- 1Natural Resource Research Directorate, Oromia Agricultural Research Institute, Addis Ababa, Ethiopia
- 2Department of Plant and Horticulture Science, College of Agriculture, Hawassa University, Hawassa, Ethiopia
- 3Soil Extension Team, International Fertilizer Development Centre (IFDC), Addis Ababa, Ethiopia
- 4Chemistry Department, College of Natural and computational sciences, Hawassa University, Hawassa, Ethiopia
- 5Institute of Agricultural Sciences in the Tropics (Hans-Ruthenberg-Institute), University of Hohenheim, Stuttgart, Germany
The symbiotic association between legumes and indigenous rhizobia is crucial for enhancing legume productivity. However, inconsistent results and suboptimal performance of rhizobia inoculation in promoting legume production have been observed. In this regard, we assessed the abundance and symbiotic efficiency of indigenous rhizobia nodulating faba bean and common bean, as well as the soil factors affecting rhizobia abundance in southern Ethiopia. The study also compared the performance of indigenous rhizobia with commercial strains and mineral nitrogen treatment plants. A total of 132 soil samples were collected from barley, wheat, maize, potato, common bean, faba bean, intercropped common bean and maize, enset, and grazing land. Indigenous rhizobia were isolated and enumerated from these samples. Faba bean (FB) and common bean (CB) rhizobia population ranged from 0.0 to 1.7 x 104 and 1.7 x 101 to 1.7 x 107 cells g-1 soil, respectively. Rhizobia populations were significantly influenced by soil pH, EC, OC, TN, CEC, exchangeable acidity, aluminium, and the host crop occurrence. The isolated indigenous rhizobia demonstrated significant potential in enhancing nodulation, shoot dry weight, and TN accumulation in plants. Symbiotic efficiency indices revealed that over 95% of the indigenous rhizobia were effective in nodulation and shoot dry matter accumulation, indicating that naturally occurring rhizobia are efficient and may reduce the need for commercial inoculants in areas with abundant indigenous populations. However, in areas where rhizobia populations are low, strains isolated from faba bean (33FB, 84FB) and common bean (44CB, 102CB), which outperformed commercial strains should be further evaluated. The results suggest that soil rhizobia population levels should be assessed prior to inoculation to optimize nodulation and crop performance. To this end, it is emphasized to evaluate soil rhizobia strains to assess their stability and competitiveness relative to commercial inoculants across different agroecological conditions.
1 Introduction
Legumes, such as faba bean (Vicia faba L.) and common bean (Phaseolus vulgaris L.), are crucial for enhancing soil fertility and promoting sustainable agriculture in Ethiopia (1, 2). Despite their importance, legume productivity in smallholder farms is often constrained by poor soil fertility, as reflected by low levels of organic carbon (OC), nitrogen (N), and phosphorus (P), and unadjusted agronomic practices (3–5). To overcome this dilemma, the symbiotic relationship between legumes and indigenous rhizobia is considered pivotal for boosting legume productivity and improving soil fertility. Besides, it can also reduce reliance on chemical fertilizers (6–9). In this context legumes play an important agronomic role in integrated cropping systems (10, 11). The incorporation of faba bean and common bean into crop rotations and intercropping systems has often been associated with significant benefits for nitrogen cycling in agricultural systems (12, 13).
The specific rhizobial species that nodulate faba bean are primarily from the genus Rhizobium: Rhizobium leguminosarum (14), Rhizobium fabae (15) and Rhizobium laguerreae (16), and common beans are typically nodulated by rhizobium species, including Rhizobium etli and Rhizobium phaseoli (17). Once nodulated, rhizobia survive in the soil through various mechanisms. They can persist as free-living cells or in a dormant state in the form of nitrogen-rich, resilient spores (18). These bacteria are capable of forming stable nodules on their host plants, where they remain active during the growing season, facilitate nitrogen fixation. Outside of their symbiotic relationships, rhizobia can survive in the soil as part of the broader microbial community, particularly in soils with high OC or in legume-cropped fields where they have access to carbon sources from decaying plant material (19, 20). However, their survival can be affected by factors such as soil pH, moisture levels, and the presence of competing microorganisms (21, 22).
The abundance of rhizobia was not only significantly linked to the integration of legumes in cropping systems; soil factor were also major determinants of rhizobia abundance. Although legume rhizobia can survive and persist in the soil, their populations may not increase significantly through rotation practices alone, particularly when rotations include other leguminous or non-leguminous crops (22, 23). Furthermore, the availability of suitable environmental conditions, such as optimal soil pH, acidity, salinity and OC, is essential for sustaining viable rhizobial populations (24). In many crop rotations, the soil conditions may not always be ideal for the proliferation of rhizobia specific to legumes, especially when the rotation includes crops that support different rhizobial species.
Research in Ethiopia has explored the use of rhizobia inoculants to improve legume yields. Various rhizobia strains have been isolated and tested, showing significant improvements in the productivity of faba beans and common beans (25–28). However, the adoption of these inoculants by farmers remains limited. Reports indicate inconsistencies and suboptimal performance of both indigenous and commercial rhizobia strains in nodulating legumes (29–31). This suggests that existing indigenous rhizobia populations in agricultural soils, potentially exceeding 102 cells per gram of soil (32, 33), may possess high symbiotic efficiency that can support legume productivity (21, 34). Yet, despite this potential, there is a lack of comprehensive data on the abundance and effectiveness of indigenous rhizobia in the diverse agroecological zones of southern Ethiopia. This region, with its variation in soil types, climate conditions, and farming practices, offers a valuable context for understanding how environmental factors influence legume–rhizobia interactions. Several previous studies have overlooked the assessment of indigenous rhizobia populations in agricultural soils prior to seed inoculation, potentially diminishing the benefits of external inoculants in regions, where indigenous rhizobia populations are already abundant (30, 34, 35).
Giller (36) outlines three scenarios where introducing rhizobia is crucial: [a] when compatible rhizobia in soils are absent, [b] when their population is insufficient for effective nodulation, or [c] when indigenous rhizobia are less effective compared to selected inoculants. The competition between introduced and indigenous rhizobia can affect the proliferation of introduced strains (37–39). The necessity for re-inoculation depends on soil conditions and the compatibility of rhizobia strains (36, 40, 41). Soil factors, such as pH, OC, nutrient status, and salinity, have been shown to impact the abundance and effectiveness of indigenous rhizobia (33, 39, 42). However, these scenarios has not been thoroughly investigated in Ethiopian agricultural soils.
Southern Ethiopia, with its diverse soils, climates, and farming practices, provides an ideal setting to explore how these factors influence legume–rhizobia interactions. This study hypothesizes that indigenous rhizobia in soils of southern Ethiopia are sufficiently abundant and effective, reducing the need for external inoculants by outcompeting introduced rhizobia, particularly under unfavorable soil conditions. To test this hypothesis, the study addresses the lack of comprehensive data by assessing the abundance and symbiotic efficiency of indigenous rhizobia nodulating faba bean and common bean. It also evaluates key soil factors affecting these populations. The results are expected to inform more sustainable legume management strategies and contribute to improved soil fertility and food security in the region.
2 Materials and methods
2.1 Description of the study areas
Soil samples were collected from the Cheha, Hula, Boricha, and Hawassa Zuria districts of southern Ethiopia, which are known for their extensive production of faba bean and common bean. Cheha and Hula were chosen as representative areas for faba bean cultivation, while Boricha and Hawassa Zuria were selected for their widespread common bean production. Soil samples for enumerating and isolating indigenous rhizobia were collected from various land use types, including enset, grazing, potato, faba bean, wheat, and barley fields in Hula and Cheha, and from enset, grazing, common bean, intercropping, and maize fields in Boricha and Hawassa Zuria.
Cheha is located between 37° 37’ 20” - 38° 30’ E and 8° 00’ 00” - 8° 18’ 40” N, with an elevation ranging from 1639 to 2840 meters above sea level (m.a.s.l). Hula lies between 38° 20’ 20” - 38° 40’ 20” E and 6° 24’ 20” - 6° 36’ N with elevation ranging from 2440 to 3267m.a.s.l. Boricha is situated between 38° 30’ 00” - 38° 24’ 00” E and 6° 45’ 20” - 7° 1’ 40” N, with altitudes between 1321 and 2085 m.a.s.l. Hawassa Zuria district is located between 38° 12′ 20′′ - 38° 28′ 40′′ E and 6° 57′ 00” - 07° 8′ 40′′ N with elevation ranging from 1670 and 2210 m.a.s.l (Figure 1). The mean atmospheric temperatures of Cheha, Hula, Boricha and Hawassa Zuria districts are 20.0°C, 14.0°C, 23.5°C, and 23.5°C, respectively. The mean annual rainfall is 1,061 mm in Boricha, 1,428 mm in Cheha, 1,576 mm in Hula, and 1,023 mm Hawassa Zuria. According to agro-ecological classification, Hula and Cheha are categorized as cool humid mid-highlands and tepid sub-humid mid-highlands, respectively. In contrast, Hawassa Zuria and Boricha fall under the tepid moist mid-highlands category (43). This agro-ecological heterogeneity significantly influences the types of crops cultivated. The dominant soil types in Hula are Eutric Nitisols and Lithosols, while Cheha is characterized by Eutric Nitisols and Pellic Vertisols. In Hawassa Zuria the predominant soils are Mollic Andosols and Eutric Fluvisols, whereas Boricha is mainly composed of Lithosols and Chromic Luvisols (44).
Figure 1. Location map of the study areas: Map of Ethiopia (A), showing the Gurage Zone and Sidama Region (B), along with the study locations in the Hula, Boricha, Hawassa Zuria, and Cheha districts (C).
2.2 Cropping and inoculation history
The five-year cropping and inoculation history for each sampling site was recorded using structured questionnaires administered to the participating farmers. The questionnaires were designed to gather detailed information about the crop rotations and inoculation practices employed over the past five years. These questionnaires included both closed and open-ended questions, allowing the respondents to provide specific details while also ensuring the collection of quantifiable data. The questionnaires were structured to cover key aspects of the cropping system, including the types of crops grown, the sequence and frequency of rotations, and any inoculation practices related to leguminous crops like faba bean and common bean. Farmers were asked to list the crops grown by type and year, as well as details about the use of inoculants (e.g., whether rhizobial inoculants were applied). To ensure the accuracy and reliability of the responses, the information provided by the farmers was validated through cross-referencing with actual field observations. This involved visiting farms to verify the types of crops grown in recent years. Further details of the collected information are provided in the Supplementary Material (Supplementary Table S1).
2.3 Soil sampling and analysis
Soil samples were collected from various land use types at 0–20 cm soil depth. In each land use type, a 10 x 10 m quadrant was laid out, and nine soil samples were collected within each quadrant. The individual soil samples from each quadrant were bulked to form one composite sample. Each land use type was replicated three times per district. A total of 60 composite soil samples were collected from the common bean production districts, while 72 composite soil samples were collected from the faba bean production districts. The composite soil samples were immediately transported to the lath house at the College of Agriculture, Hawassa University, and filled into3-liter plastic pots for trapping nodules from faba bean and common bean. The soil samples were also used for enumerating indigenous rhizobia and analyzing selected soil parameters. These included soil pH and EC, determined in a water suspension (H2O) (45), soil organic carbon (SOC) content, determined by wet oxidation (46); Total N measured by Kjeldahl method (47), available phosphorous, determined by the Olsen method (48), and cation exchange capacity (CEC), measured using ammonium acetate extraction (49). For soils with pH < 5.5, exchangeable acidity and aluminium were measured by titration with NaOH after displacement with 1 N KCl solution (50). Additionally, undisturbed soil samples were collected for bulk density determination.
2.4 Enumeration of indigenous rhizobia
The most probable number (MPN) plant infection technique was used to estimate the abundance of rhizobia in each soil sample (18). A tenfold serial dilution was prepared by diluting 10g of soil in 90 ml of sterilized distilled water. Four replicated seedlings of common bean (Phaseolus vulgaris L., Hawassa dume variety) and faba bean (Vicia faba L., Tumsa variety) were inoculated with 1 ml of each dilution (10-1 – 10-10). These varieties were selected for their adaptability and widespread cultivation in the study area. Surface sterilized seeds were planted in acid-treated and sterilized sand using 300 ml plastic pots in a lath house. The seedlings were supplied with 100 ml of sterilized pH adjusted (6.8) Jenson’s modified N-free solution (51) and examined for the presence of nodules after 5 weeks. Nodulation was recorded as “+” for the presence of nodules and “-” for their absence. The count of nodulated (+) plants was noted for each dilution. The total number of nodulated units was calculated by summing the nodulated plants across all dilution levels. The rhizobial population in each soil sample was then estimated. Based on the results, rhizobia populations in the soil samples were categorized into three groups: (i) low (<102 rhizobia g-1 of soil), (ii) medium (102 to 103 rhizobia g-1 of soil), and (iii) high (>103 rhizobia g-1 of soil) (52).
