- 1Postgrado Facultad de Ciencias Forestales, Universidad de Concepción, Concepción, Chile
- 2Iniciativa Foresta Nativa, Universidad de Concepción, Concepción, Chile
- 3Department of Soil and Crop Sciences, Texas A&M University, College Station, TX, United States
- 4Departamento de Planificación Territorial y Sistemas urbanos, Facultad de Ciencias Ambientales, Universidad de Concepción, Concepción, Chile
- 5Laboratorio de Ecología de Paisaje, Facultad de Ciencias Forestales, Universidad de Concepción, Concepción, Chile
Introduction: Anthropic disturbances are driving unprecedented changes in forest ecosystem functions and biogeochemical processes, hindering the forests’ benefits to society. Litter decomposition is one of the most critical processes that regulate forests’ carbon and nutrient cycling. However, how forest degradation affects litter decomposition and elemental dynamics requires further examination. The main objective of this study was to evaluate the effect of forest degradation on the production and decomposition of litter and C,N, and P dynamics in a temperate forest in south-central Chile.
Methods: Litter traps and litter bags were installed in three Long Term Research Forest Plots (LTER) representing different conservation states: mature, secondary, and degraded Nothofagus forests.
Results and Discussion: The total litter input varied between 3.5 to 1.1 Mg ha–1 year–1 in the mature and degraded forests, respectively. We found the highest lignin and nutrient levels in the degraded forest and the lowest in the mature forest. In the mature forest, 44% of the initial litter was decomposed, while in the degraded forest it only reached 7%. Decomposing litter showed the lowest C:N and C:P ratios in the mature forest most of the year. The balance between inputs and outputs yielded a more substantial litter accumulation in the mature forests.
Conclusion: Our results strongly suggest that anthropogenic degradation altered litter quality and nutrient dynamics while decreasing litter production and decomposition.
1. Introduction
Loss and degradation of forests are driving unprecedented changes in biodiversity and ecosystem functioning (1). A critical soil process is litter decomposition, which, together with litterfall, represents one of the most important pathways for the flow of nutrients in forests and soil fertility (2). Litter nutrient dynamics are closely related to the litter decomposition rate, which directly determines the nutritional status of the ecosystem (3). For this reason, research on litter decomposition has become a relevant aspect of the study of forest functioning (4). However, the effect of anthropogenic disturbance on these processes in natural forests has been poorly studied (5).
Litter decomposition rates depend on the interaction of three major factors: climatic conditions, litter quality, and soil organisms (6). Climate has been considered the main factor on a global scale as it directly affects decomposition rates through temperature and humidity (7). On a smaller scale, decomposition rates are strongly influenced by litter quality. Higher litter quality (i.e., high N, P), high N:P ratio, and low lignin concentrations and C:N led to a higher decomposition rate and mineralization (8–10). Soil organisms, such as bacteria, fungi, and fauna, mediate litter decomposition by degrading complex compounds such as lignin and cellulose. However, their contribution to decomposition at a local scale depends on their composition and abundance, which vary with litter quality and microclimate (1, 11).
Forest degradation due to human activities, like logging, livestock grazing, and fire, can directly or indirectly alter the composition and structure of forests (12). For example, a decrease in tree basal area, an increase in canopy opening, a change in species composition, and loss of species have been documented in degraded forests (1). These can modify the environmental conditions and litter quality due to the changes in species composition and traits (13). Since litter decomposition and dynamics are related to humidity and temperature, they can be affected by forest management and disturbances (14). Despite its relevance, the effect of anthropogenic disturbance on these processes in natural forests has been poorly studied. Studies have demonstrated that changes in microclimatic conditions associated with canopy openness have led to an increase in soil temperature and changes in humidity (15). In particular, it has been shown that higher temperatures and lower soil moisture in tropical secondary forests can lead to slower decomposition compared to primary forests (16). Similarly, other studies in disturbed tropical forests have reported a decrease in litter decomposition due to reduced biological activity in these forests (1, 13). However, the responses of decomposition rates and nutrient dynamics have proven to be challenging to predict, as decomposition rates have been reported to increase, decrease, or even remain unchanged in different forest ecosystem types across the world (5, 14, 15).
The Nothofagus forests of South America, which occupy from 37°S to around 55°S in Chile and Argentina (17), are a relevant component of the Andean landscape as they grow in areas with large-scale disturbances and at high altitudes that other species are unable to colonize. The Nothofagus genus is an ecologically significant group severely threatened by human activities that are endangering many of its species (18). The These forests have been strongly degraded by selective logging of tree individuals (19) and cattle grazing (12). These processes currently affect large extents of Nothofagus forests (20, 21). The effect of forest degradation on litter dynamics of Nothofagus forests is poorly understood. It has been documented that the clearing of Nothofagus forests in the extreme south of Chile can cause a decrease in litter production and an increase in decomposition rates due to the rise of surface temperature (15), while other studies report inconsistent trends in decomposition rates (22). In addition, litter nutrient cycling in more septentrional Nothofagus forests has been rarely evaluated as most studies have focused on well-preserved evergreen rainforests or colder temperate forests further south (~38°- 52° LS) or compared different litter qualities (23).
Hence, this study aims to assess the dynamics of litter stoichiometry, production, decomposition, and mineralization of C, N, and P in Nothofagus forests displaying different conservation states. We hypothesize that degraded forests have a significantly lower input of C, N, and P by litterfall, lower litter decomposition rate, and mineralization than better-conserved forests.
2. Methods
2.1. Study area
The study area corresponded to the Ranchillo Alto estate, located in the Andes foothills, 33 km away from the town of Yungay, Ñuble Region (37°04’ S and 71°39’ W) (Figures 1A, B). It has a humid temperate Mediterranean climate with an average annual rainfall of 3000 mm, with rains concentrated between May and September in the autumn and winter seasons. The mean annual temperature is 13.5°C, with July being the coldest month with a mean annual temperature of 3°C, and January is the warmest month with a mean of 22.5°C. The area presents an extended season of low temperatures, frequent frosts, snowfall, and the presence of snow for 3 to 5 months. The soils have been described and correlated to the Yungay series Pachic Melanudands (Andisols), formed from thick recent volcanic ashes deposited over a glacio-fluvial material. These soils are very deep and well-drained, with high organic matter content, dominant pseudo-crystalline mineralogy, and a silty loam texture (24, 25).

Figure 1 Map of Chile (A). Location of the study area in central Chile (B) (black point). The red line shows the boundary of the Ranchillo Alto estate, and the red squares delineate the location of the Long-Term Research Forest (LTER) plots (C). Image source: Esri, Maxar, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AeroGRID, IGN, and the GIS User Community.
Nothofagus temperate forests are documented to be the result of large-scale natural disturbances, including volcanic eruptions, landslides, and floods, and on small-scale disturbances, such as windthrow (26, 27). When canopy gaps become large enough to foster seedling development, shade-intolerant species such as Nothofagus alpina (Poepp. & Endl.) Oerst. And Nothofagus obliqua (Mirb.) Oerst. Can recruit and establish secondary forests (28, 29).
The study plots are located at 1300-1400 m.a.s.l., with 10-20% predominant western-facing slopes. The dominating tree species corresponded to Nothofagus species, which formed nearly pure stands (Table 1). Due to different regimes in human disturbances, the three Nothofagus forests stands differed in terms of composition, structure, and conservation states i) mature forest of N. dombeyi (Mirb) Oerst., which represent a well-preserved forest form mainly by trees 10 to 20 m tall and with a majority diameter at breast height (DBH) between 16 to 52 cm, with individuals exceeding 100 cm DBH, and a composition of 97% perennial trees; ii) secondary forest of N. alpina originated from selective logging for firewood, charcoal, and timber since 1950, it has a composition of 48% evergreen and 52% deciduous trees; and iii) degraded forest dominated by N. obliqua altered by tree cutting, fire, cattle browsing, and grazing for approximately 65 years, which is composed of 100% deciduous trees (Figure 1C). Illegal intensive logging has occurred since the 1950s, affecting all Nothofagus forest stands, which can be verified by the presence of stumps across the area (Table 1). However, the degraded forest has experienced substantial felling of the largest and healthy tree individuals, yielding a largely coetaneous N. obliqua forest with a few smaller clusters of N. dombeyi trees. Forest regeneration in the degraded site has been further limited by fire and continuous and non-systemic grazing (21, 30). In 2015, a forest management plan began, gradually stalling illegal logging and regulating cattle grazing in the area.
