- Department of Food and Nutrition, Institute of Home Economics, University of Delhi, New Delhi, India
Multiple factors affect cognitive health, such as age-related changes in the brain, injuries, mood disorders, substance abuse, and diseases. While some cannot be changed, evidence exists of many potentially possibly modifiable lifestyle factors: diet, physical activity, cognitive and social engagement, smoking and alcohol consumption which may stabilize or improve declining cognitive function. In nutrition, the focus has been mainly on its role in brain development in the early years. There is a strong emerging need to identify the role of diet and nutrition factors on age-related cognitive decline, which will open up the use of new approaches for prevention, treatment or management of age-related disorders and maintaining a good quality of life among older adults. While data on effect of high protein diets is not consistent, low-fat diets are protective against cognitive decline. Several micronutrients like B group vitamins and iron, as well as many polyphenols play a crucial role in cognitive health. Mediterranean, Nordic, DASH, and MIND diets are linked to a lower risk of cognitive decline and dementia. The relationship between the gut microbiome and brain function through the gut-brain axis has led to the emergence of data on the beneficial effects of dietary fibers and probiotics through the management of gut microbes. A “whole diet” approach as well as macro- and micro-nutrient intake levels that have protective effects against cardiovascular diseases are most likely to be effective against neurodegenerative disorders too. Young adulthood and middle age are crucial periods for determining cognitive health in old age. The importance of cardio metabolic risk factors such as obesity and hypertension, smoking and physical inactivity that develop in middle age suggest that preventive approaches are required for target populations in their 40s and 50s, much before they develop dementia. The commonality of dementia risk with cardiovascular and diabetes risk suggests that dementia could be added to present non-communicable disease management programs in primary healthcare and broader public health programs.
1. Introduction
According to Hendrie et al. (1) “cognitive health is the development and preservation of the multidimensional cognitive structure that allows older people to maintain social connectedness, an ongoing sense of purpose and the abilities to function independently, to permit functional recovery from illness or injury, and to cope with residual functional deficits.” The main features include mental abilities, acquired skills, and the ability to apply these to complete a purposeful task/activity (2).
Cognitive health involves thinking, learning, and remembering, besides other aspects such as the motor function of making and controlling movements, including balance; emotional function of interpreting and responding to emotions and tactile function like feeling and responding to sensations of touch. Good brain health enables individuals to comprehend their abilities and adjust their cognitive, psychological, emotional, and behavioral functioning according to various life events to cope optimally.
Multiple factors affect the brain’s health, such as age-related changes in the brain, injuries, mood disorders, substance abuse and diseases. While some cannot be changed, evidence exists of many modifiable lifestyle factors: diet and physical activity, social engagement, and cognitive activity, smoking and alcohol consumption which may stabilize or improve declining cognitive function (3). These factors work via multiple mechanisms and are thought to either increase or reduce the risk of dementia. Another tool that scientists have proposed is that of cognitive reserve, that is the brain’s capacity to provide a buffer against any brain pathology. Cognitive decline or dementia symptoms may manifest only when there is a more significant threshold/burden of pathology (4).
With fast-evolving evidence on the various dimensions of nutrition and cognitive health covering the human lifespan, there is a need to document the scientific evidence-based data available. So far, the focus has been mainly on the role of nutrition in brain development in the early years. There is a strong emerging need to identify the role of various lifestyle factors, including diet, nutrition, and physical activity, on age-related cognitive decline, which will open the use of new approaches for prevention, treatment or management of age-related disorders and maintaining a good quality of life among older adults.
2. Methodology
This review encompasses the role of dietary and nutritional factors on cognitive health through the life course. The scope of this study is to review developments published at the national and international levels, particularly over the last 10 years.
2.1. Search strategy
Studies were searched in PubMed over the last 10 years, i.e., 2011–2021. Over 50 keywords, finalized after brainstorming among a few experts, were included in the search, and the investigators repeated the search using all suitable search term combinations. We used MeSH terms for the keywords for searching.
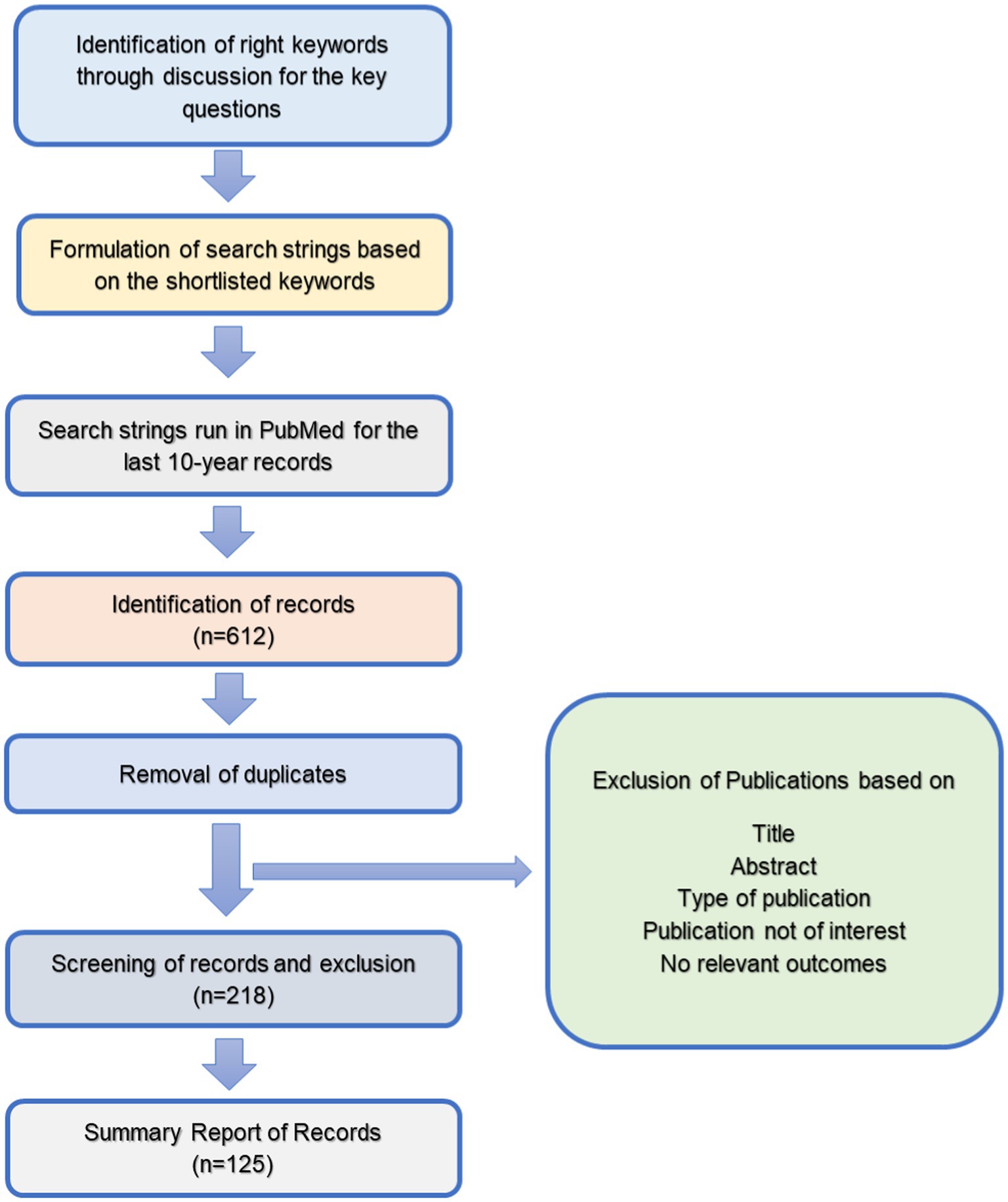
Studies selected for the present review were observational and intervention studies, review articles, systematic reviews and meta-analysis studies conducted on humans published in MEDLINE. These articles could be accessed with free full text in the English language. Articles that mentioned brain health, cognitive health or cognition and corresponding variables in either the title or abstract were initially included for full-text review. Based on this search, 612 articles were identified. In the second stage, the exclusion criteria, as given below, were applied, and 218 studies were selected. It was decided to examine a maximum of 75–100 full-text articles for in-depth analysis. Hence, finally, 125 full-text articles, after further filtering, were included for review (Figure 1).
The exclusion criteria included data older than 2010, records with words like case reports, rats, sheep, lamb or animal studies etc., in publication title, documents with only basic research or conference proceedings, and records of studies on psychological health like depression, anxiety, emotions, moods etc.