2.5 Nodule trapping and isolation of indigenous rhizobia
Faba bean and common bean were selected as a host crop for indigenous rhizobia trapping, following earlier established protocols (52). Seeds were allowed to germinate at room temperature for 3 days (53) before being transferred to pots filled with soil. Water was added every three days based on observation to provide optimum soil moisture during the growing period. After 45 days, the plants were carefully uprooted, washed with tap water, and the pink-colored, undamaged nodules were selected for further analysis. Upon cracking the nodules, the pink coloration was confirmed and used as a criterion for selecting nodules for rhizobia isolation. Pink-colored nodules were selected because of their coloration strongly indicates active nitrogen fixation. The pink color in legume nodules results from the presence of leghemoglobin, a molecule produced by the plant in response to rhizobial infection (52). Prior isolation, nodules were stored in screw-capped vials containing silica gel at the bottom, separated from the nodules by a cotton plug, kept at -4 °C. Rhizobia were isolated following standard procedures (18, 51). The acronyms CB and FB were used to uniquely identify the isolates from common bean and faba bean, respectively. Each isolate was labeled using the acronym of the host crop followed by a number linked to the soil sample.
2.6 Confirmatory test
The isolates were classified as rhizobia using established methodologies (18). Consequently, four confirmatory tests were performed, such as a congo red dye absorption, a ketolactose test, growth on Peptone Glucose Agar (PGA), and gram staining to confirm isolates as rhizobium rather than agrobacterium or other bacteria, which are frequently found as contaminants.
2.7 Authentication of the isolates
The purified isolates were inoculated onto faba bean and common bean to test the compatibility following established procedures (18, 51). Briefly, three pre-germinated seeds of uniform size were selected and aseptically transferred to sterilized 300 ml modified Leonard jars using sterile forceps. The jars, filled with sand, were autoclaved at 121°C for 15 minutes. A week after planting, the seedlings were thinned to one per jar and each was individually treated with 1 ml of rhizobial broth culture at exponential growth phase, containing approximately 109 cells ml-1 (52).
Fifty-three rhizobial isolates of faba bean and 51 common bean rhizobia were considered as treatment. The treatments were arranged in a complete randomized design (CRD) with three replications in the lath house. Two controls were included in the study. The negative control consisted of uninoculated and unfertilized seeds, while the positive control comprised of seeds treated with a potassium nitrate fertilizer solution (0.5 g KNO3 per 1 L).
The seeds were surface-sterilized and allowed to germinate on moistened filter paper in sterilized Petri dishes for 2–3 days. After germination, the seedlings were transferred into pots filled with sterile sand and inoculated with 1 ml of rhizobial culture in the exponential growth phase, delivering approximately 109 cells ml-1 per pot.
Commercial rhizobial inoculants were included as reference treatments. For common bean, the strain Rhizobium etli HB-429 was used, and for faba bean, the strain Rhizobium leguminosarum biovar viceae EAL-110 was applied. Both inoculants were obtained from Menagesha Biotech Industry P.L.C. (Addis Ababa, Ethiopia) and were supplied as sterile peat-based formulations containing approximately 109 viable cells per gram. For seed inoculation, 10 g of a peat-based inoculant was thoroughly mixed with 1 kg of seed using a sugar solution as an adhesive to enhance adhesion and maintain rhizobial viability. The treated seeds were air-dried for 30 minutes before sowing to ensure even distribution of the inoculant. The seedlings were supplied weekly with 100 ml of sterilized Jenson’s modified nitrogen-free solution, adjusted to pH 6.8. Seedlings were grown and isolates verified using modified Leonard containers, made by combining two plastic cups. The top cups contained river sand, while the bottom cups were connected with cotton wicks, which absorbed and transported water and nutrients to the upper cups through capillary action. The jars containing the tested isolates were routinely monitored for moisture levels and watered with sterilized water as needed.
2.8 Growth data collection
After 45 days of planting, the plants were carefully uprooted from the jars. The root and shoot fractions were immediately separated at harvest. From the roots, nodules were picked off and spread on the sieve to wash the soil adhered to their surfaces. The nodule numbers per plant data was then recorded. The nodules, roots, and shoots were oven dried at 70°C for 48 hrs to determine their dry matter accumulation.
2.9 Estimation of symbiotic efficiency
In this study, Relative Symbiotic Effectiveness (RSE), Absolute Symbiotic Effectiveness (ASE), and Symbiotic Potential (SP) were employed as key indices to evaluate the symbiotic efficiency of rhizobial strains (18, 19, 54). These metrics were selected because they provide integrative, plant-level assessments of nitrogen-fixing performance based on easily measurable growth parameters, particularly shoot dry weight (55). They are widely accepted in rhizobia-legume research, as these indices offer a practical and reliable estimation of rhizobial effectiveness under controlled conditions (18, 55). Moreover, they strike a balance between scientific rigor and operational feasibility, enabling effective comparison across treatments with minimal technical complexity (18). Given the scale and objectives of this study, RSE, ASE, and SP were appropriate, robust tools for capturing the functional outcomes of the symbiosis.
Relative symbiotic effectiveness percentage (RSE %): Compared the potential of rhizobia isolates against N fertilized plants (56) using Equation 1.
SDW: Shoot dry weight, RSE was categorized as highly effective (SE > 80%), effective (SE = 50- 80%), poorly effective (SE = 35-50% and ineffective (SE <35%).
Absolute symbiotic effectiveness percentage (ASE %): Compared the efficiency of rhizobia isolates against unfertilized and uninoculated plants (54) using Equation 2.
Symbiotic performance (SP%): The performance of isolates over reference inoculants (commercial inoculants used by farmers) were tested by the formula outlined by (19) and categorized as optimal (>70%) and sub-optimal (<70%) using Equation 3.
2.10 Data analysis
One way analysis of variance (ANOVA) was conducted to test the effects of rhizobial isolates on nodule number, nodule dry weight, root dry weight, shoot dry weight and shoot total nitrogen concentration in faba bean and common bean using R software version 4.3.2 (57). The data were Box and Cox transformed to stabilize variances and make the dataset more closely approximate a normal distribution. Means comparisons were performed using the least significant difference (LSD) at a 5% probability level. The influences of selected soil parameters on MPN, which was transformed using log function [log10(MPN+1)], was quantified through linear regression analysis. Boxplots were generated using R software to graphically represent the distribution and variability of the data set.
3 Results
3.1 Selected soil properties of the study area
The mean bulk density values of the soils studied ranged from 0.89 g cm-3 in enset fields to 1.30 g cm-3 in grazing lands, which was optimal for most crops. Soils from faba bean-based land in Hula and Cheha were acidic, with pH values from 4.6 to 5.5. The pH of soils in common bean fields in Boricha and Hawassa Zuria ranged between 5.75 and 6.81. Exchangeable acidity ranged from 1.40 to 2.60 cmolc kg-1 across different land uses with the lowest acidity observed in enset fields and the highest in barley fields in Hula. The levels of exchangeable acidity and aluminum were higher in Hula than in Cheha, which correlated with the differences in soil pH observed between the two sites across all land uses. Soils under enset fields had higher SOC content (3.81 to 4.54%), which contributed to increased levels of TN (0.28 to 0.39%), available P (17.21 to 31.38 mg kg-1), and CEC (24.38 to 42.27 meq100g-1) compared to soils under other land use types (Supplementary Table S2).
3.2 Abundance of indigenous rhizobia nodulating faba bean and common bean
The abundance of indigenous rhizobia nodulating faba bean and common bean varied significantly across land uses in different locations (Figure 2; p<0.05). In Hula, rhizobia population for faba bean ranged from undetectable to high (>log10(103))while in Cheha they ranged from low (<log10(102)) to high (>log10(103)), regardless of land use types. Similarly, common bean rhizobia in Hawassa Zuria and Boricha ranged from low to high, irrespective of land use type. In Hula, 13.9% of the soil lacked faba bean rhizobia. Low populations were observed in 61.1% of Hula soils and 47.2% of Cheha soils. In contrast, high faba bean rhizobia populations were found in 5.6% of Hula soils and 19.4% of Cheha soils. For common bean, high rhizobia populations were detected in 40.0% of Boricha soils and 46.7% of Hawassa Zuria soils while low populations were present in 26.7% of Boricha soil and 23.3% of Hawassa Zuria soil respectively.
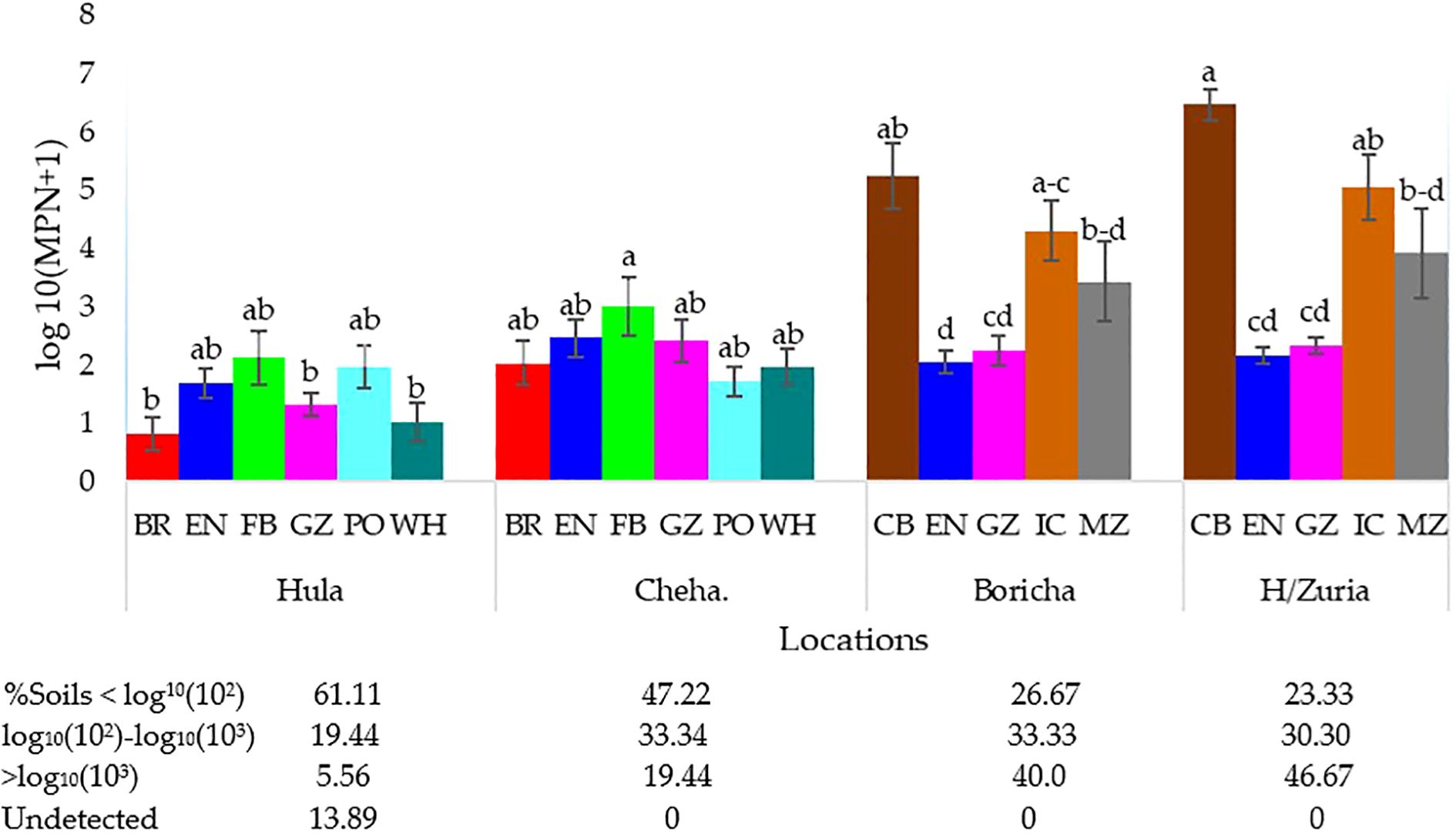
Figure 2. Interaction effect of land use type and location on rhizobia number in 132 collected soils (Hula, n = 36), (Cheha, n = 36), (Boricha, n = 30) and (Hawassa Zura, n = 30). Where: BR; Barley field, EN; Enset field, FB; Faba bean field, GZ; Grazing land, PO; Potato field, WH; Wheat field, CB; Common bean field, IC; Intercropping (maize + common bean) field, MZ; Maize field. Vertical lines on bars represent S.E of the means. The letters in a bars indicate the significant differences between land use and location.
