- 1Department of Urology, the Second Affiliated Hospital of ZunYi Medical University, Zunyi, China
- 2Department of Thoracic Oncology, the Second Affiliated Hospital of ZunYi Medical University, Zunyi, China
Sarcomatoid renal cell carcinoma (SRCC), a manifestation of sarcomatoid dedifferentiation in renal cell carcinoma, is characterized by elevated invasiveness and a grim prognosis. Typically, SRCC patients present with advanced or metastatic conditions and survival rates rarely extend beyond one year. In this study, we describe a case of SRCC characterized by the patient exhibiting right flank pain without hematuria. Initially, imaging interpretations led to a diagnosis of severe hydronephrosis. Subsequently, an open right nephrectomy post-surgery confirmed the pathology of sarcomatoid renal cell carcinoma.
Introduction
Renal cell carcinoma (RCC) is recognized as one of the most lethal malignancies affecting the urinary system (1). Sarcomatoid renal cell carcinoma (SRCC), which constitutes a mere 5% of RCC cases, represents a highly resistant and deadly manifestation of kidney cancer (2). Patients diagnosed with SRCC commonly present advanced or metastatic conditions, resulting in an exceptionally dismal prognosis (3). The absence of distinct clinical symptoms and imaging characteristics can contribute to delayed diagnoses or misinterpretations. The occurrence of sarcomatoid renal cell carcinoma masquerading as severe renal hydronephrosis is exceedingly uncommon. In this report, we detail a clinical case that exemplifies this rarity. The patient initially sought medical attention due to persistent lumbar pain of unidentified origin, which led to an initial diagnosis of severe right renal hydronephrosis based on imaging findings. Consequently, an open right nephrectomy was performed, unveiling sarcomatoid renal cell carcinoma through postoperative pathology.
Case report
A 52-year-old male presented with recurrent lumbar pain persisting for one month, lacking an identifiable cause and hematuria, while maintaining satisfactory mental and dietary conditions. External CT imaging revealed a massive cystic lesion in the right upper abdomen, originating from the right kidney, suggestive of severe right renal hydronephrosis. The patient was admitted to our hospital for further evaluation and treatment. Upon admission, he reported a history of fracture surgery with no infectious or chronic diseases. A palpable mass was identified in the right upper abdomen; CT results revealed severe right renal hydronephrosis accompanied by multiple stones (Figure 1A). Consequently, the provisional diagnosis was severe right renal hydronephrosis, attributed to right ureteral stricture. Following the exclusion of surgical contraindications, an open right nephrectomy was conducted. Intraoperatively, exposure of the renal fascia revealed a significantly enlarged right kidney with thin cyst walls measuring approximately 20cm*15cm (Figure 1B), from which 1000 ml of pale yellow turbid fluid was drained. Pathological examination of the excised tumor revealed histological features indicative of a spindle cell tumor, including neutrophil infiltration in the right kidney (Figures 1C, D). Immunohistochemical staining showed positive vimentin, PAX8 in epithelial and occasional spindle cells, CK in epithelial cells, CK5/6 in epithelial cells, CD99, CD10, focally weak EMA, CD68 in histiocytic cells, 40% Ki67; negative SMA, Myogenin, ALK, CD34, MyoD1, Bcl-2, ETV6, S100, Desmin, and PAS (Figure 2). Based on the comprehensive pathology and immunohistochemistry findings, the diagnosis was confirmed as sarcomatoid renal cell carcinoma with local ossification and vascular invasion. The patient received immune-targeted therapy consisting of Atezolizumab (1200 mg intravenous infusion on day 1) and Bevacizumab (600 mg intravenous infusion on day 1) two months post-surgery, followed by a second round of immune-targeted therapy in the third month post-surgery. Subsequently, the patient underwent regular follow-up examinations. As of the manuscript revision (15 months post-surgery), the patient remains alive.
