- Department of OBGYN and Reproductive Sciences, University of California, San Diego, La Jolla, CA, United States
A fundamental principle in reproductive neuroendocrinology is sex steroid feedback: steroid hormones secreted by the gonads circulate back to the brain to regulate the neural circuits governing the reproductive neuroendocrine axis. These regulatory feedback loops ultimately act to modulate gonadotropin-releasing hormone (GnRH) secretion, thereby affecting gonadotropin secretion from the anterior pituitary. In females, rising estradiol (E2) during the middle of the menstrual (or estrous) cycle paradoxically “switch” from being inhibitory on GnRH secretion (“negative feedback”) to stimulating GnRH release (“positive feedback”), resulting in a surge in GnRH secretion and a downstream LH surge that triggers ovulation. While upstream neural afferents of GnRH neurons, including kisspeptin neurons in the rostral hypothalamus, are proposed as critical loci of E2 feedback action, the underlying mechanisms governing the shift between E2 negative and positive feedback are still poorly understood. Indeed, the precise cell targets, neural signaling factors and receptors, hormonal pathways, and molecular mechanisms by which ovarian-derived E2 indirectly stimulates GnRH surge secretion remain incompletely known. In many species, there is also a circadian component to the LH surge, restricting its occurrence to specific times of day, but how the circadian clock interacts with endocrine signals to ultimately time LH surge generation also remains a major gap in knowledge. Here, we focus on classic and recent data from rodent models and discuss the consensus knowledge of the neural players, including kisspeptin, the suprachiasmatic nucleus, and glia, as well as endocrine players, including estradiol and progesterone, in the complex regulation and generation of E2-induced LH surges in females.
Introduction
A fundamental tenet of hypothalamic-pituitary-gonadal (HPG) axis regulation is sex steroid feedback: the ability of gonadal steroid hormones (estrogens, androgens, and progestins) to circulate back into the brain and regulate the neural circuits, including gonadotropin-releasing hormone (GnRH) neurons, that govern the HPG neuroendocrine axis. Although feedback loops were proposed decades ago, the detailed mechanisms by which gonadal sex steroids act in the brain to inhibit (“negative feedback”) or stimulate (“positive feedback”) GnRH secretion still remain poorly understood, in part because GnRH cells themselves lack the requisite sex steroid receptors for steroid feedback. Thus, other “upstream” brain cells communicating with GnRH neurons are posited to serve as loci of sex steroid feedback action. Though advances were made in recent years with the discovery of the neuropeptide kisspeptin, the precise brain cells, neural signaling factors and receptors, and physiological and molecular mechanisms by which ovarian-derived estrogen acts in the brain to stimulate GnRH release (“estrogen positive feedback”) still remain major gaps in knowledge. In the present review, we summarize essential background on neuroendocrine mechanisms of estrogen positive feedback, highlight recent advances on this topic, and discuss some critical gaps in knowledge that need addressing to better understand how the LH surge is both generated and modulated. Given other recent in-depth reviews on this and related topics (Herbison, 2008; Christian and Moenter, 2010; Uenoyama et al., 2021; Goodman et al., 2022; Tonsfeldt et al., 2022), we will focus herein on historical and recent data gleaned primarily from rodent models. Readers interested in comparative aspects of estrogen feedback and kisspeptin biology in other species are referred to several other informative reviews (Berga and Naftolin, 2012; Plant, 2012; Lehman et al., 2013; Matsuda et al., 2019; Goodman et al., 2022).
Estrogen positive feedback and the LH surge
GnRH neurons in the forebrain project fibers to the median eminence to secrete pulsatile GnRH, which activates pituitary secretion of gonadotropin hormones (LH and FSH), in turn driving the synthesis and secretion of gonadal sex steroids [estradiol (E2) and testosterone (T)]. Besides regulating reproductive physiology and behavior, circulating E2 and T also provide feedback loops to the brain to modulate GnRH secretion. During most of the female cycle, lower levels of ovarian E2 provide negative feedback on pulsatile GnRH release, keeping it within a proper homeostatic range (Sarkar and Fink, 1980; Chongthammakun and Terasawa, 1993; Evans et al., 1994; Freeman, 2006; Goodman and Inskeep, 2006; Herbison, 2020). However, rising E2 levels at the end of the follicular phase (proestrus in rodents) paradoxically “switch” from being inhibitory to stimulatory, providing positive feedback activation of GnRH cells. This E2 positive feedback induces a massive increase in GnRH secretion (the “GnRH surge”; Sarkar et al., 1976; Moenter et al., 1990, 1992; Freeman, 2006; Herbison, 2008, 2020) which causes a large corresponding “LH surge” from the pituitary to trigger ovulation (Freeman, 1994; Goodman and Inskeep, 2006). The mechanisms governing the critical switch between E2 negative and positive feedback are still poorly understood.
Importantly, GnRH neurons lack the sex steroid receptors [estrogen receptor α (ERα), androgen receptor, progesterone receptor] that mediate both sex steroid positive and negative feedback (Lubahn et al., 1993; Couse et al., 2003; Wintermantel et al., 2006; Christian et al., 2008; Herbison, 2008; Cheong et al., 2015). Thus, sex steroid control of GnRH secretion is indirect, occurring in other upstream steroid-sensitive brain cells that communicate with GnRH cells. Classic studies identified the medial basal hypothalamus as the key region for sex steroid negative feedback in both sexes, whereas the hypothalamic anteroventral periventricular nucleus (AVPV), located in the preoptic area (POA), was identified as a critical area for E2 positive feedback, especially in rodents [reviewed in Herbison (2008), Christian and Moenter (2010), Khan and Kauffman (2012)]. Unlike GnRH cells, the AVPV region contains many ERα-expressing cells (Bloch et al., 1992; Herbison and Theodosis, 1992; Shughrue et al., 1997; Merchenthaler et al., 2004), and many historical studies functionally implicated the AVPV as a key site for E2 induction of the LH surge in rodents (Kalra and McCann, 1975; Goodman, 1978; Wiegand et al., 1978, 1980; Wiegand and Terasawa, 1982; Ronnekleiv and Kelly, 1988; Petersen and Barraclough, 1989; Petersen et al., 1989; Le et al., 1997, 1999, 2001; Smith et al., 2005a). More recently, it was determined that virtually all preoptic ERα + afferents to GnRH neurons reside in the AVPV and immediately adjacent rostral periventricular nucleus, a small anatomical continuum now termed the “rostral periventricular nucleus of the 3rd ventricle” (RP3V; Wintermantel et al., 2006; Herbison, 2008).
Estrogen regulation of kisspeptin neurons
The neuropeptide kisspeptin, encoded by the Kiss1 gene, directly stimulates GnRH neurons. Humans and rodents lacking either kisspeptin or its receptor, KISS1R, are completely infertile, with low LH and gonadal sex steroids due to diminished GnRH secretion (de Roux et al., 2003; Seminara et al., 2003; d’Anglemont de Tassigny et al., 2007; Lapatto et al., 2007; Topaloglu et al., 2012). KISS1R is expressed in GnRH cells (Irwig et al., 2004; Messager et al., 2005; Semaan and Kauffman, 2015), and kisspeptin potently stimulates GnRH neuron activation and GnRH secretion, thereby causing LH secretion (Gottsch et al., 2004; Irwig et al., 2004; Dhillo et al., 2005; Han et al., 2005; Messager et al., 2005; Navarro et al., 2005; Kauffman et al., 2007c; d’Anglemont de Tassigny et al., 2008). Importantly, pharmacologically blocking kisspeptin signaling inhibits the LH surge (Kinoshita et al., 2005; Pineda et al., 2010; Smith et al., 2011), and E2-treated Kiss1r- or Kiss1-null female mice do not exhibit LH surges or GnRH neuron activation (Clarkson et al., 2008; Dror et al., 2013), indicating that kisspeptin signaling is critical for the LH surge generation.
In all mammals, kisspeptin-synthesizing neurons primarily reside in two distinct hypothalamic areas. In rodents, the largest kisspeptin population is in the arcuate nucleus (ARC) while another more anterior population is in the RP3V (Gottsch et al., 2004; Smith et al., 2005a; Kauffman et al., 2007b; Clarkson et al., 2009; Kauffman, 2010a; Lehman et al., 2013). The RP3V kisspeptin population is one and the same as the originally identified kisspeptin neurons in the AVPV and PeN nuclei (and is therefore often referred to as the AVPV/PeN kisspeptin population). In the present review, we will use the designations RP3VKISS and ARCKISS neurons. Importantly, while both kisspeptin populations stimulate GnRH neurons, RP3VKISS and ARCKISS neurons directly project to different anatomical parts of the GnRH neuron, with the former targeting GnRH soma in the OVLT and POA and the latter targeting GnRH fiber terminals in the MBH and median eminence (Lehman et al., 2013; Yip et al., 2015, 2021). This is no small point, as the anatomical and physical separation of kisspeptin synthesis and release between RP3VKISS and ARCKISS neurons may in fact underlie the ability of estrogen to provide both positive and negative feedback on GnRH neurons by acting on kisspeptin neurons in different brain locations for each process (Wang et al., 2020). Indeed, and to this point, Kiss1 gene expression is strongly regulated by gonadal sex steroids (E2 and T) in a region-specific manner: for both sexes, E2 or T increases Kiss1 mRNA levels in the RP3V whereas these sex steroids reduce Kiss1 mRNA levels in the ARC (Smith et al., 2005a,b; Kauffman et al., 2007b). Conversely, when circulating sex steroids are low or absent [e.g., diestrus or ovariectomized (OVX) females], Kiss1 mRNA levels decrease in the RP3V and increase in the ARC (Smith et al., 2005a,b; Kauffman et al., 2007b,2009). Both RP3VKISS and ARCKISS neurons express high levels of ERα (Smith et al., 2005a; Adachi et al., 2007; Poling et al., 2017), and we and others, including seminal experiments from the Steiner lab (Smith et al., 2005a), have shown that E2’s effects on both RP3V and ARC Kiss1 mRNA levels are direct and occur specifically via ERα signaling (Smith et al., 2006; Gottsch et al., 2009; Dubois et al., 2015, 2016; Stephens et al., 2016; Poling et al., 2017; Stephens and Kauffman, 2021).
The seemingly simple finding that E2 regulates the Kiss1 gene differently in the two hypothalamic Kiss1-expressing populations has ultimately proven to be a crucial discovery in understanding sex steroid feedback loops. Indeed, this finding led to the proposal that differential effects of E2 on Kiss1 mRNA in the ARC and RP3V reflect different roles of the two kisspeptin populations (Smith et al., 2005a), with ARCKISS neurons participating in E2 negative feedback and RP3VKISS neurons participating in E2 positive feedback. The proposed role of ARCKISS neurons in negative feedback control of GnRH pulse secretion has been reviewed in detail elsewhere (Herbison, 2018; Moore et al., 2018; Goodman et al., 2022) and will not be discussed here other than by simply summarizing that sex steroids are known to exert negative feedback regulation indirectly on GnRH pulses by acting in the ARC region (Ferin et al., 1974; Smith and Davidson, 1974; Scott et al., 1997), and direct inhibition of ARCKISS neurons, which comprise the “GnRH pulse generator” (Han et al., 2015, 2019; Qiu et al., 2016; Clarkson et al., 2017; McQuillan et al., 2019), may be how E2 and T regulate the frequency and amplitude of GnRH (and LH) pulses.
Contrasting the role of ARCKISS neurons in driving GnRH pulses and mediating steroid feedback, it is now widely believed that RP3VKISS neurons comprise the neural conduit mediating E2 positive feedback on GnRH neurons in female rodents (thereby triggering the GnRH and LH surges and subsequent ovulation; Kauffman et al., 2007a; Herbison, 2008). As noted above, RP3VKISS cells express abundant ERα and strongly increase Kiss1 gene expression in response to elevated E2. We and others have also shown that RP3VKISS neurons display increased neuronal activation (cfos mRNA or Fos protein) at the time of the LH surge in proestrus or OVX + E2-treated females (Figures 1, 2), but not in diestrus or OVX females with insufficient E2 (Smith et al., 2006; Adachi et al., 2007; Clarkson et al., 2008; Robertson et al., 2009; Figure 2). Moreover, female mice lacking ERα in kisspeptin neurons do not generate LH surges in response to E2 (Dubois et al., 2015); however, because ERα was deleted from all kisspeptin cells, that study was not able to pinpoint the effect specifically to RP3VKISS neurons. However, another study implementing AAV-mediated partial knockdown of ERα in RP3VKISS cells lowered the LH surge magnitude in proestrus and OVX + E2 mice (Wang et al., 2019). Finally, as discussed further below, the LH surge is gated by a circadian clock (de la Iglesia and Schwartz, 2006), and RP3VKISS neurons display a circadian pattern of neuronal activation in perfect synchrony with the timing of the LH surge (Robertson et al., 2009).
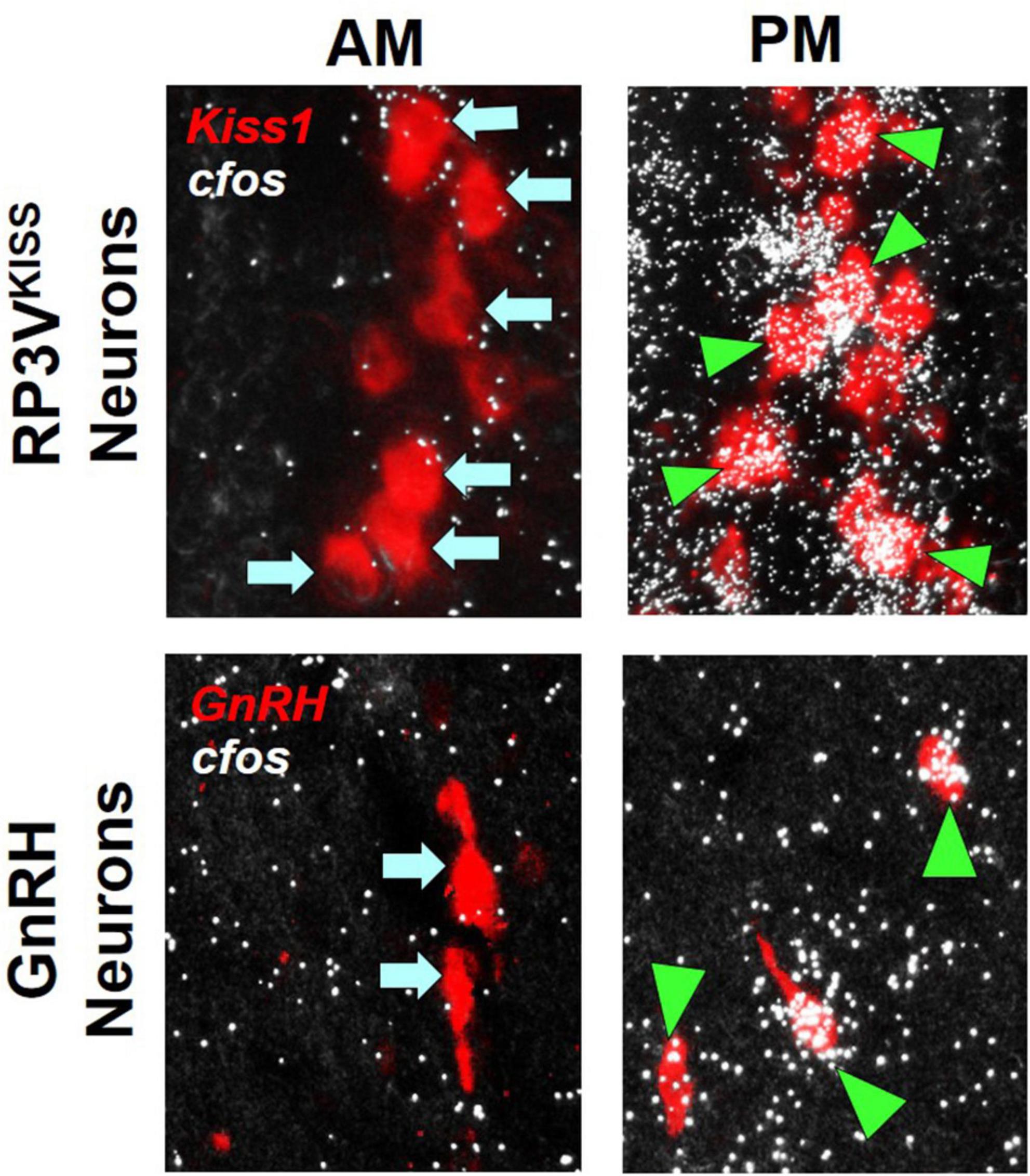
Figure 1. Examples of RP3VKISS and GnRH neuron activation, as indicated by robust cfos mRNA co-expression (white “silver grains”; assayed with radiolabeled in situ hybridization), during an E2-induced LH surge in female mice. The surge typically occurs in the late afternoon/early evening (PM) but not in the morning (AM), matching the higher degree of cfos mRNA induction in both RP3VKISS and GnRH neurons in the PM than AM. Green triangles denote example “activated” RP3VKISS or GnRH cells co-expressing cfos; blue arrows denote example non-activated cells lacking cfos. Adapted from Poling et al. (2017).