3. Nutrition and brain development
Based on brain imaging, it is known that the brain develops throughout childhood and young adulthood. Moreover, different brain structures follow different development and maturation trajectories (5). Genetic predisposition is crucial for brain development. Early childhood experiences have powerful effects on brain function, leading to individual differences that could contribute to behavioral dysfunction and an increased lifetime risk for chronic diseases (6). Nutrition is an essential modulator for early brain development, often over and above the environment to which the child is exposed.
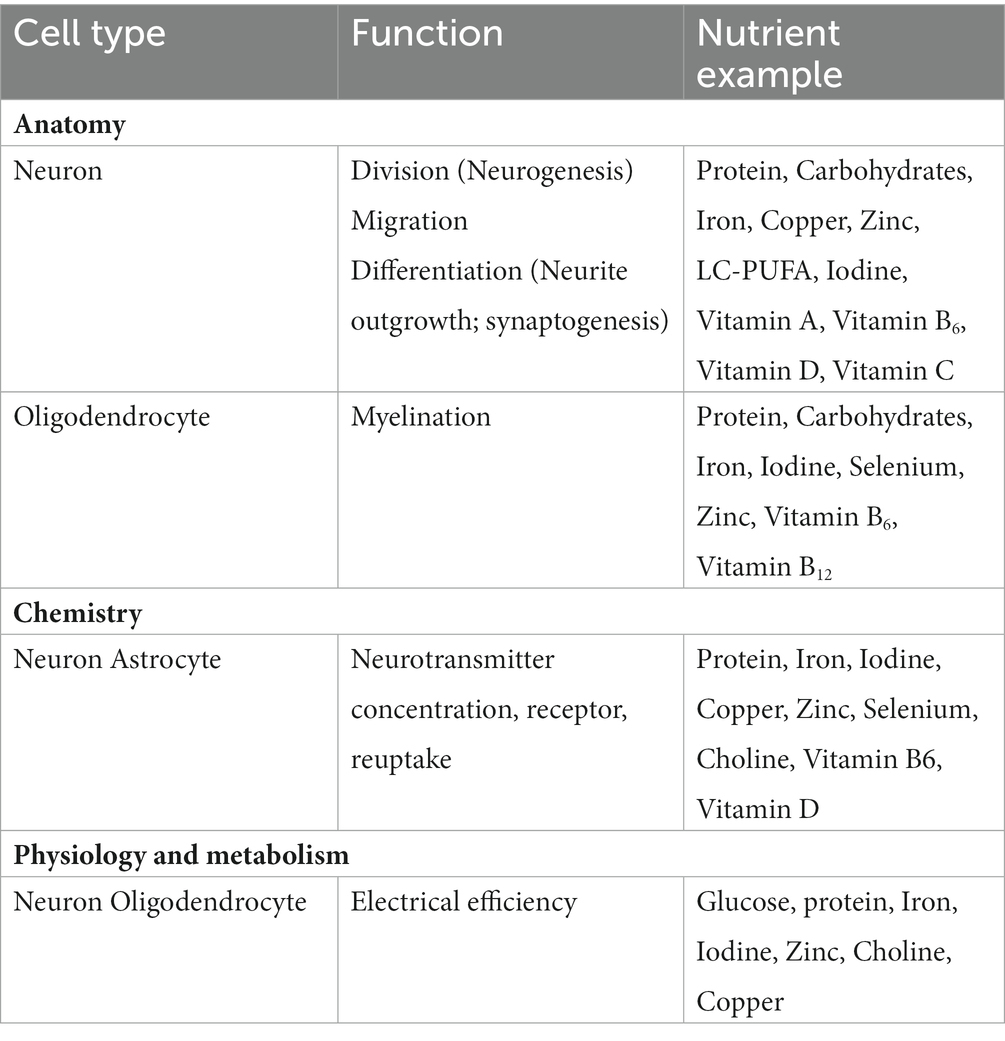
Besides generalized macronutrient under-nutrition, deficiencies of specific nutrients may significantly affect neurodevelopment, with lifelong implications. Several nutrients play an essential role in brain development during pregnancy, including protein, iron, copper, zinc, iodine, folate and certain fats (7). Requirement for these nutrients continues till later life as brain development is an ongoing process (Table 1).
Infancy is also a time of rapid brain growth and development, mainly supported by the baby’s nourishment. Breastfeeding may influence cognitive development through several mechanisms associated with breast milk composition and breastfeeding experience (8). Across all income levels among children and adolescents, breastfeeding is associated with higher performance on intelligence tests (9). The cognitive benefits of breastfeeding reportedly continue into adulthood.
General malnutrition during fetal development and the first few months after birth show life-long deleterious effects on brain development that manifest in learning difficulties (e.g., self-regulation difficulties and lower academic achievement) (10). During early and middle childhood, synapses are rapidly created and later during adolescence are removed selectively; hence, needing a constant supply of nutrients (8). Brain development particularly related to higher cognitive functioning continues through adolescence (11). During adulthood, evidence points to the need for several nutrients to support neuroplasticity and brain performance and to lessen the adverse effects of aging on the brain (12).
3.1. Role of nutrition and lifestyle in maintaining cognitive health
In healthy individuals, the decline of cognitive abilities occurs with age and is spread throughout life. The mechanisms which contribute to normal ageing, such as oxidative stress and free radical damage, neuro-inflammation and vascular dysfunction are similar to those contributing to the development of neurological diseases. Due to various genetic or environmental factors, these mechanisms get aggravated in pathological conditions.
Age-related cognitive degeneration is among the leading causes of lost Disability-Adjusted Life Years in people over 65. Addressing the age-related degeneration in neural function is a key to preserving the autonomy and well-being of older people. Besides non-modifiable risk factors, like age and genetic profile which play a significant role in the development of dementia, there is increasing evidence towards the role of modifiable risk factors that enhance or diminish the risk of developing dementia later in life. Factors identified as aggravating risk include depression; type 2 diabetes; midlife hypertension; mid-life obesity; smoking; physical inactivity and low educational attainment (13). Evidence on the role of nutrition in preventing cognitive decline in older adults is now emerging. Although the evidence is not substantial, factors that may reduce dementia risk include vegetable intake, a Mediterranean diet, and increased cognitive activity. There is also some evidence to suggest that events such as the death of a parent early in life and chronic sleep disturbances in middle age may also contribute to an enhanced risk of developing dementia (14).
3.1.1. Macronutrients that affect cognitive health
In the meta-analysis by Coelho-Junior et al. (15) no significant associations were observed between protein intake and global cognition in old age. However, as evident in three studies, there was a positive correlation between memory and protein intake. In only one study, protein intake was positively associated with visuospatial function, verbal fluency, processing speed, and sustained attention (16). A cross-sectional study by Li et al. (13) reported a positive association between protein intake from animal foods, meat, eggs and legumes and cognition.
Various studies on adults and older adults have suggested that a high-fat diet has adverse effects on cognition. A longitudinal study on 6,183 older females in the United States, reported that high amounts of saturated fatty acids were associated with worse cognitive and verbal memory trajectories. In contrast, higher MUFA intake was related to better trajectories (16). A review by Francis and Stevenson (17) reported an association between a high saturated fat, high refined carbohydrate diet and impaired cognitive function. Consumption of a high-fat diet stimulates the hippocampus to produce a neuro-inflammatory response to even a mild immune challenge, resulting in memory deficits (18). A high-fat diet increases the risk of obesity, increased chances to develop diabetes and the development of cognitive deficits and perhaps Alzheimer’s disease (AD). Insulin resistance, impaired glucose metabolism, and type 2 diabetes mellitus are well-known risk factors for AD (19). While data on effect of high protein diets is not consistent, low-fat diets are protective against cognitive decline.
Polyunsaturated fatty acids (PUFAs) regulate the function and structure of neurons, endothelial cells, and glial cells in the brain. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the omega-3 fatty acids, also modify neurotransmission, reduce neuro-inflammation and promote neuronal survival and neurogenesis (20). DHA is crucial for neurogenesis and neuronal migration, synaptogenesis, fatty acid composition of membranes, and fluidity, which affect the neurotransmitter systems, particularly the visual system. These areas of the brain regulate attention, inhibition and impulsivity (21). PUFAs have a role in maintaining cognitive function and preventing dementia due to their anti-thrombotic and anti-inflammatory properties and also affect neural processes (22). Cognitive decline in later years is associated with a high energy intake from protein and fat and a low energy intake from carbohydrates, as reported in a retrospective study in China (23). Low dietary consumption of omega-3 PUFAs can also contribute to memory loss (18).
3.1.2. Micronutrients that affect cognitive development, function and decline
Micronutrients help the body to produce enzymes, hormones, and other compounds needed for proper growth and development (24). Vitamin A, iodine and iron deficiencies are the most crucial public health concerns as they significantly impact the health of populations globally particularly young children and pregnant women in low-income countries (24). While the role of B group vitamins in brain development has been researched in recent years, not much evidence has emerged in the last decade on the association of fat-soluble vitamins and cognitive development in early life. Most studies have focused on their role in cognitive decline in later years.
