- 1The Affiliated Fuzhou Center for Disease Control and Prevention of Fujian Medical University, Fuzhou, China
- 2School of Public Health, Fujian Medical University, Fuzhou, China
Background: In recent years, an increasing number of observational studies have reported the impact of air pollution on autoimmune diseases (ADs). However, no Mendelian randomization (MR) studies have been conducted to investigate the causal relationships. To enhance our understanding of causality, we examined the causal relationships between particulate matter (PM) and nitrogen oxides (NOx) and ADs.
Methods: We utilized genome-wide association study (GWAS) data on PM and NOx from the UK Biobank in European and East Asian populations. We also extracted integrated GWAS data from the Finnish consortium and the Japanese Biobank for two-sample MR analysis. We employed inverse variance weighted (IVW) analysis to assess the causal relationship between PM and NOx exposure and ADs. Additionally, we conducted supplementary analyses using four methods, including IVW (fixed effects), weighted median, weighted mode, and simple mode, to further investigate this relationship.
Results: In the European population, the results of MR analysis suggested a statistically significant association between PM2.5 and psoriasis only (OR = 3.86; 95% CI: 1.89–7.88; PIVW < 0.00625), while a potential association exists between PM2.5–10 and vitiligo (OR = 7.42; 95% CI: 1.02–53.94; PIVW < 0.05), as well as between PM2.5 and systemic lupus erythematosus (OR = 68.17; 95% CI: 2.17–2.1e+03; PIVW < 0.05). In East Asian populations, no causal relationship was found between air pollutants and the risk of systemic lupus erythematosus and rheumatoid arthritis (PIVW > 0.025). There was no pleiotropy in the results.
Conclusion: Our results suggest a causal association between PM2.5 and psoriasis in European populations. With the help of air pollution prevention and control, the harmful progression of psoriasis may be slowed.
Background
Autoimmune diseases (ADs), as products of the intertwined effects of innate genetic factors and environmental triggers, have attracted extensive attention worldwide. These diseases cause multiple immune system disorders (1), affect nearly 5% of the global population, and their prevalence and incidence are on the rise (2). It has a broad spectrum of diseases, including inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, and psoriasis, among others. They are not congenital diseases and can develop at any age, so it is particularly critical to explore their predisposing factors. However, the pathogenesis of ADs has not yet been fully clarified, and multiple risk factors such as genetic (3), immune (4), and environmental (2) are thought to be important in increasing their risk.
As people’s health awareness increases, the threat of environmental factors to health is gradually being emphasized. Studies have shown that environmental factors account for 40–70% of the development of ADs (5, 6). In retrospect, air pollution, as one of the major risk factors for the environment, has been temporally and strongly associated with the global increase in the incidence of type 2 diabetes mellitus (7), ADs (8) and cardiovascular diseases (9). In recent years, an increasing number of studies have linked ambient air pollution to the occurrence and development of ADs, suggesting that pollutants such as PM2.5, PM2.5–10, PM10, and nitrogen oxides (NOx) may increase the risk of diseases such as systemic lupus erythematosus (10), rheumatoid arthritis (11), inflammatory bowel disease (12), and psoriasis (13). These findings have been validated not only in European populations but also in East Asian populations (14, 15).
However, on the other hand, some studies have shown no association between air pollutants and the risk of developing rheumatoid arthritis (11, 16), inflammatory bowel disease (17) or multiple sclerosis disease (18, 19). This may be related to limitations of observational studies, such as insufficient adjustment for confounders, limited follow-up time, or small sample size. Therefore, many challenges remain to establish a clear causal relationship between air pollution and ADs. To overcome these limitations, we plan to use Mendelian randomization (MR) analysis as a tool to assess the causal relationship between PM2.5, PM2.5–10, PM10 and NOx with systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, vitiligo, multiple sclerosis disease, myasthenia gravis, coeliac disease and psoriasis. This approach is less susceptible to confounding bias and risk of reverse causation, and is expected to provide us with more accurate and reliable evidence to reveal the potential link between air pollution and ADs.
Methods
Study design
MR analysis is based on three essential assumptions. The first assumption is that the genetic variants proposed as instrumental variables should have a robust association with the exposure. The second assumption states that the chosen genetic variants should not be associated with any confounding factors. The third assumption is that the selected genetic variants should only affect the risk of the outcome through risk factors (Figure 1). This MR investigation is based on publicly available GWAS, and all included studies have received approval from the respective institutional review boards and ethics committees.
