissue Res. 328, 375–382. doi: 10.1007/s00441-006-0347-5
Matsuda, K., Wada, K., Azuma, M., Leprince, J., Tonon, M. C., Sakashita, A., et al. (2011b). The Octadecaneuropeptide exerts an anxiogenic-like action in goldfish. Neuroscience 181, 100–108. doi: 10.1016/j.neuroscience.2011.02.058
Matsuda, K., Wada, K., Miura, T., Maruyama, K., Shimakura, S. I., Uchiyama, M., et al. (2007b). Effect of the diazepam-binding inhibitor-derived peptide, octadecaneuropeptide, on food intake in goldfish. Neuroscience 150, 425–432. doi: 10.1016/j.neuroscience.2007.09.012
Matsuda, K., Yoshida, T., Nagano, Y., Kashimoto, K., Yatohgo, T., Shimomura, H., et al. (1998). Purification and primary structure of pituitary adenylate cyclase activating polypeptide (PACAP) from the brain of an elasmobranch, stingray, Dasyatis akajei. Peptides 19, 1489–1495. doi: 10.1016/S0196-9781(98)00091-6
Matty, A. J. (1986). Nutrition, hormones and growth. Fish Physiol. Biochem. 2, 141–150. doi: 10.1007/BF02264082
Mawhinney, R. M. S. (2007). The Effect of Intracerebroventricular Injections of Kisspeptin and Human RFRP-3 on the Feeding Behaviour of Goldfish, Carassius auratus. Honours thesis, Memorial University of Newfoundland.
McRory, J. E., Parker, D. B., Ngamvongchon, S., and Sherwood, N. M. (1995). Sequence and expression of cDNA for pituitary adenylate cyclase activating polypeptide (PACAP) and growth hormone-releasing hormone (GHRH)-like peptide in catfish. Mol. Cell. Endocrinol. 108, 169–177. doi: 10.1016/0303-7207(94)03467-8
Mennigen, J. A., Sassine, J., Trudeau, V. L., and Moon, T. W. (2010). Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish Carassius auratus. Aquat. Toxicol. 100, 128–137. doi: 10.1016/j.aquatox.2010.07.022
Mensah, E. T., Volkoff, H., and Unniappan, S. (2010). Galanin systems in non-mammalian vertebrates with special focus on fishes. EXS 102, 243–262. doi: 10.1007/978-3-0346-0228-0_17
Merchenthaler, I. (2010). Galanin and the neuroendocrine axes. EXS 102, 71–85. doi: 10.1007/978-3-0346-0228-0_7
Micale, V., Campo, S., D'Ascola, A., Guerrera, M. C., Levanti, M. B., Germana, A., et al. (2012). Cholecystokinin in white sea bream: molecular cloning, regional expression, and immunohistochemical localization in the gut after feeding and fasting. PLoS ONE 7:e52428. doi: 10.1371/journal.pone.0052428
Micale, V., Campo, S., D'Ascola, A., Guerrera, M. C., Levanti, M. B., Germana, A., et al. (2014). Cholecystokinin: how many functions? Observations in seabreams. Gen. Comp. Endocrinol. 205, 166–167. doi: 10.1016/j.ygcen.2014.02.019
Michel, M., Page-McCaw, P. S., Chen, W., and Cone, R. D. (2016). Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proc. Natl Acad. Sci. U.S.A 113, 3084–3089. doi: 10.1073/pnas.1513212113
Mikwar, M., Navarro-Martin, L., Xing, L., Volkoff, H., Hu, W., and Trudeau, V. L. (2016). Stimulatory effect of the secretogranin-11 derived peptide secretoneurin on food intake and locomotion in female goldfish (Carassius auratus). Peptides 78, 42–50. doi: 10.1016/j.peptides.2016.01.007
Miyata, A., Arimura, A., Dahl, R. R., Minamino, N., Uehara, A., Jiang, L., et al. (1989). Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 164, 567–574. doi: 10.1016/0006-291X(89)91757-9
Mizusawa, K., Amiya, N., Yamaguchi, Y., Takabe, S., Amano, M., Breves, J. P., et al. (2012). Identification of mRNAs coding for mammalian-type melanin-concentrating hormone and its receptors in the scalloped hammerhead shark Sphyrna lewini. Gen. Comp. Endocrinol. 179, 78–87. doi: 10.1016/j.ygcen.2012.07.023
Moen, A.-G. G., and Finn, R. N. (2013). Short-term, but not long-term feed restriction causes differential expression of leptins in Atlantic salmon. Gen. Comp. Endocrinol. 183, 83–88. doi: 10.1016/j.ygcen.2012.09.027
Montero, M., Yon, L., Rousseau, K., Arimura, A., Fournier, A., Dufour, S., et al. (1998). Distribution, characterization, and growth hormone-releasing activity of pituitary adenylate cyclase-activating polypeptide in the European eel, Anguilla anguilla. Endocrinology 139, 4300–4310. doi: 10.1210/en.139.10.4300
Moore, W. G. (1941). Studies on the feeding habits of fishes. Ecology 22, 91–96. doi: 10.2307/1930015
Morley, J. E., Horowitz, M., Morley, P. M. K., and Flood, J. F. (1992). Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides 13, 1133–1135. doi: 10.1016/0196-9781(92)90019-Y
Mukherjee, A., Subhedar, N. K., and Ghose, A. (2012). Ontogeny of the cocaine- and amphetamine-regulated transcript (CART) neuropeptide system in the brain of zebrafish, Danio rerio. J. Comp. Neurol. 520, 770–797. doi: 10.1002/cne.22779
Mulley, J. F., Hargreaves, A. D., Hegarty, M. J., Heller, R. S., and Swain, M. T. (2014). Transcriptomic analysis of the lesser spotted catshark (Scyliorhinus canicula) pancreas, liver and brain reveals molecular level conservation of vertebrate pancreas function. BMC Genomics 15:1074. doi: 10.1186/1471-2164-15-1074
Murashita, K., and Kurokawa, T. (2011). Multiple cocaine- and amphetamine-regulated transcript (CART) genes in medaka, Oryzias latipes: cloning, tissue distribution and effect of starvation. Gen. Comp. Endocrinol. 170, 494–500. doi: 10.1016/j.ygcen.2010.11.005
Murashita, K., Fukada, H., Hosokawa, H., and Masumoto, T. (2006). Cholecystokinin and peptide Y in yellowtail (Seriola quinqueradiata): molecular cloning, real-time quantitative RT-PCR, and response to feeding and fasting. Gen. Comp. Endocrinol. 145, 287–297. doi: 10.1016/j.ygcen.2005.09.008
Murashita, K., Fukada, H., Hosokawa, H., and Masumoto, T. (2007). Changes in cholecystokinin and peptide Y gene expression with feeding in yellowtail (Seriola quinqueradiata): relation to pancreatic exocrine regulation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 146, 318–325. doi: 10.1016/j.cbpb.2006.11.009
Murashita, K., Jordal, A. E. O., Nilsen, T. O., Stefansson, S. O., Kurokawa, T., Bjornsson, B. T., et al. (2011). Leptin reduces Atlantic salmon growth through the central pro-opiomelanocortin pathway. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 158, 79–86. doi: 10.1016/j.cbpa.2010.09.001
Murashita, K., Kurokawa, T., Ebbesson, L. O. E., Stefansson, S. O., and Ronnestad, I. (2009a). Characterization, tissue distribution, and regulation of agouti-related protein (AgRP), cocaine- and amphetamine-regulated transcript (CART) and neuropeptide Y (NPY) in Atlantic salmon (Salmo salar). Gen. Comp. Endocrinol. 162, 160–171. doi: 10.1016/j.ygcen.2009.03.015
Murashita, K., Kurokawa, T., Nilsen, T. O., and Rønnestad, I. (2009b). Ghrelin, cholecystokinin, and peptide YY in Atlantic salmon (Salmo salar): molecular cloning and tissue expression. Gen. Comp. Endocrinol. 160, 223–235. doi: 10.1016/j.ygcen.2008.11.024
Mustonen, A. M., Nieminen, P., and Hyvarinen, H. (2002). Leptin, ghrelin, and energy metabolism of the spawning burbot (Lota lota, L.). J. Exp. Zool. 293, 119–126. doi: 10.1002/jez.10142
Nakamachi, T., Matsuda, K., Maruyama, K., Miura, T., Uchiyama, M., Funahashi, H., et al. (2006). Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. J. Neuroendocrinol. 18, 290–297. doi: 10.1111/j.1365-2826.2006.01415.x
Nam, B. H., Moon, J. Y., Kim, Y. O., Kong, H. J., Kim, W. J., Kim, D. G., et al. (2013). Structural and functional characterization of pituitary adenylyl cyclase-activating polypeptide (PACAP)/PACAP-related peptide (PRP) and its receptor in olive flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 164, 18–28. doi: 10.1016/j.cbpb.2012.09.003
Narnaware, Y. K., Peyon, P. P., Lin, X., and Peter, R. E. (2000). Regulation of food intake by neuropeptide Y in goldfish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R1025–R1034.
