- 1Department of Biophysics, Physiology, and Pathophysiology, Medical University of Warsaw, Warsaw, Poland
- 2Department of Health Studies, Institute of Biomedical Science, FH Joanneum University of Applied Sciences, Graz, Austria
- 3Centro Integrativo de Biología y Química Aplicada (CIBQA), Universidad Bernardo O'Higgins, Santiago, Chile
- 4Women's Health Research Institute and BC Women's Hospital, University of British Columbia (BC), Vancouver, BC, Canada
- 5Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 6Faculty of Mathematics & Information Science, Warsaw University of Technology, Warsaw, Poland
- 7CEAUL – Centro de Estatística e Aplicações da Universidade de Lisboa, Lisbon, Portugal
1. Introduction
The end of the COVID-19 pandemic is generating a wide interest on long COVID (LC) (1), a heterogeneous medical condition known by many alternative names, such as post-COVID-19 syndrome (2), post-acute COVID-19 syndrome (3), and post-acute sequelae of SARS-CoV-2 infection (4), and persistent post-COVID-19 syndrome (5). This condition is a top priority in the current biomedical research agenda due to its great impact on public health (6). The clinical manifestation of LC varies from mild and temporary symptoms, such as anosmia and ageusia, to highly debilitating and chronic fatigue and post-exertional malaise (PEM) (7). This spectrum of symptoms might be explained by immune dysregulation, microbiota dysbiosis, autoimmunity and immune priming, abnormal blood clotting and endothelial-related problems, and neurological signaling dysfunction, among other pathological mechanisms (1, 8).
The real burden of LC remains elusive even though systematic reviews aggregate data from hundreds of studies and thousands of individuals (9–11). The underlying problems are the reliance on self-reporting of symptoms for the LC diagnosis and the challenge of conducting studies without any sources of sampling bias (10). The same problems emerge in the few epidemiological studies on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) (12, 13). This disease remains without a specific biomarker (14), but might share some pathological mechanisms and symptoms with LC (4, 15–18). According to a recent meta-analysis (13), the pooled estimate of ME/CFS prevalence across multiple studies is 0.89% [95% CI = (0.60%−1.33%)]. This estimate shows some variations according to gender (1.36% in women vs. 0.86% in men), age (0.65% in adults vs. 0.55% in children and adolescents), or study setting (0.76% in community-based study vs. 0.63% in primary care studies). This disease inflicts dramatic individual and societal costs, such as health deterioration, reduced productivity, earnings and employment, mental health problems, and burnout (19).
Given the global urgency of managing and treating LC, several studies (20–23) are combining basic research on this disease and ME/CFS with the idea of accelerating knowledge of the underlying pathological mechanisms. However, similar combined approach remains to be adopted in epidemiological studies of LC. Therefore, these studies could expand their objectives to include the estimation of ME/CFS prevalence as well. These additional data offer a better quantification of the real burden of LC due to people that develop ME/CFS-related symptoms. Such a quantification provides the foundation for using consensual guidelines for ME/CFS healthcare to LC case management (15) that could be adopted and adapted for the specificities of a given national health system.
Such an expansion of objectives comes at a minimal cost by simply incorporating standard symptom questionaries used for the ME/CFS diagnosis and then running a diagnostic algorithm based on consensual case definitions. With this in mind, we reviewed the most consensual case definitions of ME/CFS. We also compared the symptoms assessed in the UK ME/CFS Biobank (UKMEB) (24, 25) with those documented in recent LC epidemiological studies. Finally, we provided some practical recommendations for future studies.
2. Brief review of ME/CFS and LC diagnostic criteria
ME/CFS has more than 20 proposed case definitions (26, 27). Among these definitions, the 1994 CDC (28), the 2003 Canadian Consensus Criteria (CCC) (29), and the 2015 Institute of Medicine (IOM) criteria have been used as diagnostic tools for research purposes (30). These criteria are also used for patients' enrollment in the UKMEB (24, 25).
