- 1Department of Anatomy, Faculty of Medicine, Toho University, Tokyo, Japan
- 2International Institute for Integrative Sleep Medicine (WPI-IIIS), University of Tsukuba, Ibaraki, Japan
The preoptic area (POA) has long been recognized as a sleep center, first proposed by von Economo. The POA, especially the medial POA (MPOA), is also involved in the regulation of various innate functions such as sexual and parental behaviors. Consistent with its many roles, the MPOA is composed of subregions that are identified by different gene and protein expressions. This review addresses the current understanding of the molecular and cellular architecture of POA neurons in relation to sleep and reproductive behavior. Optogenetic and pharmacogenetic studies have revealed a diverse group of neurons within the POA that exhibit different neural activity patterns depending on vigilance states and whose activity can enhance or suppress wake, non-rapid eye movement (NREM) sleep, or rapid eye movement (REM) sleep. These sleep-regulating neurons are not restricted to the ventrolateral POA (VLPO) region but are widespread in the lateral MPOA and LPOA as well. Neurons expressing galanin also express gonadal steroid receptors and regulate motivational aspects of reproductive behaviors. Moxd1, a novel marker of sexually dimorphic nuclei (SDN), visualizes the SDN of the POA (SDN-POA). The role of the POA in sleep and other innate behaviors has been addressed separately; more integrated observation will be necessary to obtain physiologically relevant insight that penetrates the different dimensions of animal behavior.
Introduction
The preoptic area (POA), the most anterior part of the hypothalamus, is a brain region that has a complex structure consisting of different groups of neurons that control various functions and behaviors essential for the survival of individuals and species. The POA, especially the medial POA (MPOA), plays a crucial role in the regulation of sleep and reproduction-associated behavior, such as parenting and male mating (Tsuneoka et al., 2013; Hull and Dominguez, 2015; Tan et al., 2016; Chung et al., 2017; Kohl et al., 2018). The MPOA is also involved in aggression, predation, feeding, and body temperature regulation (Hashikawa et al., 2016; Han et al., 2017; Ishii et al., 2017; Park et al., 2018; Morrison and Nakamura, 2019). Importantly, some of the MPOA nuclei show distinct sexual dimorphism (Simerly et al., 1984; Bloch and Gorski, 1988; Alexander et al., 1991; Orikasa and Sakuma, 2010; Tsuneoka et al., 2017a). The POA’s various important roles attracted researchers, but at the same time, many researchers believe that the POA is complex and difficult to understand. One of the reasons why there is so much confusion surrounding the POA is the inconsistent nomenclature of its subregions/nuclei. Even the two most commonly used mouse brain atlases are not consistent regarding the terminology and subnucleus structure of the POA (Paxinos et al., 2012; Allen Brain Atlas1). Therefore, in this review, we first describe the organization of the POA and then discuss how this region regulates sleep/wakefulness and various other behaviors.
Cellular Organization and Gene Expression in the POA
In mammals, the MPOA is the brain region between the anterior commissure and the optic chiasm in the vicinity of the anterior third ventricle (Gurdjian, 1927; Paxinos et al., 2012; Simerly, 2015). The lateral POA (LPOA) is an area flanked by the MPOA medially, the bed nucleus of the stria terminalis (BNST) dorsally, and the substantia innominata laterally. The ventrolateral side of the LPOA is bordered by the nucleus of the diagonal band. The LPOA contains the medial forebrain bundle, and the medial border of the bundle corresponds to the medial border of the LPOA (Simerly, 2015). Several genes are differentially expressed between the MPOA and LPOA. For example, neurons expressing the neuropeptide galanin, which are involved in the regulation of sleep/wakefulness and reproductive behavior, are abundant in the MPOA but not in the LPOA (Figures 1, 2). Similarly, neurotensin-expressing neurons are abundant in the MPOA but not in the LPOA (Figures 1, 2). Estrogen receptor (ER) α and androgen receptor (AR), which play an important role in reproductive behavior, are also plentiful in the MPOA but not in the LPOA. Conversely, choline acetyltransferase (ChAT)-positive neurons are present in the LPOA but not in the MPOA (Uschakov et al., 2007). Therefore, although both LPOA and MPOA contain the region name POA, there are significant differences in their constituent cells and gene expression.
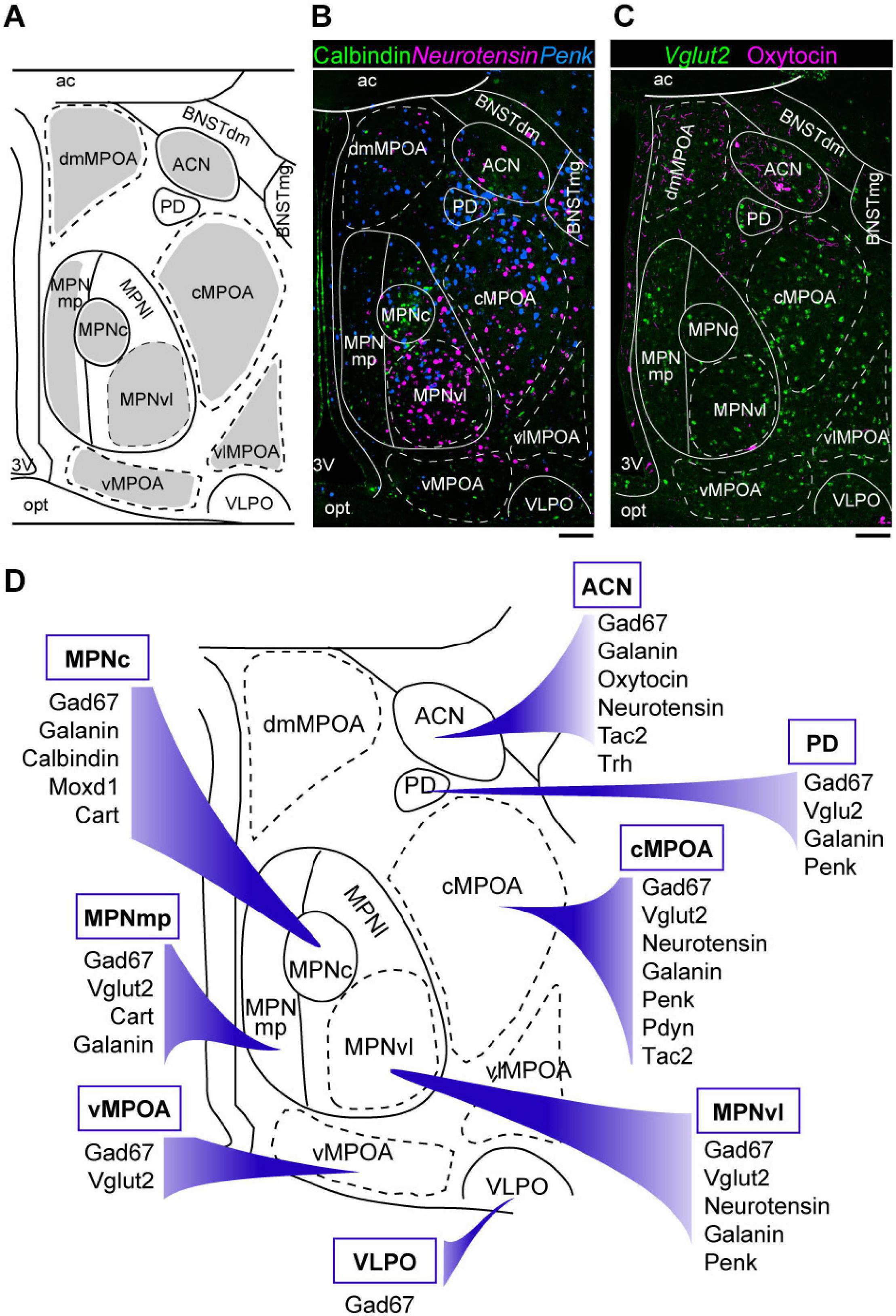
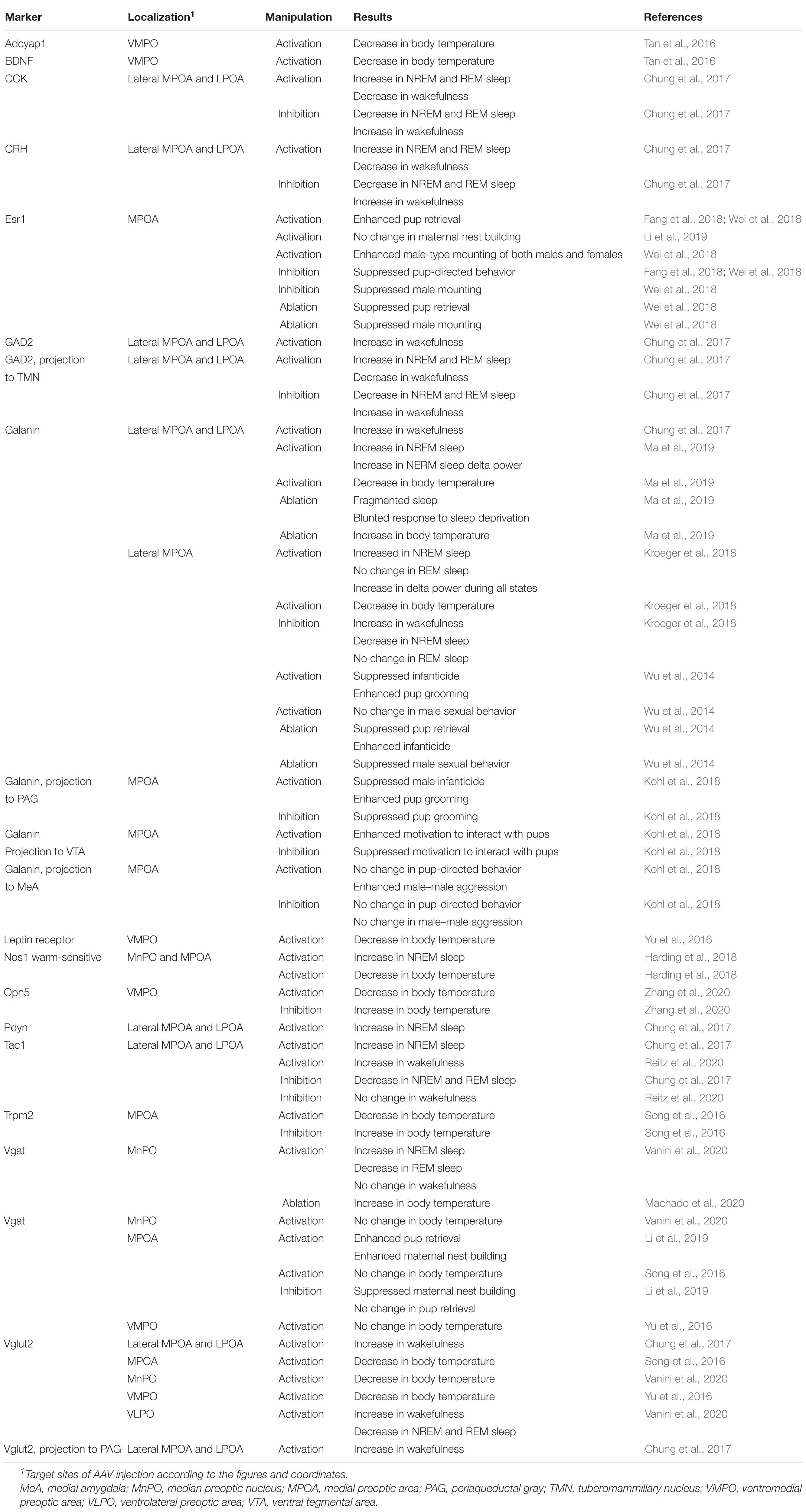
Figure 1. Neuroanatomy and heterogeneity of the medial preoptic area. (A) Coronal mouse brain diagram showing the MPOA subregions at the level of the MPNc (bregma, –0.02 mm). (B) Double ISH for Penk (blue) and neurotensin (magenta) mRNAs with immunostaining for calbindin (green). (C) ISH for Vglut2 (green) mRNA with immunostaining for oxytocin (magenta). (D) Summary of marker genes expression in selected MPOA subregions. Each subregion has a characteristic pattern of marker expression. Scale bars: 100 μm. 3v, third ventricle; ac, anterior commissure; ACN, anterior commissural nucleus; BNSTdm, dorsomedial nucleus of the BNST; BNSTmg, magnocellular nucleus of the BNST; cMPOA, central part of the MPOA; dmMPOA, dorsomedial part of the MPOA; MPNc, central part of the MPN; MPNl, lateral part of the MPN; MPNmp, posteromedial part of the MPN; MPNvl, ventrolateral part of the MPN; opt, optic tract; PD, posterodorsal preoptic nucleus; vMPOA, ventral part of the MPOA; vlMPOA, ventrolateral part of the MPOA; VLPO, ventrolateral preoptic nucleus. Modified from Tsuneoka et al. (2017b).
