- 1Department of Geriatric Neurology, Nanjing Brain Hospital Affiliated to Nanjing Medical University, Nanjing, China
- 2School of Pharmacy, Nanjing Medical University, Nanjing, China
- 3Institute of Brain Science, Nanjing Brain Hospital Affiliated to Nanjing Medical University, Nanjing, China
- 4Institute of Neuropsychiatric Diseases, Nanjing Brain Hospital Affiliated to Nanjing Medical University, Nanjing, China
Background: Rapid eye movement sleep behavior disorder (RBD) and minor hallucinations (MHs) are prevalent nonmotor symptoms in Parkinson’s disease (PD). The purpose of this study was to explore the association of MHs in PD patients with probable RBD (pRBD).
Methods: This cross-sectional study included 291 patients diagnosed with PD. Patients who scored 6 or higher on the Rapid Eye Movement Behavior Disorder (RBD) Screening Questionnaire were defined as pRBD. A comprehensive evaluation was performed for all patients, including the collection of demographic information, clinical assessment, and MH features.
Results: Among the 291 PD patients, 69 (23.7%) had pRBD. MHs were observed in 35 (50.7%) patients with pRBD, significantly higher than 29.7% in patients without RBD (p = 0.015). The main type of MHs in pRBD was presence hallucinations with variable content. Patients with pRBD and MHs tended to be older, had a longer disease duration, and were more likely to take levodopa or dopamine-receptor agonists. Besides, the pRBD with MHs group had higher scores on the Nonmotor Symptoms Questionnaire (NMS-Quest) and Hamilton Anxiety Scale (HAMA). Binary logistic regression analysis revealed that longer disease duration and higher NMS-Quest scores were associated with MHs in PD patients with pRBD.
Conclusion: A high prevalence of MHs was observed in PD patients with pRBD. The main type of MHs in pRBD was presence hallucinations. MHs in PD with RBD are mainly associated with disease duration and severity of nonmotor symptoms. These findings provide new insights into the interaction between MHs and RBD.
1. Introduction
Parkinson’s disease (PD) is a common chronic neurodegenerative disorder characterized by motor symptoms (Bloem et al., 2021). However, patients with PD suffer greatly from various nonmotor symptoms, such as sleep disturbances, psychotic symptoms, and autonomic dysfunction (Schapira et al., 2017). Rapid eye movement sleep behavior disorder (RBD) is the most frequent sleep disorder in PD patients and is characterized by dream-enacting behavior and loss of normal muscle atonia during rapid eye movement (REM) sleep (Dauvilliers et al., 2018; Postuma et al., 2019). Previous studies have shown that PD patients with RBD may experience more severe autonomic and psychiatric symptoms (Sixel-Döring et al., 2011; Fujita et al., 2022), and PD with RBD may be a more aggressive subtype of the disease (Liu et al., 2021).
Minor hallucinations (MHs) and well-structured visual hallucinations (VHs) are the most common manifestations of psychotic symptoms in PD (Ffytche et al., 2017; Clegg et al., 2018). Previous studies have reported a significant association of RBD with VHs in PD patients (Onofrj et al., 2006; Wu et al., 2016). A 2-year prospective study reported that patients with PD and RBD have a higher risk of developing VHs than those without RBD (Sinforiani et al., 2008). The interactions between RBD and VHs have been extensively explored. MHs consist of presence hallucinations, passage hallucinations, and illusions, which usually precede the onset of VHs (Lenka et al., 2019). Several studies have revealed that RBD is an independent predictor of MHs in PD patients (Lenka et al., 2017; Zhong et al., 2021). However, few studies have investigated MHs in PD patients with RBD. The characteristics of MHs in PD with RBD remain unclear.
The aim of this study was to explore the association of MHs in PD patients with probable RBD (pRBD), especially the characteristic spectrum of MHs, and to investigate the potential risk factors for MHs in this population. These findings may offer new insights into the interaction between RBD and MHs in Parkinson’s disease.
2. Materials and methods
2.1. Patients
From April 2020 to October 2022, 384 patients with PD were consecutively recruited from the Department of Geriatrics at Nanjing Brain Hospital, affiliated with Nanjing Medical University. The diagnosis of PD was made by at least two specialists in movement disorder disease according to the United Kingdom Parkinson’s Disease Society Brain Bank criteria (Hughes et al., 1992). The exclusion criteria for this study were as follows: (1) history of major psychiatric diseases (or use of any antipsychotic medication) and focal brain injury (according to MRI evidence); (2) significant cognitive impairment, based on a Montreal Cognitive Assessment (MoCA) score ≤ 20 (Gill et al., 2008; Dalrymple-Alford et al., 2010); (3) Scored over 1 point on the MDS-UPDRS part 1 hallucination & psychosis item; (4) abnormal version or corrected vision, or (5) other serious medical diseases. We excluded six patients for cerebrovascular disease, cerebral atrophy, and malignant tumors; 35 for major psychiatric diseases or use of antipsychotic medication; 43 for significant cognitive impairment; eight for formed hallucinations or delusions; and one for glaucoma. Finally, a total of 291 patients were included in this cross-sectional study.
We applied a classification of probable RBD (pRBD) according to the REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ) as used in several previous studies (Long et al., 2020; Ashraf-Ganjouei et al., 2021; Barasa et al., 2021; Pavelka et al., 2022). Assignment of pRBD to idiopathic PD individuals used the criterion RBDSQ score ≥ 6 to optimize the specificity and sensitivity for pRBD in line with the Oxford Discovery Study (Liu et al., 2021).
