- Faculty of Agriculture and Life Sciences, Lincoln University, Christchurch, New Zealand
The objective of this experiment was to determine if providing ewes in the final trimester of pregnancy with choice from diverse diet components would reduce markers of oxidative and metabolic stress in ewes and improve lamb birth weights relative to ewes offered only one forage species, repetitively. Fifty-four, twin bearing Coopworth ewes [initial live weight (LW) = 69.82 ± 1.16 kg] were blocked by weight onto iso-energetic diets with either choice from taxonomically diverse plants [DIV; spatially separated strips of ryegrass (Lolium perenne L.), chicory (Cichorium intybus L.), plantain (Plantago lanceolata L.), red clover (Trifolium pratense L.), and alfalfa (Medicago sativa L.)] or a ryegrass only diet (RYE) for the final third of gestation. The ewes offered the DIV diet birthed 8.9% heavier lambs (5.64 ± 0.20 kg) than RYE ewes (5.18 ± 0.20 kg; P = 0.03). In addition, the DIV ewes had greater (P < 0.01) glutathione peroxidase and total antioxidant status, and lower (P = 0.01) plasma non-esterified fatty acid concentrations than the RYE fed ewes 24 h after lambing. The results indicate that the DIV diet can improve antioxidant status and reduce some markers of oxidative and metabolic stress at lambing compared to a conventional RYE diet.
Introduction
Pregnant ewes and their fetuses, experience oxidative stress caused by the increased production of reactive oxygen species (ROS) (Myatt and Cui, 2004; Garrel et al., 2010; Caroprese et al., 2019; Bouroutzika et al., 2020). These ROS are involved with normal pregnancy and developmental processes, such as implantation and embryo development, fetal defense against uterine infections, pregnancy maintenance, and lambing (Caroprese et al., 2019). Excessive production of ROS can have negative developmental effects or result in abortion (Bouroutzika et al., 2020), with several metabolic disorders experienced in the periparturient period being linked to oxidative stress (e.g., immune dysregulation, mastitis, and metritis) (Lykkesfeldt and Svendsen, 2007; Sordillo and Aitken, 2009). Animals undergo several hormonal and metabolic changes during the periparturient period, which increase nutritional demands and stress (Goff and Horst, 1997; Sordillo and Mavangira, 2014). Further, nutritional stress (e.g. excessive fermentable carbohydrates) also may induce oxidative stress (Seyidoglu and Aydin, 2020; Beck et al., 2021b). Metabolic, oxidative, and physiological stress are closely related and are thought to act in a mutual reinforcement cycle (Ando and Fujita, 2009; Beck and Gregorini, 2020). Diets that exacerbate nutritional imbalances could be elevating stress and causing greater metabolic and physiological issues as animal's transition from non-lactating to lactation. Further, maternal nutrition in late gestation influences lamb birth weight, which is a predictor of lamb mortality (Roca Fraga et al., 2018), and thereby also affecting the production of the next generation.
Repeated allocation of a single dietary material may induce nutritional imbalances subsequently compromising animal production, health, and welfare (Ralphs et al., 1995; Provenza et al., 2007; Hogan and Phillips, 2008; Gregorini et al., 2017; Beck and Gregorini, 2020, 2021). Such repeated diet allocations are frequently implemented in temperate pastoral systems to fulfill basic nutritional requirements and provide ease of pastoral management. Perennial ryegrass is one such species that is fed repetitively despite its unbalanced nutritional profile at the rumen level, namely an imbalance between nitrogen (N) and energy availability (Taweel, 2004; Edwards et al., 2007; Gregorini et al., 2016). Thereby, the objective of this experiment was to determine if providing dietary diversity to ewes in the final trimester of pregnancy would improve total antioxidant status (TAS) and reduce markers of oxidative stress experienced by the ewe and lamb, which would therefore improve lamb birth weights relative to ewes offered a monotony of ryegrass.
Materials and methods
Ethics statement
The study was conducted at the Johnstone Memorial Laboratory at Lincoln University (43°38′57″S, 172°27′01″E), according to the methods approved by the Lincoln University Animal Ethics Committee (AEC 2019-34A) prior to experiment initiation.
Herbage establishment
The experimental area was comprised of six paddocks, with three sown in ryegrass (Lolium perenne L.) and three planted as separated strips of equal area, of chicory (Cichorium intybus L.), plantain (Plantago lanceolata L.), alfalfa (Medicago sativa L.), red clover (Trifolium pretense L.), or ryegrass: the arrangement within the paddock was randomized (Figure 1). Before planting in October 2019, paddocks were grazed and then prepared for planting by applying glyphosphate (Weedmaster Ts540; Nufarm, Auckland, NZ; 4 L/h), fluroxypyr (Starane Xtra Herbicide, Dow AgroScienes. New Plymouth, NZ; 1 L/ha), Carfentrazone-E (Hammer Force, FMC, Auckland, NZ; 0.1 L/ha), and Polyalkyleneoxide (Slikka, Etec Crop Solutions, Auckland, NZ; 0.15 L/ha). The area was plowed and power harrowed 7 days after spraying. The areas to be planted in red clover, alfalfa, and chicory had Trifluralin (Genfarm Trifluralin 480 Selective Herbicide, Nutrien Ag Solutions, New South Wales, AUS; 2 L/ha) sprayed and incorporated two days prior to planting. Planting occurred on the 26th of October 2019, using a direct drill with 7.6 cm row spacing, 14 days after paddocks were sprayed. The drill was calibrated for each forage species to provide a seeding rate of 25, 12, 14, 16, and 14 kg/ha for ryegrass (cv. Legion), chicory (cv. Choice), red clover (cv. Relish), alfalfa (cv. Titan), and plantain (cv. Agritonic), respectively. Once pastures were established and weeds were at the three-leaf stage, Dicamba (Kamba 500; 0.4 L/ha) was applied onto ryegrass and plantain sown areas, and Flumetsulum (Preside; 60 g/ha) and mineral oil (Uptake; 1 L/ha) were applied to the chicory, clover, and alfalfa. The area was fertilized with 250 kg di ammonium phosphate approximately one and a half months after planting.
