- 1Knowledge Management & Discovery Lab, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
- 2Tinnitus Center, Charité-Universitaetsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 3Department of Psychiatry and Psychotherapy, University of Regensburg, Regensburg, Germany
Background: Chronic tinnitus is a clinically multidimensional phenomenon that entails audiological, psychological and somatosensory components. Previous research has demonstrated age and female gender as potential risk factors, although studies to this regard are heterogeneous. Moreover, whilst recent research has begun to identify clinical “phenotypes,” little is known about differences in patient population profiles at geographically separated and specialized treatment centers. Identifying such differences might prevent potential biases in joint randomized controlled trials (RCTs) and allow for population-specific treatment adaptations.
Method: Two German tinnitus treatment centers were compared regarding pre-treatment data distributions of their patient population bases. To identify overlapping as well as center-specific factors, juxtaposition-, similarity-, and meta-data-based methods were applied.
Results: Between centers, significant differences emerged. One center demonstrated some predictive power of the patients of the other center with regard to questionnaire score after treatment, indicating similarities in treatment response across center populations. Furthermore, adherence to the completion of the questionnaires was found to be an important factor in predicting post-treatment data.
Discussion: Differential age and gender distributions per center should be considered as regards RCT design and individualized treatment planning.
1. Introduction
Tinnitus can be described as a phantom auditory perception (Jastreboff, 1990). When investigating tinnitus prevalence, age and gender are commonly analysed variables. While age is considered to be an important factor in predicting tinnitus severity, the effect of gender does not reach consensus yet among the tinnitus research community (Biswas and Hall, 2020). There is also no conclusive evidence linking tinnitus or patient characteristics to treatment outcome (Schlee et al., 2021).
The goal of this study is to expand on previous research by investigating not only tinnitus patient characteristics within a clinical center, but also whether patients from one center are predictive of patient post-treatment data from another center. Therefore, we investigate how transferable the knowledge gathered in one center is to the other. In summary, the following research questions are tackled:
RQ1: To what extent do age and gender distributions within clinical centers reflect the age and gender distributions of the general population?
RQ2: How are the similarities and differences in both centers with respect to age, gender, and questionnaire scores?
RQ3: To what extent are pre-treatment data of one center predictive of post-treatment data of patients from the same center and from the other center? Does gender improve post-treatment data predictions?
To investigate the interplay between the age variables from the general German population and the tinnitus patients (RQ1), we firstly use a method to juxtapose the two distributions (age in Germany and age in the center) using a kernel density estimation plot. This method helps highlighting the parts of the distributions that are very similar and those which are not. We also analyse the differences in age distributions per gender and per center. As a result, the age distribution of one center's female tinnitus patients is compared to the age distribution of the other center's female tinnitus patients. The same reasoning is used to the male tinnitus patients. Gender is also compared between centers and with the distribution in Germany.
Our approach to uncover similarities in distributions of questionnaire scores by gender and clinical center (RQ2) is network-based. We design the network with nodes as patients and edges as a distance function that measures the difference between the questionnaire scores of each pair of patients. We use the same representation as in a recent work (Puga et al., 2021). After mapping the data into a network representation, we use the netLSD distance to compute and compare the distance across networks. These distances can then be rated, with shorter distances suggesting higher similarity between patients.
Finally, we train models to predict post-treatment questionnaire data (i) within each center and then (ii) with the other center's data (RQ3). These models are computed with the complete sample and per gender. There are two main goals for such analysis: (i) to detect if a center is predictive of the other and (ii) to detect if same-gender patients from one center are predictive of the same-gender patients of the other center. The adherence behavior of the patients to filling out the questionnaires is included as different sets of features in the models. Hence, the extent to which these adherence features improve the prediction of the post-treatment score is also investigated.
The remainder of this article is organized as follows: section 2 presents the dataset properties used for the analyses, section 3 describes the methodology to find the relationship of age and gender with tinnitus prevalence organized per research question, section 4 shows the results of our methods to respond to each research question. In section 5 the results are discussed with respect to the available literature and section 6 summarizes the main findings.
2. Materials
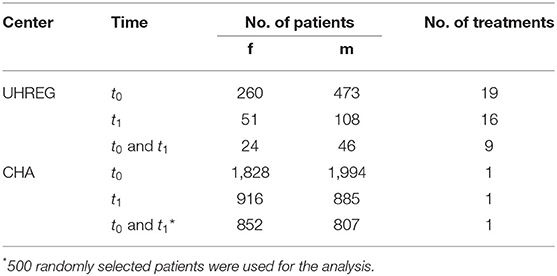
The data used in this study are from patients with chronic tinnitus who were admitted to the University Hospital of Regensburg and to the Tinnitus Center, Charité - Universitätsmedizin Berlin. The Regensburg University Hospital collected the data between January 3, 2016 and May 28, 2020. The data from the Tinnitus Center, Charité - Universitätsmedizin Berlin were gathered between January 1, 2011 and October 15, 2015. Despite the fact that both datasets contain data from many questionnaires and socio-demographic data, some variables appear only in one dataset and vice versa. Table 1 shows the number of patients with available data at different time points as well as the number of different treatments that were assigned. Two time points were considered: t0 and t1. The former denotes the time point at admission, while the latter represents the time at the final visit of the patient to the clinical center. For the sake of simplicity, University Hospital of Regensburg is denoted by UHREG and the Tinnitus Center, Charité - Universitätsmedizin Berlin is denoted by CHA.
Specific data pre-processing steps are required depending on the type of analysis performed. The three research questions require different filters. I.e., for RQ1 we consider all patients from both centers who filled out the age and gender question among all questionnaires, at admission. RQ2 and RQ3 require a match on the questionnaire data that are being compared. This phase is critical for the juxtaposition of the medical centers' questionnaires and ensuring the feasibility of the comparison.