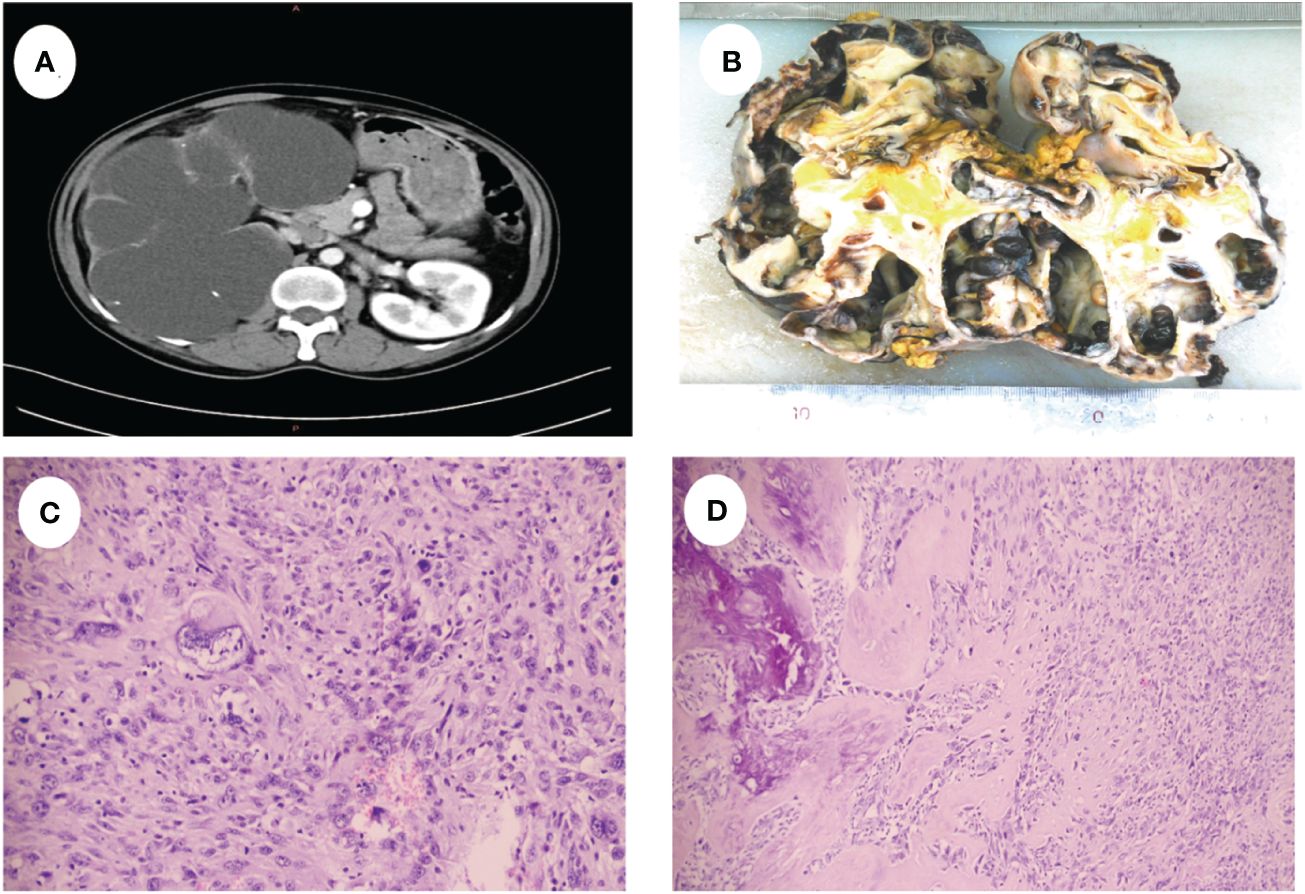
Figure 1 (A) CT shows severe hydronephrosis of the right kidney. (B) Shows the gross appearance of the right kidney, the size was about 20cm x 15cm. (C) The tumor cells were disorganized and showed sarcomatoid changes with marked heterogeneity. (D) Tumor cells are spindle-shaped with ossification formation.