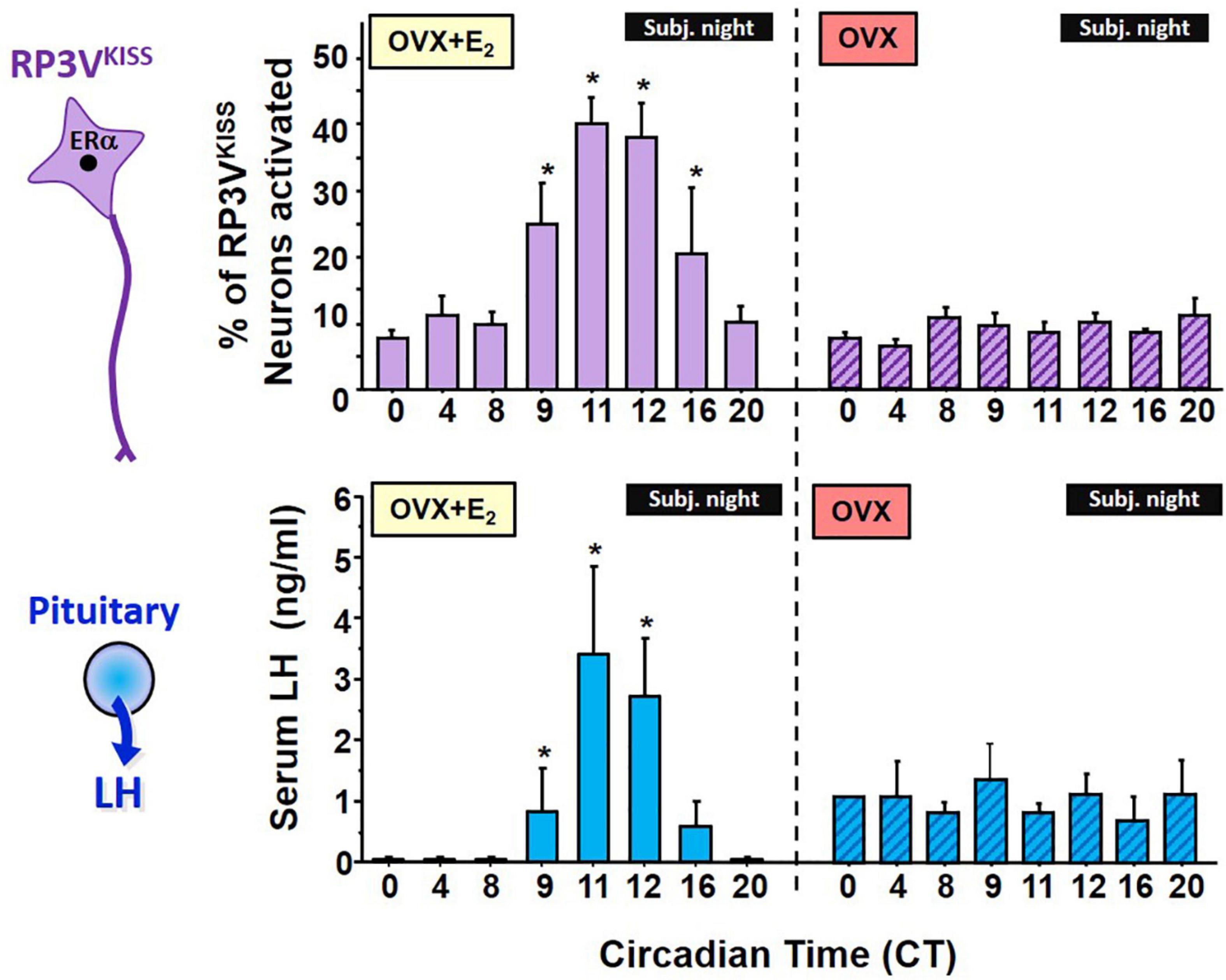
Figure 2. Circadian changes in RP3VKISS neuron activation, measured by cfos mRNA induction, and serum LH levels in OVX + E2 female mice housed in constant darkness (DD). The onset and peak of RP3VKISS neuron activation mirrors the rise and peak of LH surges, which occur right before the onset of subjective night (defined as the daily onset of locomotor activity, a robust circadian behavioral measure). For comparison, a separate cohort of OVX females not receiving E2 were similarly studied but showed no circadian increases in either RP3VKISS neuron activation or serum LH levels. Note that baseline LH levels in the OVX females are higher than in OVX + E2 due to lack of gonadal steroid negative feedback in the former group. *, Significantly different than baseline levels at CT 0. Adapted from Robertson et al. (2009).
Additional electrophysiological evidence that supports that RP3VKISS neurons are positively regulated by estrogen, including reported stimulatory effects on ion currents in these cells in mice (Piet et al., 2013; Zhang et al., 2015; Wang et al., 2016; Starrett et al., 2021). Collectively, such studies conclude that normal estrous cycle-driven rises in circulating E2 induce increases in overall action potential generation and burst firing in RP3VKISS neurons on proestrus (when E2 is elevated) by regulating multiple intrinsic currents in these kisspeptin cells (Wang et al., 2016). Moreover, evidence that increased electrical activity in these cells is sufficient to drive kisspeptin release and, consequently, electrical activity in GnRH neurons and downstream LH secretion provides further support for RP3VKISS neurons mediating and conveying E2 positive feedback information to GnRH neurons. Indeed, either continuous or bursting mode optogenetic activation of RP3VKISS neurons in female mouse brain slices reliably generated long-lasting activation of GnRH neuron firing, and optogenetic activation of RP3VKISS neurons in vivo generated large increases in LH secretion resembling the endogenous LH surge (Piet et al., 2018). This was supported by a similar report in mice that high-frequency photostimulation of RP3VKISS neurons evokes increased electrical activity in GnRH neurons in several in vitro slice orientations (Qiu et al., 2016). Importantly, although RP3VKISS neurons co-express many additional releasable signaling factors along with kisspeptin itself (Stephens and Kauffman, 2021), similar in vivo optogenetic activation of RP3VKISS neurons in which kisspeptin had been deleted did not induce LH secretion (Piet et al., 2018), indicating that kisspeptin peptide is a required signaling factor being released from RP3VKISS neurons to induces surges. This does not rule out important involvement of other co-released peptides or neurotransmitters, but clearly designates kisspeptin as one essential player for the surge process.
In rodents, E2’s positive effects on both LH surge generation and RP3VKISS cells are sexually dimorphic: E2-treated male rodents have lower RP3V Kiss1 mRNA levels than E2-treated females (Kauffman et al., 2007b; Poling et al., 2017), and male RP3VKISS neurons are not activated by elevated levels E2 (Poling et al., 2017), correlating with male rodents’ inability to generate E2-induced LH surges (Buhl et al., 1978; Homma et al., 2009; Poling et al., 2017). Interestingly, developmental manipulations that reverse the sexual differentiation of RP3VKISS neurons (Kauffman et al., 2007b; Homma et al., 2009) similarly reverse the ability to generate LH surges, such that males with female-like RP3VKISS neurons can produce an LH surge whereas females with male-like RP3VKISS neurons can no longer generate LH surges (Homma et al., 2009). Overall, these data, along with the findings in the preceding paragraph, suggest that RP3VKISS neurons participate in generating the sex-specific LH surge during E2 positive feedback. Conversely, as noted further below, rodent ARCKISS cells are strongly inhibited by E2 and are not highly activated during the LH surge (Smith et al., 2005a; Adachi et al., 2007) [though both ARCKISS and POA kisspeptin neurons may participate in the LH surge in some species (Smith, 2009; Smith et al., 2010; Watanabe et al., 2014; Vargas Trujillo et al., 2017)].
Circadian regulation of the LH surge and RP3VKISS neurons
The E2-induced LH surge is under temporal control (Alleva et al., 1971; Norman and Spies, 1974; Legan and Karsch, 1975; Seibel et al., 1982; Cahill et al., 1998; Kerdelhue et al., 2002; Mahoney et al., 2004; de la Iglesia and Schwartz, 2006). In female rodents, the LH surge is timed to occur exclusively in the late afternoon/early evening of proestrus, thereby aligning subsequent ovulation and female mating and ensuring reproductive success (Kauffman, 2010b). In mammals, the primary circadian clock located in the hypothalamic suprachiasmatic nucleus (SCN) governs circadian rhythms of many biological processes, from gene expression to physiology and behavior. Evidence in rodents indicates the circadian clock in the SCN governs the LH surge timing. First, historical studies showed that not only does the LH surge consistently and predictably occur at a specific time of day, but barbiturate treatment delays the occurrence of the LH surge exactly 24 h until the late afternoon of the next day, suggesting an internal time-keeping component for the surge event (Everett et al., 1949; Sawyer et al., 1949; Everett and Sawyer, 1950; Siegel et al., 1976; Stetson and Watson-Whitmyre, 1977). Second, OVX females do not surge, emphasizing the requisite role for E2 in surge induction, but OVX females given elevated E2 (OVX + E2) display an LH surge which occurs solely around the time of lights off and which repeats daily at the same time as long as E2 is elevated (Caligaris et al., 1971; Norman et al., 1973; Norman and Spies, 1974; Legan and Karsch, 1975; Legan et al., 1975). Third, experimental phase shifts or genetic alterations of behavioral circadian rhythms known to be timed by the SCN (e.g., circadian locomotor activity) phase shift the timing of the LH surge, with the onset and timing of the new surge always coupled to the new timing of locomotor activity onset (Alleva et al., 1971; Fitzgerald and Zucker, 1976; Moline and Albers, 1988; Lucas et al., 1999; Smarr et al., 2012). Fourth, experimentally induced “splitting” of the lateral SCN hemispheres of hamsters by exposure to constant light elicits 2 daily LH surges ∼12 h apart, along with lateralized GnRH neuron activation (Swann and Turek, 1985; de la Iglesia et al., 2003). These two daily “split” LH surges are thought to be separately caused by activation of left and right hemisphere GnRH neuron populations alternatingly activated 12 h apart by the two sides of the SCN. Further supporting this possibility, SCN neurons are activated right before the onset of the LH surge, as measured by increased Fos expression (Tsukahara, 2006). Lastly, in female rats and hamsters, physical destruction of the SCN (Bishop et al., 1972; Brown-Grant and Raisman, 1977; Gray et al., 1978; Kawakami et al., 1980; Wiegand et al., 1980; Wiegand and Terasawa, 1982; Ronnekleiv and Kelly, 1986, 1988; Palm et al., 1999), prevents LH surges, even in the presence of elevated E2, a finding supported by similar observations of impaired LH surges and reproductive dysfunction in clock gene knock out mice (Miller et al., 2004; Boden et al., 2010; Chu et al., 2013).
If RP3VKISS neurons participate in the LH surge process, do these neurons demonstrate a circadian component that may relate to the timing of the LH surge? Anatomically, the RP3V region receives SCN axonal projections (de la Iglesia et al., 1995; Watson et al., 1995), suggesting there could be SCN-derived circadian input on neurons there. Our lab therefore first tested whether RP3VKISS neurons of female mice exhibit circadian changes and if such changes occur in synchrony with the timing of the LH surge (Robertson et al., 2009). We found that OVX + E2 mice housed in constant darkness (to remove light cues and allow their circadian clock to free-run on its endogenous period) showed circadian increases in both R3PV Kiss1 mRNA levels and RP3VKISS neuron activation (Figure 2). Moreover, these temporal changes in RP3VKISS measures peaked just before the onset of subjective night [defined as the onset of daily locomotor activity; circadian time (CT) 12], with lower levels earlier in the subjective morning or later in the subjective night. Importantly, in these females, the observed circadian increases in RP3V Kiss1 levels and RP3VKISS neuron activation occurred synchronously with the circadian onset and duration of the LH surge (Robertson et al., 2009; Figure 2). These data were later confirmed by us and others in subsequent studies of female rodents housed in light-dark cycles (Figure 3) or in SCN lesioned females (Williams et al., 2011; Smarr et al., 2012, 2013; Poling et al., 2017), supporting the notion that circadian activation of RP3VKISS may underlie the circadian nature of the LH surge.
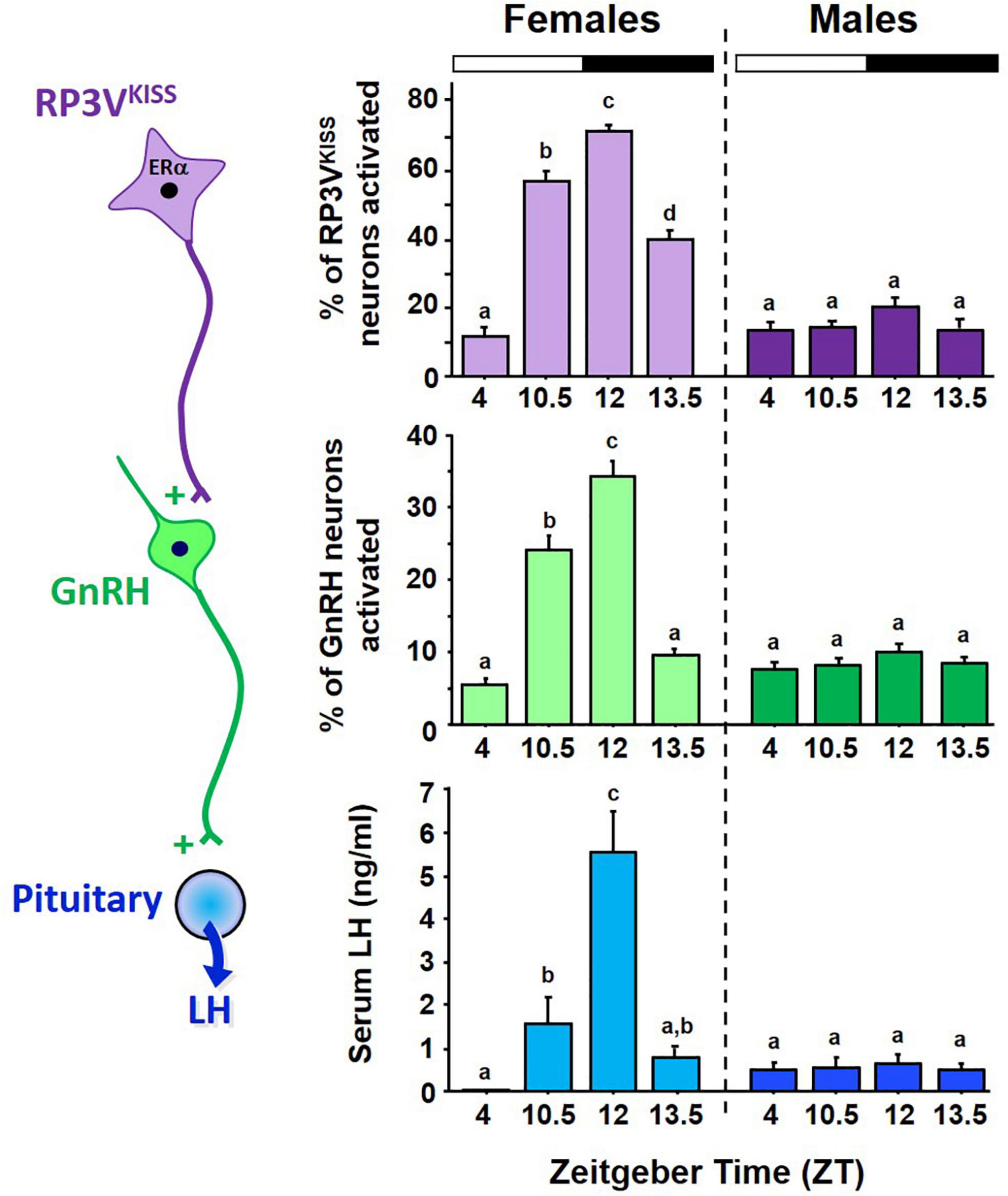
Figure 3. Circadian changes in RP3VKISS and GnRH neuron activation, measured by cfos induction using double ISH, and serum LH in female mice housed in 12:12 LD cycle. All females were OVX + E2. The onset and peak of neuron activation occurs in synchrony with the rise and peak of LH levels indicative of LH surges. For comparison, male littermates were housed in same 12:12 conditions and similarly GDX + E2, yet showed no circadian increases in RP3VKISS neuron activation, GnRH neuron activation, or serum LH levels. Bars with different letters above them are significantly different from each other. Adapted from Poling et al. (2017).
