- 1Division of Child and Adolescent Neuropsychiatry, University Hospital of Siena, Siena, Italy
- 2Division of Child and Adolescent Neuropsychiatry, Basic Medical Sciences, Neuroscience and Sense Organs Department, University Hospital of Bari, Bari, Italy
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders characterized by social and communication abnormalities. Heterogeneity in the expression and severity of the core and associated symptoms poses difficulties in classification and the overall clinical approach. Synaptic abnormalities have been observed in preclinical ASD models. They are thought to play a major role in clinical functional abnormalities and might be modified by targeted interventions. An imbalance in excitatory to inhibitory neurotransmission (E/I imbalance), through altered glutamatergic and GABAergic neurotransmission, respectively, is thought to be implicated in the pathogenesis of ASD. Glutamatergic and GABAergic agents have been tested in clinical trials with encouraging results as to efficacy and tolerability. Further studies are needed to confirm the role of E/I modulators in the treatment of ASD and on the safety and efficacy of the current agents.
Introduction
Characterizing Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a group of complex neurodevelopmental disorders characterized by the presence of multiple and persistent deficits in social communication and social interaction associated with restricted interests and stereotyped and repetitive behaviors (American Psychiatric Association, 2013; Grzadzinski et al., 2013). The prevalence of ASD is on the rise, as documented in recent surveys (Baio et al., 2018). There are more males than females with ASD (a 4:1 ratio), and there are relevant clinical differences between the sexes (Tillmann et al., 2018).
The etiology of ASD is still poorly understood, but it appears highly genetically heritable. Indeed, there are identified genetic mutations—although not fully penetrant—in approximately 15% of the cases. Non-specific environmental risks factors, such as parental age, fetal exposure to alcohol or valproate, and premature birth/low birth weight, have also been identified (Murdoch and State, 2013; De Rubeis and Buxbaum, 2015; Sestan and State, 2018; Han et al., 2021a,b). The most frequent comorbidities and associated symptoms are learning/intellectual disabilities, sleep disturbances, epilepsy, attention deficit with hyperactivity, irritability (including self-injuries behavior, and temper tantrums), anxiety, and depression. Frequently used medications include methylphenidate and atomoxetine for attention disorders, melatonin for sleep disorders, and selective serotonin reuptake inhibitors (SSRIs) for anxious-depressive symptoms. Second-generation antipsychotics, namely risperidone, and aripiprazole have demonstrated clinical efficacy in behavioral disorders of ASD, and the United States Food and Drug Administration (FDA) has approved them for this use (Accordino et al., 2016). The presence and the severity of these symptoms are very heterogeneous among patients. Usual care includes psychosocial interventions as well as educational measures, patient and family support, and behavioral therapies such as applied behavioral analysis.
The considerable heterogeneity in the expression and severity of the core and associated symptoms have been an obstacle toward understanding ASD. Variability in the social domain ranges from a near absence of interest in interacting with others to more subtle difficulties managing complex social interactions that require an understanding of other people’s goals and intentions and social context cues (American Psychiatric Association, 2013; Grzadzinski et al., 2013). Similarly, repetitive behaviors range from simple motor stereotypies and/or a preference for sameness to much more complex and elaborate rituals accompanied by emotional dysregulation or tantrums when these rituals are interrupted. Some individuals with ASD lack basic speech abilities, while others can have language deficits that are mild and limited to language pragmatics. While a majority of individuals with ASD exhibit some level of intellectual impairment, intelligence quotients vary from the severe and profoundly impaired to above-average (Frye, 2018).
Models of Excitatory/Inhibitory Imbalance in Autism Spectrum Disorder
A theory of excitatory/inhibitory imbalance in ASD was proposed by Rubenstein and Merzenich (2003). This neurobiological model is based on the theory that autism and related disorders might reflect an increase in the ratio between excitation and inhibition leading to hyper-excitability of cortical circuits. The theory was attractive because it provided a potential explanation for the frequent observation of reduced GABAergic signaling in ASD (Cellot and Cherubini, 2014; Brondino et al., 2016). In addition, since inhibition was known or believed to contribute to sharpening the selectivity of excitatory responses in many brain areas, the loss of inhibition could lead to enhanced “noise” and imprecision in learning and cognition (Rubenstein and Merzenich, 2003).
Since this initial formulation, however, other studies have suggested a nearly opposite hypothesis; namely, that at least some ASDs might be characterized by a reduction in the ratio between excitation and inhibition (Vignoli et al., 2010; Spooren et al., 2012). An excitatory/inhibitory (E/I) neurotransmission imbalance in cortical neurons, using dysfunctional glutamatergic and GABAergic neurotransmission, has been reported in multiple studies, and altered glutamine levels and/or abnormal GABA/creatine levels in brain tissue or plasma are the relevant findings (Vignoli et al., 2010; Carlson, 2012; Spooren et al., 2012; Gao and Penzes, 2015; Dickinson et al., 2016; Lee et al., 2017; Al-Otaish et al., 2018; Goel and Portera-Cailliau, 2019; Port et al., 2019; Bruining et al., 2020; Culotta and Penzes, 2020). Moreover, a recent study showed that high glutamate/glutamine levels have also been associated with altered functional connectivity in key brain regions for ASD, such as the dorsal anterior cingulate cortex, and insular, limbic, and parietal regions (Siegel-Ramsay et al., 2021).