The presence of hallucinations was assessed based on the “Hallucinations and Psychosis” item of the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part 1. Response options were as follows: 0 = no hallucinations or psychotic behavior, 1 = minor hallucinations, 2 = formed hallucinations without loss of insight, 3 = formed hallucinations with loss of insight, or 4 = delusions (Goetz et al., 2008). Patients with a score of 1 and whose MHs appeared stable in the last month were included in the MH+ group.
This study was approved by the Ethics Committee of Nanjing Brain Hospital affiliated with Nanjing Medical University, and written informed consent was obtained from all participants.
2.2. Clinical assessment
Structured interviews were conducted using a standardized questionnaire to collect general information such as name, gender, age, body mass index (BMI), education level, age at PD onset, disease duration, use of antiparkinsonian drugs, and levodopa-equivalent daily dose (LEDD; Tomlinson et al., 2010). Based on our previous research, a more comprehensive questionnaire was applied to obtain the detailed characteristics of MHs (Zhong et al., 2021). Disease severity was rated using the Hoehn and Yahr stage (H-Y stage; Hoehn and Yahr, 1967). Motor symptoms were assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS) part III score while nonmotor symptoms were measured by the Nonmotor Symptom Questionnaire (NMS-Quest; Fahn, 1987; Chaudhuri et al., 2006). Cognitive function was assessed by the Montreal Cognitive Assessment (MoCA). The Parkinson’s Disease Sleep Scale (PDSS) was used to measure sleep quality, and the Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire (RBDSQ) was used to further screen for probable RBD (Chaudhuri et al., 2002; Stiasny-Kolster et al., 2007). Besides, the 15th item (also the last) of the PDSS scale was separately calculated to evaluate daytime sleepiness symptoms. The item was the question “Have you unexpectedly fallen asleep during the day?,” and the score for this item ranged from 0 to 10 (10 represents excellent/never responses, and 0 illustrates the worst score). All patients completed the Hamilton Anxiety Rating Scale (HAMA) and the Hamilton Rating Scale for Depression (HAMD) to evaluate the severity of anxiety and depression, respectively (Hamilton, 1959; Williams, 1988). The quality of life of our patients was assessed using the 39-Item Parkinson’s Disease Questionnaire (PDQ-39; Jenkinson et al., 1997).
2.3. Statistical analysis
IBM SPSS 26.0 software was used for statistical analysis. Measurement data are expressed as the mean ± standard deviation (SD) or as number (N) or percentage (%). The Kolmogorov–Smirnov test was used to check the normality of the measurement data. A two-sample t test was used for normally distributed data, and the Mann–Whitney U test was used for nonnormally distributed data. Categorical variables are shown as percentages and were analyzed using a chi-square test. The MH (%), NMS-Quest, PDSS, MoCA, HAMA, HAMD, and PDQ-39 scores were compared by one-way analyses of covariance (ANCOVA) with adjustments for confounding factors, including age and disease duration. A logistic regression model was used to identify possible factors associated with hallucinations in patients with probable RBD. Univariate regression analysis was first performed to screen statistically significant variables. After excluding the effects of confounding factors, including age and disease duration, all significant variables were then entered into the multivariate regression model. Values of p < 0.05 were deemed statistically significant.
3. Results
3.1. Comparison of demographic information and clinical scale data between PD-pRBD and PD-nRBD groups
A total of 291 patients with PD were recruited for this study, including 69 (23.7%) PD-pRBD patients and 222 (76.3%) PD-nRBD patients. Among them, 101 (34.6%) patients experienced MHs, including 35 (50.7%) patients in the PD-pRBD group and 66 (29.7%) patients in the PD-nRBD group. As shown in Table 1, there were no differences in age, sex, BMI, education level, age of onset, H-Y stage, UPDRS-III scores, or LEDD between the two groups. However, the PD-pRBD group had a longer disease duration and a higher proportion of individuals that used dopamine receptor agonists than PD-nRBD, while other drug types did not differ between the two groups. The differences between the PD-pRBD and PD-nRBD groups were not significant regarding PDSS-item15, MoCA, HAMD, and PDQ-39 scores, while the differences in NMS-Quest, PDSS, and HAMA scores were significant. Besides, there was a significantly higher proportion of patients in pRBD group who had MH than those without RBD (p = 0.015).