Figure 2. Galanin and neurotensin mRNA expression in the preoptic area. (A,B) Double ISH for (A) galanin and (B) neurotensin of a coronal section of the mouse brain (bregma + 0.10 mm). (C,D) Double ISH for (C) galanin and (D) neurotensin of a coronal section of the mouse brain (bregma –0.02 mm). (E,F) Double ISH for (E) galanin and (F) neurotensin of a coronal section of the mouse brain (bregma, –0.14 mm). (G,H) Double ISH for (G) galanin and (H) neurotensin of a coronal section of the mouse brain (bregma, –0.24 mm). Scale bars: 200 μm. 3v, third ventricle; ac, anterior commissure; ACN, anterior commissural nucleus; AH, anterior hypothalamus; BNST, bed nucleus of the stria terminalis; fx, fornix; LAN, lateroanterior hypothalamic nucleus LPOA, lateral preoptic area; MPOA, medial preoptic area; MPN, medial preoptic nucleus; NDB, nucleus of the diagonal band; opt, optic tract; PVPOA, periventricular preoptic area; SCN, suprachiasmatic nucleus; SI, substantia innominata; SON, supraoptic nucleus; VLPO, ventrolateral preoptic nucleus. Adapted from Tsuneoka et al. (2013).
The MPOA contains several nuclei, such as the medial preoptic nucleus (MPN), median preoptic nucleus (MnPO), and posterodorsal preoptic nucleus (PD), according to regional differences in terms of neuron density and the expressions of various neuropeptides, neuropeptide receptors, and gonadal steroid receptors (Figure 1; Simerly et al., 1986, 1988, 1990; Ju and Swanson, 1989; Simerly, 2015; Tsuneoka et al., 2017b). The MPN is a cell-dense, highly conspicuous structure located in the medial MPOA. The MnPO is a dense cluster of small cells located on the dorsal midline of the anterior third ventricle. The PD is a small region containing large neurons close to the BNST, which abundantly expresses proenkephalin (Penk). The ventrolateral POA (VLPO) is a small region located on the ventrolateral margin of the MPOA, adjacent to the nucleus of the diagonal band (Simerly, 2015).
We have recently proposed additionally subdividing the MPOA apart from the established nuclei MPN, PD, and VLPO into four regions, namely, the dorsomedial part of the MPOA (dmMPOA), the central part of the MPOA (cMPOA), the ventral part of the MPOA (vMPOA), and the ventrolateral part of the MPOA (vlMPOA), based on gene expression (Tsuneoka et al., 2017b) (Figure 1). The cMPOA and vMPOA show higher expression of gonadal steroid receptors than the dmMPOA or the vlMPOA. The central part of the MPN (MPNc) has been shown to predominantly overlap with the sexually dimorphic nucleus of the POA in rats (Gorski et al., 1978; Bloch and Gorski, 1988), which can be identified as a dense cluster of calbindin-positive cells in rats and mice (Sickel and McCarthy, 2000; Orikasa and Sakuma, 2010; Jahan et al., 2015; Tsuneoka et al., 2017a) or by the expression of Moxd1 in mice (Tsuneoka et al., 2017a).
The lateral subdivision of the MPN (MPNl) has a cluster of neurotensin-positive cells (Simerly et al., 1986; Tsuneoka et al., 2013). The anterior commissural nucleus (ACN) is characterized by a population of oxytocinergic neurons in the dorsal MPOA. In the mouse ACN, there are also many thyrotropin-releasing hormone (TRH)-positive neurons (Biag et al., 2012; Tsuneoka et al., 2017b). The MPNm is divided into the anterior part (MPNma), which contains a cluster of Penk-expressing cells, and the posterior part (MPNmp), which has a high density of cocaine- and amphetamine-regulated transcript (Cart)-expressing cells. The most posterior part of the MPNl (MPNp) was different from the main part of the MPNl in the densities of neurotensin-, Penk-, prodynorphin (Pdyn)-, and tachykinin 1 (Tac1)-positive cells. Thus, as summarized in Figure 1D, the MPOA is not randomly populated with neurons that show different gene expressions but is composed of subregions that have characteristic gene expression patterns.
Recent single-cell RNA-seq analysis of the mouse MPOA demonstrated that the messenger RNA (mRNA) expression of neuropeptides such as neurotensin, galanin, Tac1, Tac2, Penk, Pdyn, Cart, and Trh contributed to the clustering of 23 excitatory subpopulations and 43 inhibitory subpopulations (Moffitt et al., 2018). In addition to single-cell RNA-seq, multiplexed error-robust fluorescent in situ hybridization (MERFISH), which visualizes hundreds of mRNAs at single-cell resolution with position information (Moffitt et al., 2016; Shah et al., 2016), showed that the locations of 30% of cell clusters matched each of the MPOA subnuclei (Moffitt et al., 2018). In other words, each MPOA subnucleus contains cell cluster(s) that represent and are limited to the subnucleus; in addition, there are also cell clusters that are broadly distributed across the MPOA.
Reflecting the fact that MPOA is associated with sex-hormone-related behaviors, ER and AR are highly expressed in the MPOA (Simerly et al., 1990; Murphy and Hoffman, 2001; Merchenthaler et al., 2004; Jahan et al., 2015). At the subregion level, gonadal steroid receptors are abundantly expressed in the cMPOA, MPNvl, and MPNma (Tsuneoka et al., 2017b), which are involved in sexual, parental, and aggressive behavior (Tsuneoka et al., 2015). More than 70% of galanin-positive neurons show ERα and AR immunoreactivity in the cMPOA and MPN (Tsuneoka et al., 2017b). Neurotensin-, Penk-, and Tac2-positive cell groups also showed high proportions of ERα and AR immunoreactivity, but Trh-positive cells did not (Tsuneoka et al., 2017b). Through region- and neuron group-specific expression of their receptors, gonadal hormones are thought to regulate sex differences in various behaviors, including sleep.
The boundaries of the MPOA can also be defined according to its characteristic gene expression. ER and AR are abundant in the MPOA, but posterior to the anterior commissure, their expression decreases sharply and is observed in only a few dorsal hypothalamic regions. Similarly, galanin and neurotensin are abundantly expressed in the MPOA with a distribution pattern similar to that of gonadal steroid receptors, but their expression is sharply reduced posterior to the anterior commissure (Tsuneoka et al., 2017b; Figure 2). Thus, we consider the oblique plane containing the posterior end of the anterior commissure and the center of the SCN to be the caudal limit of the POA. This view is also supported by the expression region of the transcription factor Nkx2.1, which plays an important role in the development of various hypothalamic structures and is abundantly expressed in the embryonic and infant POA in mice (García-López et al., 2008; Puelles et al., 2012). Thus, the MPOA subregions and LPOA can be defined according to gene expressions.
Diverse POA Neurons Regulate Sleep/Wakefulness
The POA has long been recognized as being crucial for sleep induction (Saper et al., 2005; Liu and Dan, 2019). In the winter of 1916, there was a sudden increase in the number of patients suffering from high fever, fatigue, double vision, sleep problems, and catatonia in Vienna. Thereafter, an epidemic of this disease recurred every winter until approximately 1926. Austrian psychiatrist von Economo noticed a certain pattern of brain damage in these patients and named it encephalitis lethargica. He further found that patients who suffered from insomnia had damage in the anterior hypothalamus, while those who suffered from somnolence had broad damage rostral to the oculomotor nucleus level (von Economo, 1930). His pioneering findings provided the first argument for the existence of sleep centers in the brain. Subsequent lesion studies added to the findings supporting a role of the POA in the regulation of sleep (Nauta, 1946; McGinty and Sterman, 1968; Szymusiak and McGinty, 1986b; Sallanon et al., 1989; John and Kumar, 1998; Lu et al., 2000). For example, lesions of the MPOA using N-methyl-D-aspartate (NMDA) in rats reduced deep non-rapid eye movement (NREM) and rapid eye movement (REM) sleeps, shortened NREM sleep episodes, and increased body temperature (John and Kumar, 1998).
Consistent with its role in sleep induction, unit recordings of POA neurons showed the presence of sleep-active neurons (Kaitin, 1984; Szymusiak and McGinty, 1986a; Ogawa and Kawamura, 1988; Koyama and Hayaishi, 1994; Szymusiak et al., 1998; Takahashi et al., 2009; Alam et al., 2014). For example, out of 98 neurons in the rat POA, 14 neurons were active specifically during NREM sleep, and 26 neurons were most active during REM sleep (Koyama and Hayaishi, 1994). Seventy-six percent of rat MnPO neurons were sleep active, and most of them showed a gradual increase in firing rates before sleep onset (Suntsova et al., 2002). Single-unit activity of rat MnPO neurons increased during sleep deprivation but decreased during recovery sleep (Alam et al., 2014), suggesting that the activity of sleep-active POA neurons is closely related to NREM sleep delta power generation. In mice, among 872 single units in the POA and adjacent region, 552 were sleep active (Takahashi et al., 2009), and 60% of sleep-active neurons were active during both NREM and REM sleep and resting during wakefulness. Sleep-active neurons were broadly distributed within the MPOA and LPOA, and different types of sleep-active neurons, such as NREM sleep-specific, REM sleep-specific, and NREM/REM sleep-specific neurons, were intermingled in the POA. Importantly, wake-active neurons were also found in the MPOA and LPOA and were more restricted to the middle and ventral POA than sleep-active neurons (Takahashi et al., 2009). Wake-active neurons ceased firing immediately before sleep onset and started firing around 0.5 s before the onset of wakefulness. Thus, the POA contains different sets of neurons that are most active during each vigilance state.
