- 1CHUV, Department of Clinical Neurosciences, University Hospital Lausanne, Lausanne, Switzerland
- 2Bertarelli Foundation Chair in Translational Neural Engineering, Neuro-X Institute, Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland
- 3Modular Implantable Neurotechnologies (MINE) Laboratory, Università Vita Salute San Raffaele & Scuola Superiore Sant'Anna, Milan, Italy
Restoring the ability to walk is a priority for individuals with neurological disorders or neurotraumatic injuries, given its significant impact on independence and quality of life. Multimodal closed-loop strategies that integrate robotic assistance and neuromodulation present promising avenues for personalized and physiological gait recovery. These approaches capitalize on residual motor activity, fostering neuroplasticity and motor relearning. This narrative review emphasizes the importance of mobile brain/body imaging (MoBI) for guiding the development of closed-loop systems that integrate volitional brain signals with residual motor activity in stroke and spinal cord injury patients. We explore the potential of rehabilitative and assistive interventional strategies based on robotic devices, such as exoskeletons and powered orthoses, and neuromodulation techniques like functional electrical stimulation and spinal cord stimulation. We highlight the limitations of the single interventional strategies and the potential of the synergistic combination of MoBI, robotics, and neuromodulation for gait recovery. By leveraging residual motor functions and integrating multimodal data from the different domains involved in motor recovery (i.e., brain, muscle, and biomechanics), the complementarity of these interventional strategies has the potential to enable dynamic patient-specific interventions. We outline a perspective framework on how future directions can exploit such integration to promote physiological recovery of lower limb functions and personalized therapies that are both challenging and feasible. Advancing along this path holds the promise of enhancing rehabilitative strategies, ultimately promoting functional recovery and long-term independence for individuals with neuromotor disorders.
1 Introduction
Neurological disorders and neurotraumatic injuries often result in severe motor impairments that significantly impact patients' independence (Oczkowski and Barreca, 1993; Catz et al., 1997; Scivoletto et al., 2013) and quality of life (King, 1996; Dijkers, 1997; Westgren and Levi, 1998). However, residual motor activity, which is preserved in many affected patients, can be harnessed to promote neuroplasticity and enhance muscle strength, ultimately resulting in significant functional improvements (Dobkin, 2004; van Hedel and Dietz, 2010; Langhorne et al., 2011; Nas et al., 2015; Stinear et al., 2020; Somers and Bender-Burnett, 2024). For instance, spinal cord injuries (SCI) are typically subdivided into complete and incomplete, with incomplete SCIs sparing at least some sensorimotor functions (Kang et al., 2018). Even in the case of complete SCIs, where no residual sensorimotor function is observable, some studies have suggested that electrical stimulation and intensive rehabilitation may lead to the restoration of voluntary movements (Angeli et al., 2014). Thus, the absence of observable residual function does not necessarily correspond to a complete lack of neural traffic through the cortico-spinal tract, which may be leveraged through rehabilitation (Wahlgren et al., 2021). Similarly, even though stroke typically causes limb paresis contralateral to the produced brain lesion, substantial functional recovery can be attained by exploiting residual motor functions and the plasticity of nearby brain regions (Virani et al., 2020).
When SCI or stroke results in lower limb paralysis, restoring the ability to walk safely and independently becomes a primary goal for affected individuals. In particular, individuals with SCI-related paraplegia consistently rank gait restoration among their highest priorities, second only to the recovery of bladder, bowel, and sexual functions (Simpson et al., 2012). Although stroke more commonly causes unilateral motor impairments, it is estimated that approximately one-third of stroke survivors do not regain independent ambulation (Hendricks et al., 2002). Among those who do, many continue to exhibit pathological gait asymmetries and reduced walking speed (Veerbeek et al., 2014). Furthermore, even in patients who retain some degree of mobility, residual muscle weakness can contribute to balance deficits, a problem exacerbated by advanced age (Beyaert et al., 2015). While regaining the ability to walk is a central aim of rehabilitation in individuals with lower limb paralysis, even achieving upright standing can yield systemic benefits. These include improvements in cardiovascular regulation (Dunn et al., 1998; Eng et al., 2001; Edwards and Layne, 2007), as well as enhanced bowel (Dunn et al., 1998; Walter et al., 1999; Eng et al., 2001; Hoenig et al., 2001; Netz et al., 2007) and urinary function (Dunn et al., 1998; Walter et al., 1999; Eng et al., 2001).
In recent years, a variety of technology-based interventional strategies have been explored as add-ons to traditional physical therapy for the rehabilitation of lower limb function. In this context, here we focus on two key approaches: powered orthoses and exoskeletons, robotic devices designed to provide mechanical support and passive movement to paralyzed limbs (Herr, 2009), and neuromodulation, which facilitates muscle contractions through the electrical stimulation of the neuromuscular system (Hamid and Hayek, 2008; Popović et al., 2009). Initial proof-of-concept studies have demonstrated the potential of these technologies to restore gait (Asselin et al., 2016; Wagner et al., 2018; Haufe et al., 2020; Romeni et al., 2025) and to support the execution of basic functional tasks such as standing (Hankov et al., 2025), sit-to-stand transitions (Li et al., 2023; Romeni et al., 2025), and stair climbing (Hankov et al., 2025; Romeni et al., 2025). These encouraging results have catalyzed efforts to translate such technologies into real-world applications and activities of daily living (van Dijsseldonk et al., 2020; Rowald et al., 2022), which require more sophisticated control strategies as well as improvements in portability and ease of use in unstructured environments.
Over time, various control strategies have been developed with the dual aim of enabling intuitive and continuous user-driven control to enhance device usability and acceptance (Semprini et al., 2022) and promoting activity-dependent plasticity in the nervous system to maximize neurological recovery (Roy et al., 2012; Mrachacz-Kersting et al., 2019). Wearable mobile brain/body imaging (MoBI) systems, capable of continuously capturing high-density brain and muscle signals along with body movement's kinematics, offer a comprehensive way to optimize and adapt the control of interventional devices (He et al., 2018b) (Figure 1).
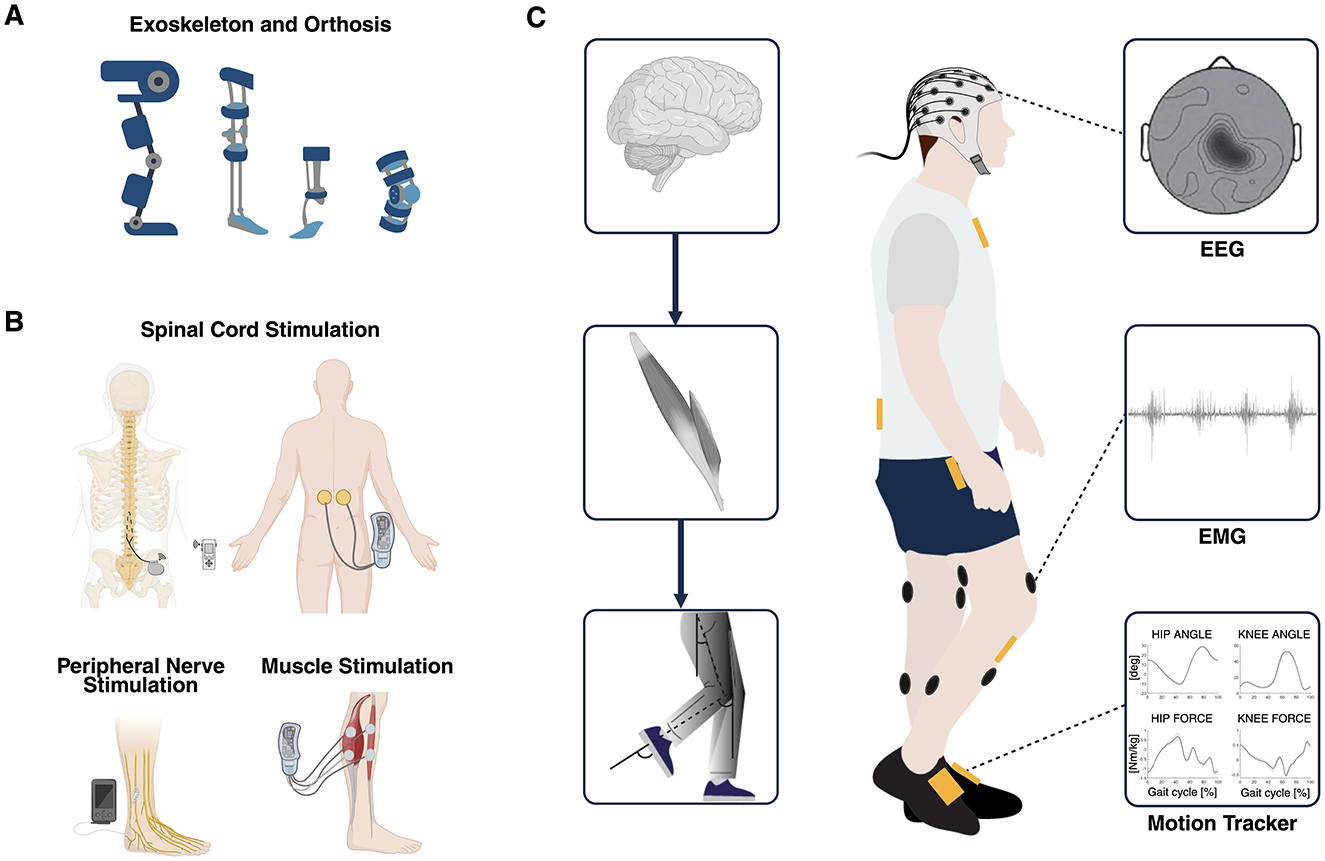
Figure 1. (A) Overview of robot-assisted devices such as exoskeletons and powered orthoses used as established interventional strategies for gait recovery. (B) Overview of electrical stimulation of the spinal cord, the nerves, and the muscles used as established interventional strategies for gait recovery. (C) Multimodal data used to simultaneously explore different domains of the hierarchical organization of the neuromusculoskeletal system: brain, muscles, and biomechanics (MoBI framework). Multimodal biomarkers of residual motor activity can be extracted from EEG, EMG, and kinematics signals and can be exploited to control in real-time different closed-loop interventions aimed at recovering walking through personalized assistance and therapy. MoBI, mobile brain/body imaging; EEG, electroencephalography; EMG, electromyography. Created with BioRender. de Seta V and Romeni S (2025). https://BioRender.com.
In this narrative review, we aim to explore how robotic devices and neuromodulation can be controlled for assistive and rehabilitative interventions, and how different interventional strategies can be integrated to provide personalized gait rehabilitation, leveraging complementary mechanisms of action. First, we will present the state-of-the-art in robotic devices and neuromodulation for lower limb movement restoration; then, we will describe how patients' residual motor activity recorded through various approaches exploiting kinematic, muscle, and neural signals has been used in the past to control such technologies. Finally, we will provide indications and highlight potential issues in the integration of MoBI techniques with different combinations of robotic devices and neuromodulation technologies to achieve functional and physiological recovery of lower limb abilities. The main goal of this review is to provide an overview of rehabilitative interventions for the recovery of lower limb motor functions through the exploitation of residual motor functions, robotic devices, neuromodulation, and monitoring of neurophysiological correlates of movement.
2 Interventional strategies for gait recovery
2.1 Rehabilitative and assistive strategies
Functional gait restoration can be achieved through a wide range of interventional approaches, broadly categorized as either rehabilitative or assistive. Rehabilitative interventions aim to improve the patient's motor function, promoting the reorganization of the nervous system, with benefits that persist even in the absence of the intervention. In contrast, assistive technologies are designed to support the execution of specific motor tasks, such as walking, without necessarily promoting long-term neuroplastic changes or functional recovery.
Rehabilitative interventions can be further divided into active and passive approaches. Active rehabilitative interventions include all strategies and technologies that enable and promote motor learning/recovery of impaired functions by engaging patients with neurological disorders in functional tasks within a longitudinal training path that leads to generalized motor improvement. Both neuromodulation and exoskeletons might fall into this category. For instance, a gait exoskeleton routinely used for robot-assisted gait training (RAGT), such as the Lokomat Pro (Hocoma, Switzerland) (Jezernik et al., 2003), is used in patients with mild to severe walking impairment to provide therapy by promoting activity-dependent modulation of the proprioceptive pathways under controlled conditions while supporting the patient's body. Indeed, by longitudinally adjusting the level of robotic assistance throughout the rehabilitative path, the patients are challenged to actively contribute to the walking pattern, and this promotes the reorganization of the nervous system, maximizing functional recovery (Riener et al., 2005; Calabrò et al., 2018). Likewise, neuromodulation technologies facilitate the performance of rehabilitative tasks (Romeni et al., 2025), but they also have an impact on synaptic and structural terms (Anderson et al., 2022; Kathe et al., 2022).
On the other hand, passive rehabilitative interventions refer to the ability of electrical stimulation to induce plastic changes in neural pathways, as well as in muscle contractile and structural properties, even when the stimulation is not activity-dependent (Gargiulo et al., 2011; Carraro et al., 2015, 2017). Powered orthoses do not activate paralyzed muscles, but rather mobilize them, and thus they do not exhibit this passive rehabilitation potential.
Whereas, assistive interventions consist of providing constant support during the task by powered orthoses or a stimulation device. Even if the patient improves their performance, the aim is not to get rid of the device, but just to get improved motor performance with the device on (Tyson and Rogerson, 2009; Yan et al., 2015). For example, an active ankle orthosis could deal with foot drop in a purely assistive fashion, thus allowing the foot not to drag during walking, without the need for a targeted rehabilitative effort to remove the orthosis at a later moment.
Rehabilitative and assistive interventions should not be intended as mutually exclusive but should be combined according to the needs and deficits of the patient and the rehabilitative target set by the clinicians, leveraging the complementarity of robotics devices and neuromodulation.
Several maladaptive changes, such as compensatory movements and spasticity, have been described along the process of motor recovery after a stroke or a SCI, addressing them is critical for the effective design and application of both rehabilitative and assistive technologies. Spasticity denotes a set of motor symptoms including increased muscle tone (hypertonia), increased reflexes (hyper-reflexia), involuntary muscle contractions (spasms and ankle clonus), and pathological co-contraction patterns, that have an important prevalence both in stroke (Thibaut et al., 2013) and SCI patients (Sköld et al., 1999; Adams and Hicks, 2005). The integration of robotic devices and neuromodulation should therefore aim not only to restore the motor function but also to promote physiological motor recovery by actively discouraging the emergence or reinforcement of such maladaptive mechanisms.
To better understand the potential of each component to support functional gait recovery and neuromuscular reactivation, the following paragraphs will show the main characteristics of robotic devices and electrical stimulation when used independently.