An E/I imbalance might be the consequence of an increased glutamatergic activity alongside with a decrease in the GABAergic signaling. Previous studies found that genes involved in the regulation of the neurogenesis or the synaptogenesis may be linked to the E/I imbalance (Vignoli et al., 2010). In neurodevelopmental disorders, an E/I imbalance could arise directly through alterations in genes coding for glutamatergic receptors or synaptic proteins (Lee et al., 2017; Culotta and Penzes, 2020). The synapse organizers, neurexins and their binding neuroligins, are implicated in the formation and maintenance of excitatory and inhibitory synapses. Heterozygous deletions eliminating exons of the neurexin-1α gene in patients with ASD have been detected, and the functional significance of this recurrent deletion is still unclear (Frye, 2018). However, the role of neurexins/neuroligins in the pathogenesis of neurodevelopmental disorders has been demonstrated (Dickinson et al., 2016; Al-Otaish et al., 2018; Wang et al., 2018; Goel and Portera-Cailliau, 2019; Port et al., 2019; Siegel-Ramsay et al., 2021). The final effect of changes in glutamatergic and GABAergic systems in ASD may be an overall increase in the ratio of excitation to inhibition (E/I) (Howell and Smith, 2019). The E-I ratio in neocortical structures is determined by pyramidal glutamatergic neurons and inhibitory GABAergic parvalbumin (PV)-positive interneurons that are modulated by minicolumns, i.e., groups of functionally autonomous neurons whose afferent and efferent connections influence the functioning of microcircuits), which are abnormal in ASD (Nelson and Valakh, 2015; Uzunova et al., 2016). There are several candidate mechanisms for glutamatergic hyperexcitability. Neuroligins (NL1-4) and neurexins (Nrxns 1–3) have been linked with ASD via point mutations and truncations, as well as chromosomal rearrangements that have been identified in the region of interest (Snijders et al., 2013; Port et al., 2014; Dickinson et al., 2016). SHANK1, SHANK2, and SHANK3 are scaffolding proteins that influence the postsynaptic density of glutamatergic synapses and are of primary importance in ASD. SHANK3 is reported to be involved in Phelan-McDermid syndrome (PMS) a form of ASD associated with moderate to severe intellectual disability (ID). Regarding SHANK2 and SHANK1, they were found altered in ASD associated with mild ID as well as in high functioning individuals (Zikopoulos and Barbas, 2013; Cochran et al., 2015; Howell and Smith, 2019; Bruining et al., 2020; Culotta and Penzes, 2020; Siegel-Ramsay et al., 2021). As to the mechanisms of GABAergic inhibitory dysfunction, the link with core ASD symptoms in humans is still under investigation. The deficit in binocular rivalry, a visual function that is thought to rely on the balance of excitation/inhibition in the visual cortex, has been observed in ASD individuals (Dunn and Jones, 2020). The link between GABA and binocular rivalry dynamics was found specifically absent in ASD pointing to an insufficient GABA inhibitory function (Dunn and Jones, 2020).
Based on these findings, research efforts have been directed to identify agents that have the potential to reverse this abnormal signaling, namely, excitation to inhibition (E/I) imbalance from preclinical models to clinical trials.
Modulators of E/I Imbalance in Autism Spectrum Disorder
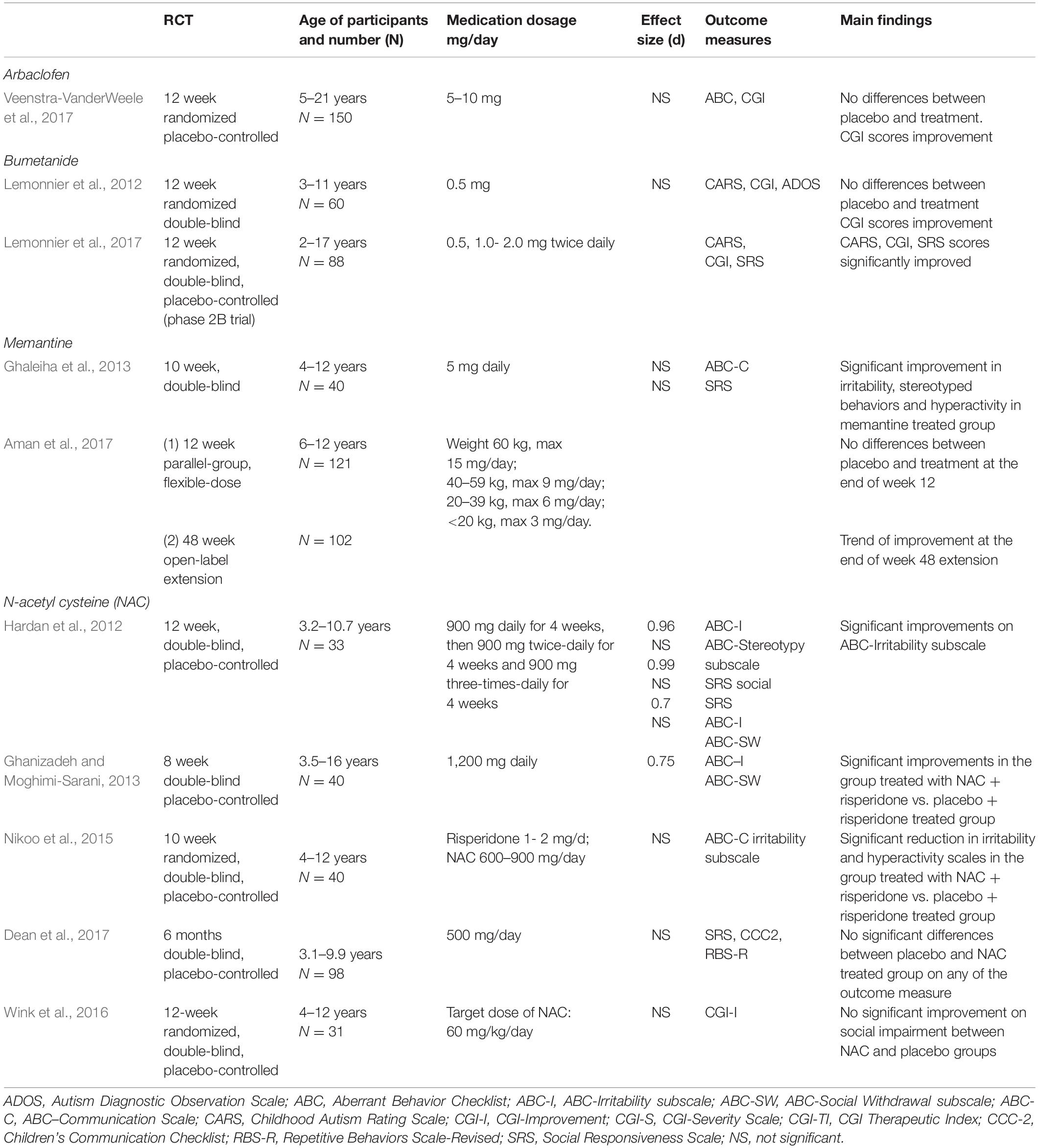