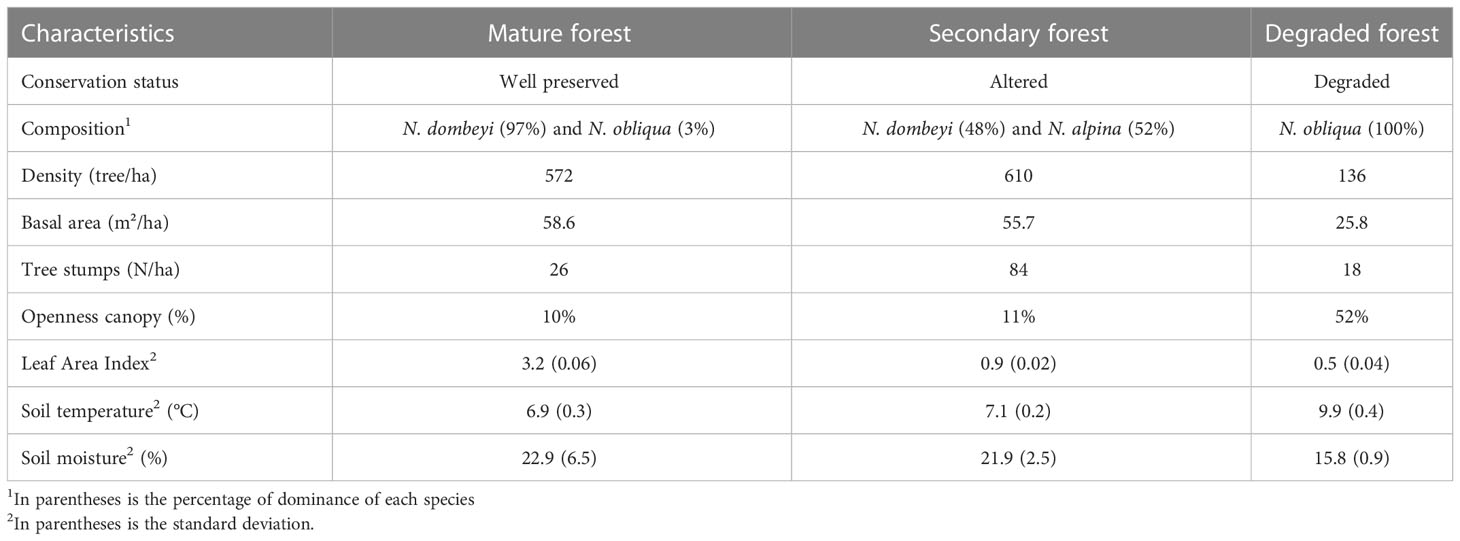
Table 1 Characteristics of the study forest stands in the LTER plots: mature, secondary, and degraded forests. Yungay, Chile.
2.2. Sampling design
Long-Term Ecological Research (LTER) plots of 100 x 100 m were established in each of the three forest stands. In each plot, we sampled litterfall and litter decomposition, for which sub-sampling plots were installed based on the GEM field manual (31) for intensive census plots, which are explained in detail below.
2.3. Sampling of litter biomass
Twenty-five 20x20 m subplots were delimited in each LTER plot (Supplementary Figure 1). In each subsampling plot, a 1x1 m litter collection trap was placed in April 2018 (75 total traps). The collection was carried out monthly from December 2018 until December 2019. The collection of samples during July 2019 was suspended due to adverse climatic conditions that impeded fieldwork (i.e., snowfall). The biomass collected in each trap was stored in hermetic bags. The content was transferred to paper bags and placed on a convection stove at 65°C for 48 to 72 hours until a constant weight was reached. Subsequently, the dry weight of each sample was determined.
2.4. Sampling of litter decomposition bags
Sixteen 25x25 m subplots were established in each LTER plot. Twelve correlatively numbered decomposition bags were placed at the center of each subplot (576 total bags) (32). Recently fallen litter was collected directly from the forest floor in each LTER plot during April and May 2018. The litter was dried at 65°C to constant weight, and a homogeneous sample was generated per plot. An aliquot of approximately 10 ± 1 g was removed and placed in 20x20 cm 1 mm mesh bags. The bags were installed in November 2018 between the litter layer and the mineral soil, simulating natural litter decomposition processes. The litter bag collection started in December 2018, after which a bag of each subplot was collected monthly for a year. Each bag was independently stored in airtight bags until delivery to the laboratory, where the material was dried at 65°C until constant weight. Posteriorly, the decomposition rate (k) was determined with the exponential model described by Olson (33) using Eq. (1).
Where X0 are initial and X1 final litter weight in a time t (33).
2.5. Remaining Litter stock
The remaining litter stock was calculated by subtracting the total annual decomposed litter to the annual input due to litterfall. The decomposed annual litter was calculated by multiplying the percentage of decomposed litter at the end of the study year by the annual input of litter. The remaining litter stock should approximate the total amount of litter remaining on the forest floor in each forest plot after a year.
2.6. Carbon, nutrient, and litter quality analysis
The carbon and nutrient content of the fallen litter and litter of decomposition bags was determined. An aliquot of the sampled material from each bag was taken at each sampling date and combined into a composite sample for each plot. These were pre-grounded in a chipper to 2mm and then pulverized in an 8000M Mixer/Mill® steel pearl mill from SPEX SamplePrep. Posteriorly 2.00 ± 0.1 mg of each sample in tin capsules were weighed in a Sartorius model ME36S microbalance (Sartorius AG, Germany). The total C and nitrogen (N) contents were determined by the Dumas-TCD dry combustion method (SERCON® Limited, UK). Total phosphorus (P) was determined by the calcination method for plant tissue described by Sadzawka et al. (34). With these results, we calculated C:N, C:P, and N:P on a mass basis for litterfall and decomposing litter. The lignin concentration was determined following the methodology used by Mendonça et al. (35). For this, extractables were removed with ethanol/toluene; then hydrolysis was carried out with 72% H2SO4 in a water bath at 30°C for 1 hour. The acid was then diluted to 3% with water, and the mixture was autoclaved for one hour at 121°C. The residual material was cooled and filtered, and the solids dried to constant weight at 105°C and determined as insoluble lignin. Soluble lignin was determined by measuring the absorbance of the solution at 205 nm (35).
Fourier-transform infrared (FTIR) band indices were calculated as a complementary measure to characterize litter quality. Index I (Eq. 2) have been used to indicate differences in the degree of decomposition. In this study, we used it to show the degree of aromaticity of the litter material (i.e., aromatic versus aliphatic bonds). Similarly, Index II (Eq. 3) was used as a proxy for organic matter recalcitrance (36). These indices are based on the intensities of the FTIR bands representing various functional groups, which are detailed below:
According to Veum et al. (37), Index I is the ratio of aromatic C=C (bands 1650 and 920) to aliphatic and CH (bands 2924, 2850, and 1470) functional groups; this index has been shown to increase with the degree of soil organic matter decomposition. Index II represents relative recalcitrance as the ratio between C (bands 2924, 2850, 1650,1470 and 920) and O (bands 3400 and 1080) functional groups, which is higher in more recalcitrant organic matter (37). For this analysis, original litter samples used for the litter bags were ground in a chipper at 2 mm, milled, and analyzed in an FT-IR Spectrometer (Thermo Scientific, Nicole iS5) with attenuated total reflectance (ATR) and automatic baseline correction. Spectra were obtained in triplicates, each based on the mean of 64 scans at 4000–400 cm-1 with a resolution of 4 cm-1. Based on the spectrum’s prominent peaks and shoulders, seven bands representing organic functional groups were identified, and the indices I and II were calculated. Peaks were selected, and absorbance intensity was measured after background removal using Essential FTIR (v3.50.205).