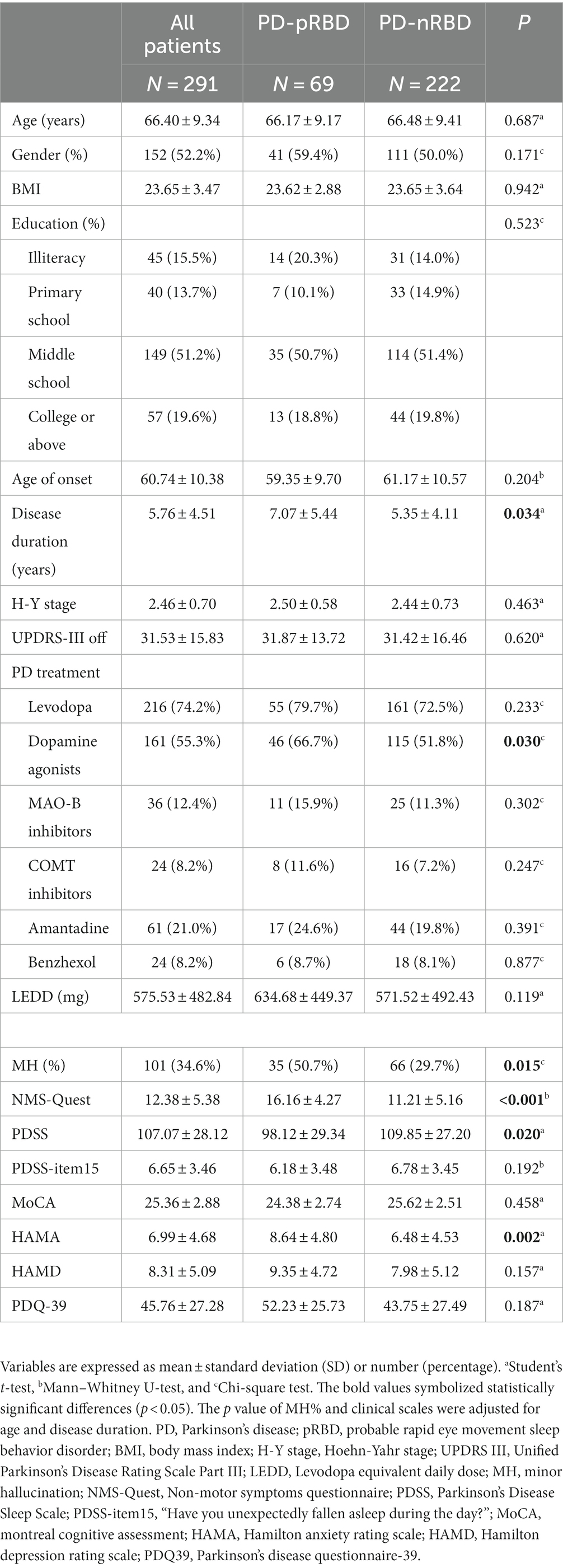
Table 1. Comparison of demographic information and clinical scale data between PD-pRBD and PD-nRBD groups.
3.2. Comparison of demographic information and clinical scale data in PD-pRBD patients based on the presence/absence of MHs
Table 2 shows the comparison between the pRBD-MH and pRBD-NH groups. Among all 69 PD-pRBD patients, 35 (50.7%) experienced MHs. Patients in the PD-pRBD group with MHs were older and had a longer disease duration. There were significant differences in the use proportion of levodopa and dopamine agonists between groups. Levodopa was used by 32 (91.4%) pRBD-MH patients and 23 (67.6%) pRBD-NH patients. Dopamine agonists were used in 28 (80%) pRBD-MH patients and 18 (52.9%) pRBD-NH patients. No significant differences were found in sex, BMI, education level, age of onset, H-Y stage, UPDRS-III score, or LEDD. We observed that NMS-Quest and HAMA scores were significantly higher in pRBD-MH patients. There were no significant group differences in PDSS, PDSS-item15, MoCA, HAMD, and PDQ-39 scores.
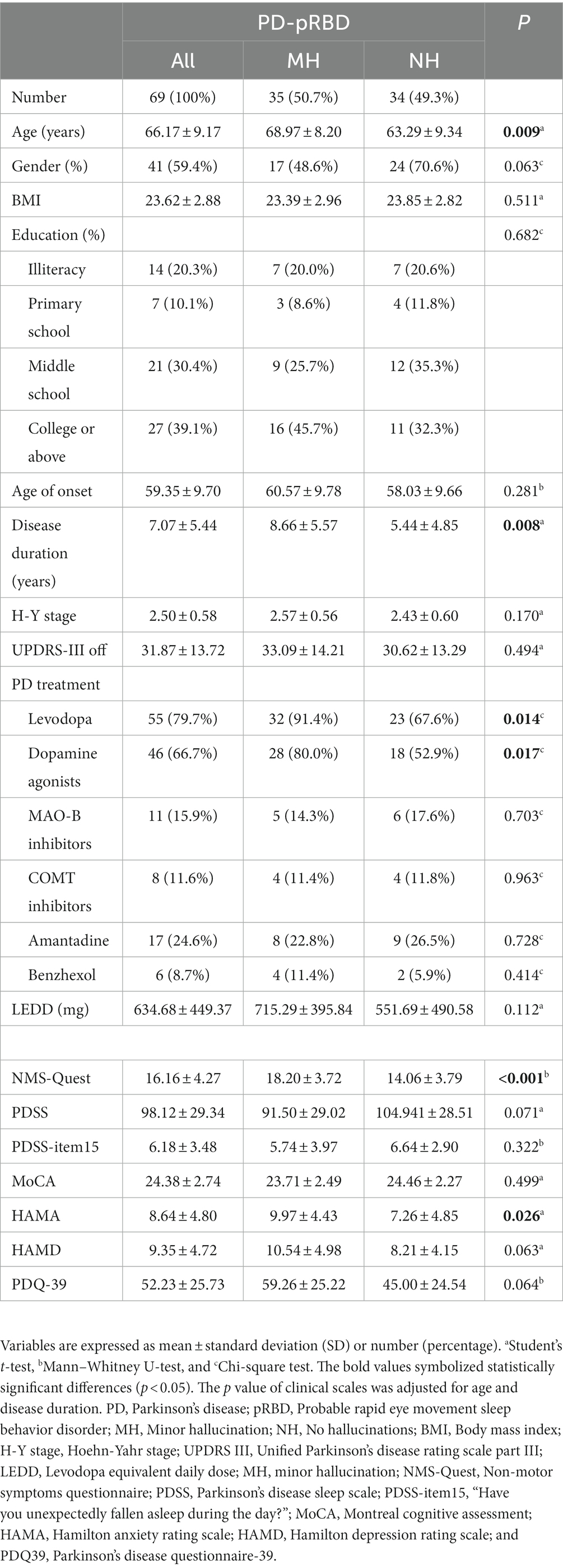
Table 2. Comparison of demographic information and clinical scale data in PD-pRBD patients based on the presence/absence of MHs.