Modulators of E/I imbalance are a group of agents that are used to restore the balance of excitation and inhibition in brain cortical regions that are dysfunctional in ASD patients. These agents include compounds that target metabotropic glutamate receptors (mGluR, e.g., mGluR5 antagonists), NMDA receptors (e.g., the NMDA receptor antagonist memantine), or AMPA receptors (with receptor potentiating drugs such as ampakines). The search of articles was performed on PubMed online database, published until May 2021 on peer-reviewed journals. Studies identified had to meet predetermined inclusion criteria: (1) randomized clinical trial; (2) written only in English; (3) patients diagnosed with ASD; (4) E/I imbalance modulators in ASD; (5) full-text availability. Only few randomized clinical trial placebo-controlled studies have been performed to date and will be dealt with in the current article (Table 1).
Glutamatergic and GABAergic Modulators in Autism Spectrum Disorder
Dysfunctional glutamatergic transmission occurs through different pathways of altered excitatory transmission: downregulation of AMPA receptors, abnormalities in NMDA-receptor-mediated plasticity, and altered mGluR signal transduction. Research has implicated glutamate hyperactivation and GABAergic system hypofunction in the pathogenesis of ASD. In neurons and glia, glutamate is involved in intracellular transduction mechanisms at NMDA and mGlu receptors (Uzunova et al., 2014). AMPA receptors are thought to be implicated in behavioral disorders associated with the core symptoms in children and adolescents with ASD (Lipton, 2006; Uzunova et al., 2014).
NMDA Receptor Antagonist: Memantine Treatment Studies
NMDA receptor antagonists, mainly memantine, have been employed in clinical trials for children and adolescents with ASD. Memantine is an uncompetitive, low-affinity glutamate NMDA receptor antagonist used for this purpose (Lipton, 2006; Wei et al., 2012). Owley et al. (2006) enrolled 14 subjects, aged 3–12 years, in an 8-week open-label study of memantine for ASD. The patients received up to 20 mg/day memantine. There were significant improvements in memory, irritability, lethargy, stereotypies, hyperactivity, and inappropriate speech (Owley et al., 2006). A retrospective study by Erickson et al. (2007) evaluated the use of memantine in 18 ASD patients (6–19 years old). The maximum dose of memantine was 20 mg/day. The outcome measures included the Clinical Global Impression (CGI)–Severity and CGI–Improvement subscales and Aberrant Behavior Checklist (ABC) data. They reported a significant reduction in hyperactivity. Chez et al. (2007) conducted an open-label study of 151 patients with autism or “Pervasive Developmental Disorder–Not Otherwise Specified” with an age range of 2.5–26.3 years. The patients received a maximum dosage of 30 mg/day of memantine. There was a clinical improvement in 70% of patients, including language, social behavior, and stereotypes as reported by the CGI scale. There was worsened irritability, hyperactivity, and manic-type behaviors in 18/150 [11.84%] of patients. A double-blind multisite study was carried out to assess the safety, tolerability, and efficacy of memantine [once-daily extended-release (ER)] in children with autism in a randomized, placebo-controlled, 12-week trial and a 48-week open-label extension (Aman et al., 2017). A group of 121 children (6–12 years) with autistic disorder (based on the DSM-IV-TR) were randomized to placebo or memantine ER for 12 weeks; 104 children entered the subsequent extension trial. The maximum memantine doses were determined by body weight; they ranged from 3 to 15 mg/day. There was no significant between-group difference on the primary efficacy outcome of caregiver/parent ratings on the Social Responsiveness Scale (SRS). This trial did not demonstrate the clinical efficacy of memantine ER in autism; however, the tolerability and safety were good.
