- 1Laboratory of Neurophysiology of the Multidisciplinary Institute of Cell Biology [IMBICE, Argentine Research Council (CONICET) and Scientific Research Commission, Province of Buenos Aires (CIC-PBA), National University of La Plata], La Plata, Buenos Aires, Argentina
- 2Department of Surgical Sciences, Functional Pharmacology and Neuroscience, University of Uppsala, Uppsala, Sweden
The liver is recognized for its central role in energy metabolism, yet emerging evidence highlights its function as an endocrine organ, secreting a variety of proteins—hepatokines—that influence distant tissues. Hepatokines not only regulate metabolic processes by acting on peripheral tissues but also exert direct effects on brain function. In this mini-review, we discuss the existing literature on the role of “brain-acting” hepatokines including IGF-1, FGF21, LEAP2, GDF15, and ANGPTLs, and their impact on energy balance and metabolism. We review the existing evidence regarding their roles in metabolism through their action in the brain, and their potential implications in metabolic disturbances. By integrating insights from recent studies, we aim to provide a comprehensive understanding of how liver-derived signals can modulate energy balance and metabolism.
Introduction
Energy metabolism encompasses the complex biochemical pathways by which organisms extract, convert, and store energy from nutrients such as lipids, carbohydrates, and proteins. The energy obtained from nutrients is expended in numerous physiological processes (i.e., resting, activity-induced and diet-induced energy expenditure), and the difference between the energy obtained and the energy expended makes up energy balance. When the amount of energy obtained continuously surpasses the amount expended, there is an excessive storage of energy that ends with an increase in body weight, leading to overweight and obesity. In recent decades, the world has witnessed an alarming rise in the prevalence of overweight and obesity. These conditions are hallmarked by persistent energy imbalance and profound metabolic dysregulation, and they now represent major public health challenges. Obesity, in particular, is a well-established risk factor for a spectrum of metabolic diseases, most notably type 2 diabetes mellitus (T2DM; Schnurr et al., 2020) and metabolic-associated steatotic liver disease (MASLD; Younossi et al., 2016). While lifestyle and environmental factors play critical roles, growing evidence highlights the contribution of endogenous signals in the development and maintenance of these metabolic disorders. Among these, liver-derived peptides—collectively known as hepatokines—have recently emerged as key regulators of systemic metabolism (Zhang et al., 2023). Not only do hepatokines influence peripheral tissues, but a subset also communicates directly with the brain to modulate metabolism, appetite, and energy expenditure. Here, we aim to synthesize current knowledge on brain-acting hepatokines (Figure 1), emphasizing their emerging roles as metabolic integrators and potential therapeutic targets in the context of obesity and related disorders.
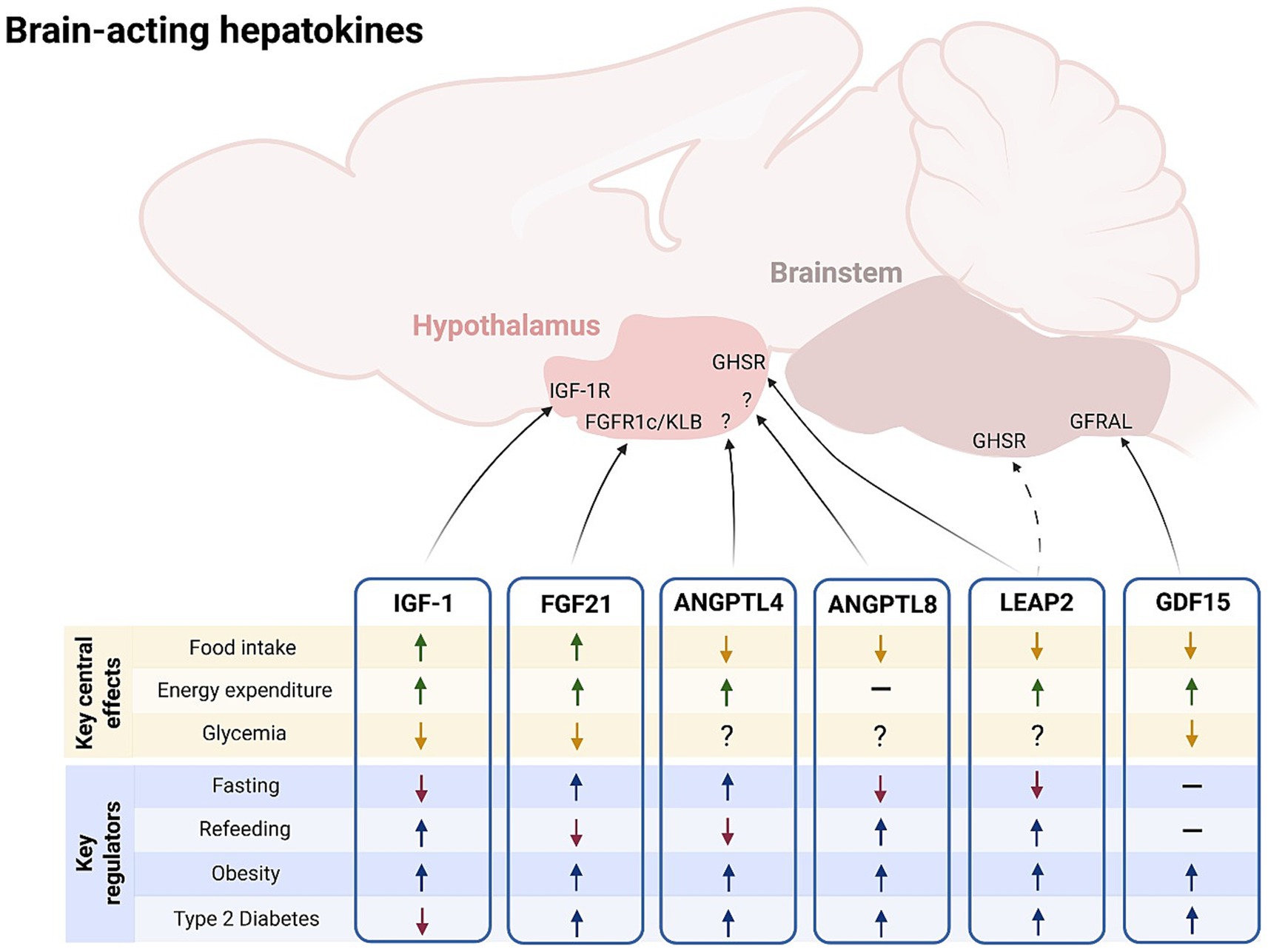
Figure 1. Brain-acting hepatokines: key central effects and key regulators. Liver-derived hepatokines FGF21, IGF-1, LEAP2, GFD15, ANGPTL4 and ANGPTL8 act on the central nervous system to modulate energy balance and metabolism (key central effects). Circulating levels of these brain-acting hepatokines vary according to the energetic status and in certain metabolic disorders (key regulators). ANGPTL, angiopoietin-like protein; FGF21, fibroblast growth factor 21; GDF15, growth differentiation factor 15; IGF-1, insulin-like growth factor 1; LEAP2, liver-expressed antimicrobial peptide 2. Figure created under the license of BioRENDER (https://BioRender.com/zelorai, license number: SH285C03YT).
Liver-to-brain communication
The liver plays a central role in regulating multiple aspects of energy metabolism, acting as a metabolic hub that coordinates nutrient processing, glycogen storage, lipid synthesis, glucose and lipid homeostasis, and hormonal signaling in response to feeding and fasting. The liver also has a predominant endocrine function since up to 40% of liver transcripts encode secreted peptides with multiple regulatory functions (Uhlén et al., 2015). Hepatocytes, the main cellular type of the liver, produce and secrete a variety of peptides called hepatokines, which serve as key mediators through which the liver communicates metabolic information to distant tissues, including the brain. In Table 1, we listed the main recognized hepatokines, indicated their receptors or target systems and organs, and also cited evidence implicating each hepatokine in the modulation of metabolism and energy balance.
Hepatokines are one of the ways by which the liver and the brain communicate. The liver also receives sympathetic and parasympathetic innervations that allow the brain to regulate liver function, and also the liver to convey metabolic information to the brain. Of note, multisynaptic brain projections to the liver comprise hypothalamic and medullary brain regions (Stanley et al., 2010), and sensory information from the hepatic portal vein reaches medullary brain centers through vagal afferents (Zsombok et al., 2024; Garcia-Luna et al., 2021). Interestingly, pharmacogenetic and optogenetic manipulation of specific hypothalamic neuronal populations modulates the expression of hepatic enzymes involved in glucose homeostasis (Kwon et al., 2020; Coutinho et al., 2017).
Hepatokines are constitutively secreted into circulation and reach distant tissues, including the brain, to modulate multiple aspects of metabolism (Schulze et al., 2019). Like other circulating factors, hepatokines can access the brain through distinct mechanisms: by crossing the blood–brain barrier, by diffusing through fenestrated capillaries in specialized brain regions, or by crossing the blood-cerebrospinal fluid (CSF) barrier constituted by hypothalamic tanycytes and choroid plexus cells (Rawal et al., 2022). In the brain, two essential hubs in the control of metabolism are the hypothalamus and the medulla. In the hypothalamus, the arcuate nucleus (ARH) plays a pivotal role in sensing circulating factors and transmitting peripheral information to other hypothalamic and extra-hypothalamic nuclei. The ARH comprises different neuronal subtypes (Campbell et al., 2017), including those co-expressing neuropeptide Y (NPY) and agouti-related peptide (AgRP), and neurons co-expressing pro-opiomelanocortin (POMC) and cocaine-and amphetamine-regulated transcript. Together with the ARH, the hypothalamic paraventricular (PVH), dorsomedial and ventromedial nuclei, and also the lateral hypothalamic area are all interconnected and form a neural network with key regulatory roles in metabolism (Jais and Brüning, 2022). In the medulla, the dorsal vagal complex (DVC)–comprising the dorsal motor nucleus of the vagus, the area postrema (AP), and the nucleus of the solitary tract (NTS)–contains multiple neuronal populations that are crucial for interpreting and relaying peripheral signals to the hypothalamus, and also to elicit autonomic responses via the vagus nerve (Abdalla, 2017). Emerging evidence suggests that hepatokines can act on these brain regions directly or indirectly, influencing central pathways that control metabolism, food intake, and energy expenditure.