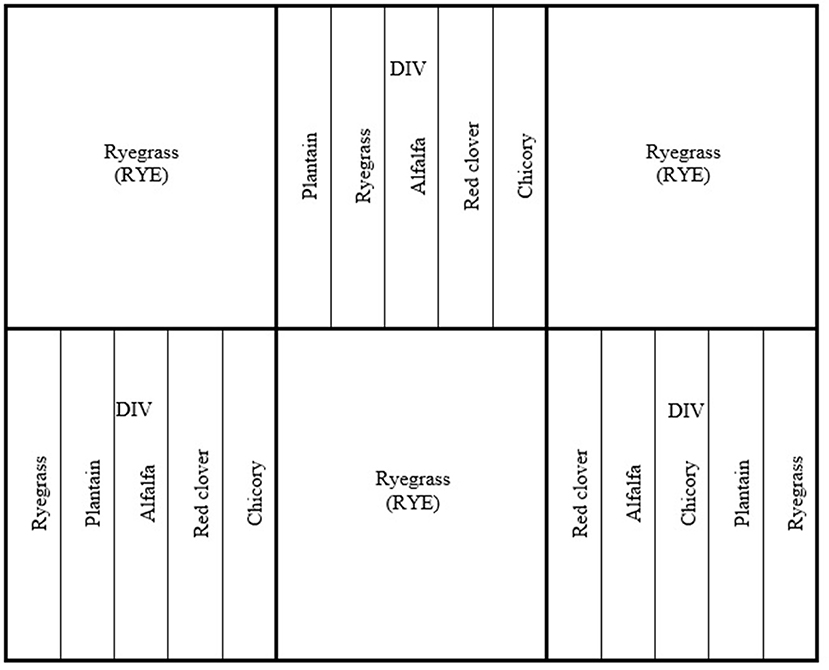
Figure 1. Experimental area and paddock layout grazed by ewes with access to only ryegrass (Lolium perenne L.; RYE) or ewes allocated a diverse diet (DIV) of spatially separated strips of equal area, of chicory [ryegrass, chicory (Cichorium intybus L.), alfalfa (Medicago sativa L.), plantain (Plantago lanceolata L.), and red clover (Trifolium pretense L.)].
Animal management and dietary treatments
Prior to experiment initiation in the first two-thirds of gestation ewes grazed ryegrass pastures. Twin-bearing, Coopworth Ewes (n = 54) in the last third of gestation were stratified by initial live weight (LW; 69.8 ± 1.16 kg; Mean ± SEM). Within stratification, ewes were randomly assigned to treatments: a diverse diet (DIV) or a ryegrass-only diet (RYE) to give equal numbers per treatment (n = 27). The last third of gestation was targeted as ~90% of fetal growth occurs during this time (Redmer et al., 2004; Pillai et al., 2017). The ewes were introduced to pastures on the 16th of July 2020 and had all finished lambing 69 days later on the 23rd of September 2020. The animals offered the DIV diet had free access to spatially separated strips of ryegrass, chicory plantain, red clover, and alfalfa. Animals had free access to water from a trough at all times. Animals strip grazed and were allocated fresh herbage every 7 days. Weekly pre-grazing quadrat measurements were collected by hand-clipping forage within a 0.25-m2 quadrat within three locations per forage species. Pre-grazing forage mass was used to determine DM availability, so that the quantity of DM allocated could be altered weekly to match the changing metabolizable energy (ME) requirements of the ewes, following the equations of Rattray et al. (2007). Pre-grazing herbage snip cuts were taken for chemical composition and nutritive value analysis every two weeks. Between the 16th of July and the 10th of August all ewes were supplemented with 300 g/head of crushed barley grain to supplement herbage and meet animal energy demands. On average, ewes were allocated 1.8 ± 0.12 kg herbage DM/head per day throughout the study assuming a 65% utilization of ryegrass and 90% utilization of the other species. There was no difference between treatments for average DM or ME allocated (P > 0.10).
Herbage sampling and analysis
Snip cut samples were obtained by clipping hand grab samples to ground level at 10 random locations within each forage species in the next area to be grazed. The snip cuts were mixed and sub-sampled into three equal parts, with these parts being randomly selected to determine the botanical and morphological composition, DM content, and herbage chemical composition. The sample used to determine the chemical composition of herbage was stored at −20°C and then freeze-dried and ground to pass through a 1-mm screen by a centrifugal mill (ZM200; Retsch, Haan, Germany). The botanical sample was sorted according to sown species into stem, leaf, reproductive, weeds, and dead material. Quadrat samples were collected by hand-clipping a 0.25-m2 area in three locations per forage species to ground level using electric clippers. Dry matter percentage was calculated for both snip-cut and quadrat samples by weighing the sample fresh, followed by oven-drying at 60°C for 7 days before measuring the sample dry weight.
