- 1Department of Environmental and Occupational Health and Safey, Abu Dhabi Publich Health Center, Abu Dhabi, United Arab Emirates
- 2Department of Pathology and Infectious Diseases, Khalifa University, Abu Dhabi, United Arab Emirates
- 3Al Kuwait Hospital Dubai, Emirates Health Services Establishment (EHS), Dubai, United Arab Emirates
- 4Department of Epidemiology and Public Health, Khalifa University, Abu Dhabi, United Arab Emirates
- 5Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
- 6Department of Pathology, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates
- 7Manchester Fungal Infection Group, The University of Manchester, Manchester, United Kingdom
- 8Department of Pediatrics, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates
- 9Department of Medical Microbiology and Immunology, Ras Al Khaimah Medical and Health Sciences University, Ras Al Khaimah, United Arab Emirates
- 10College of Natural and Health Sciences, Zayed University, Dubai, United Arab Emirates
- 11College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, United Arab Emirates
- 12School of Dentistry, Cardiff University, Cardiff, United Kingdom
- 13Biotechnology Research Center, Khalifa University, Abu Dhabi, United Arab Emirates
- 14Infection Research Unit, Khalifa University, Abu Dhabi, United Arab Emirates
Introduction: The Centers for Disease Prevention and Control lists Candida auris, given its global emergence, multidrug resistance, high mortality, and persistent transmissions in health care settings as one of five urgent threats. As a new threat, the need for surveillance of C. auris is critical. This is particularly important for a cosmopolitan setting and global hub such as the United Arab Emirates (UAE) where continued introduction and emergence of resistant variant strains is a major concern.
Methods: The United Arab Emirates has carried out a 12 years of antimicrobial resistance surveillance (2010–2021) across the country, spanning all seven Emirates. A retrospective analysis of C. auris emergence from 2018–2021 was undertaken, utilising the demographic and microbiological data collected via a unified WHONET platform for AMR surveillance.
Results: Nine hundred eight non-duplicate C. auris isolates were reported from 2018–2021. An exponential upward trend of cases was found. Most isolates were isolated from urine, blood, skin and soft tissue, and the respiratory tract. UAE nationals nationals comprised 29% (n = 186 of 632) of all patients; the remainder were from 34 other nations. Almost all isolates were from inpatient settings (89.0%, n = 809). The cases show widespread distribution across all reporting sites in the country. C. auris resistance levels remained consistently high across all classes of antifungals used. C. auris in this population remains highly resistant to azoles (fluconazole, 72.6% in 2021) and amphotericin. Echinocandin resistance has now emerged and is increasing annually. There was no statistically significant difference in mortality between Candida auris and Candida spp. (non-auris) patients (p-value: 0.8179), however Candida auris patients had a higher intensive care unit (ICU) admission rate (p-value <0.0001) and longer hospital stay (p < 0.0001) compared to Candida spp. (non-auris) patients.
Conclusion: The increasing trend of C. auris detection and associated multidrug resistant phenotypes in the UAE is alarming. Continued C. auris circulation in hospitals requires enhanced infection control measures to prevent continued dissemination.
Introduction
Invasive candidiasis which encompasses Candida bloodstream infections and deep-seated candidiasis is a significant cause of morbidity and mortality (1–6), and remains a significant healthcare-associated problem in several countries (7, 8). Within the candidemia grouping, the first known case of Candida auris was in an ear infection in Japan in 2009 (9). C. auris has now become a major public health threat, due to its propensity for horizontal transmission (10–13) and its continued nosocomial spread in long-term and acute care healthcare facilities (6, 11, 14).
C. auris has quickly developed into a global concern and cemented its place as a superbug within just a decade after its first isolation in 2009 (9). Since its emergence, it has been identified in hospitals across five continents, particularly increasing in incidence during the COVID-19 pandemic (4, 15, 16). The role played by the coronavirus disease (COVID-19) pandemic in this increase is difficult to ascertain, while restricted travel may have decreased the risk of importation of C. auris, difficult-to-control outbreaks of C. auris have continued to be reported in units caring for COVID-19 patients worldwide (17–20). C. auris presents diagnostic challenges because of difficulty in identifying strains using common microbiological procedures and challenges in treatment given its resistance to multiple anti-fungal agents, including azoles, echinocandins, and polyenes, making it a critical antibiotic resistance threat (21, 22).
C. auris is now listed among five urgent threats defined in the U.S. Centers for Disease Prevention and Control’s (CDC) 2019 Antibiotic Resistance Threats Report due to its global emergence, multidrug resistance, high mortality, and persistent transmissions in health care settings (9, 10, 23–26). A systematic review and meta-analysis that included cases between 2009 and 2019 from different countries reported an average crude mortality of 45% (95% CI: 39–51%) for C. auris bloodstream infections (21). However, mortality attributable to C. auris remains unclear. The vast majority of strains are fluconazole resistant, with variable proportions resistant to amphotericin B, echinocandins and flucytosine. Reports of antifungal susceptibility data from different geographic locations are varied and some C. auris strains exhibit elevated MICs for three major classes of antifungal drugs. The CDC has suggested tentative breakpoints, and these have been used in most studies, EUCAST and CLSI have yet to recommend clinical breakpoints or epidemiological cut-offs (27–29).
An astonishing aspect in relation to the rapid emergence of C. auris is the simultaneous but independent appearance of genetically distinct clades on different continents (4, 15). The whole-genome sequence (WGS) analysis of clinical isolates of C. auris collected from South Asia (India/Pakistan), South Africa and East Asia (Korea/Japan) has shown four highly clonal phylogenetic and geographically distinct clades that have emerged seemingly independent of one another, specifically, the South Asian clade (clade I), the East Asian clade (clade II), the South African clade (clade III), and the South American clade (clade IV) (4, 15, 30). In 2018, a fifth clade, which is exclusively found in Iran (Iranian clade), was identified (10, 24, 31).
Antifungal resistance is widespread in C. auris in the South Asia clade I isolates. These isolates are resistant to fluconazole, variably resistant to amphotericin B, and also acquire resistance to echinocandins (32–35). C. auris South America clade IV includes isolates with variable resistance to amphotericin B (36, 37), while South Africa clade III isolates are frequently resistant to azoles antifungals (38). Multidrug resistant C. auris isolates to three major classes of antifungal agents have also emerged (10, 39, 40). This severely limits treatment options, making infection control and prevention in healthcare settings essential (5).
The global number of C. auris cases has been rapidly increasing in the past few years particularly in blood cultures from patients with serious underlying medical conditions and in hospitalized patients with invasive medical devices, such as urinary tract catheters and parenteral nutrition, who have also received broad-spectrum antibiotics (1, 3). Mortality in C. auris-associated infections has been reported from 33.3% to 100% worldwide (21), and more recent data has indicated a similar (high) mortality compared to other Candida bloodstream infections (41–43).
Since the time of its first isolation in Japan, C. auris infections have been reported from several countries including South Korea, Malaysia, Kenya, South Africa, India, Pakistan, Colombia, Venezuela, Panama, United States, Canada, China, Russia and Europe (21). Among 17 countries listed under the MENA region, invasive C. auris infections have only been reported from Kuwait in (44–46), Israel (3), Oman (47, 48), Saudi Arabia (49), United Arab Emirates (50), Iran (51) and Qatar (52, 53) to date. The real prevalence and epidemiology of C. auris remains unknown in this region.
United Arab Emirates
Currently, the country hosts a population of nearly 10 million people of which 1 million are Emirati citizens, and the rest are mixed expatriates from various nationalities. The majority of this population resides in Abu Dhabi and Dubai, the two biggest Emirates of the seven that form the UAE (54). The first UAE report of C. auris was in a female patient with persistent candidemia who was admitted to Cleveland Clinic Abu Dhabi Hospital in 2018 (50). The patient had a protracted hospital stay over 1 year with several co-morbid conditions including chronic renal failure on hemodialysis, severe psoriasis, chronic atrial fibrillation and hypertension. During hospitalization the patient was admitted to intensive care unit (ICU) repeatedly and developed multiple infections (bloodstream infections, pneumonia, urinary tract infections) due to several bacterial and fungal pathogens. The patient deteriorated over the next month and died 3 months after the first isolation of C. auris from her blood (50). This has been the only reported case of C. auris in the UAE.
Here we present the first comprehensive UAE wide retrospective epidemiological analysis of all reported C. auris data to date. Thus this study aimed to investigate the trend in the incidence of C. auris over a 4 years period from 2018 to 2021.
Methods
The UAE has been carrying out a national AMR surveillance program over the past 12 years (2010–2021). A retrospective study of emerging C. auris was conducted from 2018 to 2021, using data from the UAE national AMR surveillance program. This data is gathered through a unified WHONET platform (https://whonet.org/). Data collected included demographic and microbiological parameters from all participating centers across the country. The participating sites were managed by trained personnel who gathered AMR surveillance data from routine patient care and submitted it to the National AMR surveillance program. Data was generated, collected, cleaned and analyzed through the national AMR surveillance program as described by Thomsen et al. (55).
Identification of Candida auris
C. auris identification was performed at the national AMR surveillance sites by medical professionals. C. auris isolates were identified and tested for antifungal susceptibility using mostly commercial, automated systems including VITEK® (BioMérieux SA, Craponne, France), BD Phoenix™ (Becton Dickinson, New Jersey, United States), and MicroScan™ (Beckman Coulter, California, United States). A few laboratories used Sensititre YeastOne™ (Thermo Scientific, Massachusetts, United States) plates for susceptibility. Only one laboratory (out of 45 labs) relied on a manual API® (Analytical Profile Index. BioMérieux SA, Craponne, France) system for identification, and only two labs conducted susceptibility testing by manual disc diffusion.
Antimicrobial resistance trends in Candida auris
This was assessed by analysis of routine national level AMR surveillance data. This data, which covers a spectrum of AMR pathogens including C. auris, was obtained from across a network of 317 participating hospitals (n = 84), centers and clinics (n = 233), and 45 diagnostic laboratories in the country. These participating centers include primary, secondary and tertiary care facilities as well as public and private entities. All data are routinely collected and analysed using a unified platform (WHONET) and training on data collection is provided to ensure quality assurance, standardization and accuracy. The fully anonymized data includes demographic data (age, gender, nationality, hospital site/location etc.), clinical and microbiological data such as specimen source and antifungal susceptibility testing results. For the purpose of this analysis, we applied the CDC tentative breakpoints to determine susceptibility of our isolates (29). Resistance MIC breakpoints were as follows: fluconazole ≥ 32 µg/mL; amphotericin B ≥ 2; caspofungin ≥2; anidulafungin and micafungin ≥4.
