- 1School of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Beijing Institute of Pharmacology and Toxicology, Beijing, China
- 3State Key Laboratory of Toxicology and Medical Countermeasures, Beijing, China
Adult neurogenesis plays a crucial role in cognitive function and mood regulation, while aberrant adult neurogenesis contributes to various neurological and psychiatric diseases. With a better understanding of the significance of adult neurogenesis, the demand for improving adult neurogenesis is increasing. More and more research has shown that traditional Chinese medicine (TCM), including TCM prescriptions (TCMPs), Chinese herbal medicine, and bioactive components, has unique advantages in treating neurological and psychiatric diseases by regulating adult neurogenesis at various stages, including proliferation, differentiation, and maturation. In this review, we summarize the progress of TCM in improving adult neurogenesis and the key possible mechanisms by which TCM may benefit it. Finally, we suggest the possible strategies of TCM to improve adult neurogenesis in the treatment of neuropsychiatric disorders.
1. Introduction
Adult neurogenesis is the process of generating functional neurons from neural stem cells (NSCs) (Ming and Song, 2011), which is involved in learning, memory, and emotion and may also be involved in the remodeling of the central nervous system (Taupin, 2005; Lledo et al., 2006; Toda and Gage, 2018). Adult neurogenesis abnormalities play an important role in a variety of neurodegenerative disorders, such as Alzheimer’s disease (AD), Huntington’s disease (HD), and Parkinson’s disease (PD) (Winner and Winkler, 2015; Horgusluoglu et al., 2017; Berger et al., 2020). In addition, adult neurogenesis is associated with emotional illnesses, such as depression (Sahay and Hen, 2007; Vaidya et al., 2007; Berger et al., 2020) and anxiety (Cheung et al., 2016; Toda and Gage, 2018). Stress (Odaka et al., 2017; Schoenfeld et al., 2017) and stroke (Rahman et al., 2021) are also associated with abnormal adult neurogenesis. Considering the role of adult neurogenesis in the pathophysiology of neurological and psychiatric diseases, restoring neurological function by improving adult neurogenesis is one of the main directions in the field of neuroscience.
A lot of work has gone into finding effective medications to boost adult neurogenesis. Recent progress in adult neurogenesis represents a potentially promising target for the treatment of neurological (Taupin, 2008; Matsuda and Nakashima, 2021) and mental conditions (DeCarolis and Eisch, 2010; Jun et al., 2012). Traditional Chinese medicine (TCM) has been used for centuries in China and other Asian countries, such as Korea and Japan. In recent years, TCM, including TCM prescription drugs (TCMPs), Chinese herbal medicine (CHM), and bioactive components extracted from TCM, have been found to have great potential for improving adult neurogenesis in the treatment of neuropsychiatric disorders. In this review, we summarize the effects of TCM on regulating adult neurogenesis and their potential mechanisms and provide the basis for TCM targeting adult neurogenesis in the treatment of neuropsychiatric diseases.
2. Adult neurogenesis: From neural stem cells to therapy
2.1. Biological significance of adult neurogenesis
Neurogenesis is the process by which NSCs proliferate and differentiate to produce new neurons (this process can be seen in Figure 1), which is essential for the development of the brain and the establishment of functional connections. The nervous system of adult mammals has long been considered a non-regenerative tissue. However, in 1965, Altman and Das (Altman and Das, 1965) first observed neurogenesis in adult rats, subsequently, in 1998, Eriksson et al (Eriksson et al, 1998) provided evidence for the existence of adult neurogenesis of human. Over the next decade, the evidence for adult human neurogenesis has been refined (Boldrini et al., 2018; Sorrells et al., 2018; Moreno-Jimenez et al., 2021), confirming that adult neurogenesis exists throughout life (Zhou et al., 2022). With the deepening of adult neurogenesis research, it has been confirmed that adult neurogenesis occurs in two regions of the adult brain: the subgranular zone of the hippocampus (SGZ) and the subventricular zone (SVZ) of the lateral ventricles of adult mammals (Gould, 2007).
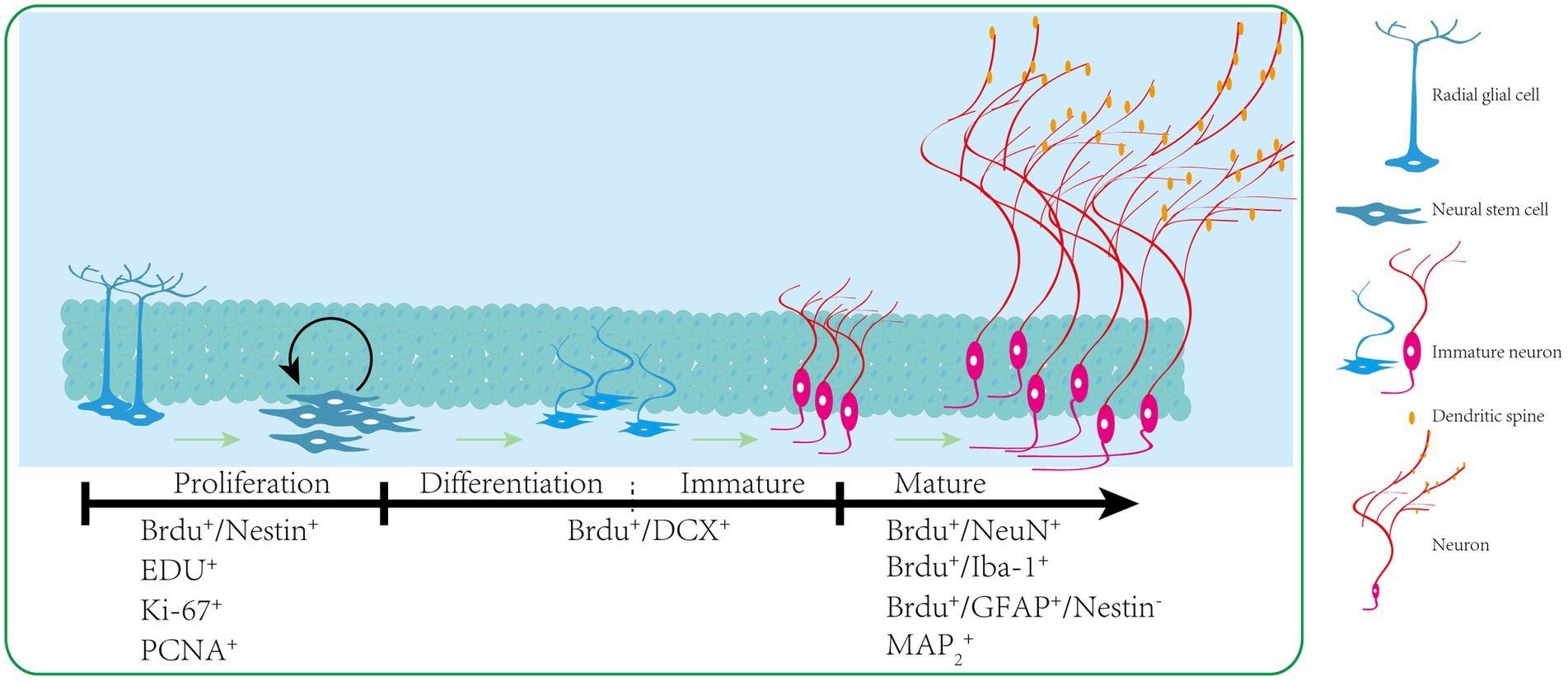
Figure 1. Adult hippocampus of the dentate gyrus. Newly formed neurons in the sub-granular zone of the dentate gyrus pass through several consecutive developmental stages. The radial glial cells can generate proliferating NSCs with transient amplifying characteristics. These NSCs can give rise to neuroblasts that subsequently differentiate into dentate granule neurons. The developmental trajectory is accompanied by the subsequent expression of stage-specific molecular markers.
There is growing evidence that adult neurogenesis is essential for central nervous system (CNS) function. Adult neurogenesis is associated with cognition and emotion (Anacker and Hen, 2017; Alam et al., 2018). Adult neurogenesis is involved in cognition, including memory interference and indexing (Miller and Sahay, 2019), learning (Yau et al., 2015), and forgetting (Akers et al., 2014). Adult neurogenesis is also involved in the regulation of mood (Anacker and Hen, 2017), reduced neurogenesis has been implicated in the pathogenesis of anxiety and depression (Snyder et al., 2011), and increasing adult neurogenesis is sufficient to reduce anxiety and depression-like behaviors (Hill et al., 2015). Meanwhile, researchers have found that adult neurogenesis confers stress resilience (Anacker et al., 2018), and this resilience is necessary for the body to adapt to new environments. In addition, after a brain injury, the new neurons generated by adult neurogenesis are essential for the recovery of neural function (Marques et al., 2019).
It is known that NSCs progress through distinct stages before they become mature neurons, and this process is tightly controlled by cell-intrinsic factors and signals in the neurogenic niche (Johnson et al., 2009; Suh et al., 2009). In short, adult neurogenesis is tightly regulated by cell-intrinsic molecules and extrinsic signaling. Intrinsic signaling involves phosphoinositide 3-kinase (PI3K)/Akt, Notch-Hairy, and enhancer of split (Notch-Hes) signaling, Hedgehog signaling, bone morphogenetic protein signaling, and Wingless/Integrated signaling (Goncalves et al., 2016; Matsubara et al., 2021). Extracellular signaling is mainly from the NSC niche that creates a favorable microenvironment and architecture to sustain NSCs and neurogenesis (Li and Guo, 2021). Such factors as growth factors, neurotrophic factors, and neurotransmitters have also been reported to be part of the regulatory signaling within the hippocampal niche (Goncalves et al., 2016). Importantly, intrinsic and extrinsic signaling crosstalk and act on the CNS to regulate neurogenesis.
As its existence has been questioned in the past, studies have sought to understand how adult neurogenesis affects the human brain in both health and disease. Researchers have also looked at the factors that may affect this process. The above developments greatly promote our understanding of adult neurogenesis and how it might be used to enhance CNS performance and for the prevention and treatment of diseases that affect it.
2.2. Adult neurogenesis and neuropsychiatric diseases
With the growing understanding of the role of adult neurogenesis in the regulation of cognitive function, emotion, and brain repair after injury, the study of the relationship between this process and neuropsychiatric diseases has also made progress. Changes in adult neurogenesis were observed in neurological (such as AD, PD, HD, and stroke) and psychiatric (depression and post-stroke depression) diseases, and adult neurogenesis has been found to be involved in the pathological mechanisms of these diseases. Improving adult neurogenesis has been tried as a means of alleviating neurological and psychiatric disorders.
Alterations in adult neurogenesis have been reported in most neurological disorders, including neurodegenerative diseases and stroke. Since adult neurogenesis is involved in the regulation of cognition (Anacker and Hen, 2017), modulating adult neurogenesis may help to improve cognitive deficits in some neuropsychiatric disorders (Berger et al., 2020). In fact, abnormal adult neurogenesis has been observed in neurodegenerative diseases such as AD, HD, PD, and amyotrophic lateral sclerosis (ALS) (Jordan et al., 2006; Vivar, 2015; Horgusluoglu et al., 2017), which manifest as cognitive decline (Winner and Winkler, 2015; Terreros-Roncal et al., 2021). In contrast to the reduction of neurogenesis in neurodegenerative diseases, the proliferation of NSCs and the production of neuroblasts were activated after stroke. These neuroblasts migrate to the infarcted area, contribute to the repair of the infarcted brain, and form glial scar tissue (Koh and Park, 2017). However, based on comparisons between the density of BrdU-stained cells colabeled with a neuronal marker at 2 and 6 weeks post-ischemia about 80% or more of the new neurons died during this time interval (Arvidsson et al., 2002). Meanwhile, the effect of compensatory neurogenesis in repairing and restoring neural function has been limited. Fortunately, exogenous transplantation of NSCs (Hassani et al., 2012) and drugs (Chen et al., 2016) can be beneficial for neurogenesis and contribute to the recovery of brain function (such as motor balance and cognition) after stroke (Jin et al., 2006), and promoting adult neurogenesis has become an important direction for post-stroke recovery treatment (Marques et al., 2019).
Alterations in adult neurogenesis and reduced size of the hippocampus were reported in most psychiatric disorders, including schizophrenia, major depression, addiction, and anxiety, and in a significant subpopulation of patients with depression (Goncalves et al., 2016). Depressive disorders may be caused by impaired adult hippocampal neurogenesis in adults (Miller and Hen, 2015), and the effects of antidepressants have been found to relate to neurogenesis (Santarelli et al., 2003). Currently, medicine uses antidepressants such as fluoxetine, sertraline, and paroxetine, which could improve impaired cognitive, emotional, and motor function by promoting adult neurogenesis (Li et al., 2009).
However, to date, there isno clinical evidence of an isolated impairment of adult hippocampal neurogenesis in the absence of other abnormalities,but numerous studies have reported alterations in adult neurogenesis that are associated with several neurological and psychiatric disorders, providing a link between adult neurogenesis and human disease (Goncalves et al., 2016).
Since neurogenesis is related to a variety of neurological and psychiatric diseases, researchers have begun to try to alleviate diseases by influencing neurogenesis and have made some progress. The amelioration of diseases by neurogenesis mainly includes intracerebral transplantation and endogenous activation of NSCs (Chrostek et al., 2019; Wang J. et al., 2021). Although clinical data or evidence of a causal relationship between adult neurogenesis and disease are still lacking, a growing body of evidence in rodents and non-human primates indicates that improving adult neurogenesis contributes to restoring brain function in neuropsychiatric disorders. On this basis, research was carried out on the treatment of diseases with NSC transplantation or endogenous activation of NSCs. NSC transplantation could improve AD, PD, depression (Bao and Song, 2018), stroke, and other diseases (Boese et al., 2018). Promoting adult neurogenesis through endogenous activation of NSCs may also have good application prospects through lifestyle interventions or drugs. In lifestyle practice, exercise, environmental enrichment, and even dietary factors have been shown to enhance adult neurogenesis in animal models and can effectively alleviate depression and cognitive decline associated with animal models of mental illness (Hueston et al., 2017; Ma C. L. et al., 2017; Gronska-Peski et al., 2021). Adult neurogenesis is improved by medication for the symptoms of depression (Elder et al., 2006; Zeng et al., 2022), AD (Ye et al., 2016; Stazi and Wirths, 2021), and stroke (Chen et al., 2016), with TCM having the greatest effects on the aforementioned adult neurogenesis-related diseases. Together, these findings show that improving adult neurogenesis is indeed one of the most important ways to treat diseases. TCM has a variety of clinical procedures to treat neurological and psychiatric diseases and brain injuries, and these procedures have a proven track record of success. Improving adult neurogenesis may also be one of the key mechanisms underlying these procedures’ efficacy.
3. The effects of TCM on adult neurogenesis in neurological and psychiatric diseases
TCM has good clinical effects in the treatment of CNS diseases. Some researchers suggest that adult neurogenesis may be the mechanism of TCM for CNS diseases (Ren and Zuo, 2012; Yang et al., 2017; Wang J. et al., 2021; Feng et al., 2022). Thus, TCM has great potential for targeting adult neurogenesis to improve CNS diseases. Indeed, it has been observed that TCM prescriptions (TCMPs), CHMs, and bioactive components derived from TCM could affect adult neurogenesis and improve cognition, alleviate mood, and restore brain function in the animal model. In addition, different TCM may be involved in the regulation of different stages of adult neurogenesis.
3.1. The effects of TCM prescriptions on adult neurogenesis
In recent years, more and more researchers have focused on TCM’s improvement of CNS diseases by targeting adult neurogenesis. Table 1 and Figure 2 show that 28 kinds of TCMPs were reported to improve abnormal adult neurogenesis, which may be related to neurological and psychiatric diseases. Besides, two kinds of TCMPs were also reported to improve adult neurogenesis under normal physiological conditions.