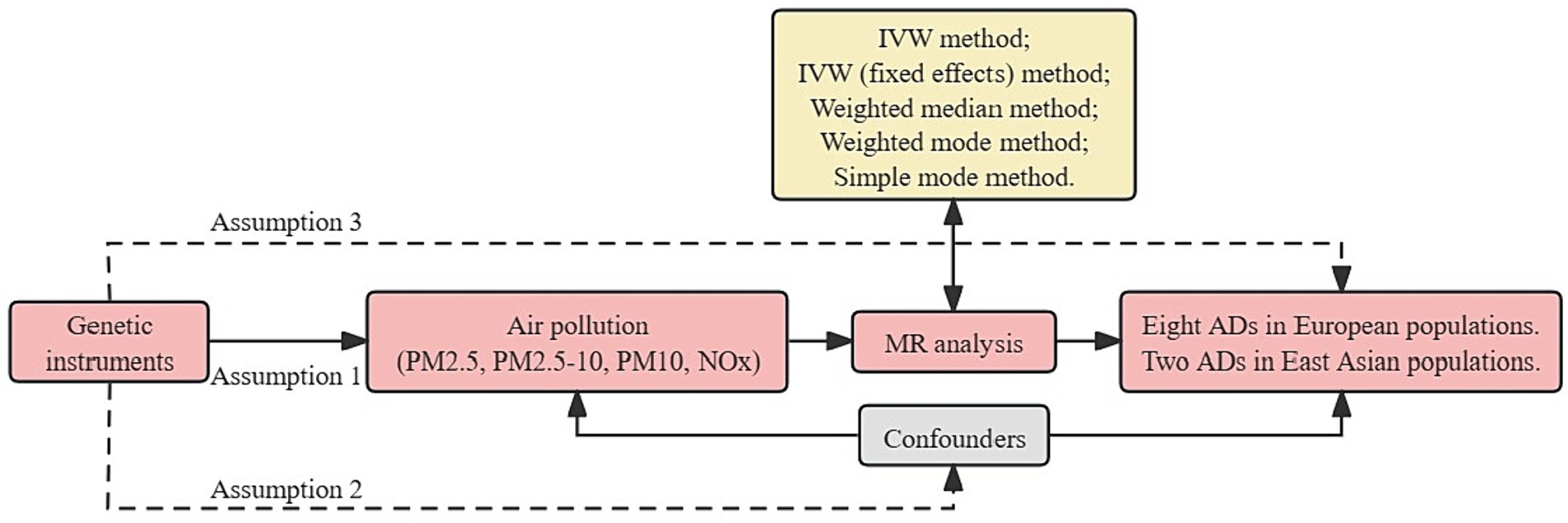
Figure 1. Overview flowchart of assumptions and schematic design. SNPs associated with air pollution were used as genetic instruments to study the causal effect of air pollution on frailty. Lines with arrows indicate that genetic instruments (SNPs) are associated with exposure and can only influence the outcome through exposure. Dashed lines indicate that the genetic tools (SNPs) are not associated with confounders between the results. ADs, autoimmune diseases; IVW, inverse variance weighted; MR, Mendelian randomization; NOx, nitrogen oxides; PM, particulate matter; SNPs, single nucleotide polymorphisms.
Data source
The dataset containing information on air pollutants was acquired from the UK Biobank’s Metadata of Environmental Exposures. To estimate air pollutants levels for the year 2010 at each address, a Land Use Regression (LUR) model developed as part of the European Study of Cohorts for Air Pollution Effects (ESCAPE) was utilized. The ESCAPE project received funding under the EU 7th Framework Program. The LUR model is based on monitoring conducted between January 26, 2010, and January 18, 2011, and the resulting air pollution estimates are representative of the year 2010.
Genetic associations for the eight ADs were obtained from the latest summary-level genetic data for European populations from the FinnGen study. In FinnGen, genome-wide association analysis was adjusted for gender, age, genetic ancestry, and genotyping batch. Genetic associations for two ADs in East Asian populations were also acquired from the Japan Biobank research and Wang YF (Supplementary Table 1).
Screening of genetic instruments
Genetic instrumental variables for environmental pollutants in European and East Asian populations were extracted from the latest GWAS data. The Medical Research Council Integrative Epidemiology Unit (MRC-IEU) conducted meta-analysis on GWAS data for environmental pollutants from the UK Biobank (Supplementary Table 1). A threshold of p < 5 × 10−8 was used to identify Single Nucleotide Polymorphisms (SNPs) significantly associated with PM2.5, PM10, and NOx exposure in European populations. Due to an insufficient number of SNPs, a threshold of p < 5 × 10−6 was used for PM2.5–10 exposure in European populations and all exposures in East Asian populations. SNPs were defined as not in linkage disequilibrium if r2 > 0.01 and clump distance >10,000 kb. Weak instrumental variable bias was assessed using F-statistics, ensuring that all SNPs had F-statistics greater than 10, thus confirming a strong correlation between instrumental variables and all exposures (Supplementary Table 2).
Statistical analysis
We evaluated the causal relationship between air pollutants and ADs using five MR methods. The primary method for MR analysis was the inverse variance weighted (IVW) method (20). Mendelian randomization Multi-Phenotype Residual Sum and Outlier (MR-PRESSO) was used to detect outliers in IVW linear regression and correct MR estimates after their removal. Supplementary methods included IVW (fixed effects), weighted median, weighted mode, and simple mode (Figure 1). Also, when the IVW method is statistically significant and the other methods are not, the OR value of the other methods must be in the same direction as the IVW, otherwise it is considered not statistically significant (21).
Sensitivity analyses were performed using various methods to confirm the robustness and validity of the results. Firstly, to assess heterogeneity between SNP estimates, Cochran’s Q-statistic was utilized. Secondly, to assess horizontal pleiotropy among SNP estimates, MR-Egger regression (22) and MR-PRESSO (20) global tests were employed for outlier detection. After removing outliers, IVW estimates without pleiotropy had a statistical threshold of p > 0.05. Finally, we also assessed bias due to individual SNP influence on outcomes using a leave-one-out analysis.
Bonferroni-corrected p-values included p = 0.05/8 = 0.00625 for adjusting multiple tests in European MR and p = 0.05/2 = 0.025 for East Asian MR. All statistical tests were two-sided, and R software version 4.3.0, along with the TwoSampleMR and MR-PRESSO packages, were used for analysis.