3.3. Characteristics of minor hallucinations in the PD-pRBD and PD-nRBD groups
Table 3 summarizes the characteristics of MHs. In this study, 101 PD patients experienced MHs, including 35 (50.7%) patients in the PD-pRBD group and 66 (29.7%) patients in PD-nRBD. There were significant differences in the types of MHs between groups. Presence hallucinations (54.3%) were more common in the PD-pRBD group (p = 0.007), while in PD-nRBD, passage hallucinations (47.7%) were more frequent (p = 0.005). The prevalence of visual hallucinations was 42.8% in the PD-pRBD group and 35.4% in PD-nRBD. Additionally, compared with the stereotyped MHs in the PD-nRBD group, MHs in PD-pRBD patients were more variable (p = 0.035). MHs could appear at any time of the day and tended to appear suddenly and last for only a few seconds in the two groups. The frequency of MHs ranged from daily to less than once a week, but daily episodes were less common in the two groups. Also, the hallucinated images were both normal and monochromatic in the two groups. The characteristics of MHs in the PD-pRBD group are shown in Figure 1.

Figure 1. Characteristics of minor hallucinations in PD patients with probable RBD. Of the 69 PD patients with probable RBD recruited in our study, 35 (50.7%) experienced MHs. Among them, 19 (54.3%) had presence hallucinations, 7 (20.0%) had passage hallucinations, and 15 (42.8%) had visual illusions. In terms of appearance time of MHs, 17 (48.6%) patients appeared at daytime, 11 (31.4%) patients appeared at nighttime, and 7 (20%) patients appeared at both times. MHs of 31 (88.6%) patients appeared suddenly, and 3 (8.6%) patients appeared gradually. MHs of 28 (80%) patients lasted seconds, and six patients lasted minutes. Only five patients had MHs daily, 14 patients had MHs 1–6 times per week, and 16 patients had MHs less than one time per week. As for the size of MHs, 28 (80%) patients had normal size images, 4 (11.4%) patients had miniaturized images, and 3 (8.6%) patients had magnified images. Hallucinatory images were stereotyped in 13 (37.1%) patients and variable in 22 (62.9%). The color of MHs was black and white in 24 (68.6%) patients and colorful in 10 (28.8%) patients.
3.4. Risk factors for MHs in PD patients with probable RBD
As shown in Table 4, the binary logistic regression analysis revealed that a longer disease duration [odds ratio (OR) 1.136, 95% confidence interval (CI) 1.012–1.274] was significantly associated with a greater risk of MHs in PD patients with probable RBD. Higher scores on the NMS-Quest (OR 1.276; 95% CI 1.076–1.514) may also be a risk factor. However, age and depression symptoms were not closely related to MHs in PD-pRBD.
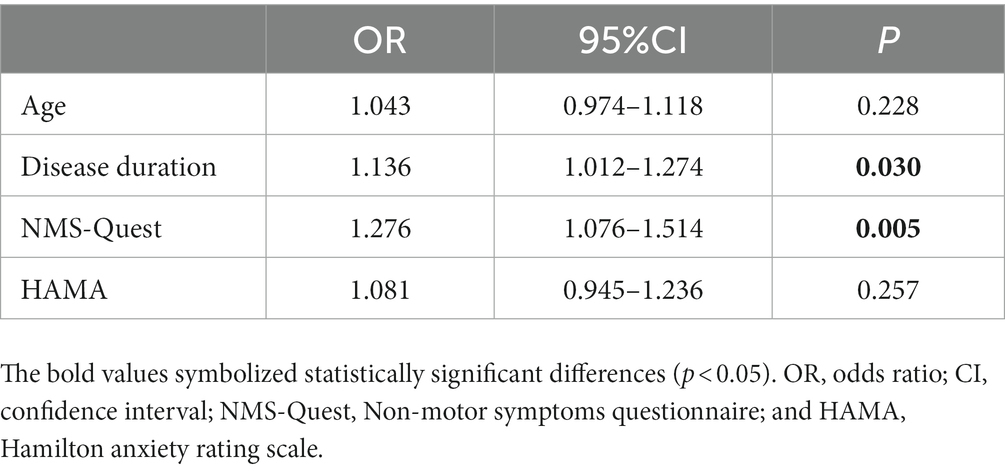
Table 4. Binary logistic regression analysis for independent predictors of minor hallucinations in PD patients with probable RBD.
4. Discussion
In this cross-sectional study, MHs were detected in 34.6% of patients in our cohort. We found that the prevalence of minor hallucinations (MHs) in patients with pRBD was up to 50.7%, the main type of which was presence hallucinations. The association between self-reported RBD and MHs was explained by longer disease duration and more severe nonmotor symptoms. To the best of our knowledge, this is the first study to explore the associations of MHs in PD patients with RBD.
Numerous articles have proved significant associations between RBD and MHs (Forsaa et al., 2010; Pagonabarraga et al., 2016; Barrett et al., 2017; Omoto et al., 2021). Zhong et al. (2021) reported that patients with MHs have higher RBDSQ scores and RBD is a risk factor for MHs in PD patients. In our study, the prevalence of MHs in pRBD patients was up to 50.7%, significantly higher than 29.7% in nRBD. This finding also showed that RBD and MHs were related. Cholinergic dysfunction may account for this relation. The cholinergic magnocellular nuclei of the basal forebrain complex are affected by Lewy body pathology in Braak stage 3 (Braak et al., 2003). Notably, the basal forebrain complex has strong interconnections with the brainstem nuclei regulating sleep and atonia during REM. Therefore, RBD symptoms may signal cholinergic system degeneration (Nardone et al., 2017). Although the pathophysiology of MHs and VHs is still under exploration, they are thought to be related to the disruption of visual processing and cholinergic dysfunction (Collerton et al., 2005).
