Abstract
Different acupoints exhibiting similar therapeutic effects are a common phenomenon in acupuncture clinical practice. However, the mechanism underlying this phenomenon remains unclear. This study aimed to investigate the similarities and differences in cerebral activities elicited through stimulation of CV12 and ST36, the two most commonly used acupoints, in the treatment of gastrointestinal diseases, so as to partly explore the mechanism of the different acupoints with similar effects. Thirty-eight eligible functional dyspepsia (FD) patients were randomly assigned into either group A (CV12 group) or group B (ST36 group). Each patient received five acupuncture treatments per week for 4 weeks. The Symptom Index of Dyspepsia (SID), Nepean Dyspepsia Symptom Index (NDSI), and Nepean Dyspepsia Life Quality Index (NDLQI) were used to assess treatment efficacy. Functional MRI (fMRI) scans were performed to detect cerebral activity changes at baseline and at the end of the treatment. The results demonstrated that (1) improvements in NDSI, SID, and NDLQI were found in both group A and group B (p < 0.05). However, there were no significant differences in the improvements of the SID, NDSI, and NDLQI scores between group A and group B (p > 0.05); (2) all FD patients showed significantly increased amplitude of low-frequency fluctuation (ALFF) in the left postcentral gyrus after acupuncture treatment, and the changes of ALFF in the left postcentral gyrus were significantly related to the improvements of SID scores (r = 0.358, p = 0.041); and (3) needling at CV12 significantly decreased the resting-state functional connectivity (rsFC) between the left postcentral gyrus and angular gyrus, caudate, middle frontal gyrus (MFG), and cerebellum, while needling at ST36 significantly increased the rsFC between the left postcentral gyrus with the precuneus, superior frontal gyrus (SFG), and MFG. The results indicated that CV12 and ST36 shared similar therapeutic effects for dyspepsia, with common modulation on the activity of the postcentral gyrus in FD patients. However, the modulatory pattern on the functional connectivity of the postcentral gyrus was different. Namely, stimulation of CV12 primarily involved the postcentral gyrus–reward network, while stimulation of ST36 primarily involved the postcentral gyrus–default mode network circuitry.
Introduction
Functional dyspepsia (FD) is a common functional gastrointestinal disease (FGID) with clinical incidence ranging from 8 to 40% (Ghoshal et al., 2011; Mahadeva and Ford, 2016). The main clinical manifestations of FD are epigastric pain, epigastric burning, early satiety, and postprandial fullness, which cannot be attributed to organic and metabolic causes (Tack and Talley, 2013; Drossman, 2016). FD not only significantly affects the quality of life (QoL) of patients but also creates severe socioeconomic burden (Talley et al., 2006). Due to the complex etiology of FD, there is currently a lack of effective pharmaceuticals (Tack and Camilleri, 2018; Yamawaki et al., 2018; Tack et al., 2019), so non-pharmaceutical therapies are sought out by doctors and patients alike.
Acupuncture, as the most commonly used alternative and complementary treatment modality worldwide, has been accepted as an effective therapy for FD (Yang et al., 2020; Wang et al., 2021). A number of clinical studies have shown that acupuncture not only can relieve symptoms of dyspepsia but can also improve patient QoL and emotional states (Xu et al., 2006; Ma et al., 2012; Zeng et al., 2012; Yang et al., 2020). For example, our previous results indicated that genuine acupuncture can significantly improve QoL and symptoms of FD patients when compared to sham acupuncture and oral itopride (Ma et al., 2012). A data mining-based review found that more than 20 acupoints can be used for treating FD in clinical practice. Among them, Zusanli (ST36) and Zhongwan (CV12) are the most commonly used (Cao et al., 2016a,b). Despite the two points exhibiting treatment efficacy, their mechanisms in the treatment of FD remain to be further studied.
In the last decade, a number of neuroimaging studies have demonstrated that FD patients exhibit significant functional and structural alterations in multiple brain regions, including the frontal cortex, somatosensory cortex, postcentral gyrus, precuneus, and caudate tail (Zeng et al., 2009; Nan et al., 2014; Lee et al., 2016; Qi et al., 2020), and these abnormal functional activities can be regulated by acupuncture, to some degree (Zeng et al., 2012; Chen et al., 2021). For example, a previous functional MRI (fMRI) study found that acupuncture at Weishu (BL21) and Zhongwan (CV12) can modulate the disrupted functional connectivity (FC) between the insula and other brain regions in rat models of FD (Chen et al., 2021). Our previous studies also indicated that needling acupoints on the stomach meridian could significantly reduce abnormally high glucose metabolism in the homeostatic afferent network of FD patients and that this regulatory effect is different from needling sham acupoints and from needling acupoints not used for treating FD (Zeng et al., 2012, 2015). However, the question of whether different acupoints with similar therapeutic effects share similar influences on brain function in patients with FD has not been explored in previous studies.
Therefore, on the basis of verifying similar clinical effects of ST36 and CV12 in the treatment of FD, our study aimed to 1) observe the influence of acupuncture on brain functional activities of all FD patients using fMRI, and attempt to determine potential target brain areas related to acupuncture efficacy, and 2) investigate the effects of needling at ST36 and CV12 on FC of the target brain region, in order to explore the mechanism(s) governing similar effects exhibited by different acupoints.
Materials and Methods
This was a randomized controlled neuroimaging trial. The FD patients were recruited from the campus of Chengdu University of Traditional Chinese Medicine (CDUTCM) and the Digestive Department of the Affiliated Hospital of CDUTCM between January 2016 and May 2018. All patients were diagnosed by clinicians in the Digestive Department of the Affiliated Hospital of CDUTCM.
This study was performed according to the principles of the Declaration of Helsinki (Version Edinburgh 2000). The study protocol was approved by the Ethics Committee of the Affiliated Hospital of CDUTCM (No. 2014KL-028) and registered at the Clinical Trial Registry (registration number: ChiCTR-IOR-15006402).
Participants
Patients were enrolled if they fulfilled all of the following inclusion criteria: (1) were aged 18 to 45 years; (2) were right-handed; (3) matched the Rome III diagnosis criteria for FD; (4) had not taken any gastrointestinal drugs or received acupuncture treatment for at least 15 days before entering the study; and (5) provided assigned informed consent. Patients were excluded if they (1) were pregnant or lactating; (2) had a history of head trauma with loss of consciousness or gastrointestinal surgery; (3) were currently taking drugs promoting gastrointestinal dynamics; (4) had any contraindications to acupuncture; or (5) had any MRI contraindications, such as pacemakers, fixed metal dentures, or severe claustrophobia.
