- Department of Biology and Center for Neuroscience, Miami University, Oxford, OH, United States
Rhythmic behaviors (e.g., walking, breathing, and chewing) are produced by central pattern generator (CPG) circuits. These circuits are highly dynamic due to a multitude of input they receive from hormones, sensory neurons, and modulatory projection neurons. Such inputs not only turn CPG circuits on and off, but they adjust their synaptic and cellular properties to select behaviorally relevant outputs that last from seconds to hours. Similar to the contributions of fully identified connectomes to establishing general principles of circuit function and flexibility, identified modulatory neurons have enabled key insights into neural circuit modulation. For instance, while bath-applying neuromodulators continues to be an important approach to studying neural circuit modulation, this approach does not always mimic the neural circuit response to neuronal release of the same modulator. There is additional complexity in the actions of neuronally-released modulators due to: (1) the prevalence of co-transmitters, (2) local- and long-distance feedback regulating the timing of (co-)release, and (3) differential regulation of co-transmitter release. Identifying the physiological stimuli (e.g., identified sensory neurons) that activate modulatory projection neurons has demonstrated multiple “modulatory codes” for selecting particular circuit outputs. In some cases, population coding occurs, and in others circuit output is determined by the firing pattern and rate of the modulatory projection neurons. The ability to perform electrophysiological recordings and manipulations of small populations of identified neurons at multiple levels of rhythmic motor systems remains an important approach for determining the cellular and synaptic mechanisms underlying the rapid adaptability of rhythmic neural circuits.
1. Introduction
Rhythmic motor behaviors are generated by central nervous system (CNS) circuits called central pattern generators (CPGs) (Bucher et al., 2015). Although CPGs can produce rhythmic output without rhythmic input, modulatory input is often required to configure CPGs into an active state. Additionally, beyond simply turning on or off, CPGs are often “multifunctional,” in that they produce different outputs to adapt to changes in the internal and external environments (Briggman and Kristan, 2008; Benjamin, 2012; Daur et al., 2016; Marder et al., 2022). In some cases, the source of modulation is intrinsic to the CPG and a necessary component of motor output (Katz, 1998). However, many sources originate outside the CPG, including sensory inputs, hormones, and modulatory projection neurons (PNs), i.e., neurons which originate in higher order CNS regions and project to CPGs (Rosen et al., 1991; Briggman and Kristan, 2008; Nusbaum, 2008; Hsu and Bhandawat, 2016).
Small circuits, particularly those underlying rhythmic behaviors, with their identified neurons, have enabled many important insights into circuit function and plasticity (Calabrese et al., 2016; Cropper et al., 2018; Katz and Quinlan, 2019; Marder et al., 2022). Similar to the accessibility of identified circuit neurons, several invertebrate preparations also have relatively small populations of modulatory PNs which are accessible to electrophysiological approaches (Rosen et al., 1991; Heinrich, 2002; Mesce et al., 2008; Nusbaum, 2008). PN populations range from ∼20 pairs in crab and mollusk feeding systems to ∼200–500 pairs targeting the insect ventral nerve cord (Rosen et al., 1991; Coleman et al., 1992; Hsu and Bhandawat, 2016; Namiki et al., 2018). Comparable PN populations in vertebrates are typically larger, include heterogeneous types, and can be distributed across multiple nuclei (Garcia et al., 2011; Sharples et al., 2014; Ruder and Arber, 2019; Flaive et al., 2020). While technological advances are increasing the ability to control vertebrate neuron populations in vitro and in vivo, cellular-level experimental access to modulatory PNs and a fully described motor circuit connectome remains challenging in many vertebrate preparations (Kim et al., 2017; Leiras et al., 2022). Here, I will focus on lessons learned from several small, invertebrate motor systems, regarding the cellular mechanisms by which modulatory PNs alter CPG output, and how their activity is regulated. Much additional work on descending motor control, including fast activation of escape behaviors, and large-scale genetic approaches investigating insect descending neurons is beyond the scope of this article (Cande et al., 2018; Herberholz, 2022).
2. Modulatory projection neurons alter CPG output
2.1. Bath-application vs. neuronal-release
Early studies primarily using bath-applied neuromodulators, but also stimulation of identified modulatory PNs, demonstrated that there is considerable flexibility in the strength and pattern of neuronal activity, as well as in which CPG(s) the neurons are participating (Hooper and Marder, 1984; Kuhlman et al., 1985; Flamm and Harris-Warrick, 1986; Dickinson et al., 1990; Harris-Warrick and Marder, 1991; Ramirez and Pearson, 1991; Marder, 2012). Although bath-application continues to provide insights into circuit modulation, bath-applied modulator actions range from very similar to neuronally-released modulator, to only mimicking some effects, to having distinct, even opposite effects (Marder, 2012; Nusbaum et al., 2017). The small numbers and exceptional experimental access afforded by invertebrate modulatory neurons have revealed several explanations for distinctions between bath-applied and neuronally-released modulators. The crustacean stomatogastric nervous system (STNS), is particularly useful because the transmitters, intrinsic properties, and synaptic connections are identified for the ∼30 neurons comprising two feeding-related CPGs (pyloric, gastric mill) (Figure 1A; Marder and Bucher, 2007; Daur et al., 2016). Additionally, identified modulatory PNs are amenable to intra-somatic and intra-axonal recordings, and identification of their (co-)transmitter content allows for direct comparison of bath-applied vs. neuronally-released neuromodulators (Figure 1A; Nusbaum and Marder, 1989a; Coleman and Nusbaum, 1994; Stein, 2009; Kwiatkowski et al., 2013; Nusbaum et al., 2017).
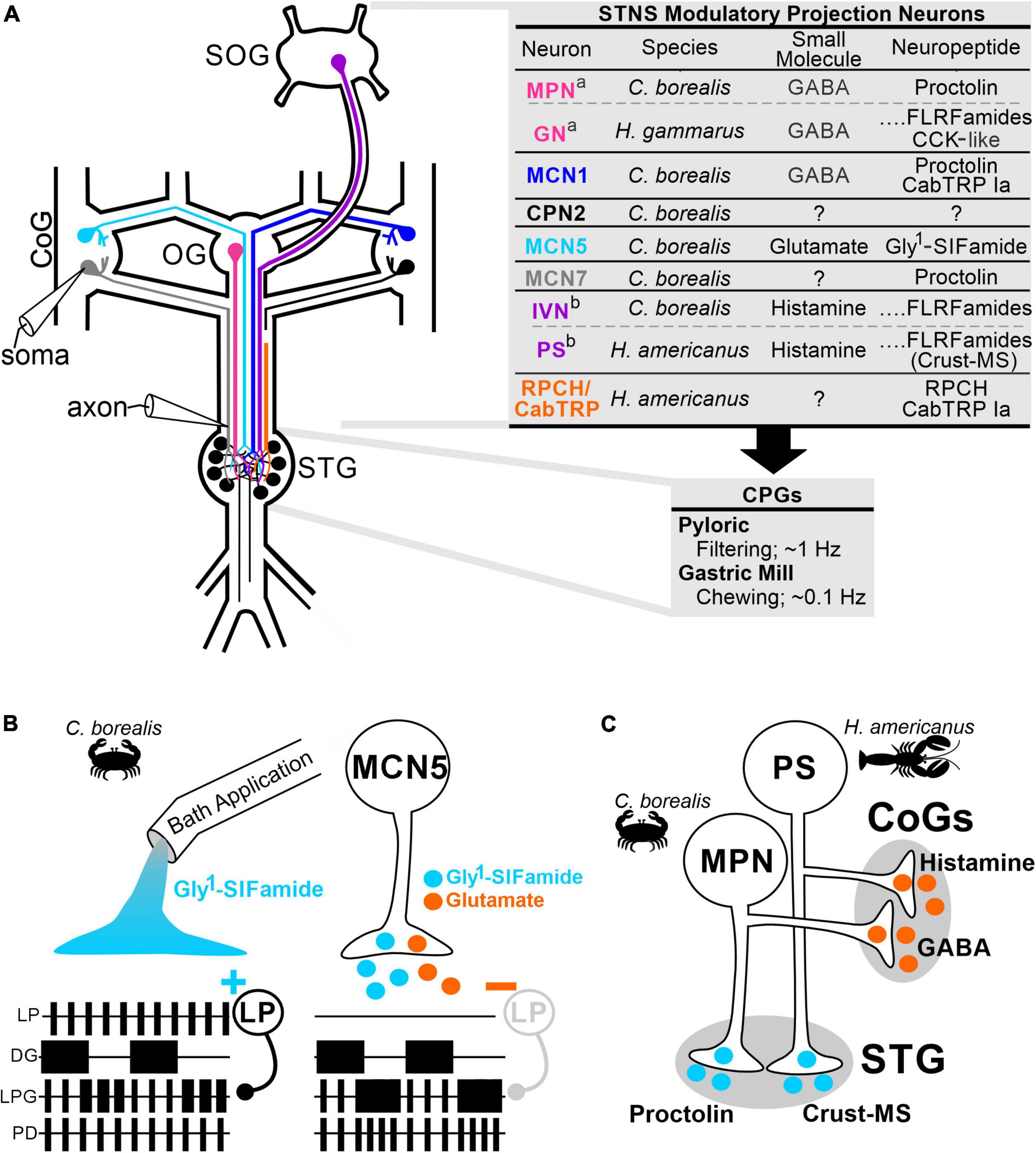