Brain-acting hepatokines and metabolism
Insulin-like growth factor 1 (IGF-1)
IGF-1 is a peptide with structural homology to insulin produced in the liver, where its production is stimulated by growth hormone (GH), and is strongly implicated in the modulation of cell growth and differentiation. Plasma IGF-1 is bound to one of the six IGF-1 binding proteins (IGF-1BP), which modulate its effects. IGF-1 production is regulated by nutrient availability, with hepatic IGF-1 mRNA and plasma levels declining with fasting and increasing with refeeding (Straus and Takemoto, 1990; Clemmons, 2012). IGF-1 mainly signals through IGF-1 receptor (IGF-1R), which is ubiquitously expressed in peripheral organs and the brain. The net effect of IGF-1 is determined by the modulation of IGF-1 production, IGF-1BPs levels, and IGF-1R expression. We recommend consulting in-depth reviews for a comprehensive understanding of these regulatory mechanisms (Yuen et al., 2024; Clemmons, 2018). For instance, fasting decreases plasma IGF-1 levels in healthy subjects (Rahmani et al., 2019), whereas altered plasma IGF-1BPs levels are observed in obese patients, producing a subtle increase in free IGF-1 (Clemmons, 2012). Also, disturbed levels of circulating IGF-BPs and free IGF-1 are detected in T2DM patients, with changes depending on the progression of the pathology (Clemmons, 2018).
IGF-1 is produced in the brain during development, and some studies have shown that central IGF-1 modulates energy metabolism and energy balance in adulthood. Intra-cerebro-ventricular (ICV) IGF-1 injection lowers hepatic glucose production in hyperinsulinemic-clamped mice (Muzumdar et al., 2006) and improves insulin sensitivity of aged rats (Huffman et al., 2016). ICV IGF-1 treatment in mice increases food intake and enhances insulin sensitivity (Hong et al., 2017). Moreover, deletion of IGF-1R from kisspeptin-expressing neurons produces a decrease in food intake and an increase in energy expenditure exclusively in female mice (Wang et al., 2024). However, partial deletion of neuronal IGF-1R causes subtle metabolic phenotypes, including higher circulating triglycerides and free fatty acids and a moderate hyperglycemia (Kappeler et al., 2008). Thus, the complexity of the IGF-1 system makes it difficult to dissect a specific modulatory role for central IGF-1 on energy balance and metabolism.
Fibroblast growth factor 21 (FGF21)
FGF21 belongs to the fibroblast growth factor family, a group of proteins involved in regulating multiple processes such as angiogenesis and embryonic development. FGF21 decreases blood glucose and improves insulin sensitivity in diabetic rodents (Kharitonenkov et al., 2005). FGF21 mRNA levels increase in the liver of high-sucrose diet consuming and diet-induced obese (DIO) mice (Fisher et al., 2010; Maekawa et al., 2017). Circulating FGF21 levels increase in fasted mice (Markan et al., 2014), in mice chronically fed high-sucrose diets (Maekawa et al., 2017), in DIO mice (Fisher et al., 2010), and in a mouse model of T2DM (Spolcová et al., 2014). In humans, circulating FGF21 increases during fasting (Fazeli et al., 2015). Also, circulating FGF21 is increased in overweight/obese individuals (Zhang et al., 2008; Ďurovcová et al., 2010), and in patients with MASLD (Dushay et al., 2010) and T2DM (Cheng et al., 2011), and in women with gestational diabetes (Tan et al., 2013). Moreover, plasma levels of FGF21 increase after gastric sleeve surgery (Al-Regaiey et al., 2024), whereas a decrease is detected in individuals with obesity/overweight and MASLD after weight loss (Erdem et al., 2024).
FGF21 is primarily produced in the liver and regulates energy balance and metabolism by acting on the brain, particularly in the hypothalamus (Hsuchou et al., 2007; Liang et al., 2014). In humans, FGF21 was detected in the CSF (Tan et al., 2013; Tan et al., 2011; Li et al., 2016). FGF21 acts via the FGF receptor 1c (FGFR1c), which is widely distributed in the mouse brain, whereas its co-receptor Klotho-β (KLB, (Ogawa et al., 2007)) is specifically present in the hypothalamic suprachiasmatic nucleus, the DVC, and also in the amygdala (Bookout et al., 2013; Bono et al., 2022; Claflin et al., 2022). KLB genetic deletion in hypothalamic neurons abolishes the reductions in body weight and plasma insulin observed in FGF21-overexpressing mice (Bookout et al., 2013). Moreover, hypothalamic KLB deletion blunts the increase in food consumption and energy expenditure and the decrease in plasma glucose and cholesterol observed in DIO FGF21-overexpressing mice (Owen et al., 2014). Interestingly, pharmacogenetic activation of KLB-expressing neurons increases energy expenditure and decreases body weight of DIO mice, and genetic deletion of KLB from glutamatergic neurons prevents FGF21 effects on energy expenditure and body weight (Claflin et al., 2022).
Studies performing central infusions of FGF21 also demonstrate its central effects. Chronic ICV injection of FGF21 improves insulin sensitivity of lean and DIO rats (Sarruf et al., 2010), whereas FGF21 ICV administrations lower body weight, percent body fat, and plasma glucose and cholesterol concentrations of DIO mice, effects dependent on hypothalamic KLB expression (Owen et al., 2014). Interestingly, ICV FGF21 treatment induces the expression of thermogenic genes and also increases sympathetic nerve activity in brown adipose tissue of DIO mice, both effects dependent on hypothalamic KLB expression (Owen et al., 2014). Moreover, ICV FGF21 administration to hypoglycemic FGF21-deficient mice normalizes their glycemia, which depends on the presence of FGFR1c in the PVH (Liang et al., 2014). Furthermore, ICV FGF21 administrations increase ERK1/2 expression in the mouse hypothalamus (Yang et al., 2012), whereas intra-PVH FGF21 injection increases ERK1/2 and CREB phosphorylation (Liang et al., 2014). Thus, the literature indicates that FGF21 impacts and depends on the activity of hypothalamic brain nuclei to modulate energy balance and metabolism, which supports the notion of FGF21 as a brain-acting hepatokine.
Liver-expressed antimicrobial peptide 2 (LEAP2)
LEAP2 was recently described as a ligand of the GH secretagogue receptor (GHSR) (Ge et al., 2018; M’Kadmi et al., 2019), which triggered a great interest on this hepatokine (Perelló, 2025). LEAP2 mRNA is predominantly found in liver hepatocytes and jejunal enterocytes of both mice and humans (Ge et al., 2018; Krause et al., 2003; Englund et al., 2024). LEAP2 is secreted via the constitutive secretory pathway, thereby its production is controlled at the gene expression level, with metabolic status as a key regulatory factor (Perelló, 2025). Liver LEAP2 mRNA levels decrease with fasting (Holm et al., 2022; Islam et al., 2020), and increase in DIO mice (Holm et al., 2022). Interestingly, liver LEAP2 mRNA decreases in mice fed with a ketogenic diet (Holm et al., 2022), whereas it increases in mice orally administered with glucose and corn oil (Islam et al., 2024). Circulating LEAP2 levels in mice decrease with fasting (Ge et al., 2018; Islam et al., 2020; Mani et al., 2019; Fernandez et al., 2022), whereas plasma LEAP2 is increased in DIO (Mani et al., 2019; Holá et al., 2023; Casado et al., 2024) and ob/ob mice (Lugilde et al., 2022), and in a mouse model of type 1 diabetes mellitus (T1DM) (Mani et al., 2019). Interestingly, LEAP2-overexpressing mice show enhanced body weight loss and impaired maintenance of glycemia under caloric restriction (Ge et al., 2018). Conversely, female LEAP2-deficient mice display increased food intake and enhanced body weight gain when fed a high fat diet (HFD) (Shankar et al., 2021). In humans, obese adults and children show increased plasma LEAP2 levels (Holm et al., 2022; Mani et al., 2019; Andreoli et al., 2024; Fittipaldi et al., 2020), which positively correlate with body mass index, percentage of body fat, and homeostatic model assessment of insulin resistance, among other parameters (Mani et al., 2019; Stark et al., 2023). Circulating LEAP2 levels are also increased and positively correlate with glycosylated hemoglobin in T2DM patients (Li et al., 2022). Interestingly, decreased plasma LEAP2 levels are detected in healthy men after exercise (Holm et al., 2022) and also in obese subjects after a short calorie restriction (Ragland and Malin, 2023).