The nutritive value of herbage samples was determined using near-infrared spectrophotometry (NIRS; Model: FOSS NIRS Systems 5000, Maryland, USA). Nutritive values used for NIRS calibration were derived prior to sample analysis for DM (AOAC, 1990; method 930.15), organic matter (OM; 100%-ash%; AOAC, 1990; method 942.05), neutral detergent fiber (NDF; Van Soest et al., 1991), acid detergent fiber (ADF; AOAC, 1990; method 973.18), water-soluble carbohydrates (WSC; MAFF, 1986), digestible OM in DM (DOMD), DM digestibility (DMD), and OM digestibility (OMD; Iowerth et al., 1975), and crude protein (CP) by combustion (Variomax CN Analyser; Elementar Analysensysteme, Hanau, Germany). The NIRS calibration equations all had R2 values >0.90 and were within the calibration range. Herbage metabolizable energy (ME) was estimated using the Primary Industries Standing Committee (2007) equation:
Animal sampling and measurements
Ewe blood samples were collected prior to treatment allocation, which was after transportation to the farm and the associated feed with-holding period, and then again 24 h after lambing (average lambing date was 10th September 2020 ± 6 days) via jugular venipuncture into a heparinized blood tube (~10 ml, Greiner Bio-One International GmbH, Kremsmünster, Austria). From each blood tube a subsample of whole blood and plasma was collected and stored at −20°C until analysis. Plasma was obtained by centrifuging (Megafuge 1.0R; Heraeus Holding GmbH; Hanau, Germany) the remaining whole blood at 2,300 × g at 4°C for 15 min. Twenty-four hours after birth, lambs were weighed using a bucket and a handheld scale (Rapala RDS50, Helsinki, Finland), after weighing a wool sample (~2 g) was shorn from their necks using portable handheld clippers.
Glutathione peroxidase (GPx) content of the whole blood samples was determined using an enzymatic-based protocol (RANSEL; Cat. No. RS504) and a clinical analyzer (Randox Rx Daytona, Crumlin, Co. Antrim, UK). Plasma total antioxidant status (TAS; Cat. No. NX2332) and non-esterified fatty acid (NEFA; Cat. No. FA115) were analyzed as per the instructions of their respective Randox kit manual using a clinical analyzer (Randox Rx Daytona mlin, Co. Antrim, UK). Wool samples were analyzed as per the methodology described in Nejad et al. (Nejad et al., 2020) using a salimetrics high sensitivity salivary cortisol, enzyme immune assay kit (No. 1-3002, State College, Pennsylvania, USA).
Statistical analysis
Statistical analysis was conducted using R (R Core Team, 2020, v.3.6.0). The ewe weights were normally distributed (P > 0.10; Shapiro-Wilk test) and had homogenous variance (P > 0.10; Bartlett's test), thereby meeting the assumptions of an analysis of variance (ANOVA), and were analyzed using the “aov” function. Other normally distributed data (e.g., TAS and GPx) were analyzed using the “lmer” function and non-normally distributed data (i.e. lamb birth weight and NEFA) were analyzed with the “glmer” function with the distribution used for the model selected based on the Q-Q-plots of the residuals, which was determined to be a Gamma distribution. The Day 1 TAS, GPx, and NEFA values were not different by treatment (P > 0.10; Table 1), and were used as covariates as they explained a significant amount of variation for the values for 24h after lambing. Lamb sex was explored as a factor effecting lamb birth weight, however, there was no interaction with treatment or effect of sex on birth weight (P > 0.05), therefore sex was included in the model as a random effect. The “lmer” and “glmer” models included day lambed as a random factor and dietary treatment as fixed effects (R Core Team, 2020). The wool cortisol values of the lambs were transformed using a natural log and analyzed using generalized liner mixed model ANOVA using the “lme4” package for R. The model was evaluated for goodness of fit, residuals vs. fitted plot, a Q–Q plot of the residuals, and Cooks distance and results indicated the model fit the data well. Pearson's correlation co-efficient between ewe weight and lamb birth weight was determined using the cor.test function of R using ewe weights from 26 days prior to the average lambing day. Least squares means were generated using the “emmeans” package (Lenth, 2019). Forage cover and nutritive quality were analyzed using the “glm” function, with repeated measures for fixed-effects. Statistical significance was declared at P ≤ 0.05 and tendencies are discussed at 0.05 < P ≤ 0.10.

Table 1. Initial measurements from ewes arriving to the trial used as covariates in the analysis of data, values were not different by treatment thereby an average value is reported.
Results
The herbages had different primary nutrient (Table 2) and botanical compositions (Table 3). Chicory and plantain had greater ME than alfalfa (P < 0.05), none of which were different to either ryegrass or red clover (P > 0.05). The DM content of the ryegrass and alfalfa, which were not different (P > 0.05), were greater than that of chicory, plantain, and red clover. Plantain had a lower DM content (P < 0.05) than ryegrass and alfalfa; which, was intermediate and not different (P > 0.05) to either chicory or red clover. The WSC content of alfalfa was lower than that of the chicory (P < 0.05); however, there were no other differences between the WSC content of herbages. The CP content of the alfalfa and red clover (P > 0.05) were greater than that of chicory, plantain, and ryegrass (P < 0.05) and the CP content of chicory was greater than that of plantain and ryegrass (P < 0.05), which were not different to one another (P > 0.05). The NDF content was greatest in ryegrass, then alfalfa, then plantain and red clover (P > 0.05), and then chicory (P > 0.05). The barley grain offered for the first few weeks had an as-fed DM% of 85%, ME of 15.56 MJ/ kg DM, and a CP (%DM) of 10.41. Ryegrass, chicory, red clover, and alfalfa were all in a vegetative state with leafy herbage comprising 76.1, 92.6, 89.3, and 64.8% of total DM. Plantain was in an early reproductive state, with 9.4% of plantain DM being comprised of reproductive stem.