Naumann, E. A., Kampff, A. R., Prober, D. A., Schier, A. F., and Engert, F. (2010). Monitoring neural activity with bioluminescence during natural behavior. Nat. Neurosci. 13, 513–520. doi: 10.1038/nn.2518
Nieminen, P., Mustonen, A.-M., and Hyvarinen, H. (2003). Fasting reduces plasma leptin- and ghrelin-immunoreactive peptide concentrations of the burbot (Lota lota) at 2C but not at 10C. Zool. Sci. 20, 1109–1115. doi: 10.2108/zsj.20.1109
Nisembaum, L. G., de Pedro, N., Delgado, M. J., and Isorna, E. (2014). Crosstalking between the “gut-brain” hormone ghrelin and the circadian system in the goldfish. Effects on clock gene expression and food anticipatory activity. Gen. Comp. Endocrinol. 205, 287–295. doi: 10.1016/j.ygcen.2014.03.016
Nishiguchi, R., Azuma, M., Yokobori, E., Uchiyama, M., and Matsuda, K. (2012). Gonadotropin-releasing hormone 2 suppresses food intake in the zebrafish, Danio rerio. Front. Endocrinol. 3:122. doi: 10.3389/fendo.2012.00122
Nishio, S., Gibert, Y., Berekelya, L., Bernard, L., Brunet, F., Guillot, E., et al. (2012). Fasting induces CART down-regulation in the zebrafish nervous system in a cannabinoid receptor 1-dependent manner. Mol. Endocrinol. 26, 1316–1326. doi: 10.1210/me.2011-1180
O'Carroll, A.-M., Lolait, S. J., Harris, L. E., and Pope, G. R. (2013). The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 219, R13–R35. doi: 10.1530/JOE-13-0227
Ohga, H., Matsumori, K., Kodama, R., Kitano, H., Nagano, N., Yamaguchi, A., et al. (2015). Two leptin genes and a leptin receptor gene of female chub mackerel (Scomber japonicus): molecular cloning, tissue distribution and expression in different obesity indices and pubertal stages. Gen. Comp. Endocrinol. 222, 88–98. doi: 10.1016/j.ygcen.2015.06.002
Olsson, C., and Holmgren, S. (2000). PACAP and nitric oxide inhibit contractions in the proximal intestine of the Atlantic cod, Gadus morhua. J. Exp. Biol. 203, 575–583.
Ortega, V. A., Lovejoy, D. A., and Bernier, N. J. (2013). Appetite-suppressing effects and interactions of centrally administered corticotropin-releasing factor, urotensin I and serotonin in rainbow trout (Oncorhynchus mykiss). Front. Neurosci. 7:196. doi: 10.3389/fnins.2013.00196
Osborne, N. N., Patel, S., Terenghi, G., Allen, J. M., Polak, J. M., and Bloom, S. R. (1985). Neuropeptide Y (NPY)-like immunoreactive amacrine cells in retinas of frog and goldfish. Cell Tissue Res. 241, 651–656. doi: 10.1007/BF00214587
O'Shea, M., Hansen, M. J., Tatemoto, K., and Morris, M. J. (2003). Inhibitory effect of apelin-12 on nocturnal food intake in the rat. Nutr. Neurosci. 6, 163–167. doi: 10.1080/1028415031000111273
Osugi, T., Son, Y. L., Ubuka, T., Satake, H., and Tsutsui, K. (2016). RFamide peptides in agnathans and basal chordates. Gen. Comp. Endocrinol. 227, 94–100. doi: 10.1016/j.ygcen.2015.06.012
Pagotto, U., Marsicano, G., Cota, D., Lutz, B., and Pasquali, R. (2006). The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 27, 73–100. doi: 10.1210/er.2005-0009
Palermo, F. A., Cardinaletti, G., Cocci, P., Tibaldi, E., Polzonetti-Magni, A., and Mosconi, G. (2013). Effects of dietary nucleotides on acute stress response and cannabinoid receptor 1 mRNAs in sole, Solea solea. Comp. Biochem. Physiol. Part A 164, 477–482. doi: 10.1016/j.cbpa.2012.12.005
Panula, P. (2010). Hypocretin/orexin in fish physiology with emphasis on zebrafish. Acta Physiol. 198, 381–386. doi: 10.1111/j.1748-1716.2009.02038.x
Papoutsoglou, S. E. (2012). The role of the brain in farmed fish. Rev. Aquac. 4, 1–10. doi: 10.1111/j.1753-5131.2012.01056.x
Park, H.-K., and Ahima, R. S. (2015). Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 64, 24–34. doi: 10.1016/j.metabol.2014.08.004
Parker, D. B., Coe, I. R., Dixon, G. H., and Sherwood, N. M. (1993). Two salmon neuropeptides encoded by one brain cDNA are structurally related to members of the glucagon superfamily. Eur. J. Biochem. 215, 439–448. doi: 10.1111/j.1432-1033.1993.tb18051.x
Paullada-Salmerón, J. A., Cowan, M., Aliaga-Guerrero, M., Gomez, A., Zanuy, S., Mananos, E., et al. (2016). LPXRFa peptide system in the European sea bass: a molecular and immunohistochemical approach. J. Comp. Neurol. 524, 176–198. doi: 10.1002/cne.23833
Pavlidis, M., Theodoridi, A., and Tsalafouta, A. (2015). Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Prog. Neuropsychopharmacol. Biol. Psychiatry 60, 121–131. doi: 10.1016/j.pnpbp.2015.02.014
Peng, W., Xu, J., Zhang, Y., Feng, J., Dong, C., Jiang, L., et al. (2016). An ultra-high density linkage map and QTL mapping for sex and growth-related traits of common carp (Cyprinus carpio). Sci. Rep. 6:26693. doi: 10.1038/srep26693
Penney, C. C., and Volkoff, H. (2014). Peripheral injections of cholecystokinin, apelin, ghrelin and orexin in cavefish (Astyanax fasciatus mexicanus): effects on feeding and on the brain expression levels of tyrosine hydroxylase, mechanistic target of rapamycin and appetite-related hormones. Gen. Comp. Endocrinol. 196, 34–40. doi: 10.1016/j.ygcen.2013.11.015
Pereira, R. T., Costa, L. S., Oliveira, I. R., Araujo, J. C., Aerts, M., Vigliano, F. A., et al. (2015). Relative distribution of gastrin-, CCK-8-, NPY- and CGRP-immunoreactive cells in the digestive tract of dorado (Salminus brasiliensis). Tissue Cell 47, 123–131. doi: 10.1016/j.tice.2015.01.009
Pérez Sirkin, D. I., Suzuki, H., Cánepa, M. M., and Vissio, P. G. (2013). Orexin and neuropeptide Y: tissue specific expression and immunoreactivity in the hypothalamus and preoptic area of the cichlid fish Cichlasoma dimerus. Tissue Cell 45, 452–459. doi: 10.1016/j.tice.2013.09.001
Peter, R. E. (1979). “The brain and feeding behavior,” in Fish Physiology, Vol. VIII, eds. H. Ws, R. Dj, and B. Jr. (New York, NY: Academic Press), 121–159.