2.7. Data analysis
Litterfall mass was compared between forest stands and through time by a non-parametric Kruskal-Wallis test, as normality was not met according to Shapiro-Wilk tests. If the Kruskal-Wallis test indicated at least one significant difference between groups, Wilcoxon signed-rank tests were used. The same approach was used to compare the mass of C, N, and P, for which the “RSTATIX” R (38) and the “CAR” R (39) packages were used. Indices I and II and initial concentrations of C, N, and P were compared using Welch’s T-tests because the data met the normality assumptions but not homoscedasticity.
The decomposition rate (k) was transformed to 1/k, fulfilling the normality and homogeneity assumption. An ANOVA was performed to identify significant differences in the k-mean values between forest types. Because significant effects of forest stands were found, a Tukey’s test was carried out. The remaining litter mass was compared with a Kruskal-Wallis test because the homogeneity assumption was met, but not normality. If this indicated at least one significant difference between groups, Wilcoxon rank-sum post hoc tests were performed, a non-parametric alternative to two-sample t-tests. All the statistical and graphic analyses were executed in R version 3.2.1 (40). Averages and standard error were reported in all the analyses, and p< 0.05 were considered significant.
3. Results
The initial concentration of N and P in the litter differed between forest stands, from lowest to highest: mature forest, secondary forest, and degraded forest. At the same time, the degraded forest had a significantly lower concentration of C and the highest concentration of lignin. Lignin was the lowest in the secondary forest (Table 2). The Index I was lower in the litter of both the mature and degraded forests, and highest in the secondary forest, indicating greater aromaticity of litter in the secondary forest. On the other hand, the Index II was higher in the mature and degraded forests, suggesting greater potential recalcitrance of the material compared to the litter of the secondary forest, which is consistent with its lowest lignin and total C contents (Table 2).
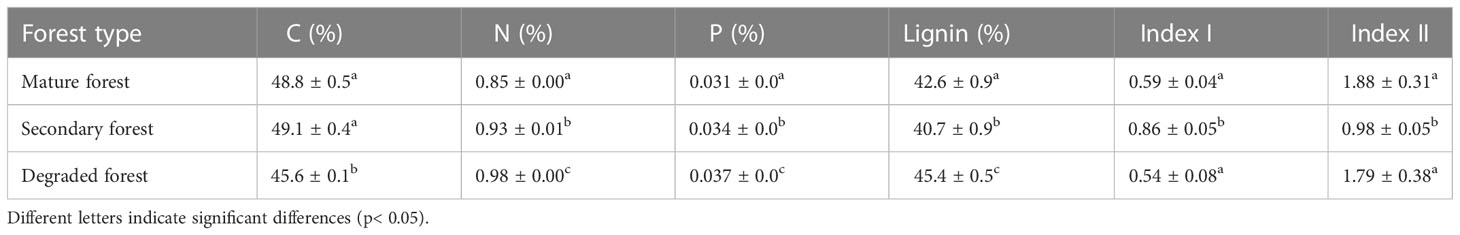
Table 2 The initial concentration of litter C, N, P, lignin and Index I and II in mature, secondary, and degraded Nothofagus forests LTER plots.
The annual litterfall ranged from 1.2 Mg ha−¹ year−¹ in the degraded forest to 3.8 Mg ha−¹ year−¹ in the mature forest (Table 3). Mature and secondary forests had a significantly higher litterfall than the degraded forest (p< 0.05). The three forest plots followed the same pattern of litterfall across the year, increasing during the autumn (Figure 2A) and with a minimum during the summer and spring months.
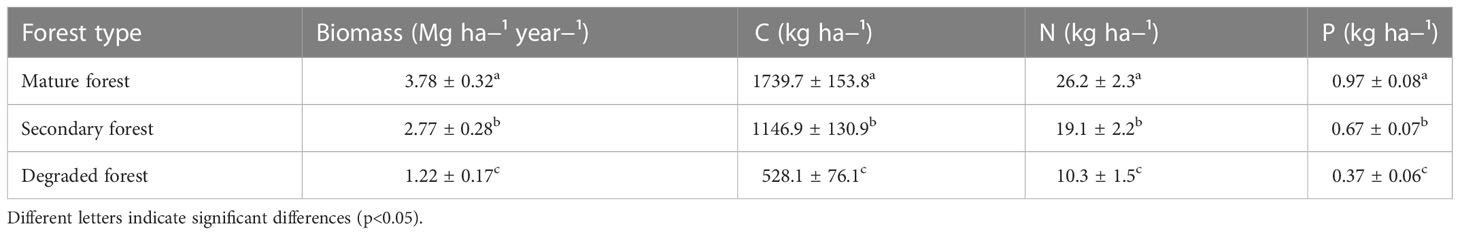
Table 3 Mean annual litterfall (Mg ha−¹ year−¹) and C, N and P (kg ha−¹) in mature, secondary, and degraded Nothofagus forests LTER plots from December 2018 to December 2019.

Figure 2 Litter biomass (A), molar C:N (B), C:P (C) and N:P (D) stoichiometric ratios, and Carbthe (E), Nitrogen (F) and Phosphorous (G) average inputs from December 2018 to December 2019 in mature (dark blue), secondary (light blue), and degraded (red) Nothofagus forests LTER plots. For (A, E, F, G) the error bars represent the standard deviation. For A significant differences within each month are shown with an asterisk.
The C:N ratio of litterfall varied throughout the year and between forest plots, from lowest to highest: degraded forest, secondary forest, and mature forest (between March and August) (Figure 2B). The litterfall C:P and N:P ratios followed a similar tendency for all forest plots over time, with a considerable increase in spring between September and December (Figures 2C, D). The mature forest showed a sharper increment in C:P and N:P in spring than the other forest types; however, litterfall production during this period abruptly declined.
The amounts of litter C, N, and P inputs varied throughout the year and between forest plots (Figure 2E–G respectively). Annually, the total amount of C in the litter went between 528.1 and 1739.7 kg ha−¹ year−¹ for degraded and mature forests, respectively. Similarly, the quantity of N ranged from 10.3 kg ha−¹ year−¹ in the degraded forest to 26.2 in mature forest, and P from 0.37 kg ha−¹ year−¹ in the degraded forest to 0.97 in the mature forest (Table 3). The mean annual amount of C, N, and P contributed by the mature forest was significantly higher than the other forest stands (p< 0.05).
After one year, the remaining litter mass was 56% for the mature forest, 65% for the secondary forest, and 93% for the degraded forest. The decomposition constant (k) was significantly higher in the mature forest, followed by the secondary, which was also considerably higher than k in the degraded forest (p< 0.05). In the same way, the mass of remaining litter differed substantially between the forest conservation states (Table 4). Regarding temporal variation, the highest decomposition rates were observed in the first month after installation and in the last month, both corresponding to December, and varied significantly between forest plots (Figure 3A). In the case of secondary forest, a high decomposition rate was also observed in September (spring).

Table 4 Mean decomposition rate and remaining mass (%) after 390 days of decomposition in mature, secondary, and degraded Nothofagus forests LTER plots.
