- 1School of Nursing, Changchun University of Chinese Medicine, Changchun, China
- 2Department of Rehabilitation, The Second People’s Hospital of Dalian, Dalian, China
Background: Sleep disorders are prevalent, affecting 27% of the population and linked to health issues like cognitive impairments and cardiovascular diseases. Current treatments have limitations, prompting interest in sensory stimulation therapy as an alternative approach.
Objective: This scoping review aims to explore the effectiveness of sensory stimulation therapy as an intervention for improving sleep disorders, as well as its range of applicability, by synthesizing existing research.
Methods: Using the methodological framework of Arksey and O’Malley and following the PRISMA guidelines, both keywords and free-text searches were conducted. Articles meeting the inclusion criteria were then selected from the China National Knowledge Infrastructure Database, Wanfang Knowledge Service Platform Database, PubMed, Embase, Web of Science and Cochrane Library databases. Each article was screened and analyzed according to specific research elements.
Results: A total of 20 randomized controlled trials, involving 1489 participants across 2 types of sensory stimulation therapy, namely multi-sensory and single-sensory stimulations, were included in the study. The main sensory stimuli applied were auditory, visual, olfactory and tactile, while the intervention methods included music therapy, light therapy, aromatherapy and massage therapy. The intervention duration, frequency and cycles varied considerably, but most studies implementing individual sessions lasting 20–60 min, at least three sessions per week. Fourteen methods were used to assess sleep quality, and the majority of studies applied two or more assessment methods. Completion rates were also high, with 85%–100% of patients completing over 80% of the intervention protocol. Interestingly, 19 studies reported no adverse events. Overall, sensory stimulation therapy had a positive impact on sleep quality, particularly in aspects such as sleep latency, total sleep time, and subjective sleep experience.
Conclusion: The results indicated that sensory stimulation therapy is a safe, feasible and beneficial intervention for improving sleep quality in patients with sleep disorders. However, several limitations were identified in the included studies. Future large-sample, multi-center, high-quality RCTs are needed to further verify the efficacy of this therapy, and provide stronger evidence for their clinical application.
Systematic review registration: https://doi.org/10.17605/OSF.IO/KQ2XT
1 Background
Sleep disorders refer to abnormalities in sleep quality and quantity as a result of different factors, such as insufficient sleep, heightened awareness of sleep or abnormal movements during sleep, that impair daytime functioning (Pavlova and Latreille, 2019). The International Classification of Sleep Disorders (ICSD) classifies sleep disorders into seven main categories, namely insomnia, sleep-related movement disorders, sleep-related breathing disorders, circadian rhythm sleep-wake disorders, central sleep apnea, atypical sleep and other sleep-related disorders (Sateia, 2014). According to the World Health Organization, the prevalence of sleep disorders is as high as 27%, with an upward trend being observed every year (Brewster et al., 2022). Research indicates that poor sleep quality or insufficient sleep disrupts the body’s circadian rhythm, thereby increasing the risk of developing various health conditions, such as cognitive impairments and cardiovascular diseases (Redline et al., 2023). Additionally, with increasing age, the natural decline in the function of the endogenous circadian rhythm system often results in typical problems such as difficulty falling asleep, sleep fragmentation, and early morning awakening, due to decreased melatonin secretion and phase advance of the circadian rhythm. Consequently, sleep disorders are particularly prevalent in the elderly population (Taillard et al., 2021).
The main treatments for sleep disorders currently include pharmacological interventions and cognitive-behavioral therapy (Wilson et al., 2019). Specifically, medication options encompass benzodiazepines, sedatives and melatonin, amongst others, although their prolonged use may lead to adverse effects such as tolerance, dependence and addiction (Asarnow, 2020). In particular, extended use of certain drugs may also impair the cognitive functions of patients. On the other hand, cognitive behavioral therapy has shown significant therapeutic efficacy, but its long treatment duration, high demands on patient self-management and inconsistent outcomes have limited its widespread clinical application (Liu et al., 2024).
In this context, sensory stimulation therapy offers a promising alternative, especially due to its safety, affordability, broad applicability, flexibility and the potential to avoid the adverse effects associated with medications. Originating in the 1950s, sensory stimulation can be divided into single-sensory and multi-sensory stimulation, often involves various methods, such as light therapy, aromatherapy, music therapy and massage that use light effects, relaxing scents, soothing music and tactile stimuli to engage one or more of a patient’s senses (i.e., sight, smell, hearing, touch and taste) (Lombardi et al., 2002). In fact, it can effectively regulate central nervous system activity to improve patients’ negative behaviors and psychological symptoms. Therefore, it is widely used in the symptomatic management and clinical care of patients with Alzheimer’s disease, cognitive impairment, and other conditions. As far as sleep disorders are concerned, studies have further demonstrated that this therapy can enhance sleep structure and quality in patients, thereby improving their quality of life and yielding better therapeutic effects compared with conventional drug therapy (Wang and Cheng, 2024).
Despite the growing number of sensory stimulation interventions for sleep disorders, there is limited comprehensive analysis regarding their types, duration, evaluation methods and effectiveness. Additionally, systematic application summaries and practical guidelines are also lacking. Therefore, this study employs a scoping review methodology to systematically examine and analyze relevant studies from both domestic and international sources in order to provide useful insights and reference points for healthcare professionals engaged in clinical practice and related research.
2 Methods
This review used the methodological framework of Arksey and O’Malley (2005) which consists of the following five steps: (1) identifying the research question, (2) retrieving relevant studies, (3) selecting studies, (4) creating data graphs, (5) organizing, summarizing and reporting the results. It also follows the guidelines for the preferred reporting items for systematic reviews and meta-analyses (PRISMA) (Tricco et al., 2018). This review has been registered on the OSF platform under the registration number https://doi.org/10.17605/OSF.IO/KQ2XT.
2.1 Formulation of research questions
The research questions guiding this study were as follows: (1) What types of sensory stimulation therapy interventions are used for patients with sleep disorders, and what are their main components? (2) How should sensory stimulation therapy interventions for sleep disorders be scheduled in terms of timing, frequency and duration? (3) What methods are used to assess the sleep quality of patients undergoing sensory stimulation therapy and what are the outcomes of these interventions?
2.2 Inclusion and exclusion criteria for literature
The following inclusion criteria were applied: (1) The research participants had to meet the diagnostic criteria for sleep disorders (American Academy of Sleep Medicine, 2014). (2) Patients with sleep disorders aged 18 years or older. (3) The exposures or interventions, including combined ones, involved sensory stimulation therapy. (4) The study design was a randomized controlled trial (RCT).
Studies were excluded if: (1) The literature did not describe the content and efficacy of the sensory stimulation therapy intervention or lacked a detailed description of the intervention methods. (2) The literature was not published in Chinese or English. (3) The literature was not accessible in full. (4) They were reviews, guidelines, conference abstracts, symposium abstracts and research protocols.
2.3 Search the database
The databases China National Knowledge Infrastructure Database (CNKI), Wanfang Knowledge Service Platform Database (WFSD), PubMed, Embase, Web of Science and the Cochrane Library for randomized controlled clinical trials were searched using only human trials and RCTs as the search criteria. The search period extended from the databases’ inception to May 1, 2025.
2.4 Search strategies
A combination of subject terms and free terms was used, with the search scope set to title, abstract and keyword fields to ensure effective and comprehensive results. The search terms included “sleep disorders,” “insomnia,” “restless legs syndrome,” “sleep apnea hypopnea syndrome,” “aromatherapy,” “massage,” “music therapy,” “light therapy,” and “audio-visual.” The complete and detailed information of all search strategies is provided in the Supplementary Appendix A.
2.5 Literature screening and data extraction
All retrieved literature was imported into EndNote 20 software to remove duplicates. Two trained researchers then independently screened the titles and abstracts, after which they reviewed the full texts of literature that met the inclusion criteria before cross-checking each other’s findings. The following data were then jointly extracted from the included literature by both researchers: author, country and year of publication, study type, sample size, participant age, sensory modalities involved, intervention method, duration of intervention and outcome assessment methods. In cases of disagreement, a third researcher was consulted until a consensus was reached. Additionally, researchers referred to supplemental information provided in the included studies (e.g., clinical trial registration numbers) to obtain more detailed data.
2.6 Literature quality evaluation
The quality of the literature was independently assessed and cross-checked by two researchers. The evaluation, which was performed using the risk bias assessment tools from the Cochrane Systematic Review Manual (Cumpston et al., 2019), included the following six assessment criteria: (1) randomization methods; (2) concealment of allocation; (3) blinding of participants and intervention providers; (4) completeness of outcome data; (5) selective reporting; and (6) other potential biases. In this case, each criterion was rated as “low risk,” “high risk,” or “unclear.”