Figure 1. Identified modulatory projection neurons reveal cooperative and divergent actions contributing to distinctions between bath-applied and neuronally-released modulator. (A) The crustacean stomatogastric nervous system (STNS) includes the pyloric (food filtering, ∼1 Hz) and gastric mill (food chewing, ∼0.1 Hz) CPGs within the stomatogastric ganglion (STG). Modulatory PNs originating in the oesophageal (OG), the paired commissural ganglia (CoGs), and the supraoesophageal ganglion (SOG) project to and modulate the CPGs. Intracellular recordings of modulatory PNs can be made at the soma in the SOG, CoG, or OG, and axon terminals near the entrance to the STG (electrode symbols). Most modulatory PNs contain small molecule and neuropeptide co-transmitters as listed in the upper table. a,bSome analogous modulatory neurons in different species (lobster, Homarus gammarus, H. americanus; crab, Cancer borealis) contain the same co-transmitters, and others contain different complements. All PNs listed occur as pairs, either as a single copy in each CoG (MCN1/5/7, CPN2), or in the same location (OG: MPN/GN; SOG: IVN/PS), however they are drawn as single neurons for clarity. (B) Ionotropic co-transmitter actions are necessary for full expression of metabotropic actions. In C. borealis, the modulatory PN MCN5 elicits a motor pattern that includes dual-network activity in the LPG neuron (shorter duration, faster pyloric-timed bursts alternating with slower gastric mill-timed bursts). Pyloric network activity is evident in LP and PD neuron activity, gastric mill network activity is represented by DG neuron activity. Neuron activity is schematized as extracellular recordings with each box representing a burst of action potentials. Bath application of the MCN5 neuropeptide Gly1-SIFamide mimics some but not all MCN5 actions. In particular, Gly1-SIFamide excites the pyloric LP neuron (+) whereas MCN5 inhibits LP (–). The increased LP activity during Gly1-SIFamide application inhibits the LPG neuron, preventing it from fully participating in the slower gastric mill network, note the extended duration LPG bursts alternating with DG that do not fully merge into a gastric mill-timed burst. MCN5 inhibits LP (gray) via its co-transmitter glutamate, which is essential for LPG to fully participate in the gastric mill network via Gly1-SIFamide effects (Blitz et al., 2019; Fahoum and Blitz, 2021). (C) Spatially divergent co-transmitter actions occur in modulatory PNs in the STNS. The MPN and PS neurons use their peptide transmitters (proctolin and crust-MS, respectively) on pyloric and gastric mill CPGs in the STG, but their small molecule transmitters (GABA and histamine, respectively) in the CoGs. It is not known whether there is differential trafficking or other explanations for these segregated co-transmitter actions (Nusbaum and Marder, 1989a; Blitz and Nusbaum, 1999; Kwiatkowski et al., 2013). Species used in the referenced studies are indicted in each panel. Neuron/transmitter identification in panel (A): (Nusbaum and Marder, 1989a; Coleman and Nusbaum, 1994; Norris et al., 1994, 1996; Blitz and Nusbaum, 1999; Blitz et al., 1999, 2019; Meyrand et al., 2000; Swensen et al., 2000; Thirumalai and Marder, 2002; Christie et al., 2004; Kwiatkowski et al., 2013; Fahoum and Blitz, 2021).
2.2. Co-transmission
Modulatory CPG inputs, including PNs, use metabotropic receptors and second messenger signaling to alter intrinsic and synaptic properties of circuit neurons to select different outputs (Katz and Calin-Jageman, 2009; Nadim and Bucher, 2014). However, they often also use rapid ionotropic transmission. Co-transmission is ubiquitous and a likely contributor to distinctions between modulatory neuron activation and bath-application. Co-transmitter complements include neuropeptide plus classical and/or amine small molecule transmitters, or multiple small molecule transmitters (Nusbaum et al., 2017; Nässel, 2018; Trudeau and El Mestikawy, 2018; Svensson et al., 2019; Eiden et al., 2022). One or more neuropeptides plus a small molecule transmitter is common in modulatory PNs targeting CPGs (Figure 1A; Schlegel et al., 2016; Nusbaum et al., 2017; Nässel, 2018).
Neuropeptide and small molecule co-neurotransmitter actions range from varying degrees of convergence, to complementary, to entirely divergent (Thirumalai and Marder, 2002; Nusbaum et al., 2017; Nässel, 2018; Florman and Alkema, 2022). In the crab STNS, a modulatory PN (MCN5) switches the CPG neuron LPG from pyloric-only network participation to dual-network (pyloric plus gastric mill) activity via its neuropeptide Gly1-SIFamide (Figure 1B; Fahoum and Blitz, 2021; Snyder and Blitz, 2022). However, bath applied Gly1-SIFamide excites the pyloric CPG neuron LP, which inhibits LPG and prevents it from fully expressing dual-network activity. This Gly1-SIFamide excitation of LP is opposite of MCN5 actions (Figure 1B; Fahoum and Blitz, 2021). MCN5-released Gly1-SIFamide can elicit the switch in LPG activity due to co-released glutamate inhibiting the LP neuron that would otherwise interfere with LPG switching into dual-network activity (Figure 1B). Thus, ionotropic classical transmitter actions are essential for metabotropic neuropeptide actions to be fully expressed. Conversely, in Aplysia feeding, ionotropic actions are enhanced by metabotropic receptor-mediated co-transmitter actions. The feeding motor pattern activated by the modulatory PN CBI-2 changes over time, due to CBI-2 modulation of its cholinergic synaptic transmission onto feeding motor neurons (Koh et al., 2003). The time-dependent effects on the motor pattern and enhanced fast cholinergic synaptic transmission are mimicked by either of the CBI-2 peptide co-transmitters (CP2, FCAP). However, the cooperative peptide effects are distinct, with CP2 and FCAP increasing quantal content versus size, respectively (Koh et al., 2003). Intracellular recordings from identified modulatory PNs such as MCN5 and CBI-2, with identified co-transmitters, revealed co-transmitter cooperativity necessary for motor pattern selection that would be missed in bath-application studies.
In some cases, neuropeptide and small molecule actions appear partially redundant. In the nematode Caenorhabditis elegans, serotonin or NLP-3 neuropeptide release from a modulatory PN is sufficient to activate egg-laying, however their combined actions elicit additional egg-laying. Further work is necessary to determine whether their actions converge onto the same targets (Brewer et al., 2019). Co-transmitters may converge onto the same cellular or even subcellular targets (Nadim and Bucher, 2014), however without cellular-level access to the full CPG circuit, similar network level actions may hide cellular divergence. In Aplysia feeding, three neuropeptides released from modulatory neuron CBI-12, each have the same circuit level effect, shortening the protraction phase of an ingestive motor pattern (Jing and Weiss, 2005; Zhang et al., 2018). However, the peptides act on different CPG neurons to mediate the same circuit effect (Zhang et al., 2018). Such redundancy may ensure a particular adjustment to circuit output even when some targets are unresponsive.
