- 1Hangzhou Lin’an Traditional Chinese Medicine Hospital, Affiliated Hospital, Hangzhou City University, Hangzhou, Zhejiang, China
- 2Key Laboratory of Novel Targets and Drug Study for Neural Repair of Zhejiang Province, School of Medicine, Hangzhou City University, Hangzhou, China
- 3Department of Orthopedics, Suzhou Municipal Hospital, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, China
- 4Institute of Traditional Medicine and Technology of Mongolia, Ulaanbaatar, Mongolia
Objectives: In this study, we evaluated the key features of the 100 most-cited publications on optic nerve regeneration from 2005 to 2025 employing bibliometric and visual analysis.
Methods: The data for this study were obtained from a comprehensive search across multiple databases, including the Web of Science, Scopus, and Dimensions. We identified the top 100 most-cited articles published in each database from 2005 to 2025, merged and deduplicated the results, and selected the 100 most-cited papers on optic nerve regeneration. After extracting key details such as titles, authors, keywords, publication information, and institutional affiliations, a bibliometric analysis was conducted.
Results: The top 100 most cited papers on optic nerve regeneration published between 2005 and 2025, accumulating 34,636 total citations with a median of 346 citations per paper. Prof. Zhigang He emerged as the most prolific author with 19 publications. The United States contributed 59 papers, while Harvard University led institutions with 30 publications. Key research themes included optic nerve regeneration, CNTF, gene therapy, and retinal ganglion cells.
Conclusion: Our analysis of top-cited optic nerve regeneration research reveals sustained United States leadership in output and innovation. Early work focused on neuronal signaling pathways (PTEN/mTOR, KLF family), while current studies explore novel targets and biomaterials. Global collaboration among the United States, China, and European nations has accelerated progress. Key challenges remain in achieving functional long-distance regeneration. Future direction should prioritize the development of multi-target therapeutic methods, precise drug delivery, and the control of inflammation to improve nerve regeneration efficiency.
1 Introduction
The optic nerve, comprising the axons of retinal ganglion cells (RGCs), is the only pathway through which visual signals travel from the retina to the brain, and whose functional integrity is essential for maintaining visual perception (Laha et al., 2017). The optic nerve is a crucial component of the central nervous system (CNS) and, as such, shares the limited regenerative capacity characteristic of the mature CNS of most mammals. Additionally, its axons are prone to irreversible degenerative changes after injury, and their regenerative capacity is significantly lower than that of axons in the peripheral nervous system (PNS) (Benowitz et al., 2017). Traumatic optic neuropathy, genetic disorders, and diseases such as glaucoma, can result in damage to the optic nerve (Chen et al., 2022). Such damage can not only severely impair visual function, but can also directly lead to the apoptosis of RGCs, and, eventually, irreversible blindness.
Optic nerve regeneration is an important prerequisite for the recovery of visual function. Despite this, how to promote this process remains a major challenge in the field of neuroscience. Current research efforts are focused on decoding the intrinsic regulatory mechanisms of RGCs (Moore et al., 2009; Park et al., 2008), the modulation of the neural microenvironment (Yin et al., 2006; Yin et al., 2009), guiding axon growth, and the restoration of visual function. A range of methods are employed at present for inducing optic nerve regeneration, including multi-gene therapy (Kurimoto et al., 2010), immune system modulation (Baldwin et al., 2015), neurotrophic factor therapy (Bei et al., 2016; Jacobi et al., 2022; Müller et al., 2007), cell therapy (Mead et al., 2015; Mead et al., 2017), and bioactive material-based strategies (Pan et al., 2024).
Bibliometric analysis, supported by visualization methods, can help researchers understand the development and research hotspots within academic fields. Bibliometric methods, mainly involving literature quantity, collaboration, influence, and keyword analysis, are increasingly used in medicine. However, bibliometric studies relating to optic nerve research are relatively scarce, and there is a need to clarify the current situation, hot spots, and trends in this field. The aim of this study was to comprehensively analyze the 100 most frequently cited papers on optic nerve regeneration published between 2005 and 2025,and construct a relevant multi-dimensional knowledge map comprising an international cooperation network, a core author cluster, data on high-impact journal distribution, and a keyword co-occurrence network.
2 Materials and methods
2.1 Search strategies and data extraction
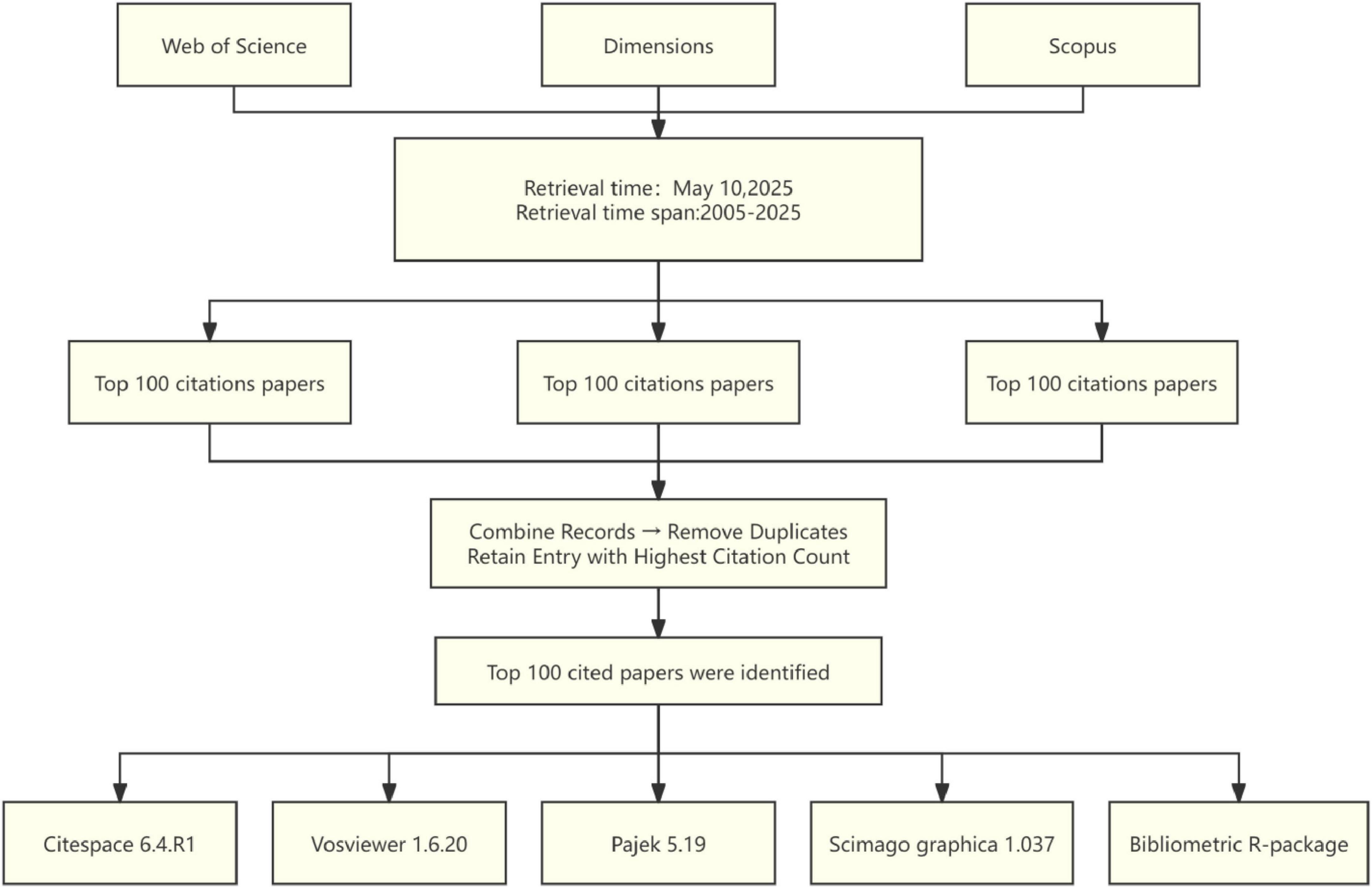
The data for this study were sourced from three authoritative databases: the Web of Science Core Collection (WoSCC), Scopus, and Dimensions. WoSCC is distinguished by its stringent journal selection criteria, while Scopus provides the most extensive disciplinary coverage. Additionally, Dimensions integrate various types of research data. The combined utilization of these databases ensures comprehensive and representative literature retrieval process. Articles and reviews published between 2005 and 2025, were retrieved using the following search terms: (optic nerve regeneration*) or (Optic nerve repair*) or (Optic nerve recovery*) or (Optic nerve regrowth*) or (Axonal regeneration of retinal ganglion cells); the language was limited to English. The top 100 most-cited articles were retrieved separately from each database, with data downloaded on 10 May 2025, to prevent potential bias from subsequent database updates. After merging and removing duplicate records, the final list of top 100 most-cited articles was determined based on citation frequency ranking. In addition, two researchers independently screened the titles, abstracts, and document types. In case of disagreement, the full text of the manuscript was reviewed, and consensus was reached through discussion. The final dataset was exported in the “Complete Record and Cited References” format for subsequent analysis, including title, authors, keywords, journal, publication year, country, and institutional affiliation (Figure 1).
2.2 Data analysis and visualization
In this study, several bibliometric tools were used to systematically analyze the data. Initially, descriptive statistical analysis of basic data and visual chart generation were performed using Microsoft Excel 2019. Then, a keyword co-occurrence network and an author cooperation network were constructed using VOS viewer, a document measurement tool developed by van Eck and Waltman (2010). These visualizations intuitively illustrate the clustering of research hotspots and academic cooperation relationships, respectively. Furthermore, in conjunction with the network analysis capabilities of Pajek (Batagelj and Mrvar, 2002), a domain focus map was generated to reveal the core direction of current research. Meanwhile, a map of global academic influence was drawn using Scimago Graphica (Hassan-Montero et al., 2022), showing the citation frequency of the literature in different countries/regions in the form of geographical distribution. Additionally, CiteSpace (Chen, 2004) software was employed for multi-dimensional dynamic analysis, enabling the identification of academic communities based on author cooperation networks, the detection of keywords with high burst intensity to track the research frontier, and the visualization of the evolution of research hotspots through a timeline graph. In the network graph generated by CiteSpace, institutions, authors, and keywords are represented as nodes. The thickness of the lines between nodes reflects the cooperation intensity and co-occurrence frequency. Clustering groups, distinguished by different colors, represent subdivisions within the research topic. In addition, Bibliometrix, an R language toolkit, was used for bibliometric index analysis, enabling the rapid identification of the field’s founding literature, high-impact researchers, and emerging research directions. The tool also supports the generation of Sankey diagrams, where the arrow or line width represents the magnitude of knowledge flow paths, such as cross-disciplinary strength or inheritance of research topics, thereby providing visual support for data-driven interpretation of academic trends.