3 Results
3.1 Results of literature search
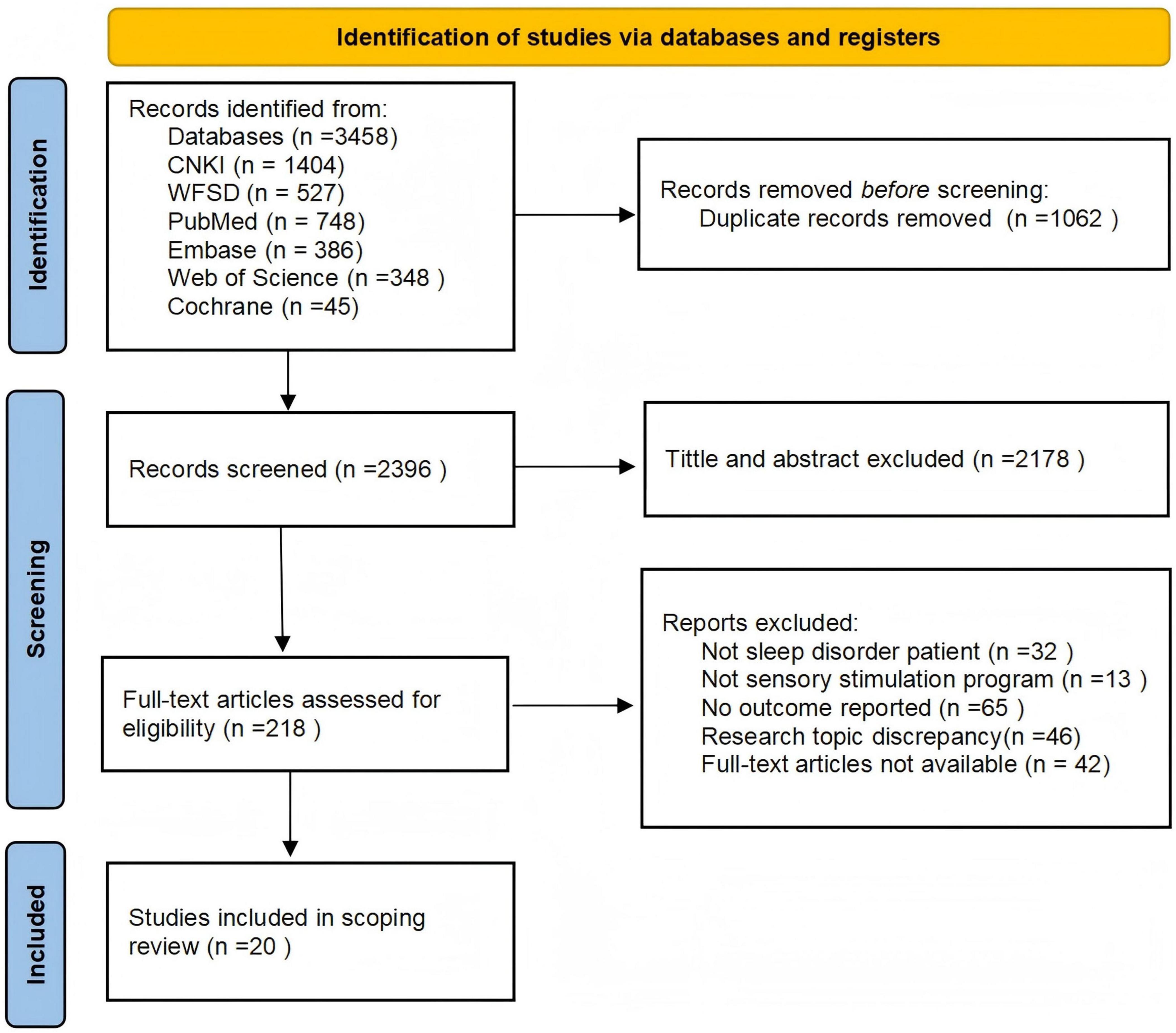
The initial search identified 3458 articles: 1404 from the CNKI database, 527 from the Wanfang Knowledge Service Platform database, 748 from PubMed, 386 from Embase, 348 from Web of Science and 45 from the Cochrane Library. From these, 1062 duplicate articles were removed, and after reviewing the titles, abstracts, and full texts of the remaining 2396 ones, review articles, guidelines, conference abstracts, articles on unrelated topics as well as those without full-text access were excluded. Eventually, a total of 20 articles were included (Ayik and Özden, 2018; Bean et al., 2022; Cheraghbeigi et al., 2019; Dos Reis Lucena et al., 2021; Genç et al., 2020; Ghasemi et al., 2021; Jespersen et al., 2019; Kawabata et al., 2020; Kim et al., 2022; Li et al., 2023; Lund et al., 2020; Oliveira et al., 2012; Oshvandi et al., 2021; Pattison et al., 2024; Rafii et al., 2020; Tang H. J. et al., 2021; Wang and Zhong, 2021; Wang Y. et al., 2024; Yang et al., 2024; Yoon et al., 2023). A flowchart illustrating the literature selection process is provided in Figure 1.
3.2 Inclusion criteria and quality assessment of the literature
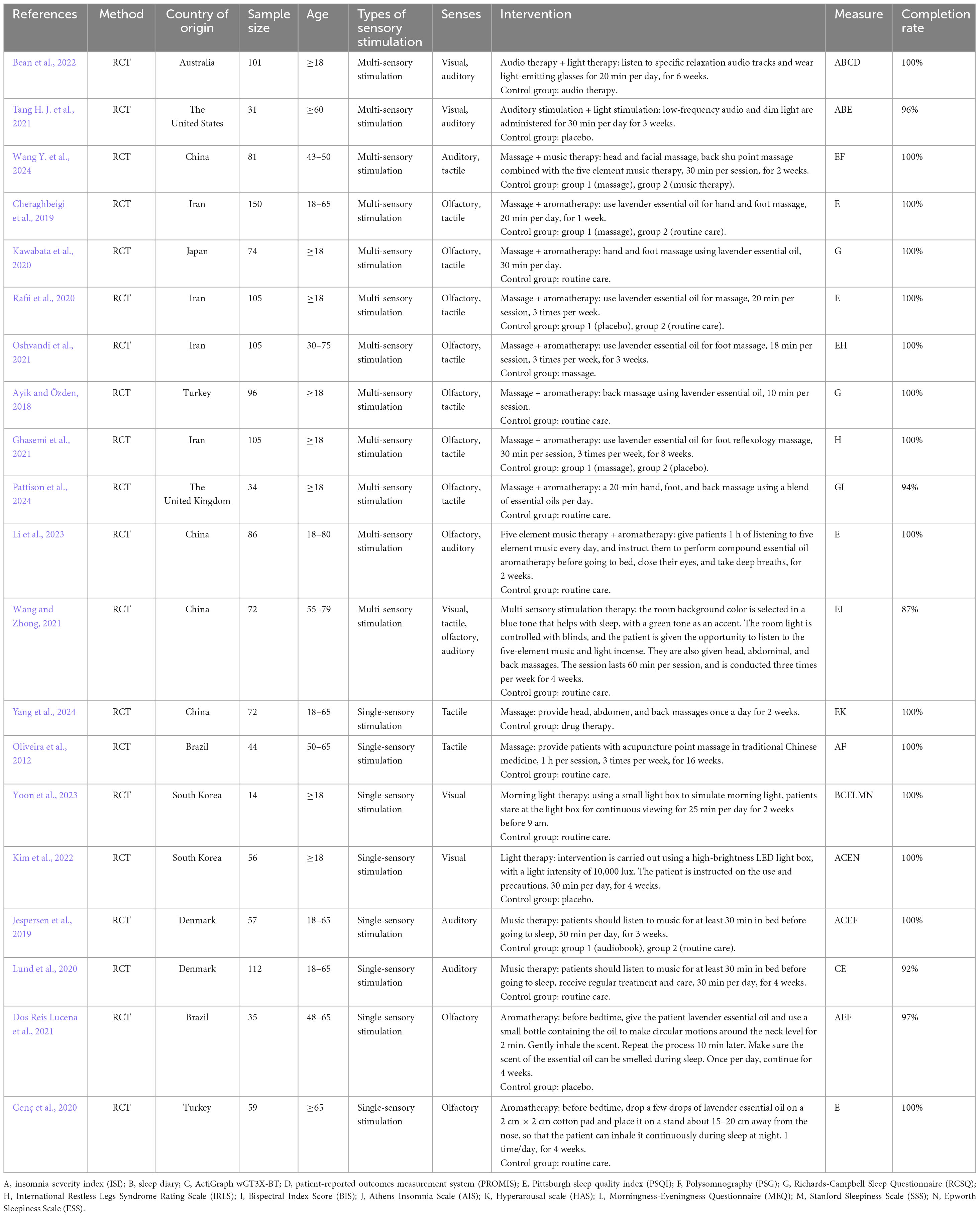
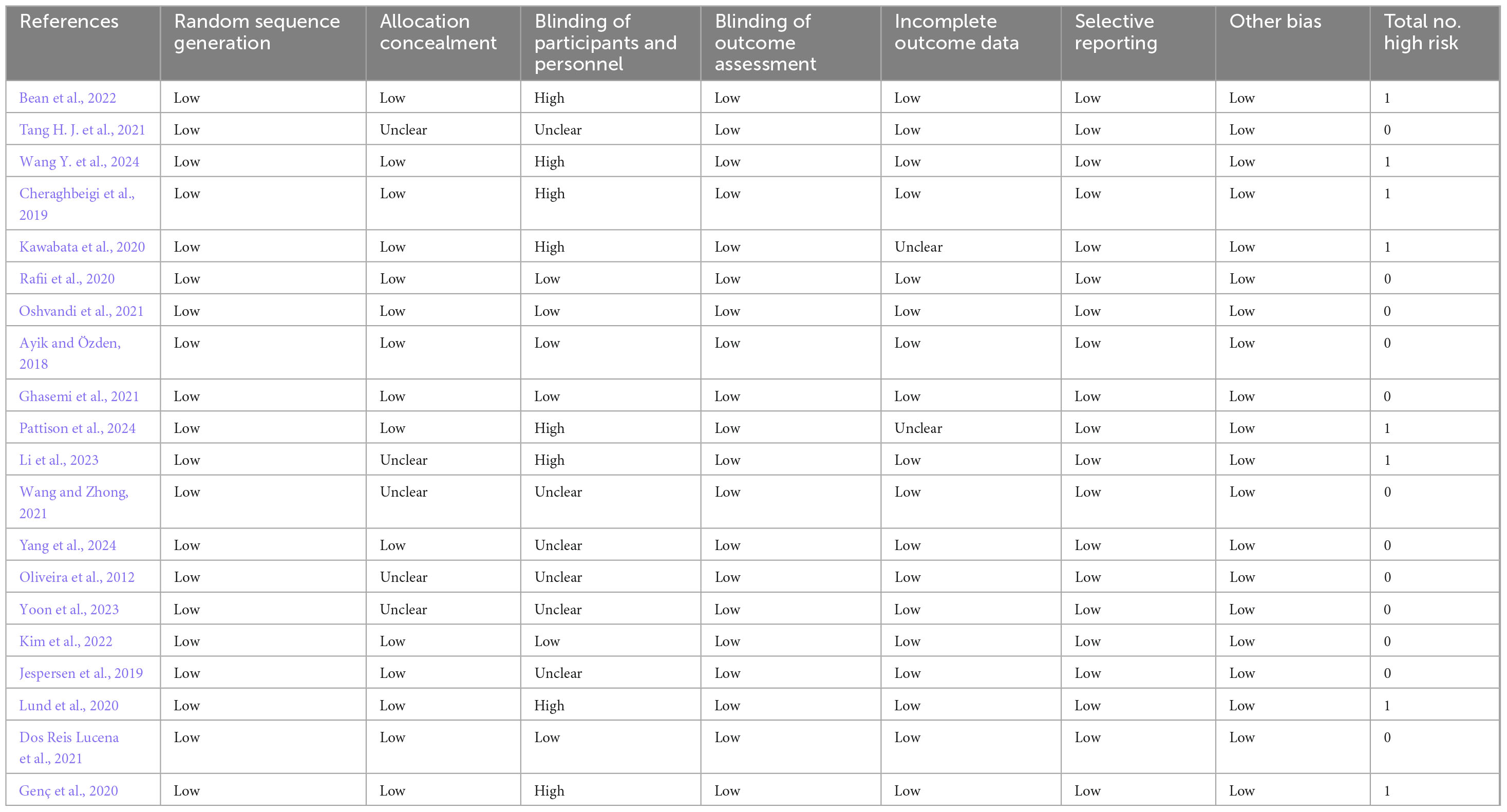
Following a comprehensive review of the literature, 20 studies conducted across various countries were included: Iran (n = 4) (Cheraghbeigi et al., 2019; Ghasemi et al., 2021; Oshvandi et al., 2021; Rafii et al., 2020), China (n = 4) (Li et al., 2023; Wang and Zhong, 2021; Wang Y. et al., 2024; Yang et al., 2024), Turkey (n = 2) (Ayik and Özden, 2018; Genç et al., 2020), South Korea (n = 2) (Kim et al., 2022; Yoon et al., 2023), Brazil (n = 2) (Dos Reis Lucena et al., 2021; Oliveira et al., 2012), Denmark (n = 2) (Jespersen et al., 2019; Lund et al., 2020), the United States (n = 1) (Tang H. J. et al., 2021), Australia (n = 1) (Bean et al., 2022), the United Kingdom (n = 1) (Ghasemi et al., 2021) and Japan (n = 1) (Cheraghbeigi et al., 2019). All studies involved participants aged 18 years or older, with five focusing on middle-aged or elderly patients (Bean et al., 2022; Li et al., 2023; Oliveira et al., 2012; Tang H. J. et al., 2021; Yang et al., 2024). In addition, all studies involved randomized controlled trials, and most had sample sizes between 30 and 150 participants (n = 19) (Ayik and Özden, 2018; Bean et al., 2022; Cheraghbeigi et al., 2019; Dos Reis Lucena et al., 2021; Genç et al., 2020; Ghasemi et al., 2021; Jespersen et al., 