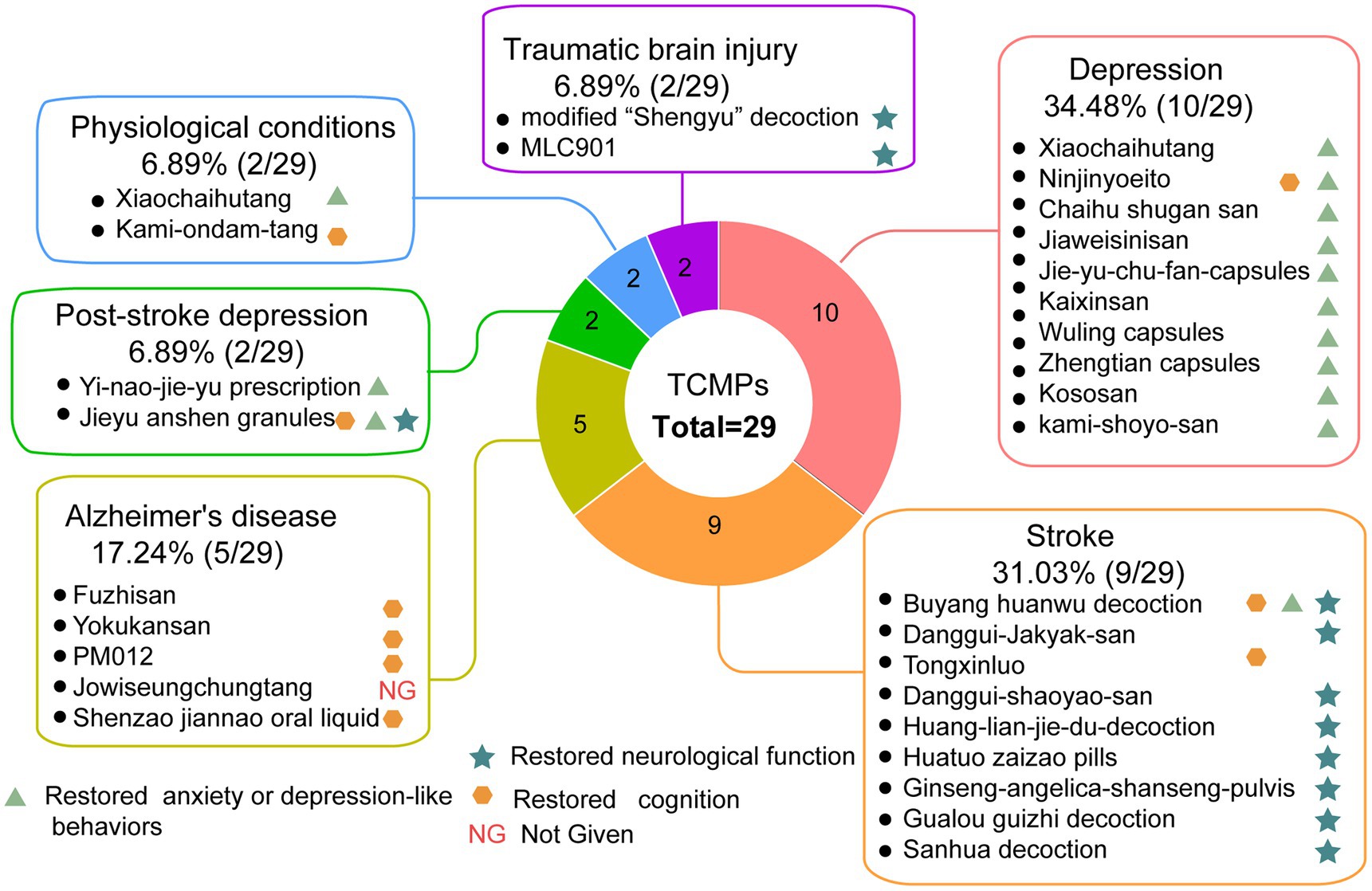
Figure 2. Pie chart of TCMPs improving adult neurogenesis and brain function, n = 29. Xiaochaihutang improves adult neurogenesis in stroke and physiological conditions, so it was counted in two cases for two situations.
Traditional Chinese medicine prescriptions could improve neurological diseases, some of which were found to be related to adult neurogenesis. At present, it has been reported that the major neurological diseases improved by TCMPs mainly include AD, stroke, and traumatic brain injury (TBI), and these diseases are all related to abnormalities of neurogenesis. From a functional perspective, promoting adult neurogenesis plays an important role in structural plasticity and network maintenance in AD (Mu and Gage, 2011). Currently, five types of TCMPs are being used in the treatment of AD; four of them improved the cognitive function in AD, and one reduced amyloid-β (Aβ) aggregation and Aβ-mediated pathology. Different TCMPs may improve the behavioral or pathological abnormalities of AD by acting at different stages of neurogenesis. Fuzhisan (Yang et al., 2011) and yokukansan (Azuma et al., 2018) acted at the proliferation level, Shenzao Jiannao oral liquid acted at the proliferation and maturation stages (Xiao et al., 2020), and herbal formula PM012 acted at the differentiation and maturation stages (Ye et al., 2016). Moreover, Jowiseungchungtang (Shin et al., 2018) inhibited Aβ-mediated pathology in an AD animal model (5XFAD) and restored adult neurogenesis in the proliferation and differentiation stages. Nine types of TCMPs have been shown to be effective in treating stroke, which is another common neurological disease. Seven of these TCMPs increased post-stroke brain function, and two of them improved both brain function and cognition after stroke. The above-mentioned nine TCMPs improved the restoration of brain function after stroke by promoting neurogenesis proliferation, differentiation, and maturation. Danggui Shaoyao San (Ren et al., 2015), Huatuo zaizao pill (Duan et al., 2017), and Sanhua decoction (Fu et al., 2020) acted at the differentiation stage. Tongxinluo (Chen L. et al., 2014; Chen et al., 2016) and Gualou Guizhi decoction (Han et al., 2018) all had an effect on the proliferation and differentiation levels. Huang-Lian-Jie-Du decoction (Zou et al., 2016) and Danggui jakyak San (Song et al., 2013) targeted the stages of proliferation, differentiation, and maturation of neurogenesis. Significantly, Buyang Huanwu decoction (Wang et al., 2011; Liu et al., 2013; Chen H. J. et al., 2015; Chen et al., 2020; Zhuge et al., 2020) and Ginseng Angelica shansheng pulvis (Liu et al., 2019) not only improved post-stroke brain function but also improved cognition, which may be related to the action of these two TCMPs on the proliferation, differentiation, and maturation stages of neurogenesis. In addition, after TBI, modified “Shengyu” decoction (Chen M. M. et al., 2015) improved neurological function, and MLC901 (Quintard et al., 2014) restored cognitive function, which may be related to the fact that these two TCMPs promoted the proliferation of the NCS (Figure 2).
The most important psychiatric disorder improved by TCMPs is depression. Depression is associated with impairments in adult neurogenesis in the dentate gyrus, while the effects of antidepressants are mediated by increased neurogenesis. Increasing adult hippocampal neurogenesis could reduce anxiety and depression-like behaviors (Hill et al., 2015; Tunc-Ozcan et al., 2019). At present, a total of ten TCMPs alleviated the mental symptoms of depression, with nine of them significantly improving anxiety and depression-like mood after depression; one TCMP not only alleviated mood but also improved cognition. The aforementioned TCMPs that improve cognition and mood in depression may act on different stages of neurogenesis. Kami-shoyo-san (Park et al., 2007), Kososan (Ito et al., 2017), and Wuling capsules (Li et al., 2010) promoted the proliferation stage; Jiaweisinisan promoted the differentiation stage (Wang H. Z. et al., 2021); Kaixinsan promoted the maturation stage (Yan et al., 2016; Dong et al., 2020); Jie Yu Chu fan capsules (Ji et al., 2020), Xiaochaihutang (Zhang et al., 2015, 2016; Ma J. et al., 2017) and Zhengtian capsules (Yang et al., 2020) promoted the proliferation and differentiation stages; Chaihu-Shugan-San (Chen et al., 2018; Zhang et al., 2021) promoted the differentiation and maturation stages. In addition to reducing depressive symptoms, Ninjinyoeito (Murata et al., 2018) also improved cognitive performance, which may be connected to promoting the proliferation and differentiation stages of neurogenesis. Meanwhile, Kososan (Ito et al., 2017) improved mood, but it simply tended to advance the stage of proliferation. In addition, post-stroke depression (PSD) is a significant social and public health issue, and antidepressant preventive and curative treatments are worth investigating (Villa et al., 2018). TCMs not only ameliorated depression by affecting neurogenesis but also alleviated the symptoms of PSD by promoting the maturation of neurogenesis. Both Yi-nao-jie-yu (Tian et al., 2018) and Jieyu Anshen granules (Du et al., 2020) relieved the mood after PSD, restored brain function, and improved cognitive function; this may be related to the fact that these two TCMPs promoted NSC maturation.
In addition, under physiological conditions, Kami-ondam-tang (Hong et al., 2011) is good for cognition, and Xiaochaihutang (Zhang et al., 2015) is beneficial for emotion, which may be related to the fact that these TCMPs are able to promote the differentiation of neural stem cells.
The application of each of the above 29 types of TCMPs is based on the theory of TCM and has been consistently enhanced through the process of practice. As a result, neurogenesis has been improved in a variety of situations. It is evident that each TCMP contains several different herbs, but identifying which ones are the most important may be difficult to explain. Future research will focus on those factors that support adult neurogenesis and have either antagonistic or synergistic effects.
3.2. The effects of Chinese herbal medicine on adult neurogenesis
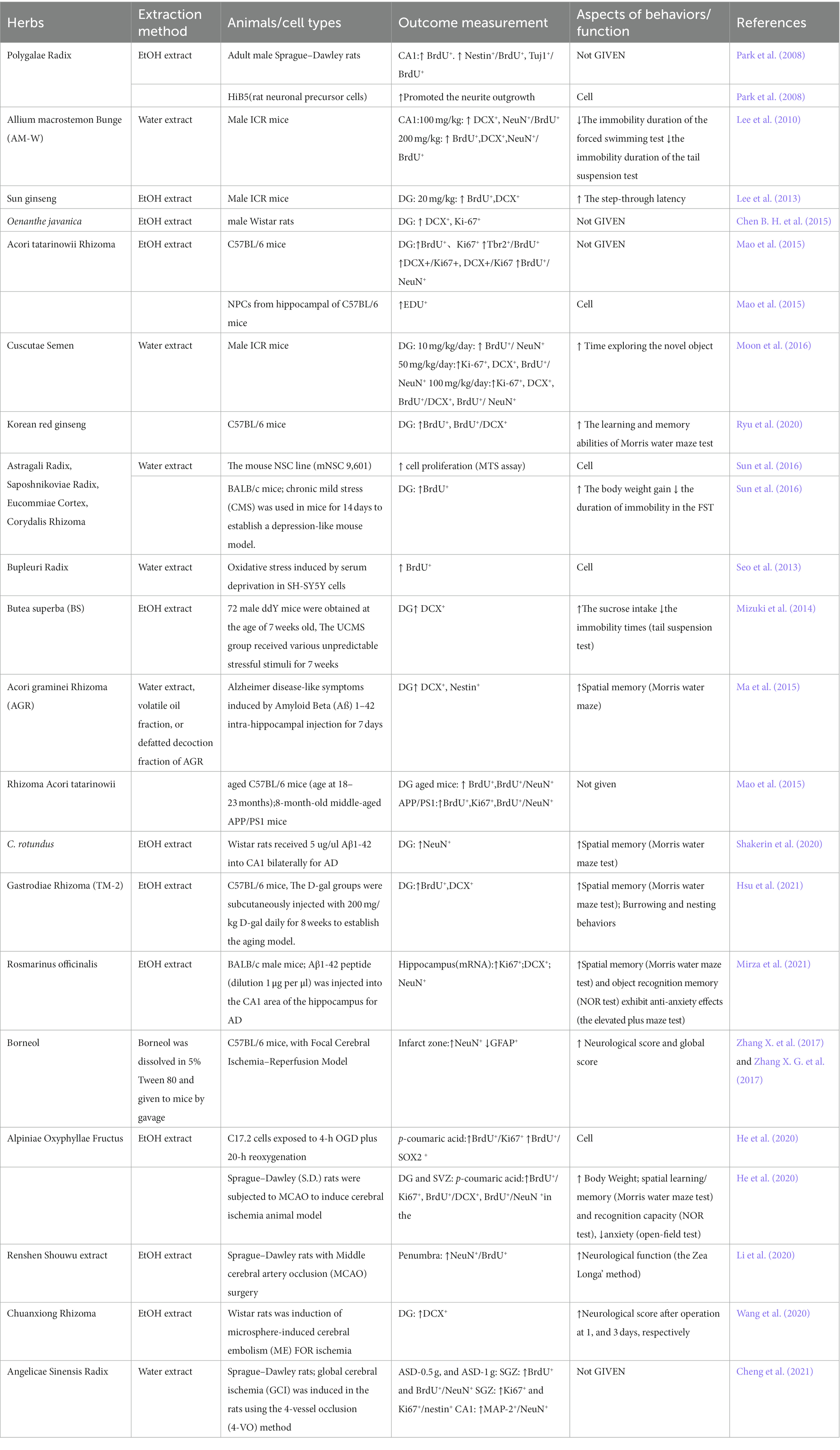
According to the “jun-chen-zuo-shi” principle of TCM, each CHM in a TCMP is essential and has a specific function (Zhang et al., 2014). The advancement of modern pharmacology has made it easier to further study the active components in TCM that promote adult neurogenesis. Therefore, the effects of CHMs on adult neurogenesis have been widely studied. Table 2 and Figure 3 summarizes the impact of CHMs on adult neurogenesis under different pathological and physiological situations.
The main neurological diseases that CHMs could improve are AD and stroke, and this improvement in neurological symptoms may be related to neurogenesis. Four CHMs promoted neurogenesis in AD animal models, and three of them improved the cognition of AD animals, but they had different effects on neurogenesis. Acori graminei rhizoma mainly acted on proliferation and differentiation (Ma et al., 2015), Rosmarinus officinalis mainly acted on differentiation (Mirza et al., 2021), and Cyperus rotundus mainly acted on maturation (Shakerin et al., 2020). In addition, Acori tatarinowii rhizoma (Mao et al., 2015) promoted the proliferation and maturation of neurogenesis in AD animal models, but its effect on cognition has not been shown. Five CHMs have improved brain function after an ischemic stroke. The restored brain function after stroke may be related to chuanxiong rhizome-stimulated differentiation (Wang et al., 2020), Borneol (Zhang X. G. et al., 2017), and Renshen Shouwu extract stimulated maturation (Li et al., 2020). Meanwhile, Alpiniae oxyphyllae fructus improved cognition and mood after stroke, which may be related to its promotion of cell proliferation, differentiation, and maturation (He et al., 2020).
Three reports indicate that CHMs improved the neurogenesis of depression Butea superba (Mizuki et al., 2014); Astragali radix, Saposhnikoviae radix, Eucommiae cortex, and Corydalis rhizoma (Sun et al., 2016) all have the potential to lessen depression, and one of the possible mechanisms is the promotion of the proliferation of neurogenesis. In addition, Bupleuri radix is a key component in a number of oriental herbal medicines used to treat stress and other psychiatric illnesses, and these seem to have proliferative effects (Seo et al., 2013).
Six CHMs promote neurogenesis under physiological conditions, and three of these improved learning and memory, with one CHM alleviating emotion and two others promoting neurogenesis (however, their effects on cognition and emotion were not demonstrated). The following three CHMs have been shown to improve learning and memory. Their potential mechanisms may involve sun ginseng, which promotes NSC proliferation and survival (Lee et al., 2013), Korean red ginseng, which promotes NSC differentiation (Ryu et al., 2020), and Cuscuta japonica Choisy, which promotes NSC proliferation, differentiation, and maturation (Moon et al., 2016). Allium macrostemon Bunge (Lee et al., 2010) was beneficial to antidepressant-like activity and promoted the proliferation, differentiation, and maturation of neurogenesis. In addition, Oenanthe javanica encouraged neurogenesis proliferation and differentiation (Chen B. H. et al., 2015), and the root of Polygala tenuifolia encouraged neurogenesis proliferation and maturation (Park et al., 2008), although their effects on cognition and emotion under physiological situations were not demonstrated. In Asia, certain CHMs, such as Korean red ginseng, are used as both medicine and food.
3.3. The effects of bioactive components on adult neurogenesis
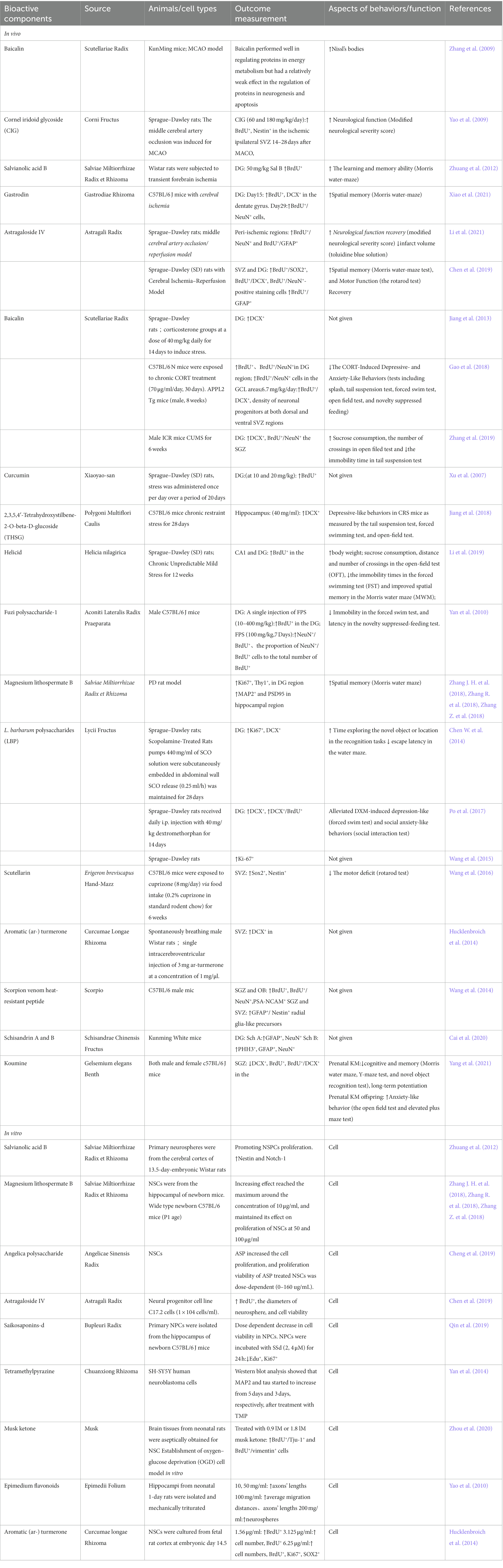
The bioactive components were extracted from CHMs due to their structural diversity and biological activities, which make them important sources of clinical drugs. Although there are fewer components in CHMs than in TCMPs, it is still very difficult to determine which components are effective. Therefore, the separation and extraction of bioactive components from CHMs for research provide a more stable outcome and may be conducive to the study of pharmacological mechanisms. The destiny of NSCs may be influenced by bioactive components (Zhang Z. et al., 2018), and bioactive components’ support of adult neurogenesis has attracted extensive attention. Table 3 and Figure 4A show the 17 kinds of bioactive components that were reported to improve adult neurogenesis in connection to physiological or pathological conditions. The effects of bioactive components on adult neurogenesis have been studied at the cellular and animal levels (Table 3).