3 Results
3.1 Analysis of publications and citations
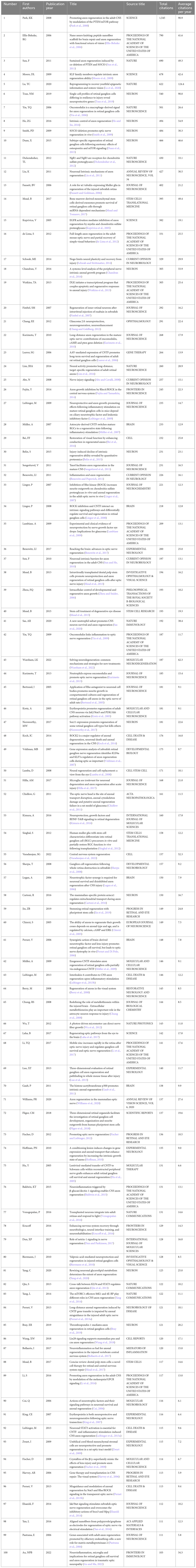
Figure 2A presents the number of published articles and citation counts from 2005 to 2025. Figure 2B shows that among these top 100 most cited articles, research articles account for approximately three quarters and reviews for about one quarter. The details of the top 100 most-cited articles on optic nerve regeneration from 2005 to 2025 are presented in Table 1. The top 100 articles accumulated between 103 and 1,545 citations, with a median of 169.5 and an average of 346.4 citations per article. The most cited article, “Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/mTOR Pathway” (Park et al., 2008), was published in SCIENCE in 2008 and has been cited 1,545 times. The second most cited paper, “Nano neuro knitting: Peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision” (Ellis-Behnke et al., 2006), appeared in the journal PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA in 2009 and has garnered 790 citations. The third most-cited paper, “Sustained axon regeneration induced by co-deletion of PTEN and SOCS3” (Sun et al., 2011), was published in NATURE in 2011 and accumulated 690 citations. While earlier publications have higher total citation counts, when ranked according to the average number of citations per year, some later publications were found to have a greater impact. For example, “Reprogramming to recover youthful epigenetic information and restore vision” (Tran et al., 2019), published in NATURE in 2020, ranked Fifth in total citations and First in average citations per year (124.0).
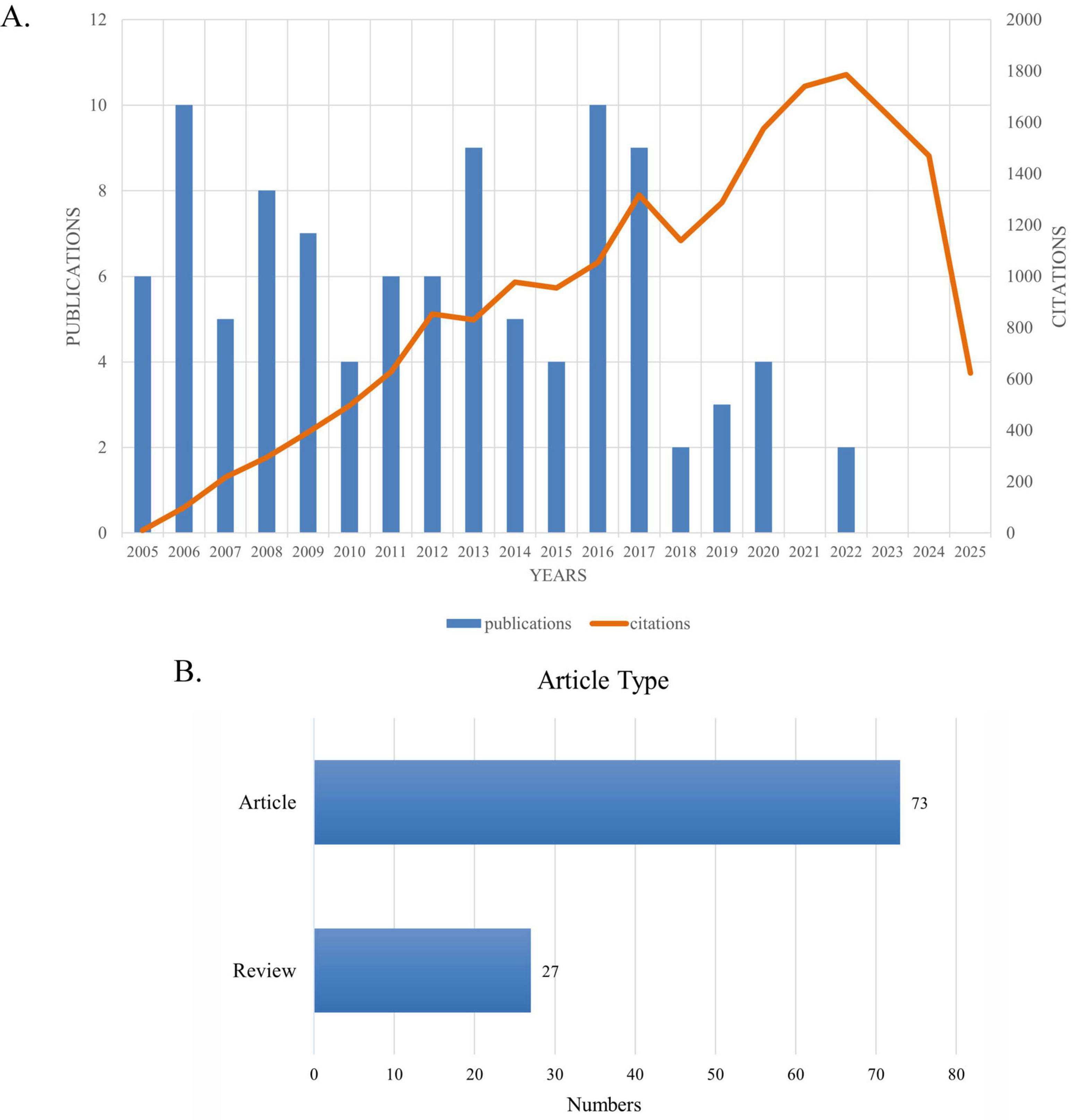
Figure 2. (A) Number and citations of top-cited publications from 2005 to 2025. (B) Article types and quantities.
3.2 Analysis of the most productive countries
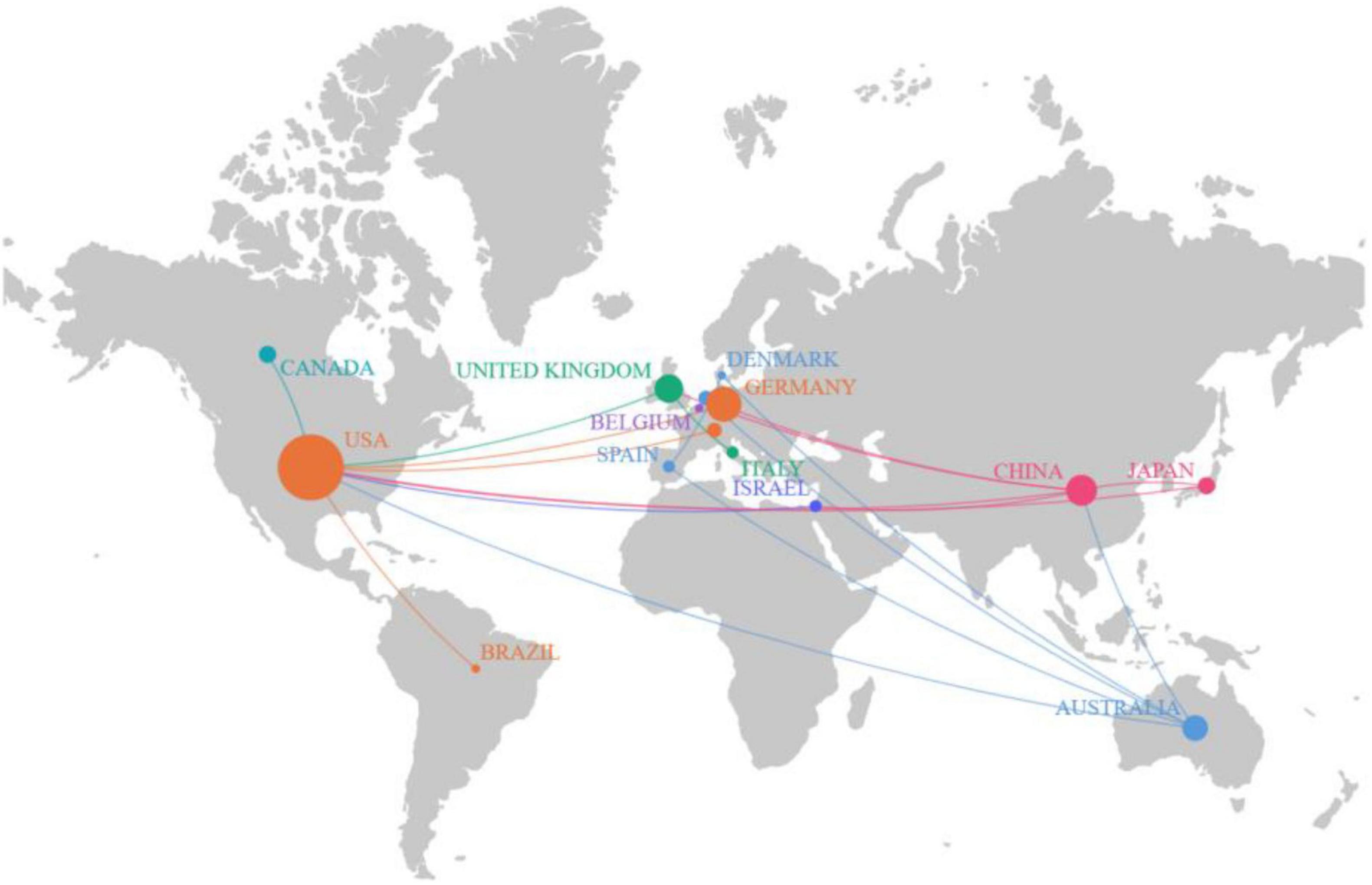
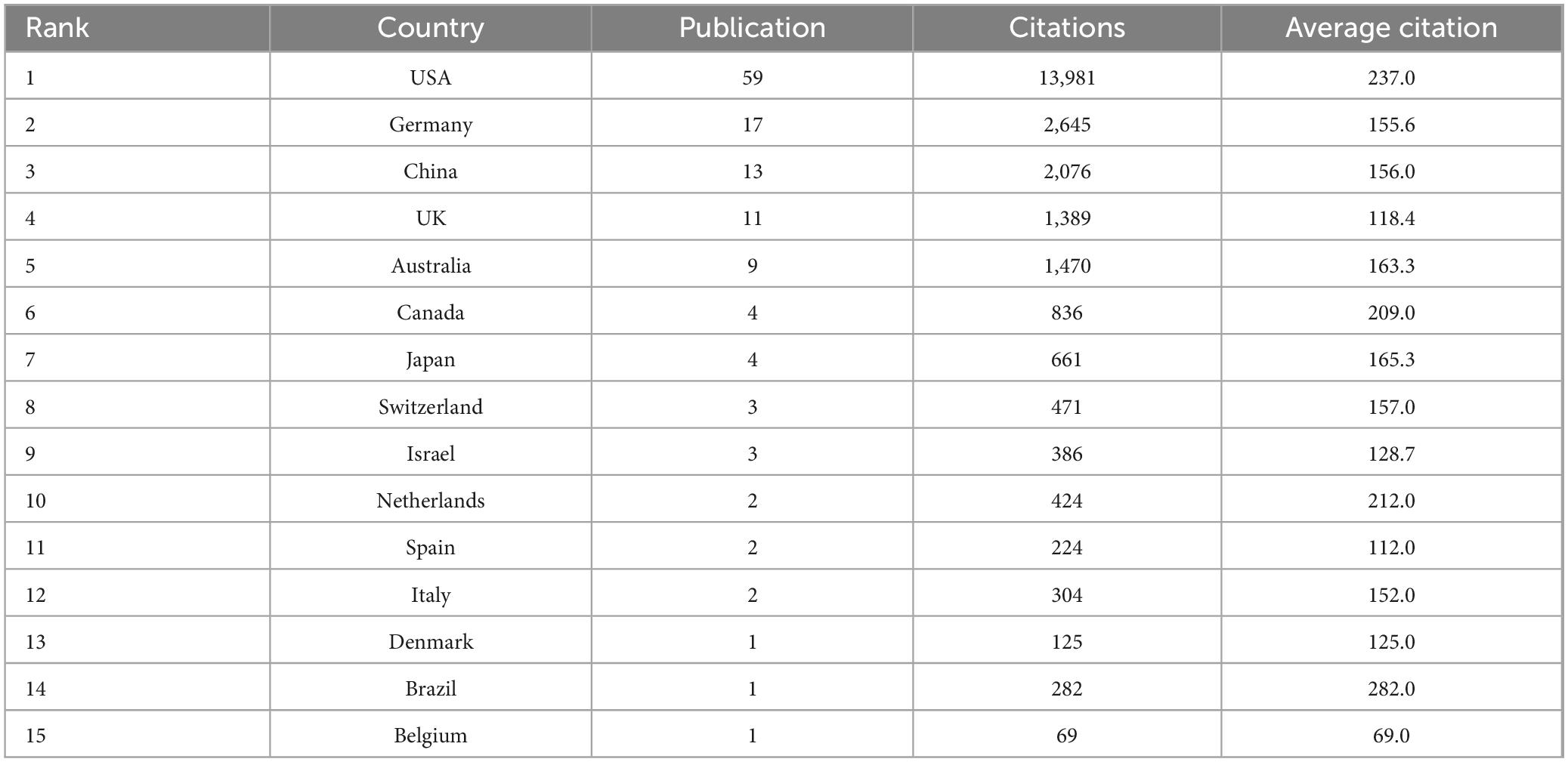
An analysis of the pattern of international collaboration in optic nerve regeneration research showed that a total of 15 countries/regions participated in the 100 most-cited papers. Figure 3 illustrates the national collaborative network in this field through a world map generated using Scimago Graphica software. The size of the bubbles represents the number of publications in each country, while the thickness of the connecting lines represents the closeness of cooperation between countries. The field of optic nerve regeneration was dominated by the United States, accounting for 59 of the 100 most-cited papers. These publications received a total of 13,981 citations, averaging 237.0 per paper (Table 2). Germany ranked second, contributing 17 papers that received 2,645 citations, resulting in an average of 155.6 citations per paper. China was third, contributing 13 articles. These accumulated a total of 2,076 citations, with an average of 156.0 citations per article. The United States, China, Germany, and the United Kingdom have established significant research collaborations in this area. While other countries also have partnerships, these connections are relatively weak and more fragmented.