Results
In the European population, there is a correlation between PM2.5 levels and psoriasis (Pivw < 0.00625), while a potential association exists between PM2.5–10 and vitiligo, as well as between PM2.5 and systemic lupus erythematosus (Pivw < 0.05). Our results also show no support for the causal hypothesis that the remaining diseases are related to air pollution (Pivvw > 0.00625) (Figures 2, 3). Furthermore, there is no evidence of significant horizontal pleiotropy (Ppleiotropy > 0.05) (Supplementary Table 3). When heterogeneity is present, MR-PRESSO was used to remove heterogeneous SNPs (PCochrane’s Q > 0.05 and PMR-PRESSO > 0.05) (Supplementary Tables 3, 4). Leave-one-out analysis (Supplementary Table 5) indicates that removing each SNP one by one had little impact on the results, suggesting that no single SNP significantly influenced the overall causal effect estimate.
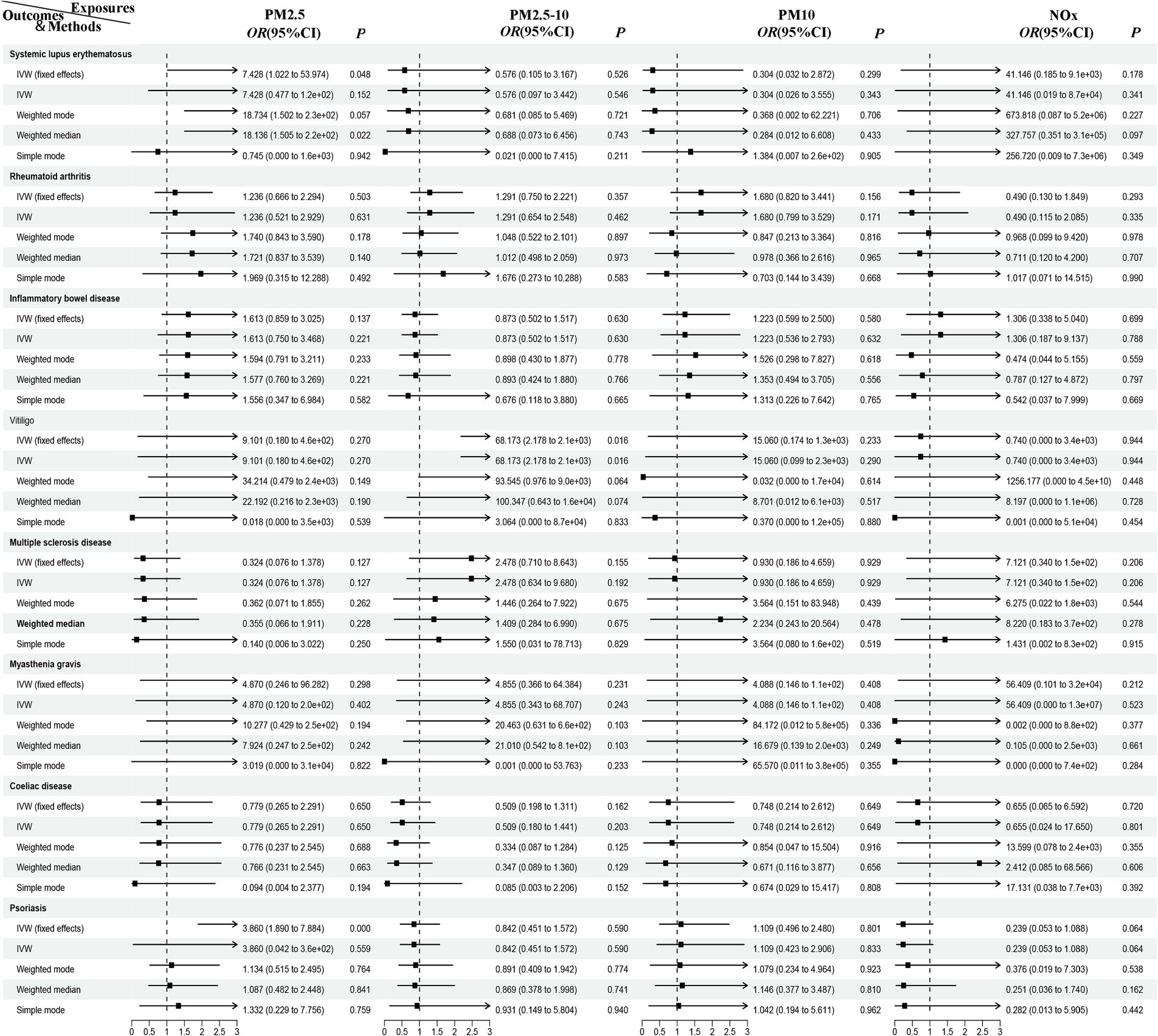
Figure 2. MR analysis of air pollution to ADs in European population. PM, particulate matter; NOx, nitrogen oxides; OR, odds radio; CI, confidence interval; IVW, inverse variance weighted; MR, Mendelian randomization; ADs, autoimmune diseases.