After an initial 2-week baseline evaluation, 38 eligible patients were randomly assigned to two groups using a computer-generated randomization sequence. The randomization information was concealed from the researchers until the completion of statistical analysis. Patients were blinded to the group assignment.
Acupuncture Intervention
The acupoint used for group A was Zhongwan (CV12), while the acupoint used for group B was Zusanli (ST 36). The locations of the acupoints are shown in Figure 1. Acupuncture treatment was administered by two licensed acupuncturists with more than 6 years of clinical experience and who had received specialized acupuncture training in the selection of acupoints and standard acupuncture operating procedures. Manual acupuncture treatment was administered using disposable sterile filiform needles (25–40 × 0.25 mm, Huatuo Medical Instrument Co., Ltd., Jiangsu, China). The acupuncture treatment protocol used is outlined in our previous study (Yin et al., 2017). All patients received five acupuncture treatments per week for 4 weeks.
FIGURE 1
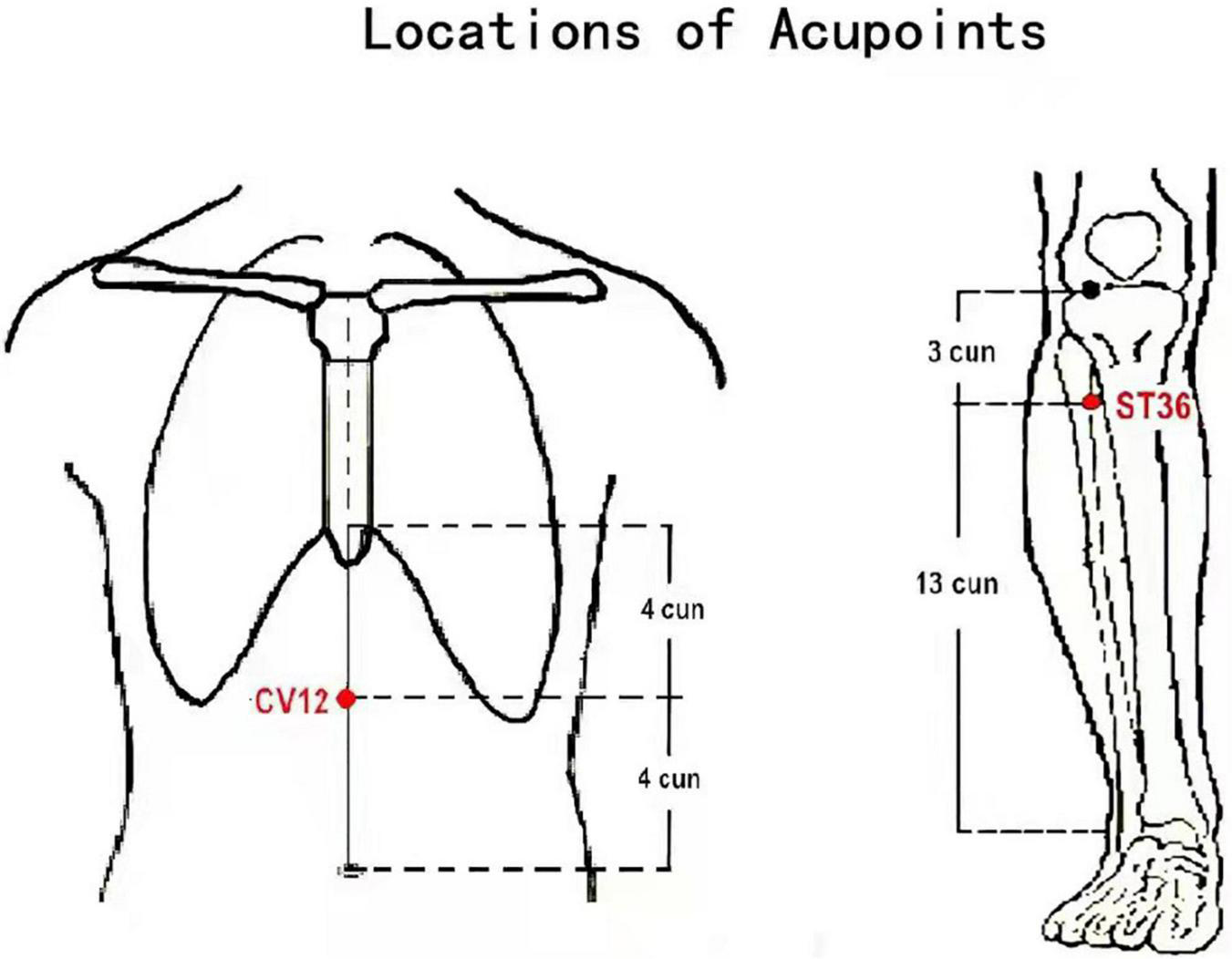
Acupoint locations of He-Mu point combination. Zhongwan (CV12): on the anterior median line of the upper abdomen, 4 cun above the navel. Zusanli (ST36): on the anterior lateral side of the shank, 3 cun below Dubi (ST35), one horizontally placed finger distance lateral to the anterior border of the tibia.
Outcome Measurement
The Symptom Index of Dyspepsia (SID) and the Nepean dyspepsia index (NDI) were used to assess the efficacy of acupuncture for treating FD. The SID focused on the four chief symptoms of FD: postprandial fullness discomfort, early satiety, epigastric pain, and epigastric burning. Each symptom was graded as follows: asymptomatic (0 points), mild (1 point), moderate (2 points), or severe (3 points; Ma et al., 2012). The NDI is composed of the Nepean Dyspepsia Symptom Index (NDSI) and Nepean Dyspepsia Life Quality Index (NDLQI) (Talley et al., 1999). The NDSI evaluates the clinical symptoms of patients by measuring the frequency, intensity, and level of discomfort for 15 upper gastrointestinal symptoms. The NDLQI is an FD-specific questionnaire to assess patients’ QoL from four dimensions, including interference (13 items), knowledge/control (7 items), eat/drink (3 items), and sleep/disturb (2 items). Furthermore, the Self-Rating Anxiety Scale (SAS) (Zung, 1971) and Self-Rating Depression Scale (SDS) (Zung et al., 1965) were used to evaluate the emotional states of patients. The SID, NDSI, NDLQI, SAS, and SDS were measured at baseline and the end of the treatment.
Functional MRI Scan
All patients received MRI scans at baseline and the end of the treatment. MRI data were acquired with a 3.0-T magnetic resonance scanner (Siemens, Munich, Germany) at Huaxi Magnetic Resonance Research Center, West China Hospital of Sichuan University, Chengdu, China. The scanning procedure contained a localizer, a high-resolution three-dimensional T1-weighted imaging (3D-T1WI), and a blood oxygenation level-dependent fMRI (BOLD-fMRI). According to previous studies, resting-state fMRI (rsfMRI) signals can vary when awake versus asleep, while the arousal level is closely related to rsfMRI signals at the sensorimotor region (Gu et al., 2019). Therefore, patients were told to maintain wakefulness during the scan to avoid the effect of falling asleep on brain activity in this study.