B-vitamins are essential for brain development and function through many mechanisms. Venkatramanan et al. (25) emphasized the importance of adequate vitamin B-12 status, particularly during pregnancy and early childhood, as vitamin B-12 has a role in neural myelination, brain development, fetal and child growth.
Studies assessing the association of B vitamins and cognition in older adults were inconclusive (26–28). Only one study on United States adults showed an association of B-vitamins (niacin, folate, B6, and B12) with better cognitive function in midlife. In postmenopausal women free of Mild Cognitive Impairment (MCI), low folate intake may increase the risk of MCI/dementia in later life.
The role of vitamin D in brain health and cognition is emerging with reports of lower serum 25-hydroxyvitamin D (25(OH) D) levels in those with impaired cognitive function and AD than in healthy controls (29, 30). Moreover, low vitamin D levels increased AD risk 7 years later (31). In an 18-week study which compared high-dose vitamin D3 (4,000 IU/day) to a low dose supplement (400 IU/day) in healthy adults, the high-dose supplementation improved only visuospatial memory and no other cognitive domain (32). In a review by Annweiler et al. (33) executive dysfunction was predicted by lower serum 25(OH) D concentrations, while its association with episodic memory was inconclusive (33). A meta-analysis observed cognitive impairment in patients deficient in vitamin D (34). A systematic review by van der Schaft et al. (35) reported that poor outcome in various cognitive tests was linked to a higher dementia incidence risk in patients with low 25 (OH) D levels. Hypovitaminosis D was also associated with a subjective cognitive complaint that predicts cognitive decline and dementia (36).
While many studies show that the intake of antioxidant vitamins (E and C) results in reduced risk of cognitive decline, mixed results are reported. The use of vitamin E and C supplements resulted in a reduced risk of cognitive decline in a prospective cohort in Canada (37). Studies also reported no association (38) or inverse association (39) between antioxidants and cognition. A cross-sectional United States-based study associated a high vitamin E intake with a higher score on verbal memory, immediate recall, and better language/verbal fluency performance (40). A cross-sectional study by Chouet et al. (41) on 192 French older adults reported that higher dietary phylloquinone (vitamin K) was associated with better cognition related behavior among older adults.
Iron is essential for transport of oxygen to all the body’s organs including the brain, as it is a constituent of hemoglobin. Iron deficiency anemia (IDA) is a risk factor for short and long-term cognitive impairment. IDA is associated with poor mental and motor development in infancy and with poor cognition and school achievement during later childhood (8). There is a consensus that prevention is preferable to treatment of iron deficiency and that it is better to protect the brain from suboptimal iron status at an early stage, e.g., in the prenatal period and early infancy (21). Iron deficiency in the brain is associated with disordered neurophysiological mechanisms and hence, compromise motor and cognitive development (e.g., impaired coordination, executive function, attention, and memory) (42).
On the other hand, iron overload in the brain also impairs neurophysiological mechanisms (e.g., enhanced oxidative stress, neuronal cell death) and is associated with decline in motor and cognitive functions (e.g., slow motor function, altered feedback processing and sensitivity, memory loss, and impaired decision-making) (43).
3.1.3. Dietary patterns and food groups
Nutritional components, as well as diet as a composite, affects brain maintenance and function. Healthy diets may protect against dementia and mild cognitive impairment. Smyth et al. (42) have reiterated that a higher intake of healthy foods is a powerful potential method for reducing the global burden of cognitive decline. Wright et al. (44) demonstrated that better cognitive performance, particularly in verbal retention and memory is associated with a higher diet quality irrespective of race and poverty status. There is evidence to support the ‘whole diet approach’ theory, i.e., a balanced diet, as a whole, rather than single nutrients is beneficial for brain health. Specific dietary patterns that may prove more valuable than consuming individual food/food groups include the Mediterranean diet, the Nordic Diet, and the DASH Diet.
The Mediterranean diet refers to the diet of people in Greece, Spain, France, Italy, Egypt, Algeria, and Libya. The key features of this diet are that unrefined carbohydrates and starches are consumed in large amounts along with cheese, yoghurt, fruits, and vegetables. Chicken, fish, and eggs are consumed a few times in a week, while red meat intake is not more than a few times in a month. Fat content varies from 28 to 40 percent, mainly from an unsaturated source, olive oil (45). Studies have shown that this diet is linked to a low risk of cognitive decline (46–48), lower prevalence of dementia, depression, (49, 50) and reduced risk of Alzheimer’s disease (48). A PREDIMED sub-study assessed cognitive performance at baseline and after 4 years. There was improvement in cognitive function in subjects on the Mediterranean diet and a decline in cognitive function among those on the control diet (51). There is evidence of a moderate protective effect of the Mediterranean diet against cognitive decline and Alzheimer’s disease based on large longitudinal observational studies (48, 52, 53). Another systematic review by van de Rest et al. (54) reported that greater adherence to a Mediterranean diet is associated with lesser cognitive decline, dementia, or Alzheimer’s disease, as evident in several cross-sectional, longitudinal studies, trials, and meta-analyses.
The Nordic Diet is based on the types of food consumed in Scandinavian countries (55). The emphasis is on foods and nutrients such as fruits and vegetables, fish, canola oil, and several types of meat. A 4-year study to examine the associations of Nordic Diet with cognitive function was conducted on 1,140 men and women with normal cognition. It revealed that subjects who followed the guidelines of the Nordic Diet had enhanced levels of cognitive functioning compared to baseline (56).
The Dietary Approaches to Stop Hypertension (DASH) Diet is characterized by low sodium content and small portion sizes, which have significant health benefits. The DASH diet improved cardiovascular risk factors and had greater beneficial effects in subjects with an increased cardio metabolic risk (57). MIND or Mediterranean-Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay includes specific guidelines beneficial for brain health. The foods included in the MIND diet are antioxidant-rich to enhance cognition; green leafy vegetables to prevent cognitive decline (55), and blueberries to improve memory (58–60) fish to help maintain cognitive function due to high amounts of EPA and DHA present (61). More research is warranted to establish the role of this diet in maintaining brain health.
Asian plant-based dietary patterns are based on foods like whole grains, soy, green leafy and other vegetables, green tea, mushrooms, and seaweed. Evidence strongly suggests that these dietary patterns are associated with a lower risk of cognitive impairment, a slower rate of cognitive decline and better logical memory or higher global cognitive assessment scores (62).
According to van de Rest et al. (57) other healthy dietary patterns derived both a priori (e.g., Healthy Diet Indicator and Healthy Eating Index) and a posteriori (e.g., factor analysis, cluster analysis, and reduced rank regression), were also associated with reduced cognitive decline and/or a reduced risk of dementia (54).
3.1.4. Importance of breakfast
Literature has addressed the effects of consuming breakfast on cognition. Breakfast composition has a profound impact on multiple cognitive domains: attention capacity (63); processing speed (64); working memory (65); immediate recall, delayed recall, recognition (66). Adolphus et al. (67) reported that breakfast consumption in children and adolescents (4–18 years), compared to fasting, had a short-term (same morning) positive effect on cognition tasks requiring attention, executive function, and memory. In adults over 18 years, breakfast consumption showed a small but robust advantage for memory (particularly delayed recall), but the effect on attention and motor and executive function was not well established. No effects of breakfast on language were evident (68).
3.1.5. Food group intake
The role of different food groups on brain function and cognitive decline is well documented. Consumption of refined cereals and grains was associated with worse cognitive function and decline (69), while unrefined cereals and whole-grain consumption was associated with better cognitive function (47, 70, 71). An inverse relationship was observed between refined carbohydrate intake and non-verbal intelligence (72). As seen in both cross-sectional and longitudinal studies, a positive association was noted between fish intake and cognition (51, 69, 73, 74). Fish consumption lowered the risk of cognitive decline, MCI, and dementia (69, 70, 73, 74). One study reported better attention, visual memory, episodic verbal memory, working memory and executive function in both adults and older adults who consumed fish, but worse cognitive and executive function was reported in subjects on the consumption of red meat (74). Dairy consumption (high-fat milk) was associated with worse cognitive function (74) and cognitive decline (69), while no association was seen with cheese (75) and ice-cream intake (69). In addition, foods like avocados (76), berries (60) and extra-virgin olive oil (77) were associated with the delay of cognitive decline (78).