Supplementary Table S3 presents the rhizobia populations associated with faba bean and common bean across different land use types. In Hula, the highest faba bean rhizobia population (1.7 x 103 cells g−1 soil) was found in soils from faba bean and potato fields. At Cheha the highest population (1.7 x 104 cells g−1 soil) occurred in soils under faba bean fields. Soils from barley and wheat fields in Hula showed low faba bean rhizobia populations (0–58 cells g−1 soil), while soils under enset and grazing lands exhibited low to moderate faba bean rhizobia populations. In Cheha, soils from enset (5.8 x 10¹ - 5.8 x 103 cells g−1 soil), grazing (10 - 3.1 x 103 cells g−1 soil), faba bean (1.7 x 10¹ - 1.7 x 104 cells g−1 soil), and wheat (1.7 x 10¹ - 1.7 x 103 cells g−1 soil) fields showed low to high faba bean rhizobia abundance, while soils of potato and barley fields contained low to moderate rhizobia populations (10² - 103 cells g−1 soil).
The rhizobia population associated with common beans increased in soils under maize-common bean rotation and intercropping systems (Supplementary Table S3). Following sole common bean fields, the highest abundance of indigenous rhizobia (>103 cells g-1 of soil) was found in the soils of intercropping fields (maize with common bean). Soils under enset and grazing land use types also harbored common bean rhizobia, though within a low to moderate range (<102 to <103 cells g-1 of soil).
3.3 Abundance of indigenous rhizobia and its association with soil properties and host crops
The abundance of faba bean and common bean nodulating rhizobia showed different associations with soil properties. Faba bean rhizobia abundance was positively associated with higher soil pH, EC, total N, and CEC (p<0.0001), but negatively associated with higher exchangeable acidity and Al+3 (p<0.0001) (Table 1). The top three factors responsible for low faba bean rhizobia populations were high exchangeable acidity and Al+3 and, low soil pH (Table 1). The abundance of faba bean rhizobia was not linked to the integration of faba bean in crop rotations (p>0.05). For common bean associated rhizobia, SOC and total N were negatively related to their population (Table 1), while the occurrence of the host crop in the cropping system was positively associated with rhizobia abundance (<0.0001).
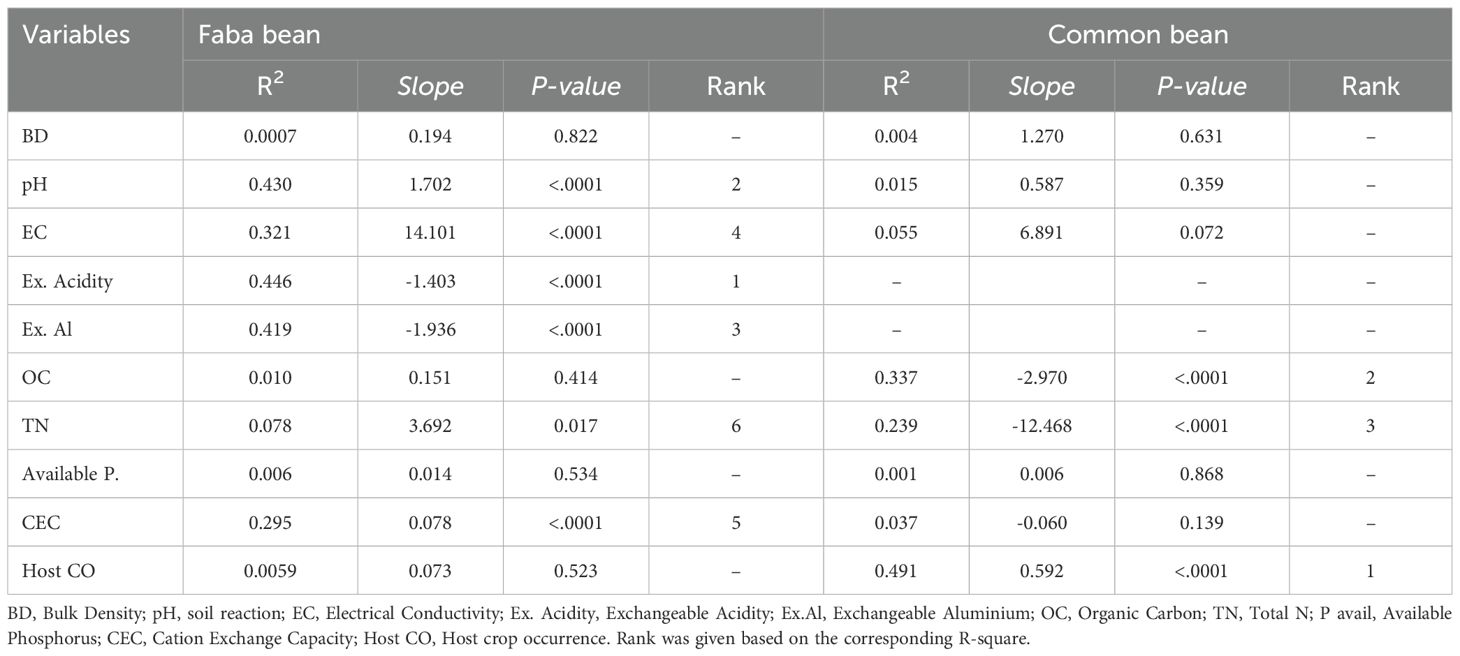
Table 1. Soil properties and host crops association with abundance of rhizobium populations [log10(MPN+1)] in Southern Ethiopia.
3.4 Screening and presumptive test of indigenous rhizobia isolates for authentication
The absence of rhizobia in five soil samples prevented faba bean nodulation, whereas common bean rhizobia successfully formed nodules in all soil samples. As a result, 60 indigenous common bean rhizobia and 67 faba bean rhizobia isolates were obtained from various land use types in southern Ethiopia. Among these, 14 faba bean and 9 common bean rhizobia isolates were Gram positive, absorbed Congo-red in dark conditions, grew on peptone glucose agar medium (PGA), and turned yellow on keto-lactose media with Benedict’s reagent. However, true rhizobia that nodulate legumes are typically Gram negative. Therefore, based on isolate characterization and confirmatory tests, 51 common bean and 53 faba bean isolates were confirmed as true root-nodule rhizobia (Table 2).
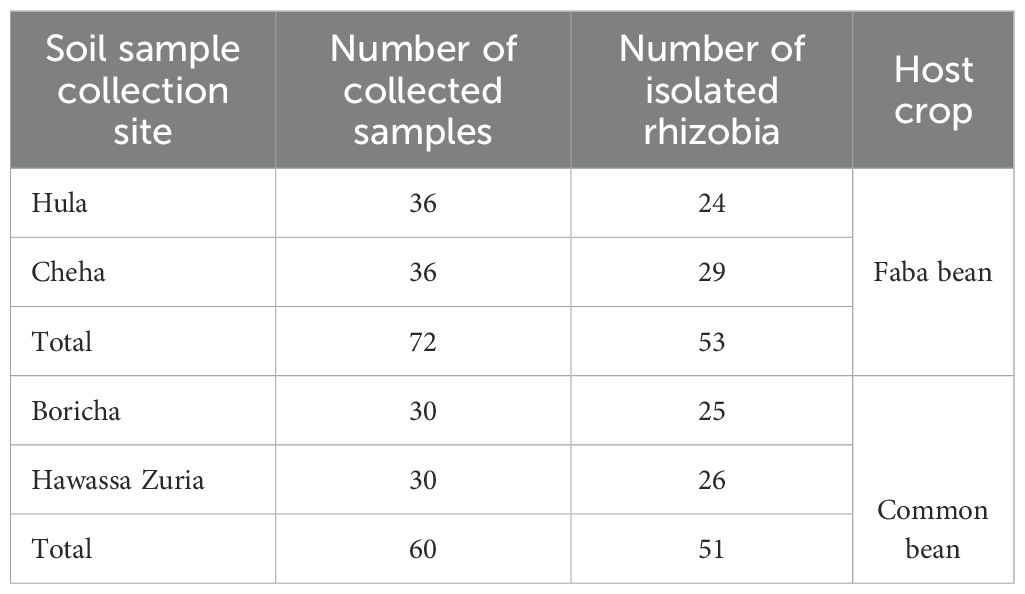
Table 2. Number of collected soil samples and indigenous rhizobia isolated from various location using host crops.
3.5 Evaluation of symbiotic effectiveness of indigenous rhizobia isolates
3.5.1 Nodule numbers and nodule dry weight per plant
Re-inoculation of indigenous rhizobia strains isolated from various land use types highly significantly influenced nodule number and dry weight (P < 0.01; Supplementary Table S4). The highest nodule number (91.0) and nodule dry weight per plant (0.093 g plant−1) for faba bean were recorded with application of 84FB and 33FB isolates, respectively (Figure 3). For common bean, the highest nodule number (53.0) and dry weight (0.068 g plant−1) were achieved with application of 44CB and 102CB isolates, respectively. All indigenous rhizobia isolates produced significantly higher nodule numbers and dry weights compared to uninoculated and non-N fertilized control plants (p<0.001). Compared to commercial inoculants, faba bean seeds inoculated with 84FB and common bean seeds inoculated with 102CB, 44CB, and 100CB isolates increased nodule numbers per plant (p<0.001).
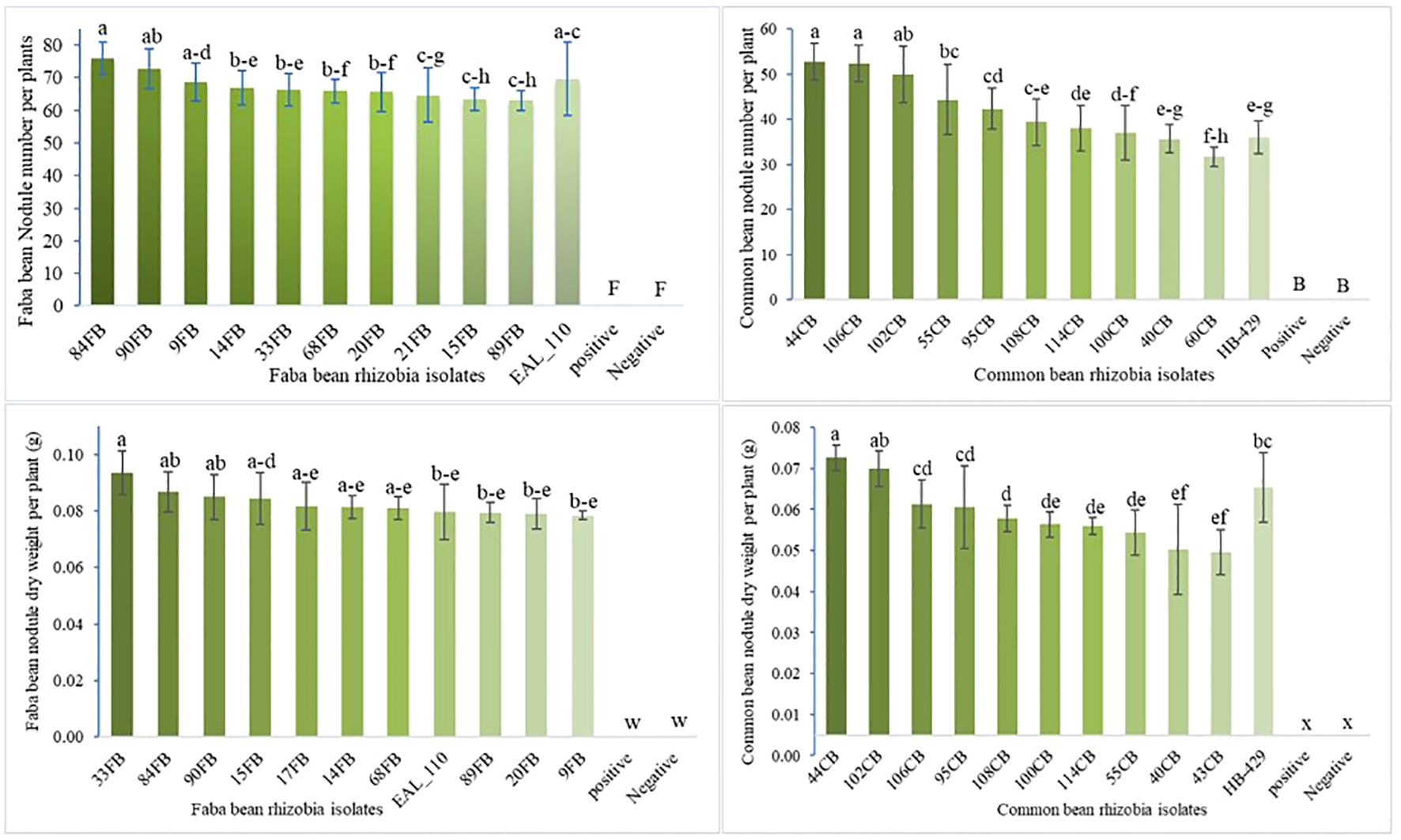
Figure 3. Faba bean and common bean nodule number and its dry weight response to rhizobia inoculation. Notice: The graph showcases the top 10 most effective indigenous rhizobia. Supplementary Table S4 details the responses of faba bean and common bean to all indigenous rhizobia. Vertical lines on bars represent S.E of the means. Different letters above the bars indicate statistically significant differences between isolates (p < 0.05).