2.3. Spatial segregation of co-transmitter actions
Divergent co-transmitter actions may result from spatial segregation. In the crustacean STNS, modulatory PNs (MPN, PS) each use their peptide transmitter on CPG neurons within the stomatogastric ganglion (STG), but their small molecule transmitters act at distinct arbors, in different ganglia [commissural ganglia (CoGs)] (Figure 1C; Nusbaum and Marder, 1989b; Blitz and Nusbaum, 1999; Kwiatkowski et al., 2013). Spatially distinct actions could occur due to distinct trafficking of transmitter vesicles, differential receptor expression on postsynaptic targets, or differential sensitivity of transmitter release to neuronal activity (Kueh and Jellies, 2012; Nusbaum et al., 2017; Cropper et al., 2018; Cifuentes and Morales, 2021). Where determined, the low end of physiological firing frequencies is sufficient to release both peptide and small molecule transmitters (Cropper et al., 2018). On a finer scale, peptidases can constrain the actions of neuronally-released peptides, enabling distinct effects even when released into the same densely overlapping neuropil regions (Christie et al., 1997; Blitz et al., 1999; Nusbaum, 2002; Wood and Nusbaum, 2002; Nässel, 2009). Although neuromodulators are often considered to act via relatively non-specific “volume transmission,” it is becoming increasingly clear that there is also spatial constraint of neuromodulator actions (Disney and Higley, 2020; Liu et al., 2021; Nässel and Zandawala, 2022). Localization of reuptake and degradative machinery, and constrained release/receptor distributions beyond anatomically-defined synapses can limit the sphere of neuromodulator influence (Nusbaum, 2002; Disney and Higley, 2020; Liu et al., 2021; Eiden et al., 2022).
2.4. Local presynaptic feedback onto modulatory projection neurons
The ability to record from modulatory PN axon terminals revealed local presynaptic regulation of their transmission (Nusbaum, 1994). For example, rhythmic presynaptic inhibition from a circuit neuron onto modulatory PN terminals in the crab STNS and the subsequent waxing and waning of modulatory effects is essential to elicit a chewing pattern (Coleman et al., 1995). Further, the system is tuned such that this local feedback inhibition results in a more coordinated motor pattern when both PN copies are coactive compared to the same cumulative activity in a single PN copy (Colton et al., 2020). The presynaptic regulation occurs at terminals that are ∼1–2 cm distant from the soma (Figure 1A) and due to electrotonic decay, is not present in somatic recordings and does not alter PN activity initiating in the PN ganglion of origin (Nusbaum et al., 1992; Coleman and Nusbaum, 1994; Coleman et al., 1995). Local synaptic input includes chemical transmission between circuit neurons and PNs and between PNs, plus extensive electrical coupling between circuit neurons and PN terminals (Perrins and Weiss, 1998; Hurwitz et al., 2005; Stein et al., 2007; Marder et al., 2017; Blitz et al., 2019). Local feedback actions may generally alter transmission, or be more specific, including decreasing chemical but not electrical transmission (Coleman et al., 1995), or decreasing peptide but not small molecule transmitter release (DeLong et al., 2009). Rhythmic presynaptic regulation from CPG elements can also cause modulatory PN actions to occur via distinct mechanisms (e.g., electrical vs. chemical transmission) during different phases of motor output (Coleman et al., 1995; Hurwitz et al., 2005). Long-distance synaptic feedback also regulates PN transmission, however through changes in PN activity (see Section “3.3. Long-distance CPG feedback”). While much continues to be learned from bath-application studies, studies discussed above provide a note of caution, as even co-transmitter bath application may not mimic neuronal release due to the lack of spatial and temporal control that occurs with neuronally-released neuromodulators.
3. Regulation of modulatory projection neuron activity
Modulatory PNs serve as a link between sensory and/or higher-order inputs, and the motor circuits responsible for behavior. Thus, understanding how PN activity is controlled is important to understanding how sensory information and higher-order decisions are converted to appropriate behavioral responses.
3.1. State-dependence
In vitro and in vivo, single modality sensory input can be sufficient to initiate relevant behaviors via activation of identified modulatory PNs (Willard, 1981; Rosen et al., 1991; Horn et al., 1999; Jing and Weiss, 2005; Hedrich et al., 2011). However, PN activity is often regulated by multiple sources. In particular, inputs relaying behavioral state information can alter PN sensitivity to other inputs during ongoing behaviors, or result in different behavioral versions, on multiple time scales (Kristan and Shaw, 1997; Staudacher, 2001; Beenhakker et al., 2007; Barrière et al., 2008; White et al., 2017; Ache et al., 2019; Cook and Nusbaum, 2021). State-dependent PN activity may be a consequence of inputs specifically targeting PNs, such as courtship-promoting neurons converging with visual input onto the Drosophila P9 PN, to elicit courtship locomotor behavior (Bidaye et al., 2020). Behavioral state can also be conveyed to PNs through broadly-acting hormones (Willard, 1981; Mesce and Pierce-Shimomura, 2010; Flood et al., 2013). In the medicinal leech, circulating serotonin increases with hunger, coincident with a decreased threshold for swimming. Although serotonin does not activate swim-activating cell 204, it modulates its intrinsic properties, making it easier for other inputs to activate this neuron and elicit swimming (Angstadt and Friesen, 1993; Kristan et al., 2005). Even if the responsiveness of a modulatory PN does not change, the consequences of its activity may be state-dependent. The leech R3b1 PN elicits crawling or swimming, with the decision determined by the surrounding fluid level (Esch and Kristan, 2002). “Shallow water detector” sensory neurons appear to select motor output downstream from modulatory PNs, via actions on CPG neurons (Figure 2A). However, dopamine application biases the entire nervous system toward crawling and R3b1 only elicits crawling in this context (Figure 2A; Puhl et al., 2012), suggesting both PN- and CPG-level control of motor system state.
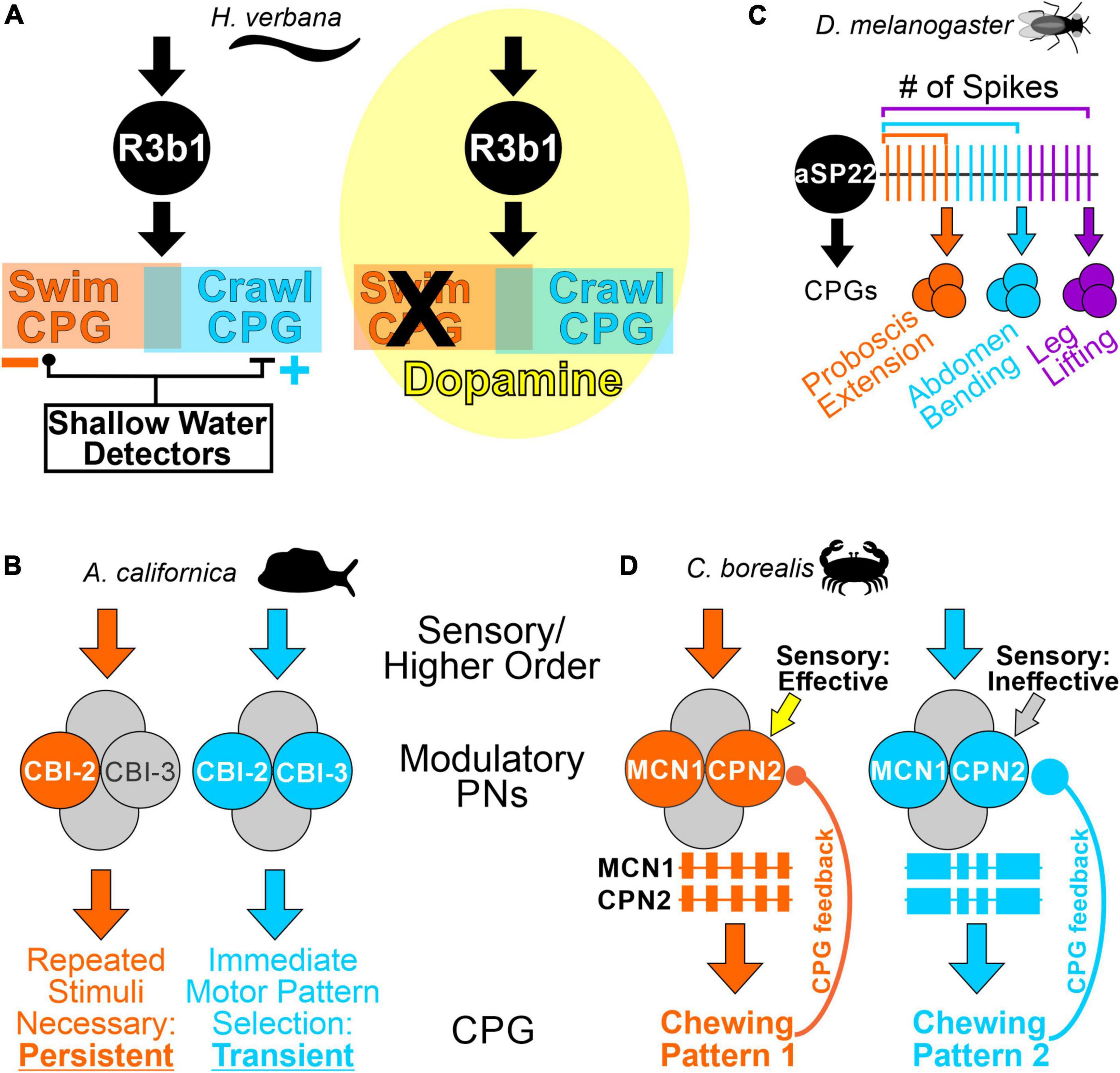