In cross-sectional as well as longitudinal studies, better cognitive function and lesser cognitive decline were seen in subjects who consumed plant-based foods (47, 73–75) olive oil (51, 74, 76) legumes (71) and walnuts (72, 74,78) Green leafy vegetable consumption did not lower the risk of cognitive impairment (75). Fruits, berries, potatoes and vegetable consumption were not associated with better cognitive function (51, 70, 74, 77, 78). The effects of a plant-based diet reviewed by Medawar et al. (79) reported the impact on cognition/cognitive processes, brain activity for language and empathy-related tasks, emotional health, and personality traits. Rajaram et al. (62) also reported that consumption of citrus fruits, grapes, berries, nuts, green tea, cocoa and coffee improved specific cognitive domains, especially the executive functions. However, they established no causal relationship between the use of a plant-based diet and its putative effects on cognitive, mental, and neurological functions. Participants in the Mediterranean diet plus nuts group improved in the memory composite compared to the control diet group (80). Two observational studies, the Doetinchem Cohort (81) and the Nurses’ Health Study (82), reported that long-term nut consumption was related to better overall cognition at an older age but not to cognitive decline during follow-up for 5 to 6 years.
Alcohol intake was not associated with cognitive function (70, 73, 74, 80, 81) except better global cognition was associated with wine intake in community-dwelling residents of Spain (51) No association with cognitive decline was seen with the intake of spirits/beers in the longitudinal study by Shakersain et al. (69). No association with cognitive decline or impairment was observed in longitudinal studies conducted on the intake of sugar/fruit juices (69), sweetened beverages, sodium intake (71), processed and fast/fried food, and sweets and pastries (75), animal-based cooking fat (83).
A systematic review to evaluate the impact of healthy diet consumption among children and adolescents on executive functioning reported that among the ten studies examined, there was a positive association between healthier foods (e.g., whole grains, fish, fruits, and vegetables) and executive function (84). In contrast, intake of less-healthy snack foods, sugar-sweetened beverages and red/processed meats were associated with poor executive functioning.
Certain herbs that may prove beneficial in enhancing cognition or delaying cognitive decline include Ashwagandha, turmeric, Brahmi etc. In a systematic review, the extract of Ashwagandha (Indian Ginseng/Winter Cherry) corrected mild cognitive impairment and enhanced executive functions in adults with MCI (85). Curcumin in turmeric reduces oxidative damage and improves cognitive functions related to senescence. It also binds with β-amyloid plaques, inhibits its aggregation, and is beneficial in Alzheimer’s disease. An RCT demonstrated that 400 mg/day of curcumin improved sustained attention and working memory functions in adults over 60 (86). Brahmi (waterhyssop/thyme-leafed gratiola/Indian pennywort) is commonly used as a memory enhancer.
3.1.6. Other dietary components
Polyphenols are secondary metabolites of plants and comprise flavonoids, lignans, stilbenes, coumarins and tannins. They are present abundantly in colorful fruits (berries, grapes, and tomatoes), vegetables, tea, spices, herbs, and olive oil. They contribute to brain health similar to antioxidants by regulating oxidative stress and mediating anti-inflammatory mechanisms (87). The extensively studied group in relation to brain health, under polyphenols is flavonoids. There is evidence for established associations between flavonoids and delayed cognitive decline (88), and enhanced language and verbal memory tasks (46). Cocoa flavonoids (in dark chocolate) enhance cognition (87, 88) however, a study on participants with cognitive impairment involving cocoa flavonoids was inconclusive (89). An RCT conducted on healthy adults aged 50–69, reported that a high cocoa flavanol-containing diet enhanced gyrus function after 3 months (90) and was associated with a 41% lower risk of cognitive decline (91).
In the review by Jirout et al., (10) high levels of carotenoids present in leafy vegetables were associated with higher scores on tests in the visual–spatial domain. Lutein, one of the three major dietary carotenoids present in the brain (92), is of functional importance to cognition and infant brain development. It is also associated with density of the macular pigment which interacts with cognitive functioning (93). Interestingly, lutein concentration was higher in the brains of children than in adults and was clearly related to cognition, i.e., executive function, language, learning, and memory (93) and improved the speed of temporal processing in young adults (94).
There are limited studies to elucidate the role of caffeine in memory and cognition enhancement. High serum levels of caffeine delayed dementia progression in a case–control study on 124 older persons with MCI (95). However, another study did not find any association with risk of cognitive impairment, overall dementia, or AD (96). Lesser cognitive decline among coffee consumers was seen in a longitudinal study but this decline was not dose related (97).
Soy isoflavones include genistein and diadzein, and their effects on cognition are variable and inconclusive (98), with an overall absence of adverse events (99). An initial positive effect in adults appears to reverse in older women; in men, the data are even more inconclusive (98).
In the Doetinchem Cohort Study, which included 2,613 participants 43–70 years old, higher consumption of allium (onion, garlic, and leek) was associated with poorer scores on cognitive flexibility and speed of cognitive processes in the cross-sectional analyses. However, in the longitudinal data, allium consumption was not seen to be associated with cognitive decline (81).
3.2. Microbiome-gut-brain Axis
According to Korecka and Arulampalam (100) the term “gut microbiome” refers to “the complex ecosystem of bacteria that colonize the gut, including their genes, proteins, and metabolites” (101). Although evidence on how the gut microbiome may modulate brain development is less, immune signaling is likely to play a critical role (102). The benefits of human-microbe symbiosis are now known to extend to human mental health, with growing evidence that the gut–brain axis plays a crucial role in maintaining brain health via bidirectional communication between the microbes and the brain (101, 102). It also affects human behavior and the pathophysiology of mental illnesses (101, 103).
The two-way interaction between the gut and the brain, i.e., the microbiome-gut-brain axis is now well recognized. Emerging evidence has revealed the importance of the gut microbiome in this bidirectional communication system, i.e., enabling the gut microbes to communicate with the brain and the brain with the gut (102).
The gut microbiome is highly influenced by negative external lifestyle factors, such as poor diet, sleep deprivation, circadian rhythm disturbances, chronic noise and sedentary behavior, which are also important risk factors for the development of Alzheimer’s and other non-communicable diseases (104). Evidence for the beneficial effects of dietary fibers and probiotics through the management of gut microbes is strongly emerging. Several published research have shown the effect of intestinal dysbiosis caused by dietary changes, the use of antibiotics, non-steroidal anti-inflammatory drugs, and the presence of pathogenic microorganisms on the brain’s cognitive functions (104).
Immune cells, cytokines, and chemokines are the microbiome’s mechanisms for interacting with the brain, regulating brain processes and vice versa (99, 105). The gut microbiota may modulate brain function and development through such immune signaling as well as endocrine and neural pathways. Nutritional components may influence each communication pathway (102). Conversely, the brain may impact the gut through neurotransmitters that affect immune function and alter cortisol levels, intestinal motility, and permeability. The gut microbiota changes dynamically across the lifespan, establishing their relationship with the host at critical periods during infancy, adolescence, and ageing. There is an increased vulnerability to external insults at these time windows, resulting in greater susceptibility to brain disorders. Disturbances of the developing gut microbiota early in life can impact neurodevelopment significantly and lead to adverse mental health outcomes later in life.
Similarly, the microbiota may contribute to ageing and the developmental course of neurodegenerative disorders The gut microbiota also regulates key central neurotransmitters by altering levels of precursors—the inhibitory neurotransmitter γ-aminobutyric acid is produced by Lactobacillus and Bifidobacterium species; noradrenaline (norepinephrine) is produced by Escherichia, Bacillus and Saccharomyces spp.; Candida, Streptococcus, Escherichia, and Enterococcus spp. produce serotonin; Bacillus produce dopamine, and certain Lactobacillus spp. can produce acetylcholine (106, 107). These microbially synthesized neurotransmitters can cross the intestines mucosal layer and possibly mediate brain physiological events (106). Short-chain fatty acids, including propionate, butyrate and acetate, are metabolic products of gut microbial activity and may exert central effects directly or indirectly (108–110).
Majority of the evidence for the involvement of the gut microbiota in cognition is provided by animal experiments on induced infections (111) antibiotic and dietary manipulations (112, 113). and probiotic interventions (114).
Dietary Recommendations for Brain Health*
• A Mediterranean-like healthy diet which contains fruits, vegetables, legumes (e.g., lentils, beans), nuts and whole grains (e.g., unrefined maize, millet, oats, wheat, brown rice).
• At least 400 g (five portions) of fruits and vegetables daily. Potatoes, sweet potatoes, and other starchy root vegetables are not included in this category of fruits or vegetables.
• Less than 10% of total energy intake from free sugars. This is equivalent to 50 g (or 10 teaspoons) for a person of healthy body weight consuming approximately 2000 calories per day. Ideally consume less than 5% of total energy intake (i.e., 25 g) for additional health benefits. Most free sugars are added to foods or drinks by the manufacturer, cook or consumer, and can also be found naturally present in honey, syrups, fruit juices and fruit juice concentrates.