3.5.2 Root and shoot dry weight per plant
The application of indigenous rhizobia highly significantly affected the root and shoot dry weight of faba bean and common bean (P < 0.01;Supplementary Table S4). Isolate 90FB produced the highest mean root dry weight of faba bean (0.555 g plant-1), while isolate 102CB resulted in the highest root dry weight for common bean (0.329 g plant-1, Figure 4).
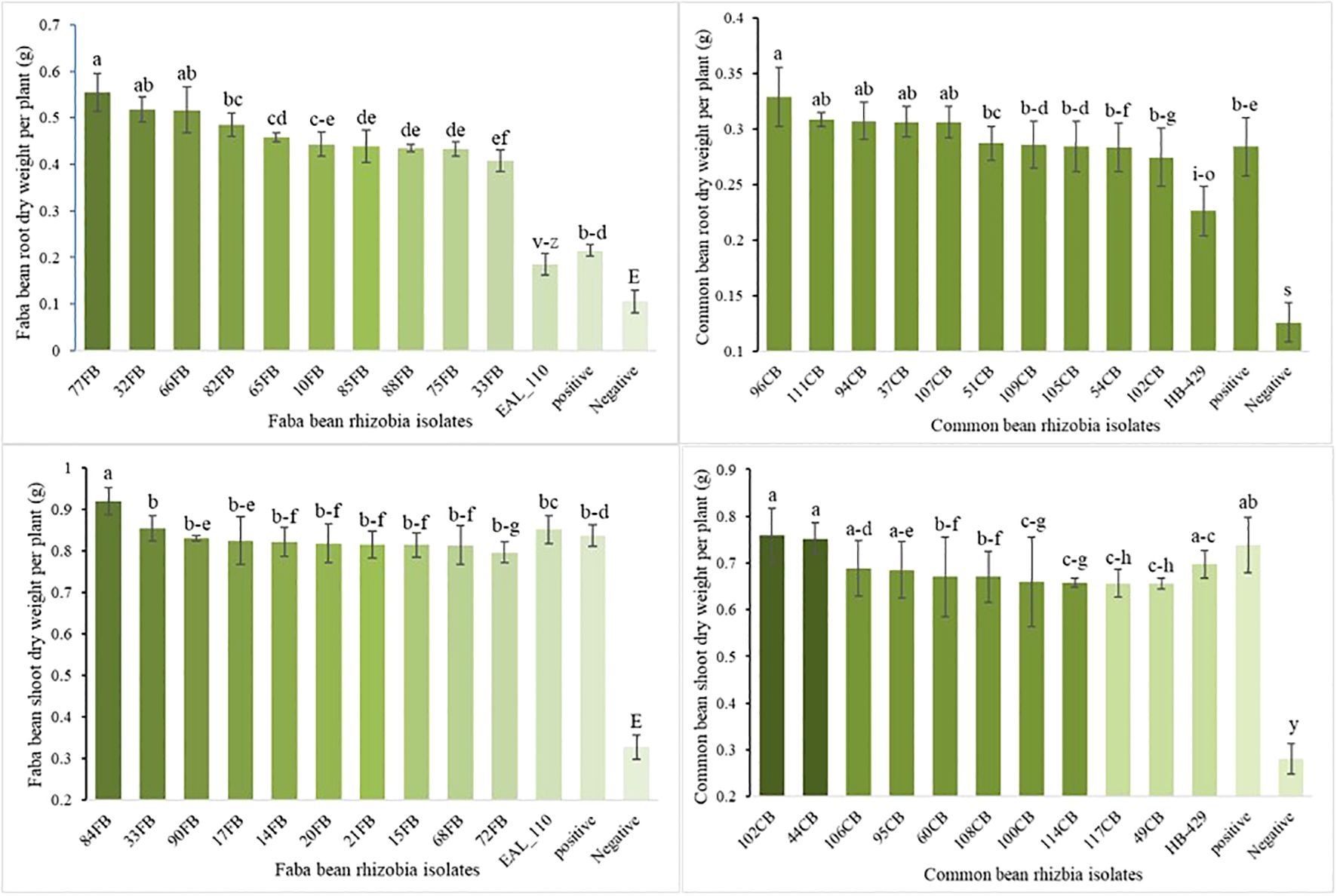
Figure 4. Root and shoot dry weight of faba bean and common bean response to rhizobia inoculation. Notice: The graph showcases the top 10 most effective indigenous rhizobia. Supplementary Table S4 details the responses of faba bean and common bean to all indigenous rhizobia. Vertical lines on bars represent S.E of the means. Different letters above the bars indicate statistically significant differences between isolates (p < 0.05).
The highest mean shoot dry weight of faba bean (0.92 g plant-1) and common bean (0.758 g plant-1) were recorded with 84FB and 102CB rhizobia isolates, respectively, which were slightly higher than commercial inoculant treated plant (P < 0.001; Supplementary Table S4). Notably, common bean isolates 102CB and44CB, and faba bean isolates 84FB and 33FB produced higher shoot dry weight than both N-fertilized and commercial inoculated plants. Overall, the results indicate that indigenous rhizobia isolates were more effective in promoting nodulation and shoot biomass accumulation than commercial inoculants and N fertilization.
3.5.3 Shoot total nitrogen (TN%) concentration
The application of rhizobia isolates highly significantly affected the accumulation of shoot total N (P < 0.01; Supplementary Table S4). Total N concentration in faba bean tissue ranged from 2.44 to 3.59%, and in common bean from 2.23 to 3.44%. The highest total N concentration was observed with isolate 33FB in faba bean and isolate 102CB in common bean (Figure 5).
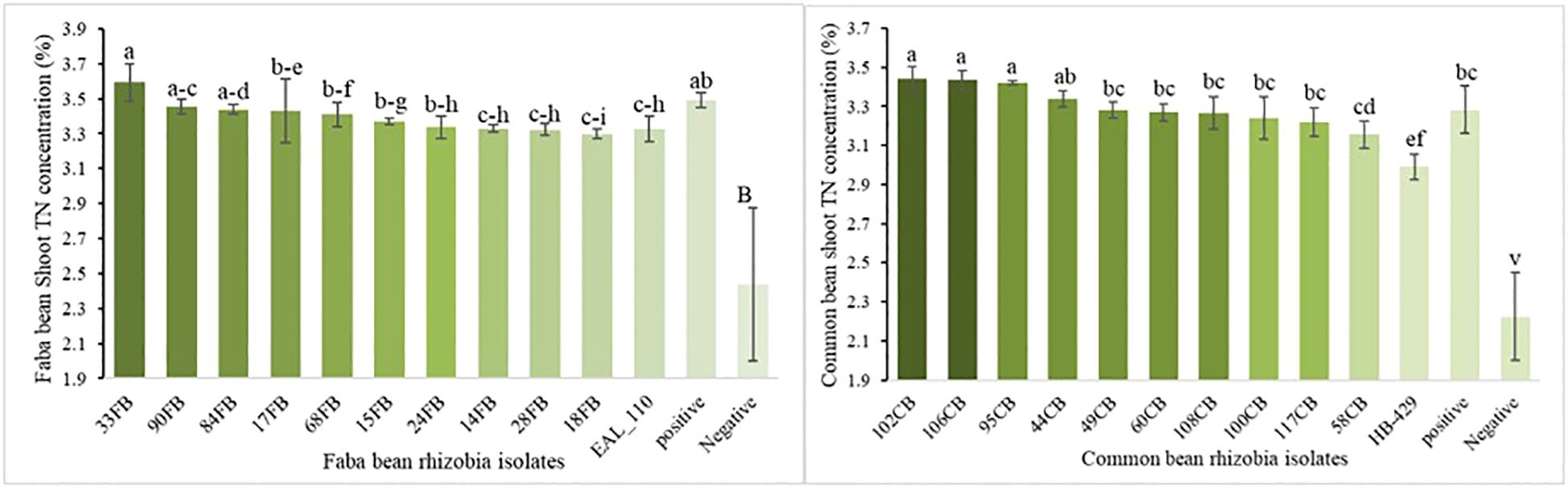
Figure 5. Shoot total N concentration of faba bean and common in response to rhizobia inoculation. Notice: The graph showcases the top 10 most effective indigenous rhizobia. Supplementary Table S4 details the responses of faba bean and common bean to all indigenous rhizobia. Vertical lines on bars represent S.E of the means. Different letters above the bars indicate statistically significant differences between isolates (p < 0.05).
3.5.4 Symbiotic efficiency indices of faba bean and common bean rhizobia isolates
The rhizobia isolates were tested for nitrogen fixation effectiveness using different symbiotic efficiency measures. The relative symbiotic efficiency (RSE) of faba bean isolates ranged from 48.5% (12FB) to 109.8% (84FB) (Figure 6a), while for common bean isolates, RSE ranged from 42.6% (41CB) to 102.8% (102CB) (Figure 6b). Among faba bean isolates, 54.7% were classified as highly effective (RSE > 80%), 43.4% as effective (50-80%), and 1.9% as poorly effective (35-50%) in comparison to the positive control (N fertilizer plants). For common bean isolates, 37.3% were highly effective, 58.8% effective, and 3.9% ineffective in terms of nitrogen fixation efficiency.
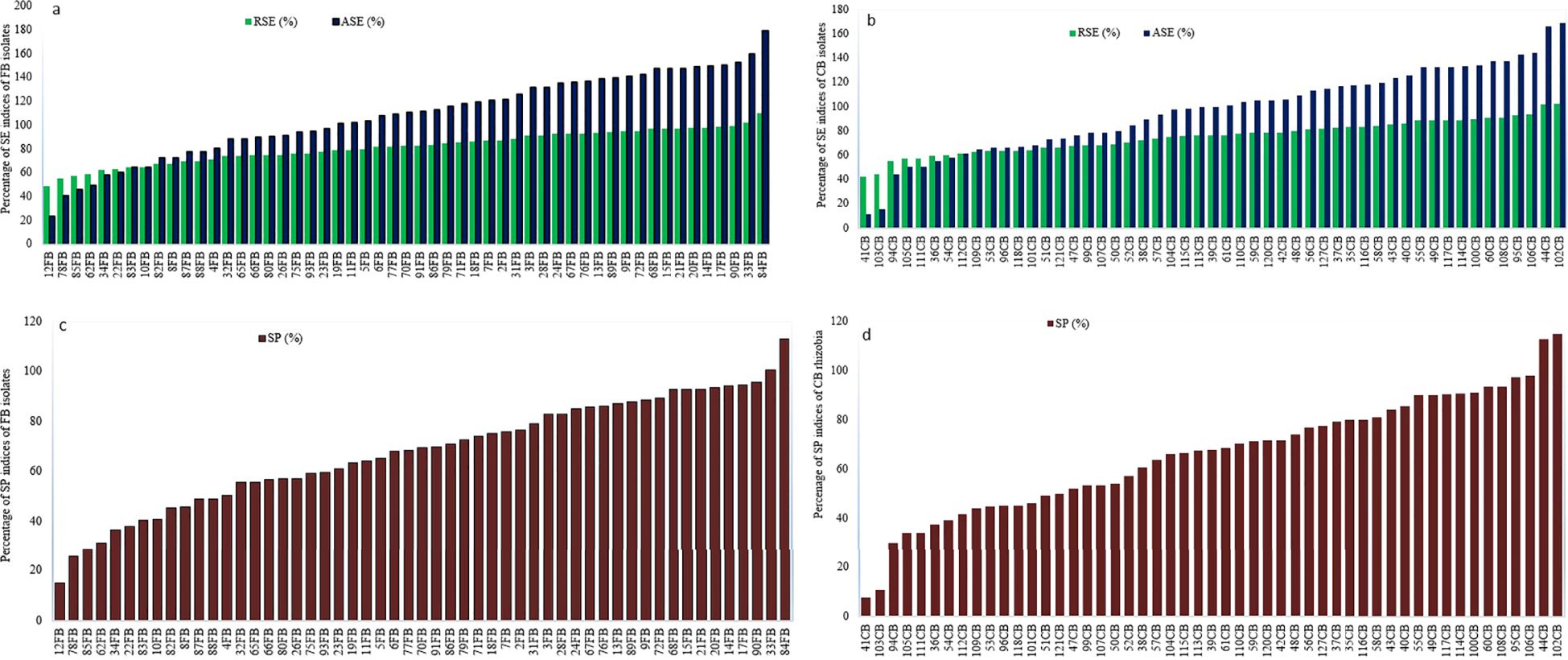
Figure 6. Mean relative symbiotic efficiency (RSE) and absolute symbiotic efficiency (ASE) of faba bean (a) and common bean rhizobia isolates (b) and symbiotic performance (SP) of the 53 and 51 indigenous rhizobia isolates of faba bean (c) and common bean (d). The mean values are calculated from triplicate pots.