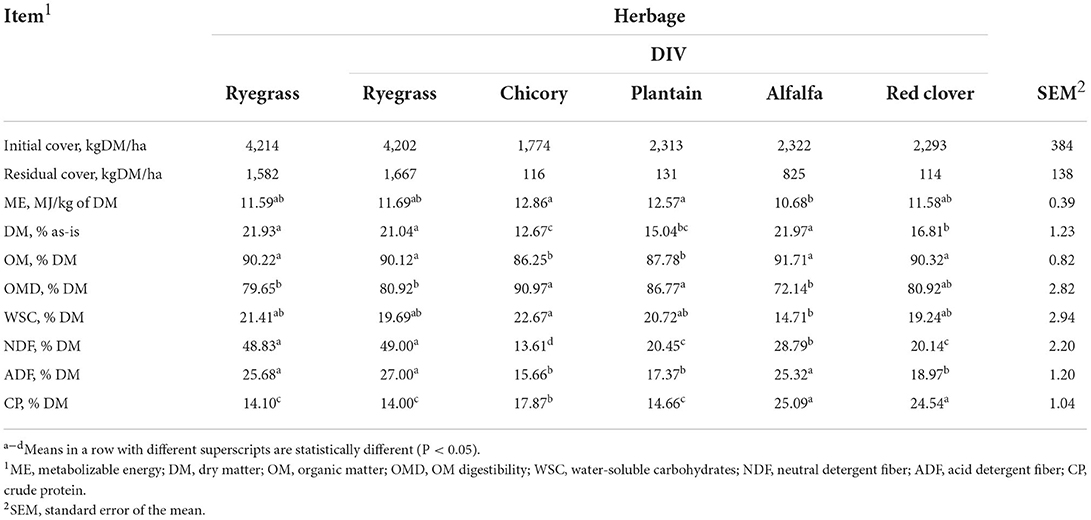
Table 2. Chemical composition of the herbage composing the single forage perennial ryegrass diet (Lolium perenne L.; Rye) or a taxonomically diverse multi-forage choice (DIV) diet of ryegrass, chicory (Cichorium intybus L.), alfalfa (Medicago sativa L.), plantain (Plantago lanceolata L.), and red clover (Trifolium pretense L.).
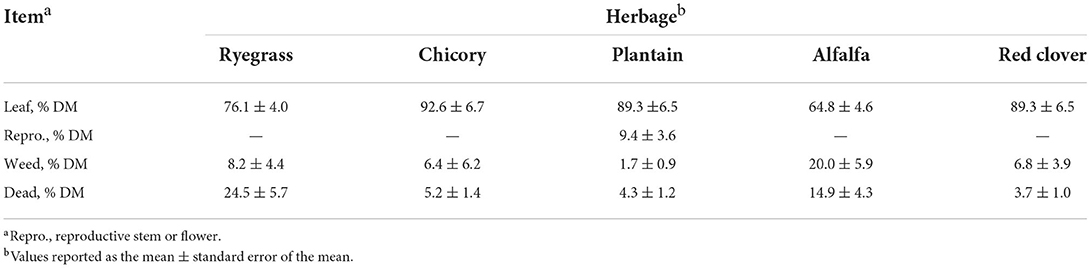
Table 3. Botanical composition of the herbage composing the single forage perennial ryegrass diet (Lolium perenne L.; RYE) or a taxonomically diverse multi-forage choice (DIV) diet of ryegrass, chicory (Cichorium intybus L.), alfalfa (Medicago sativa L.), plantain (Plantago lanceolata L.), and red clover (Trifolium pretense L.). Values for ryegrass botanical information were not different by treatment so herbage averages are presented (P > 0.10).
Twenty-six ± 6 days prior to the average lambing date, the DIV ewes (78.71 ± 1.63 kg) did not differ in weight from the RYE ewes (75.76 ± 1.60 kg; P = 0.20). There was no difference in lambing date between treatments (P = 0.95). The birth weight of lambs from the DIV ewes (5.64 ± 0.20 kg) was 9% greater than lambs born to RYE fed ewes (5.18 ± 0.20 kg; P = 0.03; Table 4). Twenty-four hours after lambing, the DIV ewes GPx concentration (16.17 ± 0.50 U/ml) was 35% greater (P < 0.01) than that of the RYE ewes (11.98 ± 0.50 U/ml). In addition, the TAS concentration of the DIV treatment (1.38 ± 0.02 mmol/L) was 8% greater than that of the RYE ewes (1.28 ± 0.02 mmol/L; P < 0.01). Further, the NEFA concentrations of the RYE ewes (0.68 ± 0.16 mmol/L) was 74% greater than DIV ewes (0.38 ± 0.07 mmol/L; P = 0.01). Finally, the lambs born to the DIV ewes tended to have lower concentrations of cortisol within their wool than the RYE lambs (P = 0.08).
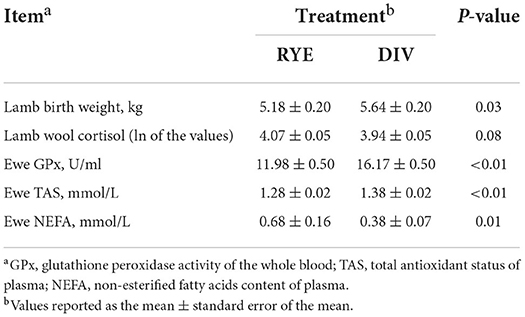
Table 4. Animal measurements for sheep allocated either a single forage perennial ryegrass diet (Lolium perenne L.; RYE) or a taxonomically diverse multi-forage choice (DIV) diet of ryegrass, chicory (Cichorium intybus L.), alfalfa (Medicago sativa L.), plantain (Plantago lanceolata L.), and red clover (Trifolium pretense L.) 24h after lambing.