Peterson, B. C., Waldbieser, G. C., Riley, L. G., Upton, K. R., Kobayashi, Y., and Small, B. C. (2012). Pre- and postprandial changes in orexigenic and anorexigenic factors in channel catfish (Ictalurus punctatus). Gen. Comp. Endocrinol. 176, 231–239. doi: 10.1016/j.ygcen.2012.01.022
Peyon, P., Lin, X. W., Himick, B. A., and Peter, R. E. (1998). Molecular cloning and expression of cDNA encoding brain preprocholecystokinin in goldfish. Peptides 19, 199–210. doi: 10.1016/S0196-9781(97)00296-9
Peyon, P., Saied, H., Lin, X., and Peter, R. E. (1999). Postprandial, seasonal and sexual variations in cholecystokinin gene expression in goldfish brain. Brain Res. Mol. Brain Res. 74, 190–196. doi: 10.1016/S0169-328X(99)00282-X
Pfundt, B., Mielenz, B., Sanver, F., Pfeffer, E., Sauerwein, H., and Mielenz, M. (2016). Effects of largely different feeding intensities on serum insulin-like growth factor-1 concentrations, quantified by enzyme immunoassay, leptin and growth hormone receptor 1 mRNA in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 22, 586–596. doi: 10.1111/anu.12282
Piccinetti, C. C., Migliarini, B., Petrosino, S., Di Marzo, V., and Carnevali, O. (2010). Anandamide and AM251, via water, modulate food intake at central and peripheral level in fish. Gen. Comp. Endocrinol. 166, 259–267. doi: 10.1016/j.ygcen.2009.09.017
Ping, H. C., Feng, K., Zhang, G. R., Wei, K. J., Zou, G. W., and Wang, W. M. (2013). Ontogeny expression of ghrelin, neuropeptide Y and cholecystokinin in blunt snout bream, Megalobrama amblycephala. J. Anim. Physiol. Anim. Nutr. doi: 10.1111/jpn.12084
Pohlenz, C., Buentello, A., Miller, T., Small, B. C., MacKenzie, D. S., and Gatlin, D. M. (2013). Effects of dietary arginine on endocrine growth factors of channel catfish, Ictalurus punctatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 166, 215–221. doi: 10.1016/J.Cbpa.2013.06.016
Qu, D., Ludwig, D. S., Gammeltoft, S., Piper, M., Pelleymounter, M. A., Cullen, M. J., et al. (1996). A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380, 243–247. doi: 10.1038/380243a0
Quillet, R., Ayachi, S., Bihel, F., Elhabazi, K., Ilien, B., and Simonin, F. (2016). RF-amide neuropeptides and their receptors in Mammals: pharmacological properties, drug development and main physiological functions. Pharmacol. Ther. 160, 84–132. doi: 10.1016/j.pharmthera.2016.02.005
Rajjo, I. M., Vigna, S. R., and Crim, J. W. (1988). Actions of cholecystokinin-related peptides on the gallbladder of bony fishes in vitro. Comp. Biochem. Physiol. C 90, 267–273. doi: 10.1016/0742-8413(88)90132-6
Renquist, B. J., Zhang, C., Williams, S. Y., and Cone, R. D. (2013). Development of an assay for high-throughput energy expenditure monitoring in the zebrafish. Zebrafish 10, 343–352. doi: 10.1089/zeb.2012.0841
Riediger, T., Zuend, D., Becskei, C., and Lutz, T. A. (2003). The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R114–R122. doi: 10.1152/ajpregu.00333.2003
Roch, G. J., Busby, E. R., and Sherwood, N. M. (2014). GnRH receptors and peptides: skating backward. Gen. Comp. Endocrinol. 209, 118–134. doi: 10.1016/j.ygcen.2014.07.025
Rønnestad, I., Nilsen, T. O., Murashita, K., Angotzi, A. R., Moen, A. G. G., Stefansson, S. O., et al. (2010). Leptin and leptin receptor genes in Atlantic salmon: cloning, phylogeny, tissue distribution and expression correlated to long-term feeding status. Gen. Comp. Endocrinol. 168, 55–70. doi: 10.1016/j.ygcen.2010.04.010
Rui, L. (2013). Brain regulation of energy balance and body weight. Rev. Endocr. Metabol. Disord. 14, 387–407. doi: 10.1007/s11154-013-9261-9
Sakamoto, T., Iwata, K., and Ando, M. (2002). Growth hormone and prolactin expression during environmental adaptation of gobies. Fish. Sci. 68, 757–760. doi: 10.2331/fishsci.68.sup1_757
Sakurai, T. (2014). Roles of orexins in the regulation of body weight homeostasis. Obes. Res. Clin. Pract. 8, e414–e420. doi: 10.1016/j.orcp.2013.12.001
Salmerón, C., Johansson, M., Angotzi, A. R., Rønnestad, I., Jonsson, E., Bjornsson, B. T., et al. (2015). Effects of nutritional status on plasma leptin levels and in vitro regulation of adipocyte leptin expression and secretion in rainbow trout. Gen. Comp. Endocrinol. 210, 114–123. doi: 10.1016/j.ygcen.2014.10.016
Schroeter, J. C., Fenn, C. M., and Small, B. C. (2015). Elucidating the roles of gut neuropeptides on channel catfish feed intake, glycemia, and hypothalamic NPY and POMC expression. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 188, 168–174. doi: 10.1016/j.cbpa.2015.06.031
Schwandt, S. E., Peddu, S. C., and Riley, L. G. (2010). Differential roles for octanoylated and decanoylated ghrelins in regulating appetite and metabolism. Int. J. Pept. 2010:275804. doi: 10.1155/2010/275804
Shanshan, L., Cuizhen, Z., and Gang, P. (2016). Effects of starvation on the expression of feeding related neuropeptides in the larval zebrafish hypothalamus. Yi Chuan 38, 821–830. doi: 10.16288/j.yczz.16-087
Shepherd, B. S., Eckert, S. M., Parhar, I. S., Vijayan, M. M., Wakabayashi, I., Hirano, T., et al. (2000). The hexapeptide KP-102 (D-ala-D-beta-Nal-ala-trp-D-phe-lys-NH(2)) stimulates growth hormone release in a cichlid fish (Ooreochromis mossambicus). J. Endocrinol. 167, R7–R10. doi: 10.1677/joe.0.167R007
Sherwood, N. M., Adams, B. A., Isaac, E. R., Wu, S., and Fradinger, E. A. (2007). Knocked down and out: PACAP in development, reproduction and feeding. Peptides 28, 1680–1687. doi: 10.1016/j.peptides.2007.03.008
Sherwood, N. M., Krueckl, S. L., and McRory, J. E. (2000). The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/Glucagon superfamily. Endocr. Rev. 21, 619–670. doi: 10.1210/er.21.6.619
Shimakura, S., Marayama, K., Miura, T., Uchiyama, M., Kawauchi, H., Takahashi, A., et al. (2006). Effect of melanin-concentrating hormone on feeding behavior and locomotor activity in the goldfish, Carassius auratus. Regul. Pept. 135, 156–156.