Interestingly, we also demonstrated that adult OVX mice without E2 supplementation lack any circadian changes in LH secretion, RP3V Kiss1 levels, or RP3VKISS neuron activation, with all measures being the same at all time points (Robertson et al., 2009; Figure 2). Thus, the circadian regulation of RP3VKISS is dependent on elevated E2, supported by similar data of dampened circadian increases in RP3V kisspeptin neuron activation in OVX female hamsters or in Kiss1 mRNA levels in diestrus versus proestrus mice (Williams et al., 2011; Chassard et al., 2015). These findings may explain, in part, why OVX (no E2) and diestrus females (low E2) do not display an LH surge, though the underlying reason for the E2-dependence of RP3V kisspeptin neuron circadian changes is still not entirely known. As noted below, the vasopressin receptor, V1a, is E2-sensitive (Kalamatianos et al., 2004), and this might contribute, in part, to the lack of RP3VKISS neuron activation in OVX and diestrus females. Indeed, vasopressin treatment stimulates RP3VKISS neuron activity in OVX + E2 but not OVX mice (Piet et al., 2015), indicating E2 may enhance the sensitivity of RP3VKISS neuron to vasopressin. In some cases, E2 also has also been shown to modulate synaptic transmission to RP3VKISS neurons, increasing stimulatory glutamate transmission and decreasing inhibitory GABAergic transmission to these neurons (DeFazio et al., 2014; Wang et al., 2018), though this has not always been consistently observed.
Because of sexual differentiation, the brains of male rodents, unlike their female counterparts, are incapable of generating LH surges in response to elevated E2. We recently showed that adult E2-treated male mice also demonstrate no circadian changes in GnRH cell activation, Kiss1 mRNA, or RP3VKISS neuron activation (Poling et al., 2017; Figure 3). Thus, in addition to synthesizing less kisspeptin in the RP3V than females (Clarkson and Herbison, 2006; Kauffman et al., 2007b; Poling et al., 2017), males’ RP3VKISS neurons also do not become activated by E2 at any point of the circadian cycle, indicating additional sexual dimorphisms beyond just Kiss1 gene expression. Interestingly, we showed that male mice have lower levels of ERα co-expression in RP3VKISS neurons than do females (Poling et al., 2017), though male RP3VKISS neurons still had a decent degree of ERα and it remains unknown if the ERα sex difference explains the complete lack of neuron activation in males. Unraveling exactly why E2-treated males lack any circadian RP3VKISS changes, or why the circadian activation of RP3VKISS neuron is E2-dependent in females, could provide valuable mechanistic insight for how E2 positive feedback normally operates.
Possible suprachiasmatic nucleus circuits regulating gonadotropin-releasing hormone and RP3VKISS neurons
The SCN is anatomically and functionally divided into the ventrolateral “core” and the dorsomedial “shell.” The ventrolateral neurons receive direct light input from the retinohypothalamic tract and relay this photic information to other SCN neurons, including dorsomedial neurons (Abrahamson and Moore, 2001). Each SCN neuron is rhythmic on its own (Welsh et al., 1995) but the nucleus as a whole synchronizes among itself to produces a single robust rhythmic output that can govern circadian changes in many other brain areas (Abrahamson and Moore, 2001; Deurveilher and Semba, 2005; Welsh et al., 2010). Different SCN neurons produce a variety of neuropeptides, including vasoactive intestinal polypeptide (VIP), arginine vasopressin (AVP), gastrin-releasing peptide, prokineticin 2, neuromedin S, and substance P, as well as GABA (Cheng et al., 2002; Antle and Silver, 2005; Lee et al., 2015; Shan et al., 2020; Wen et al., 2020). VIP and AVP are synthesized in the ventrolateral and dorsomedial SCN, respectively, (Card et al., 1988). Both Vip mRNA levels and VIP release demonstrate in vitro circadian rhythmicity in SCN slices (Shinohara et al., 1999, 2000; Dardente et al., 2004). Findings from transgenic mice lacking either VIP or VIPR2 (aka VPAC2, a VIP receptor) suggest that VIP is an important communicator to extra-SCN brain areas because these knockout mice display disrupted or altered circadian rhythms, and sometimes arrhythmicity (Harmar et al., 2002; Colwell et al., 2003). Likewise, AVP also demonstrates circadian rhythmicity, with highest levels during the subjective day (Reppert et al., 1981). Although AVP-deficient Brattleboro rats show intact circadian rhythmicity for several behaviors (Peterson et al., 1980; Groblewski et al., 1981) other studies suggest that AVP is an important SCN output signal for some physiological circadian rhythms, including daily stress hormone secretion (Kalsbeek et al., 1992, 1996a,1996b) and possibly reproductive hormone secretion (discussed below), and SCN AVP neurons project to several hypothalamic regions, including the PVN, DMN, POA, and RP3V (Dai et al., 1998; Kalsbeek et al., 2008).
How might the SCN clock communicate circadian timing information to the GnRH system to help time the occurrence of the LH surge? At least two neural anatomical pathways (1 direct, 1 indirect) may link the SCN to GnRH neurons (Figure 4). A proposed direct pathway involves VIP neurons in the ventrolateral SCN that directly target GnRH neurons (van der Beek et al., 1993; Van der Beek et al., 1997), which express the VIP receptor VIPR2 (Van der Beek et al., 1997; Smith et al., 2000; Figure 4). Early data in female rats indicated that many GnRH neurons that express Fos at the time of the LH surge also receive VIP-immunoreactive contacts (Lee et al., 1990a; van der Beek et al., 1994; Harney et al., 1996), suggesting that GnRH-surge generating neurons may be under regulation by VIP. However, on its own this remains a correlational line of evidence that does not demonstrate a functional role for VIP in activating GnRH neurons at the time of the surge, and also does not consider the likelihood that Fos-expressing GnRH neurons may also be targeted by other non-VIP neurons; that is, whether the observed Fos induction in GnRH is actually caused by SCNVIP neurons or VIP peptide signaling still requires direct demonstration. Still, the observation in rats that SCN VIP neurons anatomically project to GnRH neurons is often cited as evidence that this circuit likely plays a role in the GnRH surge.
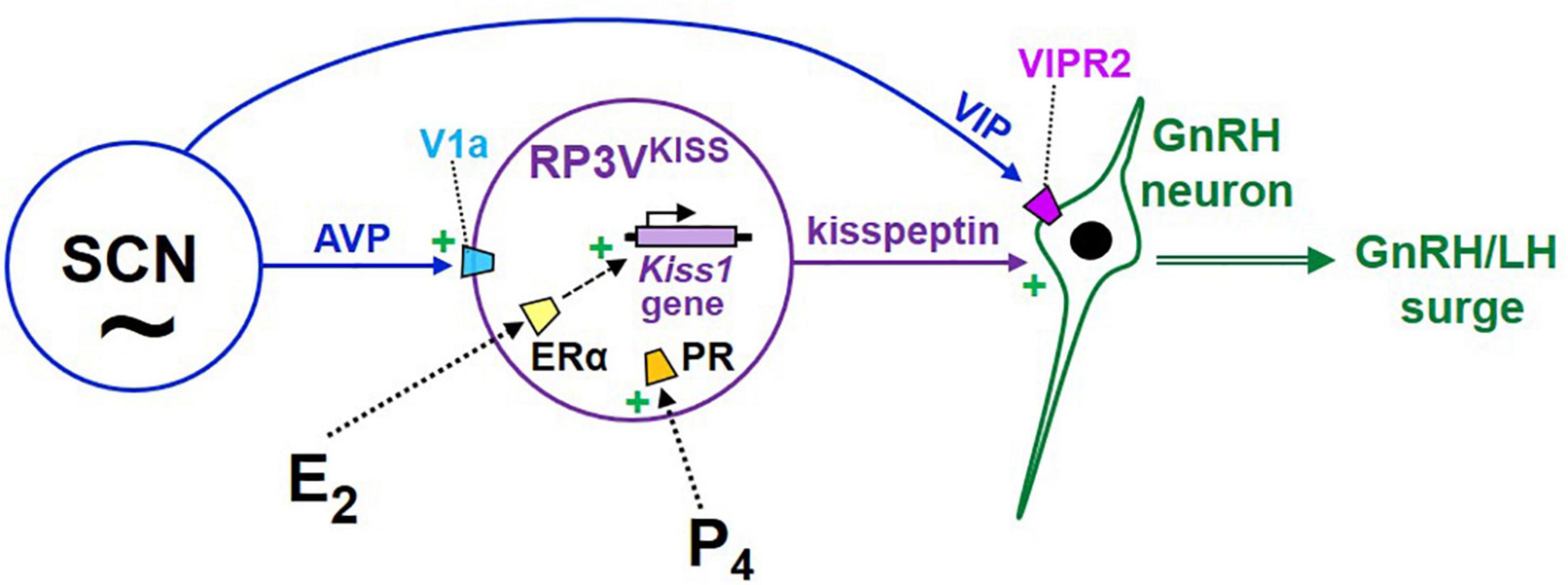
Figure 4. Cartoon model of the proposed hypothalamic neural circuits regulating the E2-induced preovulatory LH surge in female rodents. Under this model, GnRH neurons are activated by upstream kisspeptin neurons in the RP3V, which are E2-sensitive and express both ER and PR. RP3VKISS neurons are themselves also influenced by both progesterone and circadian cues, the latter likely arising from the SCN, including vasopressin neurons. In addition, VIP neurons in the SCN project to GnRH neurons and may be involved in additional modulation of GnRH secretion during E2 positive feedback.
At present, there is only limited, and mostly indirect, functional evidence that the SCNVIP→GnRH circuit is important for the LH surge: (1) in vitro VIP treatment can directly stimulate GnRH neuron electrical activity in female mouse brain slices (Christian and Moenter, 2008; Piet et al., 2016); and (2) in rats, antisense lowering of Vip mRNA levels in the SCN delays and reduces (but does not abolish) the in vivo LH surge and lowers GnRH neuron activation (Harney et al., 1996; Gerhold et al., 2005). This was echoed by another study in rats showing that centrally administered VIP antiserum blunts the amplitude of the LH surge and delays its onset (van der Beek et al., 1999), though the exact neuroanatomical site of action could not be determined in that study. Contrastingly, another study reported that central (icv) antibody blockade of VIP signaling did not alter the LH surge (Murai et al., 1989), though this may be confounded by lack of specificity in the regional and cell targets of central icv infusions. Promisingly, female Vip KO mice have circadian disruptions and are subfertile, including ovulatory deficits (Colwell et al., 2003; Loh et al., 2014), though the presence or timing of the LH surge has not been directly studied and VIP is absent from all brain areas in these KOs, limiting interpretation. Like the antisense Vip knockdown study, the Vip KO findings do not tease apart a possible direct effect of SCN-derived VIP output on GnRH neurons versus an indirect effect of disabled VIP signaling elsewhere or locally within the SCN (important for daily clock function). Still, these limited data suggest VIP may facilitate some aspect of GnRH surge timing, though more compelling direct evidence is still needed, especially in vivo. Indeed, while VIP activates GnRH neuron electrical activity in vitro, several studies report that central VIP injections in young adult female rats surprisingly inhibit the LH surge and GnRH neurons (Weick and Stobie, 1992; Harney et al., 1996; Kauffman et al., 2014), via mechanisms currently unknown. Again, a caveat is that icv VIP treatment may act on multiple brain areas and cell types, including but not limited to RP3VKISS cells, which themselves were also inhibited by icv VIP injection (Kauffman et al., 2014), complicating interpretation. Which VIP receptor that might be mediating effects is also not determined; RP3VKISS and GnRH neurons do not readily express VPIR1, only VIPR2 (Burger et al., 2018; Stephens and Kauffman, 2021), making this receptor a leading candidate. However, other neurons involved may express VPIR1 and its involvement cannot be ruled out. Moreover, electrophysiology recent evidence suggests that VIP may directly communicate with RP3VKISS cells in an excitatory manner during diestrus (Mansano et al., 2022); what the relevance of such diestrus VIP signaling is for the LH surge process and whether this also occurs on proestrus is not yet known.
An indirect SCN→GnRH circuit has been proposed with AVP neurons in the dorsomedial SCN targeting and stimulating RP3VKISS neurons (Figure 4), which then activate GnRH neurons (de la Iglesia et al., 1995; Watson et al., 1995; Leak and Moore, 2001; Kriegsfeld et al., 2004; Herbison, 2008). Anatomically, some SCNAVP neurons project to the RP3V region, including RP3VKISS neurons (Williams et al., 2011; Jamieson et al., 2021) which express V1a (AVP receptor; Williams et al., 2011; Stephens and Kauffman, 2021; Figure 4). This finding of AVP-ir fiber targeting of RP3VKISS neurons has been confirmed at the electron microscopy level in female mice (Vida et al., 2010). Importantly, lesioning the SCN in hamsters removed most AVP appositions on RP3VKISS neurons, suggesting that the primary source of AVP input is the SCN, at least in this species (Williams et al., 2011). In female mice, more kisspeptin neurons show AVP appositions with E2 treatment (Vida et al., 2010); by contrast, in the same animals, VIP connections to RP3VKISS neurons were reported to be far less prevalent, regardless of E2 treatment (Vida et al., 2010). Supporting this anatomical data are correlational findings that SCN AVP levels are circadian, peaking in the late subjective day similar to circadian increases in RP3VKISS neurons and the LH surge (Reppert et al., 1981; Krajnak et al., 1998), and E2 increases V1a levels in the POA area (which contains the RP3V; Funabashi et al., 2000; Kalamatianos et al., 2004).
Functionally, in vitro AVP treatment or optogenetic stimulation of SCNAVP neural fibers in POA brain slices is sufficient to stimulate in vitro RP3VKISS electrical activity via V1a receptor (Piet et al., 2015; Jamieson et al., 2021). This is supported by limited in vivo data that icv AVP injection increases LH secretion in arrhythmic Clock KO mice that normally lack LH surges (though RP3VKISS was not studied; Miller et al., 2006) and stimulates Fos in hamster RP3VKISS neurons (though LH was not measured; Williams et al., 2011). Although those 2 studies did not determine where in the brain the icv AVP injections acted, AVP infused directly into the POA induced an LH surge in SCN-lesioned OVX + E2 rats (Palm et al., 1999). In all cases, it was not determined if the in vivo AVP effects were due to direct or indirect action on RP3VKISS neurons (indeed, RP3VKISS was not examined in 2 of the 3 studies). However, another study in SCN-lesioned OVX + E2 rats (that do not normally show LH surges) used reverse microdialysis to increase extracellular AVP levels specifically in the POA/RP3V area which then led to LH surge-like secretion (Palm et al., 1999). Interestingly, exogenous AVP treatment is effective at inducing the LH surge in the late afternoon, but not at other times of the day, but the reason for this has not yet been determined (Palm et al., 2001).
