- 1King Abdullah International Medical Research Center, Jeddah, Saudi Arabia
- 2College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
- 3Department of Pharmaceutics, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 4College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
- 5Department of Basic Sciences, College of Science and Health Professions (KSAU-HS), King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
- 6Department of Medicine, College of Medicine, Al-Baha University, Al-Baha, Saudi Arabia
- 7College of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
- 8Department of Medicine, Ministry of the National Guard-Health Affairs, Jeddah, Saudi Arabia
- 9Department of Clinical Pharmacy, College of Pharmacy, Taif University, Taif, Saudi Arabia
- 10Department of Pharmaceutical Sciences, Jerry. H. Hodge School of Pharmacy, Texas Tech University Health Sciences Center, Amarillo, TX, United States
- 11Center for Blood Brain Barrier Research, Jerry. H. Hodge School of Pharmacy, Texas Tech University Health Sciences Center, Amarillo, TX, United States
- 12Department of Pharmaceutical Care, King Abdulaziz Medical City, Riyadh, Saudi Arabia
- 13College of Pharmacy, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 14King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
- 15Saudi Critical Care Pharmacy Research (SCAPE) Platform, Riyadh, Saudi Arabia
- 16Department of Pharmacy Practice, King Abdulaziz University, Jeddah, Saudi Arabia
- 17King Salman Center for Disability Research, Riyadh, Saudi Arabia
Background: Stroke is a leading cause of mortality and disability and one of the most common neurological conditions globally. Many studies focused on vitamin D as a stroke risk factor, but only a few focused on its serum level as a predictor of stroke initial clinical severity and recovery with inconsistent results. The purpose of this study was to assess the relationship between serum vitamin D levels and stroke clinical severity at admission and functional independence and disability at discharge in Saudi Arabia.
Methodology: A retrospective cohort study of adult ischemic stroke patients who had their vitamin D tested and admitted within 7 days of exhibiting stroke symptoms at King Abdulaziz Medical City (KAMC) Jeddah, Saudi Arabia. Based on vitamin D level, the patients were categorized into normal [25(OH)D serum level ≥ 75 nmol/L], insufficient [25(OH)D serum level is 50–75 nmol/L], and deficient [25(OH)D serum level ≤ 50 nmol/L]. The primary outcome was to assess the vitamin D serum level of ischemic stroke patients’ clinical severity at admission and functional independence at discharge. The National Institute of Health Stroke Scale (NIHSS) was used to assess the clinical severity, whereas the modified Rankin scale (mRS) was used to assess functional independence and disability.
Results: The study included 294 stroke patients, out of 774, who were selected based on the inclusion and exclusion criteria. The mean age of the participants was 68.2 ± 13.4 years, and 49.3% were male. The patients’ distribution among the three groups based on their vitamin D levels is: normal (n = 35, 11.9%), insufficient (n = 66, 22.5%), and deficient (n = 196, 65.6%). After adjusting for potential covariates, regression analysis found a significant inverse relationship of NIHSS based on 25(OH)D serum level (beta coefficient: −0.04, SE: 0.01, p = 0.003). Patients with deficient serum vitamin D level also had significantly higher odds of worse functional independence in mRS score [OR: 2.41, 95%CI: (1.13–5.16), p = 0.023] when compared to participants with normal vitamin D level.
Conclusion: Low vitamin D levels were associated with higher severity of stroke at admission and poor functional independence and disability at discharge in patients with acute ischemic stroke. Further randomized clinical and interventional studies are required to confirm our findings.
Introduction
Stroke continues to be the world’s leading cause of long-term impairment of physical, cognitive, psychosocial, and other functions (Al Shoyaib et al., 2021a; Alamri et al., 2021). Nearly 85% of stroke survivors suffer paresis after stroke, and 50% of them continue living with motor impairments through the rest of their lives (Syeara et al., 2020). Several risk factors were found to be linked to stroke, including unmodifiable risk factors such as genetics, gender, and age, and modifiable risk factors such as hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, cigarette smoking, and sedentary lifestyle (Kuriakose and Xiao, 2020; Sayed et al., 2022). Given the limited therapeutic time window for improving stroke outcomes, identifying novel risk variables and severity predictors in ischemic stroke patients can aid in identification of patients that are at higher or lower risk of poor outcomes and allow stratification of patients for more personalized care and therapy (Kwakkel et al., 1996; Vijayan et al., 2019; Al Shoyaib et al., 2021b). Therefore, the discovery and validation of new predictive prognostic biomarkers is an active area of research, and some blood biomarkers and circulating molecules such as uric acid, interleukin 6, and vitamin D have already been introduced as predictors of stroke outcomes (Tu et al., 2013; Huang et al., 2016; Ng et al., 2017; Kamtchum-Tatuene and Jickling, 2019).
The physiological roles of vitamin D include calcium absorption from the gut, bone mineralization and growth, muscle function, and modulation of immunity (Lips, 2006; Bikle, 2009). In addition, recent studies indicate the role of vitamin D in cardiovascular disease prevention (Pilz et al., 2008). Low levels of 25-hydroxyvitamin D [25(OH)D, a vitamin D biomarker], have been linked to an elevated risk of coronary artery disease, ischemic heart disease, heart failure (HF), and stroke (Saponaro et al., 2019). Low vitamin D levels have also been linked to hypertension, dyslipidemia, and diabetes, all of which have an impact on cardiovascular and cerebrovascular events (Forman et al., 2007; Song et al., 2013; Lupton et al., 2016). Based on these studies, vitamin D deficiency is deemed to be a public health concern, especially for stroke patients.
In addition, several studies have investigated the role of vitamin D in stroke pathophysiology (Takeda et al., 2010; Wong et al., 2010; Balden et al., 2012). Vitamin D deficiency has been linked to increased post-stroke inflammatory activity by dysregulating the inflammatory response (Takeda et al., 2010; Wong et al., 2010; Balden et al., 2012). Vitamin D deficiency was associated with increased levels of interleukin-6 and high-sensitivity C-reactive protein in acute stroke patients (Wang et al., 2018). Also, vitamin D was shown to induce the expression of insulin-like growth factor 1 (IGF-1), a neuroprotective hormone that prevents axon and dendritic degeneration, and has antithrombotic properties (Turetsky et al., 2015; Yalbuzdag et al., 2015; Huang et al., 2016). Furthermore, vitamin D may also improve post-ischemic stroke vasodilation and neuronal survival by stimulating nitric oxide synthase (Turetsky et al., 2015; Yalbuzdag et al., 2015). These studies indicate a possible interaction between the level of vitamin D and stroke pathophysiology.
Previous experimental and observational studies have suggested that low vitamin D levels are independently associated with larger infarct volumes (Balden et al., 2012; Wang et al., 2014; Turetsky et al., 2015; Huang et al., 2016; Nie et al., 2017). Low levels of vitamin D have been identified in several epidemiological studies as a predictor of increased risk of stroke (Poole et al., 2006; Pilz et al., 2008; Chowdhury et al., 2012; Shi et al., 2020). Furthermore, decreased serum 25(OH)D levels in ischemic stroke patients were shown to independently predict stroke recurrence and mortality at 24 months (Qiu et al., 2017).
