- 1Faculty of Physical Education, Huainan Normal University, Anhui, China
- 2International College, Krirk University, Bangkok, Bangkok, Thailand
- 3Chongqing Preschool Education College, Chongqing, China
The circadian system regulates core physiological processes, including muscle regeneration, protein synthesis, and cellular homeostasis. Disruptions in circadian rhythms contribute to impaired muscle function in older adults, with age-related declines in muscle mass and regenerative capacity serving as major contributors to sarcopenia. Emerging evidence indicates that exercise—a powerful modulator of muscle adaptation—can also influence circadian regulation, offering a potential avenue to enhance muscle repair in aging populations. This review examines how physical activity interacts with circadian mechanisms in aged skeletal muscle, emphasizing key molecular and cellular pathways involved in muscle regeneration. Central circadian regulators such as Clock, BMAL1, and PER1 are discussed in the context of muscle protein turnover, satellite cell activity, and mitochondrial function. Aligning exercise timing with circadian rhythms is proposed as a promising strategy to enhance muscle recovery and functional capacity in older individuals. Furthermore, the review highlights the therapeutic potential of chrono-exercise to delay the onset of sarcopenia and promote healthy aging. By integrating insights from chronobiology, geroscience, and exercise physiology, this analysis underscores the importance of chrono-exercise in supporting muscle health during aging.
1 Introduction
The preservation of muscle structure and function plays a crucial role in supporting healthy aging and independence. Sarcopenia, the degenerative loss of muscular strength and mass with age, is a major factor underlying elevated risks of injury, diminished mobility, and mortality among older adults (Shen et al., 2023; Kamel, 2003; Larsson et al., 2019). These physiological functions are modulated by circadian rhythms, the internal, near-24-h biological clock that orchestrates key processes such as the sleep–wake cycle, hormone secretion, and metabolic homeostasis (Zisapel, 2007). Muscle regeneration, the process by which damaged muscle tissue is repaired and rebuilt, is crucial for maintaining muscle health throughout life, and its efficiency can diminish with advancing age (Shen et al., 2023; Domingues-Faria et al., 2016; Conboy and Rando, 2005; Joanisse et al., 2016). Exercise, a well-established non-pharmacological intervention, has the potential to influence both circadian rhythms and muscle regeneration processes (Zisapel, 2007; Ciorciari, 2025; Wolff and Esser, 2019; Yamanaka et al., 2006).
Older adults often experience disruptions in their circadian rhythms and a decline in their capacity for muscle regeneration, which can significantly aid in the development of sarcopenia and cause a notable decline in quality of life (Domingues-Faria et al., 2016; Conboy and Rando, 2005; Marzetti, 2022; Colleluori and Villareal, 2021; Rodrigues et al., 2022). Given the increasing prevalence of sarcopenia in the aging population, identifying effective strategies to counteract this decline is crucial. Exercise, as a modifiable lifestyle factor, presents a promising avenue for intervention. Furthermore, the fact that disruptions in circadian rhythms are not merely a consequence of aging but can also contribute to conditions like sarcopenia suggests a potential cyclical relationship where a disturbed body clock might exacerbate muscle aging. Exercise’s potential as a non-drug treatment for circadian rhythm issues and muscle recovery underscores its importance as a vital research area, especially considering the limitations and potential side effects associated with pharmacological treatments for sarcopenia (Fernández-Martínez et al., 2023; Palmese et al., 2025; Vitale et al., 2019). This review will explore how strategically timed physical activity—a concept we will refer to as “Chrono-exercise”—can serve as a powerful intervention.
Furthermore, this review synthesizes the evidence on the interplay between exercise, circadian rhythms, and muscle regeneration in aging. Beyond summarization, we propose a novel conceptual framework—the “Chrono-Adaptive Framework for Muscle Health in Aging”—to integrate these components. This model provides a testable hypothesis on how strategically timed exercise can counteract age-related disruptions in hormonal and molecular pathways to optimize satellite cell function and muscle protein synthesis. The ultimate goal is to guide the development of targeted Chrono-exercise interventions aimed at alleviating sarcopenia and promoting healthy aging.
2 The science of circadian rhythms
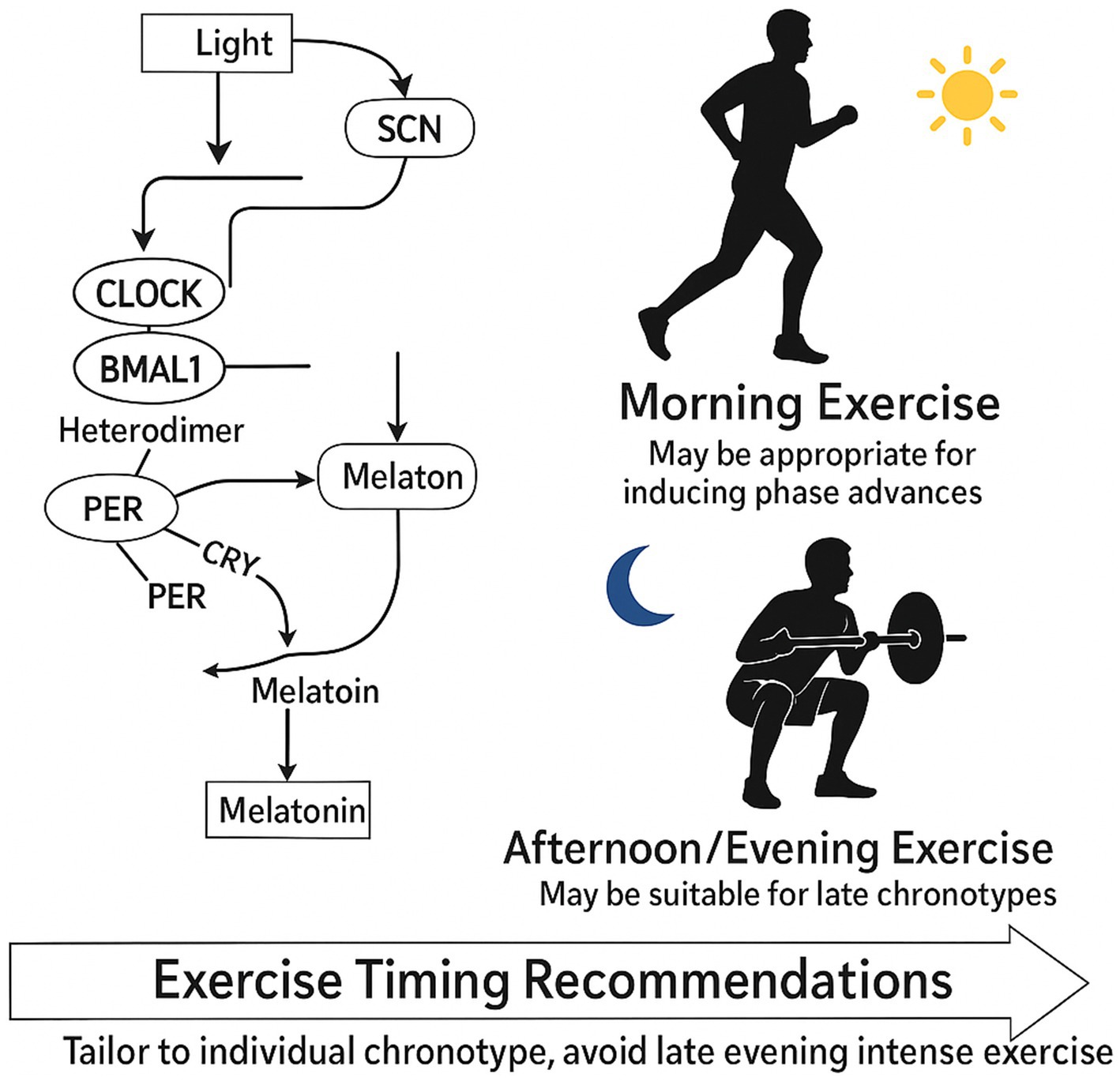
As a vital biological system, the circadian rhythm controls the timing of a wide array of physiological and behavioral activities, operating within a roughly 24-h cycle. At the center of this system is the suprachiasmatic nucleus (SCN), located in the hypothalamic region of the brain. The SCN, acting as the central pacemaker, interprets light information from the retina and ensures the coordination of the body’s internal time-regulating systems (Vitale et al., 2019; Patton and Hastings, 2018; Ono et al., 2021; Van Drunen and Eckel-Mahan, 2021; Meléndez-Fernández et al., 2023).
Circadian clocks are not limited to the central SCN; they also exist in peripheral tissues, including skeletal muscle, throughout the body (Shen et al., 2023; Zhang et al., 2009). These peripheral clocks possess the ability to function autonomously, maintaining their own rhythmic activity. However, their synchronization with the central clock and the external environment is crucial for overall physiological coordination (Dibner et al., 2010). The SCN’s role as the central pacemaker, along with the presence of local oscillators in peripheral tissues, ensures the balance between global coordination and tissue-specific responses to environmental signals (Begemann et al., 2020).
The regulation of these circadian rhythms, which approximate a 24-h cycle, is controlled by complex molecular processes that involve core clock genes such as BMAL1, CLOCK, PER (Period), and CRY (Cryptochrome). These genes contribute to the feedback loops that link transcription and translation (Andreani et al., 2015; Csép, 2021; BaHammam and Pirzada, 2023; Gabryelska et al., 2022; Costello and Gumz, 2021). BMAL1 and CLOCK proteins, by forming heterodimers, bind to targeted DNA sequences and promote the expression of the PER and CRY genes. Once the PER and CRY proteins accumulate in the cytoplasm, they translocate into the nucleus and inhibit the function of the BMAL1/CLOCK complex, effectively repressing their own gene expression (Langmesser et al., 2008; Qu et al., 2021; Schibler, 2021; Qu et al., 2023). The negative feedback cycle results in the periodic gene expression patterns that govern the biological processes of circadian rhythms (Rosbash, 2021). Disruptions in this fundamental molecular clockwork, whether occurring centrally or in the periphery, can have far-reaching consequences for health, as the precise timing of biological processes is essential for maintaining physiological balance. These kinds of misalignments have been found to be related to the occurrence of multiple diseases (Cornelissen and Hirota, 2024; Weston and Hood, 2004).