The scanning parameters were as follows: 3D-T1WI: repetition time (TR)/echo time (TE) = 1,900/2.26 ms; slice thickness = 1 mm; slices = 176; matrix size = 256 × 256; field of view (FOV) = 256 × 256 mm2. BOLD-fMRI: TR/TE = 2,000/30 ms; flip angle = 90°; slice number = 30; matrix size = 64 × 64; FOV = 240 × 240 mm2; slice thickness = 5 mm; total fMRI scans = 180, with the functional scan lasting 6 min.
Statistical Analysis
Clinical Data
Data analysis was performed using SPSS 22.0 statistic software package (IBM Corp, Somers, NY, United States). Independent-samples t-test, Mann–Whitney U-test, and chi-square tests were applied to compare the baseline demographic and clinical characteristics of FD patients. A paired t-test or Wilcoxon signed-rank test was applied to compare within-group differences, and analysis of covariance (ANCOVA) was applied for between-group analysis. Correlation analysis was performed using Pearson’s correlation analysis. A p-value < 0.05 was considered statistically significant.
Functional MRI Data
Data Preprocessing
The functional BOLD data were preprocessed using Data Processing Assistant for Resting-State fMRI (DPARSF) software1 in MATLAB (MathWorks, Inc., Natick, MA, United States). During the preprocessing phase, the first 10 timepoints were discarded to avoid instability in initial MRI signals, and slice-timing correction, head motion estimation, and realignment were performed. Then images were segmented and coregistered to each patients’ high-resolution T1 scan and normalized to the standard Montreal Neurological Institute (MNI) template. After that, images were smoothed with a Gaussian kernel of 6-mm3 full width at half maximum (FWHM) and band-pass filtered with a frequency window of 0.01–0.08 Hz. Patients with excessive head motion [with mean frame-wise displacement greater than 0.5 mm (Power et al., 2012)] were excluded from the analysis.
Amplitude of Low-Frequency Fluctuation and Functional Connectivity Analysis
After preprocessing, the amplitude of low-frequency fluctuation (ALFF) was first calculated using DPARSF. Paired t-test was used to assess the within-group differences of ALFF in all FD patients. Gaussian random field (GRF) correction was used, and voxel-level p < 0.005 and corrected cluster-level p < 0.05 were considered statistically significant. Next, correlation analysis of the ALFF change values and clinical improvement values of all FD patients was performed to obtain the brain areas associated with acupuncture efficacy. Finally, the regions that were most significantly correlated with clinical improvement value were selected as regions of interest (ROIs) for FC analysis. The paired t-test was performed to investigate the functional alterations of FD patients before and after acupuncture treatment in each group. GRF correction was made, and voxel-level p < 0.05 and corrected cluster-level p < 0.05 were considered statistically significant.
Results
Five participants were excluded due to excessive head motion (mean framewise displacement > 0.5 mm) during imaging. Thirty-three FD patients (18 in group A and 15 in group B) were included in the final clinical data and fMRI data analysis.
The Baseline Characteristics of the Two Groups
The baseline characteristics of patients in the two groups are displayed in Table 1. As shown in Table 1, except for age, there was no significant difference in baseline characteristics between these two groups (p > 0.05; Table 1).
TABLE 1
| Characteristic | Group A (n = 18) |
Group B (n = 15) |
Statistical value | p-Value |
| No. of women, n (%) | 12 (66.67%) | 13 (86.67%) | 1.782 | 0.182 |
| Age (years), mean ± SD | 22.78 ± 1.63 | 21.20 ± 2.40 | 2.243 | 0.032* |
| BMI, mean ± SD | 19.09 ± 1.48 | 19.70 ± 1.96 | –1.030 | 0.311 |
| Course of disease (M), mean ± SD | 42.83 ± 26.15 | 33.60 ± 19.18 | 1.136 | 0.265 |
| SID score, mean ± SD | 3.89 ± 1.41 | 3.80 ± 1.42 | –0.149 | 0.882 |
| NDLQI score, mean ± SD | 77.58 ± 8.41 | 74.60 ± 11.43 | 0.863 | 0.395 |
| NDSI score, mean ± SD | 45.67 ± 17.27 | 41.93 ± 14.07 | 0.671 | 0.507 |
| SAS score, mean ± SD | 40.32 ± 7.68 | 43.67 ± 9.77 | –1.104 | 0.278 |
| SDS score, mean ± SD | 41.90 ± 9.11 | 45.18 ± 10.29 | –0.971 | 0.339 |
The baseline characteristics in two groups.
BMI, body mass index; NDLQI, the Nepean Dyspepsia Life Quality Index; NDSI, the Nepean Dyspepsia Symptom Index; SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; SID, Symptom Index of Dyspepsia. *p < 0.05.
The Therapeutic Effects in the Two Groups
The within-group analyses showed that a significant increase in NDLQI scores and a significant decrease in SID scores, NDSI scores, SAS scores, and SDS scores were found in both group A and group B after acupuncture treatment (p < 0.05; Table 2).
TABLE 2
| Items | Group A |
Group B |
F-value | p | ||||||||
| Pre | Pos | Pos–Pre | Z-value | p | Pre | Pos | Pos–Pre | Z-value | p | |||
| SID score | 3.89 ± 1.41 | 1.94 ± 0.54 | −1.94 ± 1.47 | –3.573 | 0.000** | 3.80 ± 1.42 | 1.20 ± 1.08 | −2.60 ± 1.45 | –3.427 | 0.001* | 1.416 | 0.243 |
| (mean ± SD) | ||||||||||||
| NDLQI score | 77.58 ± 8.41 | 89.93 ± 5.81 | 12.35 ± 8.32 | –3.636 | 0.000** | 74.60 ± 11.43 | 88.88 ± 11.56 | 14.28 ± 8.47 | –3.408 | 0.001* | 1.224 | 0.277 |
| (mean ± SD) | ||||||||||||
| NDSI score | 45.67 ± 17.27 | 20.17 ± 10.14 | −25.50 ± 13.87 | –3.724 | 0.000** | 41.93 ± 14.07 | 14.00 ± 14.48 | −27.93 ± 14.92 | –3.352 | 0.001* | 0.988 | 0.328 |
| (mean ± SD) | ||||||||||||
| SAS score | 40.32 ± 7.68 | 34.72 ± 7.13 | −5.60 ± 9.86 | –2.329 | 0.020* | 43.67 ± 9.77 | 34.00 ± 8.77 | −9.67 ± 6.28 | –3.297 | 0.001* | 0.894 | 0.352 |
| (mean ± SD) | ||||||||||||
| SDS score | 41.90 ± 9.11 | 36.04 ± 8.43 | −5.86 ± 10.58 | –2.509 | 0.012* | 45.18 ± 10.29 | 34.67 ± 8.67 | −10.52 ± 9.13 | –3.109 | 0.002* | 0.349 | 0.559 |
| (mean ± SD) | ||||||||||||
Comparison of the therapeutic effects between group A and group B.