The 1994 CDC criteria is mainly a research tool for ME/CFS diagnosis. In these criteria, an individual receives an ME/CFS diagnosis if she (or he) experiences unexplained, persistent, or relapsing fatigue for at least 6 months. The fatigue experienced should substantially reduce the normal levels of daily activities. Resting is also insufficient to restore normal energy levels. The individual should also experience four or more of the following eight symptoms:
I Substantial impairment in short-term memory or concentration;
II Sore throat;
III Tender cervical and axillary lymph nodes;
IV Muscle pain;
V Multi-joint pain without swelling or redness;
VI Headaches of a new type, pattern, or severity;
VII Unrefreshing sleep;
VIII PEM.
Note that a group of experts recommend PEM as a hallmark rather than an optional symptom to consider the diagnosis of ME/CFS based on these criteria (30). Exclusion criteria include all medical conditions that could explain fatigue (e.g., untreated hypothyroidism or sleep apnea), alcohol or other substance abuse, and severe obesity (body mass index greater than 45 kg/m2). Other authors discussed the possibility of defining a severely obese individual by a body mass index equal to or greater than 40 kg/m2 (31).
The 2003 CCC is basically a diagnostic tool for clinical settings. However, many research studies are using this tool for ME/CFS diagnosis. This criterion also recognizes 6 months as the minimal symptom duration. The hallmark symptoms are pathological fatigue, PEM, sleep abnormalities (unrefreshing sleep, reduced sleep quality or quantity, reversed or chaotic diurnal sleep rhythms), muscle or multi-joint pain, and two or more cognitive symptoms. The diagnosis also requires the presentation of one or more symptoms belonging to at least two additional domains: autonomic, neuroendocrine, and immune. Exclusion criteria also apply.
The 2015 IOM criterion is also a primary diagnostic tool for clinical settings. It also requires the presence of fatigue for more than 6 months, PEM, and unrefreshing sleep. This case definition also requires at least one of the following manifestations: cognitive impairment and orthostatic intolerance. In contrast to the 1994 CDC and 2003 CCC criteria, the 2015 IOM criterion does not contemplate any list of exclusionary medical conditions or co-morbidities. However, one should not diagnose a patient as having ME/CFS if treatment for the alternative diagnosis eliminates all symptoms in a patient. A recent discussion about the exclusionary medical conditions can be found elsewhere (32).
According to the World Health Organization, the definition of LC is the presence of at least one unresolved symptom after 3 months of a confirmed SARS-CoV-2 infection (2). Another definition is based on an international Delphi consensus of 11 outcomes for the core symptom set of LC (33). These outcomes are: fatigue; pain; post-exertion symptoms; work or occupational and study changes; survival; and functioning, symptoms, and conditions for each of cardiovascular, respiratory, nervous system, cognitive, mental health, and physical outcomes. Alternatively, the LC diagnosis might be diagnosed by a disease scoring system based on 37 symptoms (34).
3. Symptoms reported by patients in UKMEB and in current LC prevalence studies
Given the broad clinical spectrum of LC patients, prevalence studies of LC typically estimate the individual prevalence of a large number of symptoms. The basic question is whether these studies collect symptom data that would also allow them conducting a possible diagnosis of ME/CFS in the study participants.
Previously, we reported the prevalence of each of 47 symptoms evaluated in 222 ME/CFS patients upon their enrollment in the UKMEB, as reported elsewhere (35). Excluding fatigue (with 100% prevalence), the prevalence per symptom varied from 33.9% (palpitations) to 98.7% (unrefreshing sleep) with an average prevalence of 72.0%. Therefore, we can conclude that these 47 symptoms are highly prevalent in patients with ME/CFS complying with the 1994 CDC or the 2003 CCC criteria.