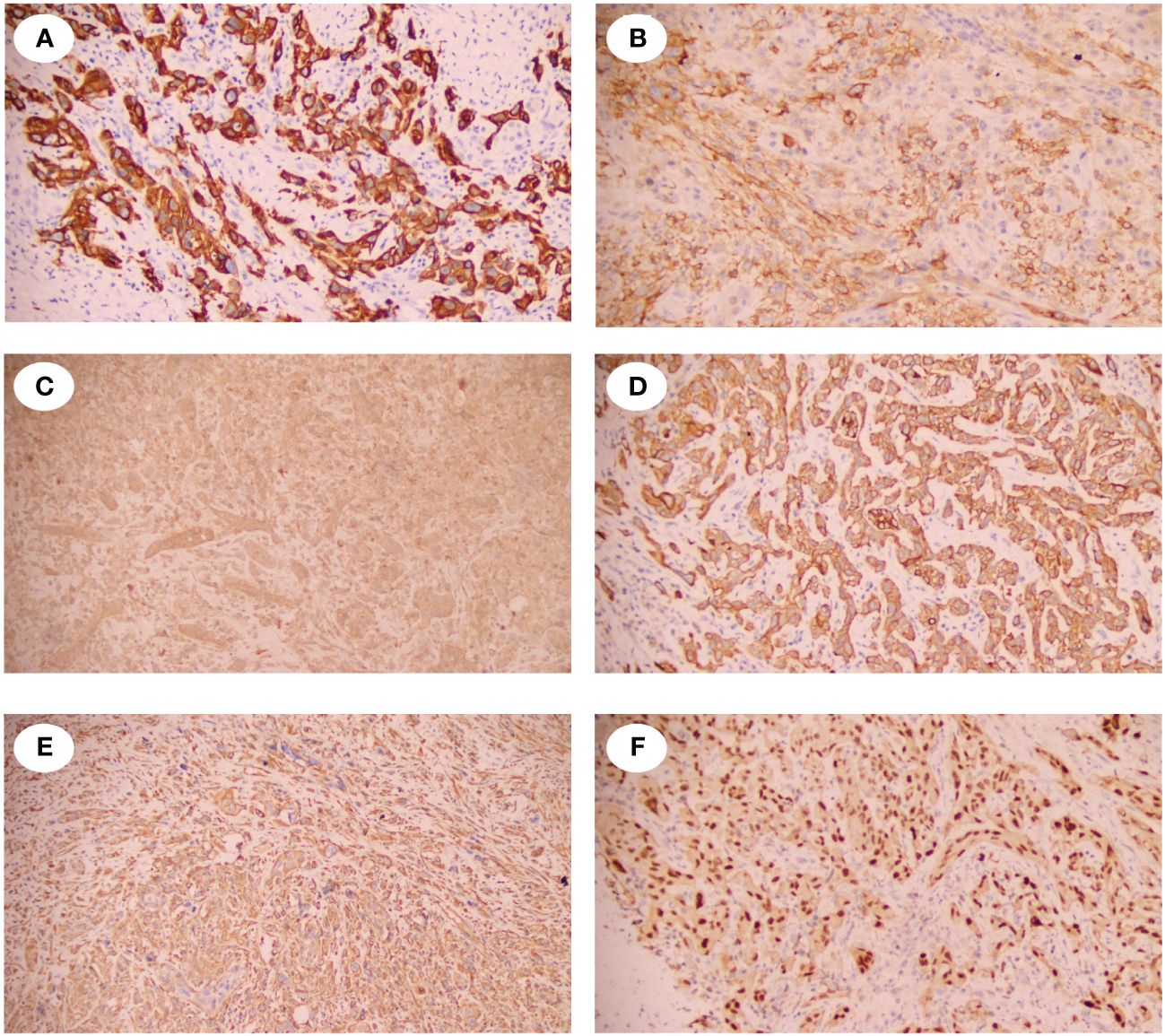
Figure 2 Immunohistochemical staining of the SRCC. (A) showed the spindle tumor cells were cytoplasmic positive for CD5/6. (B) showed the spindle tumor cells were cytoplasmic positive for CD10. (C) showed the spindle tumor cells were cytoplasmic positive for CD99. (D) showed the spindle tumor cells were cytoplasmic positive for CK. (E) showed the spindle tumor cells were cytoplasmic positive for Vinentin. (F) showed the spindle tumor cells were nuclear positive for PAX-8.