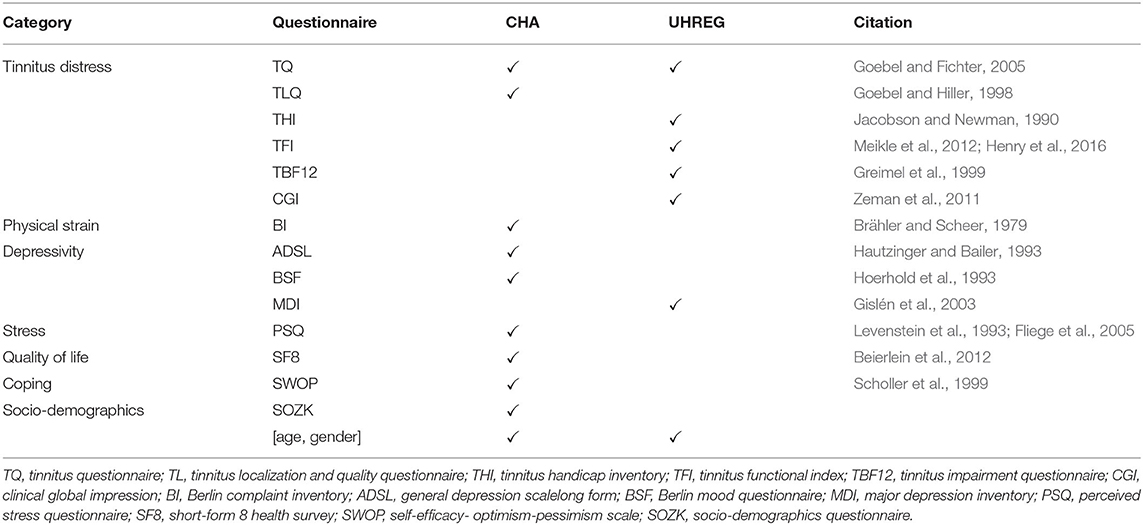
Table 2 summarizes the available questionnaires per center. In order to compute the treatment outcome, the scores at admission and after treatment are used. As a consequence, the shared questionnaire (in this case, TQ) has to be available at both time points (t0 and t1) in order to learn a model that predicts the treatment outcome.
3. Methods
3.1. Our Approach to Answer RQ1
To juxtapose the centers to each other and to the general population, we combine statistical testing and visualization tools.
According to RQ1, we compare the age distributions of our two samples s1 and s2 (one per center) for each gender separately and correct it for multiple testing over the 6 comparisons that are performed, using Bonferroni correction. In particular, we apply the Shapiro-Wilk test (Shapiro and Wilk, 1965) to check whether the samples follow a normal distribution: if yes, student's test (Gosset, 1908) is applied on the means of the samples; if not, MannWhitney U test (Neuhäuser, 2011) is conducted instead. All tests are performed with α = 0.05.
To visualize the distributions, we use kernel density estimation plots. In particular, we plot the two age distributions—of the German population and of the tinnitus patients from both clinical centers. For comparison purposes, we computed the percentage of people at a certain age for both data sets.
Secondly, we identified the regions of the age distributions that have similar shapes. I.e., we plot both distributions in a kernel density estimation plot and then interpret the zones where both distributions behave similarly—when there is a high ratio of German population for a certain age and a high percentage of tinnitus patients too.
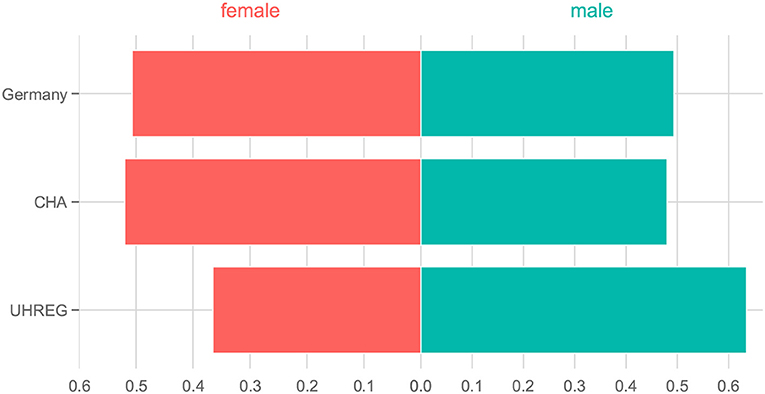
This visual tool enables the identification of intervals of age in which both distributions show a high or low percentage of people. On the other hand, we may also identify age intervals in which one distribution has a high percentage of people and the other has lower values. The goal of this analysis is to identify age intervals in both populations (residents in Germany and tinnitus patients) where the percentage of people inside that interval is similar and where it differs. By identifying these differences and similarities, we explore the extent to which the two distributions agree. Concerning gender, the female and male ratio in Germany and in each clinical center are plotted using a bi-directional bar plot.
3.2. Our Approach to Answer RQ2
3.2.1. Modeling Center Similarity With Respect to Questionnaire Score and Gender
A network-based analysis is performed to compute and interpret the similarity of tinnitus patients per gender and clinical center. In this network, nodes represent patients and edges represent the distance between them. The questionnaire score used to compute the edge weights is the TQ score. As a first step we standardize the questionnaire scores, as in Equation (1).
where pi,X denotes a patient i from the clinical center X, qpi,X is the questionnaire score of patient pi,X, μq,X and σq,X are the mean and standard deviation of the questionnaire scores q in clinical center X, respectively.
The higher the score difference between patients, the weaker the connection between them. To account for that, a transformation 1/x is applied as shown in Equation (2), representing the edge weight.
where wpi, pj, X as the edge weight between patient pi and pj in clinical center X.
For the graph (or network) comparison, Tsitsulin et al. (2018) states that there are three main approaches: (i) direct methods, (ii) kernel methods, and (iii) statistical representations. Their approach falls in a different category, which is based on spectral representation. Tsitsulin et al. (2018) introduce the NetLSD (network laplacian spectral descriptor) method which creates a vector for each network using the “heat equation.” The difference between these vectors is computed and the final distance is the NetLSD metric. In comparison to the other approaches this method is able to meet three properties simultaneously: permutation invariance, scale-adaptivity, and size-invariance.
The permutation invariance property guarantees that the distance between two isomorphic graphs is equal to zero. The scale-adaptivity property is based on the representation including both local and global graph properties. Finally, the size-invariance property takes into account the magnitude of the graphs and can distinguish between graphs with comparable features but different magnitudes.
Datasets from different clinical centers vary in size and hence each graph that represents a different clinical center is of different size. The size-invariance property of NetLSD is critical in this work since it enables graph comparison between graphs of different sizes.