The absolute symbiotic efficiency (ASE) of faba bean inoculated with rhizobia isolates varied from ineffective to highly effective in fixing atmospheric nitrogen, compared to uninoculated plants. Specifically, 77.4% of faba bean isolates were classified as highly effective, 15.1% as effective, 5.7% as poorly effective, and 1.9% as ineffective in atmospheric nitrogen fixation (Figure 6a). For common bean isolates, 62.8% were highly effective, 31.4% effective, 2.0% poorly effective, and 3.9% ineffective compared to the uninoculated plants (negative control) (Figure 6b).
The mean symbiotic performance (SP) indicated that 50.9% of the inoculated faba bean isolates showed optimal SP (SP>70%), while 49.1% showed sub-optimal SP (SP<70%) (Figure 6c). Similarly, 58.8% and 41.2% of the inoculated common bean isolates showed optimal and sub-optimal SP, respectively, when compared with commercial inoculants (Figure 6d).
3.6 Variability in nodulation and plant growth induced by rhizobia from soils subjected to different land use types
Our results showed that the mean number of nodules per plant (NNPP) and shoot dry weight of faba bean induced by isolates from different land use types did not significantly vary, unlike nodule dry weight (P > 0.05: Figures 7a, c, e). However, there was a considerable variability among isolates across land use types in inducing faba bean nodulation and its dry weight. The boxplots indicated high variability among isolates from the same land use types in forming the number of nodules per plant.
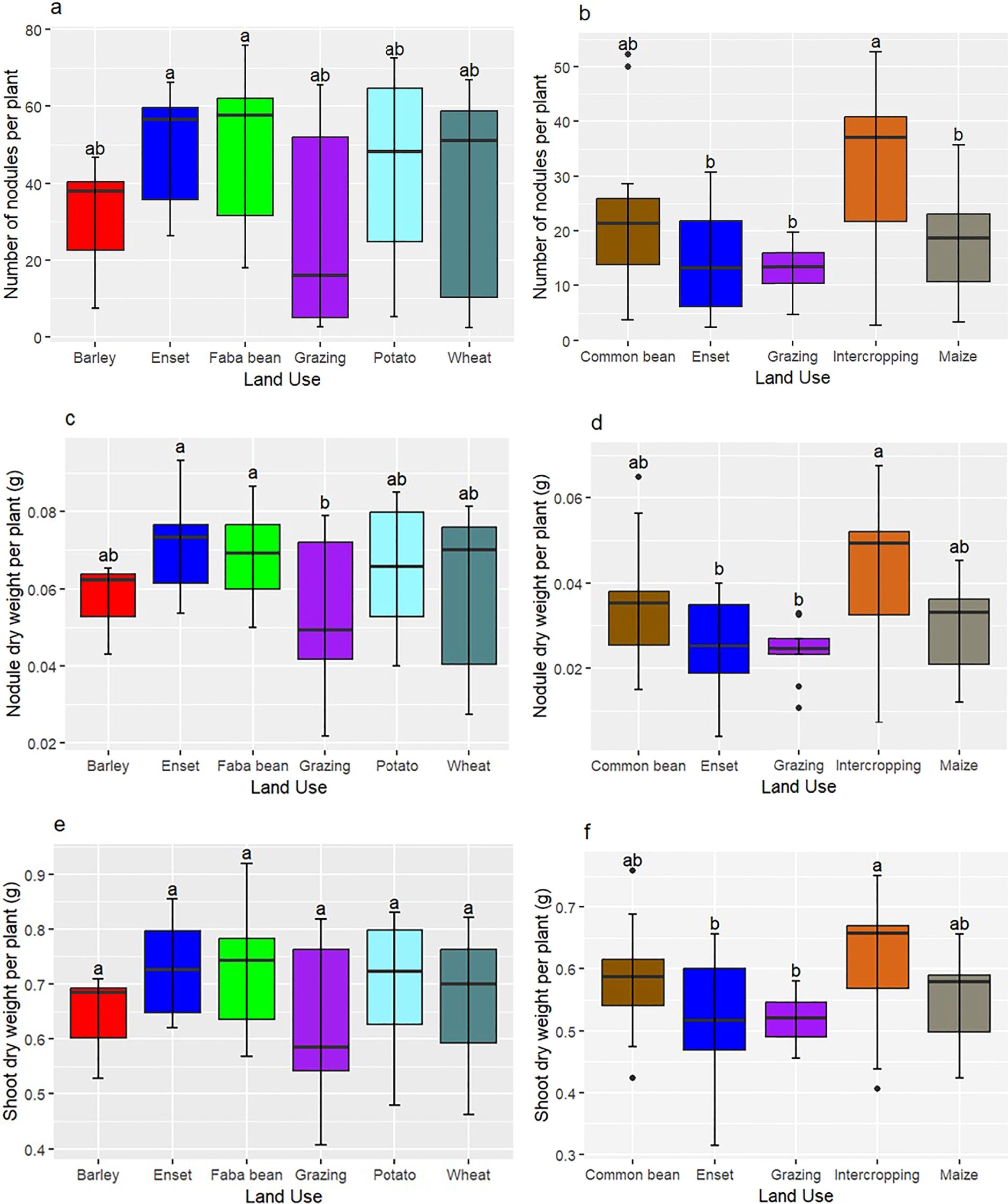
Figure 7. Boxplots showing the nodule numbers on faba bean (a) and common bean (b); nodule dry weight on faba bean (c) and common bean (d); shoot dry weight of faba bean (e) and common bean (f) inoculated with indigenous rhizobia isolates from various land uses. Lines in the box indicate the median; box limits indicate the lower and upper quartiles. Mean values shared the same lower-case letters on each box plot are not significantly different at 5% probability level based on Bonferroni pair wise comparison of means. BR, Barley field; EN, Enset field; FB, Faba bean field; GZ, Grazing land; PO, Potato field; WH, Wheat field; CB, Common bean field; IC, Intercropping (maize + common bean) field; MZ, Maize field.
The number of nodules formed by isolates from soils of grazing land, wheat, and potato fields showed high variability, with interquartile ranges of 47.0, 48.4, and 39.9 nodules per plant, respectively. In contrast, isolates from soils under faba bean, enset, and barley fields exhibited comparatively lower variability, with interquartile ranges of 30.3, 24.0, and 17.7 nodules per plant, respectively (Figure 7a). Isolates from faba bean field soils also showed wide variation in nodule numbers, ranging from 18 (88FB) to 76 (84FB) (Figures 3, 7a). Nodule dry weight variability followed a similar trend to that of nodule number across different land use types (Figure 7c).
The mean values of nodule traits and shoot dry weight of common bean induced by isolates from different land use types varied significantly (P < 0.05; Figures 7b, d, f). Nodule numbers of common bean (NNPP) formed by isolates from intercropped field soils showed a higher interquartile range (19.2 NNPP) compared to the isolates obtained from soils under grazing, enset land, common bean, and maize fields (Figure 7b). Isolates from intercropped fields also exhibited considerable variability in nodulation, ranging from 2.7 to 52.7 NNPP, with a mean of 30.4 NNPP (Figure 7b). Nodule dry weight of common bean followed a similar trend to NNPP across different land use types (Figure 7d).
Faba bean shoot dry weight was influenced by rhizobia isolates obtained from soils of grazing land, which showed the highest interquartile range (0.22 g plant−1), followed by isolates from wheat and potato field soils (both 0.17 g plant−1), compared to the smaller interquartile range observed for barley field soil isolates (0.09 g plant−1) (Figure 7e). However, isolates from faba bean field soils promoted high plant growth, with shoot biomass ranging from 0.57 to 0.92 g plant−1, particularly isolates 8FB and 84FB yielding a mean shoot dry weight of 0.72 g plant−1 (Figures 4, 7e).
The common bean data showed high variability in shoot dry weight induced by isolates from soils under enset, as indicated by a relatively high interquartile range, followed by isolates from intercropping field soils (Figure 7f). In contrast, isolates from soils of common bean fields exhibited less variability, with a smaller interquartile range of 0.074 g plant−1 shoot dry weight. The highest shoot dry weights in soils from common bean fields ranged from 0.42 to 0.76 g plant−1, induced by isolates 105CB and 102CB, respectively. However, the highest overall mean shoot dry weight (0.61 g plant−1) was obtained for isolates from intercropping field soils (Figure 7b).
4 Discussion
4.1 Effect of land use/cropping system and soil property on rhizobia abundance
The results indicate that rhizobia abundance was significantly influenced by both land use types/cropping systems and soil properties. Land use types, particularly enset fields and grazing lands showed a marked decrease in rhizobia populations compared to legume fields. Soils from wheat, barley and potato field soils also showed very low faba bean rhizobia populations relative to those from faba bean fields. Similarly, rhizobia population associated with common bean were significantly lower in soils under maize and potato compared to common bean fields. These findings suggest that the continuous cultivation of non-legume crops such as barley, wheat, potato, and maize negatively affects the abundance of rhizobia. This is further supported by the positive correlation observed between the presence of host legumes and rhizobia populations.
The type of crop grown was identified as key factors influencing rhizobia abundance. Notably, the presence of host legumes was positively correlated with higher rhizobia populations, supporting the hypothesis that indigenous rhizobia, particularly those associated with common bean, thrive in such soils and may reduce the need for external inoculants. In contrast, soils planted with non-leguminous crops, such as enset, grass and cereals exhibited significantly lower rhizobia abundance, likely due to the absence of symbiotic relationships. However, the inclusion of legumes in cropping systems can significantly boost rhizobia populations through their symbiotic relationships with host plants, as shown in previous studies (19, 21). When a particular legume is cultivated continuously over multiple years, the rhizobia species that form successful symbiotic relationships with that legume are likely to become more abundant, as they adapt and proliferate in response to the consistent presence of their host (58). Consequently, the high rhizobia populations observed in common bean production areas can be attributed to the integration of legumes in the cropping systems, which promotes the growth and persistence of rhizobia through these beneficial symbiotic interactions (21, 22, 59).
In contrast, the soils under enset, grazing land, and cereal crops, where legumes are absent, exhibited significantly lower rhizobia abundance, likely due to the lack of symbiotic relationships. In such environments, rhizobia rely on free-living survival, which leads to slower growth rates and reduced population sizes (60–63). Additionally, non-leguminous plants provide minimal benefit to rhizobia, contributing to a natural decline in their populations over time (64). Therefore, land management practices such as crop rotation and intercropping with legumes can enhance rhizobia abundance, while cereal monocropping may lead to a reduction in these beneficial bacteria. In our study, fields intercropped with common bean exhibited higher rhizobia populations compared to those with sole maize cropping.
Moreover, continuous cultivation of cereal crops can suppress rhizobia populations due to factors such as soil compaction, nutrient depletion, and low organic carbon levels (21, 22, 59). Our study found that several soil properties particularly pH, exchangeable acidity, exchangeable Al, EC, OC, TN, and CEC significantly influenced rhizobia abundance. Notably, we observed a positive correlation between soil pH, EC, TN, CEC with rhizobial populations, while a negative correlation was found with exchangeable acidity and Al. Soil pH emerged as a key factor, with acidic soils (pH < 5.5) generally exhibiting reduced rhizobia populations. This aligns with prior research indicating that low pH conditions can disrupt rhizobial metabolism and cell membrane function, reduce bacterial survival, and interfere with the nodulation process (64, 65). Additionally, acidic soils often contain elevated levels of exchangeable acidity and Al, which is toxic to both rhizobia and host plant roots (66, 67). Aluminum can damage root tips and inhibit infection thread formation, thereby limiting effective symbiosis and nitrogen fixation (67, 68).