Shpilman, M., Hollander-Cohen, L., Ventura, T., Gertler, A., and Levavi-Sivan, B. (2014). Production, gene structure and characterization of two orthologs of leptin and a leptin receptor in tilapia. Gen. Comp. Endocrinol. 207, 74–85. doi: 10.1016/j.ygcen.2014.05.006
Silverstein, J. T., and Plysetskaya, E. M. (2000). The effects of NPY and insulin on food intake regulation in fish. Am. Zool. 40, 296–308. doi: 10.1093/icb/40.2.296
Silverstein, J. T., Breininger, J., Baskin, D. G., and Plisetskaya, E. M. (1998). Neuropeptide Y-like gene expression in the salmon brain increases with fasting. Gen. Comp. Endocrinol. 110, 157–165. doi: 10.1006/gcen.1998.7058
Sinha, A. K., Liew, H. J., Diricx, M., Kumar, V., Darras, V. M., Blust, R., et al. (2012). Combined effects of high environmental ammonia, starvation and exercise on hormonal and ion-regulatory response in goldfish (Carassius auratus L.). Aquat. Toxicol. 114, 153–164. doi: 10.1016/j.aquatox.2012.02.027
Sinnett, P. M., and Markham, M. R. (2015). Food deprivation reduces and leptin increases the amplitude of an active sensory and communication signal in a weakly electric fish. Horm. Behav. 71, 31–40. doi: 10.1016/j.yhbeh.2015.03.010
Smith, S. M., and Vale, W. W. (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 8, 383–395.
Sobrino Crespo, C., Perianes Cachero, A., Puebla Jimenez, L., Barrios, V., and Arilla Ferreiro, E. (2014). Peptides and food intake. Front. Endocrinol. 5:58. doi: 10.3389/fendo.2014.00058
Söderberg, C., Pieribone, V. A., Dahlstrand, J., Brodin, L., and Larhammar, D. (1994). Neuropeptide role of both peptide YY and neuropeptide Y in vertebrates suggested by abundant expression of their mRNAS in a cyclostome brain. J. Neurosci. Res. 37, 633–640. doi: 10.1002/jnr.490370510
Sohn, J. W. (2015). Network of hypothalamic neurons that control appetite. BMB Rep. 48, 229–233. doi: 10.5483/BMBRep.2015.48.4.272
Song, Y., and Cone, R. D. (2007). Creation of a genetic model of obesity in a teleost. FASEB J. 21, 2042–2049. doi: 10.1096/fj.06-7503com
Song, Y., Golling, G., Thacker, T. L., and Cone, R. D. (2003). Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine 22, 257–265. doi: 10.1385/ENDO:22:3:257
Song, Y.-F., Wu, K., Tan, X.-Y., Zhang, L.-H., Zhuo, M.-Q., Pan, Y.-X., et al. (2015). Effects of recombinant human leptin administration on hepatic lipid metabolism in yellow catfish Pelteobagrus fulvidraco: in vivo and in vitro studies. Gen. Comp. Endocrinol. 212, 92–99. doi: 10.1016/j.ygcen.2015.01.022
Sterling, M. E., Karatayev, O., Chang, G. Q., Algava, D. B., and Leibowitz, S. F. (2015). Model of voluntary ethanol intake in zebrafish: effect on behavior and hypothalamic orexigenic peptides. Behav. Brain Res. 278, 29–39. doi: 10.1016/J.Bbr.2014.09.024
Striberny, A., Ravuri, C. S., Jobling, M., and Jorgensen, E. H. (2015). Seasonal differences in relative gene expression of putative central appetite regulators in Arctic charr (Salvelinus alpinus) do not reflect its annual feeding cycle. PLoS ONE 10:e0138857. doi: 10.1371/journal.pone.0138857
Subhedar, N. K., Nakhate, K. T., Upadhya, M. A., and Kokare, D. M. (2014). CART in the brain of vertebrates: circuits, functions and evolution. Peptides 54, 108–130. doi: 10.1016/j.peptides.2014.01.004
Subhedar, N., Barsagade, V. G., Singru, P. S., Thim, L., and Clausen, J. T. (2011). Cocaine- and amphetamine-regulated transcript peptide (CART) in the telencephalon of the catfish, Clarias gariepinus: distribution and response to fasting, 2-deoxy-D-glucose, glucose, insulin, and leptin treatments. J. Comp. Neurol. 519, 1281–1300. doi: 10.1002/cne.22569
Suda, A., Kaiya, H., Nikaido, H., Shiozawa, S., Mishiro, K., and Ando, H. (2012). Identification and gene expression analyses of ghrelin in the stomach of Pacific bluefin tuna (Thunnus orientalis). Gen. Comp. Endocrinol. 178, 89–97. doi: 10.1016/J.Ygcen.2012.04.026
Sun, J., He, S., Liang, X. F., Li, L., Wen, Z., Zhu, T., et al. (2014). Identification of SNPs in NPY and LEP and the association with food habit domestication traits in mandarin fish. J. Genet. 93, e118–e122. doi: 10.1007/s12041-014-0442-4
Sundarrajan, L., Blanco, A. M., Bertucci, J. I., Ramesh, N., Canosa, L. F., and Unniappan, S. (2016). Nesfatin-1-Like peptide encoded in nucleobindin-1 in goldfish is a novel anorexigen modulated by sex steroids, Macronutrients and daily rhythm. Sci. Rep. 6:28377. doi: 10.1038/srep28377
Sundström, G., Larsson, T. A., Xu, B., Heldin, J., and Larhammar, D. (2013). Interactions of zebrafish peptide YYb with the neuropeptide Y-family receptors Y4, Y7, Y8a, and Y8b. Front. Neurosci. 7:29. doi: 10.3389/fnins.2013.00029
Suzuki, H., Miyoshi, Y., and Yamamoto, T. (2007). Orexin-A (hypocretin 1)-like immunoreactivity in growth hormone-containing cells of the Japanese seaperch (Lateolabrax japonicus) pituitary. Gen. Comp. Endocrinol. 150, 205–211. doi: 10.1016/j.ygcen.2006.08.008
Suzuki, H., and Yamamoto, T. (2013). Orexin-B-like immunoreactivity localizes in both luteinizing-hormone-containing cells and melanin-concentrating hormone-containing fibers in the red-bellied piranha (Pygocentrus nattereri) pituitary. Cell Tissue Res. 351, 175–182. doi: 10.1007/S00441-012-1516-3
Tachibana, T., and Sakamoto, T. (2014). Functions of two distinct prolactin-releasing peptides evolved from a common ancestral gene. Front. Endocrinol. 5:170. doi: 10.3389/fendo.2014.00170
Takahashi, A. (2016). “Chapter 16 - proopiomelanocortin family A2 - Takei, Yoshio,” in Handbook of Hormones, eds H. Ando and K. Tsutsui (San Diego, CA: Academic Press), e116–e113.
Takahashi, A., Amano, M., Itoh, T., Yasuda, A., Yamanome, T., Amemiya, Y., et al. (2005). Nucleotide sequence and expression of three subtypes of proopiomelanocortin mRNA in barfin flounder. Gen. Comp. Endocrinol. 141, 291–303. doi: 10.1016/j.ygcen.2005.01.010
Takahashi, A., Tsuchiya, K., Yamanome, T., Amano, M., Yasuda, A., Yamamori, K., et al. (2004). Possible involvement of melanin-concentrating hormone in food intake in a teleost fish, barfin flounder. Peptides 25, 1613–1622. doi: 10.1016/j.peptides.2004.02.022
Takeuchi, S. (2016). “Subchapter 8B - Agouti-Related Protein A2 - Takei, Yoshio,” in Handbook of Hormones, eds. H. Ando and K. Tsutsui (San Diego, CA: Academic Press), 70–71.