3.3 Institution analysis
A total of 134 research institutions worldwide contributed to the 100 most influential papers in the field of optic nerve regeneration. As shown in the institutional ranking in Figure 4A, Harvard University stands out with 30 highly cited papers, establishing it as the most important research force in the field. Cite Space software is used to visualize the interconnections between institutions (Figure 4B). Several prominent research institutions can be identified, such as Harvard University, Boston Children’s Hospital, the University of California, and Stanford University, among others.
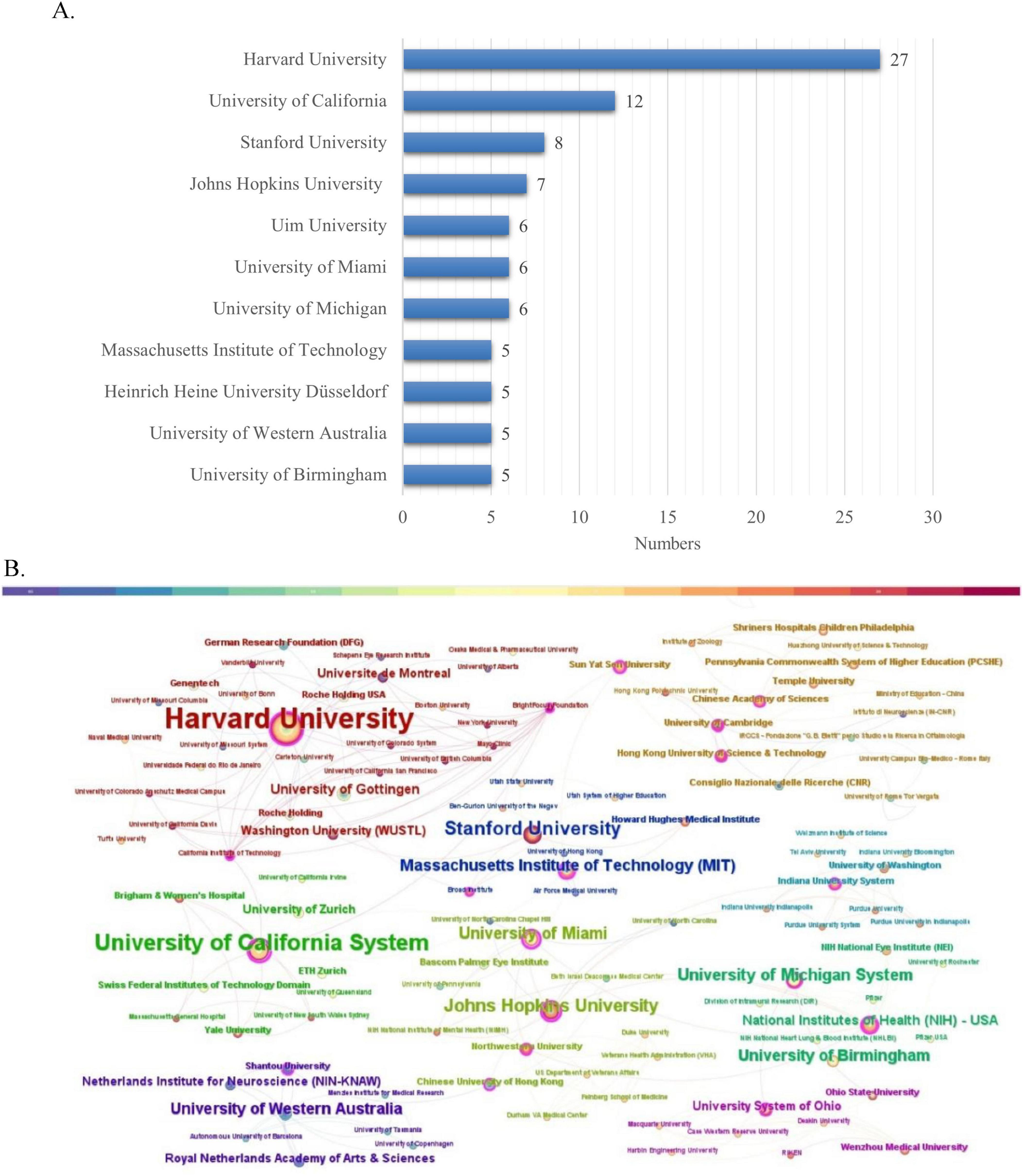
Figure 4. Institution analysis. (A) The most relevant institutions. (B) Partnerships between institutions.
3.4 Author analysis
As shown in Figure 5A, He Zhigang was the most prolific author, contributing to 19 publications. The academic activity period of the top 10 core researchers is presented through a time trend chart in Figure 5B. The cooperative network map in Figure 5C reveals the existence of a continuous and stable network of academic cooperation in this field. The Sankey diagram in Figure 5D further shows the flow of knowledge among countries, institutions, and authors, highlighting the significant academic influence of American researchers in this field.
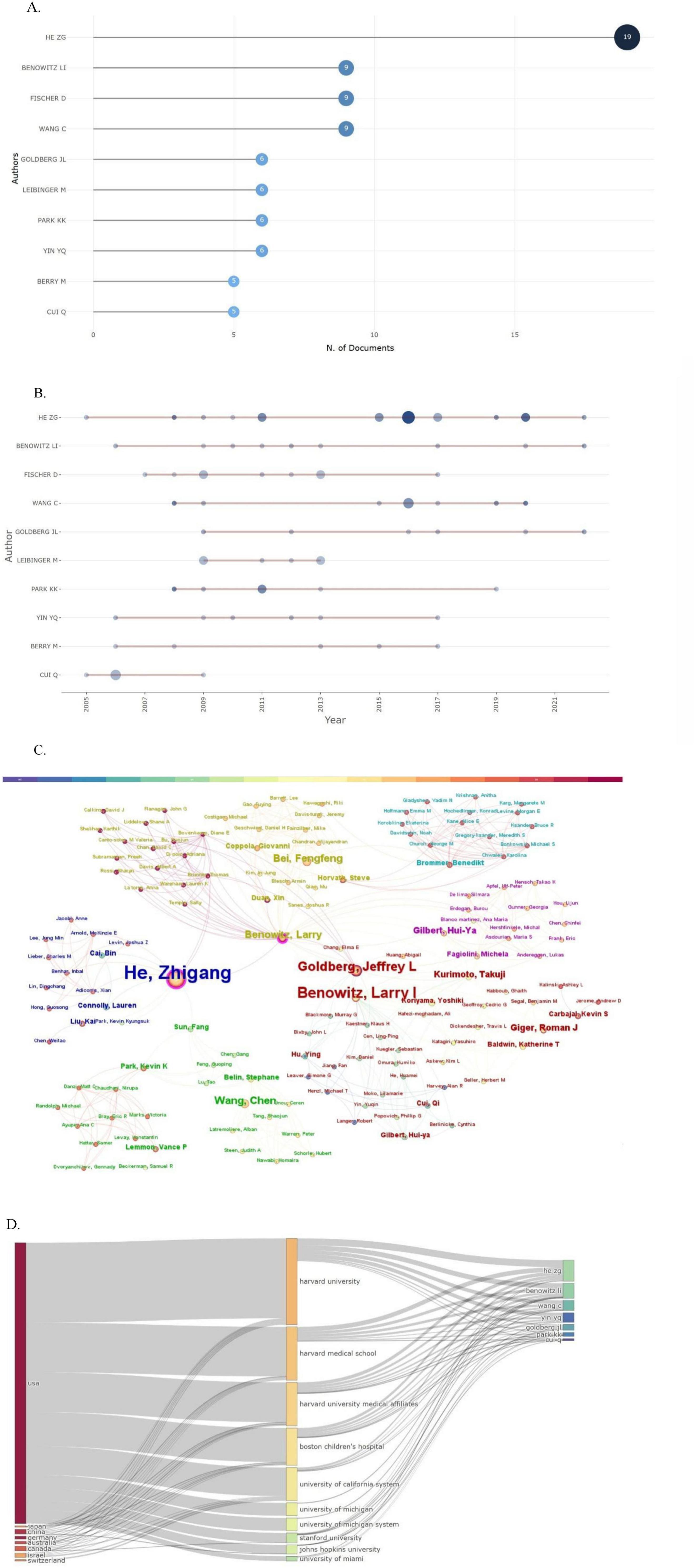
Figure 5. Author analysis. (A) Most relevant authors. (B) Authors’ production over time. (C) The author’s collaborative relationship map. (D) Three-field plot (country-affiliation-author).