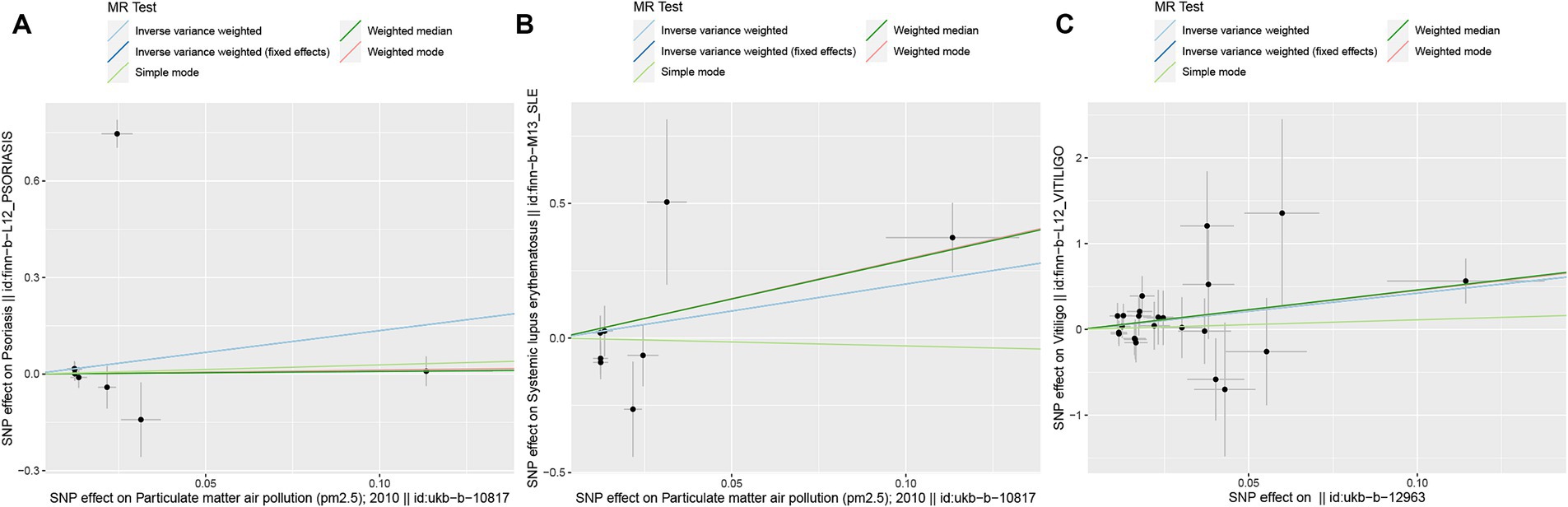
Figure 3. Scatter plots of SNPs associated with air pollution and ADs. Each black point representing each SNP on the exposure (horizontal-axis) and on the outcome (vertical-axis) is plotted with error bars corresponding to each standard error. The MR regression slopes of the lines represent the causal estimates using five approaches (IVW, IVW (fixed effects), simple mode, weighted median, and weighted mode). (A) PM2.5. (B) PM2.5. (C) PM2.5–10. MR, Mendelian randomization; SNP(s), single nucleotide polymorphism(s); ADs, autoimmune diseases; IVW, inverse variance weighted; PM, particulate matter.
Due to the lack of GWAS data for other ADs, MR analysis was only conducted for environmental pollutants and systemic lupus erythematosus and rheumatoid arthritis in the East Asian population, further enhancing the credibility of the results mentioned above. After removing the linkage disequilibrium IVs, 8, 23, 22, and 8 SNPs were identified for PM2.5, PM2.5–10, PM10, and NOx, respectively. The results show that in the East Asian population, there is no evidence of a non-causal relationship between air pollutants and the risk of systemic lupus erythematosus and rheumatoid arthritis (Pivw > 0.025). Using the IVW model, IVW fixed effects model, weighted model, weighted median model, and simple model methods, we found no evidence of a causal relationship between air pollutants and the two ADs (Pivw > 0.025) (Figure 4). However, due to the lack of sufficient instrumental variables for PM2.5–10 and rheumatoid arthritis MR, we only provided two analytical methods. Furthermore, there is no evidence of significant horizontal pleiotropy (Ppleiotropy > 0.05) and heterogeneity (PCochrane’s Q > 0.05 and PMR-PRESSO > 0.05) (Supplementary Table 3). Leave-one-out analysis (Supplementary Table 6) indicates that removing each SNP one by one had little impact on the results, suggesting that no single SNP significantly influenced the overall causal effect estimate.
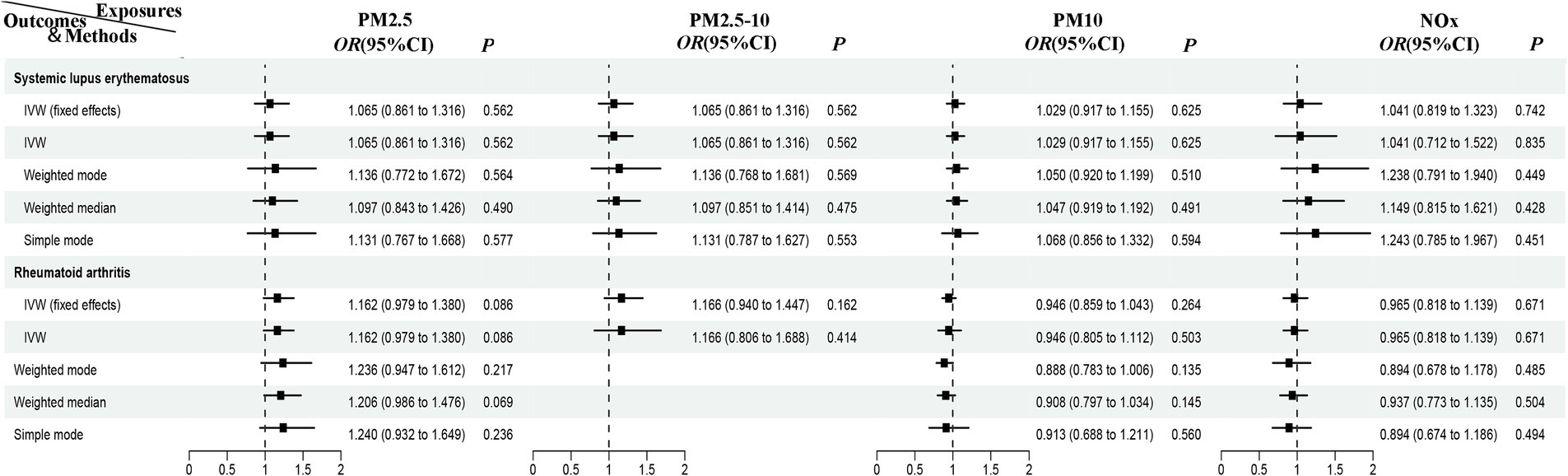
Figure 4. MR analysis of air pollution to systemic lupus erythematosus and rheumatoid arthritis in East Asian population. PM, particulate matter; NOx, nitrogen oxides; OR, odds radio; CI, confidence interval; IVW, inverse variance weighted; MR, Mendelian randomization.