2019; Kawabata et al., 2020; Kim et al., 2022; Li et al., 2023; Lund et al., 2020; Oliveira et al., 2012; Oshvandi et al., 2021; Pattison et al., 2024; Rafii et al., 2020; Tang H. J. et al., 2021; Wang and Zhong, 2021; Wang Y. et al., 2024; Yang et al., 2024), except for one in which less than 30 participants were included (Yoon et al., 2023). Table 1 presents the basic information of the included studies. Furthermore, of the included studies, five did not report allocation concealment, fourteen did not mention or apply blinding and two did not address the completeness of outcome data (Table 2). A visual representation of the risk of bias assessment is shown in Supplementary Appendix B.
3.3 Sensory stimulation therapy intervention plan for patients with sleep disorders
3.3.1 Types and contents of sensory stimulation therapy intervention for patients with sleep disorders
Among the 20 included studies, 12 employed multi-sensory stimulation therapy (Ayik and Özden, 2018; Bean et al., 2022; Cheraghbeigi et al., 2019; Ghasemi et al., 2021; Kawabata et al., 2020; Li et al., 2023; Oshvandi et al., 2021; Pattison et al., 2024; Rafii et al., 2020; Tang H. J. et al., 2021; Wang and Zhong, 2021; Wang Y. et al., 2024), while 8 used single-sensory stimulation therapy (Dos Reis Lucena et al., 2021; Genç et al., 2020; Jespersen et al., 2019; Kim et al., 2022; Lund et al., 2020; Oliveira et al., 2012; Yang et al., 2024; Yoon et al., 2023), with both targeting auditory, visual, olfactory and tactile stimuli. The multi-sensory approach commonly combined two sensory modalities, such as auditory-visual, auditory-tactile, olfactory-tactile and olfactory-auditory combinations, with only one study using a combination of more than two sensory stimuli by incorporating visual, tactile olfactory and auditory components. Intervention methods included music therapy, light therapy, aromatherapy and massage. Specifically, the music therapy techniques commonly included the Five Elements Music Therapy and slow-paced music, although participants also had the option to choose their preferred music, which enhanced acceptance and compliance. In the case of light therapy, the options included morning and bright light therapy delivered through the use of light-emitting glasses, LED light boxes and adjustments to the indoor lighting. Finally, aromatherapy interventions frequently employed essential oils, such as lavender, orange and blended oils, while massage techniques usually included hand and foot massage, head massage, back massage and acupressure massage.