Furthermore, neuroimaging studies also provided evidence. The increased psychiatric disturbances in PD with RBD were considered due to damage to key limbic regions (Matzaras et al., 2022). The typical PD-associated psychosis (PDP) is a continuous process that begins with MHs and then evolves into major hallucinations with insight, followed by gradual loss of insight and eventually delusions (Ffytche et al., 2017). Although only a few articles have been published on imaging studies of MHs, several similar structural and functional characteristics between MHs and VHs have been found. Apart from gray matter atrophy in limbic and paralimbic regions in patients with VHs (Lenka et al., 2015), morphometric studies also reported reduced gray matter volume of posterior cingulate cortex and parahippocampal gyri in patients with MHs (Bejr-Kasem et al., 2019; Zhong et al., 2022). Considering that RBD represents a more aggressive PD subtype (Fereshtehnejad et al., 2015), we conjecture that MHs in this population may be associated with damage to related brain regions in the early stage.
Previous studies reported that visual illusion was the most common type of MHs in patients with PD (Barrett et al., 2017). However, we found that over half of PD patients with pRBD had presence hallucinations. Presence hallucination refers to the vivid perception of the presence of someone other than oneself, usually to the side or behind oneself, in the absence of evidence for this perception (Fénelon, 2008). Previous studies proved that presence hallucinations did not tend to be stereotypical in nature (Fénelon et al., 2011; Wood et al., 2015). This explains why MHs of RBD patients showed more variability in our study. Besides, evidence has emerged that three minor hallucinations are associated with different risk factors (Ffytche et al., 2017). Archibald et al. (2011) reported that RBD was an independent predictor of presence hallucinations. Given that presence hallucinations are non-visual perceptions, cortical lesions rather than disorders of primary sensory pathways probably attribute to its generation (Sanchez-Ramos et al., 1996). Bernasconi et al. (2021) team combined MR-compatible robotics and revealed the pathological cortical sensorimotor processes of presence hallucinations in PD. Considering both RBD and psychosis are predictors of Lewy body pathology, the association of VHs with a high density of Lewy bodies in the temporal lobe may provide a clue as to the cortical substrate of presence hallucinations in patients with RBD (Harding et al., 2002).
Although the mechanism by which different MHs develop into VHs may vary, several studies reported a close association between presence hallucinations and VHs (Morgante et al., 2012). Fénelon et al. (2011) observed that 1/3 of PD patients with unformed VHs also had presence hallucinations, and presence hallucination could predict well-structured hallucinations (odds ratio: 4.5). Therefore, we speculate that VHs in patients with RBD, later in the course of the disease, may probably develop from the initial presence hallucinations. It is necessary to further investigate the pathogenesis of MHs in PD patients with RBD, especially the evolution of presence hallucination, and explore the relationship of MHs with VHs.
In PD-pRBD patients with MHs, we observed a higher use proportion of dopamine agonists. Although previous studies reported that dopamine agonists establish some side effects, including hallucinations and other psychosis in PD (Stowe et al., 2008; Morgante et al., 2012), research on visual hallucinations suggests that medications are only a precipitating factor for hallucinations, not their cause (Ravina et al., 2007; Powell et al., 2020). Other factors such as age, disease duration, cognitive status, and sleep–wake cycle disturbances may also contribute to this (Sanchez-Ramos et al., 1996; Fénelon et al., 2000). Pagonabarraga reported that MHs can occur in drug-naïve patients and even before the onset of motor symptoms (Pagonabarraga et al., 2016). Also, we found that medications are not the independent risk factors for MHs.
Rapid eye movement sleep behavior disorder and MHs are two common nonmotor symptoms in Parkinson’s disease that impose a substantial burden on patients and their caregivers. Arnulf et al. (2000) found an association between VHs and REM sleep episodes during daytime and proposed that the images of VHs may be attributed to REM sleep episodes intruding into wakefulness. Although we observed MHs patients performed worse on somnolence scores (PDSS-item15), we did not find any significant differences between groups. This may be due to the MHs in our study representing a better status on insights and lucidity. However, we observed aggravated anxiety in patients with MHs than those with single pRBD. Previous studies have also found that patients with MHs experienced more anxiety than depression (Zhong et al., 2021). In our sample, age, disease duration, levodopa use, dopamine receptor agonists use, NMS-Quest scores, HAMA scores, HAMD scores, and PDQ-39 scores were significantly associated with the presence of MHs in pRBD in univariate analysis. However, after excluding the confounding factors of age and disease duration, only age, disease duration, NMS-Quest scores, and HAMA scores were included in the final prediction model. We found that longer disease duration and higher NMS-Quest scores were independent predictors of MHs in pRBD patients. However, as RBD and MHs may worsen with the progression of PD, the screening and treatment of anxiety should be the focus of care.
The present study has some limitations. First, this study was a cross-sectional study. RBD is an important clinical feature in the prodromal stage of PD. Although we found that most MHs occurred after the onset of motor symptoms in PD, we did not collect supplementary data on the temporal relationship between pRBD and PD, such as the onset of pRBD and MHs. Longitudinal studies are needed in the future to explore their occurrence and development. Second, the diagnosis of RBD in this study was made based on the RBDSQ questionnaire. Although we applied a clinically more stringent score of 6 as the cutoff value, there may still have been some false-positive RBD patients. As the gold standard for the diagnosis of RBD, polysomnography should be more widely used in future studies to increase the accuracy of the research. Third, the sample size of this study was relatively small after subgroup grouping and more large-sample, multicenter studies are needed in the future. Finally, although our study involved a relatively structured and standardized survey of minor hallucinations, it is still necessary to establish a unified assessment scale to assess the clinical characteristics of MHs comprehensively. Such a scale will provide a better reference for future research on minor hallucinations in different PD subgroups.