Studies performing LEAP2 administrations demonstrate its ability to modulate energy balance. LEAP2 blunts fasting-induced food intake (Holá et al., 2022), and also diminishes food intake-induced increase in blood glucose in mice and humans (Hagemann et al., 2022). Interestingly, lipidized analogs of LEAP2 also display anorexigenic effects (Holá et al., 2022). The central effect of LEAP2 has also been addressed. ICV LEAP2 administration decreases HFD consumption of mice in a binge-like eating protocol (Cornejo et al., 2019), and also diminishes fasting-induced and spontaneous intake in rats and mice (Lugilde et al., 2022; Tufvesson-Alm et al., 2023). Chronic central administration of LEAP2 diminishes food intake and weight gain (Chu et al., 2022) and also reduces circulating triglycerides, while increasing energy expenditure and thermogenic biomarkers in brown adipose tissue of mice (Casado et al., 2024). To the best of our knowledge, only few studies have addressed the putative neuronal population mediating LEAP2 effects on food intake. LEAP2 was shown to hyperpolarize ARH NPY-expressing neurons, preventing their activation by ghrelin (Mani et al., 2019). Accordingly, LEAP2-deficient mice show enhanced ghrelin-induced ARH activation, measured as c-Fos immunoreactivity (Shankar et al., 2021), whereas food-deprived mice ICV administered with LEAP2 show decreased fasting-induced c-Fos in the ARH (Fernandez et al., 2022). Moreover, chronic central LEAP2 administration increases c-Fos immunoreactivity in ARH POMC neurons and chemogenetic inhibition of POMC neurons blunts LEAP2’s anorexigenic effect (Chu et al., 2022). Thus, experimental evidence demonstrates the ability of LEAP2 to modulate the activity of different hypothalamic neuronal populations, including ARH NPY and POMC neurons, which may mediate its effect on energy balance and metabolism.
Growth differentiation factor 15 (GDF15)
GDF15 is a divergent member of the transforming growth factor β superfamily. GDF15 mRNA is detected in multiple human and mouse tissues (Lockhart et al., 2020). Liver GDF15 mRNA levels are increased in ob/ob mice and Zucker diabetic fatty rats (Xiong et al., 2017), and also in DIO mice (Patel et al., 2019). Genetically modified rodent models have helped to understand the role of GDF15 on the regulation of energy balance. GDF15-deficient mice show increased food intake and body weight, and impaired glucose tolerance (Wang et al., 2021). Moreover, mice with genetic deletion of glial-derived neurotrophic factor receptor alpha-like (GFRAL), GDF15’s receptor, show attenuated DIO and insulin resistance (Emmerson et al., 2017; Mullican et al., 2017; Hsu et al., 2017; Yang et al., 2017). Conversely, GDF15-overexpressing mice have diminished food intake and body weight, and improved insulin sensitivity (Wang et al., 2021). In humans, increased liver mRNA and plasma levels of GDF15 are found in individuals with MASLD (Koo et al., 2018), whereas circulating GDF15 is also increased in obese and T2DM patients (Wang et al., 2021).
GFRAL mRNA is selectively detected in the mouse and human hindbrain (Mullican et al., 2017; Hes et al., 2025). Central GDF15 administration diminishes food intake and induces c-Fos expression in the AP and NTS (Tsai et al., 2014; Worth et al., 2020), and, although the identity of GDF15-responding neurons remains elusive, GFRAL expression co-localizes with cholecystokinin-expressing neurons (Worth et al., 2020). Interestingly, systemic GDF15 treatment diminishes gastric emptying in rodents, an effect dependent on the vagal efferent pathway (Xiong et al., 2017), and also causes emesis in musk shrews (Borner et al., 2020). Furthermore, GDF15 induces taste aversion (Patel et al., 2019; Worth et al., 2020), an effect consistent with c-Fos induction in the amygdala of GDF15-administered mice (Hsu et al., 2017). Thus, the effects of GDF15 as a brain-acting hepatokine that regulates energy balance seem to be secondary to its effect on other processes such as gastric emptying or taste aversion, processes that rely on different central circuits.
Angiopoietin-like protein 4 (ANGPTL4)
ANGPTL4 belongs to the ANGPTL protein family, which are involved in angiogenesis. ANGPTL4 is produced in the liver and adipose tissue, and, to a lesser extent, in the pituitary and hypothalamus (Wiesner et al., 2004; Kim et al., 2010; Vienberg et al., 2015). In mice, liver and plasma ANGPTL4 peptide levels increase with fasting and decrease after refeeding (Kim et al., 2010). Interestingly, ANGPTL4 peptide levels—but not mRNA levels—increase in the hypothalamus of fasted mice (Wiesner et al., 2004; Kim et al., 2010), likely due to elevated circulating peptide. Hypothalamic ANGPTL4 mRNA is increased in mouse models of T1DM and T2DM (Vienberg et al., 2015). ANGPTL4-deficient mice show reduced plasma triglycerides when fasted (Köster et al., 2005), whereas they show enhanced body weight gain and visceral adipose tissue mass deposit, but improved glucose tolerance when fed a HFD (Janssen et al., 2018). Strikingly, deletion of ANGPTL4 from hepatocytes enhances plasma triacylglycerol clearance and insulin sensitivity, and also diminishes weight gain of DIO mice (Singh et al., 2021). Conversely, ANGPTL4-overexpressing mice show increased serum cholesterol and triglycerides (Köster et al., 2005). In humans, circulating ANGPTL4 is increased in T2DM patients (Babapoor-Farrokhran et al., 2015; McCulloch et al., 2020) and in obese individuals (Schinzari et al., 2021) and to decrease, together with body weight and fat mass, after bariatric surgery (Bini et al., 2022).
Only one study links the central effect of ANGPTL4 to energy balance (Kim et al., 2010). ICV ANGPTL4 suppresses fasting-induced hyperphagia and increases energy expenditure, effects absent with peripheral administration (Kim et al., 2010). Moreover, ICV ANGPTL4 decreases hypothalamic AMPK phosphorylation, and the pharmacologic inhibition of AMPK signaling blunts the effect of ANGPTL4 on food intake (Kim et al., 2010). Then, ANGPTL4’s role as a brain-acting hepatokine needs further work to confirm its central effect on energy balance, although it seems evident that ANGPTL4’s effect relies on hypothalamic circuits.
Angiopoietin-like protein 8 (ANGPTL8)
ANGPTL8 is another member of the ANGPTL protein family and is exclusively produced in the human liver, whereas its mRNA is detected in mouse liver and adipose tissue (Zhang, 2012). In mice, ANGPTL8 liver mRNA levels decrease with fasting and increase with refeeding (Quagliarini et al., 2012), HFD feeding (Zhang, 2012), and also in hyperinsulinemic (Zhang et al., 2020) and fatty liver mice (Lee et al., 2016). Circulating and CSF levels of ANGPTL8 are also higher in diabetic murine models (Meng et al., 2024). Liver ANGPTL8 overexpression increases serum triacylglycerol and very-low density lipoprotein concentrations (Cox et al., 2015), whereas ANGPTL8 deficiency produces a decrease in plasma triglycerides and improves glucose tolerance of DIO mice (Zhang et al., 2020). In humans, circulating ANGPTL8 levels decrease with fasting and increase with refeeding (Quagliarini et al., 2012). Plasma ANGPTL8 levels are also increased in individuals with T1DM (Espes et al., 2014), T2DM (Espes et al., 2014; Fu et al., 2014; Hu et al., 2014) and obesity (Fu et al., 2014), and positively correlate with hepatic steatosis in MASLD patients (von Loeffelholz et al., 2017). Interestingly, a recent study showed that ANGPTL8 CSF levels are increased in T2DM patients with cognitive dysfunction (Meng et al., 2024).
Only one study addressed ANGPTL8’s central modulation of energy balance in mice. ICV ANGPTL8 administration decreases rebound feeding and body weight regain after fasting, whereas it affects fasting-induced c-Fos in hypothalamic nuclei (Wang et al., 2018). Also, chronic ICV ANGPTL8 administration decreases body weight and circulating free fatty acids (Wang et al., 2018). Thus, further studies supporting the modulatory effect of ANGPTL8 on hypothalamic brain nuclei are needed to unequivocally establish its role as a brain-acting hepatokine.
Concluding remarks
Hepatokines have emerged as key players not only in peripheral metabolism but also in the complex regulation of brain function and whole-body energy balance. In this mini-review, we introduced the concept of “brain-acting hepatokines”, those that impact on the activity of brain centers that control energy balance and metabolism. Although the current understanding of the specific roles of brain-acting hepatokines in the central nervous system remains uneven, accumulating evidence underscores their relevance in both health and disease. Their dual function—as metabolic messengers and potential therapeutic targets—highlights the urgency and promise of advancing research in this field. Furthermore, since plasma levels of brain-acting hepatokines are increased in pathological conditions implicating metabolic disturbances such as T2DM, MASLD, and obesity, their potential as biomarkers poses them as putative targets for early diagnosis, prevention, and monitoring of disease progression. Moreover, the possibility of a synergistic central effect of brain-acting hepatokines has been only incipiently studied. Continued exploration of hepatokine signaling pathways may unlock novel strategies to address metabolic disorders and neuroendocrine dysregulation.
Author contributions
LG: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. NW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. MP: Conceptualization, Writing – original draft, Writing – review & editing. MPC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. LG, MP, and MPC were supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina, and NW was supported by Agencia Nacional of Promoción Científica y Tecnológica (ANPCyT) of Argentina.