3.3.2 Time and frequency of sensory stimulation therapy for patients with sleep disorders
All studies reported the duration of each intervention session (n = 20). In particular, among the 18 articles that detailed session length, the duration ranged from 20 to 60 min (Ayik and Özden, 2018; Bean et al., 2022; Cheraghbeigi et al., 2019; Ghasemi et al., 2021; Jespersen et al., 2019; Kawabata et al., 2020; Kim et al., 2022; Li et al., 2023; Lund et al., 2020; Oliveira et al., 2012; Oshvandi et al., 2021; Pattison et al., 2024; Rafii et al., 2020; Tang H. J. et al., 2021; Wang and Zhong, 2021; Wang Y. et al., 2024; Yang et al., 2024; Yoon et al., 2023), with most specifying a 20-min duration. In addition, two studies extended their aromatherapy sessions to span the entire night’s sleep (Dos Reis Lucena et al., 2021; Genç et al., 2020), thus requiring participants to inhale continuously throughout their nighttime sleep. Regarding intervention frequency, all participants received at least three sessions per week (n = 20). However, the intervention cycle varied considerably across studies. Specifically, of the 16 studies that specified the intervention cycle, one study reported a 16-weeks cycle (n = 1) (Oliveira et al., 2012), another one an 8-weeks cycle (Ghasemi et al., 2021) and a third one a 6-weeks cycle (n = 1) (Bean et al., 2022). The remaining studies indicated cycles of 4 weeks (n = 5) (Dos Reis Lucena et al., 2021; Genç et al., 2020; Kim et al., 2022; Lund et al., 2020; Wang and Zhong, 2021), 3 weeks (n = 3) (Jespersen et al., 2019; Oshvandi et al., 2021; Tang H. J. et al., 2021), 2 weeks (n = 4) (Li et al., 2023; Wang Y. et al., 2024; Yang et al., 2024; Yoon et al., 2023) and 1 week (n = 1) (Cheraghbeigi et al., 2019).
3.4 Assessment of sleep quality in patients receiving sensory stimulation therapy
In the 20 included studies, 14 different assessment methods were used to evaluate sleep quality in patients with sleep disorders. Of these, seven studies used only one assessment method (Ayik and Özden, 2018; Cheraghbeigi et al., 2019; Genç et al., 2020; Ghasemi et al., 2021; Li et al., 2023; Rafii et al., 2020), with the rest employing two or more methods to assess sleep quality (n = 14) (Bean et al., 2022; Dos Reis Lucena et al., 2021; Jespersen et al., 2019; Kim et al., 2022; Lund et al., 2020; Oliveira et al., 2012; Oshvandi et al., 2021; Pattison et al., 2024; Tang H. J. et al., 2021; Wang and Zhong, 2021; Wang Y. et al., 2024; Yang et al., 2024; Yoon et al., 2023). Furthermore, subjective evaluation indicators included PSQI, ISI and sleep diaries, while objective ones included PSG and the ActiGraph wGT3X-BT activity monitor. PSQI is a widely used subjective measure in clinical practice for assessing sleep disorders, and it specifically evaluates components such as sleep quality, sleep latency, sleep duration, normal sleep efficiency, sleep disorders, use of sleep medication and daytime function impairment (Chen et al., 2018). In contrast, ISI assesses the nature, severity and impact of insomnia over the past 2 weeks (Yu, 2010). Additionally, a sleep diary records sleep onset and wake times, the frequency of awakenings, self-assessed sleep quality and daytime alertness, thus providing a comprehensive reflection of a patient’s sleep quality (Dietch and Taylor, 2021). PSG, recognized as the gold standard for diagnosing sleep disorders (Zhang et al., 2019), uses multi-channel monitoring to continuously record bioelectrical and physiological changes during sleep. In particular, it captures parameters, such as EEG, EMG, ECG, EOG, SpO2 and respiration to objectively assess a patient’s sleep quality (Schyvens et al., 2024). Finally, the ActiGraph wGT3X-BT is a wearable device that records body movement, allowing long-term objective data to be collected in an economical and efficient way. In 2007, the American Academy of Sleep Medicine (AASM) recognized it as an effective and reliable assessment tool for evaluating sleep and circadian rhythm disorders (Sadeh and Acebo, 2002).
3.5 Effects of sensory stimulation therapy on patients with sleep disorders
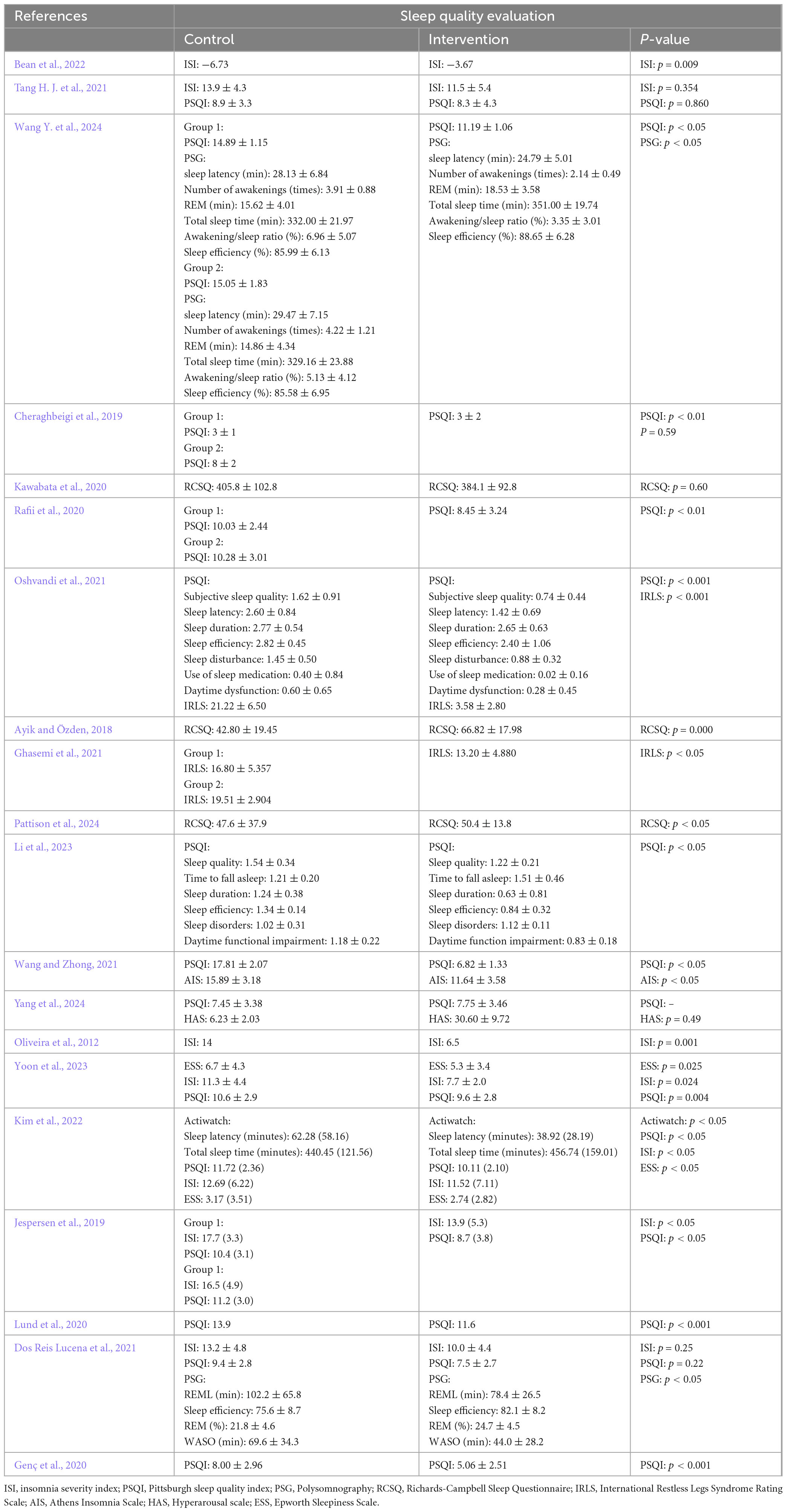
This study investigated the feasibility and effectiveness of sensory stimulation therapy in improving sleep quality for patients with sleep disorders. (1) Feasibility: Sensory stimulation therapy demonstrated good safety and feasibility for patients with sleep disorders, and for most of them, it was practical and safe to conduct at least three sessions per week during hospitalization. In fact, out of 19 studies, none reported adverse events, with the only exception being one instance of mild photophobia after bright light therapy although no other serious adverse events were noted (Yoon et al., 2023). Furthermore, the researchers set an adherence criterion of completing more than 80% of the intervention plan, with the results indicating that 85%–100% of the patients met this criterion, thus demonstrating good compliance. In this case, the main barriers to treatment completion included early discharge, employment changes, relocation, children’s educational needs, lack of energy, aversion to essential oil scents and death. (2) Sleep quality: Sensory stimulation therapy significantly improved the sleep quality of patients with sleep disorders to different extents. Common evaluation indicators included sleep latency, total sleep time, sleep structure, sleep efficiency and subjective sleep experience. Of the 20 included studies, 15 reported notable improvements in sleep latency, total sleep time and subjective sleep experience following the intervention (p < 0.05) (Table 3).