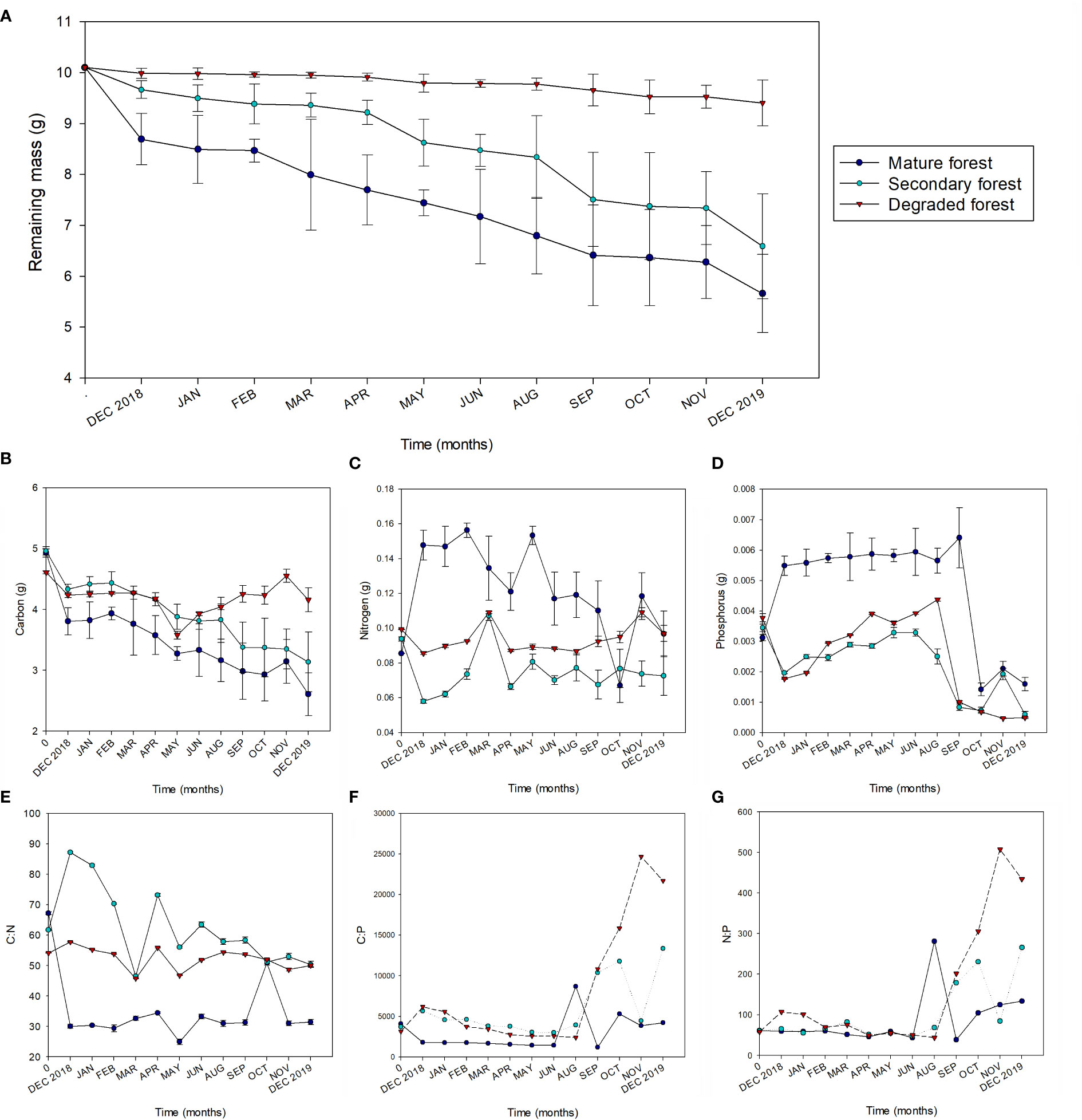
Figure 3 Remaining litter biomass (A), mass of Carbon (B), Nitrogen (C) and Phosphorus (D), and molathe:N (E), C:P (F) and N:P (G) stoichiometric ratios during litter decomposition from December 2018 to December 2019 in mature (dark blue), secondary (light blue), and degraded (red) Nothofagus forests LTER plots. For (A–D) the error bars represent the standard deviation.
Decomposing litter total C decreased significantly over time in all forest plots, but this trend was more consistent in the mature and secondary forests. In the degraded forest, total litter C tended to increase from May to November (Figure 3B). N presented an initial net accumulation in mature forest, which decreased towards the end of the year (Figure 3C). The secondary forest showed a slight decrease in N content. In contrast, in the degraded foresmount amount of N remained relatively constant between the beginning and the end of the study period. P increased substantially in the mature forest after the first month and stayed steady until the spring, when it dropped significantly until the end of the experiment (Figure 3D). On the contrary, litter P in the secondary and degraded forests dropped during the first month and then gradually increased until the spring when it fell significantly.
The C:N on the decomposing litter was lower for the mature forest, intermediate for the degraded forest, and higher for the secondary forest (Figure 3E). There was a substantial decrease in the C:N ratio in the mature forest after the first month. Conversely, C:N ratios in the secondary forest increase abruptly after the first month and then gradually decrease until the end of the experiment (except for a substantial drop observed in March). Meanwhile, the values of the degraded forest were relatively constant between the start and the end of the experiment. The C:P and N:P ratios followed a similar trend over the evaluated period, with a differential increment in spring and summer among forest plots (Figures 3F, G).
Finally, the total balance between all inputs of litter and decomposition outputs indicates that the remnant stock of litter is 2.14 Mg ha-1 in mature forest, which represents 56.6% of litter inputs; 1.81 Mg ha-1 in secondary forest, equivalent to 65.3% of litter inputs; and 1.14 Mg ha-1 in the degraded forest, equivalent to 93.4% of litter inputs (Table 5).
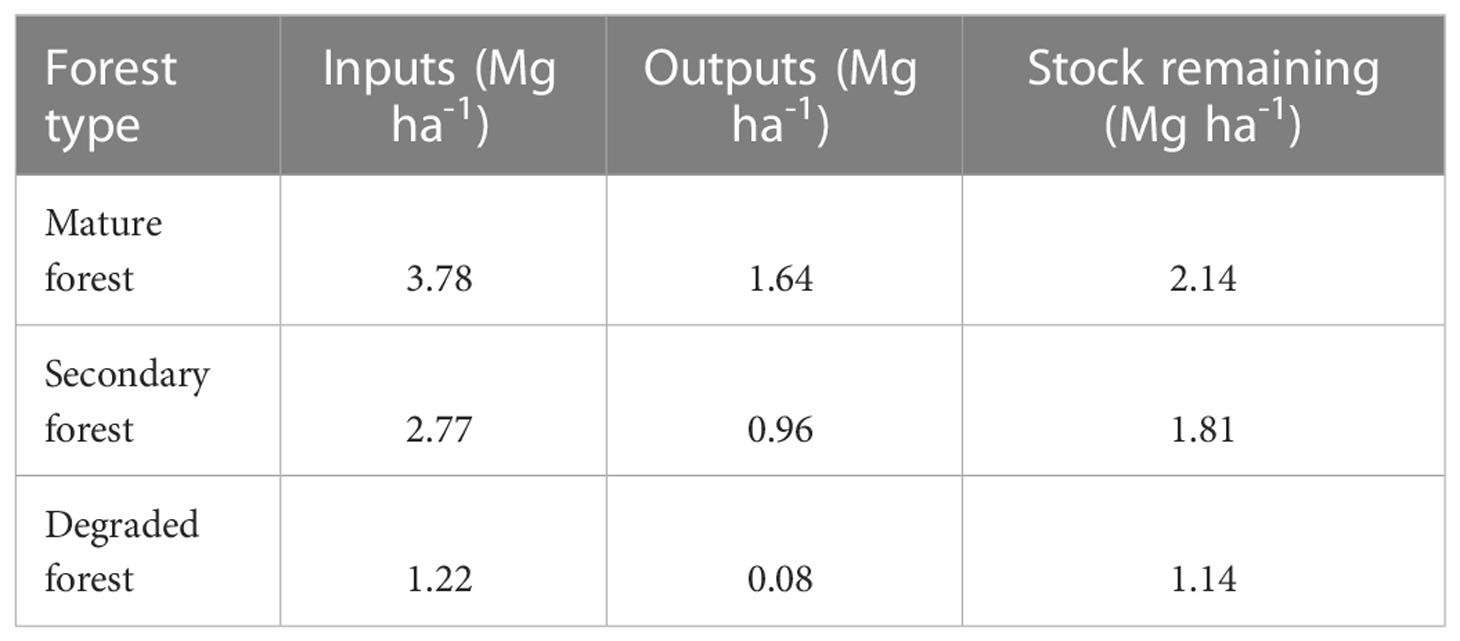
Table 5 Litter inputs, decomposition outputs, and remaining stock (Mg ha-1) between December 2018 and December 2019 in mature, secondary, and degraded Nothofagus forests LTER plots.