We then investigated whether three large systematic reviews of LC prevalence reported these symptoms. Besides the prevalence of fatigue (data not shown), there were only individual prevalence estimates for 23, 18, and 14 of the 47 UKMEB-related symptoms reported by O'Mahoney et al. (9), Woodrow et al. (10), and Natarajan et al. (11), respectively (Table 1). More importantly, these reviews did not report data related to PEM, although post-exertion symptoms are in the core outcome set of LC (33). Besides that, two large survey reported the prevalence of PEM higher than 80% in LC patients (7, 34). This lack of reporting indicates that current LC epidemiological studies have not collected sufficient symptom data to allow for a preliminary symptom assessment necessary for an ME/CFS diagnosis.
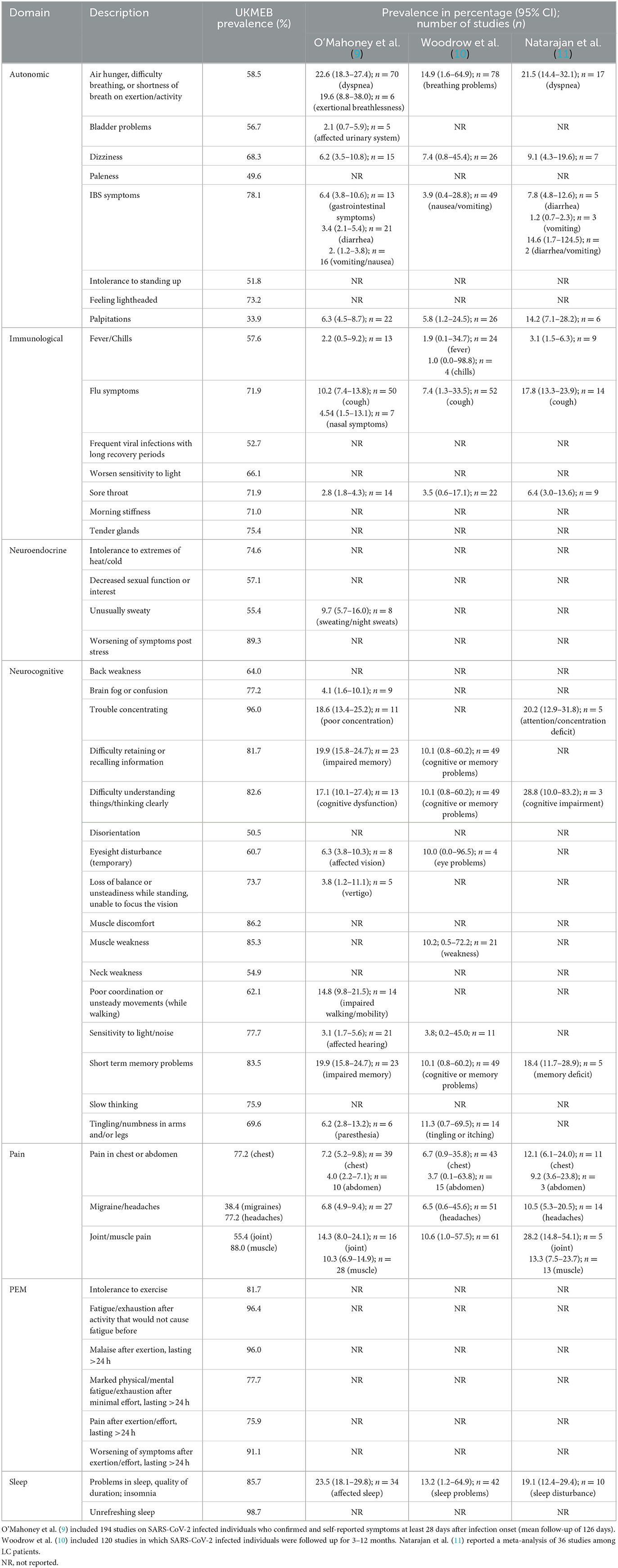
Table 1. Forty-seven symptoms in the UKMEB symptom assessment questionnaire (based on 2003 CCC) and their reporting in three systematic reviews on the prevalence of LC and its symptoms.