Data sources and statistical analysis
AMR data was extracted from the national AMR surveillance database. p < 0.05 were considered statistically significant. We performed three types of analyses. In the first analysis, binary logistic regression was used to model the proportion of positive C. auris among all reported infections. Estimates of this analysis provide evidence regarding the annual increase in the reported positive C. auris cases among all reported cases. In the second analysis, the binary logistic regression model was used to investigate the proportion of positive C. auris among reported Candida spp. cases only. Estimates of this model provide data regarding the annual increase in the reported positive C. auris cases among Candida spp. cases. One main limitation of the above two analyses is the possibility that the trend in positive C. auris cases over time could be due to a potential increase in the screening of C. auris over time. To adjust for this potential bias, the total number of tests performed to screen for C. auris should be used. Unfortunately, these metrics are not available in the database. To investigate this possibility, we conducted a large simulation study where different scenarios for the annual increase in the screening rate of C. auris are assumed (see Supplementary material for more details). For each hypothetical screening rate, a binary logistic regression model was fitted, and significance and direction of percentage change in C. auris reported. For all three analyses, odds ratio and corresponding 95% confidence intervals were derived, and provide indication of the change over time in the incidence of positive C. auris cases (increase, or decrease, or no change over time). A chi-square test was used to test the association between categorical variables including mortality and ICU admission. The weighted log rank test was used to assess differences in length of stay in hospital. Binary logistic regression analyses and chi-square test for data presented in tables was performed using the R software (R: The R Project for Statistical Computing, n.d.), chi-square test for mortality rate was performed using Epi Info™ for Windows v7.2.4.0.
Overview of the UAE national AMR surveillance
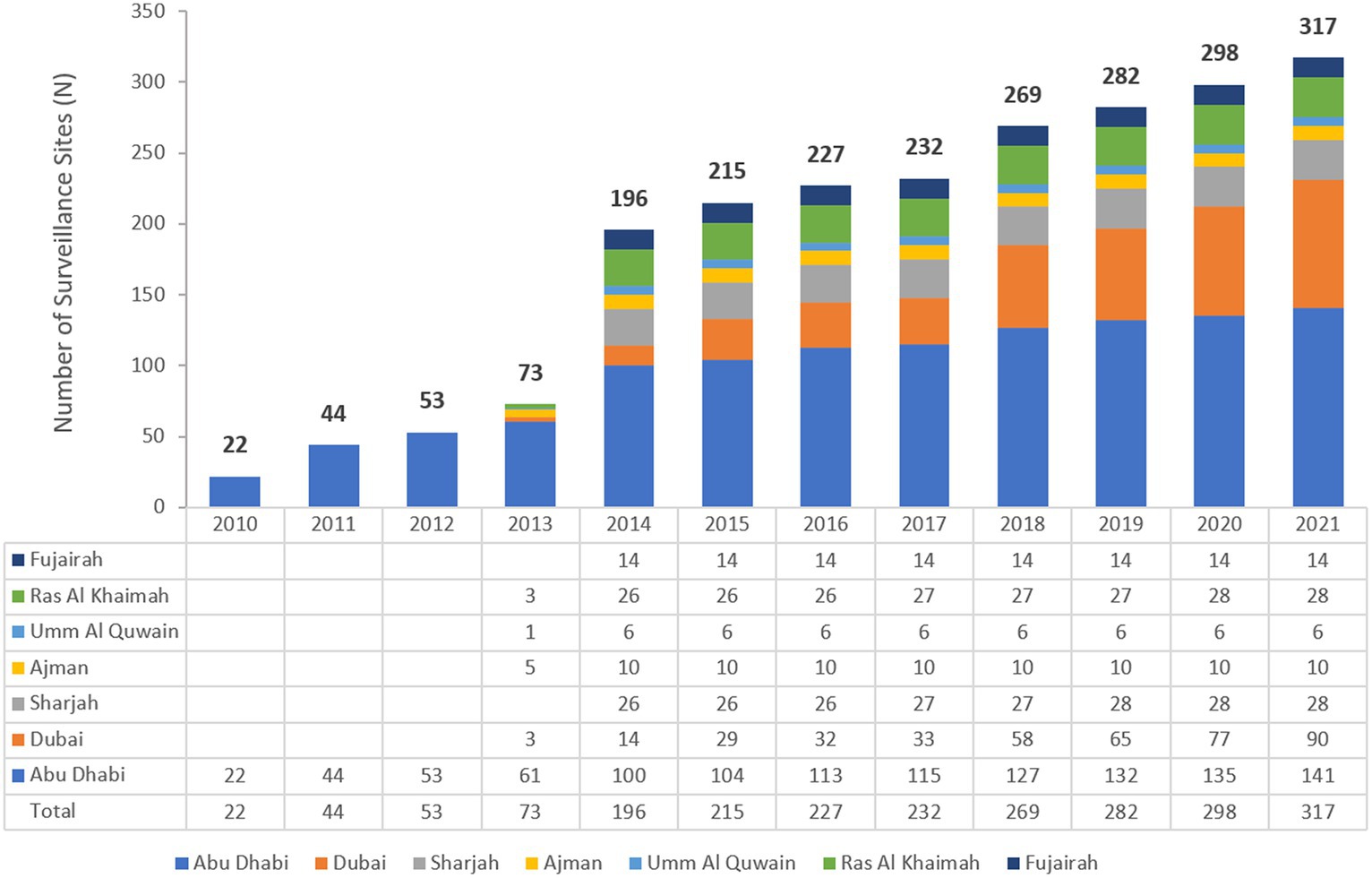
The UAE national AMR surveillance was initiated in 2010 in the Abu Dhabi Emirate where 6 hospitals and 16 Centers/Clinics adopted the WHONET 2021 Software for AMR surveillance.1 Additional sites were recruited over the years, starting with only 22 participating sites in 2010, which is the first year during which the study started, and located only in the Emirate of Abu Dhabi to reach a total of 317 surveillance sites from the 7 Emirates, including 84 hospitals and 233 centres/clinics and representing all seven Emirates of the country in 2021. Figure 1 shows the distribution of surveillance sites for National AMR Surveillance program from 2010 to 2021.
Results
Demographic, clinical and health outcomes of Candida auris
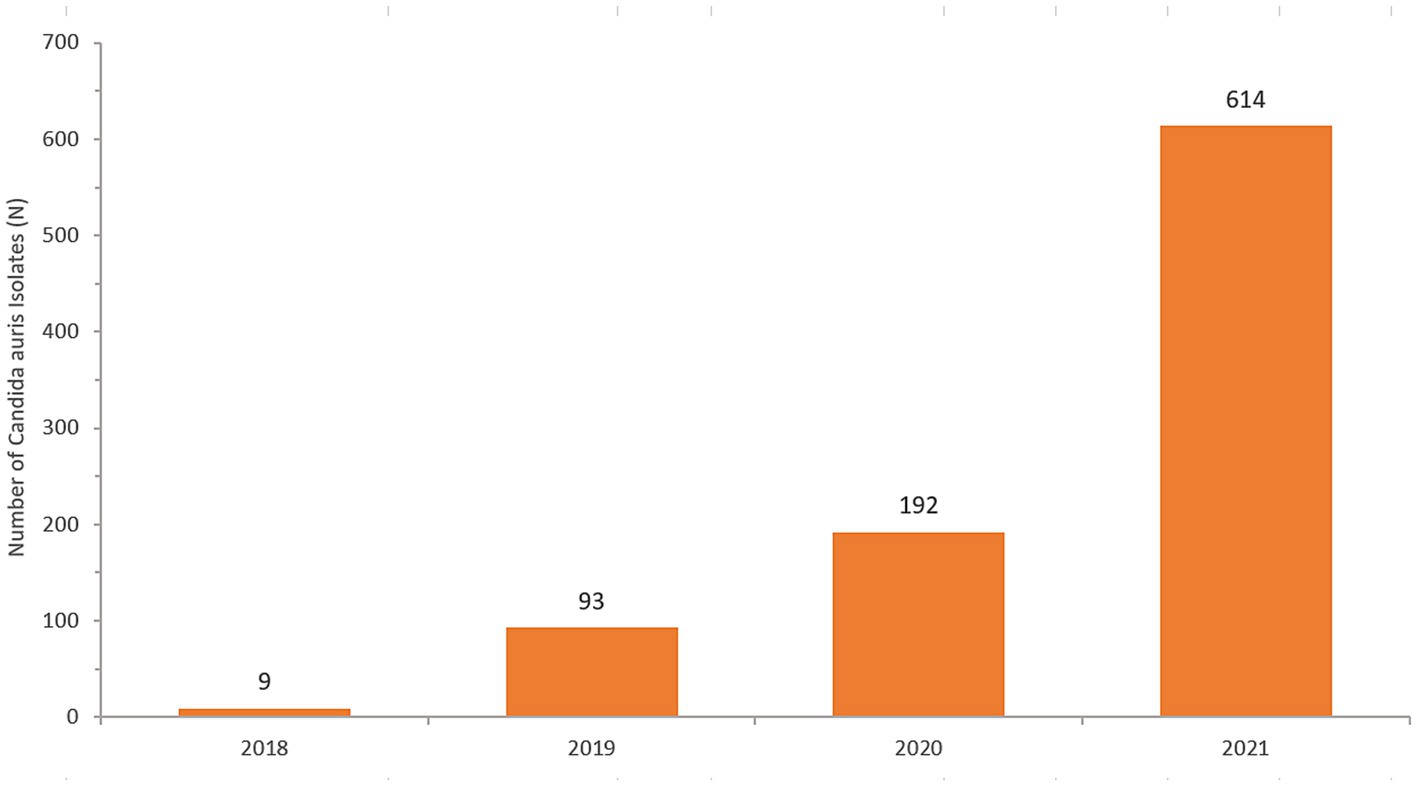
A total of 908 non-duplicate C. auris isolates were reported from 2018–2021 (2018: n = 9; 2019: n = 93; 2020: n = 192; 2021: n = 614). Most of C. auris isolates were obtained from urine (280/908, 30.8%), blood (248/908, 27.3%) and skin and soft tissue (221/908, 24.3%). This was followed by respiratory tract (142/908, 15.6%), genital tract (3/908, 0.3%), and cerebrospinal fluid (CSF) specimens (2/908, 0.2%). C. auris was isolated across a broad range of sample types, showing widespread dissemination. Figure 2 shows the distribution of specimen types where C. auris was isolated from.