Figure 2. Motor pattern selection by modulatory PNs is state-dependent, and can be encoded in the population of active PNs, or in PN activity. (A) The effects of PN R3b1 are determined by environmental and internal conditions. Left, in an in vitro or semi-intact leech preparation, the R3b1 neuron elicits either swimming or crawling in response to the same input. The swim and crawl CPGs consist of partially overlapping neurons (orange and blue boxes). Fluid depth around the animal determines which locomotor pattern is selected. The proposed mechanism is that “shallow water detector” neurons provide inhibitory input to the swim CPG and excitatory input to the crawl CPG (Esch et al., 2002). Right, in the presence of dopamine (yellow cloud), the entire nervous system is biased toward crawling, and R3b1 only elicits crawling (Puhl et al., 2012). (B) Distinct subpopulations of activated PNs select feeding patterns with different dynamics. When the modulatory PN CBI-2 alone is activated, repeated stimulation is necessary to elicit an ingestive feeding pattern which persists for ∼30 min. However, if CBI-2 and CBI-3 are co-activated, an ingestive feeding pattern is immediately selected, but it is a transient activation (Evans et al., 2021). (C) The same PN, aSP22, activates different CPGs and different behaviors based on a spike number code. In this “ramp-to-threshold” example, as an increasing number of action potentials crosses different thresholds, aSP22 progressively activates CPGs contributing to different aspects of courtship (McKellar et al., 2019). (D) In response to different stimuli, the modulatory PNs MCN1 and CPN2 elicit qualitatively different chewing patterns due to distinctions in their activity patterns and rates (Beenhakker and Nusbaum, 2004; Blitz et al., 2008; White and Nusbaum, 2011; Diehl et al., 2013). MCN1 and CPN2 activity is indicated as extracellular recordings, with each colored box representing a burst of action potentials (different firing rates are not represented in the schematics). The differences in their activity are due to different strengths of CPG feedback (CPG feedback terminal size (colored circles) is representative of relative CPG feedback strength) (Blitz, 2017). Additionally, proprioceptive sensory neurons regulate MCN1 and CPN2 activity in the “orange” state when CPG feedback is weak, but not in the “blue” state, when CPG feedback is stronger (Beenhakker et al., 2007; White et al., 2017). Species used in the referenced studies are indicated in the panels.
3.2. Long-lasting activity states
Inputs to modulatory PNs have rapid transient effects, via fast synaptic transmission, or trigger activity persisting beyond the stimulus duration, via slower metabotropic actions (Rosen et al., 1991; Beenhakker and Nusbaum, 2004; Kristan et al., 2005; Brodfuehrer et al., 2008; Benjamin, 2012). For long-lasting PN activation, a behavioral switch might require active termination of PN activity, such as a transient “stop” signal from a sensory pathway that triggers an incompatible behavior via other PNs (Esch and Kristan, 2002; Mesce and Pierce-Shimomura, 2010). Additionally, interactions between modulatory neurons, serving to either reinforce or suppress activity in other modulatory PNs, enables them to play important roles in maintaining or switching behavioral state. This includes inhibiting competing PNs to remove their drive of an alternative CPG, activating PNs which inhibit a competing CPG, or exciting complementary PNs (Blitz and Nusbaum, 1997, 1999; Crisp and Mesce, 2006; Wu et al., 2014; Pirger et al., 2021).
A persistent behavioral state can also occur without long-term PN activation, but instead due to the duration of PN modulatory actions. In Aplysia feeding, repeated CBI-2 stimulation progressively adapts CPG activity and improves behavioral output, due to second messenger accumulation in target CPG neurons (Cropper et al., 2017). As a result, the CPG is biased toward one output over another, which may stabilize the circuit when one behavior is more likely to be useful (Cropper et al., 2017). Different from this auto-regulation, in another mollusk, Lymnaea, the octopaminergic OC cells enhance CPG responses to other modulatory neurons for multiple motor pattern cycles (Benjamin, 2012). Thus, motor system state can be regulated directly at the PN level, or in circuit responsiveness to PNs, across multiple timescales.
3.3. Long-distance CPG feedback
Another source of regulation is synaptic feedback from CPG neurons to PNs, which results in PN firing being time-locked to circuit activity, including in vivo and in semi-intact preparations when PNs are activated by physiological stimuli (Gillette et al., 1978; Blitz and Nusbaum, 2008; Mesce et al., 2008; Hedrich et al., 2011; Blitz, 2017). A distinct case occurs in the stick insect Carausius morosus in which PN walking-timed activity is due to sensory feedback instead of CPG feedback (Stolz et al., 2019). Feedback to PNs contributes to inter-circuit coordination, duration of PN activity, and gating of other PN inputs (Wood et al., 2004; Antri et al., 2009; Kozlov et al., 2014). Additionally, feedback control of modulatory PN activity can be important for motor pattern selection (see Section “4.2. Activity code”).
4. Motor pattern selection
4.1. Population code
Although experimentally-induced activation of an individual PN can elicit a motor pattern, physiological stimuli often activate more than one PN type (Coleman and Nusbaum, 1994; Esch and Kristan, 2002; Beenhakker and Nusbaum, 2004; Benjamin, 2012; Follmann et al., 2018; Fahoum and Blitz, 2021). This raises the possibility that the “modulatory code” for selecting a motor output is one in which different stimuli activate distinct PN subsets, resulting in a combinatorial “population code.” Such a scenario occurs in several systems, and experimentally manipulating which PNs are active elicits switches between motor patterns (Kristan and Shaw, 1997; Combes et al., 1999; Kupfermann and Weiss, 2001; Hedrich et al., 2009; Guo et al., 2022). In Aplysia when the modulatory PN CBI-2 is active, repeated stimulations are necessary to elicit an ingestive pattern, which is persistent, but if CBI-2 and CBI-3 are both active, they immediately elicit an ingestive motor pattern without induction of a persistent state (Evans et al., 2021; Figure 2B). Thus, the population of modulatory neurons active can determine the pattern produced, and other aspects such as the dynamics of motor pattern selection.
4.2. Activity code
Quantitatively, modulatory PN firing rate can regulate motor output, although differences occur in network sensitivity (Kristan et al., 2005; Hedrich et al., 2011; Benjamin, 2012; Spencer and Blitz, 2016; Sakurai and Katz, 2019). Additionally, an “activity code,” i.e., PN pattern and/or rate can encode qualitatively distinct motor patterns and behaviors. In Drosophila courtship, the same descending PN (aSP22) uses cumulative spike count, to elicit different behaviors in a sequential fashion. In this “ramp-to-threshold” mechanism, different behavioral components of courtship are generated as the aSP22 spike count crosses a series of thresholds (Figure 2C; McKellar et al., 2019). In the crab STNS, mechanosensory neurons and neuroendocrine cells each trigger long-lasting activation of two modulatory PNs (MCN1, CPN2) (Beenhakker and Nusbaum, 2004; Blitz et al., 2008). However, differential, long-lasting, modulation of CPG feedback in these two states results in distinct MCN1/CPN2 activity patterns and rates which encode different chewing behaviors, and different sensitivity to sensory feedback (Figure 2D; Beenhakker et al., 2007; Blitz and Nusbaum, 2008, 2012; Diehl et al., 2013; Blitz, 2017; White et al., 2017). The ability to manipulate feedback synapses onto small populations of identified modulatory neurons was essential for these insights into how CPG feedback to PNs contributes to motor pattern selection. Collectively, these examples illustrate that the same PNs can use an activity code to select motor outputs, instead of a population code of different PN subsets, with both mechanisms possible even in the same system, albeit in distinct species (Beenhakker and Nusbaum, 2004; Blitz et al., 2008; Hedrich et al., 2009).