3.5 Journal analysis
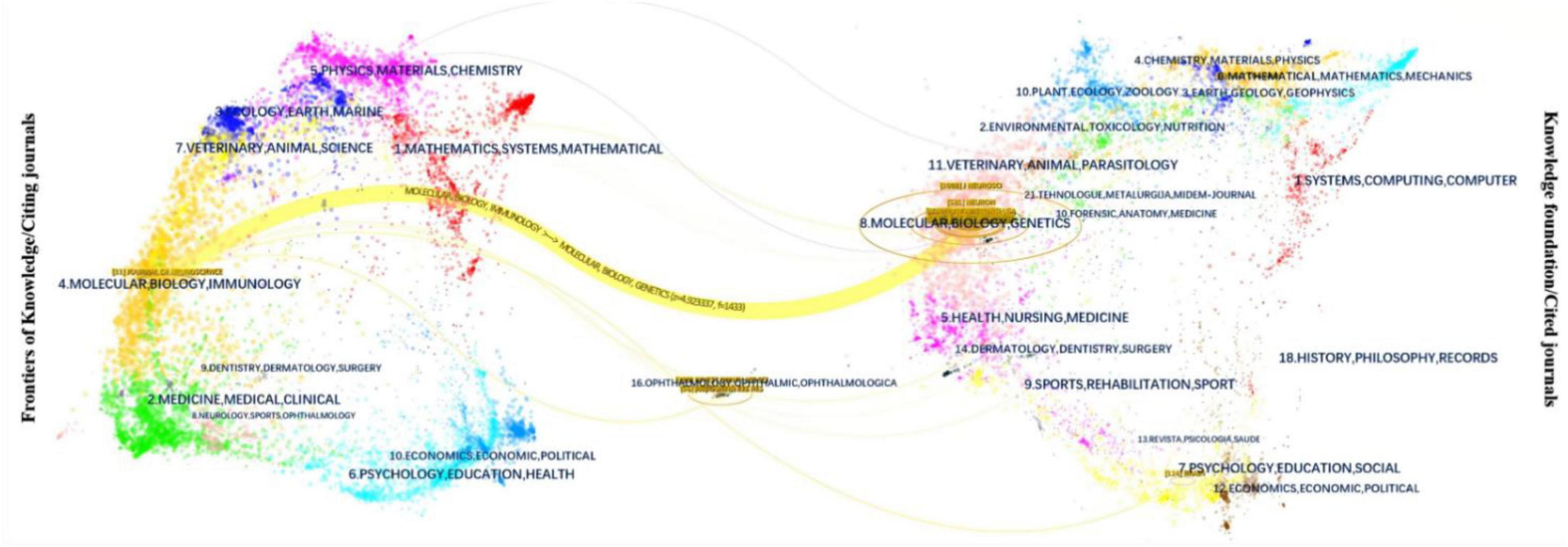
The 18 journals that have published at least two articles in the field of optic nerve regeneration and their main characteristics are listed in Table 3. The two journals with the most publications in the field of optic nerve regeneration are Journal of Neuroscience and Neuron, with 10 articles, respectively. In addition, four articles were published in Science and two in Cell. The Dual-Map Overlay journal atlas in Figure 6 demonstrates the dynamic knowledge flow from the basic disciplines on the right (represented by cited journals) to the frontier disciplines on the left (represented by citing journals). The frontier research of optic nerve regeneration, primarily concentrated in disciplines on the left such as “MOLECULAR, BIOLOGY, IMMUNOLOGY,” is supported by a solid knowledge base derived from two core disciplinary clusters on the right: one centered on “OPHTHALMOLOGY, OPHTHALMIC, OPHTHALMOLOGICA,” providing the fundamentals of Ophthalmology, and the other centered on “MOLECULAR, BIOLOGY, GENETICS,” forming the immunological basis. The overlay map also shows significant knowledge flow from domains like “CHEMISTRY, MATERIALS, PHYSICS” toward the medical frontiers. This implies that disciplines such as biomaterial, the application of nanotechnology in drug delivery systems, and biophysics provide essential technical tools and innovative solutions for the treatment strategies of optic nerve regeneration.
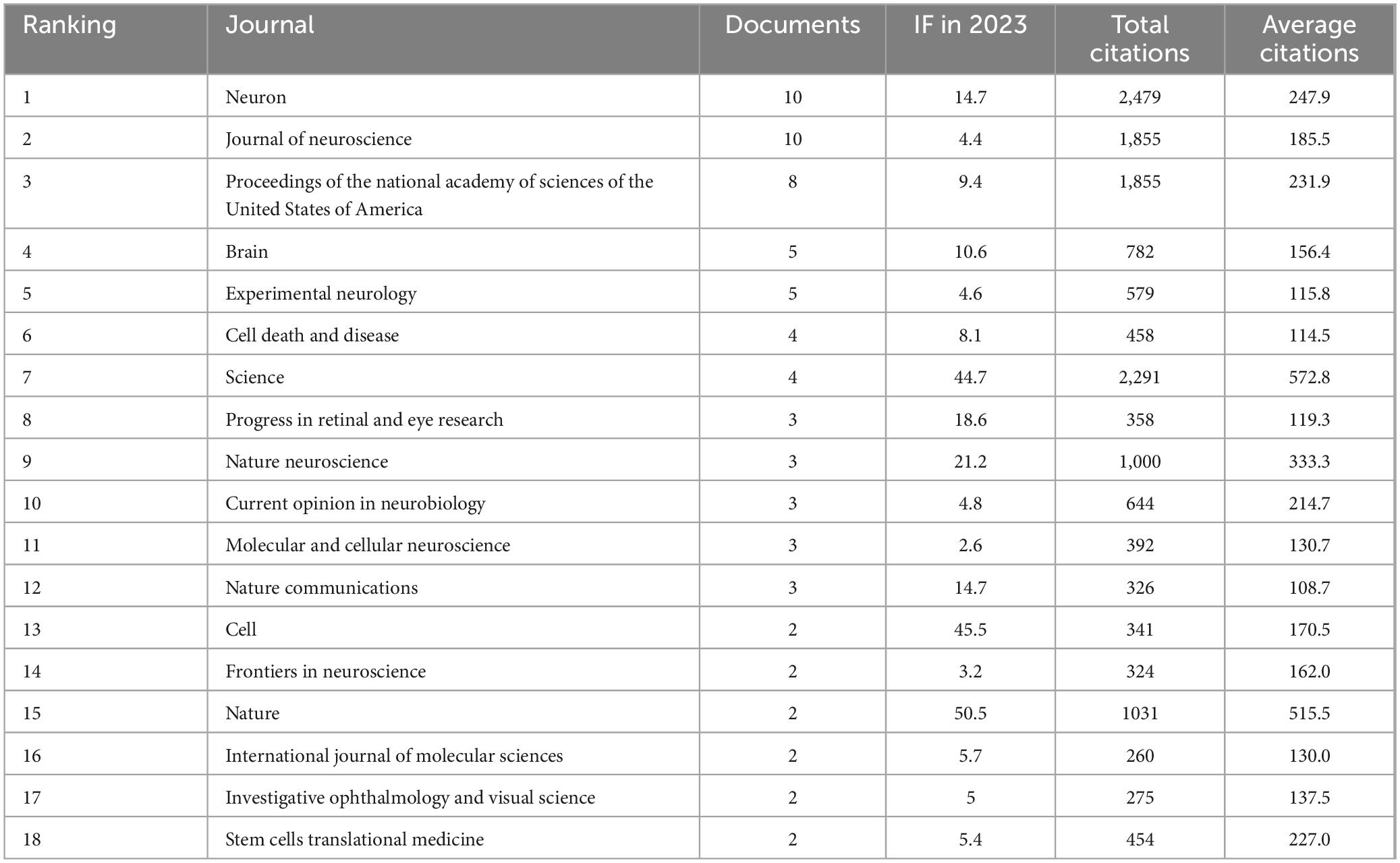
Table 3. The journals that have published the 100 most-cited articles in the field of optic nerve regeneration.
3.6 Keywords and research hot spots
Keywords play a crucial role in delineating the focus of an article, giving researchers a clear understanding of the published topic. The co-occurrence of two keywords in a given paper means that there is an intrinsic relationship between them, and the frequency of their occurrence reflects the strength of this connection. Conducting keyword co-occurrence and emergent item analyses allows the identification of the hot topics in different periods in a specific field and the consolidation of the author-provided keywords into a dataset. Keyword clustering describes the inherent knowledge structure within a particular research field and classifies its domain. In this study, cluster analysis revealed that the keywords in the field of optic nerve regeneration can be divided into the following 13 categories (Figure 7A): growth, differentiation, neurodegeneration, growth state, aav mediated expression, axonal transport, zebrafish, cells, rock2, optic nerve, growth cone, axonal regeneration and adult.
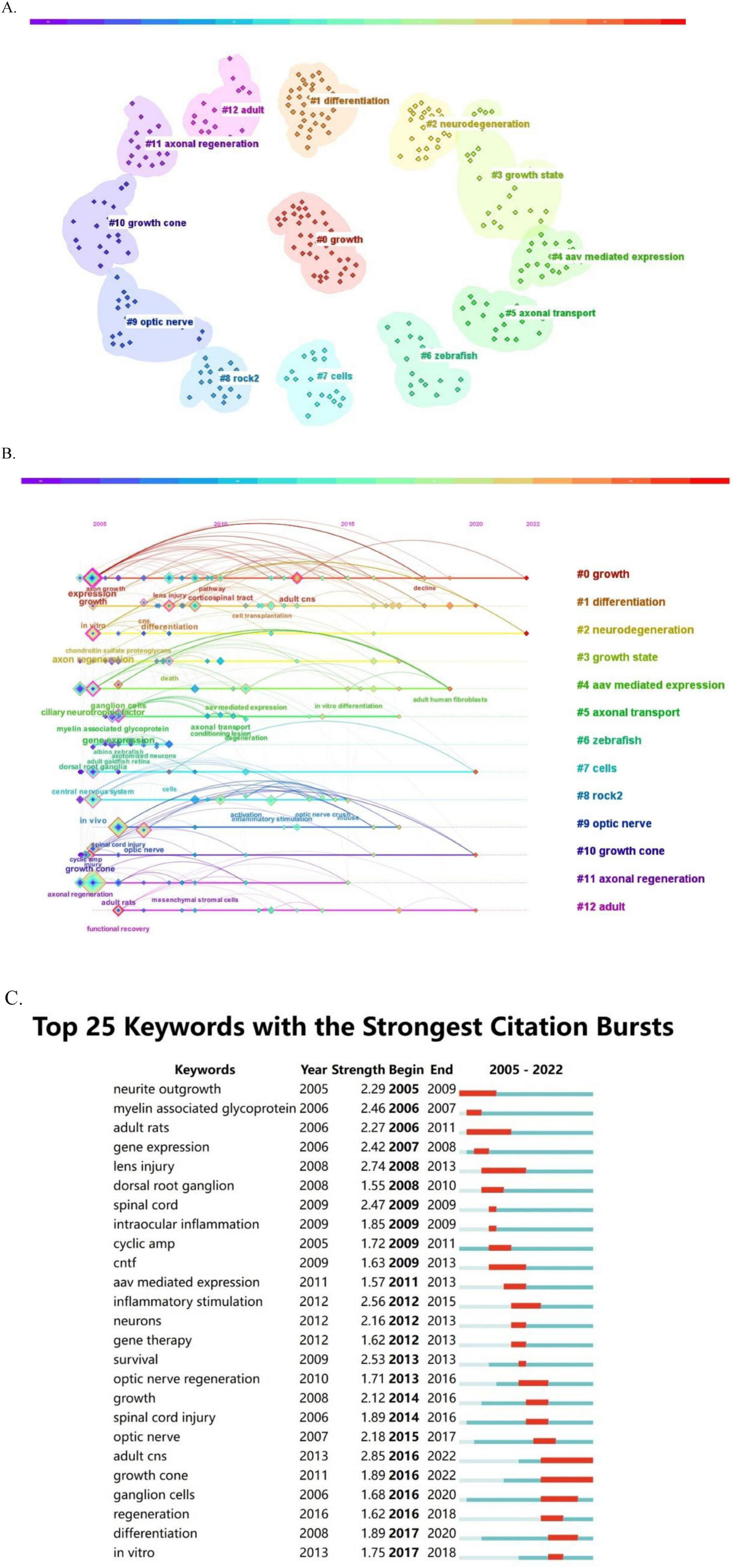
Figure 7. Keyword analysis. (A) Keyword cluster graph. (B) Timeline view of keywords. (C) Burst test of keywords.