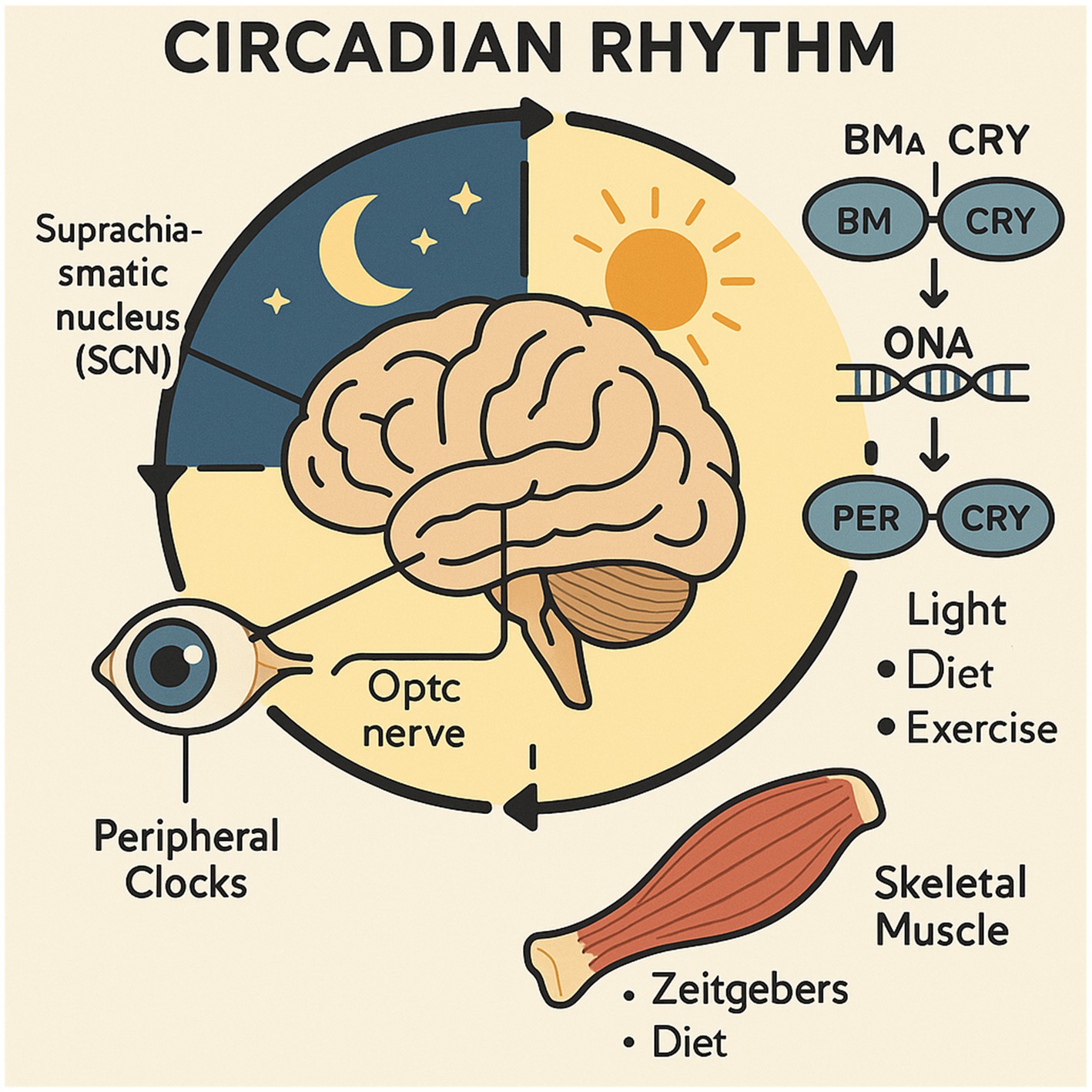
The circadian system is not solely driven by internal mechanisms; It is notably responsive to environmental cues, called zeitgebers, which serve to entrain or reset the body’s internal timekeeping mechanisms. The most potent zeitgeber is light, detected by the eyes and transmitted to the SCN. Beyond light, factors like the timing of food intake (diet) and physical activity (exercise) can effectively function as zeitgebers, with particular impact on peripheral clocks (Sharma and Chandrashekaran, 2005; Golombek and Rosenstein, 2010; Quante et al., 2019). The sensitivity of peripheral clocks to these non-photic cues, especially exercise, suggests that lifestyle interventions can directly impact tissue-specific circadian rhythms, potentially offering targeted therapeutic benefits for conditions affected by circadian disruption (Ciorciari, 2025; Fagiani et al., 2022; Neves et al., 2022; Figure 1).
The diagram illustrates the structure of the circadian rhythm system, with the brain at its core, where the suprachiasmatic nucleus (SCN), the master circadian clock, processes light input from the retina via the optic nerve. The SCN orchestrates the synchronization of peripheral timekeeping systems in the body, including those found in skeletal muscle. The core clock mechanism, detailed in the text, relies on a transcription-translation feedback loop involving key proteins like BMAL1/CLOCK and PER/CRY. External zeitgebers, such as light, diet, and physical activity, help synchronize central and peripheral clocks, ensuring the maintenance of physiological balance. Disruptions in this system may give rise to a variety of health disorders.
3 Skeletal muscle and the circadian clock
The skeletal muscle tissue features an internal circadian clock, evident in the expression of core clock genes present in muscle cells (Shen et al., 2023; Harfmann et al., 2015). The local circadian clock coordinates the timed expression of multiple genes within the muscle, including those involved in crucial processes such as muscle development, hypertrophy (growth), metabolism (the utilization of glucose and lipids for energy), and repair (Crislip et al., 2022; Schiaffino et al., 2013; Juliana et al., 2023; Morrison et al., 2022; Morena da Silva et al., 2024). Examples of these clock-controlled genes include MyoD, a key regulator of muscle differentiation, and genes involved in energy utilization like Ucp3 (Mayeuf-Louchart et al., 2015).
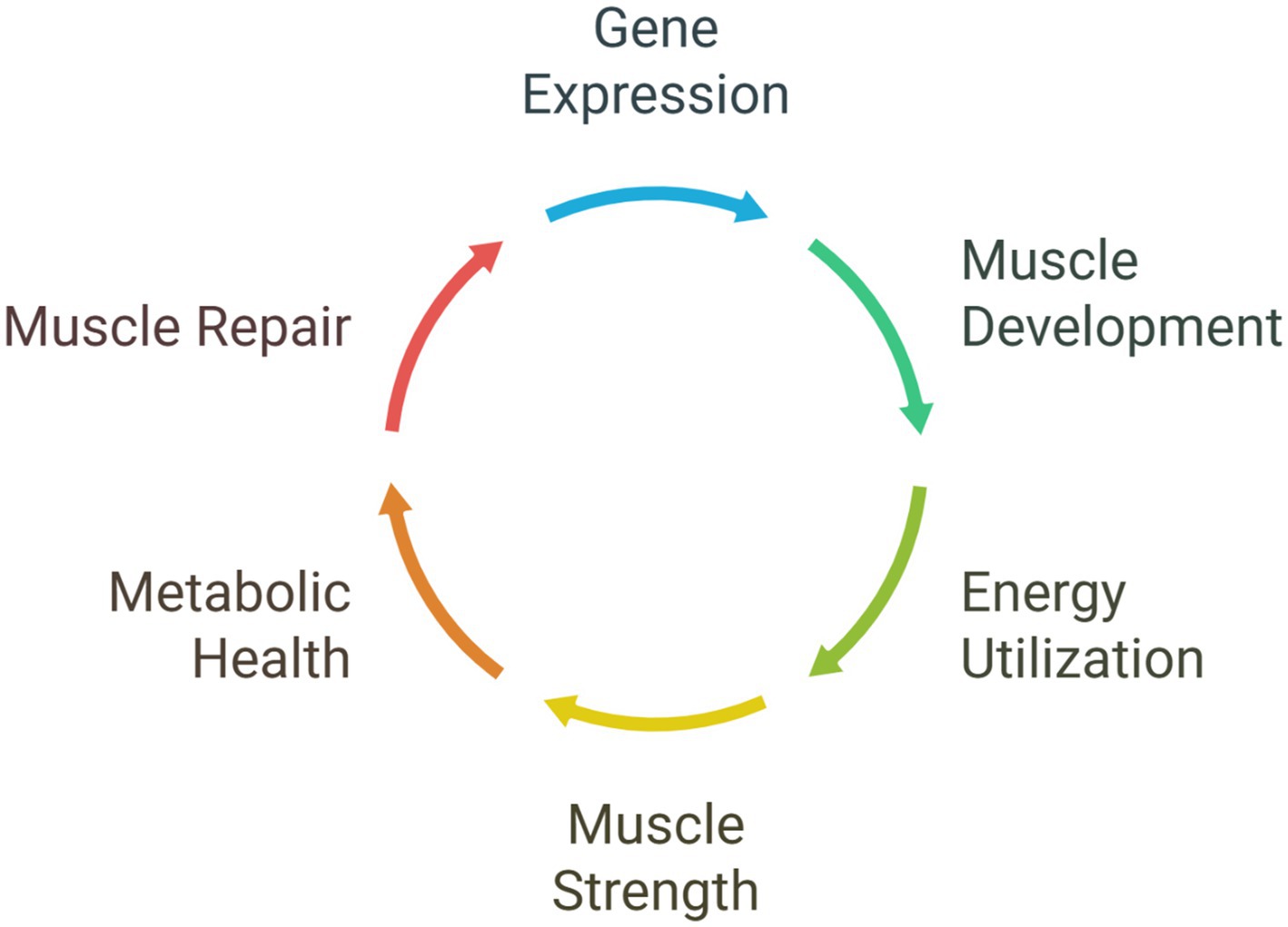
The existence of a functional circadian rhythm in skeletal muscle has substantial implications for its physiological activity. The muscle clock influences various aspects of muscle function throughout the day-night cycle, such as muscle strength and power, which tend to be at their greatest in the late afternoon or early evening (Zhang et al., 2009; Ashmore, 2019; Mirizio et al., 2020). It also regulates the metabolism of glucose and lipids, ensuring that energy substrates are available at the appropriate times to support muscle activity (Vitale et al., 2019). In addition, the muscle circadian clock is involved in the muscle tissue’s capacity to repair and regenerate after injury or exercise (Vitale et al., 2019; Tidball, 2011; Seene and Kaasik, 2013). The regulation of muscle-specific genes by circadian rhythms highlights the importance of the timing of activities, such as exercise, can profoundly impact muscle adaptation and performance by interacting with these rhythmic processes (Mansingh and Handschin, 2022; Pearson, 2000).
Beyond its local functions within the muscle tissue, a well-functioning muscle clock is also closely connected to overall metabolic health. It aids in maintaining insulin sensitivity, which enables cells to respond to insulin and uptake glucose from the bloodstream, while also regulating glucose absorption by muscle cells (Aoyama and Shibata, 2017; Merz and Thurmond, 2011). This connection between the muscle clock and metabolic health reinforces the significance of maintaining a well-regulated circadian rhythm in muscle to avoid metabolic disorders and optimize the body’s energy utilization. Disruptions to the muscle clock, conversely, can result in impaired muscle function and repair, potentially contributing to the age-associated decline in muscle mass and strength observed in sarcopenia (Shen et al., 2023; Juliana et al., 2023; Morrison et al., 2022; Morena da Silva et al., 2024). Just as a misaligned central clock can cause sleep disturbances, a disrupted muscle clock can impair the tissue’s ability to function and repair itself effectively (Morrison et al., 2022; Zelinski et al., 2014; Figure 2; Table 1).