NDLQI, the Nepean Dyspepsia Life Quality Index; NDSI, the Nepean Dyspepsia Symptom Index; SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; SID, Symptom Index of Dyspepsia.
*p < 0.05; **p < 0.001.
The between-group analyses showed that there were no significant differences in the improvements of the SID scores, NDSI scores, NDLQI scores, SAS scores, and SDS scores between the two groups (p > 0.05; Table 2).
Cerebral Activity Changes Induced by Acupuncture Treatment in Both Groups
The Amplitude of Low-Frequency Fluctuation Changes in All Functional Dyspepsia Patients
After acupuncture treatment, significantly increased ALFF values in the left postcentral gyrus were found in all FD patients. Moreover, the ALFF value changes in the left postcentral gyrus were significantly related to the improvements of SID scores with age and gender as covariates (r = 0.358, p = 0.041, uncorrected) (Figure 2).
FIGURE 2
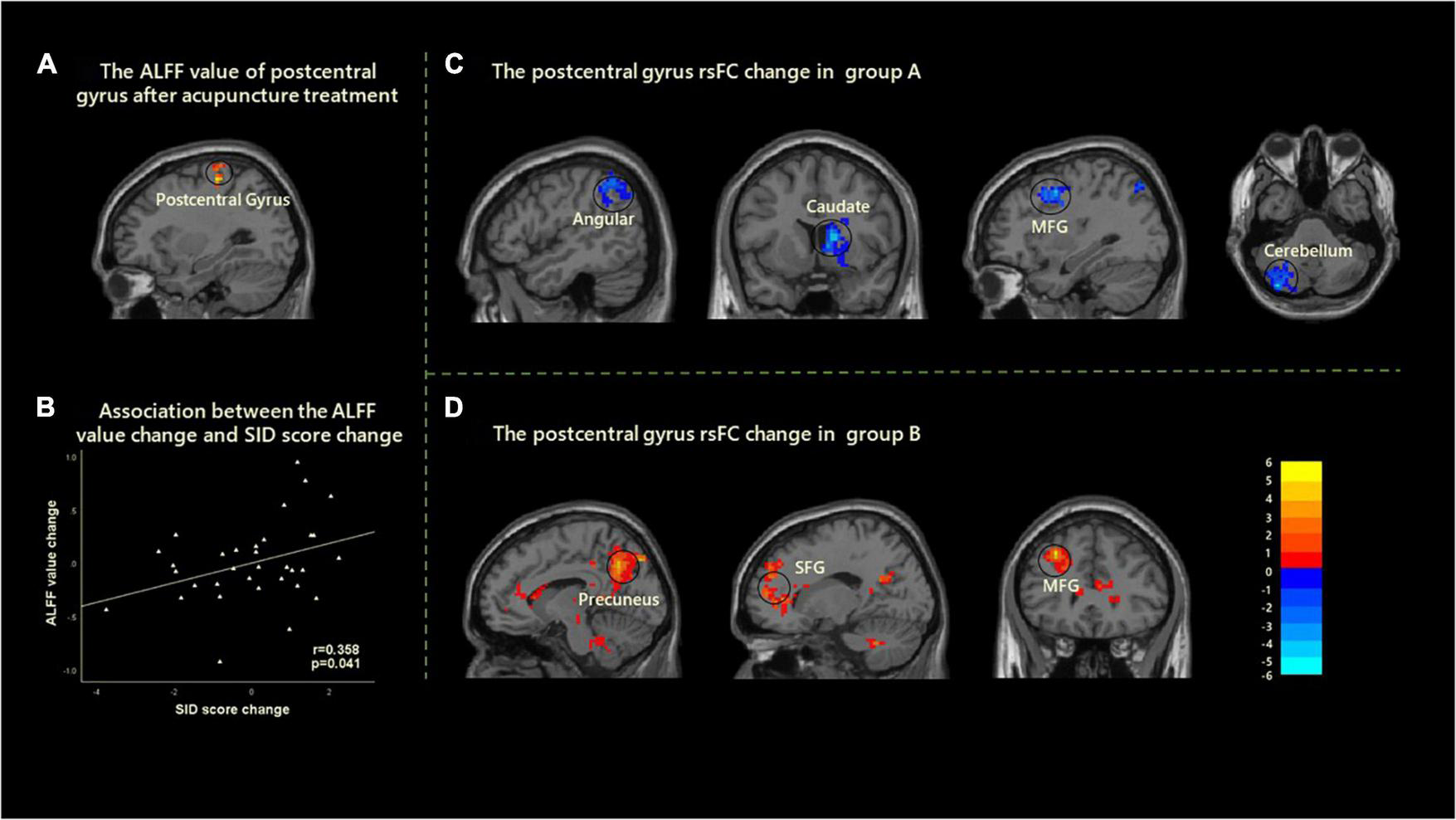
Cerebral activity changes induced by the acupuncture stimulation in two groups. (A) A significantly increased ALFF was found in the left postcentral gyrus after acupuncture treatment. (B) Correlation analysis showed that the change of ALFF value in left postcentral gyrus and the improvement of SID were positively correlated with age and gender as covariates (r = 0.358, p = 0.041). (C) After acupuncture treatment, the decreased rsFC between the left postcentral gyrus with angular, caudate, MFG, and cerebellum were found in group A. (D) After acupuncture treatment, the increased rsFC between the left postcentral gyrus with precuneus, SFG, and MFG was found in group B. ALFF, amplitude of low-frequency fluctuation; rsFC, resting-state functional connectivity; MFG, middle frontal gyrus; SID, Symptom Index of Dyspepsia; SFG, superior frontal gyrus.
The Resting-State Functional Connectivity Changes of Postcentral Gyrus in the Two Groups
The left postcentral gyrus was selected as the ROI to investigate the FC changes of FD patients in group A and group B. Within-group comparisons demonstrated that there were significantly decreased resting-state FC (rsFC) between the left postcentral gyrus and angular gyrus, caudate, middle frontal gyrus (MFG), and cerebellum after treatment in group A. In addition, there were significantly increased rsFC between the left postcentral gyrus and precuneus, superior frontal gyrus (SFG), and MFG after treatment in group B (Figure 2 and Table 3). However, there was no significant correlation between FC and clinical efficacy in group A and group B.