Acknowledgments
We thank Camila Saenz for kindly providing the BioRENDER figure license.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdalla, M. M. I. (2017). Central and peripheral control of food intake. Endocr. Regul. 51, 52–70. doi: 10.1515/enr-2017-0006
Abdelmoemen, G., Khodeir, S. A., Zaki, A. N., Kassab, M., Abou-Saif, S., and Abd-Elsalam, S. (2019). Overexpression of Hepassocin in diabetic patients with nonalcoholic fatty liver disease may facilitate increased hepatic lipid accumulation. Endocr. Metab. Immune Disord. Drug Targets 19, 185–188. doi: 10.2174/1871530318666180716100543
Al-Regaiey, K. A., Iqbal, M., Alzaid, M. A., Alkaoud, O. A., Alhadyani, M. A., Alagel, O. A., et al. (2024). Evaluating fibroblast growth factor 21 (FGF21) levels post-gastric sleeve surgery in obese patients. Cureus. 16:e66122. doi: 10.7759/cureus.66122
Andersen, G., Burgdorf, K. S., Sparsø, T., Borch-Johnsen, K., Jørgensen, T., Hansen, T., et al. (2008). AHSG tag single nucleotide polymorphisms associate with type 2 diabetes and dyslipidemia: studies of metabolic traits in 7,683 white Danish subjects. Diabetes 57, 1427–1432. doi: 10.2337/db07-0558
Andreoli, M. F., Fittipaldi, A. S., Castrogiovanni, D., De Francesco, P. N., Valdivia, S., Heredia, F., et al. (2024). Pre-prandial plasma liver-expressed antimicrobial peptide 2 (LEAP2) concentration in humans is inversely associated with hunger sensation in a ghrelin independent manner. Eur. J. Nutr. 63, 751–762. doi: 10.1007/s00394-023-03304-8
Babapoor-Farrokhran, S., Jee, K., Puchner, B., Hassan, S. J., Xin, X., Rodrigues, M., et al. (2015). Angiopoietin-like 4 is a potent angiogenic factor and a novel therapeutic target for patients with proliferative diabetic retinopathy. Proc. Natl. Acad. Sci. USA 112, E3030–E3039. doi: 10.1073/pnas.1423765112
Bini, S., D’Erasmo, L., Astiarraga, B., Minicocci, I., Palumbo, M., Pecce, V., et al. (2022). Differential effects of bariatric surgery on plasma levels of ANGPTL3 and ANGPTL4. Nutr. Metab. Cardiovasc. Dis. 32, 2647–2654. doi: 10.1016/j.numecd.2022.08.019
Bono, B. S., Koziel Ly, N. K., Miller, P. A., Williams-Ikhenoba, J., Dumiaty, Y., and Chee, M. J. (2022). Spatial distribution of beta-klotho mRNA in the mouse hypothalamus, hippocampal region, subiculum, and amygdala. J. Comp. Neurol. 530, 1634–1657. doi: 10.1002/cne.25306
Bookout, A. L., de Groot, M. H. M., Owen, B. M., Lee, S., Gautron, L., Lawrence, H. L., et al. (2013). FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, 1147–1152. doi: 10.1038/nm.3249
Borner, T., Shaulson, E. D., Ghidewon, M. Y., Barnett, A. B., Horn, C. C., Doyle, R. P., et al. (2020). GDF15 induces anorexia through nausea and Emesis. Cell Metab. 31, 351–362.e5. doi: 10.1016/j.cmet.2019.12.004
Bühler, L., Maida, A., Vogl, E. S., Georgiadi, A., Takacs, A., Kluth, O., et al. (2021). Lipocalin 13 enhances insulin secretion but is dispensable for systemic metabolic control. Life Sci. Alliance 4. doi: 10.26508/lsa.202000898
Campbell, J. N., Macosko, E. Z., Fenselau, H., Pers, T. H., Lyubetskaya, A., Tenen, D., et al. (2017). A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496. doi: 10.1038/nn.4495
Casado, S., Varela-Miguéns, M., de Oliveira, D. T., Quintela-Vilariño, C., Nogueiras, R., Diéguez, C., et al. (2024). The effects of ghrelin and LEAP-2 in energy homeostasis are modulated by thermoneutrality, high-fat diet and aging. J. Endocrinol. Investig. 47, 2061–2074. doi: 10.1007/s40618-024-02307-4
Cheng, X., Zhu, B., Jiang, F., and Fan, H. (2011). Serum FGF-21 levels in type 2 diabetic patients. Endocr. Res. 36, 142–148. doi: 10.3109/07435800.2011.558550
Chikamoto, K., Misu, H., Takayama, H., Kikuchi, A., Ishii, K. A., Lan, F., et al. (2016). Rapid response of the steatosis-sensing hepatokine LECT2 during diet-induced weight cycling in mice. Biochem. Biophys. Res. Commun. 478, 1310–1316. doi: 10.1016/j.bbrc.2016.08.117
Cho, K. W., Zhou, Y., Sheng, L., and Rui, L. (2011). Lipocalin-13 regulates glucose metabolism by both insulin-dependent and insulin-independent mechanisms. Mol. Cell. Biol. 31, 450–457. doi: 10.1128/MCB.00459-10
Chu, G., Peng, H., Yu, N., Zhang, Y., Lin, X., and Lu, Y. (2022). Involvement of POMC neurons in LEAP2 regulation of food intake and body weight. Front. Endocrinol. 13:932761. doi: 10.3389/fendo.2022.932761
Claflin, K. E., Sullivan, A. I., Naber, M. C., Flippo, K. H., Morgan, D. A., Neff, T. J., et al. (2022). Pharmacological FGF21 signals to glutamatergic neurons to enhance leptin action and lower body weight during obesity. Mol. Metab. 64:101564. doi: 10.1016/j.molmet.2022.101564
Clemmons, D. R. (2012). Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol. Metab. Clin. N. Am. 41, 425–443. doi: 10.1016/j.ecl.2012.04.017
Clemmons, DR. (2018). 40 YEARS OF IGF1: Role of IGF-binding proteins in regulating IGF responses to changes in metabolism. Available online at: https://jme.bioscientifica.com/view/journals/jme/61/1/JME-18-0016.xml (Accessed March 3, 2025).
Cooper, L. A., Page, S. T., Amory, J. K., Anawalt, B. D., and Matsumoto, A. M. (2015). The association of obesity with sex hormone-binding globulin is stronger than the association with ageing – implications for the interpretation of total testosterone measurements. Clin. Endocrinol. 83, 828–833. doi: 10.1111/cen.12768
Cornejo, M. P., Castrogiovanni, D., Schiöth, H. B., Reynaldo, M., Marie, J., Fehrentz, J., et al. (2019). Growth hormone secretagogue receptor signalling affects high-fat intake independently of plasma levels of ghrelin and LEAP 2, in a 4-day binge eating model. J. Neuroendocrinol. 31:e12785. doi: 10.1111/jne.12785
Coutinho, E. A., Okamoto, S., Ishikawa, A. W., Yokota, S., Wada, N., Hirabayashi, T., et al. (2017). Activation of SF1 neurons in the ventromedial hypothalamus by DREADD technology increases insulin sensitivity in peripheral tissues. Diabetes 66, 2372–2386. doi: 10.2337/db16-1344
Cox, A. R., Lam, C. J., Bonnyman, C. W., Chavez, J., Rios, J. S., and Kushner, J. A. (2015). Angiopoietin-like protein 8 (ANGPTL8)/betatrophin overexpression does not increase beta cell proliferation in mice. Diabetologia 58, 1523–1531. doi: 10.1007/s00125-015-3590-z
Danilova, T., Galli, E., Pakarinen, E., Palm, E., Lindholm, P., Saarma, M., et al. (2019). Mesencephalic astrocyte-derived neurotrophic factor (MANF) is highly expressed in mouse tissues with metabolic function. Front Endocrinol 10:765. doi: 10.3389/fendo.2019.00765/full
Deaton, A. M., Dubey, A., Ward, L. D., Dornbos, P., Flannick, J., Yee, E., et al. (2022). Rare loss of function variants in the hepatokine gene INHBE protect from abdominal obesity. Nat. Commun. 13:4319. doi: 10.1038/s41467-022-31757-8
Ding, E. L., Song, Y., Manson, J. E., Hunter, D. J., Lee, C. C., Rifai, N., et al. (2009). Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 361, 1152–1163. doi: 10.1056/NEJMoa0804381
Ďurovcová, V., Marek, J., Hána, V., Matoulek, M., Zikán, V., Haluzíková, D., et al. (2010). Plasma concentrations of fibroblast growth factors 21 and 19 in patients with Cushing’s syndrome. Physiol. Res. 59, 415–422. doi: 10.33549/physiolres.931801
Dushay, J., Chui, P. C., Gopalakrishnan, G. S., Varela-Rey, M., Crawley, M., Fisher Ffolliott, M., et al. (2010). Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 139, 456–463. doi: 10.1053/j.gastro.2010.04.054
Ebert, T., Kralisch, S., Loessner, U., Jessnitzer, B., Stumvoll, M., Fasshauer, M., et al. (2014). Relationship between serum levels of angiopoietin-related growth factor and metabolic risk factors. Horm. Metab. Res. 46, 685–690. doi: 10.1055/s-0034-1382078
Emmerson, P. J., Wang, F., Du, Y., Liu, Q., Pickard, R. T., Gonciarz, M. D., et al. (2017). The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 23, 1215–1219. doi: 10.1038/nm.4393
Englund, A., Gilliam-Vigh, H., Suppli, M. P., Gasbjerg, L. S., Vilsbøll, T., and Knop, F. K. (2024). Intestinal expression profiles and hepatic expression of LEAP2, ghrelin and their common receptor, GHSR, in humans. Peptides 177:171227. doi: 10.1016/j.peptides.2024.171227
Erdem, N. B., Kahramanoğlu Aksoy, E., Dikmen, D., Uçar Baş, K., Ağaçdiken, A., İlhan Esgin, M., et al. (2024). Effects of low fat diet on inflammatory parameters in individuals with obesity/overweight and non-alcoholic fatty liver disease: a cross-sectional study. Medicine (Baltimore) 103:e37716. doi: 10.1097/MD.0000000000037716