Visualization of Fos protein as a marker of active cells showed that Fos-positive neurons during sleep and after sleep deprivation were found abundantly and broadly in the MnPO and LPOA (Cirelli et al., 1995; Gong et al., 2000; Semba et al., 2001), which is consistent with a broad distribution of sleep-active neurons in the POA (Takahashi et al., 2009). Fos-positive cells during recovery sleep were also broadly distributed in the lateral MPOA and the LPOA (Zhang et al., 2015). Genetic labeling of active neurons using FosCreER mice demonstrated that the LPOA was one of a few brain regions where the number of labeled neurons increased during recovery sleep compared to sleep deprivation (Zhang et al., 2015). More than 75% of sleep-active neurons in the POA were positive for glutamic acid decarboxylase (GAD), the rate-limiting enzyme in gamma aminobutyric acid (GABA) production (Gong et al., 2004). REM sleep restriction induced Fos immunoreactivity in GAD-positive neurons in the rat POA and MnPO (Gvilia et al., 2006), consistent with REM sleep-specific neurons in the POA (Takahashi et al., 2009). These findings indicate that the POA contains neurons that are active in different aspects of sleep regulation.
Optogenetic and pharmacogenetic manipulation of POA neurons directly demonstrated their role in the regulation of sleep/wakefulness (Table 1). Activation of a group of LPOA and lateral MPOA neurons using CNO/hM3Dq induced NREM sleep (Zhang et al., 2015). Surprisingly, activation of GABAergic neurons in the LPOA and lateral MPOA enhanced wakefulness (Chung et al., 2017). However, activation of GABAergic POA neurons that projected to the tuberomammillary nucleus (TMN), where wake-promoting histaminergic neurons are located, immediately enhanced NREM sleep and a markedly increased REM sleep within 1 min, whereas inhibition of those neurons suppressed NREM and REM sleep (Chung et al., 2017). In contrast to GABAergic POA neurons projecting to the TMN, optogenetic activation of glutaminergic POA neurons projecting to the TMN enhanced wakefulness (Chung et al., 2017). Optrode recording of 17 TMN-projecting POA GABAergic neurons revealed that they were sleep active, with their highest discharge rate occurring during REM sleep. Regarding the subtype of TMN-projecting GABAergic neurons, these neurons partly overlapped with cholecystokinin (CCK)-, corticotropin-releasing hormone (CRH)-, and Tac1-positive neurons. Whereas optogenetic activation of CCK- and CRH-positive neurons increased both NREM and REM sleep (Chung et al., 2017), optogenetic activation of Tac1-positive neurons increased only NREM sleep and not REM sleep (Chung et al., 2017). However, chemogenetic activation of Tac1-positive POA neurons increased wakefulness (Reitz et al., 2020).
In conclusion, we can identify diverse inhibitory neuron populations with different neurotransmitters and projection patterns, and each population is thought to regulate sleep in different ways.
Since almost all POA neurons expressing the neuropeptide galanin are GABAergic, galanin-positive POA neurons are thought to be a subgroup of POA GABAergic neurons. Optogenetic activation of galanin-positive POA neurons at high frequencies enhanced wakefulness (Chung et al., 2017). However, another study showed that optogenetic activation of galanin-positive POA neurons at a frequency close to physiological discharge rates, such as 2–5 Hz, enhanced NREM sleep but did not change the amount of REM sleep (Kroeger et al., 2018). Accordingly, optogenetic inhibition of galanin neurons in the LPOA and lateral MPOA decreased NREM sleep but did not alter the REM sleep amount (Kroeger et al., 2018). Activation of galanin-positive neurons increased the number of sleep episodes but did not change episode length, suggesting a role of galanin-positive POA neurons in the initiation of NREM sleep episodes rather than their maintenance. In addition, sustained activation of galanin neurons in the POA using pharmacogenetic tools resulted in a mild increase in NREM sleep, a decrease in REM sleep, and prominent hypothermia (Kroeger et al., 2018). Inhibition of galanin-positive POA neurons by GABAergic neurons of the ventral lateral hypothalamic area (LHA) induced wakefulness (Venner et al., 2019), which supports a sleep-inducing role of galanin-positive POA neurons.
Importantly, when galanin-positive POA neurons were photoactivated, the electroencephalogram (EEG) showed high-amplitude slow waves entrained to photoactivation and presented an increase in NREM sleep delta power (Kroeger et al., 2018), which is often used as an indicator of sleep need (Franken et al., 2001). Ablation of galanin neurons in the LPOA and lateral MPOA (defined according to their coordinates) weakened the response to 5 h sleep deprivation; mice with ablated galanin neurons showed markedly diminished increase in sleep time and a blunted increase in delta power during recovery NREM sleep (Ma et al., 2019). These findings suggest that galanin neurons in the POA play a role in the homeostatic regulation of sleep.
Galanin-expressing neurons in the POA may also be implicated in sleep in humans and fish. The number of galaninergic neurons in the human intermediate nucleus, equivalent to the rodent POA, was correlated with sleep fragmentation in older individuals with and without Alzheimer’s disease (Lim et al., 2014). Galanin-expressing POA neurons were active during recovery sleep in zebrafish. Additionally, galanin expression increased during wakefulness and sleep deprivation in zebrafish (Reichert et al., 2019). Thus, POA neurons expressing galanin may play a conserved role in sleep promotion in diverse animal species.
Preoptic Area Fiber Connections for Sleep/Wakefulness
Microinjection of AAV-EF1α-DIO-ChR2-mCherry in the LPOA of galanin-Cre mice visualizes axon terminals in the dorsomedial nucleus (DMH), LHA, TMN, pedunculopontine tegmental nucleus, medial parabrachial nucleus, locus coeruleus, ventrolateral and lateral periaqueductal gray matter, lateral pontine tegmentum, and raphe pallidus but none in the median or MPOA (Kroeger et al., 2018). Regarding the functional connections of sleep-active neurons in the POA, neurons that express Fos during sleep and project to the paraventricular nucleus or LHA are distributed in the LPOA and MPOA (Uschakov et al., 2006). GABAergic neurons in the POA project to the POA, TMN, ventral tegmental area (VTA), locus coeruleus, orexin neurons/LHA, and laterodorsal tegmentum (Saito et al., 2013), which mainly promote wakefulness. As expected, optogenetic stimulation of GABAergic neurons in the POA inhibited orexin neuron activity in brain slices (Saito et al., 2013). Similarly, MnPO neurons send their axons to the whole MPOA and LPOA, the dorsal raphe, the locus coeruleus, and orexin neurons in the LHA (Uschakov et al., 2007). Optogenetic activation of the axons of POA GABAergic neurons in the TMN increased NREM sleep (Chung et al., 2017). In contrast to GABAergic neurons, optogenetic activation of glutamatergic POA neurons projecting to the TMN enhanced wakefulness (Chung et al., 2017).
As for the neurons upstream of sleep-regulating POA neurons, monosynaptic input to TMN-projecting LPOA neurons was found in the hypothalamus and amygdala (Chung et al., 2017). DMH neurons send their fibers to galanin-expressing GABAergic neurons in the POA (Chen et al., 2018). Optogenetic activation of these DMH neurons enhanced NREM sleep and suppressed REM sleep. Thus, there are mutual functional connections between POA GABAergic neurons and DMH neurons. In conclusion, since sleep-regulating neurons are intermingled in the POA, it is necessary to divide POA neurons into subgroups according to their afferent and efferent connections in addition to gene expression to understand how POA neurons regulate sleep/wake behavior.
Sleep-Inducing Neurons Are not Restricted to the VLPO
As discussed above, sleep-active or sleep-inducing neurons are broadly distributed in the lateral MPOA and LPOA (Figure 3A), which extends the idea that the VLPO is a sleep center (Saper et al., 2005; Saper and Fuller, 2017). Neurons that were Fos positive after sleep deprivation were found broadly in the medial and lateral POA (Cirelli et al., 1995; Gong et al., 2000; Semba et al., 2001). TMN-projecting GABAergic neurons are also broadly distributed in the lateral POA (Chung et al., 2017). AAV-based gene expression cannot be localized but rather tends to be widespread, and its distribution varies widely among injected mice (Kroeger et al., 2018). Therefore, optogenetic and chemogenetic activation of the LPOA regions led to broadly increased Fos-positive cells in both the MPOA and LPOA (Kroeger et al., 2018). CCK-, CRH-, and Tac1-positive neurons that enhance sleep are also broadly distributed across the MPOA and LPOA (Chung et al., 2017). Although GABA and galanin are sometimes used as markers for the VLPO (Sherin et al., 1998; Gaus et al., 2002), GABAergic neurons are distributed throughout the POA, and galanin is expressed abundantly in the medial MPOA but only sparsely in the VLPO regions (Figure 2). At the caudal end of the POA, galanin expression is sharply reduced and is restricted to the supraoptic nucleus (SON), which is caudally adjacent to the VLPO and induces sleep (Jiang-Xie et al., 2019). We have examined the expression of many genes and proteins in the POA but have not found any suitable marker for the VLPO. Since gene expression cannot clearly define the VLPO, we can identify the VLPO only according to its brain atlas coordinates. The problem is that these coordinates are not completely consistent between brain atlases. Neurons regulating sleep are not only found in the VLPO but throughout POA with diverse groups that can enhance or suppress wake, NREM sleep, or REM sleep, indicating that the POA is more than just a sleep-inducing center.