3.2.2. Modeling Adherence
Since the centers use partially different questionnaires, we first organize the questionnaires into topical categories that reflect the (co-)morbidity they capture, independently of the questionnaire(s) in use. Then, we model adherence with respect to a set of items I for a patient x, denoted as adh_I(x) as the percentage of items from I answered by patient x over the total number of items in I, i.e., |I|. The set of items I can be a questionnaire or a topical category that encompasses more than one questionnaire. The adherence values are computed for each patient x, but in the following, we skip (x) from the notation for simplicity.
Let be the set of questionnaires in a center and be the set of shared questionnaires between centers. Similarly, let be the set of categories in a center, where a category encompasses more than one questionnaire. Finally, let be the set of topical categories common to all centers.
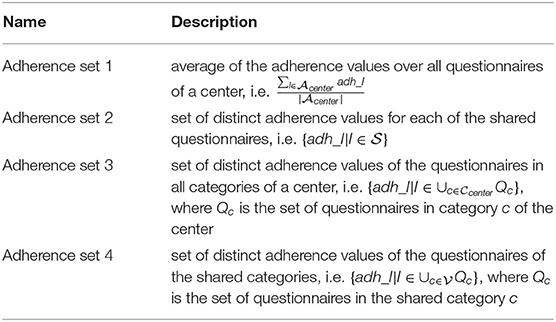
The setups—hereafter named as “adherence sets”—are subsets that represent different adherence rates expressed in percent.
On this basis, we generate sets of adherence features, as depicted on Table 3 and described hereafter. It is stressed that all sets refer to adherence at registration, i.e., at t0, since we intend to use these features to augment the original “basic set” of features used in the post-treatment predictors, i.e., at t1.
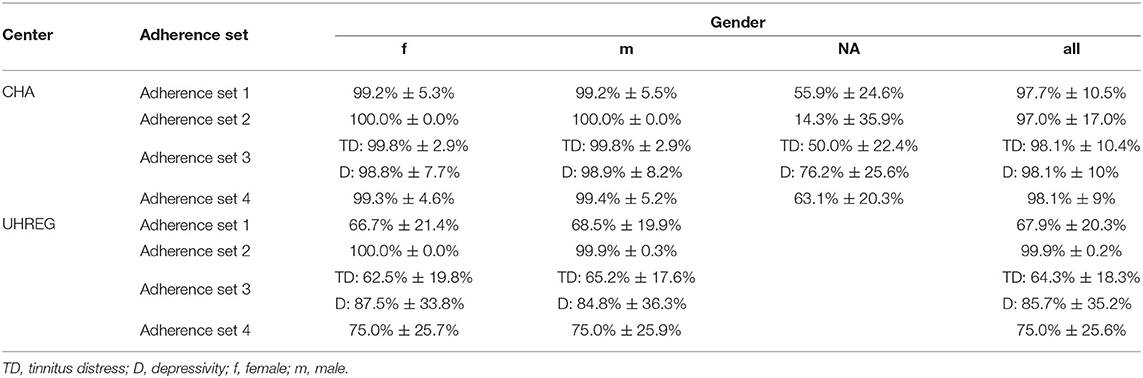
“Adherence set 1” is the average adherence behavior in percent over all questionnaires at t0. I.e., for each patient, all the adherence rates for each questionnaire at t0 are calculated as the answered questions divided by the total number of questions of the respective questionnaire. This information serves as foundation to calculate the average adherence rate by summing up the percentages and divide this sum by the number of rates. This is a rough summary of the adherence behavior of each patient and can be compared among the centers ignoring, for, e.g., different sets of questionnaires. Since the current task is a prediction task at t1, the subset must be limited to the visit at t0. Otherwise, the train set would contain information regarding the target variable (post-treatment data).
“Adherence set 2” includes the average adherence rates for each questionnaire calculated by the answer behavior of a patient at t0. It should be noted, however, that in this subset only the adherence rates of the questionnaires that are common in both centers to be compared can be used. The rest are excluded from this subset. The reason is on the one hand the availability of the questionnaire and on the other hand the feasibility of the comparison for the cross-center prediction.
“Adherence set 3” assigns all the available questionnaires at a center to a category. For instance, tinnitus distress (TD) groups all tinnitus-related questionnaires. The adherence rate is calculated as the average of the questionnaire adherence rates per category. This is also exclusively for t0. In order to enable cross-center prediction, only categories available in both centers are included.
“Adherence set 4” is the average of the categories of subset 3 after removing the categories that have no counterpart in the opposite center.
3.3. Our Approach to Answer RQ3
To understand the relationship between the pre-treatment data and the post-treatment data within centers, a regressor is trained in the data of each center with the target being the post-data treatment of interest. In our case, this refers to the TQ score at t1.
In order to extend the comprehension of the similarity between patients across centers, we fit a regression model in one center and use it to predict the score after treatment of the patients from the other center. Aside from the previously stated adherence features, we introduce a new drill-down criterion to the experiments: gender. As a result, we utilize the models trained using patients from the CHA center to predict post-treatment data of patients from the UHREG center, per gender.
The regression models used are the following: linear regressor (LR), lasso, ridge, and support vector regressor (SVR). When developing these predictors, data are divided into a test and train set, and a 10-fold cross validation is performed inside the train set to choose the best model and its hyperparameters (with a grid search approach). For evaluation, standard measures, such as MAE (mean absolute error), MSE (mean squared error), and R2 (explained variance) are used.
Firstly, we train regressor models to predict the TQ score after treatment (TQt1), per center and with different features. In total, 5 experiments are performed, per center. The first and the one with the least number of features included is constituted by age, gender, and TQt0—the basic set. Then, we add adherence features to this set. Our main goal was to understand which set of features were most predictive of post-treatment TQ score, for within-center and between-center predictions.
4. Results
4.1. Comparison of Age and Gender Distributions in Germany and in Each Clinical Center
4.1.1. Age
Despite the fact that demographics variables were analysed previously in tinnitus research (Seydel et al., 2013; Niemann et al., 2020), it was not yet explored that the age distribution may be influenced by another variable—for instance, the fact that we have less people with 70+ years in the population (due to the fact that life expectancy is within this range in Germany).
Hence, we propose to analyse the age distribution of tinnitus patients from the clinical centers along with the age distribution in Germany. Statistics from the German population age were gathered from a public data source, namely the German Federal Statistical Office (“Statistisches Bundesamt”)1.
Figure 1 shows the distribution of age in Germany and in both clinical centers and the kernel density estimate of the age distributions.