4. Discussion
Our results support the hypothesis that a degraded forest has a lower nutrient input due to a lower litterfall and decomposition rate. However, contrary to our expectations, the chemical quality of the litter (i.e., nutrient stoichiometry) did not help explain this behavior. Nutrient contents were higher in the degraded forest than in the mature and secondary forest. Although this higher nutrient content did not lead to higher litter decomposition rates, probably due to its higher lignin concentration and the adverse abiotic conditions for decomposition to proceed, especially lower soil moisture (1, 13).
4.1. Litter production and C:N:P stoichiometry
The annual litterfall in forest types was within the observed range (1.0 to 5.8 Mg ha-1 yr-1) for Nothofagus forests of central and southern Chile (22, 41), but it was slightly lower than the range observed in temperate forests in other regions (4.7 to 6.0 Mg ha-1 yr-1) (42). In the case of the degraded forest, it presented values of litterfall similar to those reported for managed Nothofagus forests (41). Litterfall in the degraded forest was lower than in mature and secondary forests, which coincides with its low basal area, high canopy opening, and low LAI (43, 44). This reduced litter production can decrease carbon and nutrient inputs to the soil, limiting plant growth and regeneration on degraded sites (45–47).
Litter C:N, C:P, and N:P ratios were high in all forest plots compared to those reported for leaf litter from other temperate forests on a global scale (48). As expected, the stoichiometric relations varied over the year. An increment was observed in the C:N ratio from autumn to winter, coinciding with the season of higher litterfall. The lower N concentration during these months may reflect preferential reabsorption of this element by vegetation (49), which contributes to internal recycling and conservative use of this nutrient (50, 51). On the other hand, the N:P and C:P ratios increased from spring to summer, suggesting a preferential relocation of phosphorus, which was particularly high in the mature forest. This contrasts with Caldentey et al. (41), who found a decrease in the concentration of P in the litter in autumn and winter. The difference between forest types may be due to the dominance of N. dombeyi in mature forests, a perennial species that has a longer leaf life span and a low N and P concentration. The observed temporal trends suggest higher recycling and more conservative nutrient use strategies in the mature forest (51, 52).
The litter C, N, and P inputs strongly depended on the quantity of litter produced, being higher in autumn for N and P in all forest plots (Figures 2E, F, G). The annual amount of nutrients provided by the litter was lower than that indicated by other studies in temperate forests of central-southern Chile, which report values between 44 to 69 kg ha-1 year-1 for N and 2.6 to 3.6 kg ha-1 year-1 for P (22). This difference could be due to the lower density of trees in the present study compared to that obtained by Staelens et al. (22), lower N and P soil availability (P-fixing andosol and low atmospheric N inputs), and the difference in species composition. It has been documented that temperate ecosystems tend to have low levels of N and P due to the low atmospheric and weathering inputs and hydrologic losses of dissolved organic P and N, all of which result in low nutrient concentrations in soils (53). Likewise, low N mass in the degraded forest may be due to episodic N losses associated with fires and removal from logging, grazing, and other local disturbances (54). The total C, N, and P mass contributed by the mature forest confirm that litter in these well-preserved forests is a more substantial C and nutrient reservoir. The greater availability and active internal cycling of these elements in mature forests sustain forest productivity and regeneration, supporting other critical ecosystem processes.
4.2. Litter decomposition and nutrient dynamics
The initial concentrations of N, and P in the degraded forest litter (Table 2) suggest that this site has a better nutritional quality than the secondary and mature forests. This may be due to the fact that the degraded forest is mostly composed of deciduous species composition, which has been shown to have a higher nutrient content compared to the litter of perennial species (55). The latter result is also consistent with previous studies in tropical forests that have shown an increase in litter quality along disturbance gradients (56). This is explained by the recruitment of fast-growing species with economic litter traits, which could lead to rapid decomposition rates (57). However, the higher nutrient concentration in the degraded forest did not yield higher decomposition rates. In fact, we found that litter from mature and secondary forest composed of a mix of deciduous and evergreen species decomposed faster than deciduous litter from degraded forest. In addition, the FTIR-derived indices I and II suggest similar aromaticity and recalcitrance levels between the litter of the mature and degraded forests. Thus, the decomposition rates are likely controlled by factors different than nutrient concentration. Our results suggest that high lignin concentrations in degraded forest litter, together with environmental factors (i.e., higher soil temperature and limited surface moisture) could lead to reduced decomposition rates, as other authors have suggested (58). Recent studies have also shown a deceleration of litter decomposition and lignin degradation in cleared forests with heightened direct solar radiation (59).
Moreover, the reduced decomposition in the degraded forest could also be due to the lower litter diversity compared to the mature and secondary forests (60), which had a greater mix of species. Synergistic effects on decomposition have been documented when litter species of different quality are mixed, which could accelerate the rate of decomposition in these forests (61). Some mechanisms that explain this effect are the interaction between the microbiomes associated with each litter type, the complementary effects of soil fauna and decomposing organisms, and the improvement in microclimatic conditions during decomposition (60), mechanisms that could be absent in the degraded forest.
The remaining mass agrees with previous studies in temperate Nothofagus forests, except for the degraded forest, which displayed a much larger remaining mass of 93%. This result differs from earlier studies in Nothofagus forests, which found increased decomposition rates in disturbed forests associated with higher temperature and humidity (15, 41). In our study, the degraded forest had a higher temperature but lower soil moisture, which could affect the decomposition process. However, our results are consistent with studies that also report a reduction in litter decomposition after clear-cutting or thinning, associated with a decrease in soil moisture and its biological activity (14, 62). Similarly, a reduction in decomposition rates has been observed in degraded tropical forests exhibits as the intensity of disturbances increases (13).
An earlier study conducted on the same study plots showed that bacterial and fungal soil communities differed at the genus level between forest types (30). Likewise, these authors reported a change in the structure of the microbial community in the most degraded forests, which could affect litter decomposition. Furthermore, other authors have reported reduced microbial activity after logging (63). The harsher conditions for microbes may have also reduced the activity of soil mesofauna. Due to the importance of these organisms for the decomposition of organic matter, particularly in the degradation of lignin (58, 64), reduced faunal activity can also explain the low decomposition rates found in the degraded forest (13). We also observed a noticeable reduction in understory coverage and plant composition, which could explain a reduction in litter decomposition driven by a decrease in mesofauna activity (65). However, this is an aspect that needs to be further studied.
The accumulation of N in all forest types, followed by short nutrient release periods, coincides with Staelens et al. (22), who reported the same trends for other deciduous species. The initial immobilization has been reported in different parts of the world for temperate and boreal climates (66, 67). The accumulation of N at the beginning of decomposition cycle may be due to microbial immobilization under low N availability (68). This explains the high accumulation of N and the lower C:N ratio in mature forests, which presented the lowest initial content of this element. Despite the higher litter quality (higher N content) in the degraded forest, it tended to accumulate more N than in the secondary forest. This N enrichment could result from external inputs from grazing livestock in the degraded forest area (69).
C:P and N:P values indicated a period of initial immobilization and high mineralization towards the end of this study. This initial accumulation may be due to external sources, for example, the precipitation and fall of new litter from the canopy (70) and also livestock grazing (69). The content of P decreased drastically starting in spring, which suggests more substantial mineralization of this element and reabsorption after the rainy season. Seasonal patterns in humidity and temperature that control microbial communities can influence changes in stoichiometry (71). During the spring (September) the conditions are more favorable for plant growth, microbial and soil fauna dispersion, which stimulates the mineralization of elements, especially P (72). This could explain the increase in N:P and C:P ratios in the remaining litter.
As the decomposition progressed, there was a decreasing trend in the C:N and C:P ratios until reaching values close to 37 - 51 and 700 - 900 (73). In our study, the C:N ratio decreased with decomposition, reaching values close to those indicated; however, the C:P ratio increased towards the end of the period, reaching values much higher than those reported by these authors. This may be due to high initial C:P values, which have led to high ratios during decomposition (74). In addition, due to the low decomposition rates found, a more extended study period may be necessary to observe a convergence toward lower C:P ratios (75, 76).