To date, limited studies have been done to investigate the impact of vitamin D level on stroke clinical severity and functional recovery with conflicting results (Bolland et al., 2010; Kuhn et al., 2013; Gupta et al., 2014; Wang et al., 2014; Park et al., 2015; Turetsky et al., 2015). Moreover, given the high prevalence (60%) of the Saudi Arabian population with vitamin D deficiency according to a recent meta-analysis (Al-Alyani et al., 2018). This study was conducted to assess the correlation between serum 25(OH)D levels and the clinical severity of ischemic stroke at admission and functional recovery at discharge in a cohort of patients in Saudi Arabia.
Materials and methods
Study design
A retrospective cohort study was conducted between June 2016 and July 2021 at King Abdulaziz Medical City (KAMC), Jeddah, Saudi Arabia. Admitted patients with acute or subacute ischemic stroke were included to assess the influence of vitamin D serum level on stroke clinical severity at admission and functional independence at discharge. Vitamin D was assessed by measuring the 25(OH)D total using chemiluminescence immunoassay (Abbott diagnostic analyzer: Architect i2000). The ischemic stroke clinical diagnosis was confirmed by brain Computed tomography (CT) and Magnetic resonance imaging (MRI) or both. The study was approved by King Abdullah International Medical Research Center (KAIMRC) Institutional Review Board in Riyadh-Saudi Arabia (Ref: RSS21J/003/06). Due to the retrospective observational nature of this study, the informed consent from the participants was waived.
Study participants
Patients were screened for eligibility using the electronic medical records system (BESTCare system 2.0) at the Ministry of National Guard-Health Affairs (MNGHA), Jeddah, Saudi Arabia. Patients with a confirmed clinical diagnosis of ischemic stroke within 7 days of symptoms onset were eligible for inclusion. Patients were excluded if aged less than 18 years old, or if vitamin D level measurements were unavailable within 1 year of admission secondary to ischemic stroke. All patients were followed until they were discharged from the hospital or died during the in-hospital stay, whichever occurred first (Figure 1).
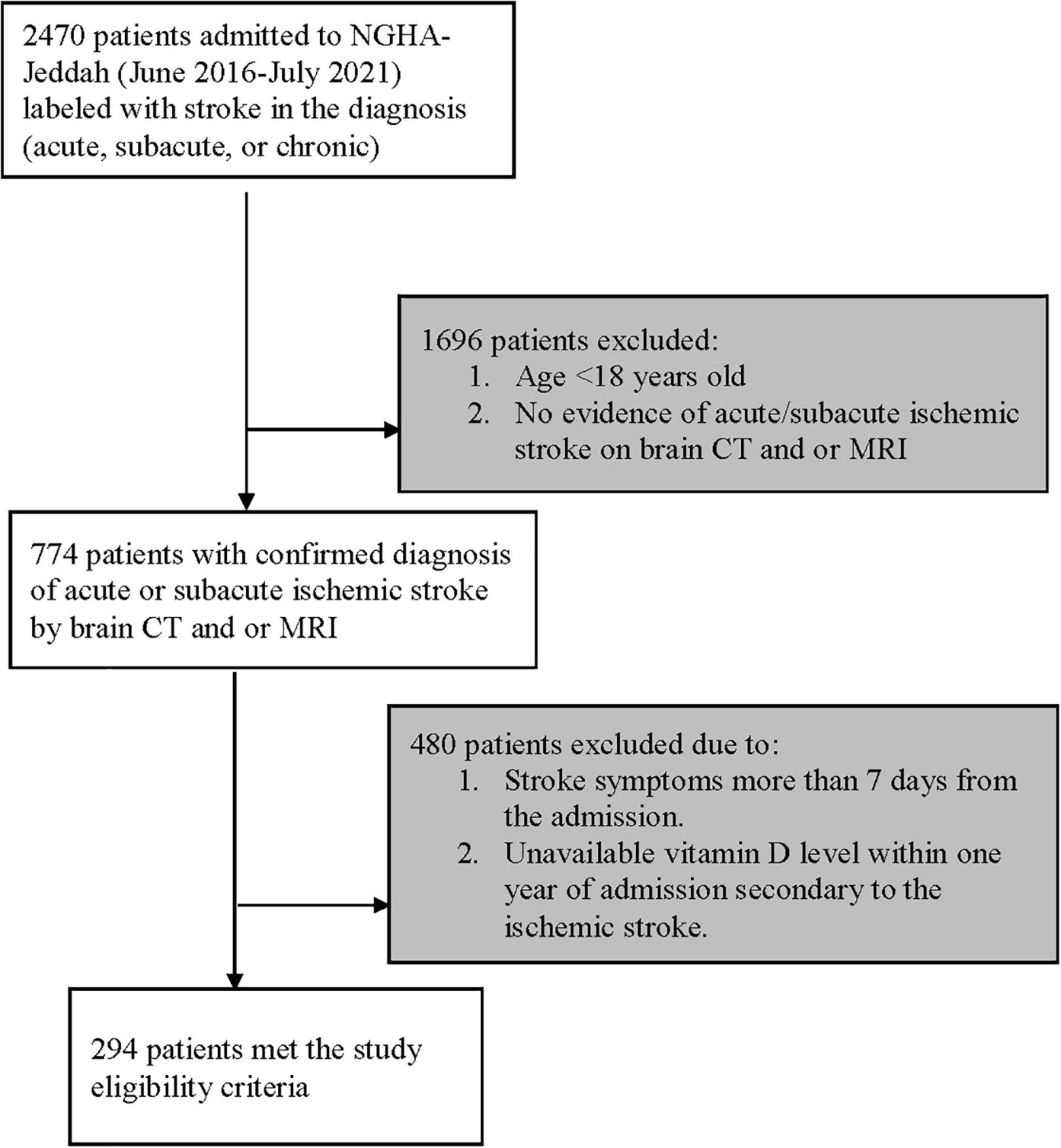
Figure 1. Flow diagram showing patients recruited with ischemic stroke. NGHA, National Guard Health Affairs; CT, Computerized tomography; MRI, Magnetic resonance imaging.
Outcomes
The primary outcome was to assess the correlation between patients’ vitamin D serum level and clinical severity of ischemic stroke at admission (NIHSS), whereas the secondary outcome was to evaluate the association of vitamin D level with functional independence and disability at discharge (mRS).
Study setting
The study was conducted at KAMC—MNGHA. KAMC is a tertiary-care academic referral hospital with 509 bed capacity in Jeddah, Saudi Arabia. This center provides all types of care to all National Guard soldiers and their families, including primary health care and specialized tertiary care.
Data collection
The collected data included demographic data (age, gender, BMI), comorbidities (hypertension, diabetes Mellitus, dyslipidemia, atrial fibrillation, dementia, and smoking), signs and symptoms of stroke (upper limb motor impairment, lower limb motor impairment, impaired consciousness, and aphasia), and complications [i.e., Pneumonia, deep vein thrombosis/pulmonary embolism (DVT/PE), hemorrhagic transformation, recurrent stroke, and death]. The presence of the aforementioned complications was accounted for if mentioned in the daily progress notes and validated by the attending physician. Diabetes mellitus, hypertension, and dyslipidemia were considered present if (1) a patient was on an antidiabetic or anti-hypertensive home medication, respectively, or (2) found in patient’s medical history, or finally, (3) found in laboratory assessment (comparing A1c and fasting glucose levels for diabetes; LDL, HDL, cholesterol, and triglyceride for dyslipidemia).