Low soil pH is a major factor that exacerbates Al toxicity, significantly impairing rhizobia survival and symbiotic efficiency. In acidic soils (pH < 5.5), aluminum becomes more soluble, leading to increased concentrations of phytotoxic Al3+ ions in the soil solution (69, 70). These ions can damage rhizobial cell membranes, disrupt enzymatic processes, and interfere with the uptake of essential nutrients such as P, Ca, and Mg (71). Moreover, aluminum toxicity adversely affects root hair development an essential step for successful rhizobial infection and nodulation by inhibiting root elongation and causing abnormal root morphology (74). Even when nodulation occurs under acidic conditions, aluminum stress can impair nodule function and reduce nitrogenase activity, leading to diminished biological nitrogen fixation (72, 73). As such, the combined effect of low pH and high aluminum availability creates a hostile soil environment that limits rhizobia proliferation, root colonization, and the establishment of effective legume–rhizobia symbioses.
To ensure the efficiency of legume-rhizobia symbiosis, rhizobia have evolved several mechanisms to mitigate the detrimental effects of low pH and Al toxicity. Rhizobia have developed adaptive acid tolerance responses (ATR), primarily regulated by two-component systems such as actR/S and chvI/exoS/exoR. These systems modulate the production of exopolysaccharides (EPS), notably succinoglycan, which play a crucial role in maintaining cell wall integrity and facilitating effective nodulation under acidic conditions (75, 76). Additionally, the synthesis of glutathione, an antioxidant, is upregulated under acidic stress, aiding in the maintenance of cytoplasmic pH (77). Aluminum toxicity, prevalent in acidic soils can disrupt cellular membranes and inhibit DNA replication. To counteract these effects, rhizobia produce EPS and siderophores, which chelate Al3+ ions, reducing their uptake and mitigating toxicity. Furthermore, Al exposure induces oxidative stress; thus, rhizobia enhance the expression of antioxidant enzymes such as superoxide dismutase and catalase to neutralize reactive oxygen species and protect cellular components (67, 78, 79). Al tolerance in symbiotic rhizobia is also attained via induction of efflux pumps resistant to heavy metals and the expression of metal-inducible (dmeRF) gene clusters in symbiotic Rhizobiaceae (67). Understanding these adaptive mechanisms is vital for developing rhizobial inoculants tailored to thrive in acidic and Al-toxic soils. Selecting or engineering strains with enhanced ATR and Al tolerance can improve the efficacy of biological nitrogen fixation, thereby promoting sustainable agricultural practices in challenging soil environments (67).
Moreover, EC is a proxy for salinity and measure of soluble salts, was associated with rhizobia abundance across sites. Moderate EC levels may indicate sufficient ionic nutrients, but high EC can signal salinity stress, which can inhibit both rhizobia and host plant performance. High salinity can cause osmotic stress in rhizobia, disrupting cell membrane integrity and metabolic processes (80, 81).
In contrast, higher levels of OC and TN were positively associated with rhizobia abundance. OC serves as a critical energy source for microbes, supporting microbial biomass and promoting favorable soil structure and moisture retention, which are conducive to rhizobia survival and activity (82). In addition, lower OC content restricts the energy supply required for the growth and metabolism of rhizobia, hindering their ability to establish and sustain symbiotic relationships with leguminous plants (83, 84). TN, particularly in the form of soil organic matter, also reflects greater microbial turnover and biological activity, creating a more favorable microenvironment for rhizobia proliferation.
Furthermore, cation exchange capacity (CEC) was positively correlated with rhizobia abundance. Soils with high CEC can retain essential cations such as calcium (Ca2+), magnesium (Mg2+), and potassium (K+), which not only improve nutrient availability for plant growth but also support rhizobial functioning and nodulation processes. These nutrients play direct roles in nodule formation, nitrogenase activity, and signaling between the host plant and rhizobia. These findings support the hypothesis that soil properties significantly influence the growth and function of rhizobia populations. Soils with optimum nutrient levels and favorable characteristics tend to support higher rhizobia populations (19, 85).
For instance, nearly neutral pH, high OC content, and a balanced nutrient profile create an environment conducive to rhizobia survival and proliferation. Conversely, lower soil pH, reduced OC, and total N levels, and elevated exchangeable acidity and aluminium content, negatively impact rhizobia populations, particularly in faba bean rhizobia. The combined effect of these factors creates a challenging environment that inhibits rhizobia growth and reduce rhizobia abundance (19, 22). These interactions suggest that the combined effect of soil properties play a crucial role in regulating rhizobia abundance and activity.
Overall, these findings demonstrate that the abundance and efficiency of indigenous rhizobia are closely linked to the chemical environment of the soil. Effective legume-rhizobia symbiosis depends not only on the presence of compatible strains but also on favorable soil conditions that enable nodulation and biological nitrogen fixation. This underscores the importance of integrating soil health management particularly addressing pH and aluminum toxicity into rhizobia inoculation strategies to enhance legume productivity and soil fertility in Ethiopian farming systems.
Land-use comparisons further revealed that soil nutrient levels in cultivated fields such as those used for wheat, barley, potato, faba bean, common bean, and maize were lower compared to enset and grazing lands. Conversely, exchangeable acidity, and exchangeable Al were higher in cultivated fields than in enset and grazing lands. These changes adversely affected rhizobia populations as previously reported (24, 86, 87). These findings support our hypothesis that soil stress negatively impacts the response of faba bean to rhizobia inoculation in production areas. Moreover, they reinforce the observation that legumes show minimal responses to rhizobia inoculation in soils with low pH and high levels of exchangeable acids and aluminium (67, 88). Overall, management strategies such as rotating and intercropping legumes with cereals, while maintaining OC and pH levels, can enhance rhizobia populations, improve soil fertility, and increase crop productivity (21, 22).
4.2 Effects of land use/cropping system and soil properties on symbiotic efficiency of indigenous rhizobia
Our analysis revealed that the inoculation with indigenous rhizobia isolates significantly enhanced nodulation, shoot dry weight, and shoot TN accumulation in both faba bean and common bean plants compared to uninoculated controls. These results suggest that the indigenous isolates exhibit high symbiotic efficiency, promoting greater N fixation and improving overall plant performance. Notably, the indigenous rhizobia isolates of faba bean (84FB and 33FB) and common bean (102CB and 44FCB) outperformed both commercial strains and N fertilizers, highlighting their potential as a cost-effective and sustainable alternative for improving legume productivity in cropping systems (89, 90). These findings align with previous research demonstrating the potential of locally adapted rhizobia to enhance plant growth under specific environmental conditions (91, 92). This effectiveness can be attributed to the strong compatibility between rhizobia and host legumes, superior competitiveness for nodule formation, and the co-evolved symbiotic efficiency developed over time (93–96).
The symbiotic efficiency indices revealed that most inoculated indigenous rhizobia isolates exhibited both relative symbiotic efficiency (RSE) and absolute symbiotic efficiency (ASE) greater than 50% compared to the N-fertilized plants and uninoculated plants. Notably, half of the isolates showed optimal performance (>70%) when compared to commercial inoculants. As hypothesized, these findings suggest that the soils of the study area harbor effective indigenous rhizobia capable of competing with commercial strains. The competitiveness of introduced rhizobia was influenced by the existing indigenous population. Specifically, indigenous rhizobia populations exceeding the threshold >10² cells g-1 soil, when coupled with high symbiotic efficiency, can lead to inconsistent responses to inoculation (37, 97). While a high rhizobia population indicates the potential for effective symbiotic nitrogen fixation, it does not guarantee it, as strains competitiveness can vary significantly (34). Therefore, it is recommended to assess both the competitiveness and effectiveness of indigenous rhizobia in addition to population enumeration (98).
The inconsistent response of legumes to rhizobia inoculation and the limited adoption of inoculants can be attributed to the presence of abundant indigenous rhizobia with high symbiotic efficiency in the soils of common bean production areas, specifically in Boricha and Hawassa Zuria. While soils in Hula and Cheha commonly used for faba bean cultivation also harbored rhizobia with high symbiotic efficiency, their population sizes were insufficient to support optimal N fixation. This discrepancy may be linked to adverse soil factors, such as low pH and high aluminium concentrations, which negatively affect rhizobia abundance and effectiveness (30, 99). Overall, the results of this study highlight the importance of evaluating indigenous rhizobia populations before relaying on commercial inoculants. Understanding the abundance, composition, and symbiotic performance of native rhizobia can lead to more targeted and efficient inoculation strategies tailored to specific local conditions. This approach ensures the use of rhizobia strains that are well-suited to the existing soil, thereby enhancing the success and sustainability of biological nitrogen fixation.
The highest common bean rhizobia population (>102 cells g-1 soil), particularly found in common bean-maize intercropping fields, showed strongest competitiveness against commercial inoculants, thereby reducing the crop’s response to inoculation in terms of nodulation, shoot dry matter and TN accumulation. When the population of effective indigenous rhizobia exceeds a threshold (>10² cells g-1 of soil), legumes often show no significant response to inoculation (33, 37). Conversely, although the indigenous rhizobia capable of nodulating faba beans in its production areas were effective in nitrogen fixation, their population was below the required threshold. This suggests that, while these rhizobia are efficient at converting atmospheric N into a plant-usable form, their limited population may constrain the overall N fixation capacity (33, 34, 37). The presence of these effective yet low-abundance indigenous rhizobia indicate a potential benefit from introducing external inoculants to boost nitrogen fixation and support faba bean growth. In general, indigenous rhizobia populations associated with faba bean grown in acidic soils exhibited reduced symbiotic efficiency, suppressed nodule formation, and lower rhizobial persistence, consistent with earlier studies (69, 99, 100).
5 Conclusions
This study highlights the significant variation in rhizobia populations across different land use types and cropping systems, as well as their crucial role in improving legume productivity through symbiotic N fixation. The findings revealed that the rhizobia population associated with common beans was not only abundant but also highly effective in enhancing N fixation in soils. This suggests that the natural presence of common bean indigenous rhizobia can effectively support nitrogen fixation without the need of external inoculation. In soils where effective rhizobia are abundant, it is essential to adopt sustainable farming practices, such as crop rotation and intercropping. These practices can help preserve and enhance the natural rhizobia population, reducing the dependence on commercial inoculant. Conversely, the effective rhizobia population associated with faba bean was found to be low, indicating the necessity of inoculating faba beans with rhizobia that are well-adapted to local soil conditions. Despite this, the various land use types and cropping systems in the study area harbored effective rhizobia populations, demonstrating their potential to sustain these beneficial microorganisms. Indigenous rhizobia isolates from faba beans (33FB and 84FB), and common bean (44CB and 102CB), outperformed both commercial strains and N-fertilized plants. This highlights the potential of indigenous rhizobia as valuable resources for improving legume productivity and soil fertility. Therefore, in areas with low indigenous rhizobia populations, further research is needed to evaluate the potential of promising indigenous rhizobia isolates, alongside commercial inoculants across diverse soils and agroecological conditions. Such studies should focus on assessing the persistence, stability, and competitiveness of these indigenous strains for successful integration into existing cropping systems. Overall, this study underscores the significant potential of indigenous rhizobia to enhance legume productivity and soil fertility, offering a sustainable alternative and reducing reliance on commercial inoculants.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
TG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. GA: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. BL: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing. FR: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This publication is part of a PhD scholarship at Hawassa University, conducted within the framework of the German-Ethiopian SDG Graduate School “Climate Change Effects on Food Security (CLIFOOD).” This program is a collaboration between the University of Hohenheim (Germany) and Hawassa University (Ethiopia), supported by DAAD with funding from the Federal Ministry for Economic Cooperation and Development (BMZ).
Acknowledgments
We would like to thank the farmers who generously allowed us to collect soil samples from their farms. We also express our gratitude to Tekle Tadale, our field technical assistant; Demere Takale, our laboratory technical assistant; and Tomas Buldudo, our project car driver, for their dedicated support and cooperation throughout the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsoil.2025.1568292/full#supplementary-material.
References
1. Meselu YK. A review on the seed sector of Ethiopia: prospects and challenges of faba bean seed supply. South Asian J Dev Res. (2019) 1:44–54. http://aiipub.com/journals/sajdr-190514-021007/.