4 Aging’s impact on circadian rhythms and muscle
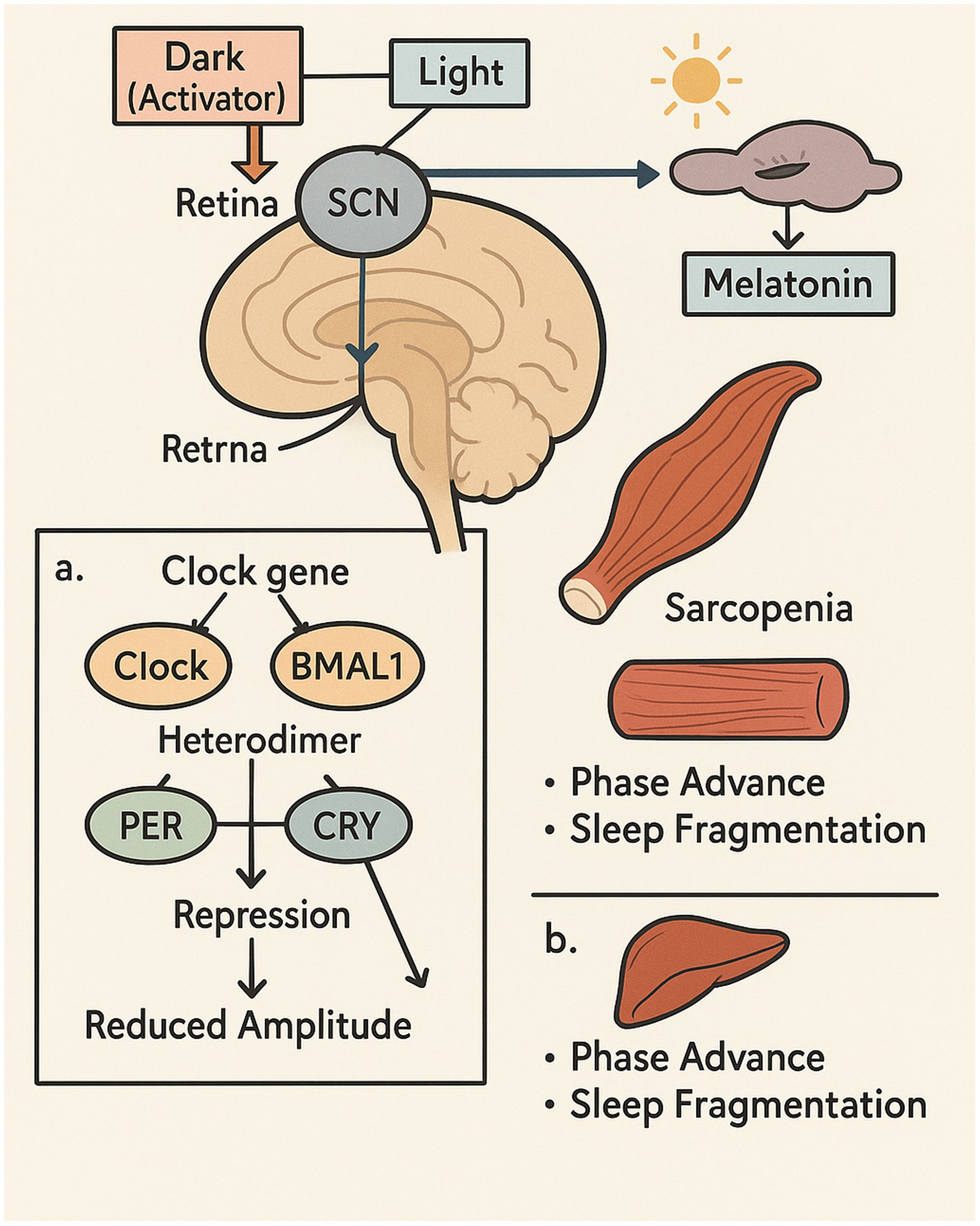
The aging process is often accompanied by significant alterations in circadian rhythms. One common change is a phase advance, where older adults tend to shift their sleep–wake cycle earlier, leading to earlier bedtimes and earlier wake-up times (Aoyama et al., 2021; Dijk et al., 2000). Additionally, the amplitude of these rhythms, describing the difference between the peak and the trough of daily fluctuations, often becomes reduced with age, resulting in less pronounced daily fluctuations in physiological processes (Brown et al., 2011). Sleep patterns in older adults also tend to become more fragmented, with increased nighttime awakenings and less time spent in deep, restorative sleep (Newsom and DeBanto, 2020). These age-related changes in circadian rhythms are thought to arise from a combination of factors, including alterations in the SCN, the central pacemaker, and a reduced sensitivity to external time cues such as light (Malhan et al., 2025; Farajnia et al., 2014).
Aging is concurrently associated with a progressive loss of skeletal muscle mass, strength, and function, a condition described as sarcopenia (Larsson et al., 2019). This decline in muscle mass can lead to major functional disabilities, increasing the likelihood of falls and limiting both mobility and independence. Various elements contribute to the progression of sarcopenia, including decreased physical activity, inadequate diet, increased inflammation, hormonal changes, and, importantly, disruptions in circadian rhythms (Shen et al., 2023; Billot et al., 2020; Dos Santos et al., 2017).
The interplay between these age-related changes in circadian rhythms and muscle can create a negative feedback loop, where disruptions in the body’s internal clock can further compromise muscle health and regeneration, potentially accelerating the progression of sarcopenia (Dudek et al., 2023). In older adults, fragmented sleep and reduced deep sleep can affect the levels of key anabolic hormones, such as growth hormone and testosterone, which play a crucial role in muscle protein synthesis and recovery (Aoyama et al., 2021; Zouhal et al., 2022). This combination of age-related impairments in both circadian rhythms and muscle regeneration underscores the significant challenge in maintaining muscle health in older adults and suggests that interventions targeting both of these aspects might be the most effective approach (Drăgoi et al., 2024; Daniels and Bonnechère, 2024). The earlier circadian phase observed in many older adults might also influence their optimal timing for physical activity and the impact of exercise on their circadian system compared to younger individuals, indicating a need for age-specific exercise recommendations. Furthermore, the reduced amplitude of circadian rhythms in older adults could potentially make them more susceptible to the negative consequences of circadian disruption and possibly less responsive to exercise as a modulator of their internal clock (Kripke et al., 2005). The interaction between age-related low-grade inflammation and the disruption of circadian rhythms can further impair muscle function and regeneration, highlighting the importance of interventions that address both of these interconnected factors (Shen et al., 2023; Colombini et al., 2022; Figure 3).
This illustration highlights the interplay between circadian rhythm alterations and muscle degeneration with aging. The SCN, the central circadian pacemaker, processes light signals from the retina, which in turn influences the secretion of melatonin from the pineal gland. With age, the circadian system undergoes a phase advance and reduced amplitude, driven by molecular disruptions in core clock genes such as CLOCK, BMAL1, PER, and CRY. These changes contribute to fragmented sleep and impaired hormonal regulation. In parallel, skeletal muscle exhibits signs of sarcopenia—characterized by reduced mass and strength—which is exacerbated by circadian misalignment. The figure emphasizes how age-induced circadian disruption impacts both central and peripheral tissues, suggesting the need for targeted interventions to support muscle health in older adults.
5 Exercise as a zeitgeber
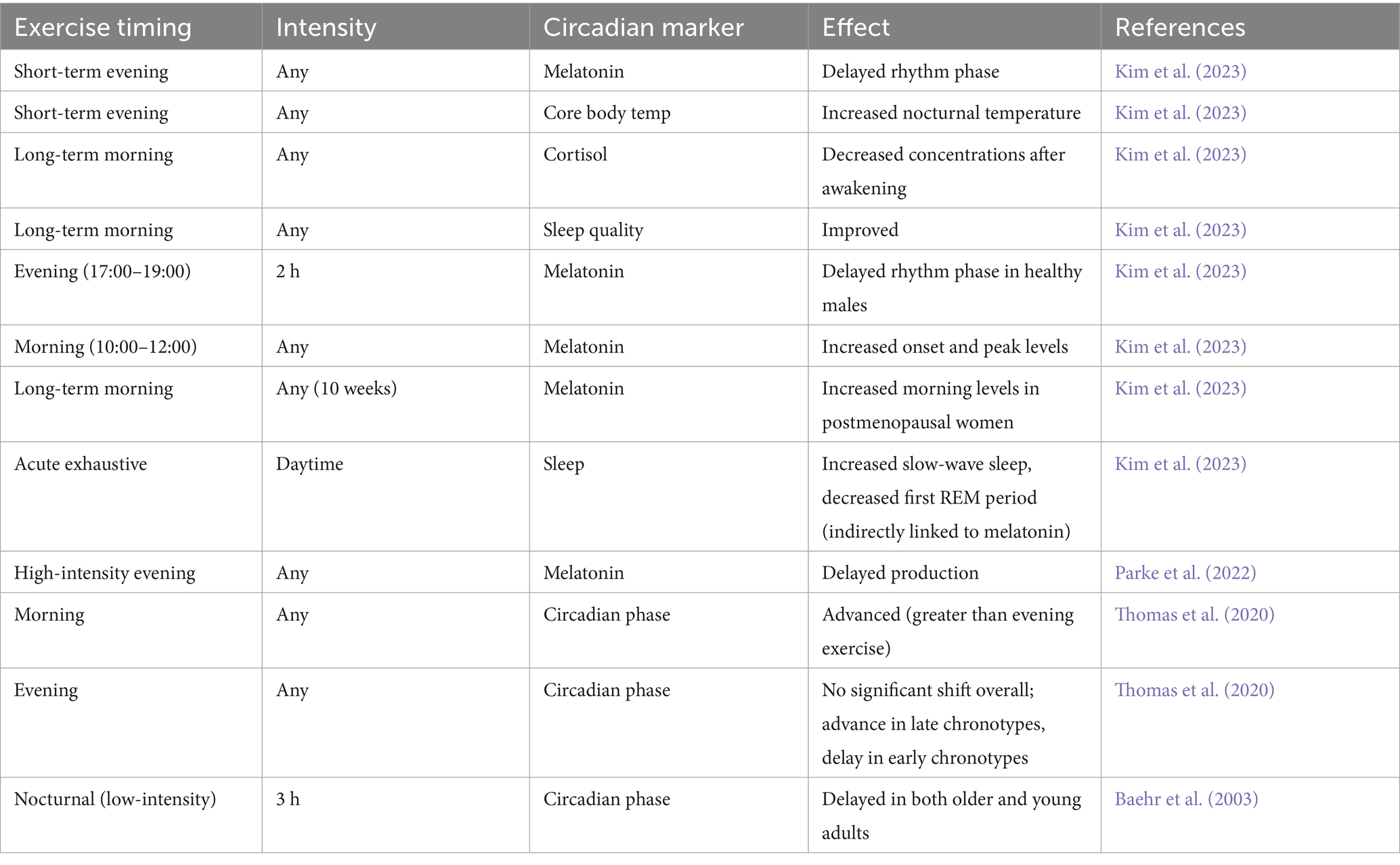
Beyond light, physical activity serves as a powerful non-photic zeitgeber capable of entraining circadian rhythms, particularly in peripheral tissues like skeletal muscle (Shen et al., 2023; Back et al., 2007; Bennett and Sato, 2023; Gabriel and Zierath, 2022; Maier et al., 2022). Different types of exercise, as well as their intensity and timing, can have varying effects on key circadian markers. For instance, while evening exercise can delay melatonin rhythm and increase core body temperature, studies consistently show that these physiological shifts often do not negatively impact, and may even improve, subsequent sleep quality (Drăgoi et al., 2024; Kim et al., 2023). Conversely, long-term engagement in morning exercise tends to lower cortisol levels after waking and enhance the quality of sleep (De Nys et al., 2022). High-intensity physical activity, even if performed just once in the evening, can inhibit the production of melatonin, a hormone that supports sleep (Parke et al., 2022).
Exercise timing is critical in influencing the direction and magnitude of changes in circadian phases. Studies have indicated that morning exercise generally induces a phase advance, shifting the circadian rhythm to an earlier time (Thomas et al., 2020; Eastman et al., 1995), although it is important to note that much of this foundational research was conducted in younger populations. The magnitude of this shift may therefore differ in older adults due to age-related changes in circadian amplitude and sensitivity to zeitgebers. Conversely, evening exercise might lead to a phase delay in some individuals, although the effects can vary depending on an individual’s chronotype (Thomas et al., 2020). For example, Conversely, evening exercise might lead to a phase delay in some individuals, although the effects can vary depending on an individual’s chronotype (Thomas et al., 2020; Glavin et al., 2021).
On a molecular scale, exercise has the potential to influence the circadian clock in both the central SCN and peripheral tissues, such as skeletal muscle. It has been shown to alter the expression of essential clock genes, including BMAL1 and PER2 (Shen et al., 2023; Schroder and Esser, 2013). Exercise-induced factors like heat, glucocorticoid release, and mechanical loading may play pivotal roles in entraining the circadian rhythm of connective tissues (Steffen et al., 2024). Exercise entrainment may trigger molecular pathways like AMPK (AMP-activated protein kinase) activation and the induction of HIF-1α (hypoxia-inducible factor 1-alpha), which can regulate the stability of clock proteins and the expression of circadian genes (Maier, 2020; Yan et al., 2025; Post et al., 2025; Gabryelska et al., 2025; Moon and Jeong, 2023; Cho et al., 2023; Pourabdi et al., 2025; Chen et al., 2025). Exercise can also act as a synchronizer for peripheral clocks in tissues like skeletal muscle, potentially helping to reset disrupted rhythms (Choi et al., 2020).