Promising in vivo evidence showed that central V1a antagonist infusion blocks the occurrence of normal circadian-timed LH surges in female rats on proestrus (Funabashi et al., 1999), implicating endogenous AVP in the LH surge process, though again the target site(s) of action of such central infusion was not determined, nor was the possible neuroanatomical source of AVP (which is also made in non-SCN cells and other brain areas). Moreover, pharmacological blockade of V1a receptors did not result in blunting of the surge in another rat study (Palm et al., 2001), leaving the issue unresolved. Indeed, a third study reported that while a V1a antagonist could prevent stimulatory effects of AVP infusion on LH secretion in Clock mutant mice, suggesting this surge-like secretion is mediated by V1a, the V1a antagonist failed to prevent endogenous proestrous LH surges in WT mice (Miller et al., 2006). That finding suggests that either exogenous AVP treatments are not triggering the actual LH surge generating circuitry or that AVP is sufficient for triggering a surge but not necessary; the latter possibility could be true if additional pathways or factors are also sufficient to activate the endogenous surge in the absence of AVP action. Clearly this is an important and complex issue that needs further addressing. Unfortunately, AVP KO mice cannot be studied for LH surges because they die in development (Zelena, 2017), but female Brattleboro rats (with a spontaneous AVP gene mutation) are subfertile, including reduced conception rates and small litters (Boer et al., 1981, 1982), perhaps due to impaired LH surges (not yet studied).
The collective findings above suggest AVP may induce LH secretion perhaps by activating RP3VKISS cells, though direct regulation of RP3VKISS neurons in vivo has not yet been determined, and “downstream” GnRH neuron activation was often not also studied. Finally, while direct SCNVIP connections to RP3VKISS neurons are reportedly uncommon (Vida et al., 2010; Williams et al., 2011), the possibility of other non-AVP SCN direct projections (or other co-released factors from AVP neurons) to RP3VKISS has not been well studied. Similarly, the SCN projects to other target brain regions besides GnRH neurons and RP3VKISS, which could possibly permit additional indirect SCN anatomical pathways to participate in the gating of the GnRH surge. Indeed, a recent study suggested that GABA signaling arising from the SCN may play a regulatory role in preventing RP3VKISS neuron activation at non-surge times (Jamieson et al., 2021); however, such GABA effects were proposed to be indirect on RP3VKISS neurons and mediated via a multi-synaptic pathway that involves one (or more) intermediary neurons. In addition to possible polysynaptic effects, extra-synaptic mechanisms are also possible, but this needs to be tested. While intriguing, this hypothesis awaits confirmatory findings and additional studies are needed to identify the location and phenotype of any possible intermediary neurons. In addition, several studies previously suggested that SCN-regulated RFRP-3 neurons in the DMN may provide inhibitory regulation on LH surge generation by acting on GnRH or kisspeptin neurons (Kriegsfeld, 2006; Anderson et al., 2009; Khan and Kauffman, 2012; Rizwan et al., 2012; Poling et al., 2013); it was demonstrated that RFRP-3 neuron activation is reduced in females in the early evening, coincident with the LH surge (Gibson et al., 2008; Poling et al., 2017), suggesting that a reduction in inhibitory signaling by RFRP-3 directly or indirectly to GnRH neurons may be a component to the LH surge process. While intriguing, a similar circadian decline in RFRP-3 also occurs in males (Poling et al., 2017), despite their lack of a surge, suggesting this temporal change may be unrelated to the surge event. Moreover, chronically activating RFRP-3 neurons in transgenic female mice to, in theory, provide long-lasting inhibitory input to the reproductive axis does not impact normal fertility or litters (Mamgain et al., 2021); it may be that RFRP-3 serves as a modulator that can blunt the surge under inhibitory physiological conditions, such as during stress or metabolic challenge, rather than being a requisite component of the normal surge process.
Possible circadian components intrinsic to RP3VKISS and gonadotropin-releasing hormone neurons
The circadian nature of the GnRH and LH surge, the demonstrated circadian pattern of RP3VKISS activation, and the abolition of the surge in SCN-lesioned females have suggested a role for the SCN in timing surge generation. However, such an involvement of the SCN does not preclude an important contribution of endogenous molecular circadian clocks in other neural populations. Molecular clocks have been demonstrated in many non-SCN cell types, leading investigators to study whether clock genes in kisspeptin neurons may also be expressed and promote intrinsic circadian rhythms. Although temporal changes in daily clock gene expression of Per1 and Bmal (also known as Arntl) mRNA were reported in the RP3V region of adult female rats (Smarr et al., 2013), that study did not examine clock gene expression specifically in kisspeptin neurons. As such, it was not possible to conclude that the observed changes were occurring in kisspeptin neurons and/or other RP3V cell types. A subsequent study in female mice examined co-expression of PER1, a core clock protein, in RP3VKISS neurons to determine if these neurons contain a circadian oscillator that helps time downstream activation of GnRH secretion (Chassard et al., 2015). Most RP3VKISS neurons were shown to express PER1 with an E2-sensitive daily rhythm (Chassard et al., 2015). However, whether this observed PER1 rhythm is functionally relevant for the surge process was not determined. Moreover, as pointed out by the authors, the presence of rhythmic PER1 in kisspeptin cells is not sufficient on its own to prove the existence of a circadian oscillator in these cells, as the observed PER1 rhythm in kisspeptin neurons could in theory be driven by upstream SCN input (Chassard et al., 2015). The same study analyzed in vitro Per2-luciferase expression in RP3V brain slices (lacking the SCN) and demonstrated a circadian rhythm, though a limitation was that the Per2 expression in this case was not specific to kisspeptin neurons and could be in any number of the many heterogeneous cells in the RP3V region. Still, it is important evidence that circadian rhythms can persist in the RP3V without the input of the SCN; future studies can ascertain if this SCN-independent rhythm occurs specifically in kisspeptin cells.
At present, no study has yet assessed clock gene expression patterns in kisspeptin neurons in the absence of a functional SCN. However, two recent studies approached this issue from another angle by selectively deleting the Bmal gene from just kisspeptin neurons using Cre/lox technology. Both studies found that conditional loss of Bmal in kisspeptin neurons does not impact fertility (Bittman, 2019; Tonsfeldt et al., 2019). One study reported unaltered LH surges at the normal circadian time (Tonsfeldt et al., 2019) while the other study observed the lack of consistent LH surges over a 5-h period (Bittman, 2019). The reason for the discrepancy between studies is unknown but regardless, overall reproductive success was unaltered, unlike global Bmal KO mice which are infertile. Thus, while RP3VKISS neurons clearly exhibit circadian patterns of Kiss1 gene expression and neuronal activation and receive anatomical input from the SCN, the possibility that RP3VKISS neurons are themselves autonomous circadian clocks still requires more compelling supporting evidence. Interestingly, global Bmal KO female mice reportedly can ovulate, though at a reduced rate (Boden et al., 2010); however, their LH surges appear to be absent (Chu et al., 2013), so the endocrine mechanism driving some ovulatory events in these KO mice is unknown and needs further addressing.
Limited evidence suggests that kisspeptin’s stimulation of GnRH cells may also be circadian-gated (Williams et al., 2011). In female hamsters, AVP treatment was shown to increase Fos expression in RP3VKISS neurons in both the morning and afternoon, but surprisingly, similar AVP treatment did not increase Fos in GnRH neurons (Williams et al., 2011). Specifically, GnRH neurons were not activated by AVP treatment in the morning and were also irresponsive in the afternoon, perhaps because they were already activated, although this remains to be studied further. The authors proposed that GnRH neurons possess an intrinsic gating mechanism that modulates their circadian responsiveness to kisspeptin input, thereby making GnRH neurons more sensitive to kisspeptin in the afternoon than the morning. Circadian differences in the ability of kisspeptin treatments to evoke LH secretion in vivo has not yet been studied, though immortalized GnRH cells demonstrate in vitro circadian changes in their responsiveness to exogenous kisspeptin (Zhao and Kriegsfeld, 2009). What permits such a GnRH neuron gating mechanism remains unknown, though GnRH neurons express circadian clock genes in vivo (Hickok and Tischkau, 2010), suggesting these cells might possess circadian machinery to possibly provide intrinsic circadian regulation, but this has not been well studied and has not been teased out from possible incoming SCN input. Interestingly, conditional deletion of Bmal from just GnRH neurons of mice did not alter fertility, C.L. numbers, or the LH surge (Tonsfeldt et al., 2019), suggesting that intrinsic clocks with GnRH cells themselves may not be necessary for proper LH surges and ovulation.
Progesterone’s role in E2 positive feedback and the LH surge
While E2 is clearly essential for positive feedback, we and others have demonstrated that progesterone (P4) and its receptor (PR) are also critical contributors to the LH surge mechanism. Classic studies showed that P4 treatment is able to amplify the magnitude, and in some cases advance the timing, of the LH surge induced by E2 (Everett, 1948; Krey et al., 1973; DePaolo and Barraclough, 1979; Levine and Ramirez, 1980; Lee et al., 1990b; Leite et al., 2016). Conversely, pharmacological blockade of progesterone signaling impaired the rodent LH surge and concurrent GnRH neuron activation (Lee et al., 1990b; Le et al., 1997; Chappell and Levine, 2000). In addition, like ERαKO mice, PR KO female mice cannot produce LH surges, even with proper sex steroid treatment (Chappell et al., 1997, 1999). Thus, P4 signaling is a required component of the LH surge process.
As with ERα, GnRH cells lack PR, indicating P4 acts on “upstream” brain circuitry to regulate the GnRH surge. One such candidate target area is the RP3V region, and specifically, RP3VKISS neurons within it. Chappell and colleagues first tested whether the obligatory P4 action might occur in the general RP3V region; the authors infused PR antisense oligonucleotides into the third ventricle adjacent to the RP3V (termed AVPV in their study) to prevent PR expression in just that area. Unlike control rats, female rats infused with PR antisense oligos near the RP3V did not exhibit any LH surges, suggesting that P4 acts somewhere in that region to influence surge generation (Chappell and Levine, 2000). Because RP3VKISS neurons highly express PR (Clarkson et al., 2008; Zhang et al., 2014; Stephens et al., 2015; Stephens and Kauffman, 2021), our group tested whether PR signaling specifically in kisspeptin cells is required for the LH surge (Stephens et al., 2015). We found that OVX + E2 transgenic mice with selective KO of PR in just kisspeptin cells (termed “KissPRKO” mice) did not show LH surges or proper RP3VKISS neuron activation, as measured by cfos coexpression (Stephens et al., 2015; Figure 5). Along with their impaired LH surges, KissPRKO females also displayed reduced numbers of corpora lutea (an indicator of ovulation) and reduced fecundity in mating trials (Stephens et al., 2015). This finding indicated that endogenous P4 signaling directly in kisspeptin cells is necessary for proper E2-induction of the LH surge, likely by facilitating RP3VKISS cell activation. A subsequent study from another group confirmed these results using a similar mouse model (Gal et al., 2016).
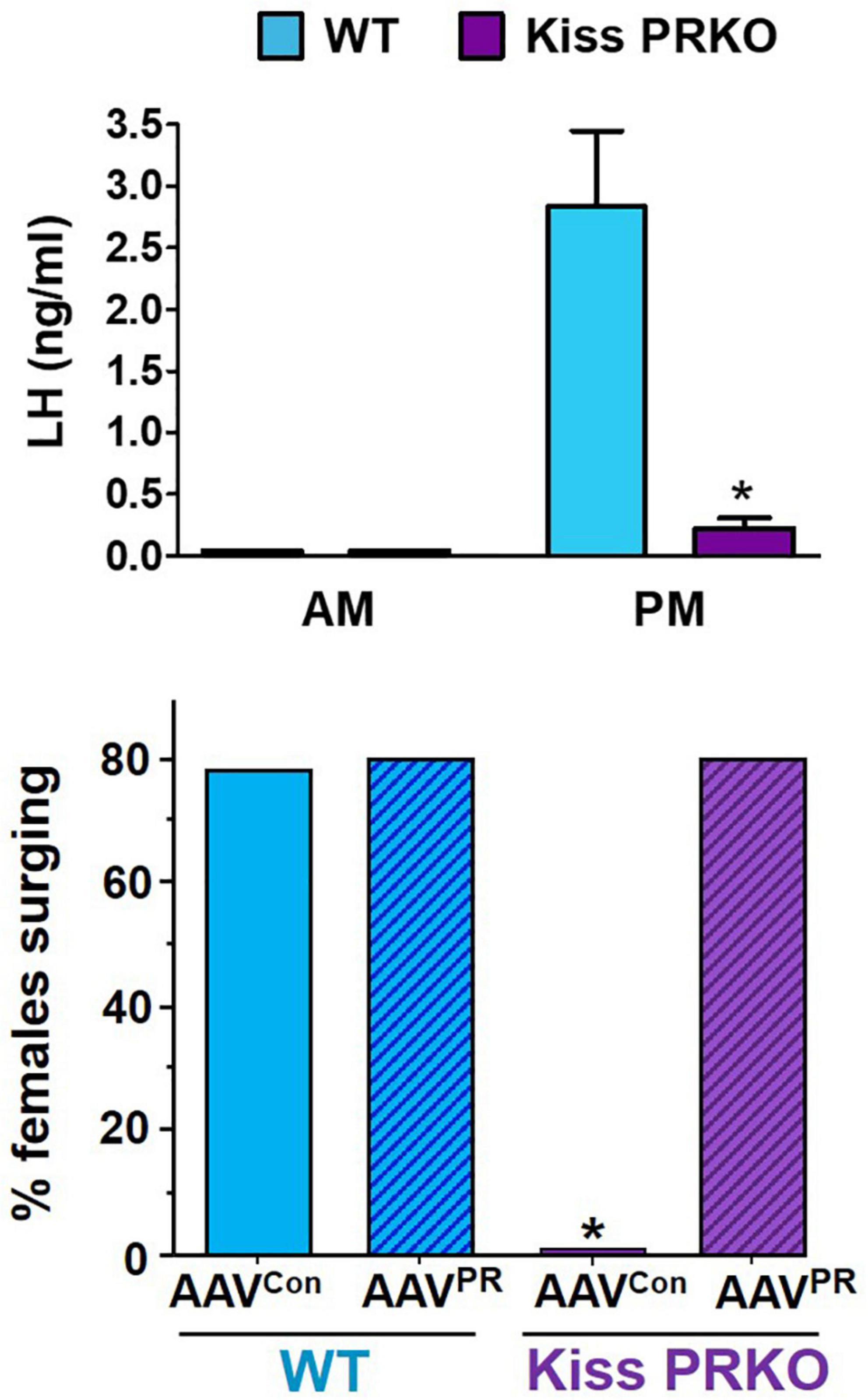
Figure 5. (Top) Importance of progesterone in the LH surge process as indicated by reduced PM LH surge generation in OVX + E2 female mice lacking PR selectively in kisspeptin cells (“Kiss PRKO”; KissCre+/PRflox). Adapted from Stephens et al. (2015); Bottom. Selective re-expression of PR in just RP3VKISS neurons using RP3V targeted AAVs rescues the LH surge occurrence in OVX + E2 Kiss PRKOs. *, Significantly different that WT controls. Adapted from Mohr et al. (2021a) AAVcon, viral delivery of control construct. AAVPR, viral delivery of Cre-dependent PR construct. WT, KissCre–/PRflox females.
While the KissPRKO studies discussed above indicated that endogenous P4 signaling in kisspeptin cells is required for the LH surge, neither study could pinpoint the specific kisspeptin neural population that is necessary for this direct P4 action because PR was deleted in all kisspeptin neurons. Thus, we recently tested if selective re-introduction of PR into just one kisspeptin population of KissPRKOs would rescue the ability to generate LH surges (Mohr et al., 2021a). Given the abundant data implicating the RP3VKISS population in the LH surge, we hypothesized that PR acts directly in those specific neurons to promote the surge. We therefore used targeted RP3V infusions of Cre-dependent AAVs to re-introduce the PR gene (Pgr) selectively in RP3VKISS neurons of KissPRKO females, while leaving PR deleted from all other kisspeptin populations (Mohr et al., 2021a). This selective re-introduction of PR into just RP3VKISS neurons was able to fully restore both the magnitude and prevalence of E2-induced LH surges (Mohr et al., 2021a; Figure 5). This exciting outcome suggests that RP3VKISS neurons are indeed direct targets of P4 action and sufficient for PR’s positive action on the LH surge process (Figure 4).