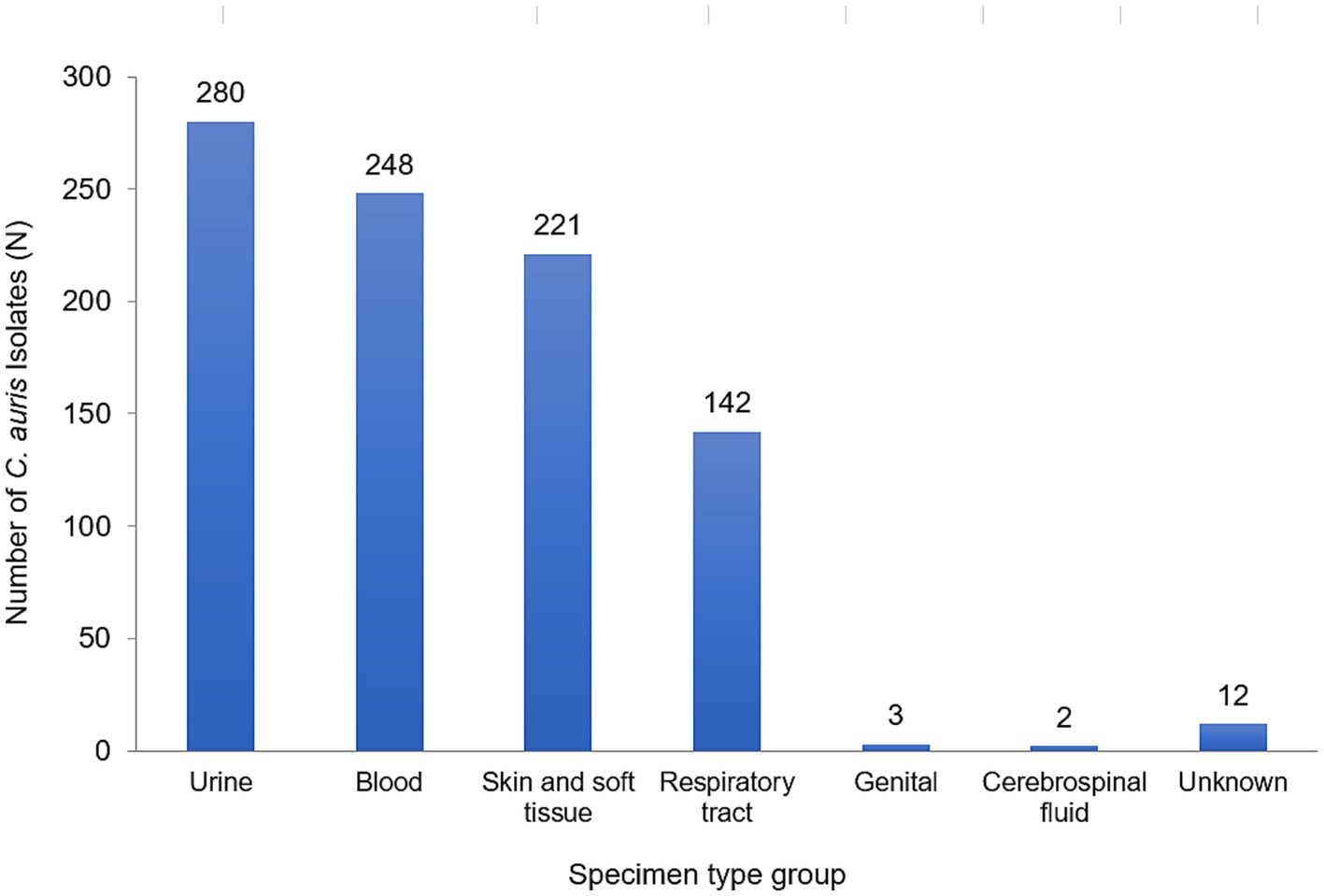
Figure 2. Distribution of Candida auris isolates/patients, by specimen type group, UAE, 2018–2021, N = 908.
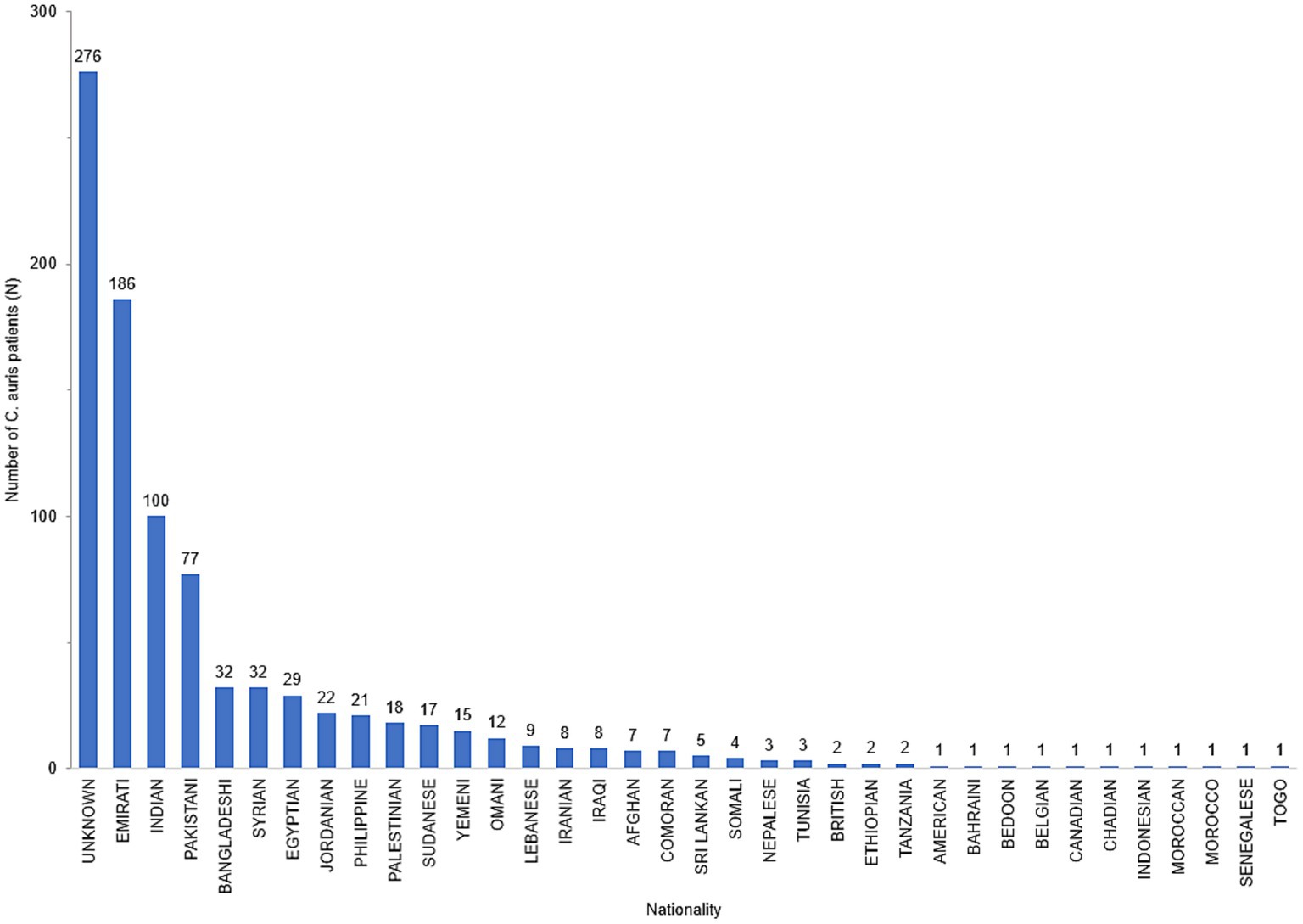
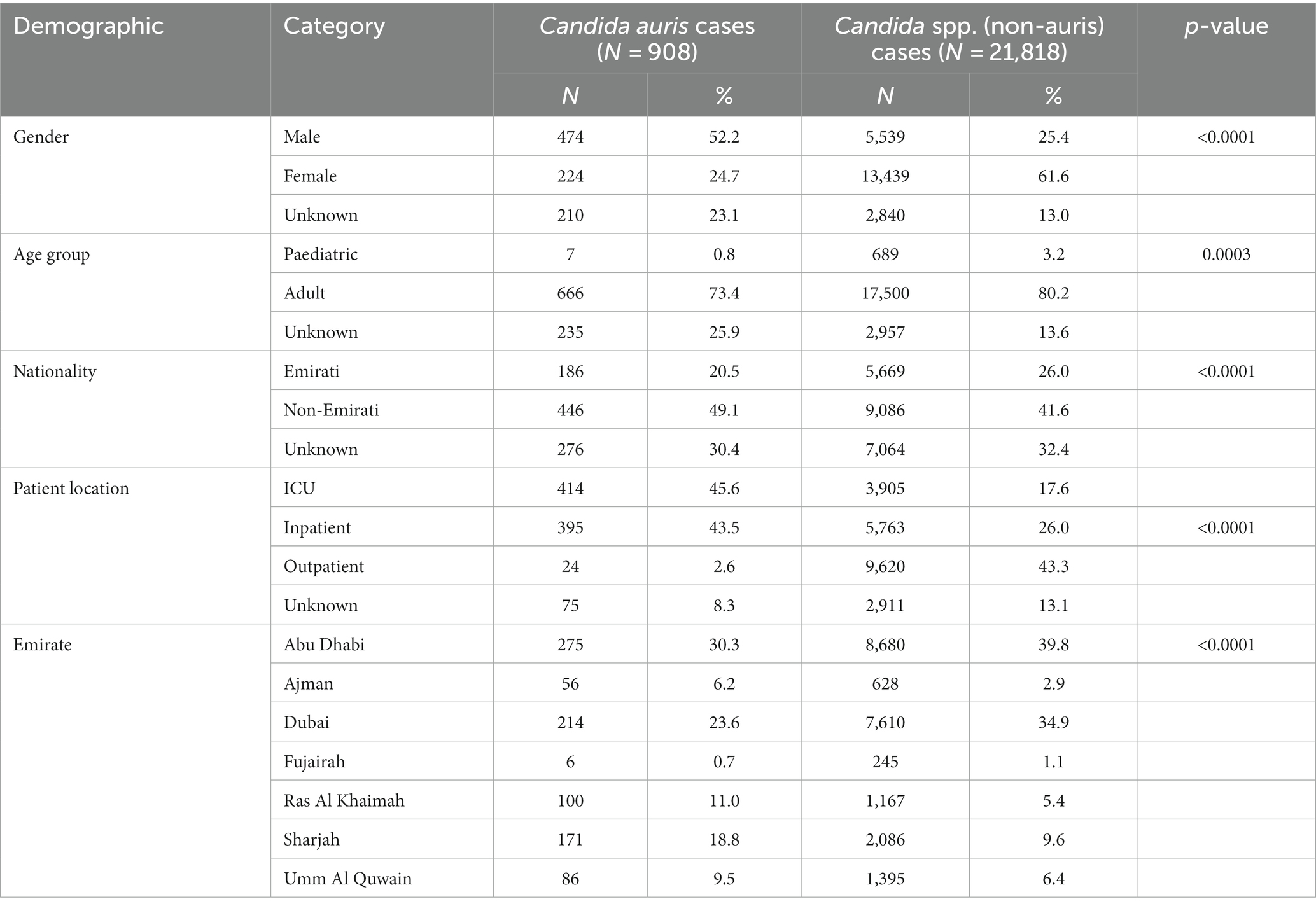
Data on nationality was available for 632 patients of whom 29.4% (n = 186) were UAE nationals and the remainder (70.6%) comprised of individuals from 34 other nationalities (Figure 3). The demographic distribution of the patients shows a heavily skewed distribution across inpatient settings (809/908, 89%) and predominantly ICU patients (414/908, 45.6%). It also revealed a male preponderance with majority of patients being in the adult age group (Table 1).
Admission to intensive care unit
A total of 19,353 patients were associated with Candida spp. (non-auris) of whom 3,905 (20.2%) patients were admitted to ICU, while a total of 835 patients were associated with Candida auris, of whom 414 (49.6%) patients where admitted to ICU. The difference in ICU admission rate is statistically significant (p < 0.0001).