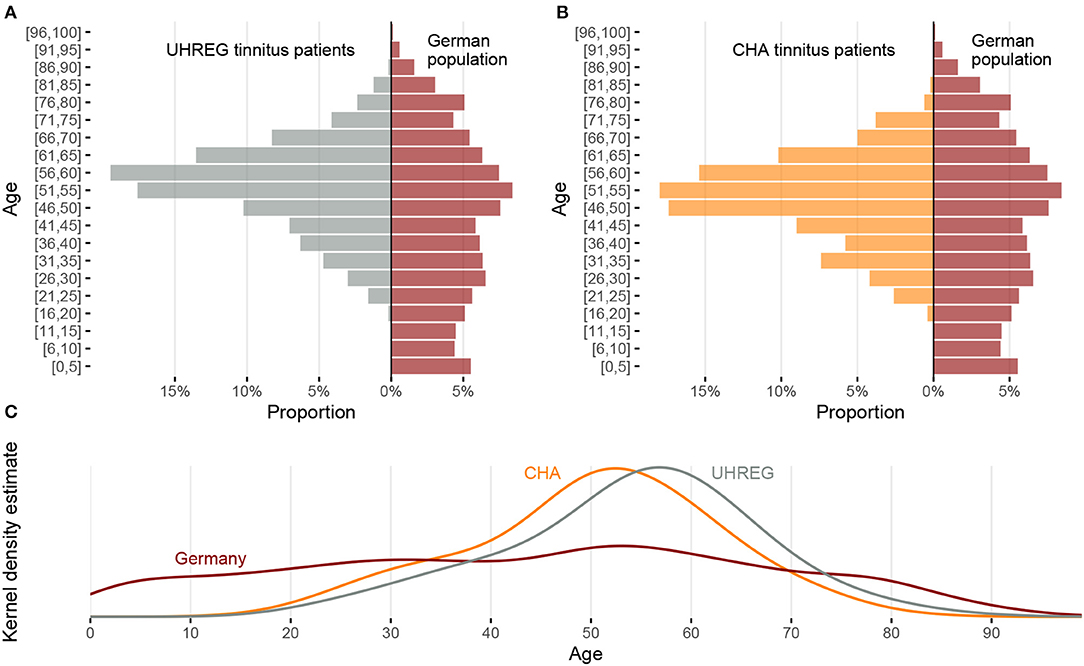
Figure 1. Comparison of age distribution between the two clinical centers and the German population. (A) Relative frequency by age intervals for UHREG (left) and the German population (right). (B) Relative frequency by age intervals for CHA (left) and the German population (right). (C) Density distributions of age for UHREG, CHA and the German population.
In the histograms of Figures 1A,B and in the kernel density plots of Figure 1C, we see that the centers of all three distributions are between 50 and 60 years but the distributions are very different. The curves of the two clinics cross the curve of the German population in the interval between 30 and 40 years of age and a little earlier than 80 years of age.
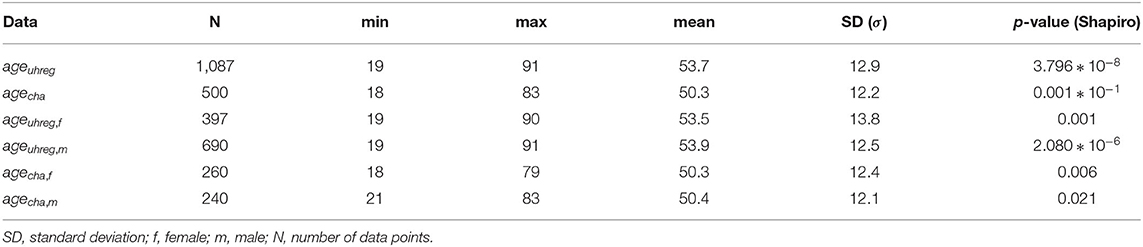
Figures 2, 3 show the distribution of age per center and gender, respectively. The descriptive statistics of these distributions are shown in Table 4, per center and per gender.
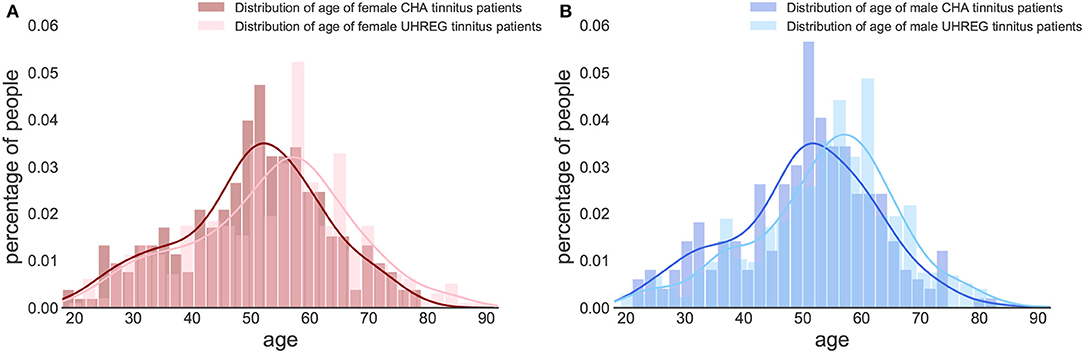
Figure 3. Age distribution per gender and clinical center. (A) Female tinnitus patients. (B) Male tinnitus patients.
We perform statistical tests to compare the age distributions between centers and determine whether there is statistical evidence to confirm that they are different. Given that the hypotheses of normality (Shapiro Wilk test) are rejected for all subsets in Table 4, we elect the Mann-Whitney test to compare location measures between samples. In particular, we test (1) whether female (first line of Table 5) vs. male tinnitus patients (second line) featured similar age distributions in the two centres and (2) whether female and male patients featured similar age distributions within each centre (3rd line for UHREG, 4th line for CHA) and, finally, whether female tinnitus patients of one centre featured similar age distributions as male patients in the other center (last two lines).