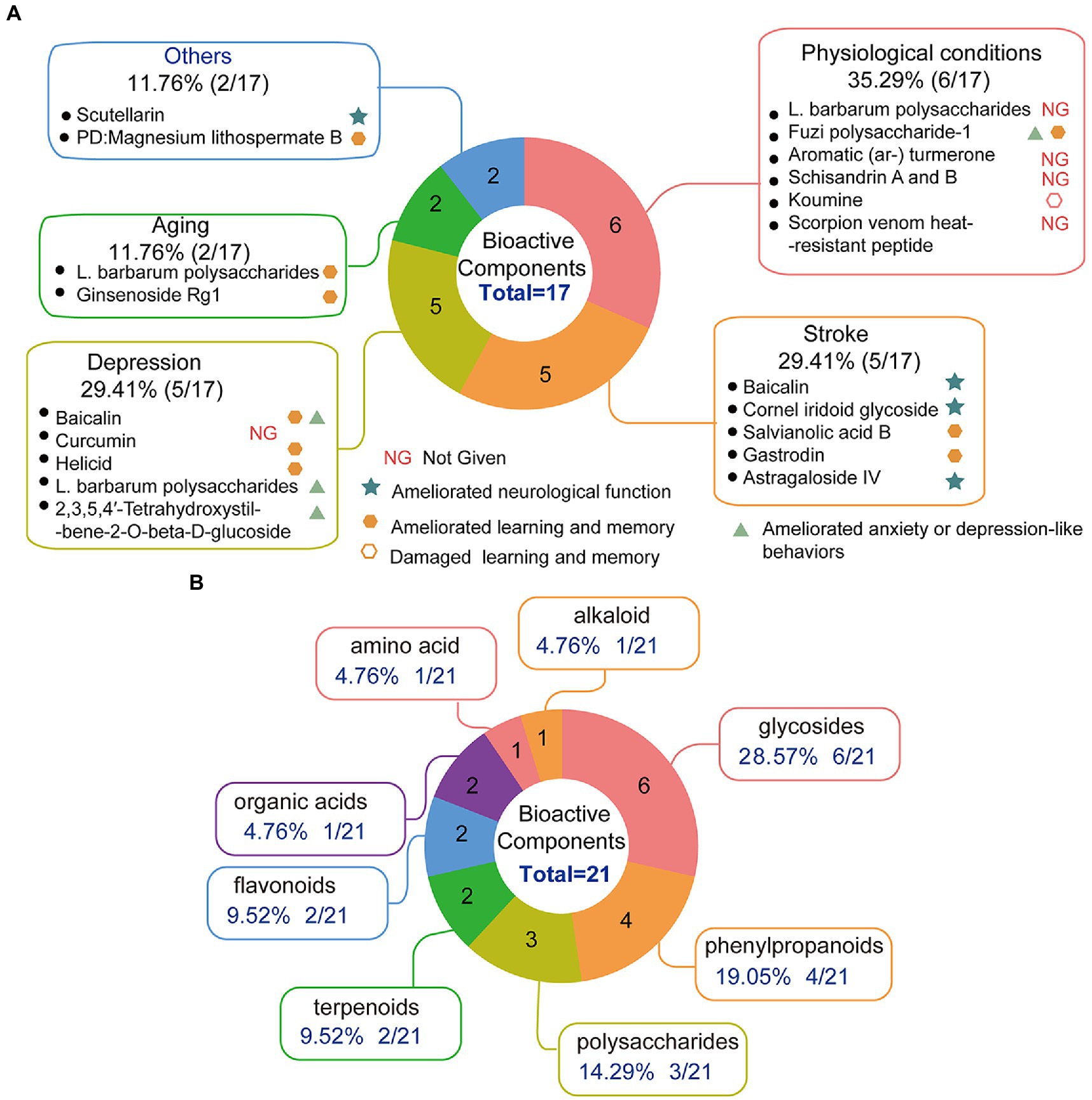
Figure 4. The effect of bioactive components on adult neurogenesis. (A) Pie chart of bioactive components improving adult neurogenesis and brain function, n = 17, L. barbarum polysaccharides improves adult neurogenesis in aging, depression, and physiological conditions, so it was counted in three cases for three situations; baicalin improves adult neurogenesis in depression and stroke conditions, so it was counted in two cases for two situations. (B) Pie chart of bioactive components according to the chemical composition that promotes adult neurogenesis (animal) or contributes to the proliferation and differentiation of NSCs, n = 21.
At present, in vivo experiments have shown that bioactive components are being used to treat neurologic diseases such as stroke, PD, multiple sclerosis, and aging. Five bioactive components improved symptoms after stroke, promoting neurogenesis. Cornel iridoid glycoside (Yao et al., 2009) restored brain function, which may be related to NSC proliferation. Learning and memory after stroke were improved by salvianolic acid B (Zhuang et al., 2012) and Gastrodin (Xiao et al., 2021). This improvement may have been caused by the stimulation of neurogenesis. Astragaloside IV (Chen et al., 2019; Li et al., 2021) not only restored brain function after stroke but also improved cognition, which may have the potential to encourage NSC proliferation, differentiation, and maturation. Baicalin also performed well in regulating proteins in energy metabolism, but had a relatively weak effect in the regulation of proteins in neurogenesis and apoptosis, according to results from proteomics to explore the various protein expression modes in mouse brains after stroke (Zhang et al., 2009). Magnesium lithospermate B (Zhang Z. et al., 2018) improved the cognitive function of PD animal models, which may be connected to NSC growth promotion. Scutellarin (Wang et al., 2016) alleviated behavioral deficits in a mouse model of multiple sclerosis, possibly by inhibiting NSC apoptosis and promoting NSC differentiation into myelin-producing oligodendrocytes. Lycium barbarum polysaccharides (LBP) (Chen W. et al., 2014) prevented cognitive and memory deficits, in addition to decreasing cell proliferation and neuroblast differentiation, in scopolamine-treated rats. Ginsenoside Rg1 prevented cognitive impairment in a rat model of aging (Zhu et al., 2014). This may be related to its ability to protect NSCs/NPCs and promote differentiation. Depression is the primary psychiatric illness alleviated by the bioactive components. Five bioactive components improved the symptoms of depression, with four of them relieving depression-like mood. This may be related to the fact that these four bioactive components can affect the neurogenesis of depression in model mice. Curcumin promoted proliferation (Xu et al., 2007), 2,3,5,4 ‘-Tetrahydroxystilbene-2-O-beta-D-glucoside (Jiang et al., 2018), and LBP (Po et al., 2017) promoted differentiation, and baicalin promoted proliferation, differentiation, and maturation (Jiang et al., 2013; Gao et al., 2018; Zhang et al., 2019). Meanwhile, Helicid (Li et al., 2019) not only relieved post-depression mood but also improved cognition, which may be associated with boosting NSC proliferation. Also, under physiological conditions, bioactive components promoted neurogenesis. For example, Fuzi polysaccharide-1 (Yan et al., 2010) improved mood, which may have been connected to its support of proliferation and maturation. The effect of LBP promoted proliferation (Wang et al., 2015), aromatic Turmerone (Hucklenbroich et al., 2014), and schisandrin A and B (Cai et al., 2020) promoted differentiation, scorpion venom heat resistant peptide promoted proliferation and differentiation (Wang et al., 2014), but the effects above five bioactive components on the behaviors of mice have not been reported.
In vitro, bioactive components mainly promoted the proliferation and differentiation of NSCs. Magnesium lithospermate B (Zhang Z. et al., 2018), angelica polysaccharide (Cheng et al., 2019), and astragaloside IV (Chen et al., 2019) all promoted the proliferation of stem cells; tetramethylpyrazine (Yan et al., 2014) and musk ketone promoted differentiation, while salvianolic acid B (Zhuang et al., 2012) and aromatic turmerone (Hucklenbroich et al., 2014) regulated both proliferation and differentiation. In addition, Epimedium flavonoids promoted axon growth, which is essential for stem cell maturation (Yao et al., 2010). Unfortunately, when pregnant rats were exposed to koumine (Zhou et al., 2020), which was isolated from Gelsemium elegans Benth, the offspring of both male and female c57BL/6 J mice showed a marked reduction in neurogenesis in the hippocampal DG. In addition, the offspring presented cognitive deficits and increased anxiety-like behavior (Yang et al., 2021). Similarly, Saikosaponin-d replicated cell viability and reduced cell growth (Qin et al., 2019).
The structural classification of the aforementioned bioactive components can be seen in Figure 4B. The indicated bioactive components are mainly concentrated in saponin (28.57%), phenylpropanoid (19.05%), and polysaccharide (14.29%), in addition to terpenoids (9.52%), organic acids (9.52%), amino acids (4.76%), alkaloids (4.76%), flavonoids (4.76%), and bioactive substances (4.76%).
As people pay more attention to the role of neurogenesis in diseases, how to improve diseases through drugs that affect neurogenesis has become a hot subject in neuroscience in recent years. TCM has outstanding clinical efficacy in the treatment of neurogenesis-related diseases and is an important source of drugs that affect neurogenesis. TCMPs have a large amount of clinical practice data, such as Xiaochaihutang (Chen et al., 2009) and Buyang Huanwu decoction (Lee et al., 2020). Some herbs, such as medlar, ginseng, and licorice can be used as both medicine and food. Surprisingly, 9 of the 21 CHMs (47.6%) were shown to enhance adult neurogenesis under physiological conditions. When it comes to the different stages of neurogenesis, TCM may regulate more than just the one biological process of adult neurogenesis mentioned above. Figure 5 shows how TCM regulates and plays a vital role in the multi-stage process of adult neurogenesis.
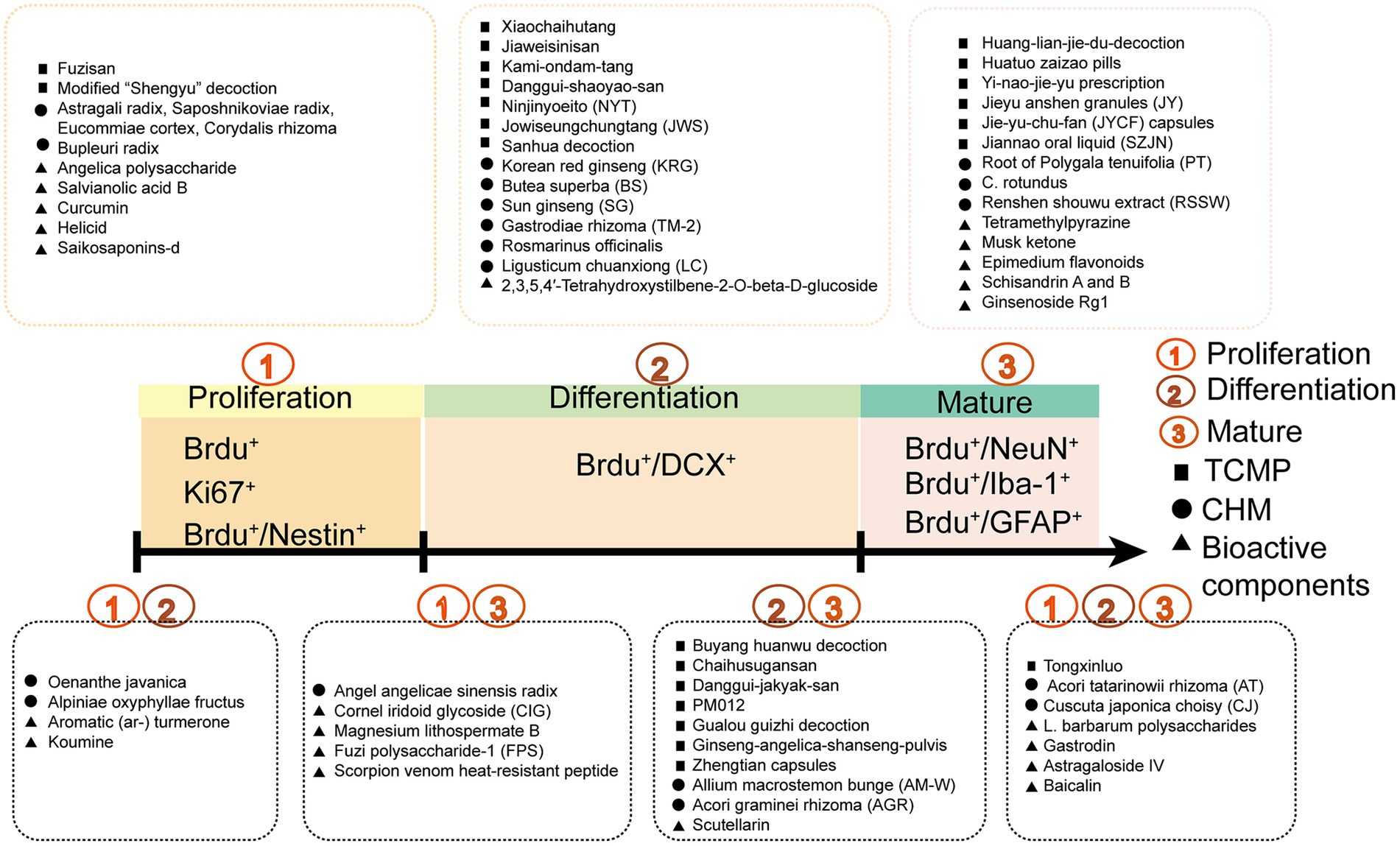
Figure 5. The influence of TCMPs, CHMs, and bioactive components on adult neurogenesis at different stages.
As shown in Figure 6, there are currently 19 CHMs that are almost present in 26 TCMPs (89.66% of the total number of TCMPs), and further analysis found that 20 CHMs contain 17 types of bioactive compounds (80.95% of the total number of bioactive compounds), which have a high potential for use before clinical application, such as baicalin, which was isolated from the root of Scutellaria baicalensis and has a great neuroprotective effect. More importantly, baicalin has shown highly promising results in two clinical trials (chiCTR180016727 and ChiCTR180016727) on mental health. If we can fully explore the mechanism of its influence on neurogenesis, its clinical application will advance even further.