Discussion
We conducted a comprehensive MR investigation of the associations between PM2.5, PM2.5–10, PM10, and NOx exposure and eight ADs in the European population. We further validated these associations with two ADs in the East Asian population. Multiple sensitivity analyses and MR analyses in two populations ensured the reliability of the results. We found a correlation between PM2.5 levels and psoriasis in individual diseases in European populations (Pivw < 0.00625); and potential associations between PM2.5–10 and vitiligo as well as between PM2.5 and systemic lupus erythematosus (Pivw < 0.05). However, overall, it appeared that PM2.5, PM2.5–10, PM10 and NOx were not associated with an increased risk of the eight ADs (except PM2.5 and psoriasis).
In a number of diseases in European populations, our findings are similar to those of previous studies (13, 23, 24). For systemic lupus erythematosus, vitiligo, and psoriasis, the biological relationship between the effects of air pollution on these diseases has not been established. The composition of PM, a major constituent of air pollutants, is more complex and varies from region to region. Previous studies (25, 26) have suggested that dysbiosis of the intestinal microflora is one of the pathogenic mechanisms of systemic lupus erythematosus. Since bacteria are also a constituent of PM, we hypothesize that airborne bacterial particles may also be involved in the immune-inflammatory response and predispose to the development of systemic lupus erythematosus. Previous studies (27, 28) have shown that oxidative stress damage to melanocytes plays an important role in vitiligo. Exposure to PM inhibits the secretion of stem cell factor (SCF) and basic fibroblast growth factor (bFGF) in keratinocytes, causing oxidative stress damage and disruption of melanocyte melanin metabolism (24). Therefore, PM may also be a risk factor for vitiligo. It has been claimed that PM treatment of keratinocytes increases cellular reactive oxygen species (ROS) production, leading to the activation of T-helper 1 (Th1) and Th17 cells (29). Specifically, PM activates aryl hydrocarbon receptors (important sensors of environmental chemicals) and further induces the production of ROS, leading to the inflammation associated with psoriasis (30, 31).
The current MR studies confirm the findings of previous epidemiological research (32, 33). However, our study results contradict some prior observational studies (10, 12, 27, 34–37). These contradictions may arise from unmeasured confounding factors, and the relatively small sample size in these studies may contribute to these discrepancies. Some studies (2) suggest that the lungs might be the initial site where PM triggers ADs. The mechanisms driving lung cancer due to fine PM are not primarily through increased genetic mutations but rather rely on altering the immune system, creating an inflammatory microenvironment, attracting macrophages to the lungs, and stimulating the release of IL-1β. Another study proposes that the mechanisms linking exposure to air pollutants with ADs primarily involve oxidative stress leading to systemic inflammation and immune imbalance. This includes the regulation of dendritic cells, regulatory T cells, and the function and phenotype of T cells, ultimately leading to the development of ADs (38). While these mechanisms seem plausible in theory, they have yet to be definitively validated through randomized controlled experiments. Furthermore, the existing observational research lacks sufficient compelling evidence, and its results exhibit variations.
Epidemiological and clinical research has provided some evidence suggesting that PM and NOx may not directly trigger ADs but could potentially prolong the duration of diseases, worsen clinical symptoms, and lead to disease relapse and other adverse effects. Therefore, implementing policies to reduce exposure to environmental pollutants, such as using filters in air conditioning systems or wearing masks in traffic, remains necessary. Some studies have indicated that PM and NOx exposure may prolong the course of systemic lupus erythematosus (39), exacerbate symptoms (40–42), and lead to complications (42). Another study has suggested that air pollution has pro-inflammatory effects on multiple sclerosis disease (43) and increases the risk of multiple sclerosis disease relapse (44, 45). Furthermore, there is also research indicating that exposure to PM and NOx may be associated with an increased risk of cancer (46), such as lung cancer (47). Additionally, the potential impact of other air pollutants (48) on ADs, including but not limited to ozone, kitchen fumes, nicotine, aldehydes, methane, and chlorofluorocarbons, should not be overlooked. Therefore, these research findings suggest that environmental pollutants may have varying degrees of impact on ADs and health issues like cancer, warranting further investigation into their mechanisms and the implementation of necessary measures to reduce pollutant exposure for public health maintenance.
