- 1Division of Biostatistics and Bioinformatics, College of Medicine, Pennsylvania State University, Hershey, PA, United States
- 2Calcutta School of Tropical Medicine, Kolkata, India
- 3Penn State Milton S. Hershey Medical Center, Hershey, PA, United States
- 4Division of Medical Oncology, The Ohio State University, Columbus, OH, United States
- 5Department of Pediatrics, University of Florida, Gainesville, FL, United States
Aims: Socioeconomic and environmental factors influence childhood asthma prevalence across the world. In-depth epidemiological research is necessary to determine the association between asthma prevalence and socio-environmental conditions, and to develop public health strategies to protect the asthmatic children against the environmental precipitators. Our research was based on aggregative data and sought to compare the asthma prevalence between children of two different age-groups across the world and to identify the association among the key socio-environmental conditions with increased childhood asthma prevalence.
Method: We included forty countries with available data on various socio-environmental conditions (2014–2015). Childhood asthma prevalence of two different age groups (6–7 and 13–14 years) were obtained from global asthma report 2014. Because of significant diversities, the selected countries were divided into two groups based on human developmental index (HDI), a well-recognized parameter to estimate the overall socioeconomic status of a country. Robust linear regression was conducted using childhood asthma prevalence as the dependent variable and female smoking prevalence, tertiary school enrollment (TSE), PM10 (particulate matter ≤10 μm in diameter) and gross domestic product (GDP) as predictors.
Results: Asthma prevalence was not different between two age groups. Among all predictors, only female smoking prevalence (reflecting maternal smoking) was associated with asthma prevalence in the countries with lower socio-economic conditions (HDI), but not in the higher HDI group. The results were unchanged even after randomization.
Conclusions: Childhood asthma prevalence did not change significantly with age. Female smoking may have a positive correlation with childhood asthma prevalence in lower HDI countries.
Introduction
Asthma is one of the most common noncommunicable diseases worldwide and has long been associated with various socioeconomic and environmental conditions. Weitzman et al. acknowledged the impact of socioenvironmental conditions on the outcome of childhood asthma in the United States as early as 1990 (1). Further, a previous study conducted by our team has demonstrated that asthma-related mortality was higher in the developing countries compared to developed ones, with lack of resources and poverty were likely to be the key contributing factors(2). However, very few global epidemiological studies have been conducted in the recent years to delineate further the impact of socio-environmental conditions on the prevalence of childhood asthma. Leading asthma researchers had previously surmised that the outcome of asthma primarily depends on IgE mediated eosinophilic inflammation or atopy (3). However, the International Study of Asthma and Allergies in Childhood (ISAAC) phase 3 study concluded that the increased childhood asthma prevalence observed in recent years, especially in developing countries, may be influenced by other environmental factors in addition to atopy1 (4). These findings constituted a conceptual shift in the asthma epidemiology and emphasized the necessity of further investigation to determine other underlying precipitators of childhood asthma1. ISAAC was terminated in 2012, and since then Global Asthma Network (GAN) has been responsible for carrying forward its mission2.
Childhood asthma prevalence varies significantly not only between affluent and poor countries2, but also differs in immigrants when compared to the inhabitants of their country of inheritance. For example, the ISAAC phase 3 trial indicated that asthma symptoms were highest among the Chinese-Canadian adolescents (born and raised in Canada), followed by the children who were born in China and later immigrated to Canada (>7 years back), while the symptoms were lowest in the Chinese adolescents who had never migrated (5). One might then infer that the Canadian socioenvironmental conditions could be accountable for the increased asthma prevalence among Canadians of Chinese origin since the asthma prevalence in Canada is higher compared to China.
Parental asthma education helps to promote greater awareness which can facilitate a favorable outcome of childhood asthma (6). Most of the developed countries with a higher gross domestic product (GDP) can offer better health care to their citizen. In contrast, children from the poorest parts of the society are worst sufferers of any chronic diseases due to lack of access to the healthcare and poor living conditions. Wisow et al. demonstrated that the rate of asthma-related admission at a Maryland hospital was higher among the African-Americans and that was largely contributed by poverty (7). Although asthma-related mortality is significantly higher in poorer countries than that of the developed ones, asthma prevalence does not follow a similar trend (2, 8). ISAAC cited a higher prevalence of wheeze among the adolescents (13–14 years) of affluent countries (9).