5. Conclusion
A high prevalence of MHs was observed in PD patients with pRBD. The main type of MHs in patients with pRBD was presence hallucinations, which may develop into VHs. This study provides evidence that MHs in PD with RBD are mainly associated with disease duration and severity of nonmotor symptoms. More neuroimaging studies and longitudinal observations focused on MHs in RBD subtypes of PD are necessary further to investigate the underlying mechanism and evolvement of MHs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by the Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University (grant numbers 2021-KY007-01). All procedures followed the Declaration of Helsinki.
Author contributions
YJ, LZ, and FH conceived and designed the study. LZ, YP, JY, and XJ obtained the funding. YJ, YW, YC, DL, JZ, XJ, and BS collected the data. YJ, JZ, and YZ conducted the data analysis. YJ drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers: 82171249 and 82101332), the Jiangsu Provincial Cadre Health Projects (grant number: BJ20005), the Special Funds of the Jiangsu Provincial Key Research and Development Program (grant number: BE2019612), the Jiangsu Province Elderly Health Project (grant numbers: LD2021013 and LR2021018), the Nanjing Medical University School Fund Project (grant number: NMUB20210223), the Nanjing Medical Science and Technology Development Foundation (grant number: QRX17026), the Nanjing Rehabilitation Medicine Center Project, and the Nanjing Industrial and Information Technology Development Special Fund Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Archibald, N. K., Clarke, M. P., Mosimann, U. P., and Burn, D. J. (2011). Visual symptoms in Parkinson's disease and Parkinson's disease dementia. Mov. Disord. 26, 2387–2395. doi: 10.1002/mds.23891
Arnulf, I., Bonnet, A. M., Damier, P., Bejjani, B. P., Seilhean, D., Derenne, J. P., et al. (2000). Hallucinations, REM sleep, and Parkinson's disease: a medical hypothesis. Neurology 55, 281–288. doi: 10.1212/WNL.55.2.281
Ashraf-Ganjouei, A., Moradi, K., Aarabi, M., Abdolalizadeh, A., Kazemi, S. Z., Kasaeian, A., et al. (2021). The association between REM sleep behavior disorder and autonomic dysfunction in Parkinson's disease. J. Parkinsons Dis. 11, 747–755. doi: 10.3233/JPD-202134
Barasa, A., Wang, J., and Dewey, R. B. (2021). Probable REM sleep behavior disorder is a risk Factor for symptom progression in Parkinson disease. Front. Neurol. 12:651157. doi: 10.3389/fneur.2021.651157
Barrett, M. J., Smolkin, M. E., Flanigan, J. L., Shah, B. B., Harrison, M. B., and Sperling, S. A. (2017). Characteristics, correlates, and assessment of psychosis in Parkinson disease without dementia. Parkinsonism Relat. Disord. 43, 56–60. doi: 10.1016/j.parkreldis.2017.07.011
Bejr-Kasem, H., Pagonabarraga, J., Martínez-Horta, S., Sampedro, F., Marín-Lahoz, J., Horta-Barba, A., et al. (2019). Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson's disease. Mov. Disord. 34, 78–86. doi: 10.1002/mds.27557
Bernasconi, F., Blondiaux, E., Potheegadoo, J., Stripeikyte, G., Pagonabarraga, J., Bejr-Kasem, H., et al. (2021). Robot-induced hallucinations in Parkinson's disease depend on altered sensorimotor processing in fronto-temporal network. Sci. Transl. Med. 13:eabc8362. doi: 10.1126/scitranslmed.abc8362
Bloem, B. R., Okun, M. S., and Klein, C. (2021). Parkinson's disease. Lancet 397, 2284–2303. doi: 10.1016/S0140-6736(21)00218-X
Braak, H., Del Tredici, K., Rüb, U., de Vos, R. A. I., Jansen Steur, E. N. H., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/S0197-4580(02)00065-9
Chaudhuri, K. R., Martinez-Martin, P., Schapira, A. H. V., Stocchi, F., Sethi, K., Odin, P., et al. (2006). International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov. Disord. 21, 916–923. doi: 10.1002/mds.20844
Chaudhuri, K. R., Pal, S., DiMarco, A., Whately-Smith, C., Bridgman, K., Mathew, R., et al. (2002). The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 73, 629–635. doi: 10.1136/jnnp.73.6.629
Clegg, B. J., Duncan, G. W., Khoo, T. K., Barker, R. A., Burn, D. J., Yarnall, A. J., et al. (2018). Categorising visual hallucinations in early Parkinson's disease. J. Parkinsons Dis. 8, 447–453. doi: 10.3233/JPD-181338
Collerton, D., Perry, E., and McKeith, I. (2005). Why people see things that are not there: a novel perception and attention deficit model for recurrent complex visual hallucinations. Behav. Brain Sci. 28, 737–757. doi: 10.1017/S0140525X05000130
Dalrymple-Alford, J. C., MacAskill, M. R., Nakas, C. T., Livingston, L., Graham, C., Crucian, G. P., et al. (2010). The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology 75, 1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9
Dauvilliers, Y., Schenck, C. H., Postuma, R. B., Iranzo, A., Luppi, P.-H., Plazzi, G., et al. (2018). REM sleep behaviour disorder. Nat. Rev. Dis. Primers 4:19. doi: 10.1038/s41572-018-0016-5
Fahn, S. (1987). Recent developments in Parkinson's disease. Macmillan Health Care Info. 2, 293–304.