Our study has several limitations that should be considered. Firstly, there is limited genetic data available for ADs in the East Asian population. While our MR analysis is based on a cross-ethnicity two-sample MR design, it only includes two diseases, namely rheumatoid arthritis and systemic lupus erythematosus. Future research should encompass a broader range of ADs in East Asian populations to validate the relationship between air pollutants and other ADs. Secondly, the exposure variance explained by the SNPs used as instruments for exposure is limited. In our study, the significance level for the SNPs associated with the four exposures in the East Asian population was 5e × 10−6. This may necessitate larger sample sizes to further validate our study’s conclusions, and future research efforts should aim to address these issues for a more comprehensive understanding of the relationship between air pollution and ADs, especially in East Asian populations.
Conclusion
In this MR study involving four pollutants and eight ADs in European populations and four pollutants and two ADs in East Asian populations, we found significant associations between PM2.5 and psoriasis as well as suggestive associations between PM2.5 and vitiligo, and PM2.5–10 and systemic lupus erythematosus in the European population only, and our study did not support the remaining associations of the causal hypothesis. Therefore, the next step needs to be taken with the help of air pollution control, which can slow down the harmful progression of psoriasis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HH: Data curation, Formal analysis, Validation, Writing – review & editing. XY: –. QC: Conceptualization, Methodology, Validation, Writing – review & editing. XH: Formal analysis, Supervision, Writing – original draft. XC: Conceptualization, Project administration, Validation, Writing – review & editing. XZ: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. YX: Conceptualization, Methodology, Resources, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Major Programs for Health and Wellness in Fujian Province (No. 2021ZD01001); Fuzhou Science and Technology Program (No. 2022-S-032); Natural Science Foundation of Fujian Province (No. 2023J01168).
Acknowledgments
We extend our heartfelt gratitude to the participants of CTG-CNCR, GEFOS, UK Biobank and PAGE for generously providing the data essential to our research. We also extend our appreciation to all the participants and researchers who actively contributed to this MR study, enabling its progress through their valuable participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1333811/full#supplementary-material
Abbreviations
ADs, autoimmune diseases; bFGF, basic fibroblast growth factor; ESCAPE, European Study of Cohorts for Air Pollution Effects; GWAS, genome-wide association study; IVW, inverse variance weighted; LUR, land use regression; MR, Mendelian randomization; MR-PRESSO, Mendelian randomization Multi-Phenotype Residual Sum and Outlier; NOx, nitrogen oxides; PM, particulate matter; ROS, reactive oxygen species; SCF, stem cell factor; SNP(s), single nucleotide polymorphism(s); Th, T helper.
References
1. Pisetsky, DS. Pathogenesis of autoimmune disease. Nat Rev Nephrol. (2023) 19:509–24. doi: 10.1038/s41581-023-00720-1
2. Zhao, CN, Xu, Z, Wu, GC, Mao, YM, Liu, LN, Qian-Wu,, et al. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev. (2019) 18:607–14. doi: 10.1016/j.autrev.2018.12.010
3. Jeong, DY, Lee, SW, Park, YH, Choi, JH, Kwon, YW, Moon, G, et al. Genetic variation and systemic lupus erythematosus: a field synopsis and systematic meta-analysis. Autoimmun Rev. (2018) 17:553–66. doi: 10.1016/j.autrev.2017.12.011
4. Sumida, TS, Cheru, NT, and Hafler, DA. The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases. Nat Rev Immunol. (2024) 1:15. doi: 10.1038/s41577-024-00994-x
5. Selmi, C, Lu, Q, and Humble, MC. Heritability versus the role of the environment in autoimmunity. J Autoimmun. (2012) 39:249–52. doi: 10.1016/j.jaut.2012.07.011
6. Ritz, SA. Air pollution as a potential contributor to the 'epidemic' of autoimmune disease. Med Hypotheses. (2010) 74:110–7. doi: 10.1016/j.mehy.2009.07.033
7. Huang, X, He, Y, Xu, H, Shen, Y, Pan, X, Wu, J, et al. Association between sociodemographic status and the T2DM-related risks in China: implication for reducing T2DM disease burden. Front Public Health. (2024) 11:1297203. doi: 10.3389/fpubh.2023.1297203
8. Piovani, D, Brunetta, E, and Bonovas, S. UV radiation and air pollution as drivers of major autoimmune conditions. Environ Res. (2023) 224:115449. doi: 10.1016/j.envres.2023.115449
9. Liang, F, Liu, F, Huang, K, Yang, X, Li, J, Xiao, Q, et al. Long-term exposure to fine particulate matter and cardiovascular disease in China. J Am Coll Cardiol. (2020) 75:707–17. doi: 10.1016/j.jacc.2019.12.031