Tang, Y., Li, H., Li, J., Yu, F., and Yu, J. (2014). Characterization and expression analysis of two distinct neuropeptide Ya paralogues in Jian carp (Cyprinus carpio var. Jian). Fish Physiol. Biochem. 40, 1709–1719. doi: 10.1007/s10695-014-9961-4
Tang, Z., Sun, C., Yan, A., Wu, S., Qin, C., Zhang, Y., et al. (2013). Genes involved in fatty acid metabolism: molecular characterization and hypothalamic mRNA response to energy status and neuropeptide Y treatment in the orange-spotted grouper Epinephelus coioides. Mol. Cell. Endocrinol. 376, 114–124. doi: 10.1016/j.mce.2013.06.020
Tatemoto, K., Carlquist, M., and Mutt, V. (1982). Neuropeptide Y: a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660. doi: 10.1038/296659a0
Tatemoto, K., Hosoya, M., Habata, Y., Fujii, R., Kakegawa, T., Zou, M.-X., et al. (1998). Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 251, 471–476. doi: 10.1006/bbrc.1998.9489
Thavanathan, R., and Volkoff, H. (2006). Effects of amylin on feeding of goldfish: interactions with CCK. Regul. Pept. 133, 90–96. doi: 10.1016/j.regpep.2005.09.025
Tian, J., He, G., Mai, K. S., and Liu, C. D. (2015). Effects of postprandial starvation on mRNA expression of endocrine-, amino acid and peptide transporter-, and metabolic enzyme-related genes in zebrafish (Danio rerio). Fish Physiol. Biochem. 41, 773–787. doi: 10.1007/s10695-015-0045-x
Tillner, R., Ronnestad, I., Dhert, P., and Ueberschar, B. (2014). The regulatory loop between gut cholecystokinin and tryptic enzyme activitcxuy in sea bass (Dicentrarchus labrax) larvae is influenced by different feeding regimes and trigger substances. Aquaculture 420, 139–146. doi: 10.1016/j.aquaculture.2013.10.046
Tillner, R., Ronnestad, I., Harboe, T., and Ueberschar, B. (2013). Hormonal control of tryptic enzyme activity in Atlantic cod larvae (Gadus morhua): involvement of cholecystokinin during ontogeny and diurnal rhythm. Aquaculture 402, 133–140. doi: 10.1016/j.aquaculture.2013.04.003
Tinoco, A. B., Näslund, J., Delgado, M. J., de Pedro, N., Johnsson, J. I., and Jonsson, E. (2014a). Ghrelin increases food intake, swimming activity and growth in juvenile brown trout (Salmo trutta). Physiol. Behav. 124, 15–22. doi: 10.1016/j.physbeh.2013.10.034
Tinoco, A. B., Nisembaum, L. G., de Pedro, N., Delgado, M. J., and Isorna, E. (2014b). Leptin expression is rhythmic in brain and liver of goldfish (Carassius auratus). Role of feeding time. Gen. Comp. Endocrinol. 204, 239–247. doi: 10.1016/j.ygcen.2014.06.006
Tinoco, A. B., Nisembaum, L. G., Isorna, E., Delgado, M. J., and de Pedro, N. (2012). Leptins and leptin receptor expression in the goldfish (Carassius auratus). Regulation by food intake and fasting/overfeeding conditions. Peptides 34, 329–335. doi: 10.1016/j.peptides.2012.02.001
Tinoco, A. B., Valenciano, A. I., Gomez-Boronat, M., Blanco, A. M., Nisembaum, L. G., De Pedro, N., et al. (2015). Two cholecystokinin receptor subtypes are identified in goldfish, being the CCKAR involved in the regulation of intestinal motility. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 187, 193–201. doi: 10.1016/j.cbpa.2015.05.027
Tonon, M.-C., Leprince, J., Gandolfo, P., Compae, V., Pelletier, G., Malagon, M. M., et al. (2006). “Chapter 111 - Endozepines,” in Handbook of Biologically Active Peptides, ed A. J. Kastin (Burlington, ON: Academic Press), 813–819.
Trombley, S., Maugars, G., Kling, P., Bjornsson, B. T., and Schmitz, M. (2012). Effects of long-term restricted feeding on plasma leptin, hepatic leptin expression and leptin receptor expression in juvenile Atlantic salmon (Salmo salar L.). Gen. Comp. Endocrinol. 175, 92–99. doi: 10.1016/j.ygcen.2011.10.001
Trombley, S., Mustafa, A., and Schmitz, M. (2014). Regulation of the seasonal leptin and leptin receptor expression profile during early sexual maturation and feed restriction in male Atlantic salmon, Salmo salar L., parr. Gen. Comp. Endocrinol. 204, 60–70. doi: 10.1016/J.Ygcen.2014.04.033
Trudeau, V. L., Martyniuk, C. J., Zhao, E., Hu, H., Volkoff, H., Decatur, W. A., et al. (2012). Is secretoneurin a new hormone? Gen. Comp. Endocrinol. 175, 10–18. doi: 10.1016/j.ygcen.2011.10.008
Tsujino, N., and Sakurai, T. (2009). Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol. Rev. 61, 162–176. doi: 10.1124/pr.109.001321
Tsutsui, K., and Ubuka, T. (2013). “Chapter 107 - gonadotropin-inhibitory hormone,” in Handbook of Biologically Active Peptides, 2nd Edn. ed A.J. Kastin. (Boston, CA: Academic Press), 802–811.