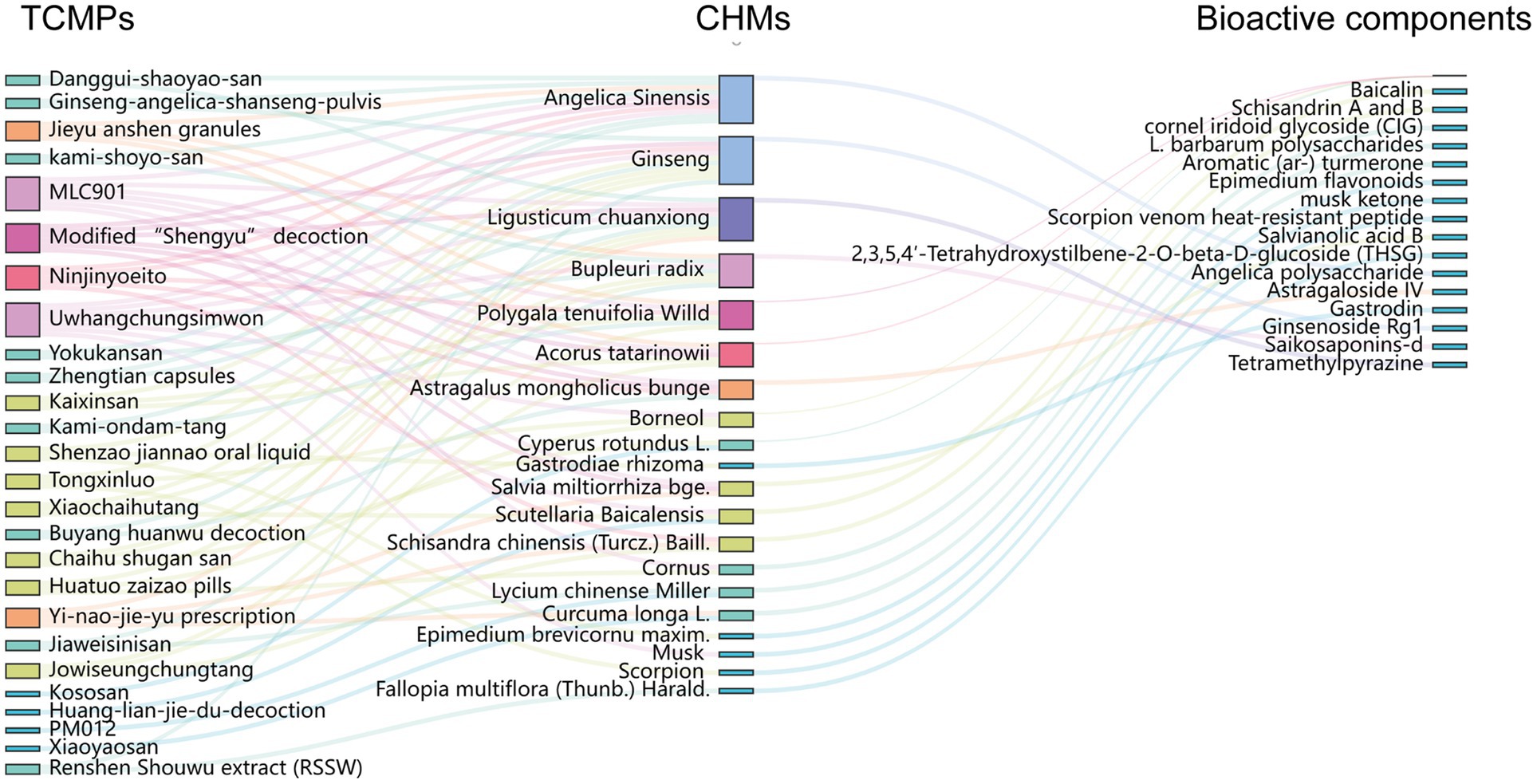
Figure 6. TCMPs and CHMs are commonly used to enhance adult neurogenesis and a network of its bioactive components.
4. Mechanism of TCM on adult neurogenesis
4.1. Increase of neurotrophic factor
Neurotrophic factors play a central role in NSC proliferation, migration, and differentiation. Their existence is crucial for maintaining neuronal function, structural integrity, and adult neurogenesis throughout life. Many TCMs (Figure 7A) show the ability to promote the secretion of neurotrophic factors, thereby enhancing hippocampal adult neurogenesis (Zhang et al., 2014).
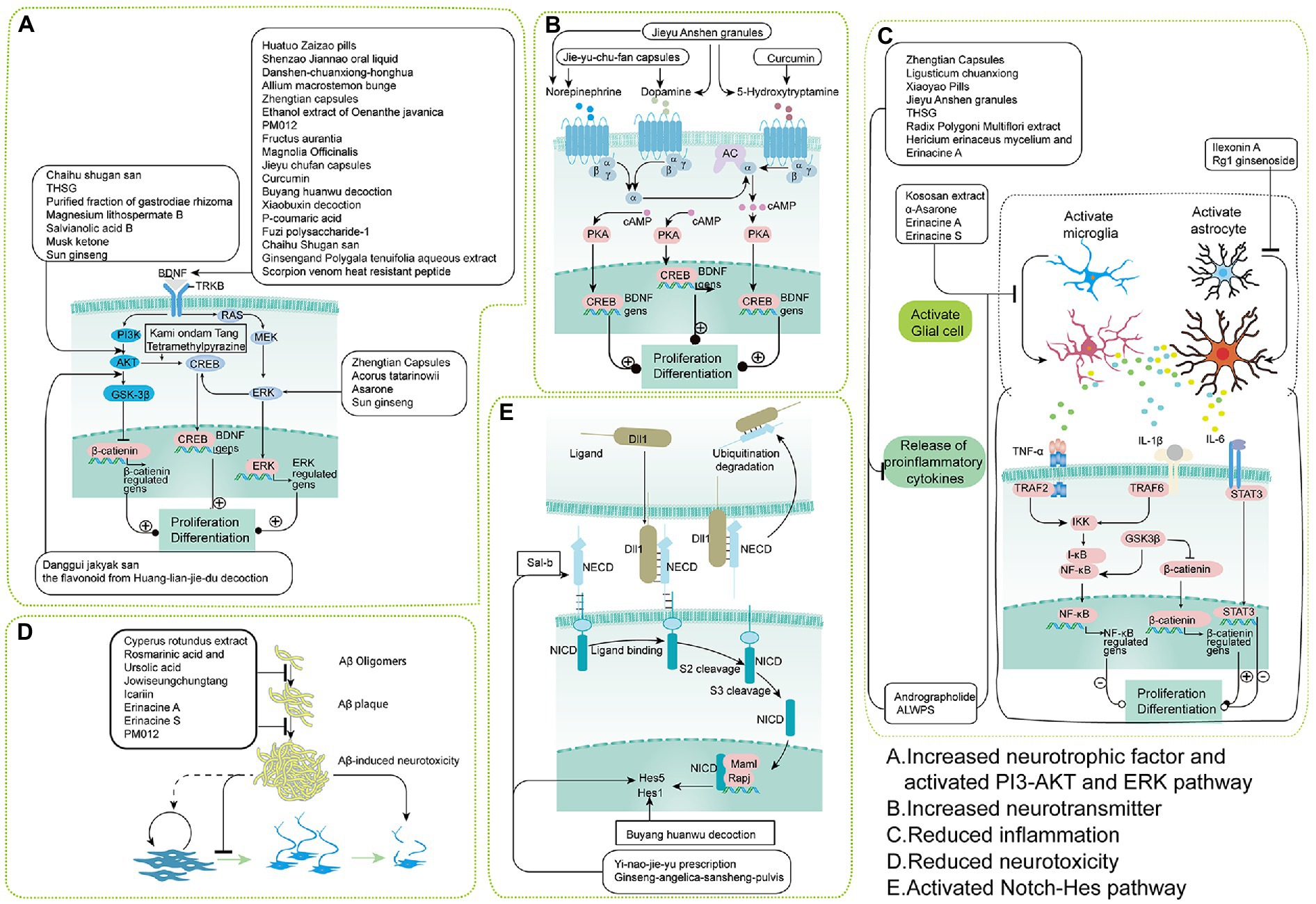
Figure 7. Mechanisms of TCMPs, CHMs, and bioactive components on adult neurogenesis. (A) TCM could increase neurotrophic factors or activate the PI3-AKT and ERK pathways for adult neurogenesis. (B) TCM could increase neurotransmitters for adult neurogenesis. (C) TCMs could reduce inflammation for adult neurogenesis. (D) TCM could reduce neurotoxicity for adult neurogenesis. (E) TCM could activate the Notch-Hes pathway for adult neurogenesis.
There are many TCMs that promote adult neurogenesis while simultaneously regulating BDNF, including Huatuo Zaizao pill (Duan et al., 2017), Shenzao jiannao oral liquid (Xiao et al., 2020), Danshen-Chuanxiong-Honghua (Zhang X. et al., 2017), and Allium macrostemon Bunge (Lee et al., 2010): these not only promote the proliferation of NSCs, but also enhance BDNF expression. Both Zhengtian capsules (Yang et al., 2020) and Oenanthe javanica ethanol extract (Chen B. H. et al., 2015) promote the proliferation and differentiation of hippocampal NSCs, while BDNF expression is also increased. PM012 promotes BDNF expression and NSC differentiation and maturation (Ye et al., 2016). In addition to the aqueous extract of gardenia, Fructus aurantii, and Magnolia officinalis also increase BDNF expression in the hippocampus of rats with chronic unpredictable mild stress and affect NSC differentiation and maturation (Xing et al., 2015). As a result, BDNF may be the target of traditional Chinese medicine to regulate neurogenesis. Thus, some researchers believe that the beneficial effect of Jieyu Chufan capsules (Ji et al., 2020) and curcumin (Xu et al., 2007) on depressed mice involves enhancing adult neurogenesis by boosting BDNF expression (Ji et al., 2020). Buyang Huanwu decoction promotes recovery from cerebral ischemia, and its mechanism may be related to the increased expression of VEGF and BDNF proteins for the differentiation and maturation of NSCs (Zhuge et al., 2020). PMC-12 (Park et al., 2016) and Xiaobuxin decoction (An et al., 2008) have beneficial effects on maturation through an increase in BDNF and p-CREB expression (An et al., 2008). Further research confirms that P-coumaric acid’s effects on BDNF/TrkB/Akt activation and NSC proliferation are eliminated when coupled with the BDNF/TrkB-specific inhibitor ANA12 (He et al., 2020). Meanwhile, K252a is an antagonist of Trk, an upstream molecule of BDNF signal transduction. TrkB inhibition blocks the transmission of the BDNF signal pathway. Although Fuzi polysaccharide-1 (Yan et al., 2010) promotes proliferation Chaihu Shugan San improves differentiation (Chen et al., 2018), and Ginseng and Polygala tenuifolia aqueous extracts (Jiang et al., 2021) enhance NSC differentiation and maturation, K252a may disrupt the function of the aforementioned TCMs on adult neurogenesis. In addition, administering Scorpion venom heat resistant peptide (SVHRP) promotes astrocytes to release BDNF and promotes the growth of axons of immature neurons. However, blocking BDNF with anti-BDNF antibodies can eliminate these SVHRP-dependent neurotrophic effects (Wang et al., 2014).
In addition to regulating BNDF to enhance adult neurogenesis, TCM can also control other neurotrophic factors to enhance adult neurogenesis. In TBI rats, “Shengyu” decoction can increase the expression of glial cell line-derived neurotrophic factor (GDNF) and nerve growth factor (NGF) for the proliferation of NSCs (Chen M. M. et al., 2015). Huatuo Zaizao extract can boost the production of newly formed neurons, and increase the levels of VEGF and BDNF (Zheng et al., 2014), in addition to BDNF, NGF, TrkB, TrkA, which are all upregulated by Xiaoyao pills (Fang et al., 2020). Additionally, Angelica sinensis (Oliv.) Diels not only promotes the proliferation and maturation of hippocampal NSCs, but can also upregulate the expression of BDNF, GDNF, and vascular endothelial growth factor A (VEGF-A) in the hippocampus in chronic cerebral ischemia models (Cheng et al., 2021).
4.2. Increase of neurotransmitters
Depression is associated with decreased adult neurogenesis and abnormal monoammonia levels (Lanni et al., 2009; Jiang et al., 2022). Importantly, monoamine neurotransmitters function to increase neurogenesis (Cameron et al., 1998). A 5-Hydroxytryptamine (5-HT) reuptake inhibitor like fluoxetine not only has an obvious antidepressant effect but can also greatly improve adult neurogenesis in depression models. The antidepressant effects of Jie Yu Chu fan capsules in depressed mice can enhance adult neurogenesis by increasing the levels of norepinephrine (NE) and dopamine (DA) (Ji et al., 2020). Jieyu Anshen granules improving the neurological and cognitive functions of PSD model mice may be related to increases in the levels of NE, DA, and 5-HT (Du et al., 2020). Curcumin increased hippocampal adult neurogenesis, which may be related to curcumin increasing 5-HT (1a) mRNA in the hippocampal subregion after stress (Xu et al., 2007). The mechanism by which the above TCMs may influence neurogenesis by affecting neurotransmitters is shown in Figure 7B.
4.2.1. Inflammation reduction
Pro-inflammatory factors IL-1β, IL-6, and NF-κB produced by activated microglia or astrocytes may impact different phases of adult neurogenesis (Ekdahl et al., 2009; Czeh et al., 2011). TCMs (Figure 7C) may alleviate abnormal adult neurogenesis by reducing glial cell activation and inflammatory factors.
One strategy for TCM to increase adult neurogenesis is to inhibit microglial activation. The influence of α-Asarone on neurogenesis may be correlated with a decline in the proportion of activated microglia, a reduction in microglial numbers, and the maintenance of velocity (Cai et al., 2016). Kososan extract can prevent the avoidance behavior of socially failed mice, which is partially mediated by the downregulation of hippocampal neuroinflammation, possibly through the regulation of increased anti-inflammatory microglia and adult hippocampal neurogenesis (Ito et al., 2017). Erinacine A and erinacine S promote hippocampal adult neurogenesis in AD mice, which may lessen glial cell activation (Tzeng et al., 2018).
Another strategy for TCM to increase adult neurogenesis at different stages is to inhibit the release of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) by microglia. In the proliferation stage, ZTC increases NSC proliferation and inhibits the expression level of NF-kB in a dose-dependent manner (Yang et al., 2020). Under the differentiation stage, Ligusticum chuanxiong (LC) significantly increased DCX in the hippocampal DG of adult rats 14 days after cerebral ischemia. Meanwhile, LC reduces IL-1β and TNF-α (Wang et al., 2020). THSG, the main active compound of the traditional Chinese herb Polygonum multiflorum, can lower TNF-α, IL-1β, and IL-6 (Jiang et al., 2018). At the maturation stage, Xiaoyao pills increase newly formed neurons and significantly decrease the levels of IL-6 and TNF-α (Fang et al., 2020). Treatment with Polygoni multiflori radix extract can greatly increase the number of new neurons after an ischemic stroke. This may be accomplished by blocking the TLR4/NF-κB/NLRP3 inflammatory signaling pathway after an ischemic stroke in rats (Li et al., 2020). Moreover, Jieyu Anshen granules (Du et al., 2020), Hericium erinaceus mycelium (HEM), and an isolated diterpenoid derivative known as erinacine A (Tsai et al., 2019) all support the development of new neurons and can reduce TNF-α and IL-1 β, which are linked to the regulation of adult neurogenesis (Du et al., 2020).
Traditional Chinese medicine can inhibit both microglia activation and the release of proinflammatory factors. Andrographolide (Lu et al., 2019) inhibits chronic stress-induced abnormalities in adult hippocampal neurogenesis by reversing microglia-mediated pro-inflammatory cytokine production. Nuclear transcription factor NF-κB level decreased, and LPS-induced IL-1β level was changed by ALWPS-regulated FAK signal. Moreover, ALWPS significantly inhibited the LPS-induced migration of BV2 microglia. Oral administration of ALWPS to C57BL/6 J mice injected with LPS can greatly improve short- and long-term memory. More importantly, oral treatment of ALWPS significantly reduced microglia activation in the hippocampus and cortex (Lee et al., 2018).
Additionally, TCM may regulate astrocyte anti-inflammation and increase NSC proliferation. Ilexonin A can enhance NSC proliferation by activating astrocytes and decreasing TNF-α and IL-1 β (Xu et al., 2020). Ginsenoside Rg1 decreased astrocyte activation and increased hippocampal cell proliferation by reducing IL-1β, IL-6, and TNF-α (Zhu et al., 2014).
4.2.2. Reduction of neurotoxicity
In the past few years, impaired adult hippocampal neurogenesis has emerged as a hallmark of AD pathophysiology along with Aβ and tau hyperphosphorylation-induced neurotoxicity, and further research has shown that Aβ-induced neurotoxicity is associated with altered neurogenesis and memory formation (Abshenas et al., 2020; Amber et al., 2020). Although Aβ causes a temporary increase in the number of neurons in younger mice, it also causes a drop in the NSC pool, which results in a lower rate of adult neurogenesis in older animals (Lopez-Toledano et al., 2010). In the existing animal model of AD, mice with Aβ intraperitoneal injections or transgenic Aβ accumulation can severely impair adult neurogenesis, and TCMs (Figure 7D) improved this situation. On the one hand, TCMs such as Cyperus rotundus extract (Shakerin et al., 2020), rosmarinic acid, and ursolic acid (Mirza et al., 2021) repaired the spatial memory damage induced by Aβ1-42 and increased adult neurogenesis. On the other hand, the transgene-induced aggregation of Aβ was also associated with an aberrant reduction of adult neurogenesis. In this situation, TCMs reduced Aβ deposition in the brain and enhanced hippocampal adult neurogenesis in AD animal models with different genetic backgrounds. For instance, Jowiseungchungtang inhibited the aggregation of Aβ and the pathology induced by Aβ in AD model mice (five family AD variants) and improved adult hippocampal adult neurogenesis in vivo (Shin et al., 2018), Icariin reduced Aβ in the brain of Tg2576 mice and enhanced adult hippocampal neurogenesis (Li et al., 2015), erinacine A and erinacine S inhibited the growth and reduced the load of Aβ plaque and promoted adult hippocampal neurogenesis in APPswe/PS1ΔE9 transgenic mice (Tzeng et al., 2018), and PM012 significantly reduced Aβ deposition and increased adult neurogenesis in 3xTG AD mice (Ye et al., 2016).