Maternal smoking and even third-hand smoking is known to affect the frequency and severity of childhood asthma (10, 11). Kabesch et al. demonstrated that second-hand smoke exposure due to maternal smoking often led to wheezing in younger children (0–6 years). However, that influence tends to decline in older children suggesting that the impact of socioeconomic conditions may differ among different age groups (younger children vs. adolescents). Uncontrolled air pollution is another important environmental factor causing increased asthma prevalence. A meta-analysis of 36 studies by Weinmayr et al. has demonstrated a strong association between higher PM10 (particulate matter ≤10 μm in diameter) and increased asthma prevalence in children (12). Association analysis between air pollution and adult asthma outcome by Maestrelli et al. suggested that PM10 concentration had influenced the asthma-related morbidity but not PM2.5 (13).
With this background, we hypothesized that in addition to atopy and racial heterogeneity, various socio-economic and environmental conditions might contribute to the disparity in asthma prevalence throughout the world. Thus, our study was aimed at determining the influences of four specific socioeconomic and environmental conditions on childhood asthma prevalence in different countries: female smoking prevalence, tertiary school enrollment, PM10, and GDP. To test this hypothesis, we developed a unique approach to analyzing and correlating international aggregative datasets obtained by various globally recognized organizations. We included group of 6–7 years old and 13–14 years old children following the categorization by GAN3.
Methods
Sample Selection
We selected 60 countries for this cross-sectional study using a random selection tool4. Forty out of those had most of the required data points available from 2014 to 2015, thus were considered for data analysis (Supplemental Figure 1).
Childhood Asthma Prevalence
Following the GAN categorization, prevalence data of two age-groups were included2: 6–7 years old and 13–14 years old.
Parameters of Socioeconomic and Environmental Conditions
GDP (per capita) and tertiary school enrollment (TSE) were included as the socio-economic conditions, while female smoking prevalence (age>15 years) and PM10 (μg/m3) were considered as environmental risk factors. We have utilized various globally recognized organizations resources for data collection (Supplemental Table 1)5,6,7,8,9, 10
Sample Size Estimation
The correlation analysis between GDP and asthma prevalence (6–7 years) was used as a model for the sample size estimation using G*power11. The correlation coefficient between those two variables was 0.47. The estimated required sample size was 32 [input criteria:0.8 of power(1-β), α = 0.05, two tails].
Stratification According to Socioeconomic Conditions (HDI)
Since we selected countries with extremely diverse conditions from six continents, stratifying them into groups according to their socioenvironmental status was necessary. Hence, a standard parameter was required to achieve that categorization. United Nations Development Program has accepted HDI as the ultimate parameter to assess the overall socioeconomic well being of a country, instead of focusing on isolated GDP12. HDI was created assimilating multiple parameters including life expectancy, education index, and GDP. Thereby, we utilized HDI to stratify selected countries into higher and lower group through the median (Supplemental Figure 1).
Statistical Analyses
We used R (3.5.1) and IBM SPSS (version 24) for data analysis and Mann Whitney U-test for two-sample comparisons. Robust linear regression was used for both the HDI groups considering non-normality of data, with asthma prevalence as the dependent variable; while female smoking prevalence, TSE, PM10, and GDP were included as the predictors. For reproducibility, 16 out of 20 countries were randomly selected 1,000 times by SRSWOR function, and robust regressions were re-run to estimate the proportion of times each predictor was significant in each HDI groups. Two-tailed p-values of <0.05 were considered statistically significant.
Results
Lower HDI Countries
Asthma prevalence in 6–7 years old children [as expressed in median (IQR)] 5.00 (6.20) was not significantly different from that of 13–14 years old children 10.80 (7.90) (p = 0.17 derived from Mann Whitney test). GDP and female smoking prevalence had strong association [correlation coefficient (r) = 0.66, p = 0.001] (Supplemental Figure 2). However, TSE did not have a significant correlation with GDP or female smoking.
Higher HDI Countries
Asthma prevalence in 6–7 years old children 11.00 (12.70) was not significantly different from that of older children 14.10 (11.20) (p = 0.51 derived from Mann Whitney test). GDP, TSE and female smoking prevalence did not have a significant correlation among themselves (Supplemental Figure 3).
Comparative Analyses (Mann Whitney U-Test- Table 1)
Low HDI countries compared to high HDI, had significantly lower asthma prevalence rates in both age groups (p = 0.043 and p = 0.023, respectively for younger and older children). However, Asthma prevalence was not significantly different between younger and older children (p-values of 0.21 and 0.75 for low and high HDI groups, respectively). Expectedly, countries with lower HDI had worse socio-economic conditions like GDP and TSE in contrast to higher HDI group (p < 0.001 for both the variables). Lower HDI countries also had a worse level of air pollution (PM10) and decreased female smoking prevalence in comparison to higher HDI countries (p < 0.001 and p = 0.005, respectively).