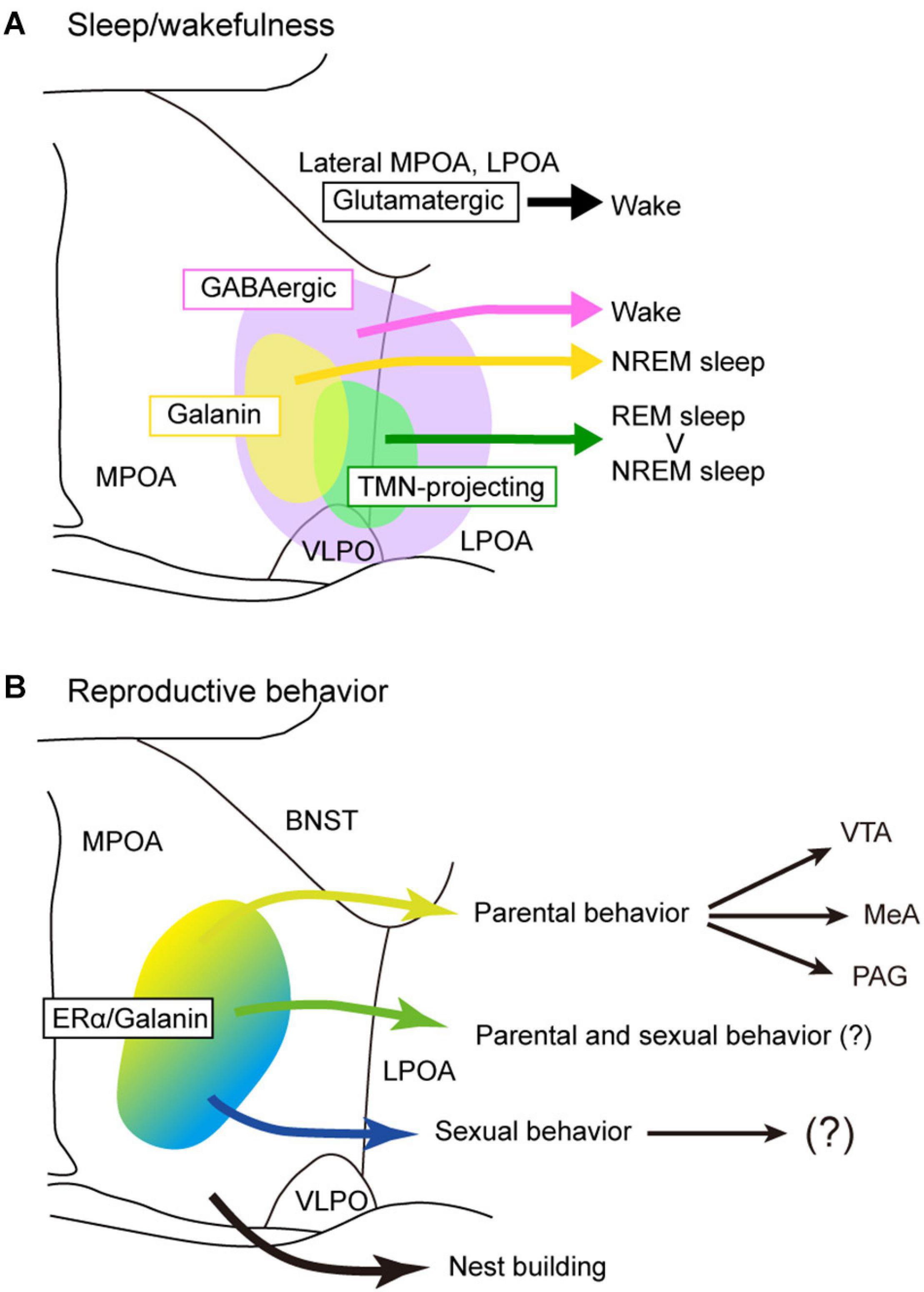
Figure 3. Models of POA neuron groups that regulate sleep and reproductive behavior. (A) Different subgroups of POA GABAergic neurons control sleep and wakefulness differently. TMN-projecting GABAergic neurons promote NREM sleep and strongly REM sleep. Galanin-positive POA neurons that are mainly GABAergic generally promote NREM sleep. POA glutamatergic neurons promote wakefulness. (B) Estrogen receptor α and/or galanin-positive neurons in the MPOA regulate parental behavior and male sexual behavior. Neurons involving nest building behavior was not estrogen receptor α positive. BNST, bed nucleus of the stria terminalis; LPOA, lateral preoptic area; MeA, medial amygdala; MPOA, medial preoptic area; PAG, periaqueductal gray; TMN, tuberomammillary nucleus; VLPO, ventrolateral preoptic nucleus; VTA, ventral tegmental area.
Parental Behavior
In addition to sleep, galanin-expression POA neurons are involved in parental behavior (Wu et al., 2014; Kohl et al., 2018). Before the identification of galanin-expressing neurons as major players in parental behaviors, it had been known that the MPOA was most critical for parental motivation, especially related to pup retrieval behavior and inhibition of aggression toward pups, known as infanticide (Febo, 2011; Pereira and Morrell, 2011; Stolzenberg and Numan, 2011).
Pup retrieval is a parental behavior in which a parent picks a pup up in the mouth and carries it to the nest in response to chemical signals and ultrasonic vocalization of the pup (Okabe et al., 2010; Isogai et al., 2018). Whereas virgin female mice retrieve pups, virgin male mice often display aggression toward pups or infanticide. However, once a virgin male mouse mates and cohabitates with a female mouse, the male will show pup retrieval behavior. In other words, female mice always show pup retrieval, whereas male mice switch from infanticide to pup retrieval in a sexual experience-dependent manner.
In rats, the dorsolateral MPOA is considered to be more important in pup retrieval behavior than the ventral or medial MPOA (Jacobson et al., 1980; Numan et al., 1990). The distribution of Fos-expressing neurons differs among MPOA subregions during maternal behavior toward pups (Numan and Numan, 1994; Lin et al., 1998; Li et al., 1999; Sheehan et al., 2000; Mathieson et al., 2002). In mice, a high density of Fos expression during parental behavior was observed in the ACN, cMPOA, and vMPOA regardless of the reproductive condition of the mice, such as virgin females, parturient females, postpartum dams, and father mice (Tsuneoka et al., 2013, 2015). Lesion ablating cMPOA neurons completely abolished pup retrieval in male mice and surprisingly led virgin and parous females to conduct infanticide (Tsuneoka et al., 2013, 2015). Fos-expressing cells in the cMPOA during parental behavior are galanin-positive neurons in both females and males (Tsuneoka et al., 2013; Wu et al., 2014). Ablation of galanin-positive POA neurons enhanced infanticide (Wu et al., 2014). These results indicate that cMPOA suppresses aggression toward pups in both sexes, possibly through the activation of galanin-positive neurons.
Estrogen and prolactin regulate maternal behavior toward pups. Pharmacological blockade of estrogen in the MPOA and small interfering RNA (siRNA) silencing of Esr1 encoding ERα suppressed maternal behaviors in mice (Ribeiro et al., 2012; Catanese and Vandenberg, 2017). Acute deletion of prolactin receptors in the mouse MPOA completely abolished pup retrieval (Brown et al., 2017). Activation of Esr1-positive MPOA neurons promoted pup retrieval and ablation of Esr1-positive cells suppressed pup retrieval (Fang et al., 2018; Wei et al., 2018). Similarly, activation of galanin-positive MPOA neurons enhanced pup grooming and suppressed infanticide (Wu et al., 2014). Since most galanin-positive neurons express ERα, MPOA neurons expressing both ERα and galanin control parental behavior under the influence of estrogen (Figure 3B). In addition to estrogen, oxytocin also regulates maternal behavior. The upregulation of oxytocin signaling in the mouse MPOA promoted experience-induced maternal motivation (Okabe et al., 2017). MERFISH showed that neuron groups expressing oxytocin receptor are specifically active during pup-directed aggression (Moffitt et al., 2018). These cell populations are distinct from parental behavior-specific cells expressing calcitonin receptor and bombesin receptor (Brs3). However, it should be noted that the analysis included the BNST subregions in addition to the MPOA (Moffitt et al., 2018) because the BNST is also involved in parental behavior (Tsuneoka et al., 2015).
Reciprocal connections between the MPOA and medial amygdala (MeA) are important for parental behavior. Galanin neurons in the MPOA send their projections to the MeA and are activated during parenting regardless of the type of parental behavior (Kohl et al., 2018). Optogenetic activation of the MPOA galanin neuron fibers in the MeA inhibited aggression toward pups in virgin male mice, although it did not affect parental behavior (Kohl et al., 2018). MPOA-projecting MeA neurons were activated during parenting behavior, especially in father mice (Kohl et al., 2018). In female mice, optogenetic stimulation of GABAergic neurons in the MeA promoted pup grooming but not pup retrieval or crouching (Chen et al., 2019). Because almost all MPOA galanin neurons are inhibitory, they may regulate interaction with pups by inhibiting negative olfactory stimuli encoded in the MeA (Kohl et al., 2018).
Projections from the MPOA to the VTA are thought to be important for maternal motivation in rats (Numan and Smith, 1984; Hansen et al., 1991; Stack et al., 2002; Numan et al., 2005). Similarly, Esr1-positive neurons in the mouse MPOA send strong inhibitory input to non-dopaminergic neurons in the VTA, and this inhibitory input promotes maternal pup retrieval through disinhibition of dopaminergic neurons (Fang et al., 2018). Since optogenetic manipulation of the axons of MPOA galaninergic neurons in the VTA did not change parental pup retrieval (Kohl et al., 2018), galanin-positive MPOA neurons projecting to the VTA may not be involved in pup retrieval. These results indicate that galanin-negative, ERα-positive GABAergic neurons may be involved in pup retrieval via the VTA (Figure 3B).
Nest Building Behavior
Many animals sleep in a certain posture in a nest. Rodents build nests for resting, sleeping, keeping warm, raising children, and hiding from enemies (Jirkof, 2014). Thus, nest building behavior is necessary for sleep and parental behavior. Lesions in the vMPOA of female mice suppressed nest building but did not affect maternal behavior toward pups (Tsuneoka et al., 2013). In contrast, cMPOA-lesioned mice showed disruption in both nest building and parental behavior (Tsuneoka et al., 2013). Activation of MPOA GABAergic neurons enhanced nest building (Li et al., 2019). Activation of agouti-related protein (AGRP) fibers from the arcuate nucleus to the MPOA markedly decreased nest building but only slightly changed pup retrieval behavior (Li et al., 2019). This suggests that different groups of neurons with specific fiber connections separately regulate nest building and retrieval of pups to the nest.
Although activation of Esr1-positive MPOA neurons enhanced maternal pup retrieval (Fang et al., 2018; Wei et al., 2018), activation of Esr1-positive MPOA neurons and local estrogen blockade by bisphenol S in the MPOA did not affect maternal nest building behavior (Catanese and Vandenberg, 2017; Li et al., 2019), suggesting that Esr1-positive MPOA neurons are not involved in nest building. Mouse build nests not only for nurturing but also for keeping warm. Mice nesting becomes inactive in warm conditions. Warm-sensitive neurons expressing pituitary adenylate cyclase-activating polypeptide (PACAP)/brain-derived neurotrophic factor (BDNF) in the MPOA drastically inhibited nest building behavior (Tan et al., 2016).
Male Sexual Behavior
Conceptually, sexual behaviors are composed of appetitive and consummatory behaviors. In male rodents, appetitive sexual behavior consists of behavioral components that increase mating opportunities, such as approaching, pursuing, and sniffing. Consummatory sexual behavior is a sequence consisting of mounting, intromission, and ejaculation. Accumulated findings indicate a crucial role of the MPOA in consummatory sexual behavior (Hull and Dominguez, 2007, 2015). Electrical stimulation of the rat MPOA during mounting facilitated ejaculation (Malsbury, 1971). Lesions in the rat MPOA abolished consummatory sexual behavior repertoires (Bermond, 1982; Hansen et al., 1982; Arendash and Gorski, 1983). Such loss of sexual performance due to MPOA lesions was not reversed even after 8 months (Ginton and Merari, 1977), suggesting that there is no alternative brain region carrying out this function of the MPOA. Importantly, the number of neurons with enhanced firing changed throughout a series of mating behaviors (Horio et al., 1986; Shimura et al., 1994), and Fos expression in the rat MPOA was increased from mounting to intromission and then to ejaculation (Coolen et al., 1996; Veening and Coolen, 1998; Yamaguchi et al., 2018). This indicates that an increasing number of MPOA cells are activated as consummatory sexual behavior progresses.