Another double-blind, placebo-controlled study explored memantine as add-on therapy in children with ASD receiving risperidone (Ghaleiha et al., 2013). The addition of memantine significantly reduced ABC subscale scores for irritability, hyperactivity, and stereotypic behavior compared to placebo. These findings suggest that memantine can be an effective adjunctive treatment for behavioral problems in children with ASD.
GABAergic Modulator: STX209 Treatment Studies
Multiple studies have suggested that GABAergic neurons and circuits may be altered in ASD (Chao et al., 2010; Port et al., 2017). GABA neurotransmission regulates several important developmental processes, including cell proliferation, differentiation, synapse maturation, and cell death. Dysfunctional GABAergic signaling early in development that leads to a severe E/I imbalance in neuronal circuits has been observed in ASD in studies conducted with different methods (Oblak et al., 2010; Ghanizadeh and Moghimi-Sarani, 2013; Gaetz et al., 2014). GABAergic modulators have been tested in ASD. An open-label trial evaluated the safety, tolerability, and efficacy of STX209, a form of arbaclofen, in ASD individuals (Erickson et al., 2014). There were improvements in several outcome measures, and Following these positive preliminary findings, a 12-week randomized controlled trial of STX209 was conducted (Veenstra-VanderWeele et al., 2017). STX209 was more efficacious compared to placebo, even if the results did not reach statistical significance.
GABAergic Modulator: Bumetanide Treatment Studies
The persistence or development of excitatory GABA neuronal signaling has been found in ASD individuals, and the E/I imbalance is hypothesized to be related to defect in GABAergic signaling (Bruining et al., 2015; Ben-Ari, 2017). Bumetanide is a loop diuretic and it could reduce intracellular chloride and thus reverse the aberrant excitatory action of GABA into inhibitory action (Lemonnier and Ben-Ari, 2010). The first use of bumetanide in ASD was a pilot study conducted in five children with ASD (3–11) years old. Bumetanide in ASD was tested in preliminary studies (Lemonnier and Ben-Ari, 2010; Lemonnier et al., 2012; Bruining et al., 2015; Hadjikhani et al., 2015), followed by a proof of concept study (Hadjikhani et al., 2015).
Lemonnier et al. (2012) conducted a randomized, double-blind, placebo-controlled trial evaluating the efficacy of bumetanide treatment in 60 French children with ASD. The group treated with the bumetanide showed a statistically significant improvement in the mean total of the Childhood Autism Rating Scale (CARS) (Schopler et al., 2009) score (5.6 ± 4 compared with placebo 1.8 ± 5.1; P = 0.0044) after 3 months. Moreover, the treatment ameliorated also the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) scores, even if the results were not statistically significant. Du et al. (2015) conducted a randomized trial in 60 Chinese children with ASD (2.5–6.5 years old), with a mean of 4.5 years, treatment with bumetanide combined with ABA training provided a better outcome than ABA training alone.
Lemonnier et al. (2017) carried out another double-blind, randomized controlled, multicenter dose-ranging study. The authors identified responders of all ages and ASD severity groups, according to CARS scores. However, it was unclear whether patients with more severe ASD symptoms have a greater response to bumetanide. Bumetanide is therefore promising in treating ASD, including core symptoms, and await for further studies.
Brain Glutamate Modulator: N-Acetylcysteine Treatment Studies
Glutathione and N-Acetylcysteine (NAC) are antioxidants that minimize oxidative stress and the downstream negative effects thought to be associated with oxidative stress. Based on magnetic resonance imaging studies, NAC modulates brain glutamate and can thus potentially modulate the E/I balance (Deepmala et al., 2015; Minarini et al., 2017).
In Hardan’s et al. 12-week double-blind, placebo-controlled randomized study of children with ASD (n = 33), 14 subjects in the NAC group and 15 in the placebo group completed the trial. Oral NAC was well tolerated with limited side effects (Hardan et al., 2012). Wink et al. (2016) reported no statistically significant difference between the NAC and placebo groups on the CGI-Improvement (P > 0.69), but glutathione was significantly higher in the NAC group (P < 0.05). NAC treatment was well tolerated but had no significant impact on social impairment in youth with ASD (Wink et al., 2016). Dean et al. (2017) examined a total of 102 children; 98 (79 males and 19 females; age range: 3.1–9.9 years) formed the study group. There were no differences between the NAC and placebo groups on any of the outcome measures for either primary or secondary endpoints. In a double-blind, placebo-controlled add-on study to risperidone (n = 40), 600 mg of NAC twice a day significantly decreased ABC-Irritability but did not change ABC-Social Withdrawal (Nikoo et al., 2015).
The limitations of the reviewed studies include the potential lack of sensitivity of the scales used to assess real improvement and behavioral changes, the absence of biological markers that would allow correlation of any clinical improvement with biological changes, and the heterogeneity of ASD symptoms. Additional randomized double-blind, placebo-controlled trials with the presented E/I modulators are required to draw firm conclusions.
Conclusion
The currently available agents that modulate E/I imbalance herein discussed act upon selective symptoms of ASD, namely irritability, and some of them (e.g., bumetanide) hold promise to modify the abnormalities in social and communication domains as well. Differences in response to the tested agents have been identified across development in subjects with ASD, and the same has been observed about tolerability. Thus, a careful distinction in age groups should be detailed when setting up study designs and evaluating treatment response. The response to placebo and the level of baseline symptomatology should be considered in the design and interpretation of future trials in ASD. It is very important to stratify the patients according to the symptom level—e.g., social avoidance and lack of interest in reciprocity may vary—so it is urgently necessary to specify it and consider the distinctly associated biomarkers.