Discussion
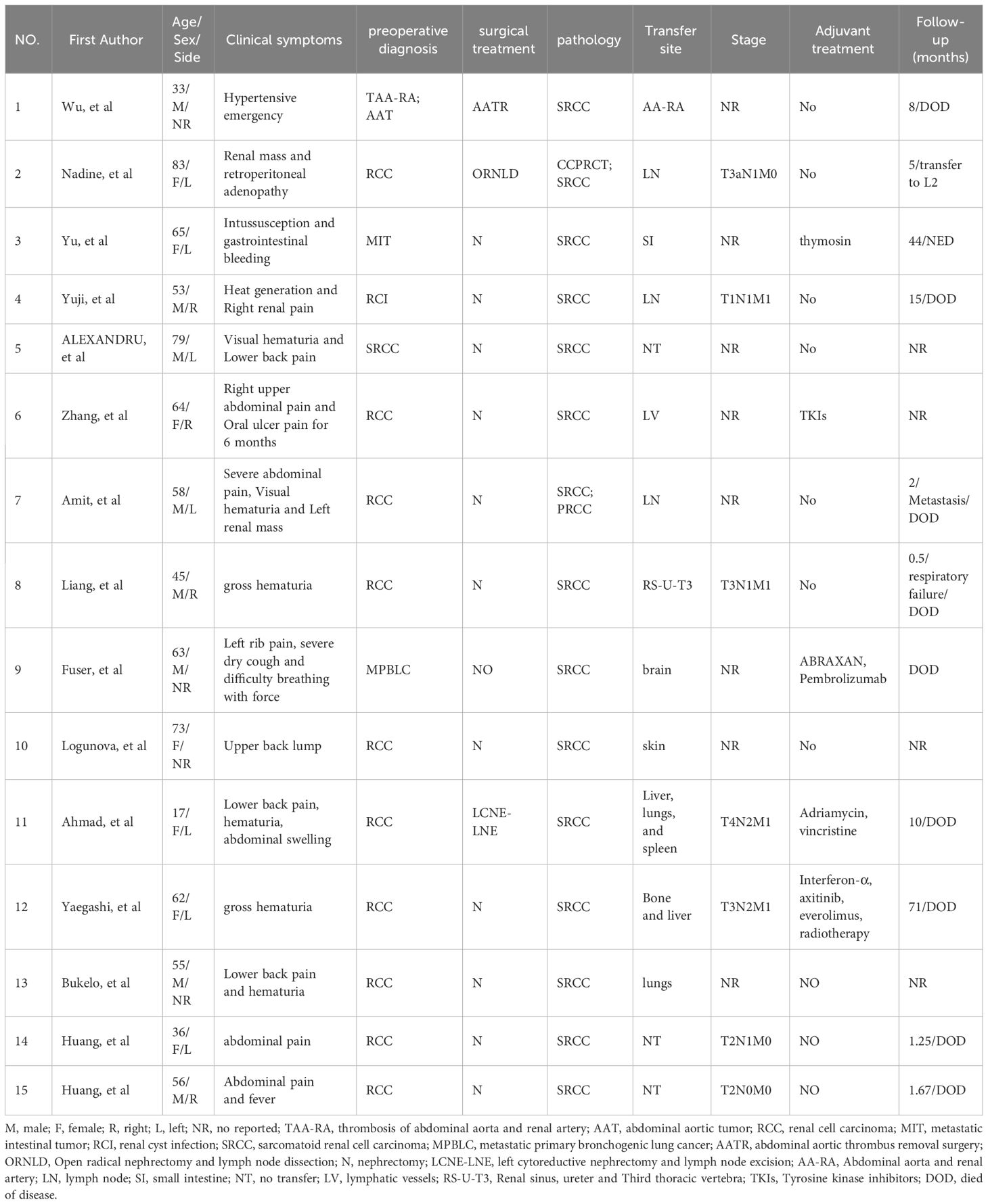
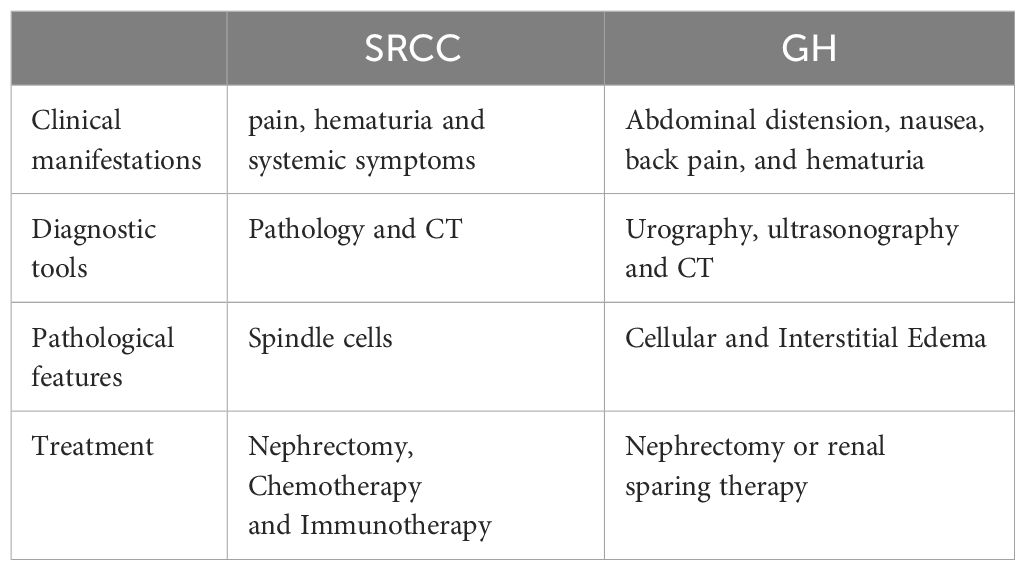
SRCC accounts for only 5% in RCC and represents a highly refractory and lethal form of kidney cancer (2). The most common symptoms among SRCC patients include pain (51–52%), hematuria (22–34%) and systemic symptoms (4), whereas cases of SRCC accompanied by severe renal hydronephrosis are exceedingly rare. In 2012, Kimura R and colleagues (5) documented the first case of SRCC with severe renal hydronephrosis. In this case, a 53-year-old male patient sought medical attention for visible hematuria and left shoulder pain. CT imaging revealed an extensive cystic lesion in the left kidney, which led to a left radical nephrectomy. Subsequent pathological analysis confirmed a diagnosis of sarcomatoid renal cell carcinoma. Similarly, another patient presented with persistent lumbar pain of unidentified origin, and CT scans indicated severe renal hydronephrosis in the right kidney. Consequently, an open right nephrectomy was performed, revealing postoperative pathology consistent with sarcomatoid renal cell carcinoma. This case represents the second documented instance of SRCC associated with severe renal hydronephrosis. A systematic review of all SRCC cases published since 2014 can be found in Table 1.