4 Discussion
4.1 Interventions of sensory stimulation therapy
4.1.1 Auditory stimulation
Soothing music is converted into neural signals via the cochlea, transmitted through the thalamus to the auditory cortex, and activates the amygdala, hippocampus, and other components of the limbic system. This process activates the parasympathetic nervous system, thereby reducing sympathetic nervous system arousal and inducing a state of relaxation (Šimić et al., 2021). In addition, auditory stimulation can also synchronize cortical activity through non-specific pathways, thereby enhancing slow-wave sleep (Bellesi et al., 2014). It is worth noting that the sleep-promoting effects of rhythmic auditory stimulation are based on a broad neurophysiological foundation. The mechanisms may involve regulating the balance of the autonomic nervous system through regular sound patterns, enhancing parasympathetic activity, and synchronizing brain electrical activity with a state of relaxation and sleep readiness (Wang et al., 2025). These mechanisms are to some extent universal across ages, which can be observed from the good response of individuals to rhythmic auditory signals in early developmental stages (Gou et al., 2025). Therefore, the application of rhythmic auditory stimulation interventions, such as music and white noise, to adult sleep disorders in clinical practice has its inherent physiological rationale. Studies have shown that music therapy, through targeted auditory stimulation, can modulate the key neurotransmitter systems involved in sleep regulation. Specifically, it promotes the release of dopamine, which is associated with pleasure and rewards and can indirectly improve sleep by alleviating psychological distress and enhancing emotional states (Wang X. et al., 2024). Additionally, music therapy can help relieve pain, stabilize emotions, and promote sleep by enhancing the secretion of endorphins and serotonin, the latter of which also serves as a precursor to melatonin. Research has also shown that music therapy can increase melatonin levels, a key hormone that directly regulates circadian rhythms and promotes sleep onset and maintenance (Dan et al., 2025; Le et al., 2025). By synergistically influencing these neurochemical pathways, music therapy can effectively reduce anxiety and depression, thereby creating a favorable neurobiological environment for improving sleep quality. For effective music therapy, slow-paced, soothing melodies at moderate volumes are recommended, while intense or fast-paced music is avoided as it may disrupt sleep.
4.1.2 Visual stimulation
Light therapy is another cost-effective and easily manageable approach, and its effects involve two key pathways. The first is the image-forming visual pathway, where photic signals are received by the rod cells responsible for scotopic vision and the cone cells responsible for photopic and color vision in the retina, which are primarily involved in visual perception (Shi and Li, 2020). The second is the non-image-forming pathway, which is crucial for the regulation of the sleep-wake cycle. This pathway is mediated by the intrinsically photosensitive retinal ganglion cells (ipRGCs) in the retina, which express melanopsin and are particularly sensitive to short-wavelength blue light. The ipRGCs receive and integrate signals from the rods and cones, and they can also directly sense light. Their axons project directly to the suprachiasmatic nucleus (SCN) of the hypothalamus, which serves as the central circadian pacemaker in humans. Through this pathway, photic signals inhibit the SCN’s daytime suppression of melatonin secretion from the pineal gland, thereby effectively regulating the circadian rhythm, correcting sleep phase shifts, and improving daytime alertness and nighttime sleep quality (Blume et al., 2019). This is particularly beneficial for elderly patients who experience decreased endogenous melatonin secretion and reduced circadian rhythm amplitude due to aging, and serves as an effective non-pharmacological means to correct their advanced sleep-wake phase (such as early morning awakening). However, patients with eye conditions, such as light sensitivity or retinal degeneration, should avoid light therapy to prevent aggravating symptoms. In fact, in one of the included studies, a participant experienced dizziness and withdrew from the light therapy (Yoon et al., 2023).
4.1.3 Tactile stimulation
Massage activates various tactile receptors, such as mechanoreceptors, through mechanical pressure on the skin and deep tissues. The resulting signals are transmitted to the somatosensory cortex in the brain via ascending pathways like the spinothalamic tract (Zhang and Wang, 2018). In addition, this stimulation can induce relaxation by promoting parasympathetic nervous system activity and reducing heart rate, blood pressure, and cortisol levels through spinal reflexes and effects on the autonomic nervous system (Diego and Field, 2009). In particular, when specific acupoints are stimulated, such as the Baihui and Taiyang points, it can help calm the mind and induce sleep.
4.1.4 Olfactory stimulation
On the other hand, aromatherapy uses natural plant essential oils to stimulate emotion-related regions of the brain through the olfactory route, thereby enhancing the release of neurotransmitters. Unlike other sensory systems, a unique pathway for olfactory signals bypasses the thalamus and directly projects to the limbic system of the brain, particularly the amygdala and hippocampus, which are closely associated with emotions and memory (Sullivan et al., 2015). This direct neural connection enables odors to rapidly and intensely influence emotional states and psychophysiological arousal levels. Furthermore, certain components, such as volatile organic compounds, in essential oils can relax muscles, improve blood circulation and induce other physiological effects in patients, thus helping to reduce fatigue and enhance sleep quality (Tang Y. et al., 2021). Despite being effective, aromatherapy should nevertheless be used with caution for certain groups, such as pregnant women, children and individuals who may be sensitive to certain components of the essential oils (Nakajima et al., 2017).
Taken together, patients with sleep disorders often experience associated physical, cognitive and emotional challenges, including anxiety and depression. As such, special requirements and higher skills may be needed from the operator for selecting and implementing sensory stimulation therapy interventions. However, a systematic and standardized training model for sensory stimulation therapy is not yet present in clinical practice. Hence, developing structured training and assessment criteria for sensory stimulation therapy would support systematic learning by healthcare professionals and maximize the intervention’s effectiveness.
4.2 Types of sensory stimulation therapy
The results of this study showed that 8 articles applied single-sensory stimulation therapy (Dos Reis Lucena et al., 2021; Genç et al., 2020; Jespersen et al., 2019; Lund et al., 2020; Oliveira et al., 2012; Yang et al., 2024; Yoon et al., 2023), while 12 used multi-sensory stimulation therapy which integrated two or more sensory modalities (Ayik and Özden, 2018; Bean et al., 2022; Cheraghbeigi et al., 2019; Ghasemi et al., 2021; Kawabata et al., 2020; Li et al., 2023; Oshvandi et al., 2021; Pattison et al., 2024; Rafii et al., 2020; Tang H. J. et al., 2021; Wang and Zhong, 2021; Wang Y. et al., 2024). Interestingly, only one study combined multiple interventions specifically for sleep disorders (Wang and Zhong, 2021). Given the complexity and varied causes of sleep disorders, single-sensory therapies providing stimuli through a single sensory channel may have limited effects due to the isolated nature of the stimulus and variability among patients. In this context, research by Wang et al. (2024b), (Cheraghbeigi et al., 2019; Ghasemi et al., 2021; Oshvandi et al., 2021; Rafii et al., 2020) suggested that multi-sensory stimulations had a stronger impact than single-sensory ones as they more effectively alleviated sleep disorder symptoms while enhancing overall sleep quality and daytime function. For elderly patients with sleep disorders in particular, their sleep issues often coexist with multiple problems such as anxiety and cognitive decline (Xu et al., 2021). This multi-target and holistic intervention strategy may be more suitable than single-sensory interventions for addressing the complex pathophysiological conditions of this population, including their unique circadian rhythm disorders. Clinical settings have also been gradually introducing “sleep rooms” with the aim of integrating multiple sensory therapies in a single space to provide patients with combined treatments, thereby maximizing therapeutic efficacy while optimizing the time of patients and medical professionals. However, studies on this approach remains limited, with a need for evidence from large sample sizes and long-term follow-ups. Therefore, further research and clinical trials are needed to validate the “sleep room” approach for treating sleep disorders, with regular follow-ups also essential to confirm its long-time benefits.