Vitamin D status was assessed by measuring 25-hydroxyvitamin D [25(OH)D] within 1 year of appearance of ischemic stroke symptoms. Eligible patients were categorized based on their 25-hydroxyvitamin D [25(OH)D] concentration into three groups: Normal (≥75 nmol/L), insufficient (50–75 nmol/L), and deficient (≤50 nmol/L) (Cuomo et al., 2019).
Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) within 24 h of admission (Toth et al., 2017). Upon discharge, patients’ independence and degree of disability were assessed by mRS (Song et al., 2019). Available NIHSS and mRS scores were collected, whereas unavailable scores were calculated using cases’ descriptions in admission and discharge data. NIHSS and mRS scores were verified by a consultant neurologist.
Statistical analysis
Univariable, bivariable, and multivariable analyses were performed. Univariable analysis was done to describe the demographic and clinical characteristics of study subjects. Frequencies and percentages were calculated for categorical variables, while means ± standard deviations (SD) or median with first and third quartiles (Q1, Q3) were calculated for numerical variables. Independent relationship with NIHSS (dependent variables) was assessed using simple and multiple linear regression. The functional outcome at discharge (mRS score) was dichotomized into good (mRS < 3) and poor (mRS ≥ 3). Simple and multiple binary logistic regression was used to identify factors associated with poor functional outcomes. Age, sex, and BMI were adjusted regardless of their bivariable association with each outcome. In addition, independent variables with p-values < 0.2 in the bivariable analysis (i.e., dyslipidemia, AF, and dementia) were included and adjusted for in the multivariable analysis. Linear regression coefficients (β) with standard error (SE) and odds ratios (OR) with 95% confidence intervals (CIs) were computed to determine the magnitude of associations of independent variables with the NIHSS and mRS. A p-value of less than 0.05 was considered statistically significant. All analyses were carried out using SAS University Edition (SAS Institute, Cary, NC, United States).
Results
Baseline characteristics of patients according to vitamin D status
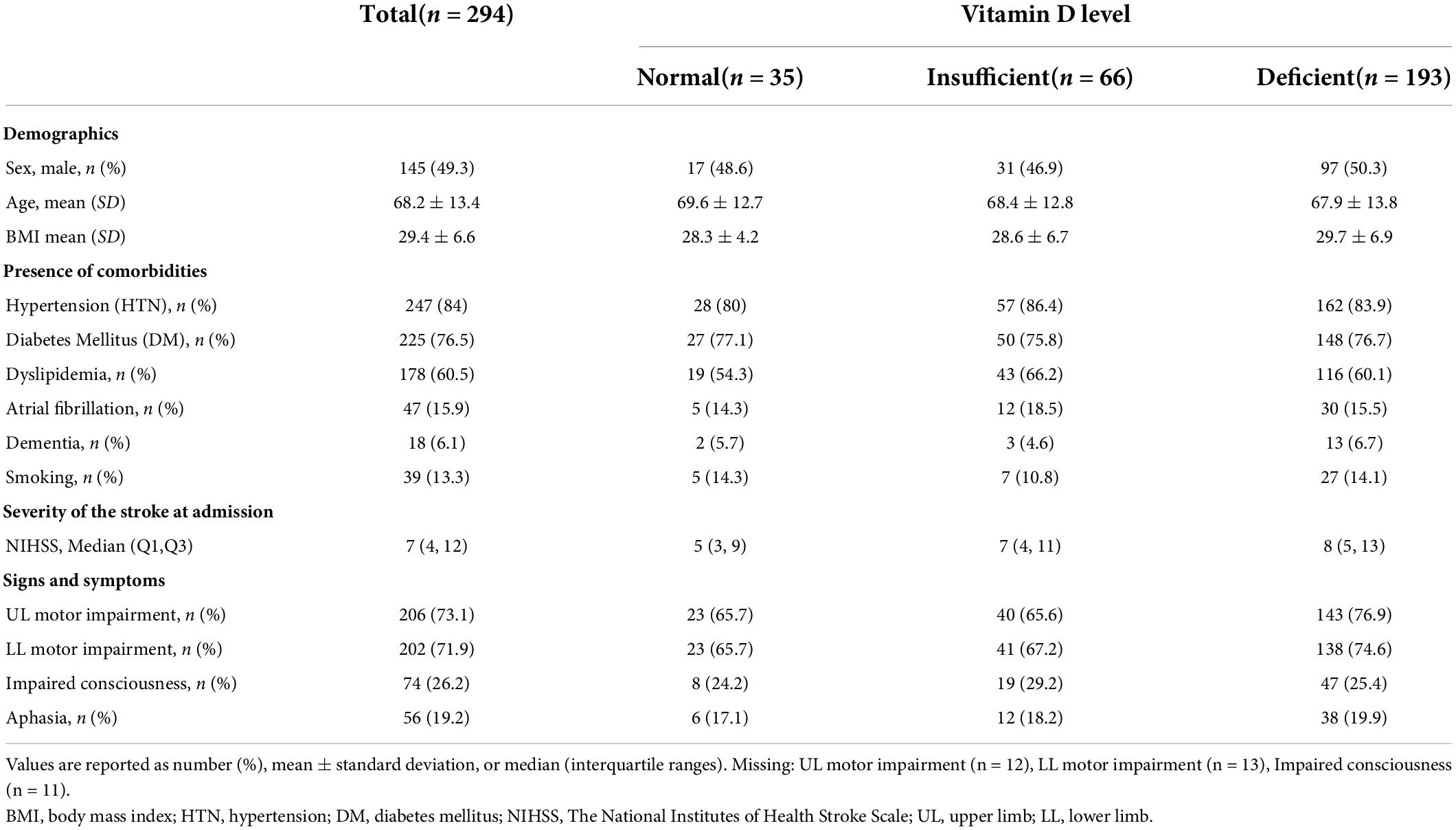
A total of 2,470 patients were screened; 294 patients admitted with stroke were included in the present study based on the eligibility criteria. The mean age of included patients was 68.2 ± 13.4 years, and 49.3% were male. The female percentage in normal, insufficient, and deficient vitamin D level subgroups were 51.4, 53.1, and 49.7%, respectively. Hypertension was reported in 84%, diabetes mellitus 76.5%, and dyslipidemia 60.5% of the patients, and similar comorbidities percentage were observed within the vitamin D level sub-groups. The median time between stroke occurrence and vitamin D level measurement was 56 (Q1,Q3: 5,164) days. The median time between stroke occurrence and discharge was 30.5 days (Q1,Q3: 6,121) (Table 1).