Fénelon, G. (2008). Psychosis in Parkinson's disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr. 13, 18–25. doi: 10.1017/S1092852900017284
Fénelon, G., Mahieux, F., Huon, R., and Ziégler, M. (2000). Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 123, 733–745. doi: 10.1093/brain/123.4.733
Fénelon, G., Soulas, T., Cleret de Langavant, L., Trinkler, I., and Bachoud-Lévi, A.-C. (2011). Feeling of presence in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 82, 1219–1224. doi: 10.1136/jnnp.2010.234799
Fereshtehnejad, S.-M., Romenets, S. R., Anang, J. B. M., Latreille, V., Gagnon, J.-F., and Postuma, R. B. (2015). New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 72, 863–873. doi: 10.1001/jamaneurol.2015.0703
Ffytche, D. H., Creese, B., Politis, M., Chaudhuri, K. R., Weintraub, D., Ballard, C., et al. (2017). The psychosis spectrum in Parkinson disease. Nat. Rev. Neurol. 13, 81–95. doi: 10.1038/nrneurol.2016.200
Forsaa, E. B., Larsen, J. P., Wentzel-Larsen, T., Goetz, C. G., Stebbins, G. T., Aarsland, D., et al. (2010). A 12-year population-based study of psychosis in Parkinson disease. Arch. Neurol. 67, 996–1001. doi: 10.1001/archneurol.2010.166
Fujita, H., Shiina, T., Sakuramoto, H., Nozawa, N., Ogaki, K., and Suzuki, K. (2022). Sleep and autonomic manifestations in Parkinson's disease complicated with probable rapid eye movement sleep behavior disorder. Front. Neurosci. 16:874349. doi: 10.3389/fnins.2022.874349
Gill, D. J., Freshman, A., Blender, J. A., and Ravina, B. (2008). The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov. Disord. 23, 1043–1046. doi: 10.1002/mds.22017
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., et al. (2008). Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170. doi: 10.1002/mds.22340
Hamilton, M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x
Harding, A. J., Broe, G. A., and Halliday, G. M. (2002). Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 125, 391–403. doi: 10.1093/brain/awf033
Hoehn, M. M., and Yahr, M. D. (1967). Parkinsonism: onset, progression and mortality. Neurology 17, 427–442. doi: 10.1212/WNL.17.5.427
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R., and Hyman, N. (1997). The Parkinson's disease questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing 26, 353–357. doi: 10.1093/ageing/26.5.353
Lenka, A., George, L., Arumugham, S. S., Hegde, S., Reddy, V., Kamble, N., et al. (2017). Predictors of onset of psychosis in patients with Parkinson's disease: who gets it early? Parkinsonism Relat. Disord. 44, 91–94. doi: 10.1016/j.parkreldis.2017.09.015
Lenka, A., Jhunjhunwala, K. R., Saini, J., and Pal, P. K. (2015). Structural and functional neuroimaging in patients with Parkinson's disease and visual hallucinations: a critical review. Parkinsonism Relat. Disord. 21, 683–691. doi: 10.1016/j.parkreldis.2015.04.005
Lenka, A., Pagonabarraga, J., Pal, P. K., Bejr-Kasem, H., and Kulisvesky, J. (2019). Minor hallucinations in Parkinson disease: a subtle symptom with major clinical implications. Neurology 93, 259–266. doi: 10.1212/WNL.0000000000007913
Liu, Y., Lawton, M. A., Lo, C., Bowring, F., Klein, J. C., Querejeta-Coma, A., et al. (2021). Longitudinal changes in Parkinson's disease symptoms with and without rapid eye movement sleep behavior disorder: the Oxford discovery cohort study. Mov. Disord. 36, 2821–2832. doi: 10.1002/mds.28763
Long, K., Wan, C., Xiang, Y., Liu, J., Xu, Q., Sun, Q., et al. (2020). Study on the clinical features of Parkinson's disease with probable rapid eye movement sleep behavior disorder. Front. Neurol. 11:979. doi: 10.3389/fneur.2020.00979
Matzaras, R., Shi, K., Artemiadis, A., Zis, P., Hadjigeorgiou, G., Rominger, A., et al. (2022). Brain neuroimaging of rapid eye movement sleep behavior disorder in Parkinson's disease: a systematic review. J. Parkinsons Dis. 12, 69–83. doi: 10.3233/JPD-212571
Morgante, L., Colosimo, C., Antonini, A., Marconi, R., Meco, G., Pederzoli, M., et al. (2012). Psychosis associated to Parkinson's disease in the early stages: relevance of cognitive decline and depression. J. Neurol. Neurosurg. Psychiatry 83, 76–82. doi: 10.1136/jnnp-2011-300043
Nardone, R., Brigo, F., Versace, V., Höller, Y., Tezzon, F., Saltuari, L., et al. (2017). Cortical afferent inhibition abnormalities reveal cholinergic dysfunction in Parkinson's disease: a reappraisal. J. Neural Transm. (Vienna) 124, 1417–1429. doi: 10.1007/s00702-017-1775-y
Omoto, S., Murakami, H., Shiraishi, T., Bono, K., Umehara, T., and Iguchi, Y. (2021). Risk factors for minor hallucinations in Parkinson's disease. Acta Neurol. Scand. 143, 538–544. doi: 10.1111/ane.13380