2.2 Lower limb-powered orthoses and exoskeletons
Many powered orthoses have been developed and commercialized to facilitate gait rehabilitation, each providing an environment optimized for the patients' current neurological status (Calabrò et al., 2021; Stampacchia et al., 2022). They are traditionally used to provide body weight support (BWS), assist lower extremity movement compensating, for example, for weak knee extension, and allow repetitive overground walking during conventional physiotherapy (Herr, 2009). According to the patient's level of impairment, they can assist multiple joints (i.e., hip, knee, and ankle) or just a single one (Figure 1A). Both multi-joint (Ortlieb et al., 2017; Laffranchi et al., 2021; Vouga et al., 2022) and single-joint (Wong et al., 2012; Lee et al., 2020) exoskeletons have been developed to assist balance and promote the recovery of physiological gait patterns (Blaya and Herr, 2004). They provide different levels of assistance through their integrated motors that can be personalized according to the patient's needs (Calabrò et al., 2021; Stampacchia et al., 2022). Moreover, the ability of exoskeletons to partially offload the patient's weight may provide BWS during early phases of rehabilitation when more complex systems like the three-dimensional overground BWS system Rysen presented by Mignardot et al. (2017) are not available. Compared to traditional BWS systems, exoskeletons can deliver targeted assistance to key joints, which may be crucial for training independent standing (Emmens et al., 2018). A comprehensive review of lower limb powered orthoses available in 2015 can be found in Yan et al. (2015). Soft exosuits have been recently proposed to address the large masses and rigid design of traditional powered orthoses, especially in unconstrained environments, reducing the metabolic cost of walking in stroke patients (Awad et al., 2017, 2020). For example, Myosuit (MyoSwiss AG, Zurich, Switzerland) is a lightweight, soft exoskeleton that actively supports weight bearing via a tendon cable assisting knee and hip extension (Schmidt et al., 2017). It was shown that it enables individuals with different movement disorders to walk faster with its assistance (Haufe et al., 2019, 2020).
Another compelling feature of powered orthoses is their ability to provide measurements of kinematic and kinetic parameters, such as joint angles, torques, step length, and temporal characteristics of gait phases, through embedded sensors. These measurements offer valuable quantitative insights into patients' motor performance, allowing for objective assessment of their progress throughout the rehabilitation process (Moeller et al., 2023). Moreover, the set of data available in real-time can be employed in a closed-loop to control the orthosis and adapt dynamically the assistance provided during gait (Baud et al., 2021). Finally, passive orthoses can be used in some cases as assistive devices to improve, for example, the foot drop or facilitate sit-to-stand transitions (Berkelman et al., 2007; Eguchi et al., 2018).
RAGT, while useful for sustaining patients during walking and promoting activity-dependent modulation of proprioceptive pathways, has significant limitations that must be addressed to optimize its rehabilitative potential. Substantial changes in muscle coordination patterns have been observed during exoskeleton-assisted walking, not only in patients but also in neurologically intact individuals (Moreno et al., 2013; Sylos-Labini et al., 2014). These alterations may reflect maladaptive changes, such as abnormal spatiotemporal integration of activity in specific spinal segments, which can impede gait recovery or even lead to gait abnormalities (Ivanenko et al., 2009).
2.3 Neuromodulation for lower limb movement restoration
Electrical stimulation acts directly on the neuromuscular system and induces muscle contraction by injecting electrical activity into the nervous system through implanted or transcutaneous electrodes (Figure 1B). In order to produce functional muscle contraction, the stimulation target and the downstream neural structures must be preserved, making different stimulation approaches optimal in different clinical scenarios.
Functional electrical stimulation (FES) is a non-invasive technique that targets muscles and neuromuscular junctions and has been investigated for over 50 years (Vodovnik et al., 1967; Ferrarin et al., 2001; Peckham and Knutson, 2005). FES can target single muscle groups to provide targeted rehabilitation or assistance, intervening on the weakness of isolated muscle groups, for example, FES reduced foot-drop and ankle plantar flexor spasticity in stroke patients (Sabut et al., 2011), or to improve standing in paraplegic patients (Hunt et al., 2001). From the early years of FES development, it became clear that gait restoration required multichannel FES systems to target the key involved muscles (Stanic et al., 1978). In Yan et al. (2005), a randomized placebo-controlled trial showed improved outcomes in 46 stroke patients when FES of tibialis anterior, quadriceps, gastrocnemius, and hamstrings muscles were activated in correspondence to the different gait phases, with respect to sham FES and no FES. An attempt to reduce the hardware complexity of FES montages has been based on the observations that superficial stimulation of the peroneal nerve allowed the elicitation of a triple flexion reflex response useful to promote swing (Stefancic et al., 1986; Bajd et al., 1999). In this way, only electrodes on the knee extensors for weight acceptance and on the peroneal nerve for inducing hip flexion can be sufficient to improve gait. A different approach employs implanted FES (iFES), reducing the cluttering of external hardware components by implanting electrodes in the target muscles and allowing for improved walking with walker and crutches, and in a few subjects, allowing stair climbing (Marsolais and Kobetic, 1987). In a study on six incomplete SCI patients, iFES training led to an improvement in gait parameters and a reduction in quadriceps spastic hypertonia (Granat et al., 1993). Standing with single-hand support was attained in SCI patients (Ho et al., 2014), and even sit-to-stand transitions (Triolo et al., 2012).
Even though it is simpler to employ in unstructured environments, iFES requires implanting stimulating electrodes in many key muscles, raising possible concerns about the invasiveness of this technological solution. Moreover, FES can lead rapidly to muscle fatigue (Vromans and Faghri, 2018). Alternatively, it is possible to target multiple muscles with a single electrode array implanted at the level of peripheral nerves, or potentially all lower limb muscles when targeting the spinal cord. Peripheral nerves can be stimulated through cuff electrodes or linear leads to improve standing and dorsiflexion/plantar flexion movements in SCI patients (Fisher et al., 2008; Delianides et al., 2020; Lemos et al., 2023). Transcutaneous spinal cord stimulation (tSCS) targets the spinal cord employing an electric current flux traversing the subject's torso. This technology was introduced in Minassian et al. (2007) and its effectiveness in eliciting motor reflexes in subjects in the supine position has been confirmed in Courtine et al. (2007), Gorodnichev et al. (2012), and Gerasimenko et al. (2015a). It has been tested in complete SCI patients to reinstate passive stepping movements (Gerasimenko et al., 2015b), and it has been shown to improve gait (Hofstoetter et al., 2013, 2020) and spasticity (Hofstoetter et al., 2020, 2021; Minassian et al., 2024). Finally, epidural electrical stimulation (EES) of the spinal cord is an emerging, fully implanted technology that recruits motor fibers primarily through monosynaptic pathways with the targeted afferent fibers in the dorsal roots of the spinal cord (Rattay et al., 2000; Capogrosso et al., 2013). EES has been employed in the past to restore or enhance lower limb functions in patients suffering from complete (Harkema et al., 2011; Angeli et al., 2014, 2018; Rowald et al., 2022) and incomplete (Wagner et al., 2018; Romeni et al., 2025) spinal cord lesions at the thoracic level. While first studies were limited to restoring standing and walking in complete SCI patients (Harkema et al., 2011), even claiming to be able to reinstate voluntary contractions despite the severity of the lesion (Angeli et al., 2014, 2018), later accounts focused more on restoring a wide range of functional activations to enable different motor tasks, including recreational activities (Rowald et al., 2022). In incomplete SCI, important longitudinal improvements have been attained in terms of voluntary muscle strength, walking distances, and walking speed (Wagner et al., 2018). Recently, high frequency EES has shown the capability to suppress spastic hyper-reflexia, hypertonia and to mitigate pathological co-contraction patterns during functional tasks (Romeni et al., 2025).
3 MoBI to control interventional strategies
3.1 Control strategies for interventional devices
For assistive devices to be effectively used in daily life activities, they must offer an intuitive control interface. Similarly, during active rehabilitation, it has been shown that synchronizing rehabilitative devices with the patient's voluntary movement intention can enhance the reorganization of the nervous system (Jackson and Zimmermann, 2012; Mrachacz-Kersting et al., 2012).
There are various modalities of controlling interventional devices: (i) manual motor state switching through device controllers; (ii) automatic motor state switching, which can be used to control the device based on patient's movement intention and to switch programs based on patient's activity (e.g., sit-to-stand transition, walking, climbing stairs); (iii) adaptive control, where the electrical stimulation parameters and, the assistance and trajectory of the exoskeletons are continuously adjusted to produce the most physiological muscle activation pattern and kinematics. For the automatic motor state switching, a high-level decoding, such as motor intention decoding, is needed, while the adaptive control of the interventional strategy requires low-level control of muscle activation and kinematics (Baud et al., 2021).
When active participation is required from the patient, the ideal solution to control interventional devices would be a so-called “neural bypass,” in which natural motor commands are extracted from brain activity and used to control the device. This is mainly used as high-level control, as it encodes the patient's movement intention rather than precise movement kinematic/kinetic features. A prominent example in the field of spinal cord stimulation is the development of a “brain-spine” interface, which decodes motor intentions from brain activity to trigger electrical stimulation of the nervous system below the injury site (Capogrosso et al., 2016). Recently, it has been demonstrated that an electrocorticography (ECoG)-based brain-spine interface can enable a patient with chronic spinal cord injury to stand and walk naturally (Lorach et al., 2022, 2023). Non-invasive brain signals such as electroencephalographic (EEG) signals have been employed in the past years to control powered orthoses and FES (Shokur et al., 2018; Tariq et al., 2018; Selfslagh et al., 2019) in the context of brain-computer interfaces (BCIs). BCI technologies provide a window into brain mechanisms, directly decoding the brain activity of the users and providing a feedback aligned with the intended action (Wolpaw and Wolpaw, 2012). In stroke patients, BCIs help to reinforce the activity of perilesional areas, enabling them to compensate for lost functions (Soekadar et al., 2015). Importantly, the brain-driven activation of impaired muscles generates sensory feedback that is both temporally and functionally aligned with the intended movement. This congruent feedback is sent back to the brain and enhances motor relearning by promoting neuroplasticity in individuals with incomplete SCI, and drives functional reorganization of the brain after a stroke (Chaudhary et al., 2016). However, EEG suffers from some structural limitations: low signal-to-noise ratio and spatial resolution, susceptibility to environmental and movement artifacts (Vaid et al., 2015). To address these challenges without requiring patients with moderate impairments to undergo surgery for an invasive brain implant, it may be necessary to integrate brain signals with other motor-related signals. The muscle activity of the legs recorded via electromyography (EMG) (Jiang et al., 2010) and lower limb kinematics through wearable motion capture technologies such as inertial measurement unit (IMU) sensors, foot switches, and force sensors can be used to leverage the overall patients' residual motor functions.
Figure 1C shows the multimodal techniques able to record signals from the three domains involved in motor recovery (i.e., brain, muscles, and kinematics). Several EEG, EMG, and kinematic parameters can be computed in real-time and used to control closed-loop interventional devices (Stauffer et al., 2009; Shokur et al., 2018; Tariq et al., 2018; Selfslagh et al., 2019). Several studies have explored different EEG correlates for decoding movement intention (López-Larraz et al., 2015; Liu et al., 2017, 2018; de Seta et al., 2024) and evaluated how to optimize the ability of EEG to non-invasively decode gait patterns (Sburlea et al., 2015; Nakagome et al., 2020; Tortora et al., 2020a). Often, the use of EEG to control interventional devices is used in combination with EMG and kinematics through the use of a hybrid approach (e.g., EEG+EMG/kinematics features) (Tortora et al., 2020b) or hybrid features (e.g., cortico-muscular coherence features) (De Seta et al., 2022) to increase the performance and facilitate the encouragement of only physiological patterns along the rehabilitation process (Sarasola-Sanz et al., 2017). EMG parameters can also be used alone to trigger electrical stimulation, to adapt in real-time the stimulation parameters (i.e., low-level control) proportionally according to the level of activation (reinforcement learning), or complementary to the residual or recovered muscular activity of the patient, reflecting the rehabilitative approach of supporting the patient through the functional recovery (Dutta et al., 2008; Jiang et al., 2010; de Seta, 2023). Whereas, IMU sensors, foot switches, and force sensors can detect gait events, kinematic (angle, speed) and kinetic (torque, force) profiles to control powered orthoses and exoskeletons (Baud et al., 2021; Figure 2).
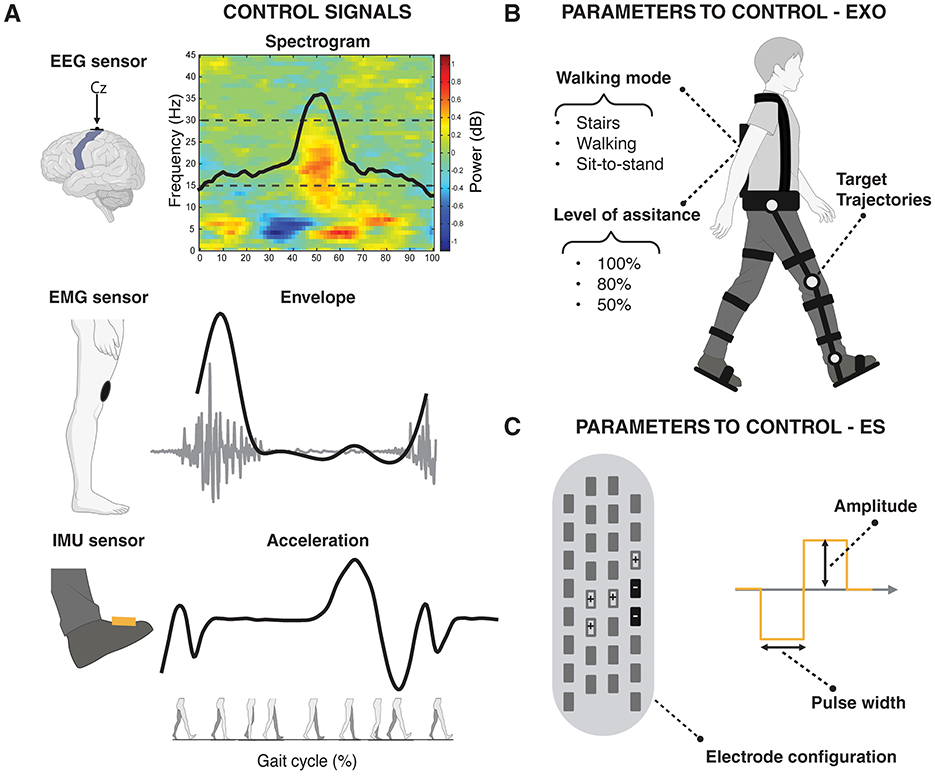
Figure 2. Conceptual representation of distinct gait-related modulations in brain, muscle, and kinematic activity, which can be leveraged as physiological signals to control (i.e., activate or deactivate) and adjust (i.e., tune parameter values of) interventional devices. (A) Modulation during a gait cycle of (Top) Brain activity can be captured via EEG over the sensorimotor cortex by the time-frequency spectral power and the related modulation curve in the beta band (15–30 Hz); (Center) Muscle activity can be captured through an EMG sensor over a knee extensor muscle by the EMG envelope; (Bottom) Kinematic data can be captured via an IMU sensor placed on the foot by the acceleration profile. (B) Examples of configurable parameters in robotic devices, such as exoskeletons and powered orthoses (EXO). These include the walking mode (e.g., stairs, speed of walking, sit-to-stand), the level of assistance (e.g., 100%, 80%, 50%), and the target joint trajectories. (C) Examples of configurable parameters in electrical stimulation (ES) technologies such as electrode configuration, stimulation amplitude, and pulse width. The figure illustrates a lead used in epidural electrical stimulation (EES) as an example, but the same parameters (with a reduced number of electrodes) apply to other ES techniques, including Functional Electrical Stimulation (FES) and transcutaneous Spinal Cord Stimulation (tSCS). EEG, electroencephalography; EMG, electromyography; IMU, inertial measurement unit.