Target symptom domains of ASD have been identified and basic mechanisms that cover distinct subsets of ASD would parallel the concept of core symptoms in ASD, based on the hypothesis that a group of protein factors converges on common pathways to be targeted.
Despite the translational issues in modeling ASD in animals—extreme phenotypic variability, lack of a biomarker, and appropriate endpoints for evaluation of changes in social behavior—animal models have made major contributions that have allowed the translation of basic research to clinical testing. The next generation of animal models carrying human mutations will be of the utmost importance in uncovering the neural and molecular bases of E/I imbalance and paving the way to clinical trials.
Future Directions
Novel modulators of E-I imbalance are under study, and currently there is a modified form of memantine—nitrosynapsin—that holds promise for this purpose. Nytrosynapsin has been tested in preclinical models of ASD and demonstrated a neuroprotective action that attenuates E/I imbalance and has the potential of modifying the core defects of ASD (Ghatak et al., 2021). In addition, oxytocin might be a promising E/I modulator agent for social impairment as the oxytocinergic system is related to GABA-mediated E/I control within the dynamic interplay of social interaction. The involvement of OXT includes both endogenous and exogenous release in a still poorly known mechanism (Lopatina et al., 2018).
However, the central role of OXT in the social brain has been further demonstrated, and future studies are anticipated to evaluate the novel modifiers of E/I imbalance in ASD.
Transcranial magnetic stimulation (TMS) can be applied as a therapeutic modality in ASD to restore the imbalance of excitation and inhibition, and modulate synaptic plasticity and gamma oscillations (Oberman et al., 2016; Uzunova et al., 2016; Khaleghi et al., 2020; Casanova et al., 2021). A recent meta-analysis showed a moderate though significant effect of TMS on repetitive behaviors and core social dysfunction in ASD (Barahona-Corrêa et al., 2018). It is worth noting that TMS may be preferred in patients who cannot take medications or are not responsive to therapies that may be combined with medications, but further studies are needed.
Limitations
The lack of preclinical data from animal models in this study is a major drawback and would have been informative, but this was out of scope since we focused on current clinical trials in ASD. Another limitation that needs to be mentioned is that the selection of the studies was based on subjective criteria based on availability of clinical trials in ASD according to our criteria that are overall in low number and need further studies.
Author Contributions
RC designed the study and wrote the manuscript. RP contributed to revise the manuscript and customized the table. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Accordino, R. E., Kidd, C., Politte, L. C., Henry, C. A., and McDougle, C. J. (2016). Psychopharmacological interventions in autism spectrum disorder. Expert Opin. Pharmacother. 17, 937–952. doi: 10.1517/14656566.2016.1154536
Al-Otaish, H., Al-Ayadhi, L., Bjørklund, G., Chirumbolo, S., Urbina, M. A., and El-Ansary, A. (2018). Relationship between absolute and relative ratios of glutamate, glutamine and GABA and severity of autism spectrum disorder. Metab. Brain Dis. 33, 843–854. doi: 10.1007/s11011-018-0186-6
Aman, M. G., Findling, R. L., Hardan, A. Y., Hendren, R. L., Melmed, R. D., Kehinde-Nelson, O., et al. (2017). Safety and efficacy of memantine in children with autism: randomized, placebo-controlled study and open-label extension. J. Child Adolesc. Psychopharmacol. 5, 403–412. doi: 10.1089/cap.2015.0146
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, TX: American Psychiatric Association.
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 67, 1–23. doi: 10.15585/mmwr.ss6706a1
Barahona-Corrêa, J. B., Velosa, A., Chainho, A., Lopes, R., and Oliveira-Maia, A. J. (2018). Repetitive transcranial magnetic stimulation for treatment of autism spectrum disorder: a systematic review and meta-analysis. Front. Integr. Neurosci. 12:27. doi: 10.3389/fnint.2018.00027
Ben-Ari, Y. (2017). NKCCl chloride importer antagonists attenuate many neurological and psychiatric disorders. Trends Neurosci. 40, 536–554. doi: 10.1016/j.tins.2017.07.001
Brondino, N., Fusar-Poli, L., Panisi, C., Damiani, S., Barale, F., and Politi, P. (2016). Pharmacological modulation of GABA function in autism spectrum disorders: a systematic review of human studies. J. Autism Dev. Disord. 46, 825–839. doi: 10.1007/s10803-015-2619-y
Bruining, H., Hardstone, R., Juarez-Martinez, E. L., Sprengers, J., Avramiea, A. E., Simpraga, S., et al. (2020). Measurement of excitation-inhibition ratio in autism spectrum disorder using critical brain dynamics. Sci. Rep. 10:9195. doi: 10.1038/s41598-020-65500-4
Bruining, H., Passtoors, L., Goriounova, N., Jansen, F., Hakvoort, B., De Jonge, M., et al. (2015). Paradoxical benzodiazepine response: a rationale for bumetanide in neurodevelopmental disorders? Pediatrics 136, e539–e543. doi: 10.1542/peds.2014-4133
Carlson, G. C. (2012). Glutamate receptor dysfunction and drug targets across models of autism spectrum disorders. Pharmacol. Biochem. Behav. 4, 850–854. doi: 10.1016/j.pbb.2011.02.003
Casanova, M. F., Shaban, M., Ghazal, M., El-Baz, A. S., Casanova, E. L., and Sokhadze, E. M. (2021). Ringing decay of gamma oscillations and transcranial magnetic stimulation therapy in autism spectrum disorder. Appl. Psychophysiol. Biofeedback 46, 161–173. doi: 10.1007/s10484-021-09509-z
Cellot, G., and Cherubini, E. (2014). GABAergic signaling as therapeutic target for autism spectrum disorders. Front. Pediatr. 2:70. doi: 10.3389/fped.2014.00070
Chao, H. T., Chen, H., Samaco, R. C., Xue, M., Chahrour, M., Yoo, J., et al. (2010). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, 263–269. doi: 10.1038/nature09582
Chez, M. G., Burton, Q., Dowling, T., Chang, M., Khanna, P., and Kramer, C. (2007). Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. J. Child Neurol. 22, 574–579. doi: 10.1177/0883073807302611
Cochran, D. M., Sikoglu, E. M., Hodge, S. M., Edden, R. A., Foley, A., Kennedy, D. N., et al. (2015). Relationship among glutamine, γ-aminobutyric acid, and social cognition in autism spectrum disorders. J. Child Adolesc. Psychopharmacol. 25, 314–322. doi: 10.1089/cap.2014.0112
Culotta, L., and Penzes, P. (2020). Exploring the mechanisms underlying excitation/inhibition imbalance in human iPSC-derived models of ASD. Mol. Autism 11:32. doi: 10.1186/s13229-020-00339-0
De Rubeis, S., and Buxbaum, J. D. (2015). Recent advances in the genetics of autism spectrum disorder. Curr. Neurol. Neurosci. Rep. 6:36.