PR KO and KissPRKO studies indicate that P4 is needed for proper LH surge generation. However, given that those PR KO and KissPRKO females were OVX + E2 with no ovarian P4 or treatment with exogenous P4, it suggests that endogenous P4 of non-ovarian origin is necessary for the surge. Where is such P4 coming from to regulate the surge? Interestingly, intriguing evidence spearheaded by the labs of Micevych and Sinchak suggests that the P4 involved in this process may be of neural origin. Pharmacological blockade of P4 synthesis or action in the brain prevents LH surges in rats (Micevych et al., 2003; Chuon et al., 2022), indicating a necessary role not just for P4 signaling but for P4 derived specifically in the brain. Indeed, insightful in vitro evidence from primary rodent astrocyte cultures shows that E2 induces de novo brain synthesis of P4 (“neuroP”) from ERα-expressing astroglia harvested from the POA region (which contains the RP3V; Sinchak et al., 2003; Micevych et al., 2007; Kuo et al., 2009, 2010a,2010b; Mohr et al., 2021b). Mirroring the in vitro data, in vivo E2 similarly increases P4 levels in the POA region of mice and rats (Mohr et al., 2019, 2021b). This “astrocrine hypothesis” posits that E2 increases cytoplasmic Ca2+ levels in RP3V astrocytes to facilitate neuroP synthesis, which then diffuses out of the glia and acts in a paracrine manner on nearby PR-expressing RP3VKISS neurons (Micevych et al., 2003; Micevych and Sinchak, 2008a,2011; Kuo and Micevych, 2012; Mittelman-Smith et al., 2017, 2018; Mohr et al., 2019; Sinchak et al., 2020). E2 is proposed to exert this effect on astroglia P4 synthesis via membrane-associated ERα (Micevych and Sinchak, 2008b; Kuo et al., 2009, 2010a,2010b; Chen et al., 2014) and not ERβ or GPR30, which are also present in astrocytes. If so, it suggests that ERα plays a dual role in the positive feedback process by acting in both astrocytes and RP3VKISS neurons.
A final thought: Do non-RP3VKISS neurons also participate in the LH surge?
The roles of the RP3V kisspeptin population and SCN has been frequently studied in relation to estrogen positive feedback control of LH surges in rodents. However, this does not exclude the possible involvement of other brain populations in driving or modulating the activation of GnRH neurons during the surge. Indeed, whether or not other kisspeptin neurons outside the RP3V are also involved in the LH surge is not entirely known. Although the evidence mounted in favor of a role of RP3VKISS in the surge event is compelling thus far, similar evidence suggesting an important direct role of ARCKISS in this process has not been abundant. ARCKISS neurons are strongly implicated in governing GnRH pulses, which then stimulate downstream tonic (pulse) LH and FSH section. Because LH and FSH are both required for ovarian E2 synthesis and secretion, the ARCKISS population is therefore indirectly required for preovulatory LH surges in so much as it is required for stimulating the tonic gonadotropin secretion that activates the ovaries. However, whether ARCKISS neurons play a more direct role in the GnRH surge mechanism within the brain, separate form stimulating GnRH pulses, is less certain. Indeed, there is some contradictory evidence on this possibility. The three most compelling pieces of data arguing against a critical role of ARCKISS in the neural GnRH surge process include are that (1) elevated E2 inhibits Kiss1 (and Tac2 and Pdyn) gene expression in the ARC (Smith et al., 2005a; Gottsch et al., 2009; Navarro et al., 2009), effectively reducing kisspeptin, NKB, and dynorphin synthesis under hormonal conditions when the surge occurs; (2) ARCKISS neurons do not show increased cfos induction during the surge or between diestrus and proestrus as occurs in AVPVKISS cells (Adachi et al., 2007), indicating ARCKISS neuron activation is not increased during the surge event; and (3) long-term fiber photometry recording of in vivo ARCKISS neuron activation (which correlates strongly with occurrence of LH pulses) over the course of the female mouse estrous cycle) demonstrates no increase in activation during the afternoon or evening of proestrus when the LH surge occurs (McQuillan et al., 2019). This collective evidence suggests that ARCKISS neurons may not be a required player in the neural LH surge mechanism in rodents. Supporting this, ablation of the majority of ARCKISS neurons in female rats does not prevent the normal occurrence of E2-induced LH surges (Helena et al., 2015; Mittelman-Smith et al., 2016).
Despite the evidence above that ARCKISS is likely not required in the LH surge neural mechanism, several studies have proposed that ARCKISS may play a modulatory role of the surge, based on limited evidence. First, ARCKISS neurons are shown to project not only to GnRH dendron terminals, but also to other brain areas, including (but not limited to) RP3VKISS neurons (Qiu et al., 2016). While purely anatomical, this evidence at least supports a possibility, yet to be tested, that ARCKISS neurons may modulate RP3VKISS neurons via the former’s ongoing “basal” activity and, perhaps, via glutamate signaling (Qiu et al., 2016); if so, enhanced activation of ARCKISS neurons at the time of the surge may not be requisite for such modulatory effects, but this requires further examination. Second, female rats sustaining ablation of their ARCKISS neurons show E2-induced LH surges of higher magnitude than females with an intact ARCKISS population (Helena et al., 2015; Mittelman-Smith et al., 2016). The authors interpret this outcome to indicate there may normally be some inhibitory factor released by ARCKISS neurons which serves to curb the amplitude of the LH surge, and in the absence of ARCKISS neurons, this inhibition is removed, resulting in a higher surge. One of these studies proposed that dynorphin released from ARCKISS neurons may be this inhibitory factor (Helena et al., 2015). However, given the reported lack of ARCKISS neuron activation at the time of the surge, it is unclear how dynorphin would be secreted from those neurons to achieve this effect. Moreover, Pdyn levels in the ARC are strongly reduced in the presence of elevated E2, and ARC dynorphin levels would therefore be low at the time of surge. Finally, a recent study using optogenetics in female mice reported that experimental activation of ARCKISS neurons for 2 h elicits a robust increase in serum LH that resembles an LH surge-like secretion (Lin et al., 2021). The authors proposed that ARCKISS neurons may therefore amplify the LH surge. Alternatively, it is also possible that experimentally forcing ARCKISS neurons to strongly fire for a sustained period of time would cause a corresponding sustained activation of GnRH neuron dendron terminals and prolonged high GnRH secretion, leading to a large secretion of LH. Such LH release may appear like an LH surge but may not represent the output of the LH surge mechanism which likely involves activation of GnRH soma rather than dendron terminals (Wang et al., 2020). Thus, while activating ARCKISS neurons is sufficient to induce strong LH secretion (as expected), this does not implicate the ARCKISS population in the normal endogenous GnRH surge mechanism. Moreover, if ARCKISS neurons provide amplification of the surge, ablation of such neurons would be predicted to reduce the surge amplitude, but the rat studies reported an enhanced surge magnitude in the absence of ARCKISS neurons (Helena et al., 2015; Mittelman-Smith et al., 2016). Finally, although ARC-specific Kiss1 mRNA knockdown in female rats caused a lower LH surge amplitude (with normal surge incidence; Hu et al., 2015), these females were ovary-intact; thus, the 32% ARC Kiss1 knockdown likely impacted downstream endogenous ovarian E2 synthesis, which may explain the observed reduction in surge magnitude.
Overall, while the current data suggesting that ARCKISS neurons are not necessary for the normal surge mechanism may be more compelling than the other side of the argument, clearly more studies are needed to directly resolve this issue, and it remains possible that ARCKISS neurons provide some non-requisite modulatory role. It should also be reiterated that in some other species, such as sheep and monkeys, ARCKISS neurons are better implicated in the LH surge mechanism, though rostral hypothalamic kisspeptin neurons (similar to RP3VKISS) also show activation in these species and may also be involved (Smith, 2009; Smith et al., 2010; Watanabe et al., 2014; Vargas Trujillo et al., 2017).
Finally, we first described the presence of a small estrogen-sensitive kisspeptin population in the medial amygdala region of rodents (Kim et al., 2011). MeAKISS neurons are more prevalent in males than females, but show moderately increased Kiss1 levels in the presence of E2 and on proestrus versus diestrus (Kim et al., 2011; Stephens et al., 2016, 2018). Whether this small population of MeAKISS neurons play a role in HPG axis regulation specifically during the LH surge remains unknown. A few studies in mice have experimentally activated MeAKISS neurons via optogenetics or chemogenetics but reported only minor increases in LH secretion (Fergani et al., 2018; Aggarwal et al., 2019; Lass et al., 2020); notably, the pattern of LH release elicited did not resemble a large LH surge profile, suggesting that MeAKISS neurons are not major players in the E2-induced LH surge mechanism. It remains possible MeAKISS may play a modulatory role in pheromone-induced LH surges induced by conspecific exposure or in aspects of socio-sexual behavior (Adekunbi et al., 2018; Aggarwal et al., 2019), though the data thus far are very limited and more supporting evidence is needed to evaluate such possibilities.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
The author’s research is supported by NIH grants R01 HD090161, R01 HD100580, and P50 HD012303.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrahamson, E. E., and Moore, R. Y. (2001). Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 916, 172–191. doi: 10.1016/s0006-8993(01)02890-6
Adachi, S., Yamada, S., Takatsu, Y., Matsui, H., Kinoshita, M., Takase, K., et al. (2007). Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 53, 367–378. doi: 10.1262/jrd.18146
Adekunbi, D. A., Li, X. F., Lass, G., Shetty, K., Adegoke, O. A., Yeo, S. H., et al. (2018). Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J. Neuroendocrinol. 30:e12572. doi: 10.1111/jne.12572
Aggarwal, S., Tang, C., Sing, K., Kim, H. W., Millar, R. P., and Tello, J. A. (2019). Medial amygdala Kiss1 neurons mediate female pheromone stimulation of luteinizing hormone in male mice. Neuroendocrinology 108, 172–189. doi: 10.1159/000496106
Alleva, J. J., Waleski, M. V., and Alleva, F. R. (1971). A biological clock controlling the estrous cycle of the hamster. Endocrinology 88, 1368–1379. doi: 10.1210/endo-88-6-1368
Anderson, G. M., Relf, H. L., Rizwan, M. Z., and Evans, J. J. (2009). Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology 150, 1834–1840. doi: 10.1210/en.2008-1359
Antle, M. C., and Silver, R. (2005). Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 28, 145–151. doi: 10.1016/j.tins.2005.01.003
Berga, S., and Naftolin, F. (2012). Neuroendocrine control of ovulation. Gynecol. Endocrinol. 28, 9–13. doi: 10.3109/09513590.2012.651929
Bishop, W., Kalra, P. S., Fawcett, C. P., Krulich, L., and McCann, S. M. (1972). The effects of hypothalamic lesions on the release of gonadotropins and prolactin in response to estrogen and progesterone treatment in female rats. Endocrinology 91, 1404–1410. doi: 10.1210/endo-91-6-1404
Bittman, E. L. (2019). Circadian function in multiple cell types is necessary for proper timing of the preovulatory LH surge. J. Biol. Rhythms 34, 622–633. doi: 10.1177/0748730419873511
Bloch, G. J., Kurth, S. M., Akesson, T. R., and Micevych, P. E. (1992). Estrogen-concentrating cells within cell groups of the medial preoptic area: sex differences and co-localization with galanin-immunoreactive cells. Brain Res. 595, 301–308. doi: 10.1016/0006-8993(92)91064-l
Boden, M. J., Varcoe, T. J., Voultsios, A., and Kennaway, D. J. (2010). Reproductive biology of female Bmal1 null mice. Reproduction 139, 1077–1090. doi: 10.1530/REP-09-0523
Boer, G. J., Boer, K., and Swaab, D. F. (1982). On the reproductive and developmental differences within the Brattleboro strain. Ann. N. Y. Acad. Sci. 394, 37–45. doi: 10.1111/j.1749-6632.1982.tb37409.x
Boer, K., Boer, G. J., and Swaab, D. F. (1981). Reproduction in Brattleboro rats with diabetes insipidus. J. Reprod. Fertil. 61, 273–280. doi: 10.1530/jrf.0.0610273
Brown-Grant, K., and Raisman, G. (1977). Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc. R. Soc. Lond. B Biol. Sci. 198, 279–296. doi: 10.1098/rspb.1977.0098
Buhl, A. E., Norman, R. L., and Resko, J. A. (1978). Sex differences in estrogen-induced gonadotropin release in hamsters. Biol. Reprod. 18, 592–597. doi: 10.1095/biolreprod18.4.592
Burger, L. L., Vanacker, C., Phumsatitpong, C., Wagenmaker, E. R., Wang, L., Olson, D. P., et al. (2018). Identification of genes enriched in GnRH neurons by translating ribosome affinity purification and RNAseq in mice. Endocrinology 159, 1922–1940. doi: 10.1210/en.2018-00001
Cahill, D. J., Wardle, P. G., Harlow, C. R., and Hull, M. G. (1998). Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil. Steril. 70, 56–59. doi: 10.1016/s0015-0282(98)00113-7