In the following subsections, we will describe how different combinations of physiological signals can be used to control powered orthoses and neuromodulation and assess patients' level of impairment. Figure 2 shows a conceptual example of brain, muscle, and kinematic modulation during gait that can be exploited in real-time as control signals to control and adjust different parameters of the interventional devices.
3.2 Mobile brain/body-controlled powered orthoses and exoskeletons
The automatic control of lower limb-powered orthoses has normally been tackled using kinematics data able to track the different phases of the gait cycles (i.e., stance and swing phase) and/or kinetics data based on the interaction forces between the user and the exoskeleton, recorded through sensors, mainly integrated in the system (Baud et al., 2021).
While neurophysiological signal-based control has not been a primary focus in research, neuromuscular control strategy based on threshold-based algorithms on EMG activity showed improvements in both functional ambulation and muscle synergies in subacute stroke patients, suggesting enhanced neuromotor control (Tan et al., 2020). Moreover, techniques like BCIs offer an intuitive way to control rehabilitative devices by leveraging remaining neural pathways and have the added value of promoting brain plasticity and maintaining high patient engagement (Daly and Wolpaw, 2008; Lebedev and Nicolelis, 2017). To optimize the effectiveness and reliability of BCI-controlled exoskeletons, a hybrid (EEG and EMG) approach can be used to control the lower limb exoskeleton to both compensate the residual or recovered muscle activation and increase decoding performances. The residual or recovered motor activity is often associated with pathological synergies, unwanted contractions, and an increase in spasticity (Beauparlant et al., 2013; Beyaert et al., 2015; Prateek et al., 2018). EEG and EMG can also be combined with different relative weights in the control strategy, depending upon the impairment of the target patient. For example, in Gordleeva et al. (2020), an EEG-based approach is proposed for very impaired patients and individuals subjected to spasticity, with an EMG-based approach entering the picture as the rehabilitation improves residual contraction. However, it has been tested only in healthy subjects operating the exoskeleton under different conditions. Moreover, the combined use of EEG and EMG signals offers a promising strategy to detect involuntary movements, such as spasms, by identifying EMG activity that occurs without corresponding cortical signals in the EEG. This information can be used to enable exoskeletons to actively counteract unwanted movements and improve the quality of assisted motion (Pons, 2010). A multimodal approach can also be used to distinguish between different types of movement-related transitions, for example, to distinguish between the movements of starting, stopping walking and independent movement to and from the centerline of the feet (Li et al., 2024). Moreover, the muscle activity can be used to confirm the decision taken by the brain decoder (Shokur et al., 2018). Such BCIs have proven to have both assistive and rehabilitative effects during gait recovery, showing how neurorehabilitation protocols that actively engage the patients' mental and physical activity while providing the patient assistance can be beneficial to regain motor control. Besides, given that safety concerns are primordial in situations where patients have little to no residual motor control, often exoskeletons require upper body engagement (e.g., crutches for balance). Shared-control strategies incorporating sensors to monitor the state of the exoskeleton have been proposed to enhance safety and mitigate the risk of falls (Vinoj et al., 2019). Brain signals can be included in such frameworks, further improving reliability, for example, including the use of perturbation-evoked potentials to develop fall-prevention mechanisms in exoskeletons (Sujatha Ravindran et al., 2022) or the integration of BCI-controlled devices, which increases the user's engagement and awareness (He et al., 2018a; Ortiz et al., 2020). Brain-controlled exoskeletons act on the affected limb to influence the central nervous system through the afferent pathways (in a bottom-up framework) and, at the same time, exploit the brain modulation to detect volition (in a top-down framework), which leads to neuroplasticity and strengthens the cortico-muscular pathway (Contreras-Vidal et al., 2018; Tariq et al., 2018; Benabid et al., 2019). However, even BCI-controlled exoskeletons face limitations, as they do not inherently become therapeutic devices. As Gharabaghi (2016) notes, for a BCI to be considered therapeutic, it must demonstrate reinforcement learning, progressive modulation of brain dynamics along the rehabilitation path, and a direct link between brain dynamics and behavioral improvements related to therapeutic goals.
The significant variability in study protocols, sample sizes, and outcome measures hinders the standardization necessary to evaluate the clinical efficacy of closed-loop lower limb powered orthoses and exoskeletons in individuals with neuromotor disorders (de Miguel-Fernández et al., 2023). This lack of consistency likely reflects the field's current focus on advancing the technological aspects, such as improving reliability, usability, and feasibility, rather than the clinical ones, given the novelty of these systems.
3.3 Mobile brain/body-controlled neuromodulation
Since FES is the older electrical stimulation technique, it is also the one where more control strategies have been thoroughly explored. Early studies primarily relied on simple mechanisms to activate or deactivate stimulation, such as foot switches, which are particularly effective in controlling the activation of stimulation to correct drop foot and are thus still largely employed (Kottink et al., 2007; Lee et al., 2014; Melo et al., 2015). More recent approaches have introduced more sophisticated control strategies based on lower limb kinematics and myoelectric signals. Closed-loop superficial FES systems using kinematic variables to promote standing were investigated in several studies (Matjacic and Bajd, 1998; Holderbaum et al., 2002; Gollee et al., 2004). For instance, Matjačić et al. (2003) used superficial FES to control ankle joint movements in paraplegic patients, monitoring balance through kinematic variables while stabilizing the hip and knee joints with an external sensorized orthosis. Similarly, Hunt et al. (2004) showed how the use of cycling-derived kinematic and dynamic data can be used to control FES targeting the quadriceps, hamstrings, and gluteal muscles [for an early review on myoelectric control, see Jiang et al. (2010)]. The feasibility of controlling FES in closed-loop led to its application in more complex and unstructured scenarios, with the impractical placement of electrodes and the lack of portability of the hardware limiting its application in realistic, clinically relevant environments (Braz et al., 2009). Solutions based on iFES were thus developed, for example, implementing EMG-controlled iFES to assist walking (Dutta et al., 2008) or to modulate walking speed (Lombardo et al., 2015). Moreover, stimulation of peripheral nerves through cuff electrodes offers the advantage of activating multiple muscle groups with a single interface, significantly simplifying the hardware requirements for intramuscular FES systems. Christie et al. (2017) reported stability results for peripheral nerve stimulation using cuff electrodes in a large cohort of patients.
The automatic control of electrical spinal cord stimulation remains relatively underdeveloped, in part because continuous subthreshold stimulation has proven sufficient to enhance muscle contractions in patients with residual motor function, both with EES (Romeni et al., 2025) and tSCS (Minassian et al., 2011, 2012). However, the development of spatiotemporal EES patterns triggered by lower limb kinematics, as demonstrated in Wagner et al. (2018), has yielded clinically significant results.
Brain control has been used mostly in rehabilitative settings to monitor subject engagement and maximize rehabilitation outcomes by driving central nervous system plasticity, thanks to the causal association of the cortical activity with the movement intention (Mrachacz-Kersting et al., 2016, 2019). However, the applications of their integration in assistive walking neuroprostheses remain infrequent. In Lorach et al. (2023), electrocorticography, a more invasive alternative to EEG was used to control EES. Moreover, EEG has been shown to successfully decode key lower limb movements involved in locomotion in a chronic SCI patient with an EES implant (Toni et al., 2024). In Atkinson et al. (2025), it was shown that EEG could be used to control tSCS, but it was tested on healthy subjects for a single movement. In Insausti-Delgado et al. (2022), transcutaneous magnetic spinal cord stimulation was controlled through EEG in healthy participants.
3.4 Mobile brain/body imaging for assessing recovery
The large number of alternative rehabilitative pathways made possible by current technologies requires the development of techniques capable of assessing the patient's condition at each stage of the rehabilitation process. These assessments should explore the full hierarchical organization of the neuromusculoskeletal pathway (i.e., brain, muscle, and biomechanics as shown in Figure 1C), given the multifaceted consequences of SCI and stroke. For example, kinematic measures such as gait asymmetry and altered walking speed can inform about compensation strategies or level of impairments, while muscular parameters (e.g., muscle synergies, EMG time- and frequency-domain parameters, inter-muscular coherence) reveal changes in motor strategies and help identify pathological co-contractions and spasticity (Barroso et al., 2017). Besides, brain activity recordings shed light on central nervous system reorganization following brain or spinal cord damage (Green et al., 1999; Cramer et al., 2005; Garro et al., 2021). MoBI systems have proven effective in characterizing physiological gait patterns across various walking conditions (Wagner et al., 2012; Artoni et al., 2017, 2023), monitoring the temporal dynamics of motor recovery (Pierella et al., 2020), and assessing the impact of closed-loop devices such as BCIs on neural activity during walking (He et al., 2018b; Tortora et al., 2023). Such multimodal approach allows to perform a comprehensive, quantitative characterization of dysfunctional and maladaptive patterns commonly observed during recovery (Gennaro and de Bruin, 2018; Brambilla et al., 2021; Pichiorri et al., 2023), facilitating patient stratification and tailoring rehabilitation strategies to meet individual needs at different stages of motor recovery (Coscia et al., 2019). Indeed, personalizing the rehabilitative treatments to address the specific needs and impairment level of each patient remains a crucial factor to optimize rehabilitation outcomes (Mignardot et al., 2017; Semprini et al., 2022; Slade et al., 2024). Thus, a multimodal approach allows a global assessment of patients' neuro-biomechanical state during rehabilitation and can inform the design of the closed-loop interventional strategies.
4 Integration of MoBI, robot-assisted technology, and neuromodulation
4.1 Complementarity of robotic devices and neuromodulation
Each one of the technologies discussed has structural limitations, making each of them only suitable to restore a subset of a patient's motor functions. Most of the available powered orthoses are characterized by bulky designs that constrain their use in controlled laboratory settings and hinder patient comfort, compromising their applicability in real-world scenarios (He et al., 2017). Moreover, the assistive use of powered orthoses and exoskeletons showed limited efficacy in promoting robust activation of the neuromuscular system in patients with neurological disorders, leading to modest motor recovery (Piira et al., 2019). Electrical stimulation, instead, can produce longitudinal motor improvements through the targeted strengthening of key muscles, which can be leveraged during rehabilitation (Roy et al., 2012; Anderson et al., 2022). On the other hand, it can be challenging to achieve the selectivity required for the restoration of a rich repertoire of lower limb movements. For example, spinal cord stimulation is unable to recruit ankle dorsiflexors without a substantial co-activation of ankle plantar flexors (Wagner et al., 2018; Romeni et al., 2025), very likely due to the fact that these muscles are innervated by the same nerve roots (Hofstoetter et al., 2020), making spatially selective stimulation protocols challenging to identify. However, foot drop can be reliably and straightforwardly managed through purely assistive interventions such as the use of an ankle and foot orthosis (Zhou et al., 2022).
Some examples of combinations of neuromodulation and robotic devices for post-stroke lower limb rehabilitation can already be found in the literature, as confirmed by the recent review by Rikhof and colleagues (Rikhof et al., 2024). Systems combining FES with powered orthoses, also referred to as “hybrid assistive systems,” are quite established for regaining motor activity after stroke or SCI (Popovic et al., 1989; Popovic and Popovic, 2006). RAGT combined with spinal cord electrical stimulation has been proposed as an efficient and physiologically relevant approach for rehabilitating people with SCI, with benefits for multiple functions, such as locomotor as well as autonomic functions (Ivanenko et al., 2023). Non-invasive spinal cord stimulation improved overground walking in an exoskeleton in a complete SCI patient, reducing the level of robotic assistance needed, increasing voluntary control over knee movement, improving cardiovascular parameters, and subjective ratings of provided assistance (Gad et al., 2017). In Hankov et al. (2025), it has recently shown that using a closed-loop activity-dependent stimulation with the support of robotic devices commonly used in rehabilitation, such as Lokomat and Myosuit, led to an increase in the lower extremity muscle strength, allowing patients to perform outdoor activities.
Finally, robotic devices can provide assistance while measuring kinetic and kinematic data that can be employed to control electrical stimulation. For example, orthoses and exoskeletons can facilitate gait initiation and stability while measuring interaction forces between the user and the system to fine-tune in real-time electrical stimulation parameters (Stauffer et al., 2009; Hankov et al., 2025), or they can allow closed-loop control of stimulation parameters to achieve a desired joint trajectory (Jezernik et al., 2003). Table 1 provides an overview of the single interventional strategies for gait recovery with their related control strategies, summarizes their key points and limitations, and highlights the potential of the synergistic combination of MoBI, robotics and neuromodulation for regaining locomotor abilities.