These systematic reviews also did not provide any data for other highly prevalent ME/CFS-related symptoms in the patients from the UKMEB, such as unrefreshing sleep (98.7%), sensitivity to light or noise (77.7%), tender lymph nodes (75.5%), and intolerance to heat and cold (74.6%; Table 1).
4. Discussion
Since the beginning of the COVID-19 pandemic in early 2020, it became clear that many people who experienced a SARS-CoV-2 infection remained ill with a clinical manifestation consistent with ME/CFS. However, most of the epidemiological studies of LC ignored this fact and, therefore, they did not assess the presence and severity of cardinal symptoms of ME/CFS diagnosis. This is an unfortunate missed opportunity, especially, in what estimating ME/CFS prevalence is concerned. One can seize this opportunity by conducting a study prospectively. These studies should be clear in the LC case definition, criteria for an acute COVID-19 episode, and the duration of follow-up, because these aspects might influence the subsequent statistical results. Studies using a retrospective design as reported in recent meta-analyses of LC should be avoided, because they might miss crucial data (e.g., PEM) unavailable from routine healthcare records.
There is evidence for a limited assessment of symptoms in LC patients related to ME/CFS, including PEM, unrefreshing sleep, and sensitivity to light or noise in epidemiological studies of LC. Most of these unreported symptoms are auxiliary rather than strictly mandatory for an ME/CFS diagnosis, but they might be useful for defining disease subtypes (36, 37). The only exceptions are the PEM-related symptoms, which are key in the 2003 CCC, the 2015 IOM criteria, and in the modified 1994 CDC criterion (30). The assessment of PEM or other symptoms is becoming more important, given that the duration of LC can reach 3 years by now in some patients (6). In the case of ME/CFS, a 2-year disease duration might reflect the transition from an early to an established disease stage (38). Hence, it is conceivable that LC cases without typical symptoms of ME/CFS at an early disease stage might develop key symptoms of this disease when the chronicity of LC symptoms becomes established.
We recommend that future epidemiological studies of LC use the DePaul Symptoms Questionnaire (DSQ) (39) or the UKMEB Symptoms Assessment Questionnaire (35), as diagnostic tools enabling the identification of cases meeting commonly used diagnostic criteria for ME/CFS. Given the cardinal importance of PEM in ME/CFS diagnosis, one should make the effort to capture accurately its different aspects, such as recovery time, frequency, and severity (40). In this scenario, one can use the DSQ dedicated to PEM specifically, the so-called DSQ-PEM (41). The use of this questionnaire is likely to be more informative than simply asking for the presence of symptoms worse after even minor physical or mental effort, as done in large study of LC (34). The reporting of the epidemiological findings could be done via a recommended guideline for the minimal data elements on ME/CFS research (42). Besides typical information about study design and demographics, one should report the case definition used, the symptom inventory, the excluded medical and psychiatric conditions and co-morbidities, and self-reported functional impairment/levels of activity.
The major difficulty to comply with the 1994 CDC and the 2003 CCC in epidemiological studies lies in the exclusion of other medical conditions that could explain fatigue. The 2015 IOM criterion, alternatively, does not impose any exclusionary conditions (32), however, they still require a clinical assessment and consideration of differential diagnosis. This case definition is already being used to report the frequency of ME/CFS cases among LC cases (34, 43). However, the use of the 2015 IOM criteria might lead to inconsistent findings across studies due to variations in frequency of co-morbidities present in different populations. It might also overestimate the prevalence of ME/CFS due to highly-frequent conditions, such as diabetes and obesity in suspected cases. For example, 43% of participants fit the 2015 IOM criterion for ME/CFS in an LC study (43). Among these compliant individuals, some had a BMI of 45 kg/m2.