Caligaris, L., Astrada, J. J., and Taleisnik, S. (1971). Release of luteinizing hormone induced by estrogen injection into ovariectomized rats. Endocrinology 88, 810–815. doi: 10.1210/endo-88-4-810
Card, J. P., Fitzpatrick-McElligott, S., Gozes, I., and Baldino, F. (1988). Localization of vasopressin-, vasoactive intestinal polypeptide-, peptide histidine isoleucine- and somatostatin-mRNA in rat suprachiasmatic nucleus. Cell Tissue Res. 252, 307–315. doi: 10.1007/BF00214373
Chappell, P. E., and Levine, J. E. (2000). Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology 141, 1477–1485. doi: 10.1210/endo.141.4.7428
Chappell, P. E., Lydon, J. P., Conneely, O. M., O’Malley, B. W., and Levine, J. E. (1997). Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 138, 4147–4152. doi: 10.1210/endo.138.10.5456
Chappell, P. E., Schneider, J. S., Kim, P., Xu, M., Lydon, J. P., O’Malley, B. W., et al. (1999). Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology 140, 3653–3658. doi: 10.1210/endo.140.8.6895
Chassard, D., Bur, I., Poirel, V. J., Mendoza, J., and Simonneaux, V. (2015). Evidence for a putative circadian kiss-clock in the hypothalamic AVPV in female mice. Endocrinology 156, 2999–3011. doi: 10.1210/en.2014-1769
Chen, C., Kuo, J., Wong, A., and Micevych, P. (2014). Estradiol modulates translocator protein (TSPO) and steroid acute regulatory protein (StAR) via protein kinase a (PKA) signaling in hypothalamic astrocytes. Endocrinology 155, 2976–2985. doi: 10.1210/en.2013-1844
Cheng, M. Y., Bullock, C. M., Li, C., Lee, A. G., Bermak, J. C., Belluzzi, J., et al. (2002). Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417, 405–410. doi: 10.1038/417405a
Cheong, R. Y., Czieselsky, K., Porteous, R., and Herbison, A. E. (2015). Expression of ESR1 in glutamatergic and GABAergic neurons is essential for normal puberty onset, estrogen feedback, and fertility in female mice. J. Neurosci. 35, 14533–14543. doi: 10.1523/JNEUROSCI.1776-15.2015
Chongthammakun, S., and Terasawa, E. (1993). Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology 132, 735–743. doi: 10.1210/endo.132.2.8425492
Christian, C. A., and Moenter, S. M. (2008). Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology 149, 3130–3136. doi: 10.1210/en.2007-1098
Christian, C. A., and Moenter, S. M. (2010). The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr. Rev. 31, 544–577. doi: 10.1210/er.2009-0023
Christian, C. A., Glidewell-Kenney, C., Jameson, J. L., and Moenter, S. M. (2008). Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 149, 5328–5334. doi: 10.1210/en.2008-0520
Chu, A., Zhu, L., Blum, I. D., Mai, O., Leliavski, A., Fahrenkrug, J., et al. (2013). Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology 154, 2924–2935. doi: 10.1210/en.2013-1080
Chuon, T., Feri, M., Carlson, C., Ondrejik, S., Micevych, P. E., and Sinchak, K. (2022). Progesterone receptor-Src kinase signaling pathway mediates neuroprogesterone induction of the luteinizing hormone surge in female rats. J. Neuroendocrinol. 34:e13071. doi: 10.1111/jne.13071
Clarkson, J., and Herbison, A. E. (2006). Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147, 5817–5825. doi: 10.1210/en.2006-0787
Clarkson, J., d’Anglemont de Tassigny, X., Moreno, A. S., Colledge, W. H., and Herbison, A. E. (2008). Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J. Neurosci. 28, 8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008
Clarkson, J., de Tassigny, X. D., Colledge, W. H., Caraty, A., and Herbison, A. E. (2009). Distribution of kisspeptin neurons in the adult female mouse brain. J. Neuroendocrinol. 21, 673–682. doi: 10.1111/j.1365-2826.2009.01892.x
Clarkson, J., Han, S. Y., Piet, R., McLennan, T., Kane, G. M., Ng, J., et al. (2017). Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. U S A. 114, E10216–E10223. doi: 10.1073/pnas.1713897114
Colwell, C. S., Michel, S., Itri, J., Rodriguez, W., Tam, J., Lelievre, V., et al. (2003). Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R939–R949. doi: 10.1152/ajpregu.00200.2003
Couse, J. F., Yates, M. M., Walker, V. R., and Korach, K. S. (2003). Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 17, 1039–1053. doi: 10.1210/me.2002-0398
Dai, J., Swaab, D. F., Van der Vliet, J., and Buijs, R. M. (1998). Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. J. Comp. Neurol. 400, 87–102. doi: 10.1002/(SICI)1096-9861(19981012)400:1<87::AID-CNE6>3.0.CO;2-P
d’Anglemont de Tassigny, X., Fagg, L. A., Carlton, M. B., and Colledge, W. H. (2008). Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149, 3926–3932. doi: 10.1210/en.2007-1487
d’Anglemont de Tassigny, X., Fagg, L. A., Dixon, J. P., Day, K., and Leitch, H. G. (2007). Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc. Natl. Acad. Sci. U S A. 104, 10714–10719. doi: 10.1073/pnas.0704114104
Dardente, H., Menet, J. S., Challet, E., Tournier, B. B., Pevet, P., and Masson-Pevet, M. (2004). Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res. Mol. Brain Res. 124, 143–151. doi: 10.1016/j.molbrainres.2004.01.010
de la Iglesia, H. O., and Schwartz, W. J. (2006). Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 147, 1148–1153. doi: 10.1210/en.2005-1311
de la Iglesia, H. O., Blaustein, J. D., and Bittman, E. L. (1995). The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport 6, 1715–1722. doi: 10.1097/00001756-199509000-00004
de la Iglesia, H. O., Meyer, J., and Schwartz, W. J. (2003). Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J. Neurosci. 23, 7412–7414. doi: 10.1523/JNEUROSCI.23-19-07412.2003
de Roux, N., Genin, E., Carel, J. C., Matsuda, F., Chaussain, J. L., and Milgrom, E. (2003). Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U S A. 100, 10972–10976. doi: 10.1073/pnas.1834399100
DeFazio, R. A., Elias, C. F., and Moenter, S. M. (2014). GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. J Neurosci. 34, 16296–16308. doi: 10.1523/JNEUROSCI.3057-14.2014
DePaolo, L. V., and Barraclough, C. A. (1979). Dose dependent effects of progesterone on the facilitation and inhibition of spontaneous gonadotropin surges in estrogen treated ovariectomized rats. Biol. Reprod. 21, 1015–1023. doi: 10.1095/biolreprod21.4.1015
Deurveilher, S., and Semba, K. (2005). Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience 130, 165–183. doi: 10.1016/j.neuroscience.2004.08.030
Dhillo, W. S., Chaudhri, O. B., Patterson, M., Thompson, E. L., Murphy, K. G., Badman, M. K., et al. (2005). Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. Clin. Endocrinol. Metab. 90, 6609–6615. doi: 10.1210/jc.2005-1468
Dror, T., Franks, J., and Kauffman, A. S. (2013). Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol. Reprod. 88, 1–8. doi: 10.1095/biolreprod.113.108555
Dubois, S. L., Acosta-Martinez, M., DeJoseph, M. R., Wolfe, A., Radovick, S., Boehm, U., et al. (2015). Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor alpha in kisspeptin neurons. Endocrinology 156, 1111–1120. doi: 10.1210/en.2014-1851
Dubois, S. L., Wolfe, A., Radovick, S., Boehm, U., and Levine, J. E. (2016). Estradiol restrains prepubertal gonadotropin secretion in female mice via activation of eralpha in kisspeptin neurons. Endocrinology 157, 1546–1554. doi: 10.1210/en.2015-1923
Evans, N. P., Dahl, G. E., Glover, B. H., and Karsch, F. J. (1994). Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 134, 1806–1811. doi: 10.1210/endo.134.4.8137746
Everett, J. W. (1948). Progesterone and estrogen in the experimental control of ovulation time and other features of the estrous cycle in the rat. Endocrinology 43, 389–405. doi: 10.1210/endo-43-6-389
Everett, J. W., and Sawyer, C. H. (1950). A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology 47, 198–218. doi: 10.1210/endo-47-3-198
Everett, J. W., Sawyer, C. H., and Markee, J. E. (1949). A neurogenic timing factor in control of the ovulatory discharge of luteinizing hormone in the cyclic rat. Endocrinology 44, 234–250. doi: 10.1210/endo-44-3-234
Fergani, C., Leon, S., Padilla, S. L., Verstegen, A. M., Palmiter, R. D., and Navarro, V. M. (2018). NKB signaling in the posterodorsal medial amygdala stimulates gonadotropin release in a kisspeptin-independent manner in female mice. eLife 7:e40476. doi: 10.7554/eLife.40476
Ferin, M., Carmel, P. W., Zimmerman, E. A., Warren, M., Perez, R., and Vande Wiele, R. L. (1974). Location of intrahypothalamic estrogen-responsive sites influencing LH secretion in the female Rhesus monkey. Endocrinology 95, 1059–1068. doi: 10.1210/endo-95-4-1059
Fitzgerald, K., and Zucker, I. (1976). Circadian organization of the estrous cycle of the golden hamster. Proc. Natl. Acad. Sci. U S A. 73, 2923–2927. doi: 10.1073/pnas.73.8.2923
Freeman, M. E. (1994). “The neuroendocrine control of the ovarian cycle of the rat,” in The Physiology of Reproduction, eds E. Knobil and J. D. Neill (New York: Raven Press).
Freeman, M. E. (2006). “Neuroendocrine control of the ovarian cycle in the rat,” in Physiology of Reproduction, 3rd Edn, ed. J. D. Neill (Carnforth: Parthenon Publishing Group). doi: 10.1016/B978-012515400-0/50048-8
Funabashi, T., Aiba, S., Sano, A., Shinohara, K., and Kimura, F. (1999). Intracerebroventricular injection of arginine-vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrous rats. Neurosci Lett. 260, 37–40. doi: 10.1016/s0304-3940(98)00940-9
Funabashi, T., Shinohara, K., Mitsushima, D., and Kimura, F. (2000). Estrogen increases arginine-vasopressin V1a receptor mRNA in the preoptic area of young but not of middle-aged female rats. Neurosci. Lett. 285, 205–208. doi: 10.1016/s0304-3940(00)01069-7
Gal, A., Lin, P. C., Cacioppo, J. A., Hannon, P. R., Mahoney, M. M., Wolfe, A., et al. (2016). Loss of fertility in the absence of progesterone receptor expression in kisspeptin neurons of female mice. PLoS One 11:e0159534. doi: 10.1371/journal.pone.0159534
Gerhold, L. M., Rosewell, K. L., and Wise, P. M. (2005). Suppression of vasoactive intestinal polypeptide in the suprachiasmatic nucleus leads to aging-like alterations in cAMP rhythms and activation of gonadotropin-releasing hormone neurons. J. Neurosci. 25, 62–67. doi: 10.1523/JNEUROSCI.3598-04.2005
Gibson, E. M., Humber, S. A., Jain, S., Williams, W. P., Zhao, S., Bentley, G. E., et al. (2008). Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 149, 4958–4969. doi: 10.1210/en.2008-0316
Goodman, R. L. (1978). The site of the positive feedback action of estradiol in the rat. Endocrinology 102, 151–159. doi: 10.1210/endo-102-1-151
Goodman, R. L., and Inskeep, E. K. (2006). “Neuroendocrine control of the ovarian cycle of the sheep,” in The Physiology of Reproduction, eds E. Knobil and J. D. Neill (New York: Raven Press Ltd). doi: 10.1016/B978-012515400-0/50049-X
Goodman, R. L., Herbison, A. E., Lehman, M. N., and Navarro, V. M. (2022). Neuroendocrine control of gonadotropin-releasing hormone: pulsatile and surge modes of secretion. J. Neuroendocrinol. 18:e13094. doi: 10.1111/jne.13094
Gottsch, M. L., Cunningham, M. J., Smith, J. T., Popa, S. M., Acohido, B. V., Crowley, W. F., et al. (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077. doi: 10.1210/en.2004-0431
Gottsch, M. L., Navarro, V. M., Zhao, Z., Glidewell-Kenney, C., Weiss, J., Jameson, J. L., et al. (2009). Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J. Neurosci. 29, 9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009
Gray, G. D., Soderstein, P., Tallentire, D., and Davidson, J. M. (1978). Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats. Neuroendocrinology 25, 174–191. doi: 10.1159/000122739
Groblewski, T. A., Nunez, A. A., and Gold, R. M. (1981). Circadian rhythms in vasopressin deficient rats. Brain Res Bull. 6, 125–130. doi: 10.1016/S0361-9230(81)80036-6
Han, S. K., Gottsch, M. L., Lee, K. J., Popa, S. M., Smith, J. T., Jakawich, S. K., et al. (2005). Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 25, 11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005
Han, S. Y., Kane, G., Cheong, I., and Herbison, A. E. (2019). Characterization of GnRH pulse generator activity in male mice using GCaMP fiber photometry. Endocrinology 160, 557–567. doi: 10.1210/en.2018-01047
Han, S. Y., McLennan, T., Czieselsky, K., and Herbison, A. E. (2015). Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc. Natl. Acad. Sci. U S A. 112, 13109–13114. doi: 10.1073/pnas.1512243112
Harmar, A. J., Marston, H. M., Shen, S., Spratt, C., West, K. M., Sheward, W. J., et al. (2002). The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109, 497–508. doi: 10.1016/s0092-8674(02)00736-5
Harney, J. P., Scarbrough, K., Rosewell, K. L., and Wise, P. M. (1996). In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology 137, 3696–3701. doi: 10.1210/endo.137.9.8756535
Helena, C. V., Toporikova, N., Kalil, B., Stathopoulos, A. M., Pogrebna, V. V., Carolino, R. O., et al. (2015). KNDy neurons modulate the magnitude of the steroid-induced luteinizing hormone surges in ovariectomized rats. Endocrinology 156, 4200–4213. doi: 10.1210/en.2015-1070
Herbison, A. E. (2008). Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res. Rev. 57, 277–287. doi: 10.1016/j.brainresrev.2007.05.006
Herbison, A. E. (2018). The gonadotropin-releasing hormone pulse generator. Endocrinology 159, 3723–3736. doi: 10.1210/en.2018-00653
Herbison, A. E. (2020). A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front. Neuroendocrinol. 57:100837. doi: 10.1016/j.yfrne.2020.100837
Herbison, A. E., and Theodosis, D. T. (1992). Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 50, 283–298. doi: 10.1016/0306-4522(92)90423-Y
Hickok, J. R., and Tischkau, S. A. (2010). In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology 91, 110–120.