The temporal evolution of keyword usage patterns is shown in Figure 7B. The size of each point in Figure 7B is related to the occurrence of the corresponding keyword. The larger the block, the higher the frequency of keyword occurrence. In addition, keywords that exhibit recent growth trends may represent hot research topics in the future. Our keyword burst analysis identified several notable words (Figure 7C), including: “lens injury,” “CNTF,” “myelin-associated glycoprotein,” “neurite outgrowth,” “optic nerve regeneration,” “AAV-mediated expression,” “intraocular inflammation,” “ganglion cells,” and “gene therapy.” Keywords with the strongest burst signal reflect the current research frontier in the field. The keywords of earlier bursts indicate that research interest was initially concentrated in these areas, while more recent bursts denote a marked increase in interest in the topic. Figure 7C highlights the five keywords with the highest burst intensity—“adult CNS,” “lens injury,” “inflammatory stimulation,” “survival,” and “spinal cord”—exhibiting burst intensities of 2.85, 2.74, 2.56, 2.53, and 2.47, respectively. The optic nerve and the spinal cord both belong to the central nervous system and are highly similar in terms of anatomical structure, cellular composition and microenvironment. The two of them share many key technological platforms and material strategies in regenerative medicine. The keywords in the earliest burst were “neurite outgrowth,” “myelin-associated glycoprotein,” “adult rats,” “gene expression,” and “lens injury,” representing the focus of initial research. Adult rats and zebrafish are often used as animal models for optic nerve regeneration and are closely related to the research on the optic nerve. Meanwhile, the keyword that recently showed a burst was “growth cone,” which reflects a new area of intense research interest.
4 Discussion
In this study, we conducted a comprehensive data and bibliometric analysis of the 100 most cited publications between 2005 and 2025 in the field of optic nerve regeneration. This analytical strategy enabled a detailed investigation of the evolution, key focus areas, and innovative trends in optic nerve regeneration research, and provided valuable quantitative insights into recent research, thereby deepening the understanding of the topic.
The top 100 publications accumulated a total of 34,636 citations, with citation counts ranging from 103 to 1,545, resulting in a median of 346.4 citations per article. He Zhigang was considered the most productive contributor, having contributed to 19 of these papers. The United States accounted for the highest number of publications (59), followed by Germany and the China with 17 and 13 publications, respectively. The Harvard University system was the most prolific institution, publishing 30 papers, followed by Children’s Hospital and Stanford University, both with eight papers. Keyword analysis identified several areas of interest, including myelin-associated glycoprotein, intraocular inflammation, CNTF, AAV-mediated expression, and gene therapy. Further keyword analysis revealed “growth cone” as an important recent keyword in the field.
4.1 Myelin-associated glycoprotein
Myelin-associated glycoprotein (MAG) is a transmembrane protein primarily expressed in myelin-forming cells (oligodendrocytes and Schwann cells) of the CNS and PNS. MAG is an important inhibitor of neurite growth. After optic nerve injury, this glycoprotein accumulates in myelin debris at the injured site, resulting in the formation of a microenvironment unfavorable to axon regeneration (David and Kottis, 1994). MAG has a bidirectional transduction mechanism, namely, myelin-to-axon and axon-to-myelin. In the former, MAG maintains the stability of the myelin–axon interface by specifically binding in trans to complex gangliosides, such as GT1b and GD1a, on the surface of the axon membrane. Furthermore, the binding of MAG dimers to sialic acid triggers axon growth cone collapse and inhibits microtubule assembly and disassembly dynamics, thus impeding nerve regeneration (Pronker et al., 2016). The latter (axon-to-myelin) involves the regulation of myelin formation and maintenance, which is dependent on the tyrosine kinase Fyn (Cafferty et al., 2010). Thus, the targeted regulation of MAG is crucial for promoting optic nerve regeneration. Evidence suggests that interventions targeting MAG alone (such as gene knockout or the use of neutralizing antibodies) only weakly promote optic axon regeneration. However, triple knockout of Nogo-A, MAG, and oligodendrocyte-myelin glycoprotein (OMgp) can significantly reduce the collapse of growth cones and extend the regeneration distance of RGC axons after optic nerve injury. (Zhang et al., 2022). Therefore, future studies should prioritize multi-target synergistic treatment strategies combined with novel delivery technologies to overcome the multiple inhibition barriers that impede regeneration in the CNS and ultimately achieve functional optic nerve regeneration.
4.2 Ciliary neurotrophic factor
Ciliary neurotrophic factor (CNTF) is mainly secreted by astrocytes during optic nerve regeneration and its endogenous expression can be significantly activated in response to inflammation or nerve injury (Kimura et al., 2016). CNTF is typically delivered via single intravitreal injection. However, its short half-life and the difficulty associated with the maintenance of effective concentrations limit its axonal regeneration effect (Müller et al., 2009). Over recent years, strategies employing subretinal injection mediated by adeno-associated virus (AAV) vectors or the delivery of genetically modified neural stem cells (CNTF-NS) have been developed. These methods have achieved continuous CNTF expression, thereby significantly prolonging the window of opportunity for axon regeneration while avoiding the inflammation induced by repeated injection (Cen et al., 2017; Dulz et al., 2020; Pernet et al., 2013b). Notably, although single CNTF treatment can induce axonal regeneration, functional recovery is limited. Studies have shown that PTEN/SOCS3 gene double-knockout or osteopontin (OPN), insulin-like growth factor 1 (IGF1), and CNTF co-expression can significantly enhance regeneration efficiency through the synergistic activation of downstream signaling pathways (Jacobi et al., 2022). Combining the administration of potassium channel blockers (such as 4-AP) with PTEN/SOCS3 gene knockout can further promote the recovery of axon electrophysiological function (Bei et al., 2016). Recently, Behtaj et al. (2024) innovatively created a bionic delivery system with gradient slow-release properties by covalently coupling CNTF to an electrospun polyglycerol sebacate/polycaprolactone (PGS/PCL) scaffold. The authors reported that this system could guide the directional migration of RGC axons toward regions with high CNTF concentrations, providing a novel therapeutic strategy for optic nerve regeneration (Behtaj et al., 2024).
4.3 Intraocular inflammation
Progress in optic nerve regeneration research has highlighted the therapeutic potential of the inflammatory response in regenerative medicine. Classical studies have shown that intraocular inflammatory stimuli, such as lens injury and yeast cell wall (zymosan) injection, can activate the regenerative program in RGCs, thereby circumventing the regenerative limitations inherent to the CNS (Yin et al., 2009). The resulting cytokine cascade triggered by immune cell infiltration is particularly critical in this process. The macrophage-/neutrophil-specific secretion of oncomodulin (OCM) is a central mediator of axonal regeneration. Its levels markedly increase after inflammatory stimulation and functional blockade experiments have shown that OCM specifically regulates axon regeneration without affecting RGC survival (Benowitz and Popovich, 2011; Yin et al., 2009). However, independent validation of these findings with OCM knockout mice has not yet been carried out. This provides a precise entry point for targeted intervention. Importantly, the microenvironment regulatory network has significant synergistic effects. For example, SDF1 enhances OCM activity by upregulating intracellular cAMP levels, thereby forming a multi-factor synergistic mechanism that overcomes the therapeutic bottleneck of single-factor therapy (Xie et al., 2022). The latest breakthrough in the field comes from the establishment of the conditioned lens injury (cLI) model, a non-genetic intervention strategy involving the implementation of mild lens injury two weeks before optic nerve compression (ONC). By recruiting CCR2+ immune cell populations, this strategy demonstrated the ability to fully regenerate axons beyond traditional approaches and even achieve functional brain innervation (Feng et al., 2023). Crucially, the regenerative effect of cLI is independent of known factors, such as OCM, suggesting the existence of novel immune regulatory pathways, a finding that may reshape existing theoretical frameworks and open up new research directions. However, the duration and nature of inflammation demonstrate a distinct “double-edged sword” characteristic. Acute inflammatory responses contribute to the regeneration-supportive microenvironment by activating microglia to remove damaged cellular debris and recruiting myeloid cells to secrete neurotrophic factors, such as regulatory proteins and cntf. In contrast, chronic inflammation, through sustained release of neurotoxic mediators, including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), promotes the activation of inhibitory A1 astrocytes and compromises the integrity of the blood-retinal barrier. These processes disrupt microenvironmental homeostasis, ultimately impeding nerve regeneration and repair (Au and Ma, 2022). This suggests that future studies need to establish an accurate immunophenotypic regulatory system to achieve a dynamic balance between the pro-regeneration mechanism and the neuroprotective effect by controlling the intensity of inflammation. This may promote a paradigm shift in optic nerve repair strategies from empirical intervention to intelligent regulation.
4.4 AAV-mediated gene modulation
Adeno-associated virus-mediated gene modulation is a technique that uses AAV as a gene delivery vector to introduce foreign genes into target cells or tissues and enable their expression (Leaver et al., 2006). More than 100 natural AAV serotypes have been identified, of which 13 (including AAV1–9 and rh10) have shown an affinity for specific subsets of retinal cells in ophthalmic studies (Carvalho et al., 2018). AAVs can carry genes encoding cytokines (e.g., CNTF, hIL-6) under the regulation of tissue-specific promoters (such as CAG and hSyn), thereby achieving directed gene expression. Studies have shown that the AAV2 serotype targets RGCs with high specificity, effectively improving their survival rate and promoting axon regeneration (Cao et al., 2019). However, its transduction range is limited to the local injection and diffusion area (Liu et al., 2020; Ross et al., 2021). Traditional invasive delivery methods, such as intravitreal or subretinal injection, are effective in targeting RGCs but can lead to complications such as retinal detachment and bleeding, as well as result in unequal viral distribution. Recent studies have attempted to target RGCs via the injection of AAV-PHP.eB through the retroorbital venous sinus. This serotype exhibits significantly enhanced transduction efficiency in mouse models. However, AAV-PHP.eB can penetrate the blood–brain barrier and thereby induce off-target effects in the CNS and also has relatively low tissue specificity (Tang et al., 2024). New AAV variants and specific promoters need to be identified to improve targeting accuracy and reduce systemic side effects. Meanwhile, combining these advancements with non-invasive delivery technology and novel cytokines holds promise for overcoming current limitations and achieving multi-gene synergistic therapy.