The bidirectional relationship between exercise and sleep quality is essential for the modulation of circadian rhythms. Regular physical activity generally promotes better sleep by helping to regulate the circadian rhythm, reducing stress, and promoting relaxation (Chennaoui et al., 2015). However, intense exercise performed too close to bedtime might be disruptive for some individuals due to increased body temperature and alertness (Parke et al., 2022). This draws attention to the need for customized exercise prescriptions that account for a person’s chronotype and intended objectives (Thomas et al., 2020). Exercise’s capacity to entrain peripheral clocks independent of the central clock suggests that tailored exercise interventions may be effective in enhancing the function of specific tissues affected by circadian rhythm disruptions. Furthermore, the interaction between exercise and sleep quality is crucial, as improved sleep can reinforce a healthy circadian cycle, and appropriately timed exercise can contribute to better sleep (Wolff and Esser, 2019; Figure 4). The effects of exercise timing on various circadian markers are summarized in Table 1. However, it is crucial to interpret these findings with caution, as the cited studies vary significantly in their methodologies. Key differences include the duration of the intervention (e.g., acute single bouts vs. long-term training), exercise intensity and modality, and the specific populations studied (e.g., healthy young men, postmenopausal women, older adults). This heterogeneity complicates direct comparisons and the formulation of universal guidelines. Furthermore, many studies rely on a limited number of central circadian markers, such as melatonin and core body temperature, and may not fully capture the influence of exercise on peripheral clocks within skeletal muscle itself. This highlights a critical gap in the literature and underscores the need for more standardized research in diverse age groups.
6 Exercise and muscle regeneration
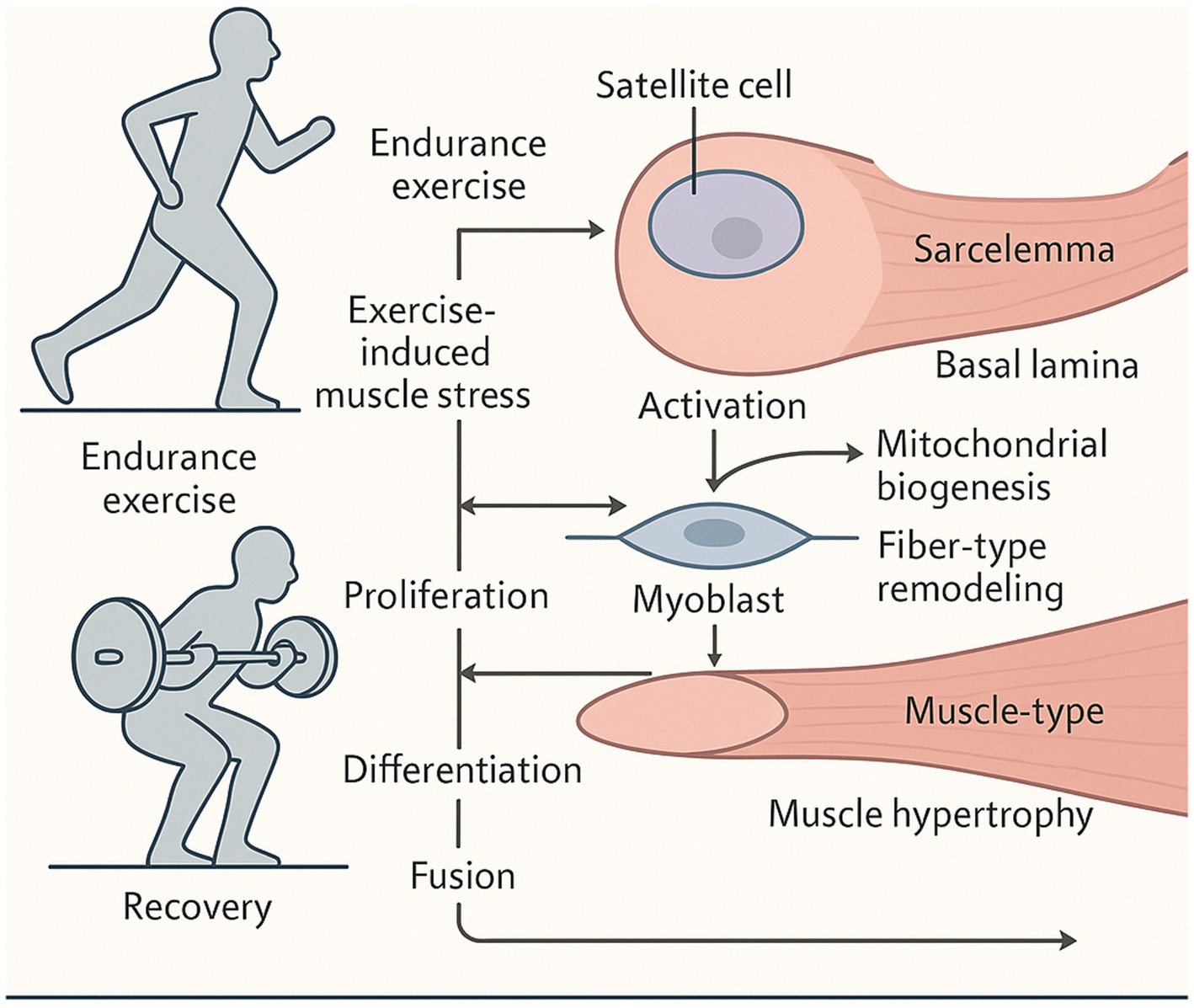
Physical activity, with a focus on resistance and endurance training, plays a significant role in stimulating muscle regeneration (Von Ruff et al., 2025). This process encompasses the activation of muscle protein synthesis, the stimulation of satellite cells (specialized muscle stem cells), and the subsequent regeneration and expansion of muscle fibers (Hu et al., 2022; Liu et al., 2023; Chen et al., 2022; Kaczmarek et al., 2021). Exercise triggers various molecular pathways that contribute to muscle regeneration, including the generation of oxidative stress, metabolic reprogramming within muscle cells, and the involvement of microRNAs (Negri et al., 2025).
Situated between the sarcolemma (muscle cell membrane) and the basal lamina of the myofiber, satellite cells are integral to the process of muscle regeneration (Dumont et al., 2015). In response to muscle damage from exercise, quiescent stem cells are activated, proliferate, and differentiate into myoblasts, which either fuse with existing damaged fibers or form new muscle fibers, facilitating muscle tissue repair and regeneration (Kaczmarek et al., 2021).
Different types of exercise can influence muscle regeneration in distinct ways. Endurance exercise, including activities like running and cycling, induces muscle fiber-type regeneration and enhances mitochondrial biogenesis within muscle cells, supporting muscle recovery after injury (Hu et al., 2022; Oliveira et al., 2021; Lippi et al., 2022; Pellegrino et al., 2022; Chatzinikita et al., 2023). Resistance training, where the body works against external resistance, is particularly efficient in stimulating muscle hypertrophy, contributing to an increase in muscle size and strength (Sayer et al., 2021).
Allowing adequate recovery time post-exercise is essential for the body to recover and adjust to the stresses introduced during the workout (Bouchard, 2021). During recovery, the body works to replenish energy supplies, repair muscle damage, and return hormone levels to baseline. Without sufficient rest, the body may not have enough time to fully recover, potentially leading to injuries or decreased performance (In and Vasanthi, 2024). Exercise, therefore, acts as a potent stimulus for muscle regeneration through multiple pathways, highlighting its therapeutic potential for combating muscle loss associated with aging and other conditions (Chen et al., 2022). The activation of satellite cells by exercise is a critical step in this process, suggesting that interventions aimed at enhancing satellite cell function could improve muscle repair capacity, especially in older adults where their activity might be impaired (Kaczmarek et al., 2021; Negri et al., 2025). The need for adequate recovery after exercise underscores the importance of balancing training load with sufficient rest to allow for muscle repair and prevent overtraining, particularly in older adults who might have slower recovery rates (Kumar and Vinayakan, 2024; Bushman, 2024; Figure 5).
This figure illustrates the mechanisms by which exercise stimulates muscle regeneration. Exercise-induced stress activates resident muscle stem cells (satellite cells), which then proliferate, differentiate, and fuse with muscle fibers to facilitate repair and growth. Different exercise types elicit distinct outcomes; endurance training enhances mitochondrial biogenesis while resistance training primarily drives hypertrophy. Key regulators include oxidative stress, metabolic shifts, and microRNAs. Adequate recovery is crucial for completing the regenerative process, highlighting exercise’s therapeutic potential against sarcopenia.
7 The interplay in older adults
The capacity for exercise to induce muscle regeneration can be influenced by age. Older adults may experience impairments in their muscle regeneration response to exercise, including a reduction in the activity of satellite cells and a blunted rate of muscle protein synthesis (Shen et al., 2023; Peake et al., 2010). Specifically, the age-related circadian disruptions discussed previously can dampen the anabolic response to exercise, thereby compromising muscle regeneration (Silva et al., 2021). For instance, the disrupted sleep patterns common in older adults can negatively impact the levels of anabolic hormones that are essential for muscle repair processes (Aoyama et al., 2021; Zouhal et al., 2022; Oliveira et al., 2021; Franzago et al., 2023; Watson et al., 2021; Sansone and Romanelli, 2021).
Given these challenges, strategically timed exercise emerges as a potential countermeasure. By carefully considering the timing of physical activity, it might be possible to modulate circadian rhythms in older adults in a way that optimizes their capacity for muscle regeneration (Dose et al., 2023). The interplay of age-related deficits in circadian rhythms and muscle regeneration represents a major challenge to sustaining muscle health in older adults, suggesting that interventions that address both aspects simultaneously might be the most effective (Agostini et al., 2023). Exercise timing appears to be a critical factor in optimizing muscle regeneration in this population by potentially aligning physical activity with more favorable circadian phases for muscle repair and growth (Drăgoi et al., 2024). Although research on the interplay of circadian rhythms, exercise, and muscle regeneration in older adults is still evolving, there is a clear need for further studies aimed at this particular population (Morrison et al., 2022).
8 A conceptual model: the Chrono-adaptive framework for muscle health in aging
To synthesize the complex interactions discussed, we propose the Chrono-Adaptive Framework for Muscle Health in Aging (Figure 6). This model provides a conceptual basis for understanding how timed exercise interventions can mitigate age-related muscle decline. The framework posits that the efficacy of exercise in older adults can be significantly enhanced by tailoring its timing to achieve specific biological goals: either reinforcing central rhythms or capitalizing on windows of peak peripheral function.