Onofrj, M., Bonanni, L., Albani, G., Mauro, A., Bulla, D., and Thomas, A. (2006). Visual hallucinations in Parkinson's disease: clues to separate origins. J. Neurol. Sci. 248, 143–150. doi: 10.1016/j.jns.2006.05.025
Pagonabarraga, J., Martinez-Horta, S., Fernández de Bobadilla, R., Pérez, J., Ribosa-Nogué, R., Marín, J., et al. (2016). Minor hallucinations occur in drug-naive Parkinson's disease patients, even from the premotor phase. Mov. Disord. 31, 45–52. doi: 10.1002/mds.26432
Pavelka, L., Rauschenberger, A., Landoulsi, Z., Pachchek, S., Marques, T., Gomes, C. P. C., et al. (2022). Body-first subtype of Parkinson's disease with probable REM-sleep behavior disorder is associated with non-motor dominant phenotype. J. Parkinsons Dis. 12, 2561–2573. doi: 10.3233/JPD-223511
Postuma, R. B., Iranzo, A., Hu, M., Högl, B., Boeve, B. F., Manni, R., et al. (2019). Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 142, 744–759. doi: 10.1093/brain/awz030
Powell, A., Muller, A. J., O'Callaghan, C., Sourty, M., Shine, J. M., and Lewis, S. J. G. (2020). Dopamine and functional connectivity in patients with Parkinson's disease and visual hallucinations. Mov. Disord. 35, 704–705. doi: 10.1002/mds.27995
Ravina, B., Marder, K., Fernandez, H. H., Friedman, J. H., McDonald, W., Murphy, D., et al. (2007). Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS. Mov. Disord. 22, 1061–1068. doi: 10.1002/mds.21382
Sanchez-Ramos, J. R., Ortoll, R., and Paulson, G. W. (1996). Visual hallucinations associated with Parkinson disease. Arch. Neurol. 53, 1265–1268. doi: 10.1001/archneur.1996.00550120077019
Schapira, A. H. V., Chaudhuri, K. R., and Jenner, P. (2017). Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450. doi: 10.1038/nrn.2017.62
Sinforiani, E., Pacchetti, C., Zangaglia, R., Pasotti, C., Manni, R., and Nappi, G. (2008). REM behavior disorder, hallucinations and cognitive impairment in Parkinson's disease: a two-year follow up. Mov. Disord. 23, 1441–1445. doi: 10.1002/mds.22126
Sixel-Döring, F., Trautmann, E., Mollenhauer, B., and Trenkwalder, C. (2011). Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 77, 1048–1054. doi: 10.1212/WNL.0b013e31822e560e
Stiasny-Kolster, K., Mayer, G., Schäfer, S., Möller, J. C., Heinzel-Gutenbrunner, M., and Oertel, W. H. (2007). The REM sleep behavior disorder screening questionnaire-a new diagnostic instrument. Mov. Disord. 22, 2386–2393. doi: 10.1002/mds.21740
Stowe, R. L., Ives, N. J., Clarke, C., van Hilten, J., Ferreira, J., Hawker, R. J., et al. (2008). Dopamine agonist therapy in early Parkinson's disease. Cochrane Database Syst. Rev. 2:CD006564. doi: 10.1002/14651858.CD006564.pub2
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 25, 2649–2653. doi: 10.1002/mds.23429
Williams, J. B. (1988). A structured interview guide for the Hamilton depression rating scale. Arch. Gen. Psychiatry 45, 742–747. doi: 10.1001/archpsyc.1988.01800320058007
Wood, R. A., Hopkins, S. A., Moodley, K. K., and Chan, D. (2015). Fifty percent prevalence of Extracampine hallucinations in Parkinson's disease patients. Front. Neurol. 6:263. doi: 10.3389/fneur.2015.00263
Wu, D. D., Li, S. H., Jin, L. Y., Jin, Y., Cui, Y. Y., Zhao, H., et al. (2016). Influencing factors of visual hallucinations in patients with Parkinson's disease and its relationship with sleep disorders. Zhonghua Yi Xue Za Zhi 96, 1016–1020. doi: 10.3760/cma.j.issn.0376-2491.2016.13.007
Zhong, M., Gu, R., Zhu, S., Bai, Y., Wu, Z., Jiang, X., et al. (2021). Prevalence and risk factors for minor hallucinations in patients with Parkinson's disease. Behav. Neurol. 2021, 1–10. doi: 10.1155/2021/3469706
Keywords: Parkinson’s disease, REM sleep behavior disorder, RBDSQ, minor hallucination, presence hallucination
Citation: Jiang Y, Zhu J, Zhao Y, Li D, Chen Y, Wang Y, Jiang X, Shen B, Pan Y, Yan J, Han F and Zhang L (2023) Minor hallucinations in Parkinson’s disease with probable rapid eye movement sleep behavior disorder. Front. Neurosci. 17:1205439. doi: 10.3389/fnins.2023.1205439
Edited by:
Maria Salsone, National Research Council (CNR), ItalyReviewed by:
Iain Duncan, King’s College, United KingdomEdoardo De Natale, University of Exeter, United Kingdom
Abhishek Lenka, Baylor College of Medicine, United States
Copyright © 2023 Jiang, Zhu, Zhao, Li, Chen, Wang, Jiang, Shen, Pan, Yan, Han and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, bmV1cm9femhhbmdsaUAxNjMuY29t
 Yinyin Jiang
Yinyin Jiang Jun Zhu1
Jun Zhu1 Dongfeng Li
Dongfeng Li Yaxi Wang
Yaxi Wang Xu Jiang
Xu Jiang Jun Yan
Jun Yan Li Zhang
Li Zhang