Discussion
We hypothesized that providing dietary diversity (i.e., DIV) would improve the TAS and reduce some potential markers of oxidative stress experienced by ewes at parturition and improve the birth weight of lambs compared with ewes grazing ryegrass (i.e., RYE). The results provide some support for this hypothesis. The kg DM/ewe per day and ME MJ/ewe per day of the diets offered were not different, thereby allowed for comparison to test this hypothesis. However, differences in CP content of dietary components available may have contributed to these results, although the innate chemistry of the diets is a product of their species composition, as well as their quality. Further, the day 1 TAS, GPx, and NEFA values and trends used as covariates are in line with existing literature (Ataollahi et al., 2020; Beck et al., 2021a).
The DIV ewes had improved antioxidant status and some evidence of potentially reduced oxidative and metabolic stress, or greater capacity to cope with lambing stress as indicated by the greater GPx, TAS, and lower NEFA 24 h after lambing. Plasma TAS describes the total balance between oxidizing species and antioxidants and therefore may be more representative of the antioxidant-to-oxidant balance than a single antioxidant (Ghiselli et al., 2000). Elevated TAS is indicative of reduced oxidative stress or increased capacity to cope with oxidative stress.
The DIV ewes had much lower NEFA concentrations than the RYE ewes – indicating the RYE ewes were mobilizing more of their fat stores. The mobilization of fat stores indicated by elevated NEFA has been associated with increased oxidative stress (Sordillo and Aitken, 2009; Sordillo and Mavangira, 2014; Li et al., 2016). While plasma GPx can be interpreted as a marker of oxidative stress, it is useful to consider alongside other markers, as elevated GPx levels can also be indicative of the presence of a stressor (Bernabucci et al., 2002; Beck et al., 2021b) or improved antioxidant status due to greater dietary supply of precursor materials (e.g. selenium) (Gerloff, 1992). Within the present study the greater TAS and lower NEFA concentration support the latter. Deeper rooting plants (e.g. alfalfa, white clover (Trifolium repens), dock (Rumex obtusifolius) can have greater selenium contents (>3 times) than grasses (Grant and Sheppard, 1983; Harrington et al., 2006). Although selenium content of herbages was not measured in this study, the literature values for selenium content of the diet components suggest a greater supply of this precursor mineral to the DIV animals, resulting in elevated GPx levels. Another theory for the greater antioxidant status could be the increased supply of protein to the diverse diets compared with the ryegrass diet. Metabolizable protein is digested post-ruminally and absorbed by the intestine to form amino acids and peptides (Schwab and Broderick, 2017; Osorio, 2018). An increased supply of amino acids such as methionine can increase antioxidant status (Coleman et al., 2020). Further, low energy and protein supply has been shown to result in reduced GPx and antioxidant capacity in sheep (Tsiplakou et al., 2017). However, there are also a number of studies that suggest elevated protein ingestion can increase oxidative stress (Tsiplakou et al., 2017; Zebrowska et al., 2019). The study by Tsiplakou et al. (2017) also found that overfeeding of energy and protein requirements saw a decline in the GPx activity and antioxidant capacity in sheep. Further, both diets in the present study likely meet the protein requirements of the twin-bearing ewes which is in the range of 14%−18% (Stevens, 1999), indicating that the lower supply of protein was likely not the cause of the reduced antioxidant status for the ryegrass ewes. Further, investigation into the causative mechanism of the increased antioxidant capacity of the DIV diet is required. In addition, further research examining normal levels of antioxidant capacity and oxidative stress for ewes at lambing is required before such conclusions can be drawn regarding the DIV diet and potential reductions in oxidative stress.
Increased antioxidant defense in dams may be transmitted in utero or in early life to offspring (Nieto et al., 2010a,b; Beck et al., 2021a). Such observations may be supported by the tendency for the lamb wool cortisol of the RYE lambs to be greater compared with the DIV lambs. Wool cortisol represents the cumulative cortisol over the time of wool growth, thereby the wool of the lambs would have been indicative of their in utero dietary experience as wool follicle development has approximately begun by day 70 of gestation (Lv et al., 2020) at which time the ewe dietary treatment was applied shortly after. Although information is available on the variation of ewe wool cortisol in the pre and post-partum period (Sawyer et al., 2021), to our knowledge there is no information on the normal levels of wool cortisol of lambs in utero during this time making it difficult to ascertain whether a tendency here is biologically meaningful. Thereby further research is still required to understand in utero wool cortisol and its links to stressors and dietary effects.
Ewes offered DIV had heavier lambs at birth than ewes fed ryegrass. This differs with the results of Kenyon et al. (2010) and Hutton et al. (2011), who reported no difference in lamb birth weight from ewes fed either ryegrass or offered a herb and legume mixed sward (chicory, plantain, red and white clover). This is despite similarities in pregnant ewe weight between Kenyon et al. (2010), Hutton et al. (2011) and the present study; there were no differences in ewe weight by treatment at day 132 of pregnancy by Kenyon et al. (2010), day 140 by Hutton et al. (2011), and day 126 of pregnancy (26 ± 6 days prior to lambing) of our study. Although, within the present study a trend appeared to be developing (P = 0.20), however, due to handling stressors weighing events were minimized in the final period prior to lambing. Ewe weight and lamb birth weight were not correlated (P = 0.14) and the percentage of lamb birthweight as a percentage of ewe weight was not different (P = 0.23), which was also been reported by Fogarty et al. (1992), who applied treatments of low and high nutrition to ewes during the mid-pregnancy period. The average lamb for both treatments were of normal to optimum weight range for lamb survival (Everett-Hincks and Dodds, 2008).