• Less than 30% of total energy intake from fats. Unsaturated fats (in fish, avocado, nuts, sunflower, canola and olive oils) are preferable to saturated fats (fatty meat, butter, palm and coconut oil, cream, cheese, ghee) and trans-fats of all kinds, including industrially produced trans-fats (in processed food, fried foods, cookies, biscuits, wafers, margarines and spreads) and ruminant trans-fats (in meat and dairy foods from ruminant animals, such as cows, sheep, goats, and others). Reduce the intake of saturated fats to less than 10% of total energy intake and trans-fats to less than 1% of total energy intake.
• Less than 5 g of iodized salt (approximately 1 teaspoon) per day.
[Source: (115).]
*For Adults with normal cognition or MCI. Several studies have reported improvements in cognitive function on the administration of probiotics, particularly in those with MCI (116). However, Louzada et al. (117) reported no effect on cognition on the administration of a symbiotic.
3.3. Nutritional interventions to reduce risk of cognitive decline and dementia
World Health Organization (115) has given detailed guidelines for reducing cognitive decline and dementia risk. The dietary approaches associated with better cognitive function include the Mediterranean diet (52, 118). Dietary Approaches to Stop Hypertension (DASH) (55) and the brain health-specific Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet. WHO’s recommendations for the diet are based on the Mediterranean-like diet.
There is a significant association between excess fat mass and cognitive impairment. Therefore, WHO (115) has outlined recommendations for weight management, including dietary advice and physical activity. Stress is also laid on the appropriate management of hypertension, dyslipidemia and diabetes.
Multiple factors affect brain health from conception till old age: modifiable and non-modifiable. The modifiable factors include diet, physical activity, social engagement, cognitive activity, smoking and alcohol consumption. Strategies to promote brain health throughout life should target individuals at each phase of life to adopt a healthy lifestyle (diet and physical activity), be engaged in cognitively stimulating activities and be socially active.
4. Conclusion
Good brain health enables an individual to comprehend their abilities and adjust their cognitive, psychological, emotional, and behavioral functioning according to various life events to cope optimally.
In addition to generalized macronutrient under-nutrition, individual nutrient deficiencies may substantially affect brain development and subsequent cognitive health. Several micronutrients like B group vitamins and iron play a crucial role in cognitive health. High protein and low-fat diets are protective against cognitive decline.
A salient potential approach for lowering the global burden of cognitive decline is to ensure a higher diet quality by ensuring a higher intake of healthy foods. Evidence supports the ‘whole diet approach’ theory that a balanced diet, rather than single nutrients, benefits cognitive health. Mediterranean, Nordic, DASH, and MIND diets are linked with a low risk of cognitive decline and dementia. A balanced diet should be encouraged via nutrition counseling in early adult life and regular physical activity to promote a healthy lifestyle.
In recent years, considerable information is available on the relationship between the gut microbiome and brain function through the gut-brain axis. The gut microbiome is highly influenced by negative external lifestyle factors, such as poor diet, sleep deprivation, circadian rhythm disturbances, chronic noise, and sedentary behavior, which are also important risk factors for the development of NCDs and Alzheimer’s. Data on the beneficial effects of dietary fibers and probiotics through the management of gut microbes is strongly emerging.
The role of nutrition and lifestyle factors in cognitive development, function and decline is an area garnering increasing attention in recent years. Several decades ago, some pioneering research was carried out to establish brain growth patterns and the influence of genetic and environmental factors on these. However, there is a dire need to examine the effect of the epidemiological and societal transition on cognitive health.
Moreover, the factors that have been derived are based on observational studies, and unidentified confounders may bias the results. These studies often lack consistency and detail in the description or categorization of lifestyle factors and sometimes in the measured cognitive outcomes. Longer duration longitudinal studies or cohorts are needed to get better insights into the lifestyle factors that affect cognition.
While a significant number of research are now focusing on old age, the period of adulthood is overlooked. Young adulthood and middle age are crucial periods for determining cognitive health in old age. Moreover, many existing studies are cross-sectional, so getting a life course perspective is difficult. It is therefore vital to conduct large longitudinal studies and studies established cohorts to examine the influence of environmental exposures on early life brain development and cognitive health through the life cycle, including adulthood.
Unlike non-communicable diseases like cardiovascular disorders, diabetes and cancer, there is a dearth of epidemiological data on cognitive impairment and dementias. Large national surveys should include these disorders to estimate their prevalence and related epidemiological data. In India, the Longitudinal Ageing Study in India (119), conducted by the International Institute of Population Studies and facilitated by the Ministry of Health and Family Welfare, did capture the reported prevalence of Alzheimer’s disease among 44 to 99-year-olds as only 0.4%. However, these were previously diagnosed cases, so there is a need to include screening and assessment for cognitive dysfunction to detect undiagnosed cases in such surveys.
Several countries have now acknowledged the importance of focusing on declining cognitive health among older adults and the need for preventive strategies targeting dementia. The importance of cardio-metabolic risk factors that develop in middle age, such as obesity and hypertension as well as smoking and physical inactivity, suggest that preventive approaches are required for target populations in their 40s and 50s, much before they develop dementia. The clear overlap with cardiovascular and diabetes risk suggests that dementia should be included in current non-communicable disease (NCD) management programs at primary health care level as well as broader public health programs.
The challenge for nutritionists is integrating existing scientific knowledge, and further advancing applied research on effective ways to attain and maintain optimal cognitive function throughout life. Of potentially far-reaching consequences is the concept that early life nutrition may program metabolic functions, leading over time to an increasing imbalance and promoting the emergence of disease states. A dietary approach as well as macronutrient and micronutrient intake that has protective effects against CVD is most likely to be effective against neurodegenerative disorders too.
Author contributions
SP and MS were responsible for the conceptualization of the manuscript, compiling the data, and writing the manuscript. SP and BG were responsible for reviewing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors acknowledge the financial support from ILSI India for compiling and publishing of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hendrie, HC, Albert, MS, Butters, MA, Gao, S, Knopman, DS, Launer, LJ, et al. The NIH cognitive and emotional health project. Report of the critical evaluation Study committee. Alzheimers Dement. (2006) 2:12–32. doi: 10.1016/j.jalz.2005.11.004
2. Centers for Disease Control and Prevention and the Alzheimer’s Association. The Healthy Brain Initiative: A National Public Health Road Map to Maintaining Cognitive Health. Chicago, IL: Alzheimer’s Association. (2007) Available at: www.cdc.gov/aging and www.alz.org
3. Anstey, KJ, Cherbuin, N, and Herath, PM. Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prev Sci. (2013) 14:411–21. doi: 10.1007/s11121-012-0313-2
4. Clare, L, Wu, Y-T, Teale, JC, Macleod, C, Matthews, F, Brayne, C, et al. Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: a cross-sectional study. PLoS Med. (2017) 14:e1002259. doi: 10.1371/journal.pmed.1002259
5. Lebel, C, Gee, M, Camicioli, R, Wieler, M, and Martin, WBC. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. (2012) 60:340–52. doi: 10.1016/j.neuroimage.2011.11.094
6. Miguel, PM, Pereira, LO, Silveira, PP, and Meaney, MJ. Early environmental influences on the development of children’s brain structure and function. Dev Med Child Neurol. (2019) 61:1127–33. doi: 10.1111/dmcn.14182
7. National Institutes of Health (NIH) (2011). Iodine: fact sheet for health professionals. Retrieved from: https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional
8. Prado, EL, and Dewey, KG. Nutrition and brain development in early life. Nutr Rev. (2014) 72:267–84. doi: 10.1111/nure.12102
9. Victora, CG, Horta, BL, de Mola, CL, Quevedo, L, Pinheiro, RT, Gigante, DP, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. (2015) 3:e199–205. doi: 10.1016/S2214-109X(15)70002-1
10. Jirout, J, LoCasale-Crouch, J, Turnbull, K, Gu, Y, Cubides, M, Garzione, S, et al. How lifestyle factors affect cognitive and executive function and the ability to learn in children. Nutrients. (2019) 11:1–29. doi: 10.3390/nu11081953
11. Black, MM, Walker, SP, Fernald, LCH, Andersen, CT, DiGirolamo, AM, Lu, C, et al. Advancing early childhood development: from science through the life course. Lancet. (2017) 389:77–90. doi: 10.1016/S0140-6736(16)31389-7
12. Goyal, MS, and Iannotti, LLRME. Brain nutrition: a life span approach. Annu Rev Nutr. (2018) 38:381–99. doi: 10.1146/annurev-nutr-082117-051652
13. Li, Y, Li, S, and Wang, WZD. Association between dietary protein intake and cognitive function in adults aged 60 years and older. J Nutr Health Aging. (2020) 24:223–9. doi: 10.1007/s12603-020-1317-4
14. O’Donnell, CA, Manera, V, and Köhler, SIK. Promoting modifiable risk factors for dementia: is there a role for general practice? Br J Gen Pract. (2015) 65:567–8. doi: 10.3399/bjgp15X687241
15. Coelho-Júnior, HJ, Calvani, R, Landi, F, Picca, A, and Marzetti, E. Protein intake and cognitive function in older adults: a systematic review and meta-analysis. Nutr Metab Insights. (2021) 14:117863882110223. doi: 10.1177/11786388211022373
16. Okereke, OI, Rosner, BA, Kim, DH, Kang, JH, Cook, NR, Manson, JE, et al. Dietary fat types and 4-year cognitive change in community-dwelling older women. Ann Neurol. (2013) 72:124–34. doi: 10.1002/ana.23593.Dietary
17. Francis, HSR. The longer-term impacts of Western diet on human cognition and the brain. Appetite. (2013) 63:119–28. doi: 10.1016/j.appet.2012.12.018
18. Spencer, SJ, Korosi, A, Layé, S, Shukitt-Hale, B, and Barrientos, RM. Food for thought: how nutrition impacts cognition and emotion. NPJ Sci Food. (2017) 1:7. doi: 10.1038/s41538-017-0008-y
19. Barbagallo, M. Type 2 diabetes mellitus and Alzheimer’s disease. World J Diabetes. (2014) 5:889–93. doi: 10.4239/wjd.v5.i6.889
20. Bazinet, RPLS. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. (2014) 15:771–85. doi: 10.1038/nrn3820
21. Cusick, SE, and Georgieff, MK. The role of nutrition in brain development: the Golden opportunity of the first 1000 days brain development in late fetal and early postnatal life. J Pediatr. (2016) 175:16–21. doi: 10.1016/j.jpeds.2016.05.013.The
22. Gillette-Guyonnet, S, Secher, M, and Vellas, B. Nutrition and neurodegeneration: epidemiological evidence and challenges for future research. Br J Clin Pharmacol. (2013) 75:738–55. doi: 10.1111/bcp.12058
23. Ding, B, Xiao, R, Ma, W, Zhao, L, Bi, Y, and Zhang, Y. The association between macronutrient intake and cognition in individuals aged under 65 in China: a cross-sectional study. BMJ Open. (2018) 8:1–8. doi: 10.1136/bmjopen-2017-018573
24. World Health Organization (WHO) (2021). Ageing and health. Available at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed on 11th October 2021.