4.2.3. Activation of PI3-AKT and ERK pathways
The ERK and PI3K / Akt pathways may regulate different stages of adult neurogenesis, including the growth, differentiation, maturation, and survival of NSCs (Shioda et al., 2009; Mellios et al., 2018). TCMs (Figure 7A) could affect adult neurogenesis by regulating the ERK and PI3K / Akt pathways of NSCs.
Salvianolic acid B maintains self-renewal and promotes the proliferation of NSCs via the PI3K / Akt signaling pathway, which is confirmed by PI3 (LY294002) inhibition eliminating this effect (Zhuang et al., 2012). Meanwhile, THSG (a primary active compound of the traditional Chinese herb Polygonum multiflorum) (Jiang et al., 2018) and the purified fraction of Gastrodiae rhizoma (Hsu et al., 2021) stimulate adult neurogenesis and regulate the PI3K/Akt pathway. Moreover, magnesium lithospermate B (Zhang Z. et al., 2018) and musk ketone (Zhou et al., 2020) promote the proliferation and differentiation of NSCs through the activation of the PI3K/Akt signaling pathway. Akti-1/2, an Akt inhibitor, also blocks the effect of musk ketone on NSCs. This suggests that Muscone promotes NSC proliferation and differentiation by activating the PI3K/Akt signaling pathway (Zhou et al., 2020). Chaihu Shugan San increases the levels of pPI3k/PI3K and pAkt/Akt in the hippocampus of stressed mice and restores the newly formed neurons. The two main active ingredients in Chaihu Shugan San, quercetin and luteolin, were then discovered to have a good docking fraction with the PI3K protein using molecular docking technology. This further confirmed that the PI3K/Akt pathway is how CSS participates in the treatment of MDD (Zhang et al., 2021).
Chaihu Shugan San not only boosted the PI3K/Akt pathway in stressed mice but also reduced the level of p-GSK3β/GSK3β to promote adult neurogenesis (Zhang et al., 2021). Danggui Jakyak San increases Akt/GSK3 β/β- Catenin signal transduction, which may be one of the mechanisms through which it promotes adult neurogenesis (Song et al., 2013). Similarly, raising p-Akt and p-GSK-3β is thought to play a factor in how alkaloids in Huanglian Jiedu decoction encourage NSC proliferation. Flavonoid treatment promotes the differentiation of cortical precursor cells into neurons rather than glial cells, which could be attributed to the upregulation of Akt and GSK-3β (Zou et al., 2016). In addition, Saikosaponin-d (SSD) inhibits cell viability and proliferation of hippocampal NPCs in a concentration-dependent manner. Subsequent research indicates that SSD suppresses adult neurogenesis and NPC proliferation via the GSK3 β/β- Catenin signaling pathway (Qin et al., 2019).
Traditional Chinese medicine could aid the PI3K/Akt/CREB pathway in NSC differentiation. Kami-ondam-tang greatly enhanced the expression of p-Akt and p-CREB in the hippocampal CA1 region and dentate gyrus, and, at the same time, the number of DCX-positive cells in the dentate gyrus increased significantly. These results suggest that Kami-ondam-tang improves cognitive ability by upregulating Akt/CREB/BDNF signaling and adult neurogenesis (Hong et al., 2011). Tetramethylpyrazine induces the release of BDNF from bone marrow mesenchymal stem cells by activating the PI3K/Akt/CREB pathway for neural differentiation. This effect could be reversed by the PI3K inhibitor LY294002 (Chen et al., 2021).
Traditional Chinese medicine could also influence NSC during development and survival via the Akt pathway. Baicalin induces neuronal development, matures them via the Akt/Foxg1 pathway, and sustains them to have an antidepressant effect (Zhang et al., 2019). By the activation of PI3K/Akt/BAD, Buyang Huanwu decoction (BHD) stimulates neurogenesis in apoptosis, proliferation, differentiation, maturation, and eventually the recovery of the function of learning and memory (Chen et al., 2020).
Another type of kinase that influences NSC proliferation, differentiation, and survival is extracellularly regulated protein kinases (ERK) (Rai et al., 2019). TCM promotes the proliferation of NSCs through ERK. Acorus tatarinowii and its components, α- asarone and β- asarone, promote NPC proliferation in vitro. Subsequent research has shown that Acorus tatarinowii and asarone activated ERK but did not activate the Akt pathway; FR180204 inhibited ERK activity and effectively blocked the promoting effect of Acorus tatarinowii or asarone on the proliferation of NPC (Mao et al., 2015). In contrast to Acorus tatarinowii, Sun ginseng increases p-ERK and p-Akt levels in addition to NSC proliferation and survival, which may be the method through which memory is enhanced (Lee et al., 2013). In addition, Zhengtian capsules promote the proliferation of hippocampal NSCs and the protein levels of phosphorylated ERK1/2 and CREB (Yang et al., 2020).
4.2.4. Activation of the Notch-Hes pathway
Activation of the Notch signaling pathway enhances the production of Hes1 and Hes5, which promote stem cell proliferation and inhibit neuronal differentiation (Mendes-da-Silva et al., 2015; Zhang R. et al., 2018; Ohtsuka and Kageyama, 2021). TCM may influence adult neurogenesis by regulating stem cell proliferation and differentiation via the Notch1/Hes pathway (Figure 7E). Zhuang et al. (2012) screened 45 bioactive components from TCM, which were widely used in the treatment of stroke in China, and evaluated their effect on the proliferation of neural stem/progenitor cells. The results showed that Sal-b promoted NSC self-renewal along with an increase in Notch1 gene expression. The Buyang Huanwu decoction increased the expression of Hes1 and promoted NSCs to differentiate into astrocytes (Chen et al., 2020). More importantly, TCMs regulate neurogenesis under different pathological conditions through the Notch1/Hes5 pathway, which may have a time effect from the Yi-nao-jie-yu prescription (Tian et al., 2018) and a dosage effect from the Ginseng-Angelica-Sansheng-pulvis combination (Liu et al., 2019).
5. Toxic and side effects
There are only a few clinical reports on the toxicity and adverse effects of TCMs regulating adult neurogenesis, whether used alone or in combination. The side effects of TCMs, including MLC901 (Kumar et al., 2020), curcumin (Asher and Spelman, 2013; Fan et al., 2013), and Polygala tenuifolia (Zhao X. et al., 2020), are largely gastrointestinal, such as nausea, vomiting, and diarrhea. The majority of side effects are mild and temporary, and after discontinuing the medication, these symptoms will gradually subside. There are also a few reports on the side effects of TCMs in other systems. Pseudoaldosteronism caused by Yokukansan (Ishida et al., 2020; Katsuki et al., 2021) causes hypertension, hypokalemia, and muscular weakness, which may lead to death. Therefore, patients must be aware of the risks when considering taking Yokukansan (Ishida et al., 2020). Curcumin may chelate dietary trace elements, and long-term supplementation of curcumin aggravates iron deficiency (Chin et al., 2014). Clinicians should pay attention to any side effects that could increase the number and function of myeloid-derived suppressor cells when using angelica polysaccharide as an immune enhancer (Shen et al., 2022). Cornus officinalis extract has shown good results in treating drug-resistant asthma, but it may cause allergic contact dermatitis (Mirsadraee et al., 2018).
The toxic and adverse effects of combining TCMs with Western medicine have also been documented and require special attention. TCMs may affect the activity of the cytochrome P450 (CYP) enzyme system, which may enhance therapeutic effects but could also lead to increased side effects. For the treatment of epilepsy, Gastrodiae rhizoma might lengthen the plasma half-life and concentration of carbamazepine and its metabolite (carbamazepine-10, 11-epoxide). However, it could also be accompanied by an expansion of the neurological signs of toxicity (Yip et al., 2020). Ginkgo stimulates both CYP3A4 and CYP2C9 and alters the AUC and Cmax of conventional medications like midazolam, tolbutamide, lopinavir, and nifedipine. Ginsenosides Re increased CYP2C9, which reduced the anticoagulant activity of warfarin (Suroowan and Mahomoodally, 2019). In addition, Glycyrrhizae radix et rhizoma replaces serum-bound cardiovascular medications and reduces the disease-treating effects of diltiazem, nifedipine, and verapamil (Suroowan and Mahomoodally, 2019). Individuals who took ginger and aprepitant together experienced more severe acute nausea than those who took only aprepitant (Zick et al., 2009). Despite the limited and contradictory results about curcumin enhancing the function of doxorubicin-induced cardiac toxicity, it is necessary to conduct carefully designed research to evaluate the safety and effectiveness of the new formulation of this compound during cancer treatment (Armandeh et al., 2022).
It should be noted that ingesting an excessive amount of TCMs, even “medicine food homologous,” will produce adverse reactions. For example, Korean red ginseng (KRG) is very popular as a dietary supplement, but its excessive intake can cause “shanghuo,” which is closely related to the acceleration of the TCA cycle and the increase of AMPK activity (Zhao T. et al., 2020). At a regular dose, Morinda officinalis has not been associated with any significant negative effects in clinical trials, but in some cases, doses greater than 1 g/kg have been linked to irritability, insomnia, and unpleasant feelings (Zhang J. H. et al., 2018). Excessive intake of curcumin may have adverse effects on the kidney, heart, liver, blood, and immune system, which is a reminder that there is still much research to be done before curcumin can be effectively used and transformed (Liu et al., 2022). High doses of baicalin improve the antioxidant system in rat liver, but at the same time, they also lead to the reduction of trace minerals, thereby decreasing the activity of some metal-containing enzymes and having negative health implications (Gao et al., 2003).
Based on the aforementioned reports, TCMs that regulate adult neurogenesis should be used with caution in clinical applications due to their toxicity and side effects. Regarding the effectiveness, toxicity, and side effects of TCMs on adult neurogenesis, quality control and reliability of TCMs are also important determining factors. Genuine traditional Chinese materia medica, processing, safety, compatibility with other medications, and dosage of TCMs used for different medical conditions should all be taken into consideration. It is also necessary to keep researching the scientific and ethical principles of TCM clinical trials on adult neurogenesis. All of these techniques can effectively protect the subjects’ rights, interests, and safety while also improving the development of TCMs on adult neurogenesis to prevent and treat nervous system disorders.
6. Conclusion and future work
Many studies have shown that adult neurogenesis plays an important role in the regulation of neurological and psychotic disorders. A better understanding of the effects and mechanisms that regulate adult neurogenesis will identify disease pathologies that drive cognitive and emotional impairments, thereby providing an avenue for the development of effective therapeutic strategies. Here, we have focused on the role of adult neurogenesis in neuropsychiatric disorders, especially the characteristics and mechanisms of the ameliorative effects of TCM resulting from its regulation of adult neurogenesis. This review provides recent evidence on the regulation of adult neurogenesis by TCM.
Extensive studies have made significant progress in the regulation of adult neurogenesis thanks to TCM, but there are still many questions and thus further studies are needed. (1) Although adult neurogenesis has been shown to exist in animals, there is insufficient evidence to date to adequately support its existence in adult humans. It is crucial for future research to explore the dynamic changes and the functional role of adult neurogenesis in the normal human brain and alterations in neuropsychiatric disorders. More accurate approaches, cell markers, and human imaging protocols that can efficiently study adult neurogenesis are the greatest necessities in this field. (2) Although many promising results have been achieved by using TCM to regulate adult neurogenesis in various animal models and in in vitro cell cultures, no clinical trials have been conducted so far. One limitation that hinders the clinical trials of drugs on adult neurogenesis is the lack of an in situ method to monitor and calculate adult neurogenesis. However, greater efforts should be made to conduct clinical research to further verify the efficacy of TCM in improving adult neurogenesis in humans. (3) The discovery of effective ingredients from TCM to improve adult neurogenesis holds great promise, but current studies on the exact targets and the pathways involved are far from sufficient. The study of the exact pharmacological targets of TCM for improving adult neurogenesis should be further conducted in the future. With the aid of new methods such as bioinformatics, it would be clarified, and then more effective agents could be designed and developed accordingly.
Although adult neurogenesis per se has not yet yielded a clinically approved compound for any indication, the target remains of interest and is under investigation for drug development. TCM is a great treasure that provides abundant sources for drug discovery to modulate adult neurogenesis. We believe that the future development of medications from TCM that can improve adult neurogenesis would bring us one step closer to its application in the treatment of human diseases.
Author contributions
WS reviewed the databases and analyzed the information on subjects. NJ and WZ designed this review and worked on the manuscript revision. WS and NJ wrote the draft and modified this article. WZ revised this article and replied to the reviewers in the modification phase. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2022YFC3500304).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abshenas, R., Artimani, T., Shahidi, S., Ranjbar, A., Komaki, A., Salehi, I., et al. (2020). Treadmill exercise enhances the promoting effects of preconditioned stem cells on memory and neurogenesis in Aβ-induced neurotoxicity in the rats. Life Sci. 249:117482. doi: 10.1016/j.lfs.2020.117482
Akers, K. G., Martinez-Canabal, A., Restivo, L., Yiu, A. P., De Cristofaro, A., Hsiang, H. L., et al. (2014). Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602. doi: 10.1126/science.1248903
Alam, M. J., Kitamura, T., Saitoh, Y., Ohkawa, N., Kondo, T., and Inokuchi, K. (2018). Adult neurogenesis conserves hippocampal memory capacity. J. Neurosci. 38, 6854–6863. doi: 10.1523/JNEUROSCI.2976-17.2018
Altman, J., and Das, G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335. doi: 10.1002/cne.901240303
Amber, S., Sumera, M. F. J., Asif, M., Hassan, D., Ahmed, T., and Zahid, S. (2020). Amyloid-beta induced neurotoxicity impairs cognition and adult hippocampal neurogenesis in a mouse model for Alzheimer's disease. Curr. Alzheimer Res. 17, 1033–1042. doi: 10.2174/1567205017666201224162730
An, L., Zhang, Y. Z., Yu, N. J., Liu, X. M., Zhao, N., Yuan, L., et al. (2008). The total flavonoids extracted from Xiaobuxin-Tang up-regulate the decreased hippocampal neurogenesis and neurotrophic molecules expression in chronically stressed rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 32, 1484–1490. doi: 10.1016/j.pnpbp.2008.05.005
Anacker, C., and Hen, R. (2017). Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat. Rev. Neurosci. 18, 335–346. doi: 10.1038/nrn.2017.45
Anacker, C., Luna, V. M., Stevens, G. S., Millette, A., Shores, R., Jimenez, J. C., et al. (2018). Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559, 98–102. doi: 10.1038/s41586-018-0262-4
Armandeh, M., Bameri, B., Samadi, M., Heidari, S., Foroumadi, R., and Abdollahi, M. (2022). A systematic review of nonclinical studies on the effect of Curcumin in chemotherapy- induced Cardiotoxicity. Curr. Pharm. Des. 28, 1843–1853. doi: 10.2174/1381612828666220513125312
Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z., and Lindvall, O. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970. doi: 10.1038/nm747
Asher, G. N., and Spelman, K. (2013). Clinical utility of curcumin extract. Altern. Ther. Health Med. 19, 20–22. doi: 10.1016/j.hermed.2012.11.003
Azuma, K., Toyama, T., Katano, M., Kajimoto, K., Hayashi, S., Suzuki, A., et al. (2018). Yokukansan ameliorates hippocampus-dependent learning impairment in senescence-accelerated mouse. Biol. Pharm. Bull. 41, 1593–1599. doi: 10.1248/bpb.b18-00359
Bao, H., and Song, J. (2018). Treating brain disorders by targeting adult neural stem cells. Trends Mol. Med. 24, 991–1006. doi: 10.1016/j.molmed.2018.10.001
Berger, T., Lee, H., Young, A. H., Aarsland, D., and Thuret, S. (2020). Adult hippocampal neurogenesis in major depressive disorder and Alzheimer's disease. Trends Mol. Med. 26, 803–818. doi: 10.1016/j.molmed.2020.03.010
Boese, A. C., Le, Q. E., Pham, D., Hamblin, M. H., and Lee, J. P. (2018). Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res Ther 9:154. doi: 10.1186/s13287-018-0913-2
Boldrini, M., Fulmore, C. A., Tartt, A. N., Simeon, L. R., Pavlova, I., Poposka, V., et al. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589–599.e5. doi: 10.1016/j.stem.2018.03.015
Cai, Q., Li, Y., Mao, J., and Pei, G. (2016). Neurogenesis-promoting natural product α-Asarone modulates morphological dynamics of activated microglia. Front. Cell. Neurosci. 10:280. doi: 10.3389/fncel.2016.00280
Cai, N. N., Wang, Z. Z., Zhu, X. C., Jiang, Y., Zhu, W. Q., Yang, R., et al. (2020). Schisandrin a and B enhance the dentate gyrus neurogenesis in mouse hippocampus. J. Chem. Neuroanat. 105:101751. doi: 10.1016/j.jchemneu.2020.101751
Cameron, H. A., Hazel, T. G., and McKay, R. D. (1998). Regulation of neurogenesis by growth factors and neurotransmitters. J. Neurobiol. 36, 287–306. doi: 10.1002/(SICI)1097-4695(199808)36:2<287::AID-NEU13>3.0.CO;2-B
Chen, B., An, J., Guo, Y. S., Tang, J., Zhao, J. J., Zhang, R., et al. (2021). Tetramethylpyrazine induces the release of BDNF from BM-MSCs through activation of the PI3K/AKT/CREB pathway. Cell Biol. Int. 45, 2429–2442. doi: 10.1002/cbin.11687
Chen, X., Chen, H., He, Y., Fu, S., Liu, H., Wang, Q., et al. (2020). Proteomics-guided study on Buyang Huanwu decoction for its Neuroprotective and neurogenic mechanisms for transient ischemic stroke: involvements of EGFR/PI3K/Akt/bad/14-3-3 and Jak2/Stat3/Cyclin D1 Signaling cascades. Mol. Neurobiol. 57, 4305–4321. doi: 10.1007/s12035-020-02016-y
Chen, F. P., Chen, F. J., Maw-Shiou, J., Hui-Lin, T., Wang, J. R., and Shinn-Jang, H. (2009). Modern use of Chinese herbal formulae from Shang-Han Lun. Chin Med J 122, 1889–1894.