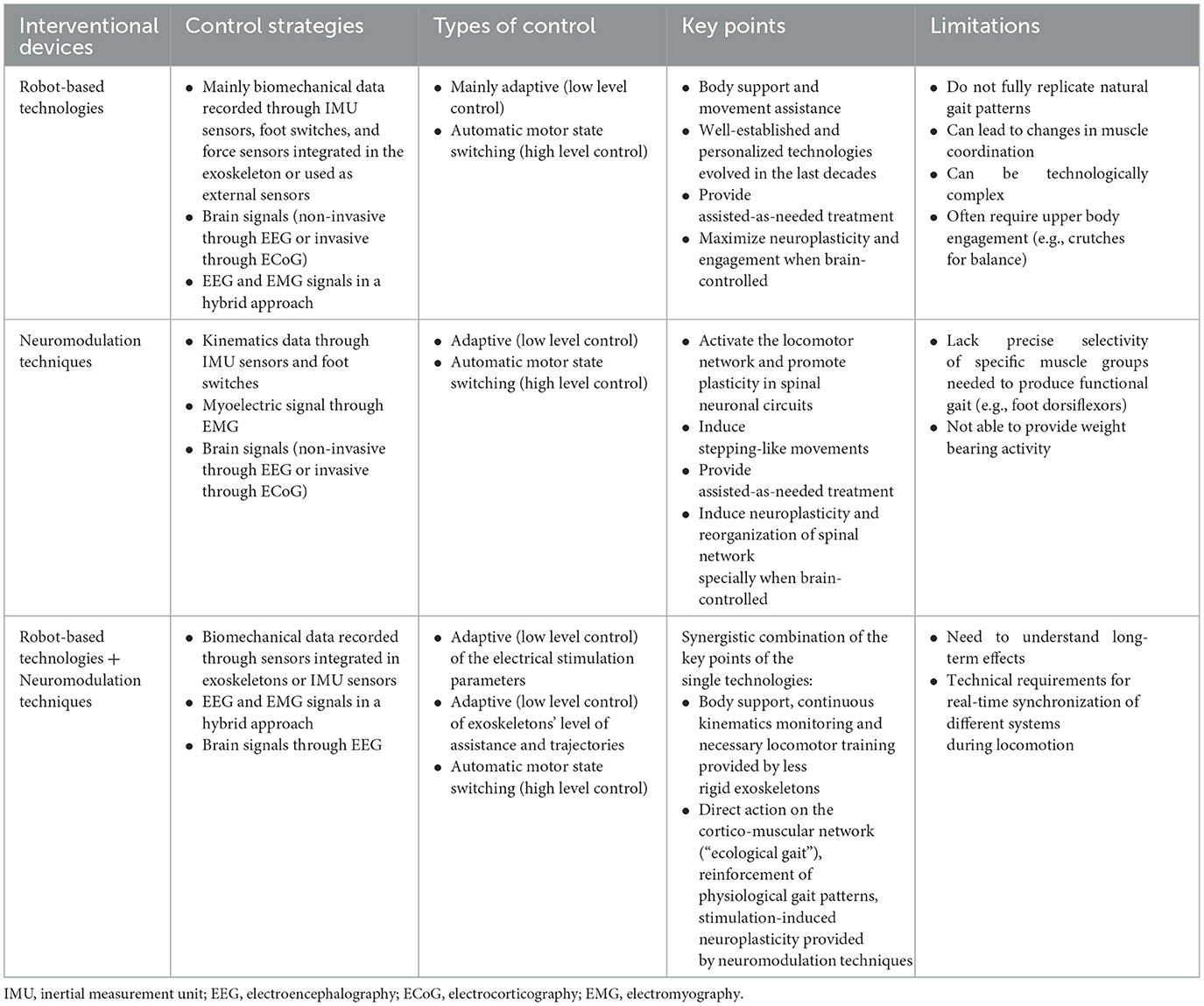
Table 1. Overview of the key points and limitations of robot-based technologies and neuromodulation techniques used separately and combined for gait recovery, with their relative control strategies and types.
The set of robotic and neuromodulation technologies employed on the same patient may change over time and can combine the rehabilitative and assistive aspects of these technologies (Hankov et al., 2025). For example, passive electrostimulation could be employed to strengthen muscles before the start of the rehabilitation, so that the residual neural drive available may be enough to produce sufficient strength. As another example, the rehabilitative path of a patient could benefit from assistive technologies to focus on rehabilitating a target function without worrying about other deficits. For instance, an ankle orthosis for foot drop could be employed when training the hip flexion for ambulation, so that the patient may focus on the rehabilitation of a single movement. When good motor outcomes have been obtained with respect to the movement target of the rehabilitation, it can be decided that it is worth/possible to undergo rehabilitation of the movement that has been passively assisted through the orthosis, Table 2.
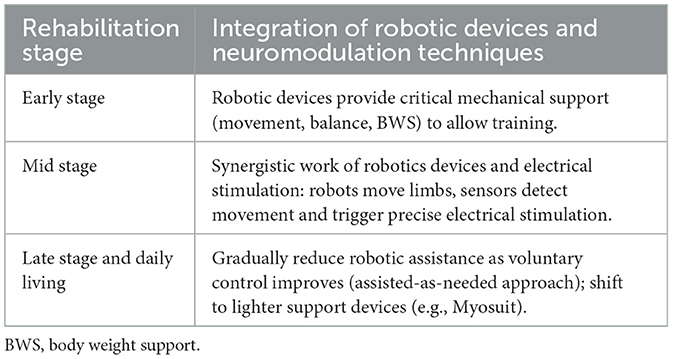
Table 2. Dynamic integration of robotic devices and neuromodulation techniques across rehabilitation stages.
4.2 Toward a multimodal framework for personalized gait recovery
Recent technological advancements have led to the definition of a large set of technology modules that can be combined according to the patient's needs, as described in Shokur et al. (2021). In such work, the idea of modularity has been introduced with the main purpose of reusing the same technology modules across different neuroprosthetic devices: for instance, Utah array electrodes to decode motor intentions from the brain (Collinger et al., 2013) or to produce tactile sensory feedback through intracortical electrical stimulation (Flesher et al., 2016), or again to be implanted into peripheral nerves of arm amputees to concurrently record movement intention decoding and stimulate sensory feedback (Wendelken et al., 2017). In the current state of rehabilitative and assistive technologies, personalization is a crucial step (Borton et al., 2013) and such modularity approach can be used to address personalization in gait recovery due to the large functional and anatomical variability between patients even inside the same condition, i.e., lesion characteristics (lesion type, area, etc.) and individual characteristics (age, gender, comorbidities, etc.). Recognizing that no single technology can address all patient needs has led to the development of multimodal frameworks where each neurotechnology module can be supported by multiple technological solutions (del-Ama et al., 2014). This modularity principle is at the core of MoBI, and allows to exploit the whole hierarchical organization of the neuromusculoskeletal system to extract more efficiently relevant biomarkers of a patient's level of impairment and exploits such information as motor commands to control and adapt the interventional device. It also inspires the creation of hybrid neuroprosthetic approaches, where multiple interventional strategies can coexist and be adapted based on the quantitative patient profile provided by MoBI to address their needs. This synergistic integration allows for personalized therapy, maximizing functional recovery and providing the patient with an intervention that resembles as much as possible the physiology of movement. Indeed, walking is a dynamic process requiring continuous integration of motor commands and sensory feedback. To replicate physiological gait control in individuals with motor impairments, an effective strategy should involve the combination of high-level (i.e., automatic motor switching) and low-level (i.e., adaptive control) control mimicking what happens during physiological movement. In healthy individuals, the brain initiates movement intentions, while spinal circuits and muscle synergies handle detailed execution without conscious effort. Recreating this hierarchical control structure in rehabilitation devices enhances both naturalness and efficacy. Therefore, high-level decoding should rely on brain signals such as EEG to capture movement intentions and drive motor state transitions (e.g., gait initiating walking). These intentions occur at relatively slow temporal scales and can trigger specific motor programs (He et al., 2018a). While low-level adaptation should rely on signals from EMG and wearable motion sensors, reflecting the spinal and muscular execution of movement (Boonstra et al., 2015; Zipser-Mohammadzada et al., 2023), and ensure physiological coordination, adjust device assistance dynamically, and counteract pathological movement patterns, all without requiring direct patient control. This dual-level framework enables closed-loop devices to simultaneously promote neuroplasticity, personalize support according to residual abilities, and facilitate challenging yet feasible rehabilitation.
Indeed, brain control has demonstrated its efficiency in controlling, in a natural way, assistive devices for gait while promoting neuroplasticity for motor recovery (Belda-Lois et al., 2011). When combined in a multimodal approach with additional physiological measures, such as monitoring functional and dysfunctional muscle patterns or comparing reference kinematics with actual joint angles, it transforms into a personalized and optimized rehabilitation tool (Selfslagh et al., 2019).
Finally, regular multimodal assessments along the rehabilitation path allow for dynamic adjustment of control parameters and contribution of each interventional strategy (i.e., robotic devices and neuromodulation) as the patient progresses based on objective and quantitative biomarkers. This supports longitudinal, personalized therapy (Coscia et al., 2019). Figure 3 shows a schematic representation of the multimodal framework for gait recovery based on the synergistic integration of MoBI, robot-based technologies, and neuromodulation techniques. However, the long-term effects of such approach need more research to translate these technologies into viable clinical solutions.
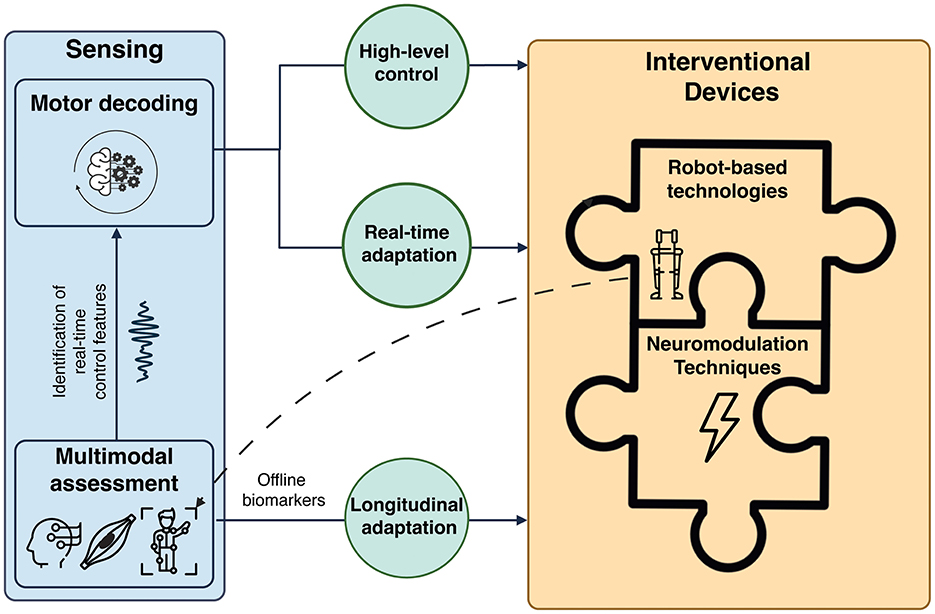
Figure 3. Multimodal framework for motor recovery based on the synergistic integration of mobile brain/body imaging (MoBI), robot-based technologies, and neuromodulation techniques. Multimodal data (i.e., brain, muscle, and kinematics signals) are collected during assessment sessions and used to identify real-time control features to use for the high-level control of the interventional technologies (automatic motor state switching) and the online adaptation of the interventional parameters (i.e., stimulation parameters, exoskeletons' levels of assistance and trajectories) based on patient's intention and residual motor activity. Exoskeletons and powered orthoses, thanks to their integrated sensors, can also be used to record kinematics data. Robot-assisted technologies and neuromodulation devices can be used in a complementary fashion as different components of an integrated interventional technology. The contribution of each single technology and its control features can be adjusted along the rehabilitation path (longitudinal adaptation) based on offline biomarkers of patient's level of impairment, providing personalized rehabilitative treatments.
4.3 Technical requirements to couple MoBI systems with interventional devices
The development of multimodal closed-loop systems capable of translating from laboratory environments to clinical and home settings presents several important challenges. First, non-invasive sensing methods should be preferred to invasive ones when possible, as they enable recordings over broader areas, providing a global picture of the patient's status and avoiding risks associated with surgical procedures. However, non-invasive technologies exhibit some disadvantages with respect to fully implantable technologies. For example, they are more sensitive to artifacts, even though they provide neuromechanical biomarkers with robustness similar to or higher than conventional clinical scales (Garro et al., 2021). Additionally, despite procedures such as sensor placement and calibration can challenge their adoption, particularly for users with motor impairments or limited caregiver support, several technological advancements have been introduced in the last decade to mitigate this problem. Dry EEG systems (Kleeva et al., 2024) or fully wireless systems (Niso et al., 2023) can facilitate quick and reliable brain data acquisition (Song and Nordin, 2021). Similarly, EMG and inertial sensors must be designed to tolerate minor placement errors and provide consistent data, even when positioned by patients. Medium-density EMG sleeves and armbands improve everyday usability, reducing sensitivity to placement errors even when positioned by patients (Tan et al., 2012; Artoni et al., 2019). Finally, sensors integrated into powered orthoses can be used for data collection in home and community settings for extensive periods of time (Bonato et al., 2024).
Moreover, the reliability of neural signals is often compromised by movement artifacts during ambulatory tasks, which can decrease decoding accuracy and affect user safety (Gorjan et al., 2022). Therefore, real-time artifact mitigation and signal processing are critical for effective deployment in real-world settings, requiring preservation of the optimal trade-off between movement classification accuracy and processing speed (De Seta et al., 2022). A multimodal approach offers a promising strategy to address the limitations of EEG-based control by leveraging additional sources of residual motor activity to improve decoding robustness. Furthermore, employing brain signals primarily for high-level control, such as triggering sequences of motor commands mimicking the physiology of movement, reduces the need for rapid, continuous signal decoding. Instead of requiring the detection of fast dynamics, such as single gait phases, this strategy enables the use of more complex processing techniques than simple filters to remove physiological artifacts, such as moving averages, thresholding of meaningful parameters (e.g., channel variance) and wavelet transform, which can improve signal reliability and reduce sensitivity to transient artifacts (Tariq et al., 2018).
Finally, real-time processing with minimal latency is often required, for example, to prevent falls or unsafe movements during overground walking (Vinoj et al., 2019), particularly during daily life activities, where patients engage different terrains and can be perturbed through interactions with the surrounding environment. Closed-loop systems require being time-locked to motor intentions and efforts, and thus, a precise time synchronization across multiple devices should be granted to avoid issues like jitter and latency, ensuring reliable solutions (Artoni et al., 2017; Iwama et al., 2024). Finally, integrated interventional devices should require minimal recalibration across different sessions, with dedicated optimization sessions in clinical settings ensuring their effective and independent use in real-world scenarios (Hankov et al., 2025). These considerations highlight broader challenges related to real-world usability, underscoring the need for future research to focus on overcoming technical, practical, and usability challenges. Addressing them will be essential to advance multimodal closed-loop systems toward becoming viable clinical and everyday solutions for gait rehabilitation and assistance.