The difference in nutrient dynamics during litter decomposition between forest conservation states may be due to the difference in litter quality (5, 77). Different authors have found an initial immobilization of N and P in low-quality litter and a more significant release of these elements during the decomposition of high-quality litter (72). In our study, we found a greater initial immobilization of N and P in the mature forest, which presented the lowest concentrations of these nutrients. However, despite the higher quality of the litter in the degraded forest, it did not show a greater nutrient release. On the other hand, it has been reported that high concentrations of lignin in the litter can increase the initial immobilization of N and P due to the formation of recalcitrant substances (78). We found a high concentration of lignin in all forest stands but the lowest in the secondary forest, which coincidentally presented the lowest initial immobilization.
4.3. Remaining litter stock
The annual litter production was three times higher in the mature forest and two times higher in the secondary than in the degraded forest. After balancing inputs and outputs by decomposition, the mature forest presents the highest accumulation of litter on the forest floor. Due to its higher decomposition rate, we could also expect a higher carbon influx into the mineral horizon and nutrient influx through mineralization. Previous studies have found less litterfall and nutrient influx in degraded Mediterranean, Temperate, and Tropical forests, along with a depletion of ecosystem carbon stocks and reduced soil nutrient availability. Both factors decrease the recycling of nutrients and limit forest productivity, soil functionality, and the provision of ecosystem services (3, 47, 79, 80).
The differences in litterfall, decomposition, and dynamics of C, N, and P showed that forest degradation in these sites altered litter production, litter quality, and the dynamics of C, N, and P mineralization. Hampering these critical biogeochemical processes limit soil fertility and thus the regenerative capacity, productivity, and ecological complexity of these forests, making them less resilient to ever-increasing biotic and abiotic disturbances driven by global change (81).
5. Conclusions
Litter dynamics and nutrient cycling of Nothofagus forests vary according to their conservation state. Forest degradation by human disturbances results in different amounts of litterfall, decomposition rates, and contrasting C, N, and P dynamics. Higher decomposition in mature Nothofagus forests indicates faster nutrient cycling than in the degraded forest. Furthermore, nutrient reabsorption in mature forests suggests a more efficient internal cycle despite their lower litter quality. On the contrary, low litterfall and low decomposition in degraded forests indicate an altered ecological functioning, which can reduce the availability and release of nutrients limiting ecosystem productivity and regeneration, as well as hindering the provision of key benefits such as carbon sequestration and nutrient cycling. These findings support the importance of preserving mature forests to maintain biogeochemical processes and, thus, the productivity and sustainability of terrestrial ecosystems. On the other hand, despite the recognized importance of litter quality for litter decomposition, we found that other factors may be co-limiting decomposition rates in degraded forests, such as changes in microclimatic conditions, which may also hinder decomposers activity. Highly dynamic C:N:P stoichiometry of litterfall and litter emphasizes the need for long-term monitoring of these parameters to fully understand the multi-elemental cycling during decomposition and transformation of litter into organic soil horizons.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FL wrote the original manuscript draft and performed the data curation and statistical analysis under the guidance of FA. FA contributed to the conception and design of the study methodology and funding acquisition. NA collected samples, performed analysis and organized the database, wrote sections of the manuscript, and implemented the methodology. CE contributed to the funding acquisition. PG provided additional data and database organization. All authors contributed to the manuscript revision, read, and approved the submitted version.
Acknowledgments
We thank the collaboration project N 73-J-21-ER2 between ENEL-University of Concepcion and Foresta Nativa, which supported this study, and the Faculty of Forestry Sciences of the University of Concepcion for allowing us to carry out this study in the Ranchillo Alto National Protected Property. FA was supported by the USDA National Institute of Food and Agriculture, Hatch project 7002883 “Pedological and Biogeochemical Implications and Mitigation of Land-Use Intensification”. We also thank all the students and colleagues who helped us in the fieldwork and processing of the samples. Special mention to Dr. Regis Teixeira and Claudia Vidal from the University of Concepcion Biotechnology Center for their support in determining lignin and FTIR analyses.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsoil.2023.1111694/full#supplementary-material
References
1. Paudel E, Dossa GG, de Blécourt M, Beckschäfer P, Xu J, Harrison RD. Quantifying the factors affecting leaf litter decomposition across a tropical forest disturbance gradient. Ecosphere (2015) 6(12):1–20. doi: 10.1890/ES15-00112.1
2. Berg B, McClaugherty C. (2014). Decomposition as a process: Some main features. In: Plant Litter. Springer, Berlin, Heidelberg. 11–34. doi: 10.1007/978-3-642-38821-7_2
3. Bohara M, Yadav RKP, Dong W, Cao J, Hu C. Nutrient and isotopic dynamics of litter decomposition from different land uses in naturally restoring taihang mountain, north China. Sustain (Switzerland) (2019) 11(6). doi: 10.3390/su11061752
4. Zhao G-F, Cai Y-B, Luo Y-Y, Li M-H, Yu M-J. Nutrient dynamics in litter decomposition in an evergreen broad-leaved forest in East China. Acta Ecologica Sin (2006) 26(10):3286–95.
5. Cowan OS, Anderson PML. Litter decomposition variation across a degradation gradient and two seasons in a critically endangered vegetation type within the fynbos biome, south Africa. South Afr J Bot (2019) 121:200–9. doi: 10.1016/j.sajb.2018.11.002
6. Lavelle P, Blanchart E, Martin A, Martin S, Spain A. A hierarchical model for decomposition in terrestrial ecosystems: application to soils of the humid tropics. Biotropica (1993) 130-150. doi: 10.2307/2389178
7. Aerts R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos (1997) 79(3):439–49. doi: 10.2307/3546886
8. Canessa R., van den Brink L., Saldaña A., Rios R. S., Hättenschwiler S., Mueller C. W., et al. (2021). Relative effects of climate and litter traits on decomposition change with time, climate and trait variability. J Ecology 109(1):447–58. doi: 10.1111/1365-2745.13516
9. Chae HM, Choi SH, Lee SH, Cha S, Yang KC, Shim JK. Effect of litter quality on needle decomposition for four pine species in Korea. Forests (2019) 10(5):371. doi: 10.3390/f10050371
10. Yao MK, Koné AW, Otinga AN, Kassin EK, Tano Y. Carbon and nutrient cycling in tree plantations vs. natural forests: implication for an efficient cocoa agroforestry system in West Africa. Regional Environ Change (2021) 21(2):1–14. doi: 10.1007/s10113-021-01776-0
11. García-Palacios P, Maestre FT, Kattge J, Wall DH. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol Lett (2013) 16(8):1045–53. doi: 10.1111/ele.12137
12. Zamorano-Elgueta C, Cayuela L, Rey-Benayas JM, Donoso PJ, Geneletti D, Hobbs RJ. The differential influences of human-induced disturbances on tree regeneration community: a landscape approach. Ecosphere (2014) 5(7):1–17. doi: 10.1890/ES14-00003.1
13. Stone MJ, Shoo L, Stork NE, Sheldon F, Catterall CP. Recovery of decomposition rates and decomposer invertebrates during rain forest restoration on disused pasture. Biotropica (2020) 52(2):230–41. doi: 10.1111/btp.12682
14. Blanco JA, Imbert JB, Castillo FJ. Thinning affects pinus sylvestris needle decomposition rates and chemistry differently depending on site conditions. Biogeochemistry (2011) 106(3):397–414. doi: 10.1007/s10533-010-9518-2
15. Bahamonde HA, Peri PL, Alvarez R, Barneix A, Moretto A, Pastur GM. Litter decomposition and nutrients dynamics in nothofagus antarctica forests under silvopastoral use in southern Patagonia. Agroforestry Syst (2012) 84(3):345–60. doi: 10.1007/s10457-012-9479-7
16. Ostertag R, Marín-Spiotta E, Silver WL, Schulten J. Litterfall and decomposition in relation to soil carbon pools along a secondary forest chronosequence in Puerto Rico. Ecosystems (2008) 11(5):701–14. doi: 10.1007/s10021-008-9152-1