The term “Giant hydronephrosis” (GH) describes a condition where the kidney’s collecting system holds over 1000 milliliters of urine or the kidney comprises at least 1.6% of total body weight (6). GH occurs more frequently in children than in adults. The primary congenital cause of GH is obstruction at the renal pelvis-ureter junction. Additional causes include ureteral ectopia, duplicated collecting systems, and abnormal vascular systems that compress at this junction, also leading to GH (7). Regarding the association between GH and SRCC, sarcomatoid tumors are typically large, averaging 9–10 cm in size (3). Consequently, the tumor’s presence can lead to local obstruction, potentially causing multiple stones in the right renal calyx and impaired urine flow, which may contribute to renal hydronephrosis. In our case, despite the use of CT imaging, distinguishing between severe renal hydronephrosis and sarcomatoid renal cell carcinoma remains challenging. Here we summarize the similarities and differences between SRCC and GH in Table 2.
The pattern and degree of pleomorphism do not influence clinical behavior; therefore, all SRCCs are classified as International Society of Urological Pathology grade 4, reflecting their poor prognosis (8). SRCC is highly invasive (1) and typically occurs in patients at advanced stages or who have already experienced metastasis (9). Numerous studies have confirmed that sarcomatoid transformation independently impacts patient prognosis, with more extensive transformations associated with poorer outcomes. Reports indicate that the median survival for SRCC patients typically ranges from 4.9 to 19 months (10–13). Presently, nephrectomy remains the predominant treatment strategy for SRCC. Michail et al. analyzed clinical data from 879 SRCC patients in the SEER database, finding that nephrectomy significantly improves patient outcomes. All patients eligible for surgery should undergo consideration for nephrectomy (14). Exploration of diverse and more effective treatment methods continues, with immunotherapy playing a pivotal role. A meta-analysis involving 467 patients suggests that immune combination therapy significantly reduces mortality and disease progression compared to sunitinib treatment (15). Robot-assisted partial nephrectomy (RAPN) is currently utilized in the treatment of renal cell carcinoma, showing promising results (16). Hanif’s retrospective study also shows that therapy with immune checkpoint inhibitors (CPI) offers superior outcomes compared to conventional treatments using molecular targeted drugs or chemotherapy (17). Therefore, immunotherapy presents a promising approach to potentially improve the prognosis of patients with SRCC.
In future clinical scenarios, it is crucial to recognize the potential for sarcomatoid renal cell carcinoma to present as significant renal edema, which may indicate a more aggressive pathological subtype and greater invasiveness.
Conclusion
SRCC represents a highly invasive malignancy with a pessimistic prognosis within the urinary system. Clinicians must remain vigilant to identify the potential presence of sarcomatoid renal cell carcinoma in patients exhibiting clinical signs and imaging findings suggestive of renal hydronephrosis, thus preventing delays or misinterpretations in diagnosing SRCC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SC: Writing – original draft. ZH: Writing – original draft. MM: Writing – review & editing. ZT: Writing – review & editing. CZ: Writing – review & editing. NF: Writing – review & editing. BY: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the grants from the plan of Science and Technology of Zunyi (grant 2022-403) and the Guizhou Provincial Health Commission (grant gzwkj2023-374).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol. (2019) 75:74–84. doi: 10.1016/j.eururo.2018.08.036
2. Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist. (2012) 17:46–54. doi: 10.1634/theoncologist.2011-0227
3. Blum KA, Gupta S, Tickoo SK, Chan TA, Russo P, Motzer RJ, et al. Sarcomatoid renal cell carcinoma: biology, natural history and management. Nat Rev Urol. (2020) 17:659–78. doi: 10.1038/s41585-020-00382-9
4. Lebacle C, Pooli A, Bessede T, Irani J, Pantuck AJ, Drakaki A. Epidemiology, biology and treatment of sarcomatoid RCC: current state of the art. World J Urol. (2019) 37:115–23. doi: 10.1007/s00345-018-2355-y
5. Kimura R, Koyama K, Abe H. [A case of sarcomatoid renal cell carcinoma associated with giant hydronephrosis]. Hinyokika Kiyo. (2012) 58:435–8.