We also recommend raising the standard of research and reporting in LC, ME/CFS, and other chronic diseases; we made a similar recommendation for genetic association studies in ME/CFS (44). Our recommendation is based on two systematic reviews of LC prevalence data. One systematic review suggested that 45% of LC cases had ME/CFS (45). However, this review incorrectly assumed that the persistence of fatigue was equivalent to ME/CFS. The other systematic review suggested that only a few epidemiological studies collected representative samples from the LC population (10). Low population representativeness, convenience sampling, and different sources of bias might be present in the remaining published studies (10). Randomness and sample representativeness are the pillars of a sound statistical inference. If a study does not minimally ensure these foundational assumptions, the subsequent statistical inference might be tricky, or even impossible (46).
Author contributions
ADG: Investigation, Validation, Writing – review & editing. FW: Investigation, Validation, Writing – review & editing. LN: Data curation, Resources, Writing – review & editing. EL: Data curation, Resources, Writing – review & editing. NS: Conceptualization, Investigation, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. LN acknowledges partial funding from the BCCDC Foundation for Population and Public Health and the BC Women's Health Foundation. EL acknowledges funding from the National Institutes of Health, UK (grant ref. 5R01AI170839-02). NS acknowledges partial funding from FCT—Fundação para a Ciência e Tecnologia, Portugal (grant ref. UIDB/00006/2020).
Acknowledgments
The authors would like to thank João Malato for proof-reading and making suggestions to the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
2. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. (2022) 22:e102–7. doi: 10.1016/S1473-3099(21)00703-9
3. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
4. Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, Reeves WB. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. (2023) 12:e86002. doi: 10.7554/eLife.86002
5. Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, et al. Review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol. (2023) 64:66–74. doi: 10.1007/s12016-021-08848-3
7. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
8. Altmann DM, Whettlock EM, Liu S, Arachchillage DJ, Boyton RJ. The immunology of long COVID. Nat Rev Immunol. (2023) 23:618–34. doi: 10.1038/s41577-023-00904-7
9. O'Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. (2022) 55:101762. doi: 10.1016/j.eclinm.2022.101762
10. Woodrow M, Carey C, Ziauddeen N, Thomas R, Akrami A, Lutje V, et al. Systematic review of the prevalence of long COVID. Open Forum Infect Dis. (2023) 10:ofad233. doi: 10.1093/ofid/ofad233
11. Natarajan A, Shetty A, Delanerolle G, Zeng Y, Zhang Y, Raymont V, et al. A systematic review and meta-analysis of long COVID symptoms. Syst Rev. (2023) 12:88. doi: 10.1186/s13643-023-02250-0
12. Estévez-López F, Mudie K, Wang-Steverding X, Bakken IJ, Ivanovs A, Castro-Marrero J, et al. Systematic review of the epidemiological burden of myalgic encephalomyelitis/chronic fatigue syndrome across Europe: current evidence and EUROMENE research recommendations for epidemiology. J Clin Med. (2020) 9:1557. doi: 10.3390/jcm9051557
13. Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. (2020) 18:100. doi: 10.1186/s12967-020-02269-0
14. Scheibenbogen C, Freitag H, Blanco J, Capelli E, Lacerda E, Authier J, et al. The European ME/CFS biomarker landscape project: an initiative of the European network EUROMENE. J Transl Med. (2017) 15:162. doi: 10.1186/s12967-017-1263-z