Homma, T., Sakakibara, M., Yamada, S., Kinoshita, M., Iwata, K., Tomikawa, J., et al. (2009). Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol. Reprod. 81, 1216–1225. doi: 10.1095/biolreprod.109.078311
Hu, M. H., Li, X. F., McCausland, B., Li, S. Y., Gresham, R., Kinsey-Jones, J. S., et al. (2015). Relative importance of the arcuate and anteroventral periventricular kisspeptin neurons in control of puberty and reproductive function in female rats. Endocrinology 156, 2619–2631. doi: 10.1210/en.2014-1655
Irwig, M. S., Fraley, G. S., Smith, J. T., Acohido, B. V., Popa, S. M., Cunningham, M. J., et al. (2004). Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80, 264–272. doi: 10.1159/000083140
Jamieson, B. B., Bouwer, G. T., Campbell, R. E., and Piet, R. (2021). Estrous cycle plasticity in the central clock output to kisspeptin neurons: implications for the preovulatory surge. Endocrinology 162:bqab071. doi: 10.1210/endocr/bqab071
Kalamatianos, T., Kallo, I., Goubillon, M. L., and Coen, C. W. (2004). Cellular expression of V1a vasopressin receptor mRNA in the female rat preoptic area: effects of oestrogen. J. Neuroendocrinol. 16, 525–533. doi: 10.1111/j.1365-2826.2004.01199.x
Kalra, P. S., and McCann, S. M. (1975). The stimulatory effect on gonadotropin release of implants of estradiol or progesterone in certain sites in the central nervous system. Neuroendocrinology 19, 289–302. doi: 10.1159/000122450
Kalsbeek, A., Buijs, R. M., van Heerikhuize, J. J., Arts, M., and van der Woude, T. P. (1992). Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 580, 62–67. doi: 10.1016/0006-8993(92)90927-2
Kalsbeek, A., van der Vliet, J., and Buijs, R. M. (1996a). Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: a reverse microdialysis study. J. Neuroendocrinol. 8, 299–307. doi: 10.1046/j.1365-2826.1996.04597.x
Kalsbeek, A., van Heerikhuize, J. J., Wortel, J., and Buijs, R. M. (1996b). A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J. Neurosci. 16, 5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996
Kalsbeek, A., Verhagen, L. A., Schalij, I., Foppen, E., Saboureau, M., Bothorel, B., et al. (2008). Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur. J. Neurosci. 27, 818–827. doi: 10.1111/j.1460-9568.2008.06057.x
Kauffman, A. S. (2010a). Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol. Cell Endocrinol. 324, 51–63. doi: 10.1016/j.mce.2010.01.017
Kauffman, A. S. (2010b). “Mammalian female sexual behavior and hormones,” in Encyclopedia of Animal Behavior, eds M. D. Breed and J. Moore (Oxford: Academic Press). doi: 10.1016/B978-0-08-045337-8.00276-X
Kauffman, A. S., Park, J. H., McPhie-Lalmansingh, A. A., Gottsch, M. L., Bodo, C., Hohmann, J. G., et al. (2007c). The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J. Neurosci. 27, 8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007
Kauffman, A. S., Gottsch, M. L., Roa, J., Byquist, A. C., Crown, A., Clifton, D. K., et al. (2007b). Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148, 1774–1783. doi: 10.1210/en.2006-1540
Kauffman, A. S., Clifton, D. K., and Steiner, R. A. (2007a). Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 30, 504–511. doi: 10.1016/j.tins.2007.08.001
Kauffman, A. S., Navarro, V. M., Kim, J., Clifton, D. K., and Steiner, R. A. (2009). Sex Differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am. J. Physiol. Endocrinol. Metab. 297, 1212–1221. doi: 10.1152/ajpendo.00461.2009
Kauffman, A. S., Sun, Y., Kim, J., Khan, A. R., Shu, J., and Neal-Perry, G. (2014). Vasoactive intestinal peptide modulation of the steroid-induced LH surge involves kisspeptin signaling in young but not in middle-aged female rats. Endocrinology 155, 2222–2232. doi: 10.1210/en.2013-1793
Kawakami, M., Arita, J., and Yoshioka, E. (1980). Loss of estrogen-induced daily surges of prolactin and gonadotropins by suprachiasmatic nucleus lesions in ovariectomized rats. Endocrinology 106, 1087–1092. doi: 10.1210/endo-106-4-1087
Kerdelhue, B., Brown, S., Lenoir, V., Queenan, J. T., Jones, G. S., Scholler, R., et al. (2002). Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology 75, 158–163. doi: 10.1159/000048233
Khan, A. R., and Kauffman, A. S. (2012). The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J. Neuroendocrinol. 24, 131–143. doi: 10.1111/j.1365-2826.2011.02162.x
Kim, J., Semaan, S. J., Clifton, D. K., Steiner, R. A., Dhamija, S., and Kauffman, A. S. (2011). Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152, 2020–2030. doi: 10.1210/en.2010-1498
Kinoshita, M., Tsukamura, H., Adachi, S., Matsui, H., Uenoyama, Y., Iwata, K., et al. (2005). Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146, 4431–4436. doi: 10.1210/en.2005-0195
Krajnak, K., Kashon, M. L., Rosewell, K. L., and Wise, P. M. (1998). Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology 139, 4189–4196. doi: 10.1210/endo.139.10.6259
Krey, L. C., Tyrey, L., and Everett, J. W. (1973). The estrogen-induced advance in the cyclic LH surge in the rat: dependency on ovarian progesterone secretion. Endocrinology 93, 385–390. doi: 10.1210/endo-93-2-385
Kriegsfeld, L. J. (2006). Driving reproduction: RFamide peptides behind the wheel. Horm. Behav. 50, 655–666. doi: 10.1016/j.yhbeh.2006.06.004
Kriegsfeld, L. J., Leak, R. K., Yackulic, C. B., LeSauter, J., and Silver, R. (2004). Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J. Comp. Neurol. 468, 361–379. doi: 10.1002/cne.10995
Kuo, J., and Micevych, P. (2012). Neurosteroids, trigger of the LH surge. J. Steroid Biochem. Mol. Biol. 131, 57–65. doi: 10.1016/j.jsbmb.2012.01.008
Kuo, J., Hamid, N., Bondar, G., Dewing, P., Clarkson, J., and Micevych, P. (2010a). Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biol. Sex Differ. 1:7. doi: 10.1186/2042-6410-1-7
Kuo, J., Hamid, N., Bondar, G., Prossnitz, E. R., and Micevych, P. (2010b). Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J. Neurosci. 30, 12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010
Kuo, J., Hariri, O. R., Bondar, G., Ogi, J., and Micevych, P. (2009). Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology 150, 1369–1376. doi: 10.1210/en.2008-0994
Lapatto, R., Pallais, J. C., Zhang, D., Chan, Y. M., Mahan, A., Cerrato, F., et al. (2007). Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 148, 4927–4936. doi: 10.1210/en.2007-0078
Lass, G., Li, X. F., de Burgh, R. A., He, W., Kang, Y., Hwa-Yeo, S., et al. (2020). Optogenetic stimulation of kisspeptin neurones within the posterodorsal medial amygdala increases luteinising hormone pulse frequency in female mice. J Neuroendocrinol. 32:e12823. doi: 10.1111/jne.12823
Le, W. W., Attardi, B., Berghorn, K. A., Blaustein, J., and Hoffman, G. E. (1997). Progesterone blockade of a luteinizing hormone surge blocks luteinizing hormone-releasing hormone Fos activation and activation of its preoptic area afferents. Brain Res. 778, 272–280. doi: 10.1016/s0006-8993(97)00971-2
Le, W. W., Berghorn, K. A., Rassnick, S., and Hoffman, G. E. (1999). Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology 140, 510–519. doi: 10.1210/endo.140.1.6403
Le, W. W., Wise, P. M., Murphy, A. Z., Coolen, L. M., and Hoffman, G. E. (2001). Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology 142, 4976–4982. doi: 10.1210/endo.142.11.8470
Leak, R. K., and Moore, R. Y. (2001). Topographic organization of suprachiasmatic nucleus projection neurons. J. Comp. Neurol. 433, 312–334. doi: 10.1002/cne.1142
Lee, I. T., Chang, A. S., Manandhar, M., Shan, Y., Fan, J., Izumo, M., et al. (2015). Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron 85, 1086–1102. doi: 10.1016/j.neuron.2015.02.006
Lee, W. S., Smith, M. S., and Hoffman, G. E. (1990a). Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc. Natl. Acad. Sci. U S A. 87, 5163–5167. doi: 10.1073/pnas.87.13.5163
Lee, W. S., Smith, M. S., and Hoffman, G. E. (1990b). Progesterone enhances the surge of luteinizing hormone by increasing the activation of luteinizing hormone-releasing hormone neurons. Endocrinology 127, 2604–2606. doi: 10.1210/endo-127-5-2604
Legan, S. J., and Karsch, F. J. (1975). A daily signal for the LH surge in the rat. Endocrinology 96, 57–62. doi: 10.1210/endo-96-1-57
Legan, S. J., Coon, G. A., and Karsch, F. J. (1975). Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology 96, 50–56. doi: 10.1210/endo-96-1-50
Lehman, M. N., Hileman, S. M., and Goodman, R. L. (2013). Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv. Exp. Med. Biol. 784, 27–62. doi: 10.1007/978-1-4614-6199-9_3
Leite, C. M., Kalil, B., Uchoa, E. T., Antunes-Rodrigues, J., Elias, L. K., Levine, J. E., et al. (2016). Progesterone-induced amplification and advancement of GnRH/LH surges are associated with changes in kisspeptin system in preoptic area of estradiol-primed female rats. Brain Res. 1650, 21–30. doi: 10.1016/j.brainres.2016.08.021
Levine, J. E., and Ramirez, V. D. (1980). In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology 107, 1782–1790. doi: 10.1210/endo-107-6-1782
Lin, X. H., Lass, G., Kong, L. S., Wang, H., Li, X. F., Huang, H. F., et al. (2021). Optogenetic activation of arcuate kisspeptin neurons generates a luteinizing hormone surge-like secretion in an estradiol-dependent manner. Front. Endocrinol. 12:775233. doi: 10.3389/fendo.2021.775233
Loh, D. H., Kuljis, D. A., Azuma, L., Wu, Y., Truong, D., Wang, H. B., et al. (2014). Disrupted reproduction, estrous cycle, and circadian rhythms in female mice deficient in vasoactive intestinal peptide. J. Biol. Rhythms 29, 355–369. doi: 10.1177/0748730414549767
Lubahn, D. B., Moyer, J. S., Golding, T. S., Couse, J. F., Korach, K. S., and Smithies, O. (1993). Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. U S A. 90, 11162–11166. doi: 10.1073/pnas.90.23.11162
Lucas, R. J., Stirland, J. A., Darrow, J. M., Menaker, M., and Loudon, A. S. (1999). Free running circadian rhythms of melatonin, luteinizing hormone, and cortisol in Syrian hamsters bearing the circadian tau mutation. Endocrinology 140, 758–764. doi: 10.1210/endo.140.2.6538
Mahoney, M. M., Sisk, C., Ross, H. E., and Smale, L. (2004). Circadian regulation of gonadotropin-releasing hormone neurons and the preovulatory surge in luteinizing hormone in the diurnal rodent, Arvicanthis niloticus, and in a nocturnal rodent, Rattus norvegicus. Biol. Reprod. 70, 1049–1054. doi: 10.1095/biolreprod.103.021360
Mamgain, A., Sawyer, I. L., Timajo, D. A. M., Rizwan, M. Z., Evans, M. C., Ancel, C. M., et al. (2021). RFamide-related peptide neurons modulate reproductive function and stress responses. J. Neurosci. 41, 474–488. doi: 10.1523/JNEUROSCI.1062-20.2020
Mansano, N. D. S., Paradela, R. S., Bohlen, T. M., Zanardi, I. M., Chaves, F. M., Silveira, M. A., et al. (2022). Vasoactive intestinal peptide exerts an excitatory effect on hypothalamic kisspeptin neurons during estrogen negative feedback. Mol. Cell Endocrinol. 542:111532. doi: 10.1016/j.mce.2021.111532
Matsuda, F., Ohkura, S., Magata, F., Munetomo, A., Chen, J., Sato, M., et al. (2019). Role of kisspeptin neurons as a GnRH surge generator: comparative aspects in rodents and non-rodent mammals. J. Obstetrics Gynaecol. Res. 45, 2318–2329. doi: 10.1111/jog.14124
McQuillan, H. J., Han, S. Y., Cheong, I., and Herbison, A. E. (2019). GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology 160, 1480–1491. doi: 10.1210/en.2019-00193
Merchenthaler, I., Lane, M. V., Numan, S., and Dellovade, T. L. (2004). Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol. 473, 270–291. doi: 10.1002/cne.20128
Messager, S., Chatzidaki, E. E., Ma, D., Hendrick, A. G., Zahn, D., Dixon, J., et al. (2005). Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. U S A. 102, 1761–1766. doi: 10.1073/pnas.0409330102
Micevych, P. E., Chaban, V., Ogi, J., Dewing, P., Lu, J. K., and Sinchak, K. (2007). Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology 148, 782–789. doi: 10.1210/en.2006-0774
Micevych, P., and Sinchak, K. (2008a). Estradiol regulation of progesterone synthesis in the brain. Mol. Cell Endocrinol. 290, 44–50. doi: 10.1016/j.mce.2008.04.016
Micevych, P., and Sinchak, K. (2008b). Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology 149, 2739–2742. doi: 10.1210/en.2008-0011
Micevych, P., and Sinchak, K. (2011). The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front. Endocrinol. 2:90. doi: 10.3389/fendo.2011.00090
Micevych, P., Sinchak, K., Mills, R. H., Tao, L., LaPolt, P., and Lu, J. K. (2003). The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology 78, 29–35. doi: 10.1159/000071703
Miller, B. H., Olson, S. L., Levine, J. E., Turek, F. W., Horton, T. H., and Takahashi, J. S. (2006). Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol. Reprod. 75, 778–784. doi: 10.1095/biolreprod.106.052845
Miller, B. H., Olson, S. L., Turek, F. W., Levine, J. E., Horton, T. H., and Takahashi, J. S. (2004). Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr. Biol. 14, 1367–1373. doi: 10.1016/j.cub.2004.07.055
Mittelman-Smith, M. A., Krajewski-Hall, S. J., McMullen, N. T., and Rance, N. E. (2016). Ablation of KNDy neurons results in hypogonadotropic hypogonadism and amplifies the steroid-induced LH surge in female rats. Endocrinology 157, 2015–2027. doi: 10.1210/en.2015-1740
Mittelman-Smith, M. A., Rudolph, L. M., Mohr, M. A., and Micevych, P. E. (2017). Rodent models of non-classical progesterone action regulating ovulation. Front. Endocrinol. 8:165. doi: 10.3389/fendo.2017.00165
Mittelman-Smith, M. A., Wong, A. M., and Micevych, P. E. (2018). Estrogen and progesterone integration in an in vitro model of RP3V kisspeptin neurons. Neuroendocrinology 106, 101–115. doi: 10.1159/000471878
Moenter, S. M., Brand, R. C., and Karsch, F. J. (1992). Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130, 2978–2984. doi: 10.1210/endo.130.5.1572305
Moenter, S. M., Caraty, A., and Karsch, F. J. (1990). The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127, 1375–1384. doi: 10.1210/endo-127-3-1375
Mohr, M. A., Esparza, L. A., Steffen, P., Micevych, P. E., and Kauffman, A. S. (2021a). Progesterone receptors in AVPV kisspeptin neurons are sufficient for positive feedback induction of the LH surge. Endocrinology 162:bqab161. doi: 10.1210/endocr/bqab161
Mohr, M. A., Keshishian, T., Falcy, B. A., Laham, B. J., Wong, A. M., and Micevych, P. E. (2021b). Puberty enables oestradiol-induced progesterone synthesis in female mouse hypothalamic astrocytes. J. Neuroendocrinol. 34:e13082. doi: 10.1111/jne.13082
Mohr, M. A., Wong, A. M., Tomm, R. J., Soma, K. K., and Micevych, P. E. (2019). Pubertal development of estradiol-induced hypothalamic progesterone synthesis. Horm. Behav. 111, 110–113. doi: 10.1016/j.yhbeh.2018.12.007
Moline, M. L., and Albers, H. E. (1988). Response of circadian locomotor activity and the proestrous luteinizing hormone surge to phase shifts of the light-dark cycle in the hamster. Physiol. Behav. 43, 435–440. doi: 10.1016/0031-9384(88)90116-3
Moore, A. M., Coolen, L. M., Porter, D. T., Goodman, R. L., and Lehman, M. N. (2018). KNDy cells revisited. Endocrinology 159, 3219–3234. doi: 10.1210/en.2018-00389
Murai, I., Reichlin, S., and Ben-Jonathan, N. (1989). The peak phase of the proestrous prolactin surge is blocked by either posterior pituitary lobectomy or antisera to vasoactive intestinal peptide. Endocrinology 124, 1050–1055. doi: 10.1210/endo-124-2-1050
Navarro, V. M., Castellano, J. M., Fernandez-Fernandez, R., Tovar, S., Roa, J., Mayen, A., et al. (2005). Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 146, 156–163. doi: 10.1210/en.2004-0836
Navarro, V. M., Gottsch, M. L., Chavkin, C., Okamura, H., Clifton, D. K., and Steiner, R. A. (2009). Regulation of GnRH Secretion by Kiss1/Dynorphin/Neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29, 11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009
Norman, R. L., and Spies, H. G. (1974). Neural control of the estrogen-dependent twenty-four-hour periodicity of LH release in the golden hamster. Endocrinology 95, 1367–1372. doi: 10.1210/endo-95-5-1367
Norman, R. L., Blake, C. A., and Sawyer, C. H. (1973). Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology 93, 965–970. doi: 10.1210/endo-93-4-965
Palm, I. F., Van Der Beek, E. M., Wiegant, V. M., Buijs, R. M., and Kalsbeek, A. (1999). Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 93, 659–666. doi: 10.1016/S0306-4522(99)00106-2
Palm, I. F., van der Beek, E. M., Wiegant, V. M., Buijs, R. M., and Kalsbeek, A. (2001). The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res. 901, 109–116. doi: 10.1016/s0006-8993(01)02309-5
Petersen, S. L., and Barraclough, C. A. (1989). Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 484, 279–289. doi: 10.1016/0006-8993(89)90371-5