The median NIHSS was 7 (Q1,Q3: 4,12). Stratified by vitamin D levels, patients with deficient vitamin D level had higher median NIHSS of 8 (Q1,Q3: 5,13) compared to those with insufficient [7 (Q1,Q3: 4,11)], or normal [5 (Q1,Q3: 3,9)] vitamin D levels (Table 1). Poor functional outcome (mRS ≥ 3) was reported in 178 subjects (60.5% of total subjects), out of which 16 subjects had normal vitamin D level (45.7% of normal vitamin D subjects), whereas 125 had deficient vitamin D level (64.8% of deficient vitamin D subject) (Table 2).
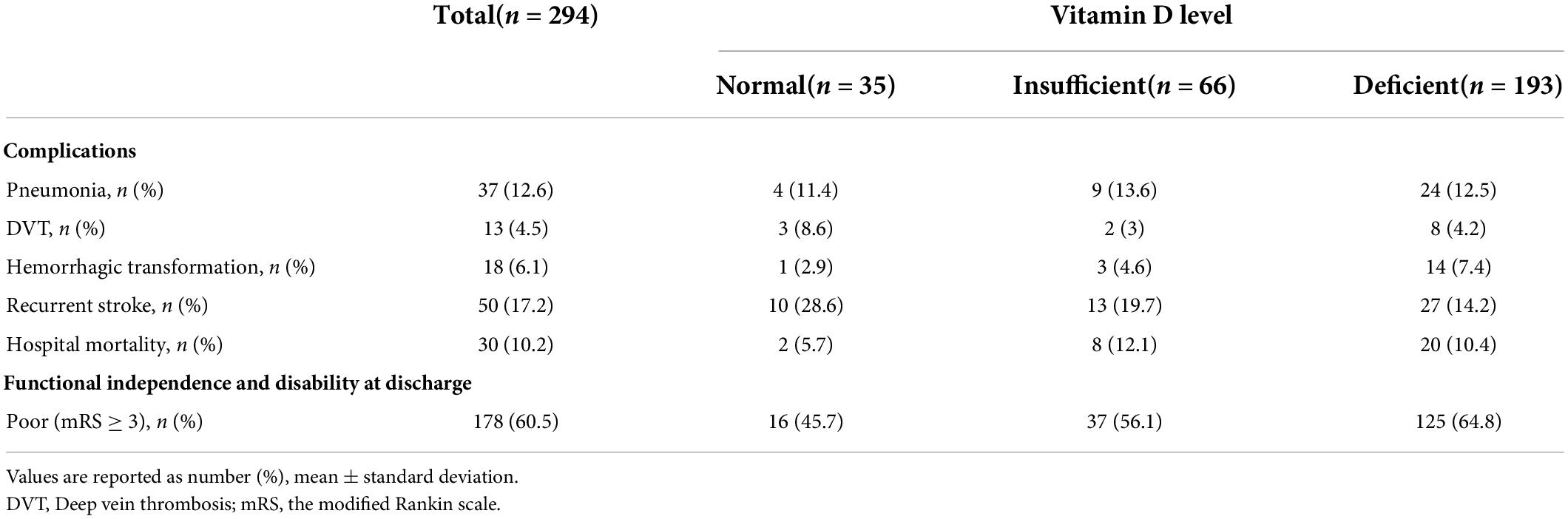
Table 2. Complications during hospital stay and functional independence at discharge according to vitamin D level.
Upper and lower limb motor impairment were observed in 65.7% of the normal vitamin D group. Similar prevalence of upper (65.6%) and lower (67.2%) motor impairment in insufficient vitamin D group but these symptoms were higher in vitamin D deficient group in that 76.9% had upper limb and 74.6% had lower limb impairment (Table 1). The majority of death cases (28 out of 30) either belong to insufficient vitamin D group (n = 8, 12.1%) or deficient vitamin D group (n = 20, 10.4%) (Table 2).
Association between the serum vitamin D level and national institute of health stroke scale
The NIHSS was significantly higher for patients with deficient vitamin D level. The results revealed that lower vitamin D levels were significantly associated with higher NIHSS scores [β (SE) = −0.03 (0.01), p = 0.007] (Figure 2 and Table 3). After adjusting for age, sex, BMI, and variables with p < 0.2 in the bivariable analysis (dyslipidemia, AF, and dementia), vitamin D level remained a significant predictor of NIHSS score [β (SE) = −0.04 (0.01), p = 0.003].
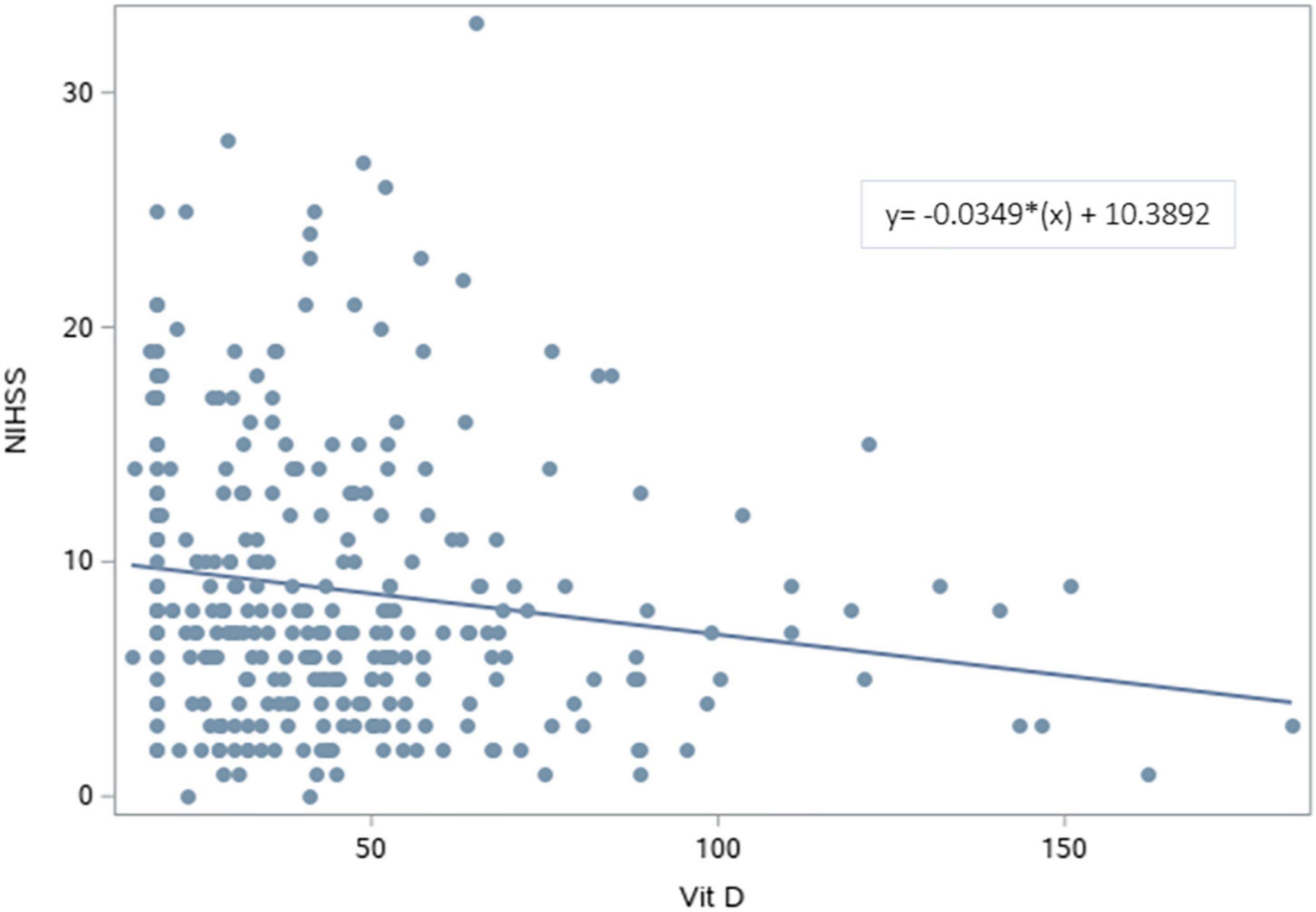
Figure 2. Scatterplot of the National Institutes of Health Stroke Scale vs. Vitamin D levels. A significant regression equation predicting NIHSS score based on vitamin D level was found (F = 7.44, p = 0.007). Patients predicted NIHSS score is equal to 10.3892–0.0349 (Vitamin D level) measured in nmol/L, with an R2 of 0.25.