2. Kebede E. Grain legumes production and productivity in Ethiopian smallholder agricultural system, contribution to livelihoods and the way forward. Cogent Food Agric. (2020) 6:1722353. doi: 10.1080/23311932.2020.1722353
3. Worku W. Haricot bean production guide: with emphasis on southern Ethiopia (English version). Sponsored by Project ‘Scaling-up Pulse Innov Food Nutrition Security South Ethiopia’. Can Int Food Secur Res Fund Hawassa. (2015), 34.
4. Abebe Z, Abdulkadir B, Mekonnen K, and Thorne PJ. Current situation of legumes production and intensification in Ethiopia: a review on experiences, challenges, opportunities and policy recommendations. Addis Ababa, Ethiopia: ILRI Res Rep (2022).
5. Zerihun A and Haile D. The effect of organic and inorganic fertilizers on the yield of two contrasting soybean varieties and residual nutrient effects on a subsequent finger millet crop. Agronomy. (2017) 7:42. doi: 10.3390/agronomy7020042
6. Stagnari F, et al. Multiple benefits of legumes for agriculture sustainability: an overview. Chem Biol Technol Agric. (2017) 4:1–13. doi: 10.1186/s40538-016-0085-1
7. Hailu H and Geremu T. Effect of sorghum-legume intercropping patterns on selected soil chemical properties and yield of sorghum at midland areas of West Hararghe Zone of Oromia Regional State, Eastern Ethiopia. Irrig Drain Syst Eng. (2021) 10:270.
8. Abd-Alla MH, Al-Amri SM, and El-Enany A-WE. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture. (2023) 13:2092. doi: 10.3390/agriculture13112092
9. Wendimu A, Yoseph T, and Ayalew T. Ditching phosphatic fertilizers for phosphate-solubilizing biofertilizers: a step towards sustainable agriculture and environmental health. Sustainability. (2023) 15:1713. doi: 10.3390/su15021713
10. Degaga J and Angasu B. Assessment of indigenous knowledge of smallholder farmers on intercropping practices in West Hararghe Zone; Oromia National Regional State, Ethiopia. J Agric Econ Rural Dev. (2017) 3:270–8.
11. Yimer T, Abera G, Beyene S, and Rasche F. Optimizing maize–bean cropping systems for sustainable intensification in southern Ethiopia. Agron J. (2022) 114:3283–96. doi: 10.1002/agj2.21143
12. Okumu OO, Otieno HM, and Okeyo GO. Production systems and contributions of grain legumes to soil health and sustainable agriculture: a review. Arch Agric Environ Sci. (2023) 8:259–67. doi: 10.26832/24566632.2023.0802024
13. Jena J, Maitra S, Hossain A, Pramanick B, Gitari HI, Praharaj S, et al. Role of legumes in cropping system for soil ecosystem improvement. In: Ecosystem Services: Types, Management and Benefits. Nova Science Publishers, Inc (2022). p. 1–21.
14. Allen ON and Allen EK. The Leguminosae, a source book of characteristics, uses, and nodulation. Univ of Wisconsin Press (1981).
15. Tian CF, Wang ET, Wu LJ, Han TX, Chen WF, Gu CT, et al. Rhizobium fabae sp. nov., a bacterium that nodulates Vicia faba. Int J Syst Evol Microbiol. (2008) 58:2871–5. doi: 10.1099/ijs.0.2008/000703-0
16. Saidi S, Ramirez-Bahena MH, Santillana N, Zuniga D, Alvarez-Martinez E, Peix A, et al. Rhizobium laguerreae sp. nov. nodulates Vicia faba on several continents. Int J Syst Evol Microbiol. (2014) 64:242–7. doi: 10.1099/ijs.0.052191-0
17. Hailu Gunnabo A, Geurts R, Wolde-Meskel E, Degefu T, Giller KE, and van Heerwaarden J. Phylogeographic distribution of rhizobia nodulating common bean (Phaseolus vulgaris L.) in Ethiopia. FEMS Microbiol Ecol. (2021) 97. doi: 10.1093/femsec/fiab046
18. Somasegaran P and Hoben HJ. Methods in legume-Rhizobium technology. University of Hawaii NifTAL Project and MIRCEN, Department of Agronomy, University of Hawaii (1985).
19. Drew EA, Denton MD, Sadras VO, and Ballard RA. Agronomic and environmental drivers of population size and symbiotic performance of Rhizobium leguminosarum bv. viciae in Mediterranean-type environments. Crop Pasture Sci. (2012) 63:467–77. doi: 10.1071/CP12032
20. Siczek A, Gryta A, Oszust K, and Frąc M. Faba bean in crop rotation shapes bacterial and fungal communities and nutrient contents under conventional tillage of triticale. Appl Soil Ecol. (2024) 202:105597. doi: 10.1016/j.apsoil.2024.105597
21. Kebede E, Amsalu B, Argaw A, and Tamiru S. Abundance of native rhizobia nodulating cowpea in major production areas of Ethiopia as influenced by cropping history and soil properties. Sustain Environ. (2021) 7:1889084. doi: 10.1080/27658511.2021.1889084
22. Yan J, Han XZ, Ji ZJ, Li Y, Wang ET, Xie ZH, et al. Abundance and diversity of soybean-nodulating rhizobia in black soil are impacted by land use and crop management. Appl Environ Microbiol. (2014) 80:5394–402. doi: 10.1128/AEM.01135-14
23. Maluk M, Ferrando-Molina F, Lopez del Egido L, Langarica-Fuentes A, Gebre Yohannes G, Young MW, et al. Fields with no recent legume cultivation have sufficient nitrogen-fixing rhizobia for crops of faba bean (Vicia faba L.). Plant Soil. (2022) 472:345–68. doi: 10.1007/s11104-021-05246-8
24. Nzeyimana F, Onwonga RN, Ayuke FO, Chemining'wa GN, Nabahungu NL, Bigirimana J, et al. Determination of abundance and symbiotic effectiveness of native rhizobia nodulating soybean and other legumes in Rwanda. Plant Environ Interact. (2024) 5:e10138. doi: 10.1002/pei3.10138
25. Argaw A and Akuma A. Rhizobium leguminosarum bv. viciae sp. inoculation improves the agronomic efficiency of N of common bean (Phaseolus vulgaris L.). Environ Syst Res. (2015) 4:1–13. doi: 10.1186/s40068-015-0036-z
26. Argaw A. Effectiveness of Rhizobium inoculation on common bean productivity as determined by inherent soil fertility status. J Crop Sci Biotechnol. (2016) 19:311–22. doi: 10.1007/s12892-016-0094-4
27. Argaw A and Mnalku A. Effectiveness of native Rhizobium on nodulation and yield of faba bean (Vicia faba L.) in Eastern Ethiopia. Arch Agron Soil Sci. (2017) 63:1390–403. doi: 10.1080/03650340.2017.1287353
28. Chimdi A, Negasa D, and Chala G. Effects of rhizobium inoculation and P fertilizer levels on selected soil properties, yield, and yield components of faba bean (Vicia faba L.): The case of Abuna Gindeberat, west Shewa Zone, Ethiopia. Appl Environ Soil Sci. (2022) 2022:3635989. doi: 10.1155/2022/3635989
29. Zerihun A and Abera T. Yield response of faba bean to fertilizer rate, rhizobium inoculation and lime rate at Gedo highland, western Ethiopia. Glob J Crop Soil Sci Plant Breed. (2014) 2:134–9.
30. Muleta D, Ryder MH, and Denton MD. The potential for rhizobial inoculation to increase soybean grain yields on acid soils in Ethiopia. Soil Sci Plant Nutr. (2017) 63:441–51. doi: 10.1080/00380768.2017.1370961
31. Getahun A, Muleta D, Assefa F, and Kiros S. Field application of rhizobial inoculants in enhancing faba bean production in acidic soils: an innovative strategy to improve crop productivity. In: Akhtar M, editor. Salt Stress, Microbes, and Plant Interactions: Causes and Solution. Springer, Singapore (2019). p. 147–80. doi: 10.1007/978-981-13-8801-9_7
32. Danso SK. Biological nitrogen fixation in tropical agrosystems: twenty years of biological nitrogen fixation research in Africa. In: Biological Nitrogen Fixation and Sustainability of Tropical Agriculture: Proc of the 4th International Conf of the African Assoc for Biological Nitrogen Fixation, held at the Int Inst of Trop Agric, Nigeria, 24-28 Sept 1990. Wiley, Chichester (1992).
33. Brockwell J, Bottomley PJ, and Thies JE. Manipulation of rhizobia microflora for improving legume productivity and soil fertility: a critical assessment. In: Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems: Extended versions of papers presented at the Symposium on Biological Nitrogen Fixation for Sustainable Agriculture at the 15th Congress of Soil Science, Acapulco, Mexico, 1994. Springer (1995).
34. Argaw A and Tsigie A. Indigenous rhizobia population influences the effectiveness of Rhizobium inoculation and need of inorganic N for common bean (Phaseolus vulgaris L.) production in eastern Ethiopia. Chem Biol Technol Agric. (2015) 2:1–13. doi: 10.1186/s40538-015-0047-z
35. Wondosen H, Dobo B, and Mikru A. The contribution of rhizobia and arbuscular mycorrhizal fungi co-inoculation on growth and yield of haricot bean (Phaseolus vulgaris). Ethiop J Appl Sci Technol. (2023) 14:33–47. https://ejhs.ju.edu.et/index.php/ejast/article/view/5076.
37. Thies JE, Singleton PW, and Bohlool BB. Influence of the size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl Environ Microbiol. (1991) 57:19–28. doi: 10.1128/aem.57.1.19-28.1991
38. Thilakarathna MS and Raizada MN. A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol Biochem. (2017) 105:177–96. doi: 10.1016/j.soilbio.2016.11.022
39. Alves LA, Ambrosini VG, de Oliveira Denardin LG, et al. Biological N2 fixation by soybeans grown with or without liming on acid soils in a no-till integrated crop-livestock system. Soil Tillage Res. (2021) 209:104923. doi: 10.1016/j.still.2020.104923
40. Albareda M, Rodríguez-Navarro DN, and Temprano FJ. Soybean inoculation: dose, N fertilizer supplementation and rhizobia persistence in soil. Field Crops Res. (2009) 113:352–6. doi: 10.1016/j.fcr.2009.05.013
41. Hungria M and Mendes IC. Nitrogen fixation with soybean: the perfect symbiosis? In: de Bruijn FJ, editor. Biological Nitrogen Fixation. Wiley (2015). p. 1009–24. doi: 10.1002/9781119053095.ch99
42. Brockwell J, Pilka A, and Holliday RA. Soil pH is a major determinant of the numbers of naturally occurring Rhizobium meliloti in non-cultivated soils in central New South Wales. Aust J Exp Agric. (1991) 31:211–9. doi: 10.1071/EA9910211
43. Ministry of Agriculture (MoA). Major Agro-Ecological Zones of Ethiopia. Addis Ababa, Ethiopia: Ministry of Agriculture and Rural Development (2005).
44. FAO. Geomorphology and Soils of Ethiopia: Assistance to Land Use Planning (1:1,000,000). Addis Ababa, Ethiopia: FAO/UNDP, ETH/78/003, LUPRD/MOA (1984).
45. van Reeuwijk LP. Procedures for Soil Analysis. Wageningen: International Soil Reference and Information Centre (ISRIC (1995).
46. Walkley A and Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. (1934) 37:29–38. doi: 10.1097/00010694-193401000-00003
47. Bremner JM and Mulvaney C. Nitrogen total. In: Page AL, Miller RH, and Keeney DR, editors. Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed. ASA and SSSA, Madison (1982). p. 595–624.
48. Olsen SR. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. Washington, DC: US Department of Agriculture (1954). USDA Circular No. 939.
49. Rhoades J. Cation exchange capacity. In: Page AL, Miller RH, and Keeney DR, editors. Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed. ASA and SSSA, Madison (1983). p. 149–57.
50. Rowell DL. Soil Science: Methods and Applications. Harlow, England: Addison Wesley Longman Ltd (1994).
51. Vincent JM. A Manual for the Practical Study of the Root-Nodule Bacteria. Oxford: Blackwell Scientific Publications (1970).
52. Howieson JG and Dilworth MJ. Working with Rhizobia. Canberra, Australia: Australian Centre for International Agricultural Research (2016).