Length of stay
We performed a length of stay (LOS) analysis and assessed the differences in duration of hospitalization using a weighted log-rank test. We included data of patients for whom the date of admission and date of discharge was known. For those patients who were associated with Candida spp. (non-auris) (n = 4,912) the median length of stay was 14.0 days, while for those patients who were associated with C. auris (n = 140) the median length of stay was 33.5 days. The observed difference in length of hospitalization between patients associated with C. auris and non-C. auris spp. was statistically significant (chi square 64.1, p < 0.0001). Based on a total of n = 908 patients during the observation period (2018–2021), a total of 17,706 excess days of hospitalization were observed, attributable to C. auris. For the year 2021 only (n = 614 C. auris cases), a total of 11,973 excess hospitalization days were observed, attributable to C. auris (see Supplementary Figure S1). Kaplan-Meier curve: probability of longer hospitalization of Candida auris patients versus Candida spp. (non-auris) patients [UAE, 2010–2021].
Mortality rate
Analysis on a subset of patients for whom the health outcome was known was performed. A total of 5,694 patients were associated with Candida spp. (non-auris) of whom 1,503 patients died (mortality rate: 26.4%). A total of 171 patients were associated with C. auris, of whom 47 patients (mortality rate: 27.5%) died. The difference in proportion of those who died between C. auris patients and Candida spp. (non-auris) patients is not statistically significant (p = 0.818). Crude mortality rate for patients with C. auris isolates from blood cultures only was 22/61 (36.1%).
Trend analysis of Candida auris among all reported infections: approach 1
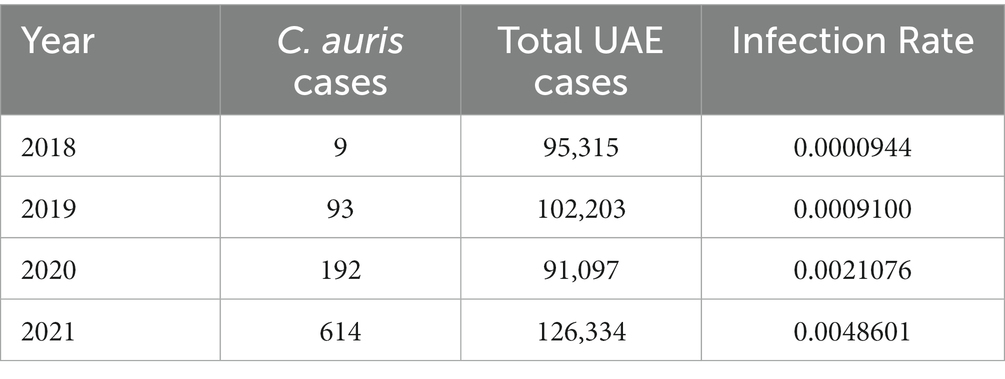
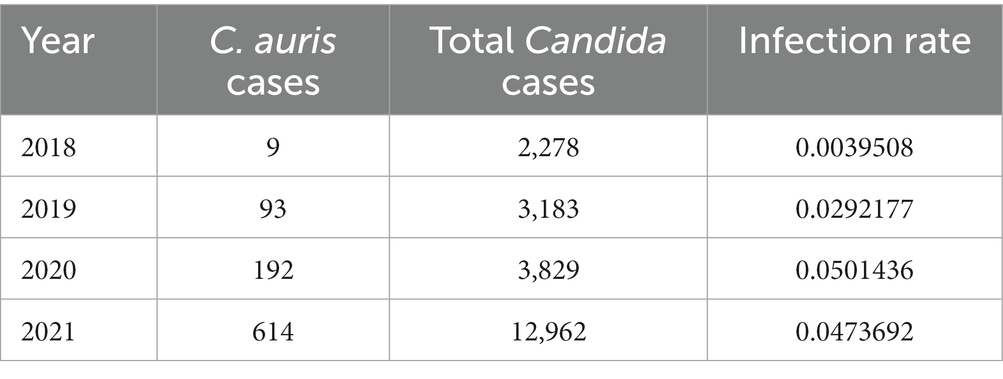
Table 2 shows the number of cases of C. auris and the total number of national AMR surveillance cases reported from 2018 up to 2021, along with the proportion of positive C. auris cases for each year. Figure 4 shows the trend over time from 2018 to 2021.
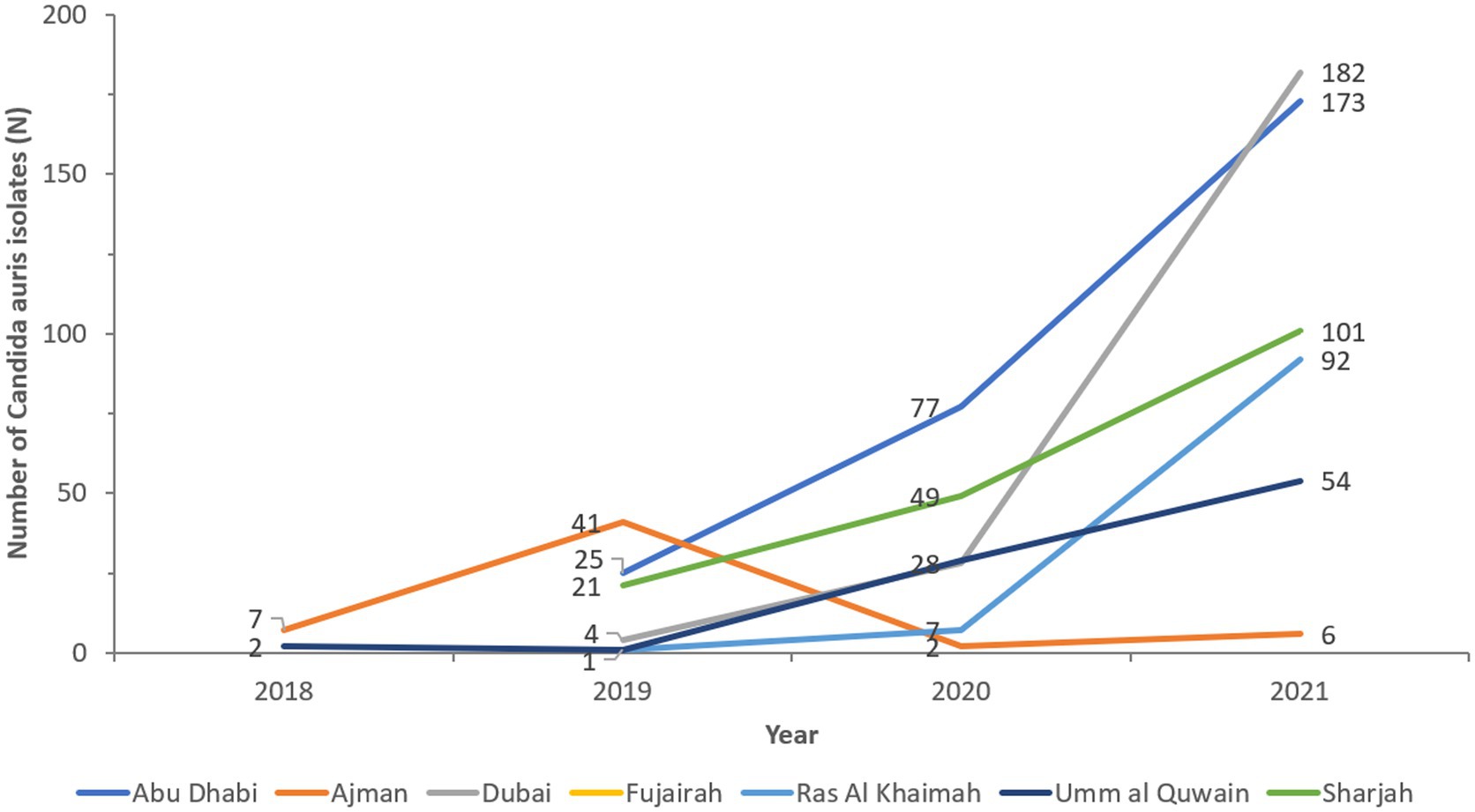
The cases show widespread distribution across all reporting sites and Emirates (Figure 5). Ajman and Umm Al Quwain first reported C. auris isolates in 2018. Emergence occurred in all other Emirates in 2019 and spread rapidly. Abu Dhabi and Sharjah have almost doubled cases annually. Dubai identified 4 cases in 2019 to 182 in 2021, representing a 4450% increase in cases in 2 years.
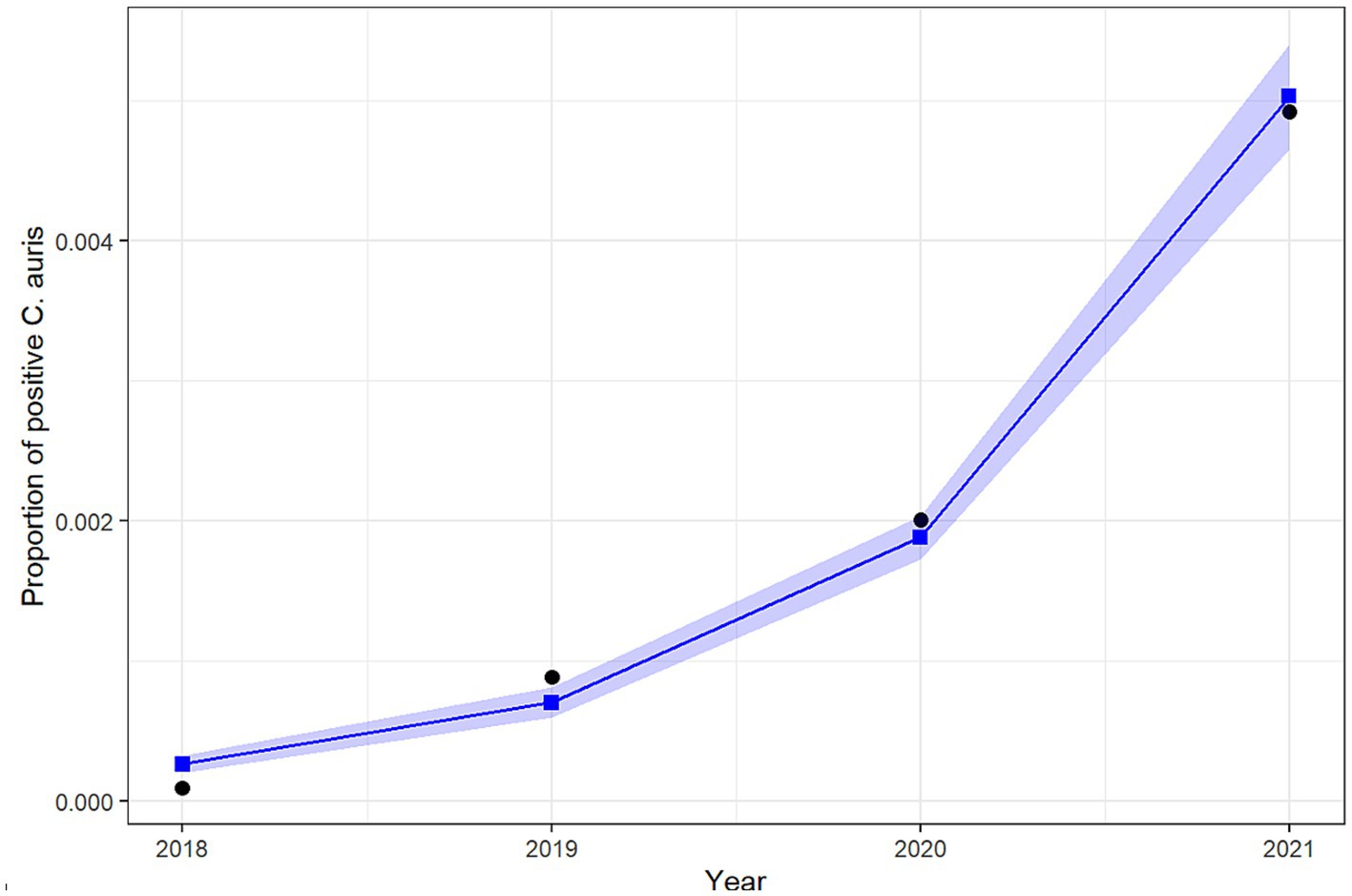
The results of the logistic regression show a significant increase over the years in the odds of reporting positive C. auris cases among all reported cases. More specifically, the odds of reporting a positive C. auris cases increases by 161.5% (95% CI: 140.6–185.1%) each year from 2018 to 2021. Figure 6 shows the predicted versus the observed counts of positive C. auris cases derived from the fit of the binary logistic regression model.