4.5 Growth cone
The growth cone is the core functional structure of axon regeneration, playing a key role in microenvironment perception, signal integration, and guidance extension during nerve injury repair (Chierzi et al., 2005). In models of optic nerve injury, the axonal ends of RGCs in adult mammals often form characteristic retractable ball structures. This pathological phenomenon is considered to be an important morphological sign of hindered axon regeneration. Recent studies have revealed that the knockout of the gene coding for non-muscle myosin IIA/B in RGCs significantly reduced the formation of retractable spheres, and successfully transformed the ends of axons with stagnant regeneration into functional growth cones with dynamic activity, thus achieving a significant improvement in axon regenerative ability (Wang et al., 2020). From a cell biological perspective, axon regeneration requires adequate membrane component support. Studies have shown that enhancing phospholipid synthesis in RGCs by modulating lipid metabolism can effectively promote growth cone membrane extension (Chen et al., 2024). Regarding microenvironment regulation, laminin significantly enhances the structural stability of the growth cone and promotes its continuous extension by activating the integrin receptor signaling pathway (Fligor et al., 2018). In addition, studies on growth cone guiding molecules have demonstrated that netrin-1 and other chemical orientation factors can both significantly improve the axon elongation rate and increase the directional elongation of growth cones by activating intracellular signaling cascades (Qiu et al., 2024).
4.6 Clinical translation
The regeneration of the optic nerve is a key part of the restoration of visual function in people with blindness. The convergence of life science and engineering technologies is driving revolutionary breakthroughs in the field of optic nerve regeneration. Multidisciplinary collaborations have led to innovations such as the development of chitosan-CNTF bioactive materials and targeted delivery systems, while gene editing techniques have precisely unlocked the regenerative potential of RGCs. Additionally, stem cell therapy can contribute to restoring the ecological balance in the damaged microenvironment. Animal studies have demonstrated that axons can exceed the regenerative limits of the CNS; however, the central challenge in clinical translation lies in bridging the “precision connectivity gap,” that is, ensuring that regenerated nerve fibers not only grow over long distances but also re-establish functional links with the visual centers of the brain. However, the regenerated axons in optic nerve are almost no myelin sheath around them, and thus cannot conduct action potentials (Bei et al., 2016; Suter et al., 2021). The myelin sheath formation is a crucial step in the recovery of visual function (Del Negro et al., 2023), and it have been demonstrated that increased myelin regeneration of the optic nerve is associated with improved visual function (Henriet et al., 2023). The standardized visual function assessments include Visual Evoked Potentials (VEP), which records electrical activity in the visual cortex to confirm the functional connectivity of the retinocortical pathway; Optomotor Response (OMR), a non-invasive behavioral assay that evaluates gross visual functions like motion perception and contrast sensitivity, essential for validating functional improvements in the cLI model; Pupillary Light Reflex (PLR), which assesses the integrity of the retinocollicular pathway and is suitable for models of proximal optic nerve injury such as traumatic optic neuropathy; and Visual Water Maze (VWM), which measures higher-order visual functions. In addition, AI navigation and intelligent biological scaffolds hold promise for resolving the challenge of the accurate docking of optic nerves, potentially leading to brain–computer interfaces or a hybrid pathway of “bio-digital vision.” The future for patients with optic nerve injury is bright and increasingly promising.
5 Limitations
This study had several limitations. Although the Web of Science is the most commonly used database for conducting a literature search, it does not contain all publications. Additionally, relying on citation frequency as a selection criterion can lead to the exclusion of recently published works that may be influential but have not yet accumulated a large number of citations. Moreover, there is a possibility of citation bias because papers from certain institutions or well-known authors may receive more citations than equally valuable works from less prominent sources.
6 Conclusion
This study systematically revealed the evolution of the research paradigm and the characteristics of the knowledge graph in the field of optic nerve regeneration through a quantitative analysis of the 100 most highly cited publications from the past 20 years. Bibliometric data showed that United States research institutions continue to lead the field in terms of publication volume, international collaboration networks, and breakthrough output. Early studies focused on identifying the mechanisms underlying endogenous neuronal signaling pathways (PTEN/mTOR, KLF family), while recent research has concentrated on identifying novel targets for optic nerve regeneration and the development of intelligent biomaterials. The transnational cooperation network formed by the United States, China, Germany, Britain, and other countries has significantly accelerated the process of knowledge transformation. The core challenge of current research is how to achieve long-distance regeneration of the optic nerve and reconnect the regenerated axons to the relevant brain regions. Future efforts should be concentrated on developing multi-target therapies against inhibitor molecules and establishing real-time immune monitoring platforms for precise control of inflammation response to improve neural regeneration. These advances will help bridge the gap between basic research and clinical treatment for optic nerve repair.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Saijilafu: Conceptualization, Project administration, Writing – review & editing, Supervision, Funding acquisition. PC: Methodology, Formal analysis, Investigation, Writing – original draft, Validation, Data curation. LY: Writing – original draft, Methodology, Visualization, Investigation. YS: Data curation, Methodology, Writing – original draft. QW: Writing – original draft, Methodology. XC: Methodology, Writing – original draft. CC: Writing – review & editing, Conceptualization. JZ: Writing – original draft, Methodology. LF: Funding acquisition, Conceptualization, Writing – review & editing. R-JX: Conceptualization, Supervision, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the start-up funding from Hangzhou City University to Saijilafu, and the Hangzhou City Health and Technology Project B20254767 (to Linjun Fang and Saijilafu).
Acknowledgments
We apologize to the authors for not being able to cite their work due to space constraints.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abe, N., and Cavalli, V. (2008). Nerve injury signaling. Curr. Opin. Neurobiol. 18, 276–283. doi: 10.1016/j.conb.2008.06.005
Au, N., and Ma, C. (2022). Neuroinflammation, microglia and implications for retinal ganglion cell survival and axon regeneration in traumatic optic neuropathy. Front. Immunol. 13:860070. doi: 10.3389/fimmu.2022.860070
Baldwin, K., Carbajal, K., Segal, B., and Giger, R. (2015). Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration. Proc. Natl. Acad. Sci. U S A. 112, 2581–2586. doi: 10.1073/pnas.1423221112
Batagelj, V., and Mrvar, A. (2002). ““Pajek - Analysis and visualization of large networks. GRAPH Drawing 2265”,” in Lecture Notes In Computer Science, Vol. 2265, eds P. Mutzel, M. Junger, and S. Leipert (Berlin: Springer-Verlag Berlin), 477–478.
Behtaj, S., Karamali, F., Najafian, S., Masaeli, E., and Rybachuk, M. (2024). Ciliary neurotrophic factor mediated growth of retinal ganglion cell axons on PGS/PCL scaffolds. Biomed. Mater. 19, doi: 10.1088/1748-605X/ad1bae
Bei, F., Lee, H., Liu, X., Gunner, G., Jin, H., Ma, L., et al. (2016). Restoration of visual function by enhancing conduction in regenerated axons. Cell 164, 219–232. doi: 10.1016/j.cell.2015.11.036
Belin, S., Nawabi, H., Wang, C., Tang, S., Latremoliere, A., Warren, P., et al. (2015). Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron 86, 1000–1014. doi: 10.1016/j.neuron.2015.03.060
Benowitz, L., and Popovich, P. (2011). Inflammation and axon regeneration. Curr. Opin. Neurol. 24, 577–583. doi: 10.1097/WCO.0b013e32834c208d
Benowitz, L., He, Z., and Goldberg, J. (2017). Reaching the brain: Advances in optic nerve regeneration. Exp. Neurol. 287, 365–373. doi: 10.1016/j.expneurol.2015.12.015
Berry, M., Ahmed, Z., Lorber, B., Douglas, M., and Logan, A. (2008). Regeneration of axons in the visual system. Restor. Neurol. Neurosci. 26, 147–174.
Bertrand, J., Winton, M., Rodriguez-Hernandez, N., Campenot, R., and McKerracher, L. (2005). Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J. Neurosci. 25, 1113–1121. doi: 10.1523/JNEUROSCI.3931-04.2005
Biermann, J., Grieshaber, P., Goebel, U., Martin, G., Thanos, S., Di Giovanni, S., et al. (2010). Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 51, 526–534. doi: 10.1167/iovs.09-3903
Bollaerts, I., Van Houcke, J., Andries, L., De Groef, L., and Moons, L. (2017). Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediators Inflamm. 2017:9478542. doi: 10.1155/2017/9478542
Bray, E., Yungher, B., Levay, K., Ribeiro, M., Dvoryanchikov, G., Ayupe, A., et al. (2019). Thrombospondin-1 mediates axon regeneration in retinal ganglion cells. Neuron 103, 642–657.e7. doi: 10.1016/j.neuron.2019.05.044
Cafferty, W., Duffy, P., Huebner, E., and Strittmatter, S. M. (2010). MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 30, 6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010
Cao, X., Yung, J., Mak, H., and Leung, C. (2019). Factors governing the transduction efficiency of adeno-associated virus in the retinal ganglion cells following intravitreal injection. Gene Ther. 26, 109–120. doi: 10.1038/s41434-019-0060-0
Cartoni, R., Norsworthy, M., Bei, F., Wang, C., Li, S., Zhang, Y., et al. (2016). The mammalian-specific protein armcx1 regulates mitochondrial transport during axon regeneration. Neuron 92, 1294–1307. doi: 10.1016/j.neuron.2016.10.060
Carvalho, L., Xiao, R., Wassmer, S., Langsdorf, A., Zinn, E., Pacouret, S., et al. (2018). Synthetic adeno-associated viral vector efficiently targets mouse and nonhuman primate retina in vivo. Hum. Gene Ther. 29, 771–784. doi: 10.1089/hum.2017.154
Cen, L., Liang, J., Chen, J., Harvey, A., Ng, T., Zhang, M., et al. (2017). AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience 343, 472–482. doi: 10.1016/j.neuroscience.2016.12.027