25. Venkatramanan, S, Armata, IE, Strupp, BJ, and Finkelstein, JL. Vitamin B-12 and cognition in children 1–3. Adv Nutr. (2016) 7:879–88. doi: 10.3945/an.115.012021
26. Agnew-Blais, JC, Wassertheil-Smoller, S, Kang, JH, Hogan, PE, Coker, LH, Snetselaar, LG, et al. NIH public access. Bone. (2008) 115:231–41. doi: 10.1016/j.jand.2014.07.006
27. Dangour, AD, Allen, E, Clarke, R, Elbourne, D, Fletcher, AE, Letley, L, et al. Effects of vitamin B-12 supplementation on neurologic and cognitive function in older people: a randomized controlled trial 1, 2. Am J Clin Nutr. (2015) 102:639–47. doi: 10.3945/ajcn.115.110775
28. Doets, EL, In’t Veld, PH, Szczecińska, A, RAM, D-R, Cavelaars, AEJM, Van’t Veer, P, et al. Systematic review on daily Vitamin B12 losses and bioavailability for deriving recommendations on Vitamin B12 intake with the factorial approach. Ann Nutr Metab. (2013) 62:311–22. doi: 10.1159/000346968
29. Goodwill, AMSCA. Systematic review and meta-analysis of the effect of low Vitamin D on cognition. J Am Geriatr Soc. (2017) 65:2161–8. doi: 10.1111/jgs.15012
30. Afzal, S, and Bojesen, SENBG. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. 2014 may; 10(3):296-302. Alzheimers Dement. (2014) 10:296–302. doi: 10.1016/j.jalz.2013.05.1765
31. Annweiler, C, Rolland, Y, Schott, AM, Blain, H, and Vellas, BBO. Serum vitamin D deficiency as a predictor of incident non-Alzheimer dementias: a 7-year longitudinal study. Dement Geriatr Cogn Disord. (2011) 32:273–8. doi: 10.1159/000334944
32. Pettersen, JA. Does high dose vitamin D supplementation enhance cognition?: A randomized trial in healthy adults. Exp Gerontol. (2017) 90:90–7. doi: 10.1016/j.exger.2017.01.019
33. Annweiler, C, and Llewellyn, DJBO. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. (2013) 33:659–74. doi: 10.3233/JAD-2012-121432
34. Etgen, T, Sander, D, Bickel, H, and Sander, KFH. Vitamin D deficiency, cognitive impairment and dementia: a systematic review and meta-analysis. Dement Geriatr Cogn Disord. (2012) 33:297–305. doi: 10.1159/000339702
35. van der Schaft, J, Koek, HL, Dijkstra, E, Verhaar, HJ, van der Schouw, YTE-VMH, van der Schaft, J, et al. The association between vitamin D and cognition: a systematic review. Ageing Res Rev. (2013) 12:1013–23. doi: 10.1016/j.arr.2013.05.004
36. Landel, V, Annweiler, C, Millet, P, Morello, M, Féron, F, Wion, D, et al. Cognition and Alzheimer’s disease: the therapeutic benefit is in the D-tails. J Alzheimers Dis. (2016) 53:419–44. doi: 10.3233/JAD-150943
37. Basambombo, LL, Carmichael, PH, and Côté, SLD. Use of Vitamin E and C supplements for the prevention of cognitive decline. Ann Pharmacother. (2017) 51:118–24. doi: 10.1177/1060028016673072
38. Nooyens, AC, Milder, IE, van Gelder, BM, Bueno-de-Mesquita, HB, and van Boxtel, MPVWM. Diet and cognitive decline at middle age: the role of antioxidants. Br J Nutr. (2015) 113:1410–7. doi: 10.1017/S0007114515000720
39. Galasko, DR, Peskind, E, Clark, CM, Quinn, JF, Ringman, JM, Jicha, GA, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. (2012) 69:836–41. doi: 10.1001/archneurol.2012.85
40. Beydoun, MA, Fanelli-Kuczmarski, MT, Kitner-Triolo, MH, Beydoun, HA, Kaufman, JS, Mason, MA, et al. Dietary antioxidant intake and its association with cognitive function in an ethnically diverse sample of US adults. Psychosom Med. (2015) 77:68–82. doi: 10.1097/PSY.0000000000000129
41. Chouet, J, Ferland, G, Féart, C, Rolland, Y, Presse, N, Boucher, K, et al. Dietary vitamin K intake is associated with cognition and behaviour among geriatric patients: the CLIP study. Nutrients. (2015) 7:6739–50. doi: 10.3390/nu7085306
42. Smyth, A, Dehghan, M, Anderson, C, Teo, K, Gao, P, Sleight, P, et al. Healthy eating and reduced risk of cognitive decline. American Acad. Neurol. (2015) 84:2258–65. doi: 10.1212/WNL.0000000000001638
43. Ferreira, A, Neves, P, and Gozzelino, R. Multilevel impacts of iron in the brain: the cross talk between neurophysiological mechanisms, cognition, and social behavior. Pharmaceuticals. (2019) 12:1–26. doi: 10.3390/ph12030126
44. Morgan, M. Rogers-Carter1 2 JAV 2 KBGAFPMTMMR, Christianson1 JP. Diet quality and cognitive function in an urban sample: findings from the healthy aging in neighborhoods of diversity across the life span (HANDLS) study. Physiol Behav. (2017) 20:92–101. doi: 10.1017/S1368980016001361
45. Aridi, YS, Walker, JL, and Wright, ORL. The association between the Mediterranean dietary pattern and cognitive health: a systematic review. Nutrients. (2017) 9:674 doi: 10.3390/nu9070674
46. Kesse-Guyot, E, Andreeva, VA, Lassale, C, Ferry, M, Jeandel, C, Hercberg, S, et al. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. (2013) 97:369–76. doi: 10.3945/ajcn.112.047993
47. Samieri, C, Grodstein, F, Rosner, BA, Kang, JH, Cook, NR, Manson, JE, et al. Mediterranean diet and cognitive function in older age. Epidemiology. (2013) 24:490–9. doi: 10.1097/EDE.0b013e318294a065
48. Lourida, I, Soni, M, Thompson-Coon, J, Purandare, N, Lang, IA, and Ukoumunne, OCLDJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. (2013) 24:479–89. doi: 10.1097/EDE.0b013e3182944410
49. Solfrizzi, VPF. Mediterranean diet and cognitive decline. A lesson from the whole-diet approach: what challenges lie ahead? J Alzheimers Dis. (2014) 39:283–6. doi: 10.3233/JAD-130831