TABLE 3
| ROI | Group | Cluster regions | L/R | Cluster sizes | Peak MNI |
T | ||
| x | y | z | ||||||
| L_Postcentral Gyrus | Group A | Angular | R | 280 | 49 | −52 | 49 | −3.74 |
| Caudate | R | 274 | 15 | 9 | 12 | −4.86 | ||
| Middle frontal gyrus | R | 245 | 33 | 12 | 48 | −4.42 | ||
| Cerebellum | L | 338 | −36 | −81 | −39 | −5.51 | ||
| Group B | Precuneus | L | 791 | −10 | −61 | 42 | 6.70 | |
| Superior frontal gyrus | R | 854 | 17 | 48 | 35 | 5.00 | ||
| Middle frontal gyrus | R | 307 | −30 | 30 | 42 | 6.37 | ||
The differences of the left postcentral gyrus rsFC in each group.
rsFC, resting-state functional connectivity; MNI, Montreal Neurological Institute; ROI, region of interest.
Discussion
The current study focused on the potential central mechanism of different acupoints sharing similar therapeutic effects for FD. The results demonstrated that needling at both CV12 and ST36 could elicit activity changes in the postcentral gyrus response, although their response patterns were relatively different. Needling at ST36 mainly affected the postcentral gyrus–default mode network (DMN) circuitry, while needling at CV12 mainly aroused the postcentral gyrus–reward network (RN) circuitry.
The Similarities in Cerebral Responses Elicited by Acupuncture in Both Group A and Group B
In this study, the clinical observations indicated that needling at either CV12 or ST36 could significantly decrease SID scores, NDSI scores, SAS scores, and SDS scores and could increase NDLQI scores and that there were no significant differences in the improvements of variables mentioned above. The results indicated that both CV12 and ST36 were effective for improving symptoms, QoL, and emotional status of FD patients and that the therapeutic effects of both acupoints were similar. The current results were consistent with previous studies, which showed the effectiveness of both acupoints in the treatment of FD (Ma et al., 2012; Sun et al., 2021a). In addition, previous studies found that acupuncture treatment provided significant relief to gastrointestinal symptoms in comparison with FD patients awaiting acupuncture treatment (Chung et al., 2019). Therefore, performing acupuncture at CV12 and ST36 is a valuable treatment option for FD.
In addition to similar therapeutic effects, significantly increased ALFF values in the left postcentral gyrus were found in all FD patients (combined group A and group B) after acupuncture treatment. The postcentral gyrus of the parietal lobe corresponds to the primary sensory cortex, receiving various sensations from the body (Yu et al., 2019; DiGuiseppi and Tadi, 2021). Multiple neuroimaging studies had previously confirmed the involvement of the postcentral gyrus in the central pathology of FD patients. For instance, a H2 (15) O-PET study found that FD patients demonstrated altered activity in the primary sensory cortex, including the postcentral gyrus, and that this altered activity corresponded to decreases in gastric distention (Van Oudenhove et al., 2010). Previous studies also found higher cerebral glucose metabolism in the postcentral gyrus in FD patients through 18 F-FDG PET-CT imaging (Zeng et al., 2011) and increased ALFF values in the postcentral gyrus through fMRI (Qi et al., 2020). These results indicated the role of the postcentral gyrus in the abnormal processing of gastrointestinal sensory signals. Furthermore, the participation of the postcentral gyrus in the integration of the regulatory effect of acupuncture on the gastrointestinal tract had been identified by several neuroimaging studies (Zeng et al., 2009; Zhou et al., 2013). Our previous studies indicated that acupuncture stimulation could decrease abnormally elevated glucose metabolism in the postcentral gyrus (Liu et al., 2012), and needling at ST36 could normalize the fMRI signals in the postcentral gyrus (Li et al., 2014). However, acupuncture does not always elicit brain responses in the postcentral gyrus, although brain responses have been observed in various acupuncture-related neuroimaging studies (Yan et al., 2005; Yang et al., 2014; Yeo et al., 2016; Zheng et al., 2018). This study indicated significant increases of ALFF values in the postcentral gyrus in all FD patients after acupuncture was performed, with increased ALFF values positively correlating with improvements in SID scores. The results indicated that the activity changes in the postcentral gyrus were related to acupuncture efficacy for FD.
The Differences in Postcentral Gyrus Responses Caused by Needling at ST36 Versus CV12
This study aimed to investigate similar effects shared by different acupoints by differentiating response modes of two acupoints on the postcentral gyrus through rsFC analysis, as all patients exhibited changes in the functional activity of the postcentral gyrus after acupuncture treatment, with changes significantly positively correlating with the curative effect of acupuncture.
The results showed that needling at ST36 resulted in significantly increased rsFC between the left postcentral gyrus with precuneus, SFG, and MFG. It is well known that the precuneus is an important hub within the DMN (Brewer et al., 2011; Utevsky et al., 2014), which regulates the affective and sensory process together with the medial prefrontal cortex (mPFC) and amygdala (Vanner et al., 2016). The precuneus is involved in episodic memory retrieval (Cavanna and Trimble, 2006; Sajonz et al., 2010), appetite control (Scharmuller et al., 2012; Tuulari et al., 2015), appraisal of food (Winter et al., 2017), and reappraisal of the benefits of eating food (Yokum and Stice, 2013). A variety of studies have identified the structural and functional abnormalities of the precuneus in FD patients (Zeng et al., 2011; Liu et al., 2013). For example, Lee et al. (2018) observed that higher rsFC between the insula and precuneus was negatively correlated with FD symptoms, food craving, and depression while in a state of hunger. Our previous study also showed that DMN in FD patients may indeed undergo dysfunctional changes, with changes in DMN found to be related to FD symptom severity (Liu et al., 2013). More importantly, a number of studies have reported that acupuncture can reverse the disrupted DMN to achieve therapeutic effects in gastrointestinal disease (Bao et al., 2017; Sun et al., 2021a,b; Zhao et al., 2021). In these studies, needling at ST36 regulated the rsFC between the left postcentral gyrus with DMN. The results indicated that promoting self-regulation and adaptation via the DMN might be one of the functions achieved by needling ST36 for FD.