Dean, O. M., Gray, K. M., Villagonzalo, K. A., Dodd, S., Mohebbi, M., Vick, T., et al. (2017). A randomised, double blind, placebo-controlled trial of a fixed dose of N-acetyl cysteine in children with autistic disorder. Aust. N Z J. Psychiatry. 51, 241–249. doi: 10.1177/0004867416652735
Deepmala, D., Slattery, J., Kumar, N., Delhey, L., Berk, M., Dean, O., et al. (2015). Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci. Biobehav. Rev. 55, 294–321. doi: 10.1016/j.neubiorev.2015.04.015
Dickinson, A., Jones, M., and Milne, E. (2016). Measuring neural excitation and inhibition in autism: different approaches, different findings and different interpretations. Brain Res. 1648(Pt A), 277–289. doi: 10.1016/j.brainres.2016.07.011
Du, L., Shan, L., Wang, B., Li, H., Xu, Z., Staal, W. G., et al. (2015). A pilot study on the combination of applied behavior analysis and bumetanide treatment for children with autism. J. Child Adolesc. Psychopharmacol. 25, 585–588. doi: 10.1089/cap.2015.0045
Dunn, S., and Jones, M. (2020). Binocular rivalry dynamics associated with high levels of self-reported autistic traits suggest an imbalance of cortical excitation and inhibition. Behav. Brain Res. 388:112603. doi: 10.1016/j.bbr.2020.112603
Erickson, C. A., Posey, D. J., Stigler, K. A., Mullett, J., Katschke, A. R., and McDougle, C. J. (2007). A retrospective study of memantine in children and adolescents with pervasive developmental disorders. Psychopharmacology (Berl) 191, 141–147. doi: 10.1007/s00213-006-0518-9
Erickson, C. A., Veenstra-Vanderweele, J. M., Melmed, R. D., McCracken, J. T., Ginsberg, L. D., Sikich, L., et al. (2014). STX209 (arbaclofen) for autism spectrum disorders: an 8-week open-label study. J. Autism Dev. Disord. 44, 958–964. doi: 10.1007/s10803-013-1963-z
Frye, R. (2018). Social skills deficits in autism spectrum disorder: potential biological origins and progress in developing therapeutic agents. CNS Drugs 32, 713–734. doi: 10.1007/s40263-018-0556-y
Gaetz, W., Bloy, L., Wang, D. J., Port, R. G., Blaskey, L., Kuschner, E. S., et al. (2014). GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage 86, 1–9. doi: 10.1016/j.neuroimage.2013.05.068
Gao, R., and Penzes, P. (2015). Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 15, 146–167. doi: 10.2174/1566524015666150303003028
Ghaleiha, A., Asadabadi, M., Mohammadi, M. R., Shahei, M., Tabrizi, M., Hajiaghaee, R., et al. (2013). Memantine as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Int. J. Neuropsychopharmacol. 16, 783–789. doi: 10.1017/S1461145712000880
Ghanizadeh, A., and Moghimi-Sarani, E. (2013). A randomized double-blind placebo controlled clinical trial of N-Acetylcysteine added to risperidone for treating autistic disorders. BMC Psychiatry 13:196. doi: 10.1186/1471-244X-13-196
Ghatak, S., Talantova, M., McKercher, S. R., and Lipton, S. A. (2021). Novel therapeutic approach for excitatory/inhibitory imbalance in neurodevelopmental and neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 61, 701–721. doi: 10.1146/annurev-pharmtox-032320-015420
Goel, A., and Portera-Cailliau, C. (2019). Autism in the balance: elevated E-I ratio as a homeostatic stabilization of synaptic drive. Neuron 101, 543–545. doi: 10.1016/j.neuron.2019.01.033
Grzadzinski, R., Huerta, M., and Lord, C. (2013). DSM-5 and autism spectrum disorders (ASDs): an opportunity for identifying ASD subtypes. Mol. Autism. 4:12. doi: 10.1186/2040-2392-4-12
Hadjikhani, N., Zürcher, N., Rogier, O., Rouest, T., Hyppolyte, L., Ben-Ari, Y., et al. (2015). Improving emotional face perception in autism with diuretic bumetanide: a proof-of concept behavioral and functional brain imaging pilot study. Autism 19, 149–157. doi: 10.1177/1362361313514141
Han, V. X., Patel, S., Jones, H. F., and Dale, R. C. (2021a). Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579. doi: 10.1038/s41582-021-00530-8
Han, V. X., Patel, S., Jones, H. F., Nielsen, T. C., Mohammad, S. S., Hofer, M. J., et al. (2021b). Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl. Psychiatry 11:71. doi: 10.1038/s41398-021-01198-w
Hardan, A. Y., Fung, L. K., Libove, R. A., Obukhanych, T. V., Nair, S., Herzenberg, L. A., et al. (2012). A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol. Psychiatry. 71, 956–961. doi: 10.1016/j.biopsych.2012.01.014