Additionally, male pursuit of females disappeared in MPOA-lesioned rats (Paredes et al., 1993, 1998; Paredes and Baum, 1995; Kindon et al., 1996), and their partner preference changed from receptive females to stud males (Paredes and Baum, 1995; Kindon et al., 1996; Paredes et al., 1998). Therefore, MPOA neurons are thought to be involved in appetitive sexual behavior as well (Veening and Coolen, 2014). MPOA neurons also mediate experience-dependent sexual arousal. Sexual experience facilitates all components of mating behaviors, and this facilitation is thought to be mediated by experience-induced changes in MPOA neurons, such as synaptic plasticity (Jean et al., 2017), dopamine sensitivity (McHenry et al., 2012; Nutsch et al., 2016), oxytocin sensitivity (Gil et al., 2013), and neuropeptide precursor expression (Maejima et al., 2018).
Testosterone plays a crucial role in the regulation of male sexual behavior. After testosterone is aromatized into estradiol, this estradiol induces male sexual behavior via ERα (Sano et al., 2016). Esr1 knockdown in MPOA neurons by short-hair RNA (shRNA) drastically suppressed a series of male sexual behaviors and Esr1 knockdown in other Esr1-expressing sites such as the VMH and MeA and suppressed specific components of male sexual behaviors (Sano et al., 2013, 2016). In vivo calcium imaging revealed that Esr1-positive POA neurons were activated immediately after the initiation of any sexual behavior, and optogenetic activation of Esr1-positive neurons enhanced male sexual behavior (Wei et al., 2018). In addition, genetic ablation of Esr1-positive POA neurons suppressed male mounting behavior, and optogenetic inhibition suppressed both mounting and intromission in a stimulus-timing-dependent manner (Wei et al., 2018). Thus, estrogen derived from testosterone may activate a subgroup of POA neurons to promote male sexual behavior.
In addition to estrogen, oxytocin also regulates male sexual behavior. Male rat copulation was facilitated by microinjection of oxytocin in the MPOA and suppressed by an oxytocin receptor antagonist. Oxytocin binding in the MPOA was correlated with the sexual performance of males (Okabe et al., 2017).
It is not clear what types of MPOA neurons are involved in male sexual behaviors, but various neuropeptides and neurotransmitters such as α-MSH, dopamine, NPY, galanin, substance P, neurokinin K, opioids, orexin, and oxytocin have been demonstrated to affect male sexual behavior through MPOA neurons in rats (Argiolas and Melis, 2013). The distributions of Fos-positive neurons were different during parental behavior and male sexual behavior (Tsuneoka et al., 2015; Moffitt et al., 2018). catFISH analysis showed that only a small percentage (∼10%) of Esr1-positive neurons were activated during both parental and sexual behavior (Wei et al., 2018), suggesting that the cell populations involved in these two behaviors rarely overlap.
Moxd1, a Novel Marker of Sexually Dimorphic Nuclei
In addition to reproductive behavior, there are also sex differences in sleep (Komiya et al., 2018). The total wake time of female mice is much longer than that of male mice (Funato et al., 2016). Although it is not clear what mechanism is responsible for the sex difference in sleep, sexually dimorphic structures in brains may be involved. To examine the role of sexual dimorphism in the brain, a good marker for sexually dimorphic nuclei (SDN) is necessary.
We identified Moxd1 as a new and specific marker gene for SDN through an in silico search of the Allen Gene Expression Atlas (Figure 4). Moxd1 is expressed highly specifically in all major SDN, such as the SDN-POA, the principal nucleus of the BNST (BNSTpr), and posterodorsal part of the medial amygdala (MePD) (Tsuneoka et al., 2017a). In the MPOA, only a few Moxd1-positive cells exist outside the SDN-POA. Moxd1-positive SDN-POA cells are more numerous in male mice than in female mice (Figure 4). Subsequently, single-cell RNA-seq and MERFISH analyses have confirmed Moxd1 as a marker for a neuron subgroup in the preoptic region (Moffitt et al., 2018). Importantly, Moxd1 expression in the SDN-POA was not affected by adult castration or ovariectomy but was affected by neonatal castration, suggesting that the expression of Moxd1 in the SDN-POA is determined by the hormonal milieu during the perinatal period and that the expression is independent of the activating effect of gonadal steroids in adulthood. Moxd1 encodes a monooxygenase, DBH-like 1, that is localized in the endoplasmic reticulum and is predicted to hydroxylate a hydrophobic substrate based on its amino acid sequence, which is similar to that of dopamine β-hydroxylase (Xin et al., 2004). Because the substrates of Moxd1 protein have not been identified, its biological role in sexually dimorphic neurons is not known. Although calbindin has been used as a marker of the SDN-POA, calbindin-positive cells are also distributed outside the SDN-POA (Tsuneoka et al., 2017a) (Figure 4). Thus, the higher specificity of Moxd1 allows us to visualize and manipulate SDN in order to better understand sexual differences in behavior.
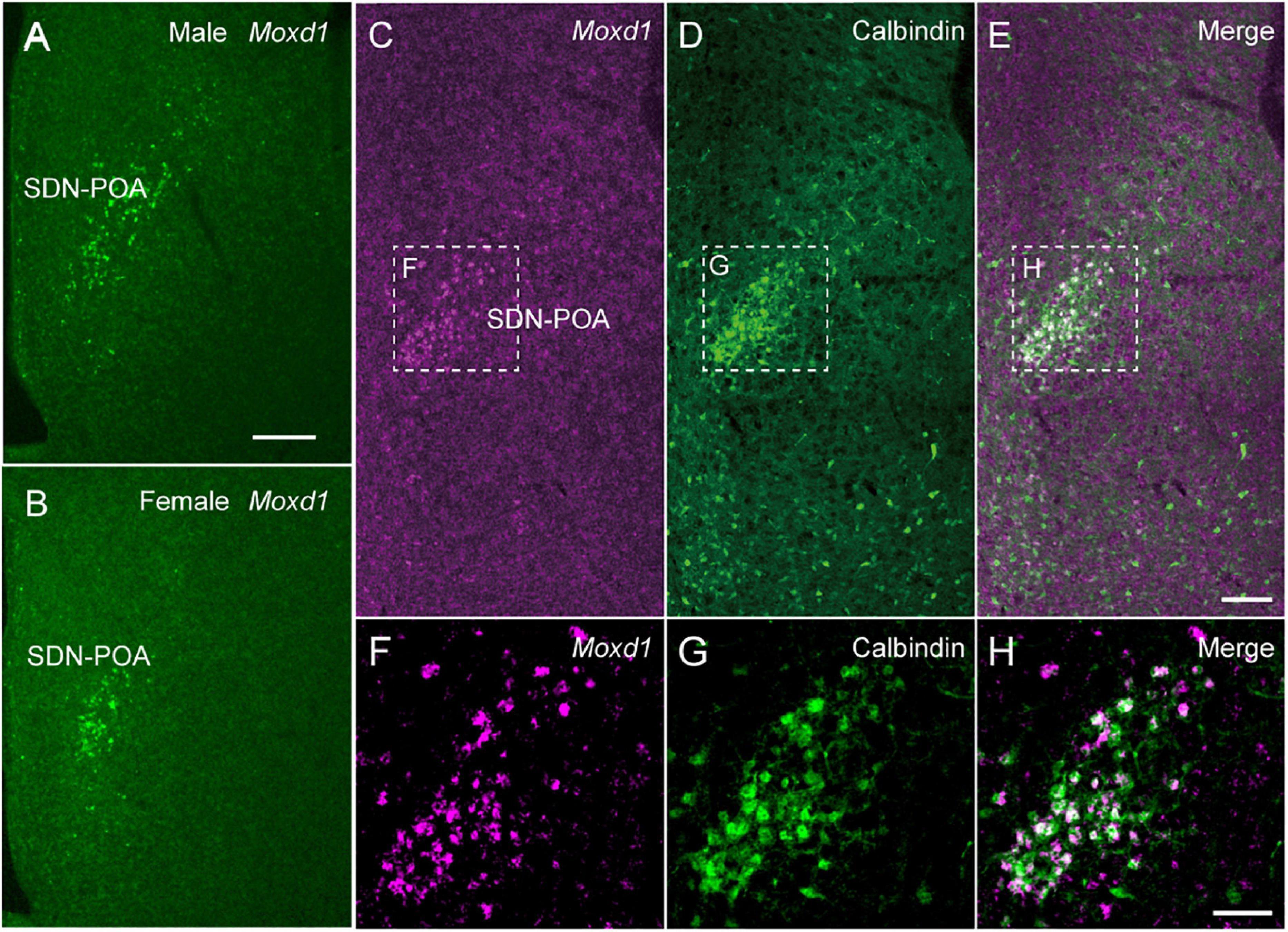
Figure 4. Moxd1 mRNA as a marker for sexually dimorphic nucleus. (A,B) Moxd1 mRNA expression in the sexually dimorphic nucleus of the POA (SDN-POA) of (A) male and (B) female mice. The number of Moxd1-positive cells of the SDN-POA are higher in males than in females. (C,D) The SDN-POA has cells expressing (C) Moxd1 and (D) calbindin. (E) Merged images of Moxd1 and Calbindin. (F,G) Moxd1, calbindin, merged image of dashed rectangles in panels (C–E). Scale bars: (A) 200 μm, (E) 100 μm, and (H) 50 μm. Modified from Tsuneoka et al. (2017a).
Future Directions
In summary, different approaches, including in vivo neurophysiology, Fos imaging, cell ablation, and optogenetic and chemogenetic manipulation, have generally shown that the POA contains a diverse group of neurons that differentially control wake, NREM sleep, and REM sleep. Detailed subgrouping of POA neurons in terms of gene markers, input/output connections, neurotransmitters, and neurophysiological properties is necessary to better understand how POA neurons regulate sleep/wakefulness.
So far, the role of the POA in sleep and other innate behaviors has been addressed separately. However, given that galanin-positive POA neurons are involved in the regulation of both sleep and parental behavior, more integrated observation will be necessary to obtain physiologically relevant insight. Since POA neurons are involved in a variety of innate behaviors associated with enhanced levels of arousal, changes in sleep/wakefulness can be secondary to increased motivation for certain behaviors. For example, whereas galanin neurons projecting to the TMN induce sleep, those projecting to the MeA promote pup-directed behavior with an increased arousal level. In this case, activation of all galanin neurons in the POA overcomes the sleep-inducing effects of a subset of galanin neurons and leads to arousal.
Although it was not the focus here, the POA serves as a hub for thermoregulation; it receives thermosensory signals from the skin and regulates downstream pathways for heat production by the brown adipose tissue and heat dissipation by the skin through vasodilation (Nakamura, 2011; Morrison et al., 2014; Harding et al., 2018; Tan and Knight, 2018; Ma et al., 2019; Morrison and Nakamura, 2019). Given that body temperature changes are associated with sleep/wake behavior, the POA may coordinately be responsible for lower body temperature during NREM sleep. The recent discovery of warm-sensing neurons in the POA expressing PACAP/Adcyap1 and BDNF demonstrated that a specific group of neurons in the POA are responsible for sensing warm environments and inducing adaptive changes that reduce heat production by brown adipose tissues and enhance heat dissipation from the skin (Tan et al., 2016). Partly overlapping neuronal populations in the midline POA have been shown to induce hypothermia (Hrvatin et al., 2020; Takahashi et al., 2020; Zhang et al., 2020).