In contrast to ST36, CV12 elicited decreased rsFC values between the left postcentral gyrus and angular gyrus, caudate, MFG, and cerebellum in this study. The results indicated that needling at CV12 mainly regulated postcentral gyrus–RN circuitry in order to achieve treatment efficacy. The caudate nucleus and cerebellum, important components of the RN, play a crucial role in viscera activities (Allen et al., 1997; Ladabaum et al., 2001). Ladabaum et al. (2001) found significant activations of the bilateral caudate nucleus during proximal gastric dilation stimulation. Our previous results indicated increased gray matter volume in the right caudate (Liu et al., 2014), decreased gray matter density in the cerebellum (Zeng et al., 2013), and higher glycometabolism in the cerebellum (Zeng et al., 2011) in FD patients compared with healthy controls. In addition, higher glycometabolism in the cerebellum was decreased after acupuncture treatment (Zeng et al., 2015). These studies confirmed the structural and functional abnormalities in the caudate nucleus and cerebellum in FD patients, as well as the favorable regulatory effect of acupuncture on cerebellum activities. In fact, some researchers have found that the expectations of acupuncture efficacy may partly be based on the self-relevant phenomenon and self-referential introspection, which was often found to be related to activation of patient self-appraisal and RN (Pariente et al., 2005; Lundeberg et al., 2007).
In this study, performing acupuncture at ST36 and CV12 can affect the function of the postcentral gyrus, although the modes of influence are different. In addition, some studies focusing on peripheral nerve activity had found that the mechanisms of acupuncture at ST36 and CV12 to regulate gastrointestinal function were different. For example, one study had found that needling of the lower limb (ST36) caused gastrointestinal muscle contractions via the somatoparasympathetic pathway, while needling in the upper abdominal area (CV12) caused gastrointestinal muscle relaxation via the somatosympathetic pathway (Takahashi, 2006). Another study had indicated that electroacupuncture stimulation at the lower limb (ST36) but not in the abdomen (ST25) can promote the vagal–adrenal anti-inflammatory axis in mice (Liu et al., 2021). In summary, although acupoints in the different regions have similar therapeutic effects, the underlying mechanisms from central to peripheral were found to be relatively different.
Limitations
There were some limitations in this study. Firstly, the sample size was relatively small. Secondly, we observed the changes in clinical symptoms and functional activities of FD patients at baseline and the end of 4 weeks of acupuncture treatment, but its long-term efficacy remains unclear due to a lack of follow-up studies. In the future, further studies are needed to assess the long-term efficacy and investigate the potential mechanism of “different acupoints exhibiting similar effects.”
Conclusion
In conclusion, acupuncture at ST36 and CV12 had similar therapeutic efficacy in the treatment of FD, and the realization of therapeutic effect may be related to the modulation of the activity of the postcentral gyrus. Meanwhile, the modulatory pattern was relatively different. Namely, ST36 mainly affected the rsFC between the postcentral gyrus and DMN, while the CV12 mainly affected the rsFC between the postcentral gyrus and RN.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Affiliated Hospital of CDUTCM (approved number. 2014KL-028). The patients/participants provided their written informed consent to participate in this study.
Author contributions
FZ and QG: experimental design. ZH, PM, YQ, SYu, XL, TZ, LH, and JL: data collection. XD and TY: data analysis. XD: manuscript preparation. YC and SYi: manuscript revision. FZ: supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of Outstanding Youth Fund in China (No. 81622052), the Ten Thousand Talent Program (W02020595), and the Youth Science and Technology Innovative Team of Sichuan Province (2019JDTD0011).
Acknowledgments
We were grateful to Peiming Feng, Tingting Ma, and Jie Wu for the patient recruitment and Xueling Suo and Du Lei for MRI data acquisition and interpretation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
1
Allen G. Buxton R. B. Wong E. C. Courchesne E. (1997). Attentional activation of the cerebellum independent of motor involvement.Science2751940–1943. 10.1126/science.275.5308.1940
2
Bao C. Wang D. Liu P. Shi Y. Jin X. Wu L. et al (2017). Effect of electro-acupuncture and moxibustion on brain connectivity in patients with Crohn’s disease: a resting-state fMRI Study.Front. Hum. Neurosci.11:559. 10.3389/fnhum.2017.00559
3
Brewer J. A. Worhunsky P. D. Gray J. R. Tang Y. Y. Weber J. Kober H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity.Proc. Natl. Acad. Sci. U.S.A.10820254–20259. 10.1073/pnas.1112029108
4
Cao F. L. T. Ha L. J. Shan C. X. Zhi M. J. Wang F. C. (2016a). Analysis of compatibility laws for acupoint selection of acupuncture in treating diabetic gastroparasis.Chin. J. Integr. Trad. West Med.36549–552.
5
Cao F. L. T. Shan C. X. Ha L. J. Li Y. Q. Wang F. C. (2016b). Study of the acupoint selection rules to treat diabetic gastroparesis with acupuncture based on the literature analysis.Chin. J. Basic Med. Trad. Chin. Med.22110–214.
6
Cavanna A. E. Trimble M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates.Brain129(Pt 3)564–583. 10.1093/brain/awl004
7
Chen Y. Zhao Y. Tan R. Y. Zhang P. Y. Long T. Shi Y. et al (2021). The influence of stomach Back-Shu and Front-Mu points on insular functional connectivity in functional dyspepsia rat models.Evid. Based Complement. Alternat. Med.2021:2771094. 10.1155/2021/2771094
8
Chung V. C. Wong C. H. Wu I. X. Ching J. Y. Cheung W. K. Yip B. H. et al (2019). Electroacupuncture plus on-demand gastrocaine for refractory functional dyspepsia: pragmatic randomized trial.J. Gastroenterol. Hepatol.342077–2085. 10.1111/jgh.14737
9
DiGuiseppi J. Tadi P. (2021). Neuroanatomy, Postcentral Gyrus.Treasure Island, FL: StatPearls.