Implementing such a multimodal approach requires the presence of multidisciplinary teams within rehabilitation clinics, comprising clinicians, engineers, and physiotherapists to ensure effective integration and individualized patient care. Moreover, there is a need for technological platforms that are easy to use by non-expert users, such as clinicians and patients, and that can seamlessly adapt and integrate different interventional technology modules.
5 Conclusions
The physiological recovery of lower limb functions is a priority after events such as a stroke or a SCI. Multimodal approaches integrating in a synergistic way different interventional and control strategies have the potential to address the challenges related to the reinstatement of complex motor behaviors. While some studies have already explored this integration, with this narrative review, we aim to contribute to the outline of a practical framework for designing personalized rehabilitative treatments that leverage patients' residual motor activity. Advances in mobile brain/body imaging, electrical stimulation, and powered orthoses open promising avenues for modular, cost-effective neuroprostheses that adapt to the patient's specific needs. These flexible systems can decode motor intention, monitor residual motor activity, and address pathological movement patterns in real-time, promoting neuroplasticity and facilitating more natural and efficient recovery processes.
With this approach, patients can be supported from the early stages of their motor disorders through an integrated technology that delivers intensive and safe rehabilitation. This is achieved by combining body support, continuous kinematic monitoring, and essential locomotor training through less rigid exoskeletons, together with direct modulation of the cortico-muscular network and reinforcement of physiological gait patterns via stimulation-induced neuroplasticity provided by neuromodulation techniques. The modularity of this strategy enables continuous optimization and personalization of the intervention according to the patient's evolving functional motor needs across multiple clinical optimization sessions.
Thus, future efforts in gait recovery after neuromotor disorders should prioritize exploiting residual motor functions where available while leveraging neuromodulation and robotics to induce physiological muscle contractions, even in cases of complete paralysis. By reinforcing movement patterns that closely resemble physiological activation, these interventions promote structural and functional reorganization of the nervous and muscular systems. Ultimately, well-powered clinical trials are needed to evaluate the therapeutic potential of this multimodal framework and to allow its clinical translation. The development of synergistic and personalized recovery systems can transform rehabilitation, enabling patients to achieve optimized outcomes through strategies that complement one another and adapt to their individual needs. These systems hold the potential to redefine the boundaries between rehabilitation and assistance, empowering patients with a pathway toward regaining independence and mobility.
Author contributions
VdS: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. SR: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work has been partially funded by the Bertarelli Foundation and by Università Vita Salute San Raffaele through the seed funding of MINE laboratory. Open access funding by the Swiss Federal Institute of Technology in Lausanne (EPFL).
Acknowledgments
The authors sincerely thank Prof. Silvestro Micera for his guidance, inspiration, and for providing the opportunity to conduct this research in his laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, M. M., and Hicks, A. L. (2005). Spasticity after spinal cord injury. Spinal Cord 43, 577–586. doi: 10.1038/sj.sc.3101757
Anderson, M. A., Squair, J. W., Gautier, M., Hutson, T. H., Kathe, C., Barraud, Q., et al. (2022). Natural and targeted circuit reorganization after spinal cord injury. Nat Neurosci. 25, 1584–1596. doi: 10.1038/s41593-022-01196-1
Angeli, C. A., Boakye, M., Morton, R. A., Vogt, J., Benton, K., Chen, Y., et al. (2018). Recovery of over-ground walking after chronic motor complete spinal cord injury. New Engl. J. Med. 379, 1244–1250. doi: 10.1056/NEJMoa1803588
Angeli, C. A., Edgerton, V. R., Gerasimenko, Y. P., and Harkema, S. J. (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409. doi: 10.1093/brain/awu038
Artoni, F., Barsotti, A., Guanziroli, E., Micera, S., Landi, A., and Molteni, F. (2017). Effective Synchronization of EEG and EMG for mobile brain/body imaging in clinical settings. Front. Hum. Neurosci. 11:652. doi: 10.3389/fnhum.2017.00652
Artoni, F., Cometa, A., Dalise, S., Azzollini, V., Micera, S., and Chisari, C. (2023). Cortico-muscular connectivity is modulated by passive and active Lokomat-assisted Gait. Sci. Rep. 13:21618. doi: 10.1038/s41598-023-48072-x
Artoni, F., Kreipe, S., and Micera, S. (2019). “Myoelectric activity imaging and decoding with multichannel surface EMG for enhanced everyday life applicability,” in 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER) (San Francisco, CA: IEEE), 690–693.
Asselin, P. K., Avedissian, M., Knezevic, S., Kornfeld, S., and Spungen, A. M. (2016). Training persons with spinal cord injury to ambulate using a powered exoskeleton. J. Vis. Exp. 16:e54071. doi: 10.3791/54071
Atkinson, C., Lombardi, L., Lang, M., Keesey, R., Hawthorn, R., Seitz, Z., et al. (2025). Development and evaluation of a non-invasive brain-spine interface using transcutaneous spinal cord stimulation. J. NeuroEngineering Rehabil. 22:95. doi: 10.1186/s12984-025-01628-6
Awad, L. N., Bae, J., O'Donnell, K., De Rossi, S. M. M., Hendron, K., Sloot, L. H., et al. (2017). A soft robotic exosuit improves walking in patients after stroke. Sci. Transl. Med. 9:eaai9084. doi: 10.1126/scitranslmed.aai9084
Awad, L. N., Kudzia, P., Revi, D. A., Ellis, T. D., and Walsh, C. J. (2020). Walking faster and farther with a soft robotic exosuit: implications for post-stroke gait assistance and rehabilitation. IEEE Open J. Eng. Med. Biol. 1, 108–115. doi: 10.1109/OJEMB.2020.2984429
Bajd, T., Kralj, A., Štefančič, M., and Lavrač, N. (1999). Use of functional electrical stimulation in the lower extremities of incomplete spinal cord injured patients. Artif. Organs 23, 403–409. doi: 10.1046/j.1525-1594.1999.06360.x
Barroso, F. O., Torricelli, D., Molina-Rueda, F., Alguacil-Diego, I. M., Cano-de-la-Cuerda, R., Santos, C., et al. (2017). Combining muscle synergies and biomechanical analysis to assess gait in stroke patients. J. Biomech. 63, 98–103. doi: 10.1016/j.jbiomech.2017.08.006
Baud, R., Manzoori, A. R., Ijspeert, A., and Bouri, M. (2021). Review of control strategies for lower-limb exoskeletons to assist gait. J Neuroeng. Rehabil. 18:119. doi: 10.1186/s12984-021-00906-3
Beauparlant, J., van den Brand, R., Barraud, Q., Friedli, L., Musienko, P., Dietz, V., et al. (2013). Undirected compensatory plasticity contributes to neuronal dysfunction after severe spinal cord injury. Brain 136, 3347–3361. doi: 10.1093/brain/awt204
Belda-Lois, J.-M., Mena-del Horno, S., Bermejo-Bosch, I., Moreno, J. C., Pons, J. L., Farina, D., et al. (2011). Rehabilitation of gait after stroke: a review towards a top-down approach. J NeuroEng. Rehabil. 8:66. doi: 10.1186/1743-0003-8-66
Benabid, A. L., Costecalde, T., Eliseyev, A., Charvet, G., Verney, A., Karakas, S., et al. (2019). An exoskeleton controlled by an epidural wireless brain-machine interface in a tetraplegic patient: a proof-of-concept demonstration. Lancet Neurol. 18, 1112–1122. doi: 10.1016/S1474-4422(19)30321-7
Berkelman, P., Rossi, P., Lu, T., and Ma, J. (2007). “Passive orthosis linkage for locomotor rehabilitation,” in 2007 IEEE 10th International Conference on Rehabilitation Robotics (Noordwijk: IEEE), 425–431.
Beyaert, C., Vasa, R., and Frykberg, G. E. (2015). Gait post-stroke: pathophysiology and rehabilitation strategies. Neurophysiol. Clin. 45, 335–355. doi: 10.1016/j.neucli.2015.09.005
Blaya, J. A., and Herr, H. (2004). Adaptive control of a variable-impedance ankle-foot orthosis to assist drop-foot gait. IEEE Trans. Neural. Syst. Rehabil. Eng. 12, 24–31. doi: 10.1109/TNSRE.2003.823266
Bonato, P., Feipel, V., Corniani, G., Arin-Bal, G., and Leardini, A. (2024). Position paper on how technology for human motion analysis and relevant clinical applications have evolved over the past decades: striking a balance between accuracy and convenience. Gait Posture 113, 191–203. doi: 10.1016/j.gaitpost.2024.06.007
Boonstra, T. W., Danna-Dos-Santos, A., Xie, H.-B., Roerdink, M., Stins, J. F., and Breakspear, M. (2015). Muscle networks: connectivity analysis of EMG activity during postural control. Sci. Rep. 5:17830. doi: 10.1038/srep17830
Borton, D., Micera, S., Millán, J., del, R., and Courtine, G. (2013). Personalized neuroprosthetics. Sci. Transl. Med. 5, 210rv2–210rv2. doi: 10.1126/scitranslmed.3005968
Brambilla, C., Pirovano, I., Mira, R. M., Rizzo, G., Scano, A., and Mastropietro, A. (2021). Combined use of EMG and EEG techniques for neuromotor assessment in rehabilitative applications: a systematic review. Sensors 21:7014. doi: 10.3390/s21217014
Braz, G. P., Russold, M., and Davis, G. M. (2009). Functional electrical stimulation control of standing and stepping after spinal cord injury: a review of technical characteristics. Neuromodulation 12, 180–190. doi: 10.1111/j.1525-1403.2009.00213.x
Calabrò, R. S., Naro, A., Russo, M., Bramanti, P., Carioti, L., Balletta, T., et al. (2018). Shaping neuroplasticity by using powered exoskeletons in patients with stroke: a randomized clinical trial. J. Neuroeng. Rehabil. 15:35. doi: 10.1186/s12984-018-0377-8
Calabrò, R. S., Sorrentino, G., Cassio, A., Mazzoli, D., Andrenelli, E., Bizzarini, E., et al. (2021). Robotic-assisted gait rehabilitation following stroke: a systematic review of current guidelines and practical clinical recommendations. Eur. J. Phys. Rehabil. Med. 57, 460–471. doi: 10.23736/S1973-9087.21.06887-8
Capogrosso, M., Milekovic, T., Borton, D., Wagner, F., Moraud, E. M., Mignardot, J.-B., et al. (2016). A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284–288. doi: 10.1038/nature20118
Capogrosso, M., Wenger, N., Raspopovic, S., Musienko, P., Beauparlant, J., Bassi Luciani, L., et al. (2013). A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J. Neurosci. 33, 19326–19340. doi: 10.1523/JNEUROSCI.1688-13.2013
Carraro, U., Kern, H., Gava, P., Hofer, C., Loefler, S., Gargiulo, P., et al. (2015). Biology of muscle atrophy and of its recovery by FES in aging and mobility impairments: roots and by-products. Eur. J. Transl. Myol. 25, 221–230. doi: 10.4081/ejtm.2015.5272
Carraro, U., Kern, H., Gava, P., Hofer, C., Loefler, S., Gargiulo, P., et al. (2017). Recovery from muscle weakness by exercise and FES: lessons from masters, active or sedentary seniors and SCI patients. Aging Clin. Exp. Res. 29, 579–590. doi: 10.1007/s40520-016-0619-1
Catz, A., Itzkovich, M., Agranov, E., Ring, H., and Tamir, A. (1997). SCIM – spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord 35, 850–856. doi: 10.1038/sj.sc.3100504
Chaudhary, U., Birbaumer, N., and Ramos-Murguialday, A. (2016). Brain–computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 12, 513–525. doi: 10.1038/nrneurol.2016.113
Christie, B. P., Freeberg, M., Memberg, W. D., Pinault, G. J. C., Hoyen, H. A., Tyler, D. J., et al. (2017). Long-term stability of stimulating spiral nerve cuff electrodes on human peripheral nerves. J. Neuroeng. Rehabil. 14, 1–12. doi: 10.1186/s12984-017-0285-3
Collinger, J. L., Wodlinger, B., Downey, J. E., Wang, W., Tyler-Kabara, E. C., Weber, D. J., et al. (2013). High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381, 557–564. doi: 10.1016/S0140-6736(12)61816-9
Contreras-Vidal, J. L., Bortole, M., Zhu, F., Nathan, K., Venkatakrishnan, A., Francisco, G. E., et al. (2018). Neural decoding of robot-assisted gait during rehabilitation after stroke. Am. J. Phys. Med. Rehabil. 97:541. doi: 10.1097/PHM.0000000000000914
Coscia, M., Wessel, M. J., Chaudary, U., Millán, J., del, R., Micera, S., et al. (2019). Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain 142, 2182–2197. doi: 10.1093/brain/awz181
Courtine, G., Harkema, S. J., Dy, C. J., Gerasimenko, Y. P., and Dyhre-Poulsen, P. (2007). Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J. Physiol. 582, 1125–1139. doi: 10.1113/jphysiol.2007.128447
Cramer, S. C., Lastra, L., Lacourse, M. G., and Cohen, M. J. (2005). Brain motor system function after chronic, complete spinal cord injury. Brain 128, 2941–2950. doi: 10.1093/brain/awh648
Daly, J. J., and Wolpaw, J. R. (2008). Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 7, 1032–1043. doi: 10.1016/S1474-4422(08)70223-0
de Miguel-Fernández, J., Lobo-Prat, J., Prinsen, E., Font-Llagunes, J. M., and Marchal-Crespo, L. (2023). Control strategies used in lower limb exoskeletons for gait rehabilitation after brain injury: a systematic review and analysis of clinical effectiveness. J. Neuroeng. Rehabil. 20:23. doi: 10.1186/s12984-023-01144-5
de Seta, V. (2023). Re-establishing cortico-muscular communication to enhance recovery: development of a hybrid brain-computer Interface for post-stroke motor rehabilitation. Available online at: https://iris.uniroma1.it/handle/11573/1673966 (accessed January 21, 2025).