Tuziak, S. M., and Volkoff, H. (2012). A preliminary investigation of the role of melanin-concentrating hormone (MCH) and its receptors in appetite regulation of winter flounder (Pseudopleuronectes americanus). Mol. Cell. Endocrinol. 348, 281–296. doi: 10.1016/j.mce.2011.09.015
Tuziak, S. M., and Volkoff, H. (2013a). Melanin-concentrating hormone (MCH) and gonadotropin-releasing hormones (GnRH) in Atlantic cod, Gadus morhua: tissue distributions, early ontogeny and effects of fasting. Peptides 50, 109–118. doi: 10.1016/j.peptides.2013.10.005
Tuziak, S. M., and Volkoff, H. (2013b). Gonadotrophin-releasing hormone in winter flounder (Pseudopleuronectes americanus): molecular characterization, distribution and effects of fasting. Gen. Comp. Endocrinol. 184, 9–21. doi: 10.1016/j.ygcen.2012.12.010
Tuziak, S. M., Rise, M. L., and Volkoff, H. (2014). An investigation of appetite-related peptide transcript expression in Atlantic cod (Gadus morhua) brain following a Camelina sativa meal-supplemented feeding trial. Gene 550, 253–263. doi: 10.1016/j.gene.2014.08.039
Unniappan, S., Cerdá-Reverter, J. M., and Peter, R. E. (2004). In situ localization of preprogalanin mRNA in the goldfish brain and changes in its expression during feeding and starvation. Gen. Comp. Endocrinol. 136, 200–207. doi: 10.1016/j.ygcen.2003.12.010
Unniappan, S., Lin, X., Cervini, L., Rivier, J., Kaiya, H., Kangawa, K., et al. (2002). Goldfish ghrelin: molecular characterization of the complementary deoxyribonucleic acid, partial gene structure and evidence for Its stimulatory role in food intake. Endocrinology 143, 4143–4146. doi: 10.1210/en.2002-220644
Upton, K. R., and Riley, L. G. (2013). Acute stress inhibits food intake and alters ghrelin signaling in the brain of tilapia (Oreochromis mossambicus). Domest. Anim. Endocrinol. 44, 157–164. doi: 10.1016/J.Domaniend.2012.10.001
Valen, R., Jordal, A. E. O., Murashita, K., and Ronnestad, I. (2011). Postprandial effects on appetite-related neuropeptide expression in the brain of Atlantic salmon, Salmo salar. Gen. Comp. Endocrinol. 171, 359–366. doi: 10.1016/j.ygcen.2011.02.027
Valenti, M., Cottone, E., Martinez, R., De Pedro, N., Rubio, M., Viveros, M. P., et al. (2005). The endocannabinoid system in the brain of Carassius auratus and its possible role in the control of food intake. J. Neurochem. 95, 662–672. doi: 10.1111/j.1471-4159.2005.03406.x
Van Nguyen, M., Jordal, A. E. O., Espe, M., Buttle, L., Lai, H. V., and Ronnestad, I. (2013). Feed intake and brain neuropeptide Y (NPY) and cholecystokinin (CCK) gene expression in juvenile cobia fed plant-based protein diets with different lysine to arginine ratios. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 165, 328–337. doi: 10.1016/j.cbpa.2013.04.004
Varricchio, E., Russo, F., Coccia, E., Mario Turchini, G., Scott Francis, D., and Paolucci, M. (2015). The orexinergic system in rainbow trout Oncorhynchus mykiss and its regulation by dietary lipids. Microsc. Res. Tech. 78, 707–714. doi: 10.1002/jemt.22528
Varricchio, E., Russo, F., Coccia, E., Turchini, G., Francis, D., De Girolamo, P., et al. (2012). Immunohistochemical and immunological detection of ghrelin and leptin in rainbow trout Oncorhynchus mykiss and murray cod Maccullochella peelii peelii as affected by different dietary fatty acids. Microsc. Res. Tech. 75, 771–780. doi: 10.1002/jemt.21124
Västermark, A., and Schioth, H. B. (2011). The early origin of melanocortin receptors, agouti-related peptide, agouti signalling peptide, and melanocortin receptor-accessory proteins, with emphasis on pufferfishes, elephant shark, lampreys, and amphioxus. Eur. J. Pharmacol. 660, 61–69. doi: 10.1016/j.ejphar.2010.10.106
Velasco, C., Librán-Pérez, M., Otero-Rodino, C., Lopez-Patino, M. A., Maguez, J. M., Cerda-Reverter, J. M., et al. (2016). Ghrelin modulates hypothalamic fatty acid-sensing and control of food intake in rainbow trout. J. Endocrinol. 228, 25–37. doi: 10.1530/JOE-15-0391
Vicentic, A., and Jones, D. C. (2007). The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J. Pharmacol. Exp. Ther. 320, 499–506. doi: 10.1124/jpet.105.091512
Vieira-Lopes, D. A., Pinheiro, N. L., Sales, A., Ventura, A., Araujo, F. G., Gomes, I. D., et al. (2013). Immunohistochemical study of the digestive tract of Oligosarcus hepsetus. World J. Gastroenterol. 19, 1919–1929. doi: 10.3748/wjg.v19.i12.1919
Vigna, S. R., and Gorbman, A. (1977). Effects of cholecystokinin, gastrin, and related peptides on coho salmon gallbladder contraction in vitro. Am. J. Physiol. 232, E485–E491.
Vikesa, V., Nankervis, L., Remo, S. C., Waagbo, R., and Hevroy, E. M. (2015). Pre and postprandial regulation of ghrelin, amino acids and IGF1 in Atlantic salmon (Salmo salar L.) at optimal and elevated seawater temperatures. Aquaculture 438, 159–169. doi: 10.1016/j.aquaculture.2014.12.021
Vivas, Y., Azpeleta, C., Feliciano, A., Velarde, E., Isorna, E., Delgado, M. J., et al. (2011). Time-dependent effects of leptin on food intake and locomotor activity in goldfish. Peptides 32, 989–995. doi: 10.1016/j.peptides.2011.01.028
Volkoff, H. (2006). The role of neuropeptide Y, orexins, cocaine and amphetamine-related transcript, cholecystokinin, amylin and leptin in the regulation of feeding in fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 144, 325–331. doi: 10.1016/j.cbpa.2005.10.026
Volkoff, H. (2011). “Control of appetite in fish,” in Encyclopedia of Fish Physiology, ed A. P. Farrell (San Diego, CA: Academic Press), 1509–1514.
Volkoff, H. (2012). Sleep and orexins in non mammalian vertebrates. Vitam. Horm. 89, 315–339. doi: 10.1016/B978-0-12-394623-2.00017-2
Volkoff, H. (2013). The effects of amphetamine injections on feeding behavior and the brain expression of orexin, CART, tyrosine hydroxylase (TH) and thyrotropin releasing hormone (TRH) in goldfish (Carassius auratus). Fish Physiol. Biochem. 39, 979–991. doi: 10.1007/s10695-012-9756-4
Volkoff, H. (2014a). Appetite regulating peptides in red-bellied piranha, Pygocentrus nattereri: cloning, tissue distribution and effect of fasting on mRNA expression levels. Peptides 56C, 116–124. doi: 10.1016/j.peptides.2014.03.022
Volkoff, H. (2014b). In vitro assessment of interactions between appetite-regulating peptides in brain of goldfish (Carassius auratus). Peptides 61, 61–68. doi: 10.1016/j.peptides.2014.09.002
Volkoff, H. (2015a). Cloning and tissue distribution of appetite-regulating peptides in pirapitinga (Piaractus brachypomus). J. Anim. Physiol. Anim. Nutr. 99, 987–1001. doi: 10.1111/jpn.12318
Volkoff, H. (2015b). Cloning, tissue distribution and effects of fasting on mRNA expression levels of leptin and ghrelin in red-bellied piranha (Pygocentrus nattereri). Gen. Comp. Endocrinol. 217–218:20–27. doi: 10.1016/j.ygcen.2015.05.004
Volkoff, H., and Peter, R. E. (2000). Effects of CART peptides on food consumption, feeding and associated behaviors in the goldfish, Carassius auratus: actions on neuropeptide Y- and orexin A-induced feeding. Brain Res. 887, 125–133. doi: 10.1016/S0006-8993(00)03001-8
Volkoff, H., and Peter, R. E. (2001a). Characterization of two forms of cocaine- and amphetamine-regulated transcript (CART) peptide precursors in goldfish: molecular cloning and distribution, modulation of expression by nutritional status, and interactions with leptin. Endocrinology 142, 5076–5088. doi: 10.1210/endo.142.12.8519
Volkoff, H., and Peter, R. E. (2001b). Interactions between orexin A, NPY and galanin in the control of food intake of the goldfish, Carassius auratus. Regul. Pept. 101, 59–72. doi: 10.1016/S0167-0115(01)00261-0
Volkoff, H., and Wyatt, J. L. (2009). Apelin in goldfish (Carassius auratus): cloning, distribution and role in appetite regulation. Peptides 30, 1434–1440. doi: 10.1016/j.peptides.2009.04.020
Volkoff, H., Bjorklund, J. M., and Peter, R. E. (1999). Stimulation of feeding behavior and food consumption in the goldfish, Carassius auratus, by orexin-A and orexin-B. Brain Res. 846, 204–209. doi: 10.1016/S0006-8993(99)02052-1
Volkoff, H., Canosa, L. F., Unniappan, S., Cerda-Reverter, J. M., Bernier, N. J., Kelly, S. P., et al. (2005). Neuropeptides and the control of food intake in fish. Gen. Comp. Endocrinol. 142, 3–19. doi: 10.1016/j.ygcen.2004.11.001
Volkoff, H., Eykelbosh, A. J., and Peter, R. E. (2003). Role of leptin in the control of feeding of goldfish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res. 972, 90–109. doi: 10.1016/S0006-8993(03)02507-1
Volkoff, H., Hamoutene, D., and Payne, J. F. (2007). Potential Effects of Tebufenozide on Feeding and Metabolism of Lake Trout (Salvelinus namaycush) Canadian Technical Report of Fisheries and Aquatic Sciences.