Chen, W., Cheng, X., Chen, J., Yi, X., Nie, D., Sun, X., et al. (2014). Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One 9:e88076. doi: 10.1371/journal.pone.0088076
Chen, X. Q., Li, C. F., Chen, S. J., Liang, W. N., Wang, M., Wang, S. S., et al. (2018). The antidepressant-like effects of Chaihu Shugan san: dependent on the hippocampal BDNF-TrkB-ERK/Akt signaling activation in perimenopausal depression-like rats. Biomed. Pharmacother. 105, 45–52. doi: 10.1016/j.biopha.2018.04.035
Chen, B. H., Park, J. H., Cho, J. H., Kim, I. H., Shin, B. N., Ahn, J. H., et al. (2015). Ethanol extract of Oenanthe javanica increases cell proliferation and neuroblast differentiation in the adolescent rat dentate gyrus. Neural Regen. Res. 10, 271–276. doi: 10.4103/1673-5374.152382
Chen, H. J., Shen, Y. C., Shiao, Y. J., Liou, K. T., Hsu, W. H., Hsieh, P. H., et al. (2015). Multiplex brain proteomic analysis revealed the molecular therapeutic effects of Buyang Huanwu decoction on cerebral ischemic stroke mice. PLoS One 10:e0140823. doi: 10.1371/journal.pone.0140823
Chen, L., Wang, X., Chen, X., Xing, S., Zhang, J., Li, J., et al. (2014). Tongxinluo attenuates neuronal loss and enhances neurogenesis and angiogenesis in the ipsilateral thalamus and improves neurological outcome after focal cortical infarction in hypertensive rats. Restor. Neurol. Neurosci. 32, 533–546. doi: 10.3233/RNN-140403
Chen, L., Wang, X., Zhang, J., Dang, C., Liu, G., Liang, Z., et al. (2016). Tongxinluo enhances neurogenesis and angiogenesis in Peri-infarct area and subventricular zone and promotes functional recovery after focal cerebral ischemic infarction in hypertensive rats. Evid. Based Complement. Alternat. Med. 2016:8549590. doi: 10.1155/2016/8549590
Chen, X., Wu, H., Chen, H., Wang, Q., Xie, X. J., and Shen, J. (2019). Astragaloside VI promotes neural stem cell proliferation and enhances neurological function recovery in transient cerebral ischemic injury via activating EGFR/MAPK Signaling cascades. Mol. Neurobiol. 56, 3053–3067. doi: 10.1007/s12035-018-1294-3
Chen, M. M., Zhao, G. W., He, P., Jiang, Z. L., Xi, X., Xu, S. H., et al. (2015). Improvement in the neural stem cell proliferation in rats treated with modified "Shengyu" decoction may contribute to the neurorestoration. J. Ethnopharmacol. 165, 9–19. doi: 10.1016/j.jep.2015.02.037
Cheng, C. Y., Huang, H. C., Kao, S. T., and Lee, Y. C. (2021). Angelica sinensis extract promotes neuronal survival by enhancing p38 MAPK-mediated hippocampal neurogenesis and dendritic growth in the chronic phase of transient global cerebral ischemia in rats. J. Ethnopharmacol. 278:114301. doi: 10.1016/j.jep.2021.114301
Cheng, X., Yao, H., Xiang, Y., Chen, L., Xiao, M., Wang, Z., et al. (2019). Effect of Angelica polysaccharide on brain senescence of nestin-GFP mice induced by D-galactose. Neurochem. Int. 122, 149–156. doi: 10.1016/j.neuint.2018.09.003
Cheung, J. S., Chan, J. N., Lau, B. W., and Ngai, S. P. (2016). Purposeful activity in psychiatric rehabilitation: is neurogenesis a key player? Hong Kong J. Occup. Ther. 27, 42–47. doi: 10.1016/j.hkjot.2016.04.002
Chin, D., Huebbe, P., Frank, J., Rimbach, G., and Pallauf, K. (2014). Curcumin may impair iron status when fed to mice for six months. Redox Biol. 2, 563–569. doi: 10.1016/j.redox.2014.01.018
Chrostek, M. R., Fellows, E. G., Crane, A. T., Grande, A. W., and Low, W. C. (2019). Efficacy of stem cell-based therapies for stroke. Brain Res. 1722:146362. doi: 10.1016/j.brainres.2019.146362
Czeh, M., Gressens, P., and Kaindl, A. M. (2011). The yin and yang of microglia. Dev. Neurosci. 33, 199–209. doi: 10.1159/000328989
DeCarolis, N. A., and Eisch, A. J. (2010). Hippocampal neurogenesis as a target for the treatment of mental illne ss: a critical evaluation. Neuropharmacology 58, 884–893. doi: 10.1016/j.neuropharm.2009.12.013
Dong, X. Z., Wang, D. X., Zhang, T. Y., Liu, X., Liu, P., and Hu, Y. (2020). Identification of protein targets for the antidepressant effects of Kai-Xin-san in Chinese medicine using isobaric tags for relative and absolute quantitation. Neural Regen. Res. 15, 302–310. doi: 10.4103/1673-5374.265555
Du, Y., Ruan, J., Zhang, L., and Fu, F. (2020). Jieyu Anshen granule, a Chinese herbal formulation, exerts effects on Poststroke depression in rats. Evid. Based Complement. Alternat. Med. 2020:7469068. doi: 10.1155/2020/7469068
Duan, S., Wang, T., Zhang, J., Li, M., Lu, C., Wang, L., et al. (2017). Huatuo Zaizao pill promotes functional recovery and neurogenesis after cerebral ischemia-reperfusion in rats. BMC Complement. Altern. Med. 17:19. doi: 10.1186/s12906-016-1516-z
Ekdahl, C. T., Kokaia, Z., and Lindvall, O. (2009). Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158, 1021–1029. doi: 10.1016/j.neuroscience.2008.06.052
Elder, G. A., De Gasperi, R., and Gama Sosa, M. A. (2006). Research update: neurogenesis in adult brain and neuropsychiatric disorders. Mt. Sinai J. Med. 73, 931–940.
Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., et al. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317. doi: 10.1038/3305
Fan, X., Zhang, C., Liu, D. B., Yan, J., and Liang, H. P. (2013). The clinical applications of curcumin: current state and the future. Curr. Pharm. Des. 19, 2011–2031. doi: 10.2174/138161213805289255
Fang, Y., Shi, B., Liu, X., Luo, J., Rao, Z., Liu, R., et al. (2020). Xiaoyao pills attenuate inflammation and nerve injury induced by lipopolysaccharide in hippocampal neurons in vitro. Neural Plast. 2020:8841332. doi: 10.1155/2020/8841332
Feng, L., Xing, H., and Zhang, K. (2022). The therapeutic potential of traditional Chinese medicine in depression: targeting adult hippocampal neurogenesis. Phytomedicine 98:153980. doi: 10.1016/j.phymed.2022.153980
Fu, D. L., Li, J. H., Shi, Y. H., Zhang, X. L., Lin, Y., and Zheng, G. Q. (2020). Sanhua decoction, a classic herbal prescription, exerts Neuroprotection through regulating phosphorylated tau level and promoting adult endogenous neurogenesis after cerebral ischemia/reperfusion injury. Front. Physiol. 11:57. doi: 10.3389/fphys.2020.00057
Gao, C., Du, Q., Li, W., Deng, R., Wang, Q., Xu, A., et al. (2018). Baicalin modulates APPL2/glucocorticoid receptor Signaling Cascade, promotes neurogenesis, and attenuates emotional and olfactory dysfunctions in chronic Corticosterone-induced depression. Mol. Neurobiol. 55, 9334–9348. doi: 10.1007/s12035-018-1042-8
Gao, Z., Xu, H., Chen, X., and Chen, H. (2003). Antioxidant status and mineral contents in tissues of rutin and baicalin fed rats. Life Sci. 73, 1599–1607. doi: 10.1016/S0024-3205(03)00487-9
Goncalves, J. T., Schafer, S. T., and Gage, F. H. (2016). Adult neurogenesis in the hippocampus: from stem cells to behavior. Cells 167, 897–914. doi: 10.1016/j.cell.2016.10.021
Gould, E. (2007). How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 8, 481–488. doi: 10.1038/nrn2147
Gronska-Peski, M., Goncalves, J. T., and Hebert, J. M. (2021). Enriched environment promotes adult hippocampal neurogenesis through FGFRs. J. Neurosci. 41, 2899–2910. doi: 10.1523/JNEUROSCI.2286-20.2021
Han, J., Zhang, J. Z., Zhong, Z. F., Li, Z. F., Pang, W. S., Hu, J., et al. (2018). Gualou Guizhi decoction promotes neurological functional recovery and neurogenesis following focal cerebral ischemia/reperfusion. Neural Regen. Res. 13, 1408–1416. doi: 10.4103/1673-5374.235296
Hassani, Z., O'Reilly, J., Pearse, Y., Stroemer, P., Tang, E., Sinden, J., et al. (2012). Human neural progenitor cell engraftment increases neurogenesis and microglial recruitment in the brain of rats with stroke. PLoS One 7:e50444. doi: 10.1371/journal.pone.0050444
He, Y., Chen, S., Tsoi, B., Qi, S., Gu, B., Wang, Z., et al. (2020). Alpinia oxyphylla Miq. And its active compound P-Coumaric acid promote brain-derived Neurotrophic factor Signaling for inducing hippocampal neurogenesis and improving post-cerebral ischemic spatial cognitive functions. Front cell. Dev. Biol. 8:577790. doi: 10.3389/fcell.2020.577790
Hill, A. S., Sahay, A., and Hen, R. (2015). Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology 40, 2368–2378. doi: 10.1038/npp.2015.85
Hong, J. G., Kim, D. H., Park, S. J., Kim, J. M., Cai, M., Liu, X., et al. (2011). The memory-enhancing effects of kami-ondam-tang in mice. J. Ethnopharmacol. 137, 251–256. doi: 10.1016/j.jep.2011.05.014
Horgusluoglu, E., Nudelman, K., Nho, K., and Saykin, A. J. (2017). Adult neurogenesis and neurodegenerative diseases: a systems biology perspective. Am. J. Med. Genet. B Neuropsychiatr. Genet. 174, 93–112. doi: 10.1002/ajmg.b.32429
Hsu, W. H., Huang, N. K., Shiao, Y. J., Lu, C. K., Chao, Y. M., Huang, Y. J., et al. (2021). Gastrodiae rhizoma attenuates brain aging via promoting neuritogenesis and neurodifferentiation. Phytomedicine 87:153576. doi: 10.1016/j.phymed.2021.153576
Hucklenbroich, J., Klein, R., Neumaier, B., Graf, R., Fink, G. R., Schroeter, M., et al. (2014). Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res Ther 5:100. doi: 10.1186/scrt500
Hueston, C. M., Cryan, J. F., and Nolan, Y. M. (2017). Stress and adolescent hippocampal neurogenesis: diet and exercise as cognitive modulators. Transl. Psychiatry 7:e1081. doi: 10.1038/tp.2017.48
Ishida, T., Kawada, K., Morisawa, S., Jobu, K., Morita, Y., and Miyamura, M. (2020). Risk factors for Pseudoaldosteronism with Yokukansan use: analysis using the Japanese adverse drug report (JADER) database. Biol. Pharm. Bull. 43, 1570–1576. doi: 10.1248/bpb.b20-00424
Ito, N., Hirose, E., Ishida, T., Hori, A., Nagai, T., Kobayashi, Y., et al. (2017). Kososan, a Kampo medicine, prevents a social avoidance behavior and attenuates neuroinflammation in socially defeated mice. J. Neuroinflammation 14:98. doi: 10.1186/s12974-017-0876-8
Ji, M., Niu, S., Mi, H., Jang, P., Li, Y., and Hu, W. (2020). Antidepressant functions of Jie Yu Chu Fan capsule in promoting hippocampal nerve cell neurogenesis in a mouse model of chronic unpredictable mild stress. Ann Transl Med 8:1020. doi: 10.21037/atm-20-5599
Jiang, C. Y., Qin, X. Y., Yuan, M. M., Lu, G. J., and Cheng, Y. (2018). 2,3,5,4'-Tetrahydroxystilbene-2-O-beta-D-glucoside reverses stress-induced depression via inflammatory and oxidative stress pathways. Oxidative Med. Cell. Longev. 2018:9501427. doi: 10.1155/2018/9501427
Jiang, N., Wang, H., Li, C., Zeng, G., Lv, J., Wang, Q., et al. (2021). The antidepressant-like effects of the water extract of Panax ginseng and Polygala tenuifolia are mediated via the BDNF-TrkB signaling pathway and neurogenesis in the hippocampus. J. Ethnopharmacol. 267:113625. doi: 10.1016/j.jep.2020.113625
Jiang, X., Xu, J., Zou, D., Yang, L., and Wang, Y. (2013). Baicalin influences the dendritic morphology of newborn neurons in the hippocampus of chronically stressed rats. Neural Regen. Res. 8, 496–505. doi: 10.3969/j.issn.1673-5374.2013.06.002
Jiang, Y., Zou, D., Li, Y., Gu, S., Dong, J., Ma, X., et al. (2022). Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals (Basel) 15, 1–19. doi: 10.3390/ph15101203
Jin, K., Wang, X., Xie, L., Mao, X. O., Zhu, W., Wang, Y., et al. (2006). Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. U. S. A. 103, 13198–13202. doi: 10.1073/pnas.0603512103
Johnson, M. A., Ables, J. L., and Eisch, A. J. (2009). Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep. 42, 245–259. doi: 10.5483/BMBRep.2009.42.5.245
Jordan, J. D., Ming, G. L., and Song, H. (2006). Adult neurogenesis as a potential therapy for neurodegenerative diseases. Discov. Med. 6, 144–147. doi: 10.1517/14712598.6.9.879
Jun, H., Hussaini, M. Q., Rigby, M. J., and Jang, M.-H. (2012). Functional role of adult hippocampal neurogenesis as a therapeutic str ategy for mental disorders. Neural Plast. :854285. doi: 10.1155/2012/854285
Katsuki, M., Kawamura, S., Kashiwagi, K., and Koh, A. (2021). Medication overuse headache successfully treated by Japanese herbal Kampo medicine. Yokukansan. Cureus 13:e18326. doi: 10.7759/cureus.18326
Koh, S. H., and Park, H. H. (2017). Neurogenesis in stroke recovery. Transl. Stroke Res. 8, 3–13. doi: 10.1007/s12975-016-0460-z
Kumar, R., Abu Bakar, A., Thanabalan, J., Paramasvaran, S., Toh, C. J., Jaffar, A., et al. (2020). Safety and use of MLC601/MLC901 (NeuroAiD(TM)) in primary Intracerebral Hemorrhage: a cohort study from the NeuroAiD safe treatment registry. Brain Sci. 10, 1–9. doi: 10.3390/brainsci10080499
Lanni, C., Govoni, S., Lucchelli, A., and Boselli, C. (2009). Depression and antidepressants: molecular and cellular aspects. Cell. Mol. Life Sci. 66, 2985–3008. doi: 10.1007/s00018-009-0055-x
Lee, J. Y., Joo, B., Nam, J. H., Nam, H. Y., Lee, W., Nam, Y., et al. (2018). An aqueous extract of herbal medicine ALWPs enhances cognitive performance and inhibits LPS-induced Neuroinflammation via FAK/NF-κB signaling pathways. Front. Aging Neurosci. 10:269. doi: 10.3389/fnagi.2018.00269
Lee, C. H., Kim, J. M., Kim, D. H., Park, S. J., Liu, X., Cai, M., et al. (2013). Effects of Sun ginseng on memory enhancement and hippocampal neurogenesis. Phytother. Res. 27, 1293–1299. doi: 10.1002/ptr.4873
Lee, S., Kim, D. H., Lee, C. H., Jung, J. W., Seo, Y. T., Jang, Y. P., et al. (2010). Antidepressant-like activity of the aqueous extract of Allium macrostemon in mice. J. Ethnopharmacol. 131, 386–395. doi: 10.1016/j.jep.2010.07.015
Lee, Y. S., Woo, S. C., Kim, S. Y., and Park, J. Y. (2020). Understanding the multi-herbal composition of Buyang Huanwu decoction: a review for better clinical use. J. Ethnopharmacol. 255:112765. doi: 10.1016/j.jep.2020.112765
Li, W. L., Cai, H. H., Wang, B., Chen, L., Zhou, Q. G., Luo, C. X., et al. (2009). Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J. Neurosci. Res. 87, 112–122. doi: 10.1002/jnr.21829