Espes, D., Lau, J., and Carlsson, P. O. (2014). Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia 57, 50–53. doi: 10.1007/s00125-013-3071-1
Fazeli, P. K., Lun, M., Kim, S. M., Bredella, M. A., Wright, S., Zhang, Y., et al. (2015). FGF21 and the late adaptive response to starvation in humans. J. Clin. Invest. 125, 4601–4611. doi: 10.1172/JCI83349
Fernandez, G., Cabral, A., De Francesco, P. N., Uriarte, M., Reynaldo, M., Castrogiovanni, D., et al. (2022). GHSR controls food deprivation-induced activation of CRF neurons of the hypothalamic paraventricular nucleus in a LEAP2-dependent manner. Cell. Mol. Life Sci. 79:277. doi: 10.1007/s00018-022-04302-5
Fisher, F. M., Chui, P. C., Antonellis, P. J., Bina, H. A., Kharitonenkov, A., Flier, J. S., et al. (2010). Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59, 2781–2789. doi: 10.2337/db10-0193
Fittipaldi, A. S., Hernández, J., Castrogiovanni, D., Lufrano, D., De Francesco, P. N., Garrido, V., et al. (2020). Plasma levels of ghrelin, des-acyl ghrelin and LEAP2 in children with obesity: correlation with age and insulin resistance. Eur. J. Endocrinol. 182, 165–175. doi: 10.1530/EJE-19-0684
Frystyk, J., Vestbo, E., Skjaerbaek, C., Mogensen, C. E., and Orskov, H. (1995). Free insulin-like growth factors in human obesity. Metabolism 44, 37–44. doi: 10.1016/0026-0495(95)90219-8
Fu, Z., Berhane, F., Fite, A., Seyoum, B., Abou-Samra, A. B., and Zhang, R. (2014). Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci. Rep. 4:5013. doi: 10.1038/srep05013
Fu, J., Malale, K., Luo, X., Chen, M., Liu, Q., Cheng, W., et al. (2021). The relationship of mesencephalic astrocyte-derived neurotrophic factor with hyperlipidemia in patients with or without type 2 diabetes mellitus. Hormones (Athens) 20, 537–543. doi: 10.1007/s42000-021-00272-8
Gaich, G., Chien, J. Y., Fu, H., Glass, L. C., Deeg, M. A., Holland, W. L., et al. (2013). The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340. doi: 10.1016/j.cmet.2013.08.005
Gao, S., Ghoshal, S., Zhang, L., Stevens, J. R., McCommis, K. S., Finck, B. N., et al. (2019). The peptide hormone adropin regulates signal transduction pathways controlling hepatic glucose metabolism in a mouse model of diet-induced obesity. J. Biol. Chem. 294, 13366–13377. doi: 10.1074/jbc.RA119.008967
Gao, S., McMillan, R. P., Jacas, J., Zhu, Q., Li, X., Kumar, G. K., et al. (2014). Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes 63, 3242–3252. doi: 10.2337/db14-0388
Garcia-Luna, C., Sanchez-Watts, G., Arnold, M., de Lartigue, G., DeWalt, N., Langhans, W., et al. (2021). The medullary targets of Neurally conveyed sensory information from the rat hepatic portal and superior mesenteric veins. eNeuro 8. doi: 10.1523/ENEURO.0419-20.2021
Ge, X., Yang, H., Bednarek, M. A., Galon-Tilleman, H., Chen, P., Chen, M., et al. (2018). LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab. 27, 461–469.e6. doi: 10.1016/j.cmet.2017.10.016
Gharipour, M., Sadeghi, M., Salehi, M., Behmanesh, M., Khosravi, E., Dianatkhah, M., et al. (2017). Association of expression of selenoprotein P in mRNA and protein levels with metabolic syndrome in subjects with cardiovascular disease: results of the Selenegene study. J. Gene Med. 19. doi: 10.1002/jgm.2945
Ghodsian, N., Gagnon, E., Bourgault, J., Gobeil, É., Manikpurage, H. D., Perrot, N., et al. (2021). Blood levels of the SMOC1 Hepatokine are not causally linked with type 2 diabetes: a bidirectional Mendelian randomization study. Nutrients 13:4208. doi: 10.3390/nu13124208
Griffin, J. D., Buxton, J. M., Culver, J. A., Barnes, R., Jordan, E. A., White, A. R., et al. (2023). Hepatic Activin E mediates liver-adipose inter-organ communication, suppressing adipose lipolysis in response to elevated serum fatty acids. Mol. Metab. 78:101830. doi: 10.1016/j.molmet.2023.101830
Hagemann, C. A., Jensen, M. S., Holm, S., Gasbjerg, L. S., Byberg, S., Skov-Jeppesen, K., et al. (2022). LEAP2 reduces postprandial glucose excursions and ad libitum food intake in healthy men. Cell Rep. Med. 3:100582. doi: 10.1016/j.xcrm.2022.100582
Hale, C., and Véniant, M. M. (2021). Growth differentiation factor 15 as a potential therapeutic for treating obesity. Mol. Metab. 46:101117. doi: 10.1016/j.molmet.2020.101117
Hansen, J., Rinnov, A., Krogh-Madsen, R., Fischer, C. P., Andreasen, A. S., Berg, R. M. G., et al. (2013). Plasma follistatin is elevated in patients with type 2 diabetes: relationship to hyperglycemia, hyperinsulinemia, and systemic low-grade inflammation. Diabetes Metab. Res. Rev. 29, 463–472. doi: 10.1002/dmrr.2415
Hashimoto, O., Funaba, M., Sekiyama, K., Doi, S., Shindo, D., Satoh, R., et al. (2018). Activin E controls energy homeostasis in both Brown and White adipose tissues as a Hepatokine. Cell Rep. 25, 1193–1203. doi: 10.1016/j.celrep.2018.10.008
Hes, C., Gui, L. T., Bay, A., Alvarez, F., Katz, P., Paul, T., et al. (2025). GDNF family receptor alpha-like (GFRAL) expression is restricted to the caudal brainstem. Mol. Metab. 91:102070. doi: 10.1016/j.molmet.2024.102070
Holá, L., Tureckiuová, T., Kuneš, J., Železná, B., and Maletínská, L. (2023). High-fat diet induces resistance to ghrelin and LEAP2 peptide analogs in mice. Physiol. Res. 72, 607–619. doi: 10.33549/physiolres.935189
Holá, L., Železná, B., Karnošová, A., Kuneš, J., Fehrentz, J. A., Denoyelle, S., et al. (2022). A novel truncated liver enriched antimicrobial Peptide-2 Palmitoylated at its N-terminal antagonizes effects of ghrelin. J. Pharmacol. Exp. Ther. 383, 129–136. doi: 10.1124/jpet.122.001322
Holm, S., Husted, A. S., Skov, L. J., Morville, T. H., Hagemann, C. A., Jorsal, T., et al. (2022). Beta-Hydroxybutyrate suppresses hepatic production of the ghrelin receptor antagonist LEAP2. Endocrinology 163. doi: 10.1210/endocr/bqac038
Hong, H., Cui, Z. Z., Zhu, L., Fu, S. P., Rossi, M., Cui, Y. H., et al. (2017). Central IGF1 improves glucose tolerance and insulin sensitivity in mice. Nutr Diabetes 7, 1–10. doi: 10.1038/s41387-017-0002-0
Hsu, J. Y., Crawley, S., Chen, M., Ayupova, D. A., Lindhout, D. A., Higbee, J., et al. (2017). Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550, 255–259. doi: 10.1038/nature24042
Hsuchou, H., Pan, W., and Kastin, A. J. (2007). The fasting polypeptide FGF21 can enter brain from blood. Peptides 28, 2382–2386. doi: 10.1016/j.peptides.2007.10.007
Hu, H., Sun, W., Yu, S., Hong, X., Qian, W., Tang, B., et al. (2014). Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care 37, 2718–2722. doi: 10.2337/dc14-0602
Huang, R. L., Li, C. H., Du, Y. F., Cheng, K. P., Lin, C. H., Hu, C. Y., et al. (2020). Discovery of a role of the novel hepatokine, hepassocin, in obesity. Biofactors 46, 100–105. doi: 10.1002/biof.1574
Huffman, D. M., Farias Quipildor, G., Mao, K., Zhang, X., Wan, J., Apontes, P., et al. (2016). Central insulin-like growth factor-1 (IGF-1) restores whole-body insulin action in a model of age-related insulin resistance and IGF-1 decline. Aging Cell 15, 181–186. doi: 10.1111/acel.12415
Islam, M. N., Mita, Y., Maruyama, K., Tanida, R., Zhang, W., Sakoda, H., et al. (2020). Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J. Endocrinol. 244, 13–23. doi: 10.1530/JOE-19-0102
Islam, M. N., Nabekura, H., Ueno, H., Nishida, T., Nanashima, A., Sakoda, H., et al. (2024). Liver-expressed antimicrobial peptide 2 is a hepatokine regulated by ghrelin, nutrients, and body weight. Sci. Rep. 14:24782. doi: 10.1038/s41598-024-74048-6
Jais, A., and Brüning, J. C. (2022). Arcuate nucleus-dependent regulation of metabolism-pathways to obesity and diabetes mellitus. Endocr. Rev. 43, 314–328. doi: 10.1210/endrev/bnab025
Janssen, A. W. F., Katiraei, S., Bartosinska, B., Eberhard, D., Willems van Dijk, K., and Kersten, S. (2018). Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia 61, 1447–1458. doi: 10.1007/s00125-018-4583-5
Jensen-Cody, S. O., and Potthoff, M. J. (2020). Hepatokines and metabolism: deciphering communication from the liver. Molec. Metab. 44:101138. doi: 10.1016/j.molmet.2020.101138
Kappeler, L., Filho, C. D. M., Dupont, J., Leneuve, P., Cervera, P., Périn, L., et al. (2008). Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 6:e254. doi: 10.1371/journal.pbio.0060254