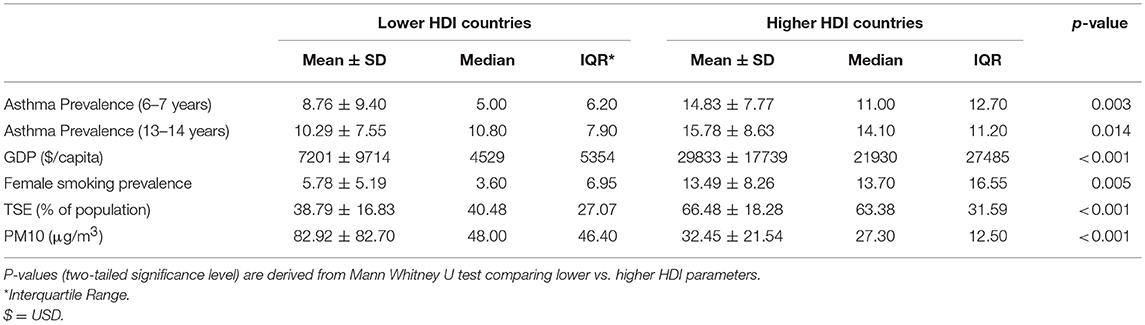
Table 1. Mann Whitney U-test demonstrating the significant difference between asthma prevalence and socio-environmental predictors between lower and higher HDI countries.
Robust Linear Regression
Low HDI Countries
Female smoking prevalence was the only predictor variable that was statistically significant for asthma prevalence in both age groups while controlling for PM10, GDP, and TSE (p < 0.01 and p = 0.01 for the younger and older age groups, respectively; Table 2).
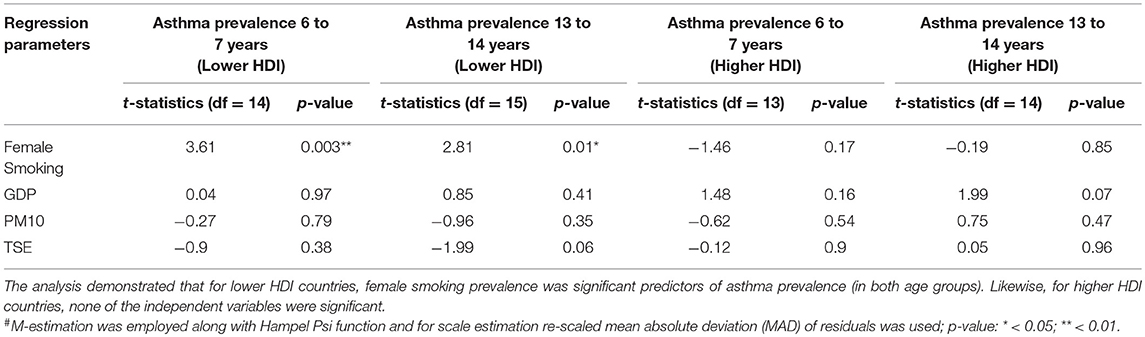
Table 2. Regression coefficients from robust linear regression# based on 20 countries in each of the lower and higher HDI groups.
High HDI Countries
None of the predictors variable including female smoking prevalence was statistically significant (Table 2).
Random Sampling Within Strata (HDI Groups)
In low HDI countries, female smoking prevalence was a significant predictor for asthma prevalence (in both age groups) in more than 80% simulations (Supplemental Figure 4). Likewise, for high HDI countries, none of the independent variables were significant in most of the simulations.
Other Methods
Regression analyses results were reproduced using linear mixed models (Supplemental Table 2) showing high conformity.
Discussion
“Growing out of childhood asthma in adolescence” is a well-known concept and the hypothesized mechanism is the reduction of asthma prevalence due to the immunological changes induced by sex hormones at puberty (14). However, according to R Zannolli, this concept was not supported by scientific evidence (15). In contrast, recent studies suggested that asthma symptoms may worsen in girls during puberty (16). We did not find a difference in asthma prevalence between younger and teenage children. However, the asthma prevalence was significantly lower in countries with low HDI compared to high HDI. The rationale behind this observation was not well understood. An inefficient data collection system and inadequate diagnostic facilities in the underdeveloped countries might be attributable for an underestimation of prevalence (2).