10. Woo, JMP, Parks, CG, Jacobsen, S, Costenbader, KH, and Bernatsky, S. The role of environmental exposures and gene-environment interactions in the etiology of systemic lupus erythematous. J Intern Med. (2022) 291:755–78. doi: 10.1111/joim.13448
11. Zhang, J, Fang, XY, Wu, J, Fan, YG, Leng, RX, Liu, B, et al. Association of Combined Exposure to ambient air pollutants, genetic risk, and incident rheumatoid arthritis: a prospective cohort study in the UK biobank. Environ Health Perspect. (2023) 131:37008. doi: 10.1289/EHP10710
12. Chen, J, Dan, L, Sun, Y, Yuan, S, Liu, W, Chen, X, et al. Ambient air pollution and risk of Enterotomy, gastrointestinal Cancer, and all-cause mortality among 4,708 individuals with inflammatory bowel disease: a prospective cohort study. Environ Health Perspect. (2023) 131:77010. doi: 10.1289/EHP12215
13. Bellinato, F, Adami, G, Vaienti, S, Benini, C, Gatti, D, Idolazzi, L, et al. Association between short-term exposure to environmental air pollution and psoriasis flare. JAMA Dermatol. (2022) 158:375–81. doi: 10.1001/jamadermatol.2021.6019
14. Lan, J, Huang, Q, Yang, L, Li, Y, Yang, J, Jiang, B, et al. Effects of ambient air pollution on outpatient visits for psoriasis in Wuhan, China: a time-series analysis. Br J Dermatol. (2023) 188:491–8. doi: 10.1093/bjd/ljac124
15. Zhao, CN, Mei, YJ, Wu, GC, Mao, YM, Wu, Q, Dan, YL, et al. Effect of air pollution on hospital admissions for systemic lupus erythematosus in Bengbu, China: a time series study. Lupus. (2019) 28:1541–8. doi: 10.1177/0961203319882503
16. De Roos, AJ, Koehoorn, M, Tamburic, L, Davies, HW, and Brauer, M. Proximity to traffic, ambient air pollution, and community noise in relation to incident rheumatoid arthritis. Environ Health Perspect. (2014) 122:1075–80. doi: 10.1289/ehp.1307413
17. Kaplan, GG, Hubbard, J, Korzenik, J, Sands, BE, Panaccione, R, Ghosh, S, et al. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol. (2010) 105:2412–9. doi: 10.1038/ajg.2010.252
18. Yuchi, W, Sbihi, H, Davies, H, Tamburic, L, and Brauer, M. Road proximity, air pollution, noise, green space and neurologic disease incidence: a population-based cohort study. Environ Health. (2020) 19:8. doi: 10.1186/s12940-020-0565-4
19. Palacios, N, Munger, KL, Fitzgerald, KC, Hart, JE, Chitnis, T, Ascherio, A, et al. Exposure to particulate matter air pollution and risk of multiple sclerosis in two large cohorts of US nurses. Environ Int. (2017) 109:64–72. doi: 10.1016/j.envint.2017.07.013
20. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
21. Hemani, G, Bowden, J, and Davey, SG. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. (2018) 27:R195–208. doi: 10.1093/hmg/ddy163
22. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
23. Pan, Y, Fang, Y, Chen, Y, Chen, C, Zhang, RD, Fang, X, et al. Associations between particulate matter air pollutants and hospitalization risk for systemic lupus erythematosus: a time-series study from Xi'an, China. Environ Geochem Health. (2023) 45:3317–30. doi: 10.1007/s10653-022-01409-3
24. Suo, D, Zeng, S, Zhang, J, Meng, L, and Weng, L. PM2.5 induces apoptosis, oxidative stress injury and melanin metabolic disorder in human melanocytes. Exp Ther Med. (2020) 19:3227–38. doi: 10.3892/etm.2020.8590
25. Xiang, K, Wang, P, Xu, Z, Hu, YQ, He, YS, Chen, Y, et al. Causal effects of gut microbiome on systemic lupus erythematosus: a two-sample Mendelian randomization study. Front Immunol. (2021) 12:667097. doi: 10.3389/fimmu.2021.667097
26. Zhang, L, Qing, P, Yang, H, Wu, Y, Liu, Y, and Luo, Y. Gut microbiome and metabolites in systemic lupus erythematosus: link Mechanisms and Intervention. Front Immunol. (2021) 12:686501. doi: 10.3389/fimmu.2021.686501
27. Ezzedine, K, Eleftheriadou, V, Whitton, M, and van Geel, N. Vitiligo. Lancet. (2015) 386:74–84. doi: 10.1016/S0140-6736(14)60763-7
28. Rodrigues, M, Ezzedine, K, Hamzavi, I, Pandya, AG, and Harris, JEVitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. (2017) 77:1–13. doi: 10.1016/j.jaad.2016.10.048
29. Jin, SP, Li, Z, Choi, EK, Lee, S, Kim, YK, Seo, EY, et al. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J Dermatol Sci. (2018) 91:175–83. doi: 10.1016/j.jdermsci.2018.04.015
30. Shi, Y, Zeng, Z, Liu, J, Pi, Z, Zou, P, Deng, Q, et al. Particulate matter promotes hyperpigmentation via AhR/MAPK signaling activation and by increasing α-MSH paracrine levels in keratinocytes. Environ Pollut. (2021) 278:116850. doi: 10.1016/j.envpol.2021.116850
31. Kim, HR, Kang, SY, Kim, HO, Park, CW, and Chung, BY. Role of aryl hydrocarbon receptor activation and autophagy in psoriasis-related inflammation. Int J Mol Sci. (2020) 21:2195. doi: 10.3390/ijms21062195