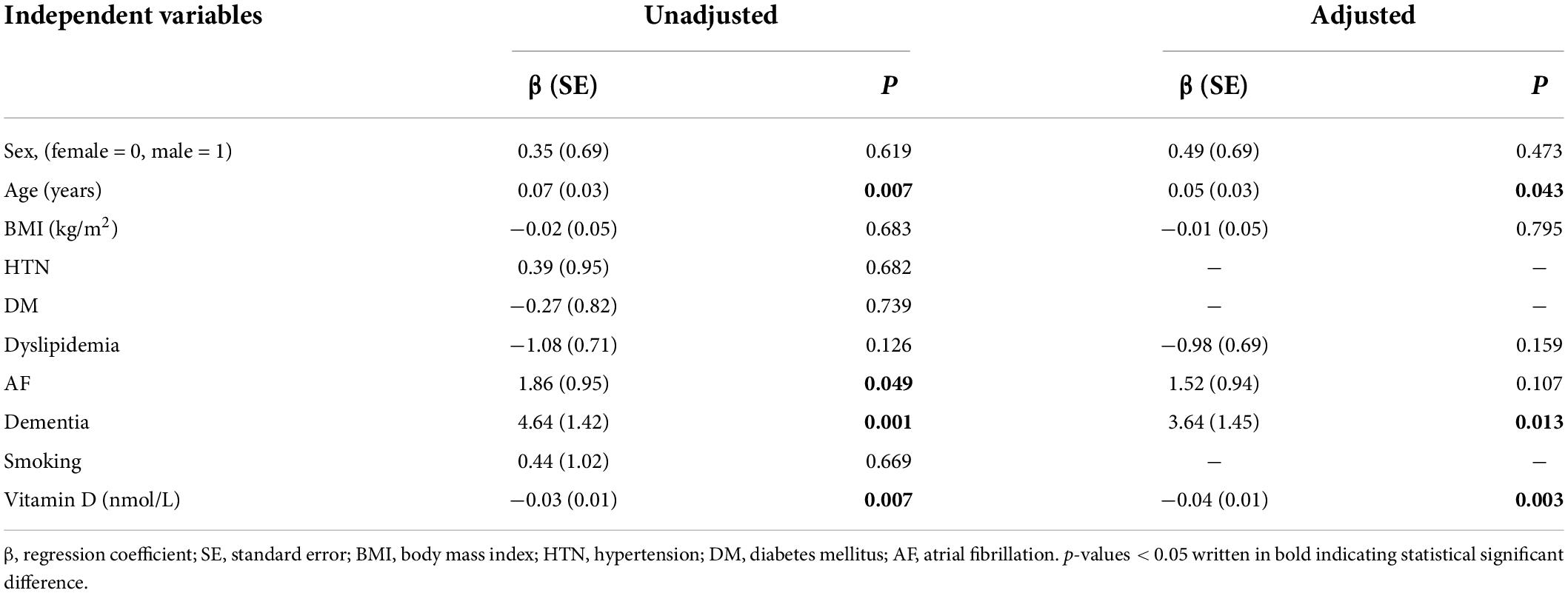
Table 3. Simple and multiple linear regression analysis for association of independent variables with the NIH Stroke score.
Association between the serum vitamin D level and functional outcome at discharge
The simple and multiple logistic regression analyses results of functional outcomes are shown in Table 4. The simple logistic regression showed that patients with deficient vitamin D levels (OR = 2.18, 95% CI: 1.05–4.51) were significantly associated with poor functional outcomes. In the adjusted model, the odds of poor functional outcome (mRS ≥ 3) were more than two times greater among patients with deficient vitamin D levels than those with normal levels (adjusted OR = 2.41, 95% CI: 1.13–5.16).
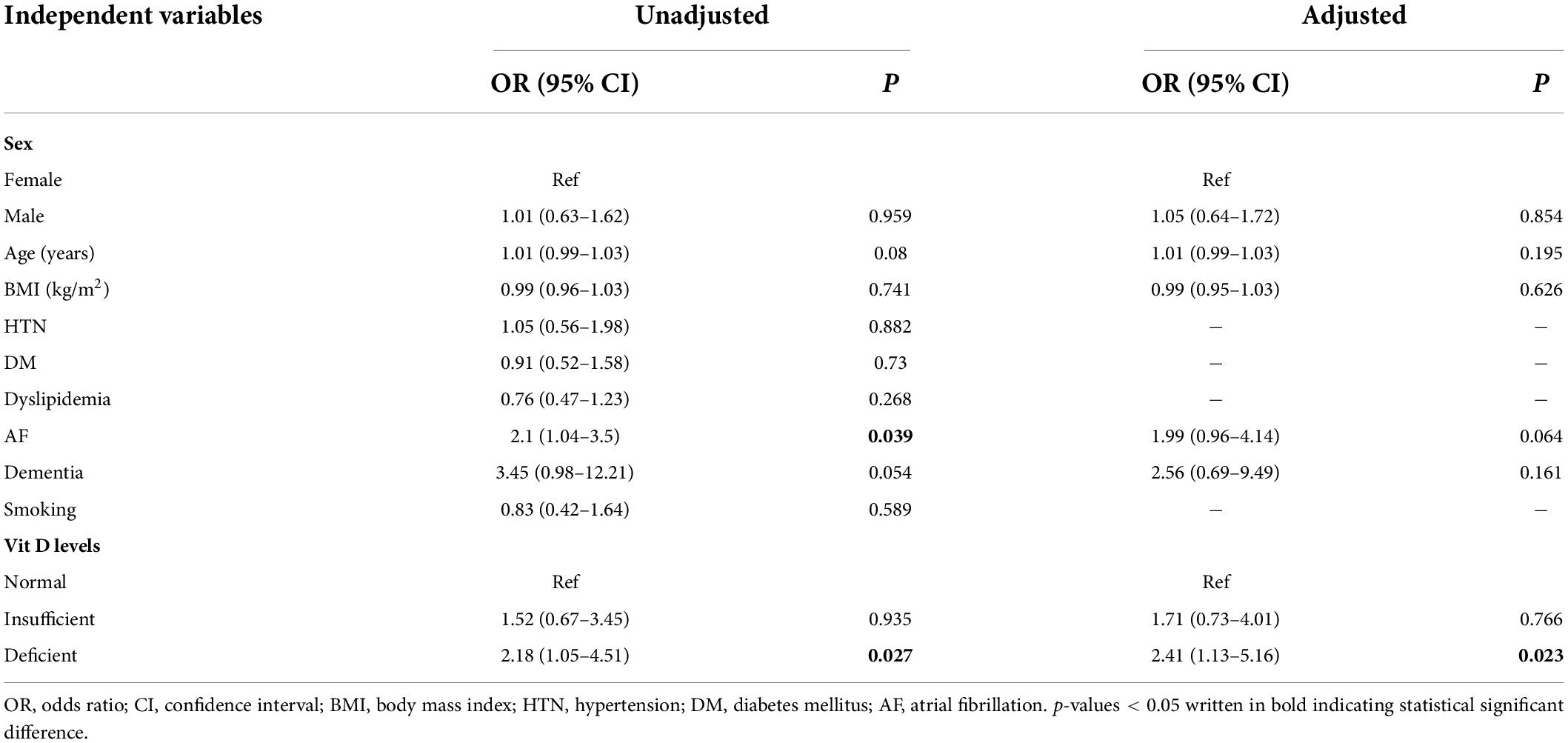
Table 4. Simple and multiple logistic regression analysis for association of independent variables with poor functional outcome (mRS ≥ 3).