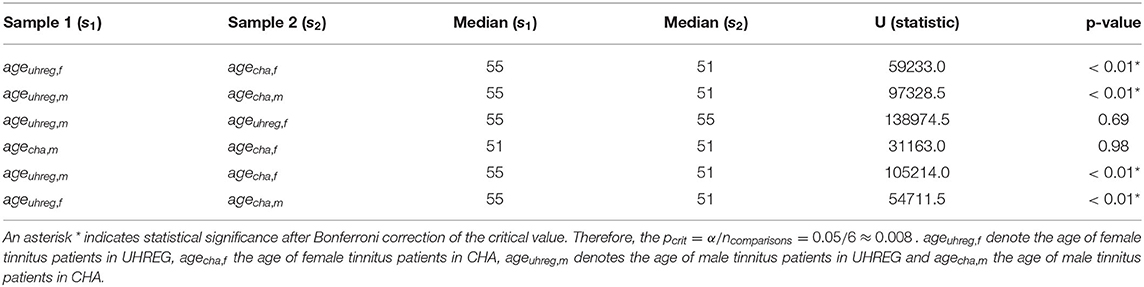
Table 5. Medians, Mann-Whitney U-statistic and p-value of a Mann-Whitney two-sided test for comparison of two samples.
As can be seen in the table, it can be stated that female and male patients within the same center follow the same age distribution (H0 cannot be rejected). The other null hypotheses are rejected. Based on these findings and complementing it with the analysis of the histograms, we can infer that the age distribution in UHREG is consistently higher than the one in CHA, regardless of gender.
4.1.2. Gender
Regarding gender, Figure 4 shows the distributions of gender in Germany [from the 'Statistisches Bundesamt' (see text footnote 1)] and in both clinical centers. We can see that distribution of gender in CHA is close to the one in Germany. In contrast, UHREG has a higher percentage of male individuals than the general German population.
4.2. Clinical Center Similarity With Respect to TQ Score Per Gender
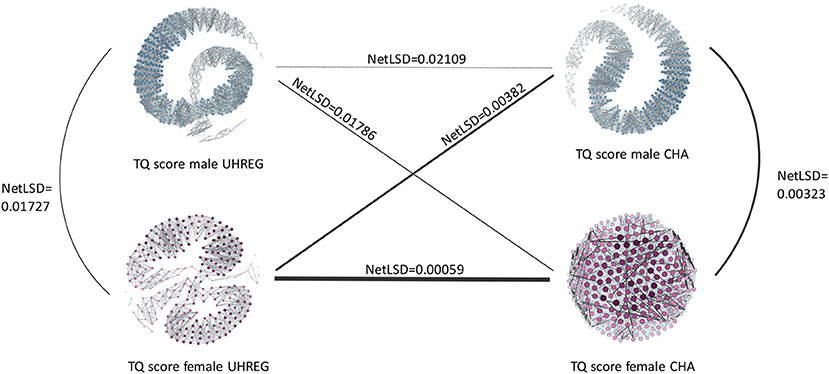
A network-based analysis is carried as a method to capture the similarity between patients, with respect to the TQ score. Four networks are generated: one per gender and one per center.
The four networks that represent distinct groups of tinnitus patients are illustrated in Figure 5. The purple networks represent TQ score data from female patients, whereas the blue networks represent TQ score data from male patients. In this analysis, the emphasis is on the TQ scores and similarity is modeled with respect to this variable.
As previously stated, the nodes represent the patients, while the edges connecting them show the similarity between them. Denser areas in each network reflect patients who are similar to one another with respect to their TQ score, whereas darker and thicker edges indicate a stronger connection between patients and therefore a high similarity.
The NetLSD score provides a measure of the distance between networks. The lower this score, the higher similarity between the networks. Given the purpose of this paper, we focus on the difference of the distance values only: Figure 5 illustrates that, compared to the respective other networks, female patient networks in UHREG and CHA are the most similar, and male patient networks in UHREG and CHA the most dissimilar with respect to the TQ score.
Irrespective of the findings in Table 5, which demonstrated statistically significant differences in the age distributions of female patients within centres, the female patients' networks were the most similar according to the TQ score. By contrast, male tinnitus patients, who also featured significant differences in the age distributions, differed most in terms of their TQ scores.
The blank regions of the graphs indicate that there is no edge linking nodes on opposite sides of the graph. This occurs following the graph pruning phase, during which non-statistically significant edges are deleted. These empty areas differ amongst graphs due to their diverse characteristics; some have more statistically significant edges than others and hence are more connected.
4.3. Adherence Feature Sets
In order to capture the diverse spectrum of patient behavior in each center, all available and applicable records are used to get the adherence meta-information. Table 2 summarizes the available questionnaires per center. This is a necessary step before computing the adherence sets, since a common feature space (in this case, pre-treatment data) is required to compute some of the sets.
The basic set of features is considered to be the common pre-treatment data between centers. In the case of the current study, this corresponds to age, gender and TQt0. For both centers the meta-information is calculated. “Adherence set 1” includes for both CHA and UHREG just a single feature with an average adherence rate. “Adherence set 2” includes the common questionnaires.
The only shared questionnaire between CHA and UHREG is the TQ. Therefore, this subset includes only one feature: the average adherence rate for TQ at t0. “Adherence set 3” focus on the average adherence rate of common categories. There are two shared categories: tinnitus distress (TD) and depressivity (D), although both centers, except for the TQ, have different sets of questionnaires per category. “Adherence set 4” is the average of both common categories, which means in fact the average of the underlying questionnaires at t0.
Table 6 shows the average and the standard deviation (SD) of the adherence features of each center. One important remark is that there are some patients with missing values for the gender from the clinical center CHA. This is also shown in Table 6 and is of importance for the prediction task.