A potential cause of this difference in lamb weight may be related to RYE-fed ewes having a lower feed conversion efficiency due to lower digestibility. This lower digestibility was likely the result of the high dead material content of the ryegrass diet components, which often occurs when covers are >3,500 kg DM. To account for this within the present study, targeted refusals were high (35%) and a secondary group of animals was brought through after grazing to graze lower and improve future pasture quality. Another potential cause may have been the differences in dietary CP between treatments, as discussed in the paragraph outlining differences in ewe TAS, NEFA, and GPx. Although the CP content in the current study was within an acceptable CP range for twin-bearing ewes [14−18% (Stevens, 1999)], it was at the lower end and is potentially a result of the lowered pasture quality through the elevated dead material content. Furthering this point, both under- and over-nutrition can cause intrauterine growth restriction (Robinson, 1977; Russel et al., 1981), making it difficult to ascertain the cause of the RYE lambs lower birth weight, considering the ewes were allocated enough feed to meet their estimated nutritional needs. Under-nutrition through incidental restriction (cessation of eating as a nutrient or plant secondary compound reaches toxic levels, while other nutritional needs remain unfulfilled) or over-nutrition through incidental augmentation (consumption continues to meet other nutritional needs, despite one nutrient having been consumed at excessive levels) may have occurred (Raubenheimer, 1992; Bailey and Provenza, 2008). Although, only primary chemistry was measured in the present study. Offering animals a range of taxonomically and implicitly phytochemically, diverse feeds allows the animal to choose plant combinations that meet their nutritional and medicinal needs, while potentially negating nutrients that are causing malaise or toxicity (Villalba et al., 2010). Perhaps, the ability of ewes to better meet their individual nutritional and nutraceutical needs on the DIV diet contributed to their greater lamb size and improved antioxidant status post lambing.
Conclusions
The current study indicates that a diverse diet fed in the final third of gestation can improve the antioxidant status and alter markers of oxidative stress of ewes at lambing. Future studies are required to test a higher quality ryegrass and to determine other indicators of oxidative stress or the incidence of transitional diseases and antioxidant defenses of ewes and lambs on each of the diets.
Data availability statement
The data supporting the findings of this study are available from the corresponding author KG, upon reasonable request.
Ethics statement
The animal study was reviewed and approved by Lincoln University Animal Ethics Committee.
Author contributions
Conceptualization: KG and PG. Methodology: KG, MB, CM, and PG. Software, investigation, resources, data curation, writing-original draft preparation, project administration, and funding acquisition: KG. Formal analysis: KG, CM, and MB. Writing-review and editing: KG, MB, CM, CL, TM, and PG. Visualization: KG and MB. Supervision: KG, CL, TM, and PG. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Agricom (Christchurch, New Zealand), Glenn Judson, and the other team members for providing the seed used for this experiment. We would also like to thank the Vernon Willey Trust Fellowship and the William Machin Doctoral Scholarship for Excellence for funding KG.s Ph.D. studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ando, K., and Fujita, T. (2009). Metabolic syndrome and oxidative stress. Free Radic. Biol. Med. 47, 213–218. doi: 10.1016/j.freeradbiomed.2009.04.030
AOAC (1990). Official Methods of Analysis: The Analysis of Agricultural Materials. Assoc. Off. Anal. Chem.
Ataollahi, G., Friend, M., McGrath, S., Dutton, G., Peters, A., Bhanugopan, M., et al. (2020). Maternal supplementation of twin bearing ewes with calcium and magnesium alters immune status and weight gain of their lambs. Vet. Anim. Sc. 9:100097. doi: 10.1016/j.vas.2020.100097
Bailey, D. W., and Provenza, F. D. (2008). “Mechanisms determining large-herbivore distribution,” in: Resource Ecology (Berlin: Springer), 7–28. doi: 10.1007/978-1-4020-6850-8_2
Beck, M. R., Garrett, K., Marshall, C. J., Olejar, K., Bunt, C., Maxwell, T. M. R., et al. (2021a). Lactobacillus fermented plant extracts provided to yearling ewes improves their lambs; antioxidant status at weaning. Anim. Feed Sci. Technol. 281:115103. doi: 10.1016/j.anifeedsci.2021.115103
Beck, M. R., Garrett, K., Olejar, K., Maxwell, T., Bunt, C., Greer, A., et al. (2021b). Negative effects of energy supplementation at peak lactation of sheep can be offset by the addition of Lactobacillus fermented plant extracts. J. Anim. Sci. 99:skab069. doi: 10.1093/jas/skab069
Beck, M. R., and Gregorini, P. (2020). How Dietary diversity enhances hedonic and eudaimonic well-being in grazing ruminants. Front. Vet. Sci. 7:191. doi: 10.3389/fvets.2020.00191
Beck, M. R., and Gregorini, P. (2021). Animal design through functional dietary diversity for future productive landscapes. Front. Sustain. Food Syst. 5, 546581. doi: 10.3389/fsufs.2021.546581
Bernabucci, U., Ronchi, B., Lacetera, N., and Nardone, A. (2002). Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 85, 2173–2179. doi: 10.3168/jds.S0022-0302(02)74296-3