Association between national institute of health stroke scale/modified rankin scale and comorbidities
The bivariable analysis showed that older age [β (SE) = 0.07 (0.03), p = 0.007] (Table 3), existence of atrial fibrillation [β (SE) = 1.86 (0.95), p = 0.049], or dementia [β (SE) = 4.64 (1.42), p = 0.001] were significant predictors of poorer NIHSS score. The presence of other comorbidities such as hypertension, diabetes mellitus, dyslipidemia, and smoking was not associated with NIHSS.
In addition, atrial fibrillation (OR = 2.1, 95% CI: 1.04–3.5) was associated with poor functional outcomes (Table 4). However, the presence of other comorbidities such as hypertension, diabetes mellitus, dyslipidemia, or smoking was not associated with poor functional outcomes at discharge.
Discussion
The current study aimed to assess the relationship between serum 25(OH)D levels and stroke clinical severity at admission, and functional independence and disability at discharge. We found that low 25(OH)D levels were associated with worsening initial stroke severity and poor functional independence in ischemic stroke patients. These associations were further strengthened after the adjustment of common covariates such as age, BMI, dementia, and other comorbidities.
Only 11.9% of the patients in our study had sufficient vitamin D levels, while 66% of our participants had vitamin D deficiency. Other studies have found a high prevalence of vitamin D deficiency among stroke patients, which is consistent with our findings. According to a study conducted in Korea, 68% of stroke patients have vitamin D deficiency. Other studies conducted in China reported vitamin D deficiency in 68–78% of stroke patients (Tu et al., 2014; Wang et al., 2014; Park et al., 2015). This high prevalence of vitamin D deficiency can be related to a lack of sunlight exposure (Holick et al., 2012), limited mobility, and malnutrition, all of which are frequent in stroke patients and can lead to vitamin D deficiency (Bouziana and Tziomalos, 2011; Pilz et al., 2011; Holick et al., 2012). Also, this could explain the association of vitamin D deficiency with poor mRS as recovery after stroke is worse when there is limited mobility and malnutrition (Bouziana and Tziomalos, 2011; Pilz et al., 2011).
Few pre-clinical and clinical studies investigated the effects of Vitamin D deficiency on experimental stroke outcomes (Balden et al., 2012; Daubail et al., 2013; Tu et al., 2014; Sayeed et al., 2019; Rad et al., 2021). In animal models, experimental stroke studies found animals having vitamin D deficiency to be associated with more severe sensorimotor impairment, larger brain infarct size, and blood–brain barrier dysfunction (Balden et al., 2012; Sayeed et al., 2019). Moreover, the relationship between 25(OH)D levels and stroke clinical severity in patients with acute ischemic stroke has only been evaluated in few studies. Our findings extend on prior research indicating 25(OH)D is a predictive factor for initial stroke severity in acute ischemic stroke patients (Daubail et al., 2013; Tu et al., 2014; Rad et al., 2021). In contrast to these findings, other studies (Park et al., 2015; Hu et al., 2019) found no association between vitamin D levels and early stroke severity as measured by NIHSS score. Besides the differences in the study population and sample size, this inconsistency could be explained in part by the fact that some studies did not adjust for the typical covariant that might influence stroke severity.
Consistent with previous findings, our results show that vitamin D level is an independent predictor of poor functional outcome, measured by mRS (Tu et al., 2014; Wajda et al., 2019). On the contrary, Hu et al. (2019) demonstrated no significant correlation between vitamin D level and stroke functional outcome. Variation in study design, population, obtained, and adjusted crucial stroke covariates may account for those differences.
This observational study has several limitations for consideration. First, the retrospective design of this study limits our ability to address causality between vitamin D level and stroke severity. Second, this is a single-center study with a small sample size which could have underpowered the study. Third, despite our adjustment of many covariates in the multivariate analysis, there is potential for residual confounders (e.g., sun exposure, diet, physical activity, and parathyroid hormone level) that could affect the observed association. Lastly, administration of vitamin D supplement was not collected, measurement timing of vitamin D levels was not fixed, and some of the outcomes could not be assessed due to the retrospective nature (e.g., size of the infarction).
The current study comes in line with reports emphasizing low 25(OH)D can serve to predict the severity and recovery from ischemic stroke. Future studies need to focus on investigating whether vitamin D supplementation can reduce clinical severity or improve functional outcomes in acute ischemic stroke patients.
Conclusion
Our findings indicate lower vitamin D level is associated with aggravating stroke clinical severity at admission and functional disability at discharge. Hence, vitamin D serum level may predict both stroke clinical severity at admission and functional disability at discharge. Future studies are required to understand the molecular pathology and whether vitamin D supplementation can be used in stroke prevention or treatment.
Data availability statement
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by the King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was funded by the King Salman Center For Disability Research through Research Group no KSRG-2022-062.