15. Komaroff AL, Lipkin WI. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. (2021) 27:895–906. doi: 10.1016/j.molmed.2021.06.002
16. Komaroff AL, Lipkin WI. ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med. (2023) 10:1187163. doi: 10.3389/fmed.2023.1187163
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-A systemic review and comparison of clinical presentation and symptomatology. Medicina. (2021) 57:418. doi: 10.3390/medicina57050418
18. Legler F, Meyer-Arndt L, Mödl L, Kedor C, Freitag H, Stein E, et al. Long-term symptom severity and clinical biomarkers in post-COVID-19/chronic fatigue syndrome: results from a prospective observational cohort. EClinicalMedicine. (2023) 63:102146. doi: 10.1016/j.eclinm.2023.102146
19. Pheby DFH, Araja D, Berkis U, Brenna E, Cullinan J, de Korwin J-D, et al. The development of a consistent Europe-Wide approach to investigating the economic impact of myalgic encephalomyelitis (ME/CFS): a report from the European Network on ME/CFS (EUROMENE). Healthcare. (2020) 8:88. doi: 10.3390/healthcare8020088
20. Haffke M, Freitag H, Rudolf G, Seifert M, Doehner W, Scherbakov N, et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J Transl Med. (2022) 20:138. doi: 10.1186/s12967-022-03346-2
21. Flaskamp L, Roubal C, Uddin S, Sotzny F, Kedor C, Bauer S, et al. Serum of post-COVID-19 syndrome patients with or without ME/CFS differentially affects endothelial cell function in vitro. Cells. (2022) 11:2376. doi: 10.3390/cells11152376
22. Hayes LD, Sanal-Hayes NEM, Mclaughlin M, Berry ECJ, Sculthorpe NF. People with long covid and ME/CFS exhibit similarly impaired balance and physical capacity: a case-case-control study. Am J Med. (2023). doi: 10.1016/j.amjmed.2023.06.028
23. Mclaughlin M, Sanal-Hayes NEM, Hayes LD, Berry EC, Sculthorpe NF. People with long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) exhibit similarly impaired vascular function. Am J Med. (2023). doi: 10.1016/j.amjmed.2023.09.013
24. Lacerda EM, Bowman EW, Cliff JM, Kingdon CC, King EC, Lee J-S, et al. The UK ME/CFS biobank for biomedical research on myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and multiple sclerosis. Open J Bioresour. (2017) 4:4. doi: 10.5334/ojb.28
25. Lacerda EM, Mudie K, Kingdon CC, Butterworth JD, O'Boyle S, Nacul L. The UK ME/CFS biobank: a disease-specific biobank for advancing clinical research into myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. (2018) 9:1026. doi: 10.3389/fneur.2018.01026
26. Brurberg KG, Fønhus MS, Larun L, Flottorp S, Malterud K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a systematic review. BMJ Open. (2014) 4:e003973. doi: 10.1136/bmjopen-2013-003973
27. Lim EJ, Son CG. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. (2020) 18:289. doi: 10.1186/s12967-020-02455-0
28. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. (1994) 121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009
29. Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lemer AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr. (2003) 11:7–115. doi: 10.1300/J092v11n01_02
30. Nacul L, Authier FJ, Scheibenbogen C, Lorusso L, Helland IB, Martin JA, et al. European network on myalgic encephalomyelitis/chronic fatigue syndrome (EUROMENE): expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina. (2021) 57:510. doi: 10.3390/medicina57050510
31. Reeves WC, Lloyd A, Vernon SD, Klimas N, Jason LA, Bleijenberg G, et al. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv Res. (2003) 3:25. doi: 10.1186/1472-6963-3-25
32. Jason LA, Ravichandran S, Katz BZ, Natelson BH, Bonilla HF. Establishing a consensus on ME/CFS exclusionary illnesses. Fatigue Biomed Heal Behav. (2022) 11:1–13. doi: 10.1080/21641846.2022.2150487