Chandran, V., Coppola, G., Nawabi, H., Omura, T., Versano, R., Huebner, E., et al. (2016). A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron 89, 956–970. doi: 10.1016/j.neuron.2016.01.034
Chang, E., and Goldberg, J. (2012). Glaucoma 2.0: Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 119, 979–986. doi: 10.1016/j.ophtha.2011.11.003
Chen, B., Zhang, H., Zhai, Q., Li, H., Wang, C., and Wang, Y. (2022). Traumatic optic neuropathy: A review of current studies. Neurosurg. Rev. 45, 1895–1913. doi: 10.1007/s10143-021-01717-9
Chen, C. (2004). Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. U S A. 101, 5303–5310. doi: 10.1073/pnas.0307513100
Chen, W., Wu, J., Yang, C., Li, S., Liu, Z., An, Y., et al. (2024). Lipin1 depletion coordinates neuronal signaling pathways to promote motor and sensory axon regeneration after spinal cord injury. Proc. Natl. Acad. Sci. U S A. 121:e2404395121. doi: 10.1073/pnas.2404395121
Chidlow, G., Ebneter, A., Wood, J., and Casson, R. (2011). The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol. 121, 737–751. doi: 10.1007/s00401-011-0807-1
Chierzi, S., Ratto, G., Verma, P., and Fawcett, J. (2005). The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur. J. Neurosci. 21, 2051–2062. doi: 10.1111/j.1460-9568.2005.04066.x
Chung, R., Penkowa, M., Dittmann, J., King, C., Bartlett, C., Asmussen, J., et al. (2008). Redefining the role of metallothionein within the injured brain: Extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J. Biol. Chem. 283, 15349–15358. doi: 10.1074/jbc.M708446200
Cui, Q. (2006). Actions of neurotrophic factors and their signaling pathways in neuronal survival and axonal regeneration. Mol. Neurobiol. 33, 155–179. doi: 10.1385/MN:33:2:155
David, S., and Kottis, V. Identification of myelin-associated glycoprotein as a major myelin-berived of neurite growth. Neuron 13, 805–811. doi: 10.1016/0896-6273(94)90247-X
de Lima, S., Koriyama, Y., Kurimoto, T., Oliveira, J., Yin, Y., Li, Y., et al. (2012). Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci. U S A. 109, 9149–9154. doi: 10.1073/pnas.1119449109
Del Negro, I., Pauletto, G., Verriello, L., Spadea, L., Salati, C., Ius, T., et al. (2023). Uncovering the genetics and physiology behind optic neuritis. Genes 14:2192. doi: 10.3390/genes14122192
Dickendesher, T., Baldwin, K., Mironova, Y., Koriyama, Y., Raiker, S., Askew, K., et al. (2012). NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 15, 703–712. doi: 10.1038/nn.3070
Duan, X., Qiao, M., Bei, F., Kim, I., He, Z., and Sanes, J. (2015). Subtype-specific regeneration of retinal ganglion cells following axotomy: Effects of osteopontin and mTOR signaling. Neuron 85, 1244–1256. doi: 10.1016/j.neuron.2015.02.017
Dulz, S., Bassal, M., Flachsbarth, K., Riecken, K., Fehse, B., Schlichting, S., et al. (2020). Intravitreal co-administration of GDNF and CNTF confers synergistic and long-lasting protection against injury-induced cell death of retinal ganglion Cells in mice. Cells 9:2082. doi: 10.3390/cells9092082
Dun, X., and Parkinson, D. (2017). Role of Netrin-1 signaling in nerve regeneration. Int. J. Mol. Sci. 18:491. doi: 10.3390/ijms18030491
Ellis-Behnke, R., Liang, Y., You, S., Tay, D., Zhang, S., So, K., et al. (2006). Nano neuro knitting: Peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc. Natl. Acad. Sci. U S A. 103, 5054–5059. doi: 10.1073/pnas.0600559103
Elsaeidi, F., Bemben, M., Zhao, X., and Goldman, D. (2014). Jak/Stat signaling stimulates zebrafish optic nerve regeneration and overcomes the inhibitory actions of Socs3 and Sfpq. J. Neurosci. 34, 2632–2644. doi: 10.1523/JNEUROSCI.3898-13.2014
Fausett, B., and Goldman, D. A. (2006). role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J. Neurosci. 26, 6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006
Feng, Q., Wong, K., and Benowitz, L. (2023). Full-length optic nerve regeneration in the absence of genetic manipulations. JCI Insight 8:e164579. doi: 10.1172/jci.insight.164579
Fimbel, S., Montgomery, J., Burket, C., and Hyde, D. (2007). Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 27, 1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007
Fischer, D., and Leibinger, M. (2012). Promoting optic nerve regeneration. Prog. Retin. Eye Res. 31, 688–701. doi: 10.1016/j.preteyeres.2012.06.005
Fischer, D., Hauk, T., Müller, A., and Thanos, S. (2008). Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol. Cell. Neurosci. 37, 471–479. doi: 10.1016/j.mcn.2007.11.002
Fligor, C., Langer, K., Sridhar, A., Ren, Y., Shields, P., Edler, M., et al. (2018). Three-dimensional retinal organoids facilitate the investigation of retinal ganglion cell development, organization and neurite outgrowth from human pluripotent stem cells. Sci. Rep. 8:14520. doi: 10.1038/s41598-018-32871-8
Fujita, Y., and Yamashita, T. (2014). Axon growth inhibition by RhoA/ROCK in the central nervous system. Front. Neurosci. 8:338. doi: 10.3389/fnins.2014.00338
Gaub, P., Joshi, Y., Wuttke, A., Naumann, U., Schnichels, S., Heiduschka, P., et al. (2011). The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain 134, 2134–2148. doi: 10.1093/brain/awr142
Harvey, A., Hu, Y., Leaver, S., Mellough, C., Park, K., Verhaagen, J., et al. (2006). Gene therapy and transplantation in CNS repair: The visual system. Prog. Retin. Eye Res. 25, 449–489. doi: 10.1016/j.preteyeres.2006.07.002
Hassan-Montero, Y., De-Moya-Anegon, F., and Guerrero-Bote, V. P. (2022). SCImago Graphica: A new too for exploring and visually communicating data. Prof. Inf. 31:e310502. doi: 10.3145/epi.2022.sep.02
He, Z., and Jin, Y. (2016). Intrinsic control of axon regeneration. Neuron 90, 437–451. doi: 10.1016/j.neuron.2016.04.022
Henriet, E., Martin, E., Jubin, P., Langui, D., Mannioui, A., Stankoff, B., et al. (2023). Monitoring recovery after CNS demyelination, a novel tool to de-risk pro-remyelinating strategies. Brain 146, 2453–2463. doi: 10.1093/brain/awad051
Hilla, A., Diekmann, H., and Fischer, D. (2017). Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J. Neurosci. 37, 6113–6124. doi: 10.1523/JNEUROSCI.0584-17.2017
Hoffman, P. N. A. (2010). conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp. Neurol. 223, 11–18. doi: 10.1016/j.expneurol.2009.09.006
Hu, Y., Leaver, S., Plant, G., Hendriks, W., Niclou, S., Verhaagen, J., et al. (2005). Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol. Ther. 11, 906–915. doi: 10.1016/j.ymthe.2005.01.016
Jacobi, A., Tran, N., Yan, W., Benhar, I., Tian, F., Schaffer, R., et al. (2022). Overlapping transcriptional programs promote survival and axonal regeneration of injured retinal ganglion cells. Neuron 110, 2625–2645.e7. doi: 10.1016/j.neuron.2022.06.002
Jin, Z., Gao, M., Deng, W., Wu, K., Sugita, S., Mandai, M., et al. (2019). Stemming retinal regeneration with pluripotent stem cells. Prog. Retin Eye Res. 69, 38–56. doi: 10.1016/j.preteyeres.2018.11.003
Kimura, A., Namekata, K., Guo, X., Harada, C., and Harada, T. (2016). Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. Int. J. Mol. Sci. 17:1584. doi: 10.3390/ijms17091584
King, C., Rodger, J., Bartlett, C., Esmaili, T., Dunlop, S., and Beazley, L. (2007). Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp. Neurol. 205, 48–55. doi: 10.1016/j.expneurol.2007.01.017
Koch, J., Tönges, L., Barski, E., Michel, U., Bähr, M., and Lingor, P. (2014). ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell. Death Dis. 5:e1225. doi: 10.1038/cddis.2014.191
Koprivica, V., Cho, K., Park, J., Yiu, G., Atwal, J., Gore, B., et al. (2005). EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310, 106–110. doi: 10.1126/science.1115462
Kretz, A., Happold, C., Marticke, J., and Isenmann, S. (2005). Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol. Cell. Neurosci. 29, 569–579. doi: 10.1016/j.mcn.2005.04.009
Krucoff, M., Rahimpour, S., Slutzky, M., Edgerton, V., and Turner, D. (2016). Enhancing nervous system recovery through neurobiologics, neural interface training, and neurorehabilitation. Front. Neurosci. 10:584. doi: 10.3389/fnins.2016.00584
Kurimoto, T., Yin, Y., Habboub, G., Gilbert, H., Li, Y., Nakao, S., et al. (2013). Neutrophils express oncomodulin and promote optic nerve regeneration. J. Neurosci. 33, 14816–14824. doi: 10.1523/JNEUROSCI.5511-12.2013
Kurimoto, T., Yin, Y., Omura, K., Gilbert, H., Kim, D., Cen, L., et al. (2010). Long-distance axon regeneration in the mature optic nerve: Contributions of oncomodulin, cAMP, and pten gene deletion. J. Neurosci. 30, 15654–15663. doi: 10.1523/JNEUROSCI.4340-10.2010
Laha, B., Stafford, B., and Huberman, A. (2017). Regenerating optic pathways from the eye to the brain. Science 356, 1031–1034. doi: 10.1126/science.aal5060
Lamba, D., Karl, M., and Reh, T. (2008). Neural regeneration and cell replacement: A view from the eye. Cell. Stem. Cell. 2, 538–549. doi: 10.1016/j.stem.2008.05.002
Lambiase, A., Aloe, L., Centofanti, M., Parisi, V., Báo, S., Mantelli, F., et al. (2009). Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. U S A. 106, 13469–13474. doi: 10.1073/pnas.0906678106
Leaver, S., Cui, Q., Plant, G., Arulpragasam, A., Hisheh, S., Verhaagen, J., et al. (2006). AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 13, 1328–1341. doi: 10.1038/sj.gt.3302791
Leibinger, M., Andreadaki, A., Diekmann, H., and Fischer, D. (2013a). Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell. Death Dis. 4:e805. doi: 10.1038/cddis.2013.310
Leibinger, M., Müller, A., Andreadaki, A., Hauk, T., Kirsch, M., and Fischer, D. (2009). Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J. Neurosci. 29, 14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009
Leibinger, M., Müller, A., Gobrecht, P., Diekmann, H., Andreadaki, A., and Fischer, D. (2013b). Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell. Death Dis. 4:e609. doi: 10.1038/cddis.2013.126
Li, S., Yang, C., Zhang, L., Gao, X., Wang, X., Liu, W., et al. (2016). Promoting axon regeneration in the adult CNS by modulation of the melanopsin/GPCR signaling. Proc. Natl. Acad. Sci. U S A. 113, 1937–1942. doi: 10.1073/pnas.1523645113
Li, Y., Andereggen, L., Yuki, K., Omura, K., Yin, Y., Gilbert, H., et al. (2017). Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc. Natl. Acad. Sci. U S A. 114, E209–E218. doi: 10.1073/pnas.1616811114
Lim, J., Stafford, B., Nguyen, P., Lien, B., Wang, C., Zukor, K., et al. (2016). Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci. 19, 1073–1084. doi: 10.1038/nn.4340
Lingor, P., Teusch, N., Schwarz, K., Mueller, R., Mack, H., Bähr, M., et al. (2007). Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J. Neurochem. 103, 181–189. doi: 10.1111/j.1471-4159.2007.04756.x
Lingor, P., Tönges, L., Pieper, N., Bermel, C., Barski, E., Planchamp, V., et al. (2008). ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain 131, 250–263. doi: 10.1093/brain/awm284
Liu, K., Tedeschi, A., Park, K., and He, Z. (2011). Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 34, 131–152. doi: 10.1146/annurev-neuro-061010-113723