Recent technical advances to visualize and manipulate specific groups of POA neurons will uncover how different groups of intermingled but largely separate POA neurons function in coordination as hubs for diverse behavioral modalities. How this system matured during ontogeny and evolved during phylogeny will be a future challenge. We believe that the diversity of POA reflects not only the diversity of individual behavior but also the diversity of the animal kingdom.
Author Contributions
YT and HF contributed to the conceptualization, writing, and funding acquisition. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Takeda Science Foundation (YT), Grant for Basic Science Research Projects from the Sumitomo Foundation (YT), the Uehara Memorial Foundation (HF), the Naito Foundation (HF), the Daiichi-Sankyo Foundation of Life Science (HF), and the JSPS KAKENHI (18K06509 to YT and 17H05583 to HF).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We were grateful to Masashi Yanagisawa, Takeshi Sakurai, Sachine Yoshida, Kimiya Narikiyo, and Satoko Oda for the helpful discussion and Kaspar Vogt for the critical reading.
Footnotes
References
Alam, M. A., Kumar, S., McGinty, D., Alam, M. N., and Szymusiak, R. (2014). Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J. Neurophysiol. 111, 287–299. doi: 10.1152/jn.00504.2013
Alexander, M. J., Kiraly, Z. J., and Leeman, S. E. (1991). Sexually dimorphic distribution of neurotensin/neuromedin N mRNA in the rat preoptic area. J. Comp. Neurol. 311, 84–96. doi: 10.1002/cne.903110107
Arendash, G. W., and Gorski, R. A. (1983). Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res. Bull. 10, 147–154. doi: 10.1016/0361-9230(83)90086-2
Argiolas, A., and Melis, M. R. (2013). Neuropeptides and central control of sexual behaviour from the past to the present: a review. Prog. Neurobiol. 108, 80–107. doi: 10.1016/j.pneurobio.2013.06.006
Bermond, B. (1982). Effects of medial preoptic hypothalamus anterior lesions on three kinds of behavior in the rat: intermale aggressive, male-sexual, and mouse-killing behavior. Aggress. Behav. 8, 335–354. doi: 10.1002/1098-2337(1982)8:4<335::aid-ab2480080403>3.0.co;2-8
Biag, J., Huang, Y., Gou, L., Hintiryan, H., Askarinam, A., Hahn, J. D., et al. (2012). Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J. Comp. Neurol. 520, 6–33. doi: 10.1002/cne.22698
Bloch, G. J., and Gorski, R. A. (1988). Cytoarchitectonic analysis of the SDN-POA of the intact and gonadectomized rat. J. Comp. Neurol. 275, 604–612. doi: 10.1002/cne.902750408
Brown, R. S. E., Aoki, M., Ladyman, S. R., Phillipps, H. R., Wyatt, A., Boehm, U., et al. (2017). Prolactin action in the medial preoptic area is necessary for postpartum maternal nursing behavior. Proc. Natl. Acad. Sci. U. S. A. 114, 10779–10784. doi: 10.1073/pnas.1708025114
Catanese, M. C., and Vandenberg, L. N. (2017). Bisphenol S (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology 158, 516–530.
Chen, K.-S., Xu, M., Zhang, Z., Chang, W.-C., Gaj, T., Schaffer, D. V., et al. (2018). A hypothalamic switch for REM and Non-REM sleep. Neuron 97, 1168–1176.e4.
Chen, P. B., Hu, R. K., Wu, Y. E., Pan, L., Huang, S., Micevych, P. E., et al. (2019). Sexually dimorphic control of parenting behavior by the medial amygdala. Cell 176, 1206–1221.e18.
Chung, S., Weber, F., Zhong, P., Tan, C. L., Nguyen, T. N., Beier, K. T., et al. (2017). Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545, 477–481. doi: 10.1038/nature22350
Cirelli, C., Pompeiano, M., and Tononi, G. (1995). Sleep deprivation and c-fos expression in the rat brain. J. Sleep Res. 4, 92–106. doi: 10.1111/j.1365-2869.1995.tb00157.x
Coolen, L. M., Peters, H. J., and Veening, J. G. (1996). Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res. 738, 67–82. doi: 10.1016/0006-8993(96)00763-9
Fang, Y.-Y., Yamaguchi, T., Song, S. C., Tritsch, N. X., and Lin, D. (2018). A hypothalamic midbrain pathway essential for driving maternal behaviors. Neuron 98, 192–207.e10.
Febo, M. (2011). A bold view of the lactating brain: functional magnetic resonance imaging studies of suckling in awake dams. J. Neuroendocrinol. 23, 1009–1019. doi: 10.1111/j.1365-2826.2011.02184.x
Franken, P., Chollet, D., and Tafti, M. (2001). The homeostatic regulation of sleep need is under genetic control. J. Neurosci. 21, 2610–2621. doi: 10.1523/jneurosci.21-08-02610.2001
Funato, H., Miyoshi, C., Fujiyama, T., Kanda, T., Sato, M., Wang, Z., et al. (2016). Forward-genetics analysis of sleep in randomly mutagenized mice. Nature 539, 378–383. doi: 10.1038/nature20142
García-López, M., Abellán, A., Legaz, I., Rubenstein, J. L. R., Puelles, L., and Medina, L. (2008). Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J. Comp. Neurol. 506, 46–74. doi: 10.1002/cne.21524
Gaus, S. E., Strecker, R. E., Tate, B. A., Parker, R. A., and Saper, C. B. (2002). Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience 115, 285–294. doi: 10.1016/s0306-4522(02)00308-1
Gil, M., Bhatt, R., Picotte, K. B., and Hull, E. M. (2013). Sexual experience increases oxytocin receptor gene expression and protein in the medial preoptic area of the male rat. Psychoneuroendocrinology 38, 1688–1697. doi: 10.1016/j.psyneuen.2013.02.002
Ginton, A., and Merari, A. (1977). Long range effects of MPOA lesion on mating behavior in the male rat. Brain Res. 120, 158–163. doi: 10.1016/0006-8993(77)90507-8
Gong, H., McGinty, D., Guzman-Marin, R., Chew, K.-T., Stewart, D., and Szymusiak, R. (2004). Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J. Physiol. 556, 935–946. doi: 10.1113/jphysiol.2003.056622
Gong, H., Szymusiak, R., King, J., Steininger, T., and McGinty, D. (2000). Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R2079–R2088.
Gorski, R. A., Gordon, J. H., Shryne, J. E., and Southam, A. M. (1978). Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 148, 333–346. doi: 10.1016/0006-8993(78)90723-0
Gurdjian, E. S. (1927). The diencephalon of the albino rat. studies on the brain of the rat. No. 2. J. Comp. Neurol. 43, 1–114. doi: 10.1002/cne.900430102
Gvilia, I., Xu, F., McGinty, D., and Szymusiak, R. (2006). Homeostatic regulation of sleep: a role for preoptic area neurons. J. Neurosci. 26, 9426–9433. doi: 10.1523/jneurosci.2012-06.2006
Han, W., Tellez, L. A., Rangel, M. J. Jr., Motta, S. C., Zhang, X., Perez, I. O., et al. (2017). Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 168, 311–324.e18.
Hansen, S., Harthon, C., Wallin, E., Löfberg, L., and Svensson, K. (1991). Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav. Neurosci. 105, 588–598. doi: 10.1037/0735-7044.105.4.588
Hansen, S., Köhler, C., Goldstein, M., and Steinbusch, H. V. (1982). Effects of ibotenic acid-induced neuronal degeneration in the medial preoptic area and the lateral hypothalamic area on sexual behavior in the male rat. Brain Res. 239, 213–232. doi: 10.1016/0006-8993(82)90843-5
Harding, E. C., Yu, X., Miao, A., Andrews, N., Ma, Y., Ye, Z., et al. (2018). A neuronal hub binding sleep initiation and body cooling in response to a warm external stimulus. Curr. Biol. 28, 2263–2273.e4.
Hashikawa, K., Hashikawa, Y., Falkner, A., and Lin, D. (2016). The neural circuits of mating and fighting in male mice. Curr. Opin. Neurobiol. 38, 27–37. doi: 10.1016/j.conb.2016.01.006
Horio, T., Shimura, T., Hanada, M., and Shimokochi, M. (1986). Multiple unit activities recorded from the medial preoptic area during copulatory behavior in freely moving male rats. Neurosci. Res. 3, 311–320. doi: 10.1016/0168-0102(86)90023-4
Hrvatin, S., Sun, S., Wilcox, O. F., Yao, H., Lavin-Peter, A. J., Cicconet, M., et al. (2020). Neurons that regulate mouse torpor. Nature 583, 115–121. doi: 10.1038/s41586-020-2387-5
Hull, E. M., and Dominguez, J. M. (2007). Sexual behavior in male rodents. Horm. Behav. 52, 45–55. doi: 10.1016/j.yhbeh.2007.03.030
Hull, E. M., and Dominguez, J. M. (2015). “Chapter 49 - male sexual behavior,” in Knobil and Neill’s Physiology of Reproduction, 5th Edn, eds T. M. Plant and A. J. Zeleznik (San Diego: Academic Press), 2211–2285.
Ishii, K. K., Osakada, T., Mori, H., Miyasaka, N., Yoshihara, Y., Miyamichi, K., et al. (2017). A labeled-line neural circuit for pheromone-mediated sexual behaviors in mice. Neuron 95, 123–137.e8.
Isogai, Y., Wu, Z., Love, M. I., Ahn, M. H.-Y., Bambah-Mukku, D., Hua, V., et al. (2018). Multisensory logic of infant-directed aggression by males. Cell 175, 1827–1841.e17.
Jacobson, C. D., Terkel, J., Gorski, R. A., and Sawyer, C. H. (1980). Effects of small medial preoptic area lesions on maternal behavior: retrieving and nest building in the rat. Brain Res. 194, 471–478. doi: 10.1016/0006-8993(80)91226-3
Jahan, M. R., Kokubu, K., Islam, M. N., Matsuo, C., Yanai, A., Wroblewski, G., et al. (2015). Species differences in androgen receptor expression in the medial preoptic and anterior hypothalamic areas of adult male and female rodents. Neuroscience 284, 943–961. doi: 10.1016/j.neuroscience.2014.11.003
Jean, A., Bonnet, P., Liere, P., Mhaouty-Kodja, S., and Hardin-Pouzet, H. (2017). Revisiting medial preoptic area plasticity induced in male mice by sexual experience. Sci. Rep. 7:17846.
Jiang-Xie, L.-F., Yin, L., Zhao, S., Prevosto, V., Han, B.-X., Dzirasa, K., et al. (2019). A common neuroendocrine substrate for diverse general anesthetics and sleep. Neuron 102, 1053–1065.e4.