5. Conclusion
Cellular-level access to modulatory PNs at their somata and axon terminals, and their CPG neuron targets in several invertebrate preparations enabled insights into regulation of PN activity, strategies for selecting an appropriate motor pattern, and significant complexity in communication between modulatory PNs and their CPG targets. Invertebrate PNs and larger vertebrate populations similarly link sensory and higher-order processing with motor circuits, and many of the insights discussed have already, or likely will be found to extend to larger circuits (Dickinson, 2006; Sharples et al., 2014; Yang et al., 2020). Technological advances are enabling recording and manipulation of genetically identified populations in organisms with barriers to electrophysiological approaches (e.g., neuronal size, accessibility, population size). However invertebrate organisms remain important for determining how modulatory PNs regulate circuits at the cellular-level, via electrophysiological recordings and manipulations that remain difficult in larger systems. Given the rapidly developing techniques making investigation in larger systems more tractable, plus the application of genetic approaches to classic neurophysiologically-accessible model organisms (Kim et al., 2017; Northcutt et al., 2018, 2019; Devineni and Scaplen, 2022; Leiras et al., 2022), diverse models and approaches are expected to continue increasing our understanding of how motor circuits rapidly adapt to the everchanging conditions in and around us.
Author contributions
DB wrote the first draft of the manuscript, revised the manuscript, read, and approved the final version.
Funding
Research in the author’s lab was supported by the National Science Foundation (IOS-1755283) and the National Institute of Neurological Disorders and Stroke (R15-NS128619).
Acknowledgments
I thank Savanna-Rae H. Fahoum and Barathan Gnanabharathi for feedback on earlier versions of the manuscript and current and former students and colleagues for their participation in work reviewed here.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ache, J. M., Namiki, S., Lee, A., Branson, K., and Card, G. M. (2019). State-dependent decoupling of sensory and motor circuits underlies behavioral flexibility in Drosophila. Nat. Neurosci. 22, 1132–1139. doi: 10.1038/S41593-019-0413-4
Angstadt, J. D., and Friesen, W. O. (1993). Modulation of swimming behavior in the medicinal leech. I. Effects of serotonin on the electrical properties of swim-gating cell 204. J. Comp. Physiol. A 172, 223–234. doi: 10.1007/BF00189398
Antri, M., Fénelon, K., and Dubuc, R. (2009). The contribution of synaptic inputs to sustained depolarizations in reticulospinal neurons. J. Neurosci. 29, 1140–1151. doi: 10.1523/JNEUROSCI.3073-08.2009
Barrière, G., Simmers, J., and Combes, D. (2008). Multiple mechanisms for integrating proprioceptive inputs that converge on the same motor pattern-generating network. J. Neurosci. 28, 8810–8820. doi: 10.1523/JNEUROSCI.2095-08.2008
Beenhakker, M. P., Kirby, M. S., and Nusbaum, M. P. (2007). Mechanosensory gating of proprioceptor input to modulatory projection neurons. J. Neurosci. 27, 14308–14316. doi: 10.1523/JNEUROSCI.4404-07.2007
Beenhakker, M. P., and Nusbaum, M. P. (2004). Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J. Neurosci. 24, 6741–6750. doi: 10.1523/JNEUROSCI.1682-04.2004
Benjamin, P. R. (2012). Distributed network organization underlying feeding behavior in the mollusk Lymnaea. Neural Syst. Circ. 2:4. doi: 10.1186/2042-1001-2-4
Bidaye, S. S., Laturney, M., Chang, A. K., Liu, Y., Bockemühl, T., Büschges, A., et al. (2020). Two brain pathways initiate distinct forward walking programs in drosophila. Neuron 108, 469–485.e8. doi: 10.1016/J.NEURON.2020.07.032
Blitz, D. M. (2017). Circuit feedback increases activity level of a circuit input through interactions with intrinsic properties. J. Neurophysiol. 118, 949–963. doi: 10.1152/jn.00772.2016
Blitz, D. M., Christie, A. E., Coleman, M. J., Norris, B. J., Marder, E., and Nusbaum, M. P. (1999). Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J. Neurosci. 19, 5449–5463. doi: 10.1523/jneurosci.19-13-05449.1999
Blitz, D. M., Christie, A. E., Cook, A. P., Dickinson, P. S., and Nusbaum, M. P. (2019). Similarities and differences in circuit responses to applied Gly1-SIFamide and peptidergic (Gly1 -SIFamide) neuron stimulation. J. Neurophysiol. 121, 950–972. doi: 10.1152/jn.00567.2018
Blitz, D. M., and Nusbaum, M. P. (1997). Motor pattern selection via inhibition of parallel pathways. J. Neurosci. 17, 4965–4975. doi: 10.1523/jneurosci.17-13-04965.1997
Blitz, D. M., and Nusbaum, M. P. (1999). Distinct functions for cotransmitters mediating motor pattern selection. J. Neurosci. 19, 6774–6783. doi: 10.1523/jneurosci.19-16-06774.1999
Blitz, D. M., and Nusbaum, M. P. (2008). State-dependent presynaptic inhibition regulates central pattern generator feedback to descending inputs. J. Neurosci. 28, 9564–9574. doi: 10.1523/JNEUROSCI.3011-08.2008
Blitz, D. M., and Nusbaum, M. P. (2012). Modulation of circuit feedback Specifies motor circuit output. J. Neurosci. 32, 9182–9193. doi: 10.1523/JNEUROSCI.1461-12.2012
Blitz, D. M., White, R. S., Saideman, S. R., Cook, A., Christie, A. E., Nadim, F., et al. (2008). A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J. Exp. Biol. 211, 1000–1011. doi: 10.1242/jeb.015222
Brewer, J. C., Olson, A. C., Collins, K. M., and Koelle, M. R. (2019). Serotonin and neuropeptides are both released by the HSN command neuron to initiate Caenorhabditis elegans egg laying. PLoS Genet. 15:e1007896. doi: 10.1371/JOURNAL.PGEN.1007896
Briggman, K. L., and Kristan, W. B. (2008). Multifunctional pattern-generating circuits. Annu. Rev. Neurosci. 31, 271–294. doi: 10.1146/annurev.neuro.31.060407.125552
Brodfuehrer, P. D., McCormick, K., Tapyrik, L., Albano, A. M., and Graybeal, C. (2008). Activation of two forms of locomotion by a previously identified trigger interneuron for swimming in the medicinal leech. Invert Neurosci. 8, 31–39. doi: 10.1007/S10158-007-0064-0
Bucher, D., Haspel, G., Golowasch, J., and Nadim, F. (2015). Central pattern generators. eLs 1–12. doi: 10.1002/9780470015902.a0000032.pub2
Calabrese, R. L., Norris, B. J., and Wenning, A. (2016). The neural control of heartbeat in invertebrates. Curr. Opin. Neurobiol. 41, 68–77. doi: 10.1016/J.CONB.2016.08.004
Cande, J., Namiki, S., Qiu, J., Korff, W., Card, G. M., Shaevitz, J. W., et al. (2018). Optogenetic dissection of descending behavioral control in Drosophila. Elife 7:e34275. doi: 10.7554/ELIFE.34275
Christie, A. E., Baldwin, D. H., Marder, E., and Graubard, K. (1997). Organization of the stomatogastric neuropil of the crab, Cancer borealis, as revealed by modulator immunocytochemistry. Cell Tissue Res. 288, 135–148. doi: 10.1007/S004410050801
Christie, A. E., Stein, W., Quinlan, J. E., Beenhakker, M. P., Marder, E., and Nusbaum, M. P. (2004). Actions of a histaminergic/peptidergic projection neuron on rhythmic motor patterns in the stomatogastric nervous system of the crab Cancer borealis. J. Comp. Neurol. 469, 153–169. doi: 10.1002/CNE.11003