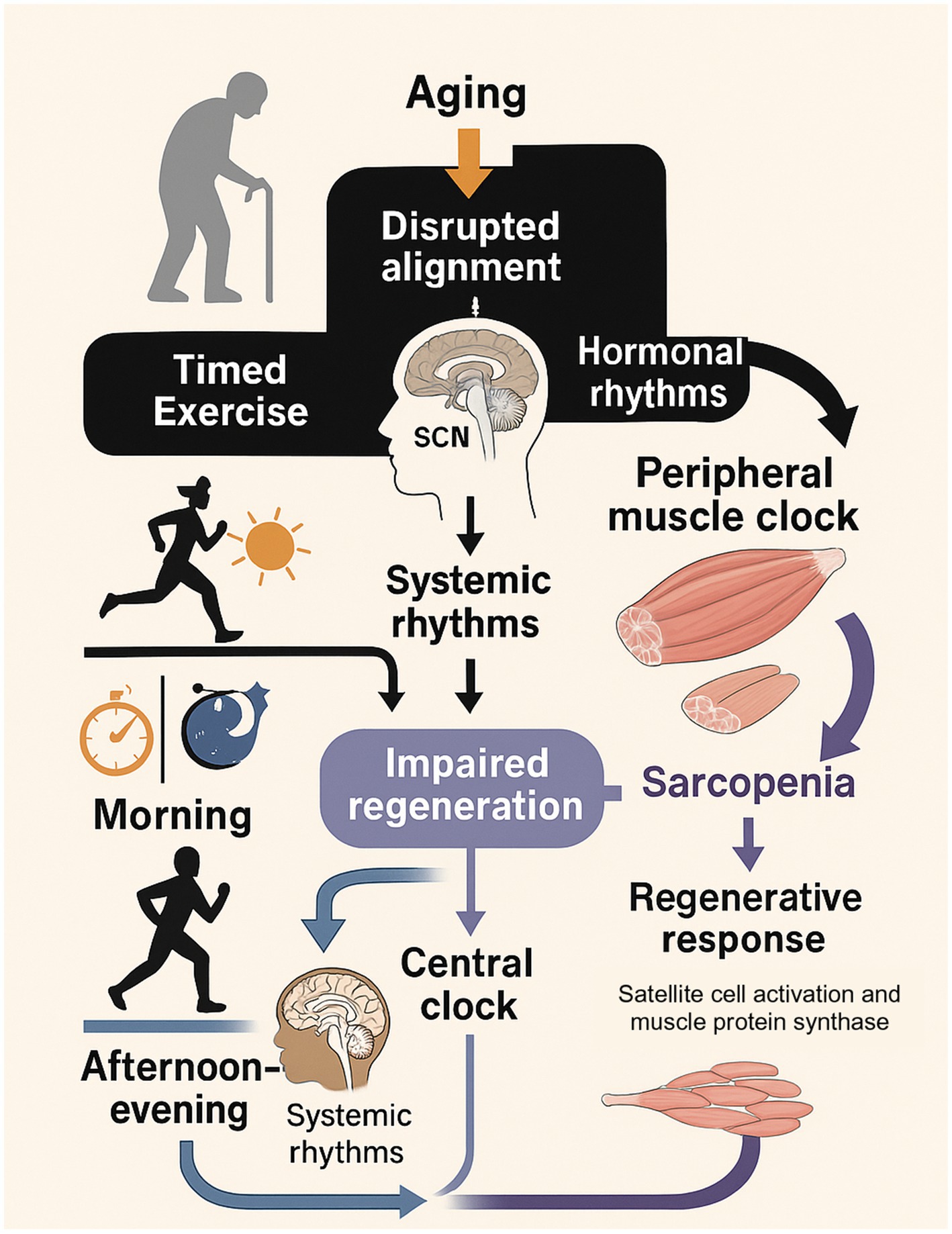
Figure 6. The Chrono-adaptive framework for muscle health in aging. This diagram illustrates how aging disrupts the alignment between the central clock (SCN), peripheral muscle clocks, and hormonal rhythms, leading to impaired muscle regeneration and sarcopenia. Timed exercise is presented as a key intervention. Morning exercise primarily targets the central clock and systemic rhythms to improve sleep and hormonal profiles. Afternoon/evening exercise is shown to align with peaks in peripheral muscle clock function and performance, directly optimizing the local regenerative response (satellite cell activation and muscle protein synthesis). The model proposes that by strategically timing exercise, it is possible to counteract age-related disruptions and enhance the overall adaptive response, thereby promoting muscle health. Draw scientific illustration for this.
The framework is built on two primary pathways:
1. Central Rhythm Reinforcement: Morning exercise is proposed to act as a powerful zeitgeber for the central SCN clock. This helps to correct the phase-advances and dampened amplitudes common in aging, leading to improved sleep architecture and the restoration of a more robust anabolic hormonal milieu (e.g., nocturnal growth hormone release) (Hower et al., 2018; Shen et al., 2023). This systemic effect creates a more favorable internal environment for muscle repair.
2. Peripheral Anabolic Optimization: Afternoon/evening exercise is proposed to align with the natural peaks in peripheral muscle clock function, neuromuscular performance, and core body temperature. This timing may directly maximize the local stimulus for muscle protein synthesis and satellite cell activation by capitalizing on the period when the muscle is most physiologically prepared for an anabolic challenge (Silva et al., 2021; Augsburger et al., 2025; Procopio and Esser, 2025).
By strategically choosing exercise timing based on these pathways, the model suggests it is possible to counteract the anabolic resistance of aging and promote more effective muscle regeneration.
9 Chrono-exercise for muscle health in older adults
It is crucial to state upfront that the following recommendations are largely extrapolated from mechanistic studies, short-term trials, and research in younger populations. Robust, long-term clinical trials that definitively establish the optimal exercise timing to combat sarcopenia in older adults are currently lacking. Therefore, these suggestions should be interpreted as a theoretical framework to guide future research and personalized approaches, rather than as definitive clinical guidelines.
Based on current research, the optimal timing for exercise in older adults may depend on the desired physiological outcome, which is rooted in molecular rhythms. For instance, morning exercise may be particularly effective for reinforcing the central clock in the SCN. On a molecular level, this strong zeitgeber input helps stabilize the expression of core clock genes like Per2, leading to a robust cortisol awakening response and properly timed melatonin suppression. This central alignment improves sleep quality, which in turn promotes the nocturnal release of anabolic hormones like growth hormone, creating a favorable systemic environment for muscle repair (Hower et al., 2018; Shen et al., 2023). Furthermore, exercising during the early active phase aligns with the natural peak expression of the transcriptional activator BMAL1, a master regulator of metabolic pathways essential for muscle energy utilization (Ehrlich et al., 2025; Viggars et al., 2024).
Conversely, scheduling resistance training in the late afternoon or early evening may better align with the peripheral muscle clock’s rhythms. This period often corresponds to the peak expression of genes involved in glycolysis, mitochondrial function, and muscle contractility, providing a molecular basis for the observed afternoon peak in strength and power (Ashmore, 2019; Mirizio et al., 2020; Negri et al., 2025; Mansingh and Handschin, 2022; Smith et al., 2023). Moreover, some evidence suggests that the key anabolic signaling pathway mTORC1, a critical driver of muscle protein synthesis, may be more responsive to the stimulus of resistance exercise later in the active phase, potentially leading to greater hypertrophic gains (Ashmore, 2019). A study in older adults also found that afternoon exercise was more effective in improving postural control, highlighting a functional benefit to this timing (Shen et al., 2023).
The importance of individual chronotype cannot be overstated when recommending exercise timing (Vitale and Weydahl, 2017). Late chronotypes, who naturally have a later sleep–wake cycle, might find evening exercise more suitable and potentially beneficial for inducing phase advances (Rutkowska et al., 2024). Conversely, early chronotypes might experience better outcomes with morning activity and should possibly avoid intense evening workouts that could further delay their circadian rhythm (Goldin et al., 2020).
For older adults seeking to optimize muscle regeneration and align their circadian rhythms, some practical recommendations can be made (Del Río, 2025). Performing moderate-intensity aerobic exercise regularly, such as brisk walking for 30 min or more on most days, is usually beneficial for both general health and sleep patterns (Li et al., 2025). Regular resistance training involving the activation of principal muscle groups no fewer than 2 days per week is integral to the maintenance and augmentation of muscle tissue (Von Ruff et al., 2025). The timing of these workouts can be experimented with to see what feels best for the individual and how it affects their sleep and energy levels. The recommendation to avoid strenuous exercise close to bedtime is common, based on its potential to interfere with sleep onset (Parke et al., 2022; Driver and Taylor, 2000). However, this view is not universally supported, and the literature presents conflicting evidence. In fact, some studies report that evening exercise can be performed without detriment to sleep quality and may even be beneficial for older adults by enhancing deep, slow-wave sleep (Kim et al., 2023). This discrepancy highlights that individual responses can vary significantly, likely influenced by factors such as personal chronotype, fitness level, and exercise intensity. Therefore, while low-impact activities like yoga or light stretching are excellent for evening relaxation (World Health Organization, 2023), the suitability of higher-intensity evening exercise should be determined on an individual basis rather than broadly discouraged.
To maintain muscle function in older adults, established exercise protocols recommend participating in muscle-strengthening routines at least 2 days each week, focusing on comprehensive engagement of major muscle groups (Liu, 2025). The intensity should be moderate to high, using weights or resistance that allows for 6–12 repetitions per set (Hurst et al., 2022). Consistency in adhering to a regular exercise routine is more critical than the specific time of day for many individuals (Gaesser et al., 2025). Tailoring exercise timing to the natural circadian rhythms of older adults could enhance the effectiveness of exercise interventions for muscle health and potentially improve adherence by aligning with their energy levels and preferences (Woodard and Berry, 2001). While general recommendations can be made, the optimal exercise timing for muscle regeneration in older adults likely depends on a complex interplay of individual factors, including chronotype, sleep patterns, health status, and lifestyle. While the potential benefits outlined in Table 2 are promising, it is important to acknowledge that current recommendations are largely extrapolated from studies on younger individuals or from short-term interventions in older adults with varied health statuses. The direct, long-term impact of Chrono-exercise on sarcopenia-related outcomes remains under-investigated. Defining robust, evidence-based exercise timing protocols tailored to support muscle regeneration in older adults—considering the heterogeneity in chronotypes, comorbidities, and baseline fitness levels—requires further systematic, longitudinal research with clinically relevant endpoints such as muscle mass, strength, and physical function (Izquierdo et al., 2021; Figure 7).
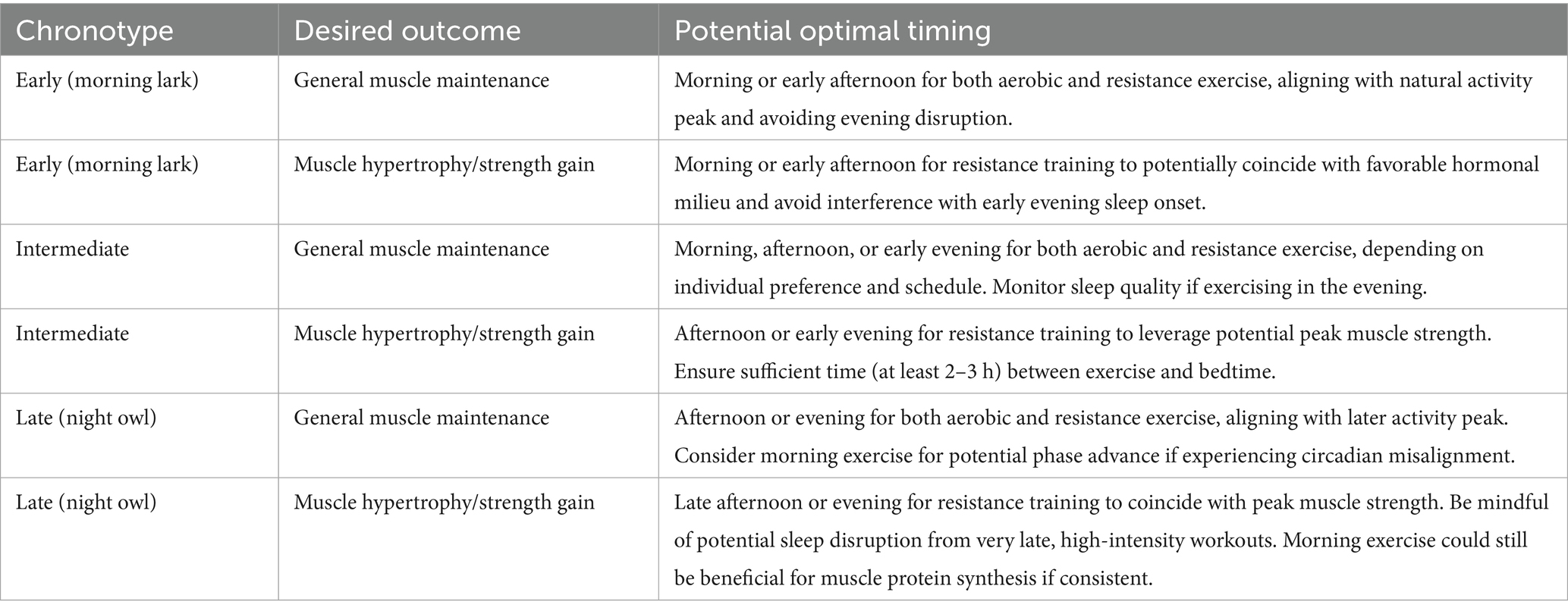
Table 2. Potential optimal exercise timings for muscle regeneration in older adults based on chronotype and desired outcome.
This illustration shows how light signals regulate the circadian clock via the SCN, influencing CLOCK–BMAL1 activity and melatonin production in a ~ 24-h cycle. On the right, morning exercise is recommended for phase advancement and improved sleep, especially in early chronotypes, while afternoon/evening exercise may suit late chronotypes and support strength gains. Tailoring exercise timing to chronotype and avoiding late evening intensity can optimize muscle health and circadian alignment.