4.4. Prediction of Questionnaire Score After Treatment
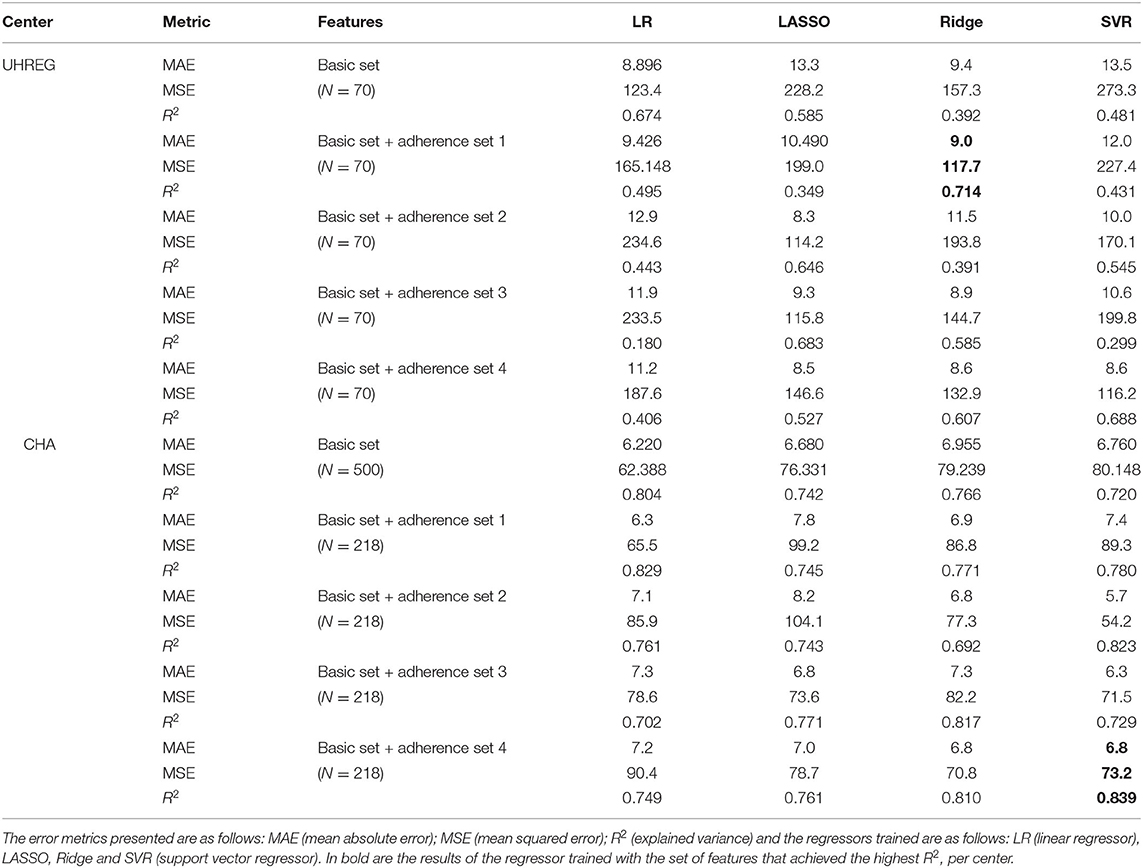
Table 7 shows the predictions of the TQt1 per center, using different feature sets. These sets differ in their features. The age, gender, and TQt0 are denoted as basic set of features. Then, four adherence feature sets are added - sets 1, 2, 3 and 4. The target variable is set to be the TQ score at t1, which we refer to as TQt1. Patients with missing values for the used features (for example, missing values of gender in CHA as reported in Table 6) are excluded from this study.
For UHREG, the ridge regressor achieves the best results using the basic feature set and and the “adherence feature set 1”. For CHA, the SVR (support vector regressor) regressor achieves the best results using the basic feature and “adherence set 4”.
To predict UHREG patients' treatment outcome, three models are trained with three different datasets: (i) all CHA patients, (ii) female CHA patients only and (iii) male CHA patients only. These models are then tested on UHREG patient data. Table 8 illustrates the results.
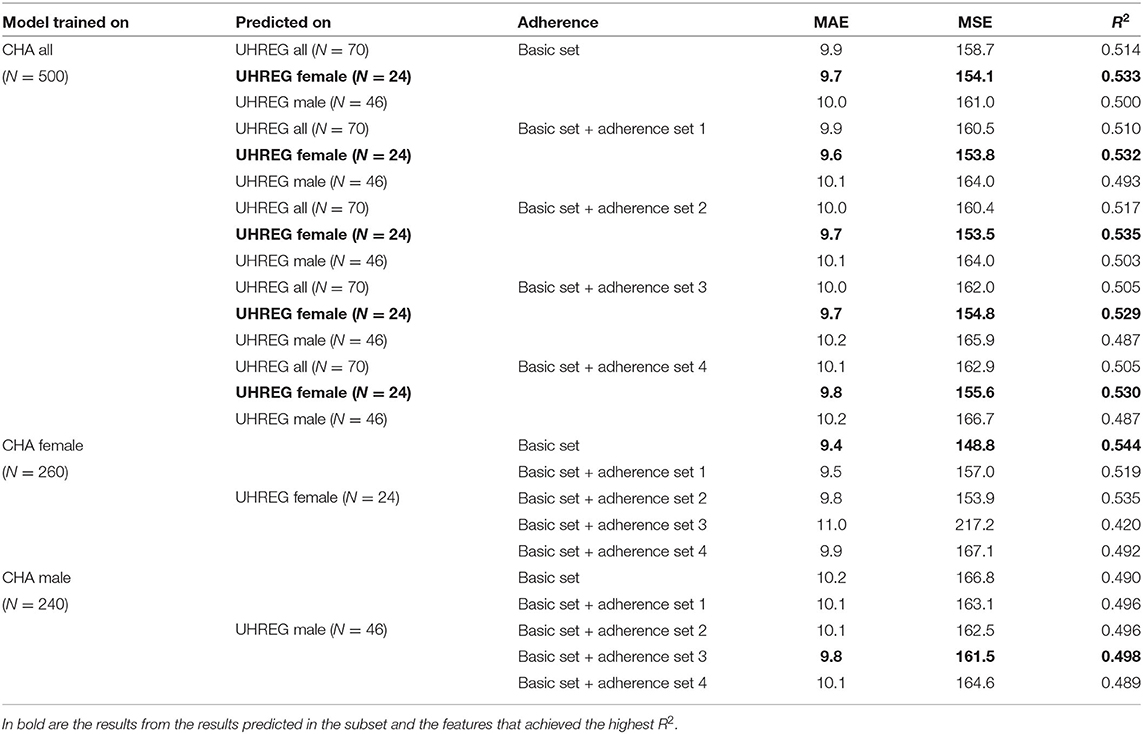
Table 8. Prediction of TQ at t1 on UHREG tinnitus patients with model trained on CHA tinnitus patients (results from the model with the highest R2 are shown).
We can conclude that the female tinnitus patients from UHREG are the most predictable among all learners from CHA trained with all their patients. The basic set and the “adherence 2” set of features are the most successful in terms of R2.