4.3 Duration and frequency of sensory stimulation therapy interventions
Currently, there are considerable variations in the duration and frequency of sensory stimulation therapy used to treat sleep disorders. While all studies required a minimum of three complete interventions per week, the length of each training session varied between 20 and 60 min, possibly due to differences in intervention methods. Similarly, there was a wide range of intervention cycles, with most studies implementing cycles of 2–4 weeks, although some extended over several months. Given the diversity of patients in terms of physical conditions, recovery rates, and underlying causes of sleep disorders, establishing a standardized duration and frequency for interventions may help enhance the safety and efficacy of treatments. However, such standardization needs to be flexibly adjusted according to individual differences to ensure that patients do not receive excessive stimulation or insufficient intervention that could affect the treatment outcomes. Additionally, standardized, individualized intervention criteria would enable healthcare professionals to assess treatment effectiveness more accurately for developing precise treatment plans. Future research should therefore aim to define standard intervention durations and cycles for sensory stimulation therapy.
4.4 Assessment methods for sensory stimulation therapy
This study’s primary outcome measure was sleep quality, assessed across 20 studies using 14 different methods. The results clearly highlighted a lack of standardized tools for evaluating the impact of sensory stimulation therapy in patients with sleep disorders. The evaluation methods consisted of both subjective and objective measures, but only 8 of the included studies used a combination of both types of tools (Bean et al., 2022; Dos Reis Lucena et al., 2021; Jespersen et al., 2019; Lund et al., 2020; Oliveira et al., 2012; Wang and Zhong, 2021; Wang Y. et al., 2024; Yoon et al., 2023), with the remaining 12 relying solely on subjective ones (Ayik and Özden, 2018; Cheraghbeigi et al., 2019; Genç et al., 2020; Ghasemi et al., 2021; Kawabata et al., 2020; Kim et al., 2022; Li et al., 2023; Oshvandi et al., 2021; Pattison et al., 2024; Rafii et al., 2020; Tang H. J. et al., 2021; Yang et al., 2024). Among the subjective tools, the PSQI is arguably the most widely used internationally for assessing sleep quality during both daytime and night time in healthy individuals as well as those with sleep or mental health-related sleep issues (Chen et al., 2021). ISI and AIS are also commonly used to gauge the severity of sleep disorders, with the ISI and the AIS focusing on the past 2 weeks (Dietch and Taylor, 2021) and the past month, respectively (Chung et al., 2011). Finally, subjective measures, such as the SSS and ESS, assess a patient’s level of drowsiness, but while SSS assesses a patient’s current sleepiness level (Chiu et al., 2017), ESS measures the patient’s general daytime sleepiness (Gonçalves et al., 2023). Relying solely on subjective assessments is prone to bias due to individual perception, emotions, and past experiences. If assessors lack standardized training, this subjectivity may be further amplified, leading to a reduction in the consistency of the assessment results. This study found that 13 articles used two or more assessment methods to assess sleep quality (Bean et al., 2022; Dos Reis Lucena et al., 2021; Jespersen et al., 2019; Kim et al., 2022; Lund et al., 2020; Oliveira et al., 2012; Oshvandi et al., 2021; Pattison et al., 2024; Tang H. J. et al., 2021; Wang and Zhong, 2021; Wang Y. et al., 2024; Yang et al., 2024; Yoon et al., 2023), while 7 relied on a single tool (Ayik and Özden, 2018; Cheraghbeigi et al., 2019; Genç et al., 2020; Ghasemi et al., 2021; Kawabata et al., 2020; Li et al., 2023; Rafii et al., 2020). Despite the common use of multiple evaluation methods, significant heterogeneity remains in their scope and emphasis. Selecting sleep quality assessment tools should therefore be individualized and based on each patient’s specific symptoms and needs. Furthermore, to enhance the objectivity, accuracy and reliability of sleep quality assessment tools, large-scale studies are needed to develop and standardize outcome evaluation tools that align with clinical requirements.
4.5 Efficacy and safety of sensory stimulation therapy
The results of this study show that sensory stimulation therapy significantly improves sleep quality in patients with sleep disorders while being highly safe. As a non-invasive intervention, sensory stimulation therapy circumvents the side effects and risks of dependency associated with medication, while allowing patients to receive treatment in a relatively safe environment. This characteristic makes it particularly suitable for the elderly population, who have decreased physical function and metabolic capacity and are more sensitive to adverse drug reactions, providing a highly promising option for managing their age-related sleep and circadian rhythm disorders. Of the 20 studies that were included, most reported no adverse events, with only one noting a minor adverse effect related to the intervention (Yoon et al., 2023). Additionally, the equipment and materials required for sensory stimulation therapy are relatively simple, and the procedures are straightforward, thus making this approach highly feasible for clinical applications. The results further suggested that patients who received sensory stimulation therapy demonstrated high adherence to the treatment, with an overall high completion rate for the intervention. Common barriers during the intervention period included early discharge, job changes, relocation, children’s schooling needs, lack of energy, rejection of essential oil aroma and death. However, it should be noted that, except for aversion to the scent of essential oil, none of the barriers were directly related to the sensory stimulation therapy itself.
4.6 Quality assessment of studies
Most studies included in this review did not use or report blinding methods, which may introduce biases in publication and implementation. Blinding is critical for minimizing subjective bias and improving the reliability of clinical trial results, and hence, a lack of blinding undoubtedly weakens the credibility of research findings (Burkhardt et al., 2010). Sensory stimulation therapy poses challenges for effective concealment due to its distinctive characteristics. Future research should therefore seek to develop innovative blinding methods and techniques to enhance the objectivity and reliability of outcomes. For instance, in music therapy, sound processing technology could combine different melodies to create a “composite music” that is difficult to differentiate. Alternatively, a “white noise” lacking rhythm and melody could be used as a placebo.
5 Limitations
This study had several limitations: Firstly, the included participants were all adults, although adolescents may also frequently experience sleep disorders. For instance, Rezaei et al. (2023) conducted an intervention study on infants with sleep disorders using tactile stimulation, and the results showed that the latter could increase vagal activity, lower heart rate and cortisol levels, reduce the number of nighttime awakenings, extend continuous sleep and improve overall sleep quality. While some studies have confirmed that sensory stimulation can enhance sleep quality in adolescents with sleep disorders, research on the application of the therapy for this particular population remains limited. Future studies should consider the unique characteristics and needs of adolescents with sleep disorders in order to develop suitable sensory stimulation therapy intervention programs that are tailored to them. Secondly, the limited number and quality of clinical studies on the use of sensory stimulation therapy for sleep disorders, coupled with inconsistencies in intervention protocols and assessment tools, present challenges. Indeed, of the 20 included studies, variations in their intervention durations and frequencies precluded a comparison of their respective effects on sleep quality, leading to heterogeneous results. Future research could consider including large-sample randomized controlled trials to develop comprehensive sensory stimulation intervention programs that use both subjective and objective evaluation tools to assess effectiveness. Thirdly, the studies included in this review cover a diverse range of cultural and geographical backgrounds from multiple regions, including the Middle East, Europe, China, Australia, and others. While this enhances the generalizability of the results, it also implies the potential for cultural and lifestyle differences (such as diet and daily routines) that may shape distinct sleep patterns and influence patients’ preferences and acceptance of specific interventions. Future research should delve deeper into cultural adaptation adjustments, such as developing music libraries or aromatherapy formulations that are in line with local cultural contexts, to further improve the acceptability and effectiveness of the interventions.
6 Conclusion
Sensory stimulation therapy is a safe, feasible and beneficial intervention for improving sleep quality in patients with sleep disorders. However, research on its application is still in its exploratory stages, and standardized, scientifically-validated intervention protocols have yet to be established. Additionally, there is a need for evaluation frameworks that combine subjective and objective measures. Future research should focus on developing structured and evidence-based intervention strategies, establishing uniform evaluation criteria and conducting large-sample, multi-center RCTs to assess the efficacy of sensory stimulation therapy for patients with sleep disorders.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZQ: Writing – review & editing, Writing – original draft, Data curation, Conceptualization, Investigation. XS: Writing – review & editing, Writing – original draft, Investigation, Data curation. XZ: Writing – review & editing, Formal analysis, Writing – original draft. JY: Project administration, Writing – review & editing, Writing – original draft. XyZ: Writing – original draft, Writing – review & editing, Supervision. HZ: Writing – review & editing. HsZ: Writing – review & editing, Visualization, Supervision, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Natural Science Foundation of Jilin Province (No. YDZJ202301ZYTS109) and National Natural Science Foundation of China (No. 82074569).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1682267/full#supplementary-material
References
American Academy of Sleep Medicine (2014). International classification of sleep disorders, 3rd Edn. Darien, IL: American Academy of Sleep Medicine.