Our study indicates that the female smoking prevalence (reflecting maternal smoking) was reduced in low HDI countries compared to higher HDI, which could have been influenced by sociocultural practices, stigma, and lack of financial freedom among women in under-developed countries (2). More importantly, the regression analyses showed that female smoking prevalence, was the only predictor of childhood asthma prevalence in countries with lower socioeconomic conditions. It was surprising to find the lack of association between asthma prevalence and any of the socio-environmental factors in higher HDI countries, despite an increased asthma prevalence and higher values of the predictors (GDP, TSE, and female smoking prevalence). A likely explanation could be that the increased asthma prevalence in high HDI countries was a result of multi-factorial contribution and none of the predictors individually was the single most important defining factor. Nonetheless, this result came out of a study based on aggregative data and could not oppose the impact of maternal smoking in the developed countries.
Female smoking, especially smoking during pregnancy, has a deleterious effect on the offspring, as it can induce epigenetic changes resulting in the increased risk of asthma and persistent wheezing in children (17, 18). Further, Tager et al. conducted a prospective study and described the progressive decline in lung functions in children who were persistently exposed to the maternal smoking as they grow older (19). Thus, based on the observations from several prospective studies, GAN recommended strict implementation of policies to reduce tobacco smoking and to avoid second-hand smoke exposure in children at both government and national organizational level to achieve the goal of reduced asthma prevalence by 50% by 20252.
Limitations of this study include lack of applicability to any specific region since our research was based on aggregative data. Thus, a causal relationship could not be derived from such data, and it would require a prospective study. Also, sex-specific asthma prevalence analysis could not be done due to lack of available data. Reproducibility of regression results after 1,000 times randomization was a strength of this study and indicated that a similar outcome should be expected from future prospective studies.
In summary, this study enabled us to recognize that among various socioeconomic and environmental factors studied, female smoking prevalence possibly had the strongest association with increased asthma prevalence in countries with lower socio-economic conditions. Our result thus supports the recommendation of GAN report 2014, that national strategies to prevent secondhand smoke exposure in general and maternal smoking, in particular, would be helpful to achieve reduced global childhood asthma prevalence by 20252.
Conclusion
Asthma prevalence in children was not significantly different between younger children and adolescents. Female smoking was found to be one of the major socio-environmental predictors of increased asthma prevalence in children of different age groups from the countries with lower socio-economic conditions.
Author Contributions
PM was the principal investigator and was primarily responsible for manuscript writing. VM helped with concept, design of the study along-with PM. VM, PM and AS performed statistical analysis. PM, SP, JD and AS has was responsible for data collection. SP helped with study design and concept. HR, JD and MA-H helped to contribute with the clinical aspects along with PM. All the authors including PM, VM, SP, HR, JD and MA-H helped the final draft of the manuscript and approved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Ms. Rachael Mills, Department of Pediatrics, Penn State College of Medicine, for her contribution to prepare the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2018.00295/full#supplementary-material
Supplemental Figure 1. Consort diagram delineating the outline of the study design.
Supplemental Figure 2. Scatter diagrams demonstrating significant associations between childhood asthma prevalence in two different age groups (6–7 years and 13–14 years of age) and female smoking prevalence in countries with lower socio-economic conditions (lower HDI; with p < 0.01 in both age groups, and r = 0.79 and r = 0.70, respectively).
Supplemental Figure 3. Scatter diagrams demonstrating lack of associations between childhood asthma prevalence in two different age groups and female smoking prevalence in countries with higher socioeconomic conditions (higher HDI; p > 0.05 for both age groups).
Supplemental Figure 4. Thousand times randomization using the SRSWOR function in R software demonstrating that for low HDI countries, female smoking prevalence was significant predictors for asthma prevalence (in both age groups) in more than 80% simulations. Likewise, for high HDI countries, none of the independent variables were significant in most of the simulations.
Footnotes
1. ^http://isaac.auckland.ac.nz/
2. ^http://www.globalasthmanetwork.org/news/GAR2014.php [Internet].
3. ^http://www.globalasthmanetwork.org/news/GAR2014.php
4. ^www.randomlists.com/random-country
5. ^https://www.cia.gov/library/publications/the-world-factbook/rankorder/2004rank.html
6. ^http://databank.worldbank.org/data/reports.aspx?source=2&series=SE.TER.ENRR&country=# [Internet].
7. ^National Accounts Main Aggregates Database for GDP. Available from: https://unstats.un.org/unsd/snaama/selbasicFast.asp
8. ^http://apps.who.int/gho/data/node.main.65
9. ^The tobacco Atlas. Available from: https://tobaccoatlas.org/topic/prevalence/
10. ^http://aqicn.org/faq/2015-05-16/world-health-organization-2014-air-pollution-ranking/. [Internet].