In the second part of Table 8, only female patients from CHA are used to train a model and this model is used to predict the female patients from UHREG. In this example, the basic feature set provides the highest R2.
Finally, a learner is trained only on the male patients from CHA and used to predict the TQt1 on patients from UHREG. The best set of features is the basic set with the “adherence set 3” set of features. This means that these features were the ones that best predicted TQt1 on patients from UHREG and can thus be deemed the most useful for understanding the post-treatment TQ score (in comparison to the other set of features).
5. Discussion
In tinnitus research, age and gender have been two variables of interest to analyse. Recently, Niemann et al. (2020) showed that women present a higher level of depression and tinnitus-related distress. Another study by Seydel et al. (2013) recognized age and gender as the most relevant factors to predict tinnitus distress. Rodrigo et al. (2021) investigated the impact of several features for the success of internet-based CBT (cognitive behavioral therapy) on tinnitus patients. In this study, age and gender were used as features but the feature that proved the highest impact on the outcome of the treatment was the education level of the patients. Their findings indicated that patients with a higher level of education were more likely to succeed after treatment.
In the present study, we found that the age distribution of the general German population is partly reflected in the age distributions of the two centers' tinnitus patients. All three distributions reflect a drop in the age window 75+. An explanation could be that elderly patients are less mobile, especially those in rural areas. Another explanation could be that the likelihood of other conditions increases with age - which might be associated with a perception of tinnitus as relatively less distressing. These are hypotheses and more investigation is needed to understand this pattern of chronic elderly tinnitus patients. Middle-aged people, on the other hand, were considerably over-represented in the tinnitus centers relative to the general German population. Therefore, the percentage of patients that seek medical care for tinnitus within middle-aged people is higher than for the other age ranges and it cannot be explained by the German population characteristics.
Within, but not between centers, same-gender patients were found to differ significantly in age. While this is true for age, the results from the netLSD distances show that female patients from different centers are, among all pairs of networks, the most similar according to their TQ score. Another finding was the fact that age of female and male patients from different centers were not statistically different.
Another intriguing finding was that models trained with adherence features outperformed baseline models that did not include these features. The model trained with female CHA tinnitus patients was more predictive of female UHREG patients than the model trained with male CHA patients and tested on male UHREG patients. As a result, UHREG female patients are better predicted with CHA data than male patients. This means that treatment-improvement rates and patterns improve more similarly in females than males, when centers are compared. This result can be cross-checked with the NetLSD distance results. In this analysis, female tinnitus patients from different centers were also found to be more similar than male patients with respect to questionnaire score at t0. Hence, we can conclude that both results from different methods agree in terms of similarity. Despite the heterogeneity of clinical centers and the fact that these are preliminary results, there may be an indication that patients across clinical centers share similar characteristics. It is worth noting that the amount of available data for analysis varies between clinical centers, which may have an impact on how representative they are of their patients. As a result, we consider the reported findings to be preliminary and the analysis should be expanded to larger datasets.
The adherence features that summarize the information the most, “adherence 1” and “adherence 4,” produced the greatest improvement in the R2 value in the gender-agnostic intra-center analysis. In the inter-center prediction using all the data from the training center, however, “adherence 2” outperforms the other adherence sets. Nonetheless, the “basic” set outperforms the other 3 adherence features for all, as well as for female and male. Thus, in the cross-center prediction scenario, information on adherence in the common questionnaire(s) seems to be a useful addition.
6. Conclusion
In this article, we performed various analyses to compare tinnitus patients from two different large clinical centers. These comparisons were carried mainly focusing on the questionnaire scores before and after treatment, socio-demographics and adherence to the filling of the questionnaires. For that, visualization and prediction methods were implemented along with a network-based representation of the data.
The main findings can be organized into three. The first one being that the distribution of age in Germany agrees, in some age ranges, to the ones from the tinnitus patients from both centers. This indicates good and representative reach of the specialist treatment centers in offering care for their target populations. The second, that female tinnitus patients from one center (CHA) are more predictive of the female patients of the other center (UHREG) than male patients. This result is complemented by the fact that our network-based approach to compute the similarity between networks also agreed that female tinnitus patients were more similar across centers than male patients, with respect to treatment score at t0. The third, that including meta-information about the adherence of the patients to the filling of the questionnaires improved the baseline predictions of post-treatment data.
The evaluation's findings could be supported further by finding datasets with more overlapping questionnaires but also closer in terms of sample size. Finding such datasets will be a future task. Incorporating information about gender and adherence could improve the prediction task. Future research should place these findings to the test by applying intra- and inter-center predictions to other centers.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets for this article are not publicly available because no consent of the patients to publish their data was obtained. Requests to access these datasets should be directed to BM, YmlyZ2l0Lm1henVyZWtAY2hhcml0ZS5kZQ== for the CHA dataset and WS, d2luZnJpZWQuc2NobGVlQGdtYWlsLmNvbQ== for the UHREG dataset.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the University Medicine Charité Berlin for the CHA Dataset and the Ethics Committee of the University Regensburg for the UHREG Dataset. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CP and MSc designed and performed the data analysis and wrote the original draft. CP designed and performed the network-based analysis. MSc modeled adherence. CP and UN designed the visualizations. UN optimized the visualizations on interpretability. VU supported on questionnaire interpretation and data preparation. BB, PB, and BM curated the dataset with patients admitted to the Tinnitus Center, Charité – Universitätsmedizin Berlin. JS, BL, and WS worked on data curation and model interpretation. BB, PB, BM, WS, and MSp supervised the data analysis and reviewed and edited the manuscript. BM and MSp led the project. All authors contributed to the article and approved the submitted version.
Funding
Part of this project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement number 848261.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://www.destatis.de, site accessed in March 2020.
References
Beierlein, V., Morfeld, M., Bergelt, C., Bullinger, M., and Brähler, E. (2012). Messung der gesundheitsbezogenen lebensqualität mit dem sf-8. Diagnostica. 58, 3. doi: 10.1026/0012-1924/a000068
Biswas, R., and Hall, D. A. (2020). “Prevalence, incidence, and risk factors for tinnitus,” in The Behavioral Neuroscience of Tinnitus (Cham: Springer International Publishing), 3–28.
Brähler, E., and Scheer, J. W. (1979). Scaling of psychosomatic by means of the giessen inventory (gbb)(author's transl). Psychotherapie Medizinische Psychologie 29, 14.