Li, F., Dong, H. X., Gong, Q. H., Wu, Q., Jin, F., and Shi, J. S. (2015). Icariin decreases both APP and Aβ levels and increases neurogenesis in the brain of Tg2576 mice. Neuroscience 304, 29–35. doi: 10.1016/j.neuroscience.2015.06.010
Li, L., Gan, H., Jin, H., Fang, Y., Yang, Y., Zhang, J., et al. (2021). Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats. Int. Immunopharmacol. 92:107335. doi: 10.1016/j.intimp.2020.107335
Li, Y., and Guo, W. (2021). Neural stem cell niche and adult neurogenesis. Neuroscientist 27, 235–245. doi: 10.1177/1073858420939034
Li, D. Q., Li, X. J., Duan, J. F., and Cai, W. (2010). Wuling capsule promotes hippocampal neurogenesis by improving expression of connexin 43 in rats exposed to chronic unpredictable mild stress. Zhong Xi Yi Jie He Xue Bao 8, 662–669. doi: 10.3736/jcim20100710
Li, Y., Liang, W., Guo, C., Chen, X., Huang, Y., Wang, H., et al. (2020). Renshen Shouwu extract enhances neurogenesis and angiogenesis via inhibition of TLR4/NF-κB/NLRP3 signaling pathway following ischemic stroke in rats. J. Ethnopharmacol. 253:112616. doi: 10.1016/j.jep.2020.112616
Li, X. Y., Qi, W. W., Zhang, Y. X., Jiang, S. Y., Yang, B., Xiong, L., et al. (2019). Helicid ameliorates learning and cognitive ability and activities cAMP/PKA/CREB Signaling in chronic unpredictable mild stress rats. Biol. Pharm. Bull. 42, 1146–1154. doi: 10.1248/bpb.b19-00012
Liu, B., Cai, G., Yi, J., and Chen, X. (2013). Buyang Huanwu decoction regulates neural stem cell behavior in ischemic brain. Neural Regen. Res. 8, 2336–2342. doi: 10.3969/j.issn.1673-5374.2013.25.004
Liu, S., Liu, J., He, L., Liu, L., Cheng, B., Zhou, F., et al. (2022). A comprehensive review on the benefits and problems of Curcumin with respect to human health. Molecules 27, 1–27. doi: 10.3390/molecules27144400
Liu, B., Zhang, Q., Ke, C., Xia, Z., Luo, C., Li, Y., et al. (2019). Ginseng-Angelica-Sansheng-Pulvis boosts neurogenesis against focal cerebral ischemia-induced neurological deficiency. Front. Neurosci. 13:515. doi: 10.3389/fnins.2019.00515
Lledo, P. M., Alonso, M., and Grubb, M. S. (2006). Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 7, 179–193. doi: 10.1038/nrn1867
Lopez-Toledano, M. A., Ali Faghihi, M., Patel, N. S., and Wahlestedt, C. (2010). Adult neurogenesis: a potential tool for early diagnosis in Alzheimer's disease? J. Alzheimers Dis. 20, 395–408. doi: 10.3233/JAD-2010-1388
Lu, J., Ma, Y., Wu, J., Huang, H., Wang, X., Chen, Z., et al. (2019). A review for the neuroprotective effects of andrographolide in the central nervous system. Biomed. Pharmacother. 117:109078. doi: 10.1016/j.biopha.2019.109078
Ma, C. L., Ma, X. T., Wang, J. J., Liu, H., Chen, Y. F., and Yang, Y. (2017). Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 317, 332–339. doi: 10.1016/j.bbr.2016.09.067
Ma, Y., Tian, S., Sun, L., Yao, S., Liang, Z., Li, S., et al. (2015). The effect of acori graminei rhizoma and extract fractions on spatial memory and hippocampal neurogenesis in amyloid beta 1-42 injected mice. CNS Neurol. Disord. Drug Targets 14, 411–420. doi: 10.2174/1871527314666150225124348
Ma, J., Wang, F., Yang, J., Dong, Y., Su, G., Zhang, K., et al. (2017). Xiaochaihutang attenuates depressive/anxiety-like behaviors of social isolation-reared mice by regulating monoaminergic system, neurogenesis and BDNF expression. J. Ethnopharmacol. 208, 94–104. doi: 10.1016/j.jep.2017.07.005
Mao, J., Huang, S., Liu, S., Feng, X. L., Yu, M., Liu, J., et al. (2015). A herbal medicine for Alzheimer's disease and its active constituents promote neural progenitor proliferation. Aging Cell 14, 784–796. doi: 10.1111/acel.12356
Marques, B. L., Carvalho, G. A., Freitas, E. M. M., Chiareli, R. A., Barbosa, T. G., Di Araujo, A. G. P., et al. (2019). The role of neurogenesis in neurorepair after ischemic stroke. Semin. Cell Dev. Biol. 95, 98–110. doi: 10.1016/j.semcdb.2018.12.003
Matsubara, S., Matsuda, T., and Nakashima, K. (2021). Regulation of adult mammalian neural stem cells and neurogenesis by cell extrinsic and intrinsic factors. Cells 10, 1–15. doi: 10.3390/cells10051145
Matsuda, T., and Nakashima, K. (2021). Natural and forced neurogenesis in the adult brain: mechanisms and the ir possible application to treat neurological disorders. Neurosci. Res. 166, 1–11. doi: 10.1016/j.neures.2020.05.011
Mellios, N., Feldman, D. A., Sheridan, S. D., Ip, J. P. K., Kwok, S., Amoah, S. K., et al. (2018). MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol. Psychiatry 23, 1051–1065. doi: 10.1038/mp.2017.86
Mendes-da-Silva, C., Lemes, S. F., Baliani Tda, S., Versutti, M. D., and Torsoni, M. A. (2015). Increased expression of Hes5 protein in notch signaling pathway in the hippocampus of mice offspring of dams fed a high-fat diet during pregnancy and suckling. Int. J. Dev. Neurosci. 40, 35–42. doi: 10.1016/j.ijdevneu.2014.11.005
Miller, B. R., and Hen, R. (2015). The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 30, 51–58. doi: 10.1016/j.conb.2014.08.012
Miller, S. M., and Sahay, A. (2019). Functions of adult-born neurons in hippocampal memory interference and indexing. Nat. Neurosci. 22, 1565–1575. doi: 10.1038/s41593-019-0484-2
Ming, G. L., and Song, H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702. doi: 10.1016/j.neuron.2011.05.001
Mirsadraee, M., Tavakoli, A., Ghorani, V., and Ghaffari, S. (2018). Effects of Rosmarinus officinalis and Platanus orientalis extracts on asthmatic subjects resistant to routine treatments. Avicenna J. Phytomed. 8, 399–407. doi: 10.22038/AJP.2018.25278.1925
Mirza, F. J., Amber, S., Sumera, H. D., Ahmed, T., and Zahid, S. (2021). Rosmarinic acid and ursolic acid alleviate deficits in cognition, synaptic regulation and adult hippocampal neurogenesis in an Aβ(1-42)-induced mouse model of Alzheimer's disease. Phytomedicine 83:153490. doi: 10.1016/j.phymed.2021.153490
Mizuki, D., Matsumoto, K., Tanaka, K., Thi Le, X., Fujiwara, H., Ishikawa, T., et al. (2014). Antidepressant-like effect of Butea superba in mice exposed to chronic mild stress and its possible mechanism of action. J. Ethnopharmacol. 156, 16–25. doi: 10.1016/j.jep.2014.08.014
Moon, M., Jeong, H. U., Choi, J. G., Jeon, S. G., Song, E. J., Hong, S. P., et al. (2016). Memory-enhancing effects of Cuscuta japonica Choisy via enhancement of adult hippocampal neurogenesis in mice. Behav. Brain Res. 311, 173–182. doi: 10.1016/j.bbr.2016.05.031
Moreno-Jimenez, E. P., Terreros-Roncal, J., Flor-Garcia, M., Rabano, A., and Llorens-Martin, M. (2021). Evidences for adult hippocampal neurogenesis in humans. J. Neurosci. 41, 2541–2553. doi: 10.1523/JNEUROSCI.0675-20.2020
Mu, Y., and Gage, F. H. (2011). Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol. Neurodegener. 6:85. doi: 10.1186/1750-1326-6-85
Murata, K., Fujita, N., Takahashi, R., and Inui, A. (2018). Ninjinyoeito improves behavioral abnormalities and hippocampal neurogenesis in the corticosterone model of depression. Front. Pharmacol. 9:1216. doi: 10.3389/fphar.2018.01216
Odaka, H., Adachi, N., and Numakawa, T. (2017). Impact of glucocorticoid on neurogenesis. Neural Regen. Res. 12, 1028–1035. doi: 10.4103/1673-5374.211174
Ohtsuka, T., and Kageyama, R. (2021). Hes1 overexpression leads to expansion of embryonic neural stem cell pool and stem cell reservoir in the postnatal brain. Development 148, 1–15. doi: 10.1242/dev.189191
Park, H. R., Kim, J. Y., Lee, Y., Chun, H. J., Choi, Y. W., Shin, H. K., et al. (2016). PMC-12, a traditional herbal medicine, enhances learning memory and hippocampal neurogenesis in mice. Neurosci. Lett. 617, 254–263. doi: 10.1016/j.neulet.2016.02.036
Park, S. W., Kim, Y. K., Lee, J. G., Kim, S. H., Kim, J. M., Yoon, J. S., et al. (2007). Antidepressant-like effects of the traditional Chinese medicine kami-shoyo-san in rats. Psychiatry Clin. Neurosci. 61, 401–406. doi: 10.1111/j.1440-1819.2007.01676.x
Park, H. J., Lee, K., Heo, H., Lee, M., Kim, J. W., Whang, W. W., et al. (2008). Effects of Polygala tenuifolia root extract on proliferation of neural stem cells in the hippocampal CA1 region. Phytother. Res. 22, 1324–1329. doi: 10.1002/ptr.2488
Po, K. K., Leung, J. W., Chan, J. N., Fung, T. K., Sánchez-Vidaña, D. I., Sin, E. L., et al. (2017). Protective effect of Lycium Barbarum polysaccharides on dextromethorphan-induced mood impairment and neurogenesis suppression. Brain Res. Bull. 134, 10–17. doi: 10.1016/j.brainresbull.2017.06.014
Qin, T., Fu, X., Yu, J., Zhang, R., Deng, X., Fu, Q., et al. (2019). Modification of GSK3β/β-catenin signaling on saikosaponins-d-induced inhibition of neural progenitor cell proliferation and adult neurogenesis. Toxicology 424:152233. doi: 10.1016/j.tox.2019.06.004
Quintard, H., Lorivel, T., Gandin, C., Lazdunski, M., and Heurteaux, C. (2014). MLC901, a traditional Chinese medicine induces neuroprotective and neuroregenerative benefits after traumatic brain injury in rats. Neuroscience 277, 72–86. doi: 10.1016/j.neuroscience.2014.06.047
Rahman, A. A., Amruta, N., Pinteaux, E., and Bix, G. J. (2021). Neurogenesis after stroke: a therapeutic perspective. Transl. Stroke Res. 12, 1–14. doi: 10.1007/s12975-020-00841-w
Rai, S. N., Dilnashin, H., Birla, H., Singh, S. S., Zahra, W., Rathore, A. S., et al. (2019). The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox. Res. 35, 775–795. doi: 10.1007/s12640-019-0003-y
Ren, C., Wang, B., Li, N., Jin, K., and Ji, X. (2015). Herbal formula Danggui-Shaoyao-san promotes neurogenesis and angiogenesis in rat following middle cerebral artery occlusion. Aging Dis. 6, 245–253. doi: 10.14336/AD.2014.1126
Ren, Z. L., and Zuo, P. P. (2012). Neural regeneration: role of traditional Chinese medicine in neurological diseases treatment. J. Pharmacol. Sci. 120, 139–145. doi: 10.1254/jphs.12R06CP
Ryu, S., Jeon, H., Kim, H. Y., Koo, S., and Kim, S. (2020). Korean red ginseng promotes hippocampal neurogenesis in mice. Neural Regen. Res. 15, 887–893. doi: 10.4103/1673-5374.268905
Sahay, A., and Hen, R. (2007). Adult hippocampal neurogenesis in depression. Nat. Neurosci. 10, 1110–1115. doi: 10.1038/nn1969
Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809. doi: 10.1126/science.1083328
Schoenfeld, T. J., McCausland, H. C., Morris, H. D., Padmanaban, V., and Cameron, H. A. (2017). Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol. Psychiatry 82, 914–923. doi: 10.1016/j.biopsych.2017.05.013
Seo, M. K., Cho, H. Y., Lee, C. H., Koo, K. A., Park, Y. K., Lee, J. G., et al. (2013). Antioxidant and proliferative activities of Bupleuri radix extract against serum deprivation in SH-SY5Y cells. Psychiatry Investig. 10, 81–88. doi: 10.4306/pi.2013.10.1.81
Shakerin, Z., Esfandiari, E., Razavi, S., Alaei, H., Ghanadian, M., and Dashti, G. (2020). Effects of Cyperus rotundus extract on spatial memory impairment and neuronal differentiation in rat model of Alzheimer's disease. Adv. Biomed. Res. 9:17. doi: 10.4103/abr.abr_173_19
Shen, J., Zhang, M., Zhang, K., Qin, Y., Liu, M., Liang, S., et al. (2022). Effect of angelica polysaccharide on mouse myeloid-derived suppressor cells. Front. Immunol. 13:989230. doi: 10.3389/fimmu.2022.989230
Shin, S. J., Jeong, Y. O., Jeon, S. G., Kim, S., Lee, S. K., Nam, Y., et al. (2018). Jowiseungchungtang inhibits amyloid-β aggregation and amyloid-β-mediated pathology in 5XFAD mice. Int. J. Mol. Sci. 19:4026. doi: 10.3390/ijms19124026
Shioda, N., Han, F., and Fukunaga, K. (2009). Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int. Rev. Neurobiol. 85, 375–387. doi: 10.1016/S0074-7742(09)85026-5
Snyder, J. S., Soumier, A., Brewer, M., Pickel, J., and Cameron, H. A. (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. doi: 10.1038/nature10287
Song, M. D., Kim, D. H., Kim, J. M., Lee, H. E., Park, S. J., Ryu, J. H., et al. (2013). Danggui-Jakyak-san ameliorates memory impairment and increase neurogenesis induced by transient forebrain ischemia in mice. BMC Complement. Altern. Med. 13:324. doi: 10.1186/1472-6882-13-324
Sorrells, S. F., Paredes, M. F., Cebrian-Silla, A., Sandoval, K., Qi, D., Kelley, K. W., et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. doi: 10.1038/nature25975
Stazi, M., and Wirths, O. (2021). Chronic memantine treatment ameliorates behavioral deficits, neuron loss, and impaired neurogenesis in a model of Alzheimer's disease. Mol. Neurobiol. 58, 204–216. doi: 10.1007/s12035-020-02120-z
Suh, H., Deng, W., and Gage, F. H. (2009). Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 25, 253–275. doi: 10.1146/annurev.cellbio.042308.113256
Sun, G. G., Shih, J. H., Chiou, S. H., Hong, C. J., Lu, S. W., and Pao, L. H. (2016). Chinese herbal medicines promote hippocampal neuroproliferation, reduce stress hormone levels, inhibit apoptosis, and improve behavior in chronically stressed mice. J. Ethnopharmacol. 193, 159–168. doi: 10.1016/j.jep.2016.07.025
Suroowan, S., and Mahomoodally, M. F. (2019). Herbal medicine of the 21st century: a focus on the chemistry, pharmacokinetics and toxicity of five widely advocated phytotherapies. Curr. Top. Med. Chem. 19, 2718–2738. doi: 10.2174/1568026619666191112121330
Taupin, P. (2005). Adult neurogenesis in the mammalian central nervous system: functionality and potential clinical interest. Med. Sci. Monit. 11, Ra247–Ra252. doi: 10.1016/j.lfs.2005.02.003
Taupin, P. (2008). Adult neurogenesis pharmacology in neurological diseases and disorders. Expert. Rev. Neurother. 8, 311–320. doi: 10.1586/14737175.8.2.311
Terreros-Roncal, J., Moreno-Jimenez, E. P., Flor-Garcia, M., Rodriguez-Moreno, C. B., Trinchero, M. F., Cafini, F., et al. (2021). Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science 374, 1106–1113. doi: 10.1126/science.abl5163
Tian, H., Li, X., Tang, Q., Zhang, W., Li, Q., Sun, X., et al. (2018). Yi-nao-jie-yu prescription exerts a positive effect on neurogenesis by regulating notch signals in the hippocampus of post-stroke depression rats. Front. Psych. 9:483. doi: 10.3389/fpsyt.2018.00483