Liu, Y., Huang, S., Ng, T., Liang, J., Xu, Y., Chen, S., et al. (2020). Longitudinal evaluation of immediate inflammatory responses after intravitreal AAV2 injection in rats by optical coherence tomography. Exp. Eye Res. 193:107955. doi: 10.1016/j.exer.2020.107955
Logan, A., Ahmed, Z., Baird, A., Gonzalez, A., and Berry, M. (2006). Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain 129, 490–502. doi: 10.1093/brain/awh706
Lu, Y., Brommer, B., Tian, X., Krishnan, A., Meer, M., Wang, C., et al. (2020). Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129. doi: 10.1038/s41586-020-2975-4
Luo, X., Salgueiro, Y., Beckerman, S., Lemmon, V., Tsoulfas, P., and Park, K. (2013). Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp. Neurol. 247, 653–662. doi: 10.1016/j.expneurol.2013.03.001
Mead, B., and Tomarev, S. (2017). Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl. Med. 6, 1273–1285. doi: 10.1002/sctm.16-0428
Mead, B., Berry, M., Logan, A., Scott, R., Leadbeater, W., and Scheven, B. (2015). Stem cell treatment of degenerative eye disease. Stem Cell. Res. 14, 243–257. doi: 10.1016/j.scr.2015.02.003
Mead, B., Logan, A., Berry, M., Leadbeater, W., and Scheven, B. (2017). Concise review: Dental pulp Stem Cells: A novel cell therapy for retinal and central nervous system repair. Stem Cells 35, 61–67. doi: 10.1002/stem.2398
Mead, B., Logan, A., Berry, M., Leadbeater, W., and Scheven, B. (2013). Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest. Ophthalmol. Vis. Sci. 54, 7544–7556. doi: 10.1167/iovs.13-13045
Moore, D., Blackmore, M., Hu, Y., Kaestner, K., Bixby, J., Lemmon, V., et al. (2009). KLF family members regulate intrinsic axon regeneration ability. Science 326, 298–301. doi: 10.1126/science.1175737
Müller, A., Hauk, T., and Fischer, D. (2007). Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain 130, 3308–3320. doi: 10.1093/brain/awm257
Müller, A., Hauk, T., Leibinger, M., Marienfeld, R., and Fischer, D. (2009). Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol. Cell. Neurosci. 41, 233–246. doi: 10.1016/j.mcn.2009.03.002
Norsworthy, M., Bei, F., Kawaguchi, R., Wang, Q., Tran, N., Li, Y., et al. (2017). Sox11 expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron 94, 1112–1120.e4. doi: 10.1016/j.neuron.2017.05.035
Pan, T., Huang, Y., Wei, J., Lai, C., Chen, Y., Nan, K., et al. (2024). Implantation of biomimetic polydopamine nanocomposite scaffold promotes optic nerve regeneration through modulating inhibitory microenvironment. J. Nanobiotechnol. 22:683. doi: 10.1186/s12951-024-02962-y
Park, K., Liu, K., Hu, Y., Smith, P., Wang, C., Cai, B., et al. (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966. doi: 10.1126/science.1161566
Pastrana, E., Moreno-Flores, M., Gurzov, E., Avila, J., Wandosell, F., and Diaz-Nido, J. (2006). Genes associated with adult axon regeneration promoted by olfactory ensheathing cells: A new role for matrix metalloproteinase 2. J. Neurosci. 26, 5347–5359. doi: 10.1523/JNEUROSCI.1111-06.2006
Pernet, V., and Di Polo, A. (2006). Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain 129, 1014–1026. doi: 10.1093/brain/awl015
Pernet, V., Joly, S., Dalkara, D., Jordi, N., Schwarz, O., Christ, F., et al. (2013a). Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol. Dis. 51, 202–213. doi: 10.1016/j.nbd.2012.11.011
Pernet, V., Joly, S., Jordi, N., Dalkara, D., Guzik-Kornacka, A., Flannery, J., et al. (2013b). Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell. Death Dis. 4:e734. doi: 10.1038/cddis.2013.266
Pronker, M., Lemstra, S., Snijder, J., Heck, A., Thies-Weesie, D., Pasterkamp, R., et al. (2016). Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat. Commun. 7:13584. doi: 10.1038/ncomms13584
Qin, S., Zou, Y., and Zhang, C. (2013). Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat. Commun. 4:2633. doi: 10.1038/ncomms3633
Qiu, Z., Minegishi, T., Aoki, D., Abe, K., Baba, K., and Inagaki, N. (2024). Adhesion-clutch between DCC and netrin-1 mediates netrin-1-induced axonal haptotaxis. Front. Mol. Neurosci. 17:1307755. doi: 10.3389/fnmol.2024.1307755
Ross, A., McDougald, D., Khan, R., Duong, T., Dine, K., Aravand, P., et al. (2021). Rescue of retinal ganglion cells in optic nerve injury using cell-selective AAV mediated delivery of SIRT1. Gene Ther. 28, 256–264. doi: 10.1038/s41434-021-00219-z
Sas, A., Carbajal, K., Jerome, A., Menon, R., Yoon, C., Kalinski, A., et al. (2020). A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 21, 1496–1505. doi: 10.1038/s41590-020-00813-0
Schwab, M., and Strittmatter, S. (2014). Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 27, 53–60. doi: 10.1016/j.conb.2014.02.011
Sengottuvel, V., Leibinger, M., Pfreimer, M., Andreadaki, A., and Fischer, D. (2011). Taxol facilitates axon regeneration in the mature CNS. J. Neurosci. 31, 2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011
Sherpa, T., Fimbel, S., Mallory, D., Maaswinkel, H., Spritzer, S., Sand, J., et al. (2008). Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev. Neurobiol. 68, 166–181. doi: 10.1002/dneu.20568
Singhal, S., Bhatia, B., Jayaram, H., Becker, S., Jones, M., Cottrill, P., et al. (2012). Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem. Cells Transl. Med. 1, 188–199. doi: 10.5966/sctm.2011-0005
Smith, P., Sun, F., Park, K., Cai, B., Wang, C., Kuwako, K., et al. (2009). SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 64, 617–623. doi: 10.1016/j.neuron.2009.11.021
Sun, F., and He, Z. (2010). Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr. Opin. Neurobiol. 20, 510–518. doi: 10.1016/j.conb.2010.03.013
Sun, F., Park, K., Belin, S., Wang, D., Lu, T., Chen, G., et al. (2011). Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480, 372–375. doi: 10.1038/nature10594
Suter, T., Wang, J., Meng, H., and He, Z. (2021). Utilizing mouse optic nerve crush to examine CNS remyelination. STAR Protoc. 2:100796. doi: 10.1016/j.xpro.2021.100796
Tang, M., Zhong, L., Rong, H., Li, K., Ye, M., Peng, J., et al. (2024). Efficient retinal ganglion cells transduction by retro-orbital venous sinus injection of AAV-PHP.eB in mature mice. Exp. Eye Res. 244:109931. doi: 10.1016/j.exer.2024.109931
Tran, N., Shekhar, K., Whitney, I., Jacobi, A., Benhar, I., Hong, G., et al. (2019). Single-Cell profiles of retinal ganglion cells differing in resilience to injury reveal neuroprotective genes. Neuron 104, 1039–1055.e12. doi: 10.1016/j.neuron.2019.11.006
van Eck, N., and Waltman, L. (2010). Software survey: Vosviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
Varadarajan, S., Hunyara, J., Hamilton, N., Kolodkin, A., and Huberman, A. (2022). Central nervous system regeneration. Cell 185, 77–94. doi: 10.1016/j.cell.2021.10.029
Veldman, M., Bemben, M., Thompson, R., and Goldman, D. (2007). Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev. Biol. 312, 596–612. doi: 10.1016/j.ydbio.2007.09.019
Venugopalan, P., Wang, Y., Nguyen, T., Huang, A., Muller, K., and Goldberg, J. (2016). Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 7:10472. doi: 10.1038/ncomms10472
Wang, X., Li, Q., Liu, C., Hall, P., Jiang, J., Katchis, C., et al. (2018). Lin28 signaling supports mammalian PNS and CNS axon regeneration. Cell. Rep. 24, 2540–2552.e6. doi: 10.1016/j.celrep.2018.07.105
Wang, X., Yang, S., Zhang, C., Hu, M., Qian, J., Ma, J., et al. (2020). Knocking out non-muscle myosin II in retinal ganglion cells promotes long-distance optic nerve regeneration. Cell. Rep. 31:107537. doi: 10.1016/j.celrep.2020.107537
Wareham, L., Liddelow, S., Temple, S., Benowitz, L., Di Polo, A., Wellington, C., et al. (2022). Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 17:23. doi: 10.1186/s13024-022-00524-0
Watkins, T., Wang, B., Huntwork-Rodriguez, S., Yang, J., Jiang, Z., Eastham-Anderson, J., et al. (2013). DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl. Acad. Sci. U S A. 110, 4039–4044. doi: 10.1073/pnas.1211074110
Williams, P., Benowitz, L., Goldberg, J., and He, Z. (2020). Axon regeneration in the mammalian optic nerve. Annu. Rev. Vis. Sci. 6, 195–213. doi: 10.1146/annurev-vision-022720-094953
Wu, T., Nieminen, T., Mohanty, S., Miotke, J., Meyer, R., Rubinszein, D., et al. (2012). A photon-driven micromotor can direct nerve fibre growth. Nat. Photonics 6, 62–67. doi: 10.1038/NPHOTON.2011.287
Xie, L., Cen, L., Li, Y., Gilbert, H., Strelko, O., Berlinicke, C., et al. (2022). Monocyte-derived SDF1 supports optic nerve regeneration and alters retinal ganglion cells’ response to Pten deletion. Proc. Natl. Acad. Sci. U S A. 119:e2113751119. doi: 10.1073/pnas.2113751119
Yan, L., Zhao, B., Liu, X., Li, X., Zeng, C., Shi, H., et al. (2016). Aligned nanofibers from polypyrrole/graphene as electrodes for regeneration of optic nerve via electrical stimulation. ACS Appl. Mater. Interfaces 8, 6834–6840. doi: 10.1021/acsami.5b12843
Yang, C., Wang, X., Wang, J., Wang, X., Chen, W., Lu, N., et al. (2020). Rewiring neuronal glycerolipid metabolism determines the extent of axon regeneration. Neuron 105, 276–292.e5. doi: 10.1016/j.neuron.2019.10.009
Yang, L., Miao, L., Liang, F., Huang, H., Teng, X., Li, S., et al. (2014). The mTORC1 effectors S6K1 and 4E-BP play different roles in CNS axon regeneration. Nat. Commun. 5:5416. doi: 10.1038/ncomms6416
Yin, Y., Cui, Q., Gilbert, H., Yang, Y., Yang, Z., Berlinicke, C., et al. (2009). Oncomodulin links inflammation to optic nerve regeneration. Proc. Natl. Acad. Sci. U S A. 106, 19587–19592. doi: 10.1073/pnas.0907085106
Yin, Y., Henzl, M., Lorber, B., Nakazawa, T., Thomas, T., Jiang, F., et al. (2006). Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 9, 843–852. doi: 10.1038/nn1701
Zhang, Q., Li, Y., and Zhuo, Y. (2022). Synaptic or non-synaptic? Different intercellular interactions with retinal ganglion cells in optic nerve regeneration. Mol. Neurobiol. 59, 3052–3072. doi: 10.1007/s12035-022-02781-y
Zhou, F., and Snider, W. (2006). Intracellular control of developmental and regenerative axon growth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1575–1592. doi: 10.1098/rstb.2006.1882
Keywords: optic nerve regeneration, bibliometric analysis, web of science core collection, VOS viewer, CiteSpace
Citation: Saijilafu, Chen P, Ye L, Shen Y, Wang Q, Chen X, Chimedtseren C, Zhang J, Fang L and Xu R (2025) Frontiers of optic nerve regeneration research: an analysis of the top 100 most influential articles in the field from 2005 to 2025. Front. Neurosci. 19:1634999. doi: 10.3389/fnins.2025.1634999
Received: 28 May 2025; Accepted: 11 September 2025;
Published: 25 September 2025.
Edited by:
Shimon Rochkind, Tel Aviv University, IsraelReviewed by:
Matthew B. Veldman, Medical College of Wisconsin, United StatesRoman J. Giger, University of Michigan, United States
LaiYang Zhou, University of Chinese Academy of Sciences, China
Copyright © 2025 Saijilafu, Chen, Ye, Shen, Wang, Chen, Chimedtseren, Zhang, Fang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renjie Xu, ZnJlZHh1cmpAcGt1Lm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
 Saijilafu
Saijilafu Peng Chen
Peng Chen Lingchen Ye3
Lingchen Ye3 Renjie Xu
Renjie Xu