11. ^http://www.gpower.hhu.de/
12. ^United Nations Development Programme and Human Development Index. Available from: http://hdr.undp.org/en/content/human-development-index-hdi
References
1. Weitzman M, Gortmaker S, Sobol A. Racial, social, and environmental risks for childhood asthma. Am J Dis Child. (1990) 144:1189–94.
2. Sinharoy A, Mitra S, Mondal P. Socioeconomic and environmental predictors of asthma-related mortality. J Environ Public Health (2018) 2018:7. doi: 10.1155/2018/9389570
4. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet (2006) 368:733–43. doi: 10.1016/S0140-6736(06)69283-0
5. Wang H-Y, Wong GW, Chen Y-Z, Ferguson AC, Greene JM, Ma Y, et al. Prevalence of asthma among Chinese adolescents living in Canada and in China. Can Med Assoc J. (2008) 179:1133–42. doi: 10.1503/cmaj.071797
6. Cesaroni G, Farchi S, Davoli M, Forastiere F, Perucci CA. Individual and area-based indicators of socioeconomic status and childhood asthma. Eur Resp J. (2003) 22:619–24. doi: 10.1183/09031936.03.00091202
7. Wissow LS, Gittelsohn AM, Szklo M, Starfield B, Mussman M. Poverty, race, and hospitalization for childhood asthma. Am J Public Health (1988) 78:777–82. doi: 10.2105/AJPH.78.7.777
8. Grant EN, Lyttle CS, Weiss KB. The relation of socioeconomic factors and racial/ethnic differences in US asthma mortality. Am J Public Health (2000) 90:1923.
9. Stewart AW, Mitchell EA, Pearce N, Strachan DP, Weiland SK. The relationship of per capita gross national product to the prevalence of symptoms of asthma and other atopic diseases in children (ISAAC). Int J Epidemiol. (2001) 30:173–9. doi: 10.1093/ije/30.1.173
10. Strachan DP, Cook DG. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax (1998) 53:204–12.
11. Drehmer JE, Walters BH, Nabi-Burza E, Winickoff JP. Guidance for the clinical management of thirdhand smoke exposure in the child health care setting. J Clin Outcomse Manag. (2017) 24:551.
12. Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. (2010) 118:449. doi: 10.1289/ehp.0900844
13. Maestrelli P, Canova C, Scapellato M, Visentin A, Tessari R, Bartolucci G, et al. Personal exposure to particulate matter is associated with worse health perception in adult asthma. J Invest Allergol Clin Immunol. (2011) 21:120–8.
14. Balfour-Lynn L. Childhood asthma and puberty. Arch Dis Child. (1985) 60:231–5. doi: 10.1136/adc.60.3.231
16. Lieberoth S, Gade EJ, Brok J, Backer V, Thomsen SF. Age at menarche and risk of asthma: systematic review and meta-analysis. J Asthma (2014) 51:559–65. doi: 10.3109/02770903.2014.903966
17. Hallit S, Leynaert B, Delmas MC, Rocchi S, De Blic J, Marguet C, et al. Wheezing phenotypes and risk factors in early life: the ELFE cohort. PloS ONE (2018) 13:e0196711. doi: 10.1371/journal.pone.0196711
18. Zacharasiewicz A. Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res. (2016) 2:00042–2016. doi: 10.1183/23120541.00042-2016
Keywords: childhood asthma prevalence, socioenvironmental predictors of asthma, global asthma network, pediatric asthma, female smoking, human developmental index
Citation: Midya V, Pal S, Sinharoy A, Das JK, Rao H, Abu-Hasan M and Mondal P (2018) The Association Between Female Smoking and Childhood Asthma Prevalence–A Study Based on Aggregative Data. Front. Public Health 6:295. doi: 10.3389/fpubh.2018.00295
Received: 10 July 2018; Accepted: 28 September 2018;
Published: 17 October 2018.
Edited by:
Richard Eugene Frye, Phoenix Children's Hospital, United StatesReviewed by:
Piotr Rzymski, Poznan University of Medical Sciences, PolandJamie Lynn Sturgill, University of Kentucky, United States
Wilfried Joachim Juergen Karmaus, University of Memphis, United States
Copyright © 2018 Midya, Pal, Sinharoy, Das, Rao, Abu-Hasan and Mondal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pritish Mondal, pmondal@pennstatehealth.psu.edu
 Vishal Midya
Vishal Midya Shekhar Pal2
Shekhar Pal2 Ankita Sinharoy
Ankita Sinharoy Jishu K. Das
Jishu K. Das Pritish Mondal
Pritish Mondal