de Seta, V., Colamarino, E., Pichiorri, F., Savina, G., Patarini, F., Riccio, A., et al. (2024). Brain and muscle derived features to discriminate simple hand motor tasks for a rehabilitative BCI: comparative study on healthy and post-stroke individuals. J. Neural Eng. 21:066015. doi: 10.1088/1741-2552/ad8838
De Seta, V., Toppi, J., Colamarino, E., Molle, R., Castellani, F., Cincotti, F., et al. (2022). Cortico-muscular coupling to control a hybrid brain-computer interface for upper limb motor rehabilitation: a pseudo-online study on stroke patients. Front. Hum. Neurosci. 16:1016862. doi: 10.3389/fnhum.2022.1016862
del-Ama, A. J., Gil-Agudo, Á., Pons, J. L., and Moreno, J. C. (2014). Hybrid FES-robot cooperative control of ambulatory gait rehabilitation exoskeleton. J. Neuroeng. Rehabil. 11, 27. doi: 10.1186/1743-0003-11-27
Delianides, C., Tyler, D., Pinault, G., Ansari, R., and Triolo, R. (2020). Implanted high density cuff electrodes functionally activate human tibial and peroneal motor units without chronic detriment to peripheral nerve health. Neuromodulation 23, 754–762. doi: 10.1111/ner.13110
Dijkers, M. (1997). Quality of life after spinal cord injury: a meta analysis of the effects of disablement components. Spinal Cord 35, 829–840. doi: 10.1038/sj.sc.3100571
Dobkin, B. H. (2004). Strategies for stroke rehabilitation. Lancet Neurol. 3, 528–536. doi: 10.1016/S1474-4422(04)00851-8
Dunn, R. B., Walter, J. S., Lucero, Y., Weaver, F., Langbein, E., Fehr, L., et al. (1998). Follow-up assessment of standing mobility device users. Assist Technol. 10, 84–93. doi: 10.1080/10400435.1998.10131966
Dutta, A., Kobetic, R., and Triolo, R. J. (2008). Ambulation after incomplete spinal cord injury with EMG-triggered functional electrical stimulation. IEEE Trans. Biomed. Eng. 55, 791–794. doi: 10.1109/TBME.2007.902225
Edwards, L. C., and Layne, C. S. (2007). Effect of dynamic weight bearing on neuromuscular activation after spinal cord injury. Am. J. Phys. Med. Rehabil. 86, 499–506. doi: 10.1097/PHM.0b013e31805b764b
Eguchi, Y., Kadone, H., and Suzuki, K. (2018). Standing mobility device with passive lower limb exoskeleton for upright locomotion. IEEE/ASME Trans. Mechatron. 23, 1608–1618. doi: 10.1109/TMECH.2018.2799865
Emmens, A., van Asseldonk, E., Masciullo, M., Arquilla, M., Pisotta, I., Tagliamonte, N. L., et al. (2018). “Improving the standing balance of paraplegics through the use of a wearable exoskeleton,” in 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Enschede: IEEE), 707–712.
Eng, J. J., Levins, S. M., Townson, A. F., Mah-Jones, D., Bremner, J., and Huston, G. (2001). Use of prolonged standing for individuals with spinal cord injuries. Phys. Ther. 81, 1392–1399. doi: 10.1093/ptj/81.8.1392
Ferrarin, M., Palazzo, F., Riener, R., and Quintern, J. (2001). Model-based control of FES-induced single joint movements. IEEE Trans. Neural Syst. Rehabil. Eng. 9, 245–257. doi: 10.1109/7333.948452
Fisher, L. E., Miller, M. E., Bailey, S. N., Davis, J. A., Anderson, J. S., Murray, L. R., et al. (2008). Standing after spinal cord injury with Four-contact Nerve-Cuff electrodes for quadriceps stimulation. IEEE Trans. Neural. Syst. Rehabil. Eng. 16, 473–478. doi: 10.1109/TNSRE.2008.2003390
Flesher, S. N., Collinger, J. L., Foldes, S. T., Weiss, J. M., Downey, J. E., Tyler-Kabara, E. C., et al. (2016). Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 8, 361ra141–361ra141. doi: 10.1126/scitranslmed.aaf8083
Gad, P., Gerasimenko, Y., Zdunowski, S., Turner, A., Sayenko, D., Lu, D. C., et al. (2017). Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete Paraplegia. Front. Neurosci. 11:333. doi: 10.3389/fnins.2017.00333
Gargiulo, P., Jens Reynisson, P., Helgason, B., Kern, H., Mayr, W., Ingvarsson, P., et al. (2011). Muscle, tendons, and bone: structural changes during denervation and FES treatment. Neurol. Res. 33, 750–758. doi: 10.1179/1743132811Y.0000000007
Garro, F., Chiappalone, M., Buccelli, S., De Michieli, L., and Semprini, M. (2021). Neuromechanical biomarkers for robotic neurorehabilitation. Front. Neurorobot. 15:742163. doi: 10.3389/fnbot.2021.742163
Gennaro, F., and de Bruin, E. D. (2018). Assessing brain–muscle connectivity in human locomotion through mobile brain/body imaging: opportunities, pitfalls, and future directions. Front. Public Health 6:39. doi: 10.3389/fpubh.2018.00039
Gerasimenko, Y., Gorodnichev, R., Moshonkina, T., Sayenko, D., Gad, P., and Reggie Edgerton, V. (2015a). Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 58, 225–231. doi: 10.1016/j.rehab.2015.05.003
Gerasimenko, Y. P., Lu, D. C., Modaber, M., Zdunowski, S., Gad, P., Sayenko, D. G., et al. (2015b). Noninvasive reactivation of motor descending control after paralysis. J. Neurotrauma 32, 1968–1980. doi: 10.1089/neu.2015.4008
Gharabaghi, A. (2016). What turns assistive into restorative brain-machine interfaces? Front. Neurosci. 10:456. doi: 10.3389/fnins.2016.00456
Gollee, H., Hunt, K. J., and Wood, D. E. (2004). New results in feedback control of unsupported standing in paraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 73–80. doi: 10.1109/TNSRE.2003.822765
Gordleeva, S. Yu, Lobov, S. A., Grigorev, N. A., Savosenkov, A. O., Shamshin, M. O., et al. (2020). Real-time EEG–EMG human–machine interface-based control system for a lower-limb exoskeleton. IEEE Access 8, 84070–84081. doi: 10.1109/ACCESS.2020.2991812
Gorjan, D., Gramann, K., De Pauw, K., and Marusic, U. (2022). Removal of movement-induced EEG artifacts: current state of the art and guidelines. J. Neural. Eng. 19:011004. doi: 10.1088/1741-2552/ac542c
Gorodnichev, R. M., Pivovarova, E. A., Puhov, A., Moiseev, S. A., Savochin, A. A., Moshonkina, T. R., et al. (2012). Transcutaneous electrical stimulation of the spinal cord: a noninvasive tool for the activation of stepping pattern generators in humans. Hum. Physiol. 38, 158–167. doi: 10.1134/S0362119712020065
Granat, M. H., Ferguson, A. C. B., Andrews, B. J., and Delargy, M. (1993). The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury - observed benefits during gait studies. Spinal Cord 31, 207–215. doi: 10.1038/sc.1993.39
Green, J. B., Sora, E., Bialy, Y., Ricamato, A., and Thatcher, R. W. (1999). Cortical motor reorganization after paraplegia. Neurology 53, 736–736. doi: 10.1212/WNL.53.4.736
Hamid, S., and Hayek, R. (2008). Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: an overview. Eur. Spine J. 17, 1256–1269. doi: 10.1007/s00586-008-0729-3
Hankov, N., Caban, M., Demesmaeker, R., Roulet, M., Komi, S., Xiloyannis, M., et al. (2025). Augmenting rehabilitation robotics with spinal cord neuromodulation: a proof of concept. Sci. Robot. 10:eadn5564. doi: 10.1126/scirobotics.adn5564
Harkema, S., Gerasimenko, Y., Hodes, J., Burdick, J., Angeli, C., Chen, Y., et al. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947. doi: 10.1016/S0140-6736(11)60547-3
Haufe, F. L., Kober, A. M., Schmidt, K., Sancho-Puchades, A., Duarte, J. E., Wolf, P., et al. (2019). User-driven walking assistance: first experimental results using the MyoSuit. IEEE Int. Conf. Rehabil. Robot. 2019, 944–949. doi: 10.1109/ICORR.2019.8779375
Haufe, F. L., Schmidt, K., Duarte, J. E., Wolf, P., Riener, R., and Xiloyannis, M. (2020). Activity-based training with the Myosuit: a safety and feasibility study across diverse gait disorders. J. Neuroeng. Rehabil. 17:135. doi: 10.1186/s12984-020-00765-4
He, Y., Eguren, D., Azorín, J. M., Grossman, R. G., Luu, T. P., and Contreras-Vidal, J. L. (2018a). Brain–machine interfaces for controlling lower-limb powered robotic systems. J. Neural Eng. 15:021004. doi: 10.1088/1741-2552/aaa8c0
He, Y., Eguren, D., Luu, T. P., and Contreras-Vidal, J. L. (2017). Risk management and regulations for lower limb medical exoskeletons: a review. Med. Devices 10, 89–107. doi: 10.2147/MDER.S107134
He, Y., Luu, T. P., Nathan, K., Nakagome, S., and Contreras-Vidal, J. L. (2018b). A mobile brain-body imaging dataset recorded during treadmill walking with a brain-computer interface. Sci. Data 5:180074. doi: 10.1038/sdata.2018.74
Hendricks, H. T., van Limbeek, J., Geurts, A. C., and Zwarts, M. J. (2002). Motor recovery after stroke: a systematic review of the literature. Arch. Phys. Med. Rehabil. 83, 1629–1637. doi: 10.1053/apmr.2002.35473
Herr, H. (2009). Exoskeletons and orthoses: classification, design challenges and future directions. J. Neuroeng. Rehabil. 6:21. doi: 10.1186/1743-0003-6-21
Ho, C. H., Triolo, R. J., Elias, A. L., Kilgore, K. L., DiMarco, A. F., Bogie, K., et al. (2014). Functional electrical stimulation and spinal cord injury. Phys. Med. Rehabil. Clin. N Am. 25, 631–ix. doi: 10.1016/j.pmr.2014.05.001
Hoenig, H., Murphy, T., Galbraith, J., and Zolkewitz, M. (2001). Case study to evaluate a standing table for managing constipation. Sci. Nurs. 18, 74–77.
Hofstoetter, U. S., Freundl, B., Danner, S. M., Krenn, M. J., Mayr, W., Binder, H., et al. (2020). Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J. Neurotrauma 37, 481–493. doi: 10.1089/neu.2019.6588
Hofstoetter, U. S., Freundl, B., Lackner, P., and Binder, H. (2021). Transcutaneous spinal cord stimulation enhances walking performance and reduces spasticity in individuals with multiple sclerosis. Brain Sci. 11:472. doi: 10.3390/brainsci11040472
Hofstoetter, U. S., Hofer, C., Kern, H., Danner, S. M., Mayr, W., Dimitrijevic, M. R., et al. (2013). Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Tech. 58 Suppl 1:/j/bmte.2013.58.issue-s1-A/bmt-2013-4014/bmt-2013-4014.xml. doi: 10.1515/bmt-2013-4014
Holderbaum, W., Hunt, K. J., and Gollee, H. (2002). H∞ robust control design for unsupported paraplegic standing: experimental evaluation. Control Eng. Pract. 10, 1211–1222. doi: 10.1016/S0967-0661(02)00082-5
Hunt, K. J., Gollee, H., and Jaime, R.-P. (2001). Control of paraplegic ankle joint stiffness using FES while standing. Med. Eng. Phys. 23, 541–555. doi: 10.1016/S1350-4533(01)00089-3
Hunt, K. J., Stone, B., Negard, N.-O., Schauer, T., Fraser, M. H., Cathcart, A. J., et al. (2004). Control strategies for integration of electric motor assist and functional electrical stimulation in paraplegic cycling: utility for exercise testing and mobile cycling. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 89–101. doi: 10.1109/TNSRE.2003.819955
Insausti-Delgado, A., López-Larraz, E., Nishimura, Y., Ziemann, U., and Ramos-Murguialday, A. (2022). Non-invasive brain-spine interface: continuous control of trans-spinal magnetic stimulation using EEG. Front. Bioeng. Biotechnol. 10:975037. doi: 10.3389/fbioe.2022.975037
Ivanenko, Y., Shapkova, E. Y., Petrova, D. A., Kleeva, D. F., and Lebedev, M. A. (2023). Exoskeleton gait training with spinal cord neuromodulation. Front. Hum. Neurosci. 17:1194702. doi: 10.3389/fnhum.2023.1194702
Ivanenko, Y. P., Poppele, R. E., and Lacquaniti, F. (2009). Distributed neural networks for controlling human locomotion: Lessons from normal and SCI subjects. Brain Res. Bull. 78, 13–21. doi: 10.1016/j.brainresbull.2008.03.018
Iwama, S., Takemi, M., Eguchi, R., Hirose, R., Morishige, M., and Ushiba, J. (2024). Two common issues in synchronized multimodal recordings with EEG: Jitter and latency. Neurosci. Res. 203, 1–7. doi: 10.1016/j.neures.2023.12.003
Jackson, A., and Zimmermann, J. B. (2012). Neural interfaces for the brain and spinal cord—restoring motor function. Nat. Rev. Neurol. 8, 690–699. doi: 10.1038/nrneurol.2012.219
Jezernik, S., Colombo, G., Keller, T., Frueh, H., and Morari, M. (2003). Robotic orthosis lokomat: a rehabilitation and research tool. Neuromodulation 6, 108–115. doi: 10.1046/j.1525-1403.2003.03017.x
Jiang, N., Falla, D., d'Avella, A., Graimann, B., and Farina, D. (2010). Myoelectric control in neurorehabilitation. Crit. Rev. Biomed. Eng. 38, 381–391. doi: 10.1615/CritRevBiomedEng.v38.i4.30
Kang, Y., Ding, H., Zhou, H., Wei, Z., Liu, L., Pan, D., et al. (2018). Epidemiology of worldwide spinal cord injury: a literature review. J. Neurorestoratol. 6, 1–9. doi: 10.2147/JN.S143236
Kathe, C., Skinnider, M. A., Hutson, T. H., Regazzi, N., Gautier, M., Demesmaeker, R., et al. (2022). The neurons that restore walking after paralysis. Nature 611, 540–547. doi: 10.1038/s41586-022-05385-7
King, R. B. (1996). Quality of life after stroke. Stroke 27, 1467–1472. doi: 10.1161/01.STR.27.9.1467
Kleeva, D., Ninenko, I., and Lebedev, M. A. (2024). Resting-state EEG recorded with gel-based vs. consumer dry electrodes: spectral characteristics and across-device correlations. Front. Neurosci. 18:1326139. doi: 10.3389/fnins.2024.1326139
Kottink, A. I., Hermens, H. J., Nene, A. V., Tenniglo, M. J., van der Aa, H. E., Buschman, H. P., et al. (2007). A randomized controlled trial of an implantable 2-channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch. Phys. Med. Rehabil. 88, 971–978. doi: 10.1016/j.apmr.2007.05.002
Laffranchi, M., D'Angella, S., Vassallo, C., Piezzo, C., Canepa, M., De Giuseppe, S., et al. (2021). User-centered design and development of the modular TWIN lower limb exoskeleton. Front. Neurorobot. 15:709731. doi: 10.3389/fnbot.2021.709731
Langhorne, P., Bernhardt, J., and Kwakkel, G. (2011). Stroke rehabilitation. Lancet 377, 1693–1702. doi: 10.1016/S0140-6736(11)60325-5
Lebedev, M. A., and Nicolelis, M. A. L. (2017). Brain-machine interfaces: from basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 97, 767–837. doi: 10.1152/physrev.00027.2016
Lee, S.-H., Lee, H.-J., Shim, Y., Chang, W. H., Choi, B.-O., Ryu, G.-H., et al. (2020). Wearable hip-assist robot modulates cortical activation during gait in stroke patients: a functional near-infrared spectroscopy study. J. Neuroeng. Rehabil. 17:145. doi: 10.1186/s12984-020-00777-0
Lee, Y.-H., Yong, S. Y., Kim, S. H., Kim, J. H., Shinn, J. M., Kim, Y., et al. (2014). Functional electrical stimulation to ankle dorsiflexor and plantarflexor using single foot switch in patients with hemiplegia from hemorrhagic stroke. Ann. Rehabil. Med. 38, 310–316. doi: 10.5535/arm.2014.38.3.310