53. Rashid MHO, Schäfer H, Gonzalez J, and Wink M. Genetic diversity of rhizobia nodulating lentil (Lens culinaris) in Bangladesh. Syst Appl Microbiol. (2012) 35:98–109. doi: 10.1016/j.syapm.2011.11.008
54. dos Santos JGD, Aguiar ADCF, Silva Junior EM, Dadalto DL, Sousa MR, Xavier GR, et al. Soil management and efficiency of rhizobia strains of cowpea Vigna unguiculata (L.) Walp. in the tropics. Chil J Agric Res. (2011) 71:594–600. doi: 10.4067/S0718-58392011000400016
55. Kebede E, Amsalu B, Argaw A, and Tamiru S. Symbiotic effectiveness of cowpea (Vigna unguiculata (L.) Walp.) nodulating rhizobia isolated from soils of major cowpea producing areas in Ethiopia. Cogent Food Agric. (2020) 6:1763648. doi: 10.1080/23311932.2020.1763648
56. Purcino H, Festin P, and Elkan G. Identification of effective strains of Bradyrhizobium for Arachis pintoi. Proc 11th Nitrogen Fixation Conference Trop Agric. (2000) 77:226–31.
57. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2011). Available at: https://www.r-project.org.
58. Kumar V, Rawat A, and Rao D. Population ecology of soybean-rhizobia in diverse crop rotations in Central India. Agric Ecosyst Environ. (2017) 240:261–8. doi: 10.1016/j.agee.2017.02.011
59. Patil A, Kale A, Ajane G, Sheikh R, and Patil S. Plant growth-promoting Rhizobium: mechanisms and biotechnological prospective. In: Rhizobium Biology and Biotechnology. Springer, Cham (2017). p. 105–34. doi: 10.1007/978-3-319-68460-4_6
60. Abaidoo RC, Keyser HH, Singleton PW, Dashiell KE, and Sanginga N. Population size, distribution, and symbiotic characteristics of indigenous Bradyrhizobium spp. that nodulate TGx soybean genotypes in Africa. Appl Soil Ecol. (2007) 35:57–67. doi: 10.1016/j.apsoil.2006.05.006
61. Walley FL, Clayton GW, Miller PR, Carr PM, and Lafond GP. Nitrogen economy of pulse crop production in the Northern Great Plains. Agron J. (2007) 99:1710–8. doi: 10.2134/agronj2006.0314s
62. Wekesa CS, Furch AC, and Oelmüller R. Isolation and characterization of high-efficiency rhizobia from Western Kenya nodulating with common bean. Front Microbiol. (2021) 12:697567. doi: 10.3389/fmicb.2021.697567
63. DiCenzo GC, Zamani M, Checcucci A, Fondi M, Griffitts JS, Finan TM, et al. Multidisciplinary approaches for studying rhizobium–legume symbioses. Can J Microbiol. (2019) 65:1–33. doi: 10.1139/cjm-2018-0377
64. Zhang F and Smith D. Inter-organismal signaling in suboptimum environments: the legume–rhizobia symbiosis. In: Biotic Interactions in Plant–Pathogen Associations, vol. 76. Advances in Agronomy (2002). p. 125–61. doi: 10.1016/S0065-2113(02)76004-5
65. Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev. (1999) 63:968–89. doi: 10.1128/MMBR.63.4.968-989.1999
66. Soares BL, Ferreira PAA, Oliveira-Longatti SMD, Marra LM, Rufini M, Andrade MJB, et al. Cowpea symbiotic efficiency, pH and aluminum tolerance in nitrogen-fixing bacteria. Sci Agric. (2014) 71:171–80. doi: 10.1590/S0103-90162014000300001
67. Jaiswal SK, Naamala J, and Dakora FD. Nature and mechanisms of aluminium toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol Fertil Soils. (2018) 54:309–18. doi: 10.1007/s00374-018-1262-0
68. Sujkowska-Rybkowska M, Borucki W, and Znojek E. Structural changes in Medicago truncatula root nodules caused by short-term aluminum stress. Symbiosis. (2012) 58:161–70. doi: 10.1007/s13199-012-0192-2
69. Munyaneza V, Zhang W, Haider S, Xu F, Wang C, and Ding G. Strategies for alleviating aluminum toxicity in soils and plants. Plant Soil. (2024), 1–24. doi: 10.1007/s11104-024-06322-6
70. Ahmed B, Rizvi A, Syed A, Rajput VD, Elgorban AM, Al-Rejaie SS, et al. Understanding the phytotoxic impact of Al³+, nano-size, and bulk Al2O3 on growth and physiology of maize (Zea mays L.) in aqueous and soil media. Chemosphere. (2022) 300:134555. doi: 10.1016/j.chemosphere.2022.134555
71. Rout GR, Samantaray S, and Das P. Aluminium toxicity in plants: a review. Agronomie. (2001) 21:3–21. doi: 10.1051/agro:2001105
72. Mendoza-Soto AB, Naya L, Leija A, and Hernández G. Responses of symbiotic nitrogen-fixing common bean to aluminum toxicity and delineation of nodule responsive microRNAs. Front Plant Sci. (2015) 6:587. doi: 10.3389/fpls.2015.00587
73. Balestrasse KB, Gallego SM, and Tomaro ML. Aluminium stress affects nitrogen fixation and assimilation in soybean (Glycine max L.). Plant Growth Regul. (2006) 48:271–81. doi: 10.1007/s10725-006-0017-2
74. Hungria M and Vargas MA. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res. (2000) 65:151–64. doi: 10.1016/S0378-4290(99)00084-2
75. Hawkins JP, Geddes BA, and Oresnik IJ. Succinoglycan production contributes to acidic pH tolerance in Sinorhizobium meliloti Rm1021. Mol Plant Microbe Interact. (2017) 30:1009–19. doi: 10.1094/MPMI-07-17-0176-R
76. Maillet F, Fournier J, Mendis HC, Tadege M, Wen J, Ratet P, et al. Sinorhizobium meliloti succinylated high-molecular-weight succinoglycan and the Medicago truncatula LysM receptor-like kinase MtLYK10 participate independently in symbiotic infection. Plant J. (2020) 102:311–26. doi: 10.1111/tpj.14625
77. Hawkins JP and Oresnik IJ. The rhizobium-legume symbiosis: co-opting successful stress management. Front Plant Sci. (2022) 12:796045. doi: 10.3389/fpls.2021.796045
78. Draghi WO, Del Papa MF, Hellweg C, Watt SA, Watt TF, Barsch A, et al. A consolidated analysis of the physiologic and molecular responses induced under acid stress in the legume-symbiont model-soil bacterium Sinorhizobium meliloti. Sci Rep. (2016) 6:29278. doi: 10.1038/srep29278
79. Wekesa C, Muoma JO, Reichelt M, Asudi GO, Furch AC, and Oelmüller R. The cell membrane of a novel Rhizobium phaseoli strain is the crucial target for aluminium toxicity and tolerance. Cells. (2022) 11:873. doi: 10.3390/cells11050873
80. Niste M, Vidican R, Pop R, and Rotar I. Stress factors affecting symbiosis activity and nitrogen fixation by Rhizobium cultured in vitro. Pro Environment. (2013) 6:54–8.
81. Kajić S, Hulak N, and Sikora S. Environmental stress response and adaptation mechanisms in rhizobia. Agric Conspec Sci. (2016) 81:15–9.
82. Li G, Tang X, Hou Q, Li T, Xie H, Lu Z, et al. Response of soil organic carbon fractions to legume incorporation into cropping system and the factors affecting it: a global meta-analysis. Agric Ecosyst Environ. (2023) 342:108231. doi: 10.1016/j.agee.2023.108231
83. Bonilla I and Bolanos L. Mineral nutrition for legume-rhizobia symbiosis: B, Ca, N, P, S, K, Fe, Mo, Co, and Ni: A review. In: Lichtfouse E, editor. Organic Farming, Pest Control and Remediation of Soil Pollutants. Springer, Dordrecht (2010). p. 253–74. doi: 10.1007/978-90-481-8741-6_10
84. Kimiti JM and Odee DW. Integrated soil fertility management enhances population and effectiveness of indigenous cowpea rhizobia in semi-arid eastern Kenya. Appl Soil Ecol. (2010) 45:304–9. doi: 10.1016/j.apsoil.2010.05.008
85. Martyniuk S and Oroń J. Survival of rhizobia in two soils as influenced by storage conditions. Pol J Microbiol. (2008) 57:257–60.
86. Richardson AE and Simpson RJ. Acid-tolerance and symbiotic effectiveness of Rhizobium trifolii associated with a Trifolium subterraneum L.-based pasture growing in an acid soil. Soil Biol Biochem. (1989) 21:87–96. doi: 10.1016/0038-0717(89)90016-3
87. Cheng Y, Waktin ELJ, Howieson JG, and O’Hara GW. Root and root hair mechanisms that confer symbiotic competence for nodulation in acidic soils within Medicago species: a holistic model. Aust J Exp Agric. (2005) 45:231–40. doi: 10.1071/EA03150
88. Wigley K, Ridgway HJ, Humphries AW, Ballard RA, and Moot DJ. Increased lucerne nodulation in acid soils with Sinorhizobium meliloti and lucerne tolerant to low pH and high aluminium. Crop Pasture Sci. (2018) 69:1031–40. doi: 10.1071/CP18124
89. Koskey G, Mburu SW, Njeru EM, Kimiti JM, Ombori O, and Maingi JM. Potential of native rhizobia in enhancing nitrogen fixation and yields of climbing beans (Phaseolus vulgaris L.) in contrasting environments of Eastern Kenya. Front Plant Sci. (2017) 8:443. doi: 10.3389/fpls.2017.00443
90. Seenivasagan R and Babalola OO. Utilization of microbial consortia as biofertilizers and biopesticides for the production of feasible agricultural product. Biology. (2021) 10:1111. doi: 10.3390/biology10111111
91. Nyaga JW and Njeru EM. Potential of native rhizobia to improve cowpea growth and production in semiarid regions of Kenya. Front Agron. (2020) 2:606293. doi: 10.3389/fagro.2020.606293
92. Tena W, Wolde-Meskel E, and Walley F. Symbiotic efficiency of native and exotic Rhizobium strains nodulating lentil (Lens culinaris Medik.) in soils of Southern Ethiopia. Agronomy. (2016) 6:11. doi: 10.3390/agronomy6010011
93. Clúa J, Roda C, Zanetti ME, and Blanco FA. Compatibility between legumes and rhizobia for the establishment of a successful nitrogen-fixing symbiosis. Genes. (2018) 9:125. doi: 10.3390/genes9030125
94. Lindström K and Mousavi SA. Effectiveness of nitrogen fixation in rhizobia. Microb Biotechnol. (2020) 13:1314–35. doi: 10.1111/1751-7915.13517
95. Thrall PH, Laine AL, Broadhurst LM, Bagnall DJ, and Brockwell J. Symbiotic effectiveness of rhizobial mutualists varies in interactions with native Australian legume genera. PloS One. (2011) 6:e23545. doi: 10.1371/journal.pone.0023545
96. Walker L, Lagunas B, and Gifford ML. Determinants of host range specificity in legume-rhizobia symbiosis. Front Microbiol. (2020) 11:585749. doi: 10.3389/fmicb.2020.585749
97. Segovia L, Pinero D, Palacios R, and Martinez-Romero E. Genetic structure of a soil population of nonsymbiotic Rhizobium leguminosarum. Appl Environ Microbiol. (1991) 57:426–33. doi: 10.1128/aem.57.2.426-433.1991
98. Argaw A and Tesso B. Effectiveness of Rhizobium inoculation on productivity of common bean (Phaseolus vulgaris L.): Investigating the effect of indigenous rhizobia population. Pol J Nat Sci. (2017) 32:593–614.
99. Peoples MB, Brockwell J, Herridge DF, Rochester IJ, Alves BJR, Urquiaga S, et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis. (2009) 48:1–17. doi: 10.1007/BF03179980
Keywords: abundance, indigenous rhizobia, Vicia faba, Phaseolus vulgaris, inoculants, symbiosis
Citation: Geremu T, Abera G, Lemma B and Rasche F (2025) Abundance and symbiotic efficiency of indigenous rhizobia nodulating faba bean and common bean in southern Ethiopia. Front. Soil Sci. 5:1568292. doi: 10.3389/fsoil.2025.1568292
Received: 29 January 2025; Accepted: 13 June 2025;
Published: 11 July 2025.
Edited by:
Amitava Rakshit, Banaras Hindu University, IndiaReviewed by:
Mustapha Mohammed, University for Development Studies, GhanaVishal Tripathi, Graphic Era University, India
Copyright © 2025 Geremu, Abera, Lemma and Rasche. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadele Geremu, YmVrZ2VyZW11QGdtYWlsLmNvbQ==
†Present address: Frank Rasche, International Institute of Tropical Agriculture (IITA), Nairobi, Kenya
 Tadele Geremu
Tadele Geremu Girma Abera3
Girma Abera3