Volkoff, H., Sabioni, R. E., and Cyrino, J. E. P. (2016). Appetite regulating factors in dourado, Salminus brasiliensis: cDNA cloning and effects of fasting and feeding on gene expression. Gen. Comp. Endocrinol. 237, 34–42. doi: 10.1016/j.ygcen.2016.07.022
Volkoff, H., Sabioni, R. E., Coutinho, L. L., and Cyrino, J. E. (2017). Appetite regulating factors in pacu (Piaractus mesopotamicus): tissue distribution and effects of food quantity and quality on gene expression. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 203, 241–254. doi: 10.1016/j.cbpa.2016.09.022
Volkoff, H., Unniappan, S., and Kelly, S. P. (2009a). “The endocrine regulation of food intake,” in Fish Physiology, eds N. J. Bernier, G. Van Der Kraak, A. P. Farrell, and C. J. Brauner (Cambridge, MA: Academic Press), 421–465.
Volkoff, H., Xu, M., MacDonald, E., and Hoskins, L. (2009b). Aspects of the hormonal regulation of appetite in fish with emphasis on goldfish, Atlantic cod and winter flounder: notes on actions and responses to nutritional, environmental and reproductive changes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 153, 8–12. doi: 10.1016/j.cbpa.2008.12.001
Wahlestedt, C., and Reis, D. J. (1993). Neuropeptide Y-related peptides and their receptors–are the receptors potential therapeutic drug targets? Annu. Rev. Pharmacol. Toxicol. 33, 309–352. doi: 10.1146/annurev.pa.33.040193.001521
Walewski, J. L., Ge, F., Lobdell, H., Levin, N., Schwartz, G. J., Vasselli, J., et al. (2014). Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity 22, 1643–1652. doi: 10.1002/oby.20725
Wall, A., and Volkoff, H. (2013). Effects of fasting and feeding on the brain mRNA expressions of orexin, tyrosine hydroxylase (TH), PYY and CCK in the Mexican blind cavefish (Astyanax fasciatus mexicanus). Gen. Comp. Endocrinol. 183, 44–52. doi: 10.1016/j.ygcen.2012.12.011
Wan, Y., Zhang, Y., Ji, P., Li, Y., Xu, P., and Sun, X. (2012). Molecular characterization of CART, AgRP, and MC4R genes and their expression with fasting and re-feeding in common carp (Cyprinus carpio). Mol. Biol. Rep. 39, 2215–2223. doi: 10.1007/s11033-011-0970-4
Wang, K., Chen, X. T., Zhao, R. W., Hao, Q. R., Zhang, H., Xu, G. F., et al. (2013). Cloning and analysis of leptin in Culter alburnus in Xingkai Lake and down regulating its expression compared to cultured population. J. Anim. Vet. Adv. 12, 128–134. doi: 10.3923/javaa.2013.128.134
Wang, Q., Tan, X., Du, S., Sun, W., You, F., and Zhang, P. (2015). Characterization, tissue distribution, and expression of neuropeptide Y in olive flounder Paralichthys olivaceus. Chinese J. Oceanol. Limnol. 33, 553–558. doi: 10.1007/s00343-015-4090-1
Wang, T., Yuan, D., Zhou, C., Lin, F., Wei, R., Chen, H., et al. (2016). Molecular characterization of melanin-concentrating hormone (MCH) in Schizothorax prenanti: cloning, tissue distribution and role in food intake regulation. Fish Physiol. Biochem. 42, 883–893. doi: 10.1007/s10695-015-0182-2
Wang, T., Zhou, C., Yuan, D., Lin, F., Chen, H., Wu, H., et al. (2014). Schizothorax prenanti corticotropin-releasing hormone (CRH): molecular cloning, tissue expression, and the function of feeding regulation. Fish Physiol. Biochem. 40, 1407–1415. doi: 10.1007/s10695-014-9935-6
Wei, R., Yuan, D., Wang, T., Zhou, C., Lin, F., Chen, H., et al. (2013). Characterization, tissue distribution and regulation of agouti-related protein (AgRP) in a cyprinid fish (Schizothorax prenanti). Gene 527, 193–200. doi: 10.1016/j.gene.2013.06.003
Wei, R., Zhou, C., Yuan, D., Wang, T., Lin, F., Chen, H., et al. (2014). Characterization, tissue distribution and regulation of neuropeptideY in Schizothorax prenanti. J. Fish Biol. 85, 278–291. doi: 10.1111/jfb.12413
White, S. L., Volkoff, H., and Devlin, R. H. (2016). Regulation of feeding behavior and food intake by appetite-regulating peptides in wild-type and growth hormone-transgenic coho salmon. Horm. Behav. 84, 18–28. doi: 10.1016/j.yhbeh.2016.04.005
Won, E. T., and Borski, R. J. (2013). Endocrine regulation of compensatory growth in fish. Front. Endocrinol. 4:74. doi: 10.3389/fendo.2013.00074
Won, E. T., Baltzegar, D. A., Picha, M. E., and Borski, R. J. (2012). Cloning and characterization of leptin in a Perciform fish, the striped bass (Morone saxatilis): control of feeding and regulation by nutritional state. Gen. Comp. Endocrinol. 178, 98–107. doi: 10.1016/j.ygcen.2012.04.019
Won, E. T., Douros, J. D., Hurt, D. A., and Borski, R. J. (2016). Leptin stimulates hepatic growth hormone receptor and insulin-like growth factor gene expression in a teleost fish, the hybrid striped bass. Gen. Comp. Endocrinol. 229, 84–91. doi: 10.1016/j.ygcen.2016.02.003
Wong, M. K., Sze, K. H., Chen, T., Cho, C. K., Law, H. C., Chu, I. K., et al. (2013). Goldfish spexin: solution structure and novel function as a satiety factor in feeding control. Am. J. Physiol. Endocrinol. Metabol. 305, E348–E366. doi: 10.1152/ajpendo.00141.2013
Woods, I. G., Schoppik, D., Shi, V. J., Zimmerman, S., Coleman, H. A., Greenwood, J., et al. (2014). Neuropeptidergic signaling partitions arousal behaviors in zebrafish. J. Neurosci. 34, 3142–3160. doi: 10.1523/JNEUROSCI.3529-13.2014
Woods, S. C., and Begg, D. P. (2016). “Regulation of the motivation to eat,” in Behavioral Neuroscience of Motivation, eds E. H. Simpson and P. D. Balsam (Cham: Springer International Publishing), 15–34.