6. Yang WT, Metreweli C. Giant hydronephrosis in adults: the great mimic. Early diagnosis with ultrasound. Postgrad Med J. (1995) 71:409–12. doi: 10.1136/pgmj.71.837.409
7. Hu G, Luo M, Xu Y. Giant hydronephrosis secondary to ureteropelvic junction obstruction in adults: report of a case and review of literatures. Int J Clin Exp Med. (2015) 8:4715–7.
8. Wei S, Al-Saleem T. The pathology and molecular genetics of sarcomatoid renal cell carcinoma: A mini-review. J Kidney Cancer VHL. (2017) 4:19–23. doi: 10.15586/jkcvhl.2017.70
9. Arnoux V, Lechevallier E, Pamela A, Long JA, Rambeaud JJ. [Sarcomatoid renal cell carcinoma]. Prog Urol. (2013) 23:430–7. doi: 10.1016/j.purol.2013.01.003
10. Cheville JC, Lohse CM, Zincke H, Weaver AL, Leibovich BC, Frank I, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. (2004) 28:435–41. doi: 10.1097/00000478-200404000-00002
11. Shuch B, La Rochelle JC, Wu J, Klatte T, Riggs SB, Kabbinavar F, et al. Performance status and cytoreductive nephrectomy: redefining management in patients with poor performance. Cancer. (2008) 113:1324–31. doi: 10.1002/cncr.23708
12. Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. (2000) 89:604–14. doi: 10.1002/(ISSN)1097-0142
13. Yu Q, Zhang YT, Feng H, Li Z. A rare case of metastatic sarcomatoid renal cell carcinoma to the small intestine. Asian J Surg. (2022) 45:1898–9. doi: 10.1016/j.asjsur.2022.03.131
14. Alevizakos M, Gaitanidis A, Nasioudis D, Msaouel P, Appleman LJ. Sarcomatoid renal cell carcinoma: population-based study of 879 patients. Clin Genitourin Cancer. (2019) 17:e447–53. doi: 10.1016/j.clgc.2019.01.005
15. Iacovelli R, Ciccarese C, Bria E, Bracarda S, Porta C, Procopio G, et al. Patients with sarcomatoid renal cell carcinoma - re-defining the first-line of treatment: A meta-analysis of randomized clinical trials with immune checkpoint inhibitors. Eur J Cancer. (2020) 136:195–203. doi: 10.1016/j.ejca.2020.06.008
16. Pandolfo SD, Cerrato C, Wu Z, Franco A, Del Giudice F, Sciarra A, et al. A systematic review of robot-assisted partial nephrectomy outcomes for advanced indications: Large tumors (cT2-T3), solitary kidney, completely endophytic, hilar, recurrent, and multiple renal tumors. Asian J Urol. (2023) 10:390–406. doi: 10.1016/j.ajur.2023.06.001
Keywords: misdiagnosis, rare disease, aggressive renal cell carcinoma, hydronephrosis, sarcomatoid renal cell carcinoma
Citation: Chen S, He Z, Meng M, Tian Z, Zhu C, Fu N and Yu B (2024) Case report: Sarcomatoid renal cell carcinoma masquerading as hydronephrosis. Front. Oncol. 14:1361250. doi: 10.3389/fonc.2024.1361250
Received: 25 December 2023; Accepted: 10 May 2024;
Published: 22 May 2024.
Edited by:
Salvatore Siracusano, University of L’Aquila, ItalyReviewed by:
Jiwen Shang, Shanxi Medical University, ChinaSavio Domenico Pandolfo, Federico II University Hospital, Italy
Copyright © 2024 Chen, He, Meng, Tian, Zhu, Fu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ni Fu, 18985601188@163.com; Bo Yu, 308913894@qq.com
†These authors have contributed equally to this work and share first authorship
 Shicheng Chen
Shicheng Chen Zhongcong He1†
Zhongcong He1† Mi Meng
Mi Meng Bo Yu
Bo Yu