Lemos, N., Fernandes, G. L., Ribeiro, A. M., Maia-Lemos, P. S., Contiero, W., Croos-Bezerra, V., et al. (2023). Rehabilitation of people with chronic spinal cord injury using a laparoscopically implanted neurostimulator: impact on mobility and urinary, anorectal, and sexual functions. Neuromodulation 26, 233–245. doi: 10.1016/j.neurom.2022.01.010
Li, W., Ma, Y., Shao, K., Yi, Z., Cao, W., Yin, M., et al. (2024). The human–machine interface design based on sEMG and motor imagery EEG for lower limb exoskeleton assistance system. IEEE Trans. Inst. Meas. 73, 1–14. doi: 10.1109/TIM.2024.3375980
Li, Y.-A., Chen, Z.-J., He, C., Wei, X.-P., Xia, N., Gu, M.-H., et al. (2023). Exoskeleton-assisted sit-to-stand training improves lower-limb function through modifications of muscle synergies in subacute stroke survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 31, 3095–3105. doi: 10.1109/TNSRE.2023.3297737
Liu, D., Chen, W., Lee, K., Chavarriaga, R., Bouri, M., Pei, Z., et al. (2017). Brain-actuated gait trainer with visual and proprioceptive feedback. J. Neural Eng. 14:056017. doi: 10.1088/1741-2552/aa7df9
Liu, D., Chen, W., Lee, K., Chavarriaga, R., Iwane, F., Bouri, M., et al. (2018). EEG-based lower-limb movement onset decoding: continuous classification and asynchronous detection. IEEE Trans. Neural Syst. Rehabil. Eng. 26, 1626–1635. doi: 10.1109/TNSRE.2018.2855053
Lombardo, L. M., Bailey, S. N., Foglyano, K. M., Miller, M. E., Pinault, G., and Triolo, R. J. (2015). A preliminary comparison of myoelectric and cyclic control of an implanted neuroprosthesis to modulate gait speed in incomplete SCI. J. Spinal Cord Med. 38, 115–122. doi: 10.1179/2045772314Y.0000000262
López-Larraz, E., Montesano, L., Gil-Agudo, Á., Minguez, J., and Oliviero, A. (2015). Evolution of EEG motor rhythms after spinal cord injury: a longitudinal study. PLoS ONE 10:e0131759. doi: 10.1371/journal.pone.0131759
Lorach, H., Charvet, G., Bloch, J., and Courtine, G. (2022). Brain–spine interfaces to reverse paralysis. Natl. Sci. Rev. 9:nwac009. doi: 10.1093/nsr/nwac009
Lorach, H., Galvez, A., Spagnolo, V., Martel, F., Karakas, S., Intering, N., et al. (2023). Walking naturally after spinal cord injury using a brain–spine interface. Nature 618, 126–133. doi: 10.1038/s41586-023-06094-5
Marsolais, E. B., and Kobetic, R. (1987). Functional electrical stimulation for walking in paraplegia. J. Spinal Cord Med. 69:728. doi: 10.2106/00004623-198769050-00014
Matjacic, Z., and Bajd, T. (1998). Arm-free paraplegic standing. I. Control model synthesis and simulation. IEEE Trans. Rehabil. Eng. 6, 125–138. doi: 10.1109/86.681178
Matjačić, Z., Hunt, K., Gollee, H., and Sinkjaer, T. (2003). Control of posture with FES systems. Med. Eng. Phys. 25, 51–62. doi: 10.1016/S1350-4533(02)00115-7
Melo, P. L., Silva, M. T., Martins, J. M., and Newman, D. J. (2015). Technical developments of functional electrical stimulation to correct drop foot: sensing, actuation and control strategies. Clin. Biomechan. 30, 101–113. doi: 10.1016/j.clinbiomech.2014.11.007
Mignardot, J.-B., Le Goff, C. G., van den Brand, R., Capogrosso, M., Fumeaux, N., Vallery, H., et al. (2017). A multidirectional gravity-assist algorithm that enhances locomotor control in patients with stroke or spinal cord injury. Sci. Transl. Med. 9:eaah3621. doi: 10.1126/scitranslmed.aah3621
Minassian, K., Freundl, B., Lackner, P., and Hofstoetter, U. S. (2024). Transcutaneous spinal cord stimulation neuromodulates pre- and postsynaptic inhibition in the control of spinal spasticity. Cell Rep. Med. 5:101805. doi: 10.1016/j.xcrm.2024.101805
Minassian, K., Hofstoetter, U., Tansey, K., and Mayr, W. (2012). Neuromodulation of lower limb motor control in restorative neurology. Clin. Neurol. Neurosurg. 114, 489–497. doi: 10.1016/j.clineuro.2012.03.013
Minassian, K., Persy, I., Rattay, F., Dimitrijevic, M. R., Hofer, C., and Kern, H. (2007). Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 35, 327–336. doi: 10.1002/mus.20700
Minassian, K., Hofstoetter, U., and Rattay, F. (2011). “Transcutaneous lumbar posterior root stimulation for motor control studies and modification of motor activity after spinal cord injury,” in Restorative Neurology of Spinal Cord Injury, eds. M. R. Dimitrijevic, B. A. Kakulas, W. B. McKay, and G. Vrbova (Oxford University Press). doi: 10.1093/acprof:oso/9780199746507.003.0010
Moeller, T., Moehler, F., Krell-Roesch, J., DeŽman, M., Marquardt, C., Asfour, T., et al. (2023). Use of lower limb exoskeletons as an assessment tool for human motor performance: a systematic review. Sensors 23:3032. doi: 10.3390/s23063032
Moreno, J. C., Barroso, F., Farina, D., Gizzi, L., Santos, C., Molinari, M., et al. (2013). Effects of robotic guidance on the coordination of locomotion. J. Neuroeng. Rehabil. 10:79. doi: 10.1186/1743-0003-10-79
Mrachacz-Kersting, N., Jiang, N., Stevenson, A. J. T., Niazi, I. K., Kostic, V., Pavlovic, A., et al. (2016). Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J. Neurophysiol. 115, 1410–1421. doi: 10.1152/jn.00918.2015
Mrachacz-Kersting, N., Kristensen, S. R., Niazi, I. K., and Farina, D. (2012). Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J. Physiol. 590, 1669–1682. doi: 10.1113/jphysiol.2011.222851
Mrachacz-Kersting, N., Stevenson, A. J. T., Jørgensen, H. R. M., Severinsen, K. E., Aliakbaryhosseinabadi, S., Jiang, N., et al. (2019). Brain state–dependent stimulation boosts functional recovery following stroke. Ann. Neurol. 85, 84–95. doi: 10.1002/ana.25375
Nakagome, S., Luu, T. P., He, Y., Ravindran, A. S., and Contreras-Vidal, J. L. (2020). An empirical comparison of neural networks and machine learning algorithms for EEG gait decoding. Sci. Rep. 10:4372. doi: 10.1038/s41598-020-60932-4
Nas, K., Yazmalar, L., Sah, V., Aydin, A., and Öneş, K. (2015). Rehabilitation of spinal cord injuries. World J. Orthop. 6, 8–16. doi: 10.5312/wjo.v6.i1.8
Netz, Y., Argov, E., Burstin, A., Brown, R., Heyman, S. N., Dunsky, A., et al. (2007). Use of a device to support standing during a physical activity program to improve function of individuals with disabilities who reside in a nursing home. Disabil. Rehabil. Assist Technol. 2, 43–49. doi: 10.1080/17483100601143371
Niso, G., Romero, E., Moreau, J. T., Araujo, A., and Krol, L. R. (2023). Wireless EEG: a survey of systems and studies. NeuroImage 269:119774. doi: 10.1016/j.neuroimage.2022.119774
Oczkowski, W. J., and Barreca, S. (1993). The functional independence measure: its use to identify rehabilitation needs in stroke survivors. Arch. Phys. Med. Rehabil. 74, 1291–1294. doi: 10.1016/0003-9993(93)90081-K
Ortiz, M., Ferrero, L., Iáñez, E., Azorín, J. M., and Contreras-Vidal, J. L. (2020). Sensory integration in human movement: a new brain-machine interface based on gamma band and attention level for controlling a lower-limb exoskeleton. Front. Bioeng. Biotechnol. 8:735. doi: 10.3389/fbioe.2020.00735
Ortlieb, A., Bouri, M., Baud, R., and Bleuler, H. (2017). An assistive lower limb exoskeleton for people with neurological gait disorders. IEEE Int. Conf. Rehabil. Robot. 2017, 441–446. doi: 10.1109/ICORR.2017.8009287
Peckham, P. H., and Knutson, J. S. (2005). Functional electrical stimulation for neuromuscular applications. Ann. Rev. Biomed. Eng. 7, 327–360. doi: 10.1146/annurev.bioeng.6.040803.140103
Pichiorri, F., Toppi, J., de Seta, V., Colamarino, E., Masciullo, M., Tamburella, F., et al. (2023). Exploring high-density corticomuscular networks after stroke to enable a hybrid brain-computer interface for hand motor rehabilitation. J. Neuroeng. Rehabil. 20:5. doi: 10.1186/s12984-023-01127-6
Pierella, C., Pirondini, E., Kinany, N., Coscia, M., Giang, C., Miehlbradt, J., et al. (2020). A multimodal approach to capture post-stroke temporal dynamics of recovery. J. Neural Eng. 17:045002. doi: 10.1088/1741-2552/ab9ada
Piira, A., Lannem, A. M., Sørensen, M., Glott, T., Knutsen, R., Jørgensen, L., et al. (2019). Robot-assisted locomotor training did not improve walking function in patients with chronic incomplete spinal cord injury: a randomized clinical trial. J. Rehabil. Med. 51, 385–389. doi: 10.2340/16501977-2547
Pons, J. L. (2010). Rehabilitation exoskeletal robotics. IEEE Eng. Med. Biol. Mag. 29, 57–63. doi: 10.1109/MEMB.2010.936548
Popovic, D., Tomovic, R., and Schwirtlich, L. (1989). Hybrid assistive system-the motor neuroprosthesis. IEEE Trans. Biomed. Eng. 36, 729–737. doi: 10.1109/10.32105
Popovic, D. B., and Popovic, M. B. (2006). Hybrid assistive systems for rehabilitation: lessons learned from functional electrical therapy in hemiplegics. Int. Conf. IEEE Eng. Med. Biol. Soc. 2006, 2146–2149. doi: 10.1109/IEMBS.2006.259550
Popović, D. B., Sinkjær, T., and Popović, M. B. (2009). Electrical stimulation as a means for achieving recovery of function in stroke patients. Neurorehabilitation 25, 45–58. doi: 10.3233/NRE-2009-0498
Prateek, G. V., Skog, I., McNeely, M. E., Duncan, R. P., Earhart, G. M., and Nehorai, A. (2018). Modeling, detecting, and tracking freezing of gait in parkinson disease using inertial sensors. IEEE Trans. Biomed. Eng. 65, 2152–2161. doi: 10.1109/TBME.2017.2785625
Rattay, F., Minassian, K., and Dimitrijevic, M. R. (2000). Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord 38, 473–489. doi: 10.1038/sj.sc.3101039
Riener, R., Lunenburger, L., Jezernik, S., Anderschitz, M., Colombo, G., and Dietz, V. (2005). Patient-cooperative strategies for robot-aided treadmill training: first experimental results. IEEE Trans. Neural Syst. Rehabil. Eng. 13, 380–394. doi: 10.1109/TNSRE.2005.848628
Rikhof, C. J. H., Feenstra, Y., Fleuren, J. F. M., Buurke, J. H., Prinsen, E. C., Rietman, J. S., et al. (2024). Robot-assisted support combined with electrical stimulation for the lower extremity in stroke patients: a systematic review. J. Neural Eng. 21:021001. doi: 10.1088/1741-2552/ad377c
Romeni, S., Losanno, E., Emedoli, D., Albano, L., Agnesi, F., Mandelli, C., et al. (2025). High-frequency epidural electrical stimulation reduces spasticity and facilitates walking recovery in patients with spinal cord injury. Sci. Trans. Med. 17:eadp9607. doi: 10.1126/scitranslmed.adp9607
Rowald, A., Komi, S., Demesmaeker, R., Baaklini, E., Hernandez-Charpak, S. D., Paoles, E., et al. (2022). Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat. Med. 28, 260–271. doi: 10.1038/s41591-021-01663-5
Roy, R. R., Harkema, S. J., and Edgerton, V. R. (2012). Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch. Phys. Med. Rehabil. 93, 1487–1497. doi: 10.1016/j.apmr.2012.04.034
Sabut, S. K., Sikdar, C., Kumar, R., and Mahadevappa, M. (2011). Functional electrical stimulation of dorsiflexor muscle: effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. Neurorehabilitation 29, 393–400. doi: 10.3233/NRE-2011-0717
Sarasola-Sanz, A., Irastorza-Landa, N., López-Larraz, E., Bibián, C., Helmhold, F., Broetz, D., et al. (2017). A hybrid brain-machine interface based on EEG and EMG activity for the motor rehabilitation of stroke patients. IEEE Int. Conf. Rehabil. Robot. 2017, 895–900. doi: 10.1109/ICORR.2017.8009362
Sburlea, A. I., Montesano, L., Cuerda, R. C., de la, Diego, I. M. A., Miangolarra-Page, J. C., and Minguez, J. (2015). Detecting intention to walk in stroke patients from pre-movement EEG correlates. J. Neuroeng. Rehabil. 12:113. doi: 10.1186/s12984-015-0087-4
Schmidt, K., Duarte, J. E., Grimmer, M., Sancho-Puchades, A., Wei, H., Easthope, C. S., et al. (2017). The Myosuit: bi-articular anti-gravity exosuit that reduces hip extensor activity in sitting transfers. Front. Neurorobot. 11:57. doi: 10.3389/fnbot.2017.00057
Scivoletto, G., Tamburella, F., Laurenza, L., and Molinari, M. (2013). The spinal cord independence measure: how much change is clinically significant for spinal cord injury subjects. Disabil. Rehabil. 35, 1808–1813. doi: 10.3109/09638288.2012.756942
Selfslagh, A., Shokur, S., Campos, D. S. F., Donati, A. R. C., Almeida, S., Yamauti, S. Y., et al. (2019). Non-invasive, brain-controlled functional electrical stimulation for locomotion rehabilitation in individuals with paraplegia. Sci. Rep. 9:6782. doi: 10.1038/s41598-019-43041-9
Semprini, M., Lencioni, T., Hinterlang, W., Vassallo, C., Scarpetta, S., Maludrottu, S., et al. (2022). User-centered design and development of TWIN-Acta: a novel control suite of the TWIN lower limb exoskeleton for the rehabilitation of persons post-stroke. Front. Neurosci. 16:95707. doi: 10.3389/fnins.2022.915707
Shokur, S., Donati, A. R. C., Campos, D. S. F., Gitti, C., Bao, G., Fischer, D., et al. (2018). Training with brain-machine interfaces, visuo-tactile feedback and assisted locomotion improves sensorimotor, visceral, and psychological signs in chronic paraplegic patients. PLoS ONE 13:e0206464. doi: 10.1371/journal.pone.0206464
Shokur, S., Mazzoni, A., Schiavone, G., Weber, D. J., and Micera, S. (2021). A modular strategy for next-generation upper-limb sensory-motor neuroprostheses. Medicine 2, 912–937. doi: 10.1016/j.medj.2021.05.002
Simpson, L. A., Eng, J. J., Hsieh, J. T. C., and Wolfe, D. L. (2012). The health and life priorities of individuals with spinal cord injury: a systematic review. J. Neurotrauma 29, 1548–1555. doi: 10.1089/neu.2011.2226
Sköld, C., Levi, R., and Seiger, Å. (1999). Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch. Phys. Med. Rehabil. 80, 1548–1557. doi: 10.1016/S0003-9993(99)90329-5
Slade, P., Atkeson, C., Donelan, J. M., Houdijk, H., Ingraham, K. A., Kim, M., et al. (2024). On human-in-the-loop optimization of human–robot interaction. Nature 633, 779–788. doi: 10.1038/s41586-024-07697-2
Soekadar, S. R., Birbaumer, N., Slutzky, M. W., and Cohen, L. G. (2015). Brain-machine interfaces in neurorehabilitation of stroke. Neurobiol. Dis. 83, 172–179. doi: 10.1016/j.nbd.2014.11.025
Somers, M., and Bender-Burnett, J. (2024). Spinal Cord Injury: Functional Rehabilitation. F.A. Davis.