10 The role of sleep and nutrition
Ensuring adequate sleep contributes significantly to the physiological processes involved in muscle healing and regeneration (Webhofer, 2025). Growth hormone activity during sleep is a critical mechanism underlying the recovery and structural renewal of muscle tissue (Lambert, 2025). Conversely, sleep disturbances can disrupt circadian rhythms, potentially hindering the muscle regeneration process (Shen et al., 2023; Morrison et al., 2022). Sustaining appropriate sleep hygiene—characterized by steady sleep timing and tranquil pre-sleep activities—is fundamental for promoting muscle health in older adults (Winegar, 2024).
The intake of adequate protein as part of a balanced diet is fundamental to promoting muscle protein synthesis and the restoration of muscle tissue (Lu et al., 2021). Consuming 25 to 30 grams of protein with each meal may help prevent sarcopenia (Li et al., 2025). Furthermore, the timing of nutrient intake, a concept known as Chrono-nutrition, can influence circadian rhythms and muscle metabolism (Negri et al., 2025). Strategically timed protein intake—whether aligned with circadian cues or following exercise—plays a role in activating the pathways responsible for initiating muscle protein synthesis (Drăgoi et al., 2024; Thomas et al., 2020). Restricting eating to biologically active times has the potential to reinstate proper temporal patterns of gene expression in skeletal muscle (Cardinali, 2019). Optimizing both sleep and nutrition are essential complementary strategies to exercise for promoting muscle health in older adults by supporting both muscle regeneration and a healthy circadian rhythm. According to the principles of Chrono-nutrition, harmonizing nutrient timing with the circadian system could further optimize muscle regeneration and metabolic well-being in elderly individuals (Negri et al., 2025).
11 Conclusion and future directions
In summary, the interplay between exercise, circadian rhythms, and muscle regeneration is critical for healthy aging. As outlined in our proposed Chrono-Adaptive Framework, timed exercise serves as a powerful, non-pharmacological tool to counteract age-related disruptions in circadian and anabolic signaling. By aligning physical activity with internal biological rhythms, it may be possible to enhance muscle repair and combat sarcopenia. Age-related changes in both circadian rhythms and muscle physiology create a challenging scenario, but one that may be overcome with strategic intervention. Time-specific exercise interventions, designed according to chronotype and therapeutic objectives, could act as viable non-pharmacological tools to recalibrate disrupted circadian patterns and facilitate the muscle healing process. The interplay between exercise, circadian rhythms, sleep, and nutrition underscores the need for a holistic approach to promoting muscle health in the aging population.
While acknowledging that the supporting evidence remains preliminary, insights derived from this report present meaningful applications that could guide practical interventions for promoting health in the elderly population. Tailoring exercise routines to align with their natural circadian rhythms, such as considering morning exercise for phase advancement and potential sleep benefits, or afternoon/early evening exercise for capitalizing on peak muscle strength, could enhance the effectiveness of physical activity. Paying attention to individual chronotype and avoiding intense exercise close to bedtime are also important considerations for optimizing both circadian alignment and sleep quality, which are crucial for muscle health.
Ongoing research should aim to explore essential dimensions of the interaction between exercise, biological rhythms, and muscle restoration in older individuals to provide clearer insights into this complex relationship. Future longitudinal research should focus on evaluating the long-term effects of exercise with consistent timing on both muscular health and circadian markers in older populations. Further clinical trials are essential to examine the role of Chrono-exercise interventions in both preventing and managing sarcopenia. Continued research into the molecular pathways that link exercise, circadian rhythm modulation in muscle, and the resulting effects on muscle stem cell behavior and regeneration during aging is necessary for a comprehensive understanding. Research on the combined benefits of timed exercise, sleep optimization strategies, and Chrono-nutrition on muscle health in older adults could lead to more comprehensive and effective interventions. Finally, studies investigating the influence of individual chronotype on the response to Chrono-exercise interventions in older populations will be crucial for developing personalized recommendations (Tables 1, 2).
Author contributions
ZS: Conceptualization, Investigation, Validation, Writing – review & editing, Supervision, Methodology, Visualization, Data curation, Writing – original draft. LX: Writing – review & editing, Methodology, Writing – original draft, Project administration, Data curation, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Research Project of Humanities and Social Sciences, Ministry of Education in 2024 (24YJE890001) and Science Research Key Project of Anhui Provincial Department of Education in 2023 (2023AH051526).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agostini, D., Gervasi, M., Ferrini, F., Bartolacci, A., Stranieri, A., Piccoli, G., et al. (2023). An integrated approach to skeletal muscle health in aging. Nutrients 15:1802. doi: 10.3390/nu15081802
Andreani, T. S., Itoh, T. Q., Yildirim, E., Hwangbo, D.-S., and Allada, R. (2015). Genetics of circadian rhythms. Sleep Med. Clin. 10, 413–421. doi: 10.1016/j.jsmc.2015.08.007
Aoyama, S., Nakahata, Y., and Shinohara, K. (2021). Chrono-nutrition has potential in preventing age-related muscle loss and dysfunction. Front. Neurosci. 15:659883. doi: 10.3389/fnins.2021.659883
Aoyama, S., and Shibata, S. (2017). The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front. Neurosci. 11:63. doi: 10.3389/fnins.2017.00063
Ashmore, A. (2019). Timing resistance training: Programming the muscle clock for optimal performance : Human Kinetics.
Augsburger, G. R., Sobolewski, E. J., Escalante, G., and Graybeal, A. J. (2025). Circadian regulation for optimizing sport and exercise performance. Clocks Sleep 7. doi: 10.3390/clockssleep7020018
Back, F. A., Fortes, F. S., Santos, E. H. R., Tambelli, R., Menna-Barreto, L. S., and Louzada, F. M. (2007). Non-photic synchronization: the effect of aerobic physical exercise. Rev. Bras. Med. Esporte 13, 138–142.
Baehr, E. K., Eastman, C. I., Revelle, W., Olson, S. H. L., Wolfe, L. F., and Zee, P. C. (2003). Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. Am. J. Phys. Regul. Integr. Comp. Phys. 284, R1542–R1550. doi: 10.1152/ajpregu.00761.2002
BaHammam, A. S., and Pirzada, A. (2023). Timing matters: the interplay between early mealtime, circadian rhythms, gene expression, circadian hormones, and metabolism—a narrative review. Clocks Sleep 5, 507–535. doi: 10.3390/clockssleep5030034
Begemann, K., Neumann, A. M., and Oster, H. (2020). Regulation and function of extra-SCN circadian oscillators in the brain. Acta Physiol. 229:e13446. doi: 10.1111/apha.13446
Bennett, S., and Sato, S. (2023). Enhancing the metabolic benefits of exercise: is timing the key? Front. Endocrinol. 14:987208. doi: 10.3389/fendo.2023.987208
Billot, M., Calvani, R., Urtamo, A., Sánchez-Sánchez, J. L., Ciccolari-Micaldi, C., Chang, M., et al. (2020). Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin. Interv. Aging 15, 1675–1690. doi: 10.2147/CIA.S253535
Bouchard, D. R. (2021). Exercise and physical activity for older adults : Human Kinetics Publishers.
Brown, S. A., Schmitt, K., and Eckert, A. (2011). Aging and circadian disruption: causes and effects. Aging 3, 813–817. doi: 10.18632/aging.100366
Cardinali, D. P. (2019). Melatonin as a chronobiotic/cytoprotector: its role in healthy aging. Biol. Rhythm. Res. 50, 28–45. doi: 10.1080/09291016.2018.1491200
Chatzinikita, E., Maridaki, M., Palikaras, K., Koutsilieris, M., and Philippou, A. (2023). The role of mitophagy in skeletal muscle damage and regeneration. Cells 12:716. doi: 10.3390/cells12050716
Chen, J., Xiang, J., Zhou, M., Huang, R., Zhang, J., Cui, Y., et al. (2025). Dietary timing enhances exercise by modulating fat-muscle crosstalk via adipocyte AMPKα2 signaling. Cell Metab. 37, 1364–1380.e6. doi: 10.1016/j.cmet.2025.02.007
Chen, J., Zhou, R., Feng, Y., and Cheng, L. (2022). Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct. Target. Ther. 7:383. doi: 10.1038/s41392-022-01233-2
Chennaoui, M., Arnal, P. J., Sauvet, F., and Léger, D. (2015). Sleep and exercise: a reciprocal issue? Sleep Med. Rev. 20, 59–72. doi: 10.1016/j.smrv.2014.06.008
Cho, Y.-H., Lee, J.-Y., Seo, T.-B., Cho, Y.-H., Lee, J.-Y., and Seo, T.-B. (2023). Effects of exercise sequence and circadian rhythms on molecular mechanisms of muscle hypertrophy and mitochondrial biogenesis in obese rat. Exerc. Sci. 32, 347–353. doi: 10.15857/ksep.2023.00367
Choi, Y., Cho, J., No, M.-H., Heo, J.-W., Cho, E.-J., Chang, E., et al. (2020). Re-setting the circadian clock using exercise against sarcopenia. Int. J. Mol. Sci. 21:3106. doi: 10.3390/ijms21093106
Ciorciari, A. M. From clocks to exercise, from metabolism to performance: a chronophysiological approach. (2025).
Colleluori, G., and Villareal, D. T. (2021). Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 155:111561. doi: 10.1016/j.exger.2021.111561
Colombini, B., Dinu, M., Murgo, E., Lotti, S., Tarquini, R., Sofi, F., et al. (2022). Ageing and low-level chronic inflammation: the role of the biological clock. Antioxidants. 11:2228. doi: 10.3390/antiox11112228
Conboy, I. M., and Rando, T. A. (2005). Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 4, 407–410. doi: 10.4161/cc.4.3.1518
Cornelissen, G., and Hirota, T. (2024). Chronobiology and chronomedicine: from molecular and cellular mechanisms to whole body interdigitating networks : Royal Society of Chemistry.
Costello, H. M., and Gumz, M. L. (2021). Circadian rhythm, clock genes, and hypertension: recent advances in hypertension. Hypertension 78, 1185–1196. doi: 10.1161/HYPERTENSIONAHA.121.14519
Crislip, G. R., Johnston, J. G., Douma, L. G., Costello, H. M., Juffre, A., Boyd, K., et al. (2022). Circadian rhythm effects on the molecular regulation of physiological systems. Compr. Physiol. 12, 2769–2798. doi: 10.1002/j.2040-4603.2022.tb00198.x
Csép, K. (2021). Transcription factors of the core feedback loop in the molecular circadian clock machinery: internal timekeeping and beyond. Acta Marisiensis Ser. Med. 67.