Petersen, S. L., Cheuk, C., Hartman, R. D., and Barraclough, C. A. (1989). Medial preoptic microimplants of the antiestrogen, keoxifene, affect luteinizing hormone-releasing hormone mRNA levels, median eminence luteinizing hormone-releasing hormone concentrations and luteinizing hormone release in ovariectomized, estrogen-treated rats. J. Neuroendocrinol. 1, 279–283. doi: 10.1111/j.1365-2826.1989.tb00116.x
Peterson, G. M., Watkins, W. B., and Moore, R. Y. (1980). The suprachiasmatic hypothalamic nuclei of the rat. VI. vasopressin neurons and circadian rhythmicity. Behav. Neural. Biol. 29, 236–245. doi: 10.1016/s0163-1047(80)90573-7
Piet, R., Boehm, U., and Herbison, A. E. (2013). Estrous cycle plasticity in the hyperpolarization-activated current ih is mediated by circulating 17beta-estradiol in preoptic area kisspeptin neurons. J. Neurosci. 33, 10828–10839. doi: 10.1523/JNEUROSCI.1021-13.2013
Piet, R., Dunckley, H., Lee, K., and Herbison, A. E. (2016). Vasoactive intestinal peptide excites GnRH neurons in male and female mice. Endocrinology 157, 3621–3630. doi: 10.1210/en.2016-1399
Piet, R., Fraissenon, A., Boehm, U., and Herbison, A. E. (2015). Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. J. Neurosci. 35, 6881–6892. doi: 10.1523/JNEUROSCI.4587-14.2015
Piet, R., Kalil, B., McLennan, T., Porteous, R., Czieselsky, K., and Herbison, A. E. (2018). Dominant neuropeptide cotransmission in Kisspeptin-GABA regulation of GnRH neuron firing driving ovulation. J. Neurosci. 38, 6310–6322. doi: 10.1523/JNEUROSCI.0658-18.2018
Pineda, R., Garcia-Galiano, D., Roseweir, A., Romero, M., Sanchez-Garrido, M. A., Ruiz-Pino, F., et al. (2010). Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 151, 722–730. doi: 10.1210/en.2009-0803
Plant, T. M. (2012). A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, old world monkey and rodent. Front. Neuroendocrinol. 33, 160–168. doi: 10.1016/j.yfrne.2012.02.002
Poling, M. C., Luo, E. Y., and Kauffman, A. S. (2017). Sex Differences in steroid receptor coexpression and circadian-timed activation of kisspeptin and RFRP-3 neurons may contribute to the sexually dimorphic basis of the LH surge. Endocrinology 158, 3565–3578. doi: 10.1210/en.2017-00405
Poling, M. C., Quennell, J. H., Anderson, G. M., and Kauffman, A. S. (2013). Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J Neuroendocrinol. 25, 876–886. doi: 10.1111/jne.12084
Qiu, J., Nestor, C. C., Zhang, C., Padilla, S. L., Palmiter, R. D., Kelly, M. J., et al. (2016). High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife 5:e16246. doi: 10.7554/eLife.16246
Reppert, S. M., Artman, H. G., Swaminathan, S., and Fisher, D. A. (1981). Vasopressin exhibits a rhythmic daily pattern in cerebrospinal fluid but not in blood. Science 213, 1256–1257. doi: 10.1126/science.7268432
Rizwan, M. Z., Poling, M. C., Corr, M., Cornes, P. A., Augustine, R. A., Quennell, J. H., et al. (2012). RFamide-related peptide-3 receptor gene expression in GnRH and kisspeptin neurons and GnRH-dependent mechanism of action. Endocrinology 153, 3770–3779. doi: 10.1210/en.2012-1133
Robertson, J. L., Clifton, D. K., de la Iglesia, H. O., Steiner, R. A., and Kauffman, A. S. (2009). Circadian regulation of Kiss1 neurons: implications for timing the preovulatory GnRH/LH surge. Endocrinology 150, 3664–3671. doi: 10.1210/en.2009-0247
Ronnekleiv, O. K., and Kelly, M. J. (1986). Luteinizing hormone-releasing hormone neuronal system during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology 43, 564–576. doi: 10.1159/000124583
Ronnekleiv, O. K., and Kelly, M. J. (1988). Plasma prolactin and luteinizing hormone profiles during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology 47, 133–141. doi: 10.1159/000124903
Sarkar, D. K., and Fink, G. (1980). Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J. Endocrinol. 86, 511–524. doi: 10.1677/joe.0.0860511
Sarkar, D. K., Chiappa, S. A., Fink, G., and Sherwood, N. M. (1976). Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264, 461–463. doi: 10.1038/264461a0
Sawyer, C. H., Everett, J. W., and Markee, J. E. (1949). A neural factor in the mechanism by which estrogen induces the release of luteinizing hormone in the rat. Endocrinology 44, 218–233. doi: 10.1210/endo-44-3-218
Scott, C. J., Kuehl, D. E., Ferreira, S. A., and Jackson, G. L. (1997). Hypothalamic sites of action for testosterone, dihydrotestosterone, and estrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology 138, 3686–3694. doi: 10.1210/endo.138.9.5401
Seibel, M. M., Shine, W., Smith, D. M., and Taymor, M. L. (1982). Biological rhythm of the luteinizing hormone surge in women. Fertil. Steril. 37, 709–711. doi: 10.1016/S0015-0282(16)46288-6
Semaan, S. J., and Kauffman, A. S. (2015). Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol. Cell Endocrinol. 401, 84–97. doi: 10.1016/j.mce.2014.11.025
Seminara, S. B., Messager, S., Chatzidaki, E. E., Thresher, R. R., Acierno, J. S. Jr., Shagoury, J. K., et al. (2003). The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 349, 1614–1627. doi: 10.1056/NEJMoa035322
Shan, Y., Abel, J. H., Li, Y., Izumo, M., Cox, K. H., Jeong, B., et al. (2020). Dual-color single-cell imaging of the suprachiasmatic nucleus reveals a circadian role in network synchrony. Neuron 108, 164–179.e7. doi: 10.1016/j.neuron.2020.07.012
Shinohara, K., Funabashi, T., and Kimura, F. (1999). Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res. Mol. Brain Res. 63, 262–267. doi: 10.1016/s0169-328x(98)00289-7
Shinohara, K., Honma, S., Katsuno, Y., and Honma, K. (2000). Circadian release of excitatory amino acids in the suprachiasmatic nucleus culture is Ca(2+)-independent. Neurosci. Res. 36, 245–250. doi: 10.1016/s0168-0102(99)00131-5
Shughrue, P. J., Lane, M. V., and Merchenthaler, I. (1997). Comparative distribution of estrogen receptor-alpha and –beta mRNA in the rat central nervous system. J. Comp. Neurol. 388, 507–525. doi: 10.1016/S0169-328X(98)00289-7
Siegel, H. I., Bast, J. D., and Greenwald, G. S. (1976). The effects of phenobarbital and gonadal steroids on periovulatory serum levels of luteinizing hormone and follicle-stimulating hormone in the hamster. Endocrinology 98, 48–55. doi: 10.1210/endo-98-1-48
Sinchak, K., Mills, R. H., Tao, L., LaPolt, P., Lu, J. K., and Micevych, P. (2003). Estrogen induces de novo progesterone synthesis in astrocytes. Dev. Neurosci. 25, 343–348. doi: 10.1159/000073511
Sinchak, K., Mohr, M. A., and Micevych, P. E. (2020). Hypothalamic astrocyte development and physiology for neuroprogesterone induction of the luteinizing hormone surge. Front. Endocrinol. 11:420. doi: 10.3389/fendo.2020.00420
Smarr, B. L., Gile, J. J., and de la Iglesia, H. O. (2013). Oestrogen-independent circadian clock gene expression in the anteroventral periventricular nucleus in female rats: possible role as an integrator for circadian and ovarian signals timing the luteinising hormone surge. J. Neuroendocrinol. 25, 1273–1279. doi: 10.1111/jne.12104
Smarr, B. L., Morris, E., and de la Iglesia, H. O. (2012). The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology 153, 2839–2850. doi: 10.1210/en.2011-1857
Smith, E. R., and Davidson, J. M. (1974). Location of feedback receptors: effects of intracranially implanted steroids on plasma LH and LRF response. Endocrinology 95, 1566–1573. doi: 10.1210/endo-95-6-1566
Smith, J. T. (2009). Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides 30, 94–102. doi: 10.1016/j.peptides.2008.08.013
Smith, J. T., Cunningham, M. J., Rissman, E. F., Clifton, D. K., and Steiner, R. A. (2005a). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146, 3686–3692. doi: 10.1210/en.2005-0488
Smith, J. T., Dungan, H. M., Stoll, E. A., Gottsch, M. L., Braun, R. E., Eacker, S. M., et al. (2005b). Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146, 2976–2984. doi: 10.1210/en.2005-0323
Smith, J. T., Li, Q., Yap, K. S., Shahab, M., Roseweir, A. K., Millar, R. P., et al. (2011). Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 152, 1001–1012. doi: 10.1210/en.2010-1225
Smith, J. T., Popa, S. M., Clifton, D. K., Hoffman, G. E., and Steiner, R. A. (2006). Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 26, 6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006
Smith, J. T., Shahab, M., Pereira, A., Pau, K. Y., and Clarke, I. J. (2010). Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol. Reprod. 83, 568–577. doi: 10.1095/biolreprod.110.085407
Smith, M. J., Jiennes, L., and Wise, P. M. (2000). Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology 141, 4317–4320. doi: 10.1210/endo.141.11.7876
Starrett, J. R., DeFazio, R. A., and Moenter, S. M. (2021). Reciprocal changes in voltage-gated potassium and subthreshold inward currents help maintain firing dynamics of AVPV kisspeptin neurons during the estrous cycle. eNeuro 8, 1–18. doi: 10.1523/ENEURO.0324-21.2021
Stephens, S. B. Z., and Kauffman, A. S. (2021). Estrogen regulation of the molecular phenotype and active translatome of AVPV kisspeptin neurons. Endocrinology 162:bqab080. doi: 10.1210/endocr/bqab080
Stephens, S. B. Z., Di Giorgio, N. P., Liaw, R. B., Parra, R. A., Yang, J. A., Chahal, N., et al. (2018). Estradiol-dependent and –independent stimulation of kiss1 expression in the amygdala, BNST, and lateral septum of mice. Endocrinology 159, 3389–3402. doi: 10.1210/en.2018-00583
Stephens, S. B., Chahal, N., Munaganuru, N., Parra, R. A., and Kauffman, A. S. (2016). Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-alpha but not estrogen receptor-beta. Endocrinology 157, 4021–4031. doi: 10.1210/en.2016-1431
Stephens, S. B., Tolson, K. P., Rouse, M. L. Jr., Poling, M. C., Hashimoto-Partyka, M. K., Mellon, P. L., et al. (2015). Absent progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology 156, 3091–3097. doi: 10.1210/en.2015-1300
Stetson, M. H., and Watson-Whitmyre, M. (1977). The neural clock regulating estrous cyclicity in hamsters: gonadotropin release following barbiturate blockade. Biol. Reprod. 16, 536–542.
Swann, J. M., and Turek, F. W. (1985). Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science 228, 898–900. doi: 10.1126/science.4001926
Tonsfeldt, K. J., Mellon, P. L., and Hoffmann, H. M. (2022). Circadian rhythms in the neuronal network timing the luteinizing hormone surge. Endocrinology 163:bqab268.
Tonsfeldt, K. J., Schoeller, E. L., Brusman, L. E., Cui, L. J., Lee, J., and Mellon, P. L. (2019). The contribution of the circadian gene bmal1 to female fertility and the generation of the preovulatory luteinizing hormone surge. J. Endocrine Soc. 3, 716–733. doi: 10.1210/js.2018-00228
Topaloglu, A. K., Tello, J. A., Kotan, L. D., Ozbek, M. N., Yilmaz, M. B., Erdogan, S., et al. (2012). Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Engl. J. Med. 366, 629–635.
Tsukahara, S. (2006). Increased Fos immunoreactivity in suprachiasmatic nucleus before luteinizing hormone surge in estrogen-treated ovariectomized female rats. Neuroendocrinology 83, 303–312. doi: 10.1159/000095341
Uenoyama, Y., Inoue, N., Nakamura, S., and Tsukamura, H. (2021). Kisspeptin neurons and estrogen-estrogen receptor alpha signaling: unraveling the mystery of steroid feedback system regulating mammalian reproduction. Int. J. Mol. Sci. 22:9229. doi: 10.3390/ijms22179229
Van der Beek, E. M., Horvath, T. L., Wiegant, V. M., Van den Hurk, R., and Buijs, R. M. (1997). Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 384, 569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0
van der Beek, E. M., Swarts, H. J., and Wiegant, V. M. (1999). Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology 69, 227–237. doi: 10.1159/000054423
van der Beek, E. M., van Oudheusden, H. J., Buijs, R. M., van der Donk, H. A., van den Hurk, R., and Wiegant, V. M. (1994). Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology 134, 2636–2644. doi: 10.1210/endo.134.6.8194489
van der Beek, E. M., Wiegant, V. M., van der Donk, H. A., van den Hurk, R., and Buijs, R. M. (1993). Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J. Neuroendocrinol. 5, 137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x
Vargas Trujillo, M., Kalil, B., Ramaswamy, S., and Plant, T. M. (2017). Estradiol upregulates kisspeptin expression in the preoptic area of both the male and female rhesus monkey (Macaca mulatta): implications for the hypothalamic control of ovulation in highly evolved primates. Neuroendocrinology 105, 77–89. doi: 10.1159/000448520
Vida, B., Deli, L., Hrabovszky, E., Kalamatianos, T., Caraty, A., Coen, C. W., et al. (2010). Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J. Neuroendocrinol. 22, 1032–1039. doi: 10.1111/j.1365-2826.2010.02045.x
Wang, L., Burger, L. L., Greenwald-Yarnell, M. L., Myers, M. G., and Moenter, S. M. (2018). Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor alpha in adult female mice. J. Neurosci. 38, 1061–1072. doi: 10.1523/JNEUROSCI.2428-17.2017
Wang, L., DeFazio, R. A., and Moenter, S. M. (2016). Excitability and burst generation of AVPV kisspeptin neurons are regulated by the estrous cycle via multiple conductances modulated by estradiol action. eNeuro 3, 1–20. doi: 10.1523/ENEURO.0094-16.2016
Wang, L., Vanacker, C., Burger, L. L., Barnes, T., Shah, Y. M., Myers, M. G., et al. (2019). Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife 8:e43999. doi: 10.7554/eLife.43999
Wang, L., Guo, W., Shen, X., Yeo, S., Long, H., Wang, Z., et al. (2020). Different dendritic domains of the GnRH neuron underlie the pulse and surge modes of GnRH secretion in female mice. eLife 9:e53945. doi: 10.7554/eLife.53945
Watanabe, Y., Uenoyama, Y., Suzuki, J., Takase, K., Suetomi, Y., Ohkura, S., et al. (2014). Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J. Neuroendocrinol. 26, 909–917. doi: 10.1111/jne.12227
Watson, R. E., Langub, M. C., Engle, M. G., and Maley, B. E. (1995). Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res. 689, 254–264. doi: 10.1016/0006-8993(95)00548-5
Weick, R. F., and Stobie, K. M. (1992). Vasoactive intestinal peptide inhibits the steroid-induced LH surge in the ovariectomized rat. J. Endocrinol. 133, 433–437.
Welsh, D. K., Logothetis, D. E., Meister, M., and Reppert, S. M. (1995). Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706.
Welsh, D. K., Takahashi, J. S., and Kay, S. A. (2010). Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 72, 551–577.
Wen, S., Ma, D., Zhao, M., Xie, L., Wu, Q., Gou, L., et al. (2020). Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat. Neurosci. 23, 456–467.
Wiegand, S. J., and Terasawa, E. (1982). Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34, 395–404. doi: 10.1159/000123335
Wiegand, S. J., Terasawa, E., and Bridson, W. E. (1978). Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology 102, 1645–1648. doi: 10.1210/endo-102-5-1645
Wiegand, S. J., Terasawa, E., Bridson, W. E., and Goy, R. W. (1980). Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31, 147–157. doi: 10.1159/000123066
Williams, W. P., Jarjisian, S. G., Mikkelsen, J. D., and Kriegsfeld, L. J. (2011). Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology 152, 595–606. doi: 10.1210/en.2010-0943
Wintermantel, T. M., Campbell, R. E., Porteous, R., Bock, D., Grone, H. J., Todman, M. G., et al. (2006). Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52, 271–280. doi: 10.1016/j.neuron.2006.07.023
Yip, S. H., Boehm, U., Herbison, A. E., and Campbell, R. E. (2015). Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology 156, 2582–2594. doi: 10.1210/en.2015-1131
Yip, S. H., Campos, P., Liu, X., Porteous, R., and Herbison, A. E. (2021). Innervation of GnRH neuron distal projections and activation by kisspeptin in a new GnRH-Cre rat model. Endocrinology 162:bqaa186. doi: 10.1210/endocr/bqaa186
Zelena, D. (2017). Comparison of natural and artificial vasopressin deficiency: why is the latter lethal? Russian J. Genet. Appl. Res. 7, 243–248.
Zhang, C., Bosch, M. A., Qiu, J., Ronnekleiv, O. K., and Kelly, M. J. (2015). 17beta-Estradiol increases persistent Na(+) current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol. Endocrinol. 29, 518–527. doi: 10.1210/me.2014-1392
Zhang, J., Yang, L., Lin, N., Pan, X., Zhu, Y., and Chen, X. (2014). Aging-related changes in RP3V kisspeptin neurons predate the reduced activation of GnRH neurons during the early reproductive decline in female mice. Neurobiol. Aging 35, 655–668. doi: 10.1016/j.neurobiolaging.2013.08.038
Keywords: Kiss1, GnRH, RP3V, AVPV, kisspeptin, SCN, ovulation, reproduction
Citation: Kauffman AS (2022) Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. Front. Neurosci. 16:953252. doi: 10.3389/fnins.2022.953252
Received: 26 May 2022; Accepted: 08 July 2022;
Published: 27 July 2022.
Edited by:
Víctor M. Navarro, Harvard Medical School, United StatesReviewed by:
Stephanie Constantin, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH), United StatesRichard Piet, Kent State University, United States
Copyright © 2022 Kauffman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander S. Kauffman, YWthdWZmbWFuQHVjc2QuZWR1
 Alexander S. Kauffman
Alexander S. Kauffman