Kersten, S. (2021). ANGPTL3 as therapeutic target. Curr. Opin. Lipidol. 32, 335–341. doi: 10.1097/MOL.0000000000000789
Kharitonenkov, A., Shiyanova, T. L., Koester, A., Ford, A. M., Micanovic, R., Galbreath, E. J., et al. (2005). FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635. doi: 10.1172/JCI23606
Kim, H. K., Youn, B. S., Shin, M. S., Namkoong, C., Park, K. H., Baik, J. H., et al. (2010). Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes 59, 2772–2780. doi: 10.2337/db10-0145
Koishi, R., Ando, Y., Ono, M., Shimamura, M., Yasumo, H., Fujiwara, T., et al. (2002). Angptl3 regulates lipid metabolism in mice. Nat. Genet. 30, 151–157. doi: 10.1038/ng814
Koo, B. K., Um, S. H., Seo, D. S., Joo, S. K., Bae, J. M., Park, J. H., et al. (2018). Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int. 38, 695–705. doi: 10.1111/liv.13587
Köster, A., Chao, Y. B., Mosior, M., Ford, A., Gonzalez-DeWhitt, P. A., Hale, J. E., et al. (2005). Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146, 4943–4950. doi: 10.1210/en.2005-0476
Krause, A., Sillard, R., Kleemeier, B., Klüver, E., Maronde, E., Conejo-García, J. R., et al. (2003). Isolation and biochemical characterization of LEAP-2, a novel blood peptide expressed in the liver. Protein Sci. 12, 143–152. doi: 10.1110/ps.0213603
Kumar, K. G., Trevaskis, J. L., Lam, D. D., Sutton, G. M., Koza, R. A., Chouljenko, V. N., et al. (2008). Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 8, 468–481. doi: 10.1016/j.cmet.2008.10.011
Kutlu, O., Altun, Ö., Dikker, O., Aktaş, Ş., Özsoy, N., Arman, Y., et al. (2019). Serum Adropin levels are reduced in adult patients with nonalcoholic fatty liver disease. Med. Princ. Pract. 28, 463–469. doi: 10.1159/000500106
Kwon, E., Joung, H. Y., Liu, S. M., Chua, S. C., Schwartz, G. J., and Jo, Y. H. (2020). Optogenetic stimulation of the liver-projecting melanocortinergic pathway promotes hepatic glucose production. Nat. Commun. 11:6295. doi: 10.1038/s41467-020-20160-w
Lam, S., Lee, C. H., Fong, C. H. Y., Wong, Y., Shiu, S. W. M., Mak, L. Y., et al. (2024). Serum Tsukushi level is associated with the severity of liver fibrosis independent of type 2 diabetes. J. Clin. Endocrinol. Metab. 109, e1048–e1054. doi: 10.1210/clinem/dgad650
Lan, F., Misu, H., Chikamoto, K., Takayama, H., Kikuchi, A., Mohri, K., et al. (2014). LECT2 functions as a Hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 63, 1649–1664. doi: 10.2337/db13-0728
Lee, Y. H., Lee, S. G., Lee, C. J., Kim, S. H., Song, Y. M., Yoon, M. R., et al. (2016). Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Sci. Rep. 6:24013. doi: 10.1038/srep24013
Li, Y., Deng, X., Wu, X., Zhao, L., Zhao, Z., Guo, C., et al. (2023). Association of serum Tsukushi level with metabolic syndrome and its components. Endocrine 79, 469–476. doi: 10.1007/s12020-022-03285-4
Li, J., Huang, P., Xiong, J., Liang, X., Li, M., Ke, H., et al. (2022). Serum levels of ghrelin and LEAP2 in patients with type 2 diabetes mellitus: correlation with circulating glucose and lipids. Endocr. Connect. 11:e220012. doi: 10.1530/EC-22-0012
Li, Y., Jin, L., Yan, J., Huang, Y., Zhang, H., Zhang, R., et al. (2021). Tsukushi and TSKU genotype in obesity and related metabolic disorders. J. Endocrinol. Investig. 44, 2645–2654. doi: 10.1007/s40618-021-01572-x
Li, Q., Yu, Y., Kang, Y., Xu, J., Lin, H., Wang, X., et al. (2016). Correlations of cerebrospinal fluid/plasma fibroblast growth factor 21 ratio with metabolic parameters in Chinese individuals of Normal weight. Clin. Lab. 62, 893–899. doi: 10.7754/Clin.Lab.2015.150926
Li, H., Zhang, C., Liu, J., Xie, W., Xu, W., Liang, F., et al. (2019). Intraperitoneal administration of follistatin promotes adipocyte browning in high-fat diet-induced obese mice. PLoS One 14:e0220310. doi: 10.1371/journal.pone.0220310
Liang, Q., Zhong, L., Zhang, J., Wang, Y., Bornstein, S. R., Triggle, C. R., et al. (2014). FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63, 4064–4075. doi: 10.2337/db14-0541
Lindahl, M., Danilova, T., Palm, E., Lindholm, P., Võikar, V., Hakonen, E., et al. (2014). MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep. 7, 366–375. doi: 10.1016/j.celrep.2014.03.023
Lockhart, S. M., Saudek, V., and O’Rahilly, S. (2020). GDF15: a hormone conveying somatic distress to the brain. Endocr. Rev. 41. doi: 10.1210/endrev/bnaa007
Lugilde, J., Casado, S., Beiroa, D., Cuñarro, J., Garcia-Lavandeira, M., Álvarez, C. V., et al. (2022). LEAP-2 counteracts ghrelin-induced food intake in a nutrient, growth hormone and age independent manner. Cells 11:324. doi: 10.3390/cells11030324
Luo, M., and Peng, D. (2018). ANGPTL8: an important regulator in metabolic disorders. Front Endocrinol. 9:169. doi: 10.3389/fendo.2018.00169
M’Kadmi, C., Cabral, A., Barrile, F., Giribaldi, J., Cantel, S., Damian, M., et al. (2019). N-terminal liver-expressed antimicrobial peptide 2 (LEAP2) region exhibits inverse agonist activity toward the ghrelin receptor. J. Med. Chem. 62, 965–973. doi: 10.1021/acs.jmedchem.8b01644
Maekawa, R., Seino, Y., Ogata, H., Murase, M., Iida, A., Hosokawa, K., et al. (2017). Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice. J. Nutr. Biochem. 49, 71–79. doi: 10.1016/j.jnutbio.2017.07.010
Maïmoun, L., Mura, T., Attalin, V., Dupuy, A. M., Cristol, J. P., Avignon, A., et al. (2020). Modification of muscle-related hormones in women with obesity: potential impact on bone metabolism. J. Clin. Med. 9:1150. doi: 10.3390/jcm9041150
Mani, B. K., Puzziferri, N., He, Z., Rodriguez, J. A., Osborne-Lawrence, S., Metzger, N. P., et al. (2019). LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Invest. 129, 3909–3923. doi: 10.1172/JCI125332
Mani, B. K., Shankar, K., and Zigman, J. M. (2019). Ghrelin’s relationship to blood glucose. Endocrinology 160, 1247–1261. doi: 10.1210/en.2019-00074
Markan, K. R., Naber, M. C., Ameka, M. K., Anderegg, M. D., Mangelsdorf, D. J., Kliewer, S. A., et al. (2014). Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63, 4057–4063. doi: 10.2337/db14-0595
Mathews, S. T., Singh, G. P., Ranalletta, M., Cintron, V. J., Qiang, X., Goustin, A. S., et al. (2002). Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 51, 2450–2458. doi: 10.2337/diabetes.51.8.2450
McCulloch, L. J., Bramwell, L. R., Knight, B., and Kos, K. (2020). Circulating and tissue specific transcription of angiopoietin-like protein 4 in human type 2 diabetes. Metabolism 106:154192. doi: 10.1016/j.metabol.2020.154192
Meng, X., Li, D., Kan, R., Xiang, Y., Pan, L., Guo, Y., et al. (2024). Inhibition of ANGPTL8 protects against diabetes-associated cognitive dysfunction by reducing synaptic loss via the PirB signaling pathway. J. Neuroinflammation 21:192. doi: 10.1186/s12974-024-03183-8
Misu, H., Takamura, T., Takayama, H., Hayashi, H., Matsuzawa-Nagata, N., Kurita, S., et al. (2010). A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 12, 483–495. doi: 10.1016/j.cmet.2010.09.015
Montgomery, M. K., Bayliss, J., Devereux, C., Bezawork-Geleta, A., Roberts, D., Huang, C., et al. (2020). SMOC1 is a glucose-responsive hepatokine and therapeutic target for glycemic control. Sci. Transl. Med. 12. doi: 10.1126/scitranslmed.aaz8048
Mouchiroud, M., Camiré, É., Aldow, M., Caron, A., Jubinville, É., Turcotte, L., et al. (2019a). The Hepatokine TSK does not affect brown fat thermogenic capacity, body weight gain, and glucose homeostasis. Molec. Metab. 30, 184–191. doi: 10.1016/j.molmet.2019.09.014
Mouchiroud, M., Camiré, É., Aldow, M., Caron, A., Jubinville, É., Turcotte, L., et al. (2019b). The hepatokine Tsukushi is released in response to NAFLD and impacts cholesterol homeostasis. JCI Insight 4:129492. doi: 10.1172/jci.insight.129492
Mullican, S. E., Lin-Schmidt, X., Chin, C. N., Chavez, J. A., Furman, J. L., Armstrong, A. A., et al. (2017). GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 23, 1150–1157. doi: 10.1038/nm.4392
Muzumdar, R. H., Ma, X., Fishman, S., Yang, X., Atzmon, G., Vuguin, P., et al. (2006). Central and opposing effects of IGF-I and IGF-binding Protein-3 on systemic insulin action. Diabetes 55, 2788–2796. doi: 10.2337/db06-0318
Namkung, J., Koh, S. B., Kong, I. D., Choi, J. W., and Yeh, B. I. (2011). Serum levels of angiopoietin-related growth factor are increased in metabolic syndrome. Metabolism 60, 564–568. doi: 10.1016/j.metabol.2010.05.013