10
Drossman D. A. (2016). Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV.Gastroenterology1501262–1279. 10.1053/j.gastro.2016.02.032
11
Ghoshal U. C. Singh R. Chang F. Y. Hou X. Wong B. C. Kachintorn U. et al (2011). Epidemiology of uninvestigated and functional dyspepsia in Asia: facts and fiction.J. Neurogastroenterol. Motil.17235–244. 10.5056/jnm.2011.17.3.235
12
Gu Y. Han F. Liu X. (2019). Arousal contributions to resting-state fMRI connectivity and dynamics.Front. Neurosci.13:1190. 10.3389/fnins.2019.01190
13
Ladabaum U. Minoshima S. Hasler W. L. Cross D. Chey W. D. Owyang C. (2001). Gastric distention correlates with activation of multiple cortical and subcortical regions.Gastroenterology120369–376. 10.1053/gast.2001.21201
14
Lee I. S. Kullmann S. Scheffler K. Preissl H. Enck P. (2018). Fat label compared with fat content: gastrointestinal symptoms and brain activity in functional dyspepsia patients and healthy controls.Am. J. Clin. Nutr.108127–135. 10.1093/ajcn/nqy077
15
Lee I. S. Wang H. Chae Y. Preissl H. Enck P. (2016). Functional neuroimaging studies in functional dyspepsia patients: a systematic review.Neurogastroenterol. Motil.28793–805. 10.1111/nmo.12793
16
Li Z. Zeng F. Yang Y. Chen Y. Zhang D. Sun J. et al (2014). Different cerebral responses to puncturing at ST36 among patients with functional dyspepsia and healthy subjects.Forsch. Komplementmed.2199–104. 10.1159/000360804
17
Liu M. L. Liang F. R. Zeng F. Tang Y. Lan L. Song W. Z. (2012). Cortical-limbic regions modulate depression and anxiety factors in functional dyspepsia: a PET-CT study.Ann. Nucl. Med.2635–40. 10.1007/s12149-011-0537-4
18
Liu P. Zeng F. Yang F. Wang J. Liu X. Wang Q. et al (2014). Altered structural covariance of the striatum in functional dyspepsia patients.Neurogastroenterol. Motil.261144–1154. 10.1111/nmo.12372
19
Liu P. Zeng F. Zhou G. Wang J. Wen H. von Deneen K. M. et al (2013). Alterations of the default mode network in functional dyspepsia patients: a resting-state fmri study.Neurogastroenterol. Motil.25e382–e388. 10.1111/nmo.12131
20
Liu S. Wang Z. Su Y. Qi L. Yang W. Fu M. et al (2021). A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis.Nature598641–645. 10.1038/s41586-021-04001-4
21
Lundeberg T. Lund I. Naslund J. (2007). Acupuncture–self-appraisal and the reward system.Acupunct. Med.2587–99. 10.1136/aim.25.3.87
22
Ma T. T. Yu S. Y. Li Y. Liang F. R. Tian X. P. Zheng H. et al (2012). Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia.Aliment. Pharmacol. Ther.35552–561. 10.1111/j.1365-2036.2011.04979.x
23
Mahadeva S. Ford A. C. (2016). Clinical and epidemiological differences in functional dyspepsia between the East and the West.Neurogastroenterol. Motil.28167–174. 10.1111/nmo.12657
24
Nan J. Liu J. Zhang D. Yang Y. Yan X. Yin Q. et al (2014). Altered intrinsic regional activity and corresponding brain pathways reflect the symptom severity of functional dyspepsia.Neurogastroenterol. Motil.26660–669. 10.1111/nmo.12311
25
Pariente J. White P. Frackowiak R. S. Lewith G. (2005). Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture.Neuroimage251161–1167. 10.1016/j.neuroimage.2005.01.016
26
Power J. D. Barnes K. A. Snyder A. Z. Schlaggar B. L. Petersen S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion.Neuroimage592142–2154. 10.1016/j.neuroimage.2011.10.018
27
Qi R. Shi Z. Weng Y. Yang Y. Zhou Y. Surento W. et al (2020). Similarity and diversity of spontaneous brain activity in functional dyspepsia subtypes.Acta Radiol.61927–935. 10.1177/0284185119883391
28
Sajonz B. Kahnt T. Margulies D. S. Park S. Q. Wittmann A. Stoy M. et al (2010). Delineating self-referential processing from episodic memory retrieval: common and dissociable networks.Neuroimage501606–1617. 10.1016/j.neuroimage.2010.01.087
29
Scharmuller W. Ubel S. Ebner F. Schienle A. (2012). Appetite regulation during food cue exposure: a comparison of normal-weight and obese women.Neurosci. Lett.518106–110. 10.1016/j.neulet.2012.04.063
30
Sun R. He Z. Ma P. Yin S. Yin T. Liu X. et al (2021a). The participation of basolateral amygdala in the efficacy of acupuncture with deqi treating for functional dyspepsia.Brain Imaging Behav.15216–230. 10.1007/s11682-019-00249-7
31
Sun R. Ma P. He Z. Yin T. Qu Z. Yin S. et al (2021b). Changed ACC-DMN functional connectivity after acupunture with deqi for functional dyspepsia treatment.World J. Acupunct. Moxibustion316–15.
32
Tack J. Camilleri M. (2018). New developments in the treatment of gastroparesis and functional dyspepsia.Curr. Opin. Pharmacol.43111–117. 10.1016/j.coph.2018.08.015
33
Tack J. Talley N. J. (2013). Functional dyspepsia–symptoms, definitions and validity of the Rome III criteria.Nat. Rev. Gastroenterol. Hepatol.10134–141. 10.1038/nrgastro.2013.14
34
Tack J. Masuy I. Van Den Houte K. Wauters L. Schol J. Vanuytsel T. et al (2019). Drugs under development for the treatment of functional dyspepsia and related disorders.Expert Opin. Investig. Drugs28871–889. 10.1080/13543784.2019.1673365
35
Takahashi T. (2006). Acupuncture for functional gastrointestinal disorders.J. Gastroenterol.41408–417. 10.1007/s00535-006-1773-6
36
Talley N. J. Haque M. Wyeth J. W. Stace N. H. Tytgat G. N. Stanghellini V. et al (1999). Development of a new dyspepsia impact scale: the Nepean Dyspepsia index.Aliment. Pharmacol. Ther.13225–235. 10.1046/j.1365-2036.1999.00445.x
37
Talley N. J. Locke G. R. III Lahr B. D. Zinsmeister A. R. Tougas G. Ligozio G. et al (2006). Functional dyspepsia, delayed gastric emptying, and impaired quality of life.Gut55933–939. 10.1136/gut.2005.078634
38
Tuulari J. J. Karlsson H. K. Hirvonen J. Salminen P. Nuutila P. Nummenmaa L. (2015). Neural circuits for cognitive appetite control in healthy and obese individuals: an fMRI study.PLoS One10:e0116640. 10.1371/journal.pone.0116640
39
Utevsky A. V. Smith D. V. Huettel S. A. (2014). Precuneus is a functional core of the default-mode network.J. Neurosci.34932–940. 10.1523/JNEUROSCI.4227-13.2014
40