Wu, C. S., Wu, G. C., Zhang, G. Q., Wang, Q., Luo, J., and Chen, G. H. (2016). Single nucleotide polymorphisms in the leptin-a gene and associations with growth traits in the golden pompano, Trachinotus blochii. J. World Aquac. Soc. 47, 414–423. doi: 10.1111/jwas.12272
Xu, C., Li, X. F., Tian, H. Y., Jiang, G. Z., and Liu, W. B. (2016). Feeding rates affect growth, intestinal digestive and absorptive capabilities and endocrine functions of juvenile blunt snout bream Megalobrama amblycephala. Fish Physiol. Biochem. 42, 689–700. doi: 10.1007/s10695-015-0169-z
Xu, M., and Volkoff, H. (2007). Molecular characterization of prepro-orexin in Atlantic cod (Gadus morhua): Cloning, localization, developmental profile and role in food intake regulation. Mol. Cell. Endocrinol. 271, 28–37. doi: 10.1016/j.mce.2007.03.003
Xu, M., and Volkoff, H. (2009). Cloning, tissue distribution and effects of food deprivation on pituitary adenylate cyclase activating polypeptide (PACAP)/PACAP-related peptide (PRP) and preprosomatostatin 1 (PPSS 1) in Atlantic cod (Gadus morhua). Peptides 30, 766–776. doi: 10.1016/j.peptides.2008.12.010
Xu, M., Long, L., Chen, L., Qin, J., Zhang, L., Yu, N., et al. (2012). Cloning and differential expression pattern of pituitary adenylyl cyclase-activating polypeptide and the PACAP-specific receptor in darkbarbel catfish Pelteobagrus vachelli. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 161, 41–53. doi: 10.1016/j.cbpb.2011.09.005
Yan, A., Zhang, L., Tang, Z., Zhang, Y., Qin, C., Li, B., et al. (2011). Orange-spotted grouper (Epinephelus coioides) orexin: molecular cloning, tissue expression, ontogeny, daily rhythm and regulation of NPY gene expression. Peptides 32, 1363–1370. doi: 10.1016/j.peptides.2011.05.004
Yan, A.-F., Chen, T., Chen, S., Ren, C.-H., Hu, C.-Q., Cai, Y.-M., et al. (2016). Goldfish leptin-AI and leptin-AII: function and central mechanism in feeding control. Int. J. Mol. Sci. 17:783. doi: 10.3390/ijms17060783
Yang, L., Sun, C., and Li, W. (2014). Neuropeptide B in Nile tilapia Oreochromis niloticus: molecular cloning and its effects on the regulation of food intake and mRNA expression of growth hormone and prolactin. Gen. Comp. Endocrinol. 200, 27–34. doi: 10.1016/j.ygcen.2014.01.016
Yang, S., Xu, G., Du, F., and Xu, P. (2016). Molecular cloning, tissue and embryonic development expression, and appetite regulatory effect of pancreatic peptide Y in Coilia nasus. Fish. Sci. 82, 347–355. doi: 10.1007/s12562-015-0960-x
Yokobori, E., Azuma, M., Nishiguchi, R., Kang, K. S., Kamijo, M., Uchiyama, M., et al. (2012). Neuropeptide Y stimulates food intake in the zebrafish, Danio rerio. J. Neuroendocrinol. 24, 766–773. doi: 10.1111/j.1365-2826.2012.02281.x
Yokobori, E., Kojima, K., Azuma, M., Kang, K. S., Maejima, S., Uchiyama, M., et al. (2011). Stimulatory effect of intracerebroventricular administration of orexin A on food intake in the zebrafish, Danio rerio. Peptides 32, 1357–1362. doi: 10.1016/j.peptides.2011.05.010
Yuan, D., Zhou, C., Wang, T., Lin, F., Chen, H., Wu, H., et al. (2014). Molecular characterization and tissue expression of peptide YY in Schizothorax prenanti: effects of periprandial changes and fasting on expression in the hypothalamus. Regul. Pept. 190–191, 32–38. doi: 10.1016/j.regpep.2014.03.004
Yuan, X., Cai, W., Liang, X. F., Su, H., Yuan, Y., Li, A., et al. (2015). Obestatin partially suppresses ghrelin stimulation of appetite in “high-responders” grass carp, Ctenopharyngodon idellus. Comp. Biochem. Physiol. Part A 184, 144–149. doi: 10.1016/j.cbpa.2015.02.019
Yuan, X., Li, A., Liang, X.-F., Huang, W., Song, Y., He, S., et al. (2016). Leptin expression in mandarin fish Siniperca chuatsi (Basilewsky): regulation by postprandial and short-term fasting treatment. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 194, 8–18. doi: 10.1016/j.cbpa.2016.01.014
Zhang, H., Chen, H., Zhang, Y., Li, S., Lu, D., Zhang, H., et al. (2013). Molecular cloning, characterization and expression profiles of multiple leptin genes and a leptin receptor gene in orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 181, 295–305. doi: 10.1016/j.ygcen.2012.09.008
Zhang, J., Ma, W., He, Y., Wu, J., Dawar, F. U., Ren, F., et al. (2016a). Sex biased expression of ghrelin and GHSR associated with sexual size dimorphism in yellow catfish. Gene 578, 169–176. doi: 10.1016/j.gene.2015.12.017
Zhang, J., Sun, P., Yang, F., Kong, T., and Zhang, R. (2016b). Tributyltin disrupts feeding and energy metabolism in the goldfish (Carassius auratus). Chemosphere 152, 221–228. doi: 10.1016/j.chemosphere.2016.02.127
Zhang, L., Nguyen, A. D., Lee, I. C. J., Yulyaningsih, E., Riepler, S. J., Stehrer, B., et al. (2012). NPY modulates PYY function in the regulation of energy balance and glucose homeostasis. Diabetes Obes. Metabol. 14, 727–736. doi: 10.1111/j.1463-1326.2012.01592.x
Zhang, X., Wu, Y., Hao, J., Zhu, J., Tang, N., Qi, J., et al. (2016c). Intraperitoneal injection urocortin-3 reduces the food intake of Siberian sturgeon (Acipenser baerii). Peptides 85, 80–88. doi: 10.1016/j.peptides.2016.09.007
Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., and Friedman, J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432. doi: 10.1038/372425a0
Zhao, E., Zhang, D., Basak, A., and Trudeau, V. L. (2009). New insights into granin-derived peptides: evolution and endocrine roles. Gen. Comp. Endocrinol. 164, 161–174. doi: 10.1016/j.ygcen.2009.01.011
Zheng, J. L., Luo, Z., Hu, W., Liu, C. X., Chen, Q. L., Zhu, Q. L., et al. (2015). Different effects of dietary Zn deficiency and excess on lipid metabolism in yellow catfish Pelteobagrus fulvidraco. Aquaculture 435, 10–17. doi: 10.1016/j.aquaculture.2014.09.011
Zhong, C., Song, Y., Wang, Y., Zhang, T., Duan, M., Li, Y., et al. (2013). Increased food intake in growth hormone-transgenic common carp (Cyprinus carpio L.) may be mediated by upregulating Agouti-related protein (AgRP). Gen. Comp. Endocrinol. 192, 81–88. doi: 10.1016/j.ygcen.2013.03.024
Zhou, C., Zhang, X., Liu, T., Wei, R., Yuan, D., Wang, T., et al. (2014). Schizothorax davidi ghrelin: cDNA cloning, tissue distribution and indication for its stimulatory character in food intake. Gene 534, 72–77. doi: 10.1016/j.gene.2013.10.012
Zhou, C., Zheng, J., Lei, L., Yuan, D., Zhu, C., Ye, H., et al. (2016). Evidence that ghrelin may be associated with the food intake of gibel carp (Carassius auratus gibelio). Fish Physiol. Biochem. [Epub ahead of print]. doi: 10.1007/s10695-016-0246-y
Keywords: fish, hormones, feeding, appetite, diversity, brain, intestine
Citation: Volkoff H (2016) The Neuroendocrine Regulation of Food Intake in Fish: A Review of Current Knowledge. Front. Neurosci. 10:540. doi: 10.3389/fnins.2016.00540
Received: 22 August 2016; Accepted: 07 November 2016;
Published: 29 November 2016.
Edited by:
Hubert Vaudry, University of Rouen, FranceCopyright © 2016 Volkoff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helene Volkoff, aHZvbGtvZmZAbXVuLmNh
 Helene Volkoff
Helene Volkoff