Arksey, H., and O’Malley, L. (2005). Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 8, 19–32. doi: 10.1080/1364557032000119616
Asarnow, L. D. (2020). Depression and sleep: What has the treatment research revealed and could the HPA axis be a potential mechanism? Curr. Opin. Psychol. 34, 112–116. doi: 10.1016/j.copsyc.2019.12.002
Ayik, C., and Özden, D. (2018). The effects of preoperative aromatherapy massage on anxiety and sleep quality of colorectal surgery patients: A randomized controlled study. Compl. Ther. Med. 36, 93–99. doi: 10.1016/j.ctim.2017.12.002
Bean, H. R., Diggens, J., Ftanou, M., Alexander, M., Stafford, L., Bei, B., et al. (2022). Light enhanced cognitive behavioral therapy for insomnia and fatigue during chemotherapy for breast cancer: A randomized controlled trial. Sleep 45:zsab246. doi: 10.1093/sleep/zsab246
Bellesi, M., Riedner, B. A., Garcia-Molina, G. N., Cirelli, C., and Tononi, G. (2014). Enhancement of sleep slow waves: Underlying mechanisms and practical consequences. Front. Syst. Neurosci. 8:208. doi: 10.3389/fnsys.2014.00208
Blume, C., Garbazza, C., and Spitschan, M. (2019). Effects of light on human circadian rhythms, sleep and mood. Somnologie 23, 147–156. doi: 10.1007/s11818-019-00215-x
Brewster, G. S., Riegel, B., and Gehrman, P. R. (2022). Insomnia in the Older Adult. Sleep Med. Clin. 17, 233–239. doi: 10.1016/j.jsmc.2022.03.004
Burkhardt, J. E., Ennulat, D., Pandher, K., Solter, P. F., Troth, S. P., Boyce, R. W., et al. (2010). Topic of histopathology blinding in nonclinical safety biomarker qualification studies. Toxicol. Pathol. 38, 666–667. doi: 10.1177/0192623310371221
Chen, D., Yin, Z., and Fang, B. (2018). Measurements and status of sleep quality in patients with cancers. Support Care Cancer 26, 405–414. doi: 10.1007/s00520-017-3927-x
Chen, S., Sun, L., and Zhang, C. (2021). Adaptation and validity of the sleep quality scale among chinese drivers. PLoS One 16:e0259813. doi: 10.1371/journal.pone.0259813
Cheraghbeigi, N., Modarresi, M., Rezaei, M., and Khatony, A. (2019). Comparing the effects of massage and aromatherapy massage with lavender oil on sleep quality of cardiac patients: A randomized controlled trial. Compl. Ther. Clin. Pract. 35, 253–258. doi: 10.1016/j.ctcp.2019.03.005
Chiu, H. Y., Chen, P. Y., Chuang, L. P., Chen, N. H., Tu, Y. K., Hsieh, Y. J., et al. (2017). Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 36, 57–70. doi: 10.1016/j.smrv.2016.10.004
Chung, K. F., Kan, K. K., and Yeung, W. F. (2011). Assessing insomnia in adolescents: Comparison of insomnia severity index, athens insomnia scale and sleep quality index. Sleep Med. 12, 463–470. doi: 10.1016/j.sleep.2010.09.019
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 10:ED000142. doi: 10.1002/14651858.ED000142
Dan, Y., Xiong, Y., Xu, D., Wang, Y., Yin, M., Sun, P., et al. (2025). Potential common targets of music therapy intervention in neuropsychiatric disorders: The prefrontal cortex-hippocampus -amygdala circuit (a review). Front. Hum. Neurosci. 19:1471433. doi: 10.3389/fnhum.2025.1471433
Diego, M. A., and Field, T. (2009). Moderate pressure massage elicits a parasympathetic nervous system response. Int. J. Neurosci. 119, 630–638. doi: 10.1080/00207450802329605
Dietch, J. R., and Taylor, D. J. (2021). Evaluation of the consensus sleep diary in a community sample: Comparison with single-channel electroencephalography, actigraphy, and retrospective questionnaire. J. Clin. Sleep Med. 17, 1389–1399. doi: 10.5664/jcsm.9200
Dos Reis Lucena, L., Dos Santos-Junior, J. G., Tufik, S., and Hachul, H. (2021). Lavender essential oil on postmenopausal women with insomnia: Double-blind randomized trial. Compl. Ther. Med. 59:102726. doi: 10.1016/j.ctim.2021.102726
Genç, F., Karadağ, S., Kılıç Akça, N., Tan, M., and Cerit, D. (2020). The effect of aromatherapy on sleep quality and fatigue level of the elderly: A randomized controlled study. Holist Nurs. Pract. 34, 155–162. doi: 10.1097/HNP.0000000000000385
Ghasemi, M., Rejeh, N., Bahrami, T., Heravi-Karimooi, M., Tadrisi, S. D., and Vaismoradi, M. (2021). Aromatherapy massage vs. foot reflexology on the severity of restless legs syndrome in female patients undergoing hemodialysis. Geriatrics 6:99. doi: 10.3390/geriatrics6040099
Gonçalves, M. T., Malafaia, S., Moutinho Dos Santos, J., Roth, T., and Marques, D. R. (2023). Epworth sleepiness scale: A meta-analytic study on the internal consistency. Sleep Med. 109, 261–269. doi: 10.1016/j.sleep.2023.07.008
Gou, Q., Li, M., Wang, X., Yuan, X., Yang, M., Li, J., et al. (2025). Meta-narrative review: The impact of music therapy on sleep and future research directions. Front. Neurol. 15:1433592. doi: 10.3389/fneur.2024.1433592
Jespersen, K. V., Otto, M., Kringelbach, M., Van Someren, E., and Vuust, P. A. (2019). randomized controlled trial of bedtime music for insomnia disorder. J. Sleep Res. 28:e12817. doi: 10.1111/jsr.12817
Kawabata, N., Hata, A., and Aoki, T. (2020). Effect of aromatherapy massage on quality of sleep in the palliative care ward: A randomized controlled trial. J. Pain Symptom Manage 59, 1165–1171. doi: 10.1016/j.jpainsymman.2020.01.003
Kim, W. H., Joa, K. L., Kim, C. B., Lee, H. S., Kang, S. G., Jung, H. Y., et al. (2022). The effect of bright light therapy on sleep and quality of life in patients with poststroke Insomnia. Psychosom. Med. 84, 123–130. doi: 10.1097/PSY.0000000000001014
Le, J., Deng, W., and Le, T. (2025). Music therapy in depression: Exploring mechanisms and efficacy in rat models. Brain Sci. 15:338. doi: 10.3390/brainsci15040338
Li, Y. Y., Xu, P. L., and Li, B. Y. (2023). Effects of corner mode five element music combined with aromatherapy on fatigue symptoms and sleep quality of patients with sleep disorder in maintenance hemodialysis. World J. Sleep Med. 10, 2593–2595. doi: 10.3969/j.issn.2095-7130.2023.11.028
Liu, Y., Sui, X., Bai, Y., Lü, D., and Yao, P. (2024). A randomized controlled trial of rTMS and CBT-I in the treatment of chronic insomnia patients. Sichuan Mental Health 37, 212–218. doi: 10.11886/scjsws20240105001
Lombardi, F., Taricco, M., De Tanti, A., Telaro, E., and Liberati, A. (2002). Sensory stimulation of brain-injured individuals in coma or vegetative state: Results of a Cochrane systematic review. Clin. Rehabil. 16, 464–472. doi: 10.1191/0269215502cr519oa
Lund, H. N., Pedersen, I. N., Johnsen, S. P., Heymann-Szlachcinska, A. M., Tuszewska, M., Bizik, G., et al. (2020). Music to improve sleep quality in adults with depression-related insomnia (MUSTAFI): Study protocol for a randomized controlled trial. Trials 21:305. doi: 10.1186/s13063-020-04247-9
Nakajima, S., Komada, Y., Sasai-Sakuma, T., Okajima, I., Harada, Y., Watanabe, K., et al. (2017). Higher sleep reactivity and insomnia mutually aggravate depressive symptoms: A cross-sectional epidemiological study in Japan. Sleep Med. 33, 130–133. doi: 10.1016/j.sleep.2016.12.023
Oliveira, D. S., Hachul, H., Goto, V., Tufik, S., and Bittencourt, L. R. (2012). Effect of therapeutic massage on insomnia and climacteric symptoms in postmenopausal women. Climacteric 15, 21–29. doi: 10.3109/13697137.2011.587557