Ogawa, Y., Kurosu, H., Yamamoto, M., Nandi, A., Rosenblatt, K. P., Goetz, R., et al. (2007). BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. USA 104, 7432–7437. doi: 10.1073/pnas.0701600104
Oike, Y., Akao, M., Yasunaga, K., Yamauchi, T., Morisada, T., Ito, Y., et al. (2005). Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat. Med. 11, 400–408. doi: 10.1038/nm1214
Olshan, D. S., and Rader, D. J. (2018). Angiopoietin-like protein 4: a therapeutic target for triglycerides and coronary disease? J. Clin. Lipidol. 12, 583–587. doi: 10.1016/j.jacl.2018.01.012
Ou, H. Y., Wu, H. T., Lin, C. H., Du, Y. F., Hu, C. Y., Hung, H. C., et al. (2017). The hepatic protection effects of Hepassocin in hyperglycemic crisis. J. Clin. Endocrinol. Metab. 102, 2407–2415. doi: 10.1210/jc.2016-3287
Owen, B. M., Ding, X., Morgan, D. A., Coate, K. C., Bookout, A. L., Rahmouni, K., et al. (2014). FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure and weight loss. Cell Metab. 20, 670–677. doi: 10.1016/j.cmet.2014.07.012
Pakarinen, E., Danilova, T., Võikar, V., Chmielarz, P., Piepponen, P., Airavaara, M., et al. (2020). MANF ablation causes prolonged activation of the UPR without neurodegeneration in the mouse midbrain dopamine system. eNeuro 7, ENEURO.0477–ENEU19.2019. doi: 10.1523/ENEURO.0477-19.2019
Patel, S., Alvarez-Guaita, A., Melvin, A., Rimmington, D., Dattilo, A., Miedzybrodzka, E. L., et al. (2019). GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 29, 707–718.e8. doi: 10.1016/j.cmet.2018.12.016
Perelló, M. (2025). Critical insights into LEAP2 biology and physiological functions: potential roles beyond ghrelin antagonism. Endocrinology 166. doi: 10.1210/endocr/bqaf011
Qaddoumi, M. G., Alanbaei, M., Hammad, M. M., Al Khairi, I., Cherian, P., Channanath, A., et al. (2020). Investigating the role of myeloperoxidase and angiopoietin-like protein 6 in obesity and diabetes. Sci. Rep. 10:6170. doi: 10.1038/s41598-020-63149-7
Quagliarini, F., Wang, Y., Kozlitina, J., Grishin, N. V., Hyde, R., Boerwinkle, E., et al. (2012). Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 109, 19751–19756. doi: 10.1073/pnas.1217552109
Ragland, T. J., and Malin, S. K. (2023). Plasma LEAP-2 following a low-calorie diet with or without interval exercise in women with obesity. Nutrients 15:655. doi: 10.3390/nu15030655
Rahmani, J., Kord Varkaneh, H., Clark, C., Zand, H., Bawadi, H., Ryan, P. M., et al. (2019). The influence of fasting and energy restricting diets on IGF-1 levels in humans: a systematic review and meta-analysis. Ageing Res. Rev. 53:100910. doi: 10.1016/j.arr.2019.100910
Rawal, S. U., Patel, B. M., and Patel, M. M. (2022). New drug delivery systems developed for brain targeting. Drugs 82, 749–792. doi: 10.1007/s40265-022-01717-z
Saez-Lopez, C., Villena, J. A., Simó, R., and Selva, D. M. (2020). Sex hormone-binding globulin overexpression protects against high-fat diet-induced obesity in transgenic male mice. J. Nutr. Biochem. 85:108480. doi: 10.1016/j.jnutbio.2020.108480
Sarruf, D. A., Thaler, J. P., Morton, G. J., German, J., Fischer, J. D., Ogimoto, K., et al. (2010). Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59, 1817–1824. doi: 10.2337/db09-1878
Schinzari, F., Vizioli, G., Campia, U., Tesauro, M., and Cardillo, C. (2021). Variable changes of circulating ANGPTL3 and ANGPTL4 in different obese phenotypes: relationship with vasodilator dysfunction. Biomedicines 9:1037. doi: 10.3390/biomedicines9081037
Schnurr, T. M., Jakupović, H., Carrasquilla, G. D., Ängquist, L., Grarup, N., Sørensen, T. I. A., et al. (2020). Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: a case-cohort study. Diabetologia 63, 1324–1332. doi: 10.1007/s00125-020-05140-5
Schulze, R. J., Schott, M. B., Casey, C. A., Tuma, P. L., and McNiven, M. A. (2019). The cell biology of the hepatocyte: a membrane trafficking machine. J. Cell Biol. 218, 2096–2112. doi: 10.1083/jcb.201903090
Sekiyama, K., Ushiro, Y., Kurisaki, A., Funaba, M., and Hashimoto, O. (2019). Activin E enhances insulin sensitivity and thermogenesis by activating brown/beige adipocytes. J. Vet. Med. Sci. 81, 646–652. doi: 10.1292/jvms.19-0036
Shankar, K., Metzger, N. P., Singh, O., Mani, B. K., Osborne-Lawrence, S., Varshney, S., et al. (2021). LEAP2 deletion in mice enhances ghrelin’s actions as an orexigen and growth hormone secretagogue. Mol. Metab. 53:101327. doi: 10.1016/j.molmet.2021.101327
Sheng, L., Cho, K. W., Zhou, Y., Shen, H., and Rui, L. (2011). Lipocalin 13 protein protects against hepatic steatosis by both inhibiting lipogenesis and stimulating fatty acid β-oxidation. J. Biol. Chem. 286, 38128–38135. doi: 10.1074/jbc.M111.256677
Shin, J. Y., Kim, S. K., Lee, M. Y., Kim, H. S., Ye, B. I., Shin, Y. G., et al. (2011). Serum sex hormone-binding globulin levels are independently associated with nonalcoholic fatty liver disease in people with type 2 diabetes. Diabetes Res. Clin. Pract. 94, 156–162. doi: 10.1016/j.diabres.2011.07.029
Singh, R., Braga, M., Reddy, S. T., Lee, S. J., Parveen, M., Grijalva, V., et al. (2017). Follistatin targets distinct pathways To promote Brown adipocyte characteristics in Brown and White adipose tissues. Endocrinology 158, 1217–1230. doi: 10.1210/en.2016-1607
Singh, A. K., Chaube, B., Zhang, X., Sun, J., Citrin, K. M., Canfrán-Duque, A., et al. (2021). Hepatocyte-specific suppression of ANGPTL4 improves obesity-associated diabetes and mitigates atherosclerosis in mice. J. Clin. Invest. 131:e140989. doi: 10.1172/JCI140989
Sousa-Victor, P., Neves, J., Cedron-Craft, W., Ventura, P. B., Liao, C. Y., Riley, R. R., et al. (2019). MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat. Metab. 1, 276–290. doi: 10.1038/s42255-018-0023-6
Spolcová, A., Holubová, M., Mikulášková, B., Nagelová, V., Stofková, A., Lacinová, Z., et al. (2014). Changes in FGF21 serum concentrations and liver mRNA expression in an experimental model of complete lipodystrophy and insulin-resistant diabetes. Physiol. Res. 63, 483–490. doi: 10.33549/physiolres.932714
Stanley, S., Pinto, S., Segal, J., Pérez, C. A., Viale, A., DeFalco, J., et al. (2010). Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proceed. Natl. Acad. Sci. U. S. A. 107, 7024–7029. doi: 10.1073/pnas.1002790107
Stark, R., Feehan, J., Mousa, A., Andrews, Z. B., and de Courten, B. (2023). Liver-expressed antimicrobial peptide 2 is associated with improved pancreatic insulin secretion in adults with overweight and obesity. Diabetes Obes. Metab. 25, 1213–1220. doi: 10.1111/dom.14968
Straus, D. S., and Takemoto, C. D. (1990). Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol. Endocrinol. 4, 91–100. doi: 10.1210/mend-4-1-91
Sugiyama, M., Kikuchi, A., Misu, H., Igawa, H., Ashihara, M., Kushima, Y., et al. (2018). Inhibin βE (INHBE) is a possible insulin resistance-associated hepatokine identified by comprehensive gene expression analysis in human liver biopsy samples. PLoS One 13:e0194798. doi: 10.1371/journal.pone.0194798
Sylow, L., Vind, B. F., Kruse, R., Møller, P. M., Wojtaszewski, J. F. P., Richter, E. A., et al. (2020). Circulating Follistatin and Activin a and their regulation by insulin in obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 105, 1343–1354. doi: 10.1210/clinem/dgaa090
Sylvers-Davie, K. L., and Davies, B. S. J. (2021). Regulation of lipoprotein metabolism by ANGPTL3, ANGPTL4, and ANGPTL8. Am. J. Physiol. Endocrinol. Metab. 321, E493–E508. doi: 10.1152/ajpendo.00195.2021
Tan, B. K., Hallschmid, M., Adya, R., Kern, W., Lehnert, H., and Randeva, H. S. (2011). Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: relationship with plasma FGF21 and body adiposity. Diabetes 60, 2758–2762. doi: 10.2337/db11-0672
Tan, B. K., Sivakumar, K., Bari, M. F., Vatish, M., and Randeva, H. S. (2013). Lower cerebrospinal fluid/plasma fibroblast growth factor 21 (FGF21) ratios and placental FGF21 production in gestational diabetes. PLoS One 8:e65254. doi: 10.1371/journal.pone.0065254
Tang, Q., Li, Y., and He, J. (2022). MANF: an emerging therapeutic target for metabolic diseases. Trends Endocrinol. Metab. 33, 236–246. doi: 10.1016/j.tem.2022.01.001
Tong, J., Cong, L., Jia, Y., He, B. L., Guo, Y., He, J., et al. (2022). Follistatin alleviates hepatic steatosis in NAFLD via the mTOR dependent pathway. Diabetes Metab. Syndr. Obes. 15, 3285–3301. doi: 10.2147/DMSO.S380053