Trend analysis of Candida auris among all Candida spp. cases: approach 2
Table 3 shows the number of positive C. auris cases and the number of positive Candida spp. cases from 2018 up to 2021, along with the proportion of positive C. auris cases for each year.
The results of the logistic regression show a significant increase over the years in the odds of reporting a positive C. auris case among Candida spp. cases. More specifically, the odds of reporting a positive C. auris case increases by 46.2% (95% CI: 35.1%–58.7.6%) each year from 2018 to 2021.
Trend analysis of Candida auris: the simulation study: approach 3
One main limitation of the above two approaches to analyse the trend is the possibility that the trend in positive C. auris cases over time could be due to a potential increase in the screening of C. auris over time. To adjust for this potential bias, and due to the non-availability of the total number of tests performed to screen for C. auris, we conducted a large simulation study where different scenarios for the yearly increase in the screening rate of C. auris were assumed. Figure 7 provides, for each hypothetical annual increase in the screening rate of C. auris, the proportion of results with non-significant change, significant increase and significant decrease in the incidence of C. auris over time.
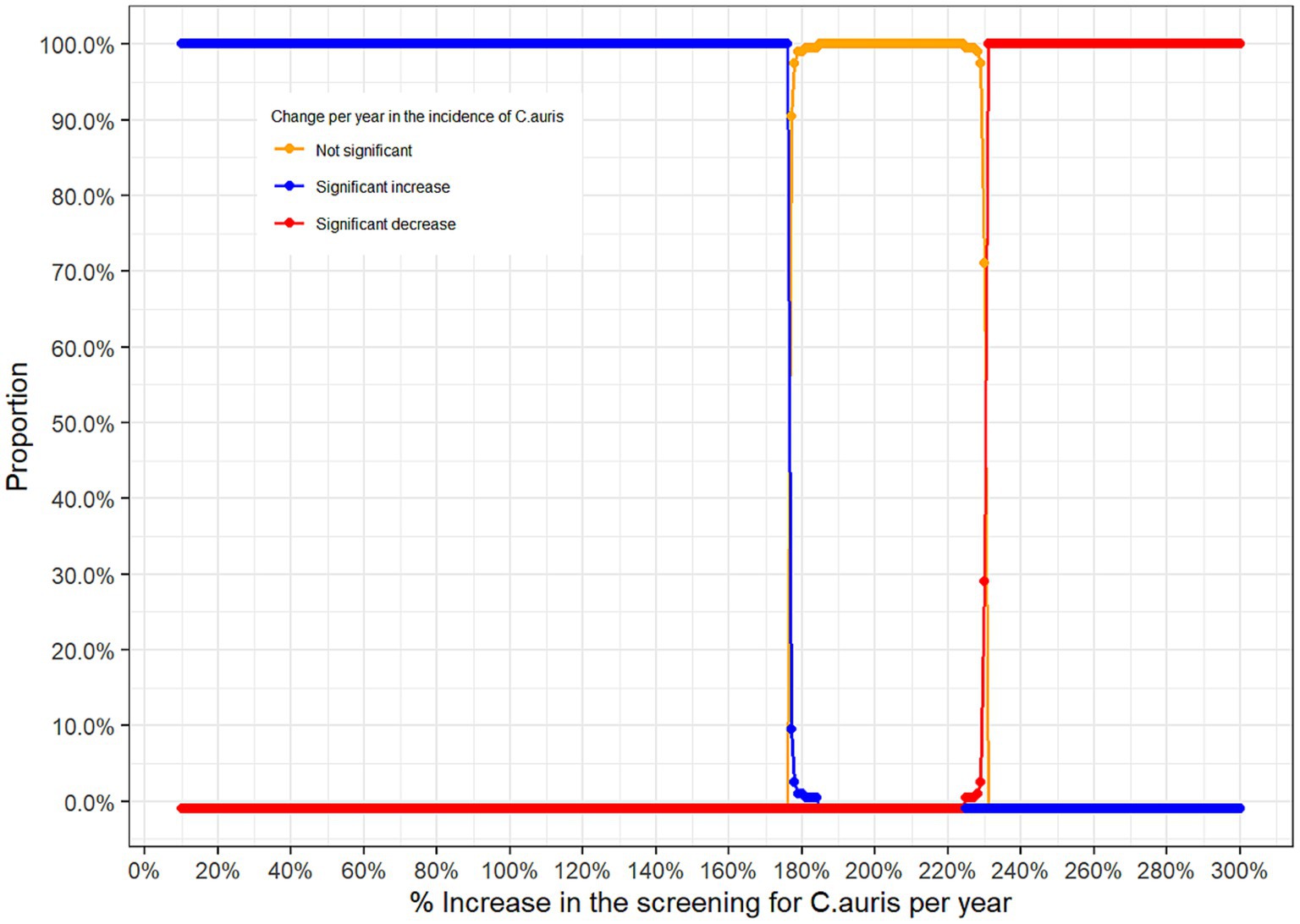
Figure 7. Proportion of significant results according to the hypothetical annual increase rate in the screening of C. auris.
From the simulation study above, one can see that positive C. auris cases observed over the 4 years reflect a statistically significant increase in the incidence of C. auris over time if the annual increase in the screening for C. auris does not exceed 176% (blue curve). If the annual increase in the screening for C. auris lies between 177% and 225% then the trend observed is not statistically significant (orange curve), however, if the annual screening rate was above 225% then the positive C. auris cases observed over the 4 years reflect a statistically significant decrease in the incidence of C. auris over time (red curve).
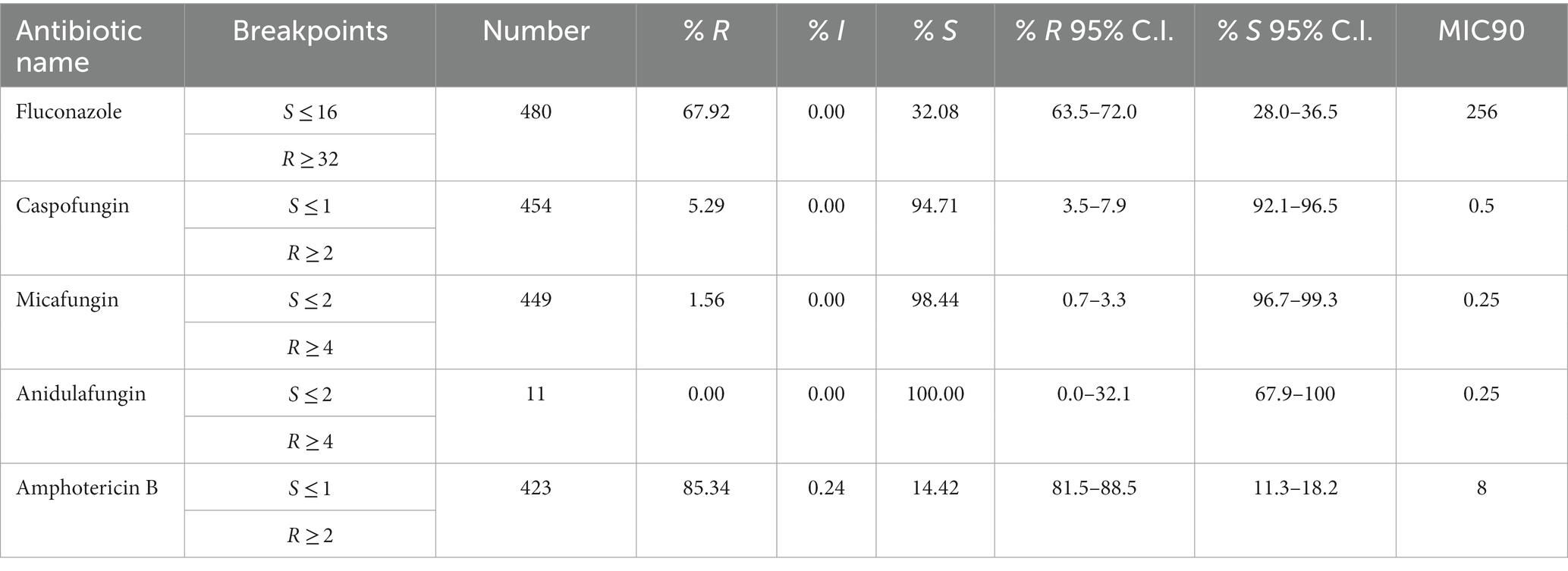
Antifungal resistance
Antifungal susceptibility testing data was available for 514 out of 809 (64.8%) non-duplicate C. auris isolates (fluconazole 480/809, 59.3%; amphotericin B 423/809, 52.3%; caspofungin 454/809, 56.1%; anidulafungin 11/809, 1.4%; micafungin 449/908, 55.5%). During the surveillance period C. auris resistance levels remained consistently high across all classes of antifungals used. C. auris in this population remains highly resistant to Azoles (fluconazole, 72.6% 2021) and rates have remained consistently high since 2019. Echinocandin resistance has now emerged and is increasing annually, from 3.8% (2019) to 7.5% (2021) for caspofungin, and from 0% (2019) to 2.2% (2021) for micafungin (Figure 8).