32. Moore, JM, Norris, JM, and Clark, ML. Exposure to air pollutants and rheumatoid arthritis biomarkers: a scoping review. Semin Arthritis Rheum. (2024) 65:152365. doi: 10.1016/j.semarthrit.2024.152365
33. Celen, H, Dens, AC, Ronsmans, S, Michiels, S, and De Langhe, E. Airborne pollutants as potential triggers of systemic autoimmune rheumatic diseases: a narrative review. Acta Clin Belg. (2022) 77:874–82. doi: 10.1080/17843286.2021.1992582
34. Ding, S, Sun, S, Ding, R, Song, S, Cao, Y, and Zhang, L. Association between exposure to air pollutants and the risk of inflammatory bowel diseases visits. Environ Sci Pollut Res Int. (2022) 29:17645–54. doi: 10.1007/s11356-021-17009-0
35. Tang, KT, Tsuang, BJ, Ku, KC, Chen, YH, Lin, CH, and Chen, DY. Relationship between exposure to air pollutants and development of systemic autoimmune rheumatic diseases: a nationwide population-based case-control study. Ann Rheum Dis. (2019) 78:1288–91. doi: 10.1136/annrheumdis-2019-215230
36. Flood-Garibay, JA, Angulo-Molina, A, and Méndez-Rojas, MÁ. Particulate matter and ultrafine particles in urban air pollution and their effect on the nervous system. Environ Sci Process Impacts. (2023) 25:704–26. doi: 10.1039/d2em00276k
37. Wu, J, Chen, H, Yang, R, Yu, H, Shang, S, and Hu, Y. Short-term exposure to ambient fine particulate matter and psoriasis: a time-series analysis in Beijing, China. Front Public Health. (2022) 10:1015197. doi: 10.3389/fpubh.2022.1015197
38. Wang, S, Zhou, Q, Tian, Y, and Hu, X. The lung microbiota affects pulmonary inflammation and oxidative stress induced by PM2.5 exposure. Environ Sci Technol. (2022) 56:12368–79. doi: 10.1021/acs.est.1c08888
39. Chen, P, Huang, J, Li, S, Tang, Y, Xiao, Y, Zou, B, et al. Nitrogen dioxide and hospital length of stay and cost for systemic lupus erythematosus in Hunan, China. Sci Total Environ. (2023) 856:159013. doi: 10.1016/j.scitotenv.2022.159013
40. Yariwake, VY, Torres, JI, Dos Santos, ARP, Freitas, SCF, De Angelis, K, Farhat, SCL, et al. Chronic exposure to PM2.5 aggravates SLE manifestations in lupus-prone mice. Part Fibre Toxicol. (2021) 18:15. doi: 10.1186/s12989-021-00407-0
41. Bernatsky, S, Fournier, M, Pineau, CA, Clarke, AE, Vinet, E, and Smargiassi, A. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE). Environ Health Perspect. (2011) 119:45–9. doi: 10.1289/ehp.1002123
42. Bai, H, Jiang, L, Li, T, Liu, C, Zuo, X, Liu, Y, et al. Acute effects of air pollution on lupus nephritis in patients with systemic lupus erythematosus: a multicenter panel study in China. Environ Res. (2021) 195:110875. doi: 10.1016/j.envres.2021.110875
43. Cortese, A, Lova, L, Comoli, P, Volpe, E, Villa, S, Mallucci, G, et al. Air pollution as a contributor to the inflammatory activity of multiple sclerosis. J Neuroinflammation. (2020) 17:334. doi: 10.1186/s12974-020-01977-0
44. Jeanjean, M, Bind, MA, Roux, J, Ongagna, JC, de Sèze, J, Bard, D, et al. Ozone, NO2 and PM10 are associated with the occurrence of multiple sclerosis relapses. Evidence from seasonal multi-pollutant analyses. Environ Res. (2018) 163:43–52. doi: 10.1016/j.envres.2018.01.040
45. Elgabsi, M, Novack, L, Yarza, S, Elgabsi, M, Shtein, A, and Ifergane, G. An impact of air pollution on moderate to severe relapses among multiple sclerosis patients. Mult Scler Relat Disord. (2021) 53:103043. doi: 10.1016/j.msard.2021.103043
46. Yu, P, Han, Y, Wang, M, Zhu, Z, Tong, Z, Shao, X, et al. Heavy metal content and health risk assessment of atmospheric particles in China: a meta-analysis. Sci Total Environ. (2023) 867:161556. doi: 10.1016/j.scitotenv.2023.161556
47. Lowe, ME, Akhtari, FS, Potter, TA, Fargo, DC, Schmitt, CP, Schurman, SH, et al. The skin is no barrier to mixtures: air pollutant mixtures and reported psoriasis or eczema in the personalized environment and genes study (PEGS). J Expo Sci Environ Epidemiol. (2023) 33:474–81. doi: 10.1038/s41370-022-00502-0
Keywords: air pollution, autoimmune diseases, Mendelian randomization, particulate matter, nitrogen oxides
Citation: Hu H, Yang X, Chen Q, Huang X, Cao X, Zhang X and Xu Y (2024) Causal association between air pollution and autoimmune diseases: a two-sample Mendelian randomization study. Front. Public Health. 12:1333811. doi: 10.3389/fpubh.2024.1333811
Edited by:
Ciro Fernando Bustillo LeCompte, Toronto Metropolitan University, CanadaReviewed by:
Shaowei Wu, Xi’an Jiaotong University Health Science Center, ChinaVinoth Kumar Ponnusamy, Kaohsiung Medical University, Taiwan
Copyright © 2024 Hu, Yang, Chen, Huang, Cao, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyang Zhang, dawnsunz@126.com; Youqiong Xu, joancoco@126.com
†These authors have contributed equally to this work
 Haiping Hu1,2†
Haiping Hu1,2† Qingquan Chen
Qingquan Chen Xinfeng Huang
Xinfeng Huang Youqiong Xu
Youqiong Xu