50. Woodside, JV, Gallagher, NE, and Neville, CEMMC. Mediterranean diet interventions to prevent cognitive decline--opportunities and challenges. Eur J Clin Nutr. (2014) 68:1241–4. doi: 10.1038/ejcn.2014.178, Epub 2014 Sep 3
51. Valls-Pedret, C, Lamuela-Raventós, RM, Medina-Remón, A, Quintana, M, Corella, D, Pintó, X, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. (2012) 29:773–82. doi: 10.3233/JAD-2012-111799
52. Fiatarone Singh, MA, Gates, N, Saigal, N, Wilson, GC, Meiklejohn, J, Brodaty, H, et al. The Study of mental and resistance training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J Am Med Dir Assoc. (2014) 15:873–80. doi: 10.1016/j.jamda.2014.09.010
53. Psaltopoulou, T, Sergentanis, TN, Panagiotakos, DB, Sergentanis, IN, and Kosti, RSN. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. (2013) 74:580–91. doi: 10.1002/ana.23944
54. van de Rest, O, Berendsen, AAM, Haveman-Nies, A, and de Groot, LCPGM. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutrition. (2015) 6:154–68. doi: 10.3945/an.114.007617
55. Morris, MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. (2017) 1367:31–7. doi: 10.1111/nyas.13047
56. Männikkö, R, Komulainen, P, Schwab, U, Heikkilä, HM, Savonen, K, Hassinen, M, et al. The Nordic diet and cognition--the DR’s EXTRA Study. Br J Nutr. (2015) 114:231–9. doi: 10.1017/S0007114515001890
57. Siervo, M, Lara, J, Chowdhury, S, Ashor, A, and Oggioni, CMJC. Effects of the dietary approach to stop hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. (2014) 113:1–15. doi: 10.1017/S0007114514003341
58. Boespflug, EL, Eliassen, JC, Dudley, JA, Shidler, MD, Kalt, W, Summer, SS, et al. In mild cognitive impairment. Nutr Neurosci. (2018) 21:297–305. doi: 10.1080/1028415X.2017.1287833.Enhanced
59. Nilsson, A, Salo, I, Plaza, M, and Björck, I. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; a randomized cross-over study in healthy older adults. PLoS One. (2017) 12:e0188173–22. doi: 10.1371/journal.pone.0188173
60. Whyte, AR, Cheng, N, Fromentin, E, and Williams, CM. A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (Thinkblue™) in maintenance of episodic and working memory in older adults. Nutrients. (2018) 10:660. doi: 10.3390/nu10060660
61. Ghasemi Fard, S, Wang, F, Sinclair, AJ, Elliott, G, and Turchini, GM. How does high DHA fish oil affect health? A systematic review of evidence. Crit Rev Food Sci Nutr. (2019) 59:1684–727. doi: 10.1080/10408398.2018.1425978
62. Rajaram, S, Jones, J, and Lee, GJ. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv Nutr. (2019) 10:S422–36. doi: 10.1093/advances/nmz081
63. An, YJ, Jung, KY, Kim, SM, and Lee, CKDW. Effects of blood glucose levels on resting-state EEG and attention in healthy volunteers. J Clin Neurophysiol. (2015) 32:51–6. doi: 10.1097/WNP.0000000000000119
64. Jones, EK, and Sünram-Lea, SIWK. Acute ingestion of different macronutrients differentially enhances aspects of memory and attention in healthy young adults. Biol Psychol. (2012) 89:477–86. doi: 10.1016/j.biopsycho.2011.12.017
65. Owen, L, Scholey, AB, Finnegan, Y, and Hu, HS-LS. The effect of glucose dose and fasting interval on cognitive function: a double-blind, placebo-controlled, six-way crossover study. Psychopharmacology. (2012) 220:577–89. doi: 10.1007/s00213-011-2510-2
66. Sünram-Lea, SI, Owen, L, and Finnegan, YHH. Dose-response investigation into glucose facilitation of memory performance and mood in healthy young adults. J Psychopharmacol. (2011) 25:1076–87. doi: 10.1177/0269881110367725
67. Adolphus, K, Lawton, CL, Claire, L, and Champ 2, LD. The effects of breakfast and breakfast composition on cognition in adults. Adv Nutrition. (2016) 7:576S–89S. doi: 10.3945/an.115.010231
68. Galioto, R, and Spitznagel, MB. The effects of breakfast and breakfast composition on cognition in adults. Adv Nutr. (2016) 7:576S–89S. doi: 10.3945/an.115.010231
69. Shakersain, B, Rizzuto, D, Larsson, SC, Faxén-Irving, G, Fratiglioni, L, and Xu, WL. The nordic prudent diet reduces risk of cognitive decline in the Swedish older adults: a population-based cohort study. Nutrients. (2018) 10:229. doi: 10.3390/nu10020229
70. Anastasiou, CA, Yannakoulia, M, Kosmidis, MH, Dardiotis, E, Hadjigeorgiou, GM, Sakka, P, et al. Mediterranean diet and cognitive health: initial results from the Hellenic longitudinal investigation of ageing and diet. PLoS One. (2017) 12:e0182048–18. doi: 10.1371/journal.pone.0182048
71. Wengreen, H, Munger, RG, Cutler, A, Quach, A, Bowles, A, Corcoran, C, et al. Prospective study of dietary approaches to stop hypertension-and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on memory, health and aging. Am J Clin Nutr. (2013) 98:1263–71. doi: 10.3945/ajcn.112.051276
72. Abargouei, AS, Kalantari, N, Omidvar, N, Rashidkhani, B, Rad, AH, Ebrahimi, AA, et al. Refined carbohydrate intake in relation to non-verbal intelligence among Tehrani schoolchildren. Public Health Nutr. (2012) 15:1925–31. doi: 10.1017/S1368980011003302
73. Bhushan, A, Fondell, E, Ascherio, A, Yuan, C, and Grodstein, FWW. Adherence to Mediterranean diet and subjective cognitive function in men. Eur J Epidemiol. (2018) 33:223–34. doi: 10.1007/s10654-017-0330-3
74. Bajerska, J, Woźniewicz, M, and Suwalska, AJJ. Eating patterns are associated with cognitive function in the elderly at risk of metabolic syndrome from rural areas. Eur Rev Med Pharmacol Sci. (2014) 18:3234–45.