Daniels, K., and Bonnechère, B. (2024). Harnessing digital health interventions to bridge the gap in prevention for older adults. Front. Public Health 11:1281923. doi: 10.3389/fpubh.2023.1281923
De Nys, L., Anderson, K., Ofosu, E. F., Ryde, G. C., Connelly, J., and Whittaker, A. C. (2022). The effects of physical activity on cortisol and sleep: a systematic review and meta-analysis. Psychoneuroendocrinology 143:105843. doi: 10.1016/j.psyneuen.2022.105843
Del Río, E. (2025). Rethinking osteoarthritis management: synergistic effects of Chronoexercise, circadian rhythm, and Chondroprotective agents. Biomedicine 13:598. doi: 10.3390/biomedicines13030598
Dibner, C., Schibler, U., and Albrecht, U. (2010). The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549. doi: 10.1146/annurev-physiol-021909-135821
Dijk, D.-J., Duffy, J. F., and Czeisler, C. A. (2000). Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 17, 285–311. doi: 10.1081/CBI-100101049
Domingues-Faria, C., Vasson, M.-P., Goncalves-Mendes, N., Boirie, Y., and Walrand, S. (2016). Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res. Rev. 26, 22–36. doi: 10.1016/j.arr.2015.12.004
Dos Santos, L., Cyrino, E. S., Antunes, M., Santos, D. A., and Sardinha, L. B. (2017). Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. J. Cachexia. Sarcopenia Muscle 8, 245–250. doi: 10.1002/jcsm.12160
Dose, B., Yalçin, M., Dries, S. P., and Relógio, A. (2023). Time teller for timing health: the potential of circadian medicine to improve performance, prevent disease and optimize treatment. Front. Digit. Health 5:1157654. doi: 10.3389/fdgth.2023.1157654
Drăgoi, C. M., Nicolae, A. C., Ungurianu, A., Margină, D. M., Grădinaru, D., and Dumitrescu, I.-B. (2024). Circadian rhythms, chrononutrition, physical training, and redox homeostasis—molecular mechanisms in human health. Cells 13:138. doi: 10.3390/cells13020138
Driver, H. S., and Taylor, S. R. (2000). Exercise and sleep. Sleep Med. Rev. 4, 387–402. doi: 10.1053/smrv.2000.0110
Dudek, M., Swift, J., and Meng, Q.-J. (2023). The circadian clock and extracellular matrix homeostasis in aging and age-related diseases. Am. J. Phys. Cell Phys. 325, C52–C59. doi: 10.1152/ajpcell.00122.2023
Dumont, N. A., Bentzinger, C. F., Sincennes, M. C., and Rudnicki, M. A. (2015). Satellite cells and skeletal muscle regeneration. Compr. Physiol. 5, 1027–1059. doi: 10.1002/cphy.c140068
Eastman, C. I., Hoese, E. K., Youngstedt, S. D., and Liu, L. (1995). Phase-shifting human circadian rhythms with exercise during the night shift. Physiol. Behav. 58, 1287–1291. doi: 10.1016/0031-9384(95)02031-4
Ehrlich, A. M., Mac Gregor, K. A., Ashcroft, S. P., Small, L., Altıntaş, A., Chibalin, A. V., et al. (2025). HIF1α mediates circadian regulation of skeletal muscle metabolism and substrate preference in response to time-of-day exercise. Proc. Natl. Acad. Sci. USA 122:e2504080122.
Fagiani, F., Di Marino, D., Romagnoli, A., Travelli, C., Voltan, D., Di Cesare Mannelli, L., et al. (2022). Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 7:41. doi: 10.1038/s41392-022-00899-y
Farajnia, S., Deboer, T., Rohling, J. H., Meijer, J. H., and Michel, S. (2014). Aging of the suprachiasmatic clock. Neuroscientist 20, 44–55. doi: 10.1177/1073858413498936
Fernández-Martínez, J., Ramírez-Casas, Y., Yang, Y., Aranda-Martínez, P., Martínez-Ruiz, L., Escames, G., et al. (2023). From chronodisruption to sarcopenia: the therapeutic potential of melatonin. Biomolecules. 13:1779. doi: 10.3390/biom13121779
Franzago, M., Alessandrelli, E., Notarangelo, S., Stuppia, L., and Vitacolonna, E. (2023). Chrono-nutrition: circadian rhythm and personalized nutrition. Int. J. Mol. Sci. 24:2571. doi: 10.3390/ijms24032571
Gabriel, B. M., and Zierath, J. R. (2022). Zeitgebers of skeletal muscle and implications for metabolic health. J. Physiol. 600, 1027–1036. doi: 10.1113/JP280884
Gabryelska, A., Turkiewicz, S., Gajewski, A., Białasiewicz, P., Strzelecki, D., Ditmer, M., et al. (2025). Elucidating the interplay of hypoxia-inducible factor and circadian clock signaling in obstructive sleep apnea patients. Int. J. Mol. Sci. 26:971. doi: 10.3390/ijms26030971
Gabryelska, A., Turkiewicz, S., Karuga, F. F., Sochal, M., Strzelecki, D., and Białasiewicz, P. (2022). Disruption of circadian rhythm genes in obstructive sleep apnea patients—possible mechanisms involved and clinical implication. Int. J. Mol. Sci. 23:709. doi: 10.3390/ijms23020709
Gaesser, G., Hall, S., Angadi, S., Poole, D., and Racette, S. (2025). Increasing the health span: unique role for exercise. J. Appl. Physiol. 138, 1285–1308. doi: 10.1152/japplphysiol.00049.2025
Glavin, E. E., Ceneus, M., Chanowitz, M., Kantilierakis, J., Mendelow, E., Mosquera, J., et al. (2021). Relationships between sleep, exercise timing, and chronotype in young adults. J. Health Psychol. 26, 2636–2647. doi: 10.1177/1359105320926530
Goldin, A. P., Sigman, M., Braier, G., Golombek, D. A., and Leone, M. J. (2020). Interplay of chronotype and school timing predicts school performance. Nat. Hum. Behav. 4, 387–396. doi: 10.1038/s41562-020-0820-2
Golombek, D. A., and Rosenstein, R. E. (2010). Physiology of circadian entrainment. Physiol. Rev. 90, 1063–1102. doi: 10.1152/physrev.00009.2009
Harfmann, B. D., Schroder, E. A., and Esser, K. A. (2015). Circadian rhythms, the molecular clock, and skeletal muscle. J. Biol. Rhythm. 30, 84–94. doi: 10.1177/0748730414561638
Hower, I. M., Harper, S. A., and Buford, T. W. (2018). Circadian rhythms, exercise, and cardiovascular health. J. Circadian Rhythms 16:7. doi: 10.5334/jcr.164
Hu, C., Ayan, B., Chiang, G., Chan, A. H., Rando, T. A., and Huang, N. F. (2022). Comparative effects of basic fibroblast growth factor delivery or voluntary exercise on muscle regeneration after volumetric muscle loss. Bioengineering 9:37. doi: 10.3390/bioengineering9010037
Hurst, C., Robinson, S. M., Witham, M. D., Dodds, R. M., Granic, A., Buckland, C., et al. (2022). Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing 51:afac 003. doi: 10.1093/ageing/afac003
In, K. V., and Vasanthi, G. (2024). Role of hormones in regulating muscle activity during exercise and their impact on recovery processes. Ж 11 Жінки, спорт і суспільство в сучасному світі: матеріали. :67.
Izquierdo, M., Merchant, R. A., Morley, J. E., Anker, S. D., Aprahamian, I., Arai, H., et al. (2021). International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J. Nutr. Health Aging 25, 824–853. doi: 10.1007/s12603-021-1665-8
Joanisse, S., Nederveen, J. P., Snijders, T., McKay, B. R., and Parise, G. (2016). Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology 63, 91–100. doi: 10.1159/000450922
Juliana, N., Azmi, L., Effendy, N. M., Mohd Fahmi Teng, N. I., Abu, I. F., Abu Bakar, N. N., et al. (2023). Effect of circadian rhythm disturbance on the human musculoskeletal system and the importance of nutritional strategies. Nutrients 15:734. doi: 10.3390/nu15030734
Kaczmarek, A., Kaczmarek, M., Ciałowicz, M., Clemente, F. M., Wolański, P., Badicu, G., et al. (2021). The role of satellite cells in skeletal muscle regeneration—the effect of exercise and age. Biology 10:1056. doi: 10.3390/biology10101056
Kim, N., Ka, S., and Park, J. (2023). Effects of exercise timing and intensity on physiological circadian rhythm and sleep quality: a systematic review. Phy. Act. Nutr. 27, 052–063. doi: 10.20463/pan.2023.0029
Kripke, D., Youngstedt, S., Elliott, J., Tuunainen, A., Rex, K., Hauger, R., et al. (2005). Circadian phase in adults of contrasting ages. Chronobiol. Int. 22, 695–709. doi: 10.1080/07420520500180439
Kumar, M. S., and Vinayakan, K. Building a sustainable fitness routine: balancing exercise, rest, and nutrition. (2024).
Lambert, C. (2025). The impact of swimming duration on exercise-induced cardiac fatigue : Liverpool John Moores University.
Langmesser, S., Tallone, T., Bordon, A., Rusconi, S., and Albrecht, U. (2008). Interaction of circadian clock proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol. Biol. 9, 41–16. doi: 10.1186/1471-2199-9-41
Larsson, L., Degens, H., Li, M., Salviati, L., Lee, Y. I., Thompson, W., et al. (2019). Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev. 99, 427–511. doi: 10.1152/physrev.00061.2017
Li, N., Chen, S., He, Y., Chen, Y., Duan, X., He, W., et al. (2025). Effects of oral supplementation of β-hydroxy-β-methylbutyrate on muscle mass and strength in individuals over the age of 50: a meta-analysis. Front. Nutr. 12:1522287. doi: 10.3389/fnut.2025.1522287
Lippi, L., de Sire, A., Mezian, K., Curci, C., Perrero, L., Turco, A., et al. (2022). Impact of exercise training on muscle mitochondria modifications in older adults: a systematic review of randomized controlled trials. Aging Clin. Exp. Res. 34, 1495–1510. doi: 10.1007/s40520-021-02073-w
Liu, C., Wu, X., Vulugundam, G., Gokulnath, P., Li, G., and Xiao, J. (2023). Exercise promotes tissue regeneration: mechanisms involved and therapeutic scope. Sports Med. Open. 9:27. doi: 10.1186/s40798-023-00573-9
Lu, W., Xiao, W., Xie, W., Fu, X., Pan, L., Jin, H., et al. (2021). The role of osteokines in sarcopenia: therapeutic directions and application prospects. Front. Cell. Dev. Biol. 9:735374. doi: 10.3389/fcell.2021.735374
Maier, G. (2020). Investigating the role of the circadian clock and timed exercise on mouse skeletal muscle function. University of Basel.
Maier, G., Delezie, J., Westermark, P. O., Santos, G., Ritz, D., and Handschin, C. (2022). Transcriptomic, proteomic and phosphoproteomic underpinnings of daily exercise performance and zeitgeber activity of training in mouse muscle. J. Physiol. 600, 769–796. doi: 10.1113/JP281535
Malhan, D., Yalçin, M., Liedtke, S., Grötsch, R., Enzmann, C., Rau, M., et al. (2025). A prospective study to investigate circadian rhythms as health indicator in women’s aging. NPJ Womens Health 3:18. doi: 10.1038/s44294-025-00057-z
Mansingh, S., and Handschin, C. (2022). Time to train: the involvement of the molecular clock in exercise adaptation of skeletal muscle. Front. Physiol. 13:902031. doi: 10.3389/fphys.2022.902031
Mayeuf-Louchart, A., Staels, B., and Duez, H. (2015). Skeletal muscle functions around the clock. Diabetes. Obes. Metab. 17, 39–46. doi: 10.1111/dom.12517
Meléndez-Fernández, O. H., Liu, J. A., and Nelson, R. J. (2023). Circadian rhythms disrupted by light at night and mistimed food intake alter hormonal rhythms and metabolism. Int. J. Mol. Sci. 24:3392. doi: 10.3390/ijms24043392
Merz, K. E., and Thurmond, D. C. (2011). Role of skeletal muscle in insulin resistance and glucose uptake. Compr. Physiol. 10, 785–809.
Mirizio, G. G., Nunes, R. S. M., Vargas, D. A., Foster, C., and Vieira, E. (2020). Time-of-day effects on short-duration maximal exercise performance. Sci. Rep. 10:9485. doi: 10.1038/s41598-020-66342-w
Moon, H. Y., and Jeong, I. C. (2023). The effect of voluntary exercise on light cycle stress-induced metabolic resistance. Phy. Act. Nutr. 27, 001–009. doi: 10.20463/pan.2023.0022
Morena da Silva, F., Esser, K. A., Murach, K. A., and Greene, N. P. (2024). Inflammation o'clock: interactions of circadian rhythms with inflammation-induced skeletal muscle atrophy. J. Physiol. 602, 6587–6607. doi: 10.1113/JP284808
Morrison, M., Halson, S. L., Weakley, J., and Hawley, J. A. (2022). Sleep, circadian biology and skeletal muscle interactions: implications for metabolic health. Sleep Med. Rev. 66:101700. doi: 10.1016/j.smrv.2022.101700
Negri, M., Pivonello, C., Amatrudo, F., Cimmino, F., Trinchese, G., Vetrani, C., et al. (2025). Effects of Chrono-exercise and Chrono-nutrition on muscle health: understanding the molecular mechanisms activated by timed exercise and consumption of proteins and carbohydrates. Nutr. Rev. 83, 1571–1593. doi: 10.1093/nutrit/nuaf007
Neves, A. R., Albuquerque, T., Quintela, T., and Costa, D. (2022). Circadian rhythm and disease: relationship, new insights, and future perspectives. J. Cell. Physiol. 237, 3239–3256. doi: 10.1002/jcp.30815
Newsom, R., and DeBanto, J. (2020). Aging and sleep: how does growing old affect sleep? Sleep Foundation.