Cifuentes, F., and Morales, M. A. (2021). Functional implications of neurotransmitter segregation. Front. Neural Circ. 15:738516. doi: 10.3389/fncir.2021.738516
Coleman, M. J., Meyrand, P., and Nusbaum, M. P. (1995). A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature 378, 502–505. doi: 10.1038/378502a0
Coleman, M. J., and Nusbaum, M. P. (1994). Functional consequences of compartmentalization of synaptic input. J. Neurosci. 14, 6544–6552. doi: 10.1523/JNEUROSCI.14-11-06544.1994
Coleman, M. J., Nusbaum, M. P., Cournil, I., and Claiborne, B. J. (1992). Distribution of modulatory inputs to the stomatogastric ganglion of the crab, Cancer borealis. J. Comp. Neurol. 325, 581–594. doi: 10.1002/CNE.903250410
Colton, G. F., Cook, A. P., and Nusbaum, M. P. (2020). Different microcircuit responses to comparable input from one versus both copies of an identified projection neuron. J. Exp. Biol. 223:jeb228114. doi: 10.1242/JEB.228114
Combes, D., Meyrand, P., and Simmers, J. (1999). Dynamic restructuring of a rhythmic motor program by a single mechanoreceptor neuron in lobster. J. Neurosci. 19, 3620–3628. doi: 10.1523/JNEUROSCI.19-09-03620.1999
Cook, A. P., and Nusbaum, M. P. (2021). Feeding state-dependent modulation of feeding-related motor patterns. J. Neurophysiol. 126, 1903–1924. doi: 10.1152/JN.00387.2021
Crisp, K. M., and Mesce, K. A. (2006). Beyond the central pattern generator: amine modulation of decision-making neural pathways descending from the brain of the medicinal leech. J. Exp. Biol. 209, 1746–1756. doi: 10.1242/JEB.02204
Cropper, E. C., Jing, J., Perkins, M. H., and Weiss, K. R. (2017). Use of the Aplysia feeding network to study repetition priming of an episodic behavior. J. Neurophysiol. 118, 1861–1870. doi: 10.1152/JN.00373.2017
Cropper, E. C., Jing, J., Vilim, F. S., Barry, M. A., and Weiss, K. R. (2018). Multifaceted expression of peptidergic modulation in the feeding system of Aplysia. ACS Chem. Neurosci. 9, 1917–1927. doi: 10.1021/ACSCHEMNEURO.7B00447
Daur, N., Nadim, F., and Bucher, D. (2016). The complexity of small circuits: the stomatogastric nervous system. Curr. Opin. Neurobiol. 41, 1–7. doi: 10.1016/J.CONB.2016.07.005
DeLong, N. D., Beenhakker, M. P., and Nusbaum, M. P. (2009). Presynaptic inhibition selectively weakens peptidergic cotransmission in a small motor system. J. Neurophysiol. 102, 3492–3504. doi: 10.1152/jn.00833.2009
Devineni, A. V., and Scaplen, K. M. (2022). Neural circuits underlying behavioral flexibility: insights from drosophila. Front. Behav. Neurosci. 15:821680. doi: 10.3389/FNBEH.2021.821680
Dickinson, P. S. (2006). Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr. Opin. Neurobiol. 16, 604–614. doi: 10.1016/J.CONB.2006.10.007
Dickinson, P. S., Mecsas, C., and Marder, E. (1990). Neuropeptide fusion of two motor-pattern generator circuits. Nature 344, 155–158. doi: 10.1038/344155A0
Diehl, F., White, R. S., Stein, W., and Nusbaum, M. P. (2013). Motor circuit-specific burst patterns drive different muscle and behavior patterns. J. Neurosci. 33, 12013–12029. doi: 10.1523/JNEUROSCI.1060-13.2013
Disney, A. A., and Higley, M. J. (2020). Diverse spatiotemporal scales of cholinergic signaling in the neocortex. J. Neurosci. 40, 720–725. doi: 10.1523/JNEUROSCI.1306-19.2019
Eiden, L. E., Hernández, V. S., Jiang, S. Z., and Zhang, L. (2022). Neuropeptides and small-molecule amine transmitters: cooperative signaling in the nervous system. Cell Mol. Life Sci. 79:492. doi: 10.1007/S00018-022-04451-7
Esch, T., and Kristan, W. B. (2002). Decision-making in the leech nervous system. Integr. Comp. Biol. 42, 716–724.
Esch, T., Mesce, K. A., and Kristan, W. B. (2002). Evidence for sequential decision making in the medicinal leech. J. Neurosci. 22, 11045–11054. doi: 10.1523/JNEUROSCI.22-24-11045.2002
Evans, C. G., Barry, M. A., Jing, J., Perkins, M. H., Weiss, K. R., and Cropper, E. C. (2021). The complement of projection neurons activated determines the type of feeding motor program in Aplysia. Front. Neural Circuits 15:685222. doi: 10.3389/FNCIR.2021.685222
Fahoum, S. R. H., and Blitz, D. M. (2021). Neuronal switching between single- And dual-network activity via modulation of intrinsic membrane properties. J. Neurosci. 41, 7848–7863. doi: 10.1523/JNEUROSCI.0286-21.2021
Flaive, A., Fougère, M., van der Zouwen, C. I., and Ryczko, D. (2020). Serotonergic modulation of locomotor activity from basal vertebrates to mammals. Front. Neural Circuits 14:590299. doi: 10.3389/FNCIR.2020.590299
Flamm, R. E., and Harris-Warrick, R. M. (1986). Aminergic modulation in lobster stomatogastric ganglion. I. Effects on motor pattern and activity of neurons within the pyloric circuit. J. Neurophysiol. 55, 847–865. doi: 10.1152/JN.1986.55.5.847
Flood, T. F., Iguchi, S., Gorczyca, M., White, B., Ito, K., and Yoshihara, M. (2013). A single pair of interneurons commands the Drosophila feeding motor program. Nature 499, 83–87. doi: 10.1038/nature12208
Florman, J. T., and Alkema, M. J. (2022). Co-transmission of neuropeptides and monoamines choreograph the C. elegans escape response. PLoS Genet. 18:e1010091. doi: 10.1371/JOURNAL.PGEN.1010091
Follmann, R., Goldsmith, C. J., and Stein, W. (2018). Multimodal sensory information is represented by a combinatorial code in a sensorimotor system. PLoS Biol. 16:e2004527. doi: 10.1371/JOURNAL.PBIO.2004527
Garcia, A. J., Zanella, S., Koch, H., Doi, A., and Ramirez, J. M. (2011). Chapter 3–networks within networks: the neuronal control of breathing. Prog. Brain Res. 188, 31–50. doi: 10.1016/B978-0-444-53825-3.00008-5
Gillette, R., Kovac, M. P., and Davis, W. J. (1978). Command neurons in Pleurobranchaea receive synaptic feedback from the motor network they excite. Science 199, 798–801. doi: 10.1126/SCIENCE.622571
Guo, L., Zhang, N., and Simpson, J. H. (2022). Descending neurons coordinate anterior grooming behavior in Drosophila. Curr. Biol. 32, 823–833.e4. doi: 10.1016/J.CUB.2021.12.055
Harris-Warrick, R. M., and Marder, E. (1991). Modulation of neural networks for behavior. Annu. Rev. Neurosci. 14, 39–57. doi: 10.1146/ANNUREV.NE.14.030191.000351
Hedrich, U. B. S., Diehl, F., and Stein, W. (2011). Gastric and pyloric motor pattern control by a modulatory projection neuron in the intact crab Cancer pagurus. J. Neurophysiol. 105, 1671–1680. doi: 10.1152/JN.01105.2010
Hedrich, U. B. S., Smarandache, C. R., and Stein, W. (2009). Differential activation of projection neurons by two sensory pathways contributes to motor pattern selection. J. Neurophysiol. 102, 2866–2879. doi: 10.1152/jn.00618.2009
Heinrich, R. (2002). Impact of descending brain neurons on the control of stridulation, walking, and flight in orthoptera. Microsc. Res. Tech. 56, 292–301. doi: 10.1002/JEMT.10033
Herberholz, J. (2022). The giant escape neurons of crayfish: past discoveries and present opportunities. Front. Physiol. 13:1052354. doi: 10.3389/FPHYS.2022.1052354
Hooper, S. L., and Marder, E. (1984). Modulation of a central pattern generator by two neuropeptides, proctolin and FMRFamide. Brain Res. 305, 186–191. doi: 10.1016/0006-8993(84)91138-7