17. Veblen TT. The ecology of the conifers of southern South America. Ecol Southern Conifers (1995), 120–155.
19. Armesto JJ, Manuschevich D, Mora A, Smith-Ramirez C, Rozzi R, Abarzúa AM, et al. From the Holocene to the anthropocene: A historical framework for land cover change in southwestern south America in the past 15,000 years. Land Use Policy (2010) 27(2):148–60. doi: 10.1016/j.landusepol.2009.07.006
20. Altamirano A, Lara A. Deforestación en ecosistemas templados de la precordillera andina del centro-sur de Chile. Bosque (Valdivia) (2010) 31(1):53–64. doi: 10.4067/S0717-92002010000100007
21. Alfaro M, Dube F, Zagal E. Soil quality indicators in an andisol under different tree covers in disturbed nothofagus forests. Chilean J Agric Res (2018) 78(1):106–16. doi: 10.4067/S0718-58392018000100106
22. Staelens J, Ameloot N, Almonacid L, Padilla E, Boeckx P, Huygens D, et al. Litterfall, litter decomposition and nitrogen mineralization in old-growth evergreen and secondar y deciduous nothofagus forests in south-central Chile. Rev Chil Hist Natural (2011) 84(1):125–41. doi: 10.4067/S0716-078X2011000100010
23. Lusk CH, Donoso C, Jiménez M, Moya C, Oyarce G, Reinoso R, et al. Descomposición de hojarasca de pinus radiata y tres especies arbóreas nativas. Rev Chil Hist Natural (2001) 74(3):705–10. doi: 10.4067/S0716-078X2001000300016
24. CIREN. Estudio agrológico VIII región: CIREN Santiago, Chile. In: Centro de información de recursos naturales. CIREN N°121 (1999). Chile.
25. Crovo O, Aburto F, Albornoz MF, Southard R. Soil type modulates the response of c, n, p stocks and stoichiometry after native forest substitution by exotic plantations. Catena (2021a) 197:104997. doi: 10.1016/j.catena.2020.104997
26. Veblen TT, Donoso C, Kitzberger T, Rebertus AJ. Ecology of southern Chilean and argentinean nothofagus forests. Ecol biogeogr Nothofagus forests (1996) 10:93–353.
27. Pollmann W, Veblen TT. Nothofagus regeneration dynamics in south-central Chile: a test of a general model. Ecol Monogr (2004) 74(4):615–34. doi: https://doi.org/10.1890/04-0004
28. Donoso P, Donoso C, Sandoval V. Proposición de zonas de crecimiento de renovales de roble (Nothofagus obliqua) y raulí (Nothofagus alpina) en su rango de distribución natural. Bosque (1993) 2):37–55%V 14. doi: 10.4206/bosque.1993.v14n2-06
29. Pollmann W. Effects of natural disturbance and selective logging on nothofagus forests in south-central Chile. J Biogeogr (2002) 29(7):955–70. doi: 10.1046/j.1365-2699.2002.00734.x
30. Atenas-Navarrete A, Aburto F, González-Rocha G, Guzmán CM, Schmidt R, Scow K, et al. Anthropogenic disturbances alter surface soil biogeochemical pools and microbial diversity in Andean temperate forests. Sci Total Environ (2021) 854. doi: 10.1016/j.scitotenv.2022.158508
31. Marthews TR, Riutta T, Menor IO. Measuring tropical forest carbon allocation and cycling (v3. 0). Manual, Global Ecosys Monitor Network. (2014)
32. Chadwick DR, Ineson P, Woods C, Piearce TG. Decomposition of pinus sylvestris litter in litter bags: influence of underlying native litter layer. Soil Biol Biochem (1998) 30(1):47–55. doi: 10.1016/S0038-0717(97)00090-4
33. Olson JS. Energy storage and the balance of producers and decomposers in ecological systems. Ecology (1963) 44(2):322–31. doi: 10.2307/1932179
34. Sadzawka A, Carrasco M, Demanet R, Flores H, Grez R, Mora M, et al. Métodos de análisis de tejidos vegetales. Serie Actas INIA (2007) 40:140.
35. Mendonça RT, Jara JF, González V, Elissetche JP, Freer J. Evaluation of the white-rot fungi ganoderma australe and ceriporiopsis subvermispora in biotechnological applications. J Ind Microbiol Biotechnol (2008) 35(11):1323. doi: 10.1007/s10295-008-0414-x
36. Margenot AJ, Calderón FJ, Bowles TM, Parikh SJ, Jackson LE. Soil organic matter functional group composition in relation to organic carbon, nitrogen, and phosphorus fractions in organically managed tomato fields. Soil Sci Soc America J (2015) 79(3):772–82. doi: 10.2136/sssaj2015.02.0070
37. Veum KS, Goyne KW, Kremer RJ, Miles RJ, Sudduth KA. Biological indicators of soil quality and soil organic matter characteristics in an agricultural management continuum. Biogeochemistry (2014) 117(1):81–99. doi: 10.1007/s10533-013-9868-7
38. Kassambara A. Rstatix: Pipe-friendly framework for basic statistical tests. In: R package version 0.7. 0, vol. 2021. (Boston, MA, USA: Free Software Foundation Inc.) (2021)
39. Fox J, Weisberg S. An {R} companion to applied regression. In: An r companion to applied regression, 3rd ed. Sage Publications United States of America. (2019). Available at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
40. R Core Team. (2021). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.R-project.org/
41. Caldentey J, Ibarra M, Hernández J. Litter fluxes and decomposition in nothofagus pumilio stands in the region of magallanes, Chile. For Ecol Manage (2001) 148(1-3):145–57. doi: 10.1016/S0378-1127(00)00532-6
42. Zhang H, Yuan W, Dong W, Liu S. Seasonal patterns of litterfall in forest ecosystem worldwide. Ecol Complexity (2014) 20:240–7. doi: 10.1016/j.ecocom.2014.01.003
43. Saarsalmi A, Starr M, Hokkanen T, Ukonmaanaho L, Kukkola M, Nöjd P, et al. Predicting annual canopy litterfall production for Norway spruce (Picea abies (L.) karst.) stands. For Ecol Manage (2007) 242(2-3):578–86. doi: 10.1016/j.foreco.2007.01.071
44. Thakur TK, Swamy S, Bijalwan A, Dobriyal MJ. Assessment of biomass and net primary productivity of a dry tropical forest using geospatial technology. J Forestry Res (2019) 30(1):157–70. doi: 10.1007/s11676-018-0607-8
45. Vesterdal L, Dalsgaard M, Felby C, Raulund-Rasmussen K, Jørgensen BB. Effects of thinning and soil properties on accumulation of carbon, nitrogen and phosphorus in the forest floor of Norway spruce stands. For Ecol Manage (1995) 77(1):1–10. doi: 10.1016/0378-1127(95)03579-Y
46. Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R, Hagedorn F, et al. How strongly can forest management influence soil carbon sequestration? Geoderma (2007) 137(3-4):253–68. doi: 10.1016/j.geoderma.2006.09.003
47. Garcia-Oliva F, Covaleda S, Gallardo JF, Prat C, Velazquez-Duran R, Etchevers JD. Firewood extraction affects carbon pools and nutrients in remnant fragments of temperate forests at the Mexican transvolcanic belt. Bosque (2014) 35(3):311–24. doi: 10.4067/s0717-92002014000300006
48. McGroddy ME, Daufresne T, Hedin LO. Scaling of c: N: P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology (2004) 85(9):2390–401. doi: 10.1890/03-0351
49. Macinnis-Ng C, Schwendenmann L. Litterfall, carbon and nitrogen cycling in a southern hemisphere conifer forest dominated by kauri (Agathis australis) during drought. Plant Ecol (2015) 216(2):247–62. doi: 10.1007/s11258-014-0432-x
50. Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr (2012) 82(2):205–20. doi: 10.1890/11-0416.1
51. Spohn M, Aburto F, Ehlers TA, Farwig N, Frings PJ, Hartmann H, et al. Terrestrial ecosystems buffer inputs through storage and recycling of elements. Biogeochemistry (2021) 156(3):351–73. doi: 10.1007/s10533-021-00848-x