Bouroutzika, E., Kouretas, D., Papadopoulos, S., Veskoukis, A. S., Theodosiadou, E., Makri, S., et al. (2020). Effects of melatonin administration to pregnant ewes under heat-stress conditions, in redox status and reproductive outcome. Antioxidants. 9:266. doi: 10.3390/antiox9030266
Caroprese, M., Ciliberti, M. G., Albenzio, M., Marino, R., Santillo, A., Sevi, A., et al. (2019). Role of antioxidant molecules in milk of sheep. Small Rumin. Res. 180, 79–85. doi: 10.1016/j.smallrumres.2019.07.011
Coleman, D. N., Lopreiato, V., Alharthi, A., and Loor, J. J. (2020). Amino acids and the regulation of oxidative stress and immune function in dairy cattle. J. Anim. Sci. 98, S175–S193. doi: 10.1093/jas/skaa138
Edwards, G. R., Parsons, A. J., Rasmussen, S., and Bryant, R. H. (2007). High sugar ryegrasses for livestock systems in New Zealand. Proc. New Zeal. Grassl. Assoc. 69, 161–171. doi: 10.33584/jnzg.2007.69.2674
Everett-Hincks, J. M., and Dodds, K. G. (2008). Management of maternal-offspring behavior to improve lamb survival in easy care sheep systems. J. Anim. Sci. 86, 259–271. doi: 10.2527/jas.2007-0503
Fogarty, N. M., Hall, D. G., and Holst, P. J. (1992). The effect of nutrition in mid pregnancy and ewe liveweight change on birth weight and management for lamb survival in highly fecund ewes. Aust. J. Exp. Agric. 32, 1–10. doi: 10.1071/EA9920001
Garrel, C., Fowler, P. A., and Al-Gubory, K. H. (2010). Developmental changes in antioxidant enzymatic defences against oxidative stress in sheep placentomes. J. Endocrinol. 205, 107–116. doi: 10.1677/JOE-09-0362
Gerloff, B. J. (1992). Effect of selenium supplementation on dairy cattle. J. Anim. Sci. 70, 3934–3940. doi: 10.2527/1992.70123934x
Ghiselli, A., Serafini, M., Natella, F., and Scaccini, C. (2000). Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic. Biol. Med. 29, 1106–1114. doi: 10.1016/S0891-5849(00)00394-4
Goff, J. P., and Horst, R. L. (1997). Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 80, 1260–1268. doi: 10.3168/jds.S0022-0302(97)76055-7
Grant, A. B., and Sheppard, A. D. (1983). Selenium in New Zealand pastures. N.Z. Vet. J. 31, 131–136. doi: 10.1080/00480169.1983.34997
Gregorini, P., Beukes, P. C., Dalley, D., and Romera, A. J. (2016). Screening for diets that reduce urinary nitrogen excretion and methane emissions while maintaining or increasing production by dairy cows. Sci. Total. Environ. 551–552, 32–41. doi: 10.1016/j.scitotenv.2016.01.203
Gregorini, P., Villalba, J. J., Chilibroste, P., and Provenza, F. D. (2017). Grazing management: setting the table, designing the menu and influencing the diner. Anim. Prod. Sci. 57, 1248–1268. doi: 10.1071/AN16637
Harrington, K. C., Thatcher, A., and Kemp, P. D. (2006). Mineral composition and nutritive value of some common pasture weeds. N. Z. Plant Prot. 59, 261–265. doi: 10.30843/nzpp.2006.59.4414
Hogan, J. P., and Phillips, C. J. C. (2008). Nutrition and the welfare of ruminants. Annu. Rev. Biomed. Sci. 10, 33–50. doi: 10.5016/1806-8774.2008.v10pT33
Hutton, P. G., Kenyon, P. R., Bedi, M. K., Kemp, P. D., Stafford, K. J., West, D. M., et al. (2011). A herb and legume sward mix increased ewe milk production and ewe and lamb live weight gain to weaning compared to a ryegrass dominant sward. Anim. Feed Sci. Technol. 164, 1–7. doi: 10.1016/j.anifeedsci.2010.11.014
Iowerth, D., Jones, H., and Hayward, M. V. (1975). The effect of pepsin pretreatment of herbage on the prediction of dry matter digestibility from solubility in fungal cellulase solutions. J. Sci. Food Agric. 26, 711–718. doi: 10.1002/jsfa.2740260518
Kenyon, P. R., Kemp, P. D., Stafford, K. J., West, D. M., and Morris, S. T. (2010). Can a herb and white clover mix improve the performance of multiple-bearing ewes and their lambs to weaning? Anim. Prod. Sci. 50, 513–521. doi: 10.1071/AN09177
Lenth, R. (2019). Emmeans: Estimated marginal means, aka least squares means. R package version 1.4.1. Available online at: https://cran.r-project.org/package=emmeans (accessed March 1, 2019).
Li, Y., Ding, H. Y., Wang, X. C., Feng, S. B., Li, X. B., Wang, Z., et al. (2016). An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 100, 844–851. doi: 10.1111/jpn.12454
Lv, X., Chen, L., He, S., Liu, C., Han, B., Liu, Z., et al. (2020). Effect of nutritional restriction on the hair follicles development and skin transcriptome of chinese merino sheep. Animals 10, 1–16. doi: 10.3390/ani10061058
Lykkesfeldt, J., and Svendsen, O. (2007). Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 173, 502–511. doi: 10.1016/j.tvjl.2006.06.005
Myatt, L., and Cui, X. (2004). Oxidative stress in the placenta. Histochem. Cell Biol. 122, 369–382. doi: 10.1007/s00418-004-0677-x
Nejad, G. J., Park, K. H., Forghani, F., Lee, H. G., Lee, J. S., and Sung, K. I. (2020). Measuring hair and blood cortisol in sheep and dairy cattle using RIA and ELISA assay: A comparison. Biol. Rhythm Res. 51, 887–97. doi: 10.1080/09291016.2019.1611335
Nieto, G., Díaz, P., Bañón, S., and Garrido, M. D. (2010a). Dietary administration of ewe diets with a distillate from rosemary leaves (Rosmarinus officinalis L.): influence on lamb meat quality. Meat Sci. 84, 23–29. doi: 10.1016/j.meatsci.2009.08.001
Nieto, G., Díaz, P., Bañón, S., and Garrido, M. D. (2010b). Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes' diet. Meat Sci. 85, 82–88. doi: 10.1016/j.meatsci.2009.12.009
Osorio, J. S. (2018). “Amino acid balancing and its role on metabolism, inflammation, and oxidative stress: future molecular implications,” in: Florida Ruminant Nutrition Symposium. University of Florida, Gainesvelle, Florida, 19–37.