Howell, B. W., and Smith, K. M. (2019). Synaptic structural protein dysfunction leads to altered excitation inhibition ratios in models of autism spectrum disorder. Pharmacol. Res. 139, 207–214. doi: 10.1016/j.phrs.2018.11.019
Khaleghi, A., Zarafshan, H., Vand, S. R., and Mohammadi, M. R. (2020). Effects of non-invasive neurostimulation on autism spectrum disorder: a systematic review. Clin. Psychopharmacol. Neurosci. 18, 527–552. doi: 10.9758/cpn.2020.18.4.527
Lee, E., Lee, J., and Kim, E. (2017). Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol. Psychiatry 81, 838–847. doi: 10.1016/j.biopsych.2016.05.011
Lemonnier, E., and Ben-Ari, Y. (2010). The diuretic bumetanide decreases autistic behaviour in five infants treated during 3 months with no side effects. Acta Paediatr. 99, 1885–1888. doi: 10.1111/j.1651-2227.2010.01933.x
Lemonnier, E., Degrez, C., Phelep, M., Tyzio, R., Josse, F., Grandgeorge, M., et al. (2012). A randomized controlled trial of bumetanide in the treatment of autism in children. Transl. Psychiatry 2:e202.
Lemonnier, E., Villeneuve, N., Sonie, S., Serret, S., Rosier, A., Roue, M., et al. (2017). Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl. Psychiatry. 7:e1056. doi: 10.1038/tp.2017.10
Lipton, S. A. (2006). Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 5, 160–170. doi: 10.1038/nrd1958
Lopatina, O. L., Komleva, Y. K., Gorina, Y. V., Olovyannikova, R. Y., Trufanova, L. V., Hashimoto, T., et al. (2018). Oxytocin and excitation/inhibition balance in social recognition. Neuropeptides 72, 1–11. doi: 10.1016/j.npep.2018.09.003
Lord, C., Risi, S., Lambrecht, L., Cook, E. H. Jr., Leventhal, B. L., DiLavore, P. C., et al. (2000). The autism diagnostic observation schedule–generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 30, 205–223.
Minarini, A., Ferrari, S., Galletti, M., Giambalvo, N., Perrone, D., Rioli, G., et al. (2017). N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin. Drug Metab. Toxicol. 13, 279–292. doi: 10.1080/17425255.2017.1251580
Murdoch, J. D., and State, M. W. (2013). Recent developments in the genetics of autism spectrum disorders. Curr. Opin. Genet. Dev. 23, 310–315. doi: 10.1016/j.gde.2013.02.003
Nelson, S. B., and Valakh, V. (2015). Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87, 684–698. doi: 10.1016/j.neuron.2015.07.033
Nikoo, M., Radnia, H., Farokhnia, M., Mohammadi, M. R., and Akhondzadeh, S. (2015). N-acetylcysteine as an adjunctive therapy to risperidone for treatment of irritability in autism: a randomized, double blind, placebo-controlled clinical trial of efficacy and safety. Clin. Neuropharmacol. 38, 11–17. doi: 10.1097/WNF.0000000000000063
Oberman, L. M., Enticott, P. G., Casanova, M. F., Rotenberg, A., Pascual-Leone, A., and McCracken, J. T. (2016). TMS in ASD consensus group. transcranial magnetic stimulation in autism spectrum disorder: challenges, promise, and roadmap for future research. Autism Res. 9, 184–203. doi: 10.1002/aur.1567
Oblak, A., Gibbs, T. T., and Blatt, G. J. (2010). Decreased GABA(B) receptor in the cingulate cortex and fusiform gyrus in autism. J. Neurochem. 114, 1414–1423. doi: 10.1111/j.1471-4159.2010.06858.x
Owley, T., Salt, J., Guter, S., and Grieve, A. (2006). A prospective, open-label trial of memantine in the treatment of cognitive, behavioral, and memory dysfunction in pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 16, 517–524. doi: 10.1089/cap.2006.16.517
Port, R. G., Gaetz, W., Bloy, L., Wang, D. J., Blaskey, L., Kuschner, E. S., et al. (2017). Exploring the relationship between cortical GABA concentrations, auditory gamma-band responses and development in ASD: Evidence for an altered maturational trajectory in ASD. Autism Res. 10, 593–597. doi: 10.1002/aur.1686
Port, R. G., Gandal, M. J., Roberts, T. P., Siegel, S. J., and Carlson, G. C. (2014). Convergence of circuit dysfunction in ASD: a common bridge between diverse genetic and environmental risk factors and common clinical electrophysiology. Front. Cell Neurosci. 8:414. doi: 10.3389/fncel.2014.00414
Port, R. G., Oberman, L. M., and Roberts, T. P. (2019). Revisiting the excitation/inhibition imbalance hypothesis of ASD through a clinical lens. Br. J. Radiol. 92:20180944. doi: 10.1259/bjr.20180944
Rubenstein, J. L., and Merzenich, M. M. (2003). Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267. doi: 10.1034/j.1601-183x.2003.00037.x
Schopler, E., Bourgondie, M E. Van, Wellman, G. J., and Love, S. R. (2009). Childhood Autism Rating Scale™, 2nd Edn. London: Pearson.