52. Yan E-R, Wang X-H, Huang J-J. Shifts in plant nutrient use strategies under secondary forest succession. Plant Soil (2006) 289(1):187–97. doi: 10.1007/s11104-006-9128-x
53. Pérez CA, Hedin LO, Armesto JJ. Nitrogen mineralization in two unpolluted old-growth forests of contrasting biodiversity and dynamics. Ecosystems (1998) 1(4):361–73. doi: 10.1007/s100219900030
54. Perakis SS, Hedin LO. Nitrogen loss from unpolluted south American forests mainly via dissolved organic compounds. Nature (2002) 415(6870):416–9. doi: 10.1038/415416a
55. Park BB, Han SH, Hernandez JO, An JY, Youn WB, Choi H-S, et al. Leaf litter decomposition of deciduous quercus acutissima carruth. and evergreen quercus glauca thunb. in an inter-site experiment in three contrasting temperate forest stands in south Korea. Ann For Sci (2021) 78(2):1–11. doi: 10.1007/s13595-021-01058-z
56. Bakker MA, Carreño-Rocabado G, Poorter L. Leaf economics traits predict litter decomposition of tropical plants and differ among land use types. Funct Ecol (2011) 25(3):473–83. doi: 10.1111/j.1365-2435.2010.01802.x
57. Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-Variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Funct Ecol (2006) 20(1):21–30. doi: 10.1111/j.1365-2435.2006.01080.x
58. Rahman MM, Tsukamoto J, Rahman MM, Yoneyama A, Mostafa KM. Lignin and its effects on litter decomposition in forest ecosystems. Chem Ecol (2013) 29(6):540–53. doi: 10.1080/02757540.2013.790380
59. Wu A, Yin R, Xu Z, Zhang L, You C, Liu Y, et al. Forest gaps slow lignin and cellulose degradation of fir (Abies faxoniana) twig litter in an alpine forest. Geoderma (2022) 424:116010. doi: 10.1016/j.geoderma.2022.116010
60. Steinwandter M, Schlick-Steiner BC, Steiner FM, Seeber J. One plus one is greater than two: mixing litter types accelerates decomposition of low-quality alpine dwarf shrub litter. Plant Soil (2019) 438(1):405–19. doi: 10.1007/s11104-019-03991-5
61. Liu J, Liu X, Song Q, Compson ZG, LeRoy CJ, Luan F, et al. Synergistic effects: a common theme in mixed-species litter decomposition. New Phytol (2020) 227(3):757–65. doi: 10.1111/nph.16556
62. Lal R. Forest soils and carbon sequestration. For Ecol Manage (2005) 220(1):242–58. doi: 10.1016/j.foreco.2005.08.015
63. Zhang Q, Zak JC. Effects of gap size on litter decomposition and microbial activity in a subtropical forest. Ecology (1995) 76(7):2196–204. doi: 10.2307/1941693
64. Wang L, Zhang J, He R, Chen Y, Yang L, Zheng H, et al. Impacts of soil fauna on lignin and cellulose degradation in litter decomposition across an alpine forest-tundra ecotone. Eur J Soil Biol (2018) 87:53–60. doi: 10.1016/j.ejsobi.2018.05.004
65. Li R, Guan X, Han J, Zhang Y, Zhang W, Wang J, et al. Litter decomposition was retarded by understory removal but was unaffected by thinning in a Chinese fir [Cunninghamia lanceolata (Lamb.) hook] plantation. Appl Soil Ecol (2021) 163:103968. doi: 10.1016/j.apsoil.2021.103968
66. Manzoni S, Trofymow JA, Jackson RB, Porporato A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr (2010) 80(1):89–106. doi: 10.1890/09-0179.1
67. Heuck C, Spohn M. Carbon, nitrogen and phosphorus net mineralization in organic horizons of temperate forests: stoichiometry and relations to organic matter quality. Biogeochemistry (2016) 131(1):229–42. doi: 10.1007/s10533-016-0276-7
68. Kiser LC, Fox TR, Carlson CA. Foliage and litter chemistry, decomposition, and nutrient release in pinus taeda. Forests (2013) 4(3):595–612. doi: 10.3390/f4030595
69. Crovo O, Aburto F, da Costa-Reidel C, Montecino F, Rodríguez R. Effects of livestock grazing on soil health and recovery of a degraded Andean araucaria forest. Land Degradation Dev (2021b) 32(17):4907–19. doi: 10.1002/ldr.4079
70. Lanuza O, Casanoves F, Delgado D, Van den Meersche K. Leaf litter stoichiometry affects decomposition rates and nutrient dynamics in tropical forests under restoration in Costa Rica. Restor Ecol (2019) 27(3):549–58. doi: 10.1111/rec.12893
71. Pandey R, Sharma G, Tripathi S, Singh A. Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in northeastern India. For Ecol Manage (2007) 240(1-3):96–104. doi: 10.1016/j.foreco.2006.12.013
72. Gautam MK, Lee K-S, Song B-Y, Lee D, Bong Y-S. Early-stage changes in natural 13C and 15N abundance and nutrient dynamics during different litter decomposition. J Plant Res (2016) 129(3):463–76. doi: 10.1007/s10265-016-0798-z
73. Moore TR, Trofymow JA, Prescott CE, Fyles J, Titus BD. Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems (2006) 9(1):46–62. doi: 10.1007/s10021-004-0026-x
74. Moore TR, Trofymow J, Prescott CE, Titus B. Nature and nurture in the dynamics of c, n and p during litter decomposition in Canadian forests. Plant Soil (2011) 339(1):163–75. doi: 10.1007/s11104-010-0563-3
75. Rustad LE. Element dynamics along a decay continuum in a red spruce ecosystem in Maine, USA. Ecology (1994) 75(4):867–79. doi: 10.2307/1939412
76. Liu D, Keiblinger KM, Leitner S, Mentler A, Zechmeister-Boltenstern S. Is there a convergence of deciduous leaf litter stoichiometry, biochemistry and microbial population during decay? Geoderma (2016) 272:93–100. doi: 10.1016/j.geoderma.2016.03.005
77. Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science (2007) 315(5810):361–4. doi: 10.1126/science.1134853
78. Osono T, Takeda H. Accumulation and release of nitrogen and phosphorus in relation to lignin decomposition in leaf litter of 14 tree species. Ecol Res (2004) 19(6):593–602. doi: 10.1111/j.1440-1703.2004.00675.x
79. Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P, Lavorel S, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr (2005) 75(1):3–35. doi: 10.1890/04-0922
80. Bravo-Oviedo A, Ruiz-Peinado R, Onrubia R, del Río M. Thinning alters the early-decomposition rate and nutrient immobilization-release pattern of foliar litter in Mediterranean oak-pine mixed stands. For Ecol Manage (2017) 391:309–20. doi: 10.1016/j.foreco.2017.02.032
Keywords: carbon, nitrogen, phosphorous, forest disturbance, nutrient mineralization
Citation: Leal F, Aburto F, Aguilera N, Echeverría C and Gatica-Saavedra P (2023) Forest degradation modifies litter production, quality, and decomposition dynamics in Southern temperate forests. Front. Soil Sci. 3:1111694. doi: 10.3389/fsoil.2023.1111694
Received: 29 November 2022; Accepted: 27 January 2023;
Published: 10 February 2023.
Edited by:
Longlong Xia, Karlsruhe Institute of Technology (KIT), GermanyReviewed by:
Enqing Hou, South China Botanical Garden (CAS), ChinaArmand W. Koné, University of Nangui Abrogoua, Côte d’Ivoire
Copyright © 2023 Leal, Aburto, Aguilera, Echeverría and Gatica-Saavedra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Felipe Aburto, ZmVsaXBlLmFidXJ0b0B0YW11LmVkdQ==
†ORCID: Fabiola Leal, orcid.org/0000-0002-1775-1607
Natalia Aguilera, orcid.org/0000-0003-0596-4856
Cristian Echeverría, orcid.org/0000-0001-6456-6431
Paula Gatica-Saavedra, orcid.org/0000-0002-3740-3538
 Fabiola Leal
Fabiola Leal Felipe Aburto
Felipe Aburto Natalia Aguilera2†
Natalia Aguilera2†