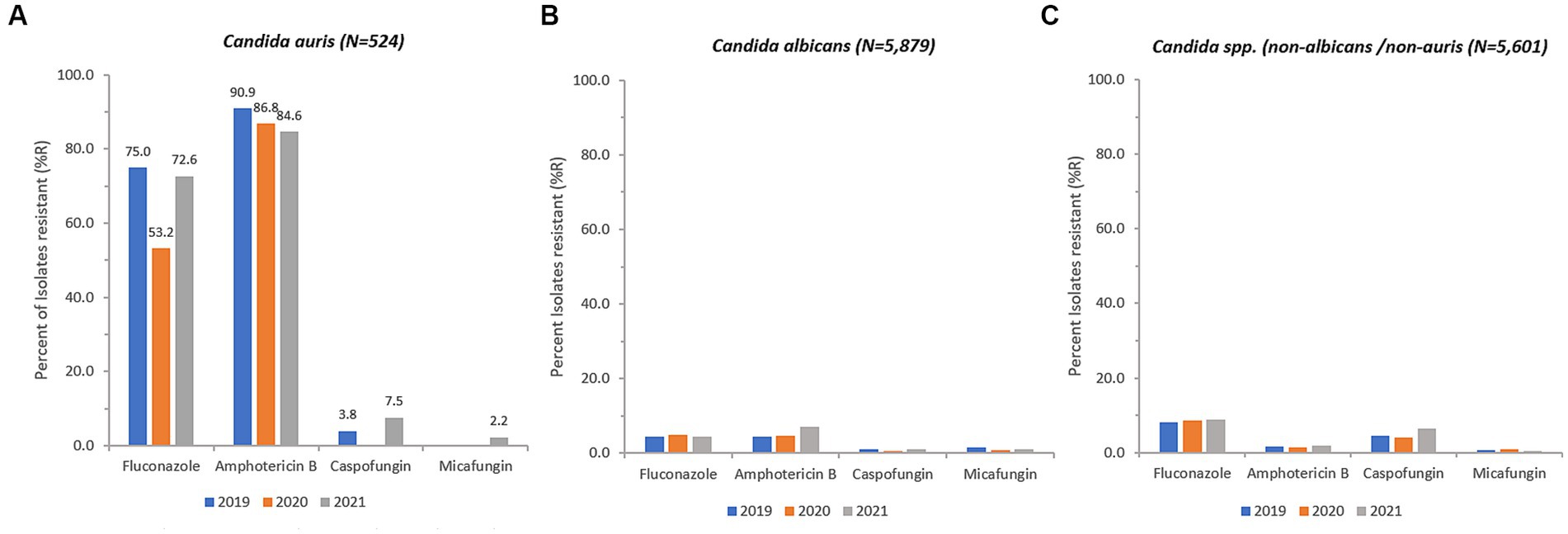
Figure 8. Annual trends for percentage of isolates resistant (% R) for C. auris (A), C. albicans (B), and Candida spp. (non-albicans/non-auris) (C), 2018–2021.
Resistance of C. auris to fluconazole was as high as 77.5% and 75.5% in isolates from skin and soft tissue, and respiratory tract, respectively, whereas fluconazole resistance was lower in isolates from urine (62.9%) and blood (64.2%). Resistance of C. auris to amphotericin B was highest in urine (87.2%), followed by respiratory tract isolates (85.1%), blood (84.8%), and skin and soft tissue (81.1%). Resistance of C. auris to caspofungin and micafungin ranged from 4.2% (blood) to 9.3% (urine), and 0% (blood) to 4.2% (urine), respectively.
Overall, 245 C. auris isolates out of 514 (47.67%) were MDR (≥ 2 antifungal classes resistant), including 20 isolates (3.89%) that were XDR (3 classes resistant, but one antifungal agent still susceptible), including 6 isolates (1.17%) that were PDR (resistant to all substances/all classes tested). The proportion of multidrug resistant C. auris isolates was 31.8% (14/44, 2019) and 28.4% (36/127, 2020), and increased in 2021 to 43.7% (150/343, 2021).
MIC distribution
MIC % RIS distributions were calculated for the collection of C. auris isolates based on the CDC tentative breakpoint recommendations and are presented below in Table 4 and Figures 9A–E.
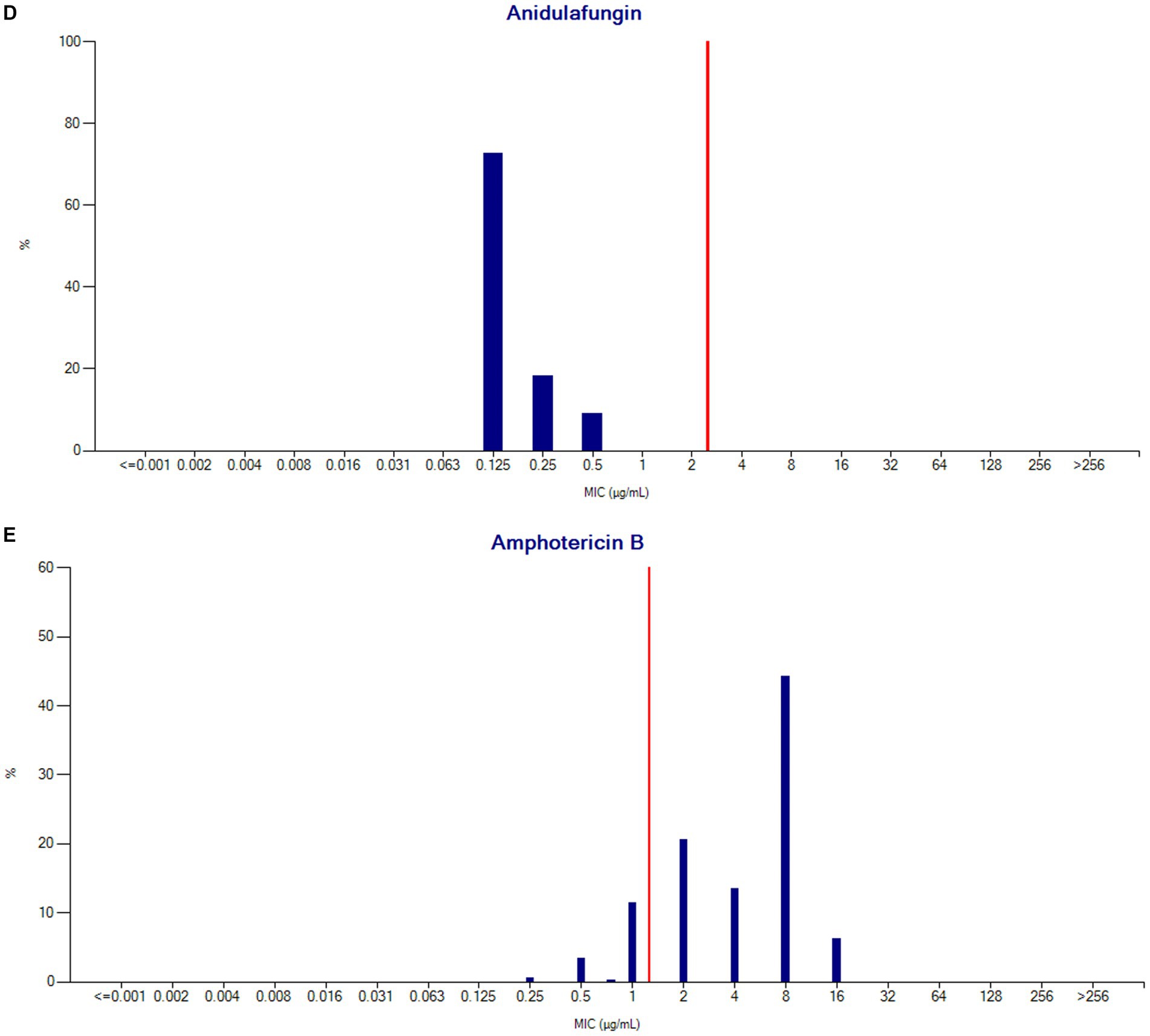
Figure 9. (A) Fluconazole MIC distribution (n = 480). (B) Caspofungin MIC distribution (n = 454). (C) Micafungin MIC distribution (n = 449). (D) Anidulafungin MIC distribution (n = 11). (E) Amphotericin B MIC distribution (n = 423).
Discussion
The growth in hospital sites reporting Candida auris, from only 2 centers in the first year to more than 34 sites towards the end of the study period, representing all 7 Emirates demonstrates considerable concern about C. auris. There is increased alertness across the country of the importance of antimicrobial resistance surveillance and mitigation.
The first cases of C. auris in UAE were detected in 2018. Since then, we have seen an alarming increase of C. auris isolations to n = 641 in 2021, especially in Abu Dhabi and Dubai. This increase is consistent with global reports of rising C. auris burden (56, 57). The COVID 19 pandemic does not seem to have impacted the dissemination of C. auris, and may have exacerbated it (58, 59). Nearly 50% of the patients were in intensive care and length of stay for these patients was extended by 19.5 days compared with patients infected with other Candida spp. Crude mortality at 27.5% (blood culture isolates: 36.1%) was similar to that for other Candida spp. and lower than seen in other countries (45% for blood culture isolates) (21).
C. auris is usually resistant to fluconazole and often to other antifungal medications (azoles, polyenes, and echinocandins). Multidrug-resistant and even pandrug-resistant C. auris isolates have also been described, which limits us to fewer and fewer treatment options (60–62). In this study, resistance rates of C. auris were high (fluconazole, 72.6% 2021, amphotericin B, 84.6% 2021), with the emergence of caspofungin and micafungin resistance in 2021, which is of great concern.
C. auris breakpoints are currently tentative. EUCAST will soon publish epidemiological cut-offs based on a global collection of isolates from which they have removed multiple epidemic or outbreak strains to minimise bias. Testing for fluconazole susceptibility shows very variable MICs, partly because of up-regulation of efflux pumps. These testing limitations may drive EUCAST to simply recommend that fluconazole is not used for C. auris infections, as they currently do for C. glabrata infections. There is general agreement that the tentative CLSI (and CDC) breakpoint for fluconazole is too high, and our finding that 27.4% of isolates were apparently susceptible to fluconazole aligns with this concern. There are also concerns about the breakpoint cut-off for amphotericin B as it bisects the wild type distribution, leading to uncertainty for MICs immediately above or below the breakpoint; an issue also described with Sensititre YeastOne testing (63). Although amphotericin resistance is high in our study, this may be an overestimation of resistance related to the susceptibility methods currently used, as highlighted in other studies (64, 65). Although we have detailed the C. auris breakpoints for the UAE for the first time, it is likely that new data and breakpoints will emerge.
There are no official guidelines for the management of C. auris infection in terms of an optimal antifungal agent(s) with dosing and duration regimen since CLSI/EUCAST breakpoints for this pathogen are yet to be defined (10, 27, 66). Echinocandins remain the first line therapy for C. auris infection, however as demonstrated by our data, resistance to all three main classes of antifungal agents remains a rising problem. Patients should be monitored closely to detect therapeutic failure and/or the development of resistance during their therapy (66).