Acknowledgments
The authors extend their appreciation to the King Salman Center For Disability Research for funding this work through Research Group no KSRG-2022-062.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al Shoyaib, A., Alamri, F. F., Biggers, A., Karamyan, S. T., Arumugam, T. V., Ahsan, F., et al. (2021a). Delayed Exercise-induced Upregulation of Angiogenic Proteins and Recovery of Motor Function after Photothrombotic Stroke in Mice. Neuroscience 461, 57–71. doi: 10.1016/j.neuroscience.2021.02.023
Al Shoyaib, A., Alamri, F. F., Syeara, N., Jayaraman, S., Karamyan, S. T., Arumugam, T. V., et al. (2021b). The Effect of Histone Deacetylase Inhibitors Panobinostat or Entinostat on Motor Recovery in Mice After Ischemic Stroke. Neuromol. Med. 23, 471–484. doi: 10.1007/s12017-021-08647-1
Al-Alyani, H., Al-Turki, H. A., Al-Essa, O. N., Alani, F. M., and Sadat-Ali, M. (2018). Vitamin D deficiency in Saudi Arabians: a reality or simply hype: a meta-analysis (2008–2015). J. Fam. Commun. Med. 25, 1–4. doi: 10.4103/jfcm.JFCM_73_17
Alamri, F. F., Al Shoyaib, A., Syeara, N., Paul, A., Jayaraman, S., Karamyan, S. T., et al. (2021). Delayed atomoxetine or fluoxetine treatment coupled with limited voluntary running promotes motor recovery in mice after ischemic stroke. Neural Regen. Res. 16, 1244–1251. doi: 10.4103/1673-5374.301031
Balden, R., Selvamani, A., and Sohrabji, F. (2012). Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology 153, 2420–2435. doi: 10.1210/en.2011-1783
Bikle, D. (2009). Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 94, 26–34. doi: 10.1210/jc.2008-1454
Bolland, M. J., Bacon, C. J., Horne, A. M., Mason, B. H., Ames, R. W., Wang, T. K., et al. (2010). Vitamin D insufficiency and health outcomes over 5 y in older women. Am. J. Clin. Nutr. 91, 82–89. doi: 10.3945/ajcn.2009.28424
Bouziana, S. D., and Tziomalos, K. (2011). Malnutrition in patients with acute stroke. J. Nutr. Metab. 2011:167898. doi: 10.1155/2011/167898
Chowdhury, R., Stevens, S., Ward, H., Chowdhury, S., Sajjad, A., and Franco, O. H. (2012). Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur. J. Epidemiol. 27, 581–591. doi: 10.1007/s10654-012-9729-z
Cuomo, A., Maina, G., Bolognesi, S., Rosso, G., Beccarini Crescenzi, B., Zanobini, F., et al. (2019). Prevalence and Correlates of Vitamin D Deficiency in a Sample of 290 Inpatients With Mental Illness. Front. Psychiat. 10:167. doi: 10.3389/fpsyt.2019.00167
Daubail, B., Jacquin, A., Guilland, J. C., Hervieu, M., Osseby, G. V., Rouaud, O., et al. (2013). Serum 25-hydroxyvitamin D predicts severity and prognosis in stroke patients. Eur. J. Neurol. 20, 57–61. doi: 10.1111/j.1468-1331.2012.03758.x
Forman, J. P., Giovannucci, E., Holmes, M. D., Bischoff-Ferrari, H. A., Tworoger, S. S., Willett, W. C., et al. (2007). Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49, 1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288
Gupta, A., Prabhakar, S., Modi, M., Bhadada, S. K., Lal, V., and Khurana, D. (2014). Vitamin D status and risk of ischemic stroke in North Indian patients. Indian J. Endocrinol. Metab. 18, 721–725. doi: 10.4103/2230-8210.139241
Holick, M. F., Binkley, N. C., Bischoff-Ferrari, H. A., Gordon, C. M., Hanley, D. A., Heaney, R. P., et al. (2012). Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 97, 1153–1158. doi: 10.1210/jc.2011-2601
Hu, W., Liu, D., Li, Q., Wang, L., Tang, Q., and Wang, G. (2019). Decreasing serum 25−hydroxyvitamin D levels and risk of early neurological deterioration in patients with ischemic stroke. Brain Behav. 9:e01227. doi: 10.1002/brb3.1227
Huang, H., Zheng, T., Wang, S., Wei, L., Wang, Q., and Sun, Z. (2016). Serum 25-hydroxyvitamin D predicts early recurrent stroke in ischemic stroke patients. Nutr. Metab. Cardiovasc. Dis. 26, 908–914. doi: 10.1016/j.numecd.2016.06.009
Kamtchum-Tatuene, J., and Jickling, G. C. (2019). Blood Biomarkers for Stroke Diagnosis and Management. Neuromol. Med. 21, 344–368. doi: 10.1007/s12017-019-08530-0
Kuhn, T., Kaaks, R., Teucher, B., Hirche, F., Dierkes, J., Weikert, C., et al. (2013). Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident myocardial infarction and stroke in the European prospective investigation into cancer and nutrition (EPIC)-Germany study. PLoS One 8:e69080. doi: 10.1371/journal.pone.0069080
Kuriakose, D., and Xiao, Z. (2020). Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 21:7609. doi: 10.3390/ijms21207609
Kwakkel, G., Wagenaar, R. C., Kollen, B. J., and Lankhorst, G. J. (1996). Predicting disability in stroke–a critical review of the literature. Age Ageing 25, 479–489. doi: 10.1093/ageing/25.6.479
Lips, P. (2006). Vitamin D physiology. Prog. Biophys. Mol. Biol. 92, 4–8. doi: 10.1016/j.pbiomolbio.2006.02.016
Lupton, J. R., Faridi, K. F., Martin, S. S., Sharma, S., Kulkarni, K., Jones, S. R., et al. (2016). Deficient serum 25-hydroxyvitamin D is associated with an atherogenic lipid profile: The Very Large Database of Lipids (VLDL-3) study. J. Clin. Lipidol. 10, 72–81.e1. doi: 10.1016/j.jacl.2015.09.006
Ng, G. J., Quek, A. M., Cheung, C., Arumugam, T. V., and Seet, R. C. (2017). Stroke biomarkers in clinical practice: a critical appraisal. Neurochem. Int. 107, 11–22. doi: 10.1016/j.neuint.2017.01.005
Nie, Z., Ji, X. C., Wang, J., and Zhang, H. X. (2017). Serum levels of 25-hydroxyvitamin D predicts infarct volume and mortality in ischemic stroke patients. J. Neuroimmunol. 313, 41–45. doi: 10.1016/j.jneuroim.2017.10.002
Park, K.-Y., Chung, P.-W., Kim, Y. B., Moon, H.-S., Suh, B.-C., Won, Y. S., et al. (2015). Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovasc. Dis. 40, 73–80. doi: 10.1159/000434691
Pilz, S., Dobnig, H., Fischer, J. E., Wellnitz, B., Seelhorst, U., Boehm, B. O., et al. (2008). Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke 39, 2611–2613. doi: 10.1161/STROKEAHA.107.513655
Pilz, S., Tomaschitz, A., Drechsler, C., Zittermann, A., Dekker, J. M., and Marz, W. (2011). Vitamin D supplementation: a promising approach for the prevention and treatment of strokes. Curr. Drug Targets 12, 88–96. doi: 10.2174/138945011793591563