Oliveira, A. N., Richards, B. J., Slavin, M., and Hood, D. A. (2021). Exercise is muscle mitochondrial medicine. Exerc. Sport Sci. Rev. 49, 67–76. doi: 10.1249/JES.0000000000000250
Ono, D., Honma, K., and Honma, S. (2021). Gabaergic mechanisms in the suprachiasmatic nucleus that influence circadian rhythm. J. Neurochem. 157, 31–41. doi: 10.1111/jnc.15012
Palmese, F., Druda, Y., Del Toro, R., Bedogni, G., Domenicali, M., and Silvani, A. (2025). The role of the circadian timing system in sarcopenia in old age: a scoping review. Eur. Geriatr. Med. 16, 447–460. doi: 10.1007/s41999-024-01129-0
Parke, S. C., Ng, A., Martone, P., Gerber, L. H., Zucker, D. S., Engle, J., et al. (2022). Translating 2019 ACSM cancer exercise recommendations for a physiatric practice: derived recommendations from an international expert panel. PM R 14, 996–1009. doi: 10.1002/pmrj.12664
Patton, A. P., and Hastings, M. H. (2018). The suprachiasmatic nucleus. Curr. Biol. 28, R816–R822. doi: 10.1016/j.cub.2018.06.052
Peake, J., Gatta, P. D., and Cameron-Smith, D. (2010). Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am. J. Phys. Regul. Integr. Comp. Phys. 298, R1485–R1495. doi: 10.1152/ajpregu.00467.2009
Pearson, K. (2000). Neural adaptation in the generation of rhythmic behavior. Annu. Rev. Physiol. 62, 723–753. doi: 10.1146/annurev.physiol.62.1.723
Pellegrino, A., Tiidus, P. M., and Vandenboom, R. (2022). Mechanisms of estrogen influence on skeletal muscle: mass, regeneration, and mitochondrial function. Sports Med. 52, 2853–2869. doi: 10.1007/s40279-022-01733-9
Post, T. E., De Gioannis, R., Schmitz, J., Wittkowski, M., Schäper, T. M., Wrobeln, A., et al. (2025). Resetting of the human circadian melatonin rhythm by ambient hypoxia. J. Pineal Res. 77:e70029. doi: 10.1111/jpi.70029
Pourabdi, R., Shahidi, F., Tabandeh, M. R., and Salehpour, M. (2025). Aerobic exercise timing affects mitochondrial dynamics and insulin resistance by regulating the circadian clock protein expression and NAD+-SIRT1-PPARα-MFN2 pathway in the skeletal muscle of high-fat-diet-induced diabetes mice. J. Physiol. Biochem. 81, 199–214. doi: 10.1007/s13105-024-01066-3
Procopio, S. B., and Esser, K. A. (2025). Clockwork conditioning: aligning the skeletal muscle clock with time-of-day exercise for cardiometabolic health. J. Mol. Cell. Cardiol. 198, 36–44. doi: 10.1016/j.yjmcc.2024.11.011
Qu, M., Qu, H., Jia, Z., and Kay, S. A. (2021). HNF4A defines tissue-specific circadian rhythms by beaconing BMAL1::CLOCK chromatin binding and shaping the rhythmic chromatin landscape. Nat. Commun. 12:6350. doi: 10.1038/s41467-021-26567-3
Qu, M., Zhang, G., Qu, H., Vu, A., Wu, R., Tsukamoto, H., et al. (2023). Circadian regulator BMAL1::CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc. Natl. Acad. Sci. USA 120:e2214829120. doi: 10.1073/pnas.2214829120
Quante, M., Mariani, S., Weng, J., Marinac, C. R., Kaplan, E. R., Rueschman, M., et al. (2019). Zeitgebers and their association with rest-activity patterns. Chronobiol. Int. 36, 203–213. doi: 10.1080/07420528.2018.1527347
Rodrigues, F., Domingos, C., Monteiro, D., and Morouço, P. (2022). A review on aging, sarcopenia, falls, and resistance training in community-dwelling older adults. Int. J. Environ. Res. Public Health 19:874. doi: 10.3390/ijerph19020874
Rosbash, M. (2021). Circadian rhythms and the transcriptional feedback loop (Nobel lecture). Angew. Chem. 133.
Rutkowska, M., Bieńko, M., Król, T., Toborek, M., Marchaj, M., Korta, K., et al. (2024). Sleep cycles and health: role of sleep stages, circadian rhythms, and lifestyle factors on optimizing physical performance and mental well-being–a literature review. Qual. Sport 18:53398. doi: 10.12775/QS.2024.18.53393
Sayer, A. A., Birkbeck, M., Cain, G., Dodds, R., Granic, A., Habiballa, L., et al. International sarcopenia translational research conference. (2021).
Schiaffino, S., Dyar, K. A., Ciciliot, S., Blaauw, B., and Sandri, M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294–4314. doi: 10.1111/febs.12253
Schibler, U. (2021). BMAL1 dephosphorylation determines the pace of the circadian clock. Genes Dev. 35, 1076–1078. doi: 10.1101/gad.348801.121
Schroder, E. A., and Esser, K. A. (2013). Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc. Sport Sci. Rev. 41, 224–229. doi: 10.1097/JES.0b013e3182a58a70
Seene, T., and Kaasik, P. (2013). Muscle damage and regeneration: response to exercise training. Health 5, 136–145.
Sharma, V. K., and Chandrashekaran, M. (2005). Zeitgebers (time cues) for biological clocks. Curr. Sci., 1136–1146.
Shen, B., Ma, C., Wu, G., Liu, H., Chen, L., and Yang, G. (2023). Effects of exercise on circadian rhythms in humans. Front. Pharmacol. 14:1282357. doi: 10.3389/fphar.2023.1282357
Silva, B. S. A., Uzeloto, J. S., Lira, F. S., Pereira, T., Coelho-E-Silva, M. J., and Caseiro, A. (2021). Exercise as a peripheral circadian clock resynchronizer in vascular and skeletal muscle aging. Int. J. Environ. Res. Public Health 18:12949. doi: 10.3390/ijerph182412949
Smith, J. A. B., Murach, K. A., Dyar, K. A., and Zierath, J. R. (2023). Exercise metabolism and adaptation in skeletal muscle. Nat. Rev. Mol. Cell Biol. 24, 607–632. doi: 10.1038/s41580-023-00606-x
Steffen, D., Kjaer, M., and Yeung, C.-Y. C. (2024). Exercise entrainment of musculoskeletal connective tissue clocks. American journal of physiology-cell. Physiology 327, C270–C277. doi: 10.1152/ajpcell.00285.2024
Thomas, J. M., Kern, P. A., Bush, H. M., McQuerry, K. J., Black, W. S., Clasey, J. L., et al. (2020). Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 5:e134270. doi: 10.1172/jci.insight.134270
Tidball, J. G. (2011). Mechanisms of muscle injury, repair, and regeneration. Compr. Physiol. 1, 2029–2062. doi: 10.1002/j.2040-4603.2011.tb00387.x
Van Drunen, R., and Eckel-Mahan, K. (2021). Circadian rhythms of the hypothalamus: from function to physiology. Clocks Sleep 3, 189–226. doi: 10.3390/clockssleep3010012
Viggars, M. R., Berko, H. E., Hesketh, S. J., Wolff, C. A., Gutierrez-Monreal, M. A., Martin, R. A., et al. (2024). Skeletal muscle BMAL1 is necessary for transcriptional adaptation of local and peripheral tissues in response to endurance exercise training. Mol Metab. 86:101980. doi: 10.1016/j.molmet.2024.101980
Vitale, J. A., Bonato, M., La Torre, A., and Banfi, G. (2019). The role of the molecular clock in promoting skeletal muscle growth and protecting against sarcopenia. Int. J. Mol. Sci. 20:4318. doi: 10.3390/ijms20174318
Vitale, J. A., and Weydahl, A. (2017). Chronotype, physical activity, and sport performance: a systematic review. Sports Med. 47, 1859–1868. doi: 10.1007/s40279-017-0741-z
Von Ruff, Z. D., Miller, M. J., Moro, T., Reidy, P. T., Ebert, S. M., Volpi, E., et al. (2025). Resistance exercise training in older men reduces ATF4-activated and senescence-associated mRNAs in skeletal muscle. Gero. Science 47, 4601–4622. doi: 10.1007/s11357-025-01564-2
Watson, M. D., Cross, B. L., and Grosicki, G. J. (2021). Evidence for the contribution of gut microbiota to age-related anabolic resistance. Nutrients 13:706. doi: 10.3390/nu13020706
Webhofer, V. The effect of circadian rhythms on medication of asthma and COPD: a systematic review. (2025)
Weston, A. D., and Hood, L. (2004). Systems biology, proteomics, and the future of health care: toward predictive, preventative, and personalized medicine. J. Proteome Res. 3, 179–196. doi: 10.1021/pr0499693
Winegar, R. (2024). Promoting healthy sleep among older adults. Geriatr. Nurs. 58, 298–303. doi: 10.1016/j.gerinurse.2024.05.032
Wolff, C. A., and Esser, K. A. (2019). Exercise timing and circadian rhythms. Curr. Opin. Physio. 10, 64–69. doi: 10.1016/j.cophys.2019.04.020
Woodard, C. M., and Berry, M. J. (2001). Enhancing adherence to prescribed exercise: structured behavioral interventions in clinical exercise programs. J. Cardiopulm. Rehabil. Prev. 21, 201–209. doi: 10.1097/00008483-200107000-00002
World Health Organization (2023). Promoting physical activity for older people: a toolkit for action : World Health Organization.
Yamanaka, Y., Honma, K.-i., Hashimoto, S., Takasu, N., Miyazaki, T., and Honma, S. (2006). Effects of physical exercise on human circadian rhythms. Sleep Biol. Rhythms 4, 199–206. doi: 10.1111/j.1479-8425.2006.00234.x
Yan, Y., Liu, F., Zhang, T., Zhao, L., Tie, Y., Wang, R., et al. (2025). Exosomal mi R-34b-3p upregulated in response to hypoxia preconditioning modulates circadian rhythms through the targeting of clock. Environ. Epigenet. 11:dvaf002. doi: 10.1093/eep/dvaf002
Zelinski, E. L., Deibel, S. H., and McDonald, R. J. (2014). The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci. Biobehav. Rev. 40, 80–101. doi: 10.1016/j.neubiorev.2014.01.007
Zhang, X., Dube, T. J., and Esser, K. A. (2009). Working around the clock: circadian rhythms and skeletal muscle. J. Appl. Physiol. 107, 1647–1654. doi: 10.1152/japplphysiol.00725.2009
Zisapel, N. (2007). Sleep and sleep disturbances: biological basis and clinical implications. Cell. Mol. Life Sci. 64, 1174–1186. doi: 10.1007/s00018-007-6529-9
Keywords: circadian rhythm, skeletal muscle regeneration, aging, exercise timing, sarcopenia
Citation: Su Z and Xiang L (2025) Exercise, circadian rhythms, and muscle regeneration: a path to healthy aging. Front. Neurosci. 19:1633835. doi: 10.3389/fnins.2025.1633835
Edited by:
Jennifer Choi Tudor, Saint Joseph’s University, United StatesReviewed by:
Geoffrey Woodard, Harvard University, United StatesStuart Hesketh, University of Central Lancashire, United Kingdom
Copyright © 2025 Su and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanguo Su, c3V6aGFuZ3VvQDEyNi5jb20=
 Zhanguo Su
Zhanguo Su Lijuan Xiang3
Lijuan Xiang3