Toda, T., and Gage, F. H. (2018). Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 373, 693–709. doi: 10.1007/s00441-017-2735-4
Tsai, Y. C., Lin, Y. C., Huang, C. C., Villaflores, O. B., Wu, T. Y., Huang, S. M., et al. (2019). Hericium erinaceus mycelium and its isolated compound, Erinacine a, ameliorate high-fat high-sucrose diet-induced metabolic dysfunction and spatial learning deficits in aging mice. J. Med. Food 22, 469–478. doi: 10.1089/jmf.2018.4288
Tunc-Ozcan, E., Peng, C. Y., Zhu, Y., Dunlop, S. R., Contractor, A., and Kessler, J. A. (2019). Activating newborn neurons suppresses depression and anxiety-like behaviors. Nat. Commun. 10:3768. doi: 10.1038/s41467-019-11641-8
Tzeng, T. T., Chen, C. C., Chen, C. C., Tsay, H. J., Lee, L. Y., Chen, W. P., et al. (2018). The cyanthin diterpenoid and sesterterpene constituents of Hericium erinaceus mycelium ameliorate Alzheimer's disease-related pathologies in APP/PS1 transgenic mice. Int. J. Mol. Sci. 19, 1–22. doi: 10.3390/ijms19020598
Vaidya, V. A., Fernandes, K., and Jha, S. (2007). Regulation of adult hippocampal neurogenesis: relevance to depression. Expert. Rev. Neurother. 7, 853–864. doi: 10.1586/14737175.7.7.853
Villa, R. F., Ferrari, F., and Moretti, A. (2018). Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol. Ther. 184, 131–144. doi: 10.1016/j.pharmthera.2017.11.005
Vivar, C. (2015). Adult hippocampal neurogenesis, aging and neurodegenerative diseases: possible strategies to prevent cognitive impairment. Curr. Top. Med. Chem. 15, 2175–2192. doi: 10.2174/1568026615666150610141524
Wang, J., Hu, J., Chen, X., Lei, X., Feng, H., Wan, F., et al. (2021). Traditional Chinese medicine monomers: novel strategy for endogenous neural stem cells activation after stroke. Front. Cell. Neurosci. 15:628115. doi: 10.3389/fncel.2021.628115
Wang, H., Lau, B. W., Wang, N. L., Wang, S. Y., Lu, Q. J., Chang, R. C., et al. (2015). Lycium barbarum polysaccharides promotes in vivo proliferation of adult rat retinal progenitor cells. Neural Regen. Res. 10, 1976–1981. doi: 10.4103/1673-5374.172315
Wang, H. W., Liou, K. T., Wang, Y. H., Lu, C. K., Lin, Y. L., Lee, I. J., et al. (2011). Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J. Ethnopharmacol. 138, 22–33. doi: 10.1016/j.jep.2011.06.033
Wang, W. W., Lu, L., Bao, T. H., Zhang, H. M., Yuan, J., Miao, W., et al. (2016). Scutellarin alleviates behavioral deficits in a mouse model of multiple sclerosis, possibly through protecting neural stem cells. J. Mol. Neurosci. 58, 210–220. doi: 10.1007/s12031-015-0660-0
Wang, H. Z., Luo, W. L., Zeng, N. X., Li, H. Z., Li, L., Yan, C., et al. (2021). Cerebrospinal fluid proteomics reveal potential protein targets of JiaWeiSiNiSan in preventing chronic psychological stress damage. Pharm. Biol. 59, 1065–1076. doi: 10.1080/13880209.2021.1954666
Wang, T., Wang, S. W., Zhang, Y., Wu, X. F., Peng, Y., Cao, Z., et al. (2014). Scorpion venom heat-resistant peptide (SVHRP) enhances neurogenesis and neurite outgrowth of immature neurons in adult mice by up-regulating brain-derived neurotrophic factor (BDNF). PLoS One 9:e109977. doi: 10.1371/journal.pone.0110617
Wang, M., Yao, M., Liu, J., Takagi, N., Yang, B., Zhang, M., et al. (2020). Ligusticum chuanxiong exerts neuroprotection by promoting adult neurogenesis and inhibiting inflammation in the hippocampus of ME cerebral ischemia rats. J. Ethnopharmacol. 249:112385. doi: 10.1016/j.jep.2019.112385
Winner, B., and Winkler, J. (2015). Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 7:a021287. doi: 10.1101/cshperspect.a021287
Wu, L., Ran, C., Liu, S., Liao, L., Chen, Y., Guo, H., et al. (2013). Jiaweisinisan facilitates neurogenesis in the hippocampus after stress damage. Neural. Regen. Res. 8, 1091–1102. doi: 10.3969/j.issn.1673-5374.2013.12.004
Xiao, H., Jiang, Q., Qiu, H., Wu, K., Ma, X., Yang, J., et al. (2021). Gastrodin promotes hippocampal neurogenesis via PDE9-cGMP-PKG pathway in mice following cerebral ischemia. Neurochem. Int. 150:105171. doi: 10.1016/j.neuint.2021.105171
Xiao, H., Li, H., Song, H., Kong, L., Yan, X., Li, Y., et al. (2020). Shenzao jiannao oral liquid, an herbal formula, ameliorates cognitive impairments by rescuing neuronal death and triggering endogenous neurogenesis in AD-like mice induced by a combination of Aβ42 and scopolamine. J. Ethnopharmacol. 259:112957. doi: 10.1016/j.jep.2020.112957
Xing, H., Zhang, K., Zhang, R., Shi, H., Bi, K., and Chen, X. (2015). Antidepressant-like effect of the water extract of the fixed combination of Gardenia jasminoides, Citrus aurantium and Magnolia officinalis in a rat model of chronic unpredictable mild stress. Phytomedicine 22, 1178–1185. doi: 10.1016/j.phymed.2015.09.004
Xu, Y., Ku, B., Cui, L., Li, X., Barish, P. A., Foster, T. C., et al. (2007). Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 1162, 9–18. doi: 10.1016/j.brainres.2007.05.071
Xu, A. L., Zheng, G. Y., Ye, H. Y., Chen, X. D., and Jiang, Q. (2020). Characterization of astrocytes and microglial cells in the hippocampal CA1 region after transient focal cerebral ischemia in rats treated with Ilexonin a. Neural Regen. Res. 15, 78–85. doi: 10.4103/1673-5374.264465
Yan, L., Hu, Q., Mak, M. S., Lou, J., Xu, S. L., Bi, C. W., et al. (2016). A Chinese herbal decoction, reformulated from Kai-Xin-san, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci. Rep. 6:30014. doi: 10.1038/srep30014
Yan, H. C., Qu, H. D., Sun, L. R., Li, S. J., Cao, X., Fang, Y. Y., et al. (2010). Fuzi polysaccharide-1 produces antidepressant-like effects in mice. Int. J. Neuropsychopharmacol. 13, 623–633. doi: 10.1017/S1461145709990733
Yan, Y., Zhao, J., Cao, C., Jia, Z., Zhou, N., Han, S., et al. (2014). Tetramethylpyrazine promotes SH-SY5Y cell differentiation into neurons through epigenetic regulation of topoisomerase IIβ. Neuroscience 278, 179–193. doi: 10.1016/j.neuroscience.2014.08.010
Yang, L., Wang, Y., Li, N., Xu, B., Duan, J., Yuan, C., et al. (2020). The anti-depression-like effects of Zhengtian capsule via induction of neurogenesis and the neurotrophic signaling pathway. Front. Pharmacol. 11:1338. doi: 10.3389/fphar.2020.01338
Yang, H., Wen, S. R., Zhang, G. W., Wang, T. G., Hu, F. X., Li, X. L., et al. (2011). Effects of Chinese herbal medicine Fuzhisan on autologous neural stem cells in the brain of SAMP-8 mice. Exp. Gerontol. 46, 628–636. doi: 10.1016/j.exger.2010.12.004
Yang, Z. H., Zhang, G. M., Chen, C. Y., He, J., and Chen, C. J. (2021). Prenatal exposure to koumine results in cognitive deficits and increased anxiety-like behavior in mice offspring. J. Chem. Neuroanat. 111:101888. doi: 10.1016/j.jchemneu.2020.101888
Yang, W. T., Zheng, X. W., Chen, S., Shan, C. S., Xu, Q. Q., Zhu, J. Z., et al. (2017). Chinese herbal medicine for Alzheimer's disease: clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 141, 143–155. doi: 10.1016/j.bcp.2017.07.002
Yao, R., Zhang, L., Li, X., and Li, L. (2010). Effects of epimedium flavonoids on proliferation and differentiation of neural stem cells in vitro. Neurol. Res. 32, 736–742. doi: 10.1179/174313209X459183
Yao, R. Q., Zhang, L., Wang, W., and Li, L. (2009). Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res. Bull. 79, 69–76. doi: 10.1016/j.brainresbull.2008.12.010
Yau, S. Y., Li, A., and So, K. F. (2015). Involvement of adult hippocampal neurogenesis in learning and forgetting. Neural Plast. 2015:717958. doi: 10.1155/2015/717958
Ye, M., Chung, H. S., An, Y. H., Lim, S. J., Choi, W., Yu, A. R., et al. (2016). Standardized herbal formula PM012 decreases cognitive impairment and promotes neurogenesis in the 3xTg AD mouse model of Alzheimer's disease. Mol. Neurobiol. 53, 5401–5412. doi: 10.1007/s12035-015-9458-x
Yip, K. L., Zhou, X., Chook, P., Leung, P. C., Schachter, S., Mok, V. C. T., et al. (2020). Herb-drug interaction of gastrodiae rhizoma on carbamazepine: a pharmacokinetic study in rats. Epilepsy Res. 165:106376. doi: 10.1016/j.eplepsyres.2020.106376
Zeng, J., Ji, Y., Luan, F., Hu, J., Rui, Y., Liu, Y., et al. (2022). Xiaoyaosan ethylacetate fraction alleviates depression-like behaviors in CUMS mice by promoting hippocampal neurogenesis via modulating the IGF-1Rbeta/PI3K/Akt signaling pathway. J. Ethnopharmacol. 288:115005. doi: 10.1016/j.jep.2022.115005
Zhang, R., Engler, A., and Taylor, V. (2018). Notch: an interactive player in neurogenesis and disease. Cell Tissue Res. 371, 73–89. doi: 10.1007/s00441-017-2641-9
Zhang, S., Lu, Y., Chen, W., Shi, W., Zhao, Q., Zhao, J., et al. (2021). Network pharmacology and experimental evidence: PI3K/AKT signaling pathway is involved in the antidepressive roles of Chaihu Shugan san. Drug Des. Devel. Ther. 15, 3425–3441. doi: 10.2147/DDDT.S315060
Zhang, R., Ma, Z., Liu, K., Li, Y., Liu, D., Xu, L., et al. (2019). Baicalin exerts antidepressant effects through Akt/FOXG1 pathway promoting neuronal differentiation and survival. Life Sci. 221, 241–248. doi: 10.1016/j.lfs.2019.02.033
Zhang, X. G., Shan, C., Zhu, J. Z., Bao, X. Y., Tong, Q., Wu, X. F., et al. (2017). Additive neuroprotective effect of borneol with mesenchymal stem cells on ischemic stroke in mice. Front. Physiol. 8:1133. doi: 10.3389/fphys.2017.01133
Zhang, E., Shen, J., and So, K. F. (2014). Chinese traditional medicine and adult neurogenesis in the hippocampus. J. Tradit. Complement. Med. 4, 77–81. doi: 10.4103/2225-4110.130372
Zhang, Z., Wang, Z., Rui, W., and Wu, S. (2018). Magnesium lithospermate B promotes proliferation and differentiation of neural stem cells in vitro and enhances neurogenesis in vivo. Tissue Cell 53, 8–14. doi: 10.1016/j.tice.2018.05.012
Zhang, K., Wang, F., Yang, J. Y., Wang, L. J., Pang, H. H., Su, G. Y., et al. (2015). Analysis of main constituents and mechanisms underlying antidepressant-like effects of Xiaochaihutang in mice. J. Ethnopharmacol. 175, 48–57. doi: 10.1016/j.jep.2015.08.031
Zhang, Z., Wu, R., Li, P., Liu, F., Zhang, W., Zhang, P., et al. (2009). Baicalin administration is effective in positive regulation of twenty-four ischemia/reperfusion-related proteins identified by a proteomic study. Neurochem. Int. 54, 488–496. doi: 10.1016/j.neuint.2009.02.005
Zhang, J. H., Xin, H. L., Xu, Y. M., Shen, Y., He, Y. Q., Hsien, Y., et al. (2018). Morinda officinalis how – A comprehensive review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 213, 230–255. doi: 10.1016/j.jep.2017.10.028
Zhang, K., Yang, J., Wang, F., Pan, X., Liu, J., Wang, L., et al. (2016). Antidepressant-like effects of Xiaochaihutang in a neuroendocrine mouse model of anxiety/depression. J. Ethnopharmacol. 194, 674–683. doi: 10.1016/j.jep.2016.10.028
Zhang, X., Zheng, W., Wang, T., Ren, P., Wang, F., Ma, X., et al. (2017). Danshen-Chuanxiong-Honghua ameliorates cerebral impairment and improves spatial cognitive deficits after transient focal ischemia and identification of active compounds. Front. Pharmacol. 8:452. doi: 10.3389/fphar.2017.00452
Zhao, X., Cui, Y., Wu, P., Zhao, P., Zhou, Q., Zhang, Z., et al. (2020). Polygalae radix: a review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia 147:104759. doi: 10.1016/j.fitote.2020.104759
Zhao, T., Yang, Z., Mei, X., Xu, L., and Fan, Y. (2020). Metabolic disturbance in Korean red ginseng-induced "Shanghuo" (excessive heat). J. Ethnopharmacol. 253:112604. doi: 10.1016/j.jep.2020.112604
Zheng, Y. Q., Li, L., Liu, J. X., Yao, M. J., Liu, S. B., Hu, Y., et al. (2014). Study on effect of huatuo zaizao extractum on focal cerebral ischemia/reperfusion neurogenesis in rats and its mechanisms. Zhongguo Zhong Yao Za Zhi 39, 891–895. doi: 10.4268/cjcmm20140526
Zhou, Z., Dun, L., Wei, B., Gan, Y., Liao, Z., Lin, X., et al. (2020). Musk ketone induces neural stem cell proliferation and differentiation in cerebral ischemia via activation of the PI3K/Akt Signaling pathway. Neuroscience 435, 1–9. doi: 10.1016/j.neuroscience.2020.02.031
Zhou, Y., Su, Y., Li, S., Kennedy, B. C., Zhang, D. Y., Bond, A. M., et al. (2022). Molecular landscapes of human hippocampal immature neurons across lifespan. Nature 607, 527–533. doi: 10.1038/s41586-022-04912-w
Zhu, J., Mu, X., Zeng, J., Xu, C., Liu, J., Zhang, M., et al. (2014). Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS One 9:e101291. doi: 10.1371/journal.pone.0101291
Zhuang, P., Zhang, Y., Cui, G., Bian, Y., Zhang, M., Zhang, J., et al. (2012). Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One 7:e35636. doi: 10.1371/journal.pone.0035636
Zhuge, L., Fang, Y., Jin, H., Li, L., Yang, Y., Hu, X., et al. (2020). Chinese medicine Buyang Huanwu decoction promotes neurogenesis and angiogenesis in ischemic stroke rats by upregulating miR-199a-5p expression. Zhejiang Da Xue Xue Bao Yi Xue Ban 49, 687–696. doi: 10.3785/j.issn.1008-9292.2020.12.03
Zick, S. M., Ruffin, M. T., Lee, J., Normolle, D. P., Siden, R., Alrawi, S., et al. (2009). Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer 17, 563–572. doi: 10.1007/s00520-008-0528-8
Keywords: adult neurogenesis, neural stem cells, traditional Chinese medicine, TCM prescriptions, Chinese herbal medicine, bioactive components
Citation: Shen W, Jiang N and Zhou W (2023) What can traditional Chinese medicine do for adult neurogenesis? Front. Neurosci. 17:1158228. doi: 10.3389/fnins.2023.1158228
Edited by:
Guibo Sun, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Kuo Zhang, Shenyang Pharmaceutical University, ChinaVivek Puri, Chitkara University, Himachal Pradesh, India
Copyright © 2023 Shen, Jiang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Jiang, amVubmlmZXItam5AMTI2LmNvbQ==; Wenxia Zhou, emhvdXd4QGJtaS5hYy5jbg==
 Wei Shen
Wei Shen Ning Jiang
Ning Jiang Wenxia Zhou
Wenxia Zhou