Jirkof, P. (2014). Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 234, 139–146. doi: 10.1016/j.jneumeth.2014.02.001
John, J., and Kumar, V. M. (1998). Effect of NMDA lesion of the medial preoptic neurons on sleep and other functions. Sleep 21, 587–598. doi: 10.1093/sleep/21.6.587
Ju, G., and Swanson, L. W. (1989). Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: i. Cytoarchitecture. J. Comp. Neurol. 280, 587–602. doi: 10.1002/cne.902800409
Kaitin, K. I. (1984). Preoptic area unit activity during sleep and wakefulness in the cat. Exp. Neurol. 83, 347–357. doi: 10.1016/s0014-4886(84)90103-1
Kindon, H. A., Baum, M. J., and Paredes, R. J. (1996). Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm. Behav. 30, 514–527. doi: 10.1006/hbeh.1996.0055
Kohl, J., Babayan, B. M., Rubinstein, N. D., Autry, A. E., Marin-Rodriguez, B., Kapoor, V., et al. (2018). Functional circuit architecture underlying parental behaviour. Nature 556, 326–331. doi: 10.1038/s41586-018-0027-0
Komiya, H., Miyoshi, C., Iwasaki, K., Hotta-Hirashima, N., Ikkyu, A., Kanno, S., et al. (2018). Sleep/wake behaviors in mice during pregnancy and pregnancy-associated hypertensive mice. Sleep 41, 1–14.
Koyama, Y., and Hayaishi, O. (1994). Firing of neurons in the preoptic/anterior hypothalamic areas in rat: its possible involvement in slow wave sleep and paradoxical sleep. Neurosci. Res. 19, 31–38. doi: 10.1016/0168-0102(94)90005-1
Kroeger, D., Absi, G., Gagliardi, C., Bandaru, S. S., Madara, J. C., Ferrari, L. L., et al. (2018). Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat. Commun. 9:4129.
Li, C., Chen, P., and Smith, M. S. (1999). Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience 94, 117–129. doi: 10.1016/s0306-4522(99)00236-5
Li, X.-Y., Han, Y., Zhang, W., Wang, S.-R., Wei, Y.-C., Li, S.-S., et al. (2019). AGRP neurons project to the medial preoptic area and modulate maternal nest-building. J. Neurosci. 39, 456–471. doi: 10.1523/jneurosci.0958-18.2018
Lim, A. S. P., Ellison, B. A., Wang, J. L., Yu, L., Schneider, J. A., Buchman, A. S., et al. (2014). Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain 137, 2847–2861. doi: 10.1093/brain/awu222
Lin, S. H., Miyata, S., Weng, W., Matsunaga, W., Ichikawa, J., Furuya, K., et al. (1998). Comparison of the expression of two immediate early gene proteins, FosB and Fos in the rat preoptic area, hypothalamus and brainstem during pregnancy, parturition and lactation. Neurosci. Res. 32, 333–341. doi: 10.1016/s0168-0102(98)00100-x
Liu, D., and Dan, Y. (2019). A motor theory of sleep-wake control: arousal-action circuit. Annu. Rev. Neurosci. 42, 27–46. doi: 10.1146/annurev-neuro-080317-061813
Lu, J., Greco, M. A., Shiromani, P., and Saper, C. B. (2000). Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 20, 3830–3842. doi: 10.1523/jneurosci.20-10-03830.2000
Ma, Y., Miracca, G., Yu, X., Harding, E. C., Miao, A., Yustos, R., et al. (2019). Galanin neurons unite sleep homeostasis and α2-adrenergic sedation. Curr. Biol. 29, 3315–3322.e3.
Machado, N. L. S., Bandaru, S. S., Abbott, S. B. G., and Saper, C. B. (2020). EP3R-expressing glutamatergic preoptic neurons mediate inflammatory fever. J. Neurosci. 40, 2573–2588. doi: 10.1523/jneurosci.2887-19.2020
Maejima, S., Abe, Y., Yamaguchi, S., Musatov, S., Ogawa, S., Kondo, Y., et al. (2018). VGF in the medial preoptic nucleus increases sexual activity following sexual arousal induction in male rats. Endocrinology 159, 3993–4005. doi: 10.1210/en.2018-00804
Malsbury, C. W. (1971). Facilitation of male rat copulatory behavior by electrical stimulation of the medial preoptic area. Physiol. Behav. 7, 797–805. doi: 10.1016/0031-9384(71)90042-4
Mathieson, W. B., Wilkinson, M., Brown, R. E., Bond, T. L. Y., Taylor, S. W., and Neumann, P. E. (2002). FOS and FOSB expression in the medial preoptic nucleus pars compacta of maternally active C57BL/6J and DBA/2J mice. Brain Res. 952, 170–175. doi: 10.1016/s0006-8993(02)03078-0
McGinty, D. J., and Sterman, M. B. (1968). Sleep suppression after basal forebrain lesions in the cat. Science 160, 1253–1255. doi: 10.1126/science.160.3833.1253
McHenry, J. A., Bell, G. A., Parrish, B. P., and Hull, E. M. (2012). Dopamine D1 receptors and phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in the medial preoptic area are involved in experience-induced enhancement of male sexual behavior in rats. Behav. Neurosci. 126, 523–529. doi: 10.1037/a0028707
Merchenthaler, I., Lane, M. V., Numan, S., and Dellovade, T. L. (2004). Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol. 473, 270–291. doi: 10.1002/cne.20128
Moffitt, J. R., Bambah-Mukku, D., Eichhorn, S. W., Vaughn, E., Shekhar, K., Perez, J. D., et al. (2018). Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362:aau5324.
Moffitt, J. R., Hao, J., Wang, G., Chen, K. H., Babcock, H. P., and Zhuang, X. (2016). High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc. Natl. Acad. Sci. U. S. A. 113, 11046–11051. doi: 10.1073/pnas.1612826113
Morrison, S. F., and Nakamura, K. (2019). Central mechanisms for thermoregulation. Annu. Rev. Physiol. 81, 285–308. doi: 10.1146/annurev-physiol-020518-114546
Morrison, S. F., Madden, C. J., and Tupone, D. (2014). Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 19, 741–756. doi: 10.1016/j.cmet.2014.02.007
Murphy, A. Z., and Hoffman, G. E. (2001). Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: a potential circuit for the initiation of male sexual behavior. J. Comp. Neurol. 438, 191–212. doi: 10.1002/cne.1309
Nakamura, K. (2011). Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1207–R1228.
Nauta, W. J. H. (1946). Hypothalamic regulation of sleep in rats; an experimental study. J. Neurophysiol. 9, 285–316. doi: 10.1152/jn.1946.9.4.285
Numan, M., and Numan, M. J. (1994). Expression of Fos-like immunoreactivity in the preoptic area of maternally behaving virgin and postpartum rats. Behav. Neurosci. 108, 379–394. doi: 10.1037/0735-7044.108.2.379
Numan, M., and Smith, H. G. (1984). Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav. Neurosci. 98, 712–727. doi: 10.1037/0735-7044.98.4.712
Numan, M., McSparren, J., and Numan, M. J. (1990). Dorsolateral connections of the medial preoptic area and maternal behavior in rats. Behav. Neurosci. 104, 964–979. doi: 10.1037/0735-7044.104.6.964
Numan, M., Numan, M. J., Schwarz, J. M., Neuner, C. M., Flood, T. F., and Smith, C. D. (2005). Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav. Brain Res. 158, 53–68. doi: 10.1016/j.bbr.2004.08.008
Nutsch, V. L., Will, R. G., Robison, C. L., Martz, J. R., Tobiansky, D. J., and Dominguez, J. M. (2016). Colocalization of mating-induced fos and D2-Like dopamine receptors in the medial preoptic area: influence of sexual experience. Front. Behav. Neurosci. 10:75.
Ogawa, Y., and Kawamura, H. (1988). Increase of multiple unit activity during slow wave sleep in the cat preoptic area. Brain Res. Bull. 20, 897–902. doi: 10.1016/0361-9230(88)90108-6
Okabe, S., Nagasawa, M., Kihara, T., Kato, M., Harada, T., Koshida, N., et al. (2010). The effects of social experience and gonadal hormones on retrieving behavior of mice and their responses to pup ultrasonic vocalizations. Zool. Sci. 27, 790–795. doi: 10.2108/zsj.27.790
Okabe, S., Tsuneoka, Y., Takahashi, A., Ooyama, R., Watarai, A., Maeda, S., et al. (2017). Pup exposure facilitates retrieving behavior via the oxytocin neural system in female mice. Psychoneuroendocrinology 79, 20–30. doi: 10.1016/j.psyneuen.2017.01.036
Orikasa, C., and Sakuma, Y. (2010). Estrogen configures sexual dimorphism in the preoptic area of C57BL/6J and ddN strains of mice. J. Comp. Neurol. 518, 3618–3629. doi: 10.1002/cne.22419
Paredes, R. G., and Baum, M. J. (1995). Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. J. Neurosci. 15, 6619–6630. doi: 10.1523/jneurosci.15-10-06619.1995
Paredes, R. G., Piña, A. L., and Bermúdez-Rattoni, F. (1993). Hypothalamic but not cortical grafts induce recovery of sexual behavior and connectivity in medial preoptic area-lesioned rats. Brain Res. 620, 351–355. doi: 10.1016/0006-8993(93)90180-u
Paredes, R. G., Tzschentke, T., and Nakach, N. (1998). Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res. 813, 1–8. doi: 10.1016/s0006-8993(98)00914-7
Park, S.-G., Jeong, Y.-C., Kim, D.-G., Lee, M.-H., Shin, A., Park, G., et al. (2018). Medial preoptic circuit induces hunting-like actions to target objects and prey. Nat. Neurosci. 21, 364–372. doi: 10.1038/s41593-018-0072-x
Paxinos, G., Keith, B. J., and Franklin, M. A. (2012). Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. Amsterdam: Elsevier Science.
Pereira, M., and Morrell, J. I. (2011). Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. J. Neuroendocrinol. 23, 1020–1035. doi: 10.1111/j.1365-2826.2011.02200.x
Puelles, L., Martinez-de-la-Torre, M., Bardet, S., and Rubenstein, J. L. R. (2012). “Hypothalamus,” in The Mouse Nervous System, eds L. Puelles, G. Paxinos, and C. Waston (Amsterdam: Elsevier), 221–312.
Reichert, S., Pavón Arocas, O., and Rihel, J. (2019). The neuropeptide galanin is required for homeostatic rebound sleep following increased neuronal activity. Neuron 104, 370–384.e5.
Reitz, S. L., Wasilczuk, A. Z., Beh, G. H., Proekt, A., and Kelz, M. B. (2020). Activation of preoptic tachykinin 1 neurons promotes wakefulness over sleep and volatile anesthetic-induced unconsciousness. Curr. Biol. 31, 394–405.e4.
Ribeiro, A. C., Musatov, S., Shteyler, A., Simanduyev, S., Arrieta-Cruz, I., Ogawa, S., et al. (2012). siRNA silencing of estrogen receptor-α expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc. Natl. Acad. Sci. U. S. A. 109, 16324–16329. doi: 10.1073/pnas.1214094109
Saito, Y. C., Tsujino, N., Hasegawa, E., Akashi, K., Abe, M., Mieda, M., et al. (2013). GABAergic neurons in the preoptic area send direct inhibitory projections to orexin neurons. Front. Neural Circuits 7:192.