75. Hosking, DE, Eramudugolla, R, and Cherbuin, NAKJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. (2019) 15:581–9. doi: 10.1016/j.jalz.2018.12.011
76. Scott, TM, Rasmussen, HM, Chen, O, and Johnson, EJ. Avocado consumption increases macular pigment density in older adults: a randomized, controlled trial. Nutrients. (2017) 9:919. doi: 10.3390/nu9090919
77. Klimova, B, Novotný, M, Kuca, K, and Valis, M. Effect of an extra-virgin olive oil intake on the delay of cognitive decline: role of secoiridoid oleuropein? Neuropsychiatr Dis Treat. (2019) 15:3033–40. doi: 10.2147/NDT.S218238
78. Klimova, B, Dziuba, S, and Cierniak-Emerych, A. The effect of healthy diet on cognitive performance among healthy seniors–a mini review. Front Hum Neurosci. (2020) 14:1–9. doi: 10.3389/fnhum.2020.00325
79. Medawar, E, Huhn, S, Villringer, A, and Veronica Witte, A. The effects of plant-based diets on the body and the brain: a systematic review. Transl Psychiatry. (2019) 9:226. doi: 10.1038/s41398-019-0552-0
80. Valls-Pedret, C, Sala-Vila, A, Serra-Mir, M, Corella, D, de la Torre, R, Martínez-González, MÁ, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. (2015) 175:1094–103. doi: 10.1001/jamainternmed.2015.1668
81. Nooyens, AC, Bueno-de-Mesquita, HB, van Boxtel, MP, van Gelder, BM, and Verhagen, HVWM. Fruit and vegetable intake and cognitive decline in middle-aged men and women: the Doetinchem cohort Study. Br J Nutr. (2011) 106:752–61. doi: 10.1017/S0007114511001024
82. O’Brien, J, Okereke, O, Devore, E, Rosner, B, Breteler, M, and Grodstein, F. Long-term intake of nuts in relation to cognitive function in older women. J Nutr Health Aging. (2014) 18:496–502. doi: 10.1007/s12603-014-0014-6
83. Qin, B, Adair, LS, Plassman, BL, Batis, C, Edwards, LJ, and Popkin, BMMMA. Dietary patterns and cognitive decline among Chinese older adults. Physiol Behav. (2017) 176:139–48. doi: 10.1097/EDE.0000000000000338.Dietary
84. Cohen, JF, Gorski, MT, Gruber, SA, and Kurdziel, LBREB. The effect of healthy dietary consumption on executive cognitive functioning in children and adolescents: a systematic review. Br J Nutr. (2016) 116:989–1000. doi: 10.1017/S0007114516002877
85. Ng, QX, Loke, W, Foo, NX, Tan, WJ, Chan, HW, and DYYWS, L. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother Res. (2020) 34:583–90. doi: 10.1002/ptr.6552
86. Cox, KH, and Pipingas, ASA. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol. (2015) 29:642–51. doi: 10.1177/0269881114552744
87. Cherniack, EP. A berry thought-provoking idea: the potential role of plant polyphenols in the treatment of age-related cognitive disorders. Br J Nutr. (2012) 108:794–800. doi: 10.1017/S0007114512000669
88. Schaffer, S, and Halliwell, B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. (2012) 7:99–109. doi: 10.1007/s12263-011-0255-5
89. Mintzer, J, Donovan, KA, Kindy, AZ, Lock, SL, Chura, LR, and Barracca, N. Lifestyle choices and brain health. Front Med (Lausanne). (2019) 6:1–11. doi: 10.3389/fmed.2019.00204
90. Adam, MB, Usman, AK, Frank, AP, Lok-Kin, Y, Wendy, S, Hagen, S, et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. (2016) 176:139–48. doi: 10.1038/nn.3850.Enhancing
91. Moreira, A, Diógenes, MJ, de Mendonça, A, and Lunet, NBH. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. (2016) 53:85–93. doi: 10.3233/JAD-160142
92. Mulder, KA, Innis, SM, Rasmussen, BF, Wu, BT, Richardson, KJ, and Hasman, D. Plasma lutein concentrations are related to dietary intake, but unrelated to dietary saturated fat or cognition in young children. J Nutr Sci. (2014) 3:e11–8. doi: 10.1017/jns.2014.10
93. Jia, YP, Sun, L, Yu, HS, Liang, LP, Li, W, Ding, H, et al. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules. (2017) 22:1–22. doi: 10.3390/molecules22040610
94. Lieblein-Boff, JC, Johnson, EJ, Kennedy, AD, Lai, CS, and Kuchan, MJ. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PLoS One. (2015) 10:1–19. doi: 10.1371/journal.pone.0136904
95. Cao, C, Loewenstein, DA, Lin, X, Zhang, C, Wang, L, Duara, R, et al. High blood caffeine levels in MCI linked to lack of progression to dementia. Physiol Behav. (2017) 176:139–48. doi: 10.3233/JAD-2012-111781.High
96. Gelber, RP, Petrovitch, H, Masaki, KH, Ross, GW, and White, LR. Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J Alzheimers Dis. (2011) 23:607–15. doi: 10.3233/JAD-2010-101428
97. Arab, L, Biggs, ML, O’Meara, ES, Longstreth, WT, and Crane ALF, PK. Gender differences in tea, coffee, and cognitive decline in the elderly: the cardiovascular health Study. Bone. (2014) 23:1–7. doi: 10.3233/JAD-2011-110431.Gender
98. Soni, M, Rahardjo, TB, Soekardi, R, Sulistyowati, Y, Lestariningsih, Y-UA, and Irsan, AHE. Phytoestrogens and cognitive function: a review. Maturitas. (2014) 77:209–20. doi: 10.1016/j.maturitas.2013.12.010
99. Alekel, DL, Genschel, U, Koehler, KJ, Hofmann, H, Van Loan, MD, Beer, BS, et al. Soy Isoflavones for reducing bone loss Study: effects of a 3-year trial on hormones, adverse events, and endometrial thickness in postmenopausal women. Menopause. (2015) 22:185–97. doi: 10.1097/GME.0000000000000280
100. Hill, JH, and Claudia Solt, MTF. Obesity associated disease risk: the role of inherent differences and location of adipose depots. Horm Mol biol. Clin Investig. (2018) 16. doi: 10.1515/hmbci-2018-0012
101. Korecka, A, and Arulampalam, V. The gut microbiome: scourge, sentinel or spectator? J Oral Microbiol. (2012) 4:9367. doi: 10.3402/jom.v4i0.9367
102. Keunen, K, Van Elburg, RM, Van Bel, F, and Benders, MJNL. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr Res. (2015) 77:148–55. doi: 10.1038/pr.2014.171
103. Mohajeri, MH, Brummer, RJM, Rastall, RA, Weersma, RK, Harmsen, HJM, Faas, M, et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. (2018) 57:1–14. doi: 10.1007/s00394-018-1703-4
104. Askarova, S, Umbayev, B, Masoud, AR, Kaiyrlykyzy, A, Safarova, Y, Tsoy, A, et al. The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front Cell Infect Microbiol. (2020) 10:1–12. doi: 10.3389/fcimb.2020.00104
105. Mayer, EA, Knight, R, Mazmanian, SK, Cryan, JF, and Tillisch, K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. (2014) 34:15490–6. doi: 10.1523/JNEUROSCI.3299-14.2014
106. Devasia, S, Kumar, S, Stephena, PS, Inoue, N, Sugihara, F, Koizumi, S, et al. A double blind, randomised, four arm clinical study to evaluate the safety, efficacy and tolerability of collagen peptide as a nutraceutical therapy in the management of Type II diabetes mellitus. J Diabetes Metab. (2020) 10:839. doi: 10.35248/2155-6156.19.10.839
107. Wall, R, Cryan, JF, Ross, RP, Fitzgerald, GF, and Dinan, TGSC. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. (2014) 817:221–39. doi: 10.1007/978-1-4939-0897-4_10
108. Dinan, TG, and Cryan, JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. (2017) 595:489–503. doi: 10.1113/JP273106
109. Stilling, RM, van de Wouw, M, Clarke, G, Stanton, C, and Dinan, TGCJF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. (2016) 99:110–32. doi: 10.1016/j.neuint.2016.06.011
110. Paul, B, Barnes, S, Demark-Wahnefried, W, Morrow, C, Salvador, C, Skibola, C, et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenetics. (2015) 7:112–1. doi: 10.1186/s13148-015-0144-7
111. Gareau, MG, Wine, E, Rodrigues, DM, Cho, JH, Whary, MT, Philpott, DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. (2011) 60:307–17. doi: 10.1136/gut.2009.202515
112. Desbonnet, L, Clarke, G, Traplin, A, O’Sullivan, O, Crispie, F, Moloney, RD, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. (2015) 48:165–73. doi: 10.1016/j.bbi.2015.04.004
113. Ohland, CL, Kish, L, Bell, H, Thiesen, A, Hotte, N, and Pankiv, EMKL. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. (2013) 38:1738–47. doi: 10.1016/j.psyneuen.2013.02.008
114. Davari, S, Talaei, SA, and Alaei, HSM. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. (2013) 240:287–96. doi: 10.1016/j.neuroscience.2013.02.055
115. Risk Reduction of Cognitive Decline and Dementia. WHO Guidelines. Geneva: World Health Organization (2019).
116. Hung, C-S, Lee, J-K, Yang, C-Y, Hsieh, H-R, Ma, W-Y, Lin, M-S, et al. Measurement of visceral fat: should we include retroperitoneal fat? PLoS One. (2014) 9:e112355. doi: 10.1371/journal.pone.0112355
117. Louzada, ERRSML. Synbiotic supplementation, systemic inflammation, and symptoms of brain disorders in elders: a secondary study from a randomized clinical trial. Nutr Neurosci. (2020) 23:93–100. doi: 10.1080/1028415X.2018.1477349
118. Wu, L, and Sun, D. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. (2017) 7:1–9. doi: 10.1038/srep41317
119. NPHCE. Longitudinal Ageing Study in India (LASI). International Institute for Population Sciences (IIPS) (2020)1–632. Available at: http://iipsindia.org/research_lasi.htm
Keywords: diet, cognitive health, dementia, healthy aging, nutrient, life course approach
Citation: Puri S, Shaheen M and Grover B (2023) Nutrition and cognitive health: A life course approach. Front. Public Health. 11:1023907. doi: 10.3389/fpubh.2023.1023907
Edited by:
Xianwen Shang, The University of Melbourne, AustraliaReviewed by:
Devin Wahl, Colorado State University, United StatesMary H. Kosmidis, Aristotle University of Thessaloniki, Greece
Copyright © 2023 Puri, Shaheen and Grover. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seema Puri, dr.seemapuri@gmail.com
 Seema Puri
Seema Puri Majida Shaheen
Majida Shaheen Bhanvi Grover
Bhanvi Grover