Horn, C. C., Benjamin, P. R., Weiss, K. R., and Kupfermann, I. (1999). Decrement of the response of a serotonergic modulatory neuron (the metacerebral cell) in Aplysia, during repeated presentation of appetitive (food) stimuli. Neurosci. Lett. 267, 161–164. doi: 10.1016/S0304-3940(99)00339-0
Hsu, C. T., and Bhandawat, V. (2016). Organization of descending neurons in Drosophila melanogaster. Sci. Rep. 6:20259. doi: 10.1038/SREP20259
Hurwitz, I., Susswein, A. J., and Weiss, K. R. (2005). Transforming tonic firing into a rhythmic output in the Aplysia feeding system: presynaptic inhibition of a command-like neuron by a CpG element. J. Neurophysiol. 93, 829–842. doi: 10.1152/JN.00559.2004
Jing, J., and Weiss, K. R. (2005). Generation of variants of a motor act in a modular and hierarchical motor network. Curr. Biol. 15, 1712–1721. doi: 10.1016/J.CUB.2005.08.051
Katz, P. S. (1998). Neuromodulation intrinsic to the central pattern generator for escape swimming in Tritonia. Ann. N. Y. Acad. Sci. 860, 181–188. doi: 10.1111/J.1749-6632.1998.TB09048.X
Katz, P. S., and Calin-Jageman, R. J. (2009). Neuromodulation. Encycl. Neurosci. 6, 497–503. doi: 10.1016/B978-008045046-9.01964-1
Katz, P. S., and Quinlan, P. D. (2019). The importance of identified neurons in gastropod molluscs to neuroscience. Curr. Opin. Neurobiol. 56, 1–7. doi: 10.1016/J.CONB.2018.10.009
Kim, L. H., Sharma, S., Sharples, S. A., Mayr, K. A., Kwok, C. H. T., and Whelan, P. J. (2017). Integration of descending command systems for the generation of context-specific locomotor behaviors. Front. Neurosci. 11:581. doi: 10.3389/FNINS.2017.00581
Koh, H. Y., Vilim, F. S., Jing, J., and Weiss, K. R. (2003). Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J. Neurophysiol. 90, 2074–2079. doi: 10.1152/JN.00358.2003
Kozlov, A. K., Kardamakis, A. A., Kotaleski, J. H., and Grillner, S. (2014). Gating of steering signals through phasic modulation of reticulospinal neurons during locomotion. Proc. Natl. Acad. Sci. U. S. A. 111, 3591–3596. doi: 10.1073/PNAS.1401459111
Kristan, W. B., Calabrese, R. L., and Friesen, W. O. (2005). Neuronal control of leech behavior. Prog. Neurobiol. 76, 279–327. doi: 10.1016/J.PNEUROBIO.2005.09.004
Kristan, W. B., and Shaw, B. K. (1997). Population coding and behavioral choice. Curr. Opin. Neurobiol. 7, 826–831. doi: 10.1016/S0959-4388(97)80142-0
Kueh, D., and Jellies, J. A. (2012). Targeting a neuropeptide to discrete regions of the motor arborizations of a single neuron. J. Exp. Biol. 215, 2108–2116. doi: 10.1242/JEB.067603
Kuhlman, J. R., Li, C., and Calabrese, R. L. (1985). FMRF-amide-like substances in the leech. II. Bioactivity on the heartbeat system. J. Neurosci. 5, 2310–2317. doi: 10.1523/JNEUROSCI.05-09-02310.1985
Kupfermann, I., and Weiss, K. R. (2001). Motor program selection in simple model systems. Curr. Opin. Neurobiol. 11, 673–677. doi: 10.1016/S0959-4388(01)00267-7
Kwiatkowski, M. A., Gabranski, E. R., Huber, K. E., Chapline, M. C., Christie, A. E., and Dickinson, P. S. (2013). Coordination of distinct but interacting rhythmic motor programs by a modulatory projection neuron using different co-transmitters in different ganglia. J. Exp. Biol. 216, 1827–1836. doi: 10.1242/JEB.082503
Leiras, R., Cregg, J. M., and Kiehn, O. (2022). Brainstem circuits for locomotion. Annu. Rev. Neurosci. 45, 63–85. doi: 10.1146/ANNUREV-NEURO-082321-025137
Liu, C., Goel, P., and Kaeser, P. S. (2021). Spatial and temporal scales of dopamine transmission. Nat. Rev. Neurosci. 22, 345–358. doi: 10.1038/S41583-021-00455-7
Marder, E. (2012). Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11. doi: 10.1016/j.neuron.2012.09.010
Marder, E., and Bucher, D. (2007). Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Ann. Rev. Physiol. 69, 291–316. doi: 10.1146/annurev.physiol.69.031905.161516
Marder, E., Gutierrez, G. J., and Nusbaum, M. P. (2017). Complicating connectomes: electrical coupling creates parallel pathways and degenerate circuit mechanisms. Dev. Neurobiol. 77, 597–609. doi: 10.1002/DNEU.22410
Marder, E., Kedia, S., and Morozova, E. O. (2022). New insights from small rhythmic circuits. Curr. Opin. Neurobiol. 76:102610. doi: 10.1016/J.CONB.2022.102610
McKellar, C. E., Lillvis, J. L., Bath, D. E., Fitzgerald, J. E., Cannon, J. G., Simpson, J. H., et al. (2019). Threshold-Based ordering of sequential actions during Drosophila courtship. Curr. Biol. 29, 426–434.e6. doi: 10.1016/J.CUB.2018.12.019
Mesce, K. A., Esch, T., and Kristan, W. B. (2008). Cellular substrates of action selection: a cluster of higher-order descending neurons shapes body posture and locomotion. J. Comp. Physiol. A Neuroethol. Sens Neural Behav. Physiol. 194, 469–481. doi: 10.1007/S00359-008-0319-1
Mesce, K. A., and Pierce-Shimomura, J. T. (2010). Shared strategies for behavioral switching: understanding how locomotor patterns are turned on and off. Front. Behav. Neurosci. 4:49. doi: 10.3389/FNBEH.2010.00049
Meyrand, P., Faumont, S., Simmers, J., Christie, A. E., and Nusbaum, M. P. (2000). Species-specific modulation of pattern-Generating circuits. Eur. J. Neurosci. 12, 2585–2596. doi: 10.1046/J.1460-9568.2000.00121.X
Nadim, F., and Bucher, D. (2014). Neuromodulation of neurons and synapses. Curr. Opin. Neurobiol. 29, 48–56. doi: 10.1016/j.conb.2014.05.003
Namiki, S., Dickinson, M. H., Wong, A. M., Korff, W., and Card, G. M. (2018). The functional organization of descending sensory-motor pathways in Drosophila. Elife 7:e34272. doi: 10.7554/ELIFE.34272
Nässel, D. R. (2009). Neuropeptide signaling near and far: how localized and timed is the action of neuropeptides in brain circuits? Invert Neurosci. 9, 57–75. doi: 10.1007/S10158-009-0090-1
Nässel, D. R. (2018). Substrates for neuronal cotransmission with neuropeptides and small molecule neurotransmitters in drosophila. Front Cell Neurosci. 12:83. doi: 10.3389/FNCEL.2018.00083/BIBTEX
Nässel, D. R., and Zandawala, M. (2022). Endocrine cybernetics: neuropeptides as molecular switches in behavioural decisions. Open Biol. 12:220174. doi: 10.1098/RSOB.220174
Norris, B. J., Coleman, M. J., and Nusbaum, M. P. (1994). Recruitment of a projection neuron determines gastric mill motor pattern selection in the stomatogastric nervous system of the crab, Cancer borealis. J. Neurophysiol. 72, 1451–1463. doi: 10.1152/JN.1994.72.4.1451
Norris, B. J., Coleman, M. J., and Nusbaum, M. P. (1996). Pyloric motor pattern modification by a newly identified projection neuron in the crab stomatogastric nervous system. J. Neurophysiol. 75, 97–108. doi: 10.1152/JN.1996.75.1.97
Northcutt, A. J., Fischer, E. K., Puhl, J. G., Mesce, K. A., and Schulz, D. J. (2018). An annotated CNS transcriptome of the medicinal leech, Hirudo verbana: De novo sequencing to characterize genes associated with nervous system activity. PLoS One 13:e0201206. doi: 10.1371/JOURNAL.PONE.0201206
Northcutt, A. J., Kick, D. R., Otopalik, A. G., Goetz, B. M., Harris, R. M., Santin, J. M., et al. (2019). Molecular profiling of single neurons of known identity in two ganglia from the crab Cancer borealis. Proc. Natl. Acad. Sci. U. S. A. 116, 26980–26990. doi: 10.1073/PNAS.1911413116
Nusbaum, M. P. (1994). Presynaptic control of neurones in pattern-generating networks. Curr. Opin. Neurobiol. 4, 909–914.