Poole, K. E., Loveridge, N., Barker, P. J., Halsall, D. J., Rose, C., Reeve, J., et al. (2006). Reduced vitamin D in acute stroke. Stroke 37, 243–245. doi: 10.1161/01.STR.0000195184.24297.c1
Qiu, H., Wang, M., Mi, D., Zhao, J., Tu, W., and Liu, Q. (2017). Vitamin D Status and the Risk of Recurrent Stroke and Mortality in Ischemic Stroke Patients: Data from a 24-Month Follow-Up Study in China. J. Nutr. Health Aging 21, 766–771. doi: 10.1007/s12603-016-0821-z
Rad, R. E., Zarbakhsh, M., and Sarabi, S. (2021). The relationship of vitamin D deficiency with severity and outcome of acute stroke. Rom. J. Intern. Med. 59, 351–358. doi: 10.2478/rjim-2021-0013
Saponaro, F., Marcocci, C., and Zucchi, R. (2019). Vitamin D status and cardiovascular outcome. J. Endocrinol. Invest. 42, 1285–1290. doi: 10.1007/s40618-019-01057-y
Sayed, A., Munir, M., Nabet, M. S., Alghamdi, B. S., Ashraf, G. M., Bahbah, E. I., et al. (2022). Galectin-3: A Novel Marker for the Prediction of Stroke Incidence and Clinical Prognosis. Med. Inflam. 2022, 1–10. doi: 10.1155/2022/2924773
Sayeed, I., Turan, N., Stein, D. G., and Wali, B. (2019). Vitamin D deficiency increases blood-brain barrier dysfunction after ischemic stroke in male rats. Exp. Neurol. 312, 63–71. doi: 10.1016/j.expneurol.2018.11.005
Shi, H., Chen, H., Zhang, Y., Li, J., Fu, K., Xue, W., et al. (2020). 25-Hydroxyvitamin D level, vitamin D intake, and risk of stroke: a dose-response meta-analysis. Clin. Nutr. 39, 2025–2034. doi: 10.1016/j.clnu.2019.08.029
Song, S. Y., Zhao, X. X., Rajah, G., Hua, C., Kang, R. J., Han, Y. P., et al. (2019). Clinical Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Patients With Ischemic Stroke or Hemorrhagic Stroke: An Updated Meta-Analysis. Front. Neurol. 10:1032. doi: 10.3389/fneur.2019.01032
Song, Y., Wang, L., Pittas, A. G., Del Gobbo, L. C., Zhang, C., Manson, J. E., et al. (2013). Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 36, 1422–1428. doi: 10.2337/dc12-0962
Syeara, N., Alamri, F. F., Jayaraman, S., Lee, P., Karamyan, S. T., Arumugam, T. V., et al. (2020). Motor deficit in the mouse ferric chloride-induced distal middle cerebral artery occlusion model of stroke. Behav. Brain Res. 380:112418. doi: 10.1016/j.bbr.2019.112418
Takeda, M., Yamashita, T., Sasaki, N., Nakajima, K., Kita, T., Shinohara, M., et al. (2010). Oral administration of an active form of vitamin D3 (calcitriol) decreases atherosclerosis in mice by inducing regulatory T cells and immature dendritic cells with tolerogenic functions. Arterioscler. Thromb. Vasc. Biol. 30, 2495–2503. doi: 10.1161/ATVBAHA.110.215459
Toth, N. K., Szekely, E. G., Czuriga-Kovacs, K. R., Sarkady, F., Nagy, O., Lanczi, L. I., et al. (2017). Elevated Factor VIII and von Willebrand Factor Levels Predict Unfavorable Outcome in Stroke Patients Treated with Intravenous Thrombolysis. Front. Neurol. 8:721. doi: 10.3389/fneur.2017.00721
Tu, W. J., Dong, X., Zhao, S. J., Yang, D. G., and Chen, H. (2013). Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J. Neuroendocrinol. 25, 771–778. doi: 10.1111/jne.12052
Tu, W. J., Zhao, S. J., Xu, D. J., and Chen, H. (2014). Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischaemic stroke. Clin. Sci. 126, 339–346. doi: 10.1042/CS20130284
Turetsky, A., Goddeau, R. P. Jr., and Henninger, N. (2015). Low Serum Vitamin D Is Independently Associated with Larger Lesion Volumes after Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 24, 1555–1563. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.051
Vijayan, M., Alamri, F. F., Al Shoyaib, A., Karamyan, V. T., and Reddy, P. H. (2019). Novel miRNA PC-5P-12969 in Ischemic Stroke. Mol. Neurobiol. 56, 6976–6985. doi: 10.1007/s12035-019-1562-x
Wajda, J., Swiat, M., Owczarek, A. J., Brzozowska, A., Olszanecka-Glinianowicz, M., and Chudek, J. (2019). Severity of Vitamin D Deficiency Predicts Mortality in Ischemic Stroke Patients. Dis. Markers 2019:3652894.
Wang, Q., Zhu, Z., Liu, Y., Tu, X., and He, J. (2018). Relationship between serum vitamin D levels and inflammatory markers in acute stroke patients. Brain Behav. 8:e00885.
Wang, Y., Ji, H., Tong, Y., and Zhang, Z. B. (2014). Prognostic value of serum 25-hydroxyvitamin D in patients with stroke. Neurochem. Res. 39, 1332–1337.
Wong, M. S., Man, R. Y., and Vanhoutte, P. M. (2010). Calcium-independent phospholipase A2 plays a key role in the endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Am. J. Physiol. Heart Circulat. Physiol. 298, H1260–H1266. doi: 10.1152/ajpheart.01068.2009
Yalbuzdag, S. A., Sarifakioglu, B., Afsar, S. I., Celik, C., Can, A., Yegin, T., et al. (2015). Is 25(OH)D Associated with Cognitive Impairment and Functional Improvement in Stroke? A Retrospective Clinical Study. J. Stroke Cerebrovasc. Dis. 24, 1479–1486. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.007
Keywords: stroke, vitamin D, 25(OH)D, modified Rankin scale (mRS), NIHSS score, disability, recovery, prevention
Citation: Alharbi AR, Alali AS, Samman Y, Alghamdi NA, Albaradie O, Almaghrabi M, Makkawi S, Alghamdi S, Alzahrani MS, Alsalmi M, Karamyan VT, Al Sulaiman K, Aljuhani O and Alamri FF (2022) Vitamin D serum level predicts stroke clinical severity, functional independence, and disability—A retrospective cohort study. Front. Neurosci. 16:951283. doi: 10.3389/fnins.2022.951283
Received: 23 May 2022; Accepted: 28 June 2022;
Published: 27 July 2022.
Edited by:
Badrah Saeed Alghamdi, King Abdulaziz University, Saudi ArabiaReviewed by:
Donald Stein, Emory University, United StatesHanan Helmy Elgendy, Cairo University, Egypt
Copyright © 2022 Alharbi, Alali, Samman, Alghamdi, Albaradie, Almaghrabi, Makkawi, Alghamdi, Alzahrani, Alsalmi, Karamyan, Al Sulaiman, Aljuhani and Alamri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faisal F. Alamri, YWxhbXJpZkBrc2F1LWhzLmVkdS5zYQ==
†These authors have contributed equally to this work
 Abdullah R. Alharbi
Abdullah R. Alharbi Amer S. Alali3†
Amer S. Alali3† Maan Almaghrabi
Maan Almaghrabi Seraj Makkawi
Seraj Makkawi Vardan T. Karamyan
Vardan T. Karamyan Faisal F. Alamri
Faisal F. Alamri