Sallanon, M., Denoyer, M., Kitahama, K., Aubert, C., Gay, N., and Jouvet, M. (1989). Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience 32, 669–683. doi: 10.1016/0306-4522(89)90289-3
Sano, K., Nakata, M., Musatov, S., Morishita, M., Sakamoto, T., Tsukahara, S., et al. (2016). Pubertal activation of estrogen receptor α in the medial amygdala is essential for the full expression of male social behavior in mice. Proc. Natl. Acad. Sci. U. S. A. 113, 7632–7637. doi: 10.1073/pnas.1524907113
Sano, K., Tsuda, M. C., Musatov, S., Sakamoto, T., and Ogawa, S. (2013). Differential effects of site-specific knockdown of estrogen receptor α in the medial amygdala, medial pre-optic area, and ventromedial nucleus of the hypothalamus on sexual and aggressive behavior of male mice. Eur. J. Neurosci. 37, 1308–1319. doi: 10.1111/ejn.12131
Saper, C. B., and Fuller, P. M. (2017). Wake-sleep circuitry: an overview. Curr. Opin. Neurobiol. 44, 186–192. doi: 10.1016/j.conb.2017.03.021
Saper, C. B., Scammell, T. E., and Lu, J. (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263. doi: 10.1038/nature04284
Semba, K., Pastorius, J., Wilkinson, M., and Rusak, B. (2001). Sleep deprivation-induced c-fos and junB expression in the rat brain: effects of duration and timing. Behav. Brain Res. 120, 75–86. doi: 10.1016/s0166-4328(00)00362-4
Shah, S., Lubeck, E., Zhou, W., and Cai, L. (2016). In situ transcription profiling of single cells reveals spatial organization of cells in the mouse hippocampus. Neuron 92, 342–357. doi: 10.1016/j.neuron.2016.10.001
Sheehan, T. P., Cirrito, J., Numan, M. J., and Numan, M. (2000). Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav. Neurosci. 114, 337–352. doi: 10.1037/0735-7044.114.2.337
Sherin, J. E., Elmquist, J. K., Torrealba, F., and Saper, C. B. (1998). Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 18, 4705–4721. doi: 10.1523/jneurosci.18-12-04705.1998
Shimura, T., Yamamoto, T., and Shimokochi, M. (1994). The medial preoptic area is involved in both sexual arousal and performance in male rats: re-evaluation of neuron activity in freely moving animals. Brain Res. 640, 215–222. doi: 10.1016/0006-8993(94)91875-9
Sickel, M. J., and McCarthy, M. M. (2000). Calbindin-D28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J. Neuroendocrinol. 12, 397–402. doi: 10.1046/j.1365-2826.2000.00474.x
Simerly, R. B. (2015). “Chapter 13 - organization of the hypothalamus,” in The Rat Nervous System, 4th Edn, ed. G. Paxinos (San Diego: Academic Press), 267–294. doi: 10.1016/b978-0-12-374245-2.00013-9
Simerly, R. B., Chang, C., Muramatsu, M., and Swanson, L. W. (1990). Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 294, 76–95. doi: 10.1002/cne.902940107
Simerly, R. B., Gorski, R. A., and Swanson, L. W. (1986). Neurotransmitter specificity of cells and fibers in the medial preoptic nucleus: an immunohistochemical study in the rat. J. Comp. Neurol. 246, 343–363. doi: 10.1002/cne.902460305
Simerly, R. B., McCall, L. D., and Watson, S. J. (1988). Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J. Comp. Neurol. 276, 442–459. doi: 10.1002/cne.902760309
Simerly, R. B., Swanson, L. W., and Gorski, R. A. (1984). Demonstration of a sexual dimorphism in the distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus of the rat. J. Comp. Neurol. 225, 151–166. doi: 10.1002/cne.902250202
Song, K., Wang, H., Kamm, G. B., Pohle, J., Reis, F. C., Heppenstall, P., et al. (2016). The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353, 1393–1398. doi: 10.1126/science.aaf7537
Stack, E. C., Balakrishnan, R., Numan, M. J., and Numan, M. (2002). A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav. Brain Res. 131, 17–36. doi: 10.1016/s0166-4328(01)00370-9
Stolzenberg, D. S., and Numan, M. (2011). Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci. Biobehav. Rev. 35, 826–847. doi: 10.1016/j.neubiorev.2010.10.003
Suntsova, N., Szymusiak, R., Alam, M. N., Guzman-Marin, R., and McGinty, D. (2002). Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J. Physiol. 543, 665–677. doi: 10.1113/jphysiol.2002.023085
Szymusiak, R., Alam, N., Steininger, T. L., and McGinty, D. (1998). Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 803, 178–188. doi: 10.1016/s0006-8993(98)00631-3
Szymusiak, R., and McGinty, D. (1986a). Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 370, 82–92. doi: 10.1016/0006-8993(86)91107-8
Szymusiak, R., and McGinty, D. (1986b). Sleep suppression following kainic acid-induced lesions of the basal forebrain. Exp. Neurol. 94, 598–614. doi: 10.1016/0014-4886(86)90240-2
Takahashi, K., Lin, J.-S., and Sakai, K. (2009). Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience 161, 269–292. doi: 10.1016/j.neuroscience.2009.02.075
Takahashi, T. M., Sunagawa, G. A., Soya, S., Abe, M., Sakurai, K., Ishikawa, K., et al. (2020). A discrete neuronal circuit induces a hibernation-like state in rodents. Nature 583, 109–114. doi: 10.1038/s41586-020-2163-6
Tan, C. L., and Knight, Z. A. (2018). Regulation of body temperature by the nervous system. Neuron 98, 31–48. doi: 10.1016/j.neuron.2018.02.022
Tan, C. L., Cooke, E. K., Leib, D. E., Lin, Y.-C., Daly, G. E., Zimmerman, C. A., et al. (2016). Warm-sensitive neurons that control body temperature. Cell 167, 47–59.e15.
Tsuneoka, Y., Maruyama, T., Yoshida, S., Nishimori, K., Kato, T., Numan, M., et al. (2013). Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. J. Comp. Neurol. 521, 1633–1663. doi: 10.1002/cne.23251
Tsuneoka, Y., Tokita, K., Yoshihara, C., Amano, T., Esposito, G., Huang, A. J., et al. (2015). Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. EMBO J. 34, 2652–2670. doi: 10.15252/embj.201591942
Tsuneoka, Y., Tsukahara, S., Yoshida, S., Takase, K., Oda, S., Kuroda, M., et al. (2017a). Moxd1 is a marker for sexual dimorphism in the medial preoptic area, bed nucleus of the stria terminalis and medial amygdala. Front. Neuroanat. 11:26.
Tsuneoka, Y., Yoshida, S., Takase, K., Oda, S., Kuroda, M., and Funato, H. (2017b). Neurotransmitters and neuropeptides in gonadal steroid receptor-expressing cells in medial preoptic area subregions of the male mouse. Sci. Rep. 7:9809.
Uschakov, A., Gong, H., McGinty, D., and Szymusiak, R. (2006). Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur. J. Neurosci. 23, 3284–3296. doi: 10.1111/j.1460-9568.2006.04860.x
Uschakov, A., Gong, H., McGinty, D., and Szymusiak, R. (2007). Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience 150, 104–120. doi: 10.1016/j.neuroscience.2007.05.055
Vanini, G., Bassana, M., Mast, M., Mondino, A., Cerda, I., Phyle, M., et al. (2020). Activation of preoptic GABAergic or glutamatergic neurons modulates sleep-wake architecture, but not anesthetic state transitions. Curr. Biol. 30, 779–787.e4.
Veening, J. G., and Coolen, L. M. (1998). Neural activation following sexual behavior in the male and female rat brain. Behav. Brain Res. 92, 181–193. doi: 10.1016/s0166-4328(97)00190-3
Veening, J. G., and Coolen, L. M. (2014). Neural mechanisms of sexual behavior in the male rat: emphasis on ejaculation-related circuits. Pharmacol. Biochem. Behav. 121, 170–183. doi: 10.1016/j.pbb.2013.12.017
Venner, A., De Luca, R., Sohn, L. T., Bandaru, S. S., Verstegen, A. M. J., Arrigoni, E., et al. (2019). An inhibitory lateral hypothalamic-preoptic circuit mediates rapid arousals from sleep. Curr. Biol. 29, 4155–4168.e5.
von Economo, C. (1930). Sleep as a problem of localization. J. Nerv. Ment. Dis. 71, 249–259. doi: 10.1097/00005053-193003000-00001
Wei, Y.-C., Wang, S.-R., Jiao, Z.-L., Zhang, W., Lin, J.-K., Li, X.-Y., et al. (2018). Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat. Commun. 9:279.
Wu, Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M., and Dulac, C. G. (2014). Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. doi: 10.1038/nature13307
Xin, X., Mains, R. E., and Eipper, B. A. (2004). Monooxygenase X, a member of the copper-dependent monooxygenase family localized to the endoplasmic reticulum. J. Biol. Chem. 279, 48159–48167. doi: 10.1074/jbc.m407486200
Yamaguchi, S., Abe, Y., Maejima, S., and Tsukahara, S. (2018). Sexual experience reduces neuronal activity in the central part of the medial preoptic nucleus in male rats during sexual behavior. Neurosci. Lett. 685, 155–159. doi: 10.1016/j.neulet.2018.08.037
Yu, S., Qualls-Creekmore, E., Rezai-Zadeh, K., Jiang, Y., Berthoud, H.-R., Morrison, C. D., et al. (2016). Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J. Neurosci. 36, 5034–5046. doi: 10.1523/jneurosci.0213-16.2016
Zhang, K. X., D’Souza, S., Upton, B. A., Kernodle, S., Vemaraju, S., Nayak, G., et al. (2020). Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nature 585, 420–425. doi: 10.1038/s41586-020-2683-0
Keywords: preoptic area, parental behavior, male sexual behavior, sexual dimorphism, galanin, Moxd1, NREM sleep, REM sleep
Citation: Tsuneoka Y and Funato H (2021) Cellular Composition of the Preoptic Area Regulating Sleep, Parental, and Sexual Behavior. Front. Neurosci. 15:649159. doi: 10.3389/fnins.2021.649159
Received: 04 January 2021; Accepted: 05 March 2021;
Published: 31 March 2021.
Edited by:
Masayuki Ikeda, University of Toyama, JapanReviewed by:
William Wisden, Imperial College London, United KingdomMatt Carter, Williams College, United States
Copyright © 2021 Tsuneoka and Funato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yousuke Tsuneoka, eW91c3VrZS50c3VuZW9rYUBtZWQudG9oby11LmFjLmpw; Hiromasa Funato, aGlyb21hc2EuZnVuYXRvQG1lZC50b2hvLXUuYWMuanA=
 Yousuke Tsuneoka
Yousuke Tsuneoka Hiromasa Funato
Hiromasa Funato