Nusbaum, M. P. (2002). Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav. Evol. 60, 378–387. doi: 10.1159/000067791
Nusbaum, M. P. (2008). “Modulatory projection neurons,” in Encyclopedia of Neuroscience, eds M. D. Binder, N. Hirokawa, and U. Windhorst (Berlin: Springer), 2385–2388. doi: 10.1007/978-3-540-29678-2_3538
Nusbaum, M. P., Blitz, D. M., and Marder, E. (2017). Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 18, 389–403. doi: 10.1038/nrn.2017.56
Nusbaum, M. P., and Marder, E. (1989a). A modulatory proctolin-containing neuron (MPN). I. Identification and characterization. J. Neurosci. 9, 1591–1599. doi: 10.1523/JNEUROSCI.09-05-01591.1989
Nusbaum, M. P., and Marder, E. (1989b). A modulatory proctolin-containing neuron (MPN). II. State-dependent modulation of rhythmic motor activity. J. Neurosci. 9, 1600–1607. doi: 10.1523/JNEUROSCI.09-05-01600.1989
Nusbaum, M. P., Weimann, J. M., Golowasch, J., and Marder, E. (1992). Presynaptic control of modulatory fibers by their neural network targets. J. Neurosci. 12, 2706–2714. doi: 10.1523/JNEUROSCI.12-07-02706.1992
Perrins, R., and Weiss, K. R. (1998). Compartmentalization of information processing in an Aplysia feeding circuit interneuron through membrane properties and synaptic interactions. J. Neurosci. 18, 3977–3989. doi: 10.1523/JNEUROSCI.18-10-03977.1998
Pirger, Z., László, Z., Naskar, S., Crossley, M., O’Shea, M., Benjamin, P. R., et al. (2021). Interneuronal mechanisms for learning-induced switch in a sensory response that anticipates changes in behavioral outcomes. Curr. Biol. 31, 1754–1761.e3. doi: 10.1016/J.CUB.2021.01.072
Puhl, J. G., Masino, M. A., and Mesce, K. A. (2012). Necessary, sufficient and permissive: a single locomotor command neuron important for intersegmental coordination. J. Neurosci. 32, 17646–17657. doi: 10.1523/JNEUROSCI.2249-12.2012
Ramirez, J. M., and Pearson, K. G. (1991). Octopaminergic modulation of interneurons in the flight system of the locust. J. Neurophysiol. 66, 1522–1537. doi: 10.1152/JN.1991.66.5.1522
Rosen, S. C., Teyke, T., Miller, M. W., Weiss, K. R., and Kupfermann, I. (1991). Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J. Neurosci. 11, 3630–3655. doi: 10.1523/JNEUROSCI.11-11-03630.1991
Ruder, L., and Arber, S. (2019). Brainstem circuits controlling action diversification. Annu. Rev. Neurosci. 42, 485–504. doi: 10.1146/ANNUREV-NEURO-070918-050201
Sakurai, A., and Katz, P. S. (2019). Command or Obey? Homologous neurons differ in hierarchical position for the generation of homologous behaviors. J. Neurosci. 39, 6460–6471. doi: 10.1523/JNEUROSCI.3229-18.2019
Schlegel, P., Texada, M. J., Miroschnikow, A., Schoofs, A., Hückesfeld, S., Peters, M., et al. (2016). Synaptic transmission parallels neuromodulation in a central food-intake circuit. Elife 5:e16799. doi: 10.7554/ELIFE.16799
Sharples, S. A., Koblinger, K., Humphreys, J. M., and Whelan, P. J. (2014). Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front. Neural Circuits 8:55. doi: 10.3389/FNCIR.2014.00055
Snyder, R. R., and Blitz, D. M. (2022). Multiple intrinsic membrane properties are modulated in a switch from single- to dual-network activity. J. Neurophysiol. 128, 1181–1198. doi: 10.1152/JN.00337.2022
Spencer, R. M., and Blitz, D. M. (2016). Network feedback regulates motor output across a range of modulatory neuron activity. J. Neurophysiol. 115, 3249–3263. doi: 10.1152/jn.01112.2015
Staudacher, E. M. (2001). Sensory responses of descending brain neurons in the walking cricket, Gryllus bimaculatus. J. Comp. Physiol. A 187, 1–17. doi: 10.1007/S003590000171
Stein, W. (2009). Modulation of stomatogastric rhythms. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 195, 989–1009. doi: 10.1007/S00359-009-0483-Y
Stein, W., DeLong, N. D., Wood, D. E., and Nusbaum, M. P. (2007). Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur. J. Neurosci. 26, 1148–1165. doi: 10.1111/j.1460-9568.2007.05744.x
Stolz, T., Diesner, M., Neupert, S., Hess, M. E., Delgado-Betancourt, E., Pflüger, H. J., et al. (2019). Descending octopaminergic neurons modulate sensory-evoked activity of thoracic motor neurons in stick insects. J. Neurophysiol. 122, 2388–2413. doi: 10.1152/JN.00196.2019
Svensson, E., Apergis-Schoute, J., Burnstock, G., Nusbaum, M. P., Parker, D., and Schiöth, H. B. (2019). General principles of neuronal co-transmission: insights from multiple model systems. Front. Neural Circuits 12:117. doi: 10.3389/fncir.2018.00117
Swensen, A. M., Golowasch, J., Christie, A. E., Coleman, M. J., Nusbaum, M. P., and Marder, E. (2000). GABA and responses to GABA in the stomatogastric ganglion of the crab Cancer borealis. J. Exp. Biol. 203, 2075–2092. doi: 10.1242/JEB.203.14.2075
Thirumalai, V., and Marder, E. (2002). Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. J. Neurosci. 22, 1874–1882. doi: 10.1523/JNEUROSCI.22-05-01874.2002
Trudeau, L. E., and El Mestikawy, S. (2018). Glutamate cotransmission in cholinergic, GABAergic and monoamine systems: contrasts and commonalities. Front. Neural Circuits 12:113. doi: 10.3389/FNCIR.2018.00113/BIBTEX
White, R. S., and Nusbaum, M. P. (2011). The same core rhythm generator underlies different rhythmic motor patterns. J. Neurosci. 31, 11484–11494. doi: 10.1523/JNEUROSCI.1885-11.2011
White, R. S., Spencer, R. M., Nusbaum, M. P., and Blitz, D. M. (2017). State-dependent sensorimotor gating in a rhythmic motor system. J. Neurophysiol. 118, 2806–2818. doi: 10.1152/jn.00420.2017
Willard, A. L. (1981). Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J. Neurosci. 1, 936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981
Wood, D. E., Manor, Y., Nadim, F., and Nusbaum, M. P. (2004). Intercircuit control via rhythmic regulation of projection neuron activity. J. Neurosci. 24, 7455–7463. doi: 10.1523/JNEUROSCI.1840-04.2004
Wood, D. E., and Nusbaum, M. P. (2002). Extracellular peptidase activity tunes motor pattern modulation. J. Neurosci. 22, 4185–4195. doi: 10.1523/jneurosci.22-10-04185.2002
Wu, J. S., Wang, N., Siniscalchi, M. J., Perkins, M. H., Zheng, Y. T., Yu, W., et al. (2014). Complementary interactions between command-like interneurons that function to activate and specify motor programs. J. Neurosci. 34, 6510–6521. doi: 10.1523/JNEUROSCI.5094-13.2014
Yang, C. F., Kim, E. J., Callaway, E. M., and Feldman, J. L. (2020). Monosynaptic projections to excitatory and inhibitory prebötzinger complex neurons. Front. Neuroanat. 14:58. doi: 10.3389/FNANA.2020.00058
Keywords: central pattern generator, neuropeptide, feedback, neuromodulation, neural circuit, modulatory projection neuron
Citation: Blitz DM (2023) Neural circuit regulation by identified modulatory projection neurons. Front. Neurosci. 17:1154769. doi: 10.3389/fnins.2023.1154769
Received: 31 January 2023; Accepted: 01 March 2023;
Published: 17 March 2023.
Edited by:
Krista Todd, Westminster College, United StatesReviewed by:
Joshua Lillvis, Janelia Research Campus, United StatesCopyright © 2023 Blitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dawn M. Blitz, blitzdm@miamioh.edu
 Dawn M. Blitz
Dawn M. Blitz