The increasing trend of C. auris detection is suggestive of continued C. auris circulation predominately in hospitals. Thus infection control measures are critical to prevent continued dissemination. Such infection control measures could include better adherence to hand hygiene, appropriate use of transmission-based precautions based on setting, cleaning and disinfecting the patient care environment and reusable equipment with recommended products, communication about patient’s C. auris status when patient is transferred, screening contacts of newly identified case patients to identify C. auris colonization, and laboratory surveillance of clinical specimens to detect additional cases (67). Newly described approaches include UV-C light inactivation of C. auris, re-formulation of chlorhexidine for superficial use and silver nanoparticles as examples (68–71).
In the MENA region, C. auris has been reported from only six countries. Since genomic studies are lacking in the UAE, it was not possible to ascertain their similarity with C. auris clades from other geographic areas. Additional extensive research is needed on C. auris in the UAE to provide insight into its genetic epidemiology. Moreover, risk factors and methods of transmission need to be exhaustively identified to guide measures for prevention and to control the spread of the pathogen.
In conclusion, the emergence of C. auris poses a global health threat primarily to hospitalized and critically ill patients and should be met with a call for urgent action given its resistant patterns to various classes of antifungals. Our analysis of the national C. auris AMR surveillance data provides insights into the evolving patterns of disease and antimicrobial resistance in the UAE. The findings highlight the need for a continued surveillance program, particularly genomic epidemiological surveillance, to guide the continued AMR monitoring and active intervention and control measures to address the growing threat of antibiotic resistance. Furthermore continued C. auris circulation in hospitals requires enhanced infection control measures to prevent continued dissemination.
Data availability statement
The national AMR Surveillance database managed by the UAE Ministry of Health and Prevention (MOHAP) contains confidential health information, and as such can only be made available upon reasonable request from the UAE Ministry of Health and Prevention (https://mohap.gov.ae).
Ethics statement
Ethical approval for this study was provided by the Ministry of Health and Prevention Research Ethics Committee (MOHAP/DXB-REC/J.J.J./No. 86/2023), Dubai Scientific Research Ethics Committee (DSREC-GL17-2023), and Abu Dhabi Health Research and Technology Ethics Committee (DOH/ZHCD/2023/1316).
Author contributions
DE, JT, NA, GM, CM, AS and the UAE AMR Surveillance Consortium: conceptualization and data collection. AO, DE, PSN, and JT: formal analysis. AO, AS, JT, NA, AA, DD, FA, GM, CM, and DE: data interpretation and manuscript review and editing. DE and JT: manuscript preparation. All authors contributed to the article and approved the submitted version.
The UAE AMR surveillance consortium
Funding
The article processing charge (APC) was funded by the Khalifa University, Abu Dhabi, United Arab Emirates.
Acknowledgments
The authors wish to thank the UAE Ministry of Health and Prevention (MOHAP), regional health and public health authorities (DHA, DoH/ADPHC), AMR Focal points at participating sites, and all healthcare professionals and other experts involved in national AMR surveillance in the UAE for their continuous collaboration and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1244358/full#supplementary-material
Footnotes
References
1. Azar, MM, Turbett, SE, Fishman, JA, and Pierce, VM. Donor-derived transmission of Candida auris during lung transplantation. Clin Infect Dis. (2017) 65:1040–2. doi: 10.1093/cid/cix460
2. Bassetti, M, Azoulay, E, Kullberg, BJ, Ruhnke, M, Shoham, S, Vazquez, J, et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin Infect Dis. (2021) 72:S121–7. doi: 10.1093/cid/ciaa1751
3. Ben-Ami, R, Berman, J, Novikov, A, Bash, E, Shachor-Meyouhas, Y, Zakin, S, et al. Multidrug-resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg Infect Dis. (2017) 23:195–203. doi: 10.3201/eid2302.161486
4. Du, H, Bing, J, Hu, T, Ennis, CL, Nobile, CJ, and Huang, G. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. (2020) 16:e1008921. doi: 10.1371/journal.ppat.1008921
5. Welsh, RM, Bentz, ML, Shams, A, Houston, H, Lyons, A, Rose, LJ, et al. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol. (2017) 55:2996–3005. doi: 10.1128/JCM.00921-17
6. Watkins, RR, Gowen, R, Lionakis, M, and Ghannoum, M. Update on the pathogenesis, virulence, and treatment of Candida auris. Pathog Immun. (2022) 7:46–65. doi: 10.20411/pai.v7i2.535
7. Bongomin, F, Gago, S, Oladele, R, and Denning, D. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. (2017) 3:57. doi: 10.3390/jof3040057
8. Qin, J, Yang, H, Shan, Z, Jiang, L, and Zhang, Q. Clinical efficacy and safety of antifungal drugs for the treatment of Candida parapsilosis infections: a systematic review and network meta-analysis. J Med Microbiol. (2021) 70:001434. doi: 10.1099/jmm.0.001434
9. Satoh, K, Makimura, K, Hasumi, Y, Nishiyama, Y, Uchida, K, and Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. (2009) 53:41–4. doi: 10.1111/j.1348-0421.2008.00083.x
10. Lockhart, SR, Etienne, KA, Vallabhaneni, S, Farooqi, J, Chowdhary, A, Govender, NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. (2017) 64:134–40. doi: 10.1093/cid/ciw691
11. Vallabhaneni, S, Kallen, A, Tsay, S, Chow, N, Welsh, R, Kerins, J, et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. MMWR Morb Mortal Wkly Rep. (2016) 65:1234–7. doi: 10.15585/mmwr.mm6544e1
12. Kim, EJ, Lee, E, Kwak, YG, Yoo, HM, Choi, JY, Kim, SR, et al. Trends in the epidemiology of candidemia in intensive care units from 2006 to 2017: results from the Korean National Healthcare-Associated Infections Surveillance System. Front Med. (2020) 7:606976. doi: 10.3389/fmed.2020.606976
13. Chowdhary, A, Sharma, C, Duggal, S, Agarwal, K, Prakash, A, Singh, PK, et al. New clonal strain of Candida auris, Delhi. India Emerg Infect Dis. (2013) 19:1670–3. doi: 10.3201/eid1910.130393
14. Schelenz, S, Hagen, F, Rhodes, JL, Abdolrasouli, A, Chowdhary, A, Hall, A, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. (2016) 5:35. doi: 10.1186/s13756-016-0132-5
15. Ahmad, S, and Alfouzan, W. Candida auris: epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities. Microorganisms. (2021) 9:807. doi: 10.3390/microorganisms9040807
16. Kanj, SS, Haddad, SF, Meis, JF, Verweij, PE, Voss, A, Rautemaa-Richardson, R, et al. The battle against fungi: lessons in antifungal stewardship from COVID 19 times. Int J Antimicrob Agents. (2023) 62:106846. doi: 10.1016/j.ijantimicag.2023.106846
17. Allaw, F, Kara Zahreddine, N, Ibrahim, A, Tannous, J, Taleb, H, Bizri, AR, et al. First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathogens. (2021) 10:157. doi: 10.3390/pathogens10020157
18. Pan American Health Organization and World Health Organization, Epidemiological alert: Candida auris outbreaks in health care services in the context of the COVID-19 pandemic. (2021). Available online at: https://www.paho.org/en/documents/epidemiological-alert-candida-auris-outbreaks-health-care-services-context-covid-19)
19. Nobrega de Almeida, J, Brandão, IB, Francisco, EC, de Almeida, SLR, de Oliveira Dias, P, Pereira, FM, et al. Axillary digital thermometers uplifted a multidrug-susceptible Candida auris outbreak among COVID-19 patients in Brazil. Mycoses. (2021) 64:1062–72. doi: 10.1111/myc.13320
20. Prestel, C, Anderson, E, Forsberg, K, Lyman, M, de Perio, MA, Kuhar, D, et al. Candida auris outbreak in a COVID-19 specialty care unit—Florida, July–August 2020. MMWR Morb Mortal Wkly Rep. (2021) 70:56–7. doi: 10.15585/mmwr.mm7002e3
21. Chen, J, Tian, S, Han, X, Chu, Y, Wang, Q, Zhou, B, et al. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect Dis. (2020) 20:827. doi: 10.1186/s12879-020-05543-0
22. Sanyaolu, A, Okorie, C, Marinkovic, A, Abbasi, AF, Prakash, S, Mangat, J, et al. Candida auris: an overview of the emerging drug-resistant fungal infection. Infect Chemother. (2022) 54:236–46. doi: 10.3947/ic.2022.0008
23. CDC. Antibiotic resistance threats in the United States. (2019) Available at: https://www.cdc.gov/drugresistance/biggest-threats.html
24. Chow, NA, Gade, L, Tsay, SV, Forsberg, K, Greenko, JA, Southwick, KL, et al. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis. (2018) 18:1377–84. doi: 10.1016/S1473-3099(18)30597-8