Van Oudenhove L. Vandenberghe J. Dupont P. Geeraerts B. Vos R. Dirix S. et al (2010). Abnormal regional brain activity during rest and (anticipated) gastric distension in functional dyspepsia and the role of anxiety: a H(2)(15)O-PET study.Am. J. Gastroenterol.105913–924. 10.1038/ajg.2010.39
41
Vanner S. Greenwood-Van Meerveld B. Mawe G. Shea-Donohue T. Verdu E. F. Wood J. et al (2016). Fundamentals of neurogastroenterology: basic science.Gastroenterology1501280–1291. 10.1053/j.gastro.2016.02.01
42
Wang X. Y. Wang H. Guan Y. Y. Cai R. L. Shen G. M. (2021). Acupuncture for functional gastrointestinal disorders: a systematic review and meta-analysis.J. Gastroenterol. Hepatol.363015–3026. 10.1111/jgh.15645
43
Winter S. R. Yokum S. Stice E. Osipowicz K. Lowe M. R. (2017). Elevated reward response to receipt of palatable food predicts future weight variability in healthy-weight adolescents.Am. J. Clin. Nutr.105781–789. 10.3945/ajcn.116.141143
44
Xu S. Hou X. Zha H. Gao Z. Zhang Y. Chen J. D. (2006). Electroacupuncture accelerates solid gastric emptying and improves dyspeptic symptoms in patients with functional dyspepsia.Dig. Dis. Sci.512154–2159. 10.1007/s10620-006-9412-x
45
Yamawaki H. Futagami S. Wakabayashi M. Sakasegawa N. Agawa S. Higuchi K. et al (2018). Management of functional dyspepsia: state of the art and emerging therapies.Ther. Adv. Chronic. Dis.923–32. 10.1177/2040622317725479
46
Yan B. Li K. Xu J. Wang W. Li K. Liu H. et al (2005). Acupoint-specific fMRI patterns in human brain.Neurosci. Lett.383236–240. 10.1016/j.neulet.2005.04.021
47
Yang J. W. Wang L. Q. Zou X. Yan S. Y. Wang Y. Zhao J. J. et al (2020). Effect of acupuncture for postprandial distress syndrome: a randomized clinical trial.Ann. Intern. Med.172777–785. 10.7326/M19-2880
48
Yang M. Yang J. Zeng F. Liu P. Lai Z. Deng S. et al (2014). Electroacupuncture stimulation at sub-specific acupoint and non-acupoint induced distinct brain glucose metabolism change in migraineurs: a PET-CT study.J. Transl. Med.12:351. 10.1186/s12967-014-0351-6
49
Yeo S. van den Noort M. Bosch P. Lim S. (2016). Ipsilateral putamen and insula activation by both left and right GB34 acupuncture stimulation: an fMRI study on healthy participants.Evid. Based Complement. Alternat. Med.2016:4173185. 10.1155/2016/4173185
50
Yin S. Chen Y. Lei D. Sun R. R. Ma T. T. Feng P. M. et al (2017). Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial.Neural Regen. Res.12831–840. 10.4103/1673-5374.206655
51
Yokum S. Stice E. (2013). Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods.Int. J. Obes. (Lond.)371565–1570. 10.1038/ijo.2013.39
52
Yu Y. Chen L. Wang Q. Hu L. Ding Q. Jia X. et al (2019). Altered amplitude of low-frequency fluctuations in inactive patients with nonneuropsychiatric systemic lupus erythematosus.Neural Plast.2019:9408612. 10.1155/2019/9408612
53
Zeng F. Lan L. Tang Y. Liu M. Liu X. Song W. et al (2015). Cerebral responses to puncturing at different acupoints for treating meal-related functional dyspepsia.Neurogastroenterol. Motil.27559–568. 10.1111/nmo.12532
54
Zeng F. Qin W. Liang F. Liu J. Tang Y. Liu X. et al (2011). Abnormal resting brain activity in patients with functional dyspepsia is related to symptom severity.Gastroenterology141499–506. 10.1053/j.gastro.2011.05.003
55
Zeng F. Qin W. Ma T. Sun J. Tang Y. Yuan K. et al (2012). Influence of acupuncture treatment on cerebral activity in functional dyspepsia patients and its relationship with efficacy.Am. J. Gastroenterol.1071236–1247. 10.1038/ajg.2012.53
56
Zeng F. Qin W. Yang Y. Zhang D. Liu J. Zhou G. et al (2013). Regional brain structural abnormality in meal-related functional dyspepsia patients: a voxel-based morphometry study.PLoS One8:e68383. 10.1371/journal.pone.0068383
57
Zeng F. Song W. Z. Liu X. G. Xie H. J. Tang Y. Shan B. C. et al (2009). Brain areas involved in acupuncture treatment on functional dyspepsia patients: a PET-CT study.Neurosci. Lett.4566–10. 10.1016/j.neulet.2009.03.080
58
Zhao T. Pei L. Ning H. Guo J. Song Y. Zhou J. et al (2021). Networks are associated with acupuncture treatment in patients with diarrhea-predominant irritable bowel syndrome: a resting-state imaging study.Front. Hum. Neurosci.15:736512. 10.3389/fnhum.2021.736512
59
Zheng W. Su Z. Liu X. Zhang H. Han Y. Song H. et al (2018). Modulation of functional activity and connectivity by acupuncture in patients with Alzheimer disease as measured by resting-state fMRI.PLoS One13:e0196933. 10.1371/journal.pone.0196933
60
Zhou S. Zeng F. Liu J. Zheng H. Huang W. Liu T. et al (2013). Influence of acupuncture stimulation on cerebral network in functional diarrhea.Evid. Based Complement. Alternat. Med.2013:975769. 10.1155/2013/975769
61
Zung W. W. (1971). A rating instrument for anxiety disorders.Psychosomatics12371–379. 10.1016/S0033-3182(71)71479-0
62
Zung W. W. Richards C. B. Short M. J. (1965). Self-rating depression scale in an outpatient clinic. Further validation of the SDS.Arch. Gen. Psychiatry13508–515. 10.1001/archpsyc.1965.01730060026004
Summary
Keywords
acupuncture, functional dyspepsia, default mode network, reward network, functional magnetic resonance imaging
Citation
Dong X, Yin T, Yu S, He Z, Chen Y, Ma P, Qu Y, Yin S, Liu X, Zhang T, Huang L, Lu J, Gong Q and Zeng F (2022) Neural Responses of Acupuncture for Treating Functional Dyspepsia: An fMRI Study. Front. Neurosci. 16:819310. doi: 10.3389/fnins.2022.819310
Received
21 November 2021
Accepted
15 February 2022
Published
02 May 2022
Volume
16 - 2022
Edited by
Chunhui Bao, Shanghai University of Traditional Chinese Medicine, China
Reviewed by
Jiliang Fang, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, China; Yang Hu, Xidian University, China; Feng Han, The Pennsylvania State University (PSU), United States
Updates
Copyright
© 2022 Dong, Yin, Yu, He, Chen, Ma, Qu, Yin, Liu, Zhang, Huang, Lu, Gong and Zeng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zeng, zengfang@cdutcm.edu.cn
†These authors have contributed equally to this work
This article was submitted to Gut-Brain Axis, a section of the journal Frontiers in Neuroscience
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.