Oshvandi, K., Mirzajani Letomi, F., Soltanian, A. R., and Shamsizadeh, M. (2021). The effects of foot massage on hemodialysis patients’ sleep quality and restless leg syndrome: A comparison of lavender and sweet orange essential oil topical application. J. Compl. Integr. Med. 18, 843–850. doi: 10.1515/jcim-2020-0121
Pattison, N., O’Gara, G., Thomas, K., Wigmore, T., and Dyer, J. (2024). An aromatherapy massage intervention on sleep in the ICU: A randomized controlled feasibility study. Nurs. Crit. Care 29, 14–21. doi: 10.1111/nicc.12957
Pavlova, M. K., and Latreille, V. (2019). Sleep disorders. Am. J. Med. 132, 292–299. doi: 10.1016/j.amjmed.2018.09.021
Rafii, F., Ameri, F., Haghani, H., and Ghobadi, A. (2020). The effect of aromatherapy massage with lavender and chamomile oil on anxiety and sleep quality of patients with burns. Burns 46, 164–171. doi: 10.1016/j.burns.2019.02.017
Redline, S., Azarbarzin, A., and Peker, Y. (2023). Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat. Rev. Cardiol. 20, 560–573. doi: 10.1038/s41569-023-00846-6
Rezaei, R., Sharifnia, H., Nazari, R., and Saatsaz, S. (2023). Bedtime massage intervention for improving infant and mother sleep condition: A randomized controlled trial. J. Neonatal. Perinatal. Med. 16, 271–278. doi: 10.3233/NPM-210964
Sadeh, A., and Acebo, C. (2002). The role of actigraphy in sleep medicine. Sleep Med. Rev. 6, 113–124. doi: 10.1053/smrv.2001.0182
Sateia, M. J. (2014). International classification of sleep disorders-third edition: Highlights and modifications. Chest 146, 1387–1394. doi: 10.1378/chest.14-0970
Schyvens, A. M., Van Oost, N. C., Aerts, J. M., Masci, F., Peters, B., Neven, A., et al. (2024). Accuracy of fitbit charge 4, garmin vivosmart 4, and WHOOP versus polysomnography: Systematic review. JMIR Mhealth Uhealth 12:e52192. doi: 10.2196/52192
Shi, X. X., and Li, Z. (2020). Research progress of phototherapy in patients with mental disorders. Chin. General Pract. 23, 1344–1348. doi: 10.12114/j.issn.1007-9572.2019.00.381
Šimić, G., Tkalčić, M., Vukić, V., Mulc, D., Španić, E., Šagud, M., et al. (2021). Understanding emotions: Origins and roles of the amygdala. Biomolecules 11:823. doi: 10.3390/biom11060823
Sullivan, R. M., Wilson, D. A., Ravel, N., and Mouly, A. M. (2015). Olfactory memory networks: From emotional learning to social behaviors. Front. Behav. Neurosci. 9:36. doi: 10.3389/fnbeh.2015.00036
Taillard, J., Gronfier, C., Bioulac, S., Philip, P., and Sagaspe, P. (2021). Sleep in normal aging, homeostatic and circadian regulation and vulnerability to sleep deprivation. Brain Sci. 11:1003. doi: 10.3390/brainsci11081003
Tang, H. J., McCurry, S. M., Pike, K. C., Riegel, B., and Vitiello, M. V. (2021). Open-loop audio-visual stimulation for sleep promotion in older adults with comorbid insomnia and osteoarthritis pain: Results of a pilot randomized controlled trial. Sleep Med. 82, 37–42. doi: 10.1016/j.sleep.2021.03.025
Tang, Y., Gong, M., Qin, X., Su, H., Wang, Z., and Dong, H. (2021). The therapeutic effect of aromatherapy on insomnia: A meta-analysis. J. Affect Disord. 288, 1–9. doi: 10.1016/j.jad.2021.03.066
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 169, 467–473. doi: 10.7326/M18-0850
Wang, D., and Zhong, J. G. (2021). Effect of TCM comprehensive rehabilitation program based on “multi sensory stimulation” theoryon sleep function of elderly patients with insomnia after ischemic stroke. J. Pract. Med. 37, 1619–1625. doi: 10.3969/j.issn.1006-5725.2021.12.021
Wang, M., Fan, S., Wang, Z., and Ren, J. (2025). A systematic review and meta-analysis of acoustic stimulation in the treatment of insomnia. Front. Neurosci. 19:1572086. doi: 10.3389/fnins.2025.1572086
Wang, X., Feng, T., Liu, S., and Ruan, J. (2024). Application of music therapy in improving the sleep quality and mental health of nurses with circadian rhythm sleep disorders caused by work shifts. Noise Health 26, 294–299. doi: 10.4103/nah.nah_32_24
Wang, Y., Zheng, Q. X., Ye, Y., Li, J., Ye, J., Liao, J., et al. (2024). The clinical therapeutic effect of two-part tuina therapy based on the theory of “Yin-invigorating Yang” combined with five-element music for liver-stagnation kidney-deficiency insomnia in perimenopause. Chin. J. Gerontol. 44, 1109–1112. doi: 10.3969/j.issn.1005-9202.2024.05.023
Wang, Y. Y., and Cheng, L. N. (2024). Application of multi-sensory stimulation therapy in elderly patients with stroke complicated with primary insomnia. Chin. J. Convalescent Med. 33, 76–79. doi: 10.13517/j.cnki.ccm.2024.02.016
Wilson, S., Anderson, K., Baldwin, D., Dijk, D. J., Espie, A., Espie, C., et al. (2019). British association for psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J. Psychopharmacol. 33, 923–947. doi: 10.1177/0269881119855343
Xu, W. Q., Lin, L. H., Ding, K. R., Ke, Y. F., Huang, J. H., Hou, C. L., et al. (2021). The role of depression and anxiety in the relationship between poor sleep quality and subjective cognitive decline in Chinese elderly: Exploring parallel, serial, and moderated mediation. J. Affect. Disord. 294, 464–471. doi: 10.1016/j.jad.2021.07.063
Yang, H. R., Wang, Y. X., Zhou, B., Lei, Y., Yang, T., Wang, J. M., et al. (2024). Randomized controlled study on the treatment of insomnia by three parts Tuina based on qi regulation theory. China J. Traditional Chin. Med. Pharmacy 39, 510–513.
Yoon, J., Heo, S., Lee, H., Sul, E., Han, T., and Kwon, Y. J. (2023). Feasibility and efficacy of morning light therapy for adults with insomnia: A pilot, randomized, open-label, two-arm study. Medicina 59:1066. doi: 10.3390/medicina59061066
Yu, D. S. (2010). Insomnia severity index: Psychometric properties with chinese community-dwelling older people. J. Adv. Nurs. 66, 2350–2359. doi: 10.1111/j.1365-2648.2010.05394.x
Zhang, J., and Wang, Y. G. (2018). Research progress on the mechanisms of tactile stimulation effects on the brain. Magnet. Resonance Imag. 9, 791–795. doi: 10.12015/issn.1674-8034.2018.10.015
Keywords: sleep disorders, sensory stimulation, music therapy, light therapy, aromatherapy, massage therapy, scoping review
Citation: Qu Z, Sun X, Zhang X, Yin J, Zhang X, Zhao H and Zhang H (2025) The effects of sensory stimulation therapy in patients with sleep disorders: a scoping review. Front. Neurosci. 19:1682267. doi: 10.3389/fnins.2025.1682267
Received: 08 August 2025; Accepted: 19 September 2025;
Published: 03 October 2025.
Edited by:
Eloy Gerardo Moreno-Galindo, University of Colima, MexicoReviewed by:
JorgeAlberto PerezLeon, Universidad Autónoma de Ciudad Juárez, MexicoJoy Perrier, PSL Université, France
Xuexing Luo, Macau University of Science and Technology, Macao SAR, China
Copyright © 2025 Qu, Sun, Zhang, Yin, Zhang, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongshi Zhang, NTUwMzU3NkBxcS5jb20=
 Zihan Qu
Zihan Qu Xuefeng Sun
Xuefeng Sun Xiaotu Zhang
Xiaotu Zhang Jiawei Yin
Jiawei Yin Xinye Zhang
Xinye Zhang Haifeng Zhao2
Haifeng Zhao2 Hongshi Zhang
Hongshi Zhang