33. Munblit D, Nicholson T, Akrami A, Apfelbacher C, Chen J, De Groote W, et al. A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study. Lancet Respir Med. (2022) 10:715–24. doi: 10.1016/S2213-2600(22)00169-2
34. Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. (2023) 329:1934. doi: 10.1001/jama.2023.15712
35. Domingues TD, Malato J, Grabowska AD, Lee JS, Ameijeiras-Alonso J, Biecek P, et al. Association analysis between symptomology and herpesvirus IgG antibody concentrations in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and multiple sclerosis. Heliyon. (2023) 9:e18250. doi: 10.1016/j.heliyon.2023.e18250
36. Huber KA, Sunnquist M, Jason LA. Latent class analysis of a heterogeneous international sample of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Fatigue Biomed Heal Behav. (2018) 6:163. doi: 10.1080/21641846.2018.1494530
37. Vaes AW, Van Herck M, Deng Q, Delbressine JM, Jason LA, Spruit MA. Symptom-based clusters in people with ME/CFS: an illustration of clinical variety in a cross-sectional cohort. J Transl Med. (2023) 21:112. doi: 10.1186/s12967-023-03946-6
38. Nacul L, O'Boyle S, Palla L, Nacul FE, Mudie K, Kingdon CC, et al. How myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) progresses: the natural history of ME/CFS. Front Neurol. (2020) 11:826. doi: 10.3389/fneur.2020.00826
39. Jason LA, Sunnquist M. The development of the DePaul symptom questionnaire: original, expanded, brief, and pediatric versions. Front Pediatr. (2018) 6:330. doi: 10.3389/fped.2018.00330
40. Jason LA, Holtzman CS, Sunnquist M, Cotler J. The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome. J Health Psychol. (2021) 26:238. doi: 10.1177/1359105318805819
41. Cotler J, Holtzman C, Dudun C, Jason LA. A brief questionnaire to assess post-exertional malaise. Diagnostics. (2018) 8:66. doi: 10.3390/diagnostics8030066
42. Jason LA, Unger ER, Dimitrakoff JD, Fagin AP, Houghton M, Cook DB, et al. Minimum data elements for research reports on CFS. Brain Behav Immun. (2012) 26:401. doi: 10.1016/j.bbi.2012.01.014
43. Bonilla H, Quach TC, Tiwari A, Bonilla AE, Miglis M, Yang PC, et al. Myalgic encephalomyelitis/chronic fatigue syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic. Front Neurol. (2023) 14:1090747. doi: 10.3389/fneur.2023.1090747
44. Grabowska AD, Lacerda EM, Nacul L, Sepúlveda N. Review of the quality control checks performed by current genome-wide and targeted-genome association studies on myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr. (2020) 8:293. doi: 10.3389/fped.2020.00293
45. Salari N, Khodayari Y, Hosseinian-Far A, Zarei H, Rasoulpoor S, Akbari H, et al. Global prevalence of chronic fatigue syndrome among long COVID-19 patients: a systematic review and meta-analysis. Biopsychosoc Med. (2022) 16:21. doi: 10.1186/s13030-022-00250-5
Keywords: ME/CFS, long COVID, prevalence, diagnostic criteria, fatigue, post-exertional malaise, disease duration, unrefreshing sleep
Citation: Grabowska AD, Westermeier F, Nacul L, Lacerda E and Sepúlveda N (2023) The importance of estimating prevalence of ME/CFS in future epidemiological studies of long COVID. Front. Public Health 11:1275827. doi: 10.3389/fpubh.2023.1275827
Received: 10 August 2023; Accepted: 23 October 2023;
Published: 03 November 2023.
Edited by:
Wakiro Sato, National Center of Neurology and Psychiatry, JapanReviewed by:
Bo Christer Bertilson, Karolinska Institutet (KI), SwedenAnthony L. Komaroff, Brigham and Women's Hospital and Harvard Medical School, United States
Copyright © 2023 Grabowska, Westermeier, Nacul, Lacerda and Sepúlveda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nuno Sepúlveda, N.Sepulveda@mini.pw.edu.pl
 Anna D. Grabowska
Anna D. Grabowska Francisco Westermeier
Francisco Westermeier Luís Nacul
Luís Nacul Eliana Lacerda
Eliana Lacerda Nuno Sepúlveda
Nuno Sepúlveda