Pillai, S. M., Jones, A. K., Hoffman, M. L., McFadden, K. K., Reed, S. A., Zinn, S. A., et al. (2017). Fetal and organ development at gestational days 45, 90. 135 and at birth of lambs exposed to under- or over-nutrition during gestation. Transl. Anim. Sci. 1:16–25. doi: 10.2527/tas2016.0002
Primary Industries Standing Committee (2007). Nutrient requirements of domesticated ruminants. CSIRO PUBLISHING. Available online at: https://ebooks.publish.csiro.au/content/9780643095106/9780643095106 (accessed October 21, 22020).
Provenza, F. D., Villalba, J. J., Haskell, J., MacAdam, J. W., Griggs, T. C., Wiedmeier, R. D., et al. (2007). The value to herbivores of plant physical and chemical diversity in time and space. Crop Sci. 47, 382–398. doi: 10.2135/cropsci2006.02.0083
R Core Team (2020). R: A Language and Environment for Statistical Computing. Available online at: https://www.r-project.org/ (accessed October, 2020).
Ralphs, M. H., Provenza, F. D., Wiedmeier, R. D., and Bunderson, F. B. (1995). Effects of energy source and food flavor on conditioned preferences in sheep. J. Anim. Sci. 73, 1651–1657. doi: 10.2527/1995.7361651x
Rattray, P. V., Brooks, I. M., and Nicol, A. M. (2007). Pasture and supplements for grazing animals,” in: New Zealand Society of Animal Production. (New Zealand Society of Animal Society).
Raubenheimer, D. (1992). Tannic acid, protein, and digestible carbohydrate : dietary imbalance and nutritional compensation in locusts. raubenheimer. Ecology. 73, 1012–1027. doi: 10.2307/1940176
Redmer, D. A., Wallace, J. M., and Reynolds, L. P. (2004). Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest. Anim. Endocrinol. 27, 199–217. doi: 10.1016/j.domaniend.2004.06.006
Robinson, J. J. (1977). The influence of maternal nutrition on ovine foetal growth. Proc. Nutr. Soc. 36, 9–16. doi: 10.1079/PNS19770003
Roca Fraga, F. J., Lagisz, M., Nakaagawa, S., Lopez-Villalobos, N., Blair, H. T., Kenyon, P. R., et al. (2018). J. Anim. Sci. 96, 1962–1977. doi: 10.1093/jas/sky072
Russel, A. J. F., Foot, J. Z., White, I. R., and Davies, G. J. (1981). The effect of weight at mating and of nutrition during mid-pregnancy on the birth weight of lambs from primiparous ewes. J. Agric. Sci. 97, 723–729. doi: 10.1017/S0021859600037096
Sawyer, G., Fox, D. R., and Narayan, E. E. (2021). Pre- and post-partum variation in wool cortisol and wool micron in Australian Merino ewe sheep (Ovis aries). PeerJ. 9, e11288. doi: 10.7717/peerj.11288
Schwab, G., and Broderick, G. A. (2017). A 100-year review: protein and amino acid nutrition in dairy cows. J. Dairy. Sci. 100, 10094–10112. doi: 10.3168/jds.2017-13320
Seyidoglu, N., and Aydin, C. (2020). “Stress, natural antioxidants and future perspectives,” in: The health benefits of food - current knowledge and further development, Vol. 32. ed L. C. Salanṭǎ (IntechOpen), 137–144, 149–165. doi: 10.5772/intechopen.91167
Sordillo, L. M., and Aitken, S. L. (2009). Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 128, 104–109. doi: 10.1016/j.vetimm.2008.10.305
Sordillo, L. M., and Mavangira, V. (2014). The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 54, 1204–1214. doi: 10.1071/AN14503
Stevens, D. R. (1999). “Ewe nutrition: decisions to be made with scanning information,” in: proceedings of the New Zealand Society of Animal Production. Vol. 59, 93−94.
Taweel, H. Z. (2004). Perennial Ryegrass for Dairy Cows: Grazing Behaviour, Intake, Rumen Function and Performance. Netherlands: Wageningen University.
Tsiplakou, E., Mitsiopoulou, M., Mavrommatis, A., Karaiskou, C., Chronopoulou, E. G., Mavridis, G., et al. (2017). Effect of under- and overfeeding on sheep and goat milk plasma enzymes activities related to oxidation. J. Anim. Physiol. Anim. Nutr. 102, 288–298. doi: 10.1111/jpn.12741
Van Soest, P. J, Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
Villalba, J. J., Provenza, F. D., and Manteca, X. (2010). Links between ruminants food preference and their welfare. Animal. 4, 1240–1247. doi: 10.1017/S1751731110000467
Keywords: functional dietary diversity, welfare, lambing, diet repetition, total antioxidant status
Citation: Garrett K, Marshall CJ, Beck MR, Maxwell TMR, Logan CM and Gregorini P (2022) A diverse diet as an alternative to ryegrass can improve the total antioxidant status of dams at lambing. Front. Sustain. Food Syst. 6:885436. doi: 10.3389/fsufs.2022.885436
Received: 28 February 2022; Accepted: 29 July 2022;
Published: 08 September 2022.
Edited by:
Gary S. Kleppel, University at Albany, United StatesCopyright © 2022 Garrett, Marshall, Beck, Maxwell, Logan and Gregorini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: K. Garrett, konagh.garrett@outlook.com
†Present Address: M. R. Beck, USDA-ARS, Conservation and Production Research Laboratory, Bushland, TX, United States
 K. Garrett
K. Garrett C. J. Marshall
C. J. Marshall M. R. Beck
M. R. Beck T. M. R. Maxwell
T. M. R. Maxwell C. M. Logan
C. M. Logan P. Gregorini
P. Gregorini