Sestan, N., and State, M. W. (2018). Lost in translation: traversing the complex path from genomics to therapeutics in autism spectrum disorder. Neuron 100, 406–423. doi: 10.1016/j.neuron.2018.10.015
Siegel-Ramsay, J. E., Romaniuk, L., Whalley, H. C., Roberts, N., Branigan, H., Stanfield, A. C., et al. (2021). Glutamate and functional connectivity - support for the excitatory-inhibitory imbalance hypothesis in autism spectrum disorders. Psychiatry Res. Neuroimaging 313:111302. doi: 10.1016/j.pscychresns.2021.111302
Snijders, T. M., Milivojevic, B., and Kemner, C. (2013). Atypical excitation-inhibition balance in autism captured by the gamma response to contextual modulation. Neuroimage Clin. 3, 65–72. doi: 10.1016/j.nicl.2013.06.015
Spooren, W., Lindemann, L., Ghosh, A., and Santarelli, L. (2012). Synapse dysfunction in autism: a molecular medicine approach to drug discovery in neurodevelopmental disorders. Trends Pharmacol. Sci. 33, 669–684. doi: 10.1016/j.tips.2012.09.004
Tillmann, J., Ashwood, K., Absoud, M., Bölte, S., Bonnet-Brilhault, F., Buitelaar, J. K., et al. (2018). Evaluating sex and age differences in ADI-R and ADOS scores in a large European multisite sample of individuals with autism spectrum disorder. J. Autism Dev. Disord. 48, 2490–2505. doi: 10.1007/s10803-018-3510-4
Uzunova, G., Hollander, E., and Shepherd, J. (2014). The role of ionotropic glutamate receptors in childhood neurodevelopmental disorders: autism spectrum disorders and fragile X syndrome. Curr. Neuropharmacol. 12, 71–98. doi: 10.2174/1570159X113116660046
Uzunova, G., Pallanti, S., and Hollander, E. (2016). Excitatory/inhibitory imbalance in autism spectrum disorders: implications for interventions and therapeutics. World J. Biol. Psychiatry. 17, 174–186. doi: 10.3109/15622975.2015.1085597
Veenstra-VanderWeele, J., Cook, E. H., King, B. H., Zarevics, P., Cherubini, M., Walton-Bowen, K., et al. (2017). Arbaclofen in children and adolescents with autism spectrum disorder: a randomized, controlled, phase 2 trial. Neuropsychopharmacology 42, 1390–1398. doi: 10.1038/npp.2016.237
Vignoli, A., Fabio, R. A., La Briola, F., Giannatiempo, S., Antonietti, A., Maggiolini, S., et al. (2010). Correlations between neurophysiological, behavioral, and cognitive function in Rett syndrome. Epilepsy Behav. 17, 489–496. doi: 10.1016/j.yebeh.2010.01.024
Wang, J., Gong, J., Li, L., Chen, Y., Liu, L., Gu, H., et al. (2018). Neurexin gene family variants as risk factors for autism spectrum disorder. Autism Res. 11, 37–43. doi: 10.1002/aur.1881
Wei, H., Dobkin, C., Sheikh, A. M., Malik, M., Brown, W. T., and Li, X. (2012). The therapeutic effect of memantine through the stimulation of synapse formation and dendritic spine maturation in autism and fragile X syndrome. PLoS One 7:e36981. doi: 10.1371/journal.pone.0036981
Wink, L. K., Adams, R., Wang, Z., Klaunig, J. E., Plawecki, M. H., Posey, D. J., et al. (2016). A randomized placebo-controlled pilot study of N-acetylcysteine in youth with autism spectrum disorder. Mol. Autism 7:26. doi: 10.1186/s13229-016-0088-6
Keywords: children and adolescents, autism spectrum disorders, excitation/inhibition imbalance, experimental treatments, excitation/inhibition modulators
Citation: Canitano R and Palumbi R (2021) Excitation/Inhibition Modulators in Autism Spectrum Disorder: Current Clinical Research. Front. Neurosci. 15:753274. doi: 10.3389/fnins.2021.753274
Received: 04 August 2021; Accepted: 08 November 2021;
Published: 30 November 2021.
Edited by:
Giovanni Provenzano, University of Trento, ItalyReviewed by:
Laura Baroncelli, National Research Council (CNR), ItalyLynn Waterhouse, The College of New Jersey, United States
Copyright © 2021 Canitano and Palumbi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Canitano, ci5jYW5pdGFub0Bhby1zaWVuYS50b3NjYW5hLml0
 Roberto Canitano
Roberto Canitano Roberto Palumbi
Roberto Palumbi