Song, S., and Nordin, A. D. (2021). Mobile electroencephalography for studying neural control of human locomotion. Front. Hum. Neurosci. 15:749017. doi: 10.3389/fnhum.2021.749017
Stampacchia, G., Gazzotti, V., Olivieri, M., Andrenelli, E., Bonaiuti, D., Calabro, R. S., et al. (2022). Gait robot-assisted rehabilitation in persons with spinal cord injury: a scoping review. Neurorehabilitation 51, 609–647. doi: 10.3233/NRE-220061
Stanic, U., Acimović-Janezic, R., Gros, N., Trnkoczy, A., Bajd, T., and Kljajić, M. (1978). Multichannel electrical stimulation for correction of hemiplegic gait. Methodology and preliminary results. Scand J. Rehabil. Med. 10, 75–92.
Stauffer, Y., Allemand, Y., Bouri, M., Fournier, J., Clavel, R., Metrailler, P., et al. (2009). The walktrainer—A new generation of walking reeducation device combining orthoses and muscle stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 38–45. doi: 10.1109/TNSRE.2008.2008288
Stefancic, M., Kralj, A., Turk, R., Bajd, T., Benko, H., and Sega, J. (1986). Neurophysiological background of the use of functional electrical stimulation in paraplegia. Electromyogr. Clin. Neurophysiol. 26, 423–435.
Stinear, C. M., Lang, C. E., Zeiler, S., and Byblow, W. D. (2020). Advances and challenges in stroke rehabilitation. Lancet Neurol. 19, 348–360. doi: 10.1016/S1474-4422(19)30415-6
Sujatha Ravindran, A., Malaya, C. A., John, I., Francisco, G. E., Layne, C., and Contreras-Vidal, J. L. (2022). Decoding neural activity preceding balance loss during standing with a lower-limb exoskeleton using an interpretable deep learning model. J. Neural Eng. 19:036015. doi: 10.1088/1741-2552/ac6ca9
Sylos-Labini, F., La Scaleia, V., d'Avella, A., Pisotta, I., Tamburella, F., Scivoletto, G., et al. (2014). EMG patterns during assisted walking in the exoskeleton. Front. Hum. Neurosci. 8:423. doi: 10.3389/fnhum.2014.00423
Tan, C. K., Kadone, H., Watanabe, H., Marushima, A., Hada, Y., Yamazaki, M., et al. (2020). Differences in muscle synergy symmetry between subacute post-stroke patients with bioelectrically-controlled exoskeleton gait training and conventional gait training. Front. Bioeng. Biotechnol. 8:770. doi: 10.3389/fbioe.2020.00770
Tan, D., Saponas, T. S., Morris, D., and Turner, J. (2012). Wearable Electromyography-Based Controllers for Human-Computer Interface. Available online at: https://patents.google.com/patent/US8170656B2/en (accessed December 23, 2024).
Tariq, M., Trivailo, P. M., and Simic, M. (2018). EEG-based BCI control schemes for lower-limb assistive-robots. Front. Hum. Neurosci. 12:312. doi: 10.3389/fnhum.2018.00312
Thibaut, A., Chatelle, C., Ziegler, E., Bruno, M.-A., Laureys, S., and Gosseries, O. (2013). Spasticity after stroke: physiology, assessment and treatment. Brain Injury 27, 1093–1105. doi: 10.3109/02699052.2013.804202
Toni, L., de Seta, V., Romeni, S., Mendez, V., Pollina, L., Emedoli, D., et al. (2024). “Decoding lower limb motor attempts from EEG signals in a spinal cord injury patient with epidural electrical implant,” in Converging Clinical and Engineering Research on Neurorehabilitation V. ICNR 2024. Biosystems & Biorobotics, vol. 32, eds. J. L. Pons, J. Tornero, and M. Akay (Cham: Springer), 68–72. doi: 10.1007/978-3-031-77584-0_14
Tortora, S., Artoni, F., Micera, S., Tonin, L., and Shokur, S. (2023). Editorial: hybrid brain-robot interfaces for enhancing mobility. Front. Neurorobot. 17:1264045. doi: 10.3389/fnbot.2023.1264045
Tortora, S., Ghidoni, S., Chisari, C., Micera, S., and Artoni, F. (2020a). Deep learning-based BCI for gait decoding from EEG with LSTM recurrent neural network. J. Neural Eng. 17:046011. doi: 10.1088/1741-2552/ab9842
Tortora, S., Tonin, L., Chisari, C., Micera, S., Menegatti, E., and Artoni, F. (2020b). Hybrid human-machine interface for gait decoding through bayesian fusion of EEG and EMG classifiers. Front. Neurorobot. 14:582728. doi: 10.3389/fnbot.2020.582728
Triolo, R. J., Bailey, S. N., Miller, M. E., Rohde, L. M., Anderson, J. S., Davis, J. A., et al. (2012). Longitudinal performance of a surgically implanted neuroprosthesis for lower-extremity exercise, standing, and transfers after spinal cord injury. Arch. Phys. Med. Rehabil. 93, 896–904. doi: 10.1016/j.apmr.2012.01.001
Tyson, S. F., and Rogerson, L. (2009). Assistive walking devices in nonambulant patients undergoing rehabilitation after stroke: the effects on functional mobility, walking impairments, and patients' opinion. Arch. Phys. Med. Rehabil. 90, 475–479. doi: 10.1016/j.apmr.2008.09.563
Vaid, S., Singh, P., and Kaur, C. (2015). “EEG signal analysis for BCI interface: a review,” in 2015 Fifth International Conference on Advanced Computing & Communication Technologies, Haryana, India (IEEE Xplore), 143–147. doi: 10.1109/ACCT.2015.72.
van Dijsseldonk, R. B., van Nes, I. J. W., Geurts, A. C. H., and Keijsers, N. L. W. (2020). Exoskeleton home and community use in people with complete spinal cord injury. Sci. Rep. 10:15600. doi: 10.1038/s41598-020-72397-6
van Hedel, H. J. A., and Dietz, V. (2010). Rehabilitation of locomotion after spinal cord injury. Restor. Neurol. Neurosci. 28, 123–134. doi: 10.3233/RNN-2010-0508
Veerbeek, J. M., Wegen, E., van, Peppen, R., van, Wees, P. J., van der, Hendriks, E., Rietberg, M., et al. (2014). What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 9:e87987. doi: 10.1371/journal.pone.0087987
Vinoj, P. G., Jacob, S., Menon, V. G., Rajesh, S., and Khosravi, M. R. (2019). Brain-controlled adaptive lower limb exoskeleton for rehabilitation of post-stroke paralyzed. IEEE Access 7, 132628–132648. doi: 10.1109/ACCESS.2019.2921375
Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2020). Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141, e139–e596. doi: 10.1161/CIR.0000000000000757
Vodovnik, L., Crochetiere, W. J., and Reswick, J. B. (1967). Control of a skeletal joint by electrical stimulation of antagonists. Med. and biol. Engng. 5, 97–109. doi: 10.1007/BF02474498
Vouga, T., Fasola, J., Baud, R., Manzoori, A. R., Pache, J., and Bouri, M. (2022). TWIICE One powered exoskeleton: effect of design improvements on usability in daily life as measured by the performance in the CYBATHLON race. J. Neuroeng. Rehabil. 19:63. doi: 10.1186/s12984-022-01028-0
Vromans, M., and Faghri, P. D. (2018). Functional electrical stimulation-induced muscular fatigue: effect of fiber composition and stimulation frequency on rate of fatigue development. J. Electromyogr. Kinesiol. 38, 67–72. doi: 10.1016/j.jelekin.2017.11.006
Wagner, F. B., Mignardot, J.-B., Le Goff-Mignardot, C. G., Demesmaeker, R., Komi, S., Capogrosso, M., et al. (2018). Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71. doi: 10.1038/s41586-018-0649-2
Wagner, J., Solis-Escalante, T., Grieshofer, P., Neuper, C., Müller-Putz, G., and Scherer, R. (2012). Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. NeuroImage 63, 1203–1211. doi: 10.1016/j.neuroimage.2012.08.019
Wahlgren, C., Levi, R., Thorell, O., Amezcua, S., and Thordstein, M. (2021). Prevalence of discomplete sensorimotor spinal cord injury as evidenced by neurophysiological methods: a cross-sectional study. J. Rehabil. Med. 53, jrm00156–jrm00156. doi: 10.2340/16501977-2774
Walter, J. S., Sola, P. G., Sacks, J., Lucero, Y., Langbein, E., and Weaver, F. (1999). Indications for a home standing program for individuals with spinal cord injury. J. Spinal Cord Med. 22, 152–158. doi: 10.1080/10790268.1999.11719564
Wendelken, S., Page, D. M., Davis, T., Wark, H. A. C., Kluger, D. T., Duncan, C., et al. (2017). Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah Slanted Electrode Arrays (USEAs) implanted in residual peripheral arm nerves. J. Neuroeng. Rehabil. 14:121. doi: 10.1186/s12984-017-0320-4
Westgren, N., and Levi, R. (1998). Quality of life and traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 79, 1433–1439. doi: 10.1016/S0003-9993(98)90240-4
Wolpaw, J., and Wolpaw, E. W. (2012). Brain-Computer Interfaces: Principles and Practice. Oxford University Press. doi: 10.1093/acprof:oso/9780195388855.001.0001
Wong, C. K., Bishop, L., and Stein, J. (2012). A wearable robotic knee orthosis for gait training: a case-series of hemiparetic stroke survivors. Prosthet. Orthot. Int. 36, 113–120. doi: 10.1177/0309364611428235
Yan, T., Cempini, M., Oddo, C. M., and Vitiello, N. (2015). Review of assistive strategies in powered lower-limb orthoses and exoskeletons. Robot. Auton. Syst. 64, 120–136. doi: 10.1016/j.robot.2014.09.032
Yan, T., Hui-Chan, C. W. Y., and Li, L. S. W. (2005). Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke. Stroke 36, 80–85. doi: 10.1161/01.STR.0000149623.24906.63
Zhou, C., Yang, Z., Li, K., and Ye, X. (2022). Research and development of ankle–foot orthoses: a review. Sensors 22:6596. doi: 10.3390/s22176596
Zipser-Mohammadzada, F., Scheffers, M. F., Conway, B. A., Halliday, D. M., Zipser, C. M., Curt, A., et al. (2023). Intramuscular coherence enables robust assessment of modulated supra-spinal input in human gait: an inter-dependence study of visual task and walking speed. Exp. Brain Res. 241, 1675–1689. doi: 10.1007/s00221-023-06635-4
Keywords: gait recovery, robotic assistance, neuromodulation, multimodal approach, closed-loop technologies, MoBI, neuromotor disorders, personalized therapy
Citation: de Seta V and Romeni S (2025) Multimodal closed-loop strategies for gait recovery after spinal cord injury and stroke via the integration of robotics and neuromodulation. Front. Neurosci. 19:1569148. doi: 10.3389/fnins.2025.1569148
Received: 31 January 2025; Accepted: 26 May 2025;
Published: 17 July 2025.
Edited by:
Mikhail A. Lebedev, Lomonosov Moscow State University, RussiaReviewed by:
Ricardo Siu, Case Western Reserve University, United StatesNinad Saraf, Integrated Rehabtech Systems Private Limited, India
Copyright © 2025 de Seta and Romeni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria de Seta, dmFsZXJpYS5kZXNldGFAZXBmbC5jaA==
 Valeria de Seta
Valeria de Seta Simone Romeni
Simone Romeni