Fliege, H., Rose, M., Arck, P., Walter, O. B., Kocalevent, R.-D., Weber, C., et al. (2005). The perceived stress questionnaire (psq) reconsidered: validation and reference values from different clinical and healthy adult samples. Psychosomatic Med. 67, 78–88. doi: 10.1097/01.psy.0000151491.80178.78
Gislén, A., Dacke, M., Kröger, R. H., Abrahamsson, M., Nilsson, D.-E., and Warrant, E. J. (2003). Superior underwater vision in a human population of sea gypsies. Curr. Biol. 13, 833–836. doi: 10.1016/s0960-9822(03)00290-2
Goebel, G., and Fichter, M. (2005). “Psychiatrische komorbidität bei tinnitus,” in Tinnitus (Heidelberg: Springer), 137–150.
Goebel, G., and Hiller, W. (1998). Tinnitus-Fragebogen:(TF); ein Instrument zur Erfassung von Belastung und Schweregrad bei Tinnitus; Handanweisung. Hogrefe, Verlag für Psychologie.
Greimel, K. V., Leibetseder, M., Unterrainer, J., and Albegger, K. (1999). Ist tinnitus messbar? methoden zur erfassung tinnitusspezifischer beeinträchtigungen und präsentation des tinnitus-beeinträchtigungs-fragebogens (tbf-12). HNO 47, 196–201.
Hautzinger, M., and Bailer, M. (1993). ADS, Allgemeine Depressions Skala. Manual. Technical Report, Beltz Test.
Henry, J. A., Griest, S., Thielman, E., McMillan, G., Kaelin, C., and Carlson, K. F. (2016). Tinnitus functional index: development, validation, outcomes research, and clinical application. Hearing Res. 334, 58–64. doi: 10.1016/j.heares.2015.06.004
Hoerhold, M., Klapp, B. F., and Schimmack, U. (1993). Testing the invariance and hierarchy of a multidimensional model of mood by means of repeated measurement with student and patient sample. Z Med. Psychol. 1, 27–35.
Jacobson, G. P., and Newman, C. W. (1990). The development of the dizziness handicap inventory. Arch. Otolaryngol. Head Neck Surg. 116, 424–427.
Jastreboff, P. J. (1990). Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res. 8, 221–254.
Levenstein, S., Prantera, C., Varvo, V., Scribano, M. L., Berto, E., Luzi, C., et al. (1993). Development of the perceived stress questionnaire: a new tool for psychosomatic research. J. Psychosomatic Res. 37, 19–32.
Meikle, M. B., Henry, J. A., Griest, S. E., Stewart, B. J., Abrams, H. B., McArdle, R., et al. (2012). The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hearing 33, 153–176. doi: 10.1097/AUD.0b013e31822f67c0
Niemann, U., Boecking, B., Brueggemann, P., Mazurek, B., and Spiliopoulou, M. (2020). Gender-specific differences in patients with chronic tinnitus—baseline characteristics and treatment effects. Front. Neurosci. 14, 1–11. doi: 10.3389/fnins.2020.00487
Puga, C., Niemann, U., Unnikrishnan, V., Schleicher, M., Schlee, W., and Spiliopoulou, M. (2021). “Discovery of patient phenotypes through multi-layer network analysis on the example of tinnitus,” in 2021 IEEE 8th International Conference on Data Science and Advanced Analytics (DSAA) (Porto), 1–10.
Rodrigo, H., Beukes, E. W., Andersson, G., and Manchaiah, V. (2021). Exploratory data mining techniques (decision tree models) for examining the impact of internet-based cognitive behavioral therapy for tinnitus: Machine learning approach. J. Med. Internet Res. 23, e28999. doi: 10.2196/28999
Schlee, W., Langguth, B., Pryss, R., Allgaier, J., Mulansky, L., Vogel, C., et al. (2021). Using Big Data to Develop a Clinical Decision Support System for Tinnitus Treatment. Cham: Springer International Publishing, 175–189.
Scholler, G., Fliege, H., and Klapp, B. F. (1999). Questionnaire of self-efficacy, optimism and pessimism: reconstruction, selection of items and validation of an instrument by means of examinations of clinical samples. Psychotherapie Psychosomatik Medizinische Psychologie 49, 275–283.
Seydel, C., Haupt, H., Olze, H., Szczepek, A. J., and Mazurek, B. (2013). Gender and chronic tinnitus. Ear and Hearing 34, 661–672. doi: 10.1097/AUD.0b013e31828149f2
Shapiro, S. S., and Wilk, M. B. (1965). An analysis of variance test for normality (complete samples). Biometrika 52, 591–611.
Tsitsulin, A., Mottin, D., Karras, P., Bronstein, A., and Müller, E. (2018). “NetLSD: hearing the shape of a graph,” in Proceedings of the ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (London), 2347–2356.
Keywords: tinnitus, networks, similarity, socio-demographics, adherence, predictive modeling
Citation: Puga C, Schleicher M, Niemann U, Unnikrishnan V, Boecking B, Brueggemann P, Simoes J, Langguth B, Schlee W, Mazurek B and Spiliopoulou M (2022) Juxtaposing Medical Centers Using Different Questionnaires Through Score Predictors. Front. Neurosci. 16:818686. doi: 10.3389/fnins.2022.818686
Received: 19 November 2021; Accepted: 01 February 2022;
Published: 23 March 2022.
Edited by:
Andreas K. Maier, University of Erlangen Nuremberg, GermanyReviewed by:
Marc Fagelson, East Tennessee State University, United StatesTijana Bojić, University of Belgrade, Serbia
Alessandra Fioretti, European Hospital, Italy
Copyright © 2022 Puga, Schleicher, Niemann, Unnikrishnan, Boecking, Brueggemann, Simoes, Langguth, Schlee, Mazurek and Spiliopoulou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clara Puga, Y2xhcmEucHVnYUBvdmd1LmRl
 Clara Puga
Clara Puga Miro Schleicher1
Miro Schleicher1 Uli Niemann
Uli Niemann Benjamin Boecking
Benjamin Boecking Petra Brueggemann
Petra Brueggemann Berthold Langguth
Berthold Langguth Winfried Schlee
Winfried Schlee Birgit Mazurek
Birgit Mazurek Myra Spiliopoulou
Myra Spiliopoulou