Tsai, V. W. W., Manandhar, R., Jørgensen, S. B., Lee-Ng, K. K. M., Zhang, H. P., Marquis, C. P., et al. (2014). The anorectic actions of the TGFβ cytokine MIC-1/GDF15 require an intact brainstem area Postrema and nucleus of the solitary tract. PLoS One 9:e100370. doi: 10.1371/journal.pone.0100370
Tufvesson-Alm, M., Zhang, Q., Aranäs, C., Sköldheden, S. B., Edvardsson, C. E., and Jerlhag, E. (2023). Decoding the influence of central LEAP2 on hedonic food intake and its association with dopaminergic reward pathways [internet]. bioRxiv. doi: 10.1038/s41398-024-03136-y
Uhlén, M., Fagerberg, L., Hallström, B. M., Lindskog, C., Oksvold, P., Mardinoglu, A., et al. (2015). Tissue-based map of the human proteome. Science 347:1260419. doi: 10.1126/science.1260419
Vienberg, S. G., Kleinridders, A., Suzuki, R., and Kahn, C. R. (2015). Differential effects of angiopoietin-like 4 in brain and muscle on regulation of lipoprotein lipase activity. Mol. Metab. 4, 144–150. doi: 10.1016/j.molmet.2014.11.003
von Loeffelholz, C., Pfeiffer, A. F. H., Lock, J. F., Lieske, S., Döcke, S., Murahovschi, V., et al. (2017). ANGPTL8 (Betatrophin) is expressed in visceral adipose tissue and relates to human hepatic steatosis in two independent clinical collectives. Horm. Metab. Res. 49, 343–349. doi: 10.1055/s-0043-102950
Wang, D., Day, E. A., Townsend, L. K., Djordjevic, D., Jørgensen, S. B., and Steinberg, G. R. (2021). GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 17, 592–607. doi: 10.1038/s41574-021-00529-7
Wang, M., Pugh, S. M., Daboul, J., Miller, D., Xu, Y., and Hill, J. W. (2024). IGF-1 acts through Kiss1-expressing cells to influence metabolism and reproduction. bioRxiv. doi: 10.1101/2024.07.02.601722
Wang, R., Yuan, J., Zhang, C., Wang, L., Liu, Y., Song, L., et al. (2018). Neuropeptide Y-positive neurons in the dorsomedial hypothalamus are involved in the anorexic effect of Angptl8. Front. Mol. Neurosci. 11:451. doi: 10.3389/fnmol.2018.00451
Wiesner, G., Morash, B. A., Ur, E., and Wilkinson, M. (2004). Food restriction regulates adipose-specific cytokines in pituitary gland but not in hypothalamus. J. Endocrinol. 180, R1–R6. doi: 10.1677/joe.0.180r001
Worth, A. A., Shoop, R., Tye, K., Feetham, C. H., D’Agostino, G., Dodd, G. T., et al. (2020). The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. eLife 9:e55164. doi: 10.7554/eLife.55164
Wu, T., Liu, Q., Li, Y., Li, H., Chen, L., Yang, X., et al. (2021). Feeding-induced hepatokine, Manf, ameliorates diet-induced obesity by promoting adipose browning via p38 MAPK pathway. J. Exp. Med. 218:e20201203. doi: 10.1084/jem.20201203
Wu, H. T., Lu, F. H., Ou, H. Y., Su, Y. C., Hung, H. C., Wu, J. S., et al. (2013). The role of hepassocin in the development of non-alcoholic fatty liver disease. J. Hepatol. 59, 1065–1072. doi: 10.1016/j.jhep.2013.06.004
Xiong, Y., Walker, K., Min, X., Hale, C., Tran, T., Komorowski, R., et al. (2017). Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci. Transl. Med. 9. doi: 10.1126/scitranslmed.aan8732
Xiong, X., Wang, Q., Wang, S., Zhang, J., Liu, T., Guo, L., et al. (2019). Mapping the molecular signatures of diet-induced NASH and its regulation by the hepatokine Tsukushi. Mol Metab. 20, 128–137. doi: 10.1016/j.molmet.2018.12.004
Yakar, S., Liu, J. L., Fernandez, A. M., Wu, Y., Schally, A. V., Frystyk, J., et al. (2001). Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes 50, 1110–1118. doi: 10.2337/diabetes.50.5.1110
Yakar, S., Setser, J., Zhao, H., Stannard, B., Haluzik, M., Glatt, V., et al. (2004). Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J. Clin. Invest. 113, 96–105. doi: 10.1172/JCI200417763
Yang, L., Chang, C. C., Sun, Z., Madsen, D., Zhu, H., Padkjær, S. B., et al. (2017). GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 23, 1158–1166. doi: 10.1038/nm.4394
Yang, S. J., Hwang, S. Y., Choi, H. Y., Yoo, H. J., Seo, J. A., Kim, S. G., et al. (2011). Serum Selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J. Clin. Endocrinol. Metabol. 96, E1325–E1329. doi: 10.1210/jc.2011-0620
Yang, C., Jin, C., Li, X., Wang, F., McKeehan, W. L., and Luo, Y. (2012). Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One 7:e33870. doi: 10.1371/journal.pone.0033870
Yoo, H. J., Hwang, S. Y., Choi, J. H., Lee, H. J., Chung, H. S., Seo, J. A., et al. (2017). Association of leukocyte cell-derived chemotaxin 2 (LECT2) with NAFLD, metabolic syndrome, and atherosclerosis. PLoS One 12:e0174717. doi: 10.1371/journal.pone.0174717
Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., and Wymer, M. (2016). Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84. doi: 10.1002/hep.28431
Yuen, K. C. J., Hjortebjerg, R., Ganeshalingam, A. A., Clemmons, D. R., and Frystyk, J. (2024). Growth hormone/insulin-like growth factor I axis in health and disease states: an update on the role of intra-portal insulin. Front Endocrinol (Lausanne). 15:1456195. doi: 10.3389/fendo.2024.1456195
Zang, H., Jiang, F., Cheng, X., Xu, H., and Hu, X. (2018). Serum adropin levels are decreased in Chinese type 2 diabetic patients and negatively correlated with body mass index. Endocr. J. 65, 685–691. doi: 10.1507/endocrj.EJ18-0060
Zhang, R. (2012). Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 424, 786–792. doi: 10.1016/j.bbrc.2012.07.038
Zhang, Y., Wang, Y., and Liu, J. (2023). Friend or foe for obesity: how hepatokines remodel adipose tissues and translational perspective. Genes Diseases 10, 825–847. doi: 10.1016/j.gendis.2021.12.011
Zhang, Z., Wu, H., Dai, L., Yuan, Y., Zhu, Y., Ma, Z., et al. (2020). ANGPTL8 enhances insulin sensitivity by directly activating insulin-mediated AKT phosphorylation. Gene 749:144707. doi: 10.1016/j.gene.2020.144707
Zhang, X., Yeung, D. C. Y., Karpisek, M., Stejskal, D., Zhou, Z. G., Liu, F., et al. (2008). Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57, 1246–1253. doi: 10.2337/db07-1476
Zhang, Z., Zeng, H., Lin, J., Hu, Y., Yang, R., Sun, J., et al. (2018). Circulating LECT2 levels in newly diagnosed type 2 diabetes mellitus and their association with metabolic parameters: an observational study. Medicine (Baltimore) 97:e0354. doi: 10.1097/MD.0000000000010354
Zsombok, A., Desmoulins, L. D., and Derbenev, A. V. (2024). Sympathetic circuits regulating hepatic glucose metabolism: where we stand. Physiol. Rev. 104, 85–101. doi: 10.1152/physrev.00005.2023
Glossary
AgRP - agouti-related peptide
AMPK - AMP-activated protein kinase
ANGPTL4 - angiopoietin-like protein 4
ANGPTL8 - angiopoietin-like protein 8
AP - area postrema
ARH - hypothalamic arcuate nucleus
CSF - cerebrospinal fluid
DIO - diet-induced obese
DVC - dorsal vagal complex
FGF-21 - fibroblast growth factor 21
FGFR1c - FGF receptor 1c
GDF15 - growth differentiation factor 15
GFRAL - glial-derived neurotrophic factor receptor alpha-like
GH - growth hormone
GHSR - GH secretagogue receptor
HFD - high fat diet
ICV - intra-cerebro-ventricular
IGF-1 - insulin-like growth factor 1
IGF-1BP - IGF-1 binding proteins
IGF-1R - IGF-1 receptor
KLB - Klotho-β
LEAP2 - liver-expressed antimicrobial peptide 2
MASLD - metabolic-associated steatotic liver disease
NPY - neuropeptide Y
NTS - nucleus of the solitary tract
POMC - pro-opiomelanocortin
PVH - paraventricular hypothalamic nucleus
T1DM - type 1 diabetes mellitus
T2DM - type 2 diabetes mellitus
Keywords: hepatokines, brain, metabolism, energy balance, liver
Citation: Giovanini L, Wanionok N, Perello M and Cornejo MP (2025) Brain-acting hepatokines: its impact on energy balance and metabolism. Front. Neurosci. 19:1589110. doi: 10.3389/fnins.2025.1589110
Edited by:
Cinthia Garcia-Luna, National Institute of Psychiatry Ramon de la Fuente Muñiz (INPRFM), MexicoReviewed by:
Francisco Díaz-Castro, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), SpainCopyright © 2025 Giovanini, Wanionok, Perello and Cornejo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Paula Cornejo, cGNvcm5lam9AaW1iaWNlLmdvdi5hcg==
†ORCID: Lucía Giovanini, orcid.org/0009-0003-0061-773X
Nahuel Wanionok, orcid.org/0009-0006-1614-6292
Mario Perello, orcid.org/0000-0003-2114-6765
Maria Paula Cornejo, orcid.org/0000-0001-5425-1946
 Lucía Giovanini
Lucía Giovanini Nahuel Wanionok
Nahuel Wanionok Mario Perello
Mario Perello Maria Paula Cornejo
Maria Paula Cornejo