25. WHO. WHO fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization (2022).
26. Hinrichs, C, Wiese-Posselt, M, Graf, B, Geffers, C, Weikert, B, Enghard, P, et al. Successful control of Candida auris transmission in a German COVID-19 intensive care unit. Mycoses. (2022) 65:643–9. doi: 10.1111/myc.13443
27. Arendrup, MC, Prakash, A, Meletiadis, J, Sharma, C, and Chowdhary, A. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother. (2017) 61:e00485. doi: 10.1128/AAC.00485-17
28. CDC. Recommendations for identification of Candida auris. Available at: https://www.cdc.gov/fungal/diseases/candidiasis/recommendations.html
29. CDC. Antifungal susceptibility testing and interpretation. (2020) Available at: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html
30. Ben Abid, F, Salah, H, Sundararaju, S, Dalil, L, Abdelwahab, AH, Salameh, S, et al. Molecular characterization of Candida auris outbreak isolates in Qatar from patients with COVID-19 reveals the emergence of isolates resistant to three classes of antifungal drugs. Clin Microbiol Infect. (2023) 29:1083.e1–7. doi: 10.1016/j.cmi.2023.04.025
31. Cernakova, L, Roudbary, M, Brás, S, Tafaj, S, and Rodrigues, CF. Candida auris: a quick review on identification, current treatments, and challenges. Int J Mol Sci. (2021) 22:4470. doi: 10.3390/ijms22094470
32. Chatterjee, S, Alampalli, SV, Nageshan, RK, Chettiar, ST, Joshi, S, and Tatu, US. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics. (2015) 16:686. doi: 10.1186/s12864-015-1863-z
33. Chowdhary, A, Prakash, A, Sharma, C, Kordalewska, M, Kumar, A, Sarma, S, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–2017) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. (2018) 73:891–9. doi: 10.1093/jac/dkx480
34. Sarma, S, Kumar, N, Sharma, S, Govil, D, Ali, T, Mehta, Y, et al. Candidemia caused by amphotericin B and fluconazole resistant Candida auris. Indian J Med Microbiol. (2013) 31:90–1. doi: 10.4103/0255-0857.108746
35. Zhu, Y, O’Brien, B, Leach, L, Clarke, A, Bates, M, Adams, E, et al. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: impact and lessons learned. J Clin Microbiol. (2020) 58:e01503. doi: 10.1128/JCM.01503-19
36. Calvo, B, Melo, ASA, Perozo-Mena, A, Hernandez, M, Francisco, EC, Hagen, F, et al. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect. (2016) 73:369–74. doi: 10.1016/j.jinf.2016.07.008
37. Escandon, P, Chow, NA, Caceres, DH, Gade, L, Berkow, EL, Armstrong, P, et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin Infect Dis. (2019) 68:15–21. doi: 10.1093/cid/ciy411
38. Maphanga, TG, Naicker, SD, Kwenda, S, Muñoz, JF, van Schalkwyk, E, Wadula, J, et al. In vitro antifungal resistance of Candida auris isolates from bloodstream infections, South Africa. Antimicrob Agents Chemother. (2021) 65:e0051721. doi: 10.1128/AAC.00517-21
39. O’Brien, B, Liang, J, Chaturvedi, S, Jacobs, JL, and Chaturvedi, V. Pan-resistant Candida auris: New York subcluster susceptible to antifungal combinations. Lancet Microbe. (2020) 1:e193–4. doi: 10.1016/S2666-5247(20)30090-2
40. Ostrowsky, B, Greenko, J, Adams, E, Quinn, M, O’Brien, B, Chaturvedi, V, et al. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. MMWR Morb Mortal Wkly Rep. (2020) 69:6–9. doi: 10.15585/mmwr.mm6901a2
41. Moin, S, Farooqi, J, Rattani, S, Nasir, N, Zaka, S, and Jabeen, K. C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med Mycol. (2021) 59:1238–42. doi: 10.1093/mmy/myab057
42. Parak, A, Stacey, SL, and Chibabhai, V. Clinical and laboratory features of patients with Candida auris cultures, compared to other Candida, at a South African Hospital. J Infect Dev Ctries. (2022) 16:213–21. doi: 10.3855/jidc.14917
43. Simon, SP, Li, R, Silver, M, Andrade, J, Tharian, B, Fu, L, et al. Comparative outcomes of Candida auris bloodstream infections: a multicenter retrospective case-control study. Clin Infect Dis. (2023) 76:e1436–43. doi: 10.1093/cid/ciac735
44. Emara, M, Ahmad, S, Khan, Z, Joseph, L, Al-Obaid, I, Purohit, P, et al. Candida auris candidemia in Kuwait, 2014. Emerg Infect Dis. (2015) 21:1091–2. doi: 10.3201/eid2106.150270
45. Khan, Z, Ahmad, S, Al-Sweih, N, Joseph, L, Alfouzan, W, and Asadzadeh, M. Increasing prevalence, molecular characterization and antifungal drug susceptibility of serial Candida auris isolates in Kuwait. PLoS One. (2018) 13:e0195743. doi: 10.1371/journal.pone.0195743
46. Khan, Z, Ahmad, S, Benwan, K, Purohit, P, Al-Obaid, I, Bafna, R, et al. Invasive Candida auris infections in Kuwait hospitals: epidemiology, antifungal treatment and outcome. Infection. (2018) 46:641–50. doi: 10.1007/s15010-018-1164-y
47. Al-Siyabi, T., Al Busaidi, I, Balkhair, A, Al-Muharrmi, Z, Al-Salti, M, Adawi, B, et al., First report of Candida auris in Oman: clinical and microbiological description of five candidemia cases. J Infect, (2017). 75: p. 373–376, doi: 10.1016/j.jinf.2017.05.016
48. Mohsin, J, Hagen, F, Al-Balushi, ZAM, de Hoog, GS, Chowdhary, A, Meis, JF, et al. The first cases of Candida auris candidaemia in Oman. Mycoses. (2017) 60:569–75. doi: 10.1111/myc.12647
49. Abdalhamid, B, Almaghrabi, R, Althawadi, S, and Omrani, A. First report of Candida auris infections from Saudi Arabia. J Infect Public Health. (2018) 11:598–9. doi: 10.1016/j.jiph.2018.05.010
50. Alatoom, A, Sartawi, M, Lawlor, K, AbdelWareth, L, Thomsen, J, Nusair, A, et al. Persistent candidemia despite appropriate fungal therapy: first case of Candida auris from the United Arab Emirates. Int J Infect Dis. (2018) 70:36–7. doi: 10.1016/j.ijid.2018.02.005
51. Abastabar, M, Haghani, I, Ahangarkani, F, Rezai, MS, Taghizadeh Armaki, M, Roodgari, S, et al. Candida auris otomycosis in Iran and review of recent literature. Mycoses. (2019) 62:101–5. doi: 10.1111/myc.12886
52. Koleri, J, Petkar, HM, Al Soub HA, RS, and AlMaslamani MA, RS. Candida auris blood stream infection—a descriptive study from Qatar. BMC Infect Dis. (2023) 23:513. doi: 10.1186/s12879-023-08477-5
53. Salah, H, Sundararaju, S, Dalil, L, Salameh, S, Al-Wali, W, Tang, P, et al. Genomic epidemiology of Candida auris in Qatar reveals hospital transmission dynamics and a South Asian origin. J Fungi. (2021) 7:240. doi: 10.3390/jof7030240
54. Population and demographic mix—the official portal of the UAE Government. Available at: https://u.ae/en/information-and-services/social-affairs/preserving-the-emirati-national-identity/population-and-demographic-mix
55. Thomsen, J, Abdulrazzaq, NM, and AlRand, H. and The UAE AMR Surveillance Consortium. Surveillance of antimicrobial resistance in the United Arab Emirates: the early implementation phase. Front. Public Health. (2023) 11:1247627. doi: 10.3389/fpubh.2023.1247627
56. CDC, Increasing threat of spread of antimicrobial-resistant fungus in healthcare facilities. (2023). Available at: https://www.cdc.gov/media/releases/2023/p0320-cauris.html#:~:text=CDC%20has%20deemed%20C.,infections%20with%20high%20death%20rates.
57. Nelson, R. Emergence of resistant Candida auris. Lancet Microbe. (2023) 4:e396. doi: 10.1016/S2666-5247(23)00143-X
58. Khojasteh, S, Jafarzdeh, J, Hosseini, SA, Haghani, I, Turki, H, Aghaei Gharehbolagh, S, et al. Candida auris and COVID-19: a health threatening combination. Curr Med Mycol. (2022) 8:44–50. doi: 10.18502/cmm.8.3.11211
59. Tsai, CS, Lee, SSJ, Chen, WC, Tseng, CH, Lee, NY, Chen, PL, et al. COVID-19-associated candidiasis and the emerging concern of Candida auris infections. J Microbiol Immunol Infect. (2022) 56:672–9. doi: 10.1016/j.jmii.2022.12.002
60. Jacobs, SE, Jacobs, JL, Dennis, EK, Taimur, S, Rana, M, Patel, D, et al. Candida auris pan-drug-resistant to four classes of antifungal agents. Antimicrob Agents Chemother. (2022) 66:e0005322. doi: 10.1128/aac.00053-22
61. Jeffery-Smith, A, Taori, SK, Schelenz, S, Jeffery, K, Johnson, EM, Borman, A, et al. Candida auris: a review of the literature. Clin Microbiol Rev. (2018) 31:e00029. doi: 10.1128/CMR.00029-17
62. Lyman, M, Forsberg, K, Reuben, J, Dang, T, Free, R, Seagle, EE, et al. Notes from the field: transmission of pan-resistant and echinocandin-resistant Candida auris in health care facilities—Texas and the District of Columbia, January–April 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1022–3. doi: 10.15585/mmwr.mm7029a2
63. Siopi, M, Peroukidou, I, Beredaki, MI, Spruijtenburg, B, de Groot, T, Meis, JF, et al. Overestimation of amphotericin B resistance in Candida auris with Sensititre YeastOne antifungal susceptibility testing: a need for adjustment for correct interpretation. Microbiol Spectr. (2023) 11:e0443122. doi: 10.1128/spectrum.04431-22
64. Kathuria, S, Singh, PK, Sharma, C, Prakash, A, Masih, A, Kumar, A, et al. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization-time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol. (2015) 53:1823–30. doi: 10.1128/JCM.00367-15
65. Keighley, C, Garnham, K, Harch, SAJ, Robertson, M, Chaw, K, Teng, JC, et al. Candida auris: diagnostic challenges and emerging opportunities for the clinical microbiology laboratory. Curr Fungal Infect Rep. (2021) 15:116–26. doi: 10.1007/s12281-021-00420-y
66. Lepak, AJ, Zhao, M, Berkow, EL, Lockhart, SR, and Andes, DR. Pharmacodynamic optimization for treatment of invasive Candida auris infection. Antimicrob Agents Chemother. (2017) 61:e00791. doi: 10.1128/AAC.00791-17
67. CDC. Infection prevention and control for Candida auris; Available at: https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html
68. Ahmad, S, and Asadzadeh, M. Strategies to prevent transmission of Candida auris in healthcare settings. Curr Fungal Infect Rep. (2023) 17:36–48. doi: 10.1007/s12281-023-00451-7
69. Elshaer, M, Herrada, J, Gamal, A, McCormick, TS, and Ghannoum, M. Efficacy of chlorhexidine in advanced performance technology formulation in decolonizing the skin using Candida auris skin colonization mouse model. Am J Infect Control. (2023) 51:836–7. doi: 10.1016/j.ajic.2022.11.010
70. AlJindan, R, and AlEraky, DM. Silver nanoparticles: a promising antifungal agent against the growth and biofilm formation of the emergent Candida auris. J Fungi. (2022) 8:744. doi: 10.3390/jof8070744
Keywords: Candida auris, surveillance, healthcare-associated infections, antifungals, antimicrobial-resistance, UAE, MENA
Citation: Thomsen J, Abdulrazzaq NM, Oulhaj A, Nyasulu PS, Alatoom A, Denning DW, Al Dhaheri F, the UAE AMR Surveillance Consortium, Menezes GA, Moubareck CA, Senok A and Everett DB (2024) Emergence of highly resistant Candida auris in the United Arab Emirates: a retrospective analysis of evolving national trends. Front. Public Health. 11:1244358. doi: 10.3389/fpubh.2023.1244358
Edited by:
Dalal Hammoudi Halat, Qatar University, QatarReviewed by:
Ifeanyi Mba, University of Nigeria, Nsukka, NigeriaTolossa Waqkene, Independent Researcher, Oromia, Ethiopia
Copyright © 2024 Thomsen, Abdulrazzaq, Oulhaj, Nyasulu, Alatoom, Denning, Al Dhaheri, the UAE AMR Surveillance Consortium, Menezes, Moubareck, Senok and Everett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dean B. Everett, RGVhbi5FdmVyZXR0QGt1LmFjLmFl
†These authors share second authorship
‡These authors share last authorship
 Jens Thomsen1,2
Jens Thomsen1,2 Najiba M. Abdulrazzaq
Najiba M. Abdulrazzaq Abderrahim Oulhaj
Abderrahim Oulhaj Adnan Alatoom
Adnan Alatoom David W. Denning
David W. Denning Fatima Al Dhaheri
Fatima Al Dhaheri Godfred Antony Menezes
Godfred Antony Menezes Abiola Senok
